
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 26 March 2025
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1570936
Fisheries Restricted Areas (FRAs), as area-based fisheries management tools, can be effective in providing protection for fisheries and biodiversity, in addition to traditional Marine Protected Areas (MPAs), and are already in effect in waters above 1,000 m of the Mediterranean and the Black Seas. Whereas in the North-Western part of the Black Sea all fishing activities are concentrated in the shallow area (at depths of maximum 90 m), where species and habitats of conservation interest are present, this restriction is completely irrelevant. In this context, given that a FRA can also be established nationally (nFRA), the main objective of this research was pre-identifying suitable area(s) at the Romanian coast and providing the scientific substantiation for such an endeavor. In addition to being a traditional fishing ground for small-scale local fishermen, the study perimeter, which includes the Northern Romanian coast (part of the Marine Zone of the Danube Delta Biosphere Reserve), was chosen for various reasons. First, because of the uniqueness of the habitats found here, it serves as a vital forage area for fish species that are valuable both from a conservation and economic standpoint. Additionally, during the past ten years, beam trawl fishing for the gastropod Rapana venosa (Valenciennes, 1846) has affected the area, potentially causing harm to benthic habitats. A variety of data sources were integrated as part of the research strategy, including the composition and spatial distribution of macrozoobenthos, an inventory of the local fish fauna conducted through scientific fishing (both for commercial fish and species of conservation interest), fish stomach content analysis (by dissection or gastric lavage), and records of fishing effort and catch. The identified Sf. Gheorghe - Sahalin nFRA, covering 272.76 km2 between the 40 m and 50 isobaths, proved to be appropriate according to both ecological and fisheries criteria, and, after public consultation, was established by law, thus becoming the first national Fisheries Restricted Area in the Black Sea, to the benefit of both nature conservation, by spillover effects to adjacent areas, and the livelihood of local coastal communities.
The latest statistics of the General Fisheries Commission for the Mediterranean (GFCM) revealed that more than half of fish stocks monitored (58%) are overexploited, including stocks in the Black Sea (FAO, 2023). Despite this being the lowest rate observed since the trend in overexploitation was first reversed a decade ago, taking alternative measures to counter overfishing and restore fish populations is essential for sustainable fisheries management. In addition to applying the general provisions of the Common Fisheries Policy (CFP), the establishment of spatial restrictions can help protect Essential Fish Habitats (EFH), thus reducing pressure on stocks and Vulnerable Marine Ecosystems (VME), by mitigating the impact on sensitive species and habitats (FAO, 2023). This can be achieved by establishing Fisheries Restricted Areas (FRAs), an approach that is fully consistent with Target 1 of the GFCM 2030 Strategy, which aims to implement effective spatial measures to reduce the impact on vulnerable species, sensitive habitats and essential fish habitats, with the purpose of meeting conservation objectives at international level (FAO, 2021). Moreover, the creation of such restricted areas also meets the objectives of the European Union’s 2030 Biodiversity Strategy, which states that 30% of the EU’s marine area should be under protection (European Commission, 2020).
Restricting fishing activities in certain critical areas for ichthyofauna (such as feeding, spawning or wintering grounds) is intended to reduce total fishing mortality and habitat destruction/impairment (Taylor et al., 2021). Properly designed and implemented fishing restricted areas provide the opportunity for improved fisheries management to mitigate potentially destructive changes to critical habitats by different fishing methods (Lauck et al., 1998). The use of such “closed” areas has been particularly promoted in tropical reef systems, where habitat attributes give structure to the ichthyological community and movements of adults outside the boundaries of these areas are limited (Bohnsack, 1994), but more recently it has also proven effective in temperate areas (Murawski et al., 2000). For example, in the Northwest Atlantic (USA), restricted areas have become important elements in fisheries management programs, and their use, combined with more aggressive measures (direct controls and sanctions), has led to major changes in the rate of fishing mortality, species abundance and distribution (Murawski et al., 2000).
In the Mediterranean Sea and the Black Sea, 1,760,000 km2 are under the protection regime as Fisheries Restricted Areas established under the GFCM (gfcmFRAs), most of this area being covered by the deep-sea zone (in waters above 1,000 m of the Mediterranean and the Black Sea, any trawling and dredging activity is prohibited, in order to protect demersal habitats) (FAO, 2021). Given that the Black Sea is anoxic and practically lifeless at depths greater than 200 m (Dubinin et al., 2024), this restriction is totally ineffective and irrelevant, as all fishing activities are concentrated in the shallow area (at depths of maximum 90 m), where species and habitats of conservation interest, such as deep-sea mussel clumps on soft substrate and sturgeons, are present (Nicolaev et al., 2018). In addition to being impacted by fisheries, the North-Western Black Sea shelf habitats in front of the Danube Delta are under continuous stress induced by the Danube’s inputs and anthropogenic influence (Teacă et al., 2020). The constant stress to fish stocks caused by the Danube’s outflow calls for careful management measures, the establishment of the FRA contributing to mitigating this impact. In this context and given that a FRA can also be established at national level (Petza et al., 2017), our research aimed at identifying suitable area(s) at the Romanian coast and providing the scientific substantiation for such an endeavor.
The study perimeter, which covers the northern part of the Romanian coast (part of the Marine Zone of the Danube Delta Biosphere Reserve ROSCI0066), was selected for several reasons: firstly, through the uniqueness of the habitats present here (Teacă et al., 2020), it represents an essential feeding area for fish species valuable both from a conservation and economic point of view (Niță et al., 2023a); moreover, it is a traditional fishing ground for small-scale local fishermen (Niță et al., 2023b), and, additionally, in the last decade, the area has been affected by beam trawl fishing for the gastropod Rapana venosa (Valenciennes, 1846), with a potentially destructive impact on benthic habitats (Danilov et al., 2019; Mureșan et al., 2019; Teacă et al., 2019; Nenciu et al., 2023). The research strategy applied involved integrating several types of data, starting from macrozoobenthos composition and spatial distribution, an inventory of the fish fauna in the area performed by scientific fishing (both for commercial fish and species of conservation interest), fish stomach content analysis (by dissection or gastric lavage), as well as catch and fishing effort records. The ultimate goal of this study was identifying an area suitable to be proposed for closure of fisheries which would benefit both nature conservation and the livelihood of local coastal communities, by potential spillover and propagation effects to adjacent fishing grounds.
The study area is located in the North-Western Black Sea, in Romania’s Exclusive Economic Zone, at depths ranging from 37 to 53 m. It encompasses the Natura 2000 sites ROSCI066 and ROSCI0413, along with adjacent areas. These marine protected areas (MPAs) were designated under the EC Habitats Directive (2009/92/EEC) in 2009 to protect unique marine ecosystems. The region is strongly influenced by the Danube River, the primary source of inorganic sediment input into the Black Sea, surpassing contributions from other major rivers such as the Dniester and Dnieper. This area represents a critical ecological transition zone, where Danube Delta habitats gradually merge with the continental shelf. Local hydrodynamics, nutrient influx, and sediment-laden freshwater shape distinct habitat types. The invertebrate fauna exhibits a mixed composition, ranging from brackish species with high salinity tolerance to strictly marine taxa (Teacă et al., 2020; Menabit et al., 2024).
The macro-zoobenthos data were obtained within the various national projects of the Romanian Nucleu Program (GEODEMAN, GEOBIOECOMAR, GEOMARDIGITAL), the Large Infrastructure Operational Program funded project “Revision of the Management Plan and Regulation of the Danube Delta Biosphere Reserve”), as well as from international grants (H2020 - BRIDGE-BS) implemented by GeoEcoMar in the last six years. During the eight scientific surveys carried out in the northern area of the Romanian continental shelf of the Black Sea with the R/V “Mare Nigrum”, during 2018-2023, 43 macrozoobenthos samples were collected from 37 stations (Figure 1), using a Van Veen boden-greifer (area 0.125 m2). To reduce the sample volume and make the sorting of organisms easier, the collected biological material was separated from the sedimentary material on board the vessel by passing the samples through a granulometric sieve with a 0.5 mm mesh size. The biological material was subsequently preserved with a 4% formaldehyde saline solution and stored in plastic containers (Băcescu et al., 1971; Holme and McIntyre, 1984; Todorova and Konsulova, 2005).
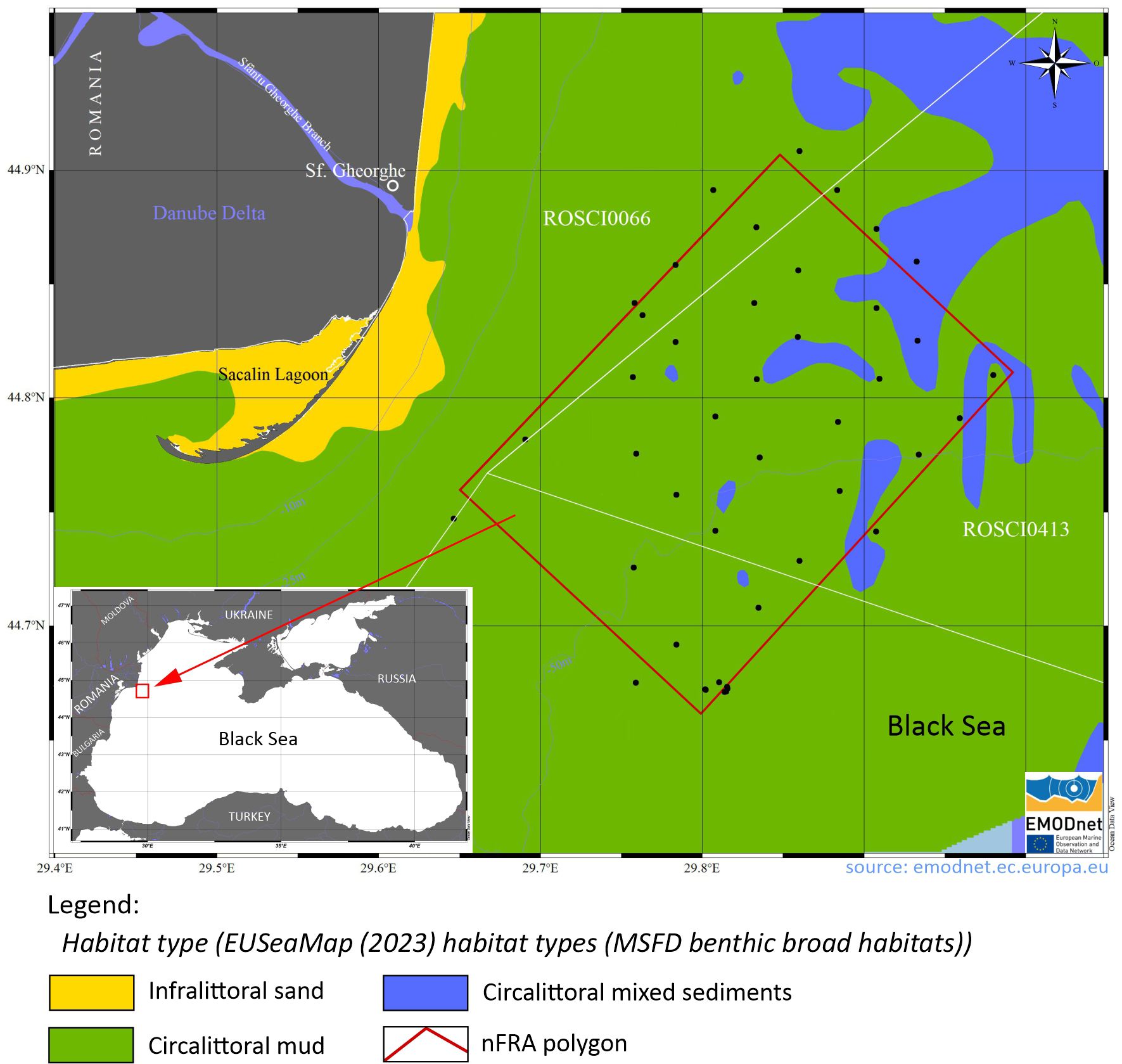
Figure 1. Map of macrozoobenthos sampling stations in the northern area of the Romanian continental shelf of the Black Sea (2018-2023).
In the laboratory, the samples were processed according to the procedure described by Todorova and Konsulova (2005), as follows: the sorting of macrobenthic organisms (e.g. mollusks) was done directly, visually (macroscopically) or by means of a low power binocular magnifier; the sorting of the rest of the macrozoobenthos organisms was carried out with a stereomicroscope; the identification of all organisms was performed under a microscope and the nomenclature of species was checked following the World Register of Marine Species (WoRMS, 2024); all data were recorded in a sorting/triage sheet and used for further processing and statistical analysis. Density and biomass (as wet weight) were referred to one square meter (ind.m-2, g.m-2), and bivalves were weighed with shells.
The macrozoobenthic community structure was analyzed based on species composition (S), density, dominance (D), frequency (F), diversity (H’), and biomass (B). Diversity was assessed using the Shannon-Wiener index (H’) with a log₂ base (Shannon and Weaver, 1963). Spatial analyses were conducted using Ocean Data View (v.5.4.0) (Schlitzer, 2018), while univariate and multivariate analyses were performed with PRIMER 7 (v.7.0.17) (Clarke et al., 2014). Multidimensional scaling (MDS) and cluster analysis were applied using density and biomass values.
The AZTI Marine Biotic Index (AMBI) (Borja et al., 2000) and multivariate AMBI (M-AMBI) (Muxika et al., 2007) were calculated using dedicated software (v.5.0) (AZTI, 2024). Species distribution across the five Ecological Groups (EG) was based on the AMBI species list (June 2017) (Borja and Muxika, 2005). The M-AMBI*(n) index was subsequently derived as the arithmetic mean of the normalized minimum and maximum AMBI, H’, and S (Sigovini et al., 2013). AMBI and related indices were applied following recommendations from national authority reports (Abaza et al., 2018) and the Romania-Bulgaria intercalibration initiative (Todorova et al., 2018). For circalittoral habitats, a threshold value of 0.68 for M-AMBI*(n) was used to distinguish between Good Ecological Status (GES) and non-GES (Abaza et al., 2018).
Scientific fishing surveys were carried out in the northern area of the Romanian continental shelf with NIMRD’s R/V “Steaua de Mare 1”, the “Thethys” motor boat and the “Kingfisher” pneumatic boat in the frame of the National Fisheries Data Collection Programme and the Large Infrastructure Operational Program funded project “Revision of the Management Plan and Regulation of the Danube Delta Biosphere Reserve”, following which an inventory of ichthyological biodiversity was made in the Marine Zone of the Danube Delta Biosphere Reserve (ROSCI0066) and the adjacent offshore area (Figure 2). Fish sampling was conducted in compliance with the Scientific Fishing License no. 05/20.02.2024, issued by the Romanian National Agency for Fisheries and Aquaculture. Concerning the vulnerable sturgeon species, all Acipenseridae specimens included in the study were by-caught (not targeted) and, after performing the gastric lavage procedure onboard the fishing vessel, all individuals were released back into the wild alive and viable.
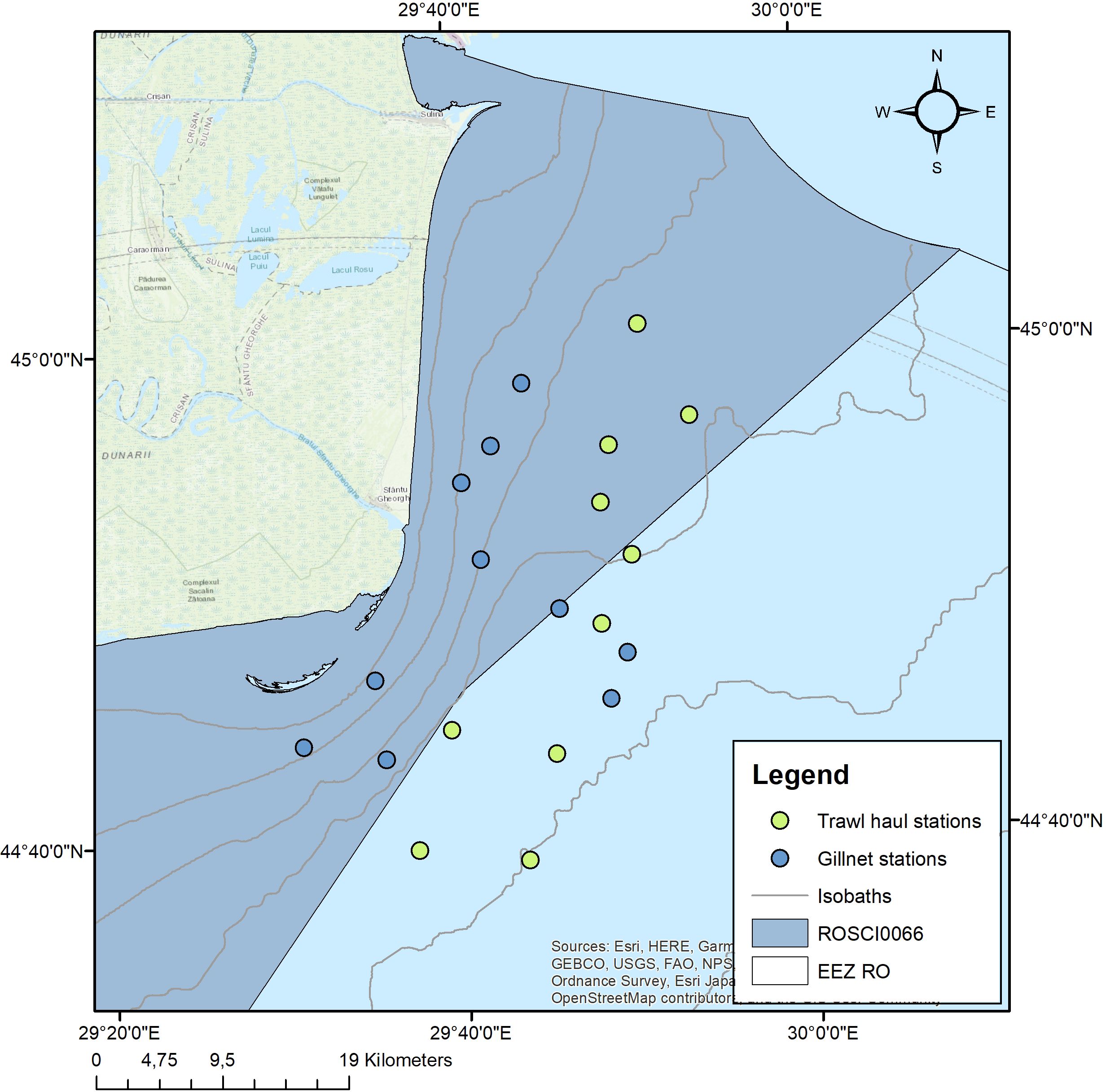
Figure 2. Map of scientific fishing stations in the Northern part of the Romanian continental shelf.
The fishing gears used were pelagic and demersal trawls (operated from the research vessel) (Supplementary Figure 1A) and fishing nets launched and recovered using the motorboats, namely gillnets (mesh size a = 32 mm, height = 4 m, length = 95 m) and trammel nets (mesh size a = 32 mm, height = 3.20 m, length = 95 m) (Supplementary Figure 1B).
The ichthyological samples taken were analyzed in the field or in the laboratory, as the case may be, both quantitatively and qualitatively (Supplementary Figure 2). Systematic classification was achieved with verification in global biodiversity information systems (FishBase, 2024; WoRMS, 2024). The inventory of the species that make up the ichthyofauna in the investigated area was represented in the form of a table with the species caught during the surveys.
In order to correlate information on essential benthic habitats for fish species, stomach content analysis was performed using two different methods: classic dissection analysis (for commercial species) and gastric lavage with subsequent release of the individuals (for protected species).
Turbot Scophthalmus maeoticus (Pallas, 1814) individuals (species of commercial interest) caught in the investigated area were measured, weighed (with and without stomach), dissected in order to extract the stomach (which was then tied at the ends); then the stomach was weighed (with and without food), the identification of food elements at microscope being subsequently performed (Supplementary Figure 1). The stomachs were preserved in 4-10% formaldehyde solution, and the contents later examined in the laboratory.
To investigate the stomach contents in sturgeons without endangering the physical integrity of the individuals, gastric lavage was performed, adapting internationally agreed methods (Haley, 1998; Brosse et al., 2000, 2002). A plastic jar was used to collect the stomach contents. A catheter was carefully inserted through the oesophagus down to the first gastric loop. The catheter was attached to a wash bottle, which was used to pump seawater into the digestive tract of the sturgeon. The pumping of seawater forced the discharge of the stomach contents into the collection jar. The whole procedure, from the retrieval of the fish from the fishing gear to the release back into the sea, took between 3 and 7 minutes, depending on the size of the specimen, as well as the amount of food in the stomach (Brosse et al., 2000, 2002). The lavage procedure itself took about 30 seconds/individual. After being subjected to gastric lavage, all sturgeon specimens were safely released into the sea (Supplementary Figure 1), and stomach contents samples were preserved in 4-10% formaldehyde solution, and later analyzed in the laboratory.
In the laboratory, two methods were used to determine the stomach contents in both turbot and sturgeons, namely: qualitative and quantitative analyses. The qualitative analysis consisted in the complete identification of the food items present in the fish stomach. The quantitative method consisted of numerical analysis, and the calculated parameters were: Frequency of Occurrence (FO), Dominance (Di%) and Index of Relative Importance (IRI).
The Frequency of Occurrence (FO%), as a numerical percentage of the food items identified, was calculated to characterize the stomach contents (Hyslop, 1980; Hansson, 1998), according to the formula:
where FOi stands for the number of stomachs where the species “i” occurs, and FOt represents the total number of stomachs analyzed.
Dominance (Di%) was calculated as a share of stomachs dominated by a certain type of food and expressed as a percentage of the total number of stomachs analyzed (Hyslop, 1980), according to the formula:
where Di stands for the total number of taxa of a group, and Dt is the total number of taxa identified in the stomach contents analyzed.
The Index of Relative Importance (IRI) represents an integrated value of number, mass and frequency of occurrence, which evaluates the relationship between different food items in the stomach (Ahlbeck et al., 2012). It is calculated according to the formula:
where Ni, Wi and FOi are the percentages of the number, mass, and frequency of occurrence, respectively, of the food item “i”.
To estimate comparisons between groups within the food array of a species, IRI was standardized to %IRI (Cortés, 1997).
Catch (kg) and fishing effort (days at sea) information for the period 2022-2024 for all commercial species in the target area were centralized from the Romanian National Agency for Fishery and Aquaculture (NAFA), covering the landing points in Sulina and Sf. Gheorghe. The purpose of collecting these data was to create a baseline for assessing the future effect of establishing a fishery restricted area on fisheries in the North-Western part of the Romanian coast. A regression model was applied to calculate the correlation between total fish catches and fishing effort using the Excel software (Microsoft Office 365 Business Pack).
A total of 107 macrobenthic taxa were identified, representing 20 major taxonomic groups. Most taxa were classified to the species level, except for Oligochaeta, treated as a superspecific taxon, and some Nemertea representatives, which remained indeterminate. The identified species belong to Porifera (6 species), Anthozoa (2), Hydrozoa (3), Turbellaria (2), Nemertea (6), Polychaeta (30), Gastropoda (7), Bivalvia (14), Pantopoda (2), Phoronida (1), Cirripedia (1), Amphipoda (15), Cumacea (4), Isopoda (1), Tanaidacea (1), Echinodermata (2), and Tunicata (4). Polychaetes constituted 28% of the total taxa, followed by mollusks, crustaceans, and other invertebrate groups (Porifera, Coelenterata, Pantopoda, Echinodermata, and Tunicata) with relatively similar representation.
Two major benthic habitats were delineated using a broad manual mapping approach based on a sampling point grid, considering community structure, the presence and coverage of ecosystem engineers’ species, and substrate type (Figure 3). These habitats include mixed circalittoral sediments with Dipolydora quadrilobata and biogenic reefs with Mytilus galloprovincialis, as well as muddy circalittoral sediments with Melinna palmata (Supplementary Figure 1).
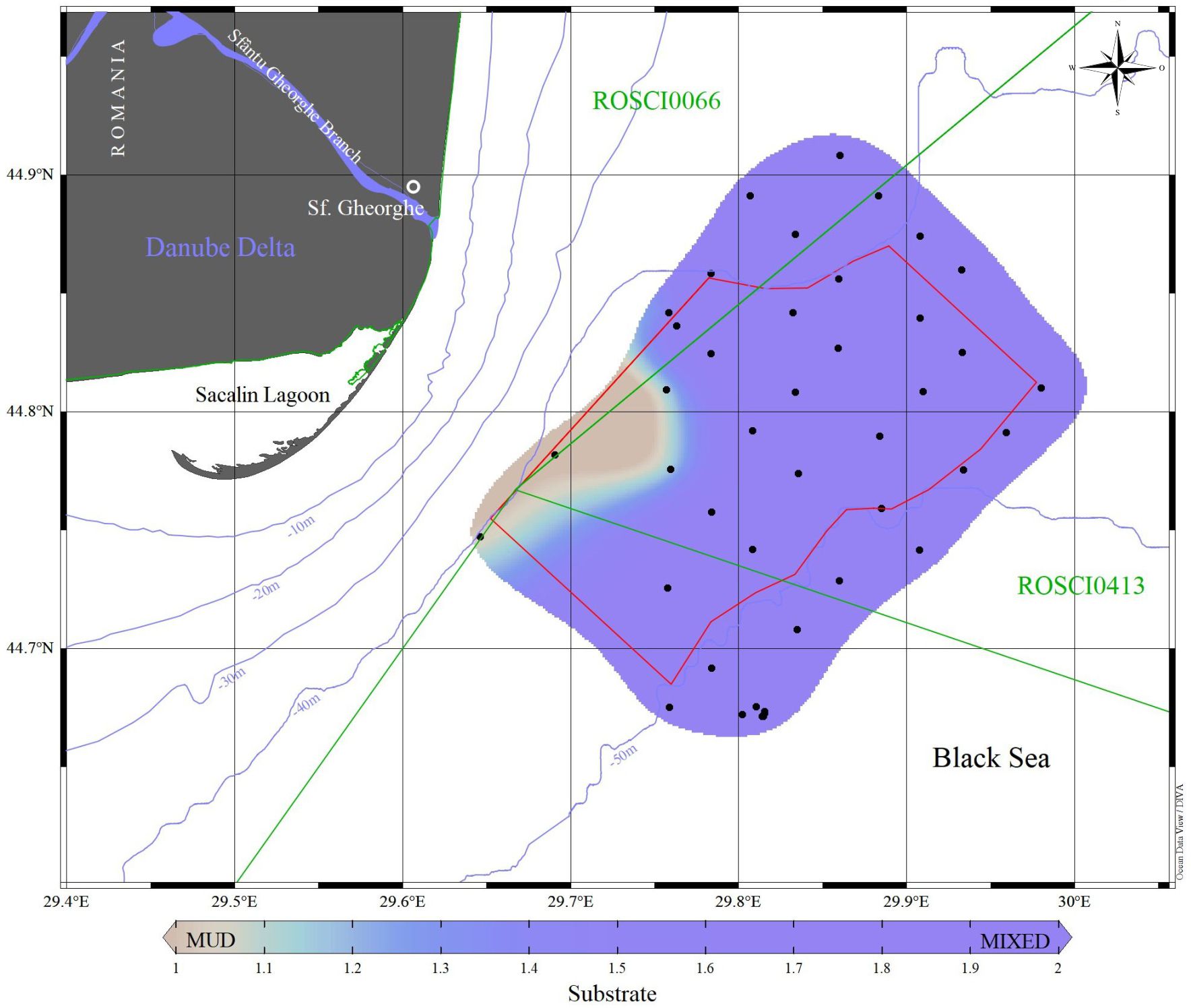
Figure 3. Spatial distribution of the benthic habitats identified in the study area (habitats type: circalittoral mixed sediments with Dipolydora quadrilobata and Mytilus galloprovincialis biogenic reefs; circalittoral mud with Melinna palmata).
A two-way analysis of similarity (ANOSIM) based on the Bray-Curtis matrix was conducted to assess differences in benthic community structure across stations, bathymetric ranges, and benthic habitats (Figure 4). The similarity analysis, based on macrozoobenthic population densities and multidimensional scaling, revealed a variation between 44% and 54% (global ANOSIM R = 0.716, p = 0.1%, permutations = 999). The dissimilarity in numerical abundances of macrobenthic species between the identified groups was 81%, indicating well-differentiated communities. Species richness differ between habitats, significantly higher species number was observed at Dipolydora habitat compared to Melinna habitat (One-Way ANOVA, F = 12.5, p = 0.007).
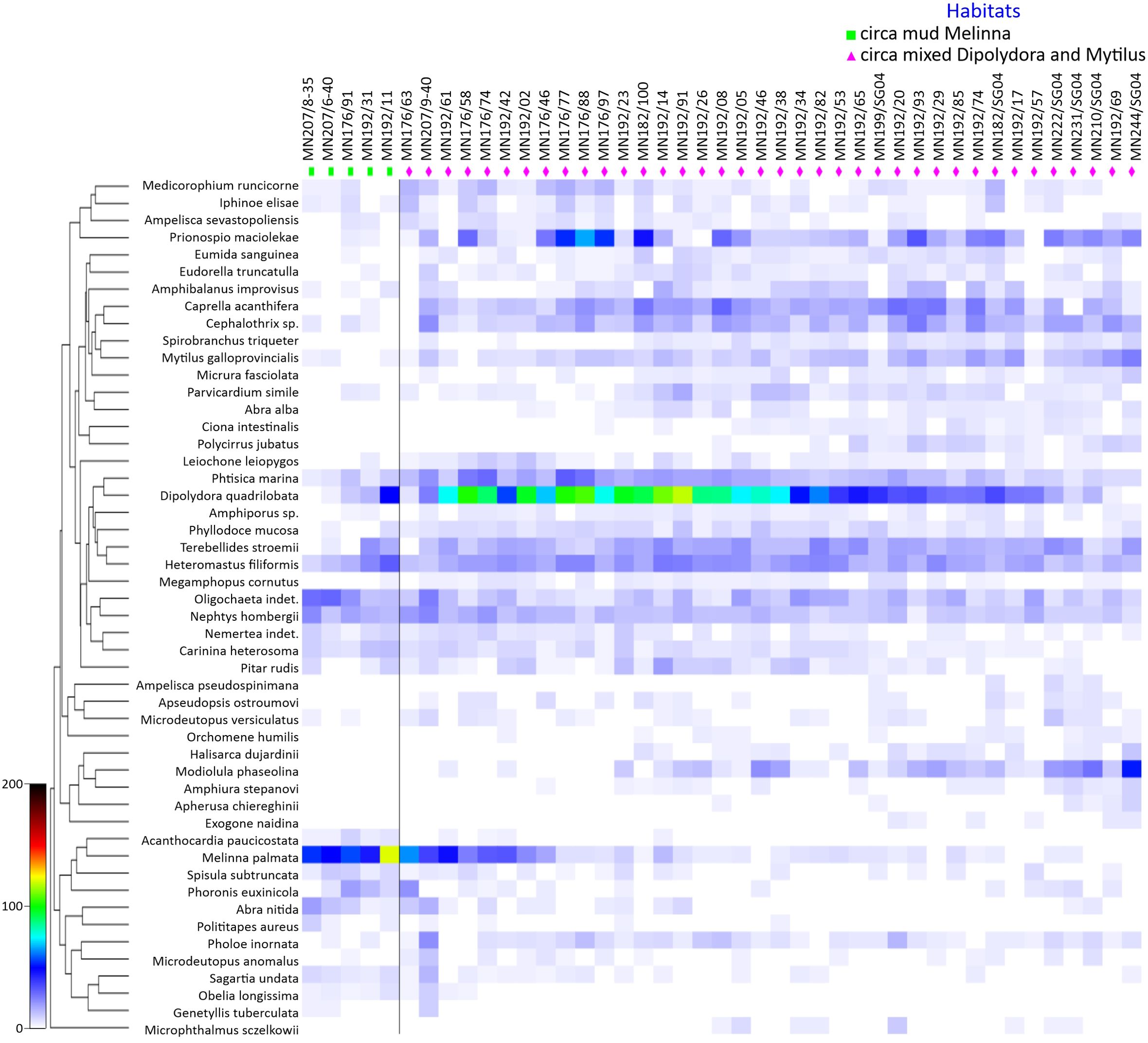
Figure 4. Benthic species association index based on numerical abundance and diversity data by habitat type.
Of the 107 taxa identified, 102 were recorded in the mixed circalittoral sediment habitat with Dipolydora quadrilobata and biogenic reefs with Mytilus galloprovincialis. This habitat represents an enclave in the northern circalittoral zone, extending from the Phyllophora habitat on the Ukrainian side. D. quadrilobata has occupied an empty ecological niche following the decline of sciaphyllous rhodophyte algae (Teacă et al., 2020). The primary factor defining this habitat is substrate composition, marking a transition to deeper circalittoral habitats. Species richness in samples ranged from 44 to 48 species (Figure 5), with a community dominated by endobenthic tube-building polychaetes (D. quadrilobata) and mussel clumps. D. quadrilobata is an opportunistic species that has recently become widespread along the Romanian coast, frequently dominating areas with high organic sediment loads. On sedimentary bottoms, it constructs tubes from sand particles bound by fine pelitic fractions, which are vertically embedded within the sediment.
The characteristic species of the mixed circalittoral sediment habitat with D. quadrilobata and biogenic reefs with M. galloprovincialis, highlighted based on ecological index calculations, included D. quadrilobata, Heteromastus filiformis, Nephtys hombergii, Phtisica marina, and various oligochaete species, all with 100% frequency. Among the top 10 species ranked by ecological significance index based on density, only two crustaceans were present: P. marina and Caprella acanthifera.
As with diversity, numerical abundance (density, ind. m-2) and biomass (g m-2) varied considerably between stations, as reflected in the population similarity indices and station clustering dendrogram. The average macrozoobenthic density in this habitat was 7,935 ind. m-2, with an average biomass of 396 g m-2. The bivalve species in mussel-dominated areas were Abra alba (F% = 66), Parvicardium simile (F% = 84), and Pitar rudis (F% = 76). M. galloprovincialis, the dominant species, exhibited an uneven mosaic distribution, with an average abundance of 43 ind. m-2 and 317 g m-2, accounting for 86% of the total macrobenthic biomass in this habitat.
The second habitat identified in the study area, the muddy circalittoral habitat with M. palmata, comprised 52 taxa - 51% fewer than the D. quadrilobata and M. galloprovincialis habitat. This lower diversity (Figure 6) results from the specific environmental conditions that favor species with high abiotic tolerance. The accumulation of detritus in the sediments, during the 1970s, facilitated the mass expansion of M. palmata populations, leading to the establishment of a new community on the Romanian coast. This community developed within areas previously occupied by deep-sea mussel habitats, with M. palmata as the dominant species. Between 1970 and 1980, M. palmata populations had an average density of approximately 2,330 ind. m-2, with peak values exceeding 17,000 ind. m-2 (Gomoiu, 1982). In the present study area, M. palmata exhibited 100% frequency, with an average density of 5,020 ind. m-2, accounting for 65% of the total macrozoobenthic density (7,672 ind. m-2). Compared to the Dipolydora-Mytilus habitat, macrozoobenthic abundances in the Melinna habitat were slightly lower (Supplementary Figure 2).
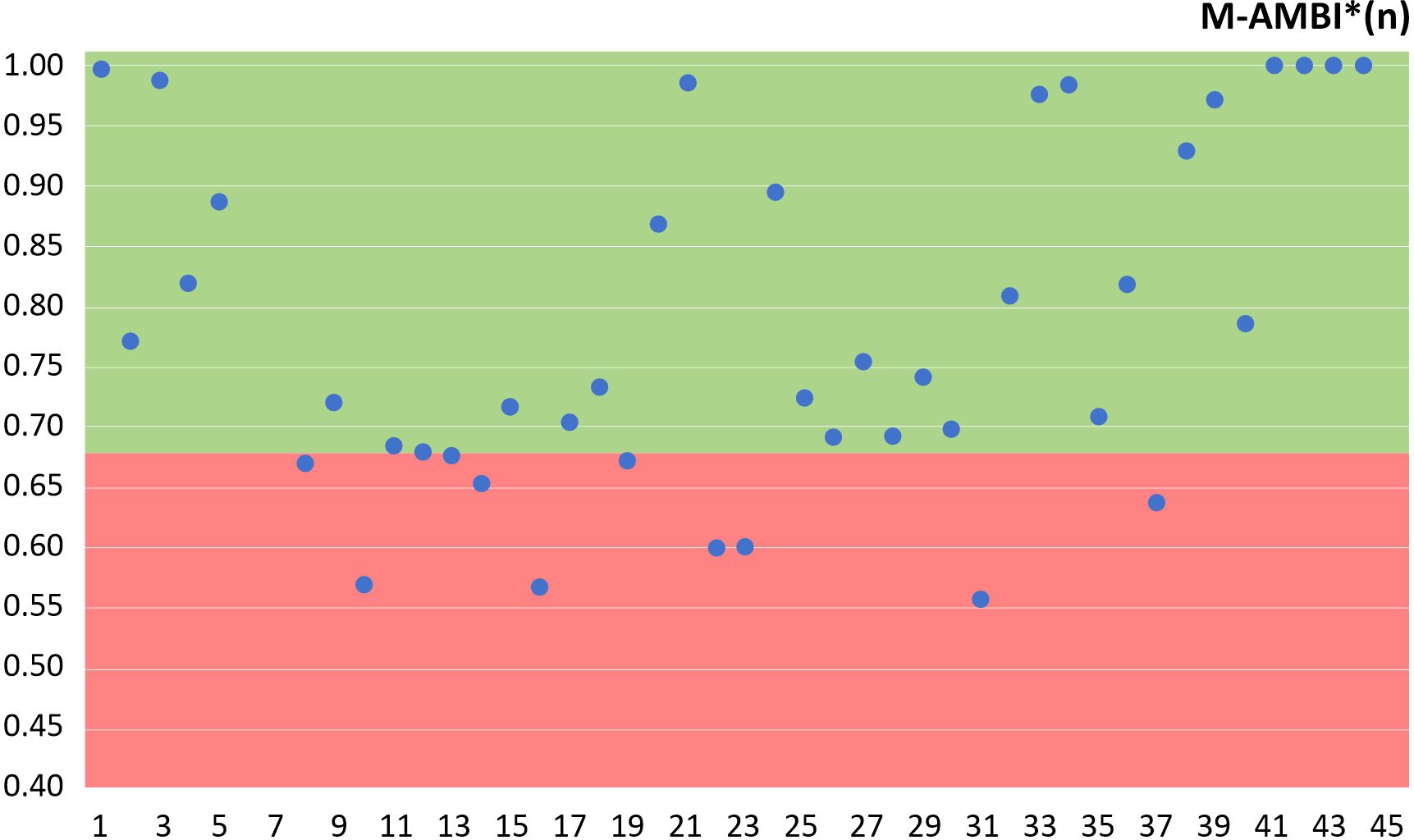
Figure 6. The ecological status of the mixed circalittoral sediment habitat with D. quadrilobata and M. galloprovincialis and the muddy circalittoral sediment habitat with M. palmata, calculated according to the M-AMBI*(n) index (green - Good Ecological Status/GES; red - non-GES).
In the Melinna habitat, biomass is mostly dominated by the bivalves Polititapes aureus, M. galloprovincialis, Spisula subtruncata, and Abra nitida, along with the large polychaetes M. palmata and N. hombergii. Spatial distribution of average biomass values indicated lower and more uniform biomass in the Melinna habitat compared to the Dipolydora-Mytilus habitat (Supplementary Figure 3).
The application of the AMBI biotic index revealed a relatively low percentage of sensitive species (EG I), generally below 10%, ranging from 1% in the Melinna habitat to 51% in a station within the Dipolydora-Mytilus habitat. In contrast, second-order opportunistic species (D. quadrilobata, M. palmata, etc.) dominated most stations (~44%) but exhibited high variability (1.6%–82%). Tolerant and indifferent species together accounted for 42%, while first-order opportunistic species (EG V) were absent in some stations, with proportions ranging from 0.07% to 21.2%.
Furthermore, based on the M-AMBI*(n) index and the GES/non-GES thresholds from Teacă et al. (2020) and Abaza et al. (2018), 80% of the investigated habitats achieved Good Ecological Status (GES) in accordance with the Marine Strategy Framework Directive (Figure 6).
Out of the 140 species of fish reported in the Black Sea (Niță et al., 2024), in the research expeditions carried out by NIMRD’s experts in the northern part of the Romanian coast, 62 fish species were identified (Supplementary Table 1), of which 20 are of commercial interest (turbot, picked dogfish, flounder, sole, Black Sea sprat, whiting, garfish, sprat, anchovy, horse mackerel, Azov shad, bluefish, golden-gray and flathead mullet, red mullet, big-scale sand smelt, Danube shad, black goby, round goby, knout goby), Moreover, species of high ecological and conservation importance were also by-caught, namely the three sturgeon species: beluga, Russian sturgeon and starry sturgeon. It should be mentioned that, in the last 5 years, a slight increase in the number of fish species identified on the Romanian coast was reported (Niță et al., 2024). Although the number of species consistently observed in the analyzed samples decreased, the occurrence of rare species had a slight upward trend (Niță et al., 2023b).
Regarding the conservation status of fish species identified following research surveys in the northern sector of the Romanian coast (according to IUCN - International Union for Conservation of Nature), out of the 62 species identified, 4 species (6%) are Critically Endangered (CR), namely the 3 sturgeons: Huso huso, Acipenser gueldenstaedtii and Acipenser stellatus, and Anguilla anguilla, 4 species (6%) are Vulnerable (VU) - Alosa immaculata, Pomatomus saltatrix, Umbrina cirrosa and Squalus acanthias, 2 species (3%) are Near Threatened (NT) - Sciaena umbra and Raja clavata, 6 species (9%) are Data Deficient (DD) - Ophidion rochei, Dasyatis pastinaca, Hippocampus guttulatus, Syngnathus schmidti, Syngnathus tenuirostris and Syngnathus variegatus, and 6 species (10%) are Not Evaluated (NE) - Sprattus sprattus, Gaidropsarus mediterraneus, Planiliza haematocheilus, Aphia minuta, Pegusa nasuta and Scophthalmus maeoticus. The remaining 40 species (65% of the total reported species) inventoried in the investigated sector are Least Concern (LC) (Figure 7).
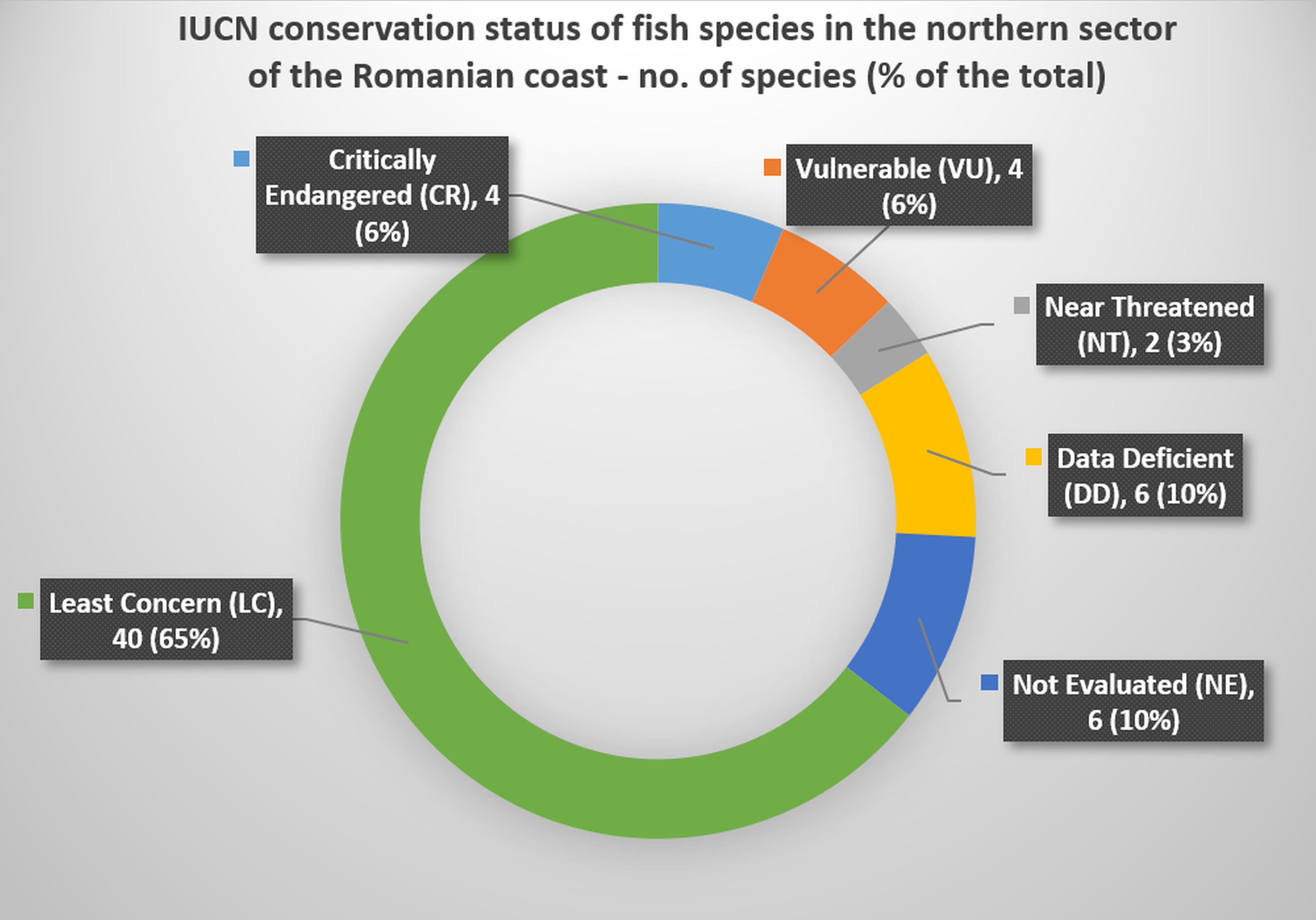
Figure 7. IUCN conservation status of fish species in the northern sector of the Romanian coast - no. of species (% of the total).
In order to substantiate the proposal of a delimited area in which fisheries are restricted at national level (nFRA), among all the fish species identified in the northern part of the Romanian coast, turbot (S. maeoticus), as a species with high economic value, and the 3 sturgeon species (H. huso, A. gueldenstaedtii and A. stellatus), representing species of conservation importance, were studied in detail, focusing mainly on their feeding behavior by stomach content analysis.
Following the scientific fishing surveys carried out, the greatest abundance of turbot (over 60 out of 100 specimens) and sturgeons (10 out of 15 accidentally caught individuals) encountered in the catch were recorded in the perimeter located off the Sahalin Peninsula - Sfântu Gheorghe, at a distance of approximately 10 km from the shore, between the 40 and 50 m isobaths, which indicates the area as extremely important for fish species, which use it mainly for feeding.
Turbot is a predatory fish that occupies an important position in the marine food web, thus, studying the stomach contents of this species contributes to the analysis of trophic interactions in the Black Sea basin (Niță et al., 2024). The 100 specimens analyzed during the study period had an average length of 51.02 cm and an average biomass of 2,629.13 g, all exceeding the legal minimum size (45 cm) for commercial fishing in Romanian waters.
Regarding the analysis of the dominance of food elements, the following results were obtained after investigating the stomach contents of turbot: the dominant group was fish (81.13%), followed by mollusks (12.85%), then crustaceans (3.15%) and polychaetes (2.87%) (Figure 8). With reference to the percentual value of the Index of Relative Importance (%IRI), the fish group was again the one that prevailed (Table 1).
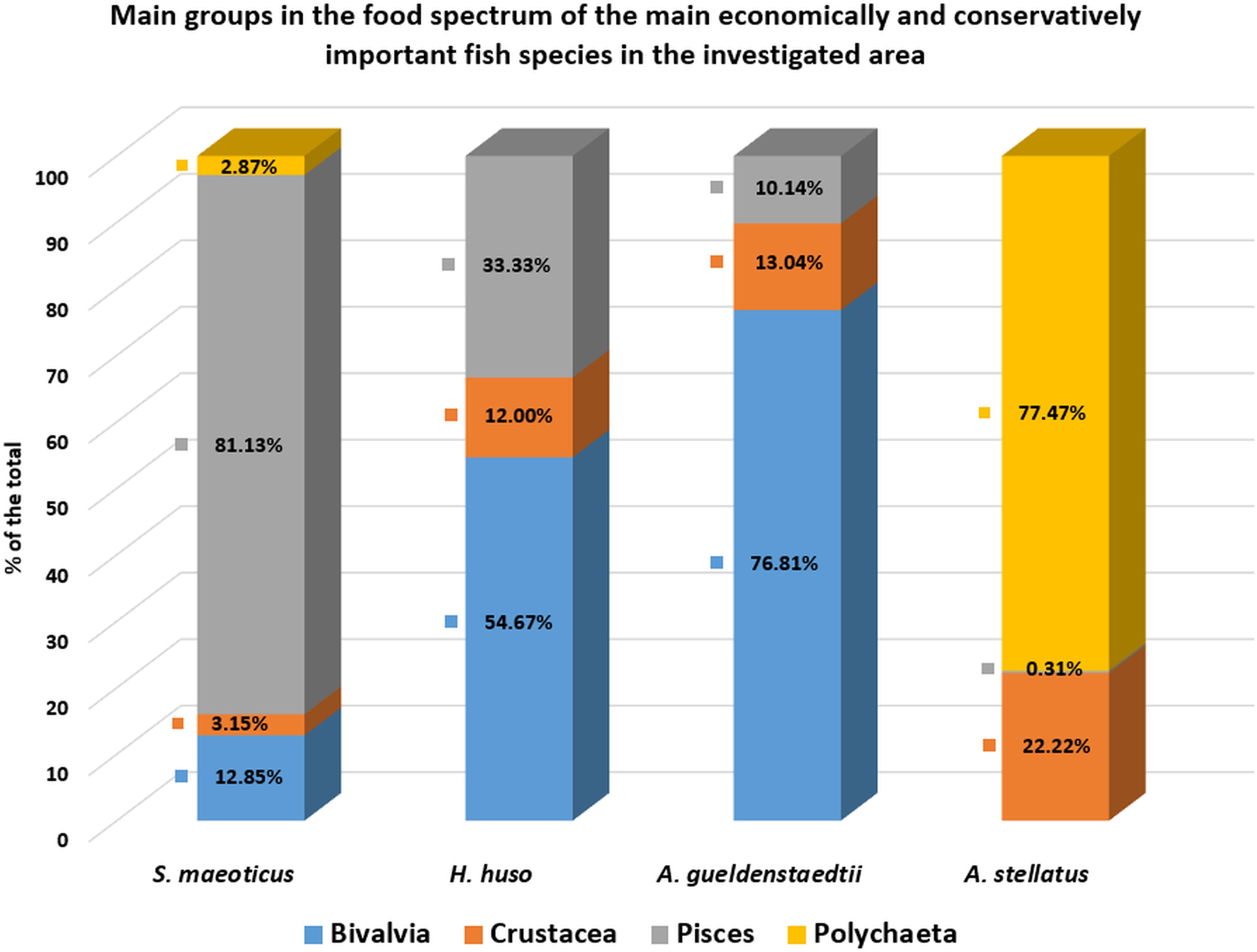
Figure 8. Dominance of food item groups in the food spectrum of the economically and conservatively important fish species caught in the study area.
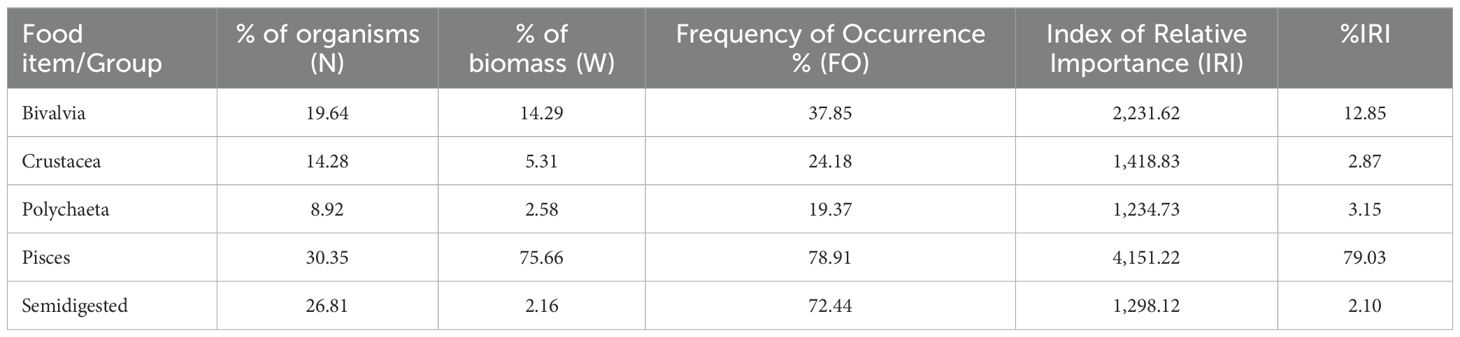
Table 1. Frequency of Occurrence (%FO), Index of Relative Importance (IRI) and %IRI of the main groups of organisms identified in the stomach contents of turbot.
It should be highlighted that a high percentage of mollusks (mussels) was also identified in the stomach contents of the analyzed turbot specimens (Supplementary Figure 1A), while the predominant fish species in the analyzed stomachs was whiting (Merlangius merlangus) (Supplementary Figure 1B). This species consumes sprat, anchovies, red mullet and mollusks (İşmen, 1995; Şensurat-Genç et al., 2019; Başçinar et al., 2023). Another fish species identified in the analyzed stomachs was horse mackerel (Trachurus trachurus), which in turn consumes anchovies, sprat, mollusks and crustaceans (Yankova et al., 2008). Red mullet, which feeds mainly on sprat, anchovies, mollusks and crustaceans (Onay and Dalgiç, 2019), has also been identified in the turbot stomachs investigated.
Regarding sturgeons, following the qualitative and quantitative analysis of the stomach contents of the 15 accidentally caught specimens, the following results were obtained: the trophic spectrum of the species H. huso was dominated by fish and organisms belonging to the group Bivalvia, in A. gueldenstaedtii bivalves predominated, and, in the case of A. stellatus, items belonging to the groups Polychaeta and Crustacea were dominant (Table 2).
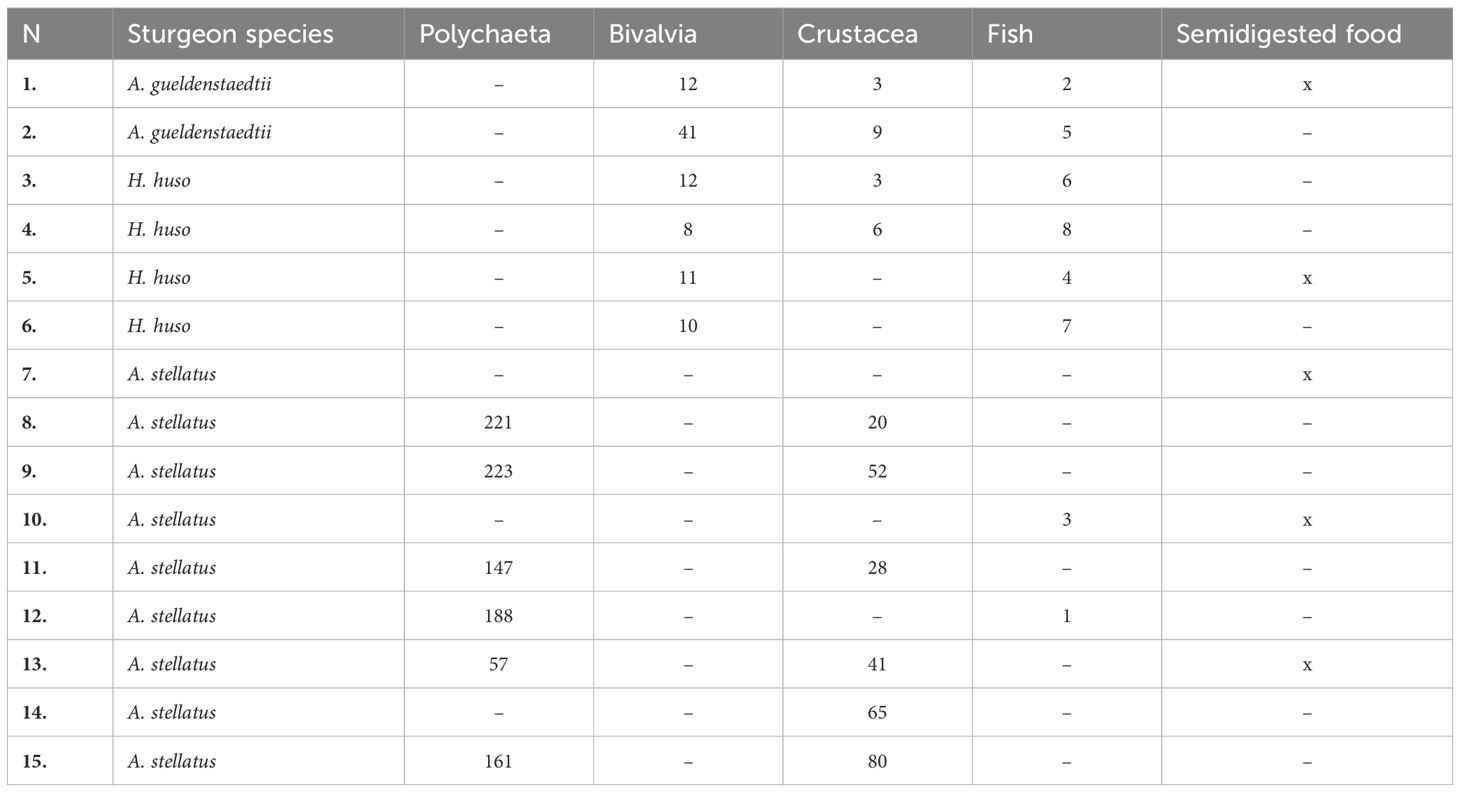
Table 2. Food items identified in the food spectrum of the three sturgeon species in the investigated area.
Following the analysis of the Frequency of Occurrence (FO%) of food items in the 15 gastric lavage samples, the most consumed type of food was Crustacea (Decapoda), followed by fish. Individuals belonging to the groups Polychaeta and Bivalvia were identified in 40% of the samples analyzed (Supplementary Figure 2). The Frequency of Occurrence (FO%) and the Index of Relative Importance (%IRI) of the main groups of organisms identified in the stomach contents of sturgeons are shown in Table 3 below.
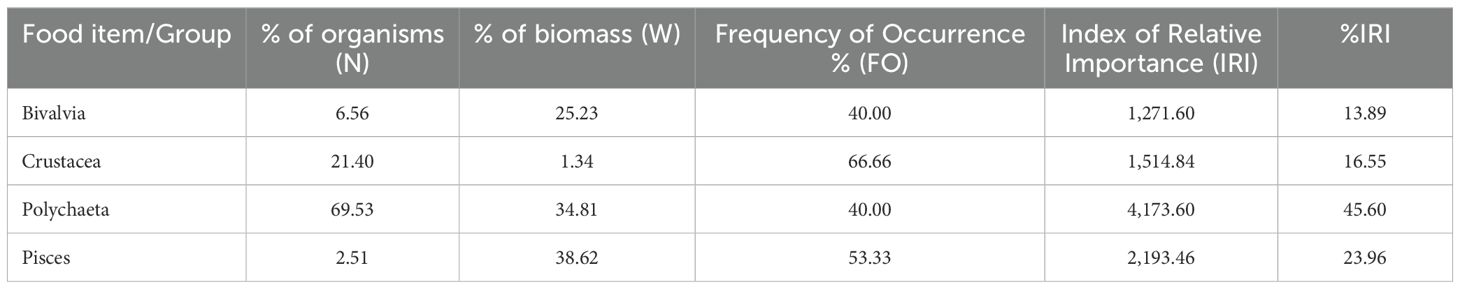
Table 3. Frequency of Occurrence (%FO), Index of Relative Importance (IRI) and %IRI of the main groups of organisms identified in the stomach contents of sturgeons.
With reference to the analysis of the dominance of food items, the following results were obtained: in the two gastric lavage samples from beluga (H. huso) the dominant items were mollusks (54.67%), followed by fish (anchovies and gobies), with 33%, then by crustaceans (12%). In the case of Russian sturgeon (A. gueldenstaedtii), the clear dominance of mollusks (76.81%) was recorded, followed by crustaceans (13.04%) and fish (10.14%). In the case of starry sturgeon (A. stellatus), however, there was a clear dominance of polychaetes (77.47%), followed by crustaceans (22.22%) and, in a lower percentage, by fish (0.31%) (Figure 8).
In summary, according to the analyses of stomach content samples, bivalves are the dominant food group for large sturgeon species (beluga and Russian sturgeon), while polychaetes are essential for smaller sturgeons (starry sturgeon), fish and crustaceans being present in the food spectrum of all sturgeon species in the investigated area.
The latest catch data from the landing points in the investigated area (Sf. Gheorghe and Sulina landing points) show an overall decreasing trend in the total catch (from 309.65 tons in 2022 to 95.57 tons 2024, which is a direct result of the drastic reduction of R. venosa landings in recent years in the area (which decreased four times during 2022-2024, from 288.06 tons to 71.10 tons). Turbot catches, the main target of fisheries, remained constant during the period investigated (around 21 tons) (Figure 9). Other commercial interest species caught included Danube shad, picked dogfish, sprat, red mullet, stingray and thornback ray, but in rather low quantities (all below 1 ton). Fishing effort, on the other hand, increased steadily, from 61 days at sea in 2022 to 235 days at sea in 2024 (Figure 10), thus a strong negative correlation between catches and effort in the investigated area is recorded (R-Squared value of 0.96).
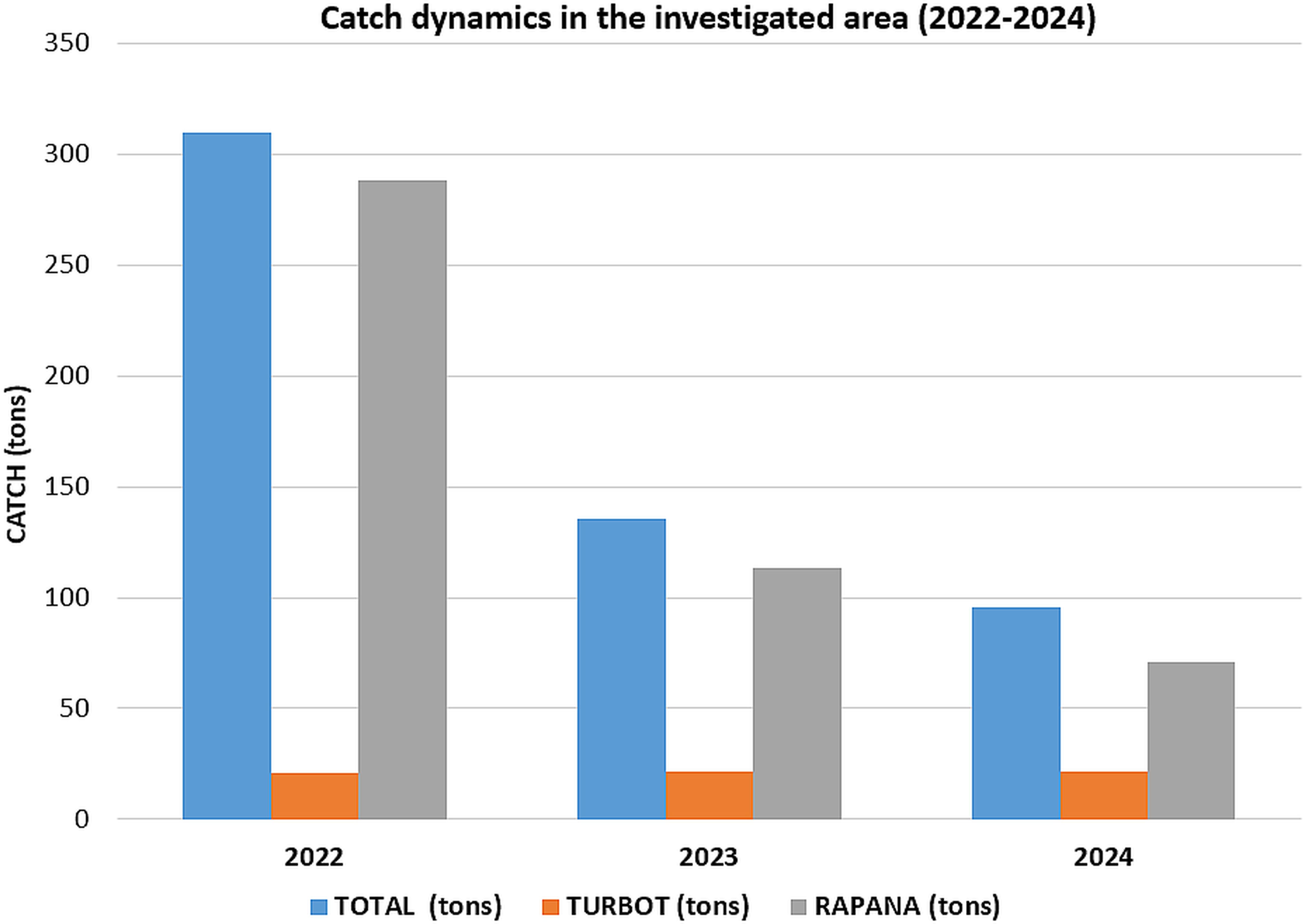
Figure 9. Evolution of catches (total catch, turbot catch, rapa whelk catch) in the investigated perimeter.
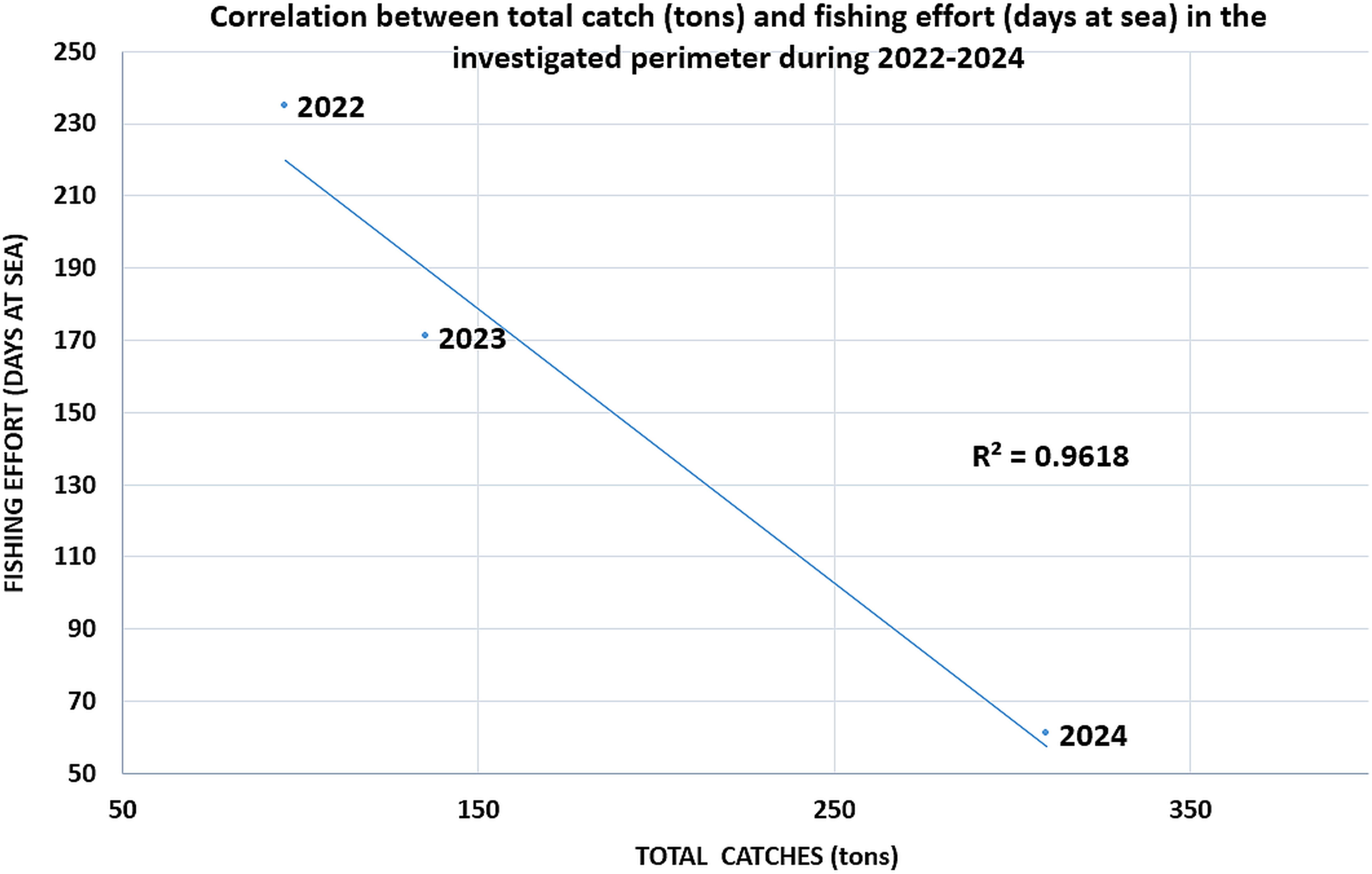
Figure 10. Fishing effort (days at sea) in the investigated area (landing points in Sf. Gheorghe and Sulina).
As a result of the investigation performed and corroborating all the acquired information, the broad area studied and identified as highly important for both vulnerable and commercial fish species was proposed to be established as the first national Fisheries Restricted Area (nFRA) at the Romanian coast. In the pursuit of a participatory approach and seeking the compliance of all stakeholders, the proposal was debated within the Romanian Fisheries Advisory Council Meeting organized by the National Agency for Fisheries and Aquaculture (NAFA). This public debate was attended by representatives of individual fishermen and fishing associations, research institutes (National Institute for Marine Research and Development “Grigore Antipa”, Danube Delta National Institute for Research and Development, Research and Development Institute for Aquatic Ecology, Fishing and Aquaculture), universities (“Lower Danube” University) and other authorities (Ministry of Environment and Forests, Romanian Waters National Administration, Danube Delta Biosphere Reserve Administration). After the legal public consultation period, during which the proposal was transparently available online for comments and suggestions, the nFRA was officially established by law: Joint Order no. 23/28.01.2025 & 297/31.01.2025 (Romanian Ministries of Agriculture and Rural Development and Environment, Waters and Forests, 2025), covering an area of 272.76 km2, between the 40 m and 50 isobaths (Table 4) (Figure 11).
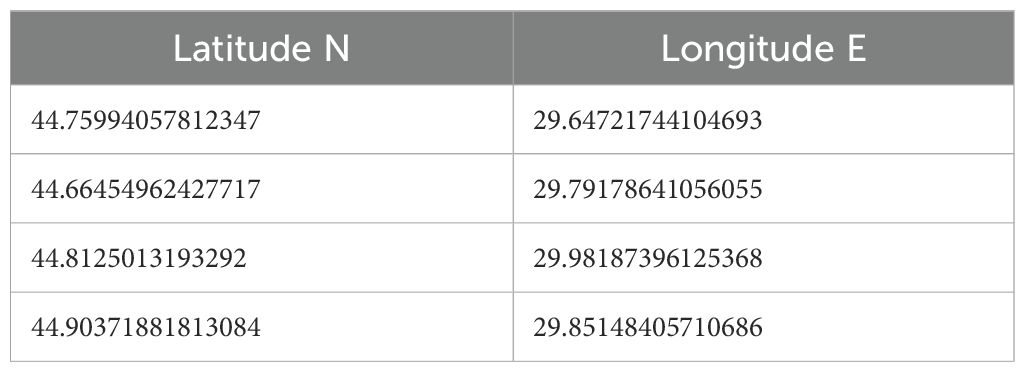
Table 4. Coordinates (decimal) of the Sfântu Gheorghe - Sahalin national Fisheries Restricted Area (nFRA).
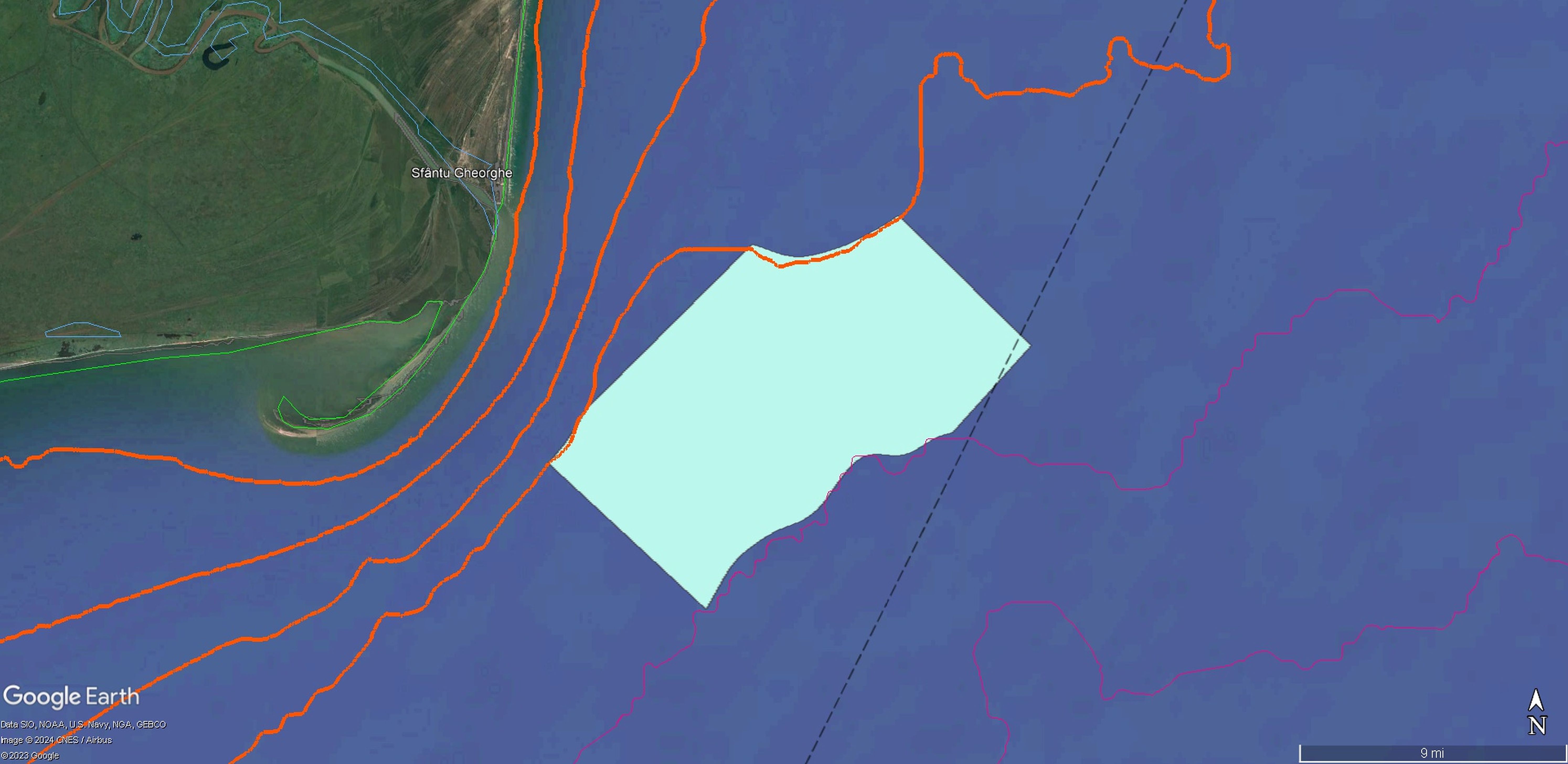
Figure 11. Integrated map of the Sfântu Gheorghe - Sahalin national Fisheries Restricted Area (nFRA): boundaries, benthic habitats and fish sampling points (source: Google Earth).
Area-based fisheries management measures are potential tools to manage fisheries (Petza et al., 2023) and can be used as an alternative to classical Marine Protected Areas (MPAs), which do not always prove effective both for fisheries and biodiversity management (Claudet et al., 2020). The first prerequisite for a marine area to be suitable for establishment as a no-take zone (restricted to fisheries) is its richness in food for valuable fish species (Clark et al., 2016), thus a sound knowledge of zoobenthos structure and diversity is essential (Tyler-Walters et al., 2009). Benthic organisms are an excellent food source of many high trophic value fish and the state of the macrozoobenthic fauna can provide crucial information on the carrying capacity of the habitat, as well as its resilience (Teacă et al., 2020; Begun et al., 2022; Chevalier et al., 2024).
The area investigated within this study overlaps with a unique biocoenosis in the Black Sea - the deep-sea mussel clumps. The deep-sea mussel biocenosis is one of the most characteristic and well-defined, both for the Romanian coast and for the entire Black Sea basin (Borcea, 1931, 1937; Băcescu et al., 1971; Băcescu, 1977). Off the Romanian coast, this biocenosis occupies a vast area between 25 - 45 m and 48 - 70 m deep, but it appears in its typical form between 30 and 50 m deep. It always occupies a vertical level difference of at least 20 m, the width of the occupied bottom strip varying between a minimum of 8 km (southeast of Sf. Gheorghe) and a maximum of 100 km east of Sulina. The total area covered in front of the Romanian coasts comprises about 7,000 km2 (Băcescu et al., 1971; Băcescu, 1977). The substrate is made of gray silt with a variable proportion of clay. Because of the very little consistent silt, the populations of the leading form - M. galloprovincialis Lamarck, 1819 - have an uneven distribution, forming small agglomerations, the so-called “mussel clumps”, included in the habitat identified within this study as mixed circalittoral sediments with D. quadrilobata and biogenic reefs with M. galloprovincialis. During the study period, the most numerically abundant mussel specimens were found in this habitat. Deep sea mussels have an essential ecological role, as they create a hard tridimensional substrate where otherwise there would only be mud/silt, thus supporting a wide biodiversity, including polychaetes, other mollusks and fish (Begun et al., 2022; Nenciu et al., 2023). These particularities render it as an Essential Fish Habitat (EFH), as it provides forage and shelter areas for valuable fish species (Katara et al., 2021). Additionally, this habitat could be a potential Vulnerable Marine Ecosystem (VME) on the North-Western Black Sea shelf, as it complies with most applicable criteria in the field: uniqueness or rarity; functional significance of the habitat; life-history traits of component species that make recovery difficult; structural complexity (FAO, 2003, 2009), to be further investigated. The second habitat identified in the study area, muddy circalittoral sediments with M. palmata, equally offers nutritious food sources for various fish species, as polychaetes are widely consumed by benthic fishes (Ross et al., 2018). Moreover, by applying ecological indices, it resulted that both investigated habitats are in Good Ecological Status (80% achieving GES) (Teacă et al., 2020), thus indicating that they can definitely provide a sound trophic support for fish communities in the area.
Ichthyofauna is a basic component of marine biodiversity, and, according to bibliographic sources, the fish fauna of the Black Sea includes over 140 species and subspecies (Niță et al., 2024). However, in the last decades, significant changes in the structure of the ichthyofauna, as well as ethological shifts in fish populations, have been highlighted. These can be correlated with the influence of numerous factors, such as: the significant input of the Danube, the increase of maritime traffic, tourism, the intensification of port activities, the urbanization of coastal areas, excessive fishing, but also with inappropriate (non-selective) gears, the penetration of alien species and other anthropogenic influences (Bănărescu, 1964; Cărăușu, 1952; Feider et al., 1964; Oțel, 2007; Radu et al., 2008). Over 60 fish species were identified in the investigated area, of which turbot (S. maeoticus), as a species with high economic value, and the 3 sturgeon species (H. huso, A. gueldenstaedtii and A. stellatus), representing species of conservation importance, are of particular interest for Fisheries Restricted Area implementation, as the perimeter is documented as an essential foraging area for these species (Niță et al., 2023a). Stomach contents analysis performed both on commercial and conservation interest fish species showed a strong connection between the zoobenthos structure in the area and the food spectrum of the species investigated.
Turbot stomach content analysis revealed that fish, mollusks, crustaceans and polychaetes make up the core of the food spectrum, fish being the preferred prey (mainly whiting, horse mackerel and red mullet), consistent with literature sources (İşmen, 1995; Yankova et al., 2008; Onay and Dalgiç, 2019; Şensurat-Genç et al., 2019; Başçinar et al., 2023). The food chain relationships between turbot as top predator and the main groups identified in its stomach contents clearly point out that the common item among the food spectrum of the above-mentioned fish species is represented by mollusks, thus it can be inferred that there is a direct connection between the top predator (turbot) and mussel clumps on the muddy substrate in the investigated area.
Bibliographic sources indicate that sturgeons, especially juveniles, feed on soft-bodied benthic organisms (Bănățean-Dunea et al., 2015). The preference for a particular food spectrum differs from one species to another but also depends on the type of food and its availability in a given habitat (Khodorevskaya et al., 1995; Polyaninova and Molodtseva, 1995; LeBreton and Beamish, 1998; Billard and Lecointre, 2001; Zengin et al., 2013). Thus, according to specialized literature, H. huso feeds especially on organisms belonging to the groups Harpacticoida, Cumacea, Nematoda, Polychaeta, Mollusca and Pisces. In the Danube, juvenile belugas feed first on Gammaridae, and later on crustaceans and insect larvae. Adult beluga feeds on mollusks, crustaceans and fish (anchovy, mullet, turbot, whiting, gobies) (Radu et al., 2008; Niță et al., 2024). As for Russian sturgeon, in the Danube the fry feed on organisms belonging to the groups Crustacea and Chironomida, and the adults on Ephemeropterae larvae, crustaceans and fish. In the sea, A. gueldenstaedtii adults prefer mollusks, crustaceans, polychaetes and fish (Radu et al., 2008; Niță et al., 2024). Starry sturgeon juveniles are documented to feed on larvae of Chironomidae, Tricopterae, Ephemeridae and small crustaceans, while adults prefer organisms belonging to the groups Mollusca, Crustacea, Polychaeta, Amphipoda, Cumacea and Pisces (Radu et al., 2008; Niță et al., 2024). Within this study, the analyses of sturgeon stomach by gastric lavage revealed that bivalves represent the dominant group of food items for large-sized sturgeon species (beluga and Russian sturgeon), while polychaetes are essential for smaller-sized sturgeons (starry sturgeon), fish and crustaceans being present in the food spectrum of all sturgeon species in the investigated area.
Spillover of adult fish and propagation of larvae to adjacent areas is one of the most expected effects of establishing a Fisheries Restricted Area (Di Lorenzo et al., 2020; Hilborn et al., 2025). Two types of spillover can be considered: ecological spillover (namely the net export of juvenile, subadult and adult biomass from the FRA outwards driven by density-dependent processes), and the fishery spillover, representing the proportion of this biomass that can be fished, taking into account regulations and accessibility (Di Lorenzo et al., 2016). In this context, catch and fishing effort data offer an overview of the fisheries in the investigated area and are to be used as a baseline to evaluate the effects of creating a restricted area in the North-Western sector of the Romanian coast and represent a valuable starting point (Batista et al., 2015). Historically, Romanian fisheries experienced a collapse after 1990, caused by a combination of environmental/ecological factors (from eutrophication to invasive species, such as the ctenophore Mnemiopsis leidyi) and the reduction of the fishing fleet (Radu et al., 2013). Catches started to increase significantly from 2013 to 2017 following the proliferation of the invasive gastropod R. venosa, which turned into an exploitable resource, becoming the main target species and accounting for 90% of the total catch (Danilov et al., 2019). It is important to emphasize that, despite the overall catch reduction in recent years caused by the drop in R. venosa landings due to stock reduction (Țiganov et al., 2023), fishing effort increased, thus the potential impacts of the valuable benthic habitats identified in the area are to be considered.
In addition to being an essential feeding area for fish species valuable both from a conservation and economic point of view (Niță et al., 2023a), the investigated perimeter is a traditional fishing ground for small-scale local fishermen (Niță et al., 2023b). Moreover, in the last decade, the area has become affected by beam trawl fishing for the gastropod R. venosa, with a potentially destructive impact on benthic habitats (Teacă et al., 2019; Danilov et al., 2019; Nenciu et al., 2023). Invasive fishing gears negatively affect zoobenthic populations in soft-substrate habitats, indirectly impacting fish stocks. Establishing a Fisheries Restricted Area (FRA) would protect both vulnerable habitats and essential fish species. The establishment of a FRA in an area where small-scale fisheries are dominant such as the northern part of the Romanian coast can bring important benefits to local communities by enhancing recruitment and fish biomass in adjacent fishing grounds, thus providing long-term sustainability for the local fishery (Chiarini et al., 2022). It is important, however, to consider alternative income sources to ensure fishermen’s livelihood and stimulate their compliance with the ban (Jones, 2009; Wintergalen et al., 2022). These alternative livelihoods should not be mistaken for a total change from one activity to another; instead, they should consider the ability to adopt multiple alternatives and options (Howard, 2003), such as eco-tourism or aquaculture initiatives, aiming at a “blue transformation” of local communities in the North-Western part of the Black Sea coast (FAO, 2021).
The endeavour of identifying, scientifically substantiating and establishing Fisheries Restricted Areas should be based both on ecological and fisheries criteria (Ortega et al., 2023), as the implementation of no-take areas to protect Vulnerable Marine Ecosystems (VME) and Essential Fish Habitats (EFH), when integrated in an ecosystem-based approach, has proven to be effective for managing fisheries and to improve overall ecosystem health (Smith et al., 2006; McConnaughey et al., 2020). For the successful endorsement of local communities and, ultimately, compliance with this fisheries management measure, it is essential to integrate socio-economic aspects in the process.
Our study was a stepping-stone for collecting and correlating essential information for identifying and finally legally establishing the first national Fisheries Restricted Area (nFRA) in the Black Sea. Thus, by integrating macro-zoobenthos composition and spatial distribution data with fish fauna and stomach content analysis, as well as catch and fishing effort records, Sf. Gheorghe - Sahalin, on the North-Western Black Sea shelf, was identified as an area suitable for closure of fisheries - beneficial both nature conservation and the livelihood of local coastal communities. Future assessments of the effects of creating this nFRA shall be performed, targeting both the evolution of the macrobenthic communities and the dynamics of fisheries in nearby fishing grounds. Evaluating the effectiveness of this spatial closure in the long-term, compared to other measures (such as quotas, gear restrictions etc.), shall generate valuable data for an efficient and sustainable fisheries management in the area.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The requirement of ethical approval was waived by the National Institute for Marine Research and Development Grigore Antipa for the studies involving animals because procedures were performed in compliance with the scientific fishing license NIMRD no. 05/20.02.24 issued by the Romanian National Agency for Fisheries and Aquaculture. The studies were conducted in accordance with the local legislation and institutional requirements.
VN: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. MN: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. TB: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. AT: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. MG: Data curation, Investigation, Writing – original draft. CD: Methodology, Investigation, Writing – original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. The authors declare financial support was received for the research which conducted to the publication of this article from the Romanian National Agency for Fisheries and Aquaculture (NAFA), in the frame of the project “Study for the Preliminary Identification of Potential National Marine Fisheries Restricted Areas in Romania” (ROnFRAs), Grant Number 6436/79 of 25.07.2024. This work was supported by the Romanian Ministry of Research, Innovation, and Digitization in the framework of the national NUCLEU Program - GEOMARDIGITAL, project: PN 23300202. The APC was covered from the NUCLEU SMART-BLUE Project PN23230301 “Ensuring Sustainable and Climate Change Challenges Resilient Food Sources through the Smart Integration of the Ecosystem Fisheries Management and the Scientific Development of Marine Aquaculture in the Blue Economy Strategy”.
The authors kindly thank Dragoș Diaconu for carefully helping with the gastric lavage operations on board the research vessel and safely releasing the sturgeon individuals back into the wild.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GT declared a shared affiliation with the authors VN, MN, MG, CD to the handling editor at the time of review.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1570936/full#supplementary-material
Abaza V., Filimon A., Dumitrache C. (2018).IV.4. D1, 6 - Biodiversitate - habitatele bentale - macrozoobentos”, in Raportul național privind starea ecologică a ecosistemului marin Marea Neagră conform cerinţelor art. Available online at: https://www.mmediu.gov.ro/app/webroot/uploads/files/STUDIU%20MSFD%20V1.9.pdf (Accessed December 11, 2024).
Ahlbeck I., Hansson S., And Hjerne O. (2012). Evaluating fish diet analysis methods by individual-based modelling. CJFAS 69 (7), 1184–1201. doi: 10.1139/f2012-051
AZTI (2024). AZTI’s Marine Biotic Index. Available online at: https://ambi.azti.es/ (Accessed December 11, 2024).
Băcescu M. (1977). Les biocenoses benthiques de la Mer Noire. Biologie des eaux saumatres la Mer Noire, Premiere partie, 128–134. (in French).
Băcescu M., Müller G. I., Gomoiu M.-T. (1971). Cercetări de ecologie bentală în Marea Neagră. Analiza cantitativă, calitativă şi comparată a faunei bentale pontice, in Ecologie Marină, vol. 4. (Bucharest, Romania: Publishing House of the Academy of the Romanian People’s Republic) (in Romanian).
Bănățean-Dunea I., Corpade A.-M., Grozea A., Nicolin A., Corpade C., Osman A., et al. (2015). Ghid sintetic de monitorizare a speciilor comunitare de peşti din România (Cluj-Napoca, România: Science Book House Publishing) (in Romanian).
Bănărescu P. (1964). “Fauna republicii populare române Vol. XIII Pisces - Osteichtyes (Pești ganoizi și osoși). (Bucharest, Romania: Romanian People’s Republic Academy Publishing) (in Romanian).
Başçinar N. S., Misir D., Altuntaş C., Genç Y., Dağtekin M., Erbay M., et al. (2023). Length-weight relationship and seasonal variations in diet composition of whiting (Merlangius merlangus) in the South-Eastern Black Sea. Mar. Biol. Res. 19, 1–12. doi: 10.1080/17451000.2023.2203499
Batista M. I., Horta e Costa B., Gonçalves L., Henriques M., Erzini K., Caselle J. E., et al. (2015). Assessment of catches, landings and fishing effort as useful tools for MPA management. Fish. Res. 172, 197–208. doi: 10.1016/j.fishres.2015.07.020
Begun T., Teacă A., Mureșan M., Quijon P. A., Menabit S., Surugiu V. (2022). Habitat and macrozoobenthic diversity in marine protected areas of the southern Romanian Black Sea coast. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.845507
Billard R., Lecointre G. (2001). Biology and conservation of sturgeon and paddlefish. Rev. Fish Biol. Fish. 10, 355–392. doi: 10.1023/A:1012231526151
Bohnsack J. A. (1994). How marine fishery reserves can improve reef fisheries. Annu. Proc. Gulf Caribb. Fish. Inst. 43, 217–241.
Borcea I. (1931). Nouvelle contributions à l’étude de la faune bentonique dans la mer Noire, près du littoral roumain. Sci. Ann. “Alexandru Ioan Cuza” Univ. Iaşi 16, 655–750.
Borcea I. (1937). Les resultats de l’expedition de recherche dans la Mer Noire de 28 aout-1 septembre 1935 sous le haut patronage de S. M. le Roi Carol II. Sci. Ann. “Alexandru Ioan Cuza” Univ. Iaşi 22, 1–26.
Borja A., Franco J., Perez V. (2000). A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Poll. Bull. 40, 1100–1114. doi: 10.1016/S0025-326X(00)00061-8
Borja A., Muxika I. (2005). Guidelines for the use of AMBI (AZTI’s marine biotic index) in the assessment of the benthic ecological quality. Mar. Pollut. Bull. 50, 787–789. doi: 10.1016/j.marpolbul.2005.04.040
Brosse L., Dumont P., Lepage M., Rochard E. (2002). Evaluation of a gastric lavage method for sturgeons. N. Am. J. Fish. Manage. 22, 955–960. doi: 10.1577/1548-8675(2002)022<0955:EOAGLM>2.0.CO;2
Brosse L., Lepage M., Dumont P. (2000). First results on the diet of the young Atlantic sturgeon Acipenser sturio L. 1758 in the Gironde estuary. Bol. Inst. Esp. Oceanogr. 16 (1/4), 75–80.
Cărăușu S. I. (1952). Tratat de ihtiologie (Bucharest, Romania: Romanian People’s Republic Academy Publishing) (in Romanian).
Chevalier S., Beauchard O., Teacă A., Soetaert K., Grégoire M. (2024). Partial recovery of macrozoobenthos on the Northwestern Shelf of the Black Sea. Mar. Pollut. Bull. 207, 116857. doi: 10.1016/j.marpolbul.2024.116857
Chiarini M., Guicciardi S., Zacchetti L., Domenichetti F., Canduci G., Angelini S., et al. (2022). Looking for a simple assessment tool for a complex task: short-term evaluation of changes in fisheries management measures in the Pomo/Jabuka pits area (Central Adriatic Sea). Sustainability 14, 7742. doi: 10.1093/icesjms/fsv123
Clark M. R., Althaus F., Schlacher T. A., Williams A., Bowden D. A., Rowden A. D. (2016). The impacts of deep-sea fisheries on benthic communities: a review. IJSM 73 (S1), i51–i69. doi: 10.1093/icesjms/fsv123
Clarke K. R., Gorley R. N., Somerfield P. J. (2014). Change in Marine Communities: An Approach to Statistical Analysis. 3rd edition (Plymouth, UK: PRIMER-E).
Claudet J., Loiseau C., Sostres M., Zupan M. (2020). Underprotected marine protected areas in a global biodiversity hotspot. One Earth 2, 380–384. doi: 10.1016/J.ONEEAR.2020.03.008
Cortés E. (1997). A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. CJFAS 54, 726–738. doi: 10.1139/f96-316
Danilov C. S., Nicolaev S., Anton E., Țiganov G., Nicolaev A. D., Păun C. V., et al. (2019). Dynamics of the fishing capacities used for the mechanized exploitation of the gastropod Rapana venosa at the Romanian coast, during 2013 - 2017. Mar. Res. J. 49, 133–140. doi: 10.55268/CM.2019.49.133
Di Lorenzo M., Claudet J., Guidetti P. (2016). Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. J. Nat. Conserv. 32, 62–66. doi: 10.1016/j.jnc.2016.04.004
Di Lorenzo M., Guidetti P., Di Franco A., Calò A., Claudet J. (2020). Assessing spillover from marine protected areas and its drivers: A meta-analytical approach. Fish Fish. 21, 906–915. doi: 10.1111/faf.12469
Dubinin A. V., Rimskaya-Korsakova M. N., Dubinina E. O., Demidova T. P., Semilova L. S., Berezhnaya E. D., et al. (2024). Light stimulation of sulfide oxidation in the Black Sea anoxic water column. Oceanology 64, 810–819. doi: 10.1134/S0001437024700541
European Commission (2020). Biodiversity strategy for 2030. Available at: https://environment.ec.europa.eu/strategy/biodiversity-strategy-2030_en (Accessed December 11, 2024).
FAO (2003). “Vulnerable marine ecosystems: processes and practices in the high seas. Fisheries management. 2,” in The Ecosystem Approach to Fisheries (Rome, Italy: Food and Agriculture Organization (FAO)).
FAO (2009). International Guidelines for the Management of Deep-sea Fisheries in the High Seas (Rome, Italy: Food and Agriculture Organization (FAO)).
FAO (2021). GFCM 2030 Strategy for Sustainable Fisheries and Aquaculture in the Mediterranean and the Black Sea (Rome, Italy: Food and Agriculture Organization (FAO)). doi: 10.4060/cb7562en
FAO (2023). The State of Mediterranean and Black Sea Fisheries 2023 - Special Edition. General Fisheries Commission for the Mediterranean. (Rome, Italy: Food and Agriculture Organization (FAO)). doi: 10.4060/cc8888en
Feider Z., Grossu A. V., Gyurko Ş., Popp. V. (1964). Zoologia Vertebratelor (Bucharest, Romania: Didactic and Pedagogic Publishing) (in Romanian).
FishBase (2024). A Global Information System on Fishes. Available online at: https://www.fishbase.se/search.php (Accessed November 22, 2024).
Gomoiu M. T. (1982). On the populations of Melinna palmata Grube at the Romanian littoral of the Black Sea. Mar. Res. J. 15, 115–131.
Haley N. (1998). A gastric lavage technique for characterizing diets of sturgeons. N. Am. J. Fish. Manage. 18, 978–981. doi: 10.1577/1548-8675(1998)018<0978:AGLTFC>2.0.CO;2
Hansson S. (1998). Methods of studying fish feeding: a comment. CJFAS 55, 2706–2707. doi: 10.1139/f98-158
Hilborn R., Fitchett M., Hampton J., Ovando D. (2025). When does spillover from marine protected areas indicate benefits to fish abundance and catch? Theor. Ecol. 18, 1–18. doi: 10.1007/s12080-024-00596-2
Holme N. A., McIntyre A. (1984). Methods for the study of marine benthos (Oxford, UK: Blackwell Science).
Howard M. (2003). When fishing grounds are closed: developing alternative livelihoods for fishing communities. SPC Women in Fish. Info. Bull. 13, 19–22.
Hyslop E. J. (1980). Stomach content analysis - a review of methods and their application. J. Fish Biol. 17, 411–429. doi: 10.1111/j.1095-8649.1980.tb02775.x
Işmen A. (1995). The biology and population parameters of the whiting (Merlangius merlangus euxinus Nordmann) in the Turkish coast of the Black Sea. Ph.D. Thesis (Ankara, Türkiye: Middle East Technical University).
Jones B. (2009). Teetering towards economic sustainability: alternatives for commercial fishermen. JEA 13, 73–77.
Katara I., Peden W. J., Bannister H., Ribeiro J., Fronkova L., Scougal C., et al. (2021). Conservation hotspots for fish habitats: a case study from english and welsh waters. Reg. Stud. Mar. Sci. 44, 101745. doi: 10.1016/j.rsma.2021.101745
Khodorevskaya R. P., Polyaninova A. A., Geraskin P. P., Romanov A. A. (1995). “A study on physiological and biochemical status of beluga sturgeon, Huso huso (L.), and its feeding habits,” in Proc. of the International Sturgeon Symposium (VNIRO, Moscow), 164–172.
Lauck T., Clark C., Mangel M., Munro G. (1998). Implementing the precautionary principle in fisheries management through marine reserves. Ecol. Appl. 8, S72–S78. doi: 10.1890/1051-0761(1998)8[S72:ITPPIF]2.0.CO;2
LeBreton G. T., Beamish F. W. H. (1998). The influence of salinity on ionic concentrations and osmolarity of blood serum in lake sturgeon, Acipenser fulvescens. Environ. Biol. Fishes 52, 477–482. doi: 10.1023/A:1007421410090
McConnaughey R. A., Hiddink J. G., Jennings S., Pitcher C. R., Kaiser M. J., Suuronen P., et al. (2020). Choosing best practices for managing impacts of trawl fishing on seabed habitats and biota. Fish Fish. 21, 319–337. doi: 10.1111/FAF.12431
Menabit S., Lavin P., Begun T., Mureșan M., Teacă A., Purcarea C. (2024). First screening of bacteria assemblages associated with the marine polychaete Melinna palmata Grube 1870 and adjacent sediments. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1279849
Murawski S. A., Brown R., Lai H.-L., Rago P. J., Hendrickson L. (2000). Large-scale closed areas as a fisheries management tool in temperate marine systems: the Georges bank experience. Bull. Mar. Sci. 66 (3), 775–798.
Mureșan M., Teacă A., Popa A., Begun T. (2019). Free-living marine nematodes community structural changes within a post-dredging site at the Romanian shelf. JEPE 20 (2), 753–760.
Muxika I., Borja A., Bald J. (2007). Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar. Pollut. Bull. 55, 16–29. doi: 10.1016/j.marpolbul.2006.05.025
Nenciu M., Niță V., Teacă A., Popa A., Begun T. (2023). An assessment of potential beam-trawling impact on North-Western Black Sea benthic habitats aiming at a sustainable fisheries management. Water 15, 2241. doi: 10.3390/w15122241
Niță V., Țoțoiu A., Nenciu M., Spînu A., Dumitrache C., Danilov C., et al. (2023a). Mapping sturgeon foraging areas in the North-Western Black Sea: scientific support for the sustainable management of marine resources. Acta Zool. Bulg. 75 (4), 527–539.
Niță V., Galațchi M., Nenciu M. (2023b). Updated overview of marine fish biodiversity: scientific support for an ecosystem-based management of the Danube Delta Biosphere Reserve. Sci. Pap. Ser. D Anim. Sci. LXVI (1), 602–612.
Niță V., Nenciu M., Galațchi M., Diaconu D. (2024). Speciile de pești de la litoralul românesc. Atlas actualizat / Fish Species of the Romanian Coast. Updated Atlas. (Bucharest, Romania: CD Press) (in Romanian and English).
Nicolaev S., Maximov V., Niță V., Zaharia T., Nenciu M. (2018). Marine protected areas management: interaction with commercial fisheries in Natura 2000 sites along the Romanian Black Sea coast. Mar. Res. J. 48 (1), 5–25.
Oțel V. (2007). Atlasul peștilor din Rezervația Biosferei Delta Dunării (Tulcea, Romania: Danube Delta Technological Information center Publishing) (in Romanian).
Onay H., Dalgiç G. (2019). Seasonal changes in the food spectrum and day-time rhythm of feeding in red mullet Mullus barbatus (Linnaeus 1758) in the South-East Black Sea. Fresenius Environ. Bull. 28 (4), 2671–2678.
Ortega M., Castro-Cadenas M. D., Steenbeek J., Coll M. (2023). Identifying and prioritizing demersal fisheries restricted areas based on combined ecological and fisheries criteria: the Western Mediterranean. Mar. Pol. 157, 105850. doi: 10.1016/j.marpol.2023.105850
Petza D., Anastopoulos P., Coll M., Garcia S. M., Kaiser M., Kalogirou S., et al. (2023). The contribution of area-based fisheries management measures to fisheries sustainability and marine conservation: a global scoping review. Rev. Fish Biol. Fisheries 33, 1049–1073. doi: 10.1007/s11160-023-09780-9
Petza D., Maina I., Koukourouvli N., Dimarchopoulou D., Akrivos D., Kavadas S., et al. (2017). Where not to fish - reviewing and mapping fisheries restricted areas in the Aegean Sea. Med. Mar. Sci. 18, 310–323. doi: 10.12681/mms.2081
Polyaninova A. A., Molodtseva A. I. (1995). “The benthos sturgeon feeding relationship for the Caspian Sea,” in Proc. of the International Sturgeon Symposium (VNIRO, Moscow), 151–157 (in Russian).
Radu G., Nicolaev S., Anton E., Maximov V. (2013). Evolution of Romanian marine fisheries following EU accession. Mar. Res. J. 43, 249–267. doi: 10.55268/CM.2013.43.249
Radu G., Radu E., Nicolaev S., Anton E. (2008). “Atlas al principalelor specii de pești din Marea Neagră,” in Pescuitul marin românesc (Constanța, Romania: Virom Publishing) (in Romanian).
Romanian Ministries of Agriculture and Rural Development and Environment, Waters and Forests (2025). Joint Order no. 23/28.01.2025 & 297/31.01.2025. Official Gazette of Romania. Available online at: https://monitoruloficial.ro/Monitorul-Oficial–PI–95–2025.html (Accessed February 3, 2025).
Ross S. D., Nielsen J. R., Gislason H., Nielsen A., Andersen N. G. (2018). Growth and food consumption of whiting Merlangius merlangus. J. Fish Biol. 9, 334–343. doi: 10.1111/jfb.13763
Şensurat-Genç T., Akyol O., Özgül A., Özden U. (2019). Food composition of whiting Merlangius merlangus, captured around the sea-cage fish farms in Ordu, South-Eastern Black Sea. JMBA 99, 1651–1659. doi: 10.1017/S0025315419000626
Schlitzer R. (2018). Ocean Data View. Available online at: https://odv.awi.de (Accessed December 11, 2024).
Shannon C. E., Weaver W. W. (1963). The Mathematical Theory of Communications (Urbana, US: University of Illinois Press).
Sigovini M., Keppel E., Tagliapietra D. (2013). M-AMBI revisited: looking inside a widely-used benthic index. Hydrobiologia 717, 41–50. doi: 10.1007/s10750-013-1565-y
Smith M. D., Zhang J., Coleman F. C. (2006). Effectiveness of marine reserves for large-scale fisheries management. Can. J. Fish. Aquat. Sci. 63, 153–164. doi: 10.1139/F05-205
Taylor N., Clarke L. J., Alliji K., Barrett C., Mcintyre R., Smith R. K., et al. (2021). Marine fish conservation: global evidence for the effects of selected interventions. Synopses of conservation evidence series. (Cambridge, UK: Apollo - University of Cambridge). doi: 10.17863/CAM.66003
Teacă A., Mureșan M., Begun T., Popa A., Ion G. (2019). Marine benthic habitats within a physical disturbed site from the Romanian coast of the Black Sea. JEPE 20 (2), 723–732.
Teacă A., Mureşan M., Menabit S., Bucșe A., Begun T. (2020). Assessment of Romanian circalittoral soft bottom benthic habitats under Danube river influence. Reg. Stud. Mar. Sci. 40, 101523. doi: 10.1016/j.rsma.2020.101523
Țiganov G., Niță V., Danilov C., Păun C., Diaconu D., Grigoraș D., et al. (2023). Status of the rapa whelk agglomerations along the Romanian Black Sea Coast. Mar. Res. J. 53 (1), 70–82. doi: 10.55268/CM.2023.53.70
Todorova V., Abaza V., Dumitrache C., Todorov E., Wolfram G., Salas Herrero F. (2018). Coastal and transitional waters Black Sea geographic intercalibration group. Benthic invertebrate fauna ecological assessment methods (Luxembourg: Publications Office of the European Union). doi: 10.2760/31396
Todorova V., Konsulova T. (2005). Manual for quantitative sampling and sample treatment of marine soft-bottom macrozoobenthos (Istanbul, Türkiye: Black Sea Commission Publications).
Tyler-Walters H., Rogers S. I., Marshall C. E., Hiscock K. (2009). A method to assess the sensitivity of sedimentary communities to fishing activities. Aquat. Conserv. 19, 285–300. doi: 10.1002/aqc.965
Wintergalen E. W., Oyanedel R., Villaseñor-Derbez J. C., Fulton S., Molina R. (2022). Opportunities and challenges for livelihood resilience in urban and rural Mexican small-scale fisheries. Ecol. Soc 27, 46. doi: 10.5751/ES-13471-270346
WoRMS. (2024). World register of marine species. Available at: https://marinespecies.org/ (Accessed December 11, 2024).
Yankova M., Raykov V., Frateva P. (2008). Diet composition of horse mackerel, Trachurus mediterraneus ponticus Aleev, 1956 (Osteichthyes: Carangidae) in the Bulgarian Black Sea Waters. TRJFAS 8 (2), 321–327.
Keywords: nFRAs, essential habitats, ichthyofauna, fisheries, spillover, management, participatory approach
Citation: Niță V, Nenciu M, Begun T, Teacă A, Galațchi M and Danilov C (2025) National fisheries restricted areas: an alternative tool for the sustainable management of Black Sea vulnerable and economically important fish populations. Front. Mar. Sci. 12:1570936. doi: 10.3389/fmars.2025.1570936
Received: 04 February 2025; Accepted: 28 February 2025;
Published: 26 March 2025.
Edited by:
Tomaso Fortibuoni, Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA), ItalyReviewed by:
Elvis Kamberi, Agricultural University of Tirana, AlbaniaCopyright © 2025 Niță, Nenciu, Begun, Teacă, Galațchi and Danilov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tatiana Begun, dGJlZ3VuQGdlb2Vjb21hci5ybw==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.