- Departamento de Química en Ciencias Farmacéuticas, Facultad de Farmacia Universidad Complutense, Instituto de Investigación Sanitaria Hospital 12 de Octubre, CIBER de Bioingeniería Biomateriales y Nanomedicina CIBER-BBN, Madrid, Spain
There is a clear need for increasingly versatile and effective implantable biomaterials, and to train qualified personnel for research and development in the field of biomaterials design and manufacturing. In all these implantable biomaterials, science and technology are imposing new designs with combinations of new biomaterials, new coatings, and new design and manufacturing technologies (biomimetic biomaterials, functional biomaterials, nanotechnology, finite element modeling, additive manufacturing, 3D printing, tissue engineering, and drug delivery) that will revolutionize the field of implants in the short term. Biomaterials are part of biomedical engineering and bring together knowledge from the world of science, engineering, biology, and medicine, being a multidisciplinary field where borders have no place.
Introduction
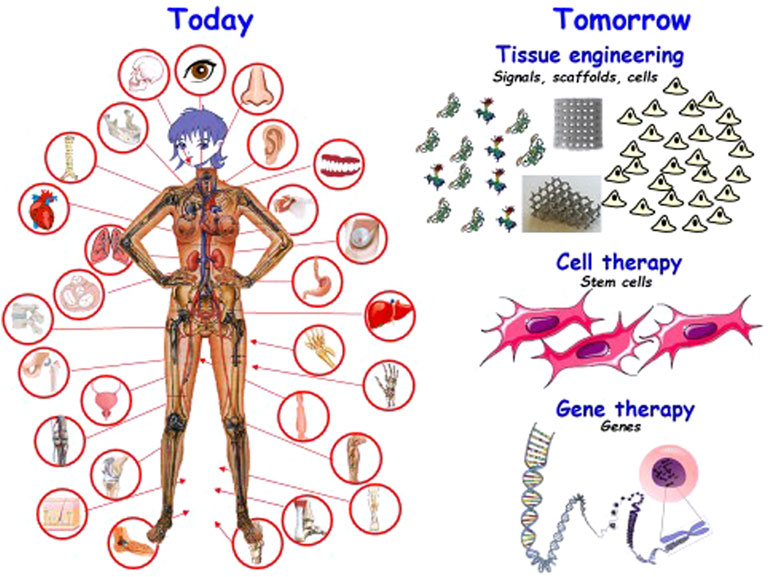
Most likely, life expectancy will increase markedly in the next few years. This fact will simultaneously bear good and bad news: the good news is, obviously, that most people will agree that the later we die, the better. The bad news, however, is that there is still a long way to go in the world of biomaterials, and the increase in medical costs of such prolonged mean life will be huge. Besides, a prolonged life should also aim to be an enhancement in life quality, both in terms of health and economy. Therefore, biomaterials must be researched and developed to achieve the right solutions. Nowadays, there is great implant availability as depicted in Figure 1. Health engineering encompasses the study of prostheses and implants, surgical instruments and equipment, and all the chemistry and biology that this entails. The field of biomaterials always implies a coordinated effort among different experts in several areas of knowledge. Without this coordination, absolutely essential, the work in biomaterials would not be such, since it would consist of the isolated study of different aspects that, although they may constitute interesting basic studies, escape the final objective of the biomaterial, which requires the realization of many stages, beginning with the manufacture of the material to be used, continuing with the processing and control of both quality and biohealth requirements, and ending with the clinical application and its follow-up, implant removal. From the first idea on what to manufacture to solve a problem, up to the insertion of an implant in the patient, there is a lot of work involving many professionals as diverse as the doctor, the research scientist, the engineer, the veterinarian, the industrial manager, and the legal counselor, among others. And during this long journey, remarkably diverse experts must therefore intervene, as befits a multidisciplinary field.
Considering biomaterials as materials independently of biology in their design and their previous evaluation in vitro and in vivo is a concept that has become obsolete. Of course, they are necessary, and we can choose with which materials to manufacture them. At first it was thought that the most important thing to manufacture these substitutes for the damaged parts of the human body were the materials themselves and that if they met the condition of being compatible with it, you could start working with them. Therefore, the important thing was the material and not the biology. However, the more biomaterials were known, the more it was favored to consider the biological world where they were going to be implanted (Ratner et al., 2013; Vallet-Regí, 2014; Van Blitterswijk and de Boer, 2015; Vallet Regí and Nanoceramics, 2016; Pawelec and Planell, 2018; Wagner et al., 2020). Thus, already at the dawn of the 21st century, the world of biomaterials was aware that the main axis of their development had to be in biology. This gives rise to tissue engineering, cell therapy, and gene transfection, where cells are in charge. In addition, already in the 21st century, nanomedicine that designs and evaluates the biomaterials at nanometric scale, is breaking out, which, without a doubt, has given rise to important applications in the world of biomaterials for use in regenerative medicine (Vallet-Regí, 2001; Vallet-Regí, 2006; Vallet-Regí, 2010; Ducheyne, 2017; Atala et al., 2019).
At this point, there is another important reflection to make: drugs are or can be intimately linked to the world of biomaterials. This is nothing more than a consequence of the interdisciplinary quality of science; it needs the knowledge of many areas for any scientific development. Biomaterials, nanomedicine, and drugs are closely linked, although nanomedicine had not yet begun its journey when biomaterials and drugs were already in their teens. But now, in the year 2022, I think it is essential to contemplate them together.
A Little Bit of History
Neanderthal man seems to have used dental implants made of wood, and in ancient Egypt it also seems that materials were used to try to repair and solve different body problems, as deduced from the study of mummies found. In Greece and Rome, from the 7th century B.C. to the 4th century A.D., materials such as metals and others of natural origin were used for the treatment of wounds and other problems. In 16th century Europe, gold and silver were used for dental repair and, later, iron threads to immobilize bone fractures. At the end of the 19th century, and because of the technological advances of those times, such as X-rays, and taking advantage of the wonderful discovery of anesthesia, along with the fact that surgery began to be practiced in sterile conditions, metals began to be used to repair internal parts of the human body. But the application of metals as they were used then began to create greater problems than the solutions they provided. These problems were mainly due to two reasons: corrosion and the lack of sufficient mechanical properties for the device to adequately fulfill the function for which it had been designed. However, research into materials useful for the manufacture of prostheses and implants emerged after World War II. At that time, a clear advance of knowledge in science and technology was combined with the need to treat countless wounded because of the war. The work in biomaterials is nucleated around scientific societies and the first scientific journals begin to appear that are collecting research and progress in biomaterials. But during the 1960s, problems caused by the presence of certain implants began to be detected, and the question arose on what to implant and how to do it and, above all, that the implant did not create more problems than the solutions it provided. For the first time, the term biocompatibility is used, which is defined as the degree of tolerance of the material by the organism. To determine biocompatibility, a series of tests must be carried out according to pre-established protocols and the subsequent statistical analysis of the results. In addition, it must be done for each specific application and for each system formed by the material under study and the biological environment with which it will be in contact. At the end of the 1960s, the world of biomaterials realized that engineers are needed to successfully develop implants and substitutes for damaged parts of the body and, in effect, engineers began to work in the laboratories of medical, surgical, and dental clinics, and their contributions began to appear in the biomedical literature. The first Biomaterials Symposium was held at Clemson University in 1969 and marked the starting point of the need to integrate complementary disciplines to engineering and medicine for the development of biomedical materials. When engineering joined the field of biomaterials, techniques began to be applied to characterize the structure and surface of materials, and to correlate them with the observed biological responses. The scientific community of biomaterials was grouped into various societies, such as the American Biomaterials Society (United States) founded in 1974 and the European Society of Biomaterials founded in 1979. In 1978, the first International Congress of Biomaterials was held, acting as the starting signal of this discipline in the whole world, an area in permanent growth since then in order to solve all the problems of damaged parts of the human body (Vallet-Regí, 2019; Wagner et al., 2020).
In terms of nomenclature, the lexicon agreed in 1986 and published in 1987 was used until 2018. In 2018, at the Chengdu Conference, the terminology used in biomaterials was revised, making necessary changes since some terms were still applicable while others had become obsolete and, most importantly, new ones that had been generated because of the great advance in the world of biomaterials had to be included. In 2019, the new definitions were published (Zhang and Williams, 2019), and are the ones that are currently being used.
Regenerative Medicine
Regenerative medicine (RM) emerged from clinical practices, such as the design of surgical implants, the use of scaffolds based on biomaterials, or the transplantation of organs or bone marrow and is closely related to tissue engineering. This practice has limitations, such as the loss of prostheses over time, inflammatory process induced by scaffolding, contamination of bone marrow aspiration, or the need to take immunosuppressive drugs after organ transplantation. The bases of RM are human stem cells that can be of adult or embryonic origin and can also be the so-called induced pluripotent stem cells (iPS) obtained by reprogramming of adult cells. Human embryos are not the ideal source. Therefore, obtaining iPS cells is an attractive approach, as it involves the transfer of genes to human cells, which brings RM closer to gene and cellular therapies.
Cell researchers generally try to use as few synthetic biomaterials as possible. However, the biomedical tissue regeneration industry is combining cell therapies with others based on genes, biomaterials, and drugs. An effective RM focused on human stem cells could replace pharmaceuticals and biomedical prostheses. For instance, RM focused on cartilage regeneration can give way to a rational development of prostheses. In addition, there is a growing link between gene therapy and RM. Cell therapy seeks to place genes in cells to implant them. Current interest in reprogramming adult cells into iPS cells is driving gene-cell linkage in the use of a genetic approach to various therapies (Vallet-Regi and Salinas, 2021).
Usually, regeneration describes the process by which lost tissue is replaced by the proliferation of specialized cells. In humans, the process is limited to some tissues, such as bone. In this sense, the aim of RM is to regenerate, mainly, by supplying cells, in particular stem cells that can stimulate regeneration. Similarly, repair is the replacement of lost tissue with granulation tissue that matures to form scar tissue. Organ regeneration is different from organ repair after an injury. Repair leads to restoration by synthesis of scar tissue without restoration of normal tissue. Since the goal of RM is to return the patient to a healthy state, repair can be considered within technologies such as surgery. In most cases, the goal of regeneration is to restore impaired function.
RM includes tissue engineering, genetic engineering, and molecular activators, and is an interdisciplinary field of research focused on the repair, replacement, or regeneration of cells, tissues, or organs to restore impairment of function resulting from birth defects, disease, trauma, or aging. RM uses several techniques that go beyond traditional biomolecule transplantation and substitution therapies, gene therapy, stem cell transplantation, tissue engineering, and reprogramming of cell and tissue types. Currently, there are commercial MR-based products to treat skin ulcers or knee cartilage lesions. These therapies usually include a scaffold made of biomaterials. It is expected that there will be more therapies with embryonic stem cells as well as more therapies with embryonic stem cells and temporary scaffolds, but in those situations where structural tissue is needed, it is hard to track results, it is hard for cells alone to succeed. Therefore, although stem cells are the central element of RM, many clinical applications need the use of scaffolds, often manufactured with bioceramics.
Regenerative medicine has gained remarkable momentum due to the aging population. The needs of tissue replacement/repair and the great developments in science and technology of the 21st century have driven an accelerated and sustained growth in the use of biomaterials for numerous clinical areas with a great economic and social impact.
Biomaterials are essential for regenerating bone in unfavorable situations such as when there are critical defects or infection occurs (García-Alvarez et al., 2017). Every year more than two million bone grafts are implanted in the world, with autografts being the “gold standard” since their pores are of different sizes and the osteogenic biomolecules and stem cells they contain make them osteoconductive, osteoinducing, and osteogenic. However, autografts have limitations such as the amount of bone available and increased surgery time, morbidity, and pain. Therefore, the aim is to develop synthetic biomaterials with the characteristics of autografts but without their limitations. Tissue engineering approaches seek to regenerate bone using stem cell and pluripotent therapies (Manzano and Vallet-Regí, 2018; Manzano and Vallet-Regí, 2019; Vallet-Regí et al., 2019; Colilla and Vallet-Regí, 2020; Vallet-Regí et al., 2020). Bone tissue engineering starts from biocompatible porous scaffolds, often manufactured with bioactive bioceramics (calcium phosphates, bioactive glasses), osteoconductive, which are decorated with osteoinductive molecules and in which the cells, osteogenic, can differentiate and proliferate. The new biomaterials, in addition to regenerating tissue, must provide mechanical support during healing and avoid possible infection processes caused by surgery. Figure 1 illustrates the situation of biomaterials from the here and now to those of the near future.
Collaboration between doctors and basic researchers is essential to reach a solution applied in medicine, but that is not always easy to achieve. First of all, it is difficult to have the necessary time to achieve this effective interaction, since clinicians have an enormous clinical workload and do not usually have much time to devote to research, and those who do it with enormous effort cannot do it systematically and constantly.
Second, the language used by both parties is different, and a joint effort must be made. The needs and priorities are different, the academic must understand well what the clinician needs to be able to carry out for an effective work and have a lot of patience to get to fruition. To all this we must add that, in general, their workplaces are far away, physically separated, which is another cause to make more difficult a continuous and systematic work that involves meeting frequently to understand mutual needs and seek solutions, as well as constantly monitoring the progress and/or failures that are being achieved.
Some Thoughts on the Biomaterials Industry
Biomedical devices have a high added value and have a small market size compared to companies in the materials sector. Lawsuits from patients affected by alleged harmful effects of a certain material are twofold. Certainly, if there has been fraud or negligence, it should be taken to court, but sometimes these actions are triggered by commercial interests, unfair competition, and so on. In addition, the abuse of lawsuits has led the companies involved to withdraw from the market. Consequently, the intention to protect the health of the patient suing for harm may have a negative effect on public health, by compromising the continuity of medical practices that require devices made from the withdrawn compound. This is a very delicate point on which to reflect.
Toward the Near Future
Future developments in biomaterials will need all size scales: pico, nano, micro, and macro, and cellular and molecular biology will provide the solutions to medical problems. Nanotechnology is developing rapidly and incessantly toward the prevention and treatment of infectious and aggressive diseases that cannot be successfully treated with conventional techniques. Incessant advances in the preparation of nanosystems with applications in the field of medicine have led to new challenges in the design of intelligent materials capable of responding to clinical demands (Manzano and Vallet-Regí, 2020). Designing devices and techniques to image tumor tissue and manufacture, when necessary, spare parts for the human body using tissue engineering and cell therapy are other major challenges to achieve. The surface of any implant plays a critical role in achieving modifications that increase biological efficiency and performance. We must bear in mind new discoveries in biological research such as CRISPR technology or human embryonic stem cells. After almost 8 years of development in biomaterials, it is possible to think that many future biomaterials will be rooted in tissue engineering and nanotechnology, but we must also emphasize what can be done, in the shorter term, so that research on biomaterials reaches the clinic as quickly as possible and improves the lives of patients, in the present. The road from the laboratory to the patient’s bed is long and tortuous. The journey that begins in research at the laboratory must end in the clinician’s hands, and therefore it is essential to perform a complex but unavoidable translational leap in the world of biomaterials if we want them to serve for what they have been designed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
MV-R acknowledges financial support from the European Research Council through ERC-2015-AdG-694160 (VERDI) project.
References
Atala, A., Lanza, R., Mikos, T., and Nerem, R. (2019). Principles of Regenerative Medicine. 3th Ed. Amsterdam: Elsevier, Academic Press.
Colilla, M., and Vallet-Regí, M. (2020). Targeted Stimuli-Responsive Mesoporous Silica Nanoparticles for Bacterial Infection Treatment. Ijms 21, 8605. doi:10.3390/ijms21228605
García-Alvarez, R., Izquierdo-Barba, I., and Vallet-Regí, M. (2017). 3D Scaffold with Effective Multidrug Sequential Release against Bacteria Biofilm. Acta Biomater. 49, 113–126. doi:10.1016/j.actbio.2016.11.028
Manzano, M., and Vallet-Regí, M. (2018). Mesoporous Silica Nanoparticles in Nanomedicine Applications. J. Mater. Sci. Mater. Med. 29, 65–14. doi:10.1007/s10856-018-6069-x
Manzano, M., and Vallet-Regí, M. (2019). Ultrasound Responsive Mesoporous Silica Nanoparticles for Biomedical Applications. Chem. Commun. 55, 2731–2740. doi:10.1039/c8cc09389j
Manzano, M., and Vallet‐Regí, M. (2020). Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 30, 1902634. doi:10.1002/adfm.201902634
Pawelec, K., and Planell, J. A. (2018). Bone Repair Biomaterials. 2nd Ed. Boca Raton: Woodhead Publish, CRC.
Ratner, B. D., Hoffman, A. S., Schoen, F. J., and Lemons, J. E. (2013). Biomaterials Science: An Introduction to Materials in Medicine. 3rd Ed. Amsterdam: Elsevier, Academic Press.
Vallet Regí, M., and Nanoceramics, A. D. (2016). Clinical Use from Materials to Applications. 2nd Ed. Cambridge: RSC Nanoscience.
Vallet-Regi, M., and Salinas, A. J. (2021). Mesoporous Bioactive Glasses for Regenerative Medicine. Mater. Today Bio. 11, 100121. doi:10.1016/j.mtbio.2021.100121
Vallet-Regí, M., González, B., and Izquierdo-Barba, I. (2019). Nanomaterials as Promising Alternative in the Infection Treatment. Int. J. Mol. Sci. 20, 3806. doi:10.3390/ijms20153806
Vallet‐Regí, M., Lozano, D., González, B., and Izquierdo‐Barba, I. (2020). Biomaterials against Bone Infection. Adv. Healthc. Mater. 9, 2000310. doi:10.1002/adhm.202070039
Vallet-Regí, M. (2001). Ceramics for Medical Applications. J. Chem. Soc. Dalton Trans., 97–108. doi:10.1039/B007852M
Vallet-Regí, M. (2006). Revisiting Ceramics for Medical Applications. Dalton Trans., 5211–5220. doi:10.1039/b610219k
Vallet-Regí, M. (2010). Evolution of Bioceramics within the Field of Biomaterials. C. R. Chim. 13, 174–185. doi:10.1016/j.crci.2009.03.004
Vallet-Regí, M. (2014). Bioceramics with Clinical Applications. Chichester, UK: John Willey & Sons Ltd.
Vallet-Regí, M. (2019). Bioceramics: From Bone Substitutes to Nanoparticles for Drug Delivery. Pure Appl. Chem. 91, 687–706. doi:10.1515/pac-2018-0505
Wagner, W., Sakiyama-Elbert, S., Zhang, G., and Yaszemski, M. (2020). Biomaterials Science: An Introduction to Materials in Medicine. London: ElsevierAcademic Press.
Keywords: biomaterials, manufacturing technologies, nanotechnology, tissue engineering, drug delivery systems
Citation: Vallet-Regí M (2022) Evolution of Biomaterials. Front. Mater. 9:864016. doi: 10.3389/fmats.2022.864016
Received: 31 January 2022; Accepted: 23 February 2022;
Published: 18 March 2022.
Edited by:
Francisca Garcia Caballero, Spanish National Research Council (CSIC), SpainReviewed by:
Vincenzo Guarino, National Research Council (CNR), ItalyCopyright © 2022 Vallet-Regí. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Vallet-Regí, dmFsbGV0QHVjbS5lcw==
 Maria Vallet-Regí
Maria Vallet-Regí