
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mater. , 02 November 2022
Sec. Polymeric and Composite Materials
Volume 9 - 2022 | https://doi.org/10.3389/fmats.2022.1003539
Purpose Acute massive pancreaticoduodenal artery (PDA) hemorrhage represents an urgent condition. Here, we report our experience in transcatheter arterial embolization (TAE) using N-butyl-2 cyanoacrylate (NBCA) Glubran® 2 for this condition. Methods A retrospective study of 10 consecutive patients (mean, 55.2 ± 15.9 years; range, 27–74 years) was conducted from April 2015 to April 2021. The data, including baseline characteristics, control of active PDA hemorrhage (the technical and clinical outcomes), volumes of Glubran 2, and related complications, were collected from medical archives. Technical success was defined as complete occlusion of bleeding vessels on the final arteriogram. Clinical success was defined as the sustained resolution of symptoms or signs of PDA hemorrhage without the need for repeat endovascular or surgical treatment after TAE. Safety was evaluated based on the occurrence of complications. Results Glubran 2 was empirically used for six patients (6/10) with PDA hemorrhage without pseudoaneurysm (PSA), and a combination of Glubran 2 and microcoils (n = 13) was used for four patients (4/10) with PSA. The concentration ratios were 1:2–1:4 mixtures of Glubran 2 and ethiodized oil, and the median total volume injected was 1.1 ml (range, 0.7–1.6 ml). Technical success based on 10 episodes of TAEs was 100% (10/10). Early rebleeding did not occur, and repeat TAE was not necessary. Clinical success was 100% (10/10). No nontarget embolization or embolization-related complications occurred. Two patients (2/10) experienced minor complications of postembolization syndrome. During a median follow-up time of 3 months, no recurrent hemorrhage was recorded. Conclusion Urgent TAE with the use of NBCA Glubran 2 plays an important role in controlling massive PDA hemorrhage. It is minimally invasive, effective, relatively safe, and likely to reduce the need for immediate traditional surgery. The condition with PSA may determine the microcoils employed.
The pancreaticoduodenal artery (PDA) arcade, which connects the celiac artery (CA) and the superior mesenteric artery (SMA), is an arterial network that encircles the pancreatic head and duodenal descending part (Mano et al., 2013; Kickuth et al., 2016). Acute massive hemorrhage of the PDA network has been reported as an infrequent but potentially life-threatening condition (Kickuth et al., 2016). Under this condition, patients with active bleeding commonly present with hypovolemic shock (HS) that requires prompt attention (Kickuth et al., 2016; Popov et al., 2017). The etiologies of acute PDA hemorrhage vary and are typically attributed to spontaneous, traumatic, iatrogenic, and protopathic causes (Gralnek et al., 2015; Popov et al., 2017). The transformation of unstable arterial hemodynamics and fragile vessel walls is thought to increase the risk of spontaneous rupture, resulting in massive hemorrhage, which is considered to be associated with a high rate of underlying mortality (Nicholson et al., 2006; Gralnek et al., 2015; Kickuth et al., 2016; Abdulmalak et al., 2018).
Patients with massive PDA hemorrhage require urgent and expeditious management. However, control of this hemorrhage may be formidable because of special collateral pathways, especially in the PDA arcades (Kickuth et al., 2016). Hence, in general, the goals of the interventions are to cease active bleeding, stabilize vital signs, and preserve as much function of feeder vessels as possible. Historically, therapies have involved conservative resuscitation with fluid or blood transfusion expansion and surgical vessel ligation (Gralnek et al., 2015); however, these approaches may not always be feasible and may be associated with recurrent bleeding (Angle et al., 2010). Transcatheter arterial embolization (TAE) has developed as an important cornerstone in the management of hemorrhage events. It seems to be technically easy, avoids open invasion and has a prophylactic effect in preventing future hemorrhage (Rösch et al., 1972; Nicholson et al., 2006; Gralnek et al., 2015; Kickuth et al., 2016; Popov et al., 2017; Abdulmalak et al., 2018; Sverdén et al., 2019).
The advent of newer coaxial microcatheters, the development of more compatible embolic materials, and the improvement of embolization techniques have raised interest in TAE for active PDA hemorrhage (Kickuth et al., 2016). Embolic materials used mainly involve particles, microcoils, and liquid agents. Each material has its specific benefits and drawbacks (Abdulmalak et al., 2018), but there are still no validated guidelines to establish strong recommendations on which appropriate material is preferred. N-butyl-2 cyanoacrylate (NBCA) Glubran 2® has emerged as a well-known liquid embolic agent. Although it has been demonstrated as an efficacy of selection (Kim et al., 2017), the outcome of this kind of embolotherapy for PDA is mainly documented from individual case. To address the need for more data, we present our experience in TAE using embolic materials (Glubran 2 and microcoils), alone or in combination, in the setting of massive PDA hemorrhage with/without PSA.
From April 2015 to April 2021, the relevant data of patients with acute massive PDA hemorrhage who underwent TAE using NBCA Glubran 2 at a single center were retrospectively reviewed. Written consent was obtained from all patients. Approval from the Institutional Review Board (IRB) was provided for this retrospective report. The data collected from the medical archives of the clinical notes, laboratory values, imaging findings, and procedure reports included the baseline characteristics, control of active hemorrhage (technical success and clinical success), volumes of NBCA Glubran 2, and related complications.
Ten consecutive patients (mean, 55.2 ± 15.9 years; range, 27–74 years) who subsequently experienced 10 episodes of TAEs to control PDA hemorrhage were included. Eight (8/10) of these patients were male. All patients (10/10) had hemodynamic instability, eight (8/10) had upper abdominal pain, five (5/10) had bleeding through an abdominal drainage tube following surgery, two (2/10) had melena, and one (1/10) had hematemesis. The mean volume of packed red blood cell transfusion prior to TAE was 5.3 U (range, 2–10 U) per patient. The demographic, clinical characteristics, and comorbidities of the patients are shown in Table 1. The signs and symptoms (hemodynamically unstable condition presented as decreased systolic blood pressure (sBP), increased heart rate and decreased hemoglobin) initially indicated acute active hemorrhage. Computed tomography (CT) scans initially confirmed hemorrhage in eight patients, and contrast-enhanced CTs were used in five patients to identify the hemorrhage and eliminate other underlying causes of hemorrhage. Two patients underwent endoscopies due to melena and/or hematemesis. The diagnosis of massive PDA hemorrhage was comprehensively based on the clinical manifestations, decreased hemoglobin levels and specific characteristics of imaging findings.
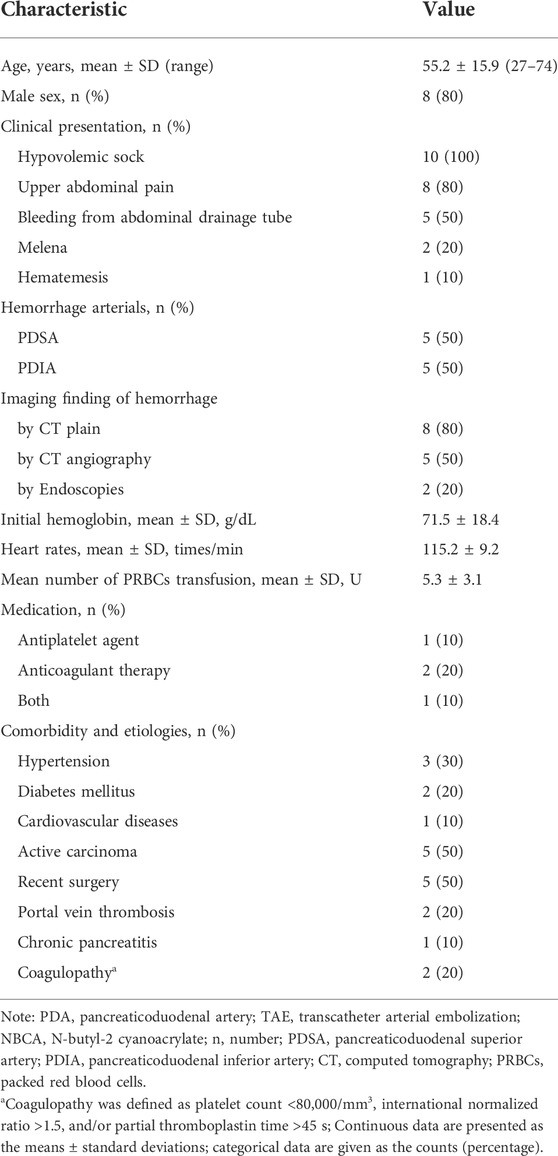
TABLE 1. Demographics, presentation and lesion characteristics, and medications of patients with PDA hemorrhage underwent TAE using NBCA Glubran-2.
The embolic materials employed in the present study consisted of microcoils (Cook, Bloomington, IN, United States) and/or Glubran 2 (N-butyl-2 cyanoacrylate; GEM, Italy), which were proposed empirically by the operators at our center. The benefits and potential risks of TAE were explained, and detailed informed consent was obtained from all patients.
Three interventional operators with at least 15 years of experience performed the procedures. Prior to TAE, to identify the arterial anatomy and localize the active bleeding sites and feeder arteries, an accurate overview angiogram of the CA and SMA was performed under local anesthesia using a 5-French (F) selective Cobra catheter (Radifocus Angiographic Catheter; Terumo, Leuven, Belgium) through a 5-F sheath via a transfemoral approach. After identifying the arterial bleeding site, a selective microcatheter was then positioned coaxially through the Cobra catheter, ensuring that the tip of the microcatheter was as close as possible to the target bleeding site. According to the operators’ experience, Glubran 2 was used solely through a 2.4-F microcatheter (Merit Medical, South Jordan, Utah) for patients without PSA. For patients with PSA, Glubran 2 was used in combination with microcoils to arrest the progression of Glubran 2 and/or to prevent glue polymerization in nontarget vessels through a 2.7-F microcatheter (Progreat; Terumo Corp., Tokyo, Japan). When microcoils were used, they were sized according to the target PSA, and under fluoroscopy, they were first released to fill the PSA cavity and then flushed with a 5% dextrose solution. Glubran 2 was mixed with ethiodized oil (Hengrui Medicine, Jiangsu, China) to make it radiopaque at a ratio of 1:2 to 1:4 to delay polymerization and then was injected as slowly as possible using thumb pressure and adjusted according to the liquid material propagation in the bleeding artery, target arterial flow speed and operator preference. If possible, the procedure was continued until feeding bleeding vessels were completely occluded to avoid undesired embolization of normal arterial branches and reduce infarcted organic parenchyma loss as much as possible. At the end of TAE, the microcatheter was rapidly withdrawn, and a final CA and SMA arteriogram via a Cobra catheter was performed to evaluate vessel occlusion. Nontarget embolization was also assessed.
The efficacy of TAE was assessed using both technical and clinical evaluations. Technical efficacy was defined as the complete cessation of target angiographic extravasation on the final arteriogram, while clinical efficacy was defined as the sustained resolution of signs or symptoms for PDA hemorrhage, which did not need repeat endovascular or surgery treatment after TAE (Angle et al., 2010; Gong et al., 2021). According to the reporting standards of the Society of Interventional Radiology, the safety of TAE was evaluated based on complications occurring mid-TAE and post-TAE (Angle et al., 2010). Clinical and laboratory values were assessed after 7 days and compared to pre-TAE values. During the follow-up, CT and/or abdominal US and clinical evaluations were performed on an outpatient basis for patients on the first and third months when clinically indicated. Any instances of rebleeding or post-TAE complications were recorded.
The SPSS statistical software package (version 23.0; SPSS statistical software, Chicago, Illinois, United States) was used for all statistical analyses. Qualitative variables are described as numbers (percentages), and continuous variables are described as the means ± standard deviations. The paired t-test was used when assessing the correlation between pre- and post-intervention variables. Findings with a p-value less than 0.05 were deemed statistically significant.
Ten patients presenting with massive PDA hemorrhage received a total of 10 episodes of TAEs. Of these, five patients underwent inferior PDA catheterization via the SMA branch, and five patients experienced superior PDA catheterization via the CA branch (the embolic materials used, TAE-related details and outcomes are listed in Table 2). Glubran 2 was successfully used as a sole embolic agent for six patients (60.0%) without PSA. Four patients (40%) diagnosed with PSA initially underwent a total of 13 microcoils (range, 2–6 mm × 2–6 mm) in the cavity of the PSA, and Glubran 2 embolization was then used in the same session (successful procedure-related details are shown in Figure 1). Four patients (4/10) experienced bleeding after pancreaticoduodenal surgery, and the exact bleeding site of one patient who underwent gastroduodenal surgery was determined by evaluation of surgical colleagues involved in corresponding surgical procedures and effective TAE. The concentration ratios used were 1:2–1:4, the total volume injected was a mean of 1.1 ml (range, 0.7–1.6 ml), and the fluoroscopy time was 20.8 ± 11.5 min. The final arteriography revealed complete occlusion of all targeted vessels, and the technical success of TAE was achieved in all patients (10/10). All patients in the pre-TAE group encountered HS with a mean sBP of 83.0 ± 9.1 mmHg, experienced a stable hemodynamic status and increased sBP following TAE to 109.6 ± 1.16 mmHg (t = 9.972, 95% CI, 20.6 to 32.6, p < 0.001). Anemia symptoms improved after blood transfusion and TAE treatment. Clinical success was achieved in all patients (10/10). All patients were free from recurrent hemorrhage after receiving embolotherapy within 30 days. At the 3-month follow-up, neither hemorrhage nor recurrent further ruptures were observed. Surgical treatment was avoided in all patients.
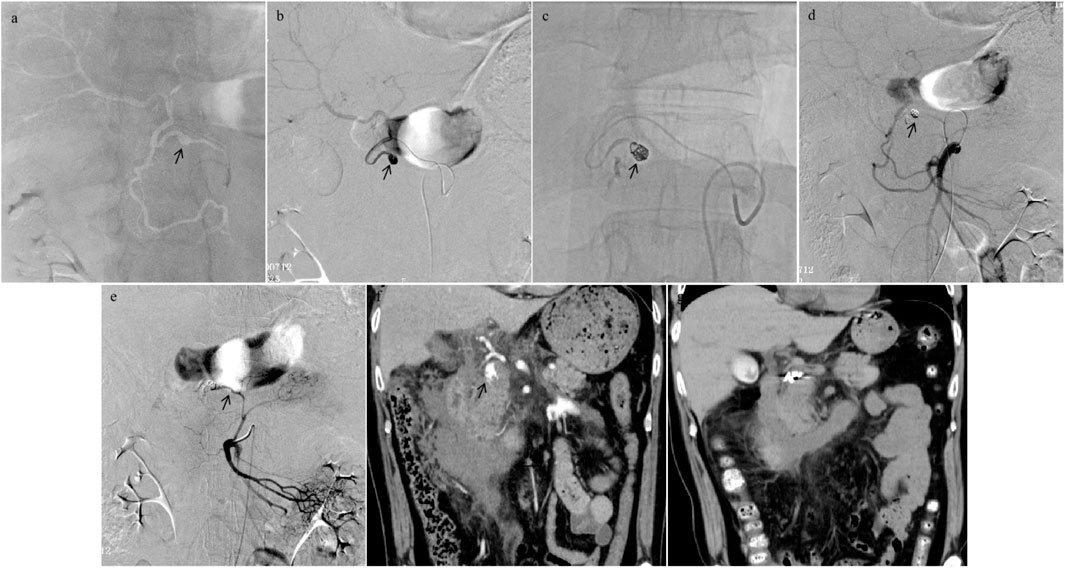
FIGURE 1. Procedural angiography images of a 59-year-old male patient who encountered pancreaticoduodenal superior arterial (PDSA) hemorrhage and underwent transcatheter arterial embolization (TAE) using Glubran 2 in combination with microcoils. (A,B) Celiac artery overview arteriogram revealed a pseudoaneurysm (PSA) artery (black arrowhead). Although a spill of contrast agent was not directly observed, irregular PSA in the microcatheter arteriogram demonstrated that it had been ruptured. (C) The microcoils (5 mm × 5.5 mm, 6 mm × 6 mm, 2 mm × 2 mm, and 2 mm × 2 mm) were released into the cavity of PSA, and the mixture of Glubran 2 and ethiodized oil (concentration ratio 1:2) was then injected as slowly as possible into the target artery, cast filling the PSA artery (black arrowhead). (D,E) The completion arteriogram from the superior mesenteric artery after TAE with Glubran 2 demonstrated PDSA feeding artery devascularization. (F) Abdominal computed tomography (CT) angiogram images of PDA hemorrhage in pre-TAE (the same patient) showed an irregular PSA (black arrowhead); (G) CT images obtained at a follow-up period of 1 week, a significant reduction area in hemorrhage size was depicted.
TAE procedures were well tolerated, and no nontarget or procedure-related complications occurred during TAE, except two patients (20.0%) who experienced minor complications of post-embolism syndrome, including aggravation of upper abdominal pain and low-grade fever (37.5°C and 37.9°C). They received conservative treatment and recovered 3 days later without permanent complications. No gangrenous duodenal ischemia or necrosis of the pancreatic head was reported after TAE.
The present study showed that TAE with NBCA Glubran 2 coupled with/without microcoils is successfully used as an endovascular technique in patients who were hemodynamically unstable secondary to acute PDA hemorrhage with/without aneurysm. Additionally, this treatment has been reported previously to be successful for patients with renal hemorrhage (Gong et al., 2021) and hemobilia (Shi et al., 2021) at our center. The limited patient population showed that this technique seems to be associated with rapid complete embolization, significant technical success and clinical success rates, and moderate minor complications. Neither massive hemorrhage nor further recurrence was observed during short-term follow-up, which demonstrated the durability of the NBCA Glubran 2 embolization.
For the embolization of acute PDA hemorrhage, many interventional operators have relied on the use of various embolic materials (Nicholson et al., 2006; Gralnek et al., 2015; Kickuth et al., 2016; Popov et al., 2017; Abdulmalak et al., 2018). Particle materials, including gelatin sponges and PVA, have been successfully applied (Kickuth et al., 2016). However, gelatin sponges are short-acting embolic agents, raising the possibility of recurrent hemorrhage in some cases, and PVA may increase the risk of organ ischemia because the important circulation of terminal collaterals might be impaired (Nicholson et al., 2006; Kickuth et al., 2016). As an advantage, coils can reduce the distal pressure of perfusion while providing enough collateral flow to prevent organ infarction. However, the sole use of coils as embolic material remains debatable, as it is associated with a high potential risk of early rebleeding from anastomotic collateral vessels and makes further rescue TAE difficult because the vessel trunk is occluded by coils (Weber et al., 2005; Loffroy et al., 2021). Therefore, we did not consider coils alone as the first choice under this urgent condition; coils were used to arrest the progression of the glue and/or to prevent its polymerization in nontarget vessels. Moreover, considering the upstream and downstream TAE of non-PSA with coils, the tip of the microcatheter should be advanced beyond the area of hemorrhage into the distal vessel, which may be technically demanding and time consuming (Kickuth et al., 2016; Loffroy et al., 2021); hence, Glubran 2 was used alone for patients without PSA.
Five patients in this study underwent PDA hemorrhage following pancreaticoduodenal or gastroduodenal surgery, and the corrosion of pancreatic juice or gastric acid to the surrounding anastomotic stoma may be associated with this hemorrhage. According to our limited experience based predominantly on operators’ judgment (Gong et al., 2021; Shi et al., 2021), the NBCA Glubran 2, rather than the more commonly advocated particles and microcoil embolic materials used alone, was selected as the primary agent in this study for the following reasons: a) expeditious embolization is required for unstable hemodynamic conditions, which are life-threatening, b) difficulty in controlling the nontarget embolization of particles embolic materials in the PDA network, c) difficulty in using microcoils successfully to access the target vessel complicated by extremely tortuous or narrow vascular anatomy, and d) challenges in achieving effective embolization in the abundant anastomoses of the PDA network and rebleeding from collateral vessels.
The successful use of cyanoacrylates in the treatment of peripancreatic hemorrhage has been described sporadically (Weber et al., 2005). Histoacryl, NBCA glues, and ethylene-vinyl alcohol copolymer were reported to have similar properties (Kickuth et al., 2016). Glubran 2, a modified NBCA in which it is combined with another comonomer, metacryloxysulfolane, has a more pliable and stable polymer with a milder exothermic reaction (45°C) that leads to less inflammation and histotoxicity than histoacryl (90°C) (Loffroy et al., 2009; Abdulmalak et al., 2018). Ethylene-vinyl alcohol copolymer is also suitable but considerably more expensive in our country and was not used in this study. Glubran 2 may be more beneficial than microcoils alone, since its application may rapidly enable TAE of different sites, even in the abundant collateral supply, which is particularly valuable in patients with life-threatening bleeding (Abdulmalak et al., 2018; Sugawara et al., 2019). In the present study, both technical success and clinical success were achieved in all patients, which is consistent with the outcomes reported in earlier studies (Gong et al., 2021; Shi et al., 2021). The success may be directly ascribable to NBCA’s penetrating ability and formation of an intravascular cast extending from the smaller vessels distally to the proximal vasculature, as well as its predictability and control lability (Gong et al., 2021). Acute massive PDA hemorrhage with a ruptured aneurysm should be treated partially as a bleeding site and partially as an aneurysm (Schenker et al., 2001). Since the aneurysm remains pressurized and at risk of repeat rupture, coil tamping is still the most efficient technique (Weber et al., 2005; Loffroy et al., 2021). The combination of occlusion of distal vascular outflow with microcoil filling of the aneurysm followed by Glubran 2 occlusion of the arterial inflow to prevent blood flow was successfully used in the present study, meaning that Glubran 2 was still considered the main embolic agent for TAE. Another advantage was that an ultrasmall lumen microcatheter of 2.4-F was used in patients without aneurysms compared with 2.7-F in patients with aneurysms.
In terms of the safety of TAE using liquid embolic materials for PDA hemorrhage, the safety of NBCA Glubran 2 remains controversial due to reported risks of migration into nontarget vessels and microcatheter blockage (Abdulmalak et al., 2018). Fortunately, no major complications of inadvertent distal embolization or undesired embolization of nontarget vessels as a result of backflow were encountered in our patients, which may be attributed to a number of precautions designed to minimize the complication rate. Before TAE, Glubran 2 was thoroughly mixed with ethiodized oil to improve polymerization time as well as fluoroscopy presentation. The concentration ratios ranged from 1:2 to 1:4, depending mainly on the arterial flow speed and the distance between the microcatheter tip and bleeding site. To avoid the adherence of the microcatheter to the vessel and occlusion, the lumen of the microcatheter was thoroughly flushed with dextrose before glue injection to remove all ionic solutions and was promptly pulled back after glue injection. As a minor complication, PES is characterized as the most frequent complication of embolization by fever and flank pain (Loffroy et al., 2009). In the present study, 20.0% of patients experienced it, presenting with aggravation of abdominal pain and low-grade fever, which were treated conservatively and resolved without permanent complications. The preservation of organic function is a crucial factor in embolization in this population, and the infraction of end-ischemia organs induced to liquefactive necrosis is a theoretical concern, whereas no case has been recorded here with these modalities of embolization.
This study has several limitations that should be acknowledged. First, it was performed at a single center involving a small patient pool, and the results should be interpreted with caution and confirmed in future studies. Nevertheless, previous studies on the management of massive PDA hemorrhage are mostly anecdotal case reports. Although our study population of 10 patients was not particularly large, to the best of our knowledge, this study may be the sole study regarding TAE using NBCA Glubran 2 for the treatment of this condition, and it may offer some experience for clinical physicians. Second, the present study was limited by its retrospective and nonrandomized inherent characteristics; hence, selection bias may have played a role, and there was no comparison with the other embolic materials, which could be an interesting issue needed in future studies. Third, most of the cases in this study were confirmed by CT plane scan due to its availability and the emergent HS condition. Fourth, our post procedural follow-up of 3 months is somewhat short; despite this, some investigators found that the probability of clinical success for patients hospitalized for less than 30 days was not significantly different from that for patients hospitalized longer than 30 days (Sugawara et al., 2019). Long-term follow-up does not seem to play a major role in clinical outcome.
In conclusion, urgent TAE with the selective use of liquid embolic material plays an important role in controlling acute massive PDA hemorrhage. It is minimally invasive, effective, relatively safe, and likely to reduce the need for immediate traditional surgery. We preferred experientially the NBCA Glubran 2 used alone for massive PDA hemorrhage without PSA in combined microcoils for massive PDA hemorrhage with PSA. This technique, however, requires further investigation with larger patient populations to determine these conclusion.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Nanjing First Hospital. The patients/participants provided their written informed consent to participate in this study.
MG and ZL contributed to this project development, manuscript writing/editing. JK contributed to data analysis. BZ and JG contributed to manuscript editing. XH and HS contributed to project development, manuscript editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdulmalak, G., Chevallier, O., Falvo, N., Di Marco, L., Bertaut, A., Moulin, B., et al. (2018). Safety and efficacy of transcatheter embolization with Glubran®2 cyanoacrylate glue for acute arterial bleeding: A single-center experience with 104 patients. Abdom. Radiol. (NY). 43 (3), 723–733. doi:10.1007/s00261-017-1267-4
Angle, J. F., Siddiqi, N. H., Wallace, M. J., Kundu, S., Stokes, L., Wojak, J. C., et al. (2010). Qua-lity improvement guidelines for percutaneous transcatheter embolization: Society of interventional Radiology standards of practice committee. J. Vasc. Interv. Radiol. 21, 1479–1486. doi:10.1016/j.jvir.2010.06.014
Gong, M., Liu, Z., Su, H., Zhao, B., Kong, J., and He, X. (2021). Urgent transcatheter arterial embolization for Wunderlich syndrome with hypovolemic shock secondary to ruptured renal angiomyolipoma. Front. Surg. 13 (8), 704478. doi:10.3389/fsurg.2021.704478
Gralnek, I. M., Dumonceau, J. M., Kuipers, E. J., Lanas, A., Sanders, D. S., Kurien, M., et al. (2015). Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European society of gastrointestinal endo-scopy (ESGE) guideline. Endoscopy 47 (10), a1–a46. doi:10.1055/s-0034-1393172
Kickuth, R., Hoppe, H., Saar, B., Inderbitzin, D., Triller, J., Raessler, S., et al. (2016). Superselective transcatheter arterial embolization in patients with acute peri-pancreatic bleeding complications: Review of 44 cases. Abdom. Radiol. (NY). 41 (9), 1782–1792. doi:10.1007/s00261-016-0772-1
Kim, P. H., Tsauo, J., Shin, J. H., and Yun, S. C. (2017). Transcatheter arterial embolization of gastrointestinal bleeding with N-butyl cyanoacrylate: A systematic review and meta-analysis of safety and efficacy. J. Vasc. Interv. Radiol. 28, 522–531.e5. doi:10.1016/j.jvir.2016.12.1220
Loffroy, R., Desmyttere, A. S., Mouillot, T., Pellegrinelli, J., Facy, O., Drouilllard, A., et al. (2021). Ten-year experience with arterial embolization for peptic ulcer bleeding: N-Butyl cyanoacrylate glue versus other embolic agents. Eur. Radiol. 31 (5), 3015–3026. doi:10.1007/s00330-020-07427-y
Loffroy, R., Guiu, B., Cercueil, J. P., and Krausé, D. (2009). Endovascular therapeutic embolisation: An overview of occluding agents and their effects on embolised tissues. Curr. Vasc. Pharmacol. 7 (2), 250–263. doi:10.2174/157016109787455617
Mano, Y., Takehara, Y., Sakaguchi, T., Alley, M. T., Isoda, H., Shimizu, T., et al. (2013). Hemodynamic assessment of celiaco-mesenteric anastomosis in patients with pancreaticoduodenal artery aneurysm concomitant with celiac artery occlusion using flow-sensitive four-dimensional magnetic resonance imaging. Eur. J. Vasc. Endovasc. Surg. 46 (3), 321–328. doi:10.1016/j.ejvs.2013.06.011
Nicholson, A. A., Patel, J., McPherson, S., Shaw, D. R., and Kessel, D. (2006). Endovascular treatment of visceral aneurysms associated with pancreatitis and a suggested classification with therapeutic implications. J. Vasc. Interv. Radiol. 17 (8), 1279–1285. doi:10.1097/01.rvi.0000231948.08617.04
Popov, M., Sotiriadis, C., Gay, F., Jouannic, A. M., Lachenal, Y., Hajdu, S. D., et al. (2017). Spontaneous intramuscular hematomas of the abdomen and pelvis: A new multilevel algorithm to direct transarterial embolization and patient manage-ment. Cardiovasc. Interv. Radiol. 40 (4), 537–545. doi:10.1007/s00270-017-1590-8
Rösch, J., Dotter, C. T., and Brown, M. J. (1972). Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology 102, 303–306. doi:10.1148/102.2.303
Schenker, M. P., Duszak, R., Soulen, M. C., Smith, K. P., Baum, R. A., Cope, C., et al. (2001). Upper gastrointestinal hemorrhage and transcatheter embolotherapy: Clinical and technical factors impacting success and survival. J. Vasc. Interv. Radiol. 12 (11), 1263–1271. doi:10.1016/s1051-0443(07)61549-8
Shi, Y., Chen, L., Zhao, B., Huang, H., Lu, Z., and Su, H. (2021). Transcatheter arterial embolization for massive hemobilia with N-butyl cyanoacrylate (NBCA) Glubran 2. Acta Radiol. 9, 360–367. doi:10.1177/0284185121992971
Sugawara, S., Arai, Y., Sone, M., Ishiguchi, T., Kitagawa, A., Aramaki, T., et al. (2019). Phase II trial of trans-arterial embolization using an n-Butyl-2-Cyanoacrylate/Lipiodol mixture (JIVROSG-0802). Cardiovasc. Interv. Radiol. 42, 534–541. doi:10.1007/s00270-018-2141-7
Sverdén, E., Mattsson, F., Lindström, D., Sondén, A., Lu, Y., and Lagergren, J. (2019). Transcatheter arterial embolization compared with surgery for uncontrolled peptic ulcer bleeding: A population-based cohort study. Ann. Surg. 269, 304–309. doi:10.1097/sla.0000000000002565
Keywords: pancreaticoduodenal hemorrhage, transcatheter arterial embolization, embolic materials, hypovolemic shock, Glubran 2
Citation: Gong M, Liu Z, Kong J, Zhao B, He X, Gu J and Su H (2022) Transcatheter arterial embolization using N-butyl-2 cyanoacrylate Glubran® 2 for acute massive pancreati coduodenal arterial hemorrhage. Front. Mater. 9:1003539. doi: 10.3389/fmats.2022.1003539
Received: 26 July 2022; Accepted: 25 October 2022;
Published: 02 November 2022.
Edited by:
Yasir Nawab, National Textile University, PakistanReviewed by:
Zhongzhi Jia, Changzhou No. 2 People’s Hospital, ChinaCopyright © 2022 Gong, Liu, Kong, Zhao, He, Gu and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Gu, Y2pyLmd1amlhbnBpbmdAdmlwLjE2My5jb20=; Haobo Su, ZG9jdG9yc3VoYW9ib0AxNjMuY29t
†ORCID: Maofeng Gong, orcid.org/0000-0002-4580-3232
‡These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.