
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 21 February 2025
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1555994
This article is part of the Research TopicAlternative Feed Ingredients and their Functional Properties in AquacultureView all 10 articles
 Hairui Yu1*
Hairui Yu1* Maida Mushtaq2,3
Maida Mushtaq2,3 Saira Razzaq3
Saira Razzaq3 Umar Ali3
Umar Ali3 Muhammad Khan2,4*
Muhammad Khan2,4* Abdur Rahman1*
Abdur Rahman1* Guobo Quan2
Guobo Quan2 Mehroze Fatima3
Mehroze Fatima3 Saima Naveed4
Saima Naveed4 Muhammad Hammad Zafar5
Muhammad Hammad Zafar5 Muhammad Aziz ur Rahman6
Muhammad Aziz ur Rahman6The study aimed to evaluate the effects of dietary supplementation of selenium (Se), vitamin C, and vitamin E on growth performance, meat quality, and antioxidant status in Hypophthalmichthys molitrix juveniles. A total of 480 juveniles (mean weight: 20 ± 0.29 g) were randomly assigned to 24 aquaria (20 fish per 100 L), which were allocated to eight dietary treatments (three aquaria per treatment) following a completely randomized design. The dietary treatments included: (1) a basal diet without supplementation (Control), (2) selenium supplementation (S), (3) vitamin C supplementation (C), (4) vitamin E supplementation (E), (5) selenium and vitamin C supplementation (SC), (6) selenium and vitamin E supplementation (SE), (7) vitamin C and vitamin E supplementation (EC), and (8) selenium, vitamin C, and vitamin E supplementation (SCE). The supplementation levels were 0.9 mg/kg Se, 300 mg/kg vitamin C, and 100 mg/kg vitamin E, respectively. After a one-week acclimatization period, a 10-week growth trial was conducted. Fish fed the SCE diet exhibited significantly higher (p< 0.05) final weight gain, body length gain, body weight gain percentage, and specific growth rates compared to other treatments. Selenium concentrations in the kidney, liver, pancreas, and muscle were significantly higher (p< 0.05) in the SCE, SC, SE, and EC groups compared to the Control and single-supplement groups. Hematological parameters, including WBC, RBC, HGB, HCT, and MCHC, were significantly higher (p< 0.05) in the SCE group compared to all other groups. Similarly, serum activities of ALT, AST, and ALP were significantly lower (p< 0.05) in the SCE group than in other treatments, while blood glucose levels were unaffected (p > 0.05) by dietary treatments. Antioxidant enzyme activities, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), were significantly enhanced (p< 0.05) in the whole body and muscle tissues of fish receiving Se, vitamin C, and E supplementation. Thiobarbituric acid reactive substances (TBARS) levels in muscle and serum were significantly higher (p< 0.05) in the Control group compared to all supplemented groups. The proximate composition of meat, including dry matter, fat, ash, and protein contents, was not significantly affected (p > 0.05) by the dietary treatments. In conclusion, dietary supplementation of selenium, vitamin C, and vitamin E significantly improved growth performance, selenium deposition in tissues, and antioxidant status of Hypophthalmichthys molitrix juveniles, without adversely affecting meat chemical composition.
Fish production systems range from extensive pond-based setups to highly controlled intensive rearing environments. Intensive aquaculture offers several advantages, including accelerated growth rates, enhanced management of the rearing environment, and early detection of diseases (Kumar and Engle, 2016). However, one significant drawback of intensive systems is the induction of stress, which often leads to oxidative stress through the overproduction of reactive oxygen species (ROS) (Mushtaq et al., 2022). Elevated ROS levels disrupt physiological processes and impair antioxidant defense mechanisms in fish, ultimately resulting in reduced performance, higher morbidity, and increased mortality (Naiel et al., 2020). The antioxidant defense system in animals comprises endogenous enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (Mushtaq et al., 2022). Exogenous antioxidants, including vitamins C and E, and micro minerals such as selenium (Se), enhance this defense system by acting as cofactors for these enzymes or through structural stabilization of biomolecules (Flora et al., 2013). Thus, formulating a balanced diet with appropriate redox-regulating nutrients is essential for promoting optimal growth performance and ensuring animal welfare in intensive aquaculture systems.
Selenium is a vital trace mineral in aquaculture due to its interaction with cysteine to form selenocysteine, a key component of selenoproteins (Arshad et al., 2021). Selenoproteins, comprising 21 amino acids, are extracellular proteins with at least 25 identified forms in animal tissues, where selenocysteine (Se-Cys) serves as a crucial cofactor for enzymatic redox processes (Yang and Liu, 2017). Extensive research highlights the role of dietary selenium in enhancing fish fertility (Naderi et al., 2017), thyroid hormone metabolism (Kohrle et al., 2005), DNA synthesis, and cellular protection against oxidative damage (Khademzade et al., 2022). Organic selenium forms, such as selenomethionine, are preferred due to their lower toxicity and higher bioavailability compared to inorganic selenium (Naderi et al., 2017).
Vitamin C, or ascorbic acid, is another essential exogenous antioxidant, recognized for its free radical scavenging properties and its role as a cofactor in various metabolic recycling pathways (Smirnoff, 2018). Dietary requirements for vitamin C vary depending on the fish species, growth stage, body size, and environmental conditions (Council et al., 2011). Furthermore, elevated levels of vitamin C can enhance immune responses beyond physiological needs, offering additional protection against stress (Trichet, 2010).
Vitamin E represents a group of lipid-soluble antioxidants, including tocopherols and tocotrienols. Among these, α-tocopherol (α-TOH) exhibits the highest biological activity (Hamre, 2011) and is preferentially retained in mammalian systems, including Atlantic salmon, likely due to its high affinity for liver tocopherol-binding proteins (Kayden and Traber, 1993). Vitamin E plays a critical role in stabilizing cellular membranes and mitigating ROS-induced oxidative stress. It interrupts lipid peroxidation chains by neutralizing fatty acid peroxide radicals, thereby preventing the oxidation of other fatty acids (Gutteridge, 1995). Numerous studies document the positive effects of vitamin E on fish growth, immune responses, health, and stress resistance (Naderi et al., 2017).
Selenium, vitamin E, and vitamin C function synergistically to enhance antioxidant defense and support immune health in fish (Harsij et al., 2020). Selenium plays a pivotal role in combating oxidative stress by targeting peroxides through selenium-dependent glutathione peroxidase (GPx), while vitamin E prevents lipid peroxidation, reducing the oxidative burden on selenium (Khademzade et al., 2022). Vitamin C complements this system by regenerating the active forms of vitamin E and selenium, ensuring sustained antioxidant activity (Gutteridge, 1995). Together, these nutrients form an integrated defense network, protecting cellular integrity, optimizing enzymatic functions, and promoting overall health and growth in fish. Their combined action highlights the importance of balanced supplementation in fish nutrition to mitigate oxidative stress and support immune function. Although considerable research exists on dietary antioxidants such as selenium, vitamin C, and vitamin E in various fish species, there is a lack of comprehensive data on the synergistic effects of these supplements in intensively reared Hypophthalmichthys molitrix. This species, commonly known as silver carp, is a significant aquaculture commodity in Asian countries, including Pakistan (Mushtaq et al., 2022). The present study aims to evaluate the impact of dietary combinations of selenium, vitamin C, and vitamin E on the growth performance and antioxidant status of intensively reared Hypophthalmichthys molitrix.
The protocols and procedures for this study were approved by the Animal Use and Care Committee of the University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan (Approval No. DR/175, dated 05-04-2022). The trial was conducted at the Fish Seed Rearing Unit, C-Block, Ravi Campus, Pattoki, UVAS. A total of 480 Hypophthalmichthys molitrix juveniles (mean weight: 20 ± 0.29 g) were randomly allocated to 24 aquaria (20 fish per 100 L), and the aquaria were assigned to eight dietary treatments in a completely randomized design (three replicates per treatment). The dietary treatments included: (1) a basal diet without supplementation (Control), (2) supplementation with selenium (S), (3) supplementation with vitamin C (C), (4) supplementation with vitamin E (E), (5) selenium and vitamin C supplementation (SC), (6) selenium and vitamin E supplementation (SE), (7) vitamin C and vitamin E supplementation (EC), and (8) selenium, vitamin C, and vitamin E supplementation (SCE). The supplementation levels were 0.9 mg/kg for selenium, 300 mg/kg for vitamin C, and 100 mg/kg for vitamin E, based on previous studies (Khan et al., 2017; Mushtaq et al., 2022). The detailed research design is given below in Figure 1. The selenium source was selenomethionine (Selisseo® 2% Se, Europe), vitamin C was provided as L-ascorbic acid-2-phosphate (Vibrell™ C, Kemin, USA), and vitamin E was supplied as OXABIOL® E (Madrid, Spain). The dosage calculations were made by considering the purity of respective supplement claimed by manufactures. To prepare the experimental diets, feed ingredients were ground to a particle size of 0.05 mm using a Kenwood grinder (AT284). The ingredients were thoroughly mixed (Kenwood, AT283), and dough was prepared by adding water. The dough was then extruded through a meat mincer (ANEX, AG 3060) to form 3 mm diameter pellets. The pellets were shade-dried to reduce moisture content to approximately 10% and stored in plastic bags until use. Diet proximate composition and feed ingredient analysis were conducted following AOAC guidelines (Horwitz, 1975). Fish were acclimatized to the experimental aquaria for two weeks before the start of the 12-week feeding trial. During the trial, fish were maintained in a controlled environment with dissolved oxygen levels of 5.8–7.3 mg/L, water temperatures of 24.9–28.7°C, and pH ranging from 7.4 to 8.6. The diets were fed ad libitum twice daily at 07:00 and 16:00 hours. Feed orts were collected and managed according to the methodology of Mushtaq et al (Mushtaq et al., 2022). to calculate feed intake. The aquaria were refilled with fresh water daily to maintain consistent water quality. The basal diet composition was based on a previous study by Mushtaq et al (Mushtaq et al., 2022), and the chemical composition on a dry matter basis is presented in Table 1. The basal diet consisted of the following ingredients on a dry matter basis: 20% fish meal, 20% soybean meal, 20% corn gluten meal-60, 15% rice polish, 14.5% wheat flour, 6% fish oil, 4% mineral-vitamin premixes, and 0.5% choline chloride. Growth performance
The fish length and live weight were recorded before the start, after every 2 weeks, and on the day of termination of the feeding trial. Growth parameters from the obtained values were calculated by using the Equations 1-4.
At the end of the feeding trial, 30 fish from each aquarium were randomly selected for weighing and anesthetized using tricaine methanesulfonate (MS-222) at a concentration of 150 mg/L, following the protocol described by Mushtaq et al (Mushtaq et al., 2022). Blood samples were collected from 15 fish by puncturing the caudal vasculature. Samples intended for serum analysis were collected in plain vacutainers, centrifuged at 3000×g for 15 minutes, and the harvested serum was stored at -20°C for further analysis. Blood samples collected in EDTA-coated tuberculin syringes were analyzed for hematological parameters using an automated hematology analyzer (MEK-6550). Blood glucose concentrations were measured with commercial kits (21503, Biosystems, Barcelona, Spain). Ten fish from each aquarium were dissected to collect organs (kidney, liver, gills, and pancreas) for determining biological indices, while the remaining five fish were minced (ANEX, AG 3060) for meat quality analysis. Collected organs and meat samples were sealed in labeled zipper bags and stored at -20°C until analysis. Dry matter contents of the feed and meat samples were determined by drying at 55°C for 48 hours in a forced-air oven. The dried samples were ground to a particle size of 1 mm using a Foss grinder (CT 293 Cyclotec, Denmark) and analyzed for crude protein content following AOAC Method 976.06. Fat content was determined using the Soxhlet procedure (Tecator, Hoganas, Sweden; AOAC Method 920.29). Ash content was measured by igniting the samples at 620°C for 3 hours in a muffle furnace.
The muscle, liver, and serum samples were analyzed for TBARS following the protocol described in Mushtaq et al (Mushtaq et al., 2022). Briefly, 1 g of each tissue sample was homogenized with 3 mL of holding solution (80 mM Tris-maleate and 11.5 g/L KCl; pH 7.4). The homogenate was mixed with 1 mL of 2 mM ascorbic acid and incubated at 37°C for 30 minutes to initiate lipid peroxidation. Subsequently, 5 mL of thiobarbituric acid (TBA) was added, followed by 5 mL of 200 g/L trichloroacetic acid. After boiling to develop the colorimetric reaction, the samples were cooled, refrigerated for 45 minutes, and centrifuged. The absorbance of the TBA complex was measured at 530 nm using a spectrophotometer.
Two grams of muscle, liver, and whole-body tissues were sliced, homogenized with 6 mL of phosphate buffer (pH 7.4), filtered through Whatman No. 1 filter paper, and centrifuged at 10,000×g for 15 minutes at 4°C to collect the supernatant. TheSOD activity was measured by its ability to inhibit nitroblue tetrazolium (NBT) reduction, following the method of Giannopolitis and Ries (1977). Catalase activity was determined by the decomposition of hydrogen peroxide at 240 nm, as per Chance and Maehly (1955). TheGPx activity was assessed by measuring the residual hydrogen peroxide concentration at 470 nm. Serum ALT, AST, and ALP activities were analyzed using commercial kits (AL1205, AS3804, AP9764; Randox Laboratories Ltd).
The collected data were assessed for normality using QQ plots with IBM SPSS Statistics 20 (Chicago, USA). One-way analysis of variance (ANOVA) was conducted using the same software to evaluate the effects of dietary treatments. The statistical model included the fixed effects of treatments:
Where Yij represents the observation, μ is the population mean, Tj denotes the treatment effect, and εij is the residual error.
Statistical significance was determined at p< 0.05, with means compared using Tukey’s test for multiple comparisons.
The means and standard errors of the means for various growth performance parameters are summarized in Table 2. The recorded survival rates were 97% for the control group, 98% for the E-fed group, and 100% for all other treatments. As shown in Table 2, growth performance indicators, including final weight, body length gain, percentage body weight gain, and specific growth rate (SGR), were significantly higher (p< 0.05) in the SCE treatment compared to the other groups. In contrast, parameters such as feed intake, initial body length, and initial body weight did not differ significantly among treatments (p > 0.05).
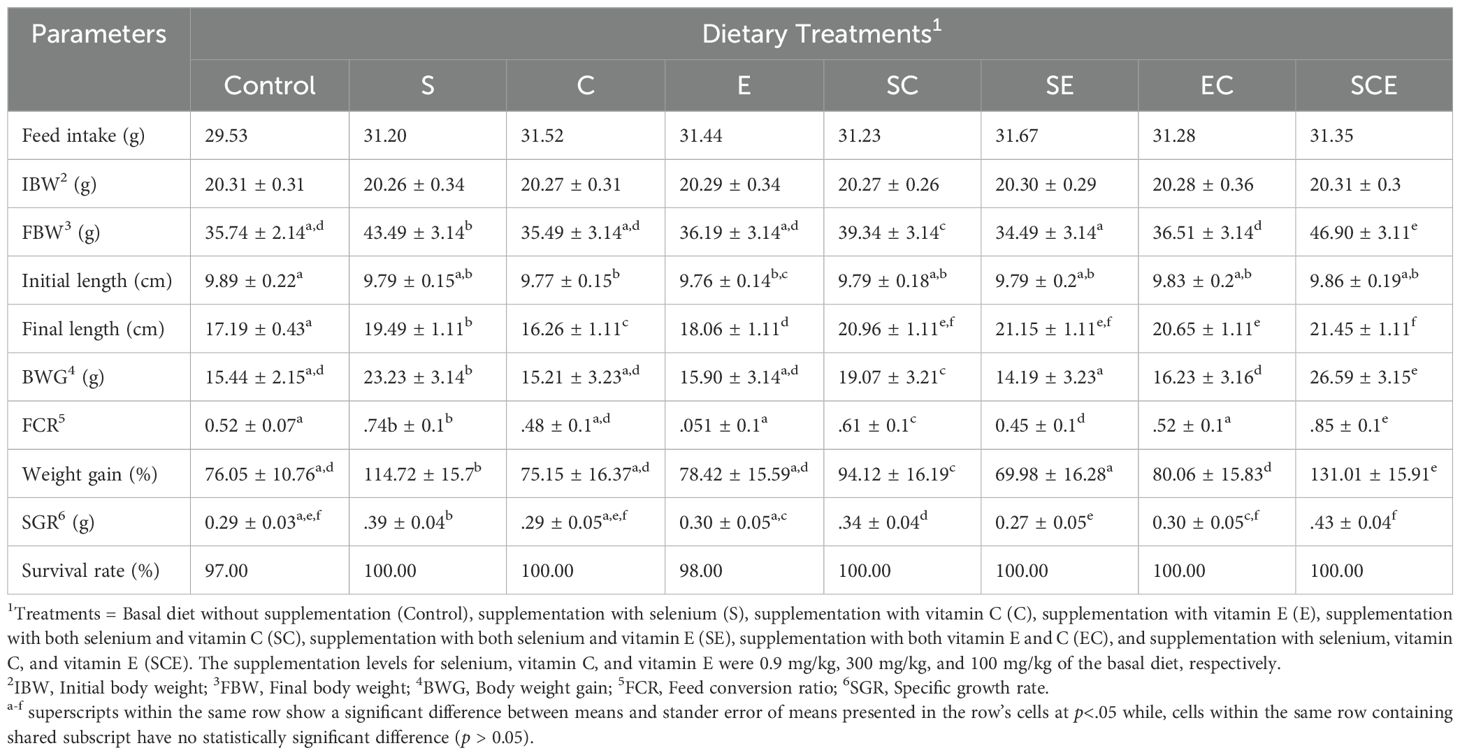
Table 2. Growth performance of juvenile Hypophthalmichthys molitrix fed on diets supplemented with methionine selenium, Vitamin C and E.
The means and standard errors of the means for hematological parameters, serum enzyme activities, and blood metabolites, including glucose concentrations, are presented in Table 3. The findings indicate that dietary supplementation with selenium (Se), vitamin C, and vitamin E significantly influenced (p< 0.05) hematological parameters such as white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (HGB) concentration, hematocrit (HCT), and mean corpuscular hemoglobin concentration (MCHC) in juvenile Hypophthalmichthys molitrix. The SCE-fed group exhibited significantly higher (p< 0.05) values for these hematological parameters compared to other dietary treatments. Similarly, the activities of ALT, AST, and ALP varied significantly (p< 0.05) among treatments, with the lowest activities observed in the SCE-fed group. In contrast, blood glucose concentrations did not differ significantly (p > 0.05) across the treatments.
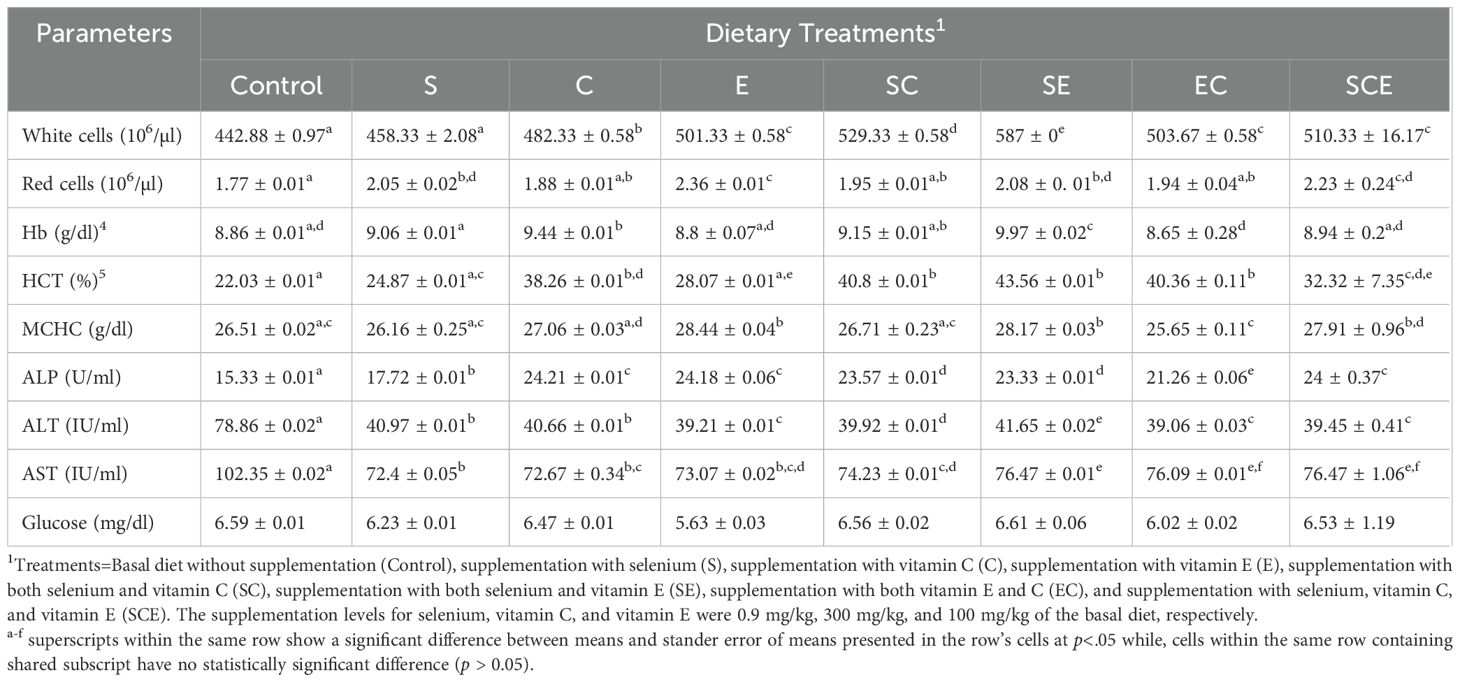
Table 3. Hematology, serum enzymes, and blood glucose analysis of juvenile Hypophthalmichthys molitrix fed on diets supplemented with methionine selenium, Vitamin C, and E.
As shown in Table 4, the means and standard errors of the means for TBARS values in muscle and serum were significantly higher (p< 0.05) in the control-fed fish group compared to those fed the other dietary treatments. Dietary supplementation with selenium, vitamin C, and vitamin E significantly enhanced (p< 0.05) the activities of SOD), CAT, and GPx in the whole body and muscle of the fish. However, changes in liver catalase activity were not statistically significant (p > 0.05) among the dietary treatments.
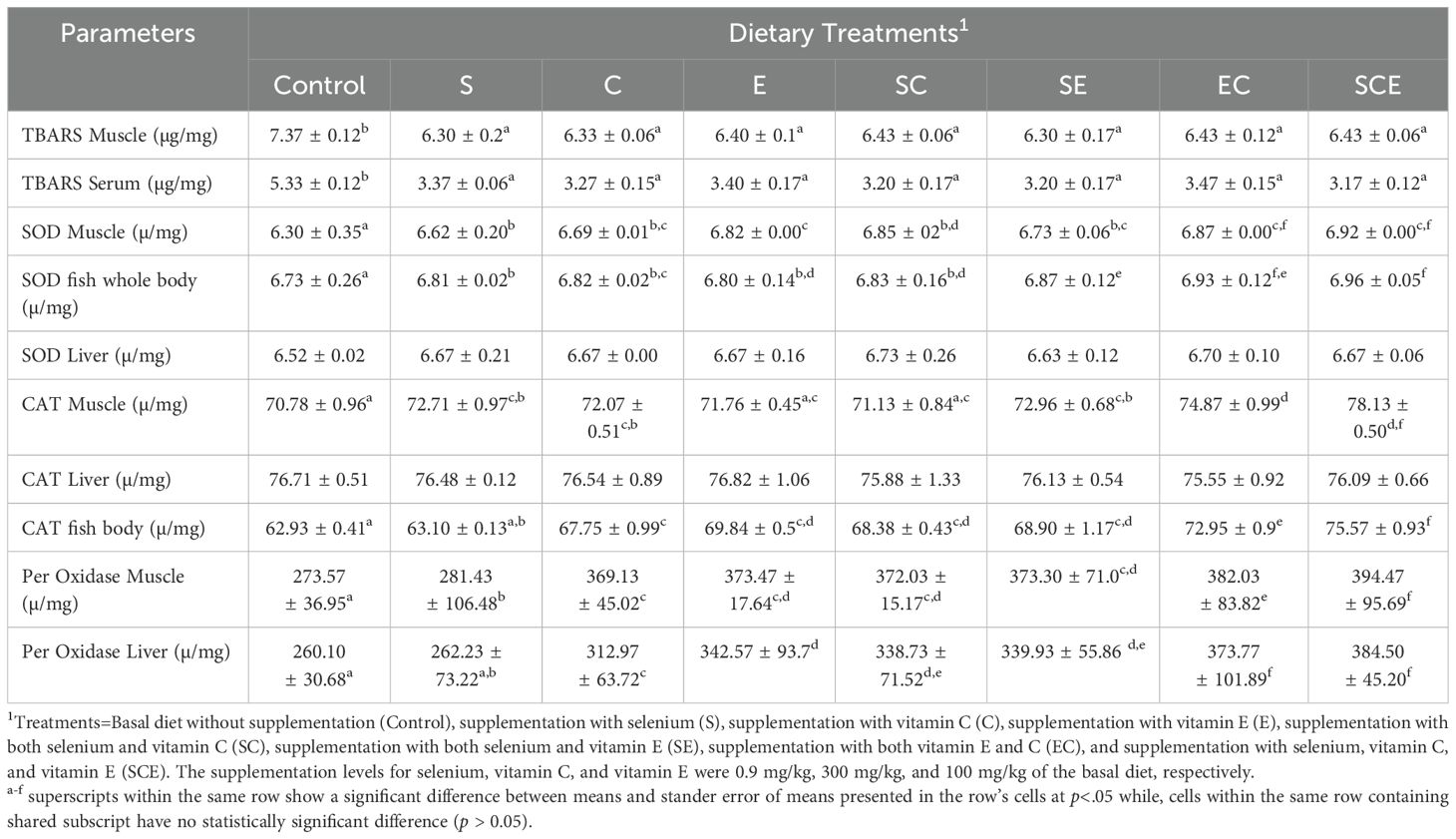
Table 4. TBARS and antioxidant enzymes in different tissues of juvenile Hypophthalmichthys molitrix fed on diets supplemented with methionine selenium, Vitamin C, and E.
The differences in means and standard errors of the means for carcass composition parameters, including dry matter, fat, ash, and protein contents, were not statistically significant (p > 0.05) among the treatments. These findings indicate that dietary supplementation with selenium (Se), vitamin C, and vitamin E did not affect the composition of fish meat (Table 5).
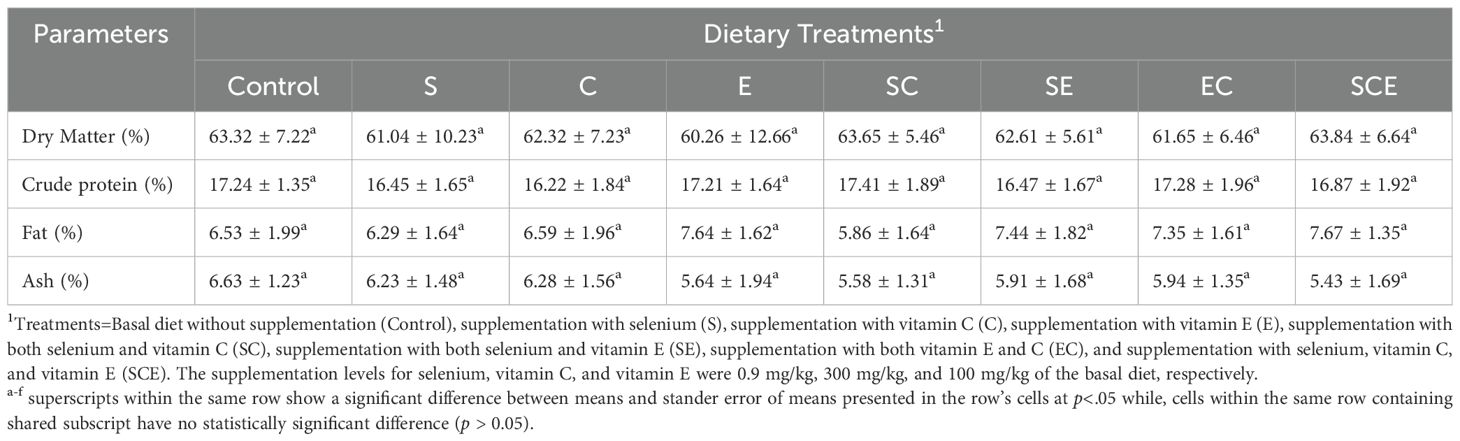
Table 5. Meat chemical composition of juvenile Hypophthalmichthys molitrix fed on diets supplemented with methionine selenium, Vitamin C, and E.
In recent years, researchers have explored various nutritional strategies to enhance the growth performance of intensively reared aquaculture species, including fish, without compromising their health or meat quality. Among these strategies, the supplementation of selenium (Se), vitamin C, and vitamin E has garnered significant attention. This discussion delves into the potential effects of these supplements on fish growth performance, their mechanisms of action, and associated health benefits. Previous studies have demonstrated that optimizing dietary Se is crucial for improving growth performance and enhancing the antioxidant system in fish species (Hoffmann and Berry, 2008; Schrauzer and Surai, 2009). Similarly, enriching diets with vitamins C and E has been shown to result in better weight gain and overall health in fish (Harsij et al., 2020). Dietary Se increases its bioaccumulation in various tissues, facilitating the formation of selenocysteine, which plays a vital role in mitigating chronic stress conditions.
In the current experiment, the results indicate that the SCE-supplemented diet significantly improved growth performance parameters such as body weight gain, specific growth rate, and feed conversion ratio in juvenile Hypophthalmichthys molitrix. These findings align with previous research on rainbow trout (Harsij et al., 2020) and tilapia (Dornelles Mello et al., 2013), which also reported enhanced weight gain and feed efficiency with diets supplemented with Se, vitamin C, and vitamin E. The superior growth performance observed in the SCE-fed group may be attributed to the additive or synergistic effects of these nutrients. Studies have revealed that simultaneous supplementation of selenium with either vitamin C or vitamin E yields synergistic benefits, enhancing growth and health outcomes (Ghazi Harsini et al., 2012; Shakeri et al., 2020). These effects can be explained by the complementary roles of these nutrients in the redox system, including the regeneration of antioxidant substances, acting as cofactors for redox enzymatic processes, reducing oxidative stress, neutralizing ROS, and protecting cellular structures to maintain integrity (Pisoschi et al., 2021).
Hematological health is influenced by various nutrients, hormones, diseases, and genetic factors (Onasanya et al., 2015). A balanced diet with diverse nutrients is essential for optimal hematological function (Pohlenz and Gatlin, 2014). In this study, hematological parameters such as WBC, RBC, HGB, HCT, and MCHC were significantly improved by individual supplementation and dietary combinations of selenium, vitamin C, and vitamin E. Research has shown that these nutrients, individually or in combination, enhance hematological parameters (Lu et al., 2016; Khan et al., 2017; Zehra and Khan, 2021). Selenium supports hematopoiesis, aiding the production and function of erythrocytes and leukocytes. It also facilitates heme synthesis, a crucial component of hemoglobin (Liao et al., 2018; Oliveira et al., 2018). Vitamin C enhances iron absorption, supporting red blood cell production (Bhadra and Deb, 2020; Godswill et al., 2020), while vitamin E prevents oxidative damage to red blood cell membranes, maintaining their structural integrity and reducing premature cell lysis (Jena et al., 2023). Thus, these supplements promote erythropoiesis and minimize cellular breakdown (Khan et al., 2017).
Liver enzyme activities, including AST, ALT, and ALP, are key biomarkers for assessing liver health. In this experiment, these enzyme activities significantly decreased with increased dietary supplementation of selenium, vitamin C, and vitamin E. These findings align with previous studies (Li et al., 2014), which reported reduced enzyme activities with antioxidant supplementation. The decrease in ALT, AST, and ALP levels can be attributed to selenium’s ability to restore proper enzyme function and reduce oxidative stress (Chen et al., 2022). Deficiencies in selenium or vitamins C and E are associated with elevated liver enzyme levels, indicating compromised liver health (Coz-Rakovac et al., 2005). Vitamins C and E, as potent antioxidants, protect liver cells from oxidative damage, normalize enzyme activities, and provide hepatoprotective effects in certain liver conditions (Uboh et al., 2012). Thiobarbituric acid reactive substances are markers of lipid peroxidation, often indicative of oxidative stress (Niki, 2021). Elevated TBARS levels in the control group in this experiment suggest oxidative stress due to overcrowding. Antioxidant supplementation with selenium, vitamins C, and vitamin E reduced TBARS levels (Chan et al., 2021), demonstrating their efficacy in mitigating oxidative stress. The reduced activities of SOD, CAT, and GPx in the whole body and muscle tissues of fish fed the SCE diet can be explained by the neutralization of free radicals facilitated by these supplements. Selenium and vitamins C and E are essential micronutrients that act as antioxidants, contributing to cellular stability and protection against ROS (Rodrigo et al., 2005). Lastly, dietary supplementation with selenium, vitamin C, and vitamin E did not alter the body chemical composition of juvenile Hypophthalmichthys molitrix. These results are consistent with previous studies on other fish species (Harsij et al., 2020; Mushtaq et al., 2022).
This study demonstrates the significant benefits of dietary supplementation with selenium (Se), vitamin C, and vitamin E on the growth performance, hematological parameters, antioxidant status, and liver health of juvenile Hypophthalmichthys molitrix. Combined supplementation improved body weight gain, specific growth rate, and feed conversion ratio without altering body composition. Enhanced antioxidant enzyme activities (SOD, CAT, GPx) and reduced TBARS levels highlight their role in mitigating oxidative stress, while improved hematological indices and liver enzyme activities (AST, ALT, ALP) indicate overall health benefits. These findings support the strategic inclusion of Se, vitamin C, and vitamin E in aquafeeds to promote growth, health, and antioxidant defenses in aquaculture species, with further research needed to optimize dosages and evaluate long-term effects.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
The animal studies were approved by the Animal Use and Care Committee of the University of Veterinary and Animal Science, Lahore, before the research trial (DR/175, dated 05-04-2022). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
HY: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Formal analysis, Funding acquisition. MM: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Project administration. SR: Investigation, Methodology, Writing – original draft, Formal analysis, Resources, Validation. UA: Formal analysis, Data curation, Visualization, Writing – review & editing. MK: Data curation, Visualization, Writing – review & editing, Conceptualization, Investigation, Methodology, Resources, Writing – original draft. AR: Conceptualization, Formal analysis, Resources, Supervision, Validation, Writing – review & editing, Writing – original draft. GQ: Software, Supervision, Validation, Writing – review & editing. MF: Conceptualization, Project administration, Resources, Supervision, Visualization, Writing – review & editing. SN: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. MZ: Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. MA: Conceptualization, Resources, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted without commercial or financial relationships that could create a conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arshad M. A., Ebeid H. M., Hassan F.-u. (2021). Revisiting the effects of different dietary sources of selenium on the health and performance of dairy animals: a review. Biol. Trace element Res. 199, 3319–3337. doi: 10.1007/s12011-020-02480-6
Chan A. M. L., Ng A. M. H., Mohd Yunus M. H., Idrus R. B. H., Law J. X., Yazid M. D., et al. (2021). Recent developments in rodent models of high-fructose diet-induced metabolic syndrome: A systematic review. Nutrients 13, 2497. doi: 10.3390/nu13082497
Chance B., Maehly A. (1955). [136] Assay of catalases and peroxidases. Methods in Enzymology. (Academic Press) 2, 764–5. doi: 10.1016/S0076-6879(55)02300-8
Chen X., Zhang J., Li H., Liu W., Xi Y., Liu X. (2022). A comprehensive comparison of different selenium supplements: Mitigation of heat stress and exercise fatigue-induced liver injury. Front. Nutr. 9, 917349. doi: 10.3389/fnut.2022.917349
Council N. R. (2011). Nutrient requirements of fish and shrimp (Washington, USA: National academies press).
Coz-Rakovac R., Strunjak-Perovic I., Hacmanjek M., Popovic N., Lipej Z., Sostaric B. (2005). Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Veterinary Res. Commun. 29, 677–687. doi: 10.1007/s11259-005-3684-z
Dornelles Mello L., Zommara M., Eweedah N. M., Helal A. I., Aboel-Darag M. A. (2013). Biosensors for antioxidant evaluation in biological systems. Combinatorial Chem. High Throughput Screening 16, 109–120.
Flora S., Shrivastava R., Mittal M. (2013). Chemistry and pharmacological properties of some natural and synthetic antioxidants for heavy metal toxicity. Curr. medicinal Chem. 20, 4540–4574. doi: 10.2174/09298673113209990146
Ghazi Harsini S., Habibiyan M., Moeini M. M., Abdolmohammadi A. R. (2012). Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Element Res. 148, 322–330. doi: 10.1007/s12011-012-9374-0
Giannopolitis C. N., Ries S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 59, 309–314. doi: 10.1104/pp.59.2.309
Godswill A. G., Somtochukwu I. V., Ikechukwu A. O., Kate E. C. (2020). Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: A systematic review. Int. J. Food Sci. 3, 1–32. doi: 10.47604/ijf.1024
Gutteridge J. (1995). Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 41, 1819–1828. doi: 10.1093/clinchem/41.12.1819
Hamre K. (2011). Metabolism, interactions, requirements and functions of vitamin E in fish. Aquaculture Nutr. 17, 98–115. doi: 10.1111/j.1365-2095.2010.00806.x
Harsij M., Kanani H. G., Adineh H. (2020). Effects of antioxidant supplementation (nano−selenium, vitamin C and E) on growth performance, blood biochemistry, immune status and body composition of rainbow trout (Oncorhynchus mykiss) under sub-lethal ammonia exposure. Aquaculture 521, 734942. doi: 10.1016/j.aquaculture.2020.734942
Hoffmann P. R., Berry M. J. (2008). The influence of selenium on immune responses. Mol. Nutr. Food Res. 52, 1273–1280. doi: 10.1002/mnfr.200700330
Horwitz W. (1975). Official methods of analysis Vol. 222 (Washington, DC: Association of Official Analytical Chemists).
Jena A. B., Samal R. R., Bhol N. K., Duttaroy A. K. (2023). Cellular Red-Ox system in health and disease: The latest update. Biomedicine Pharmacotherapy 162, 114606. doi: 10.1016/j.biopha.2023.114606
Kayden H. J., Traber M. G. (1993). Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J. Lipid Res. 34, 343–358. doi: 10.1016/S0022-2275(20)40727-8
Khademzade O., Kochanian P., Zakeri M., Alavi S. M. H., Mozanzadeh M. T. (2022). Oxidative stress-related semen quality and fertility in the male arabian yellowfin sea bream (Acanthopagrus arabicus) fed a selenium nanoparticle-supplemented plant protein-rich diet. Aquaculture Nutr. 2022. doi: 10.1155/2022/3979203
Khan K. U., Zuberi A., Nazir S., Fernandes J. B.K., Jamil Z., Sarwar H. (2017). Synergistic effects of dietary nano selenium and vitamin C on growth, feeding, and physiological parameters of mahseer fish (Tor putitora). Aquaculture Rep. 5, 70–75. doi: 10.1016/j.aqrep.2017.01.002
Kohrle J., Jakob F., Contempré́ B., Dumont J. E. (2005). Selenium, the thyroid, and the endocrine system. Endocrine Rev. 26, 944–984. doi: 10.1210/er.2001-0034
Kumar G., Engle C. R. (2016). Technological advances that led to growth of shrimp, salmon, and tilapia farming. Rev. Fisheries Sci. Aquaculture 24, 136–152. doi: 10.1080/23308249.2015.1112357
Li J., Liang X.-F., Tan Q., Yuan X., Liu L., Zhou Y. (2014). Effects of vitamin E on growth performance and antioxidant status in juvenile grass carp Ctenopharyngodon idellus. Aquaculture 430, 21–27. doi: 10.1016/j.aquaculture.2014.03.019
Liao C., Carlson. B. A., Paulson. R. F, Prabhu K. S., et al (2018). The intricate role of selenium and selenoproteins in erythropoiesis. Free Radical Biol. Med. 127, 165–171. doi: 10.1016/j.freeradbiomed.2018.04.578
Lu Y., Liang X.-P., Jin M., Sun P., Ma H.-N., Yuan Y., et al. (2016). Effects of dietary vitamin E on the growth performance, antioxidant status and innate immune response in juvenile yellow catfish (Pelteobagrus fulvidraco). Aquaculture 464, 609–617. doi: 10.1016/j.aquaculture.2016.08.009
Mushtaq M., Fatima M., Shah S. Z.H., Khan N., Naveed S., Khan M., et al. (2022). Evaluation of dietary selenium methionine levels and their effects on growth performance, antioxidant status, and meat quality of intensively reared juvenile Hypophthalmichthys molitrix. PloS One 17, e0274734. doi: 10.1371/journal.pone.0274734
Naderi M., Keyvanshokooh S., Salati A. P., Ghaedi A. (2017). Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture 474, 40–47. doi: 10.1016/j.aquaculture.2017.03.036
Naiel M. A., Shehata A.M., Negm S. S., Abd El‐Hack M. E., Amer M. S., Khafaga A. F., et al. (2020). The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: A review. Rev. aquaculture 12, 2250–2267. doi: 10.1111/raq.12432
Niki E. (2021). Lipid oxidation that is, and is not, inhibited by vitamin E: Consideration about physiological functions of vitamin E. Free Radical Biol. Med. 176, 1–15. doi: 10.1016/j.freeradbiomed.2021.09.001
Oliveira D. C., Nogueira-Pedro A., Santos E. W., Hastreiter A., Silva G. B., Borelli P., et al. (2018). A review of select minerals influencing the haematopoietic process. Nutr. Res. Rev. 31, 267–280. doi: 10.1017/S0954422418000112
Onasanya G. O., Oke F. O., Sanni T. M., Muhammad A. I. (2015). Parameters influencing haematological, serum and bio-chemical references in livestock animals under different management systems. Open J. Veterinary Med. 5, 181. doi: 10.4236/ojvm.2015.58025
Pisoschi A. M., Pop A., Iordache F., Stanca L., Predoi G., Serban A. I. (2021). Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Medicinal Chem. 209, 112891. doi: 10.1016/j.ejmech.2020.112891
Pohlenz C., Gatlin D. M. I. I. I. (2014). Interrelationships between fish nutrition and health. Aquaculture 431, 111–117. doi: 10.1016/j.aquaculture.2014.02.008
Rodrigo J., et al. (2005). The role of free radicals in cerebral hypoxia and ischemia. Free Radical Biol. Med. 39, 26–50. doi: 10.1016/j.freeradbiomed.2005.02.010
Schrauzer G. N., Surai P. F. (2009). Selenium in human and animal nutrition: resolved and unresolved issues. A partly historical treatise in commemoration of the fiftieth anniversary of the discovery of the biological essentiality of selenium, dedicated to the memory of Klaus Schwarz (1914–1978) on the occasion of the thirtieth anniversary of his death. Crit. Rev. Biotechnol. 29, 2–9. doi: 10.1080/07388550902728261
Shakeri M., Oskoueian E., Le H. H., Shakeri M. (2020). Strategies to combat heat stress in broiler chickens: Unveiling the roles of selenium, vitamin E and vitamin C. Veterinary Sci. 7, 71. doi: 10.3390/vetsci7020071
Smirnoff N. (2018). Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radical Biol. Med. 122, 116–129. doi: 10.1016/j.freeradbiomed.2018.03.033
Trichet V. V. (2010). Nutrition and immunity: an update. Aquaculture Res. 41, 356–372. doi: 10.1111/j.1365-2109.2009.02374.x
Uboh F. E., Ebong P. E., Akpan. H. D., Usoh. I. F. (2012). Hepatoprotective effect of vitamins C and E against gasoline vapor-induced liver injury in male rats. Turkish J. Biol. 36, 217–223. doi: 10.3906/biy-1004-111
Yang R., Liu Y. (2017). Structure, function, and nutrition of selenium-containing proteins from foodstuffs. Mineral Containing Proteins: Roles Nutr. p, 89–116.
Keywords: juveniles, growth performance, blood analysis, liver enzymes, antioxidants
Citation: Yu H, Mushtaq M, Razzaq S, Ali U, Khan M, Rahman A, Quan G, Fatima M, Naveed S, Zafar MH and Aziz ur Rahman M (2025) Effects of dietary organic selenium, vitamin C, and vitamin E supplementation on growth performance, serum biochemistry, and antioxidant status in juvenile Hypophthalmichthys molitrix. Front. Mar. Sci. 12:1555994. doi: 10.3389/fmars.2025.1555994
Received: 06 January 2025; Accepted: 28 January 2025;
Published: 21 February 2025.
Edited by:
Gladstone Sagada, Victory Farms, KenyaReviewed by:
Yuwen Dong, University of Pennsylvania, United StatesCopyright © 2025 Yu, Mushtaq, Razzaq, Ali, Khan, Rahman, Quan, Fatima, Naveed, Zafar and Aziz ur Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hairui Yu, eWhyNjAwM0Bob3RtYWlsLmNvbQ==; Muhammad Khan, a2hhbmJ3bjAxMUBnbWFpbC5jb20=; Abdur Rahman, YWJkdXJyZWhtYW5AdXZhcy5lZHUucGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.