
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mater., 28 June 2024
Sec. Polymeric and Composite Materials
Volume 11 - 2024 | https://doi.org/10.3389/fmats.2024.1337972
 Baodi Han1*
Baodi Han1* Lian Wang2
Lian Wang2Objective: The objective of this study is to explore the current research status, key areas, and future development trends in the field of resin materials for dental caries repair through an objective and quantitative analysis of the literature.
Methods: A search was conducted on the Web of Science Core Collection using “dental cavity” and “resin” as keywords, covering the period from 2000 to 2023. Data including author names, journals, countries, institutions, keywords, and citation rates were extracted. The collected data was subjected to statistical analysis using bibliometrics methodology, and visual knowledge maps were generated using software like CiteSpace 6.2.R4, Microsoft365, and R.
Results: A total of 4800 articles were retrieved, involving 13,423 authors, 2654 institutions, 76 countries, and 560 journals. The number of publications and cumulative publications in this field showed an increasing trend, reaching a peak in 2022. Dental Materials was the journal with the highest number of publications, cumulative publications, and citation rates. XU HHK was the most prolific author in terms of publications and citations. The University of Maryland was the institution with the highest number of publications. Brazil was the country with the highest number of publications. The USA had the highest level of collaboration with other countries. Collaboration between different authors, institutions, and countries in this field was relatively close, which contributed to the rapid development of resin materials for caries repair. The current research focus is mainly on the nature of dental caries, characteristics of resin materials, and bonding strength of adhesives. Enhancing the bioactivity and remineralization of resin materials, advanced antibacterial strategies, longevity and durability of resin restorations, nanotechnology, and material innovation, as well as digital dentistry, will receive increased attention as future research trends.
Conclusion: Resin materials for dental caries repair have received significant attention. Future research should combine nanotechnology and big data analysis to investigate the mechanisms of dental caries occurrence and development, enhance the performance and longevity of resin materials, and conduct high-quality, large-scale empirical research.
Dental caries is a chronic dental disease characterized by the interaction between bacteria in the oral environment and fermentable carbohydrates, leading to demineralization and damage of tooth structure. The World Health Organization considers dental caries as one of the three major diseases, along with cancer and cardiovascular diseases, that require focused prevention and treatment efforts (Peres et al., 2019). Dental caries has a high prevalence worldwide. According to the Global Burden of Disease Study in 2016, permanent tooth caries ranked first in terms of prevalence and second in terms of incidence among 328 diseases, with an estimated 2.44 billion people affected globally (Vos et al., 2017). In addition, the treatment rate for dental caries is low. 9% of children have untreated dental caries in their primary teeth, while the population with untreated dental caries in permanent teeth accounts for 35% of the global population (Kassebaum et al., 2015). The failure rate of dental caries treatment is also high. In the United States, the failure rates of dental restorations at 5 and 10 years are 24% and 41% respectively, resulting in an annual expenditure of over 5 billion dollars for replacing these restorations (Jokstad et al., 2001). Dental caries is considered a significant global public health issue, affecting individuals of all ages and socioeconomic backgrounds.
Currently, resin materials have become the preferred choice for dental caries repair. Compared to traditional materials like amalgam, resin materials offer several advantages, including improved aesthetics, minimal tooth structure removal, strong adhesion to tooth structure, and good biocompatibility (Cho et al., 2022). Additionally, resin materials can be used for both anterior and posterior tooth restorations, providing multiple options for addressing caries in different sizes and locations. However, resin materials may be more prone to wear and degradation, with risks of polymerization shrinkage and microleakage (Albeshir et al., 2022). Furthermore, although resin materials have good initial aesthetics, they may be more susceptible to staining or discoloration, especially in individuals who frequently consume coffee, tea, or tobacco (Amin et al., 2022). Current research is focused on overcoming the shortcomings of resin materials in dental caries repair through various innovative approaches, including the use of nanofillers for reinforcement, bioactive materials, novel bonding techniques, biocompatible monomers, smart materials, surface treatments and coatings, as well as self-healing and regenerative materials (Elfakhri et al., 2022).
In fact, the two primary strategies for preventing and treating dental caries involve inhibiting biofilm acid production and promoting remineralization. In recent years, there has been a development of dental resins with antibacterial activity by incorporating various antibacterial agents such as silver (Ai et al., 2017), chlorhexidine (Yang et al., 2021), fluoride (Zheng et al., 2021), quaternary ammonium methacrylates (Yao et al., 2022), and antimicrobial drugs (Kudou et al., 2000). Additionally, imparting remineralization capability to resins represents another crucial approach to reducing the incidence of secondary caries (Clarin et al., 2021). An ideal dental restoration material would possess physicochemical and physiomimetic properties similar to natural teeth (Liu et al., 2015). Hydroxyapatite has emerged as a promising biomaterial due to its biocompatibility and bioactivity. Amorphous calcium phosphate composites can release calcium and phosphate ions, facilitating lesion remineralization (Langhorst et al., 2009). Given their similarity to minerals in dentin and enamel, the application of hyaluronic acid in the preparation of biomimetic dental restorative materials holds significant importance in the field of dental restoration (Wang et al., 2020; Akhtar et al., 2021). Furthermore, studies have demonstrated that integrating three materials into composite materials, including two antibacterial agents (nanoparticles of silver, dimethylaminohexadecyl methacrylate) and a remineralizing agent (nanoparticles of amorphous calcium phosphate), results in superior caries-preventive effects (Melo et al., 2016).
In conclusion, there is a revolutionary change happening in the repair of dental caries using resin materials, and the number of published research studies has significantly increased. However, the current distribution of literature in terms of quantity, publication time, journals, authors, institutions, and countries, as well as the collaboration relationships, is not yet clear. The research status, hot topics, and trends in this field are also not well understood. So far, a considerable number of literature reviews and expert opinions have summarized and reviewed the research on resin materials for dental caries repair from different perspectives (Zhou et al., 2019; Guo et al., 2022; Zhang et al., 2023). However, these publications have only conducted literature reviews and qualitative studies from a single perspective, with limited analysis of the literature and a narrow scope, lacking comprehensiveness and objectivity. Bibliometrics is an emerging discipline that summarizes the characteristics of literature through qualitative and quantitative methods (Shang et al., 2023). It has been widely applied in areas such as biodegradable metallic materials (Yuan et al., 2024), nanomaterials (Ling et al., 2023; Chen and Wu, 2024; Liang et al., 2024), hydrogels (Ma et al., 2024), and tissue engineering (Liu et al., 2023; Zhu et al., 2024). These studies primarily focus on neurodegenerative diseases, osteoarthritis, tissue repair and wound healing, and cancer. However, the application of bibliometric analysis in the field of dentistry is less common, particularly in the context of resin materials for dental caries repair, where there is a notable scarcity of bibliometric analysis. Within the scope of this study, we used bibliometrics for the first time to explore the research status, hot topics, and development trends of resin materials for dental caries repair, with the aim of providing references for future in-depth research in this field.
All data in this study were obtained from the online database Science Citation Index Extension (SCI-E) within the Web of Science Core Collection (WoSCC). In order to explore the latest trends in resin materials for dental caries repair, this study searched for English-language articles and reviews published from 2000 to 2023. The search query was constructed as follows: ((((TS=(tooth demineralization OR tooth remineralization OR dental caries OR dental decay OR secondary caries OR caries dental OR dental cavity OR dental cavities OR carious lesions OR carious lesion OR carious dentin OR carious dentins)) AND TS=(nano resin OR composite resin OR light curing resin OR resin)) AND DT=(Article OR Review)) AND DOP=(2000-01-01/2023-10-02)) AND LA=(English). Two members independently conducted the search and cross-checked the results. In case of any discrepancies, expert opinions were consulted to resolve them.
The software used for data analysis in this study included Microsoft365 (Office), R software, and CiteSpace 6.2.R4 (Advanced). Microsoft365 (Office) was used for trend analysis, data organization, and the creation of relevant tables. The Bibliometrix package in R language was utilized for data statistical analysis of publication output by country, institution, and journal. CiteSpace was employed for cooperative network analysis among countries, institutions, and authors, as well as for co-occurrence and clustering analysis of keywords (Ping et al., 2017; Hou et al., 2018).
The complete records and referenced bibliographies from the literature search were exported in plain text format and stored in the input folder, named in the format of download_xxx.txt. Additionally, output, data, and project folders were created. Data from the input folder were converted into a recognizable format using CiteSpace6.2.R4 and transferred to the output folder, then copied to the data folder. The duplication checking feature in CiteSpace did not identify any repeated records. Microsoft365 (Office) was used to create spreadsheets, manage data, and generate trend graphs of the distribution over the years. The visualization analysis in CiteSpace represented different meanings through nodes, links, and colors, providing intuitive insights into the research trends in a particular field from temporal and spatial perspectives. The nodes represented various research objects such as authors, institutions, and countries, with their size typically indicating the number of publications and academic influence (Chen, 2004; Sabe et al., 2022). The links between nodes and their density reflected the level of collaboration, and the thickness of the links directly represented the strength of the connection. The circles on the nodes represented the corresponding citation rings, with thicker rings indicating a higher number of citations within a specific time period, and changes in color represented changes in citation time.
The graphs were generated based on the default settings and recommended parameters of the software, and necessary adjustments were made to the generated graphs. The following parameters were set for the CiteSpace software: 1) The time span was set from January 2000 to November 2023, with a time slice interval of “1 year per slice.” Trimming method: Pathfinder (Pruning sliced networks, Pruning the merged); Connection strength: Cosine; TopN: 50; Cluster View-Static and Show Merged Network; Clustering algorithm: Loglikelihood ratio (LLR). 2) The node types included authors, institutions, countries, keywords, journals, and references. 3) The selection criterion for “Top N%” in each time slice was to choose the most frequently cited or occurrence of the top 10% of publications.
From 2000 to present, a total of 4,800 articles and reviews on resin materials for dental caries repair have been published. The lowest number of publications was recorded in 2000, with 69 articles. The publication output reached its peak in 2022, with 415 articles. As of 2023, there have already been 285 related studies published (Figure 1A). In the past 5 years, the articles published accounted for 35% of the total publication output, indicating continuous development in this field and increasing attention it has been receiving.
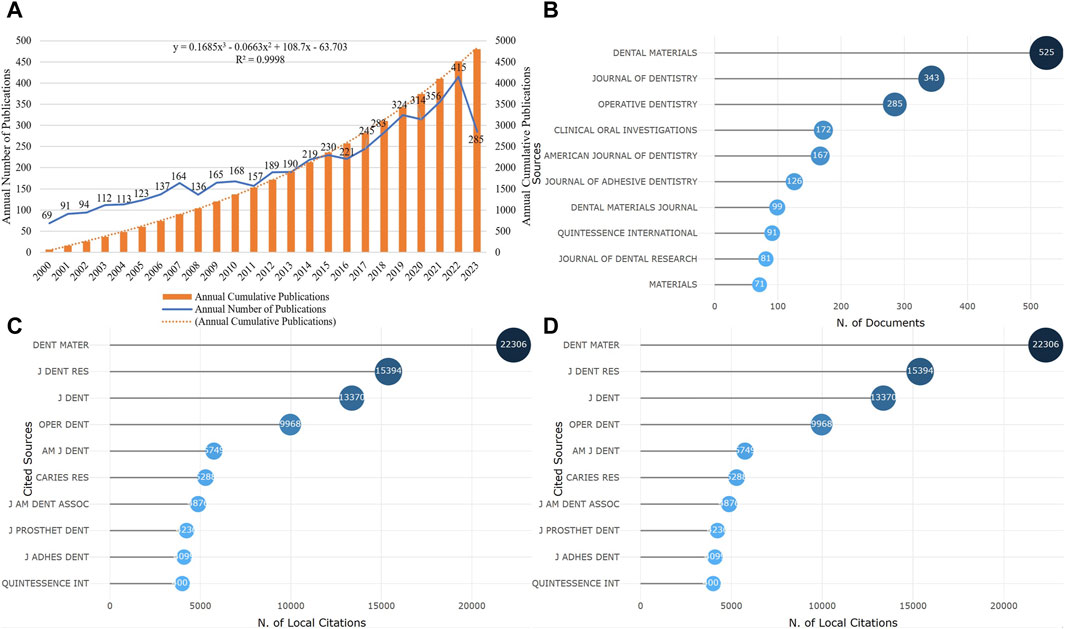
Figure 1. Publication time and core journals (A). The change of annual and cumulative number of publications (B). The top 10 journals in volume of publication (C). The top 10 journals with the fastest growth in cumulative publication (D). The top 10 journals cited).
A total of 4,800 publications were published in 560 different journals. Figure 1B shows the top 10 journals by publication output, which accounted for 40.83% of the total publication output. Figure 1C displays the top 10 journals with the fastest growth in cumulative publication output, with Dental Materials having the highest publication output of 525 articles and being the journal with the fastest growth. Figure 1D illustrates the top journals with the highest citation count, with Dental Materials being the most cited journal with a total of 22,306 citations.
A total of 13,423 authors have participated in the publication of the literature, with their publication frequencies ranging from 1 to 139 articles. Among them, authors who have published only 1 article account for 72.60% (9,745/13,423). The top 10 authors by publication output are shown in Figure 2A, with XU HHK ranking first with 139 articles, followed by WEIR MD (126 articles) and TAGAMI J (94 articles). The top 10 authors by citation count are presented in Figure 2B, with XU HHK leading with 2,649 citations, followed by WEIR MD (2,231 citations) and VAN MEERBEEK B (1,151 citations). Through co-authorship analysis, it was found that there is close collaboration among authors in the current field, with XU HHK, WEIR MD, and others taking the lead (Figure 2C).
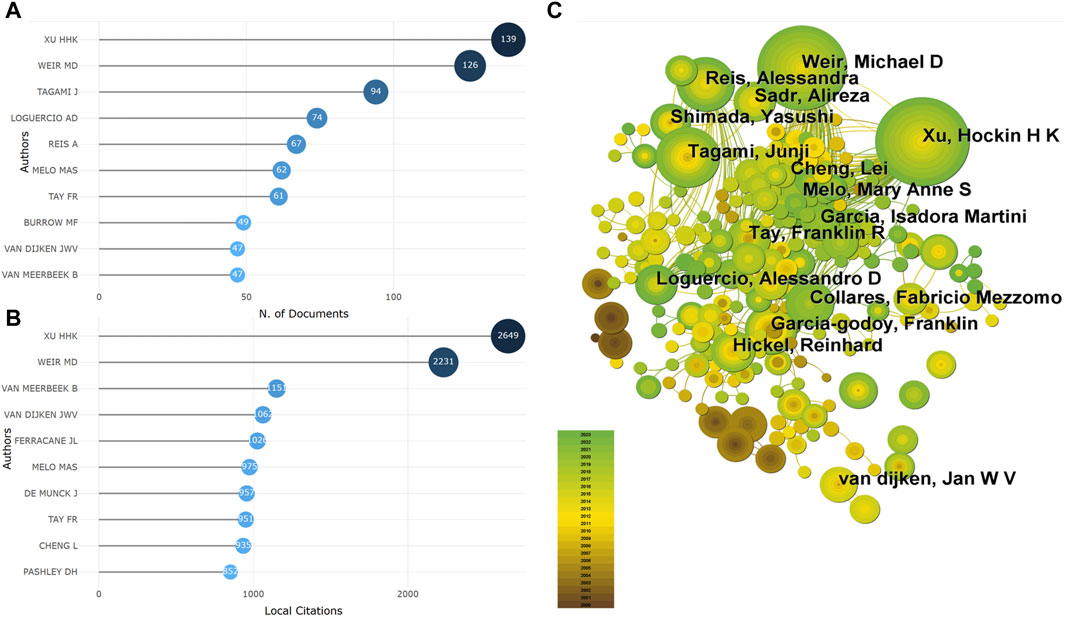
Figure 2. The core author (A). The author of the top 10 publications (B). The author of the top 10 cited amount (C). Co-occurrence analysis of the author).
A total of 2,654 institutions have contributed to the publication of the literature. The top 10 institutions by publication output are shown in Figure 3A, with the University of Maryland having the highest publication output of 447 articles, followed by the University of Sao Paulo (396 articles) and Universidade Estadual de Campinas (188 articles). Co-occurrence analysis of these institutions reveals a high degree of collaboration among different institutions, as depicted in Figure 3B. Notably, the University of Sao Paulo and the University of London are highlighted with a purple outer ring. The purple citation rings around the nodes represent the intermediary centrality, which indicates the number of times a node acts as a bridge between two other nodes in the shortest path. The thickness of the purple ring border indicates the strength of intermediary centrality, with thicker borders indicating higher intermediary centrality. This implies that these two institutions are the most active in the current research field. A total of 76 countries have participated in the publication of the literature, with BRAZIL (829 articles), USA (632 articles), and CHINA (354 articles) ranking as the top three countries by publication output. However, the strongest collaboration intensity is observed between the USA and other countries, followed by BRAZIL and CHINA, as shown in Figure 3C.
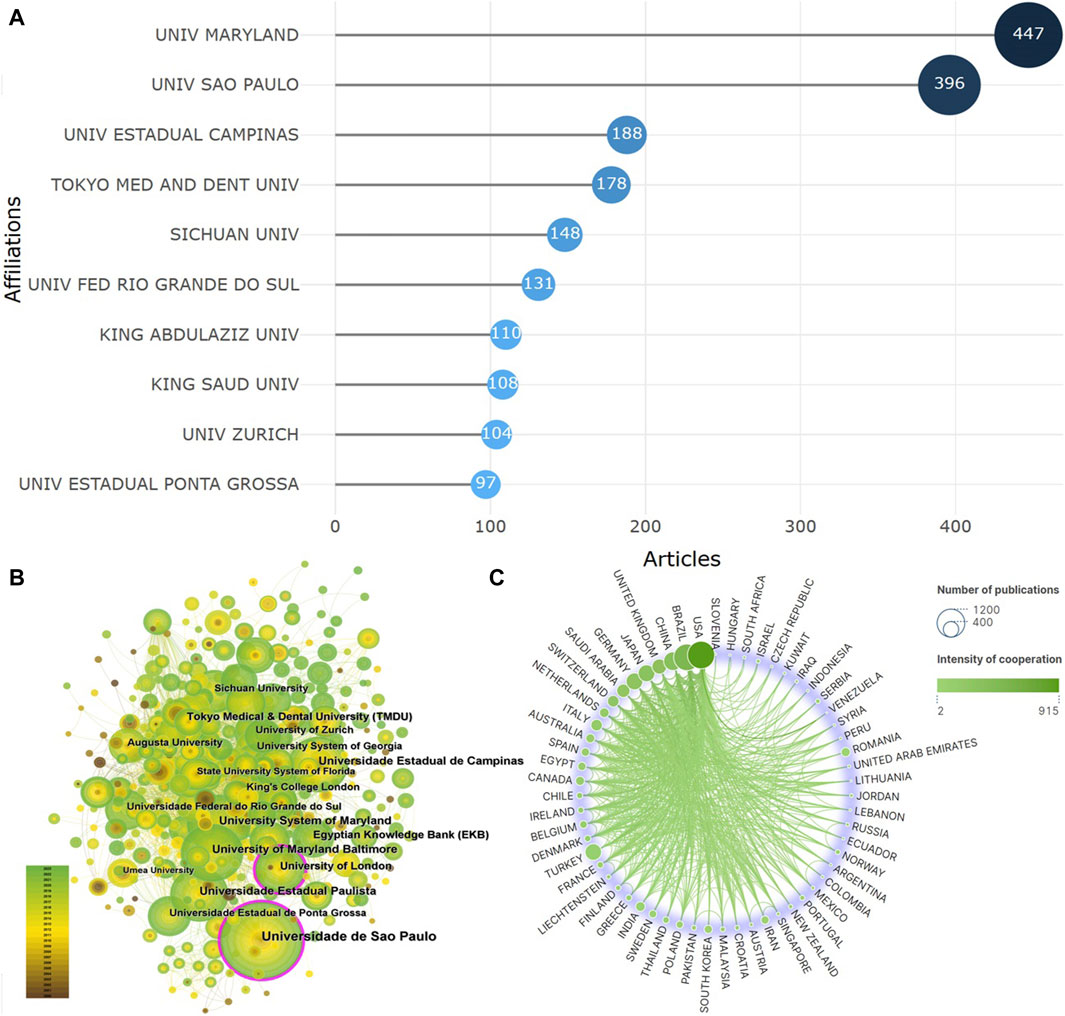
Figure 3. Core institutions and countries (A). Top 10 institutions (B). Co-occurrence analysis of institutions (C). Analysis of country cooperation).
A total of 10,213 keywords were identified, with the top five most frequently occurring keywords being resin, in-vitro, enamel, bond strength, and dentin. Co-occurrence analysis of the keywords revealed strong connections between different keywords. The keywords highlighted with a purple outer ring include surfaces, antibacterial activity, cements, adhesion, acid, dental caries, contraction stress, longevity, light, amalgam restorations, calcium hydroxide, dental caries, clinical trial, and placement. These keywords represent the research focuses in the current field (Figure 4A). After clustering all the keywords, a total of 20 clusters were formed, primarily focusing on the properties of dental caries (clusters 3, 4, 6, 8, 9, 10, 11, 12, 13), characteristics of resin materials (clusters 0, 1, 5, 7, 14, 15, 16, 17, 19, 20), and adhesive strength of adhesives (clusters 2, 18) (Figure 4B).

Figure 4. Co-occurrence and cluster analysis of keywords (A). Co-occurrence analysis (B). Cluster analysis).
Landscape figures can explore the evolution of certain keywords or clusters over time, highlighting important research directions. The results reveal that keywords such as nanomaterials, resin infiltration, dental caries, antibacterial, secondary caries, caries prevention, and photodynamic therapy have been consistently present from 2000 to 2023. These clusters mainly focus on areas such as nanomaterials, antibacterial properties, and novel therapies (Figure 5A). The emergence analysis of keywords shows that white spot lesions, antibacterial, remineralization, fluoride varnish, longevity, dental caries, bioactivity, photodynamic therapy, digital dentistry, and nanotechnology are prominent keywords in recent years. These keywords predominantly concentrate on enhanced bioactivity and remineralization, advanced antibacterial and antimicrobial strategies, longevity and durability of resin restorations, nanotechnology and material innovations, and digital dentistry (Figure 5B). They represent the current research focus in the field.
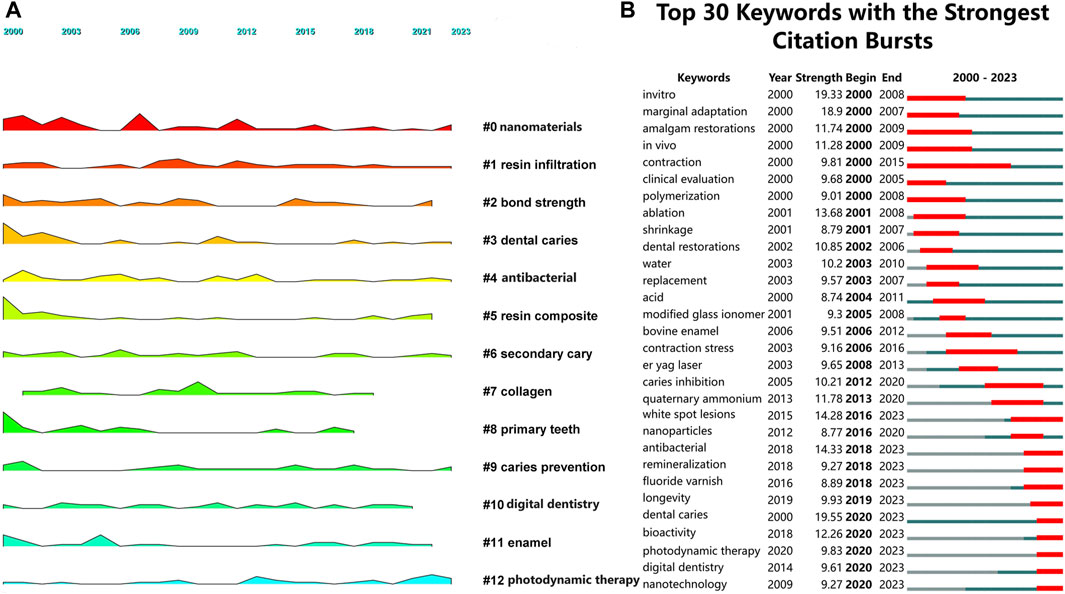
Figure 5. The analysis of the growth and decline and emergence of keywords (A). According to the analysis of growth and decline, the larger the area of shadow is, the more important the keyword is in a certain year (B). Emergent analysis shows that red represents emergence, dark green represents the year in which the keyword appears, and light green represents that the keyword does not appear).
When two or more articles appear simultaneously in the reference list of a third article, a co-citation relationship is established. Highly co-cited literature represents the fundamental theories and key findings in a field. The co-citation relationships among various documents are illustrated in Figure 6A. The study by Drummond (2008), published in 2008, exhibits the highest centrality, indicating its significant influence. This article aims to review the numerous factors affecting the mechanical properties of indirect dental resin composites containing particulate or fibrous fillers, with a focus on the impacts of aging, cyclic loading, and mixed-mode loading on their flexural strength and fracture toughness. Further cluster analysis revealed the formation of 17 clusters, primarily focusing on the nature of caries (clusters 4, 5, 9), characteristics of resin materials (clusters 0, 1, 3, 6, 7, 8, 11–17), and the adhesive strength of bonding agents (clusters 2, 10) (Figure 6B).
From 69 publications in 2000 to 415 publications in 2022, the number of publications on resin materials for dental caries repair has significantly increased each year. This indicates the growth of scientific interest, advancements in the field, and the expansion of the research community. The surge in research articles contributes to the accumulation of knowledge, promotes evidence-based practice, and ultimately benefits oral health outcomes. Among the 560 journals, the top 10 journals in publication output account for 40.83% of the total publications, indicating that the literature in this field is primarily concentrated in a few specialized journals. Among them, Dental Materials has the highest cumulative publication output, growth rate of cumulative publications, and citation count, highlighting its importance and publication of significant research findings. A total of 13,423 authors have participated in the publication of the literature, with authors who have published only 1 article accounting for 72.60%. Although these authors have contributed only one publication, they represent a group of researchers actively involved in this topic and embarking on their research journey in this field. This also indicates the continuous entry of new researchers into the field of resin materials for dental caries repair. Authors XU HHK (Zhao et al., 2010), WEIR MD (Weir et al., 2012), and TAGAMI J (Kanemura et al., 1999) have published the most papers, indicating their significant contributions to resin materials for dental caries repair. These authors may have a deeper understanding of the topic, possess specialized knowledge, or engage in more in-depth research in the field. In particular, XU HHK, as the author with the highest publication output, citation count, and collaboration intensity, represents the current state and forefront of research in the field to a large extent (Xu et al., 1998; Xu et al., 2017). A total of 2,654 institutions from 76 countries have participated in the publication of the literature, with BRAZIL, United States, and CHINA having the highest number of publications. The substantial number of publications suggests that these countries have a strong research interest, well-established research infrastructure, funding support, collaborative networks, and the potential to influence clinical practice. Among them, publications from Brazil rank high, highlighting Brazil’s significant contributions to advancing the field and shaping best practices in using resin materials for dental caries treatment. Currently, the University of Maryland has the highest publication output, indicating its strong research output and productivity in the field of resin materials for dental caries repair. The high intermediary centrality of the University of Sao Paulo and the University of London demonstrates their important role in promoting knowledge transfer and collaboration within the field. By acting as central nodes in the research network, these institutions may have stronger abilities to disseminate knowledge, guide research directions, and serve as key hubs connecting researchers and facilitating collaboration among multiple institutions. In conclusion, the extensive collaboration among different authors, institutions, and countries has fostered the vibrant development of the research field of resin materials for dental caries repair.
Keywords are the core terms that reveal the research topics and focuses. Co-occurrence analysis of keywords can illustrate the current key research areas. The clustering analysis results indicate that current research on resin materials for dental caries repair primarily focuses on three aspects. Firstly, exploring the characteristics of dental caries based on the etiology, treatment, and prevention of caries lesions. The development of dental caries is primarily caused by acidogenic microorganisms such as Streptococcus mutans, which demineralize the enamel and dentin, leading to the formation of dental plaque and complicating the caries process (Guo et al., 2022). Additionally, bacteria produce proteolytic enzymes that degrade collagen, further compromising the structural integrity of the teeth. Therefore, the filling and repair of dental caries and effective prevention are crucial research focuses at present. Secondly, the properties of resin materials, which encompass several interconnected areas. Polymerization shrinkage and microleakage of resin materials pose challenges in dental restoration. The majority of resin composite materials experience shrinkage in the early stages, with shrinkage volumes reaching 2%–6% at 30 min (Kleverlaan and Feilzer, 2005). Polymerization shrinkage weakens the bond between the tooth structure and the restoration, leading to marginal gaps, discoloration, postoperative sensitivity, and even fracture and failure of the restoration (Ferracane and Hilton, 2016; Soares et al., 2017). Current research focuses on innovating the properties of resin materials, synthesizing composite materials, and using glass ionomer cement as a replacement in order to minimize shrinkage at the interface and the resultant stress. In addition, resin infiltration into carious lesions is crucial for effective sealing and strengthening of the tooth structure (Zhou et al., 2023). Successful resin infiltration can improve the bond between the restoration and the tooth, thereby contributing to long-lasting restorations. Researchers explore strategies for enhancing resin infiltration, considering factors such as viscosity, bonding agents, and adhesive performance. This forms the third research focus in the current field, which is to improve the bonding efficacy of adhesives. By exploring new bonding techniques, adhesive systems, and innovative restoration strategies, as well as incorporating antibacterial silver nanoparticles and materials with bioactivity that can release calcium, phosphate, and fluoride ions to promote tooth remineralization, efforts are made to reduce microleakage, prolong the lifespan of restorations, and minimize the risk of caries recurrence.
Based on the high-frequency co-occurrence network of keywords, further analysis of the growth and emergence of keywords can detect those with high frequency changes and rapid growth rates, thereby analyzing the future development trends in the field.
The unique physiological and anatomical characteristics of the oral cavity make it crucial to effectively remove bacterial plaque biofilms and reduce bacterial adhesion and aggregation in order to ensure the effectiveness of caries treatment. However, due to the inherent properties of resin materials, polymerization shrinkage increases residual stress and may lead to debonding at the interface between the restoration material and the tooth structure (Loguercio et al., 2004; Oliveira et al., 2022). Fractured surfaces and microcracks provide an ideal environment for oral bacteria to grow, promoting biofilm accumulation, which is related to caries recurrence and postoperative allergies (Khvostenko et al., 2015; Maske et al., 2017). Researchers are actively exploring composite resin materials endowed with antimicrobial and bioactive properties, aiming to inhibit biofilm formation and promote the regeneration of dentin (Imazato, 2003; Jandt and Sigusch, 2009).
Bioactivity refers to the interactions at the interface between materials and biological tissues that trigger specific biological or chemical responses, thereby facilitating the formation of chemical bonds between them. This interactivity enables bioactive materials to integrate with both soft and hard bodily tissues, promoting tissue regeneration or modulating cellular processes such as proliferation, migration, differentiation, protein expression, and mineralization through the regulation of growth factor-ligand signaling pathways (Li J. et al., 2018; Li M. et al., 2018; Miguel et al., 2019; Han et al., 2022). In the context of caries repair, the introduction of bioactive additives such as calcium phosphate, bioactive glass, and fluoride releasers not only supports the remineralization process but also enhances the materials’ antimicrobial properties (Sauro et al., 2013; Milly et al., 2014). Additionally, the incorporation of antimicrobial metal ions like Zn^2+, Mg^2+, and Sr^2+ into bioactive glass or forming antimicrobial metal coatings on the material surface can significantly improve its antimicrobial efficacy (Mohseni et al., 2019; Ranga et al., 2020). However, during this process, it is crucial to carefully balance the use of bioactive additives to avoid adversely affecting the resin’s bonding strength and overall mechanical properties. The impact of bioactive materials on bond strength and durability is multifaceted. On one hand, the presence of bioactive additives can enhance the bond with tooth structures by promoting the formation of a more durable interfacial layer (Zhang et al., 2017). On the other hand, excessive dissolution or improper integration of bioactive substances can impair bond strength, reducing the wear resistance and fatigue life of the restoration (Beck, 2022). Therefore, the development of bioactive resin materials for caries repair must consider the optimal concentration and compatibility of bioactive additives to ensure they do not negatively impact the mechanical properties and durability of the resin (Chu et al., 2010).
Metal oxides, such as those of titanium, silver, and zinc—particularly nano-zinc oxide—are favored for their excellent biocompatibility, stability, and cost-effectiveness as another strategy to enhance the antimicrobial properties of resins (Tavassoli et al., 2013; Kasraei et al., 2014; Dias et al., 2019). However, the main drawback of zinc oxide nanoparticles in polymers is their incomplete dispersion and tendency to aggregate, which imparts some toxicity (Yuan et al., 2010). To overcome this issue, zinc oxide nanoparticles are often combined with other functional materials, such as graphene and other carbon-based materials (Kulshrestha et al., 2014). In recent years, research on the release of antibacterial agents has mainly focused on fluoride, chlorhexidine, and silver nanoparticles. However, as the antibacterial agents are released, their effectiveness gradually decreases, leading to the formation of pores in the composite materials, which affects their mechanical properties (Cheng et al., 2012; Ten Cate, 2012). Calcium fluoride nanoparticles (NCaF2), when incorporated as inorganic fillers in resin materials, can release fluoride and calcium ions over a long period of time, which helps inhibit tooth demineralization and bacterial growth while promoting mineralization (Xu et al., 2010; Kulshrestha et al., 2016). Therefore, resin materials containing NCaF2 are expected to protect teeth from primary and secondary caries (Ling et al., 2009; Moreau and Xu, 2010). Mitwalli et al. developed a rechargeable NCaF2 dental composite material. This novel nanocomposite material can release fluoride and calcium ions for a prolonged period, making long-lasting remineralization possible (Mitwalli et al., 2022). However, further research is needed to optimize the NCaF2 material and validate its clinical performance. Currently, there are various types of composite resin materials claiming to have good antibacterial effects; however, there is a lack of cross-comparisons on the antibacterial capabilities of different antibacterial materials. Additionally, the bacterial species in the oral cavity are extremely diverse. Some antibacterial materials can only kill specific bacteria, while having weak effects on others. Therefore, it is necessary to evaluate the impact of antibacterial resins on the oral microbiota both in vitro and in vivo. In summary, enhancing bioactivity and remineralization and improving the antibacterial properties of resin materials are among the future research directions in this field.
Resin materials have become the most popular choice for dental caries repair due to their superior aesthetic properties, ease of use, and sufficient mechanical durability, making them successful for direct restorations in prepared cavities. The longevity or durability of resin materials is a key indicator of the success of caries treatment. In the complex oral environment, occasional irreversible failures of resin restorations can occur due to microcracks caused by thermal and mechanical fatigue, as well as secondary caries (Wu et al., 2016a; Wu et al., 2016b; Sharma et al., 2019). Studies have shown that fracture of the restoration or tooth is the main cause of restoration failure, with a prevalence of over 60% within the first 3 years. Recurrent caries at the restoration margins is the second most common cause, with a prevalence of over 75% after 3 years of treatment (Sharma et al., 2019; Brunthaler et al., 2003; da Rosa et al., 2006). Therefore, the short lifespan of resin materials and potential health risks are the main drawbacks limiting their development (Bayne et al., 2019; Wu et al., 2019; Yao et al., 2020). With the continuous development of nanotechnology and material innovations, the addition of various nanoparticles such as silica-based glasses, ceramics, metals, pre-polymerized particles, natural minerals, and cellulose crystal particles can improve and enhance the mechanical properties of resin composites while achieving low shrinkage volume and stress, ideal viscosity, and opacity (Elgamily et al., 2018; Salas et al., 2018; Zhang et al., 2018; Wang et al., 2019; Fanfoni et al., 2020; Yang et al., 2020; Zhou et al., 2020; Behl et al., 2021). In conclusion, various nanoparticles greatly enhance the mechanical properties, active surface area, and bioactivity of resin materials, allowing for effective caries repair through remineralization and biofilm inhibition, thus extending the lifespan of resin restorations (Cheng et al., 2015). Studies have shown that amorphous calcium phosphate nanoparticles (NACP) have been added to resin materials for dental caries repair. NACP, due to its small size and large surface area, can effectively release calcium and phosphate ions, increasing pH levels (Moreau et al., 2011; Xu et al., 2011). This promotes remineralization of the surrounding tooth structure and helps inhibit secondary caries (Vanichvatana and Auychai, 2013; Zhang et al., 2017). Multiple studies have demonstrated the dual functionality of NACP when used in combination with antibacterial agents, achieving both remineralization and antibacterial effects (Cheng et al., 2015; Liang et al., 2019). Even after undergoing a demineralization/remineralization cycle for 30 days, NACP composite materials were able to remineralize enamel lesions (Zhang et al., 2012). Furthermore, fluoride-containing NACP has been found to have the ability to inhibit cariogenic bacteria and promote enamel remineralization. Therefore, NACP holds great potential for the preventive treatment of dental caries. However, further validation of the efficacy and safety of these nanocomposite resin materials through animal experiments and clinical research is required before they can be made available to patients. In summary, there is growing attention towards improving the lifespan and durability of resin restorations in the future, and nanotechnology and innovative materials will undoubtedly play crucial roles in this endeavor.
Currently, digital dentistry has significantly transformed traditional dental workflows and has the potential to improve the accuracy, efficiency, and aesthetics of dental caries repair. The research progress in digital dentistry for resin materials in caries restoration is rapidly advancing, with an increasing number of publications exploring its applications and outcomes. Digital imaging technologies, such as intraoral scanners, digital radiography, and cone beam computed tomography, enable precise detection of caries and its pathological features (Mai et al., 2017; Tahayeri et al., 2018; Park et al., 2019; Yeung et al., 2020). Computer-aided design and computer-aided manufacturing systems can produce precise and customized restorations from resin materials (Panayi and Tsolakis, 2021). 3D printing can enhance the mechanical properties, aesthetics, and biocompatibility of restorations, while also providing higher precision and efficiency in manufacturing complex restorations (Punia et al., 2022). Augmented reality and virtual reality technologies offer interactive and immersive visualization, planning, and simulation capabilities to enhance patient communication, education, and procedural accuracy (Ayoub and Pulijala, 2019). Integration of artificial intelligence and machine learning algorithms in caries diagnosis, treatment planning, and outcome prediction can identify patterns, improve diagnostic accuracy, and optimize treatment algorithms (Rekow, 2020; Sulaiman, 2020). In conclusion, ongoing research and innovation will drive the adoption and optimization of digital dentistry approaches, ultimately improving outcomes for patients receiving caries restorations.
In general, future research in resin materials for dental caries repair will focus on the areas of bioactivity, antibacterial strategies, durability, nanotechnology, and digital dentistry. The research in these fields is expected to drive advancements in the field and bring improvements in patient outcomes, ultimately revolutionizing the application of resin materials in caries treatment.
As the etiology of dental caries is explored in greater depth, the importance of tooth repair and remineralization is increasingly emphasized, making bioactive resin materials a focus of research. These materials, such as resins containing NCaF2, promote tooth remineralization and inhibit bacterial growth by releasing calcium and fluoride ions (Mitwalli et al., 2021). Although they possess significant theoretical potential, their actual clinical performance and long-term stability still require validation. Future studies need to assess the durability and biocompatibility of these materials in the oral environment and optimize the formulation to balance bioactivity with mechanical strength. Given the direct connection between recurrent caries and oral biofilms, developing effective and lasting antimicrobial strategies is crucial. Existing strategies have shown initial success, but the gradual release of antimicrobials may decrease efficacy over time and impair material performance. Therefore, developing new antimicrobial agents that maintain material stability and thoroughly assessing their antimicrobial effectiveness and biosafety are important directions for research. The durability of resin materials is critical for treating dental caries. Studies suggest that incorporating nano-fillers such as silica-based glass, ceramics, and metals can enhance the mechanical properties of resin materials (Kaizer et al., 2016). However, the dispersibility, compatibility, and long-term stability of nano-fillers still need optimization. Future research should improve the composition and structure of nano-fillers to ensure safety and efficacy in clinical applications. Digital dental technologies such as oral scanning, CAD/CAM, and 3D printing have already improved the precision, efficiency, and aesthetics of dental restoration (Lin et al., 2019). To promote these technologies in clinical practice, research should focus on reducing costs, enhancing user-friendliness, and developing new digital manufacturing processes through interdisciplinary knowledge to customize personalized resin repair solutions, optimize therapeutic outcomes, and improve patient experiences.
This study is the first bibliometric analysis conducted in the field of resin materials for dental caries repair. It provides an objective and quantitative analysis of the distribution of authors, institutions, countries, and journals in this field, and further investigates the current status, key areas of focus, and future trends in research. However, it is important to acknowledge the limitations of this study. Firstly, only English-language publications and reviews were included, which may overlook viewpoints expressed in literature from other languages as well as expert opinions, conference abstracts, and other types of research literature. Secondly, although the WoSCC database provides comprehensive literature coverage, relying solely on this database may omit some publications that offer important perspectives. Lastly, due to the nature of bibliometric analysis, full-text analysis of publications is not possible, leading to potential omissions of certain information.
The field of resin materials for dental caries repair is becoming increasingly important, with collaboration among different authors, institutions, and countries leading to new achievements and breakthroughs. Currently, the nature of caries, the characteristics of resin materials, and the bonding strength of adhesives are areas of focus. Enhancing the bioactivity and remineralization of resin materials, advanced antibacterial strategies, the lifespan and durability of resin restorations, nanotechnology, and material innovation, as well as digital dentistry, will receive more attention in future research trends.
BH: Conceptualization, Data curation, Project administration, Software, Supervision, Visualization, Writing–review and editing. LW: Conceptualization, Methodology, Software, Validation, Writing–original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to express our gratitude to Dr. Zhizhong Shang from Lanzhou University for his assistance in literature retrieval and screening.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ai, M., Du, Z., Zhu, S., Geng, H., Zhang, X., Cai, Q., et al. (2017). Composite resin reinforced with silver nanoparticles-laden hydroxyapatite nanowires for dental application. Dent. Mater. 33 (1), 12–22. doi:10.1016/j.dental.2016.09.038
Akhtar, K., Pervez, C., Zubair, N., and Khalid, H. (2021). Calcium hydroxyapatite nanoparticles as a reinforcement filler in dental resin nanocomposite. J. Mater. Sci. Mater. Med. 32 (10), 129. doi:10.1007/s10856-021-06599-3
Albeshir, E. G., Alsahafi, R., Albluwi, R., Balhaddad, A. A., Mitwalli, H., Oates, T. W., et al. (2022). Low-shrinkage resin matrices in restorative dentistry-narrative review. Mater. (Basel, Switz.) 15 (8), 2951. doi:10.3390/ma15082951
Amin, F., Fareed, M. A., Zafar, M. S., Khurshid, Z., Palma, P. J., and Kumar, N. (2022). Degradation and stabilization of resin-dentine interfaces in polymeric dental adhesives: an updated review. Coatings 12 (8), 1094. doi:10.3390/coatings12081094
Ayoub, A., and Pulijala, Y. (2019). The application of virtual reality and augmented reality in Oral and Maxillofacial Surgery. BMC Oral Health 19, 238–8. doi:10.1186/s12903-019-0937-8
Bayne, S. C., Ferracane, J. L., Marshall, G. W., Marshall, S. J., and van Noort, R. (2019). The evolution of dental materials over the past century: silver and gold to tooth color and beyond. J. Dent. Res. 98 (3), 257–265. doi:10.1177/0022034518822808
Beck, F. (2022). Bioactive agents and their effect on dentin bond strength: considerations for clinical applicability–an in vitro study. Dissertation. München (Germany): Ludwig-Maximilians-Universität.
Behl, S., Farahani, A. D., Rajui, R., Rajan, G., Ellakwa, A., Farrar, P., et al. (2021). Evaluation of rheological behaviour of flowable dental composites reinforced with low aspect ratio micro-sized glass fibres. Dent. Mater. official Publ. Acad. Dent. Mater. 37 (1), 131–142. doi:10.1016/j.dental.2020.10.023
Brunthaler, A., König, F., Lucas, T., Sperr, W., and Schedle, A. (2003). Longevity of direct resin composite restorations in posterior teeth: a review. Clin. oral Investig. 7 (2), 63–70. doi:10.1007/s00784-003-0206-7
Chen, C. (2004). Searching for intellectual turning points: progressive knowledge domain visualization. Proc. Natl. Acad. Sci. U. S. A. 101 (1), 5303–5310. doi:10.1073/pnas.0307513100
Chen, M., and Wu, T. (2024). Nanoparticles and neurodegeneration: insights on multiple pathways of programmed cell death regulated by nanoparticles. Sci. total Environ. 912, 168739. doi:10.1016/j.scitotenv.2023.168739
Cheng, L., Weir, M. D., Xu, H. H., Kraigsley, A. M., Lin, N. J., Lin-Gibson, S., et al. (2012). Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent. Mater. official Publ. Acad. Dent. Mater. 28 (5), 573–583. doi:10.1016/j.dental.2012.01.006
Cheng, L., Zhang, K., Weir, M. D., Melo, M. A., Zhou, X., and Xu, H. H. (2015). Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine Lond. Engl. 10 (4), 627–641. doi:10.2217/nnm.14.191
Cho, K., Rajan, G., Farrar, P., Prentice, L., and Prusty, B. G. (2022). Dental resin composites: a review on materials to product realizations. Compos. Part B Eng. 230, 109495. doi:10.1016/j.compositesb.2021.109495
Chu, C. H., Mei, M. L., and Lo, E. C. (2010). Use of fluorides in dental caries management. General Dent. 58 (1), 37–80.
Clarin, A., Ho, D., Soong, J., Looi, C., Ipe, D. S., and Tadakamadla, S. K. (2021). The antibacterial and remineralizing effects of biomaterials combined with DMAHDM nanocomposite: a systematic review. Mater. (Basel, Switz.) 14 (7), 1688. doi:10.3390/ma14071688
da Rosa, R. P. A., Cenci, M. S., Donassollo, T. A., Loguércio, A. D., and Demarco, F. F. (2006). A clinical evaluation of posterior composite restorations: 17-year findings. J. Dent. 34 (7), 427–435. doi:10.1016/j.jdent.2005.09.006
Dias, H. B., Bernardi, M. I. B., Bauab, T. M., Hernandes, A. C., and de Souza Rastelli, A. N. (2019). Titanium dioxide and modified titanium dioxide by silver nanoparticles as an anti biofilm filler content for composite resins. Dent. Mater. official Publ. Acad. Dent. Mater. 35 (2), e36–e46. doi:10.1016/j.dental.2018.11.002
Drummond, J. L. (2008). Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 87 (8), 710–719. doi:10.1177/154405910808700802
Elfakhri, F., Alkahtani, R., Li, C., and Khaliq, J. (2022). Influence of filler characteristics on the performance of dental composites: a comprehensive review. Ceram. Int. 48, 27280–27294. doi:10.1016/j.ceramint.2022.06.314
Elgamily, H. M., El-Sayed, H. S., and Abdelnabi, A. (2018). The antibacterial effect of two cavity Disinfectants against one of cariogenic pathogen: an in vitro comparative study. Contemp. Clin. Dent. 9 (3), 457–462. doi:10.4103/ccd.ccd_308_18
Fanfoni, L., De Biasi, M., Antollovich, G., Di Lenarda, R., and Angerame, D. (2020). Evaluation of degree of conversion, rate of cure, microhardness, depth of cure, and contraction stress of new nanohybrid composites containing pre-polymerized spherical filler. J. Mater. Sci. Mater. Med. 31 (12), 127. doi:10.1007/s10856-020-06464-9
Ferracane, J. L., and Hilton, T. J. (2016). Polymerization stress--is it clinically meaningful? Dent. Mater. official Publ. Acad. Dent. Mater. 32 (1), 1–10. doi:10.1016/j.dental.2015.06.020
Guo, X., Yu, Y., Gao, S., Zhang, Z., and Zhao, H. (2022). Biodegradation of dental resin-based composite-A potential factor affecting the bonding effect: a narrative review. Biomedicines 10 (9), 2313. doi:10.3390/biomedicines10092313
Han, X., Alu, A., Liu, H., Shi, Y., Wei, X., Cai, L., et al. (2022). Biomaterial-assisted biotherapy: a brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 17, 29–48. doi:10.1016/j.bioactmat.2022.01.011
Hou, J., Yang, X., and Chen, C. (2018). Emerging trends and new developments in information science: a document co-citation analysis (2009–2016). Scientometrics 115, 869–892. doi:10.1007/s11192-018-2695-9
Imazato, S. (2003). Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. official Publ. Acad. Dent. Mater. 19 (6), 449–457. doi:10.1016/s0109-5641(02)00102-1
Jandt, K. D., and Sigusch, B. W. (2009). Future perspectives of resin-based dental materials. Dent. Mater. official Publ. Acad. Dent. Mater. 25 (8), 1001–1006. doi:10.1016/j.dental.2009.02.009
Jokstad, A., Bayne, S., Blunck, U., Tyas, M., and Wilson, N. (2001). Quality of dental restorations. FDI commission project 2-95. Int. Dent. J. 51 (3), 117–158. doi:10.1002/j.1875-595x.2001.tb00832.x
Kaizer, M., Almeida, J., Gonçalves, A., Zhang, Y., Cava, S., and Moraes, R. (2016). Silica coating of nonsilicate nanoparticles for resin-based composite materials. J. Dent. Res. 95 (12), 1394–1400. doi:10.1177/0022034516662022
Kanemura, N., Sano, H., and Tagami, J. (1999). Tensile bond strength to and SEM evaluation of ground and intact enamel surfaces. J. Dent. 27 (7), 523–530. doi:10.1016/s0300-5712(99)00008-1
Kasraei, S., Sami, L., Hendi, S., Alikhani, M. Y., Rezaei-Soufi, L., and Khamverdi, Z. (2014). Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 39 (2), 109–114. doi:10.5395/rde.2014.39.2.109
Kassebaum, N. J., Bernabé, E., Dahiya, M., Bhandari, B., Murray, C. J., and Marcenes, W. (2015). Global burden of untreated caries: a systematic review and metaregression. J. Dent. Res. 94 (5), 650–658. doi:10.1177/0022034515573272
Khvostenko, D., Salehi, S., Naleway, S. E., Hilton, T., Ferracane, J., Mitchell, J., et al. (2015). Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent. Mater. official Publ. Acad. Dent. Mater. 31 (6), 702–710. doi:10.1016/j.dental.2015.03.011
Kleverlaan, C. J., and Feilzer, A. J. (2005). Polymerization shrinkage and contraction stress of dental resin composites. Dent. Mater. official Publ. Acad. Dent. Mater. 21 (12), 1150–1157. doi:10.1016/j.dental.2005.02.004
Kudou, Y., Obara, K., Kawashima, T., Kubota, M., Abe, S., Endo, T., et al. (2000). Addition of antibacterial agents to MMA-TBB dentin bonding systems--influence on tensile bond strength and antibacterial effect. Dent. Mater. J. 19 (1), 65–74. doi:10.4012/dmj.19.65
Kulshrestha, S., Khan, S., Hasan, S., Khan, M. E., Misba, L., and Khan, A. U. (2016). Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: an in vitro and in vivo approach. Appl. Microbiol. Biotechnol. 100 (4), 1901–1914. doi:10.1007/s00253-015-7154-4
Kulshrestha, S., Khan, S., Meena, R., Singh, B. R., and Khan, A. U. (2014). A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling 30 (10), 1281–1294. doi:10.1080/08927014.2014.983093
Langhorst, S. E., O'Donnell, J. N., and Skrtic, D. (2009). In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent. Mater. official Publ. Acad. Dent. Mater. 25 (7), 884–891. doi:10.1016/j.dental.2009.01.094
Li, J., Wen, J., Li, B., Li, W., Qiao, W., Shen, J., et al. (2018a). Valence state manipulation of cerium oxide nanoparticles on a titanium surface for modulating cell fate and bone formation. Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger.) 5 (2), 1700678. doi:10.1002/advs.201700678
Li, M., Xiong, P., Yan, F., Li, S., Ren, C., Yin, Z., et al. (2018b). An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 3 (1), 1–18. doi:10.1016/j.bioactmat.2018.01.001
Liang, K., Wang, S., Tao, S., Xiao, S., Zhou, H., Wang, P., et al. (2019). Dental remineralization via poly(amido amine) and restorative materials containing calcium phosphate nanoparticles. Int. J. oral Sci. 11 (2), 15. doi:10.1038/s41368-019-0048-z
Liang, L., Han, Z., Yang, R., Guo, Y., and Chen, Z. (2024). Research hotspots and trends of nanomaterials in stomatology: a bibliometric analysis from 2000 to 2023. Heliyon 10 (6), e27967. doi:10.1016/j.heliyon.2024.e27967
Lin, L., Fang, Y., Liao, Y., Chen, G., Gao, C., and Zhu, P. (2019). 3D printing and digital processing techniques in dentistry: a review of literature. Adv. Eng. Mater. 21 (6), 1801013. doi:10.1002/adem.201801013
Ling, L., Xu, X., Choi, G. Y., Billodeaux, D., Guo, G., and Diwan, R. M. (2009). Novel F-releasing composite with improved mechanical properties. J. Dent. Res. 88 (1), 83–88. doi:10.1177/0022034508328254
Ling, L. X., Ouyang, Y., and Hu, Y. (2023). Research trends on nanomaterials in gastric cancer: a bibliometric analysis from 2004 to 2023. J. nanobiotechnology 21 (1), 248. doi:10.1186/s12951-023-02033-8
Liu, F., Jiang, X., Bao, S., Wang, R., Sun, B., and Zhu, M. (2015). Effect of hydroxyapatite whisker surface graft polymerization on water sorption, solubility and bioactivity of the dental resin composite. Mater. Sci. Eng. C, Mater. Biol. Appl. 53, 150–155. doi:10.1016/j.msec.2015.04.043
Liu, Y., Wong, S., Han, Y., Li, X., Yuan, L., Xiong, M., et al. (2023). Global research trends in adipose stem cell tissue engineering: a scientometric research. Tissue Eng. Part C. Methods 29 (11), 505–525. doi:10.1089/ten.TEC.2023.0152
Loguercio, A. D., Reis, A., Schroeder, M., Balducci, I., Versluis, A., and Ballester, R. Y. (2004). Polymerization shrinkage: effects of boundary conditions and filling technique of resin composite restorations. J. Dent. 32 (6), 459–470. doi:10.1016/j.jdent.2004.02.010
Ma, H., Liu, S., Zhong, H., Zhou, M., Xing, C., Li, Y., et al. (2024). Exploring the landscape of hydrogel therapy for spinal cord injury: a bibliometric and visual analysis (1991-2023). World Neurosurg. 186, e95–e105. doi:10.1016/j.wneu.2024.03.048
Mai, H. N., Lee, K. B., and Lee, D. H. (2017). Fit of interim crowns fabricated using photopolymer-jetting 3D printing. J. Prosthet. Dent. 118 (2), 208–215. doi:10.1016/j.prosdent.2016.10.030
Maske, T. T., Kuper, N. K., Cenci, M. S., and Huysmans, M. (2017). Minimal gap size and dentin wall lesion development next to resin composite in a microcosm biofilm model. Caries Res. 51 (5), 475–481. doi:10.1159/000478536
Melo, M. A., Orrego, S., Weir, M. D., Xu, H. H., and Arola, D. D. (2016). Designing multiagent dental materials for enhanced resistance to biofilm damage at the bonded interface. ACS Appl. Mater. interfaces 8 (18), 11779–11787. doi:10.1021/acsami.6b01923
Miguel, S. P., Sequeira, R. S., Moreira, A. F., Cabral, C. S., Mendonça, A. G., Ferreira, P., et al. (2019). An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 139, 1–22. doi:10.1016/j.ejpb.2019.03.010
Milly, H., Festy, F., Watson, T. F., Thompson, I., and Banerjee, A. (2014). Enamel white spot lesions can remineralise using bio-active glass and polyacrylic acid-modified bio-active glass powders. J. Dent. 42 (2), 158–166. doi:10.1016/j.jdent.2013.11.012
Mitwalli, H., AlSahafi, R., Albeshir, E. G., Dai, Q., Sun, J., Oates, T. W., et al. (2021). Novel nano calcium fluoride remineralizing and antibacterial dental composites. J. Dent. 113, 103789. doi:10.1016/j.jdent.2021.103789
Mitwalli, H., AlSahafi, R., Alhussein, A., Oates, T. W., Melo, M. A. S., Xu, H. H., et al. (2022). Novel rechargeable calcium fluoride dental nanocomposites. Dent. Mater. official Publ. Acad. Dent. Mater. 38 (2), 397–408. doi:10.1016/j.dental.2021.12.022
Mohseni, M., Shamloo, A., Aghababaie, Z., Afjoul, H., Abdi, S., Moravvej, H., et al. (2019). A comparative study of wound dressings loaded with silver sulfadiazine and silver nanoparticles: in vitro and in vivo evaluation. Int. J. Pharm. 564, 350–358. doi:10.1016/j.ijpharm.2019.04.068
Moreau, J. L., Sun, L., Chow, L. C., and Xu, H. H. (2011). Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J. Biomed. Mater. Res. Part B, Appl. biomaterials 98 (1), 80–88. doi:10.1002/jbm.b.31834
Moreau, J. L., and Xu, H. H. (2010). Fluoride releasing restorative materials: effects of pH on mechanical properties and ion release. Dent. Mater. official Publ. Acad. Dent. Mater. 26 (11), e227–e235. doi:10.1016/j.dental.2010.07.004
Oliveira, A. A., Ribeiro, M. L. P., Costa, P. V. M., Pereira, R. D., Versluis, A., and Veríssimo, C. (2022). The effect of filling technique on the cuspal strain, polymerization shrinkage stress, enamel crack formation and depth of cure of restored molars. Dent. Mater. official Publ. Acad. Dent. Mater. 38 (8), 1404–1418. doi:10.1016/j.dental.2022.06.033
Panayi, N. C., and Tsolakis, A. I. (2021). In-house computer-aided design and 3-dimensional printing of customized orthodontic brackets using hybrid ceramic resin: is it the time for the orthodontist to take over? AJO-DO Clin. Companion 1 (3), 187–193. doi:10.1016/j.xaor.2021.07.001
Park, G. S., Kim, S. K., Heo, S. J., Koak, J. Y., and Seo, D. G. (2019). Effects of printing parameters on the fit of implant-supported 3D printing resin prosthetics. Mater. (Basel, Switz.) 12 (16), 2533. doi:10.3390/ma12162533
Peres, M. A., Macpherson, L. M. D., Weyant, R. J., Daly, B., Venturelli, R., Mathur, M. R., et al. (2019). Oral diseases: a global public health challenge. Lancet (London, Engl.) 394 (10194), 249–260. doi:10.1016/s0140-6736(19)31146-8
Ping, Q., He, J., and Chen, C. (2017). How many ways to use CiteSpace? A study of user interactive events over 14 months. J. Assoc. Inf. Sci. Technol. 68 (5), 1234–1256. doi:10.1002/asi.23770
Punia, U., Kaushik, A., Garg, R. K., Chhabra, D., and Sharma, A. (2022). 3D printable biomaterials for dental restoration: a systematic review. Mater. Today Proc. 63, 566–572. doi:10.1016/j.matpr.2022.04.018
Ranga, N., Gahlyan, S., and Duhan, S. (2020). Antibacterial efficiency of Zn, Mg and Sr doped bioactive glass for bone tissue engineering. J. Nanosci. Nanotechnol. 20 (4), 2465–2472. doi:10.1166/jnn.2020.17336
Rekow, E. D. (2020). Digital dentistry: the new state of the art - is it disruptive or destructive? Dent. Mater. 36 (1), 9–24. doi:10.1016/j.dental.2019.08.103
Sabe, M., Pillinger, T., Kaiser, S., Chen, C., Taipale, H., Tanskanen, A., et al. (2022). Half a century of research on antipsychotics and schizophrenia: a scientometric study of hotspots, nodes, bursts, and trends. Neurosci. Biobehav. Rev. 136, 104608. doi:10.1016/j.neubiorev.2022.104608
Salas, M., Lucena, C., Herrera, L. J., Yebra, A., Della Bona, A., and Pérez, M. M. (2018). Translucency thresholds for dental materials. Dent. Mater. 34 (8), 1168–1174. doi:10.1016/j.dental.2018.05.001
Sauro, S., Osorio, R., Osorio, E., Watson, T. F., and Toledano, M. (2013). Novel light-curable materials containing experimental bioactive micro-fillers remineralise mineral-depleted bonded-dentine interfaces. J. Biomaterials Sci. Polym. Ed. 24 (8), 940–956. doi:10.1080/09205063.2012.727377
Shang, Z., Wanyan, P., Wang, M., Zhang, B., Cui, X., and Wang, X. (2023). Bibliometric analysis of stem cells for spinal cord injury: current status and emerging frontiers. Front. Pharmacol. 14, 1235324. doi:10.3389/fphar.2023.1235324
Sharma, A., Alam, S., Sharma, C., Patnaik, A., and Kumar, S. R. (2019). Static and dynamic mechanical behavior of microcapsule-reinforced dental composite. Proc. Institution Mech. Eng. Part L J. Mater. Des. Appl. 233 (6), 1184–1190. doi:10.1177/1464420717733770
Soares, C. J., Faria, ESAL, Rodrigues, M. P., Vilela, A. B. F., Pfeifer, C. S., Tantbirojn, D., et al. (2017). Polymerization shrinkage stress of composite resins and resin cements - what do we need to know? Braz. oral Res. 31 (1), e62. doi:10.1590/1807-3107BOR-2017.vol31.0062
Sulaiman, T. A. (2020). Materials in digital dentistry-A review. J. Esthetic Restor. Dent. 32 (2), 171–181. doi:10.1111/jerd.12566
Tahayeri, A., Morgan, M., Fugolin, A. P., Bompolaki, D., Athirasala, A., Pfeifer, C. S., et al. (2018). 3D printed versus conventionally cured provisional crown and bridge dental materials. Dental Mater. 34 (2), 192–200. doi:10.1016/j.dental.2017.10.003
Tavassoli, H. S., Alaghemand, H., Hamze, F., Ahmadian Babaki, F., Rajab-Nia, R., Rezvani, M. B., et al. (2013). Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dental Mater. 29 (5), 495–505. doi:10.1016/j.dental.2013.03.011
Ten Cate, J. M. (2012). Novel anticaries and remineralizing agents: prospects for the future. Journal of dental research 91 (9), 813–815. doi:10.1177/0022034512455032
Vanichvatana, S., and Auychai, P. (2013). Efficacy of two calcium phosphate pastes on the remineralization of artificial caries: a randomized controlled double-blind in situ study. Int. J. Oral Sci. 5 (4), 224–228. doi:10.1038/ijos.2013.67
Vos, T., Abajobir, A. A., Abate, K. H., Abbafati, C., Abbas, K. M., Abd-Allah, F., et al. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England) 390 (10100), 1211–1259. doi:10.1016/s0140-6736(17)32154-2
Wang, Y., Hua, H., Li, W., Wang, R., Jiang, X., and Zhu, M. (2019). Strong antibacterial dental resin composites containing cellulose nanocrystal/zinc oxide nanohybrids. Journal of dentistry 80, 23–29. doi:10.1016/j.jdent.2018.11.002
Wang, Y. C., Xu, W. L., Lu, Y. P., Xu, W. H., Yin, H., and Xiao, G. Y. (2020). Investigation of nature of starting materials on the construction of hydroxyapatite 1D/3D morphologies. Materials science and engineering C, Materials for biological applications 108, 110408. doi:10.1016/j.msec.2019.110408
Weir, M. D., Chow, L. C., and Xu, H. H. (2012). Remineralization of demineralized enamel via calcium phosphate nanocomposite. Journal of dental research 91 (10), 979–984. doi:10.1177/0022034512458288
Wu, J., Weir, M. D., Melo, M. A., Strassler, H. E., and Xu, H. H. (2016a). Effects of water-aging on self-healing dental composite containing microcapsules. Journal of dentistry 47, 86–93. doi:10.1016/j.jdent.2016.01.008
Wu, J., Weir, M. D., Zhang, Q., Zhou, C., Melo, M. A., and Xu, H. H. (2016b). Novel self-healing dental resin with microcapsules of polymerizable triethylene glycol dimethacrylate and N,N-dihydroxyethyl-p-toluidine. Dental materials official publication of the Academy of Dental Materials 32 (2), 294–304. doi:10.1016/j.dental.2015.11.014
Wu, J., Xie, X., Zhou, H., Tay, F. R., Weir, M. D., Melo, M. A. S., et al. (2019). Development of a new class of self-healing and therapeutic dental resins. Polymer Degradation and Stability 163, 87–99. doi:10.1016/j.polymdegradstab.2019.02.024
Xu, H. H., Moreau, J. L., Sun, L., and Chow, L. C. (2011). Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dental materials official publication of the Academy of Dental Materials 27 (8), 762–769. doi:10.1016/j.dental.2011.03.016
Xu, H. H., Smith, D. T., Jahanmir, S., Romberg, E., Kelly, J., Thompson, V., et al. (1998). Indentation damage and mechanical properties of human enamel and dentin. Journal of dental research 77 (3), 472–480. doi:10.1177/00220345980770030601
Xu, H. H., Wang, P., Wang, L., Bao, C., Chen, Q., Weir, M. D., et al. (2017). Calcium phosphate cements for bone engineering and their biological properties. Bone research 5, 17056. doi:10.1038/boneres.2017.56
Xu, H. H., Weir, M. D., Sun, L., Moreau, J., Takagi, S., Chow, L., et al. (2010). Strong nanocomposites with Ca, PO(4), and F release for caries inhibition. Journal of dental research 89 (1), 19–28. doi:10.1177/0022034509351969
Yang, J., Shen, J., Wu, X., He, F., Xie, H., and Chen, C. (2020). Effects of nano-zirconia fillers conditioned with phosphate ester monomers on the conversion and mechanical properties of Bis-GMA- and UDMA-based resin composites. Journal of dentistry 94, 103306. doi:10.1016/j.jdent.2020.103306
Yang, Y., Xu, Z., Guo, Y., Zhang, H., Qiu, Y., Li, J., et al. (2021). Novel core-shell CHX/ACP nanoparticles effectively improve the mechanical, antibacterial and remineralized properties of the dental resin composite. Dental materials official publication of the Academy of Dental Materials 37 (4), 636–647. doi:10.1016/j.dental.2021.01.007
Yao, S., Li, T., Zhou, C., Weir, M. D., Melo, M. A. S., Tay, F. R., et al. (2020). Novel antibacterial and therapeutic dental polymeric composites with the capability to self-heal cracks and regain mechanical properties. European Polymer Journal 129, 109604. doi:10.1016/j.eurpolymj.2020.109604
Yao, S., Qin, L., Wang, Z., Zhu, L., Zhou, C., and Wu, J. (2022). Novel nanoparticle-modified multifunctional microcapsules with self-healing and antibacterial activities for dental applications. Dental materials official publication of the Academy of Dental Materials 38 (8), 1301–1315. doi:10.1016/j.dental.2022.06.012
Yeung, M., Abdulmajeed, A., Carrico, C. K., Deeb, G. R., and Bencharit, S. (2020). Accuracy and precision of 3D-printed implant surgical guides with different implant systems: an in vitro study. The Journal of prosthetic dentistry 123 (6), 821–828. doi:10.1016/j.prosdent.2019.05.027
Yuan, J. H., Chen, Y., Zha, H. X., Song, L. J., Li, C. Y., Li, J. Q., et al. (2010). Determination, characterization and cytotoxicity on HELF cells of ZnO nanoparticles. Colloids and surfaces B, Biointerfaces 76 (1), 145–150. doi:10.1016/j.colsurfb.2009.10.028
Yuan, K., Deng, C., Tan, L., Wang, X., Yan, W., Dai, X., et al. (2024). Structural and temporal dynamics analysis of zinc-based biomaterials: history, research hotspots and emerging trends. Bioactive materials 35, 306–329. doi:10.1016/j.bioactmat.2024.01.017
Zhang, K., Melo, M. A., Cheng, L., Weir, M. D., Bai, Y., and Xu, H. H. (2012). Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental materials official publication of the Academy of Dental Materials 28 (8), 842–852. doi:10.1016/j.dental.2012.04.027
Zhang, K., Zhang, N., Weir, M. D., Reynolds, M. A., Bai, Y., and Xu, H. H. (2017). Bioactive dental composites and bonding agents having remineralizing and antibacterial characteristics. Dental Clinics 61 (4), 669–687. doi:10.1016/j.cden.2017.05.002
Zhang, S., Wang, X., Yang, J., Chen, H., and Jiang, X. (2023). Micromechanical interlocking structure at the filler/resin interface for dental composites: a review. International journal of oral science 15 (1), 21. doi:10.1038/s41368-023-00226-3
Zhang, Y., Huang, C., and Chang, J. (2018). Ca-Doped mesoporous SiO(2)/dental resin composites with enhanced mechanical properties, bioactivity and antibacterial properties. Journal of materials chemistry B 6 (3), 477–486. doi:10.1039/c7tb02864d
Zhao, L., Weir, M. D., and Xu, H. H. (2010). An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 31 (25), 6502–6510. doi:10.1016/j.biomaterials.2010.05.017
Zheng, L., Li, K., Ning, C., and Sun, J. (2021). Study on antibacterial and fluoride-releasing properties of a novel composite resin with fluorine-doped nano-zirconia fillers. Journal of dentistry 113, 103772. doi:10.1016/j.jdent.2021.103772
Zhou, W., Peng, X., Zhou, X., Weir, M. D., Melo, M. A. S., Tay, F. R., et al. (2020). In vitro evaluation of composite containing DMAHDM and calcium phosphate nanoparticles on recurrent caries inhibition at bovine enamel-restoration margins. Dental materials official publication of the Academy of Dental Materials 36 (10), 1343–1355. doi:10.1016/j.dental.2020.07.007
Zhou, X., Huang, X., Li, M., Peng, X., Wang, S., Zhou, X., et al. (2019). Development and status of resin composite as dental restorative materials. Journal of Applied Polymer Science 136 (44), 48180. doi:10.1002/app.48180
Zhou, Y., Huang, X., Wu, L., Liang, Y., Huang, Y., and Huang, S. (2023). Microleakage, microgap, and shear bond strength of an infiltrant for pit and fissure sealing. Heliyon 9 (5), e16248. doi:10.1016/j.heliyon.2023.e16248
Keywords: caries, resin material, bibliometric analysis, hotspots, development trend
Citation: Han B and Wang L (2024) Global trend and hotspot of resin materials for dental caries repair: a bibliometric analysis. Front. Mater. 11:1337972. doi: 10.3389/fmats.2024.1337972
Received: 14 November 2023; Accepted: 10 June 2024;
Published: 28 June 2024.
Edited by:
Jinyang Xu, Shanghai Jiao Tong University, ChinaReviewed by:
Naji Kharouf, Université de Strasbourg, FranceCopyright © 2024 Han and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baodi Han, aGJkX29ydGhvZG9udGlzdEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.