- 1Department of Orthodontics, Shanxi Provincial People’s Hospital, Shanxi Medical University, Taiyuan, China
- 2School and Hospital of Stomatology, Shanxi Province Key Laboratory of Oral Diseases Prevention and New Materials, Shanxi Medical University, Taiyuan, China
- 3Department of Stomatology, Shanxi Bethune Hospital (Shanxi Academy of Medical Science), Taiyuan, China
In modern society, the incidence of cancer, inflammatory diseases, nervous system diseases, metabolic diseases, and cardiovascular diseases is on the rise. These diseases not only cause physical and mental suffering for patients, but also place an enormous burden on society. Early, non-invasive diagnosis of these diseases can reduce the physical and mental pain of patients and social stress. There is an urgent need for advanced materials and methods for non-invasive disease marker detection, large-scale disease screening, and early diagnosis. Biomimetic medical materials are synthetic materials designed to be biocompatible or biodegradable, then developed for use in the medical industry. In recent years, with the development of nanotechnology, a variety of biomimetic medical materials with advanced properties have been introduced. Biomimetic nanomaterials have made great progress in biosensing, bioimaging, and other fields. The latest advance of biomimetic nanomaterials in disease diagnosis has attracted tremendous interest. However, the application of biomimetic nanomaterials in disease diagnosis has not been reviewed. This review particularly focuses on the potential of biomimetic nanomaterials in non-invasive disease marker detection and disease diagnosis. The first part focuses on the properties and characteristics of different kinds of advanced biomimetic nanomaterials. In the second part, the recent cutting-edge methods using biosensors and bioimaging based on biomimetic nanomaterials for non-invasive disease diagnosis are reviewed. In addition, the existing problems and future development of biomimetic nanomaterials is briefly described in the third part. The application of biomimetic nanomaterials would provide a novel and promising diagnostic method for non-invasive disease marker detection, large-scale clinical screening, and diagnosis, promoting the exploitation of devices with better detection performance and the development of global clinical public health.
Highlights
- Emerging nanoscience and nanotechnology enable material scientists to design and manufacture biomimetic nanomaterials, which will show great advantages in early non-invasive detection and diagnosis of diseases.
- Novel biosensors and bioimaging based on biomimetic nanomaterials have guiding significance for the development of biomimetic materials application.
- This review summarized the application of biosensors with high sensitivity and stability in the detection of different disease biomarkers and the application of bioimaging with high accuracy in the diagnosis of disease.
Introduction
Serious diseases such as cancer and cardiovascular disease currently pose a major threat to human life. Therefore, non-invasive methods for the early diagnosis of serious diseases are critical for disease management and personalized medicine. Disease biomarkers are an important group of substances that indicate disease occurrence based on changes in the biomarker concentration in serum and tissues (Zhou et al., 2015). However, disease biomarkers are often found in very low concentrations and require complex biological assays for quantification. Meanwhile, traditional strategies for detecting diseases or imaging methods are frequently invasive, high in cost, insensitive, and unable to diagnosis serious diseases in their early stages. Thus, advanced materials and methods are needed to detect disease biomarkers for the early and accurate diagnosis of disease. Toward this end, biosensors and bioimaging technology based on biomimetic nanomaterials have been developed for non-invasive disease diagnosis.
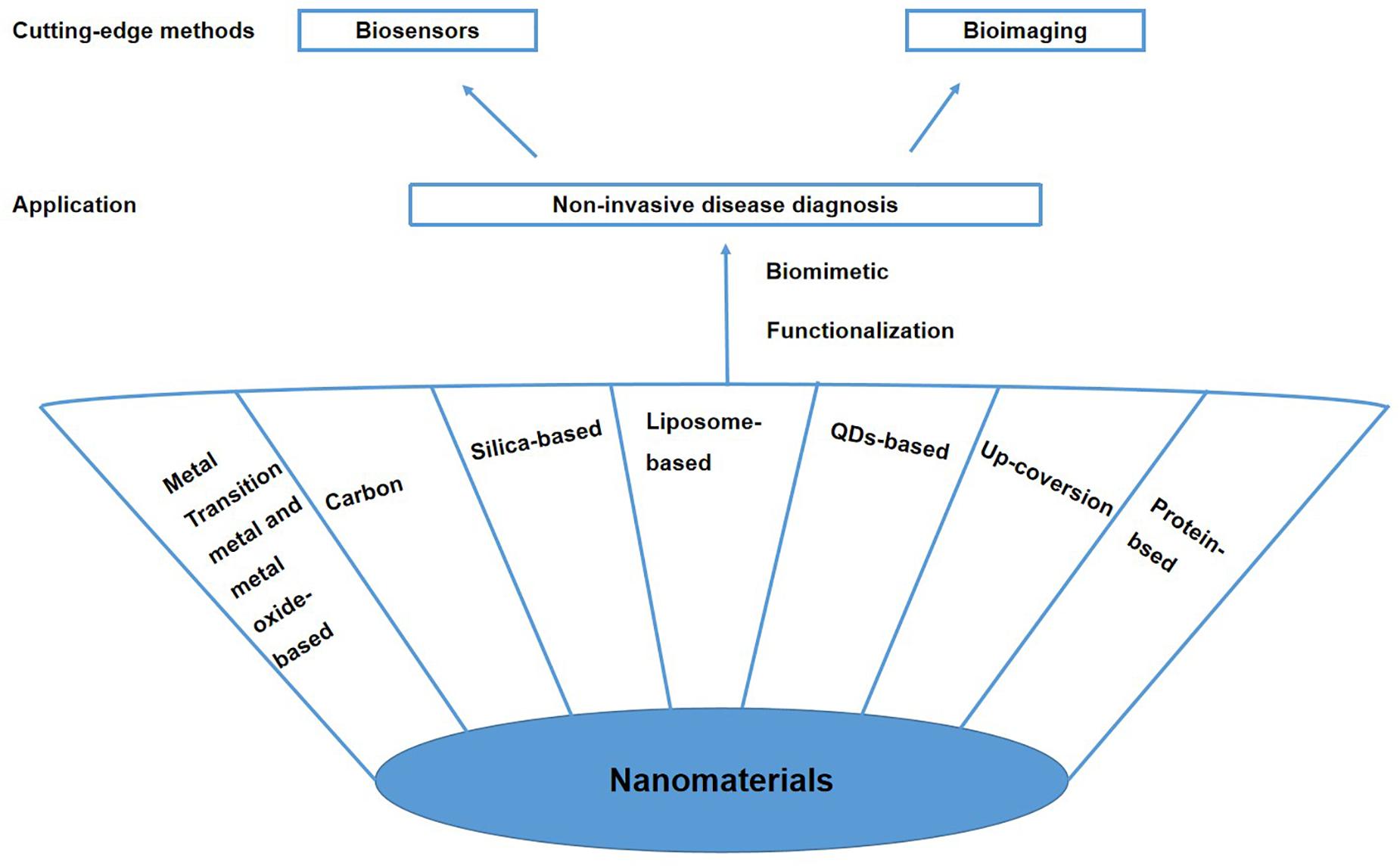
Nanomaterials generally range in size from 1 to 100 nm, making them similar in size to basic biological structures (Xiang et al., 2014). Based on their physical, chemical, and biocompatibility properties, nanomaterials are attractive for use in biomedical applications and show great potential in biomimetic medicine (Ning et al., 2017; Das and Noh, 2018; Hasanzadeh et al., 2018). Using biomimetic design principles can overcome the shortcomings of traditional nanoparticles, such as being easily recognized by the immune system and potential toxicity. Recently, with the development of biomimetic functionalized nanotechnology, nanomaterials have been modified and functionalized by biomolecules or cell membrane-derived components to form biomimetic nanomaterials with enhanced stability, targeting specificity, and biocompatibility (Das and Noh, 2018; Vijayan et al., 2018). Biomimetic nanomaterials, functional materials with biomedical applications, have made it easier to simulate biological functions and reaction processes (Das and Noh, 2018). The biomimetic functionalized modification of nanomaterials can create selectivity for disease biomarkers or tissues, suggesting that these biomimetic nanomaterials can be used for disease diagnosis. Thus, the use of biomimetic nanomaterials for the non-invasive diagnosis of diseases has aroused great interest. This paper reviews the properties of selected biomimetic nanomaterials and their cutting-edge methods for disease diagnosis in the past five years. The reviewed biomimetic nanomaterials include metal, carbon, silicon-based, liposome-based, quantum dots (QDs)-based, upconversion-based, and protein-based nanomaterials (Figure 1).
Properties of Different Advanced Biomimetic Nanomaterials
Biomimetic nanomaterials have different sizes; unique mechanical, electronic, photonic, and magnetic properties; and large surface areas (1000 m2/g), giving them a wide range of applications, including the non-invasive diagnosis of early-stage biomedical diseases (Saha et al., 2012; Thambiraj et al., 2018; Choi et al., 2019). Based on these fundamental advantages, biomimetic nanomaterials are expected to revolutionize the fields of diagnosis and personalized medicine.
Transition Metal and Metal Oxide-Based Nanomaterials
Gold Nanoparticles
Gold nanoparticles (AuNPs) have been widely used in biomedical applications. AuNPs may be constructed from colloidal gold with different morphologies, including spheres, nanorods, nanostars, nanocages, and nanoshells. Methods for preparing AuNPs include top-down (physical manipulation) and bottom-up (chemical transformation) methods (Daniel and Astruc, 2004; Singh et al., 2018). AuNPs have unique physicochemical properties that change when combined with target analytes; moreover, AuNPs are easy to synthesize and functionalize and exhibit high stability, unique photoelectric properties, high surface-to-volume ratio, good biocompatibility, and low cytotoxicity (Daniel and Astruc, 2004; Saha et al., 2012; Xiang et al., 2014). Fixing biomolecules on AuNPs does not affect their functional activity (Daniel and Astruc, 2004; Upadhyayula, 2012). Therefore, AuNPs can be used as scaffolds to fix one or more biomolecules that selectively interact with multiple receptors to detect multiple target analytes; this feature is the basis for the design of biosensors with rapid detection, simple operation, high selectivity, and high sensitivity (Daniel and Astruc, 2004; Saha et al., 2012; Upadhyayula, 2012). AuNPs modified with specific biomolecules can also target specific tumor sites in bioimaging applications (Singh et al., 2018; Thambiraj et al., 2018). AuNPs are attractive for the non-labeled, non-invasive imaging of biological samples due to their remarkable ability to generate stable optical signals through direct light scattering or intrinsic photoluminescence (PL), which is related to localized surface plasmon resonance (LSPR) (Tian F. et al., 2016).
Silver Nanoparticles
Silver nanoparticles (AgNPs) have attracted considerable attention due to their unique optical, chemical, electrical, and catalytic properties, which can be adjusted by tuning the nanoparticle surface properties, sizes, and shapes (Deshmukh et al., 2019; Lee and Jun, 2019; Padnya et al., 2020). AgNPs have been prepared in various shapes, including spheres, rods, cubes, shells, clusters, and stars (Gherasim et al., 2020). AgNPs are generally prepared via chemical reduction from relatively stable silver (I) salts using reductants, which is an easy process (Montes-García et al., 2014; Gherasim et al., 2020). The stability of AgNPs in water and air can be increased through modification (Gherasim et al., 2020). AgNPs play a vital role in the biomedical field because of their applications in imaging, sensing, diagnosis, and so on (Gherasim et al., 2020; Renu et al., 2020). Methods based on AgNPs have the advantages of facile miniaturization, rapid analytical response, and simple operation; thus, AgNPs are a good choice for point-of-care devices in clinical application and are expected to be a research hotspot in the future (Jouyban and Rahimpour, 2020).
Magnetic Nanomaterials
Magnetic nanomaterials, which exhibit unique magnetic and biological properties, have great application value in the biomedical field. Magnetic nanoparticles based on iron oxides can promote an enhanced permeability and retention effect in tumors, and functional magnetic nanoparticles can be applied in cancer localization (Knežević et al., 2019; Vangijzegem et al., 2019; Wu et al., 2019). Magnetic sensing and imaging technologies based on magnetic field sensors and magnetic particles have applications in the early screening and non-invasive diagnosis of diseases (Knežević et al., 2019; Wu et al., 2019). Magnetic nanoparticles can be applied in magnetic resonance imaging (MRI) to enhance contrast, as well as in the early diagnosis of diseases at the molecular and cellular levels (Cha and Kim, 2019).
Carbon Nanomaterials
Graphene
Graphene is an allotrope of carbon with a two-dimensional, atomic-scale hexagonal lattice (Balaji and Zhang, 2017). Graphene has attracted extensive attention in biomedical research due to its unique structure; excellent mechanical, electrical, thermal, and optical properties; and good biocompatibility (Tian W. et al., 2016; Balaji and Zhang, 2017; Wang et al., 2017). Graphene-based nanomaterials also include graphene derivatives such as graphene oxide (GO) and reduced GO (rGO), along with graphene-like nanomaterials (Liao et al., 2018). Graphene and its derivatives exhibit easy surface functionalization along with excellent electron and thermal conductivity and high mechanical strength; these advantages allow graphene and its derivatives to be used as sensors for detecting disease biomarkers at very low concentrations (Lin et al., 2016; Balaji and Zhang, 2017; Liao et al., 2018). In addition, graphene is regarded as an important material in next-generation electronic devices (Lin et al., 2016; Wang et al., 2017). Graphene-based nanomaterials also show promise as alternatives to traditional imaging agents both in vitro and in vivo due to their low toxicity and high biocompatibility (Garg et al., 2015).
Carbon Nanotubes
Due to their good electrical conductivity and luminance, carbon nanotubes (CNTs) have wide applications in biosensors, biological contrast agents, and non-invasive disease diagnosis (Sabu et al., 2019). CNTs can generally be divided into two categories: single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs) (Dizaji et al., 2020). SWCNTs are cylinders composed of one-dimensional, symmetric graphene sheets (Thess et al., 1996). MWCNTs are formed by multilayer concentric cylinders and show good electrical conductivity. Compared with MWCNTs, SWCNTs have better mechanical, structural, and optical properties (Vardharajula et al., 2012). The functionalization of the sidewalls or ends of CNTs via covalent or non-covalent bonding can improve CNTs’ biocompatibility (Niyogi et al., 2002). For example, CNTs modified by polyethylene glycol (PEG) show better solubility and biocompatibility than bare CNTs (Ghanbari et al., 2011).
Silica-Based Nanomaterials
Silica-based nanoparticles (SNPs) have many advantages, such as easy synthesis, controllable particle size and surface charge, acceptable cost-effectiveness, and ability to modify the functional groups and ligands (Albert et al., 2017). SNPs are widely used in biomedical applications due to their excellent biocompatibility and customizable physicochemical properties (Yang et al., 2020). Based on recent developments in nanomaterials research, silicon-based nanomaterials, along with combinations of silicon-based nanomaterials and other nanomaterials, have gained attention in non-invasive disease diagnosis. Mesoporous silica nanomaterials (MSNs) are ideal platforms for imaging agents because of their high specific surface areas and functionalized surfaces (Cha and Kim, 2019). MSNs can be used in a variety of imaging systems, including optical imaging (OI), MRI, positron emission tomography (PET), computed tomography (CT), and ultrasound imaging (USI) systems (Cha and Kim, 2019).
Liposome-Based Nanomaterials
Liposomes are composed of one or several layers of phospholipids and cholesterol on the outside of an aqueous core, forming a lipid bilayer (Li et al., 2017). Studies on liposomes in the biomedical field have focused primarily on their application as drug nanocarriers for disease treatment (Li et al., 2017). However, liposomes also have application value in disease diagnosis because of their cell-specific targeting ability (Lamichhane et al., 2018; Sheoran et al., 2019). Reported methods for the preparation of liposomes include ultrasonic, ethanol injection, lipid membrane hydration, and microemulsion methods (Sheoran et al., 2019). Liposomes are able to separate and dissolve both hydrophilic and hydrophobic materials. Liposomes also show good biological compatibility and biodegradability, allowing them to be used as carriers after appropriate surface modification (Lamichhane et al., 2018). Combination of liposomes with labeled probes for imaging applications is currently a research hotspot.
QDs-Based Nanomaterials
Quantum dots are nanometer-scale semiconductors with low toxicity and good biocompatibility. QDs have excellent optical properties, including high quantum yield, adjustable light emission, and chemical and optical stability. The applications of QDs in optical biosensors for detecting the activities of various target analytes and enzymes have attracted considerable attention (Matea et al., 2017). QDs can be combined with ligands to recognize specific targets and sensitively track dynamic processes over long time periods (Mahajan et al., 2012). QD toxicity is primarily related to the chemical composition of the QD and can be minimized by functionalizing the QD surface with biocompatible molecules for clinical use in vivo (Matea et al., 2017). QDs are currently being combined with other types of nanoparticles and/or bioactive molecules to develop platforms for use in disease diagnosis.
Upconversion-Based Nanomaterials
Upconversion nanoparticles (UCNPs) are a new generation of luminescent nanomaterials. Among UCNPs, those based on lanthanides have been the most studied due the special spectral properties of lanthanides, which are excited by low-energy radiation (near-infrared [NIR] light) and produce high-energy emission (visible or ultraviolet light) at high electron energy (Yao et al., 2020). UCNPs have excellent properties, such as high photostability, large emission bandwidth, easy modulation of emission color, good surface wetting characteristics, and low cytotoxicity. UCNPs modified by organic capping ligands or inorganic shell layers have broad application prospects in biomedical sensing and imaging (Yao et al., 2020). In addition, UCNPs can detect target ions with high precision based on the change in emission intensity, and the ability to use NIR excitation minimizes light damage and allows deep tissue penetration, especially for UCNPs containing rare earth ions (Sharipov et al., 2017).
Protein-Based Nanomaterials
Proteins are widely found in the body and are involved in almost all biological activities. Protein-based nanomaterials (PBNs) have good biocompatibility and abundant functional groups that can bind various functional molecules along with other metal ions. PBNs also have high bioactivity, which means that they can often be used as carriers without further surface modification (Zhang and Wang, 2019; Liang and Chen, 2020). PBNs combine the advantages of nanomaterials related to size and surface chemistry with the advantages provided by the physical and chemical properties of proteins (Zhang and Wang, 2019). PBNs can be combined with other nanoparticles for applications in bioimaging and biochip-based detection (Ye et al., 2016). Various proteins, such as human serum albumin, bovine serum albumin (BSA), ferritin, and transferrin, are being studied to construct PBNs, many of which are based on protein cages. These PBNs may find wide applications in disease imaging in the future (Liang and Chen, 2020).
Some biomimetic nanomaterials, such as metal oxides (Piro and Reisberg, 2017; Nikolova and Chavali, 2020), metal hydroxides (Sun et al., 2019) metallodendrimers (Tang et al., 2011), and polymers (Zhao et al., 2018; Regan et al., 2019) have also been applied as biomimetic nanomaterials for disease diagnosis; however, these nanomaterials are not discussed in this review.
Cutting-Edge Methods for Non-Invasive Disease Diagnosis Using Biomimetic Nanomaterials
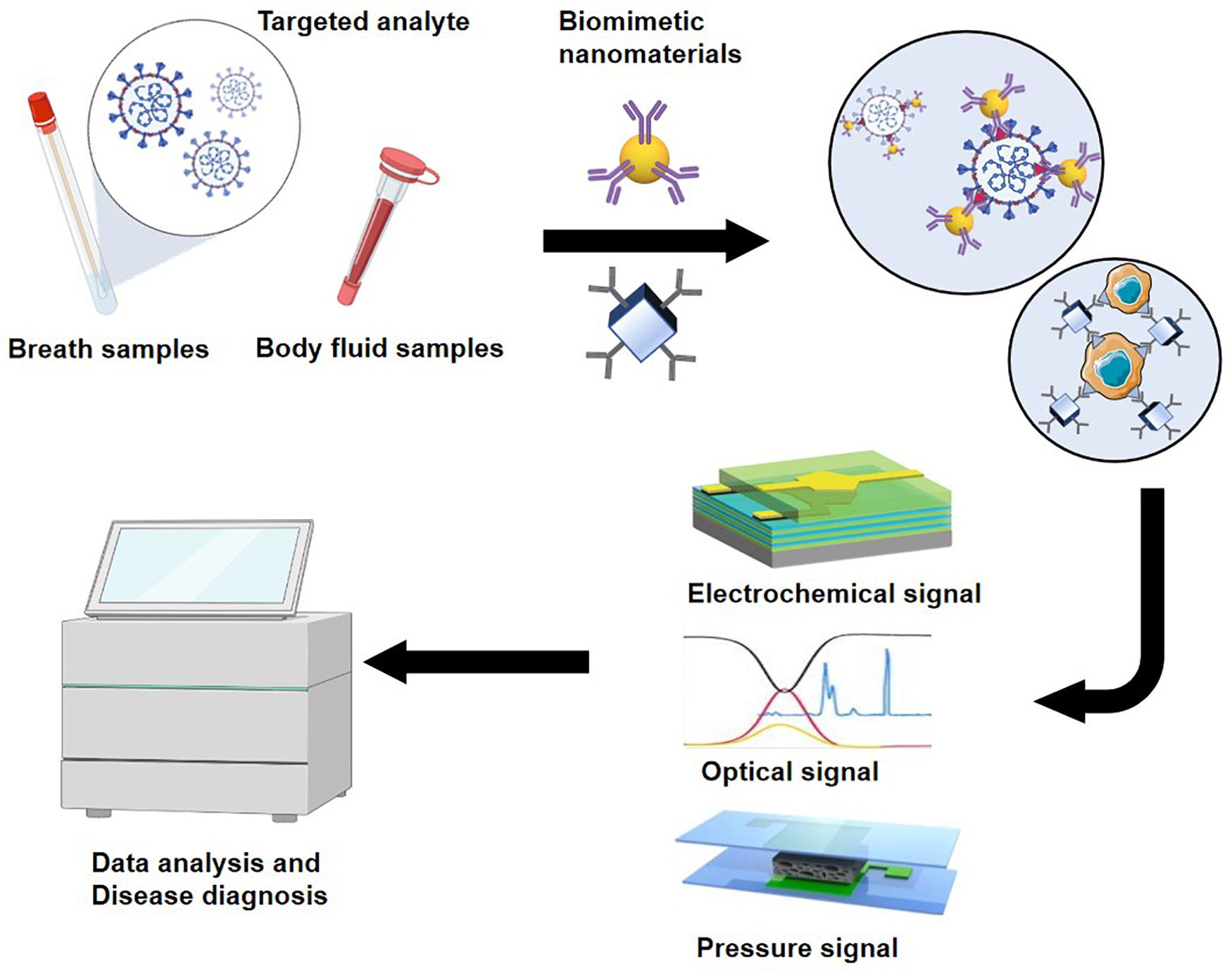
Biosensors
As a reliable strategy for early non-invasive cancer diagnosis, biosensors show great potential for development. In biosensor-based disease detection, samples of body fluids or exhaled gas are collected for non-invasive diagnosis. Biosensors have greatly changed the way diseases are diagnosed by allowing early-stage diagnosis when specific disease biomarkers first appear. Biosensors enable the sensitive and specific detection of disease markers with only a small number of samples (Takke and Shende, 2019). A typical biosensor configuration consists of three parts: a bioreceptor (e.g., an enzyme, antibody, or lipid) that is responsible for the selectivity of the device; a transducer that translates the physical or chemical change into a signal; and a signal output unit (Fitzgerald and Fenniri, 2017; Pasinszki et al., 2017; Takke and Shende, 2019). Mass biosensors, electrical biosensors, and optical biosensors have been used for non-invasive cancer screening (Fitzgerald and Fenniri, 2017). Current efforts are focused on developing biosensors with higher sensitivity and better recognition ability for various biomarkers. Biomimetic nanomaterials provide ideal platforms for the development of highly sensitive biosensors for early disease detection (Figure 2).
Biosensors for Cancer Diagnosis
Hu et al. (2019) reported urine perilipin-2 (PLIN-2) as a sensitive and specific biomarker for the non-invasive detection of early-stage renal cell carcinoma and used it to develop a plasmonic paper biosensor based on gold nanorattles. Due to the fact that gold nanorattles has a higher refractive index sensitivity than gold nanorods counterparts with similar LSPR wavelength, the reported biosensor can be used for the rapid, sensitive, economical, and non-invasive diagnosis of renal cancer, particularly in high-risk populations (Hu et al., 2019). The gold nanorattles were functionalized with a PLIN-2-specific monoclonal antibody through the bifunctional PEG approach. The remaining PEG further neutralized the non-specific binding sites. A plasmonic paper biosensor was combined with specific analyte PLIN-2, resulting in a change of LSPR wavelength. Using this method, PLIN-2 could be detected in the dynamic range of 50 pg/mL to 5 μg/mL, and the concentration of PLIN-2 was found to be positively correlated with tumor size (Pearson coefficient = 0.59, Hu et al., 2019).
Broza et al. (2019) developed a cross-reactive sensor array that combines different organic ligands on the surfaces of AuNPs to absorb volatile organic compounds in respiratory samples; the generated signal caused changes in conductivity. To evaluate the diagnostic performance of the biosensor for gastric cancer, breath samples were collected from 441 volunteers, including 99 gastric cancer patients and 342 healthy patients (control). The results of the training algorithm revealed 82% sensitivity, 78% specificity, and 79% accuracy. The method was then successfully used to classify three patients with gastric cancer and 570 healthy controls with 100% sensitivity, 79% specificity, and 79% accuracy. This cross-sensor array shows potential for the early, non-invasive detection of gastric cancer and provides a strategy for the large-scale screening of gastric cancer in the entire population (Broza et al., 2019).
Yarbakht et al. (2018) used core–shell (Au@Fe3O4) nanoparticles functionalized by mucin-1 (MUC1)-specific aptamer (Apt1) to identify tumor cells in breast, ovary, lung, and pancreas tissues based on the interaction between Apt1 and overexpressed MUC1 on tumor cell surfaces. They also used 4-thiopyridine-functionalized AuNPs to bind bovine serum protein with Apt1, resulting in a new surface-enhanced Raman scattering (SERS) nanotag (Yarbakht et al., 2018). Based on the SERS method, Bellassai used aggregates of modified AuNPs to detect cancer biomarkers at very low concentrations in clinical diagnosis (Bellassai et al., 2019).
Wang et al. (2019) developed the first non-invasive detection method based on strand displacement amplification (SDA) and AuNP-based dynamic light scattering (DLS) to diagnose bladder cancer by detecting telomerase activity in human urine samples (Wang et al., 2019). C18 spacers were introduced into primers and hairpin DNA so that two single-stranded DNA (ssDNA) fragments were present at both ends of the SDA products. The AuNPs were modified by oligopeptide acid, allowing them to specifically recognize SDA products. The ssDNA fragments were used to identify DNA-AuNP probes, induce AuNP aggregation, and realize the DLS measurement. This method can be used in the non-invasive screening and diagnosis of bladder cancer (Wang et al., 2019).
Malondialdehyde (MDA) is a biomarker for tissue damage (Siddique et al., 2012). Electrochemically polymerized dopamine can be obtained via the electrodeposition of the polymer chitosan (CS) onto the polymer dopamine (DA) on the surface of a glassy carbon electrode. Hasanzadeh et al. (2018) developed an electrochemical sensor based on AgNPs and POLY(DA-CS). In this sensor, AgNPs covalently bound to POLY(DA-CS) membranes bind MDA biomarkers. The electrochemical signal was enhanced by AgNPs, and the excellent biocompatibility and stability of CS resulted in good electrode durability. The dynamic range of the electrochemical sensor was 1.45–7.9 μm, and the lower limit of quantification was 1.45 μm. The sensor could quickly and sensitively detect MDA in exhaled breath condensate (EBC) samples. This platform has the potential to be used to diagnose lung diseases (e.g., a simple and rapid test for lung cancer) (Hasanzadeh et al., 2018).
Taurine is a non-essential amino acid that can be biosynthesized (derived) from cysteine or methionine by cysteine decarboxylase to interact with MDA. Jafari et al. (2019) proposed a new non-invasive platform for the early diagnosis of lung disease based on the quantitative analysis of MDA in exhaled gas. In this method, MDA is recognized by an electrochemical sensor containing self-assembled riboflavin–taurine as an organic substrate and AgNPs increase the rate of the electrochemical reaction. The nanomaterial complex increased the contact area with MDA and increased the electrochemical surface activity to interact with MDA. The proposed platform showed good sensitivity for the detection of MDA with a low limit of quantification of 0.59 ± 0.05 μM (Jafari et al., 2019).
Tiwari et al. (2017) reported an efficient electrochemical immunosensor with high sensitivity and a low detection limit for the non-invasive detection of Cyfra-21-1, a biomarker of oral cancer, based on the in situ synthesis of L-cysteine-capped lanthanum hydroxide nanoparticles, which immobilized anti-Cyfra21-1. The detection range of this biosensor was 0.001–10.2 ng⋅mL–1, and the detection limit was 0.001 ng⋅mL–1. The sensitivity of the biosensor reached 12.044 μ1 (ng/mL⋅cm–2), and the response time was 5 min. Although this system has not yet been applied in the analysis of actual saliva samples, it provides a new strategy for the non-invasive detection of cancer (Tiwari et al., 2017).
Zhang et al. (2019) used a fluorescent labeling system based on a graphene–peptide composite to detect tumor marker CD133. Past research has shown that peptides, an affinity molecule, can be combined with biomarkers of cancer stem cells. Therefore, the authors combined a highly specific CD133 FITC-labeled CD133-6-binding peptide (FLS7) with graphene. Graphene possesses fluorescence quenching ability. When FLS7 was combined with graphene, this fluorescence quenching ability was inactivated. In contrast, when used for the detection of CD133, the fluorescence recovered. This fluorescence change enables the detection of CD133 (Zhang et al., 2019).
In cancer cells, microRNAs (miRNAs) are abnormally expressed, suggesting that miRNAs can be used as biomarkers for early disease diagnosis. Wang et al. developed a new electrochemical biosensor with good electronic transport properties based on titanium dioxide nanorod arrays to detect changes in miRNA for cancer diagnosis. In their study, by using MSNs as carriers for loading Cu2+, MSNs were capped with a miRNA-21 responsive RNA capture probe. When the miRNA probe was combined with miRNA specifically, the released Cu2+ captured the photoelectrons generated by the photoelectrode and was reduced, thereby quantitatively analyzing the content of miRNA by detecting the change of photoelectron flow (Wang et al., 2018). Different miRNA-21 concentrations can produce different photocurrent intensities, and there is a linear relationship between photocurrent and miRNA-21 concentration. In addition, miRNA-21 produces photoelectric chemical reactions with specificity (Wang et al., 2018).
Hasanzadeh et al. (2017) developed a novel approach for detection of L-proline via an electrochemical biosensor in biological fluids. Proline dehydrogenase was immobilized on magnetic MSNs through a physical adsorption method. When L-proline was detected, proline dehydrogenase specifically combined with L-proline and an electrical catalytic reaction occurred. The proline content could then be quantified by detecting the corresponding current. The proline concentration range of detection was 0.01-0.15 μm, and the detection limit was 0.006 μm (Hasanzadeh et al., 2017).
Biosensors for Inflammatory Diseases Diagnosis
Lee et al. (2018) reported a new electrochemical immunosensor to non-invasively diagnose laryngopharyngeal reflux (LPR) by quantifying salivary gastric protein in saliva. Pepsin, a component of stomach acid, digests protein. The damage caused by LPR is related to both acid and non-acid reflux (Johnston et al., 2007). Thus, pepsin has attracted the attention of clinicians as a potential biomarker for the diagnosis of LPR. A screen-printed carbon electrode was prepared with polypyrrole nanocorals and decorated with AuNPs by electrodeposition. To construct the immunosensor, pepsin antibody was immobilized on the AuNP-decorated electrode. Cyclic voltammetry was used for electrochemical characterization, and differential pulse voltammetry was employed to determine the response of the immunosensor to different pepsin concentrations. The sensor showed a linear range of 6.25–100 ng/mL, high specificity, and a detection limit of 2.2 ng/mL (Lee et al., 2018).
Nitrites and nitrates have been reported to play important roles in inflammatory diseases (Corradi et al., 2003). Diouf et al. (2020) developed a new electrochemical sensor to detect the content of nitrite ions (NO2–) in human EBC to evaluate pulmonary inflammation in patients. Aminothiophenol (ATP) is a biological polymer with good electrical conductivity. Sodium nitrite was immobilized on 2-ATP, which was bound to a screen-printed gold electrode (Au-SPE). Meanwhile, glutaraldehyde and polyvinyl alcohol polymer nanomaterials were combined with AuNPs to cover the Au-SPE. The resulting ion-imprinted polymer sensor was used to detect the concentration of nitrite in EBC. Finally, electrochemical impedance spectroscopy (EIS) and differential pulse voltammetry were used to detect the content of NO2– in EBC. The electrochemical sensor was able to detect the concentration of NO2– in the range of 0.5–50 μg⋅mL–1, and the detection limit was 4 μmol⋅L–1 (Diouf et al., 2020).
Lipopolysaccharide (LPS) is a major component of the outer membrane of Gram-negative bacteria. Thus, LPS can be used as a biomarker for infectious diseases related to Gram-negative bacteria, including urinary tract infections and sepsis. Liu T. et al. (2018) detected the level of LPS by combining polymyxin B (PMB) with citrate-coated AgNPs. PMB is a class of antibiotics used primarily to treat Gram-negative bacterial infections. In this application, PMB also neutralizes the citrate coating the AgNPs, causing the AgNPs to cluster together (Liu T. et al., 2018). When LPS was specifically bound with AgNPs-PMB, the neutral electrical environment of the colloid was changed, and the aggregation of the AgNPs was inhibited. Therefore, the LPS concentration could be quantitatively determined by monitoring with ultraviolet–visible absorption spectroscopy (Liu T. et al., 2018).
Liu M. et al. (2018) used functionalized GO and gold nanorod (GNR) composites to detect hepatitis B surface antigen (HBsAg) using SERS immunoassays. In this method, GO coats the AuNPs and fixes anti-HBs. The GNRs were combined with 2-mercaptopyridine, the SERS probe, to enhance the SERS signal and improve the sensitivity (Jang et al., 2018). The optimal detection range of HBsAg by this biosensor was 1–1000 pg⋅mL–1, and the SERS signal intensity within this range increased with increasing HBsAg concentration. At HBsAg concentrations higher than 1000 pg⋅mL–1, the SERS intensity gradually decreased with increasing HBsAg concentration, leading to false negatives (Liu M. et al., 2018).
Biosensors for Neurological Disease Diagnosis
As cortisol is a known biomarker of emergency response, testing for cortisol can provide an objective evaluation of a patient’s stress level. Apilux et al. (2018) explored a novel immunoassay-based method to detect salivary cortisol. Gold nanomaterials were used as signal labeling, and silver irons on its surface were reduced to precipitate on gold, leading to particle enlargement and color change. The authors combined gold nanomaterials with anti-cortisol antibody resistance on the conjugate release pad of the strip. The cortisol in the samples competed with cortisol–BSA conjugate, which was fixed in the test area of the test strip. Finally, the cortisol level in saliva was monitored by visually observing the strip’s color; the redness of the strip was inversely proportional to the cortisol level. The limit of detection of this system was 0.5 ng/mL, and the detection sensitivity was 3.6 times that of the non-enhanced system (limit of detection = 1.8 ng/mL). The detection range for salivary cortisol was 0.5–150 ng/mL (R2 = 0.9984) (Apilux et al., 2018).
Delkhahi designed a biosensor based on AuNPs-DNA for the detection of miR-137 in Alzheimer’s disease (AD) (Delkhahi et al., 2017). In previous studies, miRNA in peripheral blood, especially miR-137, was found to be helpful for the early diagnosis of AD. The authors used gold nanomaterials and hybridization chain reaction to detect the miR-137 levels in plasma for AD diagnosis. DsDNA was produced by targeting miRNA as hybridization promoter. The undecorated gold nanomaterials are easily attracted by ssDNA, thereby preventing salt-induced AuNP aggregation. In contrast, double-stranded DNA (dsDNA) with a negative charge creates a repulsion between dsDNA and AuNPs; therefore, salt easily induces the aggregation of the gold nanomaterials into gold colloids, accompanied by a change in color. The miR-137 level in plasma could thus be evaluated based on the color change, which is visible to the naked eye (Delkhahi et al., 2017).
Li et al. (2016) developed a new immunomagnetic biosensor using a magnetic nitrogen-doped graphene (MNG)-modified Au electrode for the detection of amyloid-beta peptide 1–42 (Aβ42) (Coyle et al., 2019). As shown in past studies, Aβ42 is a reliable biomarker for the early diagnosis of AD (Li et al., 2016). The antibodies of Aβ 1–28 (Aβab), which were used as the specific biorecognition element for Aβ42, were conjugated on the surface of MNG, which was formed by depositing superparamagnetic magnetite (Fe3O4) nanoparticles onto N-doped graphene. MNG was successfully labeled with anti-Aβ to form magnetic immune carriers. The magnetic immune carriers were dropped onto the Au electrode, where they were trapped by placing an external magnet at the underside of the electrode for electrochemical Aβ detection toward AD diagnosis (Coyle et al., 2019).
Dopamine is an important neurotransmitter in the human body and related to Parkinson’s disease. Therefore, the detection of DA content in the body is important for the diagnosis of Parkinson’s disease. Kaya and Volkan (2012) reported a new approach for detecting DA levels based on surface-enhanced resonance Raman spectroscopy (SERRS). This method was able to detect biomarkers at femtomolar concentrations. In this approach, DA is captured by AgNPs functionalized by iron–nitrilotriacetic acid, a widely used organic chelate that forms catechins with Fe, followed by DA detection by SERRS (Kaya and Volkan, 2012). In their study, the SERRS spectra obtained by capturing DA on Ag-Fe(NTA) substrates were not only similar to those obtained by capturing DA based on the iron(III)bis (DA), but also enhanced the SERRS signal. In addition, the authors tested the rate of DA detection with the new strategy, and the SERRS signal was the same after 15 min and immediately after the addition of 1 × 10–5 M DA solution to the Ag-Fe(NTA) substrates (Kaya and Volkan, 2012).
Biosensors for Metabolic Disease Diagnosis
Zhao et al. (2019) developed a sandwich-type pressure sensor composed of AuNPs and polydimethylsiloxane (PDMS) for the detection of BSA. Due to the similar tissue structures of bovine serum protein and human serum protein, this detection method lays the foundation for the detection of nephritis in the future. The BSA recognition layer (BRL) based on AuNPs located on one side of the PDMS film. The BRL was coated with self-assembled 16-mercaptohexadecanoic acid, which was covalently bound with modified anti-BSA. Therefore, the sandwich-type pressure sensor can specifically recognize and bind BSA (Zhao et al., 2019).
Biosensors for Cardiovascular Disease Diagnosis
Overly high cholesterol levels increase the morbidity related to cardiovascular diseases. Therefore, non-invasive methods of cholesterol detection are significant for the early prevention of cardiovascular diseases. Eom et al. (2020) developed an enzyme-based electrochemical biosensor to detect low concentrations of cholesterol in saliva by immobilizing an appropriate volume of cholesterol oxidase on platinum nanoclusters. The cholesterol level was then determined by chronoamperometry. The linear range of the biosensor was 2–486 μm, the detection limit was approximately 2 μm, and the sensitivity was 132 μA mm–1⋅cm–2 (Eom et al., 2020).
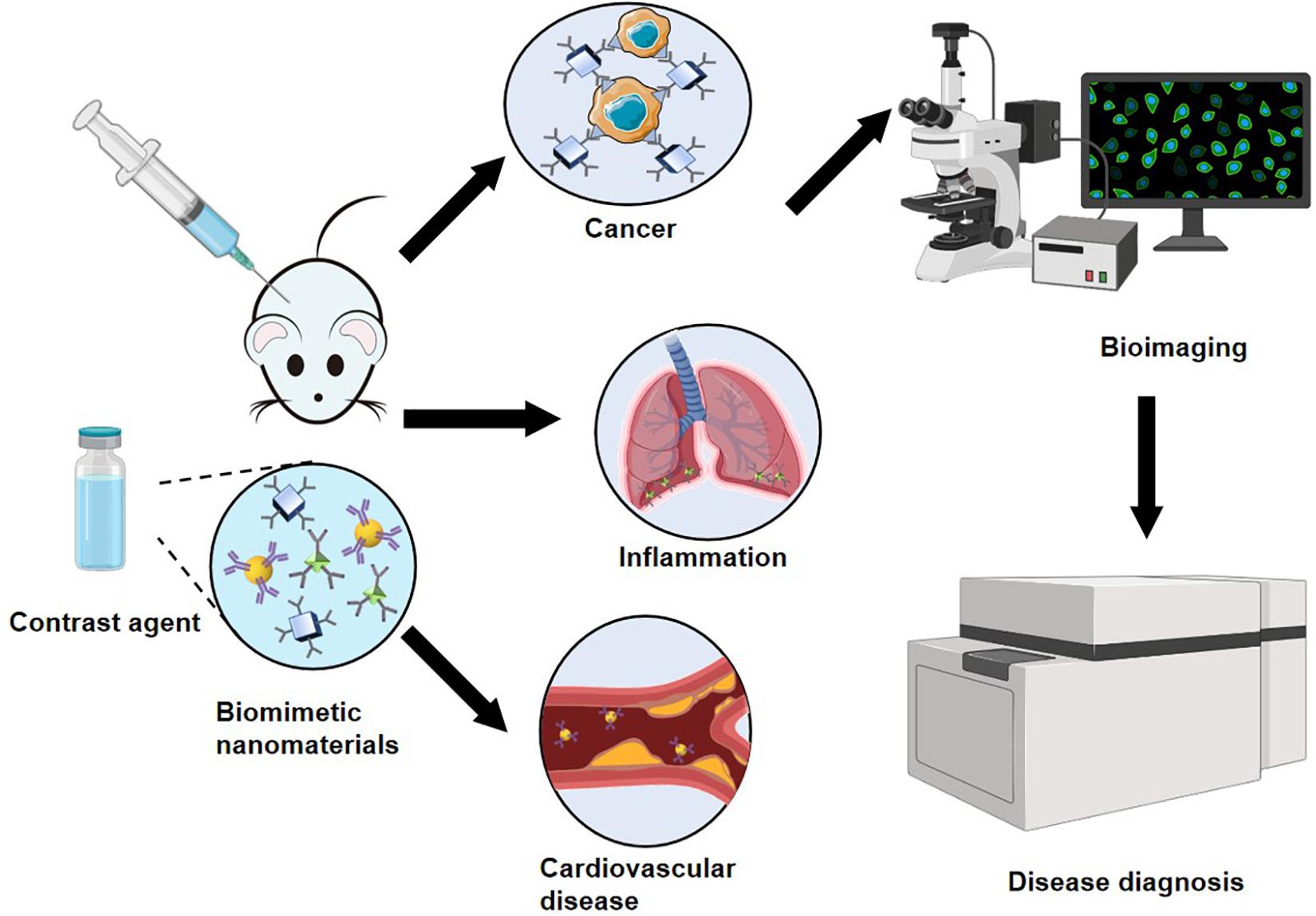
Bioimaging
As diagnostic methods improve, it is becoming possible to detect cancer early through image-based screening. Commonly used biological imaging techniques can be divided into the following four categories: (1) OI is non-invasive, non-destructive, and non-ionizing, allowing it to be used for histological imaging. (2) MRI has excellent spatial resolution, good soft tissue contrast, and deep tissue penetration. MRI does not involve ionizing radiation and thus plays an important role in non-invasive disease diagnosis. (3) USI is a well-known non-invasive diagnostic method that is based on the different responses of tissues with different ultrasound characteristics to high-frequency sound waves. Thus, USI can reveal the normal tissue structure and tumor tissues. (4) Photoacoustic imaging (PAI) is a recently developed, non-invasive, and non-ionizing imaging method based on light and sound. PAI has great potential for application in nanomedicine (Yang et al., 2019). (5) PET is a well-known, highly sensitive, and non-invasive imaging method that can achieve quantitative analysis and is not limited by tissue penetration. The application of biomimetic nanomaterials as contrast agents for non-invasive biological imaging shows great promise for improving existing methods for early disease diagnosis. With the continuous development of non-invasive imaging technology, imaging systems supported by biomimetic nanomaterials are expected to contribute to future clinical applications (Figure 3).
Bioimaging for Cancer Diagnosis
Surface-enhanced Raman scattering nanoparticles, which produce a unique “fingerprint” spectrum, can be used as contrast agents to show the precise locations of targeted cells in the body for cancer diagnosis (Nicolson et al., 2019). Nicolson et al. (2019) combined spatially offset Raman spectroscopy (SORS) with SERRS in a technique termed surface-enhanced spatially offset resonance Raman spectroscopy to image deep-seated glioblastoma multiforme tumors in a related brain tumor mouse model. This new OI technique is based on the generation of SERRS nanostars from AuNPs. The nanostars were encapsulated in a thin silicon shell and functionalized by a cyclic RGDyK peptide, which can bind to integrin receptors on tumor cell surfaces to target integrins and detect glioblastoma. This imaging method overcomes the drawbacks of traditional Raman spectroscopy-based imaging, which cannot detect tissues at depths greater than a few millimeters (Nicolson et al., 2019).
Breast cancer is a serious disease among females. The use of ultrasound molecular imaging for the early diagnosis of breast cancer is of great significance for patients. Xu et al. (2018) designed dual-targeted poly(lactide-co-glycolic acid) (PLGA) nanocapsules. The nanocapsules were coated with gold nanoshell, which bound to anti-VEGF receptor type 2 and anti-p53 (Xu et al., 2018). The novel dual-targeted ultrasound contrast agent showed high affinity and specificity for VEGFR2- and p53-positive cells. By integrating the targeted imaging of breast cancer cells with the targeted imaging of tumor angiogenic vessels, this agent could be used for the accurate and specific early detection of breast cancer in MCF-7 orthotopic mice (Xu et al., 2018).
Glycine is the simplest neutral amino acid. Glycine does not induce inflammation and can help control nanoparticle size by minimizing the extension of the ligand arms of functional groups. Chakraborty et al. (2020) combined super-paramagnetic iron oxide nanoparticles (SPIONs) with glycine to create GSPIONs for non-invasive imaging and the targeting of lung immune cells to detect lung disease. The GSPIONs were selectively absorbed by alveolar macrophages and neutrophils. Three-dimensional ultrashort echo time MRI was then used to determine the biodistribution of GSPIONs in the lung and diagnose disease. The GSPIONs have stable structures and are easy to synthesize with a particle size of 12.5 nm and a hydrodynamic diameter of 84.19 ± 18 nm. These GSPIONs may provide an accurate diagnostic tool for lung cancer (Chakraborty et al., 2020).
Yoo et al. (2017) developed an activatable MRI contrast agent based on PEG-coated superparamagnetic iron oxide nanoparticles complexed with poly(gallol). Because the polymer coating responds to oxidative stress produced by local reactive oxygen species, the activated MRI contrast agent may have potential applications in the non-invasive diagnosis of oxidative stress-related diseases such as cancer and atherosclerosis (Yoo et al., 2017).
Photoacoustic imaging is a recently developed technique that provides non-invasive and non-ionizing imaging based on light and sound. PAI has significant application potential in the field of nanomedicine (Yang et al., 2019). The photoacoustic signal is the ultrasonic signal generated by the light absorption of a biological tissue under laser irradiation. Bharathiraja et al. (2018) developed Pd@COS (chitosan oligosaccharide)-RGD (arginine-glycine-aspartic acid) as a contrast agent. They first synthesized porous palladium nanoparticles, modified the nanoparticles with CS, and functionalized them with RGD peptide. Integrin αvβ3, which is expressed in triple-negative breast cancer cells, binds with RGD peptide. The Pd@COS-RGD nanoparticles showed good biocompatibility and strong photoacoustic signal in MDA-MB-231 breast cancer cells, suggesting that they can be applied in the PAI-based diagnosis of breast cancer (Bharathiraja et al., 2018). In mice treated with the new contrast agent, the agent accumulated in the tumor region and could be used to distinguish tumor tissue.
Folate receptors are highly expressed in many cancer patients, and folic acid (FA) can combine with these folate receptors in cancer cells with specificity. Hu et al. (2013) formed hybrid nanocomposites of rGO and AgNPs and used them to detect cancer cells based on the physical adsorption of FA on the rGO-AgNPs. Due to the spectral enhancement effect of the AgNPs and the adsorption ability of graphene, Raman spectrometry could be used to detect cancer cells (Hu et al., 2013). In the experiment, HeLa cells overexpressing FRS (FRS positive) were used as model cancer cells, and A549 cells expressing a small amount of FRS (FRS negative) were used as control cells to study the targeting ability of the probe and detect cancer cells through changes in SERS signals.
Jiang et al. (2018) used a reversed-phase microemulsion method to dope silica with gadopentetic acid for use as a novel contrast agent for the specific non-invasive diagnosis of prostate-specific membrane antigen (PSMA) receptor-positive prostate cancer via MRI. In their work, amino and carboxyl groups were introduced on the surfaces of the silica nanotubes, and a monoclonal antibody to PSMA was then conjugated using the carbodiimide method. This novel specific magnetic resonance contrast agent can be used for the specific non-invasive diagnosis of PSMA receptor-positive prostate cancer via MRI (Jiang et al., 2018).
Bioimaging for Inflammatory Disease Diagnosis
Bacterial infection has high prevalence and mortality globally, and data from the United States suggest that many patients die from sepsis and infections caused by limb amputation. Bardhan et al. (2014) demonstrated a novel approach in which CNTs are employed as bacterial probes for the non-invasive diagnosis of infectious disease by fluorescence imaging. The authors conjugated SWCNTs with M13 bacteriophage to form M13-SWCNTs, which exhibited sufficient fluorescence activity for the high-quality NIR-II imaging of living subjects. In addition, because M13 has a natural binding affinity for the F’-pili of bacteria and can specifically target many F′-negative strains, M13-SWCNTs are a good candidate for the detection of bacterial infection (Bardhan et al., 2014).
Bioimaging for Cardiovascular Disease Diagnosis
Tenascin-C plays a crucial role in the formation and development of atherosclerosis; thus, it can be used as a biomarker to detect atherosclerosis. Li et al. (2018) explored a tenascin-C-targeted ultrasmall superparamagnetic iron oxide (USPIO) to detect atherosclerosis via MRI. The authors created a new MRI probe by labeling USPIO with a monoclonal antibody against mouse tenogenic protein C, which is primarily used to detect aortic arch plaque (Li et al., 2018). MRI showed more USPIO deposition in the target group than in the control group.
The safest non-invasive diagnostic tool for atherosclerosis is MRI. Chaudhary et al. (2016) developed nanocomplexes of zinc and ferrite (ZF) nanoparticles as MRI contrast agents to detect atherosclerosis. These nanoparticles were composed by bovine lactoferrin (Lf), PEG, and heat shock protein (Hsp)-70 antibody, which specifically targeted atherosclerosis. The nanocomplexes showed highly enhanced saturation magnetization and contrast of T2 and CT. Compared with ferrite nanoparticles, HSP-70 LF-PEG-ZF was slightly (1.09 times) enhanced in T1 MRI, significantly (1.22 times) enhanced in T2 MRI, and slightly (1.08 times) enhanced in CT. Kanwar et al. (2001) verified the temporal expression of heat shock proteins at atherosclerotic lesions and used Hsps-70 to diagnose atherosclerosis via MRI.
Poon et al. (2018) combined an inorganic magnetic iron oxide or manganese oxide core with an organic fibrin-targeted amphiphilic peptide to develop hybrid metal oxide–peptide amphiphile micelles (HMO-Ms) via self-assembly for the MRI of thrombosis in atherosclerotic plaques. The novel HMO-Ms could detect the vulnerable plaques. MR signal enhancement and signal brightness intensities tested the CREKA peptide functionalized hybrid nanoparticles’ targeting ability to target blood clots compared with non-targeted HMO-Ms (NT-Fe-Ms and NT-Mn-Ms) (Poon et al., 2018).
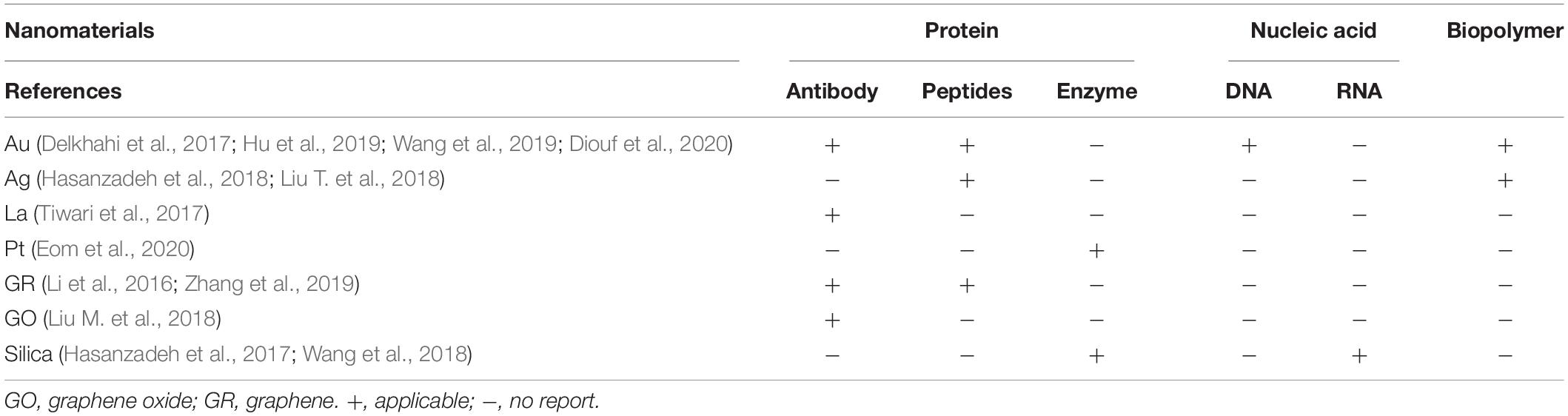
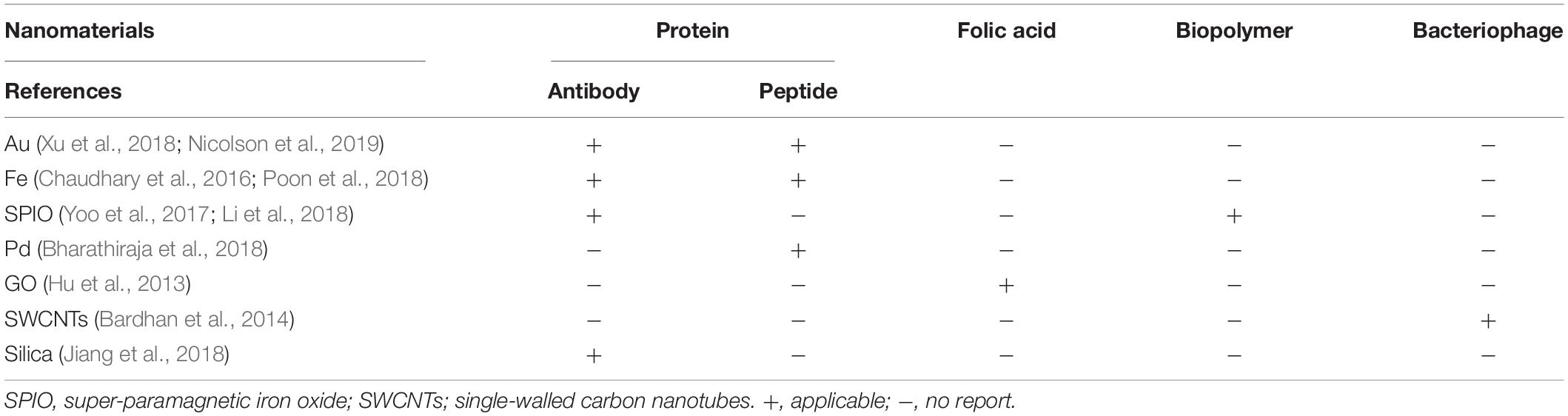
As nanotechnology evolves, biomimetic functionalization of nanomaterials using different biomolecules can be applied to biosensors and biological imaging. The biomimetic functionalization of nanomaterials by proteins and biopolymers can not only be used for biosensors but also for bioimaging. However, some biomolecules may be suitable for only one of them (Tables 1, 2).
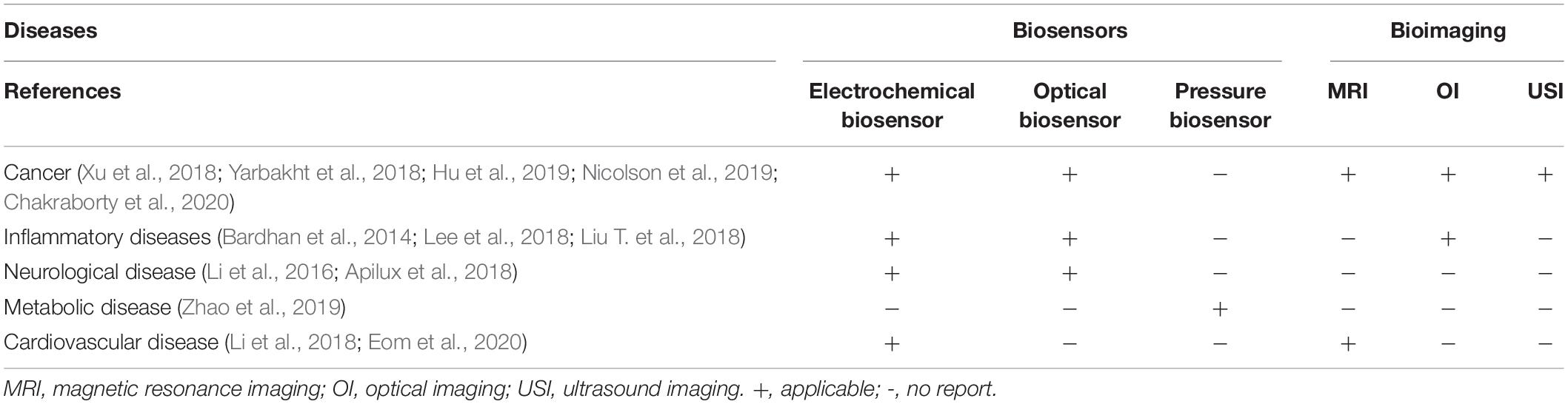
As cutting-edge methods of disease diagnosis, biosensors and bioimaging have been applied to the diagnosis of a variety of diseases. Biosensors and bioimaging have been widely used in the early diagnosis of cancer. Biosensors are more sensitive to the early diagnosis of metabolic and Neurological system diseases. Bioimaging is more accurate in the early diagnosis of cancer and cardiovascular disease. According to the characteristics of different diseases to choose the corresponding diagnostic methods, may achieve twice the result with half the effort (Table 3).
Biomimetic nanomaterials can participate in the process of biomolecular recognition, improve the rates of catalytic biological reactions, and amplify biological reactions. Therefore, the application of biomimetic nanomaterials in biomedicine has become a research hotspot. As reviewed herein, metal nanomaterials can be used to create electrochemical sensors with biological responses. Moreover, metal nanomaterials have enhanced Raman scattering properties, which is conducive to the development of diagnostic methods based on optical sensors and imaging. Based on their outstanding magnetic properties, magnetic biological nanomaterials can be modified with biological molecules and used to improve the disease detection ability and accuracy of imaging methods. Other metals and their oxides show good catalytic properties and can be used as enzyme analogs to improve biological reaction rates. In addition, carbon- and silica-based nanomaterials can be combined with other nanomaterials to form composite nanomaterials that exhibit improved biological properties compared with the single nanomaterials and thus have widespread applications in clinical diagnosis (Supplementary Appendices 1, 2).
Conclusion and Future Perspectives
Biomimetic nanomaterials have broad application prospects in biomedicine and play important roles in the non-invasive diagnosis of diseases. Metal nanomaterials, carbon-based nanomaterials, and silica-based nanomaterials have received considerable research attention due to their unique structures and biophysical and chemical properties. In particular, the application of gold, silver, and graphene nanomaterials in the early, non-invasive diagnosis of diseases has become a research hotspot. These nanomaterials interact with biomarkers of disease in vivo and create signals that can be detected by biosensors and biological imaging to diagnose diseases.
Although great progress has been made in the field of biomedicine, the application of nanomaterials still faces the following challenges: (1) Nanomaterials themselves have a significant drawback, namely their potential biotoxicity in vivo. Although the surface biomimetic modification of nanomaterials can reduce their biotoxicity, further improvements are required. (2) Some nanomaterials have components that are difficult to degrade in biological environments and cannot be metabolized and excreted from the body. Thus, the biodegradability of nanomaterials still has room for improvement. (3) There remains much room for improvement of biomimetic nanomaterials in terms of their stability and biocompatibility. (4) In the non-invasive diagnosis of diseases, the sensitivity of biomimetic nanomaterials to diseases needs to be further improved. (5) Although biomimetic nanomaterials have been used in animal studies for non-invasive disease detection, they have not been widely used in clinical practice.
Biomimetic nanomaterials based on metals, carbon, and silicon have seen great progress in the area of non-invasive disease diagnosis. In the future, we expect to see more innovative biomimetic nanomaterials based on liposomes, QDs, upconversion nanomaterials, protein, etc., generating significant progress in biomedicine.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave the final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2021.664795/full#supplementary-material
References
Albert, K., Huang, X. C., and Hsu, H. Y. (2017). Bio-templated silica composites for next-generation biomedical applications. Adv. Colloid Interface Sci. 249, 272–289. doi: 10.1016/j.cis.2017.04.011
Apilux, A., Rengpipat, S., Suwanjang, W., and Chailapakul, O. (2018). Development of competitive lateral flow immunoassay coupled with silver enhancement for simple and sensitive salivary cortisol detection. EXCLI J. 17, 1198–1209. doi: 10.17179/excli2018-1824
Balaji, A., and Zhang, J. (2017). Electrochemical and optical biosensors for early-stage cancer diagnosis by using graphene and graphene oxide. Cancer Nanotechnol. 8:10. doi: 10.1186/s12645-017-0035-z
Bardhan, N. M., Ghosh, D., and Belcher, A. M. (2014). Carbon nanotubes as in vivo bacterial probes. Nat. Commun. 5:4918. doi: 10.1038/ncomms5918
Bellassai, N., D’Agata, R., Jungbluth, V., and Spoto, G. (2019). Surface plasmon resonance for biomarker detection: advances in non-invasive cancer diagnosis. Front. Chem. 7:570. doi: 10.3389/fchem.2019.00570
Bharathiraja, S., Bui, N. Q., Manivasagan, P., Moorthy, M. S., Mondal, S., Seo, H., et al. (2018). Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 8:500. doi: 10.1038/s41598-017-18966-8
Broza, Y. Y., Khatib, S., Gharra, A., Krilaviciute, A., Amal, H., Polaka, I., et al. (2019). Screening for gastric cancer using exhaled breath samples. Br. J. Surg. 106, 1122–1125. doi: 10.1002/bjs.11294
Cha, B. G., and Kim, J. (2019). Functional mesoporous silica nanoparticles for bio-imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 11:e1515. doi: 10.1002/wnan.1515
Chakraborty, A., Royce, S. G., Selomulya, C., and Plebanski, M. (2020). A novel approach for non-invasive lung imaging and targeting lung immune cells. Int. J. Mol. Sci. 21:1613. doi: 10.3390/ijms21051613
Chaudhary, R., Roy, K., Kanwar, R. K., Walder, K., and Kanwar, J. R. (2016). Engineered atherosclerosis-specific zinc ferrite nanocomplex-based MRI contrast agents. J. Nanobiotechnol. 14:6. doi: 10.1186/s12951-016-0157-1
Choi, J. R., Yong, K. W., Choi, J. Y., and Cowie, A. C. (2019). Progress in molecularly imprinted polymers for biomedical applications. Comb. Chem. High. Throughput Screen. 22, 78–88. doi: 10.2174/1386207322666190325115526
Corradi, M., Pesci, A., Casana, R., Alinovi, R., Goldoni, M., Vettori, M. V., et al. (2003). Nitrate in exhaled breath condensate of patients with different airway diseases. Nitric Oxide 8, 26–30. doi: 10.1016/s1089-8603(02)00128-3
Coyle, V. E., Kandjani, A. E., Field, M. R., Hartley, P., Chen, M., Sabri, Y. M., et al. (2019). Co3O4 needles on Au honeycomb as a non-invasive electrochemical biosensor for glucose in saliva. Biosens. Bioelectron. 141:111479. doi: 10.1016/j.bios.2019.111479
Daniel, M. C., and Astruc, D. (2004). Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 104, 293–346.
Das, D., and Noh, I. (2018). Overviews of biomimetic medical materials. Adv. Exp. Med. Biol. 1064, 3–24. doi: 10.1007/978-981-13-0445-3_1
Delkhahi, S., Rahaie, M., and Rahimi, F. (2017). Design and fabrication a gold nanoparticle-dna based nanobiosensor for detection of microRNA involved in Alzheimer’s Disease. J. Fluoresc. 27, 603–610. doi: 10.1007/s10895-016-1988-8
Deshmukh, S. P., Patil, S. M., Mullani, S. B., and Delekar, S. D. (2019). Silver nanoparticles as an effective disinfectant: a review. Mater. Sci. Eng. C Mater. Biol. Appl. 97, 954–965. doi: 10.1016/j.msec.2018.12.102
Diouf, A., El Bari, N., and Bouchikhi, B. (2020). A novel electrochemical sensor based on ion imprinted polymer and gold nanomaterials for nitrite ion analysis in exhaled breath condensate. Talanta 209:120577. doi: 10.1016/j.talanta.2019.120577
Dizaji, B. F., Farboudi, A., Rahbar, A., Azarbaijan, M. H., and Asgary, M. R. (2020). The role of single- and multi-walled carbon nanotube in breast cancer treatment. Ther. Deliv. 11, 653–672. doi: 10.4155/tde-2020-0019
Eom, K. S., Lee, Y. J., Seo, H. W., Kang, J. Y., Shim, J. S., and Lee, S. H. (2020). Sensitive and non-invasive cholesterol determination in saliva via optimization of enzyme loading and platinum nano-cluster composition. Analyst 145, 908–916. doi: 10.1039/c9an01679a
Fitzgerald, J., and Fenniri, H. (2017). Cutting edge methods for non-invasive disease diagnosis using E-tongue and E-nose devices. Biosensors 7:59. doi: 10.3390/bios7040059
Garg, B., Sung, C. H., and Ling, Y. C. (2015). Graphene-based nanomaterials as molecular imaging agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 7, 737–758. doi: 10.1002/wnan.1342
Ghanbari, H., de Mel, A., and Seifalian, A. M. (2011). Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: a glimpse into prospective horizons. Int. J. Nanomed. 6, 775–786. doi: 10.2147/IJN.S14881
Gherasim, O., Puiu, R. A., Bîrcã, A. C., Burduşel, A. C., and Grumezescu, A. M. (2020). An updated review on silver nanoparticles in biomedicine. Nanomaterials 10:2318. doi: 10.3390/nano10112318
Hasanzadeh, M., Babaie, P., Jouyban-Gharamaleki, V., and Jouyban, A. (2018). The use of chitosan as a bioactive polysaccharide in non-invasive detection of malondialdehyde biomarker in human exhaled breath condensate: a new platform towards diagnosis of some lung disease. Int. J. Biol. Macromol. 120, 2482–2492. doi: 10.1016/j.ijbiomac.2018.09.018
Hasanzadeh, M., Nahar, A. S., Hassanpour, S., Shadjou, N., Mokhtarzadeh, A., and Mohammadi, J. (2017). Proline dehydrogenase-entrapped mesoporous magnetic silica nanomaterial for electrochemical biosensing of L-proline in biological fluids. Enzyme Microb. Technol. 105, 64–76. doi: 10.1016/j.enzmictec.2017.05.007
Hu, C., Liu, Y., Qin, J., Nie, G., Lei, B., Xiao, Y., et al. (2013). Fabrication of reduced graphene oxide and sliver nanoparticle hybrids for Raman detection of absorbed folic acid: a potential cancer diagnostic probe. ACS Appl. Mater. Interfaces 5, 4760–4768. doi: 10.1021/am4000485
Hu, R., Gupta, R., Wang, Z., Wang, C., Sun, H., Singamaneni, S., et al. (2019). Bioplasmonic paper-based assay for perilipin-2 non-invasively detects renal cancer. Kidney Int. 96, 1417–1421. doi: 10.1016/j.kint.2019.08.020
Jafari, M., Solhi, E., Tagi, S., Hasanzadeh, M., Jouyban-Gharamaleki, V., Jouyban, A., et al. (2019). Non-invasive quantification of malondialdehyde biomarker in human exhaled breath condensate using self-assembled organic-inorganic nanohybrid: a new platform for early diagnosis of lung disease. J. Pharm. Biomed. Anal. 164, 249–257. doi: 10.1016/j.jpba.2018.10.048
Jang, S. C., Kang, S. M., Lee, J. Y., Oh, S. Y., Vilian, A. E., Lee, I., et al. (2018). Nano-graphene oxide composite for in vivo imaging. Int. J. Nanomed. 13, 221–234. doi: 10.2147/IJN.S148211
Jiang, W., He, X., Fang, H., Zhou, X., Ran, H., and Guo, D. (2018). Novel gadopentetic acid-doped silica nanoparticles conjugated with YPSMA-1 targeting prostate cancer for MR imaging: an in vitro study. Biochem. Biophys. Res. Commun. 499, 202–208. doi: 10.1016/j.bbrc.2018.03.124
Johnston, N., Wells, C. W., Blumin, J. H., Toohill, R. J., and Merati, A. L. (2007). Receptor-mediated uptake of pepsin by laryngeal epithelial cells. Ann. Otol. Rhinol. Laryngol. 116, 934–938. doi: 10.1177/000348940711601211
Jouyban, A., and Rahimpour, E. (2020). Optical sensors based on silver nanoparticles for determination of pharmaceuticals: an overview of advances in the last decade. Talanta 217:121071. doi: 10.1016/j.talanta.2020.121071
Kanwar, R. K., Kanwar, J. R., Wang, D., Ormrod, D. J., and Krissansen, G. W. (2001). Temporal expression of heat shock proteins 60 and 70 at lesion-prone sites during atherogenesis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21, 1991–1997. doi: 10.1161/hq1201.100263
Kaya, M., and Volkan, M. (2012). New approach for the surface enhanced resonance Raman scattering (SERRS) detection of dopamine at picomolar (pM) levels in the presence of ascorbic acid. Anal. Chem. 84, 7729–7735. doi: 10.1021/ac3010428
Knežević, N. Ž., Gadjanski, I., and Durand, J. O. (2019). Magnetic nanoarchitectures for cancer sensing, imaging and therapy. J. Mater. Chem. B 7, 9–23. doi: 10.1039/c8tb02741b
Lamichhane, N., Udayakumar, T. S., D’Souza, W. D., Simone, C. B. II, Raghavan, S. R., Polf, J., et al. (2018). Liposomes: clinical applications and potential for image-guided drug delivery. Molecules 23:288. doi: 10.3390/molecules23020288
Lee, D., Lee, Y. J., Eun, Y. G., and Lee, G. J. (2018). Label-free detection of salivary pepsin using gold nanoparticle/polypyrrole nanocoral modified screen-printed electrode. Sensors 18:1685. doi: 10.3390/s18061685
Lee, S. H., and Jun, B. H. (2019). Silver nanoparticles: synthesis and application for nanomedicine. Int. J. Mol. Sci. 20:865. doi: 10.3390/ijms20040865
Li, S. S., Lin, C. W., Wei, K. C., Huang, C. Y., Hsu, P. H., Liu, H. L., et al. (2016). Non-invasive screening for early Alzheimer’s disease diagnosis by a sensitively immunomagnetic biosensor. Sci. Rep. 6:25155. doi: 10.1038/srep25155
Li, Y., Liu, J., Huang, J. W., Song, J. C., Ma, Z. L., and Shi, H. B. (2018). In vivo MRI detection of atherosclerosis in ApoE-deficient mice by using tenascin-C-targeted USPIO. Acta Radiol. 59, 1431–1437. doi: 10.1177/0284185118762613
Li, Z., Tan, S., Li, S., Shen, Q., and Wang, K. (2017). Cancer drug delivery in the nano era: an overview and perspectives (Review). Oncol. Rep. 38, 611–624. doi: 10.3892/or.2017.5718
Liang, K., and Chen, H. (2020). Protein-based nanoplatforms for tumor imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 12:e1616. doi: 10.1002/wnan.1616
Liao, C., Li, Y., and Tjong, S. C. (2018). Graphene nanomaterials: synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 19:3564. doi: 10.3390/ijms19113564
Lin, J., Chen, X., and Huang, P. (2016). Graphene-based nanomaterials for bioimaging. Adv. Drug Deliv. Rev. 105, 242–254. doi: 10.1016/j.addr.2016.05.013
Liu, M., Zheng, C., Cui, M., Zhang, X., Yang, D. P., Wang, X., et al. (2018). Graphene oxide wrapped with gold nanorods as a tag in a SERS based immunoassay for the hepatitis B surface antigen. Mikrochim. Acta 185:458. doi: 10.1007/s00604-018-2989-x
Liu, T., Gao, L., Zhao, J., Cao, Y., Tang, Y., and Miao, P. (2018). A polymyxin B-silver nanoparticle colloidal system and the application of lipopolysaccharide analysis. Analyst 143, 1053–1058. doi: 10.1039/c7an01788j
Mahajan, S. D., Aalinkeel, R., Law, W. C., Reynolds, J. L., Nair, B. B., Sykes, D. E., et al. (2012). Anti-HIV-1 nanotherapeutics: promises and challenges for the future. Int. J. Nanomed. 7, 5301–5314. doi: 10.2147/IJN.S25871
Matea, C. T., Mocan, T., Tabaran, F., Pop, T., Mosteanu, O., Puia, C., et al. (2017). Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 12, 5421–5431. doi: 10.2147/IJN.S138624
Montes-García, V., Pérez-Juste, J., Pastoriza-Santos, I., and Liz-Marzán, L. M. (2014). Metal nanoparticles and supramolecular macrocycles: a tale of synergy. Chemistry 20, 10874–10883. doi: 10.1002/chem.201403107
Nicolson, F., Andreiuk, B., Andreou, C., Hsu, H. T., Rudder, S., and Kircher, M. F. (2019). Non-invasive in vivo imaging of cancer using surface-enhanced spatially offset Raman spectroscopy (SESORS). Theranostics 9, 5899–5913. doi: 10.7150/thno.36321
Nikolova, M. P., and Chavali, M. S. (2020). Metal oxide nanoparticles as biomedical materials. Biomimetics 5:27. doi: 10.3390/biomimetics5020027
Ning, L., Zhu, B., and Gao, T. (2017). Gold nanoparticles: promising agent to improve the diagnosis and therapy of cancer. Curr. Drug Metab. 18, 1055–1067. doi: 10.2174/1389200218666170925122513
Niyogi, S., Hamon, M. A., Hu, H., Zhao, B., Bhowmik, P., Sen, R., et al. (2002). Chemistry of single-walled carbon nanotubes. Acc. Chem. Res. 35, 1105–1113. doi: 10.1021/ar010155r
Padnya, P., Gorbachuk, V., and Stoikov, I. (2020). The role of Calix[n]arenes and Pillar[n]arenes in the design of silver nanoparticles: self-assembly and application. Int. J. Mol. Sci. 21:1425. doi: 10.3390/ijms21041425
Pasinszki, T., Krebsz, M., Tung, T. T., and Losic, D. (2017). Carbon nanomaterial based biosensors for non-invasive detection of cancer and disease biomarkers for clinical diagnosis. Sensors 17:1919. doi: 10.3390/s17081919
Piro, B., and Reisberg, S. (2017). Recent advances in electrochemical immunosensors. Sensors 17:794. doi: 10.3390/s17040794
Poon, C., Gallo, J., Joo, J., Chang, T., Bañobre-López, M., and Chung, E. J. (2018). Hybrid, metal oxide-peptide amphiphile micelles for molecular magnetic resonance imaging of atherosclerosis. J. Nanobiotechnol. 16:92. doi: 10.1186/s12951-018-0420-8
Regan, B., Boyle, F., O’Kennedy, R., and Collins, D. (2019). Evaluation of molecularly imprinted polymers for point-of-care testing for cardiovascular disease. Sensors 19:3485. doi: 10.3390/s19163485
Renu, S., Shivashangari, K. S., and Ravikumar, V. (2020). Incorporated plant extract fabricated silver/poly-D,l-lactide-co-glycolide nanocomposites for antimicrobial based wound healing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 228:117673. doi: 10.1016/j.saa.2019.117673
Sabu, C., Henna, T. K., Raphey, V. R., Nivitha, K. P., and Pramod, K. (2019). Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 141:111201. doi: 10.1016/j.bios.2019.03.034
Saha, K., Agasti, S. S., Kim, C., Li, X., and Rotello, V. M. (2012). Gold nanoparticles in chemical and biological sensing. Chem. Rev. 112, 2739–2779. doi: 10.1021/cr2001178
Sharipov, M., Tawfik, S. M., Gerelkhuu, Z., Huy, B. T., and Lee, Y. I. (2017). Phospholipase A2-responsive phosphate micelle-loaded UCNPs for bioimaging of prostate cancer cells. Sci. Rep. 7:16073. doi: 10.1038/s41598-017-16136-4
Sheoran, R., Khokra, S. L., Chawla, V., and Dureja, H. (2019). Recent patents, formulation techniques, classification and characterization of liposomes. Recent Pat. Nanotechnol. 13, 17–27. doi: 10.2174/1872210513666181127110413
Siddique, Y. H., Ara, G., and Afzal, M. (2012). Estimation of lipid peroxidation induced by hydrogen peroxide in cultured human lymphocytes. Dose Response 10, 1–10. doi: 10.2203/dose-response.10-002.Siddique
Singh, P., Pandit, S., Mokkapati, V., Garg, A., Ravikumar, V., and Mijakovic, I. (2018). Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 19:1979. doi: 10.3390/ijms19071979
Sun, F., Wang, S., Wang, Y., Zhang, J., Yu, X., Zhou, Y., et al. (2019). Synthesis of Ni-Co hydroxide nanosheets constructed hollow cubes for electrochemical glucose determination. Sensors 19:2938. doi: 10.3390/s19132938
Takke, A., and Shende, P. (2019). Non-invasive biodiversified sensors: a modernized screening technology for cancer. Curr. Pharm. Des. 25, 4108–4120. doi: 10.2174/1381612825666191022162232
Tang, Y. H., Huang, A. Y., Chen, P. Y., Chen, H. T., and Kao, C. L. (2011). Metallodendrimers and dendrimer nanocomposites. Curr. Pharm. Des. 17, 2308–2330. doi: 10.2174/138161211797052367
Thambiraj, S., Hema, S., and Shankaran, D. R. (2018). An overview on applications of gold nanoparticle for early diagnosis and targeted drug delivery to prostate cancer. Recent Pat. Nanotechnol. 12, 110–131. doi: 10.2174/1872210511666171101120157
Thess, A., Lee, R., Nikolaev, P., Dai, H., Petit, P., Robert, J., et al. (1996). Crystalline ropes of metallic carbon nanotubes. Science 273, 483–487. doi: 10.1126/science.273.5274.483
Tian, F., Conde, J., Bao, C., Chen, Y., Curtin, J., and Cui, D. (2016). Gold nanostars for efficient in vitro and in vivo real-time SERS detection and drug delivery via plasmonic-tunable Raman/FTIR imaging. Biomaterials 106, 87–97. doi: 10.1016/j.biomaterials.2016.08.014
Tian, W., Zhang, X., Chen, Z., and Ji, H. (2016). A review of graphene on NEMS. Recent Pat. Nanotechnol. 10, 3–10. doi: 10.2174/187221051001160322151412
Tiwari, S., Gupta, P. K., Bagbi, Y., Sarkar, T., and Solanki, P. R. (2017). L-cysteine capped lanthanum hydroxide nanostructures for non-invasive detection of oral cancer biomarker. Biosens. Bioelectron. 89, 1042–1052. doi: 10.1016/j.bios.2016.10.020
Upadhyayula, V. K. (2012). Functionalized gold nanoparticle supported sensory mechanisms applied in detection of chemical and biological threat agents: a review. Anal. Chim. Acta 715, 1–18. doi: 10.1016/j.aca.2011.12.008
Vangijzegem, T., Stanicki, D., and Laurent, S. (2019). Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin. Drug Deliv. 16, 69–78. doi: 10.1080/17425247.2019.1554647
Vardharajula, S., Ali, S. Z., Tiwari, P. M., Eroðlu, E., Vig, K., Dennis, V. A., et al. (2012). Functionalized carbon nanotubes: biomedical applications. Int. J. Nanomed. 7, 5361–5374. doi: 10.2147/IJN.S35832
Vijayan, V., Uthaman, S., and Park, I. K. (2018). Cell membrane coated nanoparticles: an emerging biomimetic nanoplatform for targeted bioimaging and therapy. Adv. Exp. Med. Biol. 1064, 45–59. doi: 10.1007/978-981-13-0445-3_3
Wang, J., Zhang, J., Li, T., Shen, R., Li, G., and Ling, L. (2019). Strand displacement amplification-coupled dynamic light scattering method to detect urinary telomerase for non-invasive detection of bladder cancer. Biosens. Bioelectron. 131, 143–148. doi: 10.1016/j.bios.2019.02.014
Wang, L., Xiong, Q., Xiao, F., and Duan, H. (2017). 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 89, 136–151. doi: 10.1016/j.bios.2016.06.011
Wang, Y., Shi, H., Cui, K., Zhang, L., Ge, S., Yan, M., et al. (2018). Hierarchical hematite/TiO2 nanorod arrays coupled with responsive mesoporous silica nanomaterial for highly sensitive photoelectrochemical sensing. Biosens. Bioelectron. 117, 515–521. doi: 10.1016/j.bios.2018.06.030
Wu, K., Su, D., Liu, J., Saha, R., and Wang, J. P. (2019). Magnetic nanoparticles in nanomedicine: a review of recent advances. Nanotechnology 30:502003. doi: 10.1088/1361-6528/ab4241
Xiang, Y., Wu, P., Tan, L. H., and Lu, Y. (2014). DNAzyme-functionalized gold nanoparticles for biosensing. Adv. Biochem. Eng. Biotechnol. 140, 93–120. doi: 10.1007/10_2013_242
Xu, L., Du, J., Wan, C., Zhang, Y., Xie, S., Li, H., et al. (2018). Ultrasound molecular imaging of breast cancer in MCF-7 orthotopic mice using gold nanoshelled poly (lactic-co-glycolic acid) nanocapsules: a novel dual-targeted ultrasound contrast agent. Int. J. Nanomed. 13, 1791–1807. doi: 10.2147/IJN.S153993
Yang, Y., Wang, L., Wan, B., Gu, Y., and Li, X. (2019). Optically active nanomaterials for bioimaging and targeted therapy. Front. Bioeng. Biotechnol. 7:320. doi: 10.3389/fbioe.2019.00320
Yang, Y., Zhang, M., Song, H., and Yu, C. (2020). Silica-based nanoparticles for biomedical applications: from nanocarriers to biomodulators. Acc. Chem. Res. 53, 1545–1556. doi: 10.1021/acs.accounts.0c00280
Yao, J., Huang, C., Liu, C., and Yang, M. (2020). Upconversion luminescence nanomaterials: a versatile platform for imaging, sensing, and therapy. Talanta 208:120157. doi: 10.1016/j.talanta.2019.120157
Yarbakht, M., Nikkhah, M., Moshaii, A., Weber, K., Matthäus, C., Cialla-May, D., et al. (2018). Simultaneous isolation and detection of single breast cancer cells using surface-enhanced Raman spectroscopy. Talanta 186, 44–52. doi: 10.1016/j.talanta.2018.04.009
Ye, L., Huang, N. L., Ma, X. L., Schneider, M., Huang, X. J., and Du, W. D. (2016). Establishment of N-succinimidyl 4-(maleimidomethyl) cyclohexanecarboxylate (SMCC) modified biochip enabling concurrent detection of serum infectious antibodies in neuroborreliosis. Biosens. Bioelectron. 78, 404–410. doi: 10.1016/j.bios.2015.11.050
Yoo, E., Cheng, H. A., Nardacci, L. E., Beaman, D. J., Drinnan, C. T., Lee, C., et al. (2017). Activatable interpolymer complex-superparamagnetic iron oxide nanoparticles as magnetic resonance contrast agents sensitive to oxidative stress. Colloids Surf. B Biointerfaces 158, 578–588. doi: 10.1016/j.colsurfb.2017.07.025
Zhang, D., and Wang, Y. (2019). Functional protein-based bioinspired nanomaterials: from coupled proteins, synthetic approaches, nanostructures to applications. Int. J. Mol. Sci. 20:3054. doi: 10.3390/ijms20123054
Zhang, F. R., Lu, J. Y., Yao, Q. F., Zhu, Q. Y., Zhang, X. X., Huang, W. T., et al. (2019). Matter, energy and information network of a graphene-peptide-based fluorescent sensing system for molecular logic computing, detection and imaging of cancer stem cell marker CD133 in cells and tumor tissues. Analyst 144, 1881–1891. doi: 10.1039/c8an02115e
Zhao, D., Zhang, Q., Zhang, Y., Liu, Y., Pei, Z., Yuan, Z., et al. (2019). Sandwich-type surface stress biosensor based on self-assembled gold nanoparticles in PDMS film for BSA detection. ACS Biomater. Sci. Eng. 5, 6274–6280. doi: 10.1021/acsbiomaterials.9b01073
Zhao, W., Li, A., Zhang, A., Zheng, Y., and Liu, J. (2018). Recent advances in functional-polymer-decorated transition-metal nanomaterials for bioimaging and cancer therapy. ChemMedChem 13, 2134–2149. doi: 10.1002/cmdc.201800462
Keywords: biomarker, biomimetic nanomaterials, biosensor, bioimaging, disease diagnosis, non-invasive detection
Citation: Feng Z, Fan H, Cheng L, Zhang H, Fan H and Liu J (2021) Advanced Biomimetic Nanomaterials for Non-invasive Disease Diagnosis. Front. Mater. 8:664795. doi: 10.3389/fmats.2021.664795
Received: 06 February 2021; Accepted: 02 March 2021;
Published: 24 March 2021.
Edited by:
Lin Xu, Jilin University, ChinaReviewed by:
Quanshun Li, Jilin University, ChinaChun Hung Chu, The University of Hong Kong, Hong Kong
Copyright © 2021 Feng, Fan, Cheng, Zhang, Fan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyuan Feng, ZmVuZ3p5MDBAMTYzLmNvbQ==
 Zhiyuan Feng
Zhiyuan Feng Hao Fan
Hao Fan Lin Cheng3
Lin Cheng3