- 1Department of Chemistry, School of Science, University of Management and Technology, Lahore, Pakistan
- 2Department of Chemistry, School of Natural Sciences (SNS), National University of Science and Technology (NUST), Islamabad, Pakistan
- 3Department of Pharmaceutical Sciences, College of Pharmacy, Almaarefa University, Riyadh, Saudi Arabia
- 4Department of Pharmaceutics and Industrial Pharmacy, College of Pharmacy, Taif University, Taif, Saudi Arabia
- 5Department of Pharmaceutics and Pharmaceutical Technology, Taif University, Taif, Saudi Arabia
- 6Chemistry Department, Faculty of Science, King Khalid University, Abha, Saudi Arabia
- 7Biology Department, Faculty of Science, King Khalid University, Abha, Saudi Arabia
- 8Department of Semi Pilot Plant, Nuclear Materials Authority, Cairo, Egypt
- 9Ibn-E-Sina Institute of Technology, Islamabad, Pakistan
This work includes green synthesis of magnesium oxide nanoparticles (MgO NPs) by using Alstonia scholaris, which is indigenous to many countries such as China, Australia, Sri Lanka, Pakistan, and India. Its pharmacological activities include antidiabetic, antioxidant, anticancer, analgesic, antitussive, and anti-diarrheal activities. In this study, the antioxidant and anti-inflammatory activities of bio-inspired magnesium oxide nanoparticles, MgO NPs, were investigated. MgO NPs were prepared by using the leaf extract of Alstonia scholaris, followed by characterization using EDX, XRD, and SEM techniques. The crystallite size of magnesium oxide nanoparticles was 19.57 nm. XRD analysis confirmed the crystallinity and the purity of MgO NPs. Anti-inflammatory activity was carried out to observe inhibition of protein denaturation. Since the IC50 of MgO nanoparticles was lower than the standard, it was found to be more effective. IC50 values were compared, and results reveal that bioinspired MgO NPs undergo more scavenging of free radicals than standard (ascorbic acid) MgO NPs. These MgO nanoparticles are useful in cosmetics such as scrubs, moisturizers, and an active ingredient in microdermabrasion and in formulating effective drugs for maintaining the protein structure of the body, which will reduce inflammation.
Introduction
Nanotechnology is considered to be an extension of existing sciences into the nanoscale, having a range of 1–100 nm. It is a broad field that covers different eras, including applied physics, material sciences, colloidal science, and supramolecular chemistry, and the field of engineering. The main theme of this technology is to deal with matter on a molecular level, having a scale range less than 1 mm or usually 1–100 nm. Research and advancement in nanotechnology are subjects of interest these days across all scientific disciplines and industries. Examples of this field commonly used nowadays include the production of polymers built on molecular structure and the plan of computer chips based on surface science. Innovative applications of nanotechnology include the fields of energy, medicine and drugs, nanodevices, optical engineering, defense and security, bioengineering, cosmetics, and nanofabrics (Nasrollahzadeh et al., 2019). According to its nature, nanotechnology is considered to be a highly integrative field. The future of nanotechnology is vast as many new materials can be created at the nanoscale with a huge range of applications. The various fields where nanotechnology is playing a vital role include medicine, electronics, and material science.
The particles having a diameter of 1–100 nm in at least one spatial dimension are known as nanoparticles. The properties of nanoparticles differ from those of bulk materials. The two factors that affect the properties include the surface-to-volume ratio and size of particles. Quantum confinement, large surface area, and high surface energy are some of the reasons for nanoparticle distinctive optical, magnetic, and electrical capabilities. Nanoparticles exhibit a great variety in its chemical nature and can be made up of metals commonly reported to be Ag, Au, Cu, and Zn and made up of metal oxides, carbon, polymer, or silicates. Nanoparticles exhibit a variety of shapes such as cylindrical shapes, spheres, and sheets or in the form of tubes (Yu et al., 2014). The properties depend on their size, shape, structure, reactivity, and toughness (Khan et al., 2019). Some properties that are affected by size are bandgap, structural properties, melting point, thermal properties, mechanical properties, chemical, electronic, magnetic, and optical properties as well.
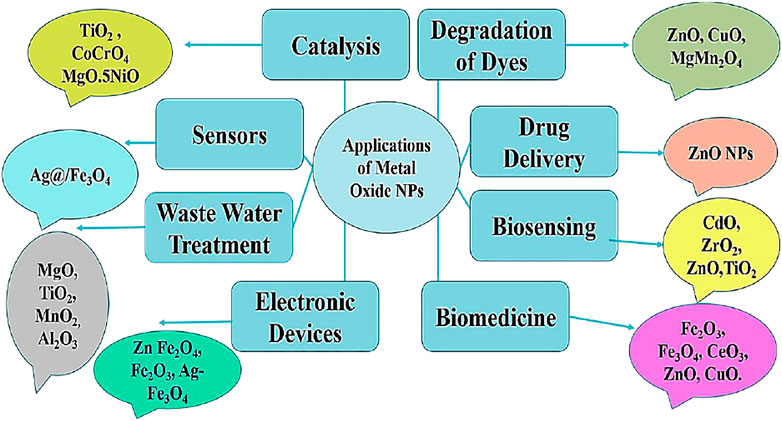
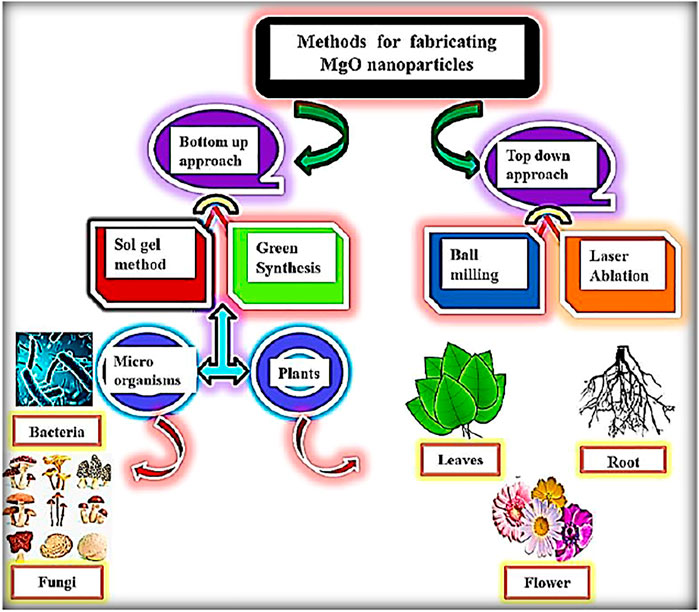
Metal oxide nanoparticles (MO NPs) are of great importance nowadays because of their wide range of applications Figure 1. Successful applications of MO NPs mainly depend on the narrow particle size (Oskam, 2006; Parashar et al., 2020). Four steps that are important in the controlled synthesis of MO NPs are the formation of the precursor, nucleation, aging, and growth (Jolivet et al., 2000). The applications of MO NPs are in the fields of catalysis, sensors, electronic devices, biosensing, degradation of dyes, biomedicine, and wastewater treatment (Taghavi Fardood et al., 2019a; Shojaei Yeganeh et al., 2020; Eskandari Azar et al., 2020; Taghavi Fardood et al., 2019b; Moradnia et al., 2020; Taghavi Fardood et al., 2020; Atrak et al., 2019; Shayegan Mehr et al., 2018; Mosallanejad et al., 2021; Moradnia et al., 2019; Singh et al., 2019; Matinise et al., 2018; George et al., 2018; Solanki et al., 2011; Parnianchi et al., 2018; Chavali and Nikolova, 2019; Naseem and Durrani, 2021a; Yang et al., 2013; Naseem and Durrani, 2021b; Lizundia et al., 2020; Dizaj et al., 2014; Vinardell and Mitjans, 2015; Rehana et al., 2017; Khan et al., 2018). MgO, MnO2, TiO2, Fe3O4, Al2O3, and CeO2 are some of the MO NPs studied by Jubai et al. for their potential application in wastewater treatment. Figure 2 shows three approaches for producing MgO nanoparticles: chemical, physical, and biological. Because of its environmentally favorable effects, green synthesis to create nanoparticles is gaining a lot of interest these days among academics. Greener technologies, including the use of biological substrates, are increasingly replacing the traditionally utilized chemical and physical approaches (Bandeira et al., 2020; Abinaya et al., 2021).
Alstonia scholaris is an evergreen tropical tree commonly known as the blackboard tree. It is abundantly available in Australia, China, India, Pakistan, and Sri Lanka. It is a medicinal plant as it is used for the treatment of various diseases (Khyade et al., 2014). The phytochemicals (alkaloids) present in this plant are picrinine, scholaricine, vallesamine, and epis-cholaricine (Zhao et al., 2021). Various plant parts such as stems, wood, bark, and leaves have their applications. It is advantageous in the sense that wood is useful in pencil fabrication, making boxes for a corpse, and tools used in the house. The outermost portion of the stem, and the root, is used to deal with constantly recurring skitters and expelling disease-causing organisms. Extract of the leaves is used for their biomedical applications. Alstonia scholaris is an effective herb against inflammatory disorders as an antioxidant drug, for catalytic degradation of dyes, and cytotoxic activity. (Shang et al., 2010; Dhruti et al., 2016; Rajasekar et al., 2021; Sarkar et al., 2021; Yaseen et al., 2021). The interesting fact revealed after the literature search is that Alstonia scholaris is effective against two or more symptoms of COVID-19 (Kumar et al., 2021). This work reports the antioxidant and anti-inflammatory response of synthesized NPs.
In this study, we synthesized MgO nanoparticles by using magnesium salt. Like some other inorganic metal oxide nanoparticles, MgO nanoparticles are nontoxic to organisms, economically feasible, and have wide industrial and biological applications (Hornak et al., 2018). Some of the unique physicochemical properties that make MgO NPs more worthwhile include an excellent refractive index, resistance to corrosion (Abinaya et al., 2021), high thermal conductance (Pilarska et al., 2017), highly pure, showing less electrical conductivity (Pendyala et al., 2019), and excellent transparency. MgO nanoparticles can be used in the fields of electronics, catalysis, ceramics, biosensors, and wastewater treatment. Before the abovementioned applications, MgO possessed biological applications such as antibacterial, antioxidant, anti-inflammatory, and anticancer properties (Dobrucka, 2018; Umaralikhan and Jamal Mohamed Jaffar, 2018; Behzadi et al., 2019; Pendyala et al., 2019; Khan et al., 2020; Ammulu et al., 2021). In this work, MgO nanoparticles were fabricated using green synthesis by Alstonia scholaris. Various plant parts of Alstonia scholaris show a variety of pharmacological activities, while in this work, the antioxidant and anti-inflammatory activity of biologically synthesized MgO nanoparticles were examined.
Experimental
All the chemicals used in this work, Mg (NO3)2.6H2O, NaOH, ethanol, DMSO, NaCl, KCl, Na2HPO4, KH2PO4, DPPH, methanol, H2SO4, ascorbic acid, and ammonium molybdate, were acquired from Sigma-Aldrich in Darmstadt, Germany, and are of analytical quality. Alstonia scholaris was discovered in Green Town, Lahore, Pakistan. Figure 3 shows the fresh leaves of Alstonia scholaris.
To eliminate unbound phyto-constituents, the leaves of A. scholars were first rinsed with tap water to remove dust and then washed three times with distilled water. The washed leaves were then dried under shade for almost 7 days or until complete drying. Completely dried leaves were then pulverized to obtain powder form, as shown in Figure 4. For the extraction of plant material, 10 g of pulverized powder was taken in a 500-ml beaker, followed by the addition of 150 ml of distilled water. It was stirred on a magnetic stirrer along with heating at 65°C for 45 min, followed by cooling at room temperature. It was filtered in a 250-ml conical flask by using Whatman filter paper.
For the green synthesis of MgO nanoparticles, 60 ml of the plant extract was taken in a 500-ml conical flask and placed on a magnetic stirrer. A 0.05 M magnesium nitrate hexahydrate solution was taken in the burette, and it was added drop by drop into the conical flask containing the extract. The color of the extract turned yellow. After that, 2 M NaOH was added and the formation of precipitate occurred rapidly. All the steps were carried out at 75°C for 1 h and 30 min. By adding sodium hydroxide, yellow colloidal particles were formed. It was filtered to extract precipitates, followed by washing with ethanol. The washed precipitates were placed in the oven for drying at 110°C for 30 min, followed by calcination in the Muffle Furnace at 600°C for 3 h. After calcination, white precipitates were obtained after calcination. The biologically synthesized nanoparticles were then characterized by spectroscopy techniques such as XRD, SEM, and EDX. The anti-inflammatory and antioxidant performances of MgO-fabricated nanoparticles were evaluated by UV–Vis spectroscopy.
Antioxidant Assessment
Antioxidant Assessment by the DPPH Method
As a standard, 50, 125, 250, and 500 ppm solutions of MgO nanoparticle and ascorbic acid were produced. To the aforementioned solution, 4 ml of 0.1 mM methanolic DPPH solution was added. After mixing the suspension, it was permitted to rest for 25 min at 26°C. Using methanol as a blank, the absorbance was observed at 516 nm. As a negative control, a methanolic DPPH emulsion was utilized. The mean value was computed after three repetitions of the operation. The following formula was used to compute the percentage scavenging of both the standard and the samples:
where Asample is the absorbance of the sample and A control is the absorbance of the control.
IC50 was calculated for both the standard (ascorbic acid) and the sample.
Antioxidant Activity by the Phosphomolybdenum Method
Following that, a reagent mixture was prepared by combining 16.7 ml H2SO4, 5.3 g of sodium phosphate, and 2.5 g of ammonium molybdate with 300 and 500 ppm of both MgO nanoparticle and standard (ascorbic acid). 4 ml reagent solution was combined with the MgO NP solution and standard and then maintained at 95°C for 1 h and 30 min in a water bath with the reagent solution in a distinct vial. Its absorbance was determined at 695 nm after cooling compared to a blank of the reagent suspension. The IC50 was determined for both the standard and the sample, and the results were compared to see which was more effective against free radicals: the standard or the MgO nanoparticles. The following formula was used to determine the percentage scavenging:
where Asample is the absorbance of the sample and A control is the absorbance of the control.
Anti-inflammatory Activity
Sample Preparation
MgO nanoparticles were diluted to 100 and 500 parts per million in 0.3 ml of egg albumin and 2.9 ml of phosphate-buffered saline at a pH of 6.4. After 20 min of incubation at 37°C, the mixture was heated for 6 min at 70°C. DMSO was used as a blank to test the absorbance after cooling at 660 nm. The following formula was used to determine protein denaturation inhibition (Dey et al., 2011; Das et al., 2019):
where V is the absorbance,
Vsample is the absorbance of the sample, and
Vcontrol is the absorbance of the control.
Standard Sample Preparation
Diclofenac sodium 100 and 500 ppm solutions were prepared. After that, 2.9 mL phosphate-buffered saline (pH 6.4) and 0.3 ml of egg albumin were added, and the mixture was incubated for 20 min at 37°C before being heated for 6 min at 70°C. DMSO was used as a blank to test the absorbance after cooling at 660 nm. The following formula was used to compute the percent inhibition of protein denaturation:
where V is the absorbance, Vsample is the absorbance of the sample, and Vcontrol is the absorbance of the control.
Results and Discussion
X-Ray Diffraction Analysis
The biologically fabricated MgO NPs were analyzed by XRD (XRD, Analytical X’PertPro). The graph is plotted between 2θ and intensity. A powder XRD was performed to analyze the crystallinity and purity of the sample. The peaks on the graph were observed at 2θ values of 29.2°, 36.8°, 42.75°, 62.15°, 74.45°, 78.4°, and 93.9°. Some more intense and some less intense peaks are observed in the XRD pattern. XRD studies confirmed the crystalline nature of biologically synthesized MgO nanoparticles. An XRD graph of synthesized MgO nanoparticles is shown in Figure 5.
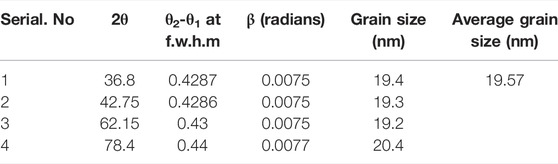
The average size of the crystal was 19.57 nm as calculated by the Debye Scherrer equation (Dz XRD= kλ/βcosθ). Using the Scherrer formula, the average size of the MgO nanoparticles was determined as shown in Table 1.
where λ is radiation wavelength, k is the constant value equal to 0.9, θ is the angle of deflection, and ß is the peak width (calculated at half the height of the peak in radian).
Calculation of grain size by XRD data.
Particle size = 0.9λ/βcosθ.
Where β(radian) = θ2-θ1 at full width, half maximum/57.3.
Scanning Electron Microscope Analysis
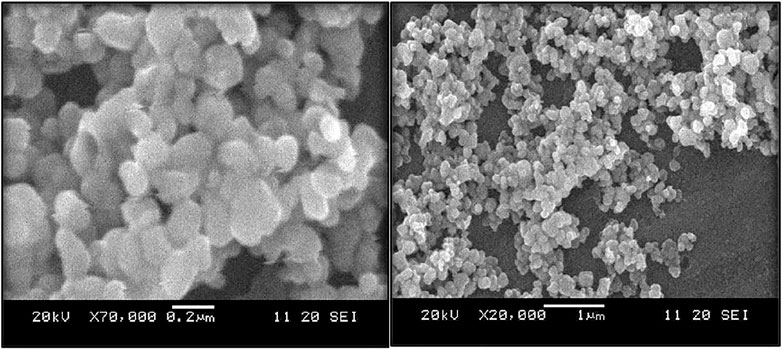
Figure 6 shows an SEM analysis of the particle size, structure, and morphology of MgO nanoparticles. The 1120 SEI Scanning Electron Microscope was used to conduct the study. The system was set to a 20 kV voltage. The particles ranged in size from 100 to 150 nm. The particles were nanosized and almost spherical. The particle distribution was consistent, and the majority of them were clumped together.
Energy-Dispersive X-Ray Analysis
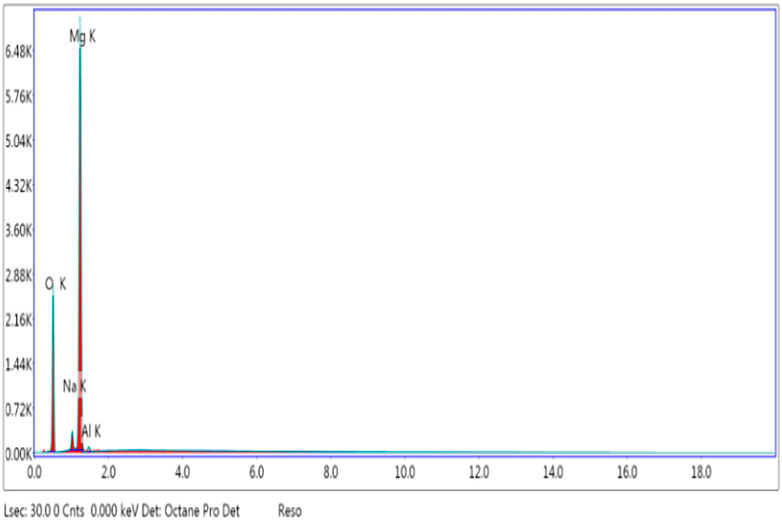
The sample’s percentage composition and purity were determined using EDX analysis. The results shown in EDX spectra Figure 7 reveal that Mg and O are present in the sample with the highest percentage, which confirms the formation of MgO nanoparticles.
Antioxidant Performance
Antioxidant Performance by the DPPH (2,2-Diphenyl-1-picrylhydrazyl) Method
Many diseases such as cancer, diabetes, cardiovascular disorders, cell toxicity, and arthritis occur due to the accumulation of free radicals in the body. Free radicals are highly unstable as they have unpaired electrons that cause damage to the cells. Production of free radicals is a chain reaction as if one radical is produced; it goes and hits other atoms or molecules. Similarly, an electron is lost which again becomes a free radical. This free radical then hits the next molecule to form another radical. In this way, the reaction continues. Antioxidants are molecules that have enough stability to donate an electron to form a stable compound and reduce the harmful effects caused by free radicals. In this work, the efficiency of biologically fabricated MgO to act as an antioxidant compound is checked by the DPPH assay. Usually, the antioxidant present in the sample reacts with DPPH and reduces it to DPPH-H. The scavenging potential of the antioxidant compound was examined by the degree of discoloration. DPPH absorbs strongly at 517 nm and has a deep purple color. This color changes to pale yellow or colorless upon reacting with the antioxidant. The results of the scavenging ability of the standard (ascorbic acid) and biologically synthesized MgO nanoparticles are listed in Tables 2, 3.
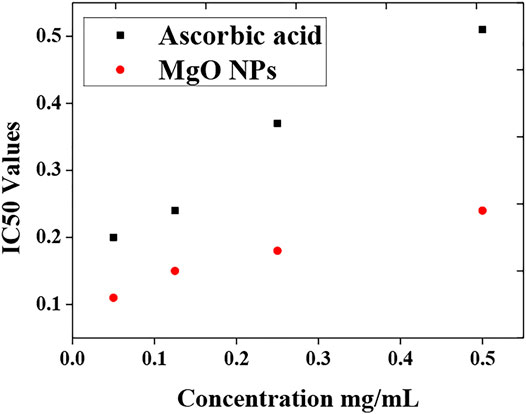
MgO nanoparticles scavenge 22.34, 42.55, 62.76, and 69.14 percent of conventional ascorbic acid at concentrations of 0.05, 0.125, 0.25, and 0.5 mg/ml, respectively, while standard ascorbic acid scavenges 12.77, 28.94, 36.49, and 46.70 percent at comparable concentrations. Biologically produced MgO nanoparticles have a higher proportion of scavenging than normal ascorbic acid. MgO nanoparticles have a lower IC50 value than other nanoparticles. The antioxidant potential is higher when the IC50 value is lower. In comparison to standard ascorbic acid, the fabricated MgO nanoparticles have higher antioxidant potential. The assessment of IC50 values of both the standard (ascorbic acid) and sample (MgO) nanoparticles is shown in Figure 8.
Phosphomolybdenum Method for Antioxidant Activity
This method is also used to evaluate the scavenging potential of biologically synthesized MgO nanoparticles. This method involves the use of phosphomolybdate reagent, which is why it is known as the phosphomolybdenum assay. When the plant extract is added to the phosphomolybdate reagent, a change in the solution color is observed that shows the reduction of phosphomolybdenum. The percentage of scavenging of both the sample (MgO) nanoparticles and standard (ascorbic acid) is given in Tables 4, 5.
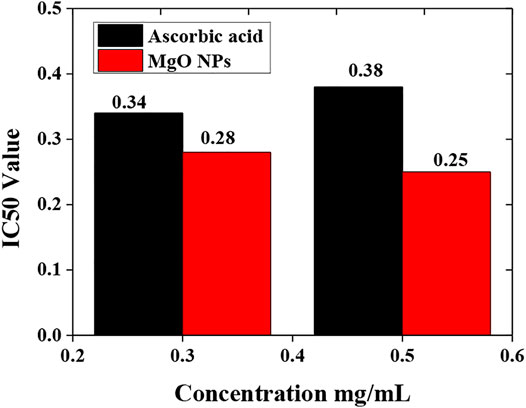
Ascorbic acid is scavenged up to 44.21 percent at a content of 0.3 mg/ml and up to 58.41 percent at a dose of 0.5 mg/ml. Furthermore, at 0.3 mg/ml, ascorbic acid had an IC50 of 0.34 mg/ml, and at 0.5 mg/ml, it had an IC50 of 0.38 mg/ml. MgO nanoparticles scavenged up to 54.36 percent at 0.31 mg/ml and 72.21 percent at 0.5 mg/ml. MgO nanoparticles had an IC50 value of 0.28 mg/ml at 0.3 mg/ml and 0.25 mg/ml at 0.5 mg/ml. The phosphomolybdenum technique was used to compare the IC50 values of the standard (ascorbic acid) and sample (MgO) nanoparticles (Figure 9).
Anti-inflammatory Assessment
The anti-inflammatory assessment of biologically fabricated MgO nanoparticles is evaluated to check their ability to reduce inflammation. The findings were then compared to those of a common anti-inflammatory medication (diclofenac sodium). The percentage inhibition of both the standard drug and MgO nanoparticles is shown in Tables 6, 7. It is observed that MgO nanoparticles synthesized by the biological method have a high value of percentage inhibition than standard diclofenac sodium.
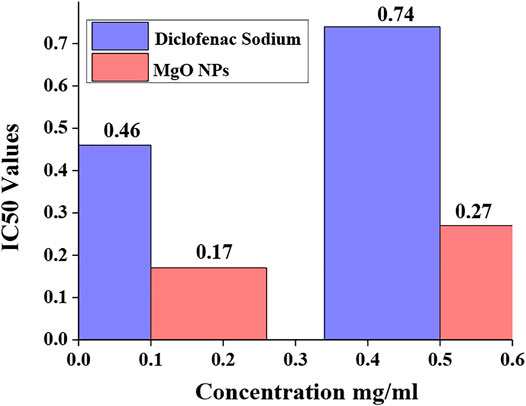
Diclofenac sodium reduced protein denaturation by up to 10.99% at 0.1 mg/ml and up to 35.37% at 0.5 mg/ml. Diclofenac sodium has an IC50 of 0.46 mg/ml at 0.1 mg/ml and 0.74 mg/ml at 0.5 mg/ml. MgO nanoparticles reduced protein denaturation up to 29.51 percent at a concentration of 0.1 mg/ml and up to 76.59 percent at a concentration of 0.5 mg/ml. MgO nanoparticles had an IC50 of 0.17 mg/ml at 0.1 mg/ml and 0.27 mg/ml at 0.5 mg/ml. Figure 10 shows the IC50 values of the standard (diclofenac sodium) and sample (MgO) nanoparticles.
Conclusion
Utilizing a leaf decoction of Alstonia scholaris, magnesium oxide nanoparticles were effectively produced using a green method and were then used to evaluate the anti-inflammatory and antioxidant potential of these MgO NPs. MgO nanoparticles displayed a spherical shape in SEM pictures and had an average crystallite size of 19.57 nm in XRD analyses. The anti-inflammatory activity of MgO nanoparticles was performed by using diclofenac sodium as a standard. The percentage inhibition was calculated, and it was observed that bio-inspired MgO NPs showed 76.59% inhibition, while at the similar concentration standard (diclofenac sodium), they showed 35.37% inhibition. Antioxidant activity was checked by two different methods, that is, DPPH and the phosphomolybdenum method. By increasing concentration, the percentage scavenging of both standards and of MgO nanoparticles increased. The biologically synthesized MgO NPs showed a scavenging potential of 69.14%, while at a similar concentration, ascorbic acid showed a 46.70% scavenging potential. In both methods, MgO nanoparticles showed more scavenging of free radicals than standard ascorbic acid, so MgO nanoparticles can be used as an effective antioxidant.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
SS: Conducted XRD analysis, performed some antibacterial experiments, acquisition of data, writing-original draft preparation. AE: Performed FTIR analysis, reviewed revised manuscript and critical revision. SM: Design of study, performed major experimental works, writing-original draft preparation. MJ: Material synthesis, visualization of data, writing reviewing and editing. SI: Conception, design of study, writing-original draft preparation and critical revision, supervision. EE: Reviewing of data and financial support. RA: Reviewed revised manuscript and critical revision. HA: Analysis and/or interpretation of data, financial funding. NA: Conception, visualization of data, writing reviewing and editing. HI: Drafting the revised manuscript and critical revision. UF: Drafting the revised manuscript and critical revision. SZ: Reviewed revised manuscript and critical revision. MN: Reviewed revised manuscript and critical revision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors express their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding this work through research groups program under grant of number R.G.P.2/276/42. RA would like to acknowledge Taif University Researchers Supporting Project Number (TURSP-2020/209), Taif University, Taif, Saudi Arabia. The authors extend their appreciation to the Research Center at AlMaarefa University for funding this work under TUMA project agreement number (TUMA-2021-4).
References
Abinaya, S., Kavitha, H. P., Prakash, M., and Muthukrishnaraj, A. (2021). Green Synthesis of Magnesium Oxide Nanoparticles and its Applications: A Review. Sust. Chem. Pharm. 19, 100368. doi:10.1016/j.scp.2020.100368
Ammulu, M. A., Viswanath, K. V., Giduturi, A. K., Vemuri, P. K., Mangamuri, U., and Poda, S. (2021). Phytoassisted Synthesis of Magnesium Oxide Nanoparticles from Pterocarpus Marsupium Rox. B Heartwood Extract and its Biomedical Applications. J. Genet. Eng. Biotechnol. 19 (1), 1–18. doi:10.1186/s43141-021-00119-0
Atrak, K., Ramazani, A., and Taghavi Fardood, S. (2019). Eco-friendly Synthesis of Mg0.5Ni0.5AlxFe2-xO4 Magnetic Nanoparticles and Study of Their Photocatalytic Activity for Degradation of Direct Blue 129 Dye. J. Photochem. Photobiol. A: Chem. 382, 111942. doi:10.1016/j.jphotochem.2019.111942
Bandeira, M., Giovanela, M., Roesch-Ely, M., Devine, D. M., and da Silva Crespo, J. (2020). Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sust. Chem. Pharm. 15, 100223. doi:10.1016/j.scp.2020.100223
Behzadi, E., Sarsharzadeh, R., Nouri, M., Attar, F., Akhtari, K., Shahpasand, K., et al. (2019). Albumin Binding and Anticancer Effect of Magnesium Oxide Nanoparticles. Int. J. Nanomedicine 14, 257–270. doi:10.2147/IJN.S186428
Chavali, M. S., and Nikolova, M. P. (2019). Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 1 (6), 1–30. doi:10.1007/s42452-019-0592-3
Das, S. K., Behera, S., Patra, J. K., and Thatoi, H. (2019). Green Synthesis of Sliver Nanoparticles Using Avicennia officinalis and Xylocarpus Granatum Extracts and In Vitro Evaluation of Antioxidant, Antidiabetic and Anti-inflammatory Activities. J. Clust Sci. 30 (4), 1103–1113. doi:10.1007/s10876-019-01571-2
Dey, P., Chatterjee, P., Chandra, S., and Bhattacharya, S. (2011). Comparative In Vitro Evaluation of Anti-inflammatory Effects of Aerial Parts and Roots from Mikania Scandens. J. Adv. Pharm. Educ. Res. 1 (6), 271–277.
Dhruti, M., Bhavika, P., and Meonis, P. (2016). Studies on Phytochemical Constituents and Antioxidant Activity of Alstonia scholaris. Int. J. Life Sci. 4 (4), 529–538.
Dizaj, S. M., Lotfipour, F., Barzegar-Jalali, M., Zarrintan, M. H., and Adibkia, K. (2014). Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Mater. Sci. Eng. C 44, 278–284. doi:10.1016/j.msec.2014.08.031
Dobrucka, R. (2018). Synthesis of MgO Nanoparticles Using Artemisia Abrotanum Herba Extract and Their Antioxidant and Photocatalytic Properties. Iran J. Sci. Technol. Trans. Sci. 42 (2), 547–555. doi:10.1007/s40995-016-0076-x
Eskandari Azar, B., Ramazani, A., Taghavi Fardood, S., and Morsali, A. (2020). Green Synthesis and Characterization of ZnAl2O4@ZnO Nanocomposite and its Environmental Applications in Rapid Dye Degradation. Optik 208, 164129. doi:10.1016/j.ijleo.2019.164129
George, J. M., Antony, A., and Mathew, B. (2018). Metal Oxide Nanoparticles in Electrochemical Sensing and Biosensing: a Review. Mikrochim Acta 185 (7), 358–426. doi:10.1007/s00604-018-2894-3
Hornak, J., Trnka, P., Kadlec, P., Michal, O., Mentlík, V., Šutta, P., Csányi, G., and Tamus, Z. (2018). Magnesium Oxide Nanoparticles: Dielectric Properties, Surface Functionalization and Improvement of Epoxy-Based Composites Insulating Properties. Nanomaterials 8 (6), 381. doi:10.3390/nano8060381
Jolivet, J. P., Henry, M., and Livage, J. (2000). Metal Oxide Chemistry and Synthesis: From Solution to Solid State. Wiley-Blackwell.
Khan, A., Shabir, D., Ahmad, P., Khandaker, M. U., Faruque, M. R. I., and Din, I. U. (2020). Biosynthesis and Antibacterial Activity of MgO-NPs Produced from Camellia-Sinensis Leaves Extract. Mater. Res. Express 8 (1), 015402. doi:10.1088/2053-1591/abd421
Khan, I., Saeed, K., and Khan, I. (2019). Nanoparticles: Properties, Applications and Toxicities. Arabian J. Chem. 12 (7), 908–931. doi:10.1016/j.arabjc.2017.05.011
Khan, S. A., Noreen, F., Kanwal, S., Iqbal, A., and Hussain, G. (2018). Green Synthesis of ZnO and Cu-Doped ZnO Nanoparticles from Leaf Extracts of Abutilon Indicum, Clerodendrum Infortunatum, Clerodendrum Inerme and Investigation of Their Biological and Photocatalytic Activities. Mater. Sci. Eng. C 82, 46–59. doi:10.1016/j.msec.2017.08.071
Khyade, M. S., Kasote, D. M., and Vaikos, N. P. (2014). Alstonia scholaris (L.) R. Br. And Alstonia Macrophylla Wall. Ex G. Don: A Comparative Review on Traditional Uses, Phytochemistry and Pharmacology. J. ethnopharmacology 153 (1), 1–18. doi:10.1016/j.jep.2014.01.025
Kumar, V., Singh, S. B., and Singh, S. (2021). Pharmacological Perspectives of Ayurvedic Herbs Viz. Alstonia scholaris L., Picrorhiza Kurroa, Swertia Chirata and Caesalpinia Crista against COVID-19: A Mini-Review. Mroc 18 (7), 841–849. doi:10.2174/1570193x17999201102200944
Lizundia, E., Armentano, I., Luzi, F., Bertoglio, F., Restivo, E., Visai, L., Torre, L., and Puglia, D. (2020). Synergic Effect of Nanolignin and Metal Oxide Nanoparticles into Poly(l-Lactide) Bionanocomposites: Material Properties, Antioxidant Activity, and Antibacterial Performance. ACS Appl. Bio Mater. 3 (8), 5263–5274. doi:10.1021/acsabm.0c00637
Matinise, N., Kaviyarasu, K., Mongwaketsi, N., Khamlich, S., Kotsedi, L., Mayedwa, N., et al. (2018). Green Synthesis of Novel Zinc Iron Oxide (ZnFe2O4) Nanocomposite via Moringa Oleifera Natural Extract for Electrochemical Applications. Appl. Surf. Sci. 446, 66–73. doi:10.1016/j.apsusc.2018.02.187
Moradnia, F., Ramazani, A., Fardood, S. T., and Gouranlou, F. (2019). A Novel green Synthesis and Characterization of Tetragonal-Spinel MgMn2O4 Nanoparticles by Tragacanth Gel and Studies of its Photocatalytic Activity for Degradation of Reactive Blue 21 Dye under Visible Light. Mater. Res. Express 6 (7), 075057. doi:10.1088/2053-1591/ab17bc
Moradnia, F., Taghavi Fardood, S., Ramazani, A., and Gupta, V. K. (2020). Green Synthesis of Recyclable MgFeCrO4 Spinel Nanoparticles for Rapid Photodegradation of Direct Black 122 Dye. J. Photochem. Photobiol. A: Chem. 392, 112433. doi:10.1016/j.jphotochem.2020.112433
Mosallanejad, B., Malek, S. S., Ershadi, M., Daryakenari, A. A., Cao, Q., Boorboor Ajdari, F., et al. (2021). Cycling Degradation and Safety Issues in Sodium-Ion Batteries: Promises of Electrolyte Additives. J. Electroanalytical Chem. 895, 115505. doi:10.1016/j.jelechem.2021.115505
Naseem, T., and Durrani, T. (2021). The Role of Some Important Metal Oxide Nanoparticles for Wastewater and Antibacterial Applications: A Review. Environ. Chem. Ecotoxicology. doi:10.1016/j.enceco.2020.12.001
Naseem, T., and Durrani, T. (2021). The Role of Some Important Metal Oxide Nanoparticles for Wastewater and Antibacterial Applications: A Review. Environ. Chem. Ecotoxicology 3, 59–75. doi:10.1016/j.enceco.2020.12.001
Nasrollahzadeh, M., Sajadi, S. M., Sajjadi, M., and Issaabadi, Z. (2019). Applications of Nanotechnology in Daily Life. Interf. Sci. Technol. 28, 113–143. doi:10.1016/b978-0-12-813586-0.00004-3
Oskam, G. (2006). Metal Oxide Nanoparticles: Synthesis, Characterization and Application. J. Sol-gel Sci. Technol. 37 (3), 161–164. doi:10.1007/s10971-005-6621-2
Parashar, M., Shukla, V. K., and Singh, R. (2020). Metal Oxides Nanoparticles via Sol-Gel Method: a Review on Synthesis, Characterization and Applications. J. Mater. Sci. Mater. Electron. 31 (5), 3729–3749. doi:10.1007/s10854-020-02994-8
Parnianchi, F., Nazari, M., Maleki, J., and Mohebi, M. (2018). Combination of Graphene and Graphene Oxide with Metal and Metal Oxide Nanoparticles in Fabrication of Electrochemical Enzymatic Biosensors. Int. Nano Lett. 8 (4), 229–239. doi:10.1007/s40089-018-0253-3
Pendyala, S. K., Thyagarajan, K., Gurusampath Kumar, A., and Obulapathi, L. (2019). Investigations on Physical Properties of Mg Ferrite Nanoparticles for Microwave Applications. J. Microwave Power Electromagn. Energ. 53 (1), 3–11. doi:10.1080/08327823.2019.1569898
Pilarska, A. A., Klapiszewski, Ł., and Jesionowski, T. (2017). Recent Development in the Synthesis, Modification and Application of Mg(OH)2 and MgO: A Review. Powder Technol. 319, 373–407. doi:10.1016/j.powtec.2017.07.009
Rajasekar, R., Samuel, M., Edison, T. N. J. I., and Raman, N. (2021). Sustainable Synthesis of Silver Nanoparticles Using Alstonia scholaris for Enhanced Catalytic Degradation of Methylene Blue. J. Mol. Struct. 1246, 131208. doi:10.1016/j.molstruc.2021.131208
Rehana, D., Mahendiran, D., Kumar, R. S., and Rahiman, A. K. (2017). In Vitro antioxidant and Antidiabetic Activities of Zinc Oxide Nanoparticles Synthesized Using Different Plant Extracts. Bioproc. Biosyst Eng 40 (6), 943–957. doi:10.1007/s00449-017-1758-2
Sarkar, K. K., Rahman, M. M., Shahriar, A. A. E., Mitra, T., Golder, M., Zilani, M. N. H., and Biswas, B. (2021). Comparative Neuropharmacological and Cytotoxic Profiles of Alstonia scholaris (L.) and Mimusops Elengi (L.) Leaves. Adv. Tradit Med. (Adtm) 21 (3), 499–506. doi:10.1007/s13596-020-00463-5
Shang, J.-H., Cai, X.-H., Feng, T., Zhao, Y.-L., Wang, J.-K., Zhang, L.-Y., Yan, M., and Luo, X.-D. (2010). Pharmacological Evaluation of Alstonia scholaris: Anti-inflammatory and Analgesic Effects. J. ethnopharmacology 129 (2), 174–181. doi:10.1016/j.jep.2010.02.011
Shayegan Mehr, E., Sorbiun, M., Ramazani, A., and Taghavi Fardood, S. (2018). Plant-mediated Synthesis of Zinc Oxide and Copper Oxide Nanoparticles by Using Ferulago Angulata (Schlecht) Boiss Extract and Comparison of Their Photocatalytic Degradation of Rhodamine B (RhB) under Visible Light Irradiation. J. Mater. Sci. Mater. Electron. 29 (2), 1333–1340. doi:10.1007/s10854-017-8039-3
Shojaei Yeganeh, M., Kazemizadeh, A. R., Ramazani, A., Eskandari, P., and Rabbi Angourani, H. (2020). Plant-mediated Synthesis of Cu0.5Zn0.5Fe2O4 Nanoparticles Using Minidium Leavigatum and Their Applications as an Adsorbent for Removal of Reactive Blue 222 Dye. Mater. Res. Express 6 (12), 1250f4. doi:10.1088/2053-1591/ab6637
Singh, J., Kumar, V., Kim, K.-H., and Rawat, M. (2019). Biogenic Synthesis of Copper Oxide Nanoparticles Using Plant Extract and its Prodigious Potential for Photocatalytic Degradation of Dyes. Environ. Res. 177, 108569. doi:10.1016/j.envres.2019.108569
Solanki, P. R., Kaushik, A., Agrawal, V. V., and Malhotra, B. D. (2011). Nanostructured Metal Oxide-Based Biosensors. NPG Asia Mater. 3 (1), 17–24. doi:10.1038/asiamat.2010.137
Taghavi Fardood, S., Forootan, R., Moradnia, F., Afshari, Z., and Ramazani, A. (2020). Green Synthesis, Characterization, and Photocatalytic Activity of Cobalt Chromite Spinel Nanoparticles. Mater. Res. Express 7 (1), 015086. doi:10.1088/2053-1591/ab6c8d
Taghavi Fardood, S., Moradnia, F., Moradi, S., Forootan, R., Yekke Zare, F., and Heidari, M. (2019a). Eco-friendly Synthesis and Characterization of α-Fe2O3 Nanoparticles and Study of Their Photocatalytic Activity for Degradation of Congo Red Dye. Nanochemistry Res. 4 (2), 140. doi:10.22036/NCR.2019.01.010
Taghavi Fardood, S., Moradnia, F., Mostafaei, M., Afshari, Z., Faramarzi, V., and Ganjkhanlu, S. (2019b). Biosynthesis of MgFe2O4 Magnetic Nanoparticles and its Application in Photo-Degradation of Malachite green Dye and Kinetic Study. Nanochemistry Res. 4 (1), 86. doi:10.22036/NCR.2019.02.005
Umaralikhan, L., and Jamal Mohamed Jaffar, M. (2018). Green Synthesis of MgO Nanoparticles and it Antibacterial Activity. Iran J. Sci. Technol. Trans. Sci. 42 (2), 477–485. doi:10.1007/s40995-016-0041-8
Vinardell, M., and Mitjans, M. (2015). Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 5 (2), 1004–1021. doi:10.3390/nano5021004
Yang, Y., Zhang, C., and Hu, Z. (2013). Impact of Metallic and Metal Oxide Nanoparticles on Wastewater Treatment and Anaerobic Digestion. Environ. Sci. Process. Impacts 15 (1), 39–48. doi:10.1039/c2em30655g
Yaseen, M. W., Sufyan, M., Nazir, R., Naseem, A., Shah, R., Sheikh, A. A., et al. (2021). Simple and Cost-Effective Approach to Synthesis of Iron Magnesium Oxide Nanoparticles Using Alstonia scholaris and Polyalthia Longifolia Leaves Extracts and Their Antimicrobial, Antioxidant and Larvicidal Activities. Appl. Nanosci 11 (9), 2479–2488. doi:10.1007/s13204-021-02051-8
Yu, D., Goh, K., Wang, H., Wei, L., Jiang, W., Zhang, Q., Dai, L., and Chen, Y. (2014). Scalable Synthesis of Hierarchically Structured Carbon Nanotube-Graphene Fibres for Capacitive Energy Storage. Nat. Nanotech 9 (7), 555–562. doi:10.1038/nnano.2014.93
Keywords: MgO nanoparticles, green synthesis, Alstonia scholaris, antioxidant activity, anti-inflammatory activity
Citation: Shahid S, Ejaz A, Javed M, Mansoor S, Iqbal S, Elkaeed EB, Alzhrani RM, Alsaab HO, Awwad NS, Ibrahium HA, Fatima U, Zaman S and Nazim Sarwar M (2022) The Anti-Inflammatory and Free Radical Scavenging Activities of Bio-Inspired Nano Magnesium Oxide. Front. Mater. 9:875163. doi: 10.3389/fmats.2022.875163
Received: 13 February 2022; Accepted: 28 March 2022;
Published: 28 April 2022.
Edited by:
Muhammad Idrees, Shenzhen University, ChinaReviewed by:
Imtiaz Khan, The University of Manchester, United KingdomShahid Ali, King Fahd University of Petroleum and Minerals, Saudi Arabia
Copyright © 2022 Shahid, Ejaz, Javed, Mansoor, Iqbal, Elkaeed, Alzhrani, Alsaab, Awwad, Ibrahium, Fatima, Zaman and Nazim Sarwar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohsin Javed, bW9oc2luLmphdmVkQHVtdC5lZHUucGs=; Shahid Iqbal, c2hhaGlkZ2NzMTBAeWFob28uY29t
 Sammia Shahid1
Sammia Shahid1 Shahid Iqbal
Shahid Iqbal