- Centro de Estudos Florestais, Instituto Superior de Agronomia, Universidade de Lisboa, Lisboa, Portugal
Tree barks are among the less studied forest products notwithstanding their relevant physiological and protective role in tree functioning. The large diversity in structure and chemical composition of barks makes them a particularly interesting potential source of chemicals and bioproducts, at present valued in the context of biorefineries. One of the valuable components of barks is cork (phellem in anatomy) due to a rather unique set of properties and composition. Cork from the cork oak (Quercus suber) has been extensively studied, mostly because of its economic importance and worldwide utilization of cork products. However, several other species have barks with substantial cork amounts that may constitute additional resources for cork-based bioproducts. This paper makes a review of the tree species that have barks with significant proportion of cork and on the available information regarding the structural and chemical characterization of their bark. A general integrative appraisal of the formation and types of barks and of cork development is also given. The knowledge gaps and the potential interesting research lines are identified and discussed, as well as the utilization perspectives.
Introduction
Trees are externally covered on their stems and branches by the bark that represents 9 to 15% of the stem volume (Harkin and Rowe, 1971). The bark is composed of several types of tissues and cells with different functions: translocation and storage of organic materials, water storage, wound healing, protection from herbivores, pathogens, and environmental factors (e.g., irradiation, desiccation, wind, flooding, hail, snow, fire), and photosynthesis in shoots (Lev-Yadun, 2011). Barks are very variable in thickness, color, and texture depending on species, age, and growing conditions, among other factors. The bark often gives a species its characteristic appearance and may be used for taxonomic purposes.
The radial growth of woody plants results from the activity of two meristems: the vascular cambium, which gives rise to the xylem (wood) and to the secondary phloem, and the cork cambium or phellogen that produces the phelloderm and phellem (cork), which together constitute the system named periderm. Bark can be defined as all the tissues formed to the outside of the vascular cambium, therefore including the phloem and the periderm (Trockenbrodt, 1990; Junikka, 1994; Richter et al., 1996; Evert, 2006). A schematic diagram of a tree stem cross section is given in Figure 1A.
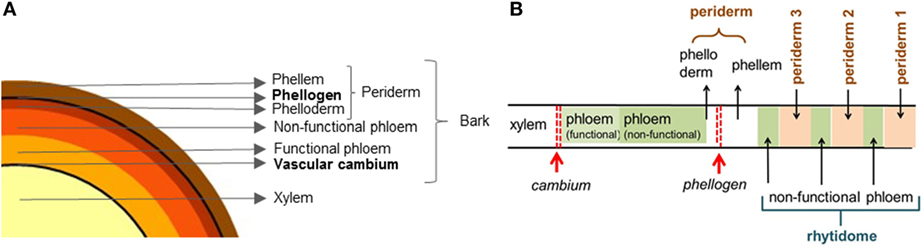
Figure 1. Schematic drawing of a cross section of a tree stem showing (A) the xylem (wood), the phloem (functional and non-functional), and the periderm and (B) the xylem (wood), the phloem (functional and non-functional), the periderm, and the rhytidome with successive periderms and phloem layers between them [adapted from Şen et al. (2015)].
Barks have been used since ancient times for several purposes: medicine, construction, chemistry, clothing, and energy. More recently, they are viewed as a potential raw material for biorefineries, given their complex structure and rich chemistry, as well as large availability (Şen et al., 2015). In fact, roundwood world production was about 3.591 million m3 in 2013 (FAO, 2015), generating over 300 million m3 of bark that are largely concentrated at processing sites and industrial mills. Nevertheless, the timber economy usually treats barks as a residue, and their main use is as fuel. Consequently, the effort undertaken to study bark development, structure, and chemistry is quite limited, e.g., only a small portion of the one given to wood (Lev-Yadun, 2011).
One exception is the bark produced by the cork oak (Quercus suber L.), and this is because of cork, a material that has attracted the curiosity of mankind for many centuries and is now the basis of an economic relevant industry. Cork is a cellular material with an outstanding set of properties, namely, low density, very little permeability to liquids and gases, chemical and biological inertia, mechanical elasticity, high friction, good insulation, and high damping capacity (Pereira, 2007). These characteristics largely justify the interest of cork as a raw material for multiple usages (Pereira, 2015). Cork is used for many products, from sealants to agglomerates and composites, suitable for diverse purposes, such as bottle stoppers for the wine industry, insulation, and surfacing panels for construction and aeronautics, pollutants absorbers, clothing, and decorative articles (Fortes et al., 2004; Pereira, 2007; Gil, 2009, 2015; Duarte and Bordado, 2015).
The cork oak produces a periderm with special characteristics of development, regularity, growth intensity, and longevity, as well as with regeneration capacity after removal, which has made this species very unique. Cork oaks have a distribution restricted to the western Mediterranean basin, with the largest areas located in Portugal and Spain, and an annual total production of cork is limited to about 200,000 tons (APCOR, 2015).
This restricted cork availability, both geographically and in quantity, conditions the development of the cork industrial sector. Therefore, the study of other species with barks containing a high cork proportion is a promising research line. Several authors report species, whose bark has high cork content, and a few have been used to replace cork from the cork oak but usually for niche markets or in times or regions with restricted access to Q. suber cork. However, the number of species that may have potential to be a source of cork and, therefore, enlarge the cork supply to the industry is not very high, and little information regarding them is available.
The present review presents a general overview of barks and of cork in particular and gathers the information available for some of the species with barks containing a substantial amount of cork, concerning the development and structural and chemical characteristics of the cork component, as well as their potential usage for cork-based bioproducts.
Bark Structure and Formation
Bark is a heterogeneous cellular material, resulting from the activity of the two radial meristems: the vascular cambium and the phellogen (Evert, 2006).
The vascular cambium encircles the stem of plants and produces xylem cells inwards and phloem cells to the outside (Figure 1). Phloem is the main food-conducting tissue and includes a functional layer near the cambium and a non-functional layer to the outside. Functional and non-functional phloem are also called, respectively, non-collapsed and collapsed phloem. The phellogen originates phellem (cork) cells to the outside and phelloderm cells to the interior. Together, phellem, phellogen, and phelloderm form the periderm, as represented in Figure 1. In most species, the phellogen has a limited lifespan, and after its death a new one is formed inside the phloem. The successive periderms, which are separated by layers of phloem are called rhytidome.
Therefore, bark consists of phloem, periderm, and rhytidome, and its macroscopic appearance and properties will depend on the structure of these tissues, their extent, and relative proportion (Huang et al., 2006).
Periderm Development
The periderm is a protective tissue formed in most dicotyledons and gymnosperms to replace the epidermis when this tissue no longer is able to accommodate radial growth and cracks. Also, in the case of an injury, a traumatic periderm may form to protect from exposure and infection.
The phellogen initials result from the dedifferentiation (i.e., return to a meristematic function) of mature parenchyma cells. The first phellogen can arise in different locations: in most situations it is formed below the epidermis, but in some cases it appears in the epidermis or in the phloem (Evert, 2006; Pereira, 2007). The phellogen mother-cells start their meristematic activity by periclinal division: the inner cell differentiates as phelloderm; the outer cell undergoes another periclinal division and originates to the exterior a phellem cell (cork) and inwards the phellogen initial that continues this meristematic activity. Sometimes, cork cells occur immediately by the first division, and no phelloderm cell is formed (Fahn, 1990; Pereira, 2007). In general, plants produce more phellem cells than phelloderm; in many cases, there is only one layer of phelloderm and several layers of phellem, although in a few species the phelloderm may be up to six layers thick (Fahn, 1990; Beck, 2010).
The first phellogen can be initiated uniformly around the stem or in localized areas and acquires continuity as the result of lateral spread due to meristematic activity (Evert, 2006). Timing and location of phellogen initiation is influenced by several factors, namely, genetics, physiology, and environment (Lev-Yadun, 2011). The phellogen has only one kind of cells that appear in transverse sections as a tangentially disposed layer of rectangular cells; in radial section, they appear flattened and in tangential view they show a polygonal structure, sometimes rather irregular (Evert, 2006; Pereira, 2007). There are no intercellular spaces between the phellogen cells, except where lenticels arise.
The phellogen activity, like that of the vascular cambium, is seasonal with periods of dormancy and of activity depending on environmental conditions, namely, light, water, and temperature (Fahn, 1990; Evert, 2006). The number of cork layers is very variable between species and with plant age and may be very large, as in the cork oak. In fact, the longevity and activity of the phellogen are decisive factors to determine the thickness and homogeneity of the cork tissue. There is also a large variability between species about the duration of the first phellogen, and in some species like the cork oak and others, the first phellogen is active throughout the entire life of the plant (Fahn, 1990; Pereira, 2007).
When one periderm ceases its functional activity and dies, it is substituted by a new functioning periderm, each time forming deeper inside the living tissues. Therefore, the first formed periderm is the outermost in the rhytidome, while the newest one (and active) is the innermost (Fahn, 1990). These successive periderms may completely encircle the stem with a cylindrical shape, or not, e.g., with lens-shaped or shell-like portions, partially overlapping each other (Beck, 2010). Trees from temperate zones usually produce more sequential periderms than tropical ones.
As phellem and phelloderm cells result from periclinal divisions of the phellogen, i.e., parallel to the tangential direction, they are disposed in well-defined radial rows. To allow for diameter increment, the phellogen cells also perform occasional anticlinal divisions, thereby increasing the number of radial rows (Pereira, 2007; Beck, 2010).
The phelloderm cells are living cells with non-suberized walls that resemble parenchyma cells but identified by their arrangement in radial rows under the phellogen initials. The phellem cells are dead cells, characterized by a cell wall containing suberin that is internally deposited onto the primary cell wall. Subsequently the phellem cells lose their protoplasm, and the cell lumen becomes empty (Pereira, 2007).
Rhytidome
In most woody species in temperate climates, the initial periderm is only functional for a few years and is replaced, in the interior, by a new functional periderm. Consequently, bark accumulates to the outside of the functioning periderm layers of dead non-functional periderms and phloem tissues between them, forming the so-called rhytidome (Evert, 2006). The term outerbark is also commonly used to designate these non-living layers, and innerbark the living tissues between cambium and the active phellogen (Pereira, 2007). Figure 1B shows a schematic diagram of a bark containing successive periderms in the rhytidome.
Along time, there is a noticeable diametric expansion of the stem because the cambium produces many xylem and phloem cell layers. Consequently, there is a compression of the outer phloem and also a substantial tangential tensile stress on the bark, leading to cracking, splitting, and wrinkling in the most external layers of the rhytidome (Beck, 2010). The structure of the rhytidome, e.g., the number of different periderms and their cellular features and development, and the cellular composition and arrangement of the phloem tissues, e.g., the proportion and arrangement of fibers, directly influence the surface morphology of the bark and often give the unique features of particular species, like depth and direction of wrinkling and the kind of exfoliation (Roth, 1981; Beck, 2010). These characteristic external features of bark can be very useful for taxonomy, especially for tropical trees.
As rhytidome is the result of the development of successive periderms, barks that have only one periderm do not have rhytidome (Evert, 2006). For instance, Q. suber, Quercus variabilis, and Kielmeyera coriacea do not have rhytidome and are some of the species analyzed in this review.
Cork Structure and Chemical Composition
Cork is formed by cells with empty lumens and suberized cell walls. The presence of suberin is the specific characteristic of cork and often used to identify cork cells in plant anatomy by applying specific suberin staining, e.g., sudan dye. Suberin confers impermeability to water and gases and resistance to acids and contributes to compressibility (Pereira, 2007, 2015).
The cork structure is compact with a very regular arrangement of the individual cells and without intercellular spaces. The cells are in general hexagonal prisms that are stacked base-to-base in radial rows, and the rows aligned in parallel; in adjacent rows, the prism bases often lay in staggered positions. When observed two-dimensionally, i.e., in sections, the arrangement has a different appearance. In the transverse section (the plane perpendicular to the plant axis), the structure is a brick-wall type with the cells cut parallel to their prism axis and appearing with a rectangular form. The radial section (the plane that contains the plant axis and a diameter) is very similar. In the tangential section (the plane perpendicular to a radius), the cork cells appear polygonal, mostly as hexagons with a honeycomb structure (Figure 2).
It is often possible to identify growth increments in cork. Macroscopically they are distinguished by the darker color of the cell layers formed at the end of the growing season, that have thicker walled cells and smaller in the radial direction (latecork cells) in contrast to the thinner walls and radially longer cells of the beginning and core of the growing season (earlycork cells) (Pereira, 2007).
The cork cells may have evenly or unevenly thickened walls, e.g., some have U-shaped wall thickenings of the inner or outer tangential wall (Evert, 2006). In some species, the phellem contains also non-suberized cells, the phelloids, which have thick or thin cell walls and differentiate as sclereids.
In addition to the typical hollow, thin-walled, and radially widened cork cells, the cork layer may include thick-walled and radially flattened cells, often filled with dark resins or tannins that occur in some species in alternating tangential bands (Fahn, 1990).
Cork is chemically very different from other plant tissues, namely, from wood and phloem. It is out-singled by the presence of suberin as a major cell wall structural component. Suberin is a large biopolymer of lipid nature formed by the esterification of glycerol and long-chain fatty acids, α,ω-diacids and ω-hydroxyacids, either saturated or with an unsaturation, epoxy, or vicinal diol substitution at mid-chain (Graça and Pereira, 1997). Suberin also includes a few aromatic monomers in most cases ferulic acid (Graça and Pereira, 1998; Marques et al., 2016). The specific composition of suberin, i.e., the proportion of monomers varies between species, as detailed in the following sections.
Lignin is the second most important structural component of cork. This macromolecule is a cross-linked aromatic polymer with strong covalent bonds disposed as a 3D-network that confers strength to the cell wall (Pereira, 2007). Lignin is usually defined as a polymer of phenylpropane units with three different aromatic units—p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S)—and the lignins are classified according to their H/G/S ratios. Lignin structural composition of barks, namely of corks, is largely unknown except for a few cases that showed that cork lignin is composed mainly of guaiacyl units with a low proportion of syringyl units (Marques et al., 1994, 1996, 1999, 2006, 2016; Marques and Pereira, 2013).
The structural polysaccharides of cell walls are cellulose and hemicelluloses. While, in wood they represent up to 80% of the structural components of the cell wall, in cork they have a much lower importance and correspond to about 20% of cork (Silva et al., 2005; Pereira, 2007). Xylans are the most important hemicelluloses in cork (Pereira, 1988).
Cork also contains non-structural components that are soluble in different solvents. Lipophilic extractives including fatty acids and alcohols, sterols, and terpenes, as well as polar compounds of phenolic nature are present in substantial amounts. The proportion and the composition of cork extractives differ substantially between species (Ferreira et al., 2015a,b, 2016a,b; Mota et al., 2016; Sen et al., 2016a).
The inorganic materials content, determined as ash, is usually below 3% (Pereira, 1988; Sen et al., 2010; Ponte-e-Sousa and Neto-Vaz, 2011; Ferreira et al., 2015a).
Much effort has been undertaken to study the variability of Q. suber cork in relation to chemical composition because this characteristic is responsible for many of its properties (Pereira, 1988, 2013; Bento et al., 2001; Sen et al., 2016a). For corks of other species, there is no systematic study of natural chemical variation.
Together, the cell structure and chemical composition determine cork properties, e.g., the solid volume ratio and the material’s density that influence elasticity and mechanical strength, as well as cork performance in insulation (Pereira, 2015). Of all mechanical properties, compression behavior is the one that has attracted most attention, due to the importance of compression in the world-known use of cork as stoppers for wine bottles (Anjos et al., 2008, 2014; Oliveira et al., 2014).
Cork-Rich Barks
The barks may be classified in two groups in relation to periderm characteristics: those that have only one superficial periderm and do not have rhytidome (Figure 1A); and those that have rhytidome (Figure 1B). This distinction is of particular relevance when a potential exploitation of the cork layer is envisaged. When only one periderm is present, the cork layer is radially and tangentially homogenous and if its thickness is adequate, it may be used for production of solid cork products, e.g., cork stoppers. In the case of a rhytidome, the cork layers of the successive periderms are separated by phloemic layers; therefore the recovery of cork will require trituration of the rhytidome and fractionation of the cork component, thereby obtaining it in a granulated form that only allows use in cork agglomerated products.
The present main commercial provider of cork is the cork oak, Q. suber, which has only one periderm and a substantial production of cork. The Chinese cork is also commercially used: it is obtained from Q. variabilis, a tree that also has only one periderm. Other species were referred as having been used for production of cork or as having potential for it. Natividade (1950) points out Q. variabilis, Phellodendron amurense, and Ulmus campestris auct. var. suberosa, as having been industrially used in a similar way as Q. suber. This author also refers that Pseudotsuga menziesii bark and the rhytidome of Abies lasiocarpa var. arizonica, Abies concolor, and Erythrina spp. were used in agglomerates. Further, he identified Pithecolobium incuriale, Enterolobium ellipticum, K. coriacea, Aspidosperma tomentosum, Zeyheria montana, and Connarus suberosus as Brazilian cork-producing species with a potential value. Melaleuca leucadendron spongy and impermeable bark was also mentioned as a possible substitute to cork from the cork oak.
Rizzini and Mors (1995) referred that Agonandra brasiliensis, Pisonia tomentosa, Aspidosperma dasycarpum, Erythrina mulungu, and Symplocos lanceolata produce enough cork to justify their commercial exploitation. Abramovay (1999) in Rios (2007) suggested Erythrina crista-galli, P. incuriale, Stryphnodendron adstringens, and Anona coriacea as promising cork species. Pereira (1988) showed that Calotropis procera has a suberous bark. Sen et al. (2010, 2011a,b) studied Quercus cerris rhytidome and its cork to evaluate its potential for agglomerates. Bhat (1982) observed the bark structure and some physical properties of Betula pendula, identifying several cork layers in the rhytidome. Recently, Mota et al. (2016) studied the cork of Plathymenia reticulata from the Brazilian cerrado.
As far as we know, only Q. suber, Q. variabilis, Q. cerris, K. coriacea, P. menziesii, B. pendula, P. reticulata, A. lasiocarpa var arizonica, and A. concolor have been studied at variable degree for their bark and potential cork utilization. Table 1 lists these species dividing them in gymnosperm and angiosperm and classifies them regarding their bark characteristics, i.e., bark with or without rhytidome.

Table 1. List of gymnosperm and angiosperm species that have been studied in relation to their cork-rich barks, classified according to their bark structure (presence/absence of rhytidome).
In the following sections, a brief explanation is given for each species about distribution area and economic importance, as well as a review of the information available about their periderm and cork characteristics, and a brief discussion about prospective utilizations.
Characterization of Some Cork-Rich Barks
There are few works on the barks that were identified as having a high cork content and on the characteristics of their cork component. One exception is the cork oak, Q. suber, the present main provider of commercial cork used by an integrated industrial chain of high economic relevance in the production regions. It has been extensively studied, and the rich array of publications was compiled in a reference book (Pereira, 2007). The barks of the other species listed in Table 1 have been much less studied, and in some cases only a few notes on bark development are available. The features of Q. suber cork, therefore, benchmark the general characterization of cork materials.
In the following sections, a synthesis of the existing knowledge on the cork of the different barks is made, organized by species, including, when available, details on periderm or rhytidome, cork cellular and chemical features, and use potential. Summary tables were prepared for comparison of corks from the different species: Table 2 summarizes cell dimensions, Table 3 summarizes chemical composition, Table 4 summarizes polysaccharide composition, and Table 5 summarizes suberin composition.
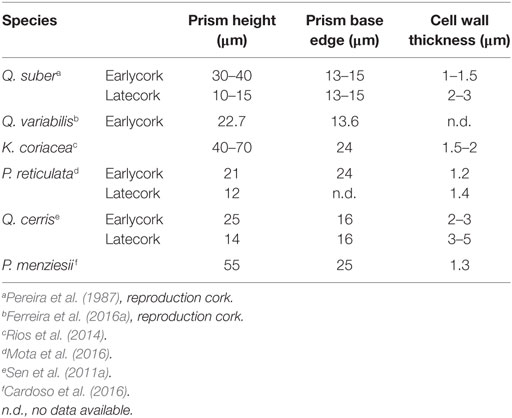
Table 2. Cellular biometry of cork of Quercus suber, Quercus variabilis, Kielmeyera coriacea, Plathymenia reticulata, Quercus cerris, and Pseudotsuga menziesii.
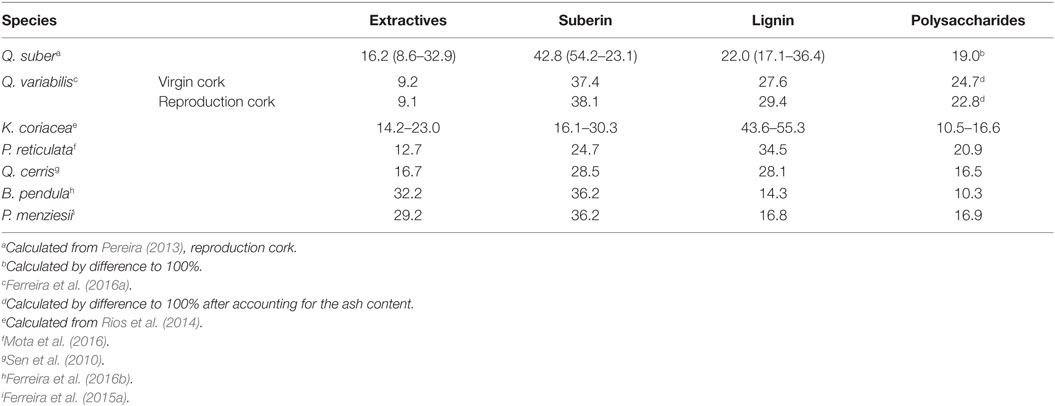
Table 3. Chemical composition (% total dry mass) of the cork of Quercus suber, Quercus variabilis, Kielmeyera coriacea, Plathymenia reticulata, Quercus cerris, Betula pendula, and Pseudotsuga menziesii.
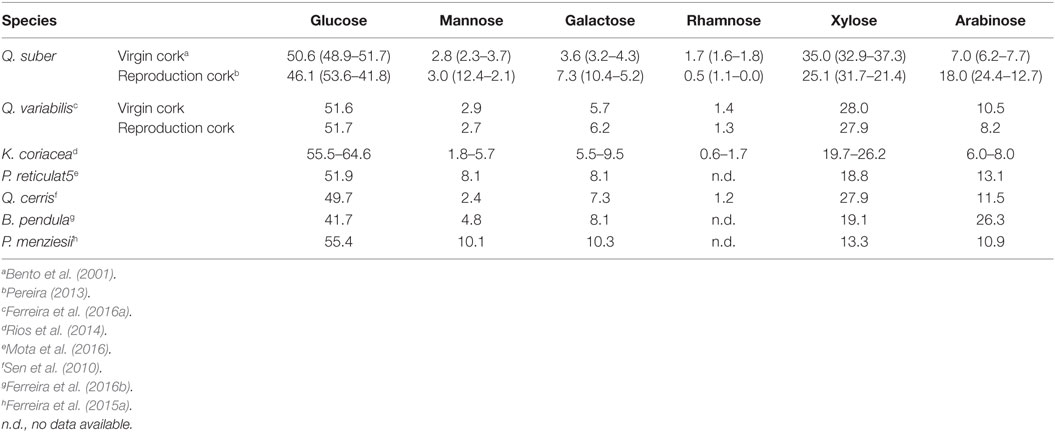
Table 4. Polysaccharides composition (% of total neutral monosaccharides) of the cork of Quercus suber, Quercus variabilis, Kielmeyera coriacea, Plathymenia reticulate, Quercus cerris, Betula pendula, and Pseudotsuga menziesii.
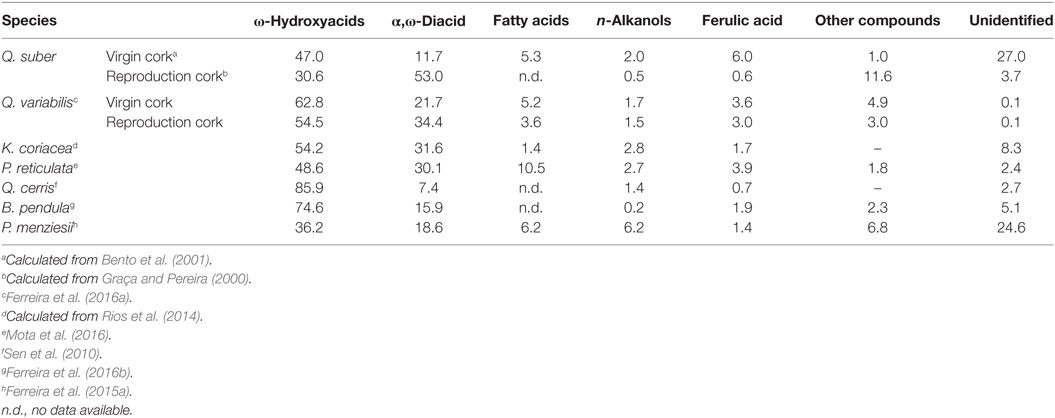
Table 5. Suberin composition (mass % of the total compounds detected by GC) of the cork of Quercus suber; Quercus variabilis, Kielmeyera coriacea, Plathymenia reticulata, Quercus cerris, Betula pendula, and Pseudotsuga menziesii.
Quercus suber
The cork oak (Q. suber) is a species native to the western Mediterranean basin, with the largest cork-producing areas situated in Portugal and Spain. The Q. suber trees are of median height (15–20 m) and may reach up to 25 m, with a broad crown and a very conspicuous bark (Figure 3).
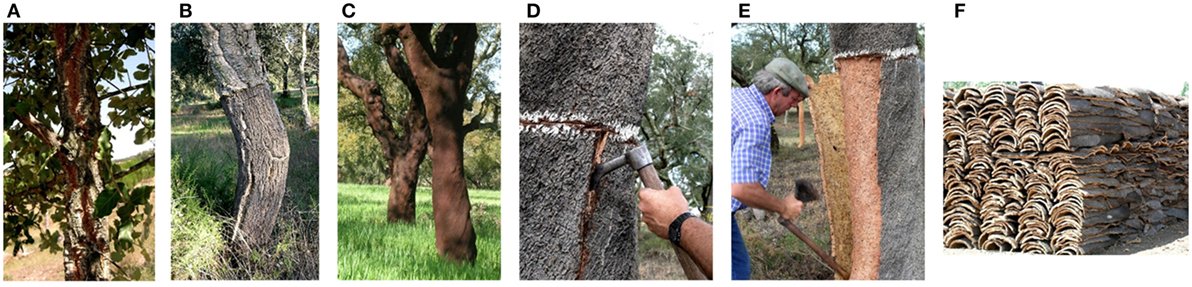
Figure 3. Quercus suber stem showing (A) virgin cork; (B) second cork; and (C) reproduction cork in mature trees and the removal of cork from Q. suber trees: (D) cutting the cork layer; (E) pulling out of the cork planks; and (F) cork piles.
Cork from the cork oak has high economic importance, mainly as a raw material for wine stoppers and also for surfacing and insulating materials. The tree is exploited using a sustainable management with periodic removals of the cork layer under a silvicultural system that has been perfected along time, integrating a multifunctional agro-forest system called montado (Natividade, 1950; Pereira and Tomé, 2004).
Periderm Development
In the cork oak, the phellogen forms a continuous layer surrounding stem and branches and may live as long as the tree, although the intensity of its activity decreases with age (Natividade, 1950).
The phellogen activity begins in the first year of the shoot; the first cork cells keep the tangential form of the phellogen initial and build up radially aligned rows (Graça and Pereira, 2004). Besides the periclinal divisions of the phellogen cells, some anticlinal divisions also occur that increase the number of phellogen initials, and therefore of the radial rows of cork cells.
The cork in the first periderm is called virgin cork (Figure 3A). It shows numerous and deep cracks that run mostly longitudinally due to the radial enlargement of the tree (Pereira, 2007).
If the initial phellogen is destroyed, as it happens by the stripping of the cork layer, a new (traumatic) phellogen is formed in the inner tissues of the phloem and begins its meristematic activity in the same way as it happened in the first periderm, thereby forming a new regular cylindrical layer of cork cells around the tree; this cork is called reproduction cork. This new periderm is covered externally by the tissues that remained to the outside of where the phellogen was formed; therefore they include the first phelloderm (formed by the initial phellogen) and a layer of the non-functional phloem. As these tissues become exposed to air, they dry out and develop thin fissures building up what is called the cork back (Figure 3B) (Taco et al., 2003).
The first reproduction cork produced by this second phellogen (also called second cork) is often longitudinally fissured due to the still high tangential growth stress of the young tree (Figure 3B). When the second cork is removed, the process is repeated as described above, and the new cork layers no longer fissure since the tangential growth stress is much smaller (Figure 3C).
In the commercial exploitation of cork oaks, this process is repeated successively and a new phellogen and periderm are formed as described. This ability to develop each time a new periderm is the basis for the sustainability of the cork production and cork oak exploitation.
Cork Cellular Structure
The cork cells are mostly hexagonal prisms that are stacked by their bases in radially aligned rows disposed in parallel without intercellular voids (Figure 4). Therefore the cork cells appear as a honeycomb structure in the tangential section (Figure 4A) and as a brick-wall structure in transverse (Figure 4B) and radial sections (Figure 4C). On average the cell prism height is 30–40 µm and the cell wall thickness 1–1.5 µm (Table 2). It is possible to observe annual rings that are marked by the presence of a layer of latecork cells at the end of the growth season with a shorter prism height (10–15 µm) and a thicker cell wall (2–3 µm) in comparison with the earlycork cells.
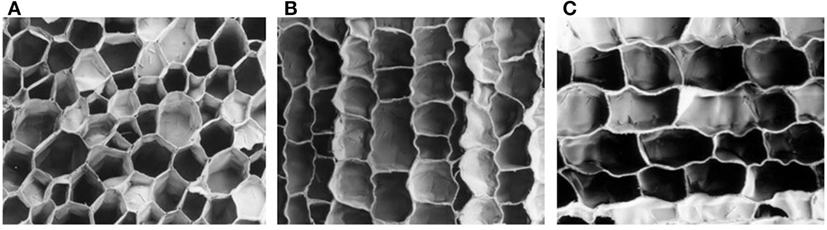
Figure 4. The cellular structure of Quercus suber cork: (A) tangential section; (B) transverse section; and (C) radial section (Pereira, 2015).
The solid fraction in the cork is 8–9% in the earlycork and 15–22% in the latecork region (Pereira, 2007), which justifies the low density of cork.
An important structural characteristic is the corrugation of the radial aligned cell walls that arise from compression stresses during cork growth, i.e., the new cell layers compress the already existing cork cells by pushing them toward the exterior. Sometimes the cell wall corrugation may be strong, especially in virgin cork (Pereira et al., 1987).
Lenticels are present and develop as lenticular channels that cross radially the cork layers; they cause the so-called porosity of cork that appears as more or less circular in tangential sections of cork and as thin strips in the other sections. Cork porosity has been extensively characterized because it is a visual quality parameter that defines the commercial quality of cork stoppers (Pereira et al., 1996; Costa and Pereira, 2007; Oliveira et al., 2012, 2015).
Chemical Composition
Reproduction cork has on average 16% extractives, 43% suberin, 22% lignin, and 19% cellulose and hemicelluloses (Table 3). The extractives include non-polar compounds that are extracted by solvents such as dichloromethane (representing on average 5.8% of cork) and polar compounds solubilized by ethanol and water (5.9 and 4.5%, respectively) (Pereira, 2013).
Suberin is the most important component of Q. suber cork. Its composition regarding the long-chain lipid monomers is shown in Table 5. In reproduction cork, the α,ω-diacids are the most abundant monomers (53.0% of the monomers), followed by ω-hydroxyacids (30.6%). The most abundant single monomer is the 9-epoxyoctadecanedioic acid (26.7%), followed by 22-hydroxydocosanoic acid (9.2%), 9,10-dihydroxyoctadecanodioic acid (9.0%), and 9-epoxy-18-hydroxyoctadecanoic acid (8.5%). In terms of chain length, most fatty acids have 18 and 22 carbons, representing, respectively, 66.2 and 14.4% of the total monomers. Suberin also contains, as a major monomer, glycerol that accounts on average to 8.5 or 14.2% of the suberin (Graça and Pereira, 2000; Pereira, 2015).
Lignin is the second most important component in the cork cell wall. Cork lignin is a G-type lignin composed by 95% guaiacyl units, 3% syringyl units, and 2% 4-hydroxyphenyl units (Marques et al., 2006; Marques and Pereira, 2013) with 80% of the inter-unit linkages as β-O-4-alkyl-aryl ether bond (Marques et al., 2016).
The hemicelluloses are mainly composed of xylose and arabinose, with smaller amount of galactose and mannose (Table 4).
There is a substantial chemical variability of Q. suber cork regarding between-tree and between-site differences that has been addressed in several studies (Pereira, 1988, 2013; Conde et al., 1998; Bento et al., 2001; Sen et al., 2016a). The content of suberin is the most important chemical attribute of cork since it is its chemical fingerprint and is directly related to most of its typical properties (Pereira, 2015).
Utilizations
Cork oak forests extend to about 2.2 million ha and produce annually up to 200,000 tons of cork that feed an important industry (APCOR, 2015). Portugal is the main producer of raw cork and of cork products that include mainly cork stoppers for the wine industry and insulation and surfacing boards (Pereira, 2007). The production of cork is based on the periodical removal of the cork layer by cutting large planks that are pulled out from the stem (Figures 3D–F). The cork removal is made usually in June–July, when the tree and the phellogen are active and allow an easy separation of the cork. The period between cork removals is usually 9 years in the major producing regions, which allows a thickness of the cork plank suitable to produce the cork stoppers (>24 mm). The cork oak silvicultural system is directed for cork production and is regulated, e.g., the minimum period between cork removals or the extension of the debarking, aiming at maintaining the overall sustainability of the forests.
The cork planks are primarily directed for the production of natural cork stoppers that are bored out from the planks in the axial direction (Costa and Pereira, 2010). If the cork planks are too thin to allow this, they are directed to produce cork discs to be used for sparkling wine stoppers. The residues of these production lines, as well as other unsuitable cork pieces (for instance, virgin cork) are triturated, and the cork granules are agglomerated with suitable adhesives for production of technical wine stoppers, insulation boards, flooring and surfacing boards, joint sealants, and damping and shock absorption layers, among other usages (Pereira, 2007).
The cork of Q. suber has been comprehensively studied. Nevertheless, areas of interest remain where more knowledge and research are needed, e.g., fundamental studies on the topochemistry and 3D architecture of the cork cell wall, the extent and causes of natural variability in chemical composition and cellular features, and how these relate with properties, as well as innovative approaches to product development and new uses.
Quercus variabilis
Quercus variabilis (Chinese cork oak) is a species with a distribution area in China, Korea, and Japan; in China, it is found mostly in the Shaanxi province and in neighboring western Hubei and eastern Sichuan provinces (Zhang and Lu, 2002; Zhou et al., 2010). In China, it is exploited for cork production but with less importance than the cork oak in Europe, although cork production has increased in the last years to approximately 50,000 tons in conjunction with the marketing of some cork products (Zhao et al., 2013; Ferreira et al., 2016a). The tree is medium-sized to large, growing up to 25–30 m, and the bark forms a continuous periderm around the stem with a thick layer of cork, as in the cork oak, and the stem appearance of these two species is similar (Figure 5).

Figure 5. Quercus variabilis stem—“Chinese cork oak bark” by Velela—own work. Licensed under Public Domain via Commons https://commons.wikimedia.org/wiki/File:Chinese_cork_oak_bark.jpg#/media/File:Chinese_cork_oak_bark.jpg.
Some research about this species can be found, mainly in China, Japan, and Korea as demonstrated by several authors [e.g., Kim et al. (1990), Kim (1993), Wei et al. (2007), YaFang et al. (2009, 2012), Zhang et al. (2009), and Miranda et al. (2013)], but with the limitation that most of it is not written in English. Recently, Ferreira et al. (2016a) analyzed the virgin and reproduction cork, comparing their chemical and cellular characteristics with those of the cork oak.
Cork Cellular Structure
The cork cells from Q. variabilis are polygonal in a honeycomb-like arrangement in the tangential section, while the transverse and radial sections show a brick-wall structure with an alignment in parallel rows (Figure 2) (YaFang et al., 2009; Miranda et al., 2013; Ferreira et al., 2016a). Cork cells are prismatic, and the cell walls present corrugations, especially the earlycork cells that form against the previous latecork cells (Miranda et al., 2013; Ferreira et al., 2016a).
Like in the cork oak, the Chinese cork has rings with larger earlycork cells and with thinner walls than latecork cells. The average ring width of Q. variabilis is significantly smaller than in Q. suber cork (0.82 vs. 2.06 mm), and each ring has two to six latecork cells representing 14% of the ring width (Kim, 1993; Miranda et al., 2013; Ferreira et al., 2016a).
Prism height in the earlycork cells of the reproduction cork of Q. variabilis is smaller than in Q. suber (23 vs. 30–40 µm, Table 2). The solid fraction in earlycork is, therefore, higher in the Chinese cork than in Q. suber cork (13 vs. 8–9%) (Miranda et al., 2013; Ferreira et al., 2016a). Consequently, Q. variabilis cork has higher density than Q. suber, what constitutes a disadvantage as an insulating material (Kim, 1993; Miranda et al., 2013). Another difference between these two corks is the appearance of the cell wall: the lumen side of the cork surface of Q. variabilis is rough with deposits of several dimensions, while in Q. suber, it is smooth and with only occasional deposits (Miranda et al., 2013).
Like in the cork oak, lenticular channels cross radially the cork layer with a circular or elliptical shape in the tangential section and with filling tissue (Kim, 1993; Ferreira et al., 2016a).
Chemical Composition
Virgin and reproduction cork of Q. variabilis are chemically very similar: on average 0.9% ash, 9.2% extractives, 37.8% suberin, and 28.5% lignin. The composition is within the variation range of reproduction cork from Q. suber (Table 3) although with less extractives, with lower levels of non-polar compounds extracted by dichloromethane (YaFang et al., 2009; Miranda et al., 2013; Ferreira et al., 2016a). The ratio suberin/lignin is 1.4 (virgin cork) and 1.3 (reproduction cork), lower than the average of 2.0 in Q. suber (Ferreira et al., 2016a). Given that most of the properties of cork result from the joint presence of suberin and lignin in the cell wall and their relative proportion, the higher proportion of lignin will give Q. variabilis cork higher compressive strength than Q. suber (Pereira, 2013).
Polysaccharides (Table 4) show a large presence of xylan-based hemicelluloses and a comparable proportion of cellulose (glucose amounts to 52% of the neutral sugars).
Suberin composition (Table 5) shows a substantial difference in relation to the reproduction cork from cork oak: Q. variabilis cork has more ω-hydroxyacids and less α,ω-diacids. The main monomers in Q. variabilis suberin are 22-hydroxydocosanoic acid (35.1–21.4%), 18-hydroxy-9-octadecenoic acid (22.0–17.1%), and 9,10-epoxy-18-hydroxyoctadecanoic acid (10.0–10.2%) (Ferreira et al., 2016a). Epoxyacids have lower contents (13.3–14.4%, Ferreira et al., 2016a) than in Q. suber suberin (30%, Graça and Pereira, 2000). The relation between the saturated and the substituted acids in Q. variabilis suberin is 1.0–0.7 (0.4 in Q. suber) (Ferreira et al., 2016a). Therefore, it is to be expected that suberin spatial development may be more compact in Q. variabilis cork. Ferulic acid is also present in the suberin extracts (3.6–3.0%), enforcing the conclusions about its function in the cross-linking between suberin and lignin (Marques et al., 2016).
Utilizations in Perspective
Cork of Q. variabilis is at present already harvested, industrially processed, and commercialized. Due to the characteristics of the raw material, it cannot be used for production of solid cork products, namely for wine stoppers; instead, it is granulated and used in agglomerates for various surfacing and insulation applications.
The cork from Q. variabilis presents lower quality than that from Q. suber, namely, higher density, compressive strength, and elasticity. However, this cork presents characteristics that are compatible with its use as an insulating, sealing, and energy absorption material and may be considered as a complementary raw material for cork from cork oak (Miranda et al., 2013; Ferreira et al., 2016a). The cork from Q. variabilis can also be a potential source of friedelin or of betulinic acid, due to the high levels of these triterpenes in the extractives (Ferreira et al., 2016a).
The exploitation of this species as a cork provider is far from being developed and optimized as it is for Q. suber and its cork, e.g., the development and improvement of Q. variabilis silviculture and forest management as well as of the respective cork industry may overcome the present constrains. More knowledge and research are certainly needed in the tree physiology related to the bark and cork formation that would complement applied silvicultural studies. The variation of cork characteristics between trees and geographical locations as well as with tree age is also important for improving products and industrial processes.
Kielmeyera coriacea
Kielmeyera coriacea is natural from the savannah-type ecosystems of the Brazilian cerrado. The trees are in general 1–4 m high (Rios, 2007) and the stem is covered by a conspicuous cork bark (Figure 6).
The bark can be several centimeters thick and has a periderm with 1.1–1.8 cm of cork and only a few inclusions of phloem (Rios et al., 2011). The cork tissue may be detached from the stem and the tree has a high regeneration capacity of the periderm and its cork; this has led to suggestions of exploitation with successive cork removals at 5- to 6-year rotations (Souza, 1974; Lima and Marcati, 1989).
The phloem inclusions appear as thin bands, approximately tangentially oriented and parallel to the phellogen, representing from 10 to 25% in area. Lenticels are present and correspond from less than 1 to 15% of the cork cross section. Together phloem inclusions and lenticels represent between 12 and 40% of the outer bark area (Rios, 2011).
Cork Cellular Structure
The cellular structure of K. coriacea cork is similar to that of Q. suber cork. The cork cells are hexagonal prisms, disposed in a honeycomb-like arrangement (Figure 2); cell wall thickness is between 1.5 and 2 µm, and cell length in the radial direction is significantly higher than in cork oak cork (Table 2).
Chemical Composition
The chemical analysis of K. coriacea virgin cork, shown in Table 3, includes not only the cork tissue but also its phloem inclusions. The content of suberin is from 16 to 30%, and this species presents higher contents of lignin than cork oak cork with 43.6–55.3% (Rios et al., 2014).
Table 4 shows that xylans are the most important hemicelluloses in the virgin cork from K. coriacea; the glucose proportion is higher than in cork of Q. suber and of all the other reported species.
Kielmeyera coriacea cork suberin (Table 5) is chemically composed mainly by ω-hydroxyacids and α,ω-diacids have a lower proportion (Rios et al., 2014). The main ω-hydroxyacids are the C18 9,10 mid-chain substituted ones, namely, with an unsaturated group (18-hydroxyoctadec-9-enoic-acid) and a vicinal diol group (9,10,18-trihydroxyoctadecanoic acid). The α,ω-diacids include the octadec-9-ene-1,18-dioic acid and 9,10-dihydroxyoctadecane-1,18-dioic acid. This suberin does not contain acid monomers with saturated chains, either ω-hydroxyacids, α,ω-diacids, or n-alkanoic acids (Rios et al., 2014).
Utilizations in Perspective
There is no industrial utilization of K. coriacea bark as a raw material for cork products. However, its exploitation as a cork provider has been already suggested (Natividade, 1950), namely, for the production of cork agglomerates (Rios et al., 2014) since the homogeneity of the cork layer does not seem suitable as a raw material for solid cork products. The cellular and chemical characteristics of K. coriacea cork are compatible with the common uses of cork, namely, as an insulation and surfacing material after trituration and agglomeration. Its use as a source for bio-based chemicals was also suggested, e.g., xantones and long-chain fatty acids from the extractives, or the bifunctional ω-hydroxyacids and α,ω-diacids monomers from suberin (Rios et al., 2014). However, any further considerations on the potential use of K. coriacea cork will require studies on its characterization as well as on tree-related aspects of cork development.
Plathymenia reticulata
Plathymenia reticulata is a species native to South America that vegetates in the Atlantic forest and, as also K. coriacea, in the cerrado, a particular savannah-type ecosystem. It grows up to 30 m but in the cerrado it is generally smaller reaching only 5 m. Its most important applications are relative to the wood which is rot-resistant and widely used as a structural timber and in high-end carpentry (Carvalho, 2009). The stem has a cork bark, as shown in Figure 7.

Figure 7. Stem cross section of Plathymenia reticulata, a tree from the Brazilian cerrado (Minas Gerais).
The only study on the cork of this species is reported here, giving data on chemical composition and cellular structure (Mota et al., 2016).
Periderm Development
The bark of this species presents an internal thick brown layer of phloem that is covered by a periderm containing a conspicuous light brown layer of cork with deep fissures that result from the tangential growth stress. The periderm develops continuously around the stem with a lifespan probably equal to the tree age and with an annual regular activity of the phellogen (Mota et al., 2016).
Cork Cellular Structure
Plathymenia reticulata cork cellular structure has no substantial difference from the one of Q. suber cork. The cork cells are polygonal, in a honeycomb-like arrangement (as in Figure 2), cell wall thickness is within the variation range observed for Q. suber but prism base edge is much higher than in cork oak (Table 2).
The cork from P. reticulata shows growth rings, with fewer and smaller latecork cells (two to four cells in a radial row) and more and larger earlycork cells (six to nine cells), although the differences between earlycork and latecork are not so pronounced as in the cork oak cork, leading to a more homogeneous material (Mota et al., 2016). Large variations of size between cells were observed, either in the tangential or in the non-tangential sections. Like in Q. suber, the cork cells of this species have undulations in the non-tangential sections as well lenticular channels, sometimes with filling tissue.
Chemical Composition
The summative chemical composition is included in Table 3 (Mota et al., 2016). The important feature is that lignin is the major structural component and not suberin (34.5 and 24.7%, respectively). This indicates a low value of the suberin/lignin ratio of 0.7; this was also found by Rios et al. (2014) for the other cerrado species K. coriacea. In comparison with the cork of Q. suber although there is a large variability in the chemical composition values, the ratio suberin:lignin is quite above this value (Pereira, 2013). This chemical difference certainly will bring differences in the chemical behavior, as discussed in Pereira (2015).
In P. reticulata cork, xylan-based hemicelluloses prevail with a considerable proportion of arabinose (Table 4), including acetyl substitutions and uronic acid monomers (Mota et al., 2016).
The suberin composition (Table 5) shows more ω-hydroxyfatty acids and less α,ω-diacids than in Q. suber reproduction cork suberin. The main individual suberin monomers are the 18-hydroxyoctadecanoic acid (saturated and substituted), the octadecanodioic acid, the tetracosanoic acid, and the hexacosanoic acid while only minor values of the 9-epoxyoctadecandioic acid (<1%) were found (Mota et al., 2016), contrarily to what happens in the suberin from cork oak where it is a main component.
Utilizations in Perspective
The cellular and chemical characteristics of P. reticulata cork are compatible with the common uses of cork, namely, as an insulation and surfacing material after trituration and agglomeration (Mota et al., 2016). Other potential utilizations were identified, namely, as a biosorbent and as a raw material for extraction of valuable compounds like lupeol, fatty acids, and terpenoids.
However, in order to fully develop the utilization of P. reticulata cork as a raw material for cork products under a sustainable cork production management, further research is needed to understand how periderm regenerates after cork removal and the characteristics of the subsequent cork layers. Should the cork be obtained as a by-product of tree exploitation for timber, i.e., the bark is produced as a residue, then its valorization for cork agglomerates is possible straightaway. In any case, studies on P. properties, e.g., density, compression, and insulation properties are needed.
Quercus cerris
Quercus cerris L. (Turkey oak) is a medium-sized tree that can reach up to 30 m. It has a distribution area from central and south-eastern Europe to Asia Minor (Sen et al., 2011b).
Quercus cerris bark (Figure 8) is thick (3–7 cm) with a brown grayish color, hard to the touch, and with short deep longitudinal furrows. It is composed of phloem, periderm, and a considerable proportion of rhytidome. The rhytidome has sequential periderms with phloem tissue between them. The cork is clearly distinguished forming patches of variable radial thickness that are non-continuous tangentially and axially. The external periderms of the rhytidome do not shed (Sen et al., 2011b).
There are some records on the utilization of Q. cerris cork as a substitute from Q. suber cork for agglomerates and stoppers (Sen et al., 2011b). Nowadays, neither the wood nor the bark of this species is economically exploited, and until recently, very little research was made on the characterization of its cork.
Periderm Development
Quercus cerris has a conspicuous cork presence in the rhytidome as layers with a radial width from about 1 to 10 mm, while the phelloderm is composed of a layer of only two or three thin-walled cells (Sen et al., 2011b).
The phellem layers present 2–5 growth rings, and each ring is composed of about 6–12 layers of phellem cells more or less radially aligned without intercellular voids. The phellem cells are suberized, with thin walls with a uniform thickness in the tangential and radial walls and are sometimes radially flattened. Like in Q. suber, at the beginning of the growth ring, cells are larger with thin walls, while at the end of the growth ring, they are smaller and have thicker walls. Despite this similarity, both species have different intensities of phellem growth. In fact, in turkey oak, each phellogen cell produces only 6–12 phellem cells/ring in each radial row (Sen et al., 2011b), and in Q. suber, this figure is about 10–20 in young plants (Graça and Pereira, 2004) and up to 100 in mature trees (Pereira et al., 1992). It is also to remark that in this species, but not in Q. suber, one or two layers of the cork cells, in the limit of each growth ring, can thicken up and become very lignified (Sen et al., 2011b).
Lenticular channels crossing radially the periderm are rare and without filling material. This feature can be explained because in turkey oak cork layers are not continuous around the stem, as it happens in cork oak, and therefore, gas exchange between the innermost living tissues and the exterior does not require the development of these channels (Sen et al., 2011b). The cork tissue also includes lignified phloem cells (fibers and sclereids).
In what concerns phellogen longevity, it is probable that its lifespan is around 25 years (Sen et al., 2011b) but, the fact that each periderm presents from two to five rings suggests that phellogen activity is not annual and that the rings cannot be considered as successive annual rings (Sen et al., 2011a).
Cork Cellular Structure
The cork cellular structure of Q. cerris is very similar to the one presented in Section “Cork Structure and Chemical Composition” for Q. suber cork (Figure 2). In fact, the cork cells form a bidimensional network of edges and vertices without intercellular voids. The cells are mostly hexagonal prisms, stacked base-to-base disposed in parallel rows aligned in the radial direction, in a compact space-filling arrangement. Frequently, in adjacent rows prism bases coincide with each other or lay in staggered positions (Sen et al., 2011a).
The cell walls also present corrugations. The intensity of corrugation is greater in Q. cerris than in Q. suber, possibly due to the thicker cell walls and to the more irregularity in the stress radial-growth distribution along the cork tissue as a consequence of the presence of phloem between the cork layers (Sen et al., 2011a).
Cork ring width is on average 201 µm and contains seven to nine earlycork cells and two to four latecork cells in each radial row (Sen et al., 2011a). Dimensions of earlycork and latecork cells are presented in Table 2. Prism base edge and cell wall thickness are higher in Q. cerris cork than in Q. suber cork, and therefore, Q. cerris cork has a higher density (Sen et al., 2011a).
Chemical Composition
The chemical composition of Q. cerris cork is given in Table 3. Suberin is the major cell wall structural component of the cork from Q. cerris but almost in the same proportion as lignin; the suberin:lignin ratio is 1.0. In the polysaccharides, that represent almost 16.7%, glucose is the major monosaccharide (Table 4) but important contents of xylose, arabinose, and galactose are also present. This composition suggests that the main hemicelluloses are xylans (Sen et al., 2010).
In Q. cerris cork suberin, ω-hydroxyacids represent almost 90% of all long-chain monomers (Table 5). The single most abundant compound is the vic-diol mid-chain substituted ω-hydroxyacid in C18: the 9,10,18-trihydroxyoctadecanoic acid represents about 54% of all long-chain monomers. The saturated ω-hydroxyacid in C22 represents 20% and the unsaturated ω-hydroxyacid 18-ω-hydroxyoctadec-9-enoic acid 11%. The α,ω-diacids are present in less than 8% of the total suberin and are constituted mostly by vic-diol substituted 9,10-dihydroxyoctadecanedioic acid with 5% of the long-chain monomers (Sen et al., 2010). Alkanoic acids in C16 and C18 as well as alkanols in C20, C22, and C24 represent a small part of the suberin (Sen et al., 2010). The C18 monomers are the most abundant, followed by C22 monomers, and their relative amounts are not very different from the ones found in cork oak (Sen et al., 2010). The major quantitative difference between Q. cerris suberin and Q. suber reproduction cork suberin lies on the relative proportion of α,ω-diacids and ω-hydroxyacids which is much lower in the first one (Table 5).
Utilizations in Perspective
Due to the characteristics of Q. cerris bark, the industrial utilization of its cork requires previous trituration and fractioning by size and density in order to obtain pure cork or cork-enriched fractions. Therefore, the use of cork is limited to granulates and the production of cork agglomerates. A pilot-scale fractioning of Q. cerris bark was already made (Sen et al., 2016b): a yield of 8.4% cork pure granulates suitable for agglomerated stoppers was obtained as well as 18.5% of cork-rich fractions usable in the production of surfacing and other cork agglomerates.
Considering the comparative cost advantage of the Q. cerris cork, it seems economically interesting to integrate it into the present cork industrial production lines, thereby allowing to enlarge the cork raw-material supply base. However, further studies are required for process optimization and for the development of Q. cerris-specific cork-based products.
Betula pendula
Birch (B. pendula) covers a large part of the Eurasian continent, from the Atlantic to eastern Siberia (except Iceland, the Iberian Peninsula, and Greece) but is present mainly in northern Europe where it is one of the most important species for pulp production (Hultén and Fries, 1986). Birch is a deciduous medium-sized tree, usually 15–25 m high but can reach 30 m.
In 2013, the European pulp and paper industry consumed about 19.6 million m3 of birch wood, thereby generating a large amount of bark (CEPI, 2013). Given a content of outer bark at 3.4% (Jensen, 1948), an annual estimate potential availability of birch outer bark at about 900,000 tons may be made.
The outer bark of birch has been used traditionally in small handicrafts, e.g., boxes, for canoe building, and for the still in-use roof coverings, but it is at present mainly used for energy.
Periderm Development
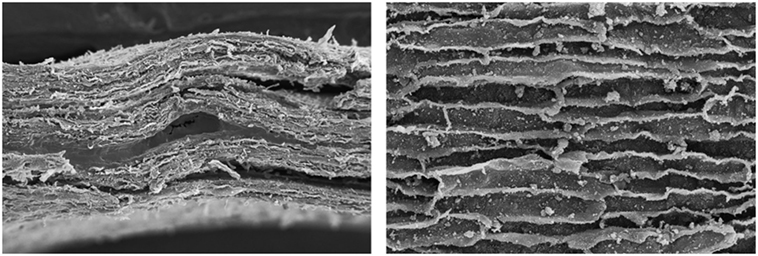
Betula pendula bark has a rhytidome with numerous tightly packed periderm layers that consist of thick-walled cork cells inwards and thin-walled cells outwards (Schönherr and Ziegler, 1980), that resemble annual increments produced in the early and late season of the growth period (Ferreira et al., 2016b). The birch periderm contains alternate layers of strongly suberized cells (five layers of cells with tangential walls up to 2 µm thickness) with a variable number of layers with little or no suberization (Schönherr and Ziegler, 1980). The cells are mostly flattened in the radial direction and stretched in the tangential direction and show many cell wall deposits on the lumen side (Ferreira et al., 2016b). Figure 9 shows SEM photographs of the cross section of birch outerbak.
Birch bark is described as having a rhytidome composed by periderms with successive cork layers and phloem layers with several sclerenchymatic tissues (fibers, sclereids, cortical, and parenchyma cells) and showing conspicuous lenticels that contain suberized and non-suberized cells disposed in stratified layers (Bhat, 1982; Trockenbrodt, 1991; Ferreira et al., 2016b). Suberized layers are continuous across the lenticels and maintain in between the loose tissue together, breaking up successively as a new phellogen is formed. Intercellular spaces with diameters up to 5 µm are seen in the tangential section across the lenticels that represent about 3% of the total periderm area (Schönherr and Ziegler, 1980).
Chemical Composition
There are a few studies on B. pendula bark chemical composition made in the 70s and 80s of last century [e.g., Holloway (1972, 1983), Holloway and Deas (1973), Ekman (1983), and Ekman and Eckerman (1985)] and more recently on suberin composition [e.g., Gandini et al. (2006) and Ferreira et al. (2013, 2016b)]. It should be noticed that several of the studies were made with the whole birch outer bark instead of only the cork fraction, thereby including the non-suberized cells.
Betula pendula cork contains a large amount of extractives of about 32% (Table 3) mostly corresponding to lipophilic extractives, i.e., 98% of the total extractives are dichloromethane solubles. Their composition shows the striking dominance of triterpenoids (90–97% of all compounds) where betulin corresponds on average to 71% of the extract; the other compounds are lupeol (on average 14%) and betulinic acid (4%) (Ferreira et al., 2016b).
Suberin represents 36% of cork (Ferreira et al., 2016b) corresponding to 53% on extractive-free cork that are in agreement with previous reported values of 59% (Holloway, 1983) and 51% (Holloway and Deas, 1973). Lignin amounts are 14% of the cork, and therefore, the suberin:lignin ratio is 2.5.
Cellulose and hemicelluloses represent only 10% of B. pendula cork with most hemicelluloses formed by arabinose and xylose (Table 4).
The suberin from B. pendula (Table 5) contains aliphatic ω-hydroxyacids of chain lengths between C16 and C26, with a large proportion of components in C18 (73% of all long-chain monomers) and C22 and with smaller quantities of fatty acids and neutral constituents (Holloway, 1972). In comparison with Q. suber reproduction cork, birch cork suberin contains much more ω-hydroxyfatty acids and much less α,ω-diacids. In fact, the main individual suberin monomers are the 9,10-epoxy-18-hydroxyoctadecanoic acid (30% of total monomers), the 22-hydroxydocosanoic acid (13%), the 9,10-dihydroxyoctadecandioic acid (12%), and the 18-hydroxyoctadecanoic acid (12%) (Ferreira et al., 2016b).
Utilization in Perspective
Birch cork does not have the structural and cellular characteristics that impart the properties that have made cork so interesting as a material. The cell biometry, e.g., the radially flattened cells and the cork thickness of the periderms, the weak tangential cohesion, and the heavy inclusion of lumen deposits do neither allow a prospective utilization for stoppers nor for the most common applications of cork as a cellular material (Ferreira et al., 2016b). Consequently, this cork main utilization and valorization will be as a chemical source.
Due to its high content in suberin, birch outer bark has been proposed as a source of monomers, e.g., of ω-hydroxyfatty acids, α,ω-dicarboxylic acids, and homologous mid-chain dihydroxy or epoxy derivatives for production of novel macromolecular materials (Gandini et al., 2006), polyesters (Olsson et al., 2007; Sousa et al., 2007), polyols (Evtiouguina et al., 2000, 2002), polyurethanes (Cordeiro et al., 1997, 1999; Evtiouguina et al., 2001) and has also been considered as a source of extractives, namely, of triterpenoids and in particular of betulinol (Pinto et al., 2009). Some patents have already addressed the possibility of using birch bark as a source of chemicals (Krasutsky et al., 2003, 2005, 2009).
Pseudotsuga menziesii
Douglas-fir (P. menziesii) is a conifer native from western North America, occupying approximately 14.4 million ha in the USA and 4.5 million ha in Canada (Weiskittel et al., 2012) and introduced into many parts of the temperate regions of the world, including Europe (Lavender and Herman, 2014). It is a very important species for timber production in its natural distribution area and also in Europe. The height of P. menziessi mature trees depends on site location, but it can reach up to 100 m (Lavender and Herman, 2014).
The bark contains a substantial proportion of cork from 25 to almost 50% (Kurth, 1953; Ross and Krahmer, 1971; Krahmer and Wellons, 1973), and cork formation occurs at a relatively early age (Hergert and Kurth, 1952). Douglas-fir bark presents a great variety in thickness, density, chemical composition, and cork content according to site quality, tree age, and axial position on the tree (Kurth and Kiefer, 1950; Hergert and Kurth, 1952; Ross and Krahmer, 1971).
The stem appearance is shown in Figure 10. The rhytidome contains alternated bands of phloem (dark brown) with cork tissues (light cream brown) very well distinguishable to the naked eye.

Figure 10. Pseudotsuga menziesii stem, cross section of the bark and SEM photograph of a transverse section of cork.
Periderm Development
The development of the first periderm in P. menziesii is similar to the process reported in Section “Bark Structure and Formation” and when it dies, a new phellogen forms inside the phloem (Grillos, 1956). The new phellogen does not form a whole circumference around the tree, but only in short portions around and along the length of the stem; therefore, the cork layer is interspersed with small parts of inactive crushed phloem (Grillos, 1956; Krahmer and Wellons, 1973). Douglas-fir rhytidome is composed by approximately 49% (in volume) of dead phloem and 51% of periderms (Patel, 1975).
Although the first phellogen can be functional for 25 to 35 years, only the deeper phellogens produce enough cork that show distinct increments (Grillos, 1956).
Most of the periderm is formed by cork cells disposed in well-oriented radial rows and the phelloderm layer is between one and three cells thick (Chang, 1954; Grillos, 1956; Krahmer and Wellons, 1973). The cork thickness is very variable between trees, in average 1.8 cm at breast height ranging from 2 cm to less than 1 mm at root collars and from 5 to 40 cell layers (Kurth, 1953; Grillos, 1956; Ross and Krahmer, 1971). At any particular tree height, the number of cork growth increments is inferior to the age of the tree (Ross and Krahmer, 1971).
One striking feature of Douglas-fir cork is the major presence of bands of compressed cork cells that build up a compact layer of crushed cells due to a severe folding of the radial cell walls (Ross and Krahmer, 1971; Cardoso et al., 2016). These bands of high solid content appear as dark layers, with a successive tangential alignment, separated by light colored layers. These layers have been called as growth increments (Chang, 1954; Ross and Krahmer, 1971; Krahmer and Wellons, 1973).
Cork Cellular Structure
Pseudotsuga menziesii cork cells are in general thin-walled and uniform in thickness. Some thick-walled cells with small pores or pits are occasionally observed either disposed in bands or scattered (Chang, 1954; Grillos, 1956); when their proportion is high the cork is referred as “woody cork” (Grillos, 1956; Krahmer and Wellons, 1973).
Cork cells are more or less isodiametric, symmetrical in shape, and disposed without intercellular voids. On the transverse (Figure 10) and radial directions, they are similar, square to rectangular (if the cells have not been crushed); on the tangential direction, the cells are mostly pentagonal or hexagonal (Krahmer and Wellons, 1973; Cardoso et al., 2016).
Douglas-fir cork cell dimensions are presented in Table 2. The cell prism height and base edge are high and cell wall thickness is low; the radial walls are wrinkled due to growth stresses after the cell walls are formed (Grillos, 1956; Krahmer and Wellons, 1973; Cardoso et al., 2016); 60% of the cells observed by Cardoso et al. (2016) were crushed.
Chemical Composition
Pseudotsuga menziesii cork has a high content of extractives (Table 3) that are mostly polar compounds soluble in ethanol and water (23.5% of the cork) (Ferreira et al., 2015a). The lipophilic extracts (5.4% of the cork) contain as major component catechin (49% of the total), as well as pinoresinol and β-sitosterol (Ferreira et al., 2015a).
The cell walls are composed mainly of suberin (Krahmer and Wellons, 1973; Graça and Pereira, 2000). Suberin represents 36.2% of the Douglas fir cork and lignin 16.8% (Ferreira et al., 2015a). The suberin:lignin ratio is 2.2, a value similar to the one in Q. suber cork (Pereira, 2013).
The monosaccharide composition (Table 4) shows that hemicelluloses contain arabinoxylans and also galactoglucomannans, e.g., galactose and mannose represented 20.4% of the sugars, and xylose and arabinose 24.2%. This differs from the compositions of the other corks and is associated to the fact that Douglas-fir is a softwood (Ferreira et al., 2015a).
Suberin composition (Table 5) shows that the most important monomers are the ω-hydroxyacids, representing 36.2% of the total, followed by the α,ω-diacids (18.6%); chain lengths of C16 and C22 are more relevant (49% of the identified long-chain monomers) (Ferreira et al., 2015a).
When comparing with Q. suber cork suberin, the Douglas-fir suberin contains more saturated α,ω-diacids (hexadecanedioic and octadecanedioic acids), less unsaturated α,ω-diacids, and no epoxyacids (Graça and Pereira, 2000; Ferreira et al., 2015a).
Utilizations in Perspetive
The potential of Douglas-fir bark as a raw material, namely, of its cork component, was recognized since the 50s of last century and several studies were performed (Kurth and Kiefer, 1950; Hergert and Kurth, 1952; Kurth, 1953; Grillos, 1956).
Although there is a considerable proportion of cork in Douglas-fir bark, the small dimensions of the cork layers and their discontinuous distribution with interspersed phloem restrict its direct use and require previous bark milling and particle size separation (Ferreira et al., 2015a,b). An integrated bark valorization approach should contemplate the separation of cork-rich fractions to be further purified and used as a raw material for cork-based products (e.g., composites), while phloem-rich fractions can be used for the extraction of polar soluble compounds (e.g., phenols and polyphenols), while the polysaccharide–lignin matrix may be considered for biorefineries (Ferreira et al., 2015b).
The utilization of the Douglas-fir cork granulates in products that rely on the properties given by the typical cork cellular structure is hindered by the high proportion of crushed cells in this cork (Cardoso et al., 2016). Studies on structural improvement, e.g., cell expansion might therefore increase its utilization potential.
Abies lasiocarpa var. arizonica
Abies lasiocarpa var. arizonica (corkbark fir) is a gymnosperm that occurs naturally in the United States of America, namely, in the Arizona, Colorado, and New Mexico at about 2400–3400 m of altitude. The wood is used for general structural purposes and pulp manufacture. This species is a medium-sized tree growing up to 20 m, exceptionally to 40–50 m.1 The bark forms a rhytidome and has a rough aspect.
The only works found on the cork from the corkbark fir were from the 50s and the 60s of last century (Chang, 1954; Mogensen, 1968).
Periderm Development
The first phellogen initiates in the outermost layer of the cortex below the epidermis. It is long lived and remains active, producing a relatively large amount of cork before a new one is formed, deeper inside the phloem, initiating the formation of a rhytidome. The rhytidome starts at 43–88 years of age, depending on sun exposure (Mogensen, 1968). Before rhytidome formation, the external bark has a soft and spongy nature given by the phellem but becomes rough and hard to the touch after the formation of the rhytidome. The cork layers do not form a complete circumference around the stem and crack due to diameter growth, and the outer layers may wear away (Mogensen, 1968).
Within the cork, there are periodic rows of cells with thick and sclerified outer tangential walls that mark the separation between the growth increments which, in young stems, can be correlated with the age of the stem. The other cork cells are thin-walled and usually slightly elongated radially.
Utilizations in Perspective
The use of this species as a raw material for cork products seems possible since the initial phellogen remains functional for a long period and produces a large quantity of cork. However, little is known on the structure, chemical composition, and properties of this cork, and research is needed to evaluate its potential utilization.
Abies concolor
Abies concolor (white fir) is a gymnosperm that occurs throughout much of the mountainous western North America and, more scattered, in Mexico (Farjon, 2013). The trees are in general 12–15 m high and are exploited for lumber (Gilman and Watson, 2014). The bark in mature trees may be 10–15 cm thick at the stem base and is deeply furrowed, hard, and resistant to fire.
The outerbark contains salmon-colored corky layers interspersed with areas of dark red phloem; cork represents from 25 to 55%, depending on age and location on the stem (Hergert, 1958). Only a few studies were performed in the 50s of last century on white fir bark and on its cork fraction that was separated from air-dried pulverized bark by suspending in 60–85°C water and skimming off the cork particles (Hergert and Kurth, 1953; Hergert, 1958).
The chemical analyses of cork gave the following composition: 26% extractives removed with methylethylketone:water and water, 18.8% hydroxy fatty acids; 26.7% polyphenolic acids, 4.7% low-molecular-weight phenols mainly ferulic acid, 8.2% polysaccharides (mainly cellulose, with glucose amounting to 58% of the neutral monosaccharides), and 15.6% lignin (Hergert, 1958). It should be noted that these results were obtained with a chemical protocol that markedly differs to the more recent summative chemical analysis.
Utilizations in Perspective
The potential commercial value of the cork fraction of white fir bark was recognized early (Hergert, 1958). However, the knowledge is very scarce, and further research should be carried out to characterize this cork and its potential application value.
Concluding Remarks
This review shows that there are some species with a high content of cork in their barks which should be scrutinized, given the importance of cork as a raw material. The species with cork-containing barks may be classified as those with only one periderm and a continuous cork layer, e.g., Q. suber, Q. variabilis, and the cerrado species K. coriacea and P. reticulata, and those who form a rhytidome, therefore with cork layers interspersed with phloem, e.g., P. menziesii and Q. cerris.
The present cork industrial chain is based on the sustainable exploitation of cork from Q. suber, a species for which a dedicated silviculture and management have been fine-tuned along the centuries allowing a sustainable exploitation of the raw material; in succession, the cork industry has developed with sound and innovative technologies, and the body of knowledge on cork and the cork oak science is very large.
The cork from Q. suber is presently the only raw material that has the characteristics necessary for production of solid cork products, e.g., of wine natural cork stoppers, and for which the trituration and production of cork agglomerates are complementary production lines.
A new cork raw material has been brought recently to the market, the Chinese cork from Q. variabilis. Presently used only in triturated form, this raw material appears to have an interesting place given the already large amounts that are harvested as well as the existing forest potential, now still far from an adequate cork-targeted management. Research is under away, and it is foreseeable that in a few years the body of knowledge on this cork will increase substantially.
The valuable properties of cork, as benchmarked by the Q. suber cork, require an adequate combination of structural and cellular features with the chemical composition. This is met by the corks of several species, e.g., K. coriacea, P. reticulata, and Q. cerris, that despite species specificities regarding cell biometry and chemistry, have characteristics that allow forecasting “corkish” properties and uses. It is clear that knowledge on the raw material and on the species has to be gathered before any utilization attempts. This knowledge is scarce for most of the cases, with the exception of Q. cerris cork that has been recently under study and for which a pilot scale test has demonstrated the feasibility of cork separation and use.
The specific cellular characteristics may hinder or limit the use of cork as a cellular material. This is the case of B. pendula cork mainly due to cell biometry, and of P. menziesii due to the large proportion of heavily corrugated or collapsed cells. The use of such corks as a chemical source is, therefore, a promising valorization route. In fact, the extractives are a chemical component group that is receiving a lot of attention in research and development in various fields, including biomedical and health care. Also suberin, the main structural component of cork cell walls, is a macromolecule with an unusual composition of long-chain fatty acids with different functional groups, e.g., hydroxyl, epoxide, and unsaturation, that is species specific and a potential source of chemical intermediates.
The available information gathered in this review on different species with cork-containing barks clearly shows that knowledge is still very limited or inexistent. Research on such barks, namely, regarding their structural and chemical characteristics, is, therefore, a first step toward their prospective valorization.
Author Contributions
All the authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ali Sen, Graciene Mota, and Sofia Cardoso for the photographs of Quercus cerris, Plathymenia reticulata, and Pseudotsuga menziesii, respectively.
Funding
This work was supported by FEDER funds through COMPETE—Programa Operacional para a Competetividade under the project NewCork 38363 and is part of the activities at the Strategic Project (UID/AGR/00239/2013) of Centro de Estudos Florestais, a research unit supported by the national funding of FCT—Fundação para a Ciência e a Tecnologia. CL acknowledges a Ph.D. grant by FCT under the SUSFOR doctoral program (PD/BD/113937/2015).
Footnote
References
Abramovay, R. (1999). Moratória para os cerrados. Elementos para uma estratégia de agricultura sustentável. São Paulo: Departamento de Economia e Programa de Ciência Ambiental da USP.
Anjos, O., Pereira, H., and Rosa, M. E. (2008). Effect of quality, porosity and density on the compression properties of cork. Holz. Roh. Werkst. 66, 295–301. doi:10.1007/s00107-008-0248-2
Anjos, O., Rodrigues, C., Morais, J., and Pereira, H. (2014). Effect of density on the compression behaviour of cork. Mater. Des. 53, 1089–1096. doi:10.1016/j.matdes.2013.07.038
Beck, C. (2010). An Introduction to Plant Structure and Development – Plant Anatomy for the Twenty-First Century. Cambridge: Cambridge University Press.
Bento, M. F. S., Pereira, H., Cunha, M. Á, Moutinho, A. M. C., Van den Berg, K. J., and Boon, J. J. (2001). A study of variability of suberin composition in cork from Quercus suber L. using thermally assisted transmethylation GC–MS. J. Anal. Appl. Pyrol. 57, 45–55. doi:10.1016/S0165-2370(00)00093-0
Bhat, K. M. (1982). Anatomy, basic density and shrinkage of birch bark. IAWA J. 3, 207–213. doi:10.1163/22941932-90000841
Cardoso, S., Ferreira, J. P. A., Quilhó, T., and Pereira, H. (2016). Cork of Douglas-fir bark: impact of structural and anatomical features on usage. Ind. Crop. Prod. (in press).
Carvalho, P. (2009). “Vinhático, Plathymenia reticulata,” in Embrapa Florestas (São Paulo: Comunicado técnico), 231.
Chang, Y. P. (1954). Bark Structure of North American Conifers. Washington, DC: US Dept. of Agriculture.
Conde, E., Cadahía, E., Garcia-Vallejo, M. C., and Gonźalez-Adrados, J. R. (1998). Chemical characterization of reproduction cork from Spanish Quercus suber. J. Wood Chem. Technol. 18, 447–469. doi:10.1080/02773819809349592
Cordeiro, N., Belgacem, M. N., Gandini, A., and Neto, C. P. (1997). Urethanes and polyurethanes from suberin: 1. kinetic study. Ind. Crop. Prod. 6, 163–167. doi:10.1016/S0926-6690(96)00212-9
Cordeiro, N., Belgacem, M. N., Gandini, A., and Neto, C. P. (1999). Urethanes and polyurethanes from suberin 2: synthesis and characterization. Ind. Crop. Prod. 10, 1–10. doi:10.1016/S0926-6690(98)00029-6
Costa, A., and Pereira, H. (2007). Influence of vision systems, black and white, colored and visual digitalization, in natural cork stopper quality estimation. J. Sci. Food Agric. 87, 2222–2228. doi:10.1002/jsfa.2947
Costa, A., and Pereira, H. (2010). Influence of cutting direction of cork planks on the quality and porosity characteristics of natural cork stoppers. For. Syst. 19, 51–60. doi:10.5424/fs/2010191-01166
Duarte, A. P., and Bordado, J. C. (2015). Cork – a renewable raw material: forecast of industrial potential and development priorities. Front. Mater. 2:2. doi:10.3389/fmats.2015.00002
Ekman, R. (1983). The suberin monomers and triterpenoids from the outer bark of Betula verrucosa Ehrh. Holzforschung 37, 205–211. doi:10.1515/hfsg.1983.37.4.205
Ekman, R., and Eckerman, C. (1985). Aliphatic carboxylic acids from suberin in birch outer bark by hydrolysis, methanolysis and alkali fusion. Paperi ja Puu 67, 255–273.
Evert, R. F. (2006). Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. New Jersey: John Wiley & Sons, Inc.
Evtiouguina, M., Barros, A. M., Cruz-Pinto, J. J., Neto, C. P., Belgacem, N., Pavier, C., et al. (2000). The oxypropylation of cork residues: preliminary results. Bioresour. Technol. 73, 187–189. doi:10.1016/S0960-8524(99)00158-3
Evtiouguina, M., Barros-Timmons, A., Cruz-Pinto, J. J., Neto, C. P., Belgace, M. N., and Gandini, A. (2002). Oxypropylation of cork and the use of the ensuing polyols in polyurethane formulations. Biomacromolecules 3, 57–62. doi:10.1021/bm010100c
Evtiouguina, M., Gandini, A., Neto, C. P., and Belgacem, N. M. (2001). Urethanes and polyurethanes based on oxypropylated cork. 1. Appraisal and reactivity of products. Polym. Int. 50, 1150–1155. doi:10.1002/pi.760
FAO. (2015). FAOSTAT – Forestry Production and Trade. Food and Agriculture Organization of the United Nations.
Farjon, A. (2013). Abies concolor. The IUCN Red List of Threatened Species 2013: e.T42276A2969061. doi:10.2305/IUCN.UK.2013-1.RLTS.T42276A2969061.en
Ferreira, J. P., Miranda, I., Sen, A., and Pereira, H. (2016a). Chemical and cellular features of virgin and reproduction cork from Quercus variabilis. Ind. Crop. Prod. 94, 638–648. doi:10.1016/j.indcrop.2016.09.038
Ferreira, J. P., Quilhó, T., and Pereira, H. (2016b). Characterization of Betula pendula outer bark regarding cork and phloem components at chemical and structural levels in view of biorefinery integration. J. Wood Chem. Technol. 1–16. doi:10.1080/02773813.2016.1224248
Ferreira, J. P. A., Miranda, I., Gominho, J., and Pereira, H. (2015a). Chemical characterization of cork and phloem from Douglas fir outer bark. Holzforschung 70, 475–483. doi:10.1515/hf-2015-0119
Ferreira, J. P. A., Miranda, I., Gominho, J., and Pereira, H. (2015b). Selective fractioning of Pseudotsuga menziesii bark and chemical characterization in view of an integrated valorization. Ind. Crop. Prod. 74, 998–1007. doi:10.1016/j.indcrop.2015.05.065
Ferreira, R., Garcia, H., Sousa, A. F., Freire, C. S., Silvestre, A. J., Rebelo, L. P. N., et al. (2013). Isolation of suberin from birch outer bark and cork using ionic liquids: a new source of macromonomers. Ind. Crop. Prod. 44, 520–527. doi:10.1016/j.indcrop.2012.10.002
Gandini, A., Neto, C. P., and Silvestre, A. J. (2006). Suberin: a promising renewable resource for novel macromolecular materials. Prog. Polym. Sci. 31, 878–892. doi:10.1016/j.progpolymsci.2006.07.004
Gil, L. (2015). Cork: sustainability and new applications. Front. Mat. 1:38. doi:10.3389/fmats.2014.00038
Gilman, E., and Watson, D. (2014). Abies concolor. ENH160 Environmental Horticulture, University of Florida/IFAS Extension, Florida.
Graça, J., and Pereira, H. (1997). Cork suberin: a glyceryl based polyester. Holzforschung 51, 225–234. doi:10.1515/hfsg.1997.51.3.225
Graça, J., and Pereira, H. (1998). Feruloyl esters of ω-hydroxyacids in cork suberin. J. Wood Chem. Technol. 18, 207–217. doi:10.1080/02773819809349577
Graça, J., and Pereira, H. (2000). Methanolysis of bark suberins: analysis of glycerol and acid monomers. Phytochem. Anal. 11, 45–51. doi:10.1002/(SICI)1099-1565(200001/02)11:1<45:AID-PCA481>3.0.CO;2-8
Graça, J., and Pereira, H. (2004). The periderm development in Quercus suber. IAWA J. 25, 325–335. doi:10.1163/22941932-90000369
Grillos, S. (1956). Structure and Development of the Bark of Douglas-fir, Pseudotsuga menziesii [PhD. Dissertation], Oregon State University.
Harkin, J. M., and Rowe, J. W. (1971). Bark and Its Possible Uses. Madison: Research Note Forest Products Laboratory 091.
Hergert, H. L., and Kurth, E. F. (1952). The chemical nature of the cork from Douglas-fir bark. Tappi 35, 59–66.
Hergert, H. L., and Kurth, E. F. (1953). The chemical nature of the extractives of white fir bark. Tappi 36, 137–144.
Holloway, P. J. (1972). The composition of suberin from the corks of Quercus suber L. and Betula pendula Roth. Chem. Phys. Lipids 9, 158–170. doi:10.1016/0009-3084(72)90011-4
Holloway, P. J. (1983). Some variations in the composition of the suberin from the cork layers of higher plants. Phytochemistry 22, 495–502. doi:10.1016/0031-9422(83)83033-7
Holloway, P. J., and Deas, A. H. B. (1973). Epoxyoctadecanoic acids in plant cutins and suberins. Phytochemistry 12, 1721–1735. doi:10.1016/0031-9422(73)80393-0
Huang, Z. K., Zheng, C. H., Du, J. X., and Wan, Y. Y. (2006). “Bark classification based on textural features using artificial neural networks,” in International Symposium on Neural Networks (Berlin, Heidelberg: Springer), 355–360. doi:10.1007/11760023_52
Hultén, E., and Fries, M. (1986). Atlas of North European Vascular Plants (North of the Tropic of Cancer), Vol. I-III. Oberreifenberg: Koeltz Scientific Books.
Jensen, W. (1948). Om ytskiktsavfallet vid framst¨ allning avbj ¨ orkfaner (About the Surface Layer Waste from Production of Birch Veneer) [PhD Dissertation], Acta Acad. Aboensis, Math. Phys. XVI. Abo.
Junikka, L. (1994). Survey of English macroscopic bark terminology. IAWA J. 15, 3–45. doi:10.1163/22941932-90001338
Kim, B. (1993). Studies on the physical and mechanical properties of imported and domestic corks. J. Korean Wood Sci. Technol. 21, 45–54 (in Korean with abstract in English).
Kim, B. R., Mishiro, A., Sugiyama, J., and Okano, T. (1990). The physical properties of virgin and reproduction corks of Quercus variabilis Blume. Bull. Tokyo Univ. For. 82, 199–217 (in Japanese).
Krahmer, R. L., and Wellons, J. D. (1973). Some anatomical and chemical characteristics of Douglas-fir cork. Wood Sci. 6, 97–105.
Krasutsky, P., Carlson, R., Kolomitsyn, I., Inventors; University of Minnesota, Assignee. (2003). Isolation of Natural Products from Birch Bark. United States patent US 2003109727.
Krasutsky, P., Carlson, R., Kolomitsyn, I., Krasutsky, D., Inventors; University of Minnesota, Assignee. (2009). Depolymerization Extraction of Compounds from Birch Bark. United States patent US 20090182158.
Krasutsky, P., Carlson, R., Nesterenko, V., Kolomitsyn, I., Edwardson, C., Inventors; University of Minnesota, Assignee. (2005). Birch Bark Processing and the Isolation of Natural Products from Birch Bark. United States patent US20050158414.
Lavender, D. P., and Herman, R. K. (2014). Douglas-Fir: The Genus Pseudotsuga. Corvallis OR: Forest Research Publications Office.
Lev-Yadun, S. (2011). “Bark,” in Encyclopedia of Life Sciences (London: Nature Publishing Group). Published in print in: 2007. Handbook of Plant Science, Vol. 1, ed. K. Roberts (Chichester: John Wiley & Sons, Ltd.), 153–156.
Lima, J. T., and Marcati, C. R. (1989). “Anatomia da madeira de Kielmeyera coriacea Mart. (Pau-santo) – Guttiferae,” in Abstract presented at the XI Congresso Nacional de Botânica (Cuiabá, Brazil), 301.
Marques, A. V., and Pereira, H. (2013). Lignin monomeric composition of corks from the barks of Betula pendula, Quercus suber and Quercus cerris determined by Py–GC–MS/FID. J. Anal. Appl. Pyrol. 100, 88–94. doi:10.1016/j.jaap.2012.12.001
Marques, A. V., Pereira, H., Meier, D., and Faix, O. (1994). Quantitative analysis of cork (Quercus suber L.) and milled cork lignin by FTIR spectroscopy, analytical pyrolysis, and total hydrolysis. Holzforschung 48, 43–50. doi:10.1515/hfsg.1994.48.s1.43
Marques, A. V., Pereira, H., Meier, D., and Faix, O. (1996). Isolation and characterization of a guaiacyl lignin from saponified cork of Quercus suber L. Holzforschung 50, 393–400. doi:10.1515/hfsg.1996.50.5.393
Marques, A. V., Pereira, H., Meier, D., and Faix, O. (1999). Structural characterization of cork lignin by thioacidolysis and permanganate oxidation. Holzforschung 53, 167–174. doi:10.1515/HF.1999.028
Marques, A. V., Pereira, H., Rodrigues, J., Meier, D., and Faix, O. (2006). Isolation and comparative characterization of a Björkman lignin from the saponified cork of Douglas-fir bark. J. Anal. Appl. Pyrol. 77, 169–176. doi:10.1016/j.jaap.2006.03.003
Marques, A. V., Rencoret, J., Gutiérrez, A. C., del Río, J. C., and Pereira, H. (2016). Ferulates and lignin structural composition in cork. Holzforschung 70, 275–289. doi:10.1515/hf-2015-0014
Miranda, I., Gominho, J., and Pereira, H. (2013). Cellular structure and chemical composition of cork from Chinese cork oak (Quercus variabilis). J. Wood Sci. 59, 1–9. doi:10.1007/s10086-012-1300-8
Mogensen, H. L. (1968). Studies on the bark of the cork bark fir: Abies lasiocarpa var. Arizonica (Merriam) Lemmon. I. Periderm ontogeny. J. Ariz. Acad. Sci. 5, 36–40. doi:10.2307/40022822
Mota, G. S., Sartori, C. J., Ferreira, J., Miranda, I., Quilhó, T., Mori, F. A., et al. (2016). Cellular structure and chemical composition of cork from Plathymenia reticulata occurring in the Brazilian Cerrado. Ind. Crop. Prod. 90, 65–75. doi:10.1016/j.indcrop.2016.06.014
Natividade, J. V. (1950). Subericultura. Lisboa: Ministério da Economia, Direcção Geral dos Serviços Florestais e Aquícolas.
Oliveira, V., Knapic, S., and Pereira, H. (2012). Natural variability of surface porosity of wine cork stoppers of different commercial classes. J. Int. Sci. Vigne Vin 46, 331–340.
Oliveira, V., Lopes, P., Cabral, M., and Pereira, H. (2015). Influence of cork defects in the oxygen ingress through wine stoppers: insights with X-ray tomography. J. Food Eng. 165, 66–73. doi:10.1016/j.jfoodeng.2015.05.019
Oliveira, V., Rosa, M. E., and Pereira, H. (2014). Variability of the compression properties of cork. Wood Sci. Technol. 48, 937–948. doi:10.1007/s00226-014-0651-2
Olsson, A., Lindström, M., and Iversen, T. (2007). Lipase-catalyzed synthesis of an epoxy-functionalized polyester from the suberin monomer cis-9, 10-epoxy-18-hydroxyoctadecanoic acid. Biomacromolecules 8, 757–760. doi:10.1021/bm060965w
Patel, R. N. (1975). Bark anatomy of radiata pine, corsican pine, and Douglas fir grown in New Zealand. New Zeal. J. Bot. 13, 149–167. doi:10.1080/0028825X.1975.10430317
Pereira, H. (1988). Chemical composition and variability of cork from Calotropis procera (Ait.) R. Br. IAWA J. 9, 53–58. doi:10.1163/22941932-90000468
Pereira, H. (2013). Variability of the chemical composition of cork. BioResources 8, 2246–2256. doi:10.15376/biores.8.2.2246-2256
Pereira, H. (2015). The rationale behind cork properties: a review of structure and chemistry. BioResources 10, 6207–6229. doi:10.15376/biores.10.3.Pereira
Pereira, H., Graça, J., and Baptista, C. (1992). The effect of growth rate on the structure and compressive properties of cork. IAWA Bull. 13, 389–396. doi:10.1163/22941932-90001294
Pereira, H., Lopes, F., and Graça, J. (1996). The evaluation of the quality of cork planks by image analysis. Holzforschung 50, 111–115. doi:10.1515/hfsg.1996.50.2.111
Pereira, H., Rosa, M. E., and Fortes, M. A. (1987). The cellular structure of cork from Quercus suber L. IAWA Bull. 8, 213–218. doi:10.1163/22941932-90001048
Pereira, H., and Tomé, M. (2004). “Cork oak and cork,” in Encyclopedia of Forest Sciences, eds J. Burley, J. Evans and J. A. Youngquist (Oxford: Elsevier), 613–620.
Pinto, P. C., Sousa, A. F., Silvestre, A. J., Neto, C. P., Gandini, A., Eckerman, C., et al. (2009). Quercus suber and Betula pendula outer barks as renewable sources of oleochemicals: a comparative study. Ind. Crop. Prod. 29, 126–132. doi:10.1016/j.indcrop.2008.04.015
Ponte-e-Sousa, J. C. A. C. C., and Neto-Vaz, A. M. (2011). Cork and metals: a review. Wood Sci. Technol. 45, 183–202. doi:10.1007/s00226-009-0288-8
Richter, H. G., Mazzoni-Viveiros, S. C., Alves, E. S., Luchi, A. E., and Costa, C. G. (1996). Padronização de critérios para a descrição anatómica da casca: lista de características e glossário de termos. IF Série Registros São Paulo 16, 1–25.
Rios, P., Cabral, V., Santos, S., Mori, F., and Graça, J. (2014). The chemistry of Kielmeyera coriacea outer bark: a potential source of cork. Eur. J. Wood Prod. 72, 509–519. doi:10.1007/s00107-014-0811-y
Rios, P., Mori, F., and Barbosa, A. (2011). Morphological characterization of Kielmeyera coriacea Mart. Cork from Brazilian Cerrado. Cerne, Lavras 17, 387–392. doi:10.1590/S0104-77602011000300013
Rios, P. D. (2007). Technological Characterization and Production of Boards of the Cork from Kielmeyera coriacea Mart. (pau-santo). [MSc Dissertation], Universidade Federal de Lavras, Minas Gerais, Brazil.
Rios, P. D. (2011). Anatomy and Chemical Characterization of Kielmeyera coriacea Mart. Cork [Ph.D. Dissertation], Universidade Federal de Lavras, Minas Gerais, Brazil.
Rizzini, C. T., and Mors, W. B. (1995). Botânica econômica brasileira. Rio de Janeiro: Âmbito Cultural.
Ross, W. D., and Krahmer, R. L. (1971). Some sources of variation in structural characteristics of Douglas-fir bark. Wood Fiber 3, 35–46.
Roth, I. (1981). Structural Patterns of Tropical Tree Barks. Berlin: Schweizerbart – Science Publishers Borntraeger.
Schönherr, J., and Ziegler, H. (1980). Water permeability of Betula periderm. Planta 147, 345–354. doi:10.1007/BF00379844
Sen, A., Miranda, I., Santos, S., Graça, J., and Pereira, H. (2010). The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind. Crop. Prod. 31, 417–422. doi:10.1016/j.indcrop.2010.01.002
Şen, A., Pereira, H., Olivella, M. A., and Villaescusa, I. (2015). Heavy metals removal in aqueous environments using bark as a biosorbent. Int. J. Environ. Sci. Technol. 12, 391–404. doi:10.1007/s13762-014-0525-z
Sen, A., Quilhó, T., and Pereira, H. (2011a). The cellular structure of cork from Quercus cerris var cerris bark in a material’s perspective. Ind. Crop. Prod. 34, 929–936. doi:10.1016/j.indcrop.2011.02.015
Sen, A., Quilhó, T., and Pereira, H. (2011b). Bark anatomy of Quercus cerris L. var cerris from Turkey. Turk. J. Bot. 35, 45–55. doi:10.3906/bot-1002-33
Sen, A., Zhianski, M., Ferreira, J., Glushova, M., Petkova, K., and Pereira, H. (2016a). Chemical composition and cellular structure of corks from Quercus suber trees planted in Bulgaria and Turkey. Wood Sci. Technol. 50, 1261–1276. doi:10.1007/s00226-016-0836-y
Sen, A., Leite, C., Lima, L., Lopes, P., and Pereira, H. (2016b). Industrial valorization of Quercus cerris bark: pilot scale fractionation. Ind. Crop. Prod. 92, 42–49. doi:10.1016/j.indcrop.2016.07.044
Silva, S. P., Sabino, M. A., Fernandes, E. M., Correlo, V. M., Boesel, L. F., and Reis, R. L. (2005). Cork: properties, capabilities and applications. Int. Mater. Rev. 50, 345–365. doi:10.1179/174328005X41168
Sousa, A. F., Pinto, P. C. R. O., Silvestre, A. J. D., Gandini, A., and Neto, C. P. (2007). “Novel biopolyesters from suberin monomers,” in Proceedings of the First International Conference on Biodegradable Polymers and Sustainable Composites, BIOPOL-2007 (Alicante, Spain).
Taco, C., Lopes, F., and Pereira, H. (2003). La variation dans l’arbre de l’épaisseur du liège et du dos des planches de liège pour des chênes-lièges en pleine production. Anais Instituto Superior de Agronomia 49, 209–221.
Trockenbrodt, M. (1990). Survey and discussion of the terminology used in bark anatomy. IAWA Bull. 11, 141–166. doi:10.1163/22941932-90000511
Trockenbrodt, M. (1991). Qualitative structural changes during bark development in Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. IAWA Bull. 12, 5–22. doi:10.1163/22941932-90001199
Wei, X., Xiang, S., and Zhou, W. (2007). Chemical composition and its effect on properties of cork from three geographical locations. China Wood Ind. 6, 17–18 (in Chinese with abstract in English).
Weiskittel, A. R., Crookston, N. L., and Rehfeldt, G. E. (2012). Projection of Douglas-fir future habitat and productivity in western North America. Schweiz. Z. Forstwes. 163, 70–78. doi:10.3188/szf.2012.0070
YaFang, L., Ting, J., and Xiaozhou, S. (2012). Chemical composition of cork from Quercus variabilis. Wood Fiber Sci. 44, 214–219.
YaFang, L., YanZhen, L., Wei, Z., and JingFeng, Z. (2009). The microstructure of cork from Quercus variabilis. Scientia Silvae Sinicae 45, 167–170 (in Chinese with abstract in English).
Zhang, L., Lei, Y., and Chang, Y. (2009). Contents of the main chemical components of cork from Quercus variabilis. J. Northwest For. Univ. 24, 163–165 (in Chinese with abstract in English).
Zhang, W. H., and Lu, Z. J. (2002). A study on the biological and ecological property and geographical distribution of Quercus variabilis population. Xibei Zhiwu Xuebao 22, 1093–1101 (in Chinese with abstract in English).
Zhao, J., Feng, D., Lei, Y., Zhang, W., and Zhang, Y. (2013). Cell structure and chemical components of sclereids and lenticels from Quercus variabilis cork. J. Northwest A & F Univ. (Nat. Sci. Ed.) 7, 22 (in Chinese with an abstract in English).
Keywords: cork, Quercus suber L., bark, periderm, rhytidome, phellem, suberin
Citation: Leite C and Pereira H (2017) Cork-Containing Barks—A Review. Front. Mater. 3:63. doi: 10.3389/fmats.2016.00063
Received: 29 September 2016; Accepted: 22 December 2016;
Published: 19 January 2017
Edited by:
Pellegrino Musto, National Research Council, ItalyReviewed by:
Ernesto Di Maio, University of Naples Federico II, ItalyMichele Galizia, University of Texas at Austin, USA
Copyright: © 2017 Leite and Pereira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Leite, Y2xlaXRlQGlzYS51bGlzYm9hLnB0
 Carla Leite
Carla Leite Helena Pereira
Helena Pereira