
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 24 March 2025
Sec. Aquatic Physiology
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1558836
Monodonta labio is exposed to prolonged periods of air exposure due to the complexity and variability of the intertidal environment, particularly the cyclical rise and fall of the tides. However, current research tends to focus on changing temperature and salinity rather than atmospheric exposure. In this study, RNA sequencing (RNA-seq) was used to analyze gene expression levels at different times of air exposure in the intertidal mollusc M. labio. Transcriptome analysis of nine individuals yielded 420.81 Mb of clean data, and the number of clean reads mapped to the genome ranged from 62.91% to 90.96%. In comparison with the control group, the 2 days and 5 days air exposure stress group groups showed 50 and 940 differentially expressed genes (DEGs), respectively. Gene Ontology (GO) enrichment analysis revealed that the DEGs were significantly enriched in enzyme activity, catalytic activity. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment revealed that the DEGs were significantly enriched in immune response, Apoptosis. Several key genes (BRAF, RAN, COL6A, DNAJA1) were related to air exposure. Among them, RAN, COL6A, DNAJA1 were differentially expressed on 2 day air exposure compared to 5 day air exposure, and BRAF was differentially expressed in all three groups. Eight differentially expressed genes were randomly selected for qRT-PCR validation, and the results showed that the transcriptomic data were of high confidence.
One of the environmental stress factors that intertidal organisms often face is air exposure (Weinrauch and Blewett, 2019). It is a condition in which aquatic animals leave the body of water for a short or long period and remain in a medium such as air, sand, or soil, mainly including water loss, hypoxia, and starvation stress (Zhang et al., 2022a). The intracellular and extracellular water balance is disrupted by the gradual evaporation of water from the organism, resulting in cell shrinkage due to water loss and damage to tissue structure (Abele et al., 2007; Paital, 2013). Aquatic organisms are unable to obtain sufficient oxygen directly from the environment and excrete carbon dioxide through water loss, limiting aerobic respiration and leading to negative effects such as oxidative/antioxidative stress damage; cellular apoptosis; water loss from tissue cells.; growth retardation; poor muscle mass; and in severe cases, even death (Li et al., 2017; Yin et al., 2017; de Andrade et al., 2021; Guan et al., 2021; Lei et al., 2023). Some genes and signaling pathways, such as the HSP gene family, antioxidant genes, and immune-related genes, are developed to adapt to air exposure conditions (Bao et al., 2019; Guan et al., 2021; Zhang et al., 2022c). So far, domestic and foreign scholars have found that organisms subjected to air exposure mainly focus on fish, crustaceans, and shellfish, and no studies have been conducted on M. labio-related adaptive molecular mechanisms (Aguilar Vitorino et al., 2019; Chhor et al., 2022; Barrios-Figueroa and Urbina, 2023).
Monodonta labio, belonging to the family Trochidae (Gastropoda, Mollusca), is a small intertidal economic mollusk commonly known as sesame snail (Tan et al., 2021). The species is widespread in the Indo-Pacific region and in intertidal areas along the coasts of East Asia, including rocky intertidal zones, gravelly intertidal zones, rocky shores and mangrove forests (Yamazaki et al., 2017; Zhao et al., 2019; Cong et al., 2020; Chiu et al., 2023). As the dominant species with the largest intertidal distribution, it is capable of adapting to large temperature (0°C-28°C) and salinity (13.23-34.95psu) variations (Nan and Cao, 2012). M. labio feed on benthic algae, their meat is tasty and rich in nutrients, favored by coastal residents, and is a common featured seafood in Zhoushan area (Jian-she et al., 2011). Currently, research on M. labio has focused on physiological characterization (Ai-yi et al., 2007; Zhao et al., 2019), genetic and cryptic species diversity (Yamazaki et al., 2017; Zhao et al., 2017; Cong et al., 2020), and analysis of metal enrichment (Yuanming, 2008).
As a dominant species in the intertidal zone, M. labio needs to be exposed to air for a long period of time during the ebb tide, which makes it vulnerable to environmental stresses such as air exposure. However, its molecular adaptation mechanism to air exposure is still unclear. Previous experiments have shown that this species can survive for at least 7 days under artificial air exposure conditions at a constant temperature of 23.5°C and humidity of 80-90% (Yinong Wang, 1994). For this reason, in this study, we compared the gene expression differences between the un-air exposure, 2 day air exposure group and 5 day air exposure group by RNA-Seq technique to reveal the mechanism of its adaptation to air exposure.
Individuals of experimental M. labio were bought in Zhoushan seafood market, Zhejiang Province, GPS coordinates: N29°59′40″, E122°12′19″. After identification by morphology, M. labio of the same level of development, vigorous growth and equal individual size were selected as experimental material. After 24 h of temporary incubation in the laboratory, 20 M. labio were removed from the tank and stored in air exposure in a thermostatic incubator at a temperature of 23.5°C and wrapped in seawater-wetted cloths and sprayed regularly with seawater-wetted to maintain humidity at 80-90%. The individuals were taken before air exposure (t0) and after 2 days of air exposure (t1) and 5 days of air exposure (t2). As biological replicates, three M. labio were taken from each group. For subsequent sequencing and comparative analysis, whole tissue homogenate were extracted as experimental material and stored in an ultra-cold freezer.
The total RNA from three sets of M. labio visceral mass individuals was extracted using the TRIzol kit (Invitrogen, Carlsbad, CA, USA). The quality of RNA was examined using agarose gel electrophoresis. RNA concentration was measured using a Qubit 2.0 Flurometer (Life Technologies, CA, USA) using the Qubit RNA Assay Kit. The purity and integrity of RNA were assessed using Nanodrop (OD 260/280) and Agilent 2100, respectively.
RNA-seq transcriptome libraries were prepared following TruSeqTM RNA sample preparation Kit from Illumina (San Diego, CA), using 1μg of total RNA. Shortly, messenger RNA was isolated with polyA selection by oligo(dT) beads and fragmented using fragmentation buffer. cDNA synthesis, end repair, A-base addition and ligation of the Illumina-indexed adaptors were performed according to Illumina’s protocol. Libraries were then size selected for cDNA target fragments of 200–300 bp on 2% Low Range Ultra Agarose followed by PCR amplified using Phusion DNA polymerase (NEB) for 15 PCR cycles. Illumina Hiseq was then used to sequence the library. Raw data were uploaded to the National Centre for Biotechnology Information (NCBI) Sequence Read Archive database.
The raw data in fastq format were quality controlled by fastp in fastqc software (Andrews, 2010; Chen et al., 2018). From the raw data, clean reads were obtained by removing reads containing adapters, ploy-N and low quality reads. Q20 and Q30 were calculated from the clean data. All of the downstream analyses were based on clean, high quality data.
The reference genome was derived from the laboratory-assembled reference genome sequence of M. labio. Paired-end pure reads were aligned to the reference genome by using STAR (Dobin et al., 2012).
The reads mapped to each gene were counted using HTseq (Anders et al., 2014). To identify DEGs between the different samples, the expression level for each gene was calculated using the fragments per kilobase of exon per million mapped reads (FRKM) method. A individual correlation heatmap of gene expression was drawn in accordance with the FPKM values of genes in all individuals. Using the DESeq2 R package, differential expression analysis was carried out on subgroups (Love et al., 2014). Genes with an adjusted P-value < 0.05 and absolute log2FC > 1 according to DESeq2 were assigned as differentially expressed. Enrichment analysis of DEGs was performed in the Gene Ontology (GO) and KEGG pathways using the clusterProfiler R package (Yu et al., 2012).
The accuracy of Illumina RNA-Seq data was validated using qRT-PCR. Eight genes were selected for validation, and primers for target and internal reference genes (18s) were designed by NCBI primer-BLAST (Table 1). To determine the temporal gene expression levels, fluorescence real-time quantitative PCR with SYBR Green fluorescent dye was used. Three replicates were performed to improve the accuracy of the data. The melting curves were analyzed to determine a single curve amplification, and the temporal expression of the target gene was analyzed using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
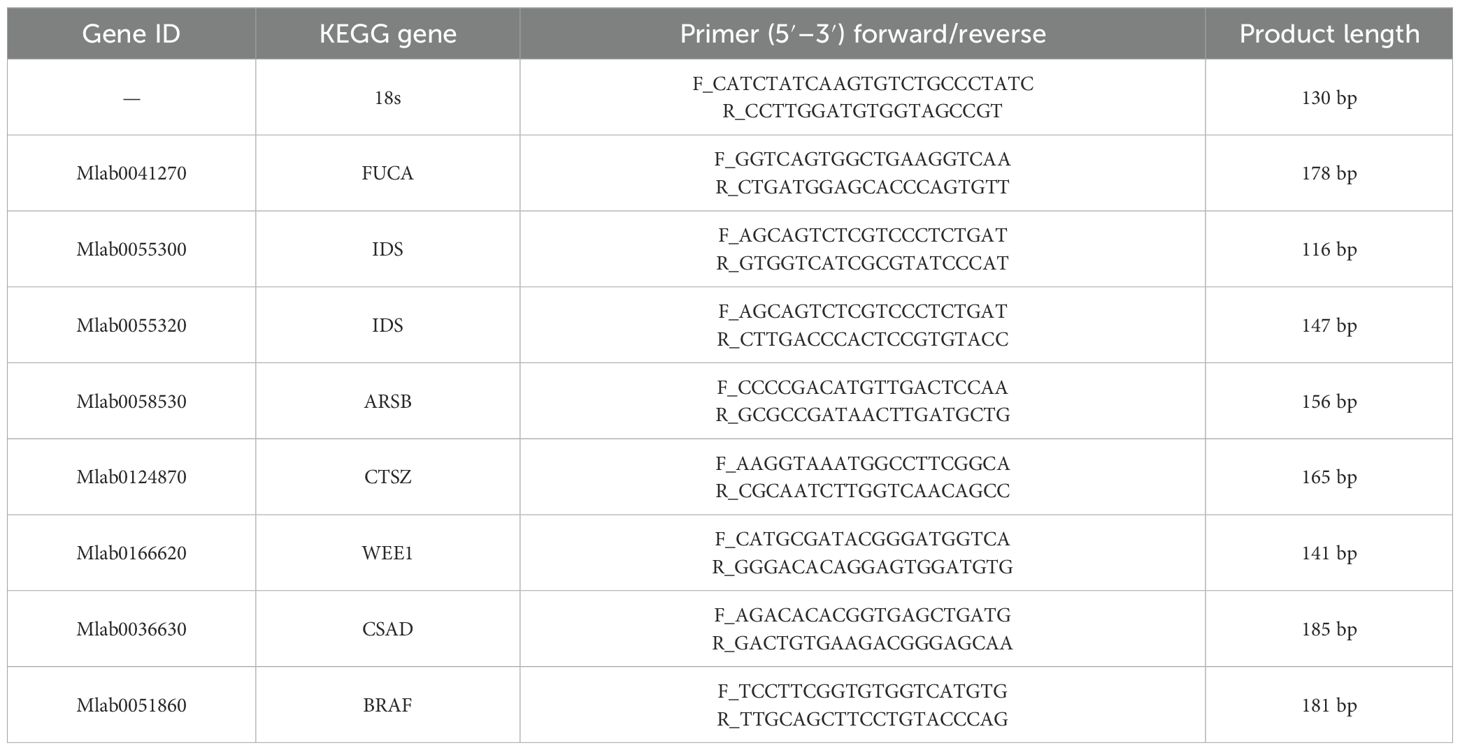
Table 1. qRT-PCR-verified primer sequences of differentially expressed genes under air exposure stress.
Nine cDNA libraries were obtained in this experiment, including air exposure stress groups (t1 and t2) and control group (t0), with three biological replicates in each group. After joints and low-quality sequences were removed, a total of 420.81 M clean reads were obtained, ranging from 44.93 M to 48.72 M. The quality analysis results showed that the Q20 values were more than 99% of the time, and the Q30 scores were over 97% (Table 2). The clean reads had a good randomness among the reference genes, and the number of clean reads mapped to the genome ranged from 62.91% to 90.96% (Table 3). And the nine sets of raw data obtained were saved to NCBI Sequence Read Archive (SRA) database under the sequence numbers SRR31938612 to SRR31938620 under biological item PRJNA1208224 and biological sample SAMN46169776.
Expression of genes was selected to be expressed as FPKM values, and the box diagram shows the gene expression of each individual (Figure 1). Differences in expression levels were observed between different individuals with different air exposure times and the same air exposure time. The dependability of the experiment and the appropriateness of individual selection can be tested by correlating gene expression levels between individuals. The individual gene expression correlation map showed that the expression pattern similarity between individuals was high, indicating that the experiment is reliable and the individual selection is reasonable (Figure 2).
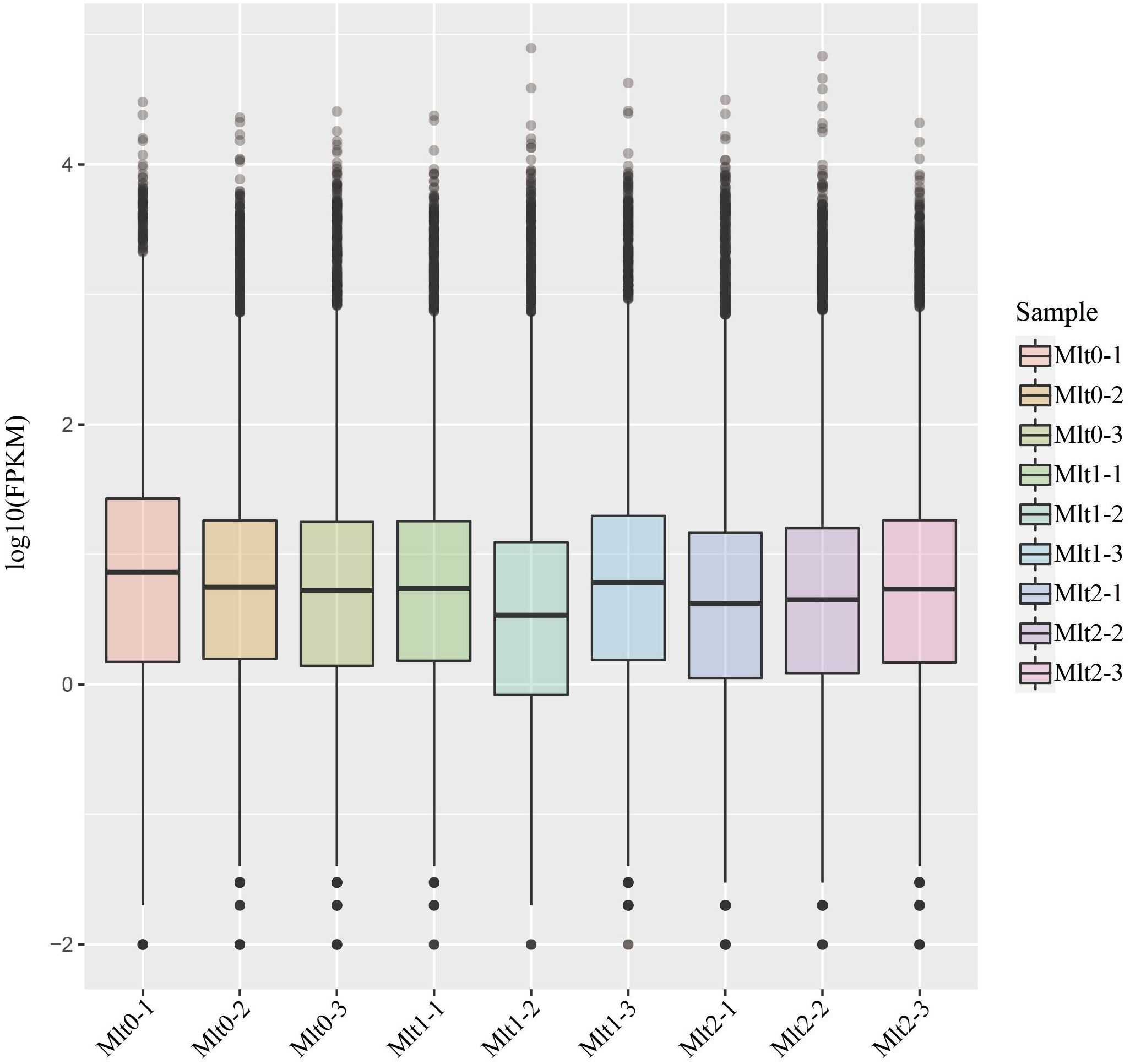
Figure 1. Gene expression levels in different treatment group (Supplementary Table S1: Gene_fpkm) The X-axis is the sample name, the Y-axis is log10(FPKM), and the box-and-line plot for each region corresponds to five statistics (upper limit, upper quartile, median, lower quartile, and lower limit, from top to bottom).
Transcripts from different days of air exposure were analyzed, in which 33 genes were up-regulated and 17 genes were down-regulated in the 2d air exposure stress group (t1) compared with the control group (t0), with a total of 50 DEGs (Figure 3A). The air exposure stress 5d group (t2) had 368 genes up-regulated and 572 genes down-regulated in comparison with the control group (t0), totaling 940 genes (Figure 3B). The air exposure stress 2d group (t1) had 364 genes up-regulated and 331 genes down-regulated in comparison to the air exposure stress 5d group (t2), totaling 695 genes (Figure 3C; Table 4).
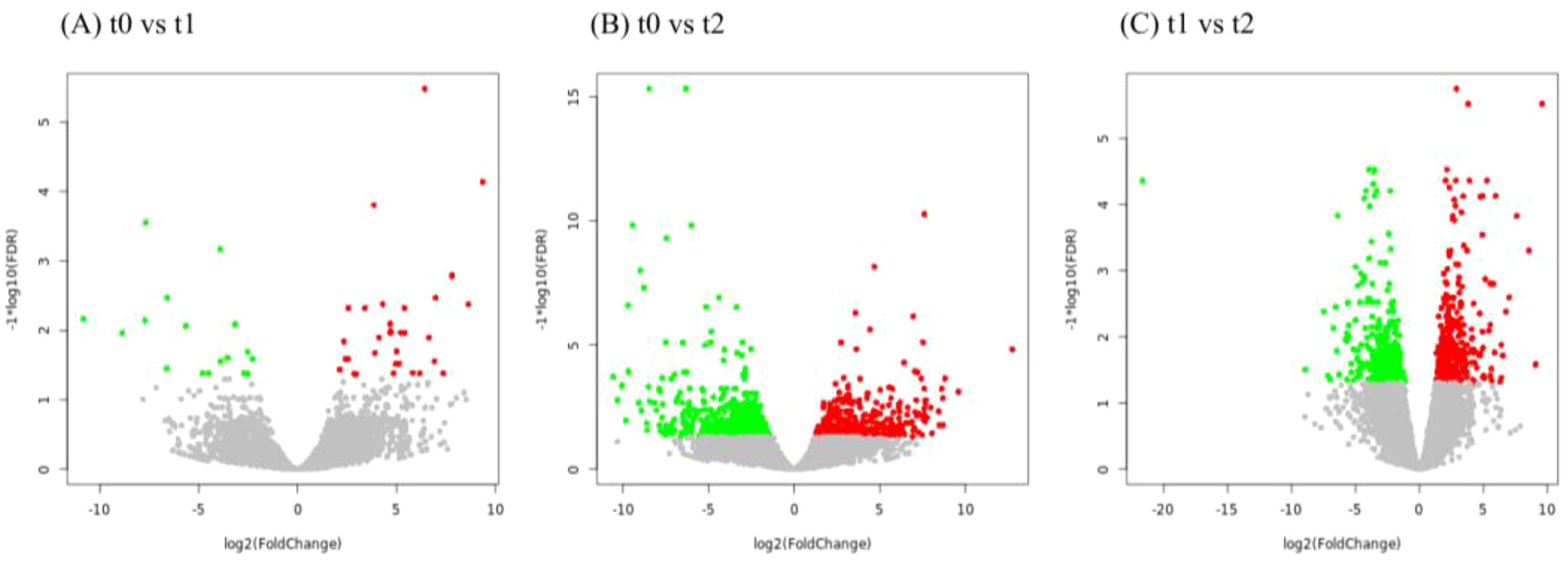
Figure 3. Volcano plot of DEGs in different air exposure comparison groups. (A) t0 versus t1 volcano plot, (B) t0 versus t2 volcano plot, and (C) t1 versus t2 volcano plot.
As shown in the Venn diagram (Figure 4), there were 13 significantly differentially expressed genes on 2 day air exposure compared to 5 day air exposure, and in addition, a total of 3 differentially expressed genes were significantly expressed in all 3 groups. Four of the 13 significantly differentially expressed genes (BRAF, RAN, COL6A, and DNAJA1) and 1 of the 3 significantly differentially expressed genes (BRAF) were annotated to several specific KEGG pathways, and the expression of their differentially expressed genes also varied according to the dry dew stress (Table 5). Ribosome biogenesis in eukaryotes(ko03008), Protein processing in endoplasmic reticulum(ko04141) and immune-related signaling pathways were mainly involved in eukaryotes. It is suggested that the adaptive mechanisms of M. labio to air exposure include immune response and apoptosis.
GO enrichment analyses were performed on the DEGs to identify the key functions affected by air exposure. The results of GO enrichment in air exposure 2d (t1) and air exposure 5d (t2) are shown in Figures 5A, B, respectively, relative to the undried dew (t0) group, and the results of GO enrichment in air exposure 5d (t2) compared with that in air exposure 2d (t1) are shown in Figure 5C. In the analysis of variance comparison between t0 versus t1 groups, catalytic activity (GO:0003824), protein kinase activity (GO:0004672), and protein phosphorylation (GO:0006468) were significantly enriched in the air exposure 2d (t1) group. In the analysis of variance comparison between t0 versus t2 groups, the air exposure 5d (t2) group showed significant enrichment in oxidoreductase activity (GO:0016491), extracellular region (GO:0005576), carbohydrate metabolism process (GO:0005975), RNA binding (GO:0003723), catalytic activity (GO:0003824), and proteolysis (GO:0006508). In the analysis of variance comparison between t1 versus t2 groups, the air exposure 5d (t2) group demonstrated significant enrichment for protein phosphorylation (GO:0006468), oxidoreductase activity (GO:0016491), protein kinase activity (GO:0004672), transmembrane transporter protein activity (GO:0022857), and calcium ion binding (GO:0005509).
The results of KEGG analysis showed the degree of gene enrichment of the signaling pathway by gene ratio, p.adjust value, and the number of genes enriched on the relevant pathway. The smaller the p.adjust is, the more significant the value. Figures 5D–F show the KEGG pathway enrichment results. Compared with the no air exposure (t0) group, the DEG in the air exposure 2d (t1) group was mainly involved in the regulation of prolactin signaling pathway (ko04917), FoxO signaling pathway (ko04068), chemokine signaling pathway (ko04062), protein processing in endoplasmic reticulum (ko04141), neurotrophic factor signaling pathway (ko04722), mitogen-activated protein kinase (MAPK) signaling pathway (ko04010), PI3K-Akt signaling pathway (ko04151), focused adhesion (ko04510), and multispecies regulation of longevity (ko04213). Compared with the no air exposure (t0) group, the DEG in the air exposure 5d (t2) group was mainly involved in the regulatory pathways of lysosome (ko04142), spliceosome (ko03040), ribosome synthesis in eukaryotes (ko03008), protein processing in the endoplasmic reticulum (ko04141), and phagosome (ko04145). The DEG in the air exposure 2d (t1) group was mainly involved in the regulation of the cell cycle (ko04110) compared with the air exposure 5d (t2) group.
Eight genes were selected from the DEGs obtained from the sequencing results, and the visceral mass tissues of M. labio without air exposure, with 2d of air exposure, and with 5d of air exposure were used as templates to test the precision of the transcriptome data by using qRT-PCR. The results found that the expressive tendencies of the eight genes were consistent with those in the transcriptome data (Figure 6), proving that the results of this sequencing are accurate and reliable.
In recent years, the molecular tolerance mechanisms of intertidal shellfish to environmental stresses have received increasing attention from researchers (Diederich et al., 2015). Air exposure is considered to be a major stressor affecting the survival of shellfish, considerably influencing their survival rate, growth, and development, and habitats. During air exposure stress, shellfish are subjected to water deprivation, oxygen deprivation, increased ambient temperature, food deprivation, sunlight radiation, and abrupt changes in body pH, all of which can cause severe tissue damage and even death, resulting in high economic losses (McMahon, 1988; Lesser, 2006; Freire et al., 2011). As a result, a portion of shellfish evolved multiple regulatory mechanisms to deal with the stress of air exposure. Previous studies have reported that air exposure stress affects immune, metabolic, and other systems of Ruditapes philippinearum and causes elicits oxidative stress; affects physiological and tissue-level functions of river clams; and affects apoptosis and redox and tissues and hemolymph in oysters (Kawabe et al., 2010; Dong et al., 2017; Nie et al., 2020; Zhang et al., 2022b). In the present study, air exposure stress in M. labio affected immunity, apoptosis. Actually, a number of functional genes regulate these cellular physiological pathways. As a result, genes associated with immunity, apoptosis have received much attention (Table 6).
The prolongation of air exposure disrupts the immune system, which leads to a weakening of defenses against bacteria and disease. The immune response plays an important role in the adaptation to air exposure changes in M. labio. The immune response was found to be regulated by the expression of the immune-related PI3K-Akt signaling pathway (ko04151), the MAPK signaling pathway (ko04010), and the genes BRAF, MAPK8IP1, MAP2K3, MAPKAPK2, GADD45, CACNA1A, EGFR,VWF, PHLPP, and COL6A.
Invertebrates have only innate immunity and are very sensitive to various biotic or abiotic stimuli (Guo et al., 2015). They adapt to changes in the external environment by modulating Immune genes and pathways in the face of external stimuli (Manfredi et al., 2008; Huang et al., 2013; Thornton et al., 2017). Involvement of VWF, CACNA1A, PHLPP and COL6A in the PI3K-Akt signaling pathway, which enables the organism’s immune response to combat air exposure stress. PHLPPs can act as immune activators or inhibitors in immune cells (Kawashima et al., 2021). When expression is upregulated, the immune response of cells is activated to maintain homeostasis in the body. Elevated plasma VWF levels are associated with cardiovascular disease, but there is growing evidence that VWF is involved in innate immunity (Jager et al., 1999; Drakeford et al., 2022). Calcium signaling is coordinated by CACNA1A (voltage-gated calcium channel), which is involved in regulation by ca2+ dependent effectors such as calmodulin (CaM) and NFAT. In aquatic organisms, the MAPK signaling pathway plays an important role in mediating cellular functions such as adaptation to various stresses. Studies have shown that the MAPK signaling pathway helps to make in a range of cellular processes such as activation by oxidative stress, inflammation, apoptosis, autophagy, and thus, it is a key regulator of immunity (Canesi et al., 2006; Geest and Coffer, 2009; Kim and Choi, 2015). In Turbo cornutus, TVE activates the MAPK signaling pathway to enhance antioxidant effects (Lee et al., 2023). The MAPK pathway regulates glycogen metabolism through the transmission of hrIGF-I signaling in Pinctada fucata (Shi and He, 2016). In this study, the expression of BRAF, MAPK8IP1, MAP2K3, MAPKAPK2, GADD45, and EGFR associated with the MAPK signaling pathway involved in the immune system’s response to resist air exposure stress. Rapid activation of the MAPK signaling pathway (ko04010) by high BRAF expression is the main defense mechanism during acute air exposure (2 days). This pathway initiates a multipronged response: clearance of ROS; enhancement of innate immunity; and GADD45 driven DNA damage repair to mitigate genotoxic stress. As the air exposure is prolonged into the chronic phase (5 days), the initial dominance of rapid activation of the MAPK signaling pathway by BRAF expression is diminished due to a decline in BRAF expression. MAPK dephosphorylates and PHLPP inactivates Akt, inhibiting glycolysis and shifting energy production to fatty acid oxidation and autophagy.
Transcriptomic data suggest that the expression of many apoptosis-related genes(BIRC2_3, XIAP, CASP8, ERN1, RAN, DNAJA1) is altered after air exposure. Similarly, Crassostrea Virginica showed changes in many genes involved in apoptosis after 7 days of exposure to air (Li et al., 2022). This proof proposes that apoptosis plays role in the response of mollusks to air exposure. Apoptosis, is a process of physiological cellular self-destruction determined by their own in-built programs (Feig and Peter, 2007; AnvariFar et al., 2016). The family of cysteinyl aspartate specific proteinases (Caspases) are mediators and executors of programmed death (Marani et al., 2002). ERN1 gene involved in endoplasmic reticulum stress-induced apoptosis and autophagosome formation [ (Babu et al., 2014). In the current study, the expression of cysteine protease CASP8, ERN1 was upregulated after air exposure. CASP8, ERN1 is a key gene on the apoptotic signaling pathway, and its upregulation could lead to an increase in the level of apoptosis as an adaptation to air exposure stress. The anti-apoptotic system antagonizes the onset of apoptosis to protect cells. Therefore, this system may be an adaptive response evolved by the organism to cope with stress, and inhibitors of apoptosis (IAPs) protect cells from massive apoptotic cell death (Deveraux et al., 1997; Dumétier et al., 2020). Upregulation of IAP is associated with cell survival and prevents apoptosis-mediated tissue damage, whereas downregulation of IAP reduces the organism’s ability to resist apoptosis (Mahalingam et al., 2009; Lu et al., 2014). In the present study, XIAP, BIRC2_3 expression was upregulated at 5 days of air exposure. This finding is compatible with the results from a transcriptome study on Ruditapes philippinarum after exposure to air and reoxygenation (Nie et al., 2020), suggesting the importance of the anti-apoptotic system in the adaptation and survival of the organism in response to air exposure stress. Ran (RAS-associated nuclear protein) is a member of the RAS superfamily of small GTPases, and disruption of Ran expression has been associated with cancer at various levels (Boudhraa et al., 2020). DNAJA1 has been shown to bind to unfolded polypeptide chains and prevent their aggregation, to import proteins into mitochondria, and to be involved in the inhibition of anti-apoptosis in cancer cells (Kanazawa et al., 1997; Terada and Oike, 2010; Stark et al., 2014). At 5 days of air exposure, the expression of RAN, DNAJA1 was down-regulated, and we speculate that it was difficult for the organism to inhibit the cancer cells from undergoing apoptosis, and that prolonged air exposure was more detrimental in M. labio.
In this study, we analyzed the transcriptome changes of M. labio under different air exposure durations to reveal the molecular regulatory network of its tolerance to air exposure stress. It was found that the duration of air exposure could significantly affect the signaling pathways related to immune stress and apoptosis. Predicting population survival risk boundaries due to climate change based on the expression of key genes can be used as a field population health assessment tool. Early warning of structural impacts of environmental stresses on M. labio populations through real-time monitoring of gene expression fluctuations. This study not only elucidates the effects of air exposure stress on mollusks, provides key data support for theoretical models of intertidal shellfish adaptation to heterogeneous environments, but also lays a scientific foundation for the development of conservation strategies based on molecular ecology.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1208224.
The animal study was approved by Ethics Committee of Zhejiang Ocean University. The study was conducted in accordance with the local legislation and institutional requirements.
ML: Data curation, Investigation, Validation, Writing – original draft. SS: Data curation, Writing – review & editing. FZ: Writing – review & editing. ZH: Funding acquisition, Project administration, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Fundamental Research Funds for Zhejiang Provincial Universities and Research Institutes (2024J004) and the National Key Research and Development Program of China (2023YFD2401901).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1558836/full#supplementary-material
Abele D., Philipp E., Gonzalez P., Puntarulo S. (2007). Marine invertebrate mitochondria and oxidative stress. J. Front. Biosci. 12, 933–946. doi: 10.2741/2115
Aguilar Vitorino H., Pastrana Alta R. Y., Ortega P. (2019). Lipid peroxidation in hepatopancreas, gill, and hemolymph of male and female crabs Platyxanthus orbignyi after air exposure. J. Mar. Sci. Eng. 7, 347. doi: 10.3390/jmse7100347
Ai-yi Z., Jia-yian X., Jin-hai C. (2007). Effects of temperature and body size on oxygen consumption rate and ammonia excretion rate of Monodonta labio. Ecol. Sci. (03), 232–236.
Anders S., Pyl P. T., Huber W. (2014). HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 31 (2), 166–169. doi: 10.1093/bioinformatics/btu638
Andrews S. (2010). FastQC: a quality control tool for high throughput sequence data (Cambridge, United Kingdom: Babraham Bioinformatics, Babraham Institute).
AnvariFar H., Amirkolaie A. K., Miandare H. K., Ouraji H., Jalali M. A., Üçüncü S.İ. (2016). Apoptosis in fish: environmental factors and programmed cell death. Cell Tissue Res. 368, 425–439. doi: 10.1007/s00441-016-2548-x
Babu S. G., Pandeya A., Verma N., Shukla N., Kumar R. V., Saxena S. (2014). Role of HCMV miR-UL70-3p and miR-UL148D in overcoming the cellular apoptosis. Mol. Cell. Biochem. 393, 89–98. doi: 10.1007/s11010-014-2049-8
Bao J., Xing Y.-N., Jiang H.-B., Li X.-D. (2019). Identification of immune-related genes in gills of Chinese mitten crabs (Eriocheir sinensis) during adaptation to air exposure stress. J.F. Immunol. S. 84, 885–893. doi: 10.1016/j.fsi.2018.10.085
Barrios-Figueroa R., Urbina M. (2023). Behavioural and physiological responses to salinization and air exposure during the ontogeny of a freshwater South American snail. J. Conserv. Physiol. 11, coac089. doi: 10.1093/conphys/coac089
Boudhraa Z., Carmona E., Provencher D., Mes-Masson A.-M. (2020). Ran GTPase: A key player in tumor progression and metastasis. Front. Cell Dev. Biol. 8, 345. doi: 10.3389/fcell.2020.00345
Canesi L., Betti M., Ciacci C., Lorusso L., Pruzzo C., Gallo G. (2006). Cell signalling in the immune response of mussel hemocytes. J. Invertebrate Survival J. 3 (1), 40–49.
Chen S., Zhou Y., Chen Y., Gu J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34 (17), i884–i890. doi: 10.1093/bioinformatics/bty560
Chhor A. D., Glassman D. M., Brownscombe J. W., Trahan A. T., Danylchuk A. J., Cooke S. J. (2022). Short-term behavioural impacts of air-exposure in three species of recreationally angled freshwater fish. J. Fisheries Res. 253, 106342. doi: 10.1016/j.fishres.2022.106342
Chiu Y.-W., Bor H., Wu J.-X., Shieh B.-S., Lin H.-D. (2023). Population Structure and Phylogeography of Marine Gastropods Monodonta labio and M. confusa (Trochidae) along the Northwestern Pacific Coast. J. Diversit 15, 1021. doi: 10.3390/d15091021
Cong H., Lei Y., Kong L. (2020). The mitochondrial genome of the toothed top shell snail Monodonta labio (Gastropoda: Trochidae): the first complete sequence in the subfamily monodontinae. Mitochondrial DNA Part B. 5, 621–622. doi: 10.1080/23802359.2019.1711221
de Andrade J. T. M., Cordeiro N. I. S., Montresor L. C., da Luz D. M. R., Viana E., Martinez C. B., et al. (2021). Tolerance of Limnoperna fortunei (Dunker 1857)(Bivalvia: Mytilidae) to aerial exposure at different temperatures. Hydrobiologia 848, 2993–3001. doi: 10.1007/s10750-020-04191-4
Deveraux Q. L., Takahashi R., Salvesen G. S., Reed J. C. (1997). X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 388 (6639), 300–304. doi: 10.1038/40901
Diederich C. M., Bashevkin S. M., Chaparro O. R., Pechenik J. A. (2015). Desiccation tolerance and lifting behavior in Crepidula fornicata (Gastropoda). Mar. Ecol. Prog. Series. 528, 235–243. doi: 10.3354/meps11284
Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. (2012). STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29 (1), 15–21. doi: 10.1093/bioinformatics/bts635
Dong W., Liu Z., Qiu L., Wang W., Song X., Wang X., et al. (2017). The modulation role of serotonin in Pacific oyster Crassostrea gigas in response to air exposure. Fish Shellfish Immunol. 62, 341–348. doi: 10.1016/j.fsi.2017.01.043
Drakeford C., Aguila S., Roche F., Hokamp K., Fazavana J., Cervantes M. P., et al. (2022). von Willebrand factor links primary hemostasis to innate immunity. Nat. Commun. 13 (1), 6320. doi: 10.1038/s41467-022-33796-7
Dumétier B., Zadoroznyj A., Dubrez L. (2020). IAP-mediated protein ubiquitination in regulating cell signaling. Cells. 9 (5), 1118. doi: 10.3390/cells9051118
Feig C., Peter M. E. (2007). How apoptosis got the immune system in shape. Eur. J. Immunol. 37 (S1), S61–S70. doi: 10.1002/eji.200737462
Freire C. A., Welker A. F., Storey J. M., Storey K. B., Hermes-Lima M. (2011). Oxidative stress in estuarine and intertidal environments (temperate and tropical). Oxidative Stress in Aquatic Ecosystems, 41–57.
Geest C. R., Coffer P. J. (2009). MAPK signaling pathways in the regulation of hematopoiesis. J. Leukocyte Biol. 86 (2), 237–250. doi: 10.1189/jlb.0209097
Guan W., Wei X., Nong W., Shao Y., Mao L. (2021). Heat shock protein 70 (HSP70) promotes air exposure tolerance of Litopenaeus vannamei by preventing hemocyte apoptosis. J.D. Immunol. C. 114, 103844. doi: 10.1016/j.dci.2020.103844
Guo X., He Y., Zhang L., Lelong C., Jouaux A. (2015). Immune and stress responses in oysters with insights on adaptation. Fish Shellfish Immunol. 46 (1), 107–119. doi: 10.1016/j.fsi.2015.05.018
Huang M., Song X., Zhao J., Mu C., Wang L., Zhang H., et al. (2013). A C-type lectin (AiCTL-3) from bay scallop Argopecten irradians with mannose/galactose binding ability to bind various bacteria 531, 31–38. doi: 10.1016/j.gene.2013.08.042
Jager A., van Hinsbergh V. W., Kostense P. J., Emeis J. J., Yudkin J. S., Nijpels G., et al. (1999). von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arteriosclerosis Thrombosis Vasc. Biol. 19 (12), 3071–3078. doi: 10.1161/01.atv.19.12.3071
Jian-she Z., Ai-yi Z., Chang-wen W. (2011). Nutritional composition analysis and evaluation of monodonta labio muscle. Food Sci. 32, 353–356.
Kanazawa M., Terada K., Kato S., Mori M. (1997). HSDJ, a human homolog of DnaJ, is farnesylated and is involved in protein import into mitochondria. J. Biochem. 121 (5), 890–895. doi: 10.1093/oxfordjournals.jbchem.a021670
Kawabe S., Takada M., Shibuya R., Yokoyama Y. (2010). Biochemical changes in oyster tissues and hemolymph during long-term air exposure. Fisheries Sci. 76, 841–855. doi: 10.1007/s12562-010-0263-1
Kawashima A. T., Wong C., Lordén G., King C. C., Lara-Gonzalez P., Desai A., et al. (2021). The PHLPP1 N-terminal extension is a mitotic cdk1 substrate and controls an interactome switch. Mol. Cell. Biol. 41 (3), e00333-20. doi: 10.1128/mcb.00333-20
Kim E. K., Choi E.-J. (2015). Compromised MAPK signaling in human diseases: an update. J. Arch. Toxicol. 89, 867–882. doi: 10.1007/s00204-015-1472-2
Lee Y.-J., Kim E.-A., Kang N., Park A., Heo S.-J. (2023). Antioxidant Effects of Turbo cornutus By-Products Visceral Extract against Hydrogen Peroxide-Induced Oxidative Stress by Regulating MAPK and Akt Signaling Pathways in Vero Cells. Foods. 12 (19), 3660. doi: 10.3390/foods12193660
Lei X., Yang L., Tan L., Yang Q., Zhou F., Jiang S., et al. (2023). Effect of air exposure and re-submersion on the histological structure, antioxidant response, and gene expression of procambarus clarkii 13, 462. doi: 10.3390/ani13030462
Lesser M. P. (2006). Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol. 68, 253–278. doi: 10.1146/annurev.physiol.68.040104.110001
Li C., Wang H., Guo X. (2022). Regulation of the cell cycle, apoptosis, and proline accumulation plays an important role in the stress response of the eastern oyster crassostrea virginica. Front. Mar. Sci. 9, 921877. doi: 10.3389/fmars.2022.921877
Li H. L., Yang S. P., Guo W. J., Tan Z. H., Chan S. F (2017). Effects of air exposure and re-submersion on oxidative stress of marine gastropod, Babylonia areolata. Israeli J. Aquaculture-Bamidgeh 69.
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. doi: 10.1006/meth.2001.1262
Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21. doi: 10.1186/s13059-014-0550-8
Lu J., Qin Q., Zhan L.-L., Liu J., Zhu H.-C., Yang X., et al. (2014). AT-406, an IAP inhibitor, activates apoptosis and induces radiosensitization of normoxic and hypoxic cervical cancer cells. J. Pharmacol. Sci. 126 (1), 56–65. doi: 10.1254/jphs.14079fp
Mahalingam D., Szegezdi E., Keane M., de Jong S., Samali A. (2009). TRAIL receptor signalling and modulation: Are we on the right TRAIL? Cancer Treat Rev. 35 (3), 280–288. doi: 10.1016/j.ctrv.2008.11.006
Manfredi A. A., Rovere-Querini P., Brunelli S., Clementi E. (2008). Cell death: tipping the balance of autoimmunity and tissue repair. J. Curr. Pharm. Des. 14, 269–277. doi: 10.2174/138161208783413275
Marani M., Tenev T., Hancock D., Downward J., Lemoine N. R. (2002). Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. J. Mol. Cell Biol. 22, 3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002
McMahon R. F. (1988). Respiratory response to periodic emergence in intertidal molluscs. J. Am. Zoologist 28, 97–114. doi: 10.1093/icb/28.1.97
Nan X., Cao Q. (2012). Toxic effects of copper on Monodonta labio. J. Ecol. rural environment 28, 277–281.
Nie H., Jiang K., Li N., Li D., Yan X. (2020). Transcriptomic analysis of Ruditapes philippinarum under aerial exposure and reimmersion reveals genes involved in stress response and recovery capacity of the Manila clam. Aquaculture 524, 735271. doi: 10.1016/j.aquaculture.2020.735271
Paital B. (2013). Antioxidant and oxidative stress parameters in brain of Heteropneustes fossilis under air exposure condition; role of mitochondrial electron transport chain. J. Ecotoxicol. Environ. Saf. 95, 69–77. doi: 10.1016/j.ecoenv.2013.05.016
Shi Y., He M.-X. (2016). PfIRR interacts with hrIGF-I and activates the MAP-kinase and PI3-kinase signaling pathways to regulate glycogen metabolism in pinctada fucata. Sci. Rep. 6 (1), 22063. doi: 10.1038/srep22063
Stark J. L., Mehla K., Chaika N., Acton T. B., Xiao R., Singh P. K., et al. (2014). Structure and function of human dnaJ homologue subfamily A member 1 (DNAJA1) and its relationship to pancreatic cancer. Biochemistry. 53 (8), 1360–1372. doi: 10.1021/bi401329a
Tan K., Zhang H., Ma H., Li S., Zheng H. (2021). Effects of tidal zones and seasons on nutritional properties of commercially importance gastropods. J. Estuarine Coast. Shelf Sci. 254, 107289. doi: 10.1016/j.ecss.2021.107289
Terada K., Oike Y. (2010). Multiple molecules of Hsc70 and a dimer of DjA1 independently bind to an unfolded protein. J. Biol. Chem. 285 (22), 16789–16797. doi: 10.1074/jbc.m110.101501
Thornton C., Leaw B., Mallard C., Nair S., Jinnai M., Hagberg H. (2017). Cell death in the developing brain after hypoxia-ischemia. J. Front. Cell Neurosci. 11, 248. doi: 10.3389/fncel.2017.00248
Weinrauch A. M., Blewett T. A. (2019). Anoxia tolerance in the sea cucumbers Parastichopus californicus and Cucumaria miniata reflects habitat use. J. Exp. Mar. Biol. Ecol. 520, 151203. doi: 10.1016/j.jembe.2019.151203
Yamazaki D., Miura O., Ikeda M., Kijima A., Van Tu D., Sasaki T., et al. (2017). Genetic diversification of intertidal gastropoda in an archipelago: the effects of islands, oceanic currents, and ecology. Mar. Biol. 164, 1–11. doi: 10.1007/s00227-017-3207-9
Yin X., Chen P., Chen H., Jin W., Yan X. (2017). Physiological performance of the intertidal Manila clam (Ruditapes philippinarum) to long-term daily rhythms of air exposure. J. Sci. Rep. 7, 41648. doi: 10.1038/srep41648
Yinong Wang G. Z. (1994). EXPERIMENTAL ECOLOGY AND DISTRIBUTION OF monodonta labio. Mar. Sci. 03), 14–16.
Yu G., Wang L.-G., Han Y., He Q.-Y. (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS: A J. Integr. Biol. 16 (5), 284–287. doi: 10.1089/omi.2011.0118
Zhang T., Wen H., Xu D., Lv G., Zhou Y. (2022a). PacBio Full-Length and Illumina Transcriptomes of the Gill Reveal the Molecular Response of Corbicula fluminea under Aerial Exposure. J. Int. J. Mol. Sci. 23, 11474. doi: 10.3390/ijms231911474
Zhang Y.-M., Xu W.-B., Cheng Y.-X., Chen D.-Y., Lin C.-Y., Li B.-Z., et al. (2022c). Effects of air exposure stress on crustaceans: Histopathological changes, antioxidant and immunity of the red swamp crayfish Procambarus clarkii. Developmental & Comparative Immunol. 135, 104480. doi: 10.1016/j.dci.2022.104480
Zhang T., Xu D., Lv G., Wang A., Wen H. (2022b). Histological, physiological, and transcriptomic responses of hepatopancreas to air exposure in asian freshwater clam Corbicula fluminea. Front. Physiol. 13, 952744. doi: 10.3389/fphys.2022.952744
Zhao D., Kong L.-F., Sasaki T., Li Q. (2019). Shell variations in the gastropod, Monodonta labio, in the North-western Pacific: the important role of temperature in the evolution process. J. Mar. Biol. Assoc. United Kingdom 99, 1591–1599. doi: 10.1017/s0025315419000481
Zhao D., Li Q., Kong L., Yu H. (2017). Cryptic diversity of marine gastropod Monodonta labio (Trochidae): did the early Pleistocene glacial isolation and sea surface temperature gradient jointly drive diversification of sister species and/or subspecies in the Northwestern Pacific? Mar. Ecol. 38 (4), e12443. doi: 10.1111/maec.12443
Keywords: Monodonta labio, air exposure, transcriptome, pathways, DEGs (differentially expressed genes)
Citation: Liu M, Sun S, Zhang F and Han Z (2025) Transcriptional response of Monodonta labio to air exposure. Front. Mar. Sci. 12:1558836. doi: 10.3389/fmars.2025.1558836
Received: 25 January 2025; Accepted: 06 March 2025;
Published: 24 March 2025.
Edited by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaReviewed by:
D. K. Meena, Central Inland Fisheries Research Institute (ICAR), IndiaCopyright © 2025 Liu, Sun, Zhang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiqiang Han, ZDYzMzkxMjRAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.