
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mater. , 15 July 2022
Sec. Environmental Degradation of Materials
Volume 9 - 2022 | https://doi.org/10.3389/fmats.2022.929639
This article is part of the Research Topic Nuclear Materials Degradation View all 6 articles
After a nuclear waste container buried 500–1,000 m underground, it gradually experiences the dual effects of groundwater infiltration and the decay heat of radioactive nuclear waste. The decay and heat release of nuclear waste will also result in temperature stress. At the same time, the groundwater will gradually saturate the buffer/backfill materials which will produce expansion stress, thus forming a typical thermal–water–stress multi-coupling environment in the geological disposal, forming the environment where the corrosion could happen. In comparison, the information obtained through laboratories, field tests, and natural simulations are limited. However, numerical simulation is very important to predict the changes of a near-field environment. On one hand, the numerical simulation can verify the corresponding experimental data in the early stages; on the other hand, it can also predict the long-term corrosion environment change. This article mainly summarizes the large-scale evolution of a typical corrosion environment obtained by numerical simulation under different deep geological conditions in various countries, focusing on the effects of temperature, saturation, oxygen content, and radiation, which provide a reference for the research on the evolution of important corrosion environments on the surface of a nuclear waste container.
Nuclear waste has attracted international attention because of its strong radioactivity, long life, high heat release, and high toxicity. How to deal with nuclear waste effectively and safely has become a key issue in nuclear waste disposal. At present, there are several disposal methods in the world, namely “freezing disposal”, “deep-sea disposal”, “hydraulic cage disposal”, “space disposal”, and “deep geological disposal” (Arup, 1985; Milnes, 1985; Bradley, 1997). It is generally approved that deep geological disposal is the most reliable and safe way (Duquette et al., 2009). In short, high-level radioactive nuclear waste is buried in a deep geological repository. Its depth is about 500–1,000 m. This “multi-barrier system” is formed as shown in Figure 1.
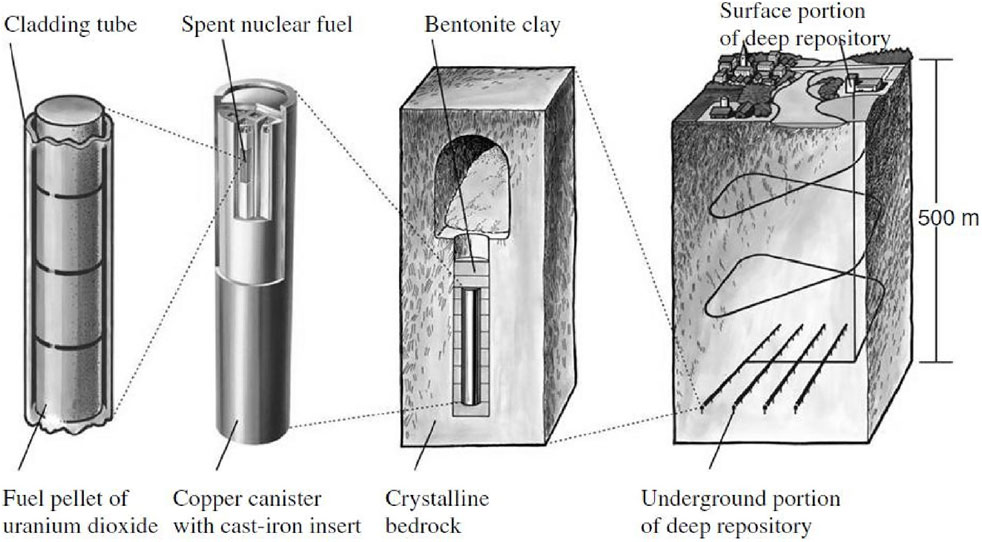
FIGURE 1. Multi-barrier system of high-level nuclear waste (Duquette et al., 2009).
The nuclear waste container is the first barrier to ensure the sealing of solidified high-level nuclear waste, which is particularly important. Various factors affecting its service life and the selection of container materials should be considered. Carbon steel is selected as the material of the nuclear waste container by South Korea, Spain, Japan, and other countries respectively, copper iron alloy is selected as the research material by Sweden and Finland, and stainless steel is selected by France, the United States, and Belgium (Rechard and Voegele, 2014). Obviously, it is impossible to carry out experimental research on container materials in the deep geological disposal environment and observe them for tens of thousands of years. At present, it is based on numerical simulations to predict the corrosion of the nuclear waste container on a large time scale. According to the production of high-level nuclear waste, each country determined the three-dimensional structure of the nuclear waste container and analyzed the time evolution of the corrosion environment on the surface of the container (Ouzounian et al., 2012).
Direct burial, bentonite buffer/backfill burial, and concrete buffer/backfill burial are the three methods for a nuclear waste container. Among them, Australia, France, and other countries (Sizgek, 2005; Kim et al., 2011; Wan et al., 2014) adopt the direct burial method. Bentonite is selected as the buffer backfill material mainly because of its good performance, strong adsorption, extremely low permeability, good expansion, and self-healing ability (Seetharam et al., 2006; Qiao et al., 2013). Sweden, Switzerland, Finland, the Czech Republic, Spain, Canada, Japan, and South Korea (Rasilainen, 2004; Zhang, 2007; Rosborg and Werme, 2008; Kim et al., 2011; Wan et al., 2014) use bentonite as the buffer backfill material. China will also choose bentonite backfill. At the same time, concrete can improve the alkalinity of the environment, so it can also be used as a buffer backfill material (Kursten and Druyts, 2008). Belgium (Kursten and Druyts, 2008; Yang et al., 2008) uses concrete as its backfill material.
Geological disposal environment is the most important factor affecting the service life and corrosion behavior of high-level nuclear waste containers. With increasing disposal time, when the groundwater is completely immersed in the container, it creates a near field environment where corrosion could happen. At the same time, the container will be subjected to the coupling action of thermal–water–stress during its service life, so that its corrosion environment is a complex environment that changes with the geological disposal year. When the disposal environment changes, the corrosive behavior of the container also has a certain causal relationship with the changes of its surrounding environment. Its environment mainly includes temperature, saturation of buffer/backfill materials, dissolved oxygen content, and radiation of nuclear waste. However, there are other factors, such as microbial influence, which are controversial. This factor is not introduced here. It is noticed that each country chooses the model according to the design of nuclear waste container, burial modes, and their geological environment. These are different in various countries. Table 1 shows the differences of the models in some countries.
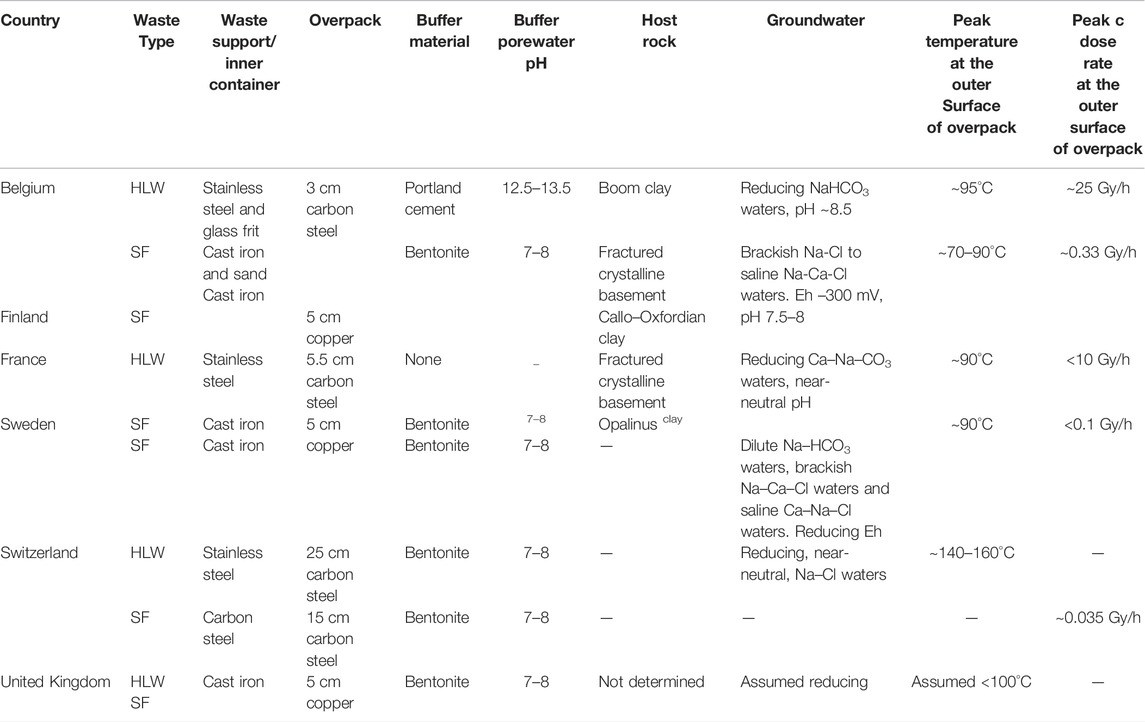
TABLE 1. Characteristics of the selected disposal systems (Bennett and Gens, 2008).
High-level nuclear waste has a long decay period, and its decay is often accompanied by a large amount of heat release, which affects the container and the surrounding environment. Studies have shown that the four main factors affecting the temperature change of deep geological environments are the buffer material and the decay heat of nuclear waste, the thermal properties, thickness, and the disposal interval of surrounding rocks (Shaw and Hund, 2009).
Finland (Rasilainen, 2004) drills holes into the nuclear waste repository with a diameter of 1.75 m and a depth of 7.5 m. They can be placed with a boiling water reactor (BWR) fuel container with an outer diameter of 1.05 m and a height of 3.55 m and a pressurized water reactor (PWR) fuel container with an outer diameter of 1.05 m and a height of 4.75 m. In this model, the spent fuel that has been temporarily stored for 30–50 years is used as the initial heat source to calculate the heat release power, and the maximum surface temperature limit of the fuel container is 373 K (Rasilainen, 2004).
P is the heat release power and ai is the coefficient of ti; t is the disposal year and ti is a disposal time, the same as below. The input parameters in the formula for the temperature evolution are related to the thermal conductivity and change with the disposal year.
Sweden (Rosborg and Werme, 2008) deals with a high-level nuclear waste container with a diameter of 1.05 m and a height of 4.833 m in vertical and horizontal disposals respectively. The difference between a horizontal and vertical disposal is that a layer of the steel container with an outer diameter of 1.765 m is added outside the bentonite buffer material for horizontal disposal. For this model, the release capacity of the nuclear waste is a time function of exponential superposition. The initial temperature of its surface is 288 K, and the maximum temperature is 373 K (Wan et al., 2014).
Canada (Wan et al., 2014) uses a container with a diameter of 1.247 m, a length of 3.909 m, and a spacing of 8 m for nuclear waste. The inner and outer shells of the container are high-pressure carbon steel and corrosion-resistant copper.
Belgium (Kursten and Druyts, 2008; Yang et al., 2008; Wan et al., 2014) first connects two containers with a glass-solidified body in series and puts them horizontally into the carbon steel container. The diameter of the container is about 0.5 m, the length is 2.8 m, and the thickness is 30 mm, and then puts them horizontally into the stainless steel super container.
Heat release capacity of the source in the model is (Wan et al.):
where Ai and λi are the correlation coefficients.
It is found that the exothermic heat decreases sharply in the first 100 years and then flattens. At the initial stage of deep geological disposal, the initial temperature of the surrounding rock is 289 K. Figure 2 summarizes the temperature evolution obtained by simulating nuclear waste storage in various countries. The temperatures were obtained according the fitting data in the model.
In Sweden, because bentonite is used as the backfill material, the maximum temperature of the container will appear in 7–10 years; the surface temperature of the Finnish container which has been buried for 2–4 years could reach 357.7 K; the maximum surface temperature of the container in Canada can reach 390.4 K in about 10 years; according to the simulation analysis, the temperature of the repository in Japan will rise rapidly at the beginning, reaching a peak of 84.3°C in about 19 years, then decrease to 54.2°C in about 100 years, and then change slowly, reaching 46.0°C in about 1,000 years.
Belgium adopts the container with concrete as the buffer backfill material. Its surface temperature rises rapidly in 3–5 years after disposal, and its peak temperature appears in about 5 years. Then, the temperature continues to decrease, but the cooling rate slows down. After about 100 years, the temperature finally flattens at about 309 K.
Researchers use different heat transfer formulas to create the models. The maximum temperature limit for nuclear waste in Australia is assumed to be 573 K. So, the temperature is the highest in Australia. But the overall trend is consistent; the maximum temperatures all appear in about 10 years.
The corrosion behavior of Q235 steel in a high pressure solid bentonite environment was studied (Zhang et al., 2019). The results show that the maximum corrosion rate occurs in the range of 343–363 K, and then decreases with the decrease of temperature. Stoulil et al. (2013) studied the effect of temperature on the corrosion rate of low carbon steel in a bentonite environment. The experiments were carried out at 313 and 363 K, respectively. The results show that the higher the temperature is, the denser the corrosion product layer is, the corrosion rate is slightly slower than that at 313 K, and the color of the corrosion products formed at different temperatures is different. Only the temperature is considered, there is an approximate prediction that the corrosion rate will first increase and then decrease.
The saturation of the buffer materials changes with the interaction of temperature and groundwater.
For the evolution of groundwater saturation, Switzerland has studied a short-term simulation by placing two heaters on the horizontal axis of the experimental disposal tunnel (Zhang and Li, 2012; Qin et al., 2013); between the heater and the disposal tunnel, the bentonite is used for backfill material, and its thickness is about 75 cm; at first, 1,200 W constant heat is provided, and finally, the maximum temperature of the contact surface between the heater and bentonite reaches 373 K; then, the temperature is controlled to be constant after 3 years, the heat source is cut off and removed after cooling for 6 months.
The temperature could reach its peak value on the 21st day after heating, and then the temperature tends to be stable. In the cooling stage (after 3 years), the temperature will drop sharply and then flatten. At the same time, the saturation decreases sharply to a certain value with time, and then reaches a stable value. When the heat source is turned off in the cooling stage, the saturation increases slowly.
Spain (Villar et al., 2005) conducted 5 years of simulated heating and 4 months of on-site temperature and humidity measurements during cooling. Bentonite is added between the container and the surrounding rocks, and its thickness is about 75 cm. In the initial heating process, the surface temperature increases sharply and reaches the peak value. Then, in the cooling process, it will take about 3 months to fall to room temperature. At first, the relative humidity decreases with the increase of temperature. In the heating stage, the relative humidity has been kept at a low level, but in the cooling stage, the relative humidity has increased. Therefore, it is found that the decay and heat release of nuclear waste and the infiltration of groundwater are important factors affecting the changes of the temperature and humidity near the container. The conclusion is similar to that of the Swiss simulation.
Japan (Zhang, 2007) conducted a long-term temperature and humidity simulation on the disposal of high-level nuclear waste and established the corresponding disposal model. Firstly, the disposal roadway with the same height and width is 5 m, and there is a distance of 10 m between the roadways. The buffer backfill material is added between the preliminarily treated solid waste and the hole, about 0.6 m thick. Finally, the tunnel needs to be sealed. The initial water content of the buffer layer and backfill material is 7% (saturation 0.28), so the water content at saturation is 25%.
Yim and Simonson (2000) studied the groundwater infiltration model. Because the seepage flow depends on the natural seepage at the site and the performance of the engineering barrier, its prediction needs to simulate the complete or partial failure components of the unsaturated water flow through the engineering barrier. The groundwater infiltration model determines the infiltration of water into the nuclear waste unit by tracking the water and its distribution at a site due to surface runoff, evaporation, and plant absorption/evaporation. The infiltration of water into nuclear waste is determined based on the flow through the covering system, concrete vault, and other barrier components. Due to the local changes of material properties and the transient characteristics of rainfall events, the accurate estimation of water flow is very complex. Therefore, it is simplified in the performance evaluation in order to obtain a reliable estimation of water flow. Darcy’s law describes the vertical steady-state flow of water infiltration in unsaturated areas (Yim and Simonson, 2000):
where K(ψ) indicates the hydraulic conductivity of the unsaturated region as a function of the indenter ψ. ∂h/∂z represents the hydraulic gradient in the vertical direction.
To describe the transient flow, the Richards equation was acquired by combining the Darcy equation with the continuity equation:
where θ is the water content of the soil medium.
Various relationships between hydraulic conductivity and water content as a function of pressure head can be obtained by the equation. Figure 3 shows Model of buffer material saturation for HLNW container in short and long terms in Japan and Switzerland.
Comparing the changes of environmental humidity near the container between Japan and Switzerland, it is found that the temperature change around the nuclear waste container has a significant impact on the water content of bentonite pore fluid through short-term simulation. However, since the heater is only used for 3 years, the subsequent saturation cannot be determined. Because the container surface environment model established by Japan is similar to the short-term simulation conducted by Switzerland in its early stage, it could be considered that Japan’s large time scale model is reasonable. The saturation of the buffer backfill material has not changed significantly (slightly decreased) for a period of time at the beginning of disposal, and increases significantly in about 3 years. And, the water content reaches saturation in about 10 years.
The simulation analysis of a near-field environment in deep geological disposal in various countries was summarized. At the same time, there are some small-scale experimental studies. It can be found that the near-field water content is not affected by groundwater infiltration after the container is just buried. The main factor affecting the water content is the sharp rise in temperature caused by heat release, which will lead to water evaporation and reduction. However, long-term groundwater infiltration continuously provides water, which can reach saturation in the later period. From the analysis results, the water content increases significantly within 3 years, and the saturation reaches the maximum value in about 10 years. The main reason for the inconsistency of the time to reach the maximum saturation is the difference of the controlled equations in the simulation process.
The amount of ions and conductivity which are related to the water content affects the corrosion behavior of the metal. Among the ions of groundwater, Cl− could destroy the passive film formed on the surface of metal materials, penetrate through the metal corrosion product layer, and react with the metal matrix. Gardiner and Melchers (2002) explained that the conductivity was the main factor determining the corrosion rate at low water content. The anodic process in the reaction is similar to the corrosion behavior in the atmosphere. Under low water content, a thick and continuous liquid film cannot be formed on the sample surface, which is greatly hindered by the difficulty of passivation and ion hydration. The electrode process is mainly controlled by the anode. There is an approximate prediction that the corrosion rate of the container material increases with the infiltration of groundwater.
The concentration of the chemical compositions of groundwater is different in various countries due to the difference of the geological environment. For example, Table 2 shows the major chemical compositions of a typical groundwater in the Beishan area of China, the preselected area for high-level nuclear waste disposal. Ions with great influence on the metal corrosion are Cl− and SO42-. There is little research about the evolution law of ions with the disposal year. Seetharam et al. (2006) studied the bentonite experiment in a small base. It was simulated that chloride and sulfate ions change with time. Figure 4 shows the concentration changes of Cl− and SO42-. The two ions both increase first and then tend to be stable, which is the result of the joint action of temperature and groundwater infiltration. However, there is no research in the effect of ion concentration change on the corrosive behavior of the nuclear waste container. Ion concentration change should be considered to assess the service life of the container accurately.
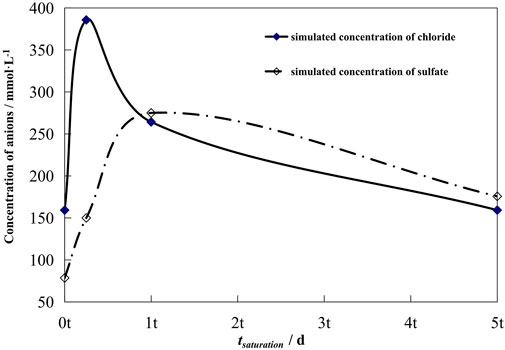
FIGURE 4. Concentration changes of Cl− and SO42- in the pore water of bentonite near the waste container (Seetharam et al., 2006).
Yang et al. (2007) studied the selected water–biological–geochemical coupling model and simulated the oxygen consumption process in the pore fluid of the buffer material after backfilling based on the Swedish nuclear waste container model. the initial saturated state of bentonite was assumed, and six cases were simulated. It is aimed to evaluate the biological and abiotic processes of oxygen absorption better. It mainly includes the diffusion of oxygen from bentonite to granite, the dissolution of chlorite in granite (ChlG), and the oxidation process of pyrite in granite and bentonite (PyrG&B) and the microbial oxygen consumption process (MicG_DocG). Figure 5 (Guo et al., 2010) shows the changes of oxygen content under different conditions.
It is obvious that it takes more than 5,000 years for the dissolved oxygen concentration to drop to a very low value only considering the diffusion effect. With the dissolution of chlorite, it will take about 1,000 years. If the dissolution of pyrite is added, it will take decades. The impact of microorganisms is ignored. However, microorganisms will affect granite, so it will take no more than 30 years. Furthermore, it only takes a few years considering the oxidation of DOC and methane in bentonite. These data indicate when oxygen will be exhausted after the closure of the nuclear waste repository, which will determinate the corrosion types including hydrogen evolution corrosion or oxygen absorption corrosion. Before the oxygen is exhausted, the cathodic reaction of corrosion is mainly oxygen absorption reaction. Once the oxygen is used up, hydrogen evolution corrosion dominates the cathodic reaction. Then, hydrogen embrittlement may happen.
Sweden (Sizgek, 2005) also analyzed the evolution of oxygen in a copper container when studying the long-term corrosion process. It was estimated that it would take about 10–300 years for the oxygen content to decrease rapidly. However, the latest research predicts that all oxygen in the groundwater will disappear 1 year after the closure of the repository.
At present, there are few studies on the evolution of oxygen content in concrete buffer materials over time. Atkinson et al. (1985) evaluated the durability of concrete as an engineering barrier in nuclear waste disposal and found that the corrosion rate of the metal container was affected by the rate of oxygen diffusion from concrete to metal materials. The diffusion coefficient of oxygen is estimated to be 10−9 m2 s−1, and the concentration of oxygen in groundwater is about 3 × 10−4 mol L−1 Bennett and Gens (2008) summarized the conceptual model of the European high-level nuclear waste repository. In the Belgian super container, the carbon steel outer package is protected by a buffer material composed of Portland cement concrete. The initial condition in the concrete buffer material is the oxidation state (the buffer material itself will be manufactured under the oxidation condition). They suggest that radiolysis has the potential to prolong the duration of the oxidation conditions in concrete buffers. Bouniol (2007) shows that when irradiation starts under oxidation conditions, the oxygen level in the concrete buffer will remain high and constant for at least more than 300 years by the radiation decomposition simulation. The model shows that this steady-state condition will be achieved quite quickly and should be relatively stable, so the oxidation condition can be maintained even when the disposal environment around the super container becomes anaerobic. It is noteworthy that the higher the temperature, the lower the solubility of oxygen. So, the oxygen content is affected by increasing temperature in the early disposal stage. The salts also influence the solubility of oxygen. According to Figure 4, if the salts’ changes are considered, oxygen will decrease faster before the groundwater is up to saturation. Once the oxygen is exhausted, hydrogen embrittlement of the container material may happen. Yasniy et al. (2011); Yasniy et al. (2013) found that a hydrogenation in the preloading stage decreased the fracture toughness of the heat-resistant steel and the crack start was a multi-level process, in which the defining role was played by the turning modes of deformation. However, Zhang et al. (2020) believed that the hydrogen permeation efficiency of titanium and its alloy decreased with the increasing geological disposal age. According to the researches (Panin et al., 2017; Maruschak et al., 2018), the long-term corrosive behavior of container steel may be based on short-term experiments. For example, the research performed the fractographic analysis of the corrosion damage on the pipe surface of the distribution gas pipeline after 40 and 56 years of operation and systematized the mechanisms of the formation of corrosion pits, laminations, and deep.
The oxygen content affects the corrosion rate of the container materials. Liu et al. (2019a); Liu et al. (2019b) respectively studied the corrosive behavior of X65 low carbon steel in the low-oxygen transition period and non-oxygen stage of geological disposal. The results show that the corrosion type is mainly uniform corrosion, the corrosion rate decreases obviously with the decrease of oxygen, and the corrosion process is controlled by diffusion. However, Winsley et al. (2011) believed that hydrogen evolution corrosion was dominant when the oxygen content around the container decreased to some degree. Therefore, the nuclear waste container may suffer from hydrogen embrittlement, which is much more severe than uniform corrosion.
The radiation of high-level nuclear waste will change its near-field environment. The radiation decomposition of groundwater is one of the most important near-field chemical effects of the nuclear waste repository (Hamilton, 1990). The International Atomic Energy Agency (IAEA) pointed out that the products of radiation decomposition of groundwater would accelerate the erosion and oxidation of radioactive waste packaging materials in 1981. Therefore, its oxidation resistance and corrosion resistance should first be considered in the selection of container materials (Roxburgh, 1987). It is well known that water decomposes due to ionizing radiation and produces free radicals (·H and OH). Its ionization occurs within 10–1–10–16 s and produces H2O+, H2O *, and e−, and these secondary electrons in turn will ionize and excite more water molecules (Dewhurst et al., 1954; Huang et al., 2013).
The number and charge of the cation and anion in the e-aq reaction determine the rate constant of the aforementioned reaction. The larger the charge of the anion, the smaller the rate constant. On the contrary, the more the charge of the cation, the higher the reactivity (Pikaev and Ershov, 1967). The strong oxidants in the radiolysis products of groundwater are H2O2, O3, HO2, and OH, and the reducing agents are H and H2 (Lu, 1981). Strong oxidants OH and H2O2 can have a wide range of chemical reactions, which can oxidize low valence metal ions in the underground environment to a high valence state, such as oxidizing Zn2+ to Zn3+; It can also oxidize anions to a high valence state, such as oxidizing Cl-to Cl2, which will change the redox properties of the system, especially H2O2, which has been considered as the most important oxidant in the process of radiation-induced dissolution of UO2-based nuclear fuel (Ekeroth et al., 2006; Lousada et al., 2013). Because H2O2 produced by the radiolysis of water will react with stable radionuclides in a geological environment, radionuclides will be oxidized to a higher valence state and will be unstable, so the fluidity will be enhanced (Huang et al., 2013). At the same time, Wei et al. researched that the corrosive behavior of the Q235 carbon steel in a groundwater simulation solution in the Beishan area of the Gansu Province under strong γ irradiation. The results showed that the irradiation decomposition of groundwater simulation solution leads to the enhancement of oxidation, the change of pH value from weak alkaline to acidic, and the decrease of conductivity (Wei et al., 2019).
Without exception, these studies sufficiently show that the highly active free radicals and molecular products produced by the radioactive decomposition of groundwater will play an important role in the chemical evolution of the near-field environment for the high-level nuclear waste in the deep geological disposal. However, the real environment is much more complex than the laboratory simulation environment. The actual deep geological disposal of high-level nuclear waste may reach tens of thousands or even millions of years, and its radiation dose also changes with time. Therefore, it is more difficult to predict the impact of radiation on groundwater on a large time scale, and most of the simulated results in the laboratory are short-term radiations at the maximum dose.
As a buffer backfill material for high-level nuclear waste containers, bentonite plays an extremely important role in fixing, supporting the container, and homogenizing the stress of the surrounding rocks. At the same time, it can also effectively delay or even prevent the infiltration and flow of groundwater near the container, and even block the migration of nuclides. According to the difference of vitrified solid waste, container, space layout, and thickness, it is predicted that the initial irradiation dose rate of the outer surface of high-level nuclear waste containers is about 0.2–2 Gy h−1 after the disposal, then it will be reduced by one order of magnitude every 100 years based on the model (Ekeroth et al., 2006; Lousada et al., 2013; Wei et al., 2019). The SI unit of the radiation dose is Gy, and 1 Gy is equivalent to 1 J kg−1. However, compared with the radiation decomposition of groundwater, the buffer material will be affected by radiation earlier in the near-field environment of the high-level nuclear waste repository, and will also be affected by radiation and decay heat for a long time. Therefore, it is also necessary to have a large-scale safety assessment of bentonite as a buffer backfill material. The safety evaluation can be carried out through the performance evolution of montmorillonite which is the main component of bentonite, focusing on some of its parameters, including water physical properties, swelling properties, and so on (Liang et al., 2015).
Researchers have studied on the denaturation of clay minerals under γ irradiation. The results show that it basically has no effect on the structure of clay even at high doses of γ irradiation, that is, the structure of clay is very stable (Plötze et al., 2003). Gu et al. studied the irradiation stability of montmorillonite and the temperature effect of its ion exchange capacity. The results showed that there was a certain relationship between the irradiation dose and the ion exchange capacity at different temperatures (Gu et al., 2001). Pusch et al. (1992) lowered the temperature between 403 and 363 K, and irradiated MX-80 bentonite at a dose rate of 456–3,972 Gy h−1 respectively for 1 year. The experimental results showed that only when it is heated to 403 K, the structure of some clays changes, but other parts do not change significantly. Liang et al. (2015) initially studied the structural changes of the modified sodium bentonite from Gaomiaozi, Inner Mongolia under different irradiation doses (1,000, 2000, 3,000, 4,000, and 5,000 kGy, respectively). The results showed although the high dose γ irradiation leads to the improvement of the heat resistance of sodium bentonite, it has little effect on the adsorbed water and interlayer water in sodium bentonite. According to the aforementioned research summary, it is found that Gaomiaozi-modified sodium bentonite has good stability. It is noticed that bentonite is selected as a buffer backfill material because of its good adsorption, very low permeability, and excellent expansion and self-healing ability. As one of the surrounding environments for the container, bentonite itself has not changed, but its saturation and oxygen contents have changed. Even under the influence of irradiation, it shows good stability.
Although there is little research on concrete as a buffer backfill material, concrete can improve the alkalinity of the environment, so it can also be used as a buffer backfill material. The most important influence of γ radiation on concrete is the hydrolysis process, that is, the radiation decomposition of concrete pore water, and the subsequent gas generation that will lead to adverse gas-pressure accumulation (Bouniol, 2004). In addition, it is more important to study the influence of γ radiation on the strength development of hardened cementitious materials or the strength of hardened samples. There are several effects: 1) It is found that 10% of the strength loss is related to the reduction of pore space due to the enhancement of the carbonization ability by gamma irradiation. 2)γ radiation can lead to the change of transport properties and the increase of carbonization depth. However, no changes in the macroscopic properties of the irradiated materials are found. 3) The use of blast furnace slag cement can lead to the formation of additional ettringite. 4) It is noted that irradiation enhances the alkali aggregate reaction if a large number of SiO2 aggregates are used. Therefore, limestone is the primary choice (Richardson et al., 1989; Tsuneki and Hitoshi, 2002; Vodák et al., 2005; Bar-Nes et al., 2008; Bart et al., 2012). In addition, the model of the radiation decomposition simulation carried out by bouniol (2007) shows that the oxidation conditions can be maintained even when the disposal environment around the nuclear waste container becomes anaerobic because of radiation.
Based on the aforementioned research, it can be considered that concrete is similar to bentonite. In most cases, the influence of γ radiation on concrete can be ignored from the perspective of mechanical properties. This is because the total radiation dose rate used in these studies is quite high (>200 Gy h−1), while the estimated radiation dose rate in the super container in Belgium is only 20 Gy h−1 (Poyet, 2007). According to the long-term evolution law of temperature and humidity in the near field environment of a nuclear waste container (Huang et al., 2018), the near-field temperature of the container reaches the highest after 10 years, and the highest temperature is 363 K in the process of deep geological disposal of high-level nuclear waste in China. Therefore, radiation will not have much effect on the structure of bentonite. At the same time, scientists have studied the effects of γ irradiation on the radiation-induced corrosion behavior of copper in a bentonite–water system (Norrfors et al., 2018), and its conditions are anaerobic. The results show that the presence of bentonite has no effect on the radiation-induced corrosion of copper in an anoxic water system. Therefore, the research on the impact of high-level nuclear waste on the medium environment of a container should focus on the situation after the buffer backfill material is saturated by groundwater.
The influences of nuclear waste radiation on the corrosion of container materials are mainly in two aspects. On one hand, the radiation decomposition of water results in an oxidizing environment. The results show the irradiation decomposition of groundwater simulation solution leads to the enhancement of oxidation, the change of pH value from weak alkaline to acidic, and the decrease of conductivity (Wei et al., 2019). On the other hand, radiation may change the electrochemical state of the container material. Smart et al. (2008) studied the effects of radiation on the corrosion behavior of carbon steel under anaerobic conditions. The corrosion rate increases most obviously at a higher dose rate. The corrosion products are mainly magnetite, some of which show that unidentified high oxidation corrosion products are formed at higher dose rates. The radiation intensity of nuclear waste decreases with the disposal year, so the corrosion rate will also decrease.
Metal corrosion is closely related to its environment. The corrosion behavior and service life of a nuclear waste container in deep geological disposal are mainly affected by temperature, saturation, oxygen content, and nuclear waste radiation.
The overall temperature follows the trend of increasing first and then decreasing. The saturation, oxygen content, and radiation intensity decrease with the increase of the disposal year. When simulating the corrosion behavior of a nuclear waste container in a deep geological environment, the change of a single influencing factor can be controlled, but the coupling effect of each influencing factor needs to be considered at the same time.
The evolution law of the surrounding environment was summarized, from which the researcher could choose the suitable nuclear waste repository environment for their country to carry out the corrosion experiments. Meanwhile, according to the evolution law, there is a rough forecast before the experiment.
At present, the influence of microorganisms on the corrosion of a nuclear waste container is controversial (the influence of microorganisms is related to the radiation of nuclear waste and container materials), so it is not introduced in the influencing factors.
The simulation research of the deep geological environment is just the beginning. There is still a lot of work to do about nuclear waste disposal.
QZ has written the draft. DX and BZ analyzed the data. YJ and JD provided guidance and funds. Other authors completed the revised manuscript, part of the experiments, and data.
This work was financially supported by the National Natural Science Foundation of China under Grant No. 51471160.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arup, O. (1985). Ocean Disposal of Radioactive Waste by Penetrator Emplacemen, Graham & Trotmon Let, for the Commission of European Communities. London.
Atkinson, A., Goult, D. J., and Hearne, J. A. (1985). An Assessment of the Long-Term Durability of Concrete in Radioactive Waste Repositories. MRS Proc. 50, 239. doi:10.1557/proc-50-239
Bar-Nes, G., Katz, A., Peled, Y., and Zeiri, Y. (2008). The Combined Effect of Radiation and Carbonation on the Immobilization of Sr and Cs Ions in Cementitious Pastes. Mater Struct. 41 (9), 1563–1570. doi:10.1617/s11527-007-9348-4
Bart, C., Geert, D. S., and Isabelle, G. (2012). Effect of Gamma Radiation and Elevated Temperatures on the Strength of Cementitious Barriers for Radwaste Disposa. J. Chin. Ceram. Soc. 40 (1), 33–38.
Bennett, D. G., and Gens, R. (2008). Overview of European Concepts for High-Level Waste and Spent Fuel Disposal with Special Reference Waste Container Corrosion. J. Nucl. Mater. 379 (1–3), 1–8. doi:10.1016/j.jnucmat.2008.06.001
Bouniol, P. (2007). Radiolysis within the Concrete of a Supercontainer Including Two Primary Waste Forms – Simulation at the Concrete/steel Interface at Variable Temperatures. French Atomic Energy Commission. Report No: RT DPC/ SCCME 07-742-A.
Bouniol, P. (2004). State of Knowledge on Radiolysis of Pore Water inside Cement Medium for Waste Containment and the Approach via Simulations. CEA. (in French), R-6069.
Bradley, D. J. (1997). Behind the Nuclear Curtain: Radioactive Waste-Management in the Former Soviet Union. USA: UN Battelle Memorial Institute.
Dewhurst, H. A., Samuel, A. H., and Magee, J. L. (1954). A Theoretical Survey of the Radiation Chemistry of Water and Aqueous Solutions. Radiat. Res. 1 (1), 62. doi:10.2307/3570180
Duquette, D. J., Latanision, R. M., Di Bella, C. A. W., and Kirstein, B. E. (2009). Corrosion Issues Related to Disposal of High-Level Nuclear Waste in the Yucca Mountain Repository-Peer Reviewers' Perspective. Corrosion 65 (4), 272–280. doi:10.5006/1.3319133
Ekeroth, E., Roth, O., and Jonsson, M. (2006). The Relative Impact of Radiolysis Products in Radiation Induced Oxidative Dissolution of UO2. J. Nucl. Mater. 355 (1-3), 38–46. doi:10.1016/j.jnucmat.2006.04.001
Gardiner, C. P., and Melchers, R. E. (2002). Corrosion of Mild Steel in Porous Media. Corros. Sci. 44 (11), 2459–2478. doi:10.1016/s0010-938x(02)00062-8
Gu, B. X., Wang, L. M., Minc, L. D., and Ewing, R. C. (2001). Temperature Effects on the Radiation Stability and Ion Exchange Capacity of Smectites. J. Nucl. Mater. 297 (3), 345–354. doi:10.1016/s0022-3115(01)00631-6
Guo, Y. H., Wang, J., Xiao, F., Wang, Z. M., Liu, S. F., Su, R., et al. (2010). Groundwater Formation in Beishan(Gansu)Preselected Area of High-Level Radioactive Waste Disposal Repository. Geol. J. China Univ. 16 (1), 13–18. doi:10.3969/j.issn.1006-7493.2010.01.003
Hamilton, E. I. (1990). Natural Analogues in Radioactive Waste Disposal. Sci. Total Environ. 92, 284–285. doi:10.1016/0048-9697(90)90340-z
Huang, X. L., Yang, B., and Liu, Y. B. (2013). Radiation Decomposition Process and Products of Water and Their Impact on the Environment. Energy Res. Manag. 4, 32–35.
Huang, Y. L., Zheng, M., Zhang, Q. C., Lu, D. Z., Wang, X. T., Sand, W., et al. (2018). Long Term Temperature and Humidity Evolution Forecast in Near Field of Nuclear Waste Container. Equip. Environ. Eng. 15 (10), 118–122. doi:10.7643/issn.1672-9242.2018.10.0018
Kim, J.-S., Kwon, S.-K., Sanchez, M., and Cho, G.-C. (2011). Geological Storage of High Level Nuclear Waste. KSCE J. Civ. Eng. 15 (4), 721–737. doi:10.1007/s12205-011-0012-8
Kursten, B., and Druyts, F. (2008). Methodology to Make a Robust Estimation of the Carbon Steel Overpack Life Time with Respect to the Belgian Supercontainer Design. J. Nucl. Mater. 379 (1), 91–96. doi:10.1016/j.jnucmat.2008.06.020
Liang, D., Liu, W., Yang, Z. T., Guo, X. L., Yang, W. B., Li, H. H., et al. (2015). Preliminary Study on Gamma Radiation Effects on Modified Sodium Bentonite. Acta Mineral. Sin. 35 (1), 103–106.
Liu, C., Tian, C., Zhang, Z., Wang, J., and Han, E. (2019a). Corrosion Behaivour of X65 Low Carbon Steel during Anaerobic Period of High Level Nuclear Waste Disposal. Corros. Sci. Prot. Technol. 31, 371–378. doi:10.11903/1002.6495.2018.239
Liu, C., Tian, C., Zhang, Z., Wang, J., and Han, E. (2019b). Corrosion Behaivour of X65 Low Carbon Steel during Redox State Transition Process of High Level Nuclear Waste Disposal. Acta Metall. Sin. 55, 849–858. doi:10.11900/0412.1961.2018.00481
Lousada, C. M., Trummer, M., and Jonsson, M. (2013). Reactivity of H2O2 towards Different UO2-Based Materials: The Relative Impact of Radiolysis Products Revisited. J. Nucl. Mater. 434 (1-3), 434–439. doi:10.1016/j.jnucmat.2011.06.003
Lu, D. R. (1981). Effect of Irradiation on Metal Corrosion. J. Chin. Soc. Corros. Prot. 1 (3), 37–47.
Maruschak, P., Poberezny, L., Prentkovskis, O., Bishchak, R., Sorochak, A., and Baran, D. (2018). Physical and Mechanical Aspects of Corrosion Damage of Distribution Gas Pipelines after Long-Term Operation. J Fail. Anal. Preven. 18 (3), 562–567. doi:10.1007/s11668-018-0439-z
Norrfors, K. K., Björkbacka, Å., Kessler, A., Wold, S., and Jonsson, M. (2018). γ-Radiation Induced Corrosion of Copper in Bentonite-Water Systems under Anaerobic Conditions. Radiat. Phys. Chem. 144, 8–12. doi:10.1016/j.radphyschem.2017.11.004
Ouzounian, G., Voinis, S., and Boissier, F. (2012). Radioactive Waste Management in France: Safety Demonstration Fundamentals. Ann. ICRP 41, 286–293. doi:10.1016/j.icrp.2012.06.026
Panin, S. V., Maruschak, P. O., Vlasov, I. V., Syromyatnikova, A. S., Bolshakov, A. M., Berto, F., et al. (2017). Effect of Operating Degradation in Arctic Conditions on Physical and Mechanical Properties of 09Mn2Si Pipeline Steel. Procedia Eng. 178, 597–603. doi:10.1016/j.proeng.2017.01.117
Pikaev, A. K., and Ershov, B. G. (1967). Primary Products of the Radiolysis of Water and Their Reactivity[J]. Russ. Chem. Rev. 36 (8), 1427–1459. doi:10.1070/rc1967v036n08abeh001675
Plötze, M., Kahr, G., and Stengele, R. H. (2003). Alteration of Clay Minerals—Gamma-Irradiation Effects on Physicochemical Properties. Appl. Clay Sci. 23 (1–4), 195–202. doi:10.1016/S0169-1317(03)00103-0
Poyet, S. (2007). Design of the ONDRAF/NIRAS Supercontainer Concept for Vitrified HLW Disposal in Belgium: Study of the Thermo-Hydrological Behaviour of the Concrete buffer[R]. Rapport. Report CEA, RT DPC/SCCME/07–741–7.
Pusch, R., Karnland, O., Lajudie, A., and Decarreau, A. (1992). MX 80 Clay Exposed to High Temperatures and Gamma Radiation (Technical Report TR-93-03). Sweden: Swedish Nuclear Fuel and Waste Management Co..
Qiao, L., Cao, S. F., Chen, L., Liu, Y. M., and Xie, J. L. (2013). Numerical Thermo-Hydro-Mechanical Modeling of Buffer Material in Mock-Up Test for Deep Geological Disposal. China Min. Mag. 22 (5), 117–121. doi:10.3969/j.issn.1004-4051.2013.05.028
Qin, A. F., Wang, H. T., and Zhao, X. L. (2013). Influence of Heat Source on Near Field Behavior of Nuclear Waste Repository. J. Shanghai Univ. 19 (5), 521–526. doi:10.3969/j.issn.1007-2861.2013.05.015
Rasilainen, K. (2004). Localisation of the SR 97 Process Report for Posiva’s Spent Fuel Repository at Olkiluoto. Finland: Posiva Oy.
Rechard, R. P., and Voegele, M. D. (2014). Evolution of Repository and Waste Package Designs for Yucca Mountain Disposal System for Spent Nuclear Fuel and High-Level Radioactive Waste. Reliab. Eng. Syst. Saf. 122, 53–73. doi:10.1016/j.ress.2013.06.018
Richardson, I. G., Groves, G. W., and Wilding, C. R. (1989). Effect of γ-Radiation on the Microstructure and Microchemistry of Ggbfs/OPC Cemet Blends. MRS Proc. 176, 31. doi:10.1557/proc-176-31
Rosborg, B., and Werme, L. (2008). The Swedish Nuclear Waste Program and the Long-Term Corrosion Behaviour of Copper. J. Nucl. Mater. 379 (1-3), 142–153. doi:10.1016/j.jnucmat.2008.06.025
Roxburgh, I. S. (1987). Geology of High-Level Nuclear Waste disposal[M]. New York, NY: Springer Science & Business Media.
Seetharam, S. C., Cleall, P. J., and Thomas, H. R. (2006). Modelling Some Aspects of Ion Migration in a Compacted Bentonitic Clay. Eng. Geol. 85 (1-2), 221–228. doi:10.1016/j.enggeo.2005.09.041
Shaw, Y. Y., and Hund, D. Y. (2009). Modeling Transient Heat Transfer in Nuclear Waste Repositories. J. Hazard. Mater. 169 (1-3), 108–112. doi:10.1016/j.jhazmat.2009.03.068
Sizgek, G. D. (2005). Three-dimensional Thermal Analysis of In-Floor Type Nuclear Waste Repository for a Ceramic Waste Form. Nucl. Eng. Des. 235 (1), 101–109. doi:10.1016/j.nucengdes.2004.09.010
Smart, N. R., Rance, A. P., and Werme, L. O. (2008). The Effect of Radiation on the Anaerobic Corrosion of Steel[J]. J. Nucl. Mater. 379 (1-3), 97–104. doi:10.1016/j.jnucmat.2008.06.007
Stoulil, J., Kaňok, J., Kouřil, M., Parschová, H., and Novák, P. (2013). Influence of Temperature on Corrosion Rate and Porosity of Corrosion Products of Carbon Steel in Anoxic Bentonite Environment. J. Nucl. Mater. 443, 20–25. doi:10.1016/j.jnucmat.2013.06.031
Tsuneki, I., and Hitoshi, K. (2002). Possibility of Radiation-Induced Degradation of Concrete by Alkali-Silica Reaction of Aggregates. J. Nucl. Sci. Technol. 39 (8), 880–884. doi:10.1080/18811248.2002.9715272
Villar, M. V., García-Siñeriz, J. L., Bárcena, I., and Lloret, A. (2005). State of the Bentonite Barrier after Five Years Operation of an In Situ Test Simulating a High Level Radioactive Waste Repository. Eng. Geol. 80 (3-4), 175–198. doi:10.1016/j.enggeo.2005.05.001
Vodák, F., Trtík, K., Sopko, V., Kapičková, O., and Demo, P. (2005). Effect of γ-irradiation on Strength of Concrete for Nuclear-Safety Structures. Cem. Concr. Res. 35 (7), 1447–1451. doi:10.1016/j.cemconres.2004.10.016
Wan, L., Sun, Q. H., Li, H. H., Liu, J., Zhao, S., and Liu, W. (2014). Investigation on the Near Field Temperature Evolution at Domestic and Overseas Nuclear Waste Repositories. Radiat. Prot. Bull. 34 (1), 12–19. doi:10.3969/j.issn.1004-6356.2014.01.003
Wei, Q. L., Liu, Y. B., Yang, B., Li, Y., and Huang, Y. (2019). Corrosion Behavior of Q235 Carbon Steel in Simulated Groundwater in Gansu Beishan Area with High-Strength γ Irradiation. Atomic Energy Sci. Technol. 53 (1), 59–66. doi:10.7538/yzk.2018.youxian.0643
Winsley, R. J., Smart, N. R., Rance, A. P., Fennell, P. A. H., Reddy, B., and Kursten, B. (2011). Further Studies on the Effect of Irradiation on the Corrosion of Carbon Steel in Alkaline Media. Corros. Eng. Sci. Technol. 46, 111–116. doi:10.1179/1743278210y.0000000010
Yang, C., Samper, J., Molinero, J., and Bonilla, M. (2007). Modelling Geochemical and Microbial Consumption of Dissolved Oxygen after Backfilling a High Level Radiactive Waste Repository. J. Contam. Hydrol. 93 (1-4), 130–148. doi:10.1016/j.jconhyd.2007.01.008
Yang, C., Samper, J., and Montenegro, L. (2008). A Coupled Non-isothermal Reactive Transport Model for Long-Term Geochemical Evolution of a HLW Repository in Clay. Environ. Geol. 53 (8), 1627–1638. doi:10.1007/s00254-007-0770-2
Yasniy, P. V., Okipnyi, I. B., Maruschak, P. O., Bishchak, R. T., and Sorochak, A. P. (2011). Toughness and Failure of Heat Resistant Steel before and after Hydrogenation. Theor. Appl. Fract. Mech. 56 (2), 63–67. doi:10.1016/j.tafmec.2011.10.001
Yasniy, P. V., Okipnyi, I. B., Maruschak, P. O., Panin, S. V., and Konovalenko, I. V. (2013). Crack Tip Strain Localisation on Mechanics of Fracture of Heat Resistant Steel after Hydrogenation. Theor. Appl. Fract. Mech. 63-64 (63-64), 63–68. doi:10.1016/j.tafmec.2013.03.007
Yim, M.-S., and Simonson, S. A. (2000). Performance Assessment Models for Low Level Radioactive Waste Disposal Facilities: A Review. Prog. Nucl. Energy 36 (1), 1–38. doi:10.1016/s0149-1970(99)00015-3
Zhang, D. B., and Li, Z. S. (2012). Numerical Analysis for Coupled Thermo-Hydro-Mechanical Processes in Near Field of Nuclear Waste Repository. J. Anhui Polytech. Univ. 27 (4), 75–79.
Zhang, Q., Huang, Y., Blackwood, D. J., Zhang, B., Lu, D., Yang, D., et al. (2020). On the Long Term Estimation of Hydrogen Embrittlement Risks of Titanium for the Fabrication of Nuclear Waste Container in Bentonite Buffer of Nuclear Waste Repository. J. Nucl. Mater. 533, 152092. doi:10.1016/j.jnucmat.2020.152092
Zhang, Q., Zheng, M., Huang, Y., Kunte, H. J., Wang, X., Liu, Y., et al. (2019). Long Term Corrosion Estimation of Carbon Steel, Titanium and its Alloy in Backfill Material of Compacted Bentonite for Nuclear Waste Repository. Sci. Rep. 9, 3195. doi:10.1038/s41598-019-39751-9
Keywords: metal corrosion, nuclear waste, environment evolution, radiation, buffer material
Citation: Zhang Q, Jiang Y, Zhao X, Chen J, Xia D, Zhang B and Duan J (2022) Research Progress on the Corrosive Environment Large-Scale Evolution for Nuclear Waste Container. Front. Mater. 9:929639. doi: 10.3389/fmats.2022.929639
Received: 27 April 2022; Accepted: 16 June 2022;
Published: 15 July 2022.
Edited by:
Hongliang Ming, Institute of Metal Research (CAS), ChinaReviewed by:
Long Hao, Corrosion and Protection Center of Materials (CAS), ChinaCopyright © 2022 Zhang, Jiang, Zhao, Chen, Xia, Zhang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jizhou Duan, ZHVhbmp6QHFkaW8uYWMuY24=; Yishan Jiang, anlzMTMwQDEyNi5jb20=; Dahai Xia, ZGFoYWl4aWFAdGp1LmVkdS5jbg==; Binbin Zhang, emhhbmdiaW5iaW5AcWRpby5hYy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.