
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mater. , 22 March 2021
Sec. Polymeric and Composite Materials
Volume 8 - 2021 | https://doi.org/10.3389/fmats.2021.646509
This article is part of the Research Topic Flame-Retardant Polymeric Materials and Polymer Composites View all 9 articles
The toxicity of CO threatens the life of people in the fire site. In this study, flame retardants of nano magnesium hydroxide particles and water-soluble flame retardant 8124 are used to be mixed into the aqueous film forming fire extinguishing agent (AFFF). Smoke-suppressed fire extinguishing agent was prepared in Waring-Blender mixing cup and then stirred at 3,000 r/min for 5 min. The new extinguishing agent shows a good performance of absorption of CO and reducing the flue gas temperature. The concentration of CO was decreased below 131 ppm and flue gas temperature was basically kept below 40°C, which was 367 ppm and 83.1°C less than that in free-fire. Using new extinguishing agent can effectively reduce the harm to the trapped personnel and firemen in the fire site. It was of great significance. The harm of CO concentration below 131 ppm and flue gas temperature below 40°C was low. The time to reach the maximum CO concentration and the maximum flue gas temperature was delayed, which ensures that people have more time to escape. Even if there was not enough time to escape, people will not be seriously threatened.
The fire typical gas carbon monoxide (CO), which can cause loss of human life, is unavoidable during combustion of fuels. It is the most lethal component of the toxic smoke (Zhao, 2012; Hampson et al., 2019). Toxic gas has brought great difficulties to personnel evacuation and fire fighting (Liu et al., 2020). Some researches indicated that myocardial infarction was associated with exposure to ambient carbon monoxide (Lee et al., 2020). Altered regional homogeneity in delayed encephalopathy, which is manifested as local brain dysfunctions, will be found after carbon monoxide poisoning (Wu et al., 2020). Reducing CO concentration and temperature of flue gas in fire site is the key to save the life of people (Zarca et al., 2015; Qiu et al., 2019; Shi et al., 2020). Therefore, it is important to develop new fire extinguishing agents for improving the CO emission inhibition performance.
Smoke suppression flame retardants have been widely used in wire and cable, indoor building decoration materials and engineering plastics (Sain et al., 2004; Tang et al., 2013; Shan et al., 2020). Therefore, smoke suppressive flame retardants are considered to be added into foam extinguishing agent. Researchers believe that solid particles can improve the stability and fire efficiency of fire foam (Xie et al., 2011; Jiang et al., 2016). Superfine magnesium hydroxide flame retardant has the advantages of low price, low toxicity, good smoke elimination and less dripping (Kuang et al., 2008; Lu et al., 2018). It shows excellent performance in smoke suppression and flame retardant, which is the result of physical and chemical actions. The superfine magnesium hydroxide powder has a great improvement in fire extinguishing efficiency compared with ordinary ammonium phosphate powder because of its large specific surface area, high activity, fast decomposition speed and strong ability to capture free radicals when heated (Wang et al., 2009). A large amount of water vapor released during the decomposition process can not only reduce the actual temperature of the flame, but also dilute the concentration of oxygen and combustible gas near the flame.
The aqueous film forming fire extinguishing agent (AFFF) used in the experiment was produced by Hebei Langfang Rongshun Fire-fighting Pharmaceutical Co., Ltd. Both nano magnesium hydroxide particles and water-soluble flame retardant 8124 are produced by Shandong Weifang Wanfeng New Material Science and Technology Co., Ltd. Nano magnesium hydroxide particles can be used as flame retardant or powder fire extinguishing agent. The water-soluble flame retardant 8124 is white water-soluble powder, which is easy to dissolve in water and dosen’t contain Nitrogen element that is harmful to the environment. It is an environmentally friendly flame retardant with properties of non decomposition, no smell, flame retardant and smoke suppression because it does not contain halogen, heavy metal and other prohibited components.
We prepared 100 ml solution containing 6% AFFF and 94% distilled water at room temperature. 10, 20, and 30 g nano magnesium hydroxide solid particles or a water-soluble flame retardant 8124 were added to the solution in order to study the effect of nano magnesium hydroxide solid particles and water-soluble flame retardant 8124 on the performance of fire extinguishing foam. The mixture was poured into Waring-Blender mixing cup and then stirred at 3,000 r/min for 5 min.
A small oil pan with a diameter of 10 cm was placed at the bottom of the cylindrical container with the diameter of 30 cm. 20 ml of gasoline was poured into the oil pan with a measuring cylinder. Xima carbon monoxide meter [(0∼1,000) ppm, ±10%, AS8700A, Shanghai Baoxin Instrument Co., Ltd.] which was fixed with an iron stand was 50 cm above the top of the cylindrical container. The detection port of the portable CO concentration meter was facing down and the instrument switch was opened for preparation. The CO concentration and flue gas temperature during the experiment were recorded by a camera fixed in front of the CO meter. Ignited the gasoline in the oil pan with the igniter, covered the circular filter screen with a diameter of 40 cm, and started timing with the stopwatch.
In order to compare the effect of AFFF foam with different flame retardants, a blank experiment of free-burning was carried out, as shown in Figure 1. A lot of CO was produced during the experiment. The CO concentration and flue gas temperature were obtained. The results are as shown in Figure 2. It was shown that CO concentration and flue gas temperature increases significantly in free-burning. Flue gas temperature begins to increase after about burning for 8 s and reaches 123.1°C at 41 s, which was the maximum temperature. The release of CO starts at 14s and the concentration of CO reaches the largest at 48 s. There was a lot of CO released in the whole process. The highest CO concentration was 498 ppm. It was believed that it was harmful if CO concentration was over 24 ppm. Therefore, it was dangerous for people to exposure to high temperature and high CO concentration.
After igniting the gasoline in the oil pan, AFFF foam was covered on the circular filter screen. The smoke passed through AFFF foam. CO was probably absorbed during the process. Figure 3 was the absorption of CO by AFFF foam and temperature curve. It can be seen from Figure 3 that the CO concentration and the flue gas temperature decrease. The maximum CO concentration which was reduced to 301 ppm at 91 s was 197 ppm less than that of 498 ppm in Figure 2. And then the CO concentration decreases rapidly. The highest temperature of flue gas drops by 10°C from 123.1 to 64.3°C. It was clear that foam was helpful for reducing flue gas temperature and absorbing CO released. AFFF foam can lead the temperature decreased because that AFFF foam contains a certain amount of water. When the flue gas spreads through the foam, the moisture in the foam can effectively reduce the temperature of the flue gas. After 20 s, the fuel burned intensely and the CO released increased sharply. But the stability of AFFF foam was reduced after heating. Bubbles burst and AFFF foam gradually lost the ability of filtering smoke, as shown in Figure 4. With the rupture of AFFF foam, the CO concentration rapidly increased. The increased flame temperature accelerated the rupture of AFFF foam until the foam completely disappeared.
Magnesium hydroxide, with good smoke suppression performance, is widely used in flame retardant materials which can release very little toxic smoke in the process of polymer combustion (Ma et al., 2020). The outer combustion product area has the function of diluting and absorbing smoke, so the smoke suppression effect of Mg(OH)2 is very obvious. Superfine magnesium hydroxide powder, which can be used as dry powder extinguishing agent, is easy to be suspended in the hot air around the flame when it is sprayed into the flame (Sener and Demirhan, 2008; Shi et al., 2019). That a large number of free radicals ·OH and H· in the flame are absorbed and transformed sharply reduces the number of free radicals and causes the chain reaction of combustion to break.
Nano magnesium hydroxide with the weight of 10, 20, and 30 g respectively were added to 100 ml AFFF foam extinguishing agent. The CO absorption experiment was also carried out and the concentration of CO was measured. Figure 5 shows the CO concentration by using foam extinguishing agent containing different weight nano magnesium hydroxide.
As shown in Figure 5, the peak value of CO concentration decreases when nano magnesium hydroxide powders of 10 g/100 ml are added into AFFF foam extinguishing agent. The maximum concentration of CO was about 294 ppm, which happens at 43 s. Compared with the foam without adding magnesium hydroxide, the peak value decreased. It indicates that nano magnesium hydroxide plays a role on filtering and absorbing CO. The stability of foam with magnesium hydroxide increases and the bubbles dose not break easily (Li et al., 2004; Qin et al., 2005; Vijayaraghavan et al., 2006; Lü et al., 2016). The time required for the smoke to pass through the foam was prolonged. The thermal stability of Mg(OH)2 was good.
Nano magnesium hydroxide particles of 10 g/100 ml added in the foam are beneficial to reduce the CO release during fuel combustion. However, it was found that when the concentration of nano magnesium hydroxide increases, the role of reducing CO concentration will decrease. The foam with magnesium hydroxide particles of 20 g/100 ml was used for the determination of CO release. The peak value of CO concentration was 333 ppm at 48 s and 340 ppm at 69 s. When the concentration of magnesium hydroxide particles was 30 g/100 ml, the CO concentration reaches a peak value of 433 ppm at 65 s. The CO concentration was higher when adding 30 g Mg(OH)2 particles than that of 10 g Mg(OH)2 particles. It indicates that the foam with too much magnesium hydroxide particles has poor absorption performance on CO released. This may be related to the structure of the foam. When a small number of particles was added, the stability of the foam increases. The bubbles broken will be replenished by liquid surrounding them. But if the concentration of particles in the foam was large, the foam viscosity increases (Li, 2018). The fluidity of the foam was poor. Bubbles burst will be caused by high temperature during the heating process. The foam around can not move to repair the cracked bubbles and the films fails to absorb CO because bubbles burst fast.
Although the effect of foam with Mg(OH)2 on absorption of CO released was not obvious, flue gas temperature decreases significantly. Figure 6 depicts the measurement result of flue gas temperature. For an easier comparison, the results obtained show that there was a remarkable decline of flue gas temperature. It drops below 55°C. And it dose not exceed 45°C if Mg(OH)2 concentration was 20 g/100 ml. Obviously, this temperature does little harm to people.
Figure 7 was the CO concentration change curve by using foam with water-soluble flame retardant 8124.
The increase of flue gas temperature and the release of CO are delayed by using foam with 8124. And the concentration of water-soluble flame retardant 8124 has a remarkable influence on CO concentration as shown in Figure 7. The CO concentration increases sharply if the concentration of flame retardant 8124 was 10 g/100 ml and the peak value reaches to 495 ppm at 55 s. The maximum CO concentration reaches 495 ppm, which was similar to that in free-burning. But it decreases with the increase of the concentration of flame retardant 8124. The maximum CO concentration was 397 ppm of 20 g/100 ml and 235 ppm of 30 g/ 100 ml.
Water-soluble flame retardant 8124 of 30 g/100 ml was obvious contributed to reducing flue gas temperature, as shown in Figure 8. After 93 s it reaches the maximum temperature of 41.6°C which was safe for people. It's almost below 38°C during burning.
This phenomenon may be related to the stability of the liquid film when heated. Images are shown in Figure 9. When a certain amount of flame retardant 8124 was added, the burning-resistance ability of the liquid film under the high temperature action was improved. After local heating, the liquid film would form a bulge and was not easy to crack, as shown in Figure 9B. Flames and smoke will spread out from the outside of the filter screen, which shows that the liquid film has strong resistance to high temperature and good coverage effect to flame. It was only under the action of high temperature for a long time that the liquid film breaks down.
In order to study the performance of compound foam, several groups of composite experiments had bee done. The result was shown in Figure 10. The interaction of Mg(OH)2 and 8124 is helpful to the inhibition and absorption of CO. It can be seen that the efficiency of compound foam was good when the concentration of Mg(OH)2 and 8124 was high.
According to the above experimental results, appropriate amount of nano magnesium hydroxide or water-soluble flame retardants 8124 was beneficial to fire-fighting foam agents. When adding 20 g/100 ml magnesium hydroxide, a good extinguishing foam with good cooling effect was obtained. Foam with water-soluble flame retardants 8124 of 30 g/100 ml has good performance of absorption of CO and cooling effect. Therefore, the combination of nano magnesium hydroxide of 20 g/100 ml and water-soluble flame retardant 8124 of 30 g/100 ml are considered to be added into extinguishing agent foam. Figure 11 shows CO concentration and flue gas temperature.
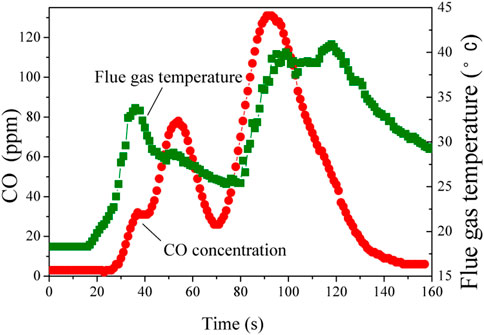
FIGURE 11. CO concentration and flue gas temperature by using compound foam with nano magnesium hydroxide of 20 g/100 ml and water-soluble flame retardant 8124 of 30 g/100 ml.
It can be seen from Figure 11 that CO concentration only reaches 131 ppm at 91 s, which was the maximum. And it only lasts for 20 s when the concentration of CO exceeds 100 ppm. It was believed that when the concentration of CO in the environment exceeds 100 ppm (100 × 10–6), the human body will have dizziness, fatigue and other discomfort. Therefore, it is safe for people that CO concentration is below 100 ppm (Dogan et al., 2019). During the whole process, it was only 9 s when the temperature exceeds 40°C. And the highest temperature was 40.9°C, then it rapidly decreases. After the oil pan fires, the foam covers on the filter net. Only a very small number of filter holes are burnt through, as shown in Figure 12.
The performance of compound foam formed by adding two kinds of flame retardants was the best. Mg(OH)2 in the foam has good smoke suppression performance. Most of free radicals OH and H in the flame were absorbed and the chain reaction of combustion was broken. The adding of water-soluble flame retardant 8124 to AFFF foam can improve effectively the viscoelasticity of the foam. Therefore the compound foam was helpful in absorbing CO and reducing the flue gas temperature. The foam has strong toughness and the effect on inhibiting CO release was very good.
In view of the problem that CO release is likely to cause death in the case of fire, this study proposes to add flame retardant nano magnesium hydroxide and water-soluble flame retardant 8124 to AFFF water film foam extinguishing agent. The concentration of CO and flue gas temperature are significantly decreased. Composite foam extinguishing agent (6% AFFF + 20 g/100 ml Mg(OH)2 + 30 g/100 ml 8124) exhibits excellent performance of inhibiting CO and reducing flue gas temperature. It can effectively reduce the harm of toxic smoke CO to the personnel at the site of fire, extinguish the fire efficiently, reduce the temperature of the fire site, and has a good application prospect.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RG designed experiments; LX carried out experiments; LX and QX analyzed experimental results. Attributed equally to the manuscript.
National Natural Science Foundation of China (No. 21704111); Cultivation Project of National Natural Science Foundation of China (No. ZKJJPY201714).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Doğan, F. S., Güneysel, Ö., Gökdağ, E., Merve Güneş, M. D., and Selin Gamze Sümen, M. D. (2019). Demographic characteristics and delayed neurological sequelae risk factors in carbon monoxide poisoning. Am. J. Emerg. Med. 38 (12), 2552–2556. doi:10.1016/j.ajem.2019.12.037
Hampson, N. B., Hauschildt, K. L., Deru, K., and Weaver, L. K. (2019). Carbon monoxide poisonings in hotels and motels: the problem silently continues. Prev. Med. Rep. 16, 100975–100977. doi:10.1016/j.pmedr.2019.100975
Jiang, X. S., Zhai, Y., Feng, J., and Lu, K. (2016). Influence factors of the formation and stability of fire-fighting three–phase foam. J. Logist. Eng. Univ. 32 (02), 41–46. doi:10.3969/j.issn.1672-7843.2016.02.008
Kuang, K., Huang, X., and Liao, G. (2008). A comparison between superfine magnesium hydroxide powders and commercial dry powders on fire suppression effectiveness. Process Saf. Environ. Prot. 86 (3), 182–188. doi:10.1016/j.psep.2007.11.002
Lee, K. K., Spath, N., Miller, M. R., Mills, N. L., and Shah, A. S. V. (2020). Short-term exposure to carbon monoxide and myocardial infarction: a systematic review and meta-analysis. Environ. Int. 143, 105901–105910. doi:10.1016/j.envint.2020.105901
Li, X. J. (2018). Study on particle concentration effect on three-phase foam property. J. Armed Police Aceade. 10, 18–23. doi:10.3969/j.issn.1008-2077.2018.10.005
Li, Z. Q., Xiang, L., and Wei, F. (2004). Fire Resistant&Smoke vanishing mechanismand production method of flame retardant type magnesium hydroxide. Haihuyan Yu Huagong 33 (2), 1–7. doi:10.1016/j.proeng.2013.02.150
Liu, C., Wu, W., Shi, Y. Q., Yang, F., Liu, M., Chen, Z., et al. (2020). Creating MXene/reduced graphene oxide hybrid towards highly fire safe thermoplastic polyurethane nanocomposites. Compos. Part B 203, 108486. doi:10.1016/j.compositesb.2020.108486
Lü, K. Z., Jiang, X. S., and Zhai, Y. (2016). The experimental study on thermal stability and oil fire extinguishing performance of three–phase foam extinguishing agent. J. Logist. Eng. Univ. 32 (06), 56–60. doi:10.3969/j.issn.1672-7843.2016.06.009
Lu, Y. H., Wu, C. F., and Xu, S. A. (2018). Mechanical, thermal and flame retardant properties of magnesium hydroxide filled poly(vinyl chloride) composites: the effect of filler shape. Compos. Part A 113, 1–11. doi:10.1016/j.compositesa.2018.07.012
Ma, J. L., Wang, X., Li, J., Chen, R., and Wei, J. (2020). Facile preparation of flame retardant cotton fabric via adhesion of Mg(OH)2 by the assistance of ionic liquid. Polymers 12 (2), 259–268. doi:10.3390/polym12020259
Qin, B. T., Wang, D. M., Chen, J. H., and Liang, X.-Y. (2005). Experimental investigation of high-performance three-phase foam for mine fire control. J. China Univ.Min. Technol. 34, 14–18. doi:10.3321/j.issn:0253-9993.2005.02.005
Qiu, X. B., Wei, Y. B., Li, N., Guo, A., Zhang, E., Li, C., et al. (2019). Development of an early warning fire detection system based on a laser spectroscopic carbon monoxide sensor using a 32-bit system-on-chip. Infrared Phys. Technol. 96, 44–51. doi:10.1016/j.infrared.2018.11.013
Sain, M., Park, S. H., Suhara, F., and Law, S. (2004). Flame retardant and mechanical properties of natural fibre-PP composites containing magnesium hydroxide. Polym. Degrad. Stab. 83 (2), 363–367. doi:10.1016/s0141-3910(03)00280-5
Sener, A. A., and Demirhan, E. (2008). The investigation of using magnesium hydroxide as a flame retardant in the cable insulation material by cross-linked polyethylene. Mater. Des. 29 (7), 1376–1379. doi:10.1016/j.matdes.2007.05.008
Shan, X. Y., Han, J., and Song, Y. (2020). Flame retardancy of epoxy resin/β-cyclodextrin @resorcinol bisdiphenyl phosphate inclusion composites. J. Wuhan Univ. Technol. Mater. Sci. Ed. 35, 455–463. 10.1007/s11595-020-2278-5.
Shi, Y. Q., Liu, C., Duan, Z. P., Yu, B., Liu, M., and Song, P. (2020). Interface engineering of MXene towards super-tough and strong polymer nanocomposites with high ductility and excellent fire safety. Chem. Eng. J. 399, 125829. doi:10.1016/j.cej.2020.125829
Shi, Y. Q., Liu, C., Liu, L., Fu, L., Yu, B., Lv, Y., et al. (2019). Strengthening, toughing and thermally stable ultra-thin MXene nanosheets/polypropylene nanocomposites via nanoconfinement. Chem. Eng. J. 378, 122267. doi:10.1016/j.cej.2019.122267
Tang, H., Zhou, X.-b., and Liu, X.-l. (2013). Effect of magnesium hydroxide on the flame retardant properties of unsaturated polyester resin. Procedia Eng. 52, 336–341. doi:10.1016/j.proeng.2013.02.150
Vijayaraghavan, K., Nikolov, A., and Wasan, D. (2006). Foam formation and mitigation in a three-phase gas-liquid-particulate system. Adv. Colloid Interf. Sci. 123–126, 49–61. doi:10.1016/j.cis.2006.07.006
Wang, L., Xu, P., and Luo, Y. (2009). The study on fire extinguishing performance of ultra-thin magnesium hydroxide powder. Fire Sci. Technol. 28 (6), 425–428. doi:10.1016/j.proeng-2017.12.035
Wu, K. F., Liu, M., Zhao, G. S., He, L., and Tan, Y. (2020). Altered regional homogeneity in delayed encephalopathy after carbon monoxide poisoning: a resting-state fMRI study. Neurosci. Lett. 729, 135002–135007. doi:10.1016/j.neulet.2020.135002
Xie, Z. H., Li, X. C., and Liu, M. M. (2011). Application of three-phase foam technology for spontaneous combustion prevention in longdong coal mine. Procedia Eng. 26, 63–69. 10.1016/j.proeng.2011.11.2140.
Zarca, G., Ortiz, I., and Urtiaga, A. (2015). Recovery of carbon monoxide from flue gases by reactive absorption in ionic liquid imidazolium chlorocuprate(I): mass transfer coefficients. Chin. J. Chem. Eng. 23 (5), 769–774. doi:10.1016/j.cjche.2014.06.040
Keywords: flame retardant, CO concentration, fire risk, magnesium hydroxide, flue gas
Citation: Li X, Guo R and Qian X (2021) Preparation and Absorption Carbon Monoxide Properties of a Novel Flame Retardants Based Fire-Fighting Foam. Front. Mater. 8:646509. doi: 10.3389/fmats.2021.646509
Received: 27 December 2020; Accepted: 08 February 2021;
Published: 22 March 2021.
Edited by:
Yongqian Shi, Fuzhou University, ChinaReviewed by:
Xin-xiao Lu, China University of Mining and Technology, ChinaCopyright © 2021 Li, Guo and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiujuan Li, bGVlbHk3OEAxNjMuY29t; Ruisong Guo, cnNndW9AdGp1LmVkdS5jbg==; Xiaodong Qian, d2p4eXF4ZEBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.