- Laboratory of Evolutionary Physiology and Behavior, Chongqing Key Laboratory of Conservation and Utilization of Freshwater Fishes, Animal Biology Key Laboratory of Chongqing Education Commission, Chongqing Normal University, Chongqing, China
Introduction: Fish in natural ecosystems face long-term environmental stressors, with anxiety being a critical stress response. This study investigates how chronic stressors—predators, social isolation, and caffeine—affect anxiety-like behavior and cortisol levels in zebrafish (Danio rerio), and examines the persistence of these effects post-stressor removal.
Methods: Zebrafish were exposed to three stressors for 14 days. Anxiety-like behavior and cortisol levels were measured for up to 28 days following the removal of the stressors.
Results: Chronic predator stress significantly increased anxiety-like behavior and cortisol levels, persisting for at least seven days post-removal. Social isolation reduced anxiety-like behavior and cortisol levels, with effects lasting no less than one day after re-socialization. Caffeine induced temporary anxiolytic effects, reversing one day post-withdrawal and persisting for at least seven days, while cortisol levels remained elevated.
Discussion: Zebrafish exhibit stressor-specific behavioral and physiological changes with varying persistence. These findings enhance understanding of fish responses to environmental stressors, offering insights into their survival strategies and ecosystem health.
Introduction
When faced with environmental stressors, organisms exhibit various physiological and behavioral stress responses (Gold, 2015; Liu et al., 2023; Yehuda et al., 2023; Zhang et al., 2023). Anxiety is a stress response to real or perceived threats. In animals, it shows through behaviors such as reduced activity, increased freezing, which are aimed at maintaining homeostasis (Bateson et al., 2011; Belzung and Griebel, 2001). It may also trigger physiological changes by activating the hypothalamic-pituitary-adrenal (HPA) axis, which releases stress hormones such as cortisol (Belzung and Griebel, 2001). Anxiety affects animal physiology and behavior in two ways. First, moderate anxiety enhances sensitivity to risks, preparing individuals physiologically, cognitively, and behaviorally to recognize and respond to survival threats (Cheng et al., 2022; Rosa et al., 2018). Conversely, excessive or prolonged anxiety can result in pathological conditions that disrupt normal physiological and behavioral functions (Cheng et al., 2022; Rosa et al., 2018). Therefore, anxiety can lead to significant consequences for population dynamics and the overall health of ecosystems (Bateson et al., 2011; Fuzzen et al., 2010; Jacobson and Roche, 2018; Perrot-Minnot et al., 2017). Understanding how anxiety-like behavior and stress hormones in animals change, especially in fast-paced and stressful environments is essential.
Fish commonly exhibit anxiety-like behavior, such as decreased exploration, increased thigmotaxis, and altered activity patterns, when they face environmental stressors (Canzian et al., 2017; Stewart et al., 2012). These behaviors help minimize their exposure to predators and other dangers (Akinrinade et al., 2023; Cachat et al., 2010; Canzian et al., 2017; Stewart et al., 2012). Zebrafish (Danio rerio) are widely studied for their low cost, rapid reproductive cycles, established behavioral patterns, and well-defined neuroendocrine stress axes (Aoki et al., 2013; Faustino et al., 2017; Green et al., 2012). Zebrafish also present genetic and physiological traits that have been conserved throughout evolution (Aoki et al., 2013; Faustino et al., 2017; Green et al., 2012). When zebrafish are placed in a new environment, they may show anxiety-like behavior, including staying at the bottom of the tank, reducing exploratory actions, and demonstrating freezing or erratic movements (Cachat et al., 2010; Canzian et al., 2017; Stewart et al., 2012). The novel tank diving test is a widely used method for assessing anxiety-like behavior and has been validated with both anxiogenic and anxiolytic-like drugs (Costa et al., 2020; Kysil et al., 2017). When studying anxiety, behavioral assessments should be combined with physiological indicators like stress hormone level (Alsop and Vijayan, 2009). Like mammals, zebrafish can activate their HPA axis when exposed to stress, resulting in the release of cortisol (Alsop and Vijayan, 2009, Alsop and Vijayan, 2008; Fuzzen et al., 2010; Ghisleni et al., 2012). Consequently, zebrafish have become widely used animal models in neuroethology and behavioral ecology research (Cachat et al., 2010; Canzian et al., 2017; Stewart et al., 2012).
Various biotic and abiotic stressors commonly found in natural environment can influence anxiety-like behavior in fish (Brachetta et al., 2015; de Carvalho et al., 2019; Shallcross et al., 2019). Predators and social isolation are biological stressors that researchers often focus on (Brachetta et al., 2015; Hegab and Wei, 2014). Studies have confirmed that exposure to predators or their chemical signals increases anxiety-like behavior in zebrafish (Brachetta et al., 2015; Hegab and Wei, 2014). Shoaling is a key characteristic of highly social fish, making social isolation particularly stressful and challenging for them (Graham et al., 2018). Research indicates that social isolation influences anxiety-like behavior and cortisol level in zebrafish (Ariyomo and Watt, 2012; Graham et al., 2018). Caffeine, a neuroactive substance, is commonly utilized as an abiotic stressor to induce anxiety models in zebrafish (de Carvalho et al., 2019). Researchers have investigated the biological significance of anxiety and potential prevention and treatment strategies for human anxiety disorders through acute exposure to moderate doses of caffeine (Amar and Ramachandran, 2023; de Carvalho et al., 2019). Furthermore, environmental caffeine, as an emerging contaminant (Dafouz et al., 2018; Li et al., 2020), serves as an abiotic environmental stressor and has been comparatively studied with biotic stressors to examine their impact on anxiety-like behavior (Amar and Ramachandran, 2023). However, existing reports show conflicting results regarding the effects of environmental stressors on anxiety-like behavior in zebrafish. While fish frequently encounter prolonged stressful conditions (Piato et al., 2011; Rambo et al., 2017), most studies have concentrated on the immediate effects of acute stressors exposure on their anxiety-like behavior (Aponte and Petrunich-Rutherford, 2019; Tran et al., 2014). In contrast, limited research has explored how prolonged exposure to environmental stressors affects anxiety-like behavior in fish, including the persistence of these effects.
Here, we investigated how three chronic environmental stressors—predators, social isolation, and caffeine—affect anxiety-like behavior in zebrafish. We also observed how long these effects lasted for 28 days after the stressors were removed. Simultaneously, we measured the level of the stress hormone cortisol. Our goal was to examine how various chronic environmental stressors influence anxiety-like behavior in zebrafish and the persistence of these effects. This provides a theoretical framework for understanding how fish behave and respond physiologically to chronic environmental stress, as well as the role of anxiety in their adaptation to different environments.
Materials and methods
Fish and ethical procedures
A total of 280 adult zebrafish (4–6 months old; ∼50:50 male: female) were obtained from a local fish farm (Yongchuan, Chongqing, China). All the fish were housed in laboratory zebrafish farming systems (tank size: length × width × height: 45 × 35 × 30 cm; 20 fish/tank). They were allowed to acclimate for one month before the experiments began. The system was maintained at 14 L:10D, the water temperature was 28 ± 1°C, and the dissolved oxygen level was >7 mg L−1. The fish were fed live brine shrimp and dried flake food (Shandong Shengsuo Feed Science and Technology Co., Ltd., Shandong, China) twice daily, comprising approximately 2% of their body weight. Before feeding, the water valves and aerator pumps were turned off. One hour after feeding, siphons were used to remove leftover food and feces to maintain water cleanliness. This study was approved by the Animal Care and Use Committee of the Chongqing Key Laboratory of Conservation and Utilization of Freshwater Fishes (permit number CKLCUFF20240520-01). All procedures were conducted in strict adherence to the guidelines outlined in the Guide for the Care and Use of Animals at the Chongqing Key Laboratory of Conservation and Utilization of Freshwater Fishes, China.
Experimental protocol
In this experiment, we randomly assigned 248 zebrafish (body mass: 0.52 ± 0.12 g; body length: 3.07 ± 0.18 cm; ∼50:50 male: female) into four groups: control, predator, social isolation, and caffeine. Each group was assigned to six identical circulating tanks (length × width × height: 45 × 35 × 30 cm), with each tank accommodating 10 to 12 zebrafish. In the control, predator and caffeine groups, two green plastic water plants (size: length × width × height: 10 × 20 × 20 cm) were present in each tank to enhance environmental enrichment. In the predator group, we placed one argus snakehead fish (Channa argus) (Engeszer et al., 2007), measuring exactly 15 cm in length in each tank, which was separated from the zebrafish by perforated transparent acrylic partitions. This design enables zebrafish to detect predators through sight and smell while avoiding direct attacks. In the predator group, the water valves for each tank were turned off, unlike in the other groups. Each tank was fitted with its own thermostatic heater and aerator pumps to maintain consistent temperature and oxygen level across all tanks. However, there was no water exchange with any of the other tanks. Each day, we added one additional zebrafish as food for the argus snakehead fish. In the social isolation group, we divided the tanks into equal-sized compartments (length × width × height: 9 cm × 9 cm × 30 cm) via opaque PVC boards, with one fish placed in each compartment. Compared to other groups of shoal zebrafish, zebrafish in the socially isolated group received only olfactory but not visual cues from their companion fish. For the caffeine group, we prepared stock solutions by dissolving caffeine (Chengdu Alfa Biotechnology Co., Ltd., Chengdu, China) in zebrafish culture water. Next, we created test solutions by diluting the stock solutions. We gently netted the fish from their tanks and placed them in a beaker with 500 mL of a 100 mg L−1 caffeine solution. They were submerged for 15 minutes each day between 9:00 AM and 12:00 PM before being returned to the circulation system (Amar and Ramachandran, 2023; Egan et al., 2009; Neri et al., 2019). To prevent bias in the results caused by operational stress, the other three groups performed the same daily operations as the caffeine group, but with the caffeine solution replaced by an equal volume of distilled water. All the fish were subjected to their respective treatments for 14 days (Figure 1). Furthermore, all other rearing conditions, such as photoperiod, temperature, and oxygen concentration, were kept consistent with those used during the acclimatization period.
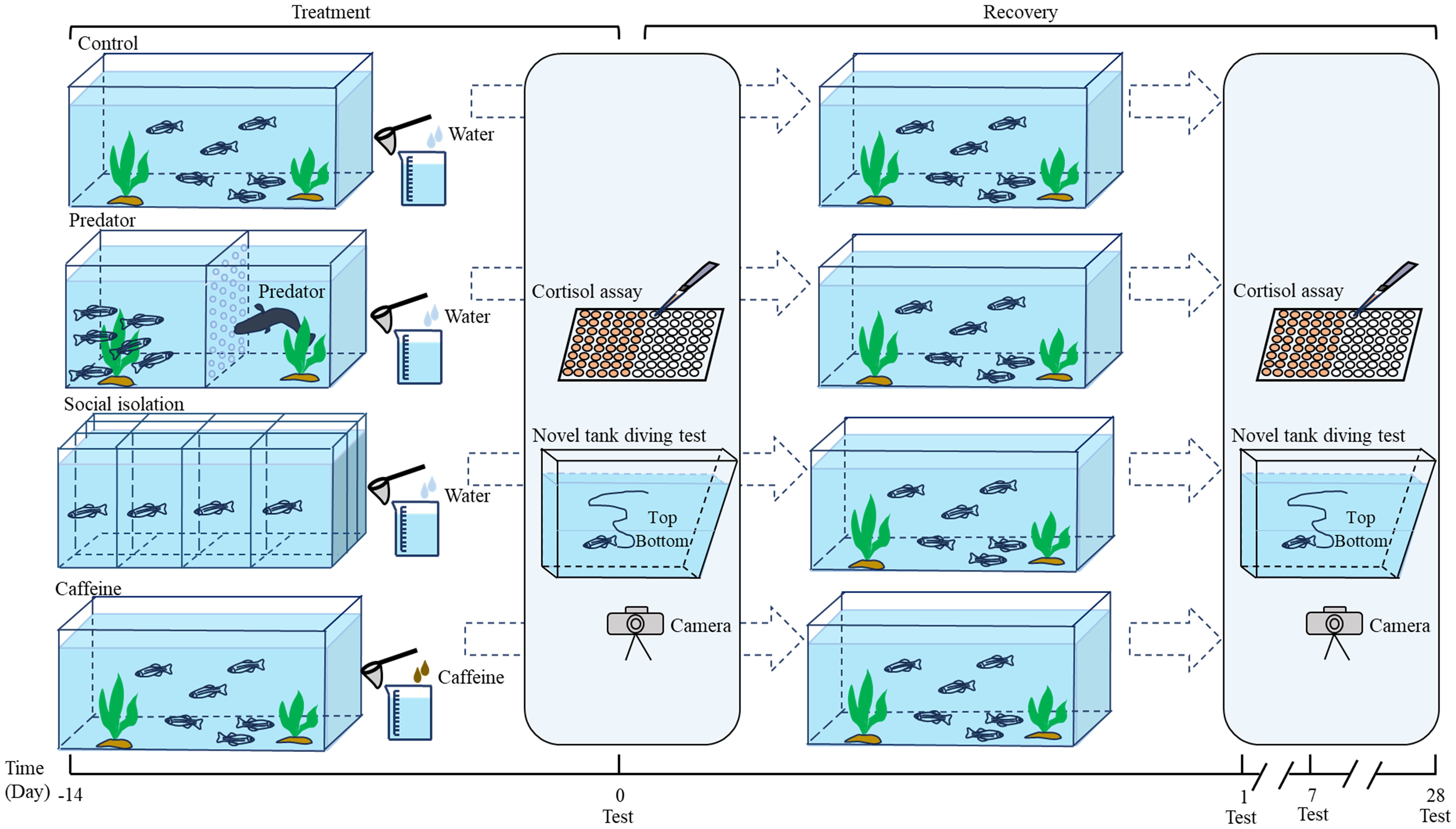
Figure 1. Diagram illustrating the experimental design. The fish were categorized into four groups: control, predator, caffeine, and social isolation. The treatment groups were subjected to predator stress, caffeine exposure, and social isolation, respectively. The same anxiety-like behavioral tests and cortisol level assessments conducted on day 0 were repeated on days 1, 7, and 28 after the removal of stressors.
At the end of the 14-day exposure period, we conducted behavioral tests and measured whole-body cortisol level, which were recorded as day 0 after exposure removal. Next, we removed all the environmental stressors from the treatment groups. The zebrafish were then kept under the same conditions as those in the control group. We repeated the behavioral tests and cortisol measurements on days 1, 7, and 28 after exposure. For each behavioral test, we selected three tanks from each group, resulting in a total of 30 zebrafish. Each tank was clearly labeled, and after the test, the fish were returned to their respective tanks, where they awaited the next round of testing, which occurred four times in total. After each behavioral test, we measured the body mass and body length of each fish. Condition factor was also measured, which was indicators of fish health and nutritional status. The remaining three tanks from each group were designated for cortisol level assessment and contained a total of 32 fish. On the day of each behavioral test, we randomly selected eight fish, with 2-3 fish taken from each tank, for euthanasia. An overdose of MS-222 (tricaine methanesulfonate, 200 mg L−1 solution) was used for this procedure. Whole-fish samples were collected and then stored at -80°C for future analysis. The sex ratio of zebrafish tested for anxiety-like behavior and whole-body cortisol level was 50:50 in each group.
Novel tank diving test
After pretreatment, the zebrafish were individually placed in a 1.5-L trapezoidal tank (height × top × bottom × width: 15.2 × 27.9 × 22.5 × 7.1 cm; Figure 1) designed to minimize lateral movement but allowing vertical and horizontal movement (Egan et al., 2009; Levin et al., 2007). The tanks were divided into two equal vertical sections (each 5 cm high) marked by a dividing line on the outside walls and filled with water to a depth of 10 cm. The tanks were thoroughly cleaned and replenished with water after each trial. A high-definition web camera (Logitech Webcam Pro 9000, 1080×720 p) was positioned 50 cm in front of the tank and connected to a computer for recording purposes. The zebrafish were individually placed into the tank, and their behaviors were recorded for 6 minutes to capture their movement trajectories. All recordings were conducted between 9:00 AM and 4:00 PM. After obtaining a video of the anxiety-like behavior, the video was converted to the AVI format using video converter software. Two-dimensional coordinate data (x-axis and y-axis pixel values) were collected for each fish at intervals of 1/15 seconds via Idtracker software (version 1.10, https://www.idtracker.es/home). We created a line graph that displays time on the horizontal axis and the fish’s y-axis pixel values on the vertical axis using Excel. We then calculated the following metrics: first entry time into the top zone, total entries to the top zone, total time spent in the top zone, freezing bouts, and total freezing time. We assessed the number of erratic movements by conducting blinded analysis while viewing the videos in pairs. The parameters related to anxiety-like behavior were finally obtained, including latency to the top zone (s), number of entries to the top zone (times), proportion of time spent in the top zone (%), freezing bouts (times), proportion of freezing time (%), and total erratic movements (times) (Egan et al., 2009; Levin et al., 2007). Freezing is defined as the complete absence of movement for at least 1 second, excluding any movement of the gills or eyes. Erratic movements exhibit sudden changes in direction or speed, often resulting in rapid and repetitive darting actions (Cachat et al., 2010). The time to reach the top zone was both longer and less frequent, leading to reduced time spent there. Additionally, the increase in both the number and duration of freezing, along with more erratic movements, reflected greater anxiety-like behavior.
Cortisol assay
Previously frozen whole-body zebrafish samples were thawed and sliced into smaller pieces. A homogenate was prepared by mixing 100 µL of phosphate-buffered saline (PBS) with 10 mg of tissue. The homogenate was then centrifuged at 3000 rpm for 20 minutes. A 50 µL sample of the supernatant was collected for further analysis.
Whole-body cortisol level was quantified via a commercial ELISA kit (FANKEW, Shanghai Kexing Trading Co., Ltd., Shanghai, China). All procedures were performed according to the manufacturer’s instructions, and the absorbance of each sample was measured at 450 nm via an ELISA reader. The plasma cortisol concentration was calculated and reported in ng g−1.
Data analysis
Data analysis was performed with SPSS 26.0 (IBM, USA). Statistical significance was determined at P < 0.05. Normally distributed data are presented as mean ± standard deviation (S.D.), whereas skewed data are shown as median (interquartile range). For parametric tests, the normality and homogeneity of variance were verified using the Shapiro-Wilk test and Levene’s test. Then the differences in body mass, body length, condition factor, and cortisol level among groups were analyzed using One-way ANOVA. Whereas a generalized linear mixed model (GLMM) was used to analyze the effects of various treatments on anxiety-like behavioral parameters (skewed distributions; non-independence of data across test times within groups; fish from different tanks, where tanks may have an effect on the results) and compare these metrics across different testing time points within the same treatment. Treatment, time, body mass, and the interaction between treatment and time were included as fixed effects, whereas tank was treated as a random effect. Pairwise comparisons were conducted for post hoc tests where necessary.
Results
Comparison of body mass, body length, and condition factor
Throughout the duration of the experiment, no fish died. One-way ANOVA revealed no statistically significant differences in body mass, body length, or condition factor among the zebrafish groups at days 0, 1, 7, and 28 after 14 days of exposure (P > 0.05; Table 1).
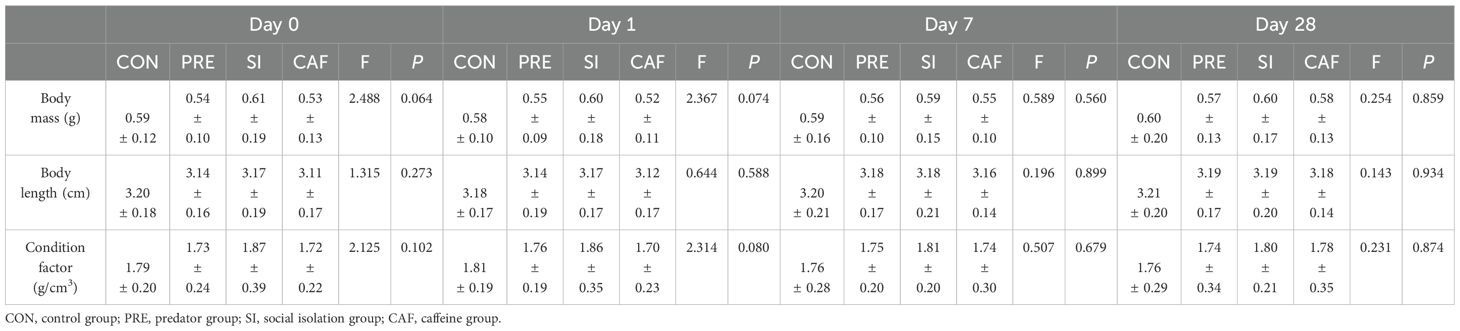
Table 1. Comparison of body mass, body length, and condition factor in zebrafish under different treatments at each test time, as analyzed via One-way ANOVA (N = 30, mean ± S.D.).
Effects of various chronic treatments on anxiety-like behavior and its persistence in zebrafish
Analysis revealed significant treatment effects for all anxiety-like behavioral parameters of interest (P < 0.05). Additionally, time effects were observed for all the parameters (P < 0.05), except for the proportions of freezing time and total erratic movements (P > 0.05). Notably, there was also a significant interaction effect between treatment and time effects on all anxiety-like behavioral parameters (P < 0.05; Table 2).
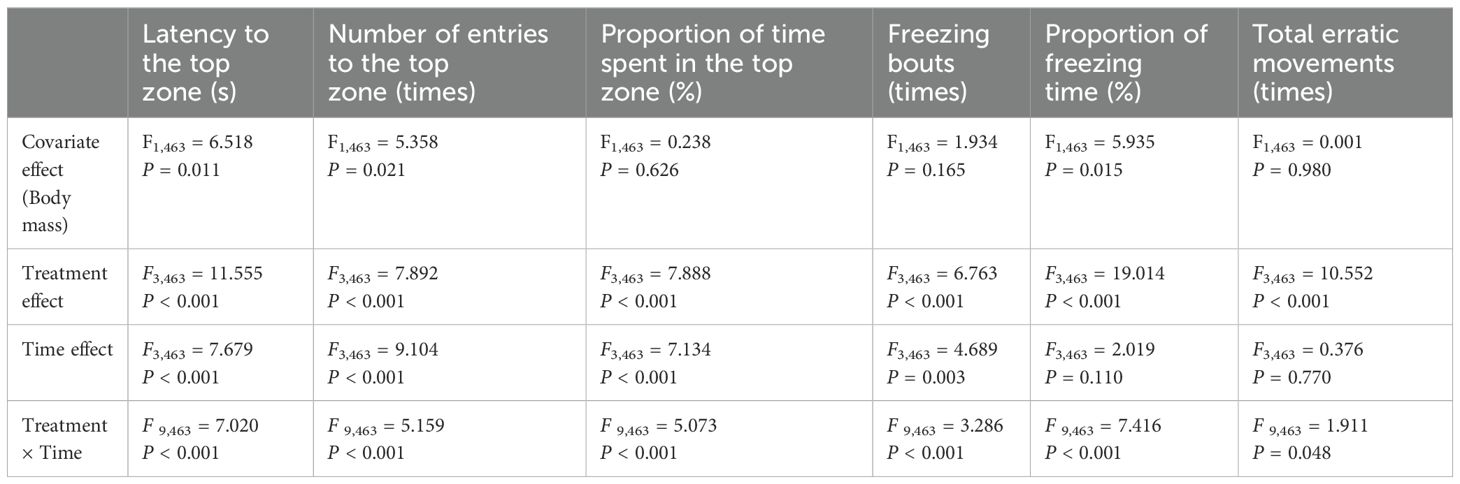
Table 2. Effects of treatment and time on anxiety-like behavior indicators in zebrafish based on a generalized linear mixed model (GLMM), with treatment, time, body mass, and the interaction between treatment and time included as fixed effects, while tank as a random effect (N=30).
Predation stress
On day 0 post-exposure to predators, zebrafish demonstrated significantly increased anxiety-like behavior compared to the control group, as indicated by a longer latency to the top zone, a greater number of freezing bouts, a higher percentage of freezing time, and increased erratic movements (P < 0.05; Figures 2A, G, I, K). Additionally, the number of entries to the top zone was significantly lower in the predator group (P < 0.05; Figure 2C). On day 1, anxiety level were still significantly greater in the predator group than in the control group (P < 0.05; Figures 2A, E, G, I, K), and no significant differences were observed compared with those on day 0 in the same group (P > 0.05; Figures 2B, D, F, H, J, L). On day 7, zebrafish in the predator group still showed greater latency to reach the top zone and a greater frequency of erratic movements than those in the control group did (P < 0.05; Figures 2A, K). However, within the same group, the proportion of freezing time significantly decreased compared with those on day 0 (P < 0.05; Figure 2J), indicating that while anxiety-like behavior persisted, but its intensity was significantly reduced. By day 28, there were no significant differences in anxiety-like behavioral parameters between the predator and control groups (P > 0.05; Figures 2A, C, E, G, I, K), suggesting that the anxiety-inducing effects of prolonged predator exposure had disappeared. These findings suggest that chronic predation stress elevates anxiety-like behavior in zebrafish, with effects lasting for at least seven days following predator removal.
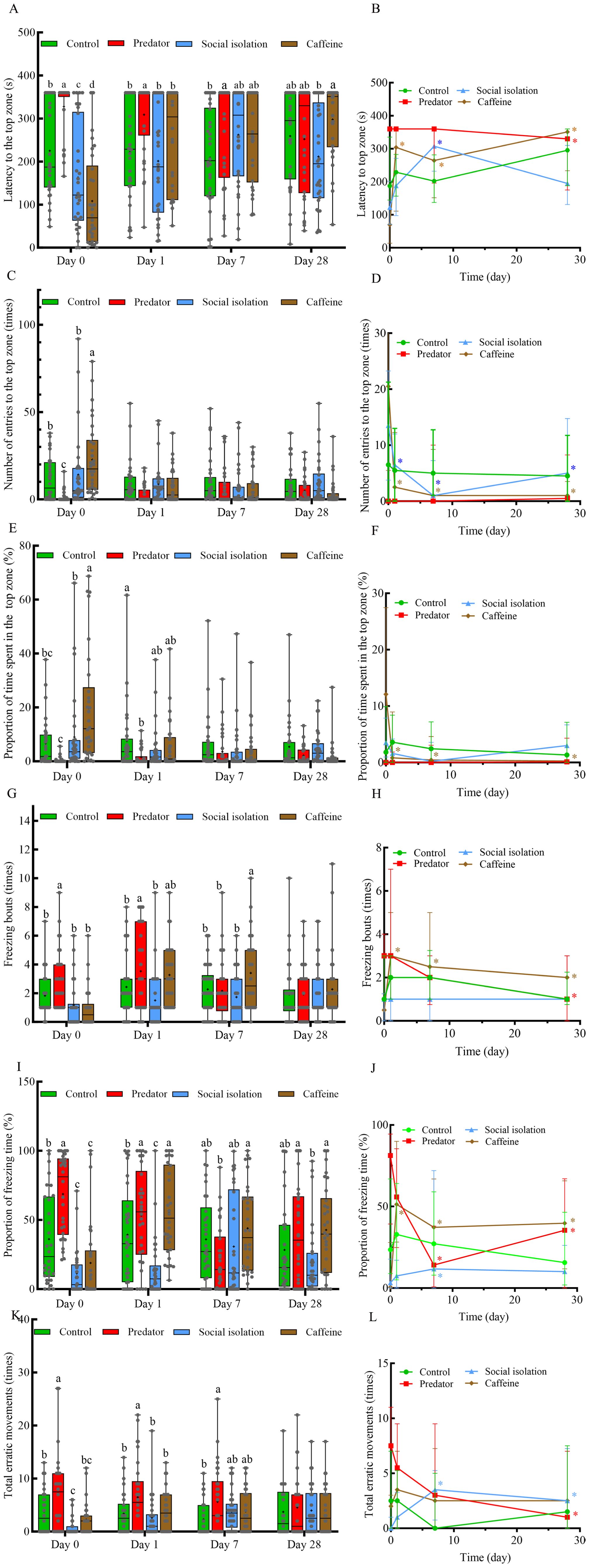
Figure 2. Effects of various chronic environmental stressors on anxiety-like behavior (A, C, E, G, I, K) and its persistence in zebrafish on days 0, 1, 7 and 28 (B, D, F, H, J, L) (N = 30). In the boxplot, the lower and upper hinges indicate the minimum and maximum values, the horizontal line inside the box represents the median, whereas the cross inside the box indicates the mean. The dots in the line graph on the right represent the median. The horizontal lines represent the 25th and 75th quartiles, respectively. Different letters indicate significant differences among treatment groups at the same time. Coloured asterisks in the line graphs indicate significant differences compared to day 0 within the same treatment group: green * for the control group, red * for the predator group, blue * for the social isolation group, and brown * represents the caffeine group. Each data point in the boxplot corresponds to an individual zebrafish.
Social isolation
On day 0 post social isolation, compared with the control zebrafish, the zebrafish in the social isolation group exhibited a shorter latency to the top zone, a lower proportion of freezing time, and fewer erratic movements (P < 0.05; Figures 2A, I, K). However, no significant differences were found in the other parameters (P > 0.05; Figures 2C, E, G), suggesting that chronic social isolation has a mild anxiolytic effect on zebrafish. On day 1 after reintroduction to the social environment, the proportion of freezing time was significantly lower than that in the control group (P < 0.05; Figure 2I), and the number of entries into the top zone was lower than that on day 0 for the same group (P < 0.05; Figure 2D). This finding indicates that anxiety level of zebrafish in the social isolation group were still lower than those in the control group, although they increased slightly. On days 7 and 28, all anxiety-like behavioral parameters in the social isolation group were not significantly different from those in the control group (P > 0.05; Figures 2A, C, E, G, I, K), suggesting that their anxiety level had returned to normal. Furthermore, on day 7 social isolation group experienced a longer time to reach the top zone, made fewer entries into it, spent more time freezing, and showed more erratic movements compared to day 0 (P < 0.05; Figures 2B, D, J, L). On day 28, zebrafish in the social isolation group had fewer entries into the top zone and more erratic movements than on day 0 (P < 0.05; Figures 2D, L). These results indicate that chronic social isolation has a mild anxiolytic effect. This reduced anxiety persisted for at least one day but to a lesser extent after the reintroduction of social interactions, and the effect disappeared completely on days 7 and 28.
Caffeine exposure
On day 0 after caffeine exposure, the anxiety-like behavior parameters revealed that zebrafish in the caffeine group had lower anxiety level than those in the control group did. This was evidenced by a shorter latency to the top zone, more entries into the top zone, a greater proportion of time spent in the top zone, and less freezing time (P < 0.05; Figures 2A, C, E, I). However, on day 1 post-caffeine withdrawal, zebrafish in the caffeine group presented a significantly greater proportion of freezing time than did those in the control group (P < 0.05; Figure 2I). In addition, zebrafish in the caffeine group on day 1 had longer latency times to enter the top zone, more freezing bouts, a higher proportion of freezing time, fewer entries to the top zone and less time spent there compared to those in the same group on day 0 (P < 0.05; Figures 2B, D, F, H, J). These findings suggest that the anxiolytic effects of chronic caffeine exposure in zebrafish quickly shifted to increased anxiety within just one day of caffeine withdrawal. On days 7, zebrafish in the caffeine group presented higher anxiety level than those in the control group did (P < 0.05; Figure 2G). On day 28, no statistically significant differences were found between the caffeine group and the control group for any of the parameters of anxiety-like behavior (P > 0.05, Figures 2A, C, E, G, I, K). The zebrafish in the caffeine group exhibited longer latency times to reach the top zone, increased freezing bouts, a higher percentage of freezing time, less entries into the top zone, and a lower percentage of time there on days 7 and 28 compared to day 0 (P < 0.05; Figures 2B, D, F, H, J). These findings indicate that although chronic caffeine treatment initially reduces anxiety, its effects are transient, and anxiety-like behavior increased sharply within one day after caffeine withdrawal and continued at least seven days.
Comparison of cortisol level
Compared with the control group, the predator group presented significantly elevated cortisol level on days 0, 1, and 7 (P < 0.05; Figure 3A), whereas no significant difference was detected on day 28 (P > 0.05; Figure 3A). Additionally, in the predator group, cortisol level did not significantly differ between day 1 and day 0 (P > 0.05; Figure 3B); however, cortisol level on days 7 and 28 was significantly lower than on day 0 (P < 0.05; Figure 3B). The social isolation group presented lower cortisol level on days 0 and 1 compared with the control group (P < 0.05; Figure 3A). The cortisol level on days 1, 7, and 28 of social isolation group was significantly higher than day 0 (P < 0.05; Figure 3B). In the caffeine group, the cortisol level was consistently greater than those in the control group at all time points (P < 0.05; Figure 3A). However, the cortisol level on days 1, 7, and 28 was significantly lower than those at baseline (day 0) in the same group (P < 0.05; Figure 3B).
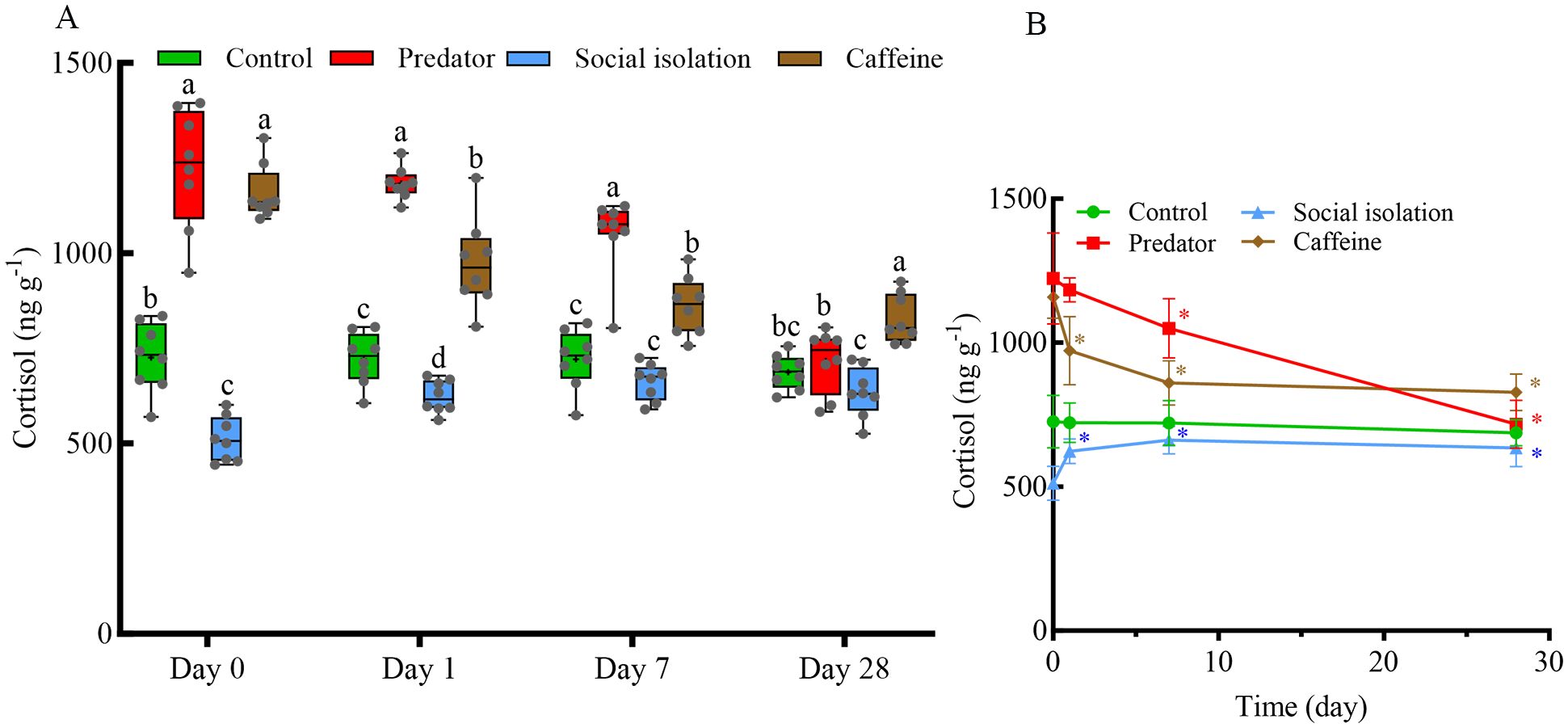
Figure 3. Effects of various chronic environmental stressors on whole-body cortisol levels in zebrafish (A) and their persistence in zebrafish on days 0, 1, 7 and 28 (B) (N=8). In the boxplot, the lower and upper hinges indicate the minimum and maximum values, the horizontal line inside the box represents the median, whereas the cross inside the box indicates the mean. The dots in the line graph on the right represent the median. The horizontal lines represent the 25th and 75th quartiles, respectively. Different letters indicate significant differences among treatment groups at the same time. Coloured asterisks in the line graphs indicate significant differences compared to day 0 within the same treatment group: green * for the control group, red * for the predator group, blue * for the social isolation group, and brown * represents the caffeine group. Each data point in the boxplot corresponds to an individual zebrafish.
Discussion
This study revealed that after 14 days of exposure, zebrafish displayed different changes in anxiety-like behavior depending on chronic environmental stressors, with variations in their persistence. Our results revealed that chronic predator stress significantly increased anxiety-like behavior and cortisol level in zebrafish, which lasted for at least seven days after predator removal. Zebrafish that experienced social isolation presented mild decreases in anxiety-like behavior and cortisol level. This reduction in anxiety-like behavior and cortisol level lasted at least one day after their re-entry into the social environment. Furthermore, exposure to caffeine led to a significant and temporary anxiolytic effect on zebrafish. A rapid reversal of the anxiety-like behavior occurred one day after withdrawal and persisted for at least seven days. However, their cortisol level was consistently higher than those of the control group until the end of the observation. The strength of this study lies in how prolonged exposure to various environmental stressors affects anxiety-like behavior in zebrafish. It also assesses how long these effects last after the stressors are removed. These findings provide significant value for predicting the impact of various environmental stressors on anxiety-like behavior in fish while also offering theoretical insights into how anxiety plays a crucial role in fish adaptation to their environment.
Bateson et al. proposed that the threshold for anxiety-like behavior depends on the risk of encountering threats in the environment and an animal’s vulnerability to those threats. An increase in either the risk of threats or an animal’s vulnerability can lower the threshold for anxiety-like behavior (Bateson et al., 2011). In the animal kingdom, encounters with predators are major stressors that can lead to death or serious health problems for prey (Brachetta et al., 2015; Liu et al., 2023). The “danger ecology hypothesis” suggests that animals living in high-predation environments have a strong antipredator response to novel cues (Greenberg, 2003). This is because unfamiliarity may indicate danger (Greenberg, 2003). Vulnerability to anxiety is often associated with the physical condition of an organism. For example, older adults are more prone to anxiety disorders (Vrach and Tomar, 2020). Our study demonstrated that 14 days of predator exposure significantly elevated anxiety-like behavior and cortisol level in zebrafish, which is consistent with our expectations and previous researches (Gerlai, 2013; Ladu et al., 2015). Following Bateson’s theory (Bateson et al., 2011), exposure to predators increases the probability of perceived environmental threats, so anxiety-like behavior is elevated. In our study, zebrafish subjected to chronic predator stress presented lower body mass and length than the control group did, but these differences were not statistically significant. Predator coercion may induce a loss of appetite in zebrafish due to fear or stress, which can lead to a decline in physical health and increased vulnerability to stress. Currently, studies investigating the persistence of the effects of chronic environmental stressors on anxiety-like behavior in zebrafish are very limited. Research has shown that early-life stress exposure at seven days postfertilization can result in anxiety-like behavior in adult zebrafish for up to three generations (Fontana et al., 2023). In our study, we found that the anxiety effects of predator exposure and elevated cortisol level persisted for at least seven days after the predator was removed. These findings suggest that predator-induced stress may cause fish to maintain increased anxiety over an extended period, potentially increasing their antipredation ability during this time.
Shoaling is a common social behavior among fish. Researchers have noted that fish often display heightened vigilance when isolated or positioned at the periphery of a group (Bateson et al., 2011; Salmeto et al., 2011). Additionally, social hierarchies within groups can influence stress level, with subordinate individuals exhibiting more pronounced anxiety-like behavior than their dominant counterparts do (Bozi et al., 2021). Social isolation has been extensively studied as a source of anxiety in social mammals, including rodents, primates, and humans (Cacioppo et al., 2011; dos Santos et al., 2009; Liang et al., 2022). Zebrafish, known for their highly social nature, naturally form tight-knit groups (Ariyomo and Watt, 2012; Graham et al., 2018) and establish social hierarchies (Spence et al., 2007). However, the impact of social isolation on anxiety-like behavior in fish remains contentious (Alnassar et al., 2023; Parker et al., 2012; Shams et al., 2015). Shams et al. found that social isolation reduces anxiety in zebrafish, possibly because it decreases their spatial stress when they are housed at high densities (Shams et al., 2017). Consequently, the influence of social isolation on anxiety-like behavior in fish can be seen as a balance among shoaling, spatial stress, and social hierarchies. In this study, 14 days of social isolation decreased anxiety-like behavior and cortisol level in zebrafish, aligning with earlier findings (Parker et al., 2012; Shams et al., 2015), but contrasts with the results reported by Alnassar et al. (2023). This occurred because, in our isolation protocol, zebrafish experienced only visual isolation while could still receive olfactory cues from their companions. This makes them subject to lower group separation pressures than zebrafish, which are both visually and olfactorily isolated. A key factor to consider is that anxiety-like behavior tests are conducted on individual subjects. This can cause stress for those housed in groups, as they must suddenly leave their companions, whereas those who are housed alone do not experience this stress (Parker et al., 2012; Shams et al., 2015). It should be clarified that we do not associate the reduction in spatial stress with the decrease in anxiety-like behavior in zebrafish, since neither the control nor social isolation groups zebrafish were subjected to the stress of high-density rearing in our study. Regarding the persistence of the anxiolytic effects of social isolation on zebrafish, we observed an increase in anxiety-like behavior and cortisol level on day 1 after returning to the social group. This suggests that the anxiolytic effects of chronic social isolation in zebrafish reversed swiftly after their return to the social group.
Caffeine acts as a psychoactive substance (de Carvalho et al., 2019). Acute exposure to 100 mg L−1 of caffeine is frequently used to create animal models of anxiety and to explore the mechanisms, prevention, and treatment of anxiety (Amar and Ramachandran, 2023; Egan et al., 2009; Neri et al., 2019). However, existing studies have primarily concentrated on immediate changes in anxiety-like behavior following acute caffeine exposure (Amar and Ramachandran, 2023; de Carvalho et al., 2019). There is limited research on the effects of chronic caffeine exposure on anxiety-like behavior, including their duration and whether they can establish a stable and lasting model of anxiety in zebrafish. Our study found that chronic intermittent exposure to caffeine at 100 mg L-1 has anxiolytic effects, in contrast to the effects of acute exposure (Amar and Ramachandran, 2023; Egan et al., 2009; Neri et al., 2019). Interestingly, anxiety-like behavior increased in zebrafish compared to controls on day 1 after caffeine withdrawal and persisted until at least day 7. This unexpected result may be related to the complex pharmacological mechanisms of caffeine, as well as the different exposure regimens and zebrafish strains. Caffeine selectively antagonizes adenosine A1 and A2 receptors, exerting dual effects on the central nervous system (CNS) (de Carvalho et al., 2019). High concentrations of caffeine block A1 receptors, which can induce anxiety, whereas low concentrations inhibit A2 receptors, resulting in anxiolytic effects (de Carvalho et al., 2019; Hughes et al., 2014; Ladu et al., 2015; El Yacoubi et al., 2000; Maximino et al., 2011). Prolonged exposure to caffeine enhances the body’s tolerance to stimulants (Nehlig et al., 1992). We hypothesized that continuous caffeine exposure would enhance tolerance in zebrafish, leading to an anxiolytic effect comparable to low caffeine concentrations. Given that cortisol level was consistently higher in the caffeine group compared to the control group, and that anxiety-like behavior decreased while cortisol increased on day 0, we speculate that caffeine might influence cortisol level through pathways unrelated to anxiety-like behavior, possibly involving norepinephrine or sympathetic nervous system activation. Moreover, caffeine influences anxiety-like behavior in various zebrafish strains in distinct ways (Rosa et al., 2018). We obtained our zebrafish from commercial aquaculture markets, aligning with the sources used in many behavioral studies. To test our hypothesis, we acquired laboratory-bred AB zebrafish and exposed them to various concentrations of caffeine. Our findings support previous studies (Amar and Ramachandran, 2023; Egan et al., 2009; Neri et al., 2019; Wong et al., 2010). Specifically, they show that acute exposure to 50 mg L-1 of caffeine produces anxiolytic effects, while exposure to 100 mg L-1 increases anxiety-like behavior, and exposure to 200 mg L-1 results in abnormal swimming patterns (see Supplementary Material). The increase in anxiety-like behavior in zebrafish during the first seven days after caffeine removal may be related to withdrawal effects. Previous studies have shown that stopping long-term cocaine use increases anxiety behaviors in zebrafish (López-Patiño et al., 2008). Similarly, moderate doses of acute alcohol exposure reduce anxiety in zebrafish. While withdrawal from alcohol after prolonged exposure raises anxiety levels (Mathur and Guo, 2011), which may resemble the results of the current study. On day 28, the caffeine group exhibited no significant differences in anxiety-like behavior compared to the control group. However, their cortisol level was higher. This suggests that anxiety-related biochemical indicators may be more stable than behavioral indicators. But these speculations require confirmation through further studies.
This study has several specific limitations. Firstly, while cortisol level is often used as a physiological indicator of stress, they may not capture all the physiological changes related to anxiety, as other hormones and neurotransmitters were not included in this study. Second, based on the observation time points established in this study, we know that chronic predator stress and caffeine exposure affect anxiety-like behaviors for a minimum of 7 days, but we do not have a precise estimate for how long these effects last. These shortcomings suggest new avenues for future research.
Conclusion
In this study, zebrafish exhibited varying anxiety-like behavior and cortisol level changes in response to chronic environmental stressors, with differing persistence. Chronic predator stress significantly increased anxiety-like behavior and cortisol level, lasting at least seven days post-stressor removal. Social isolation led to mild reductions in anxiety-like behavior and cortisol level, persisting for at least one day after re-socialization. Caffeine exposure caused a temporary anxiolytic effect, with anxiety-like behavior reversing one day post-withdrawal and persisting for seven days, though cortisol level remained elevated. This study highlights the prolonged impact of environmental stressors on zebrafish anxiety-like behavior and their persistence post-stressor removal, offering insights into the role of anxiety in fish adaptation to environmental changes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Chongqing Key Laboratory of Conservation and Utilization of Freshwater Fishes. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XL: Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Conceptualization. CF: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing, Software. XT: Data curation, Methodology, Writing – original draft. SF: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (32370509 and 31670418).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1551595/full#supplementary-material
References
Akinrinade I. D., Varela S. A. M., Oliveira R. F. (2023). Sex differences in social buffering and social contagion of alarm responses in zebrafish. Anim. Cogn. 26, 1307–1318. doi: 10.1007/s10071-023-01779-w
Alnassar N., Hillman C., Fontana B. D., Robson S. C., Norton W. H. J., Parker M. O. (2023). angptl4 gene expression as a marker of adaptive homeostatic response to social isolation across the lifespan in zebrafish. Neurobiol. Aging 131, 209–221. doi: 10.1016/j.neurobiolaging.2023.08.004
Alsop D., Vijayan M. M. (2008). Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R711–R719. doi: 10.1152/ajpregu.00671.2007
Alsop D., Vijayan M. (2009). The zebrafish stress axis: molecular fallout from the teleost-specific genome duplication event. Gen. Comp. Endocrinol. 161, 62–66. doi: 10.1016/j.ygcen.2008.09.011
Amar A., Ramachandran B. (2023). Environmental stressors differentially modulate anxiety-like behavior in male and female zebrafish. Behav. Brain Res. 450, 114470. doi: 10.1016/j.bbr.2023.114470
Aoki T., Kinoshita M., Aoki R., Agetsuma M., Aizawa H., Yamazaki M., et al. (2013). Imaging of neural ensemble for the retrieval of a learned behavioral program. Neuron 78, 881–894. doi: 10.1016/j.neuron.2013.04.009
Aponte A., Petrunich-Rutherford M. L. (2019). Acute net stress of young adult zebrafish (Danio rerio) is not sufficient to increase anxiety-like behavior and whole-body cortisol. PeerJ 7, e7469. doi: 10.7717/peerj.7469
Ariyomo T. O., Watt P. J. (2012). The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim. Behav. 83, 41–46. doi: 10.1016/j.anbehav.2011.10.004
Bateson M., Brilot B., Nettle D. (2011). Anxiety: an evolutionary approach. Can. J. Psychiatry-Revue Can. Psychiatr. 56, 707–715. doi: 10.1177/070674371105601202
>Belzung C., Griebel G. (2001). Measuring normal and pathological anxiety-like behavior in mice: a review. Behav. Brain Res. 25, 141–149. doi: 10.1016/S0166-4328(01)00291-1
Bozi B., Rodrigues J., Lima-Maximino M., de-Siqueira-Silva D. H., Soares M. C., Maximino C. (2021). Social stress increases anxiety-like behavior equally in male and female zebrafish. Front. Behav. Neurosci. 15. doi: 10.3389/fnbeh.2021.785656
Brachetta V., Schleich C. E., Zenuto R. R. (2015). Short-term anxiety response of the subterranean rodent Ctenomys talarum to odors from a predator. Physiol. Behav. 151, 596–603. doi: 10.1016/j.physbeh.2015.08.021
Cachat J., Stewart A., Grossman L., Gaikwad S., Kadri F., Chung K. M., et al. (2010). Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 5, 1786–1799. doi: 10.1038/nprot.2010.140
Cacioppo J. T., Hawkley L. C., Norman G. J., Berntson G. G. (2011). Social isolation. Ann. New York Acad. Sci. 1231, 17–22. doi: 10.1111/j.1749-6632.2011.06028.x
Canzian J., Fontana B. D., Quadros V. A., Rosemberg D. B. (2017). Conspecific alarm substance differently alters group behavior of zebrafish populations: Putative involvement of cholinergic and purinergic signaling in anxiety- and fear-like responses. Behav. Brain Res. 320, 255–263. doi: 10.1016/j.bbr.2016.12.018
Cheng R. K., Tan J. X. M., Chua K. X., Tan C. J. X., Wee C. L. (2022). Osmotic stress uncovers correlations and dissociations between larval zebrafish anxiety endophenotypes. Front. Mol. Neurosci. 15. doi: 10.3389/fnmol.2022.900223
Costa B. P. D., Moura L. A., Pinto S. A. G., Lima-Maximino M., Maximino C. (2020). Zebrafish models in neural and behavioral toxicology across the life stages. Fishes 5, 23. doi: 10.3390/fishes5030023
Dafouz R., Cáceres N., Rodríguez-Gil J. L., Mastroianni N., López de Alda M., Barceló D., et al. (2018). Does the presence of caffeine in the marine environment represent an environmental risk? A regional and global study. Sci. Total Environ. 615, 632–642. doi: 10.1016/j.scitotenv.2017.09.155
de Carvalho T. S., Cardoso P. B., Santos-Silva M., Lima-Bastos S., Luz W. L., Assad N., et al. (2019). Oxidative stress mediates anxiety-like behavior induced by high caffeine intake in zebrafish: protective effect of alpha-tocopherol. Oxid. Med. Cell. Longevity 2019, 8419810. doi: 10.1155/2019/8419810
dos Santos L., de Andrade T. G. C. S., Graeff F. G. (2009). Social separation and diazepam withdrawal increase anxiety in the elevated plus-maze and serotonin turnover in the median raphe and hippocampus. J. Psychopharmacol. 24, 725–731. doi: 10.1177/0269881109106954
Egan R. J., Bergner C. L., Hart P. C., Cachat J. M., Canavello P. R., Elegante M. F., et al. (2009). Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 205, 38–44. doi: 10.1016/j.bbr.2009.06.022
El Yacoubi M., Ledent C., Ménard J. F., Parmentier M., Costentin J., Vaugeois J. M. (2000). The stimulant effects of caffeine on locomotor behavior in mice are mediated through its blockade of adenosine A(2A) receptors. Br. J. Pharmacol. 129, 1465–1473. doi: 10.1038/sj.bjp.0703170
Engeszer R. E., Patterson L. B., Rao A. A., Parichy D. M. (2007). Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish 4, 21–40. doi: 10.1089/zeb.2006.9997
Faustino A. I., Tacao-Monteiro A., Oliveira R. F. (2017). Mechanisms of social buffering of fear in zebrafish. Sci. Rep. 7, 44329. doi: 10.1038/srep44329
Fontana B. D., Alnassar N., Parker M. O. (2023). Transgenerational effects of early-life stress on anxiety behavior in zebrafish. Behav. Processes 208, 104874. doi: 10.1016/j.beproc.2023.104874
Fuzzen M. L. M., van der Kraak G., Bernier N. J. (2010). Stirring up new ideas about the regulation of the hypothalamic-pituitary-interrenal axis in zebrafish (Danio rerio). Zebrafish 7, 349–358. doi: 10.1089/zeb.2010.0662
Gerlai R. (2013). Antipredatory behavior of zebrafish: adaptive function and a tool for translational research. Evolutionary Psychol. 11, 591–605. doi: 10.1177/147470491301100308
Ghisleni G., Capiotti K. M., Da Silva R. S., Oses J. P., Piato A. L., Soares V., et al. (2012). The role of CRH in behavioral responses to acute restraint stress in zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 36, 176–182. doi: 10.1016/j.pnpbp.2011.08.016
Gold P. W. (2015). The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 20, 32–47. doi: 10.1038/mp.2014.163
Graham C., von Keyserlingk M. A. G., Franks B. (2018). Zebrafish welfare: Natural history, social motivation and behavior. Appl. Anim. Behav. Sci. 200, 13–22. doi: 10.1016/j.applanim.2017.11.005
Green J., Collins C., Kyzar E. J., Pham M., Roth A., Gaikwad S., et al. (2012). Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 210, 266–271. doi: 10.1016/j.jneumeth.2012.07.017
Greenberg R. (2003). “The role of neophobia andneophilia in the development of innovative behavior of birds,” in Animal innovation (edsSM Reader, KN Laland) (Oxford University Press, Oxford,UK), pp 175–pp 196.
Hegab I. M., Wei W. H. (2014). Neuroendocrine changes upon exposure to predator odors. Physiol. Behav. 131, 149–155. doi: 10.1016/j.physbeh.2014.04.041
Hughes R. N., Hancock N. J., Henwood G. A., Rapley S. A. (2014). Evidence for anxiolytic effects of acute caffeine on anxiety-related behavior in male and female rats tested with and without bright light. Behav. Brain Res. 271, 7–15. doi: 10.1016/j.bbr.2014.05.038
Jacobson N. C., Roche M. J. (2018). Current evolutionary adaptiveness of anxiety: Extreme phenotypes of anxiety predict increased fertility across multiple generations. J. Psychiatr. Res. 106, 82–90. doi: 10.1016/j.jpsychires.2018.10.002
Kysil E. V., Meshalkina D. A., Frick E. E., Echevarria D. J., Rosemberg D. B., Maximino C., et al. (2017). Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 14, 197–208. doi: 10.1089/zeb.2016.1415
Ladu F., Mwaffo V., Li J., Macrì S., Porfiri M. (2015). Acute caffeine administration affects zebrafish response to a robotic stimulus. Behav. Brain Res. 289, 48–54. doi: 10.1016/j.bbr.2015.04.020
Levin E. D., Bencan Z., Cerutti D. T. (2007). Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 90, 54–58. doi: 10.1016/j.physbeh.2006.08.026
Li S., Guo J., He B., Zhu Y., Wang J. (2020). Environmental knowledge, behaviors, and attitudes regarding caffeine consumption among Chinese university students from the perspective of ecopharmacovigilance. Environ. Sci. pollut. Res. 28, 5347–5358. doi: 10.1007/s11356-020-10878-x
Liang J., Shao A. S., Al Omran A., Watanabe S. (2022). Social isolation model: A noninvasive rodent model of stress and anxiety. J. Visualized Experiments. doi: 10.3791/64567-v
Liu D., Zhu B., Liang Q., Zhang H., Dong S., Wang F. (2023). High temperatures enhance the strength of multiple predator effects in a typical crab-clam system. Mar. Pollut. Bull. 188. doi: 10.1016/j.marpolbul.2023.114670
López-Patiño M. A., Yu L., Cabral H., Zhdanova I. V. (2008). Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol. Behav. 93, 160–171. doi: 10.1016/j.physbeh.2007.08.013
Mathur P., Guo S. (2011). Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav. Brain Res. 219, 234–239. doi: 10.1016/j.bbr.2011.01.019
Maximino C., Lima M. G., Olivera K. R. M., Picanço-Diniz D. L. W., Herculano A. M. (2011). Adenosine A1, but not A2, Receptor Blockade Increases Anxiety and Arousal in Zebrafish. Basic Clin. Pharmacol. Toxicol. 109, 203–207. doi: 10.1111/j.1742-7843.2011.00710.x
Nehlig A., Daval J. L., Debry G. (1992). Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Brain Res. Rev. 17, 139–170. doi: 10.1016/0165-0173(92)90012-b
Neri D., Ruberto T., Mwaffo V., Bartolini T., Porfiri M. (2019). Social environment modulates anxiogenic effects of caffeine in zebrafish. Behav. Pharmacol. 30, 45–58. doi: 10.1097/fbp.0000000000000415
Parker M. O., Millington M. E., Combe F. J., Brennan C. H. (2012). Housing conditions differentially affect physiological and behavioral stress responses of zebrafish, as well as the response to anxiolytics. PLoS One 7, e34992. doi: 10.1371/journal.pone.0034992
Perrot-Minnot M. J., Banchetry L., Cézilly F. (2017). Anxiety-like behavior increases safety from fish predation in an amphipod crustacea. R. Soc. Open Sci. 4, 171558. doi: 10.1098/rsos.171558
Piato Â.L., Capiotti K. M., Tamborski A. R., Oses J. P., Barcellos L. J., Bogo M. R., et al. (2011). Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 561–567. doi: 10.1016/j.pnpbp.2010.12.018
Rambo C. L., Mocelin R., Marcon M., Villanova D., Koakoski G., de Abreu M. S., et al. (2017). Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiol. Behav. 171, 50–54. doi: 10.1016/j.physbeh.2016.12.032
Rosa L. V., Ardais A. P., Costa F. V., Fontana B. D., Quadros V. A., Porciúncula L. O., et al. (2018). Different effects of caffeine on behavioral neurophenotypes of two zebrafish populations. Pharmacol. Biochem. Behav. 165, 1–8. doi: 10.1016/j.pbb.2017.12.002
Salmeto A. L., Hymel K. A., Carpenter E. C., Brilot B. O., Bateson M., Sufka K. J. (2011). Cognitive bias in the chick anxiety-depression model. Brain Res. 1373, 124–130. doi: 10.1016/j.brainres.2010.12.007
Shallcross J., Hámor P., Bechard A. R., Romano M., Knackstedt L., Schwendt M. (2019). The divergent effects of CDPPB and cannabidiol on fear extinction and anxiety in a predator scent stress model of PTSD in rats. Front. Behav. Neurosci. 13. doi: 10.3389/fnbeh.2019.00091
Shams S., Chatterjee D., Gerlai R. (2015). Chronic social isolation affects thigmotaxis and whole-brain serotonin levels in adult zebrafish. Behav. Brain Res. 292, 283–287. doi: 10.1016/j.bbr.2015.05.061
Shams S., Seguin D., Facciol A., Chatterjee D., Gerlai R. (2017). Effect of social isolation on anxiety-related behaviors, cortisol, and monoamines in adult zebrafish. Behav. Neurosci. 131, 492–504. doi: 10.1037/bne0000220
Spence R., Gerlach G., Lawrence C., Smith C. (2007). The behavior and ecology of the zebrafish, Danio rerio. Biol. Rev. 83, 13–34. doi: 10.1111/j.1469-185X.2007.00030.x
Stewart A., Gaikwad S., Kyzar E., Green J., Roth A., Kalueff A. V. (2012). Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 62, 135–143. doi: 10.1016/j.neuropharm.2011.07.037
Tran S., Chatterjee D., Gerlai R. (2014). Acute net stressor increases whole-body cortisol levels without altering whole-brain monoamines in zebrafish. Behav. Neurosci. 128, 621–624. doi: 10.1037/bne0000005
Vrach I. T., Tomar R. (2020). Mental health impacts of social isolation in older people during COVID pandemic. Prog. Neurol. Psychiatry 24, 25–29. doi: 10.1002/pnp.684
Wong K., Stewart A., Gilder T., Wu N., Frank K., Gaikwad S., et al. (2010). Modeling seizure-related behavioral and endocrine phenotypes in adult zebrafish. Brain Res. 1348, 209–215. doi: 10.1016/j.brainres.2010.06.012
Yehuda H., Madrer N., Goldberg D., Soreq H., Meerson A. (2023). Inversely regulated inflammation-related processes mediate anxiety-obesity links in zebrafish larvae and adults. Cells 12, 1794. doi: 10.3390/cells12131794
Keywords: zebrafish, anxiety-like behavior, cortisol, predator, caffeine, social isolation
Citation: Li X-h, Fu C, Tan X-t and Fu S-j (2025) Responses of zebrafish to chronic environmental stressors: anxiety-like behavior and its persistence. Front. Mar. Sci. 12:1551595. doi: 10.3389/fmars.2025.1551595
Received: 26 December 2024; Accepted: 24 February 2025;
Published: 12 March 2025.
Edited by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaReviewed by:
Dapeng Liu, Ocean University of China, ChinaKe Li, Chinese Academy of Sciences (CAS), China
Copyright © 2025 Li, Fu, Tan and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shijian Fu, c2hpamlhbmZ1OUBjcW51LmVkdS5jbg==
 Xiao-hong Li
Xiao-hong Li Cheng Fu
Cheng Fu Shi-jian Fu
Shi-jian Fu