- Dipartimento di Biologia, Ecologia e Scienze Della Terra, Università Della Calabria, Rende, Italy
The protection of the stone materials represents an ongoing challenge in the field of conservation of cultural heritage. Protective coatings are used to make the stone more resistant against pollutants, biological growths, and especially against the action of water. In last decades, nanoparticles were synthetized and tested to improve the performance of such coatings. In this review, two main enhanced coatings are reported: superhydrophobic coatings and photocatalytic coatings. The first ones have a very low adhesion, so dirty, pollutants and colonies of microorganisms can be easily “washed out” by the water. Photocatalytic coatings are able to oxidize organic matter on their surface, thanks to the combination of light and photocatalyst. The state-of-art of both technologies are discussed with advantages and drawbacks.
Introduction
In the field of restoration and conservation of cultural heritage, the protection of stone materials plays a crucial role. Monuments made of stone, mainly those located outdoors can be heavily exposed to the effects of weathering, moreover, recently an increase in the rate of stone decay is detected, caused by the air pollution as well. For this reason, the understanding of the mechanisms responsible for the material decay is required, as well as the optimization of the stone protection strategies (Mosquera et al., 2002; Zàrraga et al., 2002). Stone decay pathways are driven by intrinsic factors (mineralogical and chemical composition, texture and porous structure), as well as by extrinsic factors (pollutants, humidity, wind, temperature, and biological growths) (Price and Doehne, 2011). The main weathering agent is represented by water. It can transport pollutants through the structure of the stone, causing surface erosion, disintegration and cracking, thanks to wetting-drying or freezing-thawing cycles within the pores. Soluble salts can get in the stone by water transportation, they can dissolute and crystallize inducing pressures and then, they can damage the material, moreover water can induce the hydrolysis of silicates, dissolution of carbonates and color alteration as well (Poli et al., 2004; Manoudis et al., 2007; Kim et al., 2009). Moreover, water make it possible the growth of biological patinas, which can induce further stone decay (Siegesmund et al., 2002). A barrier between the stone and the external agents, especially water, can limit the interaction of water with stone material, this is the general concept of a surface protective coating (Price and Doehne, 2011). The application of polymeric films induces a water repellence effect on the stone surface (Manoudis et al., 2009). Acrylic polymers, siloxanes, fluoropolyethers and fluorinated acrylic polymers and are usually used as hydrophobic coatings (Delgado-Rodriguez, 2001; Rizzarelli et al., 2001; Poli et al., 2004). Acrylic polymers (Paraloid B72 is one of the most popular commercial acrylic polymer) has main drawback represented by their scarce resistance to aging, especially to thermal and photooxidation processes (Lazzari and Chiantore, 2000; Chiantore and Lazzari, 2001). Conversely, siloxanes have a good chemical stability, due to the high strength Si-O bond, low surface tension, good resistance to thermal stress, they are extensively applied on different stone substrates (Lazzarini and Laurenzi Tabasso, 1994). Fluoropolymers used for restoration are chemically similar to polytetrafluoroethene (PTFE, or Teflon) which has hydrophobic and oleophobic properties. The early fluoropolymer coatings had a good potential, but a poor ability to bind to the stone, for this reason it has been developed fluoropolymers containing functional groups (such as phosphates) that can adhere to the stone surface, and then provide a more persistent protection (Aglietto et al., 1993; Piacenti et al., 1993; Gu, 2003). However, those compounds are scarcely used mainly because of its high cost. Recently more resistant fluorinated acrylics have been synthetized (Sabatini et al., 2018a,b). Beside water, biological colonization and air pollution, especially referring to particulate matter, represent a threat to the proper conservation of stone over time. All the above-mentioned products are not completely suitable to protect the stone surface against such degrading agents, in fact, their use could even worse the situation. The susceptibility of synthetic materials toward microorganisms had not been considered, when they have been firstly introduced into the field of conservation. In the last decade it has been shown that synthetic polymers can act as substrates for microorganisms such as bacteria and fungi (Cappitelli et al., 2004; Rinaldi, 2006). Microorganisms, under favorable environmental conditions, form biofilms on synthetic polymer surfaces (Flemming, 1998). For example, Cappitelli and co-workers (Cappitelli et al., 2007) have carried out a study on the Cathedral of Milan, and the shown that the synthetic coatings poly-laurylmethacrylate and poly-isobutylmethacrylate can induce damage to the monument since they represent a favorable organic substrate for biological growth.
Regarding air pollution, their impact on built heritage have been extensively studied in several case studies (La Russa et al., 2013; Barca et al., 2014; Ruffolo et al., 2015; Comite et al., 2017). Black crusts originate from deposition of atmospheric pollutants in rain-sheltered areas of stone buildings (Rodriguez-Navarro and Sebastian, 1996). Their main component is gypsum, which is included a mixture of particulate matter (PM), mineral dust (such as carbonates and clay minerals,) and biogenic particles (such as pollen, bacteria and fungi) (Camuffo et al., 1982; Schiavon et al., 1995). Several investigation of altered building stones, (Ross et al., 1989; Johnson et al., 1990; Zappia et al., 1993) have shown that, beside sulfur, non-carbonate carbon is the main anthropogenic component of atmospheric deposition in gypsum crusts, and is responsible for their typical black color. Aging tests performed on protective coatings against air pollutants on porous limestone have highlighted the decreasing of protective effect over time (Camaiti et al., 2007; Torrisi, 2008).
Nanostructured and Multifunctional Coatings
Particles commonly used in materials science, as well as those naturally present in nature, have dimensions ranging from microns to millimeters. Nanoparticles are characterized by at least one dimension below 100 nm and have generally a large surface area, which induces effect on physico-chemical properties, such as improvement of reactivity and mechanical properties, as well as quantum confinement effects (quantum dot). The making of innovative materials based on nanoparticles and nanostructures provides solutions for industrial, bio-medical and environmental applications.
Several synthetic routes have been developed in order to obtain both crystalline and amorphous particles, low size heterogeneity, high purity and stability of products. However, the production of nanoparticles still has several difficulties due to agglomeration tendency, moreover, most applications require amount of nano-sized powders. In the field of Restoration of cultural heritage, nanoparticles are successfully used. Nanosized calcium hydroxide and silica are currently used for stone consolidation purpose, because of their high reactivity, in addition thanks to their dimensions, the can deeply penetrate into the stone bulk (Baglioni and Giorgi, 2006; Rodriguez-Navarro and Ruiz-Agudo, 2017; Pozo-Antonio et al., 2019). Nanoparticles have been also used and tested to improve the performance of coatings on stone materials, in order to enhance their performance in terms of resistance against water, biological attack and pollution. Based on the features provided by the nanoparticles, these innovative coatings con be grouped in superhydrophobic coatings and photocatalytic coatings. In the first case, a self-cleaning effect is due to the fact that dirty, as well as the early colonies of microorganisms do not adhere on the surface itself and are generally “washed out” by the water. On the contrary, on a photoactive surface organic matter, can adhere on the surface, but they can be easily oxidized by the combination of light and photocatalyst.
Superhydrophobic Coatings
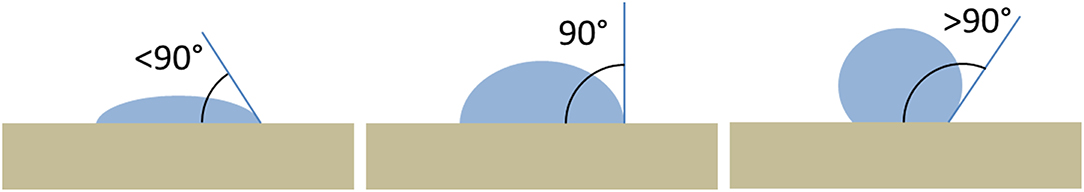
A surface, on the basis of its interaction with water drops, is classified as hydrophilic or hydrophobic. A drop of water tends to spreads on a surface with high surface energy (for example glass) and tends to form a round drop on a surface with a low surface energy (for example Teflon). A surface is hydrophilic if a water drop on it tends to spread on the surface, in this case the contact angle will be <90°. On the contrary, a surface is hydrophobic if a drop of water on it has the tendency to stick to itself more than it spreads to the surface, the contact angle will be >90° (Figure 1). The contact angle ranging from 0° (a water drop that completely wets the surface) to 180° (the drop has no interaction with the surface). Usually stone surfaces treated with a protective coating show an increasing of the contact angle with respect to the bare stone. The roll-off angle is defined as the minimum tilting angle at which a water drop rolls off on a flat surface.
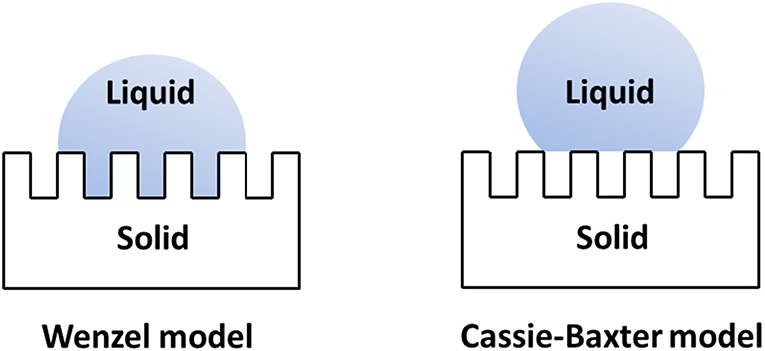
The value of contact angle is function of the liquid's surface tension and the surface's chemistry only if the surface is flat, smooth and homogeneous. On the contrary, on a surface with an increased roughness, the effective contact angle can decrease or increase depending on the chemistry of the surface. If the nature of the latter is hydrophilic, a greater roughness will decrease its contact angle. On the other hand, a surface with a hydrophobic surface chemistry will increase its contact angle as its surface is roughened (D'Urso and Simpson, 2007; Simpson et al., 2015). The increase of contact angle as a result of surface roughness and topography are described by the Wenzel model (Wenzel, 1936) and the Cassie-Baxter model (Cassie and Baxter, 1944) (Figure 2). According to the Wenzel model, a rough surface having a higher surface area than the corresponding smooth surface, the liquid droplet completely penetrates into the cavities on the surface. The apparent contact angle for a liquid droplet is related to the contact angle of the droplet on a smooth surface by the roughness factor r of the surface. The Wenzel model predicts that hydrophilicity and hydrophobicity of a surface depends on the nature of the corresponding surface. For a hydrophilic surface the hydrophilicity increases as the surface roughness increases. On the contrary, for a hydrophobic surface, surface hydrophobicity increases as the surface roughness increases. The Cassie-Baxter model does not assume a complete penetration of a liquid droplet into the surface cavities. This model suggests that the spreading of a liquid droplet on a rough surface destroys the solid–vapor interface and forms solid–liquid and liquid–vapor interfaces, causing the significant reduction in roll-off angle and contact angle hysteresis.
The micrometer-scale and nanometer-scale roughness, along with a low surface energy material can lead to contact angles >150°, a low roll off angle and the self-cleaning effect (D'Urso and Simpson, 2007). Surfaces with these properties are called “superhydrophobic” (Neinhuis and Barthlott, 1997; Gao and Jiang, 2004; Huang et al., 2006; Zheng et al., 2007; Liu et al., 2012). They are a very large ways to obtain rough surfaces which exhibit superhydrophobicity features (Onda et al., 1996; Nakajima et al., 2001; Feng et al., 2002; Martines et al., 2005). Such coatings in order to be suitable for their use on built heritage, have to induce low variations of color of coated surface (Cerimele and Cossu, 2007), do not alter significantly the breathability and the porosity of stone Pia et al., 2014.
Manoudis et al. (2009) obtained one of the first superhydrophobic coatings for stone materials following a very simple method. SiO2 nanoparticles were added to a commercial siloxane polymer (Rhodorsil 224), commonly used for restoration of stone material. This formulation has been applied on three types of sandstone with different porosity (ranging from 7 to 11 %). It was found that superhydrophobicity was achieved at concentration of silica nPs above 0.5% w/v. The type of the investigated substrate did not influence the wettability, moreover, nanoparticles induced superhydrophobicity, but they did not have any obvious effect on the water vapor permeability tests. The main drawback of this coating is the colorimetric variation induced on the treated surface, which is found up to ΔE~12. Karapanagiotis and coworker (Karapanagiotis et al., 2012) added hydrophilic alumina nanoparticles to a siloxane polymer. Those films had shown small contact angle hysteresis (5°), which was independent of the particle size. The superhydrophobic state were obtained in siloxane films by using 5–50 nm nanoparticles. However, the particle specific surface area of the particles affects dramatically the minimum critical particle concentration, which must be used in the dispersions to induce superhydrophobicity on the surfaces, since the specific surface area is related to the particle size, and therefore to the feature of the roughness induced on the surface. De Ferri et al. (2011) added different amounts of surface modified silica nanoparticles to TEOS (tetra-ethyl-orthosilicate) and they applied the product on several stones (limestone and sandstone having a low porosity, granite). TEOS is not a waterproof product, in this case the hydrophobicity has been provided by the surface modification of silica nanoparticle with 1,1,1-Trimethyl-N-(trimethylsilyl)silanamine. Static contact angle measurements of treated surfaces showed values up to 148°, although a general decrease of water repellency over time is detected. Aslanidou et al. (2018) added silica nanoparticles to an aqueous dispersion that contained alkoxy silanes, organic fluoropolymer. Such formulation, once sprayed, provided superhydrophobic, water repellent, superoleophobic and oil repellent properties to marble and sandstone. The composite coatings slightly reduced the breathability of stone substrate, and did not induce any significant chromatic effect. Cappelletti et al. (2015) has proposed a novel procedure to obtain a superhydrophobic nanostructured coating containing a siloxane polymer and no-crystalline TiO2 nanoparticles, suitable for stone protection. For this purpose three different stone materials have been considered (Angera stone, Carrara marble, and Botticino limestone). Authors had used an organic precursor of TiO2 (Ti(OC3H7)4), they added this compound to the polymer water suspension. The nanoparticles have been obtained thanks to a sol-gel process, and then the mixture has been applied on stone. The superhydrophobic state have been observed for Carrara marble, although high contact angle values (138° < θ <141°) have been detected for Angera and Botticino stone. In addition, coatings were able to reduce salts formation. This result can be ascribed to the higher hydrophobicity, which leads to a decrease of water sorption. Authors explored the behavior of such coatings against accelerated aging as well; results suggested a good stability of the product. Similar procedure has been applied for the protection of mortars (Pino et al., 2017). Organically modified silica (ORMOSIL) gels were produced and used to coat several substrates including stone (Karapanagiotis et al., 2014). Although not any nanoparticles have been used, superhydrophobicity and water repellence were induced on the surfaces of all the treated materials, as high static (>165°) and low tilt (<4°) contact angles were achieved. The treated surfaces were studied using scanning electron microscopy, which revealed the formation of a micro/nano-structured topography.
Photocatalytic Coatings
Photocatalysis is defined as the acceleration of a chemical reaction by direct irradiation or by the irradiation of a catalyst that leads to a lower activation energy of the reaction itself. Photocatalysis is a light-driven redox reaction that often requires solid-state catalysts. Efficient and cost-effective photocatalysis is obtained using semiconductors having wide bandgaps.
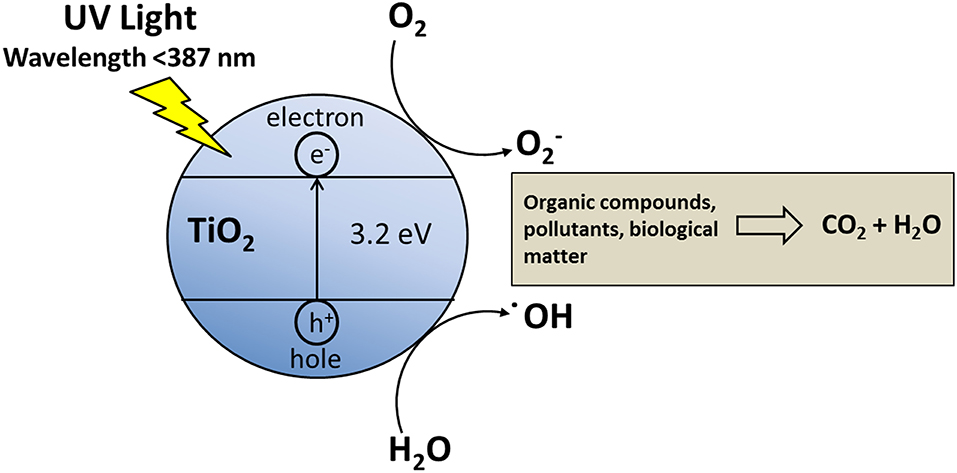
As a photocatalyst is exposed to UV, holes (h+) and excited electrons (e–) are generated. The holes are able to oxidize water or hydroxide anions into hydroxyl radicals (–OH) (Wang et al., 1998), which are able to degrade many organic compounds. The competition between hole-electron recombination, and the transfer of those to the organic compound, determines the photocatalytic efficiency (Figure 3).
Titanium dioxide (TiO2) has an energy band gap of 3.2 eV (λ < 400 nm), a chemical stability over a wide pH range and in a large number of solvents, for these reasons it has been chosen as photocatalysts for many applications. TiO2 has three main crystallographic forms (anatase, brookite and rutile) (Yonezawa et al., 1999; Yu et al., 2000; Diebold, 2003), anatase is the most photocatalytically active form. TiO2 can be deposited as thin films on glasses, Si wafers and stainless steel. The TiO2 thin films have found applications in photocatalysis (Fujishima et al., 2000; Yu et al., 2000), protective anti-reflection coatings, solar cells (O'Regan and Grätzel, 1991; Argazzi et al., 1997; Zaban et al., 1998), lithium batteries (Kavan et al., 1995), sensors (Cosnier et al., 1999) and building materials (Chen and Poon, 2009). Organic matter, such as particulate matter, as well as biological systems, can be oxidized by the oxygen, but this does not occurs easily, because of the activation energy, which can be lowered by photocatalyst.
Since the photocatalytic effect occurs on the surface, the increasing of the surface of a photocatalysts will lead to a enhancing of the photocatalytic itself. For example, one-gram single crystal of anatase has a surface area of about 2 cm2, if the same gram is divided into nanoparticles having an average size of 25 nm, the surface area will be about 300 m2. Based on this fact, several authors had started to use photocatalysts alone (Quagliarini et al., 2018), as well as added to coatings for stone materials (Scalarone et al., 2012; Pinho et al., 2013; Munafò et al., 2015; La Russa et al., 2016) in order to provide a self-cleaning and biocidal effect to the stone. For example, La Russa et al (La Russa et al., 2012) tested the photocatalytic, biocidal and hydrophobic properties of a water dispersion of acrylic polymer and titania nanoparticles, applied on limestone and marble. It has been shown a great growth inhibition efficiency against A. Niger microorganism on both lithotypes. This is due to the photo-oxidative stress induced by UV light and TiO2 (Vileno et al., 2007). The rate of methylene blue oxidation has been enhanced by photodegradation features induced by TiO2.
The color alteration the treated surface is an important issue related to the stone restoration. An excess of titania can lead to a whitening of the stone. Crupi et al. (2018) balanced the photocatalytic effect and the color variation, and they have established that about 24 g/m2 of titania on the Modica limestone (Sicily, Italy), is considered as the optimal amount of such material to assure a good performance together with a low chromatic impact. Of course, the validity of such number is limited to this lithotype or to similar ones.
An alternative process to obtain a TiO2 containing coating is to use the organic precursors of titanium oxide, instead of the ready to use nanoparticles (Kapridaki et al., 2014; Bergamonti et al., 2015; Alfieri et al., 2017). This procedure shows the advantage to avoid the dispersion procedure of nanoparticles into the binder, since nanoparticles are produced ex novo, it is possible to control the size of the particles and most of the formulated coatings are almost transparent as well.
The medium-term inhibition of microbial colonization has been studied as well in situ in the archaological site of Ercolano (Italy) (Ruffolo et al., 2017a). This study has shown that four months after the application of coatings containing TiO2, a inhibition of the recolonization has been observed. Colangiuli et al. (2019) added titania nanoparticles to a fluorinated polymer and assessed in situ the self-cleaning and photocatalytic features of such coating over time. The coated stone after natural aging is still protected, although a decrease of the overall performance was detected. The main issue related to the adding photoactive nanoparticles to an organic coating is related to the photodegradation of the coating itself. For this reason the use of organic binder should be substituted, or at least mixed with inorganic ones; the latter, of course, cannot induce a hydrophobic effect to the coating.
Crystalline titanium dioxide is the most used photocatalyst, but also other metal oxide nanoparticles were used, such as ZnO, ZnTiO3, and CuO (Ruffolo et al., 2010; Zarzuela et al., 2018; Aldosari et al., 2019) with similar results in terms of self-cleaning and biocidal efficacy. Other experiments have been carried out by doping TiO2 with elements such as silver, copper, gold and nitrogen (Ruffolo et al., 2013; La Russa et al., 2014; Banerjee et al., 2015; Bergamonti et al., 2017). Doping process consists in the introducing in the bulk of the nanoparticles, and therefore into the crystalline structure, a small amount of a metal (dopant). In some cases the metal does not goes into the crystal lattice, but it lies on the nanoparticle surface. This process can generate a variation of the band gap, and then a variation of the photoactivity of the metal oxide. Some doping process, in particular those with silver, enhanced of photocatalytic, and especially the biocidal effect, this is also due to the antimicrobial feature of the silver itself. The main drawback of the doping is related to the color variation, a widening of the energy gap could lead to an absorption into the visible region of the electromagnetic radiation, so a coating containing a doped TiO2 could provide a strong and unacceptable chromatic variation to the treated surface. Another interesting application of photocatalysts is represented by their use in underwater environment (Ruffolo et al., 2013). Stone exposed to marine underwater environment suffers the bio-colonization of underwater species, which are often related to the stone degradation (Aloise et al., 2014). The use of a photocatalyst based coating would be able to stop or slowdown such colonization (antifouling effect). The main issue related to the underwater application is the absorption of UV light by the water. However for low depths, the ultraviolet radiation which can be transmitted seems to be enough to trigger the photocatalysis leading to an antifouling effect (Ruffolo et al., 2017b), since underwater coated stones show a significant reduction of biological colonization, especially in terms of endolithic species, which is the most dangerous for the integrity of the stone.
Conclusions and Future Perspectives
In this review it has been described the use of nanoparticles to improve the performance of coatings for the protection of stone materials. In scientific literature, two well-defined features of nanostructured are reported: superhydrophobic and photocatalytic effect. In both cases, a general improvement of the protection of the stone is stated. Such coatings were successfully formulated and tested on several lithotypes. Although they are already used in other field, the use of nanostructured coatings in the field of conservation of cultural heritage is quite limited. This occurs because a long-term experimentation of the behavior of those materials is not available yet. This data is fundamental to let the stakeholders to accept the suitability of nanostructured coatings for the protection of built heritage, which has to be preserved for as long time as possible. Another issue is related to human health, the awareness of health and environmental issues related to nanoparticle exposure is rising, for this reasons, the impact of such materials has to be explored and balanced with the protective performance induced to the stone.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aglietto, M., Passaglia, E., Taburoni, E., Ciardelli, F., Botteghi, C., Matteoli, U., et al. (1993). “A new class of fluorinated acrylic polymers: protective materials for stone,” in 10th Triennial Meeting, ICOM Committee for Conservation, Washington, DC, 22–27 August 1993: Preprints, ed J. Bridgland (Paris: ICOM Committee for Conservation; Lawrence, KS: Allen Press, 553–58.
Aldosari, M. A., Darwish, S. S., Adam, M. A., Elmarzugi, N. A., and Ahmed, S. M. (2019). Using ZnO nanoparticles in fungal inhibition and self-protection of exposed marble columns in historic sites. Archaeol. Anthropol. Sci. 11, 3407–3422. doi: 10.1007/s12520-018-0762-z
Alfieri, I., Lorenzi, A., Ranzenigo, L., Lazzarini, L., Predieri, G., and Lottici, P. P. (2017). Synthesis and characterization of photocatalytic hydrophobic hybrid TiO2-SiO2 coatings for building applications. Build. Environ. 111, 72–79. doi: 10.1016/j.buildenv.2016.10.019
Aloise, P., Ricca, M., La Russa, M. F., Ruffolo, S. A., Belfiore, C. M., Padeletti, G., et al. (2014). Diagnostic analysis of stone materials from underwater excavations: the case study of the Roman archaeological site of Baia (Naples, Italy). Appl. Phys. A Mater. Sci. Proc. 114, 655–662. doi: 10.1007/s00339-013-7890-1
Argazzi, R., Bignozzi, C. A., Heimer, T. A., Castellano, F. N., and Meyer, G. J. (1997). Light-induced charge separation across Ru(II)-modified nanocrystalline TiO2 interfaces with phenothiazine donors. J. Phys. Chem. 101, 2591–2597. doi: 10.1021/jp9619393
Aslanidou, D., Karapanagiotis, I., and Lampakis, D. (2018). Waterborne superhydrophobic and superoleophobic coatings for the protection of marble and sandstone. Materials 11:585. doi: 10.3390/ma11040585
Baglioni, P., and Giorgi, R. (2006). Soft and hard nanomaterials for restoration and conservation of cultural heritage. Soft Matter 2, 293–303. doi: 10.1039/b516442g
Banerjee, S., Dionysiou, D. D., and Pillai, S. C. (2015). Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catalysis B Environ. 176–177, 396–428.
Barca, D., Comite, V., Belfiore, C. M., Bonazza, A., La Russa, M. F., Ruffolo, S. A., et al. (2014). Impact of air pollution in deterioration of carbonate building materials in Italian urban environments. Appl. Geochem. 48, 122–131. doi: 10.1016/j.apgeochem.2014.07.002
Bergamonti, L., Alfieri, I., Lorenzi, A., Predieri, G., Barone, G., Gemelli, G., et al. (2015). Nanocrystalline TiO2 coatings by sol–gel: photocatalytic activity on Pietra di Noto biocalcarenite. J. Sol Gel Sci. Technol. 75, 141–151. doi: 10.1007/s10971-015-3684-6
Bergamonti, L., Predieri, G., Paz, Y., Fornasini, L., Lottici, P. P., and Bondioli, F. (2017). Enhanced self-cleaning properties of N-doped TiO2 coating for Cultural Heritage. Microchem. J. 133, 1–12. doi: 10.1016/j.microc.2017.03.003
Camaiti, M., Bugani, S., Bernardi, E., Morselli, L., and Matteini, M. (2007). Effects of atmospheric NOx on biocalcarenite coated with different conservation products. Appl. Geochem. 22, 1248–1254. doi: 10.1016/j.apgeochem.2007.03.035
Camuffo, D., Del Monte, M., Sabbioni, C., and Vittori, O. (1982). Wetting, deterioration and visual features of stone surfaces in an urban area. Atmos. Environ. 16 2253–2259. doi: 10.1016/0004-6981(82)90296-7
Cappelletti, G., Fermo, P., and Camiloni, M. (2015). Smart hybrid coatings for natural stones conservation. Progr. Organ. Coat. 78, 511–516. doi: 10.1016/j.porgcoat.2014.05.029
Cappitelli, F., Principi, P., Pedrazzani, R., Toniolo, L., and Sorlini, C. (2007). Bacterial and fungal deterioration of the Milan Cathedral marble treated with protective synthetic resins. Sci. Total Environ. 385, 172–181. doi: 10.1016/j.scitotenv.2007.06.022
Cappitelli, F., Zanardini, E., and Sorlini, C. (2004). The biodeterioration of synthetic resins used in conservation. Macromol. Biosci. 4, 399–406. doi: 10.1002/mabi.200300055
Cassie, A. B. D., and Baxter, S. (1944). Wettability of porous surfaces. Transact. Faraday Soc. 40, 546–551. doi: 10.1039/tf9444000546
Cerimele, M. M., and Cossu, R. (2007). Decay regions segmentation from color images of ancient monuments using fast marching method. J. Cult. Herit. 8, 170–175. doi: 10.1016/j.culher.2007.01.006
Chen, J., and Poon, C. S. (2009). Photocatalytic construction and building materials: from fundamentals to applications. Build. Environ. 44, 1899–1906. doi: 10.1016/j.buildenv.2009.01.002
Chiantore, O., and Lazzari, M. (2001). Photo-oxidative stability of paraloid acrylic protective polymers. Polymer 42, 17–27. doi: 10.1016/S0032-3861(00)00327-X
Colangiuli, D., Lettieri, M., Masieri, M., and Calia, A. (2019). Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total Environ. 650, 2919–2930. doi: 10.1016/j.scitotenv.2018.10.044
Comite, V., Álvarez de Buergo, M., Barca, D., Belfiore, C. M., Bonazza, A., La Russa, M. F., et al. (2017). Damage monitoring on carbonate stones: field exposure tests contributing to pollution impact evaluation in two Italian sites. Constr. Build. Mater. 152, 907–922. doi: 10.1016/j.conbuildmat.2017.07.048
Cosnier, S., Senillou, A., Gratzel, M., Comte, P., Vlachopoulos, N., Renault, N. J., et al. (1999). Glucose biosensor based on enzyme entrapment within polypyrrole films electrodeposited on mesoporous titanium dioxide. Electroanal. Chem. 469, 176–181 doi: 10.1016/S0022-0728(99)00223-5
Crupi, V., Fazio, B., Gessini, A., Kis, Z., La Russa, M. F., Majolino, D., et al. (2018). TiO2-SiO2-PDMS nanocomposite coating with self-cleaning effect for stone material: finding the optimal amount of TiO2. Construct. Build. Mater. 166, 464–471. doi: 10.1016/j.conbuildmat.2018.01.172
De Ferri, L., Lottici, P. P., Lorenzi, A., Montenero, A., and Salvioli-Mariani, E. (2011). Study of silica nanoparticles – polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 12, 356–363. doi: 10.1016/j.culher.2011.02.006
Delgado-Rodriguez, J. (2001). “Consolidation of decayed stone. A delicate problem with few practical solutions, Historical constructions,” in Proceedings of International Seminar on Historical Constructions (Guimarães).
Diebold, U. (2003). The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229. doi: 10.1016/S0167-5729(02)00100-0
D'Urso, B., and Simpson, J. T. (2007). Emergence of superhydrophobic behavior on vertically aligned nanocone arrays. Appl. Phys. Lett. 90:044102. doi: 10.1063/1.2433039
Feng, L., Li, S., Li, S., Zhai, J., Song, Y., Jiang, L., et al. (2002). Super-hydrophobic surface of aligned polyacrylonitrile nanofibers. Angew Chem Int Ed. 41, 1221–1223. doi: 10.1002/1521-3773(20020402)41:7<1221::AID-ANIE1221>3.0.CO;2-G
Flemming, H.-C. (1998). Relevance of biofilms for the biodeterioration of surfaces of polymeric materials. Polym. Degrad. Stab. 59, 309–315. doi: 10.1016/S0141-3910(97)00189-4
Fujishima, A., Rao, T. N., and Tryk, D. A. (2000). Titanium dioxide photocatalysis. J. Photochem. Photobiol., C Photochem. 1, 1–21. doi: 10.1016/S1389-5567(00)00002-2
Gao, X., and Jiang, L. (2004). Water-repellent legs of water striders. Nature 432:36. doi: 10.1038/432036a
Gu, J.-D. (2003). Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int. Biodeterior. Biodegrad. 52, 69–91. doi: 10.1016/S0964-8305(02)00177-4
Huang, J., Wang, X., and Wang, Z. L. (2006). Controlled replication of butterfly wings for achieving tunable photonic properties. Nano Lett. 6, 2325–2331. doi: 10.1021/nl061851t
Johnson, J. B., Haneef, S. J., Hepburn, B. J., Hutchinson, A. J., Thompson, G. E., and Wood, G. C. (1990). Laboratory exposure systems to simulate atmospheric degradation of building stone under dry and wet deposition conditions. Atmos. Environ. 24, 2585–2592. doi: 10.1016/0960-1686(90)90136-B
Kapridaki, C., Pinho, L., Mosquera, M. J., and Maravelaki-Kalaitzaki, P. (2014). Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catalysis B Environ. 156 416–427. doi: 10.1016/j.apcatb.2014.03.042
Karapanagiotis, I., Manoudis, P. N., Savva, A., and Panayiotou, C. (2012). Superhydrophobic polymer-particle composite films produced using various particle sizes. Surf. Interf. Anal. 44, 870–875. doi: 10.1002/sia.4930
Karapanagiotis, I., Pavlou, A., Manoudis, P. N., and Aifantis, K. E. (2014). Water repellent ORMOSIL films for the protection of stone and other materials. Mater. Lett. 131, 276–279. doi: 10.1016/j.matlet.2014.05.163
Kavan, L., Kratochilova, K., and Gratzel, M. (1995). Study of nanocrystalline TiO2 (anatase) electrode in the accumulation regime. J. Electroanal. Chem. 394, 93–102. doi: 10.1016/0022-0728(95)03976-N
Kim, E. K., Won, J., Do, J., Kim, S. D., and Kang, Y. S. (2009). Effects of silica nanoparticle and GPTMS addition on TEOS-based stone consolidants. J. Cult. Heritage. 10, 214–221. doi: 10.1016/j.culher.2008.07.008
La Russa, M. F., Belfiore, C. M., Comite, V., Barca, D., Bonazza, A., Ruffolo, S. A., et al. (2013). Geochemical study of black crusts as a diagnostic tool in cultural heritage. Appl. Phys. A Mater. 113, 1151–1162. doi: 10.1007/s00339-013-7912-z
La Russa, M. F., Macchia, A., Ruffolo, S. A., De Leo, F., Barberio, M., Barone, P., et al. (2014). Testing the antibacterial activity of doped TiO2 for preventing biodeterioration of cultural heritage building materials. Int. Biodeteriorat. Biodegrad. 96, 87–96. doi: 10.1016/j.ibiod.2014.10.002
La Russa, M. F., Rovella, N., Alvarez De Buergo, M., Belfiore, C. M., Pezzino, A., Crisci, G. M., et al. (2016). Nano-TiO2 coatings for cultural heritage protection: the role of the binder on hydrophobic and self-cleaning efficacy. Prog. Organ. Coat. 91, 1–8. doi: 10.1016/j.porgcoat.2015.11.011
La Russa, M. F., Ruffolo, S. A., Rovella, N., Belfiore, C. M., Palermo, A. M., Guzzi, M. T., et al. (2012). Multifunctional TiO2 coatings for Cultural Heritage. Prog. Organ. Coat. 74, 186–191. doi: 10.1016/j.porgcoat.2011.12.008
Lazzari, M., and Chiantore, O. (2000). Thermal-ageing of paraloid acrylic protective polymers. Polymer 41, 6447–6455. doi: 10.1016/S0032-3861(99)00877-0
Liu, X., Liang, Y., Zhou, F., and Liu, W. (2012). Extreme wettability and tunable adhesion: biomimicking beyond nature? Soft Matter. 8, 2070–2086. doi: 10.1039/C1SM07003G
Manoudis, P. N., Karapanagiotis, I., Tsakalof, A., Zuburtikudis, I., Kolinkeovà, B., and Panayiotou, C. (2009). Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A. 97, 351–360. doi: 10.1007/s00339-009-5233-z
Manoudis, P. N., Papadopoulou, S., Karapanagiotis, I., Tsakalof, A., Zuburtikudis, I., and Panayiotou, C. (2007). Polymer-Silica nanoparticles composite films as protective coatings for stone-based monuments. J. Physics Confer. Ser. 61, 1361–1365. doi: 10.1088/1742-6596/61/1/269
Martines, E., Seunarine, K., Morgan, H., Gadegaard, N., Wilkinson, C. D. W., and Riehle, M. O. (2005). Superhydrophobicity and superhydrophilicity of regular nanopatterns. Nano Lett. 5 2097–2103. doi: 10.1021/nl051435t
Mosquera, M. J., Pozo, J., Esquivas, L., Rivas, T., and Silva, B. (2002). Application of mercury porosimetry to the study of xerogels used as stone consolidants. J. Non Cryst Solids 311, 185–194. doi: 10.1016/S0022-3093(02)01370-4
Munafò, P., Goffredo, G. B., and Quagliarini, E. (2015). TiO2-based nanocoatings for preserving architectural stone surfaces: an overview. Constr. Build. Mater. 84, 201–218. doi: 10.1016/j.conbuildmat.2015.02.083
Nakajima, A., Hashimoto, K., and Watanabe, T. (2001). Recent studies on super-hydrophobic films. Monatsh Chem. 132, 31–41. doi: 10.1007/s007060170142
Neinhuis, C., and Barthlott, W. (1997). Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Botan. 79, 667–677. doi: 10.1006/anbo.1997.0400
Onda, T., Shibuichi, S., Satoh, N., and Tsujii, K. (1996). Super-water-repellent fractal surfaces. Langmuir 12, 2125–2127. doi: 10.1021/la950418o
O'Regan, B., and Grätzel, M. (1991). A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740. doi: 10.1038/353737a0
Pia, G., Sassoni, E., Franzoni, E., and Sanna, U. (2014). Predicting capillary absorption of porous stones by a procedure based on anintermingled fractal units model. Int. J. Eng. Sci. 82, 196–204. doi: 10.1016/j.ijengsci.2014.05.013
Piacenti, F., Camaiti, M., Manganelli del, F.à, C., and Scala, A. (1993). “Fluorinated aggregating materials for stone,” in Conservation of Stone and Other Materials: Proceedings of the International RILEM/UNESCO Congress “Conservation of Stone and Other Materials: Research—Industry—Media,” held at UNESCO Headquarters, Paris, June 29–July 1, 1993, ed. M. J. Thiel (London; New York, NY: E and FN Spon; RILEM Proceedings 21, 740–47.
Pinho, L., Elhaddad, F., Facio, D. S., and Mosquera, M. J. (2013). A novel TiO2-SiO2 nanocomposite converts a very friable stone into a self-cleaning building material. Appl. Surf. Sci. 275, 389–396. doi: 10.1016/j.apsusc.2012.10.142
Pino, F., Fermo, P., La Russa, M., Ruffolo, S., Comite, V., Baghdachi, J., et al. (2017). Advanced mortar coatings for cultural heritage protection. Durability towards prolonged UV and outdoor exposure. Environ. Sci. Pollut. Res. 24, 12608–12617. doi: 10.1007/s11356-016-7611-3
Poli, T., Toniolo, L., and Chiantore, O. (2004). The protection of different Italian marbles with two partially fluorinated acrylic copolymers. Appl. Phys. A. 79, 347–351. doi: 10.1007/s00339-004-2530-4
Pozo-Antonio, J. S., Otero, J., Alonso, P., and Mas i Barberà, X. (2019). Nanolime- and nanosilica-based consolidants applied on heated granite and limestone: effectiveness and durability. Construct. Build. Mater. 201, 852–870. doi: 10.1016/j.conbuildmat.2018.12.213
Price, C. A., and Doehne, E. (2011). Stone Conservation: An Overview of Current Research. Los Angeles: Getty Conservation Institute.
Quagliarini, E., Graziani, L., Diso, D., Licciulli, A., and D'Orazio, M. (2018). Is nano-TiO2 alone an effective strategy for the maintenance of stones in Cultural Heritage? J. Cult. Herit. 30, 81–91. doi: 10.1016/j.culher.2017.09.016
Rizzarelli, P., La Rosa, C., and Torrisi, A. (2001). Testing a fluorinated compound as a protective material for calcarenite. J. Cult. Heritage. 2, 55–62. doi: 10.1016/S1296-2074(01)01109-8
Rodriguez-Navarro, C., and Ruiz-Agudo, E. (2017). Nanolimes: from synthesis to application. Pure Appl. Chem. 90, 523–550. doi: 10.1515/pac-2017-0506
Rodriguez-Navarro, C., and Sebastian, E. (1996). Role of particulate matter from vehicle exhaust on porous building stones (limestone) sulfation. Sci. Total Environ. 187 79–91. doi: 10.1016/0048-9697(96)05124-8
Ross, M., McGee, E. S., and Ross, D. R. (1989). Chemical and mineralogical effects of acid deposition on Shelburne marble and Salem limestone test samples placed at four NAPAP weather-monitoring sites. Am. Mineral. 74, 367–383.
Ruffolo, S. A., Comite, V., La Russa, M. F., Belfiore, C. M., Barca, D., Bonazza, A., et al. (2015). An analysis of the black crusts from the Seville Cathedral: a challenge to deepen the understanding of the relationships among microstructure, microchemical features and pollution sources. Sci. Total Environ. 502, 157–166. doi: 10.1016/j.scitotenv.2014.09.023
Ruffolo, S. A., De Leo, F., Ricca, M., Arcudi, A., Silvestri, C., Bruno, L., et al. (2017a). Medium-term in situ experiment by using organic biocides and titanium dioxide for the mitigation of microbial colonization on stone surfaces. Int. Biodeterior. Biodegradat. 123, 17–26. doi: 10.1016/j.ibiod.2017.05.016
Ruffolo, S. A., La Russa, M. F., Malagodi, M., Oliviero Rossi, C., Palermo, A. M., and Crisci, G. M. (2010). ZnO and ZnTiO3 nanopowders for antimicrobial stone coating. Appl. Phys. A Mater. Sci. Proc. 100, 829–834. doi: 10.1007/s00339-010-5658-4
Ruffolo, S. A., Macchia, A., La Russa, M. F., Mazza, L., Urzì, C., De Leo, F., et al. (2013). Marine antifouling for underwater archaeological sites: TiO2 and Ag-Doped TiO2. Int. J. Photoenergy 2013:251647. doi: 10.1155/2013/251647
Ruffolo, S. A., Ricca, M., Macchia, A., and La Russa, M. F. (2017b). Antifouling coatings for underwater archaeological stone materials. Progr. Organ. Coat. 104, 64–71. doi: 10.1016/j.porgcoat.2016.12.004
Sabatini, V., Cattò, C., Cappelletti, G., Cappitelli, F., Antenucci, S., Farina, H., et al. (2018a). Protective features, durability and biodegration study of acrylic and methacrylic fluorinated polymer coatings for marble protection. Progr. Organ. Coat. 114, 47–57, doi: 10.1016/j.porgcoat.2017.10.003
Sabatini, V., Farina, H., Montarsolo, A., Pargoletti, E., Ortenzi, M. A., and Cappelletti, G. (2018b). Fluorinated polyacrylic resins for the protection of cultural heritages: the effect of fluorine on hydrophobic properties and photochemical stability. Chem. Lett. 47, 280–283. doi: 10.1246/cl.171020
Scalarone, D., Lazzari, M., and Chiantore, O. (2012). Acrylic protective coatings modified with titanium dioxide nanoparticles: comparative study of stability under irradiation. Polym. Degrad. Stab. 97, 2136–2142. doi: 10.1016/j.polymdegradstab.2012.08.014
Schiavon, N., Chiavari, G., Schiavon, G., and Fabbri, D. (1995). Nature and decay effects of urban soiling on granitic building stones. Sci. Total Environ. 167 87–101. doi: 10.1016/0048-9697(95)04572-I
Siegesmund, S., Weiss, T., and Vollbrecht, A. (2002). Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies: Introduction Geological Society. London: Special Publications. doi: 10.1144/GSL.SP.2002.205.01.01
Simpson, J. T., Hunter, S. R., and Aytug, T. (2015). Superhydrophobic materials and coatings: a review. Rep. Progr. Phys. 78:086501. doi: 10.1088/0034-4885/78/8/086501
Torrisi, A. (2008). XPS study of five fluorinated compounds deposited on calcarenite stone, Part II: aged samples. Appl. Surf. Sci. 254, 7127–7136. doi: 10.1016/j.apsusc.2008.05.226
Vileno, B., Lekka, M., Sienkiewicz, A., Jeney, S., Stoessel, G., Lekki, J., et al. (2007). Stiffness alterations of single cells induced by UV in the presence of NanoTiO2. Environ. Sci. Technol. 41, 5149–5153. doi: 10.1021/es0629561
Wang, R., Hashimoto, K., Fujishima, A., Chikuni, M., Kojima, E., Kitamura, A., et al. (1998). Photogeneration of highly amphiphilic TiO2 surfaces. Adv. Mater. 10, 135–138. doi: 10.1002/(SICI)1521-4095(199801)10:2<135::AID-ADMA135>3.0.CO;2-M
Wenzel, R. N. (1936). Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 28, 988–994. doi: 10.1021/ie50320a024
Yonezawa, T., Matsune, H., and Kunitake, T. (1999). Layered nanocomposite of close-packed gold nanoparticles and TiO2 gel layers. Chem. Mater. 11, 33–35. doi: 10.1021/cm980687a
Yu, J., Zhao, X., Du, J., and Chen, W. (2000). Preparation, microstructure and photocatalytic activity of the porous TiO2 anatase coating by Sol-Gel processing. J. Sol–Gel Sci. Technol. 17, 163–171. doi: 10.1023/A:1008703719929
Zaban, A., Micic, O. I., Gregg, B. A., and Nozik, A. J. (1998). Photosensitization of nanoporous TiO2 electrodes with InP quantum dots. Langmuir 14, 3153–3156. doi: 10.1021/la9713863
Zappia, G., Sabbioni, C., and Gobbi, G. (1993). Non-carbonate carbon content on black and white areas of damaged stone monuments. Atmos. Environ. 27, 1117–1121. doi: 10.1016/0960-1686(93)90146-P
Zàrraga, R., Alvarez-Gasca, D. E., and Cervantes, J. (2002). Solvent effect on TEOS film formation in the sandstone consolidation process. Silicon Chem. 1, 397–402. doi: 10.1023/B:SILC.0000025602.64965.e7
Zarzuela, R., Moreno-Garrido, I., Blasco, J., Gil, M. L. A., and Mosquera, M. J. (2018). Evaluation of the effectiveness of CuONPs/SiO2-based treatments for building stones against the growth of phototrophic microorganisms. Construct. Build. Mater. 187, 501–509. doi: 10.1016/j.conbuildmat.2018.07.116
Keywords: stone protection, nanostructured coatings, nanoparticles, cultural heritage conservation, superhydrophobic coatings, photocatalytic coatings
Citation: Ruffolo SA and La Russa MF (2019) Nanostructured Coatings for Stone Protection: An Overview. Front. Mater. 6:147. doi: 10.3389/fmats.2019.00147
Received: 29 April 2019; Accepted: 11 June 2019;
Published: 02 July 2019.
Edited by:
P. Davide Cozzoli, University of Salento, ItalyReviewed by:
Costas Panayiotou, Aristotle University of Thessaloniki, GreeceValentina Sabatini, University of Milan, Italy
Jian Li, Northwest Normal University, China
Copyright © 2019 Ruffolo and La Russa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvestro Antonio Ruffolo, c2lsdmVzdHJvLnJ1ZmZvbG9AdW5pY2FsLml0
 Silvestro Antonio Ruffolo
Silvestro Antonio Ruffolo Mauro Francesco La Russa
Mauro Francesco La Russa