- 1Faculty of Engineering Pamukkale University, Denizli, Turkey
- 2Faculty of Engineering, Afyon Kocatepe University, Afyonkarahisar, Turkey
Diabetes-related wounds are a significant problem with serious consequences for both patients and health care systems. The aim of this study is to produce healing films that will expedite the healing of diabetic wounds in order to minimize the negative effects experienced by diabetic patients. For this purpose, films were produced by combining chitosan, which possesses the ability to accelerate wound healing, silver nanoparticles, well-known for their superior properties such as preventing the occurrence of microbiological activity and providing thermal stability, and ascorbic acid, also referred to as vitamin C, which the body requires during the treatment process. The films were evaluated by applying a series of characterization analyzes (XRD, FTIR, Transmission Electron Microscopy, DSC-TGA) to the produced films. In addition, the films were subjected to microbiological tests. Following that, the films’ swelling and ascorbic acid release behaviors were investigated in deionized water and a phosphate buffered saline solution with pH 7.4, respectively.
1 Introduction
Diabetes is one of the most serious health problems in the modern era. Diabetes mellitus is a chronic disease that occurs as a result of the deficiency or ineffectiveness of the insulin hormone. Diabetes mellitus, which occurs due to factors such as poor eating habits, a more sedentary life as a result of technological advancements, and familial hereditary factors, has a rapidly increasing incidence. According to the Diabetes Atlas of the International Diabetes Federation (IDF), 1 in every 11 adults worldwide has diabetes. In addition, according to IDF data, 1 person dies every 6 s as a result of diabetes-related complications (Zimmet et al., 2014). Insulin deficiency as a result of diabetes has a direct effect on the blood and vessels, causing significant damage to the epithelial tissue and resulting in the formation of chronic wounds, one of the most serious complications of diabetes in the body. These chronic wounds become infected very quickly and, if left untreated, produce bone inflammation (osteomyelitis) in the wound. The primary methods used in the treatment of diabetes wounds are physical methods such as hyperbaric oxygen (HBO), negative pressure wound, ozone, tropical oxygen, electrical stimulation, electromagnetic current therapy, and phototherapy. Due to the inadequacies of therapeutic physical methods, diabetic patients face a variety of complications such as various disabilities, psychosocial traumas, and an inability to meet health care costs. Moreover, the progression of an infection that can occur as a result of untreated wounds to the bone may threaten the patient’s life. In order to eliminate this danger and prevent the infection from spreading throughout the body, the patient’s injured organ patient is cut off (amputation) (Greenhalgh, 2003). While amputation prevents the infection from spreading to the rest of the body, it has been observed that such an intervention can result in various traumas to develop in some patients. Different complications have also been shown to be triggered by the elevated stress experienced by patients as a result of traumas. Chitosan is an aminopolysaccharide produced by the conversion of acetylamino (-NH-CO-CH3) groups on chitin, a natural polysaccharide obtained from shellfish, to amino (-NH2) groups, that is, via deacetylation of chitin (Fu et al., 2018). In addition to its antimicrobial properties, chitosan is a non-toxic, biocompatible, and biodegradable substance. Due to these qualities, chitosan is frequently employed in the pharmaceutical industry and medical applications (Reddy et al., 2016; Jayaramudu et al., 2020). When chitosan is applied to the bleeding site, it acts as a coagulant by attracting erythrocyte cells to the wound via electrostatic interactions. For this reason, it is utilized to block the flow of blood in surgeries or military injuries. Besides having hemostatic properties, it exhibits antimicrobial capacity. Because of its polycationic nature, it binds to negatively charged bacterial surfaces, disrupts the integrity of the bacterial cell membrane, increases cell permeability, and neutralizes bacteria. In addition, chitosan inhibits the cell’s protein synthesis by altering the DNA of microorganisms (Goy et al., 2009). Nano-sized silver particles are widely used in medical applications and implants due to their broad-spectrum antimicrobial activity and non-toxicity to bacteria, fungi and viruses (Dakal et al., 2016; Fahmy et al., 2017; Taha et al., 2022; Yin et al., 2020; Bruna et al., 2021). Studies have demonstrated that silver alters and inhibits the effects of cellular enzymes and DNA. It has been determined that Ag (I) ions bind to and neutralize electron-carrying compounds such as thiols, carboxylates, amides and imidazoles found in the enzyme and DNA structures of microorganisms (Taha et al., 2022). Ascorbic acid (AA) is used as an antioxidant due to its ability to retain oxygen. Immunity is thought to weaken during infections due to the decrease in the level of AA in white blood cells (Carr and Maggini, 2017). AA also performs a variety of functions in the body’s operation (Yin et al., 2022). Recent studies have revealed that ascorbic acid is involved in the synthesis of collagen, a connective tissue. AA is also believed to play a role in strengthening blood vessels; in the lack of AA, blood vessels weaken and bleeding may occur with small strokes. In the literature, no published studies combining these three substances with superior properties were found. Therefore, the purpose of this study is to combine these three materials with favorable and attractive properties for diabetic wound healing. Thus, although chitosan films containing Ag nanoparticles (AgN) cover the wound and prevent biological activation, it is aimed that a wound healing effect will be observed via the release of AA. In this way, it will be ensured that the healing process of the wound is accelerated in the optimum environment and that the negative effects experienced by the patient are eliminated at the lowest cost. For this reason, chitosan films containing AgN and AA were prepared for this investigation. The produced films were then subjected to several characterization analyses (XRD, FTIR, TEM, TGA, DSC) and microbiological analyses, and their swelling and the films’ AA release behavior were investigated.
2 Materials and Methods
2.1 Materials
Silver nitrate (AgNO3, Merck, extra pure) and trisodium citrate (C6H5Na3O7, Merck, %99) were utilized in the production of AgN. In the preparation of films, chitosan (Sigma Aldrich), glycerol (Sigma Aldrich), gelatin (Sigma Aldrich), and sodium bicarbonate (NaHCO3, Merck) were used. For the AA supply, Redox-C (500 mg/5 ml, Bayer) was used.
2.2 Production of Silver Nanoparticles (AgN)
The method developed by Turkevich et al. (1951) was used to synthesize AgN particles. While 60 ml of 1 mM silver nitrate solution was mixed using a magnetic stirrer, the solution was also heated. 6 ml of 10 mM trisodium citrate was added dropwise to the solution once it reached 90°C. Meanwhile, the solution was mixed and it was maintained at a constant temperature of 90°C. Following the completion of the addition process, the solution was briefly stirred and left to cool to room temperature. TEM analyses of the prepared samples were performed.
2.3 Production of Chitosan Films Loaded AgN and AA
Chitosan films were prepared by modifying the method devised by Moe et al. (Bruna et al., 2021). Chitosan (w/w, 1, 2.5 and 5%) was mixed for 1–2 h to dissolve in AA solution (1, 2.5, 5% etc.) (Liping et al., 2020). After the addition of 1 ml of the AgN solution, gelatin dissolved in 10 ml of warm water was added. While mixing continued, 7 ml of 5% aqueous sodium bicarbonate was added dropwise, and the pH was controlled during the process. It was ensured that the final solution had a pH of 6. The mixture was then added to with 2 g glycerol solution which was dissolved in 10 ml water and it was mixed for 5–10 min, after which the films were left to dry at ambient temperature. Dried films were stored at +4°C.
2.4 Characterization of Chitosan Films
Different analysis methods were used to characterize the chitosan films produced under three different conditions. X-Ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) analyzes were performed to examine the structural properties of the films, and morphological properties were determined using Transmission Electron Microscopy (TEM) for the grain size analysis of AgN particles. XRD analysis was performed using the Bruker Brand D 8 Advance instrument, FTIR analysis using a Perkin Elmer 400 spectrophotometer, and TEM analysis using the FEI Tecnai G2 Spirit Biotwin instrument. The temperature dependent behavior of the films was determined via Differential Scanning Calorimetry and Thermogravimetric Analysis (DSC/TGA, Netzsch STA 449 F3 Jupiter instrument). In addition, the antimicrobial properties of the films were examined via an antimicrobial test (Staphylococcus aureus/Escherichia coli) according to ISO 16649-2 and ISO 6888 standards. AA release studies were performed using a UV-Vis spectrophotometer (Shimadzu brand, UVmini-1240 model).
2.5 Swelling Behavior of Chitosan Films
The swelling behavior of the chitosan films was investigated using a pH 7.4 phosphate buffered saline (PBS) solution. For this, films of a certain mass were left in 50.0 ml of PBS solution and the mass of the film that was removed from the solution at certain intervals of time was measured. The % swelling values of the films were calculated using the following equation (Baş et al., 2014; Jayaramudu et al., 2017).
Where, Ws is the mass of the swollen film (g), W0 is the mass of the dry film (g).
2.6 AA Release of Films
Because skin wounds have a pH greater than 6 (Schneider et al., 2007), a phosphate buffered saline (PBS) solution with pH 7.4 was used for AA release studies. A certain amount of samples collected from the films were kept in PBS solution, and certain amounts of samples were removed from the solution at certain time intervals to determine the amount of AA passed into the solution using the UV-Vis spectrophotometric method. The data obtained were processed via calculating in terms of mass of AA released per unit mass of film (mg/g) as shown in the following equation (Şen and Yakar, 2001).
Where; CAA is the AA concentration released (mg/L), W is the weight of the film (g), V is the volume of PBS (L).
3 Results and Discussion
3.1 Production of Chitosan Films Loaded AgN and AA
Chitosan films were prepared by modifying the method devised by Moe and Khaing (2014). A series of experiments were carried out in order to optimize the preparation process of the chitosan films under laboratory conditions. 1.0, 2.5 and 5.0% chitosan concentrations were mixed for 1–2 h to dissolve in 1.0% (w/w) (2.5%, 5.0%, etc.) AA solution. However, 1.0, 2.5 and 5.0% AA solutions were observed to be insufficient to dissolve 2.5 and 5.0% (w/w) chitosan. For this reason, the percentage of chitosan was reduced to the lowest concentration available and the percentage of AA was increased. In the experiments, the rate of chitosan was kept at a constant of 1.0% (w/w) and the rate of AA varied between 5.0, 7.5, and 10.0% (w/w). In the initial studies, 1.0 ml of the AgN solution obtained was added to this solution, but it was observed that adding 1.0 ml of AgN solution did not prevent the films from developing bacterial formations. Because of that, the amount of AgN added to the chitosan films was gradually increased up to 16.0 ml. Since it was observed in the studies that the amount of gelatin added to the film was not sufficient to ensure film formation, 3.0 g of gelatin dissolved in 10.0 ml of warm water was added to the solution. During stirring, 7 ml of 5% aqueous sodium bicarbonate was added to the medium drop by drop to reduce the acidity of the solution and maintain a certain pH level. Thus, the pH of the final solution was ensured to be approximately 6.0. Then, 2.0 g glycerol solution diluted in 10.0 ml of water was added to the mixture to give the film flexibility. Following this addition, the mixture was stirred for an additional 5–10 min. The solution was transferred to Petri dishes and kept at +4°C.
3.2 Characterization Analyzes
3.2.1 Transmission Electron Microscopy Analysis of AgN
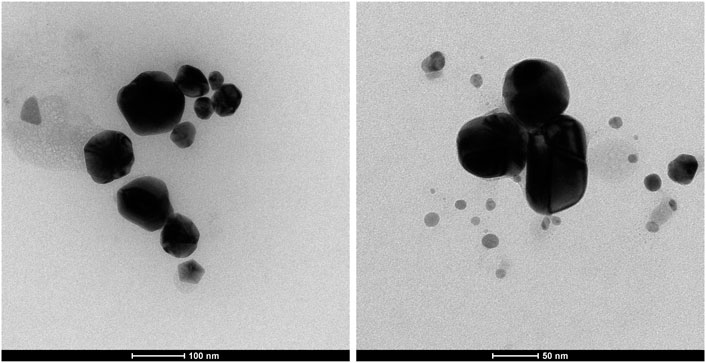
Image analysis of the produced AgN was performed by using TEM (Figure 1).
As seen in Figure 1, the sizes of AgN particles were less than 100 nm. In fact, it is demonstrated in the TEM image that the very small AgN particles grow in size as they agglomerate. The cause of this was interpreted as the particles in the sample interacting with one another and gradually increasing in size as a result (Fahmy et al., 2016; Fahmy et al., 2020). However, it was determined that the obtained silver particles were in nanoparticles.
3.2.2 X-Ray Diffraction Analysis of Chitosan Films
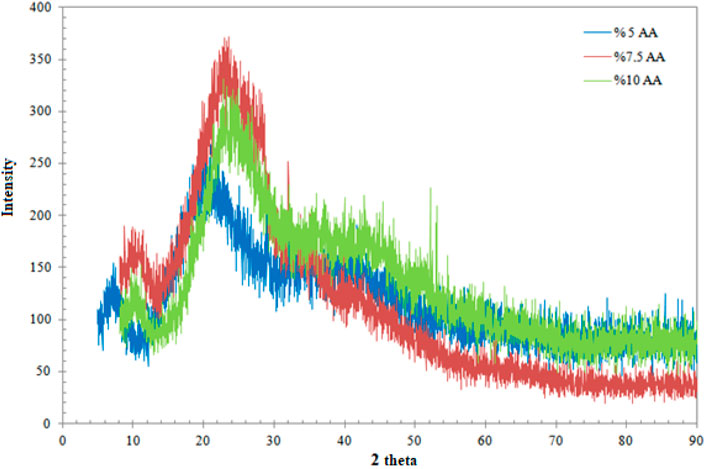
The XRD method was used in the structural analysis of chitosan films produced using three different AA compositions. The patterns obtained during the analysis are given in Figure 2.
When Figure 2 is examined, it is seen that the XRD patterns of the three films, each with different AA concentrations, are quite similar to each other. The resulting image is an amorphous structure. However, it is seen in the literature that the severe broad peak between 20 and 30 thetas is attributed to chitosan (Chen et al., 2007; Akmaz et al., 2013). Additionally, the peaks of AgN are unfortunately not visible. This is viewed as a result of the extremely low amount of AgN used in the production of the films.
3.2.3 Fourier Transform Infrared Spectroscopy Analysis of Chitosan Films
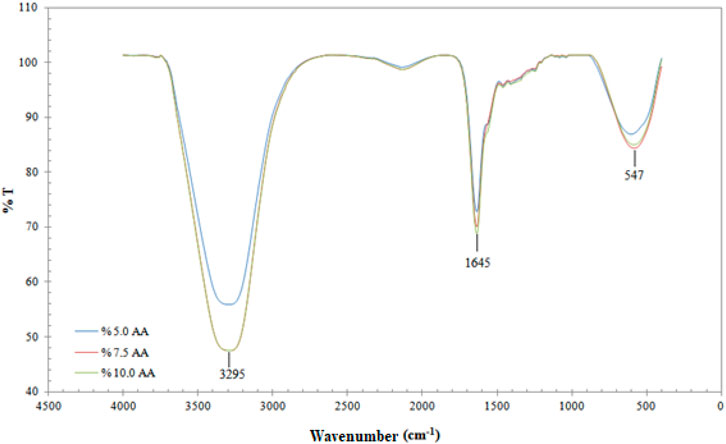
FTIR analysis was performed to determine the functional structure of the produced films. The FTIR spectra of the films are given in Figure 3.
When Figure 3 is examined, the film with three different compositions can be observed to possess sharp peaks in the same regions. The flat peak observed at 3,295 cm−1 indicates the presence of -OH. In addition, the sharp peak observed at 1,645 cm−1 can be attributed to the -NH bending vibration band (Ahmad et al., 2011). In the wavelength range of 1,600–1,000 nm, as well as the N-H bending vibration of the primary amine, vibrations of OH, CH in the ring and C-O-C and C-O stretching vibrations, regarded as fingerprints for the saccharide structure of chitosan, were detected (Kumirska et al., 2010; Sailakshmi et al., 2013; Gharaie et al., 2018; Drabczyk et al., 2020). The peaks seen at 547 cm−1 belong to the Ag-O stretching vibration band (Yong et al., 2013).
3.2.4 TGA-DSC Analysis of Chitosan Films
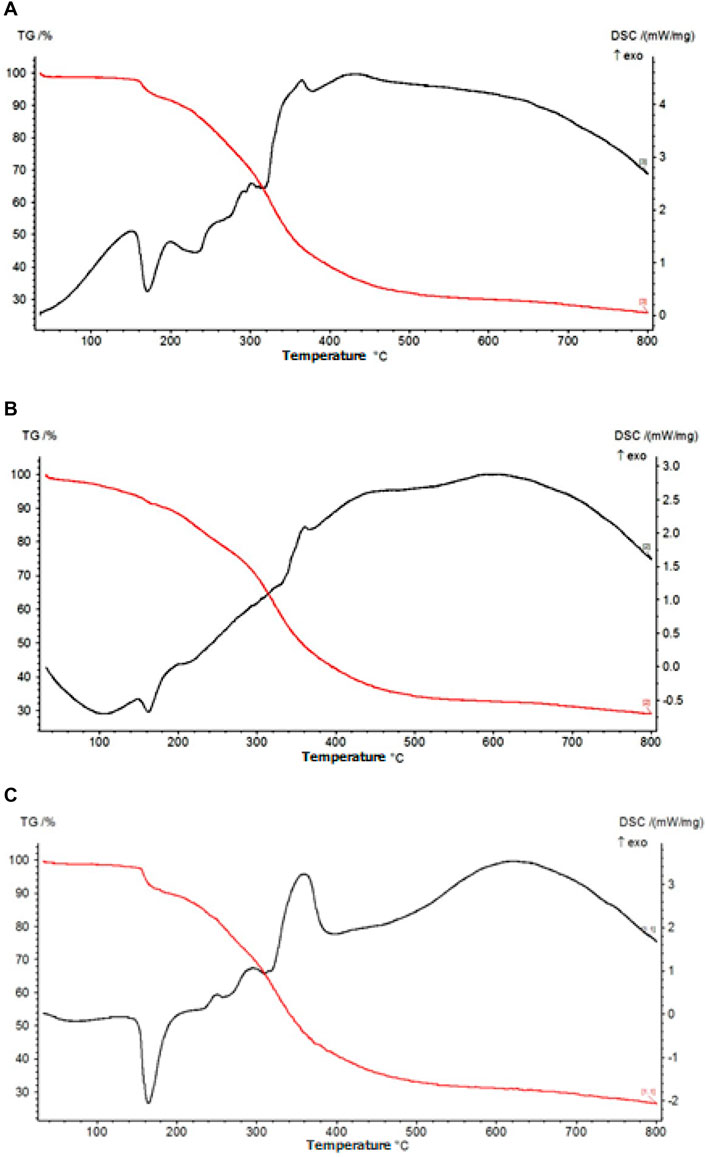
In order to examine the behavior of three different AA-containing films under varying temperature conditions, analyses were performed via the TGA and DSC methods. Measurements were taken at a heating rate of 20°C/min and in an inert atmosphere. The obtained thermograms are given in Figure 4.
When Figure 4 is examined, it is seen that the three thermograms are very similar to each other. Only a change in the peak intensities was observed due to the alteration of the composition of the films. In the TG graph, the first mass loss started at approximately 160°C and a total mass loss of 10% occurred. According to the DSC graph, this loss is due to an endothermic reaction that causes the water molecules in the films to move away from the structure (Valencia et al., 2014; Metzler et al., 2015). As illustrated in Figure 4, the second degradation occurs gradually in the range of 240–400°C. The degradation reactions occurring here are exothermic. The graph indicates that the glycosidic bonds within the body of chitosan, a polysaccharide, are broken and the chain is reduced at temperatures in the range of 240–400°C (Valencia et al., 2014; Metzler et al., 2015). At temperatures above 400°C, the structure completely deteriorated. It is seen from the TG graph that the total mass loss was approximately 75% for all three films.
3.2.5 Microbiological Analyzes of Chitosan Films
Microbiological analyzes were performed for chitosan films containing three different amounts of AA by a provincial control accredited laboratory. Microbiological analyses for Escherichia coli (E. coli) and Staphylococcus aureus (Staph. aures), which are pathological bacteria particularly found in wounds, were performed according to ISO 16649-2 and ISO 6888 standards, respectively. The results for both bacteria were negative for all three samples. Chitosan is a potent antimicrobial agent due to its cationic characteristics and antimicrobial character (Goy et al., 2009; Ahmed and Ikram 2016). Chitosan is a substance that has been approved for antimicrobial activity with three antibacterial mechanisms having been identified. One is the interaction of the chitosan ionic groups with the bacteria cell wall. Another is chitosan’s ability to penetrate the nuclei of microorganisms, initiating the inhibition of the protein synthesis. The last one is its ability to form chelating metals, suppressing the intake of essential nutrients for microbial growth (Goy et al., 2009). On the other hand, the antimicrobial effects of silver nanoparticles have been defined in 7 different ways (Yin et al., 2020). First, silver nanoparticles can adhere to and can pass through the cell wall. Secondly, silver ions interact with ribosome, denaturing them and inhibiting protein synthesis. Thirdly, silver ions activate respiratory enzymes in the cytoplasmic membrane, stopping ATP synthesis. Fourth, the membrane is damaged by the resulting reactive oxygen species. Fifth, silver nanoparticles and reactive oxygen species bind to DNA and inhibit cell proliferation. Sixth, they accumulate on the cell wall, causing denaturation of the membrane. Seventh, they directly enter the cytoplasmic membrane (Durán et al., 2016; Noronha et al., 2017; Liao et al., 2019; Yin et al., 2020).
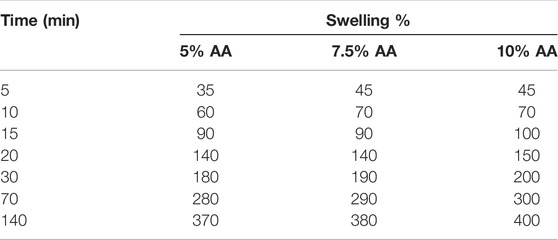
3.2.6 Swelling Behavior of Chitosan Films
The swelling behavior of the films containing three different amounts of AA in a PBS solution with a pH of 7.4 was examined over time. The obtained data are graphically presented in Table 1.
The swelling behavior of the films is critical for determining the AA release behavior. This is because the more the film swells, the more likely it is that the AA it contains will be released. However, it is possible to see structural deterioration in films that absorb a large amount of water. When Table 1 is examined, the swelling of the films, that is, the diffusion of water into them, occurs quite rapidly during the first minutes. After a length of time, the amount of water absorbed by the films reached equilibrium. All films swelled by absorbing water molecules at a rate of approximately 400% of their mass in about 140 min. The structural integrity of the films was also seen to be preserved. Kim et al. (2003) conducted their study using semi-interpenetrating polymer network hydrogels consisting of chitosan and polyacrylonitrile. In this study, the swelling behavior of the hydrogels obtained by mixing chitosan and polyacrylonitrile in different proportions were investigated. From the experimental results, they found that the swelling behavior of the hydrogels increased with the increase of the chitosan ratio in the hydrogels. They assert that this is due to the large number of sites in chitosan capable of binding to water. Felinto et al. (2007) prepared hydrogels using polyethylene glycol (PEG 300 and 400) and chitosan under ultraviolet light (UV) and c-radiation curing, at room temperature. In their study, they investigated the swelling behavior of these hydrogels. They determined that the hydrogel made from chitosan–PEG 400 exhibited a maximum swelling of 530% within 120 min at a 9.0 pH value. Findings have been interpreted as indicating that ionized carboxylic acid groups repel each other electrostatically at higher pH levels, thus increasing the swelling ratio.
3.3 AA Release Studies
AA release studies were carried out using chitosan films containing three different amounts of AA in the PBS solutions of 7.4 pH, and the data obtained were graphed and presented in Figure 5.
When Figure 5 is examined, it is seen that AA release increases with increasing AA content. At the same time, long-term release is a desirable property for controlled drug release. The polysaccharides in the chitosan structure can interact with and incorporate ascorbic acid into its structure with the help of hydrogen bonding (Ahmed and Ikram, 2016). It was determined that the amount of AA release increased over time and reached its equilibrium value (∼0.2 mg/g) in films in approximately 6 days. Desai and Park (2005) demonstrated that cross-linked chitosan microspheres exhibited nearly 96% AA release in 30 min. The obtained results will serve as a guide for future studies (Desai and Park, 2005). Kaczmarek et al. (2016) determined that the highest concentration of AA based on chitosan was released after 4 h of immersion in PBS.
4 Conclusion
In this study, preparation methods of chitosan films and their AA release behaviors were investigated. Firstly, films containing silver nanoparticles were produced to determine production conditions. Then, ascorbic acid, which was employed as a therapeutic agent in the study, was added to the films at various concentrations, and the films were characterized using different methods. Furthermore, microbiological analyzes of the films revealed that they were antibacterial against pathogenic bacteria. In the release studies, it was observed that the release kinetics occurred in a regulated manner and that drug delivery reached equilibrium in 6 days. The release investigations indicate that one of the fundamental parameters affecting the release behavior of chitosan films is their AA content. According to the experimental results, chitosan films containing silver nanoparticles loaded with AA are suitable for use as a bandage in the treatment of diabetes. To conclude, the films prepared in this study can be considered as potential healing materials for diabetic wounds and may be employed especially for local therapeutic transdermal applications.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
ND and AY manufactured the samples, performed the measurements, processed the experimental data, performed the analysis. AY also was involved in planning and supervised the work, drafted the manuscript and designed the figures, aided in interpreting the results and worked on the manuscript. All authors discussed the results and commented on the manuscript.
Funding
This study was supported by TÜBİTAK (Project No. 1919B011603227). The authors would like to thank METU Central Lab. and TUAM at Afyon Kocatepe University for characterization analyses.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, M. B., Lim, J. J., Shameli, K., Ibrahim, N. A., and Tay, M. Y. (2011). Synthesis of Silver Nanoparticles in Chitosan, Gelatin and Chitosan/Gelatin Bionanocomposites by a Chemical Reducing Agent and Their Characterization. Molecules 16, 7237–7248. doi:10.3390/molecules16097237
Ahmed, S., and Ikram, S. (2016). Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 10, 27–37. doi:10.1016/j.als.2016.04.001
Akmaz, S., Adıgüzel, E. D., Yasar, M., and Erguven, O. (2013). The Effect of Ag Content of the Chitosan-Silver Nanoparticle Composite Material on the Structure and Antibacterial Activity. Adv. Mat. Sci. Eng. 2013, 6. doi:10.1155/2013/690918
Baş, N., Yakar, A., and Bayramgil, N. P. (2014). Removal of Cobalt Ions from Aqueous Solutions by Using Poly(N,N-dimethylaminopropyl Methacrylamide/itaconic Acid) Hydrogels. J. Appl. Polym. Sci. 131 (7), 39569.
Bruna, T., Maldonado-Bravo, F., Jara, P., and Caro, N. (2021). Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 22, 7202. doi:10.3390/ijms22137202
Carr, A., and Maggini, S. (2017). Vitamin C and Immune Function. Nutrients 9, 1211. doi:10.3390/nu9111211
Chen, P., Song, L., Liu, Y., and Fang, Y.-e. (2007). Synthesis of Silver Nanoparticles by γ-ray Irradiation in Acetic Water Solution Containing Chitosan. Radiat. Phys. Chem. 76, 1165–1168. doi:10.1016/j.radphyschem.2006.11.012
Dakal, T. C., Kumar, A., Majumdar, R. S., and Yadav, V. (2016). Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 7, 1831. doi:10.3389/fmicb.2016.01831
Desai, K. G. H., and Park, H. J. (2005). Encapsulation of Vitamin C in Tripolyphosphate Cross-Linked Chitosan Microspheres by Spray Drying. J. Microencapsul. 22, 179–192. doi:10.1080/02652040400026533
Drabczyk, A., Kudłacik-Kramarczyk, S., Głąb, M., Kędzierska, M., Jaromin, A., Mierzwiński, D., et al. (2020). Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials 13, 3073. doi:10.3390/ma13143073
Durán, N., Nakazato, G., and Seabra, A. B. (2016). Antimicrobial Activity of Biogenic Silver Nanoparticles, and Silver Chloride Nanoparticles: an Overview and Comments. Appl. Microbiol. Biotechnol. 100 (15), 6555–6570. doi:10.1007/s00253-016-7657-7
Fahmy, A., Eisa, W. H., Yosef, M., and Hassan, A. (2016). Ultra-Thin Films of Poly(acrylic acid)/Silver Nanocomposite Coatings for Antimicrobial Applications. J. Spectrosc. 2016, 1–11. doi:10.1155/2016/7489536
Fahmy, A., El-Zomrawy, A., Saeed, A. M., Sayed, A. Z., El-Arab, M. A. E., Shehata, H. A., et al. (2017). One-step Synthesis of Silver Nanoparticles Embedded with Polyethylene Glycol as Thin Films. J. Adhesion Sci. Technol. 31 (13), 1422–1440. doi:10.1080/01694243.2016.1259728
Fahmy, A., Jácome, L. A., and Schönhals, A. (2020). Effect of Silver Nanoparticles on the Dielectric Properties and the Homogeneity of Plasma Poly(acrylic Acid) Thin Films. J. Phys. Chem. C 124, 22817–22826. doi:10.1021/acs.jpcc.0c06712
Felinto, M. C. F. C., Parra, D. F., da Silva, C. C., Angerami, J., Oliveira, M. J. A., and Lugão, A. B. (2007). The Swelling Behavior of Chitosan Hydrogels Membranes Obtained by UV- and γ-radiation. Nucl. Instrum. Methods Phys. Res. B 265, 418–424. doi:10.1016/j.nimb.2007.09.025
Fu, J., Yang, F., and Guo, Z. (2018). The Chitosan Hydrogels: from Structure to Function. New J. Chem. 42, 17162–17180. doi:10.1039/c8nj03482f
Gharaie, S. S., Habibi, S., and Nazockdast, H. (2018). Fabrication and Characterization of Chitosan/gelatin/thermoplastic Polyurethane Blend Nanofibers. J. Eng. Fibers Fabr. 1, 1–8. doi:10.1177/2515221118769324
Goy, R. C., Britto, D. d., and Assis, O. B. G. (2009). A Review of the Antimicrobial Activity of Chitosan. Polímeros 19, 241–247. doi:10.1590/S0104-14282009000300013
Greenhalgh, D. G. (2003). Wound Healing and Diabetes Mellitus. Clin. Plastic Surg. 30, 37–45. doi:10.1016/s0094-1298(02)00066-4
Jayaramudu, T., Varaprasad, K., Kim, H. C., Kafy, A., Kim, J. W., and Kim, J. (2017). Calcinated Tea and Cellulose Composite Films and its Dielectric and Lead Adsorption Properties. Carbohydr. Polym. 171, 183–192. doi:10.1016/j.carbpol.2017.04.077
Jayaramudu, T., Varaprasad, K., Reddy, K. K., Pyarasani, R. D., Akbari-Fakhrabadi, A., and Amalraj, J. (2020). Chitosan-pluronic Based Cu Nanocomposite Hydrogels for Prototype Antimicrobial Applications. Int. J. Biol. Macromol. 143, 825–832. doi:10.1016/j.ijbiomac.2019.09.143
Kaczmarek, B., Sionkowska, A., and Markiewicz, E. (2016). L-ascorbic Acid Release from Polymeric Matrixes Based on Blends of Chitosan, Collagen and Hyaluronic Acid. Mol. Cryst. Liq. Cryst. 640, 46–53. doi:10.1080/15421406.2016.1255509
Kim, S. J., Shin, S. R., Lee, Y. M., and Kim, S. I. (2003). Swelling Characterizations of Chitosan and Polyacrylonitrile Semi-interpenetrating Polymer Network Hydrogels. J. Appl. Polym. Sci. 87, 2011–2015. doi:10.1002/app.11699
Kumirska, J., Czerwicka, M., Kaczyński, Z., Bychowska, A., Brzozowski, K., Thöming, J., et al. (2010). Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 8, 1567–1636. doi:10.3390/md8051567
Liao, C., Li, Y., and Tjong, S. (2019). Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 20 (2), 449. doi:10.3390/ijms20020449
Liping, L., Kexin, L., Huipu, D., Jia, L., and Jie, Z. (2020). Study on Preparation of a Chitosan/Vitamin C Complex and its Properties in Cosmetics. Nat. Prod. Commun. 15, 1–9. doi:10.1177/1934578x20946876
Metzler, M., Chylińska, M., and Kaczmarek, H. (2015). Preparation and Characteristics of Nanosilver Composite Based on Chitosan-Graft-Acrylic Acid Copolymer. J. Polym. Res. 22, 146. doi:10.1007/s10965-015-0781-8
Moe, T. S., and Khaing, T. A. (2014). Lactic Acid-Chitosan Films’ Properties and Their In Vivo Wound Healing Activity. Int. J. Med. Health, Biomed. Bioeng. Pharm. Eng. 9, 633–637. doi:10.5281/zenodo.1096618
Noronha, V. T., Paula, A. J., and Durán, G. (2017). Silver Nanoparticles in Dentistry. Dent. Mater 33 (10), 1110–1126. doi:10.1016/j.dental.2017.07.002
Reddy, A. B., Manjula, B., Jayaramudu, T., Sadiku, E. R., Anand Babu, P., and Periyar Selvam, S. (2016). 5-Fluorouracil Loaded Chitosan–PVA/Na+MMT Nanocomposite Films for Drug Release and Antimicrobial Activity. Nano-Micro Lett. 8, 260–269. doi:10.1007/s40820-016-0086-4
Sailakshmi, G., Mitra, T., and Gnanamani, A. (2013). Engineering of Chitosan and Collagen Macromolecules Using Sebacic Acid for Clinical Applications. Prog. Biomater. 2, 11. doi:10.1186/2194-0517-2-11
Schneider, L. A., Korber, A., Grabbe, S., and Dissemond, J. (2007). Influence of pH on Wound-Healing: a New Perspective for Wound-Therapy? Arch. Dermatol. Res. 298, 413–420. doi:10.1007/s00403-006-0713-x
Şen, M., and Yakar, A. (2001). Controlled Release of Antifungal Drug Terbinafine Hydrochloride from poly(N-Vinyl 2-pyrrolidone/itaconic Acid) Hydrogels. Int. J. Pharm. 228, 33–41. doi:10.1016/s0378-5173(01)00804-3
Taha, I. M., Zaghlool, A., Nasr, A., Nagib, A., El Azab, I. H., Mersal, G. A. M., et al. (2022). Impact of Starch Coating Embedded with Silver Nanoparticles on Strawberry Storage Time. Polymers 14, 1439. doi:10.3390/polym14071439
Turkevich, J., Stevenson, J., and Hillier, P. C. (1951). A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 11, 55–75. doi:10.1039/df9511100055
Valencia, C. S., Castañón, A. M., Gutiérrez, M., Ruiz, F., Vázquez, J. F., Rueda, A. M., et al. (2014). Characterization and Biocompatibility of Chitosan Gels with Silver and Gold Nanoparticles. J. Nanomater. 2014, 11. doi:10.1155/2014/543419
Yin, I. X., Zhang, J., Zhao, I. Z., Mei, M. L., Li, Q., and Chu, C. H. (2020). The Antibacterial Mechanism of Silver Nanoparticles and its Application in Dentistry. Int. J. Nanomed. 15, 2555–2562. doi:10.2147/ijn.s246764
Yin, X., Chen, K., Cheng, H., Chen, X., Feng, S., Song, Y., et al. (2022). Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technolog. Antioxidants 11, 153. doi:10.3390/antiox11010153
Yong, N. L., Ahmad, A., and Mohammad, A. W. (2013). Synthesis and Characterization of Silver Oxide Nanoparticles by a Novel Method. Int. J. Sci. Eng. Res. 4, 155–158.
Keywords: ag nanoparticles, chitosan films, diabetes wounds, ascorbic acid, vitamin C
Citation: Yakar A and Dede N (2022) Production and Characterization of Healing Polymeric Films for Diabetes Patients’ Wounds. Front. Mater. 9:910761. doi: 10.3389/fmats.2022.910761
Received: 01 April 2022; Accepted: 06 June 2022;
Published: 24 June 2022.
Edited by:
Robert Li, City University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Jayaramudu Tippabattini, Centre of Polymer and Carbon Materials (PAN), PolandAlaa Fahmy, Al-Azhar University, Egypt
Copyright © 2022 Yakar and Dede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arzu Yakar, YXJ6eWFrQGdtYWlsLmNvbQ==
 Arzu Yakar
Arzu Yakar Nur Dede2
Nur Dede2