- Institute of Defense Engineering, Academy of Military Sciences, PLA, Beijing, China
In recent years, the infrared electrochromic devices based on acid doped polyaniline films have achieved much progress, which has not been summarized before. This is important to accelerate the development of this field. In this paper, we will briefly review some representing work about the infrared electrochromic properties of acid doped polyaniline films, which were reported in the last 5 years.
Introduction
With the urgent demand of optical display, smart window, and infrared camouflage, electrochromic materials with reversible color switch have become a focus of basic research and industrial applications. (Díaz-Sánchez et al., 2017; Korent et al., 2020; Naskar et al., 2021) On the basis of the first infrared electrochromic system developed by Chandrasekhar et al., a series of flexible electrochromic devices based on conducting polymers were continuously reported. (Chandrasekhar and Dooley, 1995) Among the various materials, polyaniline (PANI) is particularly prominent because of the superior electrical conductivity, low cost, good chemical stability, and excellent controllability. (Moulton et al., 2004; Bhadra et al., 2009; Li et al., 2010) As reported, the electrochromic process of PANI films is dominated by the electrochemical reaction, which involves the electron transport and ions intercalation. (Zhang et al., 2019a; Zhang et al., 2019b; Zhao et al., 2019) Usually, the increase of the conductivity, surface areas, and accessible intercalation sites can significantly facilitate the better optical performance. (Zhang et al., 2017; Hu et al., 2018; Zhang et al., 2019c) Therefore, the PANI films, with different geometric and electronic structure, have been proposed to optimize the electrochromic performances. For example, Feng Yang et al. reported the honeycomb-like polyaniline nanostructures, which exhibited an ultrafast response speed and high optical speed in the large area electrochromic display. (Zhang et al., 2020) Combing the galvanostatic with cyclic voltammetry techniques, Hao Wang et al. presented the nanostructured PANI films with remarkable flexibility, electrochromic efficiency, and multicolor feature. (Zhou et al., 2018) Nevertheless, the optical performance of pure PANI materials is not satisfactory and much effort has been devoted to the development of acid doped polyaniline composites, improving the optical contrast. Herein, we will briefly review the recent progress of PANI-based materials doped with different types of acids in infrared regulation, including the inorganic acid doped-PANI films and organic acid doped-PANI materials.
Inorganic Acid Doped-PANI Films
The IR electrochromic properties of PANI-based films largely depend on the completed transformation between the emeraldine salt (ES) state and leucoemeraldine (LE) state, which is dominated by the electron transfer and the number of the bipolarons. Acid doping can effectively induce an increase in the concentration of free carriers and bipolarons in the ES state, increasing the color sensitivity. (Zhou et al., 2018; Tian et al., 2017a) As reported in Yao Li’s work, an HClO4-doped PANI porous film was fabricated, and exhibited an optimal IR emissivity variation of 0.412 in the wavelength of 2.5–25 μm. The assembled device showed the modulation of the emittance variation between 0.735 and 0.316. The mechanism analysis pointed out the critical roles of formation/elimination of polarons/bipolarons in the corresponding coloring and fading state. (Zhang et al., 2019d) In another work, a visible-to-infrared broadband flexible device based on the H2SO4-doped PANI films (Figure 1) was also constructed by this group. Due to the increase of the carrier concentration, the IR emission change was found to be 0.4 and 0.3 in the range of 8–18 μm and 2.5–25 μm, respectively. This is much superior to other reported results. The study of response time showed that the coloring time and the bleaching time was about 9.8 s and 9.6 s, respectively. The cycling durability measurements presented that the IR emissivity of the designed device can keep stable after 500 cycles, suggesting the excellent chemical structure stability. (Xu et al., 2020a) On the basis of the electropolymerization technique, Yuge Han et al. adopted the 2:1 of aniline to sulfuric acid molar ratio to prepare the H2SO4-doped PANI films. The scanning electron microscopy showed the uncompact and disorder structure with high surface roughness. Using nylon-0.22 μm as substrates, the device based on the prepared PANI films provided the average IR emissivity change of 0.347 in the range of 3–5 μm. Furthermore, the IR emissivity modulation of the device on the different types of substrates was also reveal in this work. As presented in Table 1, the PTFE-0.22 μm substrate showed the maximum emissivity change of 0.559 and 0.390 in the wavelength of 3–5 μm and 8–14 μm, respectively. Meanwhile, the stability investigation showed that the degradation of devices reached a maximum in the 10th cycle. (Lu et al., 2021)
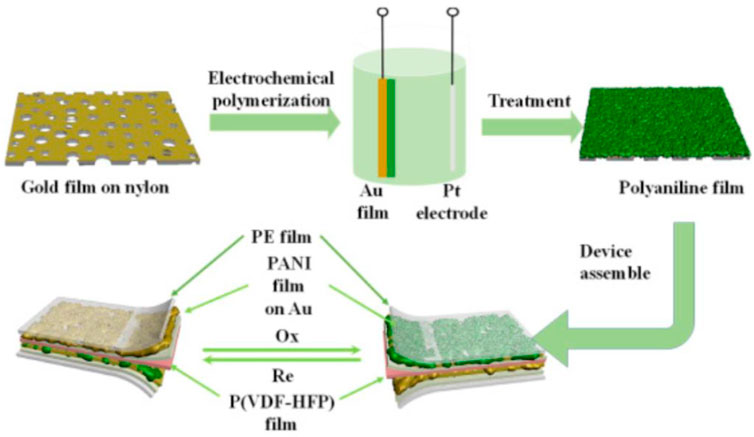
FIGURE 1. Scheme illustration for the fabrication procedure of a visible-to-infrared broadband flexible device based on the H2SO4-doped PANI films. Copy right from Xu et al. (2020a).
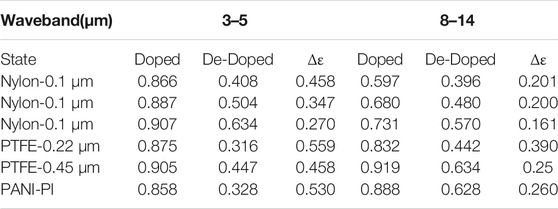
TABLE 1. The average emissivity and IR modulation value of PANI films on the different substrates in 3–5 μm and 8–14 μm, respectively. Copy right from Lu et al. (2021).
Recently, the improved electrochromic properties of multiple acid doped-PANI films were also demonstrated. For example, Xiaobai Li et al. synthesized the flexible PANI films doped by H2SO4 and HClO4. X-ray diffraction patterns and Raman analysis showed the similar signals of PANI-films polymerized at different current densities. Scanning electron microscopy and atomic force microscopy presented that the prepared PANI films doped with multiple acids exhibited a random rough surface with the surface roughness of about 100–200 nm and had abundant pores. The investigation from X-ray photoelectron energy spectroscopy indicated that the doping degree of H2SO4 is obviously higher than that of HClO4. For the infrared emittance, an increasing trend was observed at 2.5–25 μm. Moreover, the IR emissivity change value was found to be 0.47 from the constructed device with the coloring and fading time of 14.5 s and 13.2 s. Nevertheless, limited by the types of acids available, the resulting films cannot achieve arbitrary discoloration. (Xu et al., 2020b)
Organic Acid Doped-PANI Films
To show different IR reflectance and emissivity values, the organic protic acids were usually used to coordinate polyaniline through the nitrogen atoms, promoting the polarons and the bipolarons to delocalize into the whole π bond and increase the conductivity. In 2017, the dodecylbenzene sulfonate acid (DBSA) doped PANI films were prepared and utilized as the electrode layers in an electrochromic device. The IR emissivity change of device was determined to be 0.183, 0.388 and 0.315 in the wavelength of 3–5 μm, 8–12 μm and 2.5–25 μm, respectively. This tunable optical result can be attributed to the electrochemical behavior and pseudo-metallic behavior of the PANI film, which can be explained by the Drude free electron theory and the Hagen-Rubens approximation at low frequency. (Tian et al., 2017b) Also employing the DBSA-doped PANI films as the active layer, a monolithic integrated device was fabricated, which showed an ultra-high flexibility and prominent structural stability. (Song et al., 2021) Subsequently, Junpeng Zhao et al. explored the IR electrochromism of the DBSA-doped PANI films on the ITO substrate. Electrochemical results showed that the prepared PANI films displayed the IR electrochromic performance completely between the LB state and ES state. Changing the polymerization time and applied potential, the PANI films with different thickness were obtained, which played a key role in the material’s conductivity. The highest conductivity was obtained at the voltage of 0.45 V and the time of 1,500 s, indicating the abundant polarons and bipolarons. In the wavelength of 2.5–25 μm, the emissivity of PANI films exhibited the highest value at 0.45 V, and the lowest value at the potential of −0.25 V. The trend of IR emissivity change presented the first increase and then decrease, suggesting the potential of two operation modes. The maximum IR emissivity change was determined to be 0.245. (Zhang et al., 2019e) In addition, Shanxi Xiong et al. also reported a DBSA-doped PANI film with a high stable star-shaped structure and triphenylamine core. (Xiong et al., 2018)
As the polyaniline can be protonated with camphorsulfonic acid (CSA), Yao Li et al. also reported the CSA-doped polyaniline thin films. In this research, the CSA-doped PANI film was prepared by electrochemical deposition. The morphology characterization from scanning electron microscopy presented a fibrous structure with the directional growth, which facilitated the formation of ion channels and an electrochemical reaction. The investigation of electrochemical properties showed a good cycling durability and a couple of redox pair. The high IR emissivity change was calculated to be 0.225, 0.399 and 0.426 at the wavelength of 3–5 μm, 8–12 μm, and 2.5–25 μm, respectively. The switching time from coloring time to bleaching time was recorded as 30 s. (Zhang et al., 2019f) In addition, the prepared PANI films also presented the superior chromic performance in a digital camera and IR thermal imager, which showed great potential in real applications. Herein, it is worthy to mention that the doped-polymer chain is difficult to achieve homogenization, due to the memory effect of molecular structure.
Conclusion and Prospect
In this paper, the infrared electrochromic properties of acid doped-PANI films were briefly reviewed. Although much progress has been achieved, there is still a long way for the practical applications. At present, the types of acid used for doping polyaniline are relatively limited. For example, metal ions, as a kind of Lewis acids, are rarely reported to regulate the optical properties of polyaniline. Conversely, numerous metal oxide/polyaniline composites have been reported, while the related investigations mainly focus on the electrochromic properties of visible light, but not infrared chromic properties. Meanwhile, combined with some new materials, such as metal nanoclusters and metal-organic framework, more polyaniline-based chromic films still need to be developed. In the future, the present challenges, which include faster response time, better reversibility, higher sensitivity, and longer cycle life, will be overcome. Moreover, devices with excellent controllability and superior stability stay will be constructed.
Author Contributions
BL complete the draft; YC and XL checked the photographic of the article; FL helped check the English writing; JW, YL, and NL discussed and organized the manuscript, and checked the English writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the editors and reviewers for constructive comments and suggestions.
References
Bhadra, S., Khastgir, D., Singha, N. K., and Lee, J. H. (2009). Progress in Preparation, Processing and Applications of Polyaniline. Prog. Polym. Sci. 34, 783–810. doi:10.1016/j.progpolymsci.2009.04.003
Chandrasekhar, P., and Dooley, T. J. (1995). Far-IR Transparency and Dynamic Infrared Signature Control with Novel Conducting Polymer Systems. Proc.SPIE, 2528. doi:10.1117/12.219540
Díaz-Sánchez, J., Rosas-Aburto, A., Vivaldo-Lima, E., Hernández-Alcántara, J. M., Gracia-Mora, I., Vázquez-Torres, H., et al. (2017). Development and Characterization of a Flexible Electrochromic Device Based on Polyaniline and Enzymatically Synthesized Poly (Gallic Acid). Synth. Met. 223, 43–48. doi:10.1016/j.synthmet.2016.11.038
Hu, F., Xu, J., Zhang, S., Jiang, J., Yan, B., Gu, Y., et al. (2018). Core/shell Structured Halloysite/polyaniline Nanotubes with Enhanced Electrochromic Properties. J. Mater. Chem. C 6, 5707–5715. doi:10.1039/c8tc01163j
Korent, A., Žagar Soderžnik, K., Šturm, S., and Žužek Rožman, K. (2020). A Correlative Study of Polyaniline Electropolymerization and its Electrochromic Behavior. J. Electrochem. Soc. 167, 106504. doi:10.1149/1945-7111/ab9929
Li, X., Zhang, H., Wang, G., and Jiang, Z. (2010). A Novel Electrode Material Based on a Highly Homogeneous Polyaniline/titanium Oxide Hybrid for High-Rate Electrochemical Capacitors. J. Mater. Chem. 20, 10598–10601. doi:10.1039/c0jm03330h
Lu, F., Tan, P., and Han, Y. (2021). Variable Infrared Emissivity Based on Polyaniline Electrochromic Device Influenced by Porous Substrate. J. Appl. Polym. Sci 138, 49622. doi:10.1002/app.49622
Moulton, S. E., Innis, P. C., Kane-Maguire, L. A. P., Ngamna, O., and Wallace, G. G. (2004). Polymerisation and Characterisation of Conducting Polyaniline Nanoparticle Dispersions. Curr. Appl. Phys. 4, 402–406. doi:10.1016/j.cap.2003.11.059
Naskar, I., Deshagani, S., and Deepa, M. (2021). Zinc Cobaltite Micro-stars with a Zinc Oxide Nano-Stubs Overlayer Based Supercapacitor Colors a Polyaniline//tungsten Oxide Electrochromic Device. Electrochimica Acta 396, 139250. doi:10.1016/j.electacta.2021.139250
Song, S., Xu, G., Wang, B., Gu, J., Wei, H., Ren, Z., et al. (2021). Highly-flexible Monolithic Integrated Infrared Electrochromic Device Based on Polyaniline Conducting Polymer. Synth. Met. 278, 116822. doi:10.1016/j.synthmet.2021.116822
Tian, Y., Dou, S., Zhang, X., Zhang, L., Wang, L., Zhao, J., et al. (2017). Synthesis of Ordered Bowl-like Polyaniline Film with Enhanced Electrochromic Performances. Synth. Met. 232, 111–116. doi:10.1016/j.synthmet.2017.08.001
Tian, Y., Zhang, X., Dou, S., Zhang, L., Zhang, H., Lv, H., et al. (2017). A Comprehensive Study of Electrochromic Device with Variable Infrared Emissivity Based on Polyaniline Conducting Polymer. Solar Energ. Mater. Solar Cell 170, 120–126. doi:10.1016/j.solmat.2017.05.053
Xiong, S., Li, S., Zhang, X., Wang, R., Zhang, R., Wang, X., et al. (2018). Synthesis and Performance of Highly Stable Star-Shaped Polyaniline Electrochromic Materials with Triphenylamine Core. J. Elec Materi 47, 1167–1175. doi:10.1007/s11664-017-5901-2
Xu, G., Zhang, L., Wang, B., Chen, X., Dou, S., Pan, M., et al. (2020). A Visible-To-Infrared Broadband Flexible Electrochromic Device Based Polyaniline for Simultaneously Variable Optical and thermal Management. Solar Energ. Mater. Solar Cell 208, 110356. doi:10.1016/j.solmat.2019.110356
Xu, G., Zhang, L., Wang, B., Ren, Z., Chen, X., Dou, S., et al. (2020). Doping Engineering of the Flexible Polyaniline Electrochromic Material through H2SO4-HClO4 Multiple Acids for the Radiation Regulation in Snow Environment. J. Mater. Chem. C 8, 13336–13341. doi:10.1039/d0tc03177a
Zhang, L. P., Li, D. M., Li, X. B., Wang, B., Xu, G. P., Zhao, X., et al. (2019). Further Explore on the Behaviors of IR Electrochromism of a Double Layer Constructed by Proton Acid-Doped Polyaniline Film and ITO Layer. Dyes Pigm. 170, 170570. doi:10.1016/j.dyepig.2019.107570
Zhang, L., Wang, B., Li, X., Xu, G., Dou, S., Zhang, X., et al. (2019). Further Understanding of the Mechanisms of Electrochromic Devices with Variable Infrared Emissivity Based on Polyaniline Conducting Polymers. J. Mater. Chem. C 7, 9878–9891. doi:10.1039/c9tc02126d
Zhang, L., Xia, G., Li, X., Xu, G., Wang, B., Li, D., et al. (2019). Fabrication of the Infrared Variable Emissivity Electrochromic Film Based on Polyaniline Conducting Polymer. Synth. Met. 248, 88–93. doi:10.1016/j.synthmet.2019.01.007
Zhang, S., Chen, S., Hu, F., Ding, L., Gu, Y., Yan, B., et al. (2019). Patterned Flexible Electrochromic Device Based on Monodisperse Silica/Polyaniline Core/Shell Nanospheres. J. Electrochem. Soc. 166, H343–H350. doi:10.1149/2.1161908jes
Zhang, S., Chen, S., Hu, F., Xu, R., Yan, B., Jiang, M., et al. (2019). Spray-processable, Large-Area, Patterned and All-Solid-State Electrochromic Device Based on Silica/polyaniline Nanocomposites. Solar Energ. Mater. Solar Cell 200, 109951. doi:10.1016/j.solmat.2019.109951
Zhang, S., Chen, S., Yang, F., Hu, F., Yan, B., Gu, Y., et al. (2019). High-performance Electrochromic Device Based on Novel Polyaniline Nanofibers Wrapped Antimony-Doped Tin oxide/TiO2 Nanorods. Org. Electron. 65, 341–348. doi:10.1016/j.orgel.2018.11.036
Zhang, S., Fu, R., Gu, Y., Dong, L., Li, J., and Chen, S. (2017). Preparation of Nanocellulose-Based Polyaniline Composite Film and its Application in Electrochromic Device. J. Mater. Sci. Mater. Electron. 28, 10158–10165. doi:10.1007/s10854-017-6778-9
Zhang, S., Ren, J., Chen, S., Luo, Y., Bai, X., Ye, L., et al. (2020). Large Area Electrochromic Displays with Ultrafast Response Speed and High Contrast Using Solution-Processable and Patternable Honeycomb-like Polyaniline Nanostructures. J. Electroanalytical Chem. 870, 114248. doi:10.1016/j.jelechem.2020.114248
Zhao, Y., Zhang, S., Hu, F., Li, J., Chen, H., Lin, J., et al. (2019). Electrochromic Polyaniline/aramid Nanofiber Composites with Enhanced Cycling Stability and Film Forming Property. J. Mater. Sci. Mater. Electron. 30, 12718–12728. doi:10.1007/s10854-019-01636-y
Keywords: infrared, electrochromism, devices, acid-doping, PANI films
Citation: Li B, Chen Y, Liu X, Li F, Wang J, Li Y and Li N (2022) The Infrared Electrochromic Properties Based on Acid Doped-Polyaniline Films. Front. Mater. 9:857395. doi: 10.3389/fmats.2022.857395
Received: 20 January 2022; Accepted: 07 February 2022;
Published: 28 March 2022.
Edited by:
Jian Sun, Central South University, ChinaCopyright © 2022 Li, Chen, Liu, Li, Wang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jijun Wang, d2FuZ2pqZ2hzQGhvdG1haWwuY29t; Yan Li, MjE0MzYwNjE4QHFxLmNvbQ==; Ning Li, bGluaW5nbGluaW5nMDFAMTYzLmNvbQ==
 Bingzhen Li
Bingzhen Li Jijun Wang
Jijun Wang