
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mater., 10 November 2022
Sec. Biomaterials
Volume 9 - 2022 | https://doi.org/10.3389/fmats.2022.1013245
Background: Acellular dermal matrix (ADM) is currently considered as a replacement for lost extracellular matrix. Trials have been conducted on dressing materials with high contents of ADM without any impurities, such as gelatin, which only undergo the grinding process. In this study, we aimed to investigate the clinical application and wound healing effect of pure-grind ADM in lower extremity skin defect treatment.
Methods: Patients with skin defects in the lower extremities who did not achieve wound healing within 4 weeks with conservative treatment were enrolled in this study from March 2021 to July 2021. We randomized the patients into two groups. The patients in the experimental group were treated with pure-grind ADM and conventional negative-pressure wound therapy (NPWT), whereas the patients in the control group were only treated with NPWT. Every wound was followed-up for 7 weeks, and complete wound healing was confirmed when the skin defect was fully covered with epithelized tissue.
Results: A total of 41 patients were enrolled in this study. Complete wound healing was observed in 73.2% of patients, and 26.8% had an unhealed status until the end of the follow-up. The pure-grind ADM did not promote complete healing (p = 0.796) and was not associated with epithelization time but promoted the velocity of epithelization (experimental 5.58 vs. 3.50 cm2/day, p = 0.020). Considering the time of healing, the decrease in wound depth was more extensive (p = 0.021), the speed of granulation tissue formation was higher, and this difference was more evident after 5 weeks of treatment in the experimental group.
Conclusion: We demonstrated the clinical efficacy of pure-grind ADM in treatment of lower extremity skin defects. This new type of ADM, without any impurities, is important in wound healing. Its depth filling effect is powerful, and it can promote epithelization velocity and speed of granulation tissue formation.
Skin defects in the lower extremities can be caused by a spectrum of clinical situations. Regardless of the size or depth of these defects, clinicians usually face difficulty in treatment. The reasons for prolonged treatment in abnormal wound healing are rooted in three processes, including inflammation, proliferation, and maturation. Many researchers have focused on the key role of the extracellular matrix (ECM) during the healing process (Kirsner et al., 2013). The normal wound healing process is based on the interactions between several cellular components and the microenvironment. ECM plays an important role in the structural support and modulation of the wound healing process by activating signals to contribute to normal wound healing. Moreover, other factors, such as the presence of diabetes mellitus (DM), venous ulcers, peripheral arterial obstructive disease, or chronic inflammation, due to autoimmune diseases, osteomyelitis, and radiation ulcers, can affect the wound healing process and result in the formation of a chronic wound (Berrier and Yamada, 2007). The aforementioned factors can be considered as reasons for hesitation while choosing surgical treatment. In this regard, poor vascular status makes it difficult to locate a suitable recipient vessel and therefore it would be burdensome to choose flap coverage surgery. Although skin grafting can be a short and easy treatment, ensuring a proper wound dressing preparation seems to be an easier alternative (Martin et al., 2005; Cazzell et al., 2017).
In addition to surgical treatments for lower extremity skin defects, several non-surgical options have also been proposed, such as fibrin spray, negative-pressure wound therapy (NPWT), hyperbaric oxygen therapy, electrical stimulation, and ultrasound (Gould et al., 2014). Acellular dermal matrix (ADM) is currently considered as a replacement for the loss of ECM (Iorio et al., 2012; Kim et al., 2019a). In the initial processing stage, sheet-type ADM is easy to obtain. In this regard, this type of ADM is actively developed and used for breast surgery and odontogenic surgery. However, it is not suitable for use in an irregularly contoured or deep-seated wound, especially a lower extremity ulcerative wound (Ahn et al., 2019). To overcome this limitation, micronized ADM was developed and mixed with a gelatin suspension. The gelatin mixture enables easy application for any wound shape or even in deep tract-like wounds. Gelatin is a protein derived from collagen and can promote the wound healing process including epithelization, granulation, and tissue formation (Zhong et al., 2010). However, there are several limitations to gelatin use in biomedical applications. Gelatin is a hydrophilic protein with weak mechanical strength. Crosslinking with another material is normally needed to overcome these properties to enhance mechanical strength and stability (Hyun Ko et al., 2010). Moreover, gelatin can be rapidly degraded into a colloidal solution at 37°C. This also remains an obstacle in biomedical applications (Fukunishi and Shoji, 2017). Jang et al. reported that a ADM mixed with relatively high composition of gelatin has poor effects on the wound healing process compared with other materials, such as collagen (Jang et al., 2018). Additionally, higher contents of gelatin are directly linked to lower levels of ADM.
To overcome the limitations of conventional gelatin-containing ADM, trials involving dressing materials with high contents of ADM have been conducted. This article describes this new type of ADM material, which does not contain any impurities such as gelatin, and only undergoes the grinding process to achieve a maximal increase in the ADM content. We aimed to investigate the clinical application and wound healing effect of the new material, called pure-grind ADM, in lower extremity ulcerative wounds.
This study was approved by the Institutional Review Board of Ajou Hospital (approval number: AJIRB-DEV-INT-21-138) and was performed in accordance with the tenets of the Declaration of Helsinki.
The studies involving human participants were reviewed and approved by Institutional Review Board of Ajou Hospital (approval number: AJIRB-DEV-INT-21-138). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Patients with a skin defect in the lower extremities and a wound size of over 1 × 1 cm2 who were referred to our hospital from March 2021 to July 2021 were considered for inclusion. Accordingly, among these patients, only those who did not achieve wound healing within 4 weeks of conservative treatment were included in the study. All patients provided written informed consent before enrollment. Accordingly, consent to obtain and publish associated images was also obtained from all the patients, and the contents of this information were included in the additional consent form. This study was designed as a prospective clinical trial to evaluate the clinical efficacy of pure-grind ADM (CGbio, Seongnam, Seoul, Korea). Pure-grind ADM is based on human skin (Figure 1). Through de-cellularization, sheet-type ADM is acquired. Further, micronized processing is performed to produce micronizing ADM powder. The micronized ADM particles are 750 μm in size, instead of shredding the ultrastructure. Excluding the gelatin combination process, only the compression process is performed to produce pure-grind ADM.
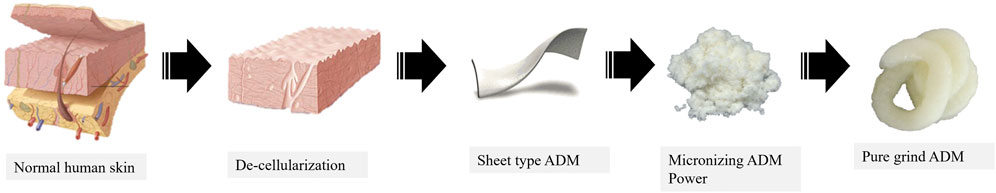
FIGURE 1. The pure-grind acellular dermal matrix (ADM). Based on normal human skin, sheet-type ADM was acquired after several procedures, including de-cellularization. Pure-grind ADM was made with powdered micronized ADM with uniform particle size.
Next, the patients were randomized into two groups. The patients in the experimental group were treated with pure-grind ADM in combination with conventional NPWT (CuraVac; Daewoong Pharmaceutical Co. Ltd., Seoul, Korea, 125 mmHg), whereas the patients in the control group were only treated with NPWT (Figure 2). Accordingly, the NPWT dressing was changed every 3–4 days based on patient conditions. In this regard, to maintain consistency, wounds were evaluated according to the planned schedule, regardless of the time of dressing change. In the experimental group, ADM was reapplied with every dressing change.
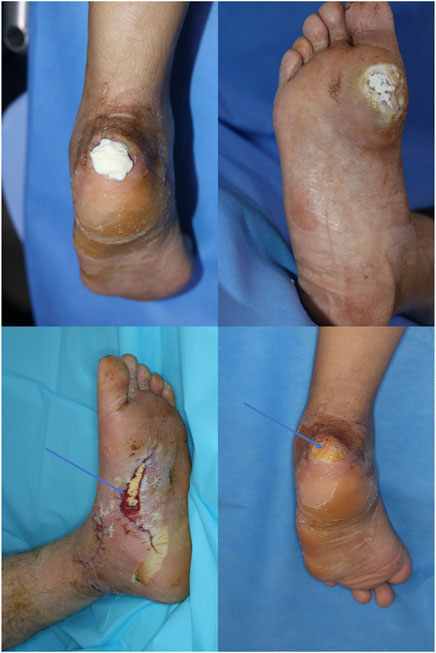
FIGURE 2. Clinical application of pure-grind ADM in lower extremity skin defects. Immediate images after application of pure-grind ADM (above, left and right). Well-taken pure-grind ADM after 3 weeks from application (below, left and right). Whitish ADM was changed to yellow color (arrow).
The wound was followed-up at the first day (day 0) and at 1, 3, 5, and 7 weeks. Every wound was followed-up until 7 weeks, but early follow-up completion was confirmed when the skin defect was fully covered with epithelized tissue. The missing value due to early completion was analyzed with a generalized estimating equation. Wound size, parameters of granulation tissue and reepithelization, and adverse events were recorded in each period. Clinical photographs were taken at a 35-cm distance using a single-lens reflex camera with constant lighting and shooting conditions. Early completion of follow up was done when an ulcer covered with fresh granulation tissue, which was suitable for performing split-thickness skin grafting.
Wound sizes were calculated by measuring the longest horizontal and vertical axes with a flexible ruler. Depth was classified into four grades: grade 1 was epithelialized skin, grade 2 was the dermis bed, grade 3 was subcutaneous fat tissue bed, and grade 4 was bone or tendon or joint exposure (Huang et al., 2021). The percentage of granulation tissue was evaluated and categorized in five steps. In this regard, step 1 indicated intact skin or partial thickness wound. Step 2 indicated a bright, beefy red granulation, which filled 75%–100% of the wound and/or tissue overgrowth. Step 3 indicated a bright, beefy red granulation, which filled <75% and >25% of the wound. Step 4 was a pink, and/or dull, dusky red granulation and/or ≤25% of the wound was filled, and step 5 was defined when no granulation tissue was present (Kim et al., 2019b). The velocity and percentage of wound contracture were calculated using the below equations. The percentage of wound contracture was calculated as the gap between the initial wound size (cm2) and the final follow-up wound size (cm2) divided by the initial wound size (cm2). The velocity of wound contracture was calculated as the gap between the initial wound size (cm2) and the final follow-up wound size (cm2) divided by full epithelization time (day).
A total of 41 patients were included in the study who underwent follow-up for the entire period. The experimental group consisted of 21 patients, and their mean age was 45.66 years. The control group consisted of 20 patients, and the mean age was 55 years. An analysis of demographical information showed that there was no significant difference between the two groups (Table 1). However, there were more trauma patients in the control group and more patients with diabetes in the experimental group (p = 0.025). Accordingly, further analysis proceeded after statistical adjustments for this difference. Considering the location of the skin defect, the foot included the heel (19.00%) and toe (9.51%).
In aspects of treatment results, complete healing occurred in 73.2% of patients, and unhealing until the final follow-up period was observed in 26.8% of patients. The chi-square test showed no statistically significant difference in complete healing between the two groups (p = 0.796). Using pure-grind ADM in treatment did not affect the rate of complete healing. The proportion of early completion before 7 weeks did not affect the rate of complete healing (p = 0.443) (Table 2).
The clinical effects on wound contracture were evaluated with full epithelization time and wound contracture size and velocity. There was no statistical difference in full epithelization time (p = 0.367) and percentage of wound contracture (p = 0.235) between the two groups. However, a statistical difference was observed in the velocity of wound contracture between the two groups. The wound contracture velocity was faster in the pure-grind ADM group than that in the control group (5.58 cm2/day vs 3.50 cm2/day, p = 0.020) (Table 3). The pure-grind ADM was not associated with epithelization time or wound contracture period but promoted the velocity of epithelization.
The interaction between time and wound depth was evaluated with generalized estimating equation statistics (Table 4). There was a statistically significant difference in time-change correlations between the groups (p = 0.021). During the follow-up period, the decrease in wound depth was more extensive in the experimental group from 3.71 grade to 1.69 grade (Figure 3). The use of pure-grind ADM did not contribute to decreasing the time or size of wound contracture but promoted an improvement in the wound depth.
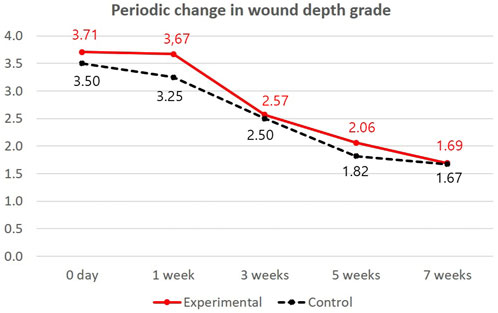
FIGURE 3. Periodic changes in wound depth grade in both groups. The red linear line indicates a periodical change in wound depth grade in the experimental group (pure-grind ADM), and the black dotted line indicates a periodic change in wound depth grade in the control group.
The analysis of the interaction between the time of granulation tissue formation and treatment time showed a statistically significant difference between the two groups (p = 0.046) (Table 5; Figure 4). The percentage of granulation tissue on the wound was evaluated according to time trends. The lower granulation tissue grade represented that the wound is filled with granulation tissue extensively. Figure 4 shows decreasing trends of granulation tissue grades. Thus, the wound was filled with granulation tissue according to time. At the final follow-up at 7 weeks, the pure-grind ADM group showed a higher-filled granulation tissue percentage than the control group (1.44 vs. 1.86). The speed of granulation tissue formation was superior in the experimental group, and this difference was more evident after 5 weeks of treatment.

TABLE 5. Statistical analysis of periodic changes in granulation tissue percentage in the two groups.
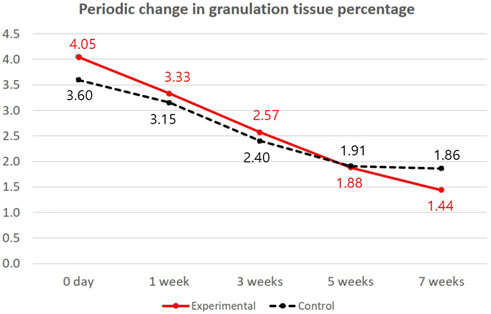
FIGURE 4. Periodic change in granulation tissue percentage in both groups. The red linear line indicates a periodical change in granulation tissue percentage in the experimental group (pure-grind ADM), and the black dotted line indicates a periodic change in granulation tissue percentage in the control group.
In this study, a comparative analysis was performed between the pure-grind ADM and control groups on the wound healing process in skin defects in the lower extremities. Unlike our hypothesis, there was no significant difference in full epithelization time and the size of wounds after contracture. However, we revealed more meaningful clinical outcomes considering the depth improvement and healing speed of the wound. It should be noted that we only included wounds which were not healed within 4 weeks with the conventional treatment. The expectation of being fully healed with epithelization tissue was a big overestimation for clinicians. However, we can assure patients that this material can improve their wound healing process by breaking the vicious cycle of chronic wound and to facilitate the formation of healthy granulation tissue. We demonstrated that pure-grind ADM promoted faster wound contracture and especially improved wound depth filling and granulation tissue formation. In the treatment of hard-to-heal wounds, the pure-grind ADM is enough to play a role as a scaffold in the wound healing process. Once the wound depth improves, the healing process starts with collagen deposition based on these scaffolds. Although within 7 weeks, we observed 28.6% of patients with unhealed wounds, the wounds were healed completely within 17 weeks (Figures 5, 6).
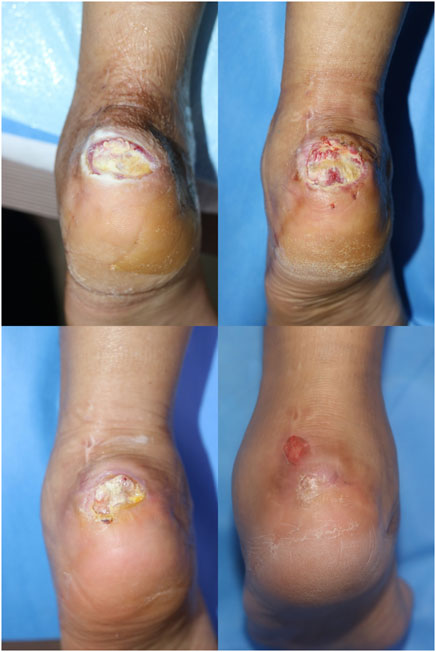
FIGURE 5. Case 1. A 34-year-old female with a history of orthostatic hypotension presented with soft tissue necrosis on the left heel due to a pressure sore (above, left). After applying pure-grind ADM, wound depth and size improved (above, right and below, left). After 7 weeks, the previous pure-grind ADM applicated wound was completely healed, and a newly abrasion wound was seen, which was not associated with the previous treatment course (below, right).
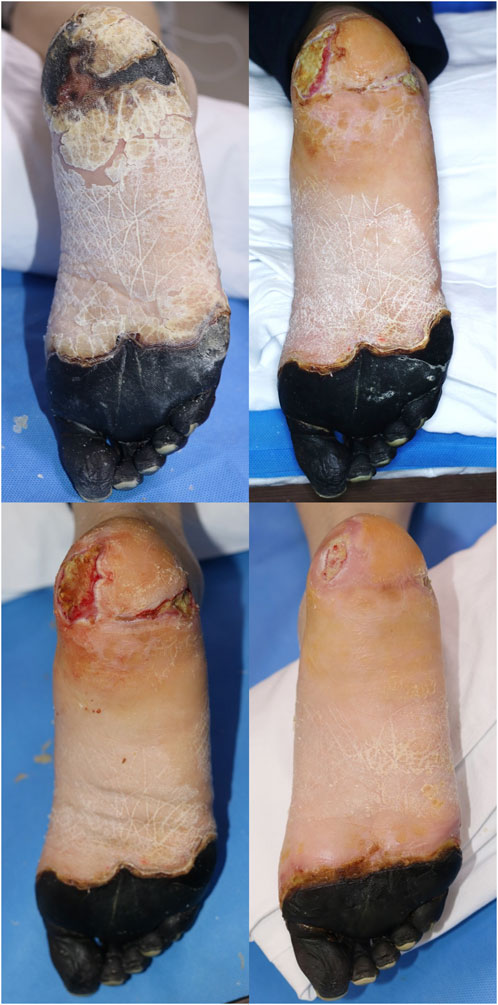
FIGURE 6. Case 2. A 53-year-old female with no medical history. Forefoot necrosis was noted owing to vasoconstrictive inotropic drug use to manage acute pyelonephritis sepsis. Surgery under general anesthesia was impossible owing to poor general conditions (above, left). There was no improvement in the wound under conservative care (above, right). Serial bedside debridement was performed. Unhealed defects remained at 7 weeks after pure-grind ADM application, but definite depth improvement was visible (below, left). This point of view is meaningful in these kinds of patients with difficult cases. After 12 weeks, wound depth and size were significantly improved (below, right).
ADM has been utilized in soft tissue reconstruction since 1995 (Kim et al., 2019a). With further developments, the popularity of ADM increased and it was used in breast reconstruction in 2005 (Vidya et al., 2017). After grafting, it is well known that ADM works as a scaffold for the adhesion of several cells associated with tissue regeneration and neovascularization (Breuing, 2007). Based on this theoretical establishment, many trials with different clinical applications have been proposed. Here, we focused on the morphological differentiation of ADM. Different from conventional sheet-type ADM, which is optimized for breast reconstruction, micronized ADM has been proposed as a wound dressing material. Further, owing to its formless features, its syringe injection type is innovative and very useful in outpatient clinics. There are many recent studies of new trials using various types of ADM in wound healing. Kim et al. in a multicenter comparative study reported the use of a paste-type ADM for chronic wounds, which was beneficial in wound size reduction, epithelization, and granulation formation (Kim et al., 2022). As we mentioned above, micronized paste-type ADM is useful in wounds with dead space, cavitary wounds, and undermining and tunneling wounds, where it can make direct contact with the surface (Tognetti et al., 2021; Taylor et al., 2008). However, in aspects of physiochemical modification of ADM, this modification can directly affect interfibrous crosslinking and degradation resistance (Ma et al., 2020). Thus, a new enzymatic process in product manufacturing has been developed that incorporates reduced graphene oxide (RGO) nanoparticles into ADM to improve its natural scaffolding properties and ensure highly efficient local transplantation in diabetic wounds (Fu et al., 2019). Moreover, addition of gelatin in micronized-type ADM is required for smaller particle-sized ADM. The reason behind conducing several of these trials is that many researchers have focused on its application as a “raw material” for the construction of biological materials. However, biocompatibility in case of gelatin addition is still concerning. Jin et al. recently reported the production of a three-dimensional bioprinting skin model using gelatin methacrylamide bioink and ADM (Jin et al., 2021). In this study, we have proposed the use of purified ADM without gelatin. Accordingly, with no concerns of other impurities and bio-incompatibility, higher contents of ADM can be transferred into the wound (Figure 7). Thus, this recently developed ADM has overcome all these limitations.
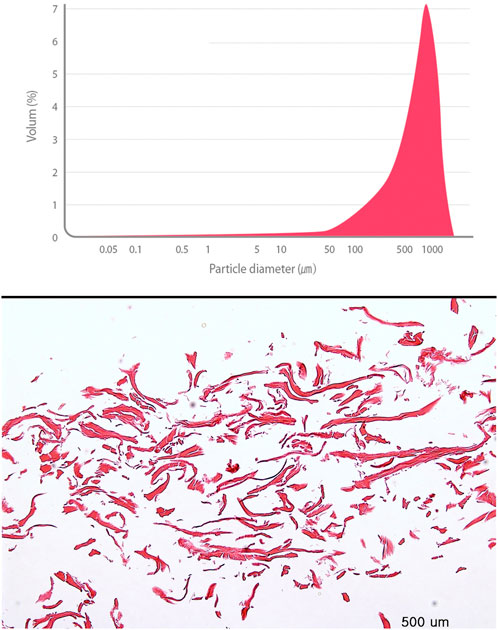
FIGURE 7. An analysis of biochemical properties of Pure-Grind Acellular Dermal Matrix Without Gelatin. Particle size distribution data. The material has the most frequent particle size, 824.5 um with 6.8%. In that, their composition is revealed evenly (above). H&E stain of material (below).
Pure-grind ADM is made with powdered micronized ADM with a constant particle size. The particle size of pure-grind ADM is uniform and 750 um in size. Using sterile distilled water, ADM is converted into a hydrated state. Without any further procedure, hydrated micronized ADM is prefilled with a syringe for use directly without additional handling, which minimizes the contamination risk. The proportion of ADM in this pure-grind ADM product is twice that of paste-type ADM (CGPaste; CGBio, Inc., Seongnam, Korea) of the same weight.
The role of the ECM, especially in hard-to-heal ulcerative lower extremity wounds, is meaningful in treatment. ECM is a key component of the wound healing process. First, the ECM provides structural support for the dermal layer (Li et al., 2011). Second, the ECM promotes the signaling proteins to activate cell adhesion and signaling (Yim et al., 2009; Pizzo et al., 2005; Hodde et al., 2005; Hodde et al., 2001). According to the above roles of ECM, a dysfunctional ECM in the wound healing process can be a significant obstacle, leading to a chronic wound. To avoid dysfunction of the ECM, many efforts have been taken to find a substitute for a dysfunctional ECM (Schultz et al., 2011; Kirsner et al., 2013). ADM has been proven beneficial as an alternative to ECM (Li et al., 2011; Brigido et al., 2009; Jeon and Kim, 2018). Conventional ADM was made in the form of a sheet. However, a chronic wound is usually irregularly contoured and deep-seated and it is not feasible to apply a sheet of ADM (Lee et al., 2022). Because of this limitation of sheet-type ADM, paste-type micronized ADM was designed mixed with gelatin suspension. Gelatin is a protein biopolymer composed in the human skin, tendons, cartilage, and bone. Gelatin has several advantages considering its biological origin, biodegradability, non-immunogenicity, biocompatibility, and relatively low cost (Zhang et al., 2006). Thus, this study trial, which enhanced the integrity of ADM within the material, facilitated the role of ADM in the wound healing process.
This study has several limitations. First, the sample size of each group was relatively small; however, it should be considered that the efficacy of this new material for lower extremity wounds was evaluated for the first time. Accordingly, despite the small sample size, we believe that our study is unique compared to other similar studies on ADM. Further, long-term and large-scale studies should be planned to establish the effectiveness of pure-grind ADM. Second, selection bias needs to be considered. This study involved a blind and random sample of patients. Accordingly, the control group included patients with more large-sized wounds, and the frequency of small-sized wounds was higher in the experimental group. Nevertheless, this issue was addressed by performing statistical correctional approaches based on the wound size. Third, the efficacy of pure-grind ADM was only evaluated compared to that of NPWT. Further evaluation is needed with large-scale studies to compare the effects of pure-grind ADM with other types of ADM and conventional dressings, such as a gauze or polyurethane foam.
In conclusion, we demonstrated the clinical efficacy of pure-grind ADM in lower extremity skin defects. Accordingly, we showed that this new type of ADM, without any impurities, is beneficial in wound healing. However, care should be taken not to overestimate its healing effect until full epithelization. It can be concluded that the expected role of the pure-grind ADM in chronic wounds is its depth filling effect. Additionally, it can promote epithelization velocity and speed of granulation tissue formation. This recently developed pure-grind ADM is promising with many expectations for future use; however, long-term clinical studies are needed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This study was approved by the Institutional Review Board of Ajou Hospital (approval number: AJIRB-DEV-INT-21-138) and was performed in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MK: conceptualization, data curation, validation, formal analysis investigation, writing-original draft, writing-reviewing and editing. YJ: data acquisition, investigation, writing—reviewing, writing-revision and rebuttal letter, and editing. TK: data curation, investigation, validation, writing-original draft, writing-reviewing, and editing. DP: formal analysis, writing- reviewing and editing. IL: conceptualization, methodology, supervision, validation, writing-reviewing, and editing. All authors read and approved the final manuscript.
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI20C2140).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahn, S., Choi, H., Lee, J., and Kim, J. H. (2019). A clinical study of micronized acellular dermal matrix collagen paste application with negative pressure wound therapy. J. Wound Manag. Res. 15, 23–30. doi:10.22467/jwmr.2018.00535
Berrier, A., and Yamada, K. M. (2007). Cell–matrix adhesion. J. Cell. Physiol. 213, 565–573. doi:10.1002/jcp.21237
Breuing, K. (2007). Undefined Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Surg. AC-A plastic 59 (3), 250–255. doi:10.1097/SAP.0b013e31802f8426
Brigido, S. A., Schwartz, E., Mccarroll, R., and Hardin-Young, J. (2009). Use of an acellular flowable dermal replacement scaffold on lower extremity sinus tract wounds: A retrospective series. Foot Ankle Spec. 2, 67–72. doi:10.1177/1938640009333474
Cazzell, S., Dean, V, and Pham, H (2017). A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active. Wiley Online Libr. 25, 483–497. doi:10.1111/wrr.12551
Fu, J., Zhang, Y., Chu, J., Wang, X., Yan, W., and Zhang, Q. (2019). Reduced graphene oxide incorporated acellular dermal composite scaffold enables efficient local delivery of mesenchymal stem cells for accelerating diabetic wound healing. ACS Biomater. Sci. Eng. 5, 4054–4066. doi:10.1021/ACSBIOMATERIALS.9B00485
Fukunishi, T., and Shoji, T. (2017). Biomedical TS-NC for, 2017 undefined Nanofiber composites in vascular tissue engineering. Biomed. Res. Int. 2017. doi:10.1155/2017/9616939
Gould, L., Abadir, P., Brem, H., Carter, M., Conner-Kerr, T., and Davidson, J. (2014). Chronic wound repair and healing in older adults: Current status and future research. Wound Repair Regen. 23, 1–13. doi:10.1111/wrr.12245
Hodde, J. P., Ernst, D. M., and Hiles, M. C. (2005). An investigation of the long-term bioactivity of endogenous growth factor in OASIS Wound Matrix. J. Wound Care 14, 23–25. doi:10.12968/JOWC.2005.14.1.26721
Hodde, J. P., Record, R. D., Liang, H. A., and Badylak, S. F. (2001). Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium 8, 11–24. doi:10.3109/10623320109063154
Huang, S., Dang, J., and Sheckter, C. (2021). A systematic review of machine learning and automation in burn wound evaluation: A promising but developing frontier. Burns 47 (8), 1691–1704. doi:10.1016/j.burns.2021.07.007
Hyun Ko, J., Yin, H., An, J., Chung, D. J., Kim, J. H., and Lee, S. B. (2010). Characterization of cross-linked gelatin nanofibers through electrospinning. Macromol. Res. 18, 137–143. doi:10.1007/s13233-009-0103-2
Iorio, M., Shuck, J., and Attinger, C. E. (2012). Undefined Wound healing in the upper and lower extremities: a systematic review on the use of acellular dermal matrices. Plast. Reconstr. Surg. 130 (5), 232–241. doi:10.1097/PRS.0b013e3182615703
Jang, H. J., Kim, Y. M., Yoo, B. Y., and Seo, Y. K. (2018). Wound-healing effects of human dermal components with gelatin dressing. J. Biomater. Appl. 32, 716–724. doi:10.1177/0885328217741758
Jeon, M., and Kim, S. Y. (2018). Application of a paste-type acellular dermal matrix for coverage of chronic ulcerative wounds. Arch. Plast. Surg. 45, 564–571. doi:10.5999/APS.2018.00605
Jin, R., Cui, Y., Chen, H., and Zhenzhen, Z. (2021). Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomater. 131, 248–261. doi:10.1016/j.actbio.2021.07.012
Kim, G. H., Lee, J. Y., Kim, J., Kim, H., and Park, J. U. (2019). Prevalence of pressure injuries nationwide from 2009 to 2015: Results from the national inpatient sample database in Korea. Int. J. Environ. Res. Public Health 16, 704. doi:10.3390/ijerph16050704
Kim, Y., Shim, H., Lee, J., and Kim, S. W. (2022). A prospective randomized controlled multicenter clinical trial comparing paste-type Acellular dermal matrix to standard care for the treatment of chronic wounds. J. ClinMed 11 (8), 2203. doi:10.3390/jcm11082203
Kim, Y. H., Hwang, K. T., Kim, K. H., Sung, I. H., and Kim, S. W. (2019). Application of acellular human dermis and skin grafts for lower extremity reconstruction. J. Wound Care 28, S12–S17. doi:10.12968/JOWC.2019.28.SUP4.S12
Kirsner, R. S., Bohn, G., Driver, V. R., Mills, J. L., Nanney, L. B., and Williams, M. L. (2013). Human acellular dermal wound matrix: Evidence and experience. Int. Wound J. 12, 646–654. doi:10.1111/iwj.12185
Lee, M. C., Jun, D. K., Choi, H. G., Kim, J. N., and Shin, D. H. (2022). Clinical efficacy of acellular dermal matrix paste in treating diabetic foot ulcers. Wounds: a compendium of clinical research and practice 32 (1), 50–56.
Li, R., Guo, W., Yang, B., and Guo, B. (2011). Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 32 (20), 4525–4528. doi:10.1016/j.biomaterials.2011.03.008
Ma, P., Wang, Y., and Li, B. (2020). Cross-linking effects of carbodiimide, oxidized chitosan oligosaccharide and glutaraldehyde on acellular dermal matrix of basa fish (Pangasius bocourti). Int. J. Biol. Macromol. 164, 677–686. doi:10.1016/j.ijbiomac.2020.07.019
Martin, B. R., Sangalang, M., Wu, S., and Armstrong, D. G. (2005). Outcomes of allogenic acellular matrix therapy in treatment of diabetic foot wounds: An initial experience. Int. Wound J. 2, 161–165. doi:10.1111/J.1742-4801.2005.00099.X
Pizzo, A. M., Kokini, K., Vaughn, L. C., Waisner, B. Z., and Voytik-Harbin, S. L. (2005). Extracellular matrix (ECM) microstructural composition regulates local cell-ECM biomechanics and fundamental fibroblast behavior: A multidimensional perspective. J. Appl. Physiol. (1985). 98, 1909–1921. doi:10.1152/JAPPLPHYSIOL.01137.2004
Schultz, G. S., Davidson, J. M., Kirsner, R. S., Bornstein, P., and Herman, I. M. (2011). Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 19, 134–148. doi:10.1111/j.1524-475X.2011.00673.x
Taylor, D., Sampaio, L., and Ferdous, Z. Decellularized matrices in regenerative medicine. Acta Biomater. 74 (1), 74–89. 2008, doi:10.1016/j.actbio.2018.04.044
Tognetti, L., Pianigiani, E., Ierardi, F., Lorenzini, G., Casella, D., and Liso, F. G. (2021). The use of human acellular dermal matrices in advanced wound healing and surgical procedures: State of the art. Dermatol. Ther. 34, e14283. doi:10.1111/dth.14283
Vidya, R., Masià, J., Cawthorn, S., Berna, G., Bozza, F., and Gardetto, A. (2017). Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J. 23, 670–676. doi:10.1111/TBJ.12810
Yim, H., Cho, Y., and Seo, C. (2009). The use of AlloDerm on major burn patients: AlloDerm prevents post-burn joint contracture. Burns 36 (3), 322–328. doi:10.1016/j.burns.2009.10.018
Zhang, Y., Venugopal, J., and Huang, Z. (2006). Crosslinking of the electrospun gelatin nanofibers. Polymer 47 (8), 2911–2917. doi:10.1016/j.polymer.2006.02.046
Keywords: acellular dermal matrix, skin defect, leg ulcer, wound healing, wounds and injuries
Citation: Kim MJ, Jeong YS, Kim TW, Park DH and Lee IJ (2022) The clinical efficacy of pure-grind acellular dermal matrix without gelatin in lower extremity skin defect treatment: A prospective randomized study. Front. Mater. 9:1013245. doi: 10.3389/fmats.2022.1013245
Received: 06 August 2022; Accepted: 13 October 2022;
Published: 10 November 2022.
Edited by:
Jiacan Su, Shanghai University, ChinaReviewed by:
Xiaoqun Li, Second Military Medical University, ChinaCopyright © 2022 Kim, Jeong, Kim, Park and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Il Jae Lee, aTAwMzI1QGxpdmUuY28ua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.