
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 14 February 2025
Sec. Marine Pollution
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1550274
This article is part of the Research Topic Fate and Effects of Sediment and Emerging Pollutants in Marine and Estuarine Environments View all 4 articles
Generally, marine dredged sediments often exhibit co-pollution with heavy metals and petroleum hydrocarbons. This work investigates the extraction-oxidation synergistic remediation treatment of heavy metals (Cu and Pb) and total petroleum hydrocarbon (TPH) in the contaminated dredged sediments. The leachability of heavy metals, the oxidation of TPH, the physicochemical properties of the treated sediment, and the fertility of the treated sediment for barley growth are compared among different combinations of extractants [ethylene diamine tetraacetic acid (EDTA), citric acid (CA) and ferric chloride (FeCl3)] and oxidant [potassium persulfate (K2S2O8)]. The results show that the extraction-oxidation synergistic remediation treatment significantly reduces the Cu and Pb contents. The total removal performances of heavy metals and TPH in the contaminated dredged sediments by each co-remediation treatment group are FeCl3+Ox > EDTA-Ox > CA-Ox. Specifically, FeCl3+Ox and EDTA-Ox achieve the highest removal rates of 91.10% and 96.95% for Cu and Pb, respectively. The extractant affects the activation of K2S2O8 by transition metals, enhancing the removal efficiency of TPH. The EDTA-Ox treatment group demonstrates the optimal treatment efficiency (37.42%) for TPH in the dredged sediment in 30 min. In the barley planting experiment, both shoot and root germinations in the EDTA-Ox treatment group reach their maximum values of 100% and 90%, respectively. Additionally, the CA-Ox treatment group exhibits maximal shoot and root lengths of 11.6 cm and 12.1 cm, respectively. The stress caused by salinity on seeds is also mitigated by the treatment. This study can provide technical support for the beneficial use of the dredged sediment.
In order to maintain the regular functionality of harbors and other coastal waterways, it is imperative to regularly dredge silted sediments, resulting in a substantial annual volume of dredged sediment. Currently, direct sea dumping is the most common disposal method for dredged sediment, which exacerbates the pressure on marine environmental protection. It is worth noting that the marine dredged sediment often contains organic matter and nutrients that can serve as the growing medium for plants (Kim et al., 2020). Effective utilization of dredged silt can be achieved by employing it as a substrate for plant growth. However, sediment has become a major aggregator and receiver of various pollutants with the development of industry and agriculture. Due to the close interaction between sediments and the aquatic environment, pollutants in the aquatic system are absorbed, deposited, and accumulated in the sediments through particulate matter (Albarano et al., 2020). Therefore, the direct application of marine dredged sediment may pose a risk of secondary environmental pollution. In order to ensure the safe and reliable use of dredged sediment, pollutants need to be removed or stabilized. Thermal treatment, biological treatment, and stabilization/solidification treatment as the frontier technologies for removing pollutants from the dredged sediment perform broad application prospect (Kim et al., 2021; Xu and Wu, 2023; Hong et al., 2023).
Generally, marine dredged sediments often exhibit co-pollution with heavy metals and petroleum hydrocarbons from diverse sources, such as wastewater discharges, shipping activities, and accidents (Doni et al., 2018; Kim et al., 2020). Heavy metals and petroleum-based contaminants sequestered in marine dredged sediments cannot be removed by self-purification (Kim et al., 2015). Consequently, a composite contamination issue arises with anomalous effects and amplified risks, posing a toxic threat to organisms in the surrounding environment through bioaccumulation. Since each pollutant has different properties and removal mechanisms, multiple remediation processes are required to remove mixed pollutants (Guarino et al., 2017; Zhang et al., 2021). Navigation channels and near-shore dredged sediments exhibit a high sand content. The chemical extraction/oxidation process is generally superior to other remediation techniques in sandy sediments (Yoo et al., 2017). Moreover, chemical extraction/oxidation remediation techniques can effectively eliminate organic compounds and heavy metals from the dredged sediment in a short period by facilitating direct contact between the contaminant and the extractant/oxidant during mixing through reagent addition, thereby effectively mitigating the leaching potential and bioavailability of heavy metals. In particular, co-contaminants, including heavy metals and organic compounds, can be removed by directly adding extracting agents or surfactants to the sediment, either sequentially or simultaneously (Yoo et al., 2017). However, the synergistic effects of the two technologies and their ecotoxicological benefits for residual contaminants have not received sufficient attention in previous studies.
HCl, HNO3, and H2SO4 are commonly used to extract heavy metals from sediments using acidic solutions. Acidic solution removal mechanisms involve increased competition between H+ ions and heavy metal ions for ligand binding sites, the dissolution of metal compounds, and the disruption of metal-containing specific components such as iron and manganese oxides to enhance desorption. Although inorganic acids are effective extractants for heavy metals, their inherent acidification and alteration of the original physicochemical properties of the sediment reduce the nutrient content and are not conducive to subsequent reuse. As an alternative to inorganic acids for the removal of heavy metals from sediments, chelates such as EDTA and CA have been widely researched and applied. Their mechanism of action primarily involves forming water-soluble chelates to extract heavy metals from sediments. Urbancl et al. (2023) employed EDTA and CA as extractants to remove Cu, Cr, and Ni from sediments. The experimental results showed that EDTA and CA achieved 60% and 80% removal of Cu from sediments, respectively. This approach highlights the effectiveness of chelating agents in remedying heavy metal contamination in sediments. As a chlorine-based extractant, ferric chloride (FeCl3) exhibits exceptional efficacy in extracting heavy metals in the solid phase (Yoo et al., 2018). FeCl3 extractant can remove heavy metals from the dredged sediment by combining chloride ions with metals, producing complexes such as [metal-Cl]+, [metal-Cl2](aq), [metal-Cl3]- and [metal-Cl4]2- (Torres et al., 2015). Yoo et al. (2018) reported that FeCl3 is the most effective extractant for Cu and Zn in marine sediment samples. Lower concentrations of FeCl3 (0.05 mol/L) extract the competitive amount of metals (70.1% Cu, 69.4% Zn) at 10 times the concentration of inorganic acids. The above three extractants (EDTA, CA, and FeCl3) all possess good potential for extracting heavy metals from the dredged sediments. In recent years, advanced oxidation processes (AOPs) have received increasing attention in the remediation of organic contaminated sediments due to their efficiency and environmental friendliness. The oxidants used include H2O2, S2O82−, MnO4−, O3, and the Fenton process. Among them, K2S2O8 is a strong oxidizing agent (E0 = 2.0V) that can be activated by heat, alkali, transition metals, and electrical energy to produce sulfate radicals (SO4•−, E0 = 2.5-3.1V), which can degrade most organic pollutants in water and sediment (Zhang et al., 2023). Persulfate has become highly emphasized in the remediation of soils contaminated with various emerging pollutants (Wang and Wang, 2018). However, its application on the contaminated dredged sediment is still insufficient.
This study investigated the changes in heavy metals (Cu and Pb) and TPH contents of the dredged marine sediments during the chemical extraction/oxidation process with different reagents (extractant: FeCl3, EDTA, and CA; oxidant: K2S2O8), as well as the effect of extraction/oxidation sequence on the overall performance. The leachability of heavy metals, oxidation of TPH, physicochemical properties of the treated sediment, and fertility for barley germination and growth were also compared for each optimal treatment combination of extractant and oxidant. This study can provide a theoretical basis and technical support for the disposal and beneficial use of the dredged sediment.
Dredged marine sediment used in this study was collected from the deep water channel of the Yangtze River in China. Three steps were taken to prepare the contaminated sediment: (1) 500 ml of hexyl hydride was used to dissolve 75 g of diesel fuel; 500 ml of deionized (DI) water was used to dissolve 1.0 g of Pb(NO3)2 and 2.0 g of Cu(NO3)2·3H2O). These solutions were added to 1000 g of the dredged sediment and uniformly stirred. The amount added was carefully calibrated, with each addition slightly exceeding the volume of the sediment present. The primary contaminated dredged sediment was carefully placed in a designated area shielded from light. (2) After complete evaporation of the hexyl hydride, the primary contaminated dredged sediment with the addition of diesel, copper, and lead was mixed with 1000 g of dredged sediment, thoroughly stirred, and left to stand for 1 d to obtain the secondary contaminated sediment. (3) According to the experimental design, appropriate amounts of secondary contaminated substrate were added to achieve the specific concentrations of diesel, copper, and lead. The mixture was then balanced at room temperature in a dark environment for one week before use, during which time the mixture was continually remixed daily to ensure homogeneity. Subsequently, the samples were subjected to a freeze-drying process for 48 h to remove moisture. After the freeze-drying, the samples were homogenized into particles of about 2 mm in size.
Sediment screening was conducted using a 2 mm sieve in this study. The particle size distribution was determined through wet sieving with 2, 0.15, and 0.075 mm sieves. Metals in sediment were extracted using aqua regia extractant, filtered with a 0.45 µm filter membrane, and the obtained filtrate was analyzed for pseudo-total metal contents using inductively-coupled plasma optical emission spectroscopy (ICP-OES, ICAP-6300, Thermo Fisher, USA). TPH in the sediment samples was extracted according to The specification for marine monitoring Part 5: Sediment analysis (GB 17378.5-2007) and analyzed using a UV spectrophotometer (UV-2600i, SHIMADZU, Japan).
The quality of the substrate as a medium for plant growth was examined by analyzing the physicochemical properties such as electrical conductivity (EC), pH, cation exchange capacity (CEC), organic matter (OM), total nitrogen (TN), and total phosphorus (TP) of the 1:5 (w:w) sediment slurry. The salt content serves as a comprehensive indicator in soil analysis, with soil EC providing a direct reflection of this salt content. Soil pH value is one of the key basic properties of soil, and is an indicator of soil formation, maturation and fertilization process. Soil CEC is the main source of soil buffering capacity and an important basis for soil improvement and rational fertilization. Among the various nutrient indicators assessed, soil OM is assessed to determine soil quality. The determination of soil TN level is an important indicator to evaluate nitrogen content, and it is very important for the evaluation and management of soil fertility. The determination of TP in soil is helpful to understand the content and distribution of phosphorus in soil and provide scientific basis for soil improvement. In summary, it is essential to evaluate these factors for assessing the effectiveness of dredged sediment improvements.
To be more specific, EC was measured using the conductivity method according to Analysis methods of water soluble salts of forest soil (LY/T 1251-1999). The pH value was measured using the potentiometric method according to the Determination of pH value in forest soil (GB7859-1987). The CEC was determined through NH4OAc atomic absorption spectrophotometry following the Determination of cation exchange capacity and exchangeable base of neutral soil (NY/T 295-1995). The OM was measured through the K2Cr2O7-H2SO4 external heating method according to Soil testing. Part 6: Method for determination of soil organic matter (NY/T 1121.6-2006). The TN was determined through the continuous flow analyzer method according to Nitrogen determination methods of forest soils (LY/T 1228-2015). The TP was measured through the acid dissolution and Mo-Sb anti-spectrophotometric methods according to Phosphorous determination methods of forest soils (LY/T 1232-2015). Crystal structures and phase compositions were analyzed by X-ray diffraction (XRD) on a Bruker D-8 diffractometer (Bruker-AXS, Germany) using Ni-filtered Cu-Kα radiation in the 2θ range 10-80°.
Heavy metal extraction experiments were conducted using FeCl3, CA, and EDTA, respectively. The experiments were designed with a controlled liquid-to-solid ratio of 10:1, an extraction period of 30 min, and a varying extractant concentration of 0.01, 0.03, 0.05, 0.1, and 0.3 mol/L. For each trial, 3.0 g of sediment (dry weight, <2.0 mm) was combined with 30 mL extractants (FeCl3, CA, or EDTA) at the specified concentrations. The mixtures were stirred in a horizontal shaking incubator at room temperature under 200 rpm. After centrifugation of the mixture for 30 min, the dredged sediment sample was filtered by a 0.45 µm filter membrane. Subsequently, the retrieved dredged sediment sample was digested using an automatic digester (DS-72, Guangzhou Genadan Instrument Co., Ltd., China). The digested sample was filtered through a 0.45 µm filter membrane, and then the heavy metal concentration was determined by ICP-OES (ICAP-6300, Thermo Fisher, USA). All experiments were conducted in duplicate, and the mean values were reported.
The chemical oxidation of TPH in the dredged sediment was performed using K2S2O8 (E0 = 2.6 V). The sediment was treated with varying concentrations of K2S2O8, specifically at 0.01, 0.03, 0.05, and 0.1 mol/L, followed by mixing at 200 rpm for 30 min. Subsequently, the obtained mixture was filtered using a 0.45 µm filter membrane. The supernatant was analyzed for transition metals using ICP-OES (ICAP-6300, Thermo Fisher, USA). After freeze-drying, the TPH content in the treated dredged sediment sample was determined using a UV-Vis spectrophotometer (UV-2600i, SHIMADZU, Japan) at 225 nm wavelength. All experiments were conducted in duplicate, and the mean values were reported.
Based on the results of the remediation experiments (according to Sections 2.3 and 2.4), the optimal concentration of extractants (FeCl3, CA, or EDTA) and oxidant (K2S2O8) was determined for investigating a synergistic sequence of remediation to enhance the removal efficiency of heavy metals and TPH from the contaminated dredged sediment. Three different cases were evaluated: extraction followed by oxidation (Ex-Ox), oxidation followed by extraction (Ox-Ex), and simultaneous oxidation and extraction (Ex+Ox). For the Ex-Ox and Ox-Ex treatment groups, the steps described in Sections 2.3 and 2.4 were followed sequentially. For the Ex+Ox treatment group, the chemical extractants (FeCl3, CA, or EDTA) and chemical oxidant (K2S2O8) were simultaneously mixed with the dredged sediment sample. Specifically, a mixture of sediment and extractant/oxidant was shaken in a horizontal shaker at 200 rpm at 20 °C for 30 min in a 1:10 weight/volume ratio. After centrifuging and washing, the contents of heavy metals (Cu and Pb), transition metals (Fe, Mn, Ni, and Ti), and TPH were determined. Finally, the mobility and bioavailability of heavy metals were characterized using the toxicity characteristic leaching procedure (TCLP) according to USEPA Method 1311.
Barley is one of the major ecotoxicity test species in international standards and is frequently employed to assess the toxic effects of organic and inorganic contaminants in sediments (Kim et al., 2023). In this study, each sediment sample (30 g) was supplemented with ten barley seeds in a 100 mL culture dish. DI water was added to the field capacity of the sediment. The barley seeds were incubated in a growth chamber that maintained consistent growth conditions (Temperature: 23°C; Daily light duration: 16 h; Humidity: 60%) for 10 d. After incubation, measurements were taken for both root and shoot germination rates as well as root and shoot lengths.
Statistical analysis was performed using SPSS version 26.0 for Windows (SPSS Inc, Chicago, IL, USA). One-way ANOVA (variance analysis) was employed to compare means of sediment physicochemical properties, contaminants (heavy metals and TPH), and barley germination and growth of different treatments. The significance level was set at P < 0.05. In this study, after the significance difference analysis, the data in the figures and tables were labeled using the significance difference letter labeling method. Generally, the lower case letter indicates the significant level α = 0.05, while the capital letter indicates the significant level α = 0.01.
The XRD analysis results are shown in Figure 1, revealing that the marine dredged sediment samples used in this study mainly comprise SiO2, MnFe2O4, Ni(SO4)(H2O), and NaFe3V3O12 (Kong et al., 2024). Notably, transition metals such as Fe and Mn in the dredged sediment samples create favorable conditions for activating persulfate (K2S2O8) to generate high oxidation capacity.
The measured physical and chemical properties of the tested dredged sediment are presented in Table 1. The particle size of the dredged sediment is predominantly below 0.075 mm. Moreover, the dredged sediment is alkaline, and the organic matter content is relatively low. Further, the high contaminant concentrations in the dredged sediment indicate that the dredged sediment has been contaminated with the corresponding contaminants (Cu, Pb, and TPH), which aligns with research requirements.
The concentration of Cu and Pb in the dredged sediment after the chemical extraction processes is illustrated in Figure 2. As expected, the removal efficiencies of Cu and Pb increase with the increasing concentration of extracting agents (Yoo et al., 2018; Xiao et al., 2019). For FeCl3, the removal efficiencies of Cu and Pb from the dredged sediment gradually increase with higher reagent concentration. The extraction of Cu and Pb exhibits a significant increase at a concentration of 0.05 mol/L, accompanied by a decrease in solution pH from 6.1 (0.01 mol/L) to 5.6 (0.05 mol/L). This variation can be attributed to the abundant protons produced by iron hydrolysis in the equilibrium reaction (Fe3++3H2O ↔ Fe(OH)3+3H+), leading to a reduction in pH within the dredged sediment. Consequently, the extraction efficiencies of Cu and Pb from the dredged sediments reach 77.77% and 67.81%, respectively. Chloride-based extractant removes Cu and Pb from the dredged sediment by forming complexes such as [metal-Cl]+, [metal-Cl2](aq), [metal-Cl3]- and [metal-Cl4]2- through the combination of chloride ions with metals (Yoo et al., 2013). As the FeCl3 concentration increases, the concentration of chloride ions in the solution increases, thereby enhancing the ability of chloride ions to form complexes with Cu and Pb. At 0.3 mol/L, FeCl3 exhibits optimal Cu and Pb removal efficiencies in the dredged sediment of 97.43% and 94.29%, respectively. In addition, FeCl3 can effectively extract Cu through the oxidative dissolution of metal sulfides (CuS+Fe3+ → Cu2++Fe2++S0) and the ion exchange between Cu organic complex and FeCl3 (Rinklebe et al., 2016). Therefore, the removal efficiency of Cu in the dredged sediment by FeCl3 slightly surpasses that of Pb.
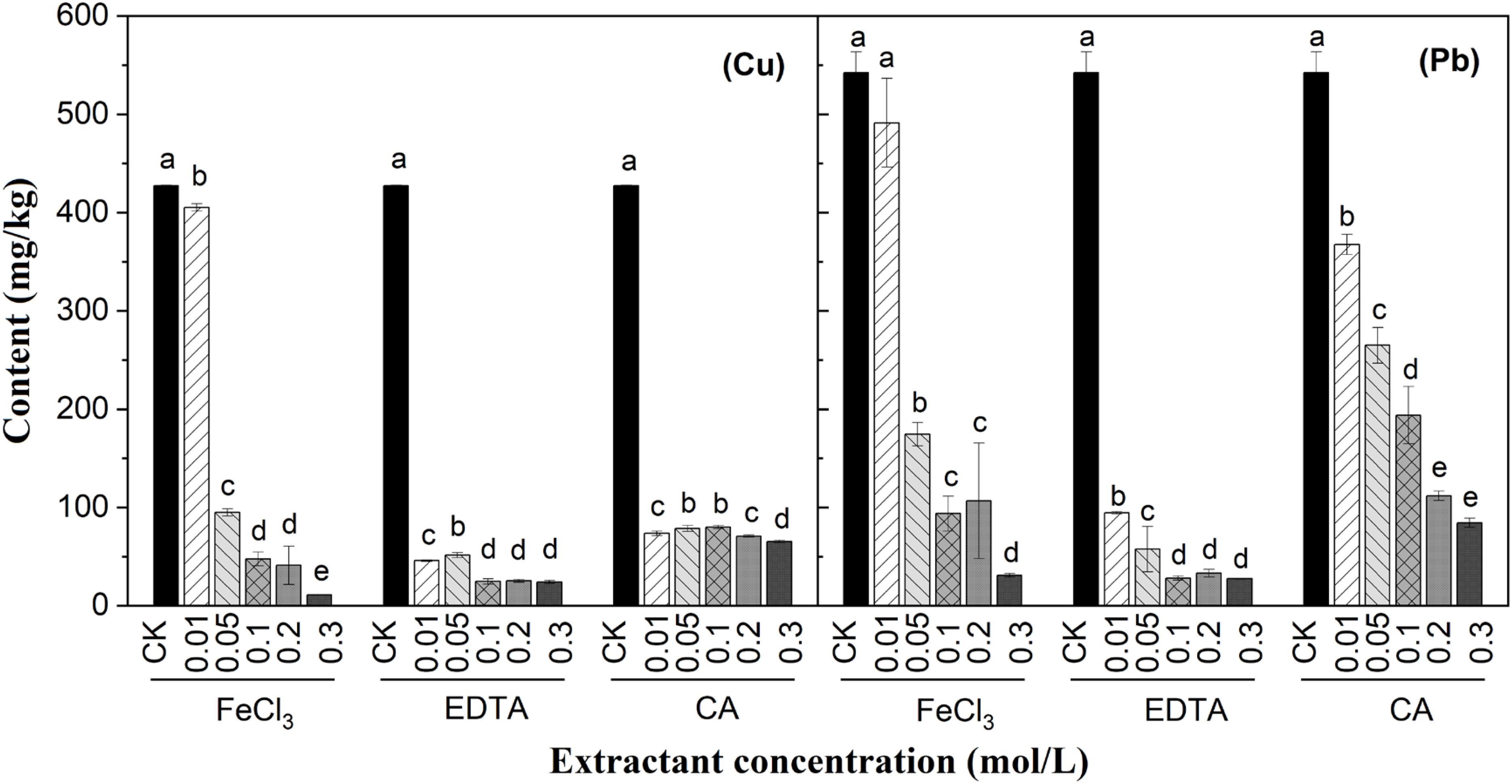
Figure 2. Effect of extracting agents on the removal efficiency of Cu and Pb in the dredged sediment. The lower case letter indicates the significant level α = 0.05. The groups with the same marked letter indicate no significant difference, while those with different marked letters indicate a significant difference.
The addition of EDTA significantly affects the removal of Cu and Pb from the dredged sediment (P > 0.05), with increasing removal efficiencies as the extraction concentration increases. At lower concentrations (0.01 mol/L), EDTA is more effective than FeCl3 and CA in removing Cu and Pb from the dredged sediment. The presence of carboxyl, amino, and other functional groups in EDTA enables the formation of complexes with heavy metals in the solid phase, thus promoting the release of heavy metal ions from the solid phase (Bolan et al., 2014). Therefore, EDTA can achieve a better removal effect of Cu and Pb in the dredged sediment at lower concentrations. Furthermore, during the formation of Cu-EDTA and Pb-EDTA metal complexes, the complexation rate of Cu with EDTA is greater than that of Pb, indicating that EDTA can achieve a high removal efficiency for Cu compared to Pb (Zhang and Lo, 2006). The pH value of the dredged sediments after EDTA extraction remains stable between 7.14 and 7.47. Within this pH range, Cu and Pb in the dredged sediment can efficiently form complexes with EDTA due to their optimal chelating ability under neutral conditions (Ahile et al., 2021). When the EDTA concentration increases from 0.1 to 0.3 mol/L, there is no statistical difference in Cu and Pb removal efficiency. Considering the cost-effectiveness, 0.1 mol/L concentration is selected as the optimal EDTA extraction concentration.
Compared with the CK group, CA demonstrates superior Cu extraction efficiency in the dredged sediment at all concentrations. However, with increasing CA concentration, there is minimal change in its removal efficiency for Cu. In contrast, the extraction effect of CA on Pb is not evident at low reagent concentrations. However, with the increase of reagent concentration, the extraction effect of Pb becomes more significant. During the extraction process, the number of functional groups involved in the reaction increases with increasing CA concentration, which in turn enhances the extraction effect on heavy metals. The CA solution contains many carboxyl groups that exhibit a high affinity for metal ions by forming metal chelates (Wang et al., 2021). At lower concentrations, the sequestration sites for Cu are occupied more rapidly than those for Pb due to the higher complexation constant of Cu with CA (KCu-CA = 6.1) compared to Pb with CA (KPb-CA = 4.1) (Palma and Mecozzi, 2007). CA solution binds to Cu by forming soluble organic complexes with carboxyl and hydroxyl functional groups, thereby inhibiting the adsorption of Cu ions on the surface of sediment particles (Ke et al., 2020). As the concentration increases, the number of chelation sites increases, leading to a decrease in the pH value of the solution. Consequently, an increased amount of H+ ions undergo ion exchange reactions with Pb in the sediment, resulting in the desorption of weakly bound Pb from the sediment surface. This phenomenon can be attributed to a reduction in the combination between Pb and acid-soluble parts, as well as some reducible parts in the sediment decreases, ultimately enhancing the extraction efficiency of Pb (Chen et al., 2016). This observation also elucidates that the removal efficiency of Cu in dredged sediments remains essentially unchanged with the addition of CA, while the removal effect for Pb in the dredged sediment increases with a higher concentration of CA. When the concentration of CA is 0.3 mol/L, the highest Cu and Pb removal efficiencies of 84.70% and 84.46% are achieved, respectively. Overall, the above results show that CA is less effective than EDTA. In addition, iron content in the contact solution significantly increases due to the great affinity between CA and Fe (KFe-CA = 11.85), which has advantages in the following synergistic remediation process (Palma and Mecozzi, 2007).
The sulfate radicals (SO4•−) produced by persulfate oxidants exhibit stronger oxidation effects and higher stability in soil/sediment environments than traditional oxidants. In addition, sulfate, the final product of persulfate oxidation, is less toxic to the ecosystem (Yen et al., 2011). Figure 3 shows the removal of TPH from the contaminated dredged sediment using K2S2O8 at different concentrations. It can be found that the removal efficiency increases with the increasing concentration. The most significant (P > 0.05) oxidation effect on TPH is achieved at an oxidation concentration of 0.1 mol/L, resulting in a removal efficiency of 13.57%. Transition metals in minerals within dredged sediments can react with persulfates and further affect the removal efficiency of pollutants by promoting activation. The specific activation mechanism can be described according to the reported equation (S2O82-+M → M++SO42-+SO4•−) (Zhou et al., 2019).
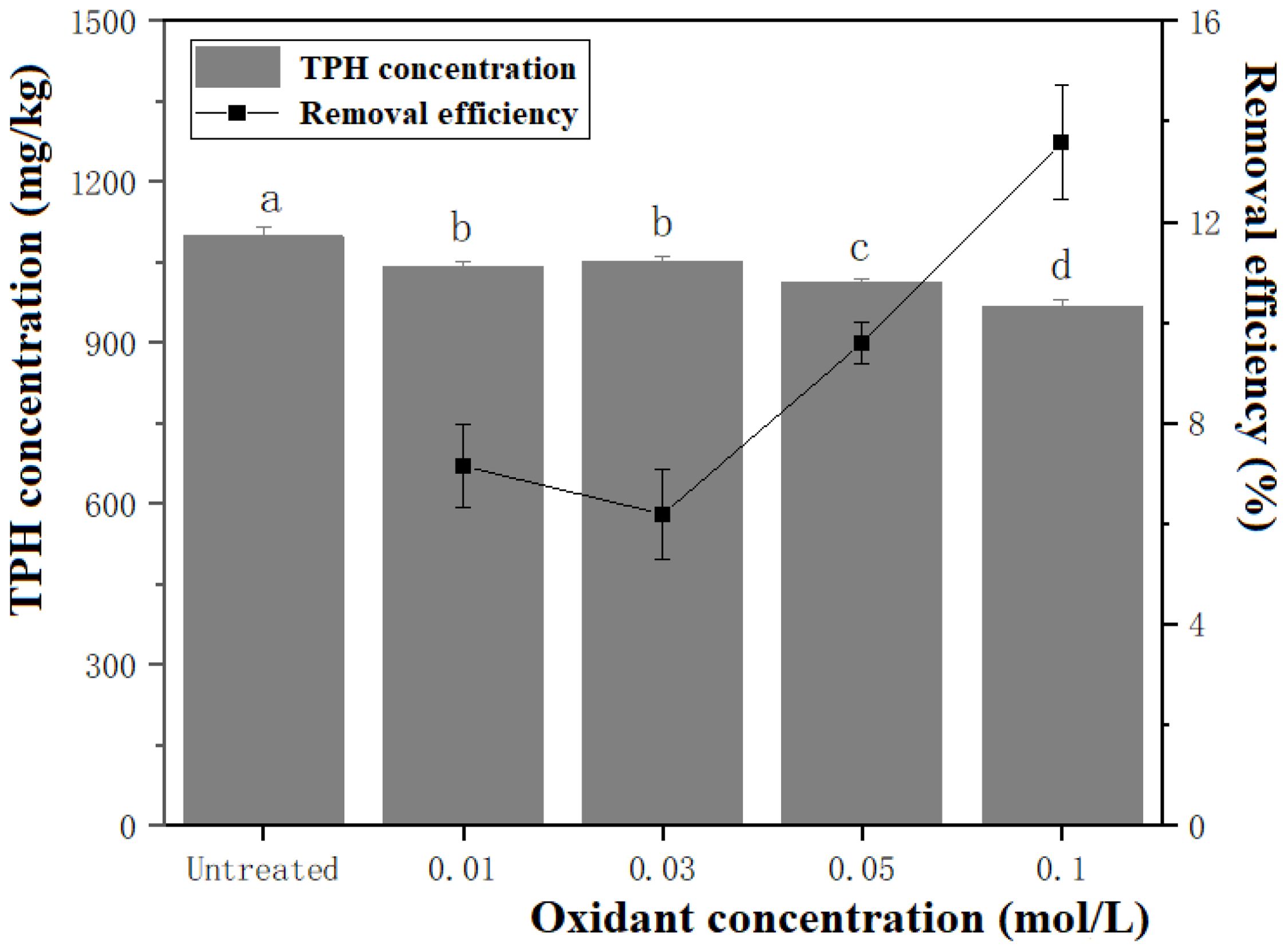
Figure 3. Effect of oxidant concentration on the removal efficiency of TPH in dredged sediment. The lower case letter indicates the significant level α = 0.05. The groups with the same marked letter indicate no significant difference, while those with different marked letters indicate a significant difference.
As shown in Table 2, the presence of transition metals in the supernatant activates the persulfate and generates SO4•− (E0 = 2.5-3.1 V) during the removal of TPH from the dredged sediments with persulfate oxidation. The redox potential of SO4•− is larger than that of persulfate (S2O82-, E0 = 2.0 V). The production of SO4•− improves the oxidation performance of persulfate for TPH, contributing to a higher removal efficiency. Although the minerals in dredged sediments have the potential to activate persulfate, the oxidative removal of TPH using persulfate may not fall short of the desired effectiveness due to the original, low mineral content of minerals (Zhou et al., 2019).
Moreover, due to the strong polarity of persulfate, oxidation mainly occurs in the water phase, greatly reducing the contact surface between persulfate and TPH adsorbed on the sediment surface. The diffusion of persulfate in sediment aggregates may also be limited, resulting in a low pollutant removal efficiency (Liu et al., 2021). In addition, due to the high adsorption capacity of TPH, it tends to closely bind to sediment particles and occupy sediment pores. Relevant studies have reported that pollutants in soil pores are relatively difficult to oxidize by oxidants, and soil adhesion can affect the release of pollutants, thus hindering restoration (Deng et al., 2015). Furthermore, results have revealed that persulfate anions could persist in sediments for several months, leading to the continuous generation of sulfate free radicals by metal activation and enhancing the contaminant oxidation (Yen et al., 2011; Zhou et al., 2019).
As shown in Figure 4, the application sequences of Ox-FeCl3, FeCl3-Ox, and FeCl3+Ox remove 89.35%, 88.69%, and 91.10% of Cu, and 83.00%, 86.85%, and 92.89% of Pb. FeCl3 application sequence experiments for removing Cu and Pb from the dredged sediments all show good performance. Specifically, the FeCl3+Ox group is slightly more effective than the remaining two groups. The primary reason for this is that FeCl3 has a low acidity coefficient (pKa) value, and anionic chloride ligands can enhance the extraction of toxic cationic heavy metals from dredged sediments (Yoo et al., 2016). These results suggest that the simultaneous application of Ex and Ox can minimize the process time and contribute to high levels of co-contaminant removal.
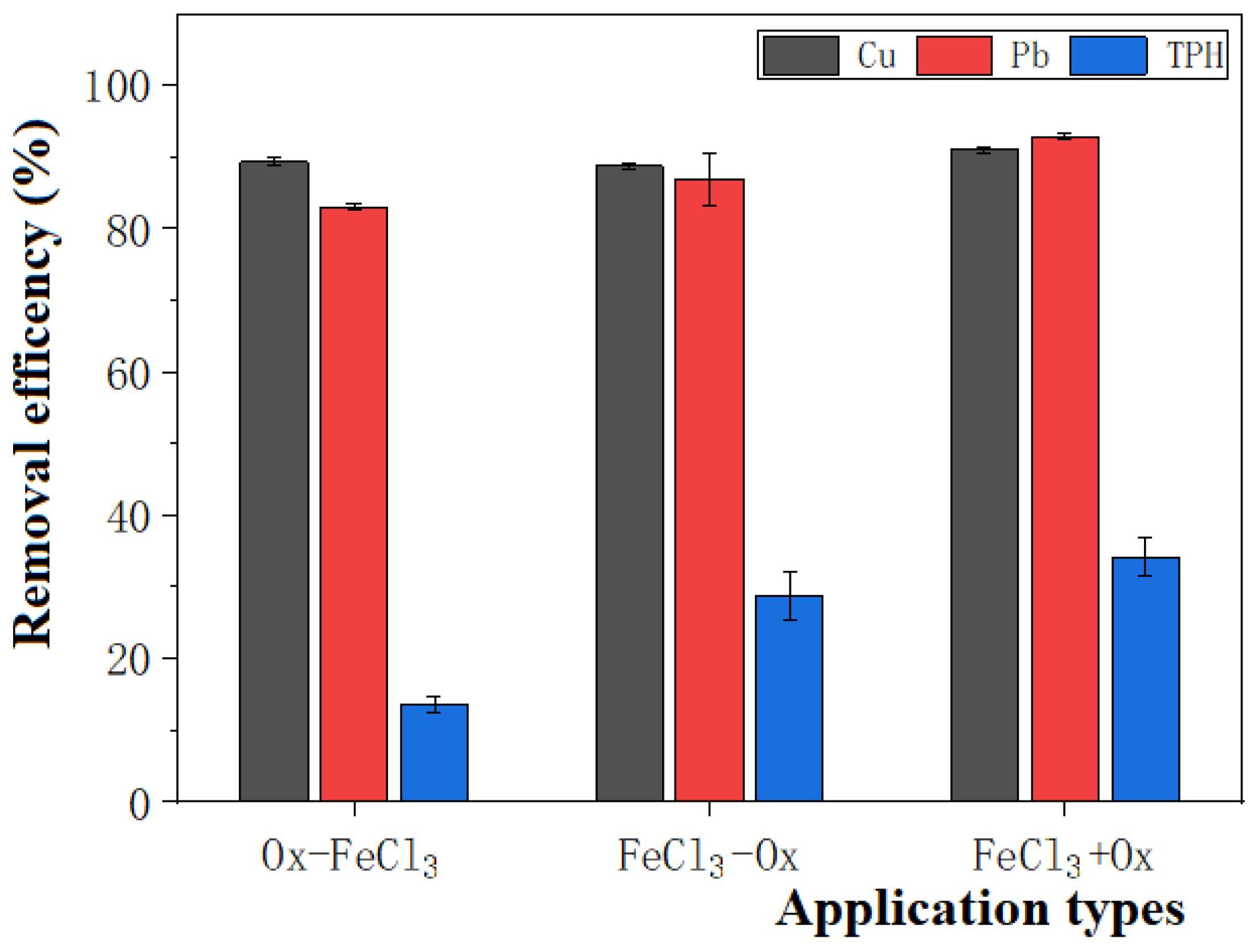
Figure 4. Effect of order of FeCl3 application on the efficiency of removal of heavy metal-petroleum hydrocarbon pollutants.
For the study of the TPH removal effect in the contaminated dredged sediment, it can be seen from Figure 4 that different treatment orders of FeCl3 and K2S2O8 greatly influence the removal of TPH. Specifically, OX-FeCl3, FeCl3-OX, and FeCl3+Ox remove 12.64%, 28.75%, and 34.11% of TPH, respectively. Yan et al. (2019) found that the dissolved Fe in the soil could promote the activation of sodium persulfate. The increase in iron content enhances the efficiency of organic pollutant removal. As illustrated in Table 3, the Fe content in FeCl3+Ox supernatant is significantly higher than that in FeCl3-Ox and Ox-FeCl3 treatment groups, substantially promoting persulfate activation. Transition metals, such as Mn, Ni, and Ti, have also been shown to activate sodium persulfate and produce sulfate radicals (Su et al., 2023). In comparison, the content of Mn, Ni, and Ti elements in the FeCl3+Ox treatment group is significantly higher than that in the FeCl3-Ox and Ox-FeCl3 treatment groups. This difference indicates that the conditions in the FeCl3+Ox group can efficiently activate persulfate to oxidize the TPH in the contaminated dredged sediment. To sum up, the FeCl3+Ox treatment group shows great advantages for TPH removal, and this superiority is mainly related to its high persulfate oxidation capacity promoted by its high transition metal (Fe, Mn, Ni, and Ti) content.
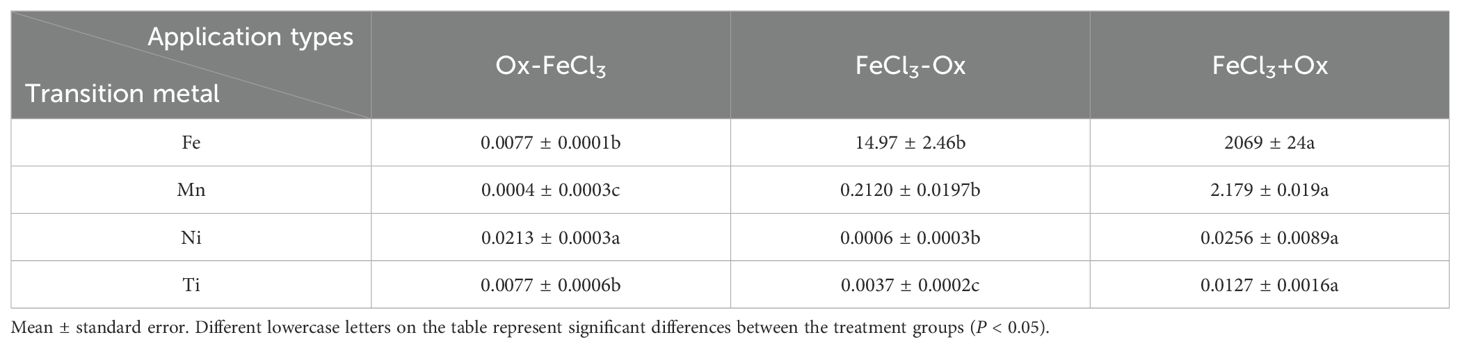
Table 3. Transition metal content of supernatants in sequential experiments with FeCl3 applications (mg/L).
As shown in Figure 5, the three application sequences minorly impact the removal of heavy metals Cu and Pb using EDTA. To be specific, Ox-EDTA, EDTA-Ox, and EDTA+Ox remove 90.22%, 90.95%, and 87.13% of Cu, and 87.44%, 96.95%, and 91.30% of Pb from the dredged sediment, respectively. When extracting Cu and Pb, EDTA mainly removes the Cu and Pb bound to Fe and Mn oxides.
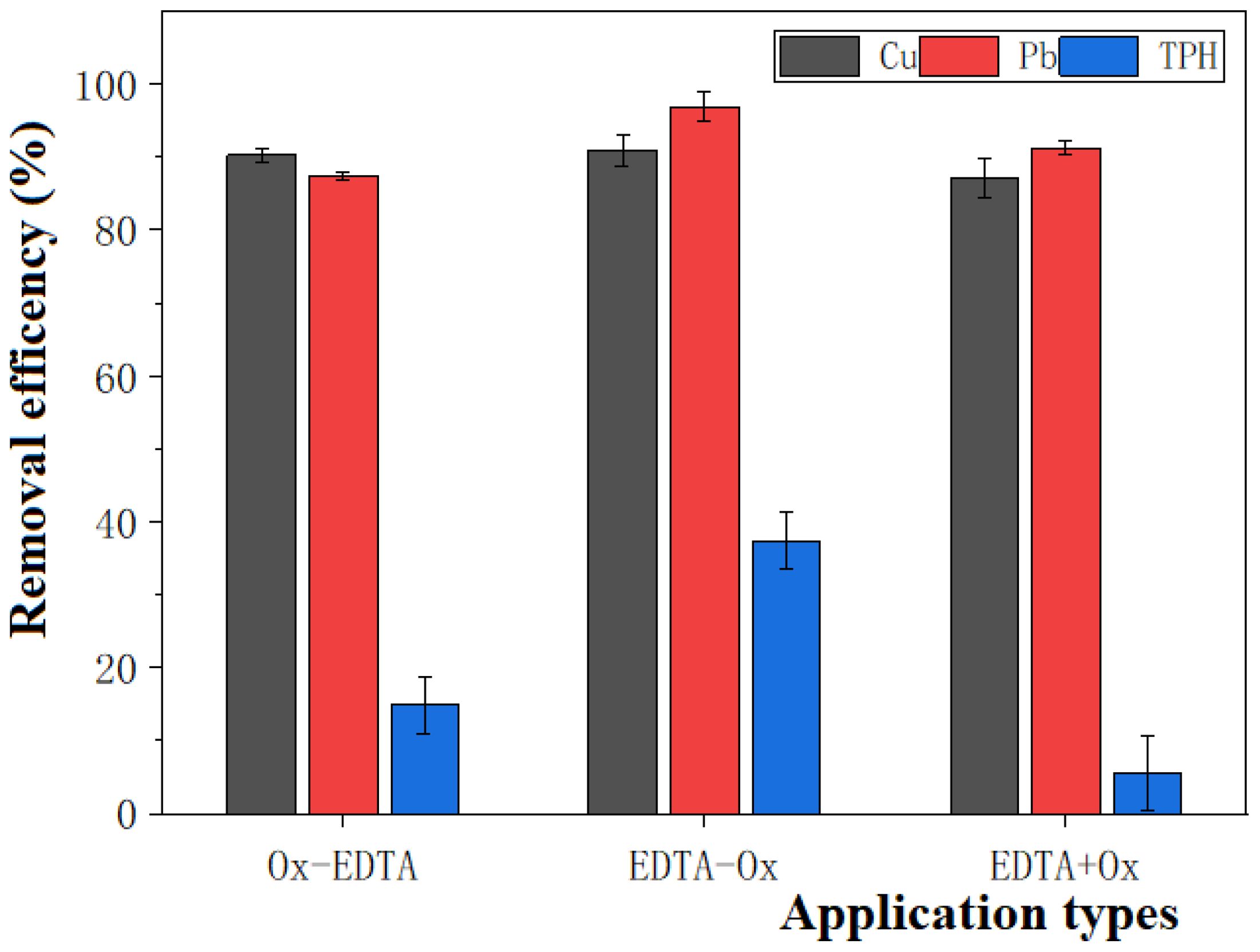
Figure 5. Effect of order of EDTA application on the efficiency of removal of heavy metal-petroleum hydrocarbon pollutants.
However, the EDTA application sequence greatly influences the oxidative removal of TPH, and adjusting the cooperative remediation order can largely increase the removal efficiency of TPH. From Figure 5, it can be seen that the oxidative removal efficiency of TPH in the EDTA-Ox treatment group (37.42%) is higher than that of Ox-EDTA (14.88%) and Ox+EDTA (5.61%) treatment groups.
As shown in Table 4, the content of transition metals in the supernatant of the EDTA+Ox treatment group is significantly higher (P < 0.05) than that of the other two treatment groups. However, EDTA+Ox does not remove more TPH in the dredged sediment. The reason is that the EDTA extractant can form complexes with Cu and Pb, as well as with the transition metals in solution (Yoo et al., 2017). Thus, the transition metals in the supernatant of the EDTA+Ox treatment group mainly exist in the form of complexes with EDTA. As a result, these transition metals cannot effectively activate persulfate to produce SO4•− with strong oxidative effects. Besides, as an organic matter, EDTA tends to consume and decompose K2S2O8 and thus competes with the target pollutant (i.e., TPH). For these reasons, the EDTA+Ox treatment cannot improve the oxidative removal of TPH from the dredged sediment.

Table 4. Transition metal content of supernatants in sequential experiments with EDTA applications (mg/L).
Figure 6 shows that the three application sequences minimally impact the removal of Cu by CA in the dredged sediment, with Ox-CA, CA-Ox, and CA+Ox treatment groups yielding 81.26%, 85.34%, and 83.75% of removal rates, respectively. In contrast, the removal efficiency of Pb is influenced by these sequences. Specifically, Ox-CA, CA-Ox, and CA+Ox treatments remove 42.83%, 61.31%, and 69.67% of Pb from the dredged sediment. In the Ox-CA sequence, the removal efficiency of Pb is relatively low. This observation is consistent with the experimental result of Yoon et al. (2018). This may be attributed to the ability of K2S2O8 to oxidize Pb in the dredged sediment into a more stable bound state (Wang et al., 2022). The reducing capacity of CA is limited, potentially converting only part of the heavy metals associated with Fe and Mn oxides into exchangeable or carbonate-bound states. As a result, the removal efficiency of Pb is relatively low in the Ox-CA treatment group.
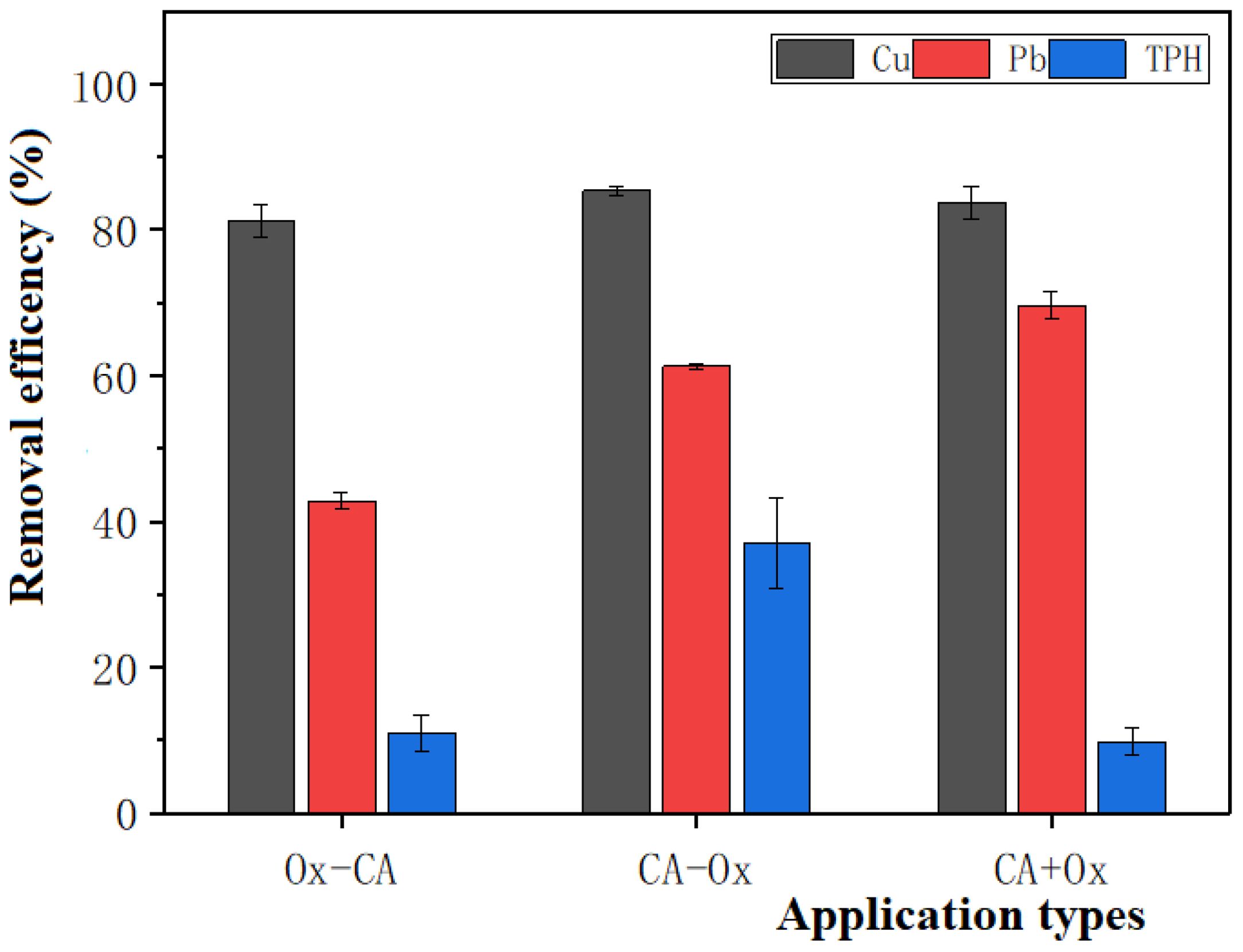
Figure 6. Effect of order of CA application on the efficiency of removal of heavy metal-petroleum hydrocarbon pollutants.
Similar to EDTA, the order of CA application has a great effect on the TPH removal by oxidation. The oxidative removal efficiency of TPH in the CA-Ox treatment group is higher than that in the Ox-CA and CA+Ox treatment groups (Figure 6). Specifically, the oxidative removal efficiency of TPH from the dredged sediment by CA-Ox, Ox-CA, and CA+Ox treatments is 37.06%, 11.03%, and 9.79%, respectively. For the CA-Ox treatment group, the addition of CA dissolves the Fe-Mn oxides in dredged sediments, and the concentration of transition metals (especially Fe) in the supernatant increases significantly during oxidation (P < 0.05) (Table 5). The high content of these transition metals significantly activates the oxidation capacity of the K2S2O8. This increases the oxidative removal efficiency of TPH in the sequence of extraction followed by oxidation (Yoon et al., 2018).
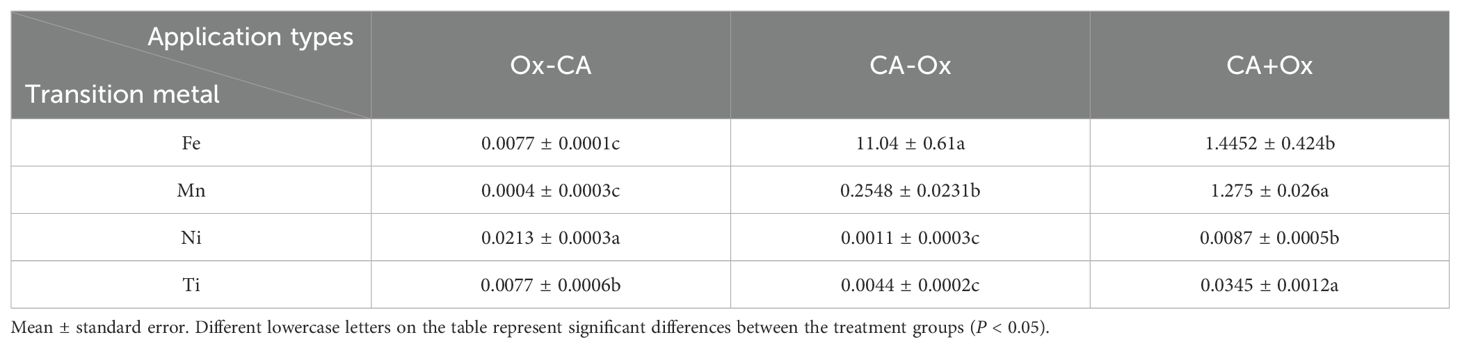
Table 5. Transition metal content of supernatants in sequential experiments with CA applications (mg/L).
Using TCLP to assess the changes in heavy metal mobility is considered a reliable tool for evaluating their potential mobility and bioavailability in sediments and solid wastes (Lang et al., 2019). The morphology of TCLP mainly reflects the biologically harmful content of heavy metal exposure to the environment, offering a more intuitive assessment than morphological analyses, such as BCR. In addition, it is more advantageous for phytotoxicity analyses.
The extraction and oxidation procedures effectively reduce heavy metals’ mobility in marine dredged sediment (Figure 7). Cu and Pb in the untreated dredged sediment possess high mobility, particularly in Cu. The reasons for the lower mobility of Pb mainly include: (1) Pb can form complexes with organic matter; (2) it can be adsorbed on oxides and silicate clay minerals; (3) Pb can be precipitated as carbonate, sulfate, or phosphate (Unda-Calvo et al., 2017). The experimental results demonstrate that FeCl3, EDTA, and CA are effective extractants. The mobility of heavy metals after CA treatment is greater than that treated with FeCl3 and EDTA, probably due to the influence of pH. The mobile fraction of heavy metals in sediment is largely influenced by the sediment pH and generally increases with decreasing pH. In the sediment, the increased pH reduces the mobility of heavy metals, thereby decreasing their bioavailability to plants.
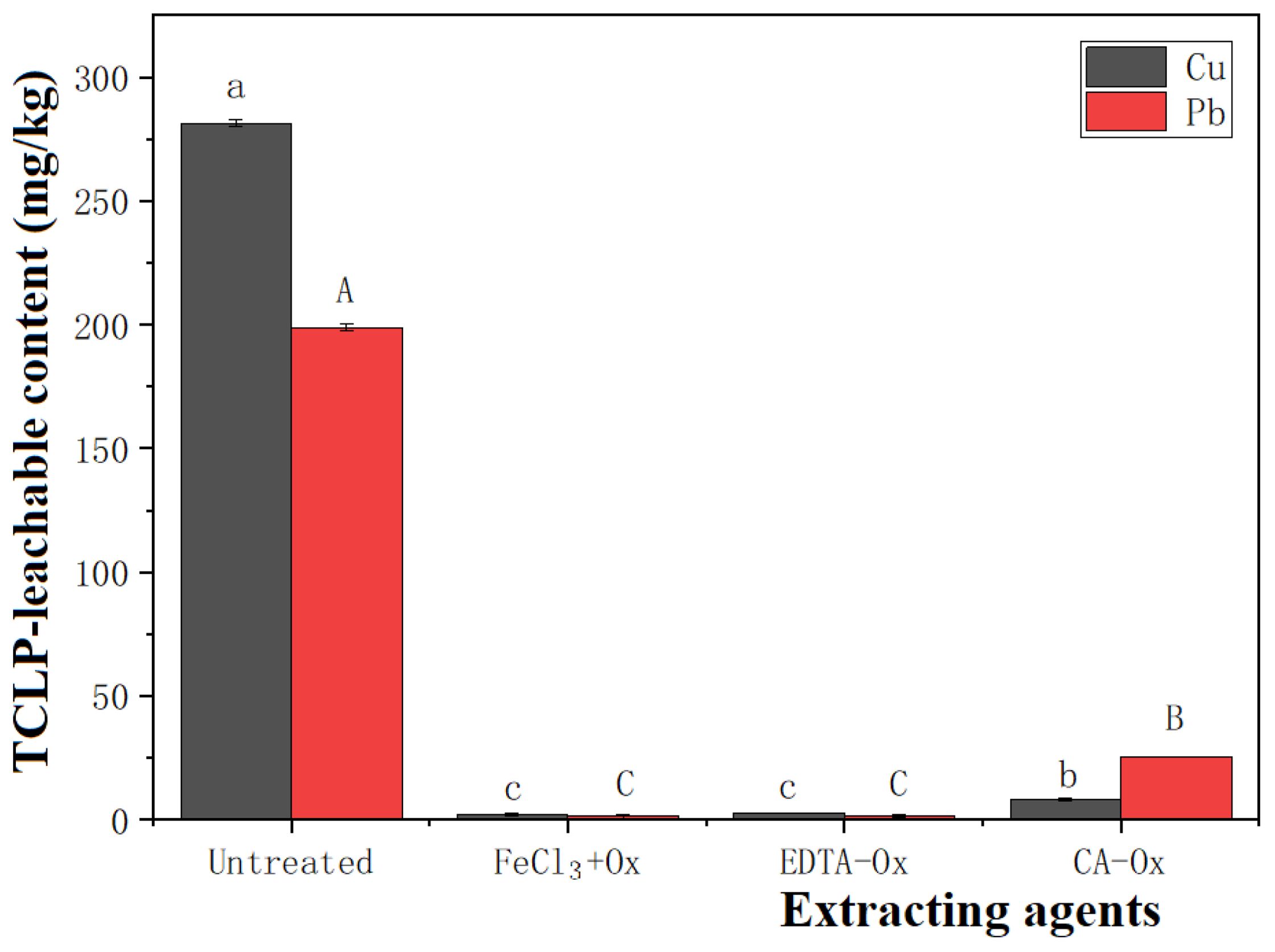
Figure 7. Heavy metal content in TCLP-leachable sediment untreated and treated with combinations of extractants. (Mean ± standard error. Different letters on the bar graphs represent significant differences between treatment groups for the same heavy metal, with upper case letters indicating differences in the Cu group and lower case letters corresponding to the Pb group (P < 0.05)).
The physicochemical properties of dredged sediments can affect plant growth. The physicochemical characteristics and nutrient composition, including EC, OM, TN, TP, and CEC of the dredged sediment, change significantly after treatments (Table 6). The pH value of the dredged sediment decreases, and the sediment environment acidifies after FeCl3 is added as a heavy metal extractant (Yoo et al., 2016). The reason for this is that the hydrolysis of Fe3+ produces considerable H+, which decreases the pH of the dredged sediment. The pH value in the EDTA-Ox treated sediment sample increases significantly, consistent with the results of the previous study (Zupanc et al., 2014).
Extraction and oxidation synergistic remediation of the contaminated dredged sediment also affects the OM content. The FeCl3+Ox and EDTA-Ox treatment groups both experience a decrease in OM content, and this is more pronounced for the FeCl3+Ox treatment group. The reason for this phenomenon is that FeCl3 acts as an acidic extractant, which is capable of forming complexes with organic substances in the dredged sediment. This interaction facilitates the extraction of a portion of the OM. However, these complexes may become soluble throughout the extraction, decreasing the organic content within the dredged sediment. In addition, after applying the synergistic remediation by the FeCl3+Ox treatment, the TN content in the dredged sediment is significantly increased, effectively improving the resistance of barley seeds. This may explain why barley seeds in the FeCl3+Ox treatment group continue to exhibit better growth despite a lower pH value.
After FeCl3+Ox, CA-Ox, and EDTA-Ox treatments, the EC content in dredged sediments undergoes significant decreases related to the substantial reduction of the soluble salts in the treated dredged sediments. The main mechanism for the decline in CEC values in the dredged sediment is due to the action of organic matter decomposition (Kim et al., 2020). After EDTA-Ox treatment, the content of TN in the dredged sediment increases, which may be related to the residual EDTA (Guo et al., 2018). The TP content decreases after FeCl3+Ox and EDTA-Ox treatments. This may be due to the dissolution of the water-soluble or readily desorbed inorganic phosphorus in the dredged sediment during synergistic remediation (Kurek et al., 2020).
The effect of the dredged sediment on plant growth after restoration can be assessed based on the growth potential of barley seed. Compared to the original dredged sediment, the treated dredged sediment is more conducive to barley germination and growth, and the planted barley has good growth potential (Figure 8). Figures 9A, C mainly illustrate the effects of different treatment conditions on the shoot and root germination rates of barley seeds, respectively. No significant difference is observed in the shoot and root germination growth rates among these groups (P > 0.05). In contrast, as shown in Figures 9B, D, different treatment groups significantly affect shoot and root lengths compared to the CK group. The influence of varied dredged sediments on the germination rate of barley seeds is small. The possible reason for this may be that the germination mainly depends on the heterotrophic growth of endosperm inside seeds.

Figure 8. Barley seed growth in each treatment group: (A) EDTA-Ox treated; (B) FeCl3+Ox treated; (C) CA-Ox treated; (D) CK.
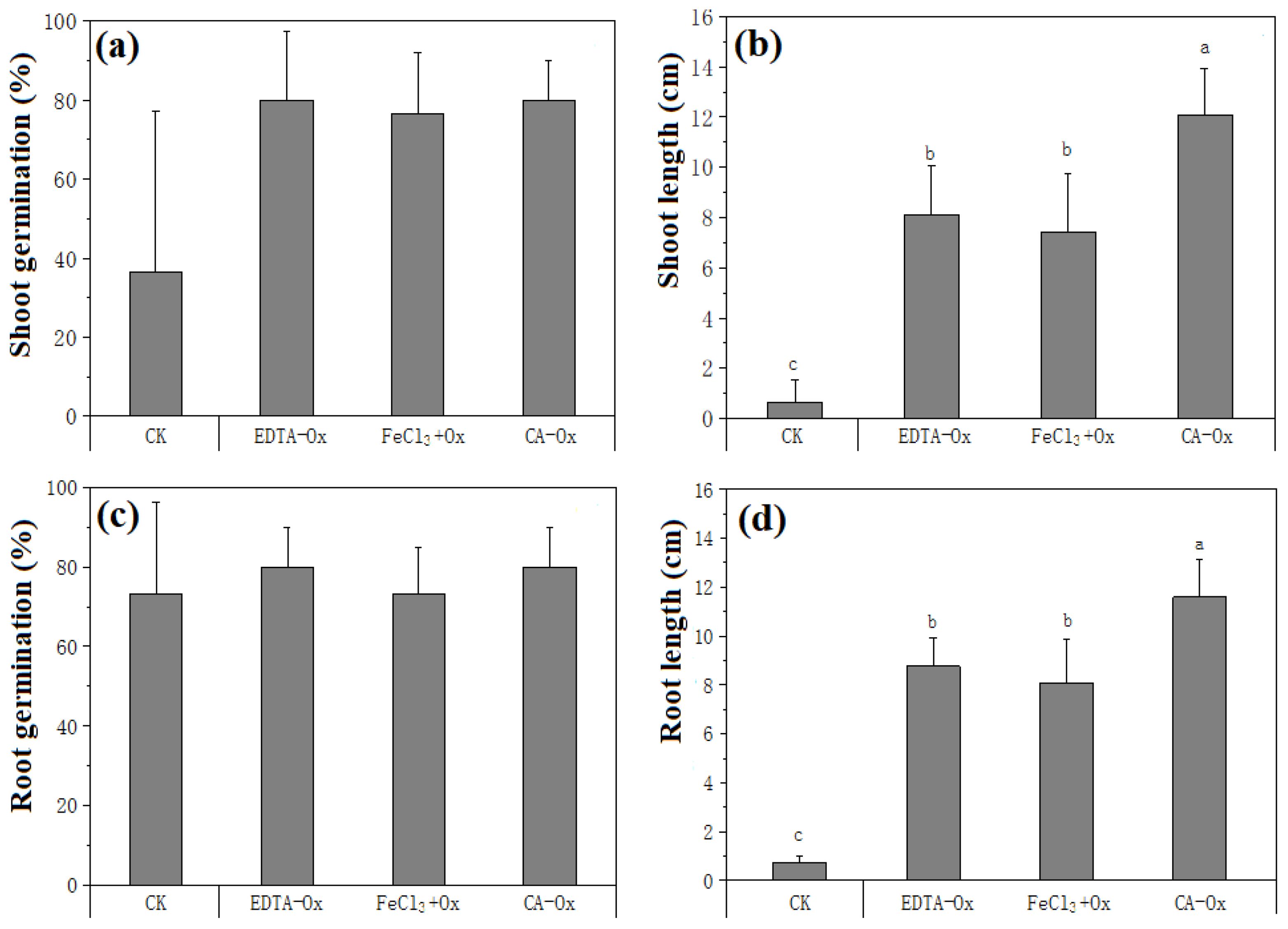
Figure 9. Barley (Hordeum vulgare L.) germination and growth test results of treated sediments: (A) shoot germination; (B) shoot length; (C) root germination; (D) root length. The lower case letter indicates the significant level α = 0.05. The groups with the same marked letter indicate no significant difference, while those with different marked letters indicate a significant difference.
The shoot and root lengths of barley are significantly greater than those of the CK group. The growth potential of barley in sediment subjected to CA-Ox remediation treatment is especially favorable, with shoot and root lengths of 11.6 cm and 12.1 cm, respectively. The reason is that adding low molecular organic acid (CA) to the contaminated dredged sediment can activate the sediment enzymes, thus promoting plant growth (Xiao et al., 2019; Manzoor et al., 2021).
Heavy metal mobility in sediments can inhibit the growth of barley, significantly reducing the remediated sediments. In addition, the bioavailability of heavy metals is reduced, as shown in Figure 7. The presence of Cu largely inhibits chlorophyll, which can affect photosynthesis and metabolic processes in plants and suppress seed germination and growth (Quan et al., 2023). Pb contamination negatively affects plant growth by interfering with ionic homeostasis, generating free radicals, and causing phytogenetic toxicity (Gul et al., 2023). Moreover, the inhibitory effect of TPH in sediments on the growth of barley seeds should be considered. Plant root elongation is more sensitive to TPH contamination in the sediment, and the inhibition of root growth becomes increasingly pronounced with rising TPH concentration (Tang et al., 2011). Due to the combined extraction-oxidation remediation, the mobility of heavy metals and the TPH concentration in the dredged sediment are reduced. Additionally, the EC results in Table 6 prove that the salinity stress on seeds is mitigated. Consequently, this treatment provides a favorable environment for the growth of barley seeds, indicating that the extraction-oxidation synergistic remediation largely reduces the disposal risk of the dredged sediment. This method effectively mitigates the environmental hazards associated with the contaminated sediments, paving the way for safer and more sustainable land use and ecological restoration.
As shown in Figures 7 and 9, compared to the CA-Ox treatment group, the mobility of Pb in the dredged sediment is reduced more significantly in the FeCl3+Ox group (P < 0.05), while the average length of barley seedlings is relatively low. Primarily, FeCl3 is an acidic extractant, and its addition decreases the pH value of the dredged sediment. This reduction leads to acidification, which is detrimental to the growth of barley. The above outcome is consistent with the experimental findings of Yoo et al. (2017).
As shown in Table 6, the TN content within the dredged sediment experiences an increase after the treatments with FeCl3+Ox or EDTA-Ox cooperative remediation. However, this increase has a minimal impact on the growth of barley malt (Figure 9). This suggests that although the treatment can alter certain chemical properties of the dredged sediment, other potential factors may contribute more to plant growth in this specific context.
The correlation between the physical and chemical properties and the nutrient content of the dredged sediments, as well as the shoot and root lengths of barley, are provided in Supplementary Figures S1 and S2, respectively. It can be found that the content of OM in the dredged sediment is positively correlated with the growth of barley seedlings. The reason is that microorganisms can decompose OM, providing nutrients for seed growth. Moreover, the presence of OM increases the water retention capacity of the dredged sediment, leading to looser and aeration-friendly conditions. It also enhances the sediment’s ability to retain moisture, preventing the adverse effects on barley growth from excessively dry or wet sediment. Moreover, as a low molecular natural organic acid, CA increases the OM in the sediment mainly by regulating the metabolites of primary and secondary metabolism. Due to the presence of CA, the plant produces more sugar, which provides greater energy to the organism. This is why barley shoot and root lengths in the CA-Ox treatment group are more prolonged than the other two treatment groups.
High salinity is an important factor in suppressing the root growth of barley seeds. As shown in Supplementary Figures S1 and S2, EC is negatively correlated with barley root length. The EC of the dredged sediment treated with FeCl3+Ox, CA-Ox, and EDTA-Ox decreases significantly. This effectively reduces the stress exerted by the dredged sediment on the barley seed roots and maintains the osmotic pressure between the environment and the roots, providing favorable conditions for plant growth (Kim et al., 2020).
As depicted in Supplementary Figures S1 and S2, the CEC, TN, and TP in the dredged sediment do not exhibit a strong correlation with the growth of barley malt. This may be attributed to the source of the original marine dredged sediment from a waterway near the Yangtze River estuary, where a high sediment load is carried. Moreover, the soil texture is characterized by sandy loam with inherently poor fertility retention capabilities. Consequently, the middle ranges of CEC, TN, and TP in the dredged sediment are relatively low. The variations in CEC, TN, and TP are insignificantly influenced by the extraction-oxidation co-remediation process.
This study investigates the effectiveness of extraction-oxidation synergistic remediation treatments for removing heavy metals (Cu and Pb) and TPH in contaminated dredged sediments. The results indicate that this treatment significantly reduces the content of Cu and Pb in the dredged sediment. The total removal performance of different treatment groups on heavy metals and TPH in the contaminated dredged sediment is ranked as FeCl3+Ox > EDTA-Ox > CA-Ox. In addition, the FeCl3+Ox and EDTA-Ox treatment groups achieve the maximal removal efficiencies for Cu and Pb, reaching 91.10% and 96.95%, respectively. Adding extractants affects the activation of the K2S2O8 through transition metals, enhancing the oxidation and removal efficiency for TPH in the dredged sediment. The EDTA-Ox treatment group exhibits the optimal extraction effect (37.42%) on TPH in the dredged sediment within 30 min. After the extraction-oxidation synergistic remediation, the mobility of heavy metals and the TPH concentration in the treated dredged sediment are reduced. During the barley planting experiment using the treated dredged sediment, the shoot and root in the EDTA-Ox treatment group exhibit the maximum germination rates of 100% and 90%, respectively. The shoot and root lengths of the CA-Ox-treated barley are the longest among samples, measuring 11.6 cm and 12.1 cm, respectively. Additionally, the treatment of interest mitigates the stress caused by salinity on seeds. This method effectively alleviates the environmental hazards associated with the contaminated dredged sediments, facilitating safer and more sustainable land use and ecological restoration. This study presents innovative approaches for the remediation of the contaminated dredged sediments. Our future research aims to enhance the effectiveness of dredged sediment improvements, facilitating practical and widespread applications in the beneficial-use of the dredged sediment.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
WY: Writing – original draft, Writing – review & editing. JL: Writing – original draft, Writing – review & editing. YS: Conceptualization, Investigation, Methodology, Writing – original draft. ZW: Formal analysis, Methodology, Project administration, Writing – review & editing. CH: Investigation, Methodology, Software, Writing – review & editing. JH: Funding acquisition, Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by Dalian outstanding youth science and technology talent program (2023RY010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1550274/full#supplementary-material
Ahile U. J., Wuana R. A., Itodo A. U., Sha’Ato R., Malvestiti J. A., Dantas R. F. (2021). Are iron chelates suitable to perform photo-Fenton at neutral pH for secondary effluent treatment? J. Environ. Manage. 278, 111566. doi: 10.1016/j.jenvman.2020.111566
Albarano L., Costantini M., Zupo V., Lofrano G., Guida M., Libralato G. (2020). Marine sediment toxicity: A focus on micro-and mesocosms towards remediation. Sci. Total Environ. 708, 134837. doi: 10.1016/j.scitotenv.2019.134837
Bolan N., Kunhikrishnan A., Thangarajan R., Kumpiene J., Park J., Makino T., et al. (2014). Remediation of heavy metal(loid)s contaminated soils – To mobilize or to immobilize? J. Hazard. Mater. 266, 141–166. doi: 10.1016/j.jhazmat.2013.12.018
Chen C., Tian T., Wang M. K., Wang G. (2016). Release of Pb in soils washed with various extractants. Geoderma 275, 74–81. doi: 10.1016/j.geoderma.2016.04.015
Deng D., Lin X., Ou J., Wang Z., Li S., Deng M., et al. (2015). Efficient chemical oxidation of high levels of soil-sorbed phenanthrene by ultrasound induced, thermally activated persulfate. Chem. Eng. J. 265, 176–183. doi: 10.1016/j.cej.2014.12.055
Doni S., Macci C., Martinelli C., Iannelli R., Brignoli P., Lampis S., et al. (2018). Combination of sediment washing and bioactivators as a potential strategy for dredged marine sediment recovery. Ecol. Eng. 125, 26–37. doi: 10.1016/j.ecoleng.2018.10.009
Guarino C., Spada V., Sciarrillo R. (2017). Assessment of three approaches of bioremediation (Natural Attenuation, Landfarming and Bioagumentation — Assisted Landfarming) for a petroleum hydrocarbons contaminated soil. Chemosphere 170, 10–16. doi: 10.1016/j.chemosphere.2016.11.165
Gul I., Manzoor M., Ahmad I., Kallerhoff J., Arshad M. (2023). Phytoaccumulation of cadmium by Pelargonium × hortorum – tolerance and metal recovery. Environ. Sci. pollut. R. 30, 32673–32682. doi: 10.1007/s11356-022-24485-5
Guo X., Zhao G., Zhang G., He Q., Wei Z., Zheng W., et al. (2018). Effect of mixed chelators of EDTA, GLDA, and citric acid on bioavailability of residual heavy metals in soils and soil properties. Chemosphere 209, 776–782. doi: 10.1016/j.chemosphere.2018.06.144
Hong S. H., Hwang S., Lee C. G., Park S. J. (2023). Stabilization of As-contaminated dredged sediment using Al- and Fe-impregnated food waste biochar. J. Soil. Sediment. 23, 2628–2640. doi: 10.1007/s11368-023-03520-z
Ke X., Zhang F. J., Zhou Y., Zhang H. J., Guo G. L., Tian Y. (2020). Removal of Cd, Pb, Zn, Cu in smelter soil by citric acid leaching. Chemosphere 255, 126690. doi: 10.1016/j.chemosphere.2020.126690
Kim H. S., Kim Y. J., Seo Y. R. (2015). An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J. Cancer Prev. 20, 232–240. doi: 10.15430/JCP.2015.20.4.232
Kim K., Kwon H. A., Joo G., Choi Y. (2021). Development of a low-temperature thermal treatment process for the production of plant-growable media using petroleum-impacted dredged sediment. Sci. Total Environ. 776, 145917. doi: 10.1016/j.scitotenv.2021.145917
Kim K., Kwon H. A., Park J., Lee H., Choi Y. (2023). Thermal treatment of petroleum-contaminated marine sediment according to oxygen availability and temperature: Product quality as a potential plant-growth medium. Chemosphere 324, 138347. doi: 10.1016/j.chemosphere.2023.138347
Kim K., Yoon S., Kwon H. A., Choi Y. (2020). Effects of treatment agents during acid washing and pH neutralization on the fertility of heavy metal-impacted dredged marine sediment as plant-growing soil. Environ. pollut. 267, 115466. doi: 10.1016/j.envpol.2020.115466
Kong X., Wang X., Zhang Z., Sun A., Yang L., Zhang F., et al. (2024). Microscopic mechanism and road performance analysis of MgO carbonation–solidification of dredged sediment. Sustainability 16, 5097. doi: 10.3390/su16125097
Kurek M. R., Harir M., Shukle J. T., Schroth A. W., Schmitt-Kopplin P., Druschel G. K. (2020). Chemical fractionation of organic matter and organic phosphorus extractions from freshwater lake sediment. Anal. Chim. Acta 1130, 29–38. doi: 10.1016/j.aca.2020.07.013
Lang Q., Chen M., Guo Y., Liu Z., Gai C. (2019). Effect of hydrothermal carbonization on heavy metals in swine manure: Speciation, bioavailability and environmental risk. J. Environ. Manage. 234, 97–103. doi: 10.1016/j.jenvman.2018.12.073
Liu J. W., Wei K. H., Xu S. W., Cui J., Ma J., Xiao X. L., et al. (2021). Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 756, 144142. doi: 10.1016/j.scitotenv.2020.144142
Manzoor M., Gul I., Manzoor A., Kallerhoff J., Arshad M. (2021). Optimization of integrated phytoremediation system (IPS) for enhanced lead removal and restoration of soil microbial activities. Chemosphere 277, 130243. doi: 10.1016/j.chemosphere.2021.130243
Palma L. D., Mecozzi R. (2007). Heavy metals mobilization from harbor sediments using EDTA and citric acid as chelating agents. J. Hazard. Mater. 147, 768–775. doi: 10.1016/j.jhazmat.2007.01.072
Quan L., Duan K., Wei Z., Li W., Chen Y., Duan W., et al. (2023). Beneficial effects of arbuscular mycorrhizae on Cu detoxification in Mimosa pudica L. grown in Cu-polluted soils. Environ. Sci. pollut. R. 30, 25755–25763. doi: 10.1007/s11356-022-23919-4
Rinklebe J., Shaheen S. M., Yu K. (2016). Release of As, Ba, Cd, Cu, Pb and Sr under pre-definite redox conditions in different rice paddy soils originating from the U.S.A. and Asia. Geoderma 270, 21–32. doi: 10.1016/j.geoderma.2015.10.011
Su R., Li Z., Cheng F., Dai X., Wang H., Luo Y., et al. (2023). Advances in the degradation of emerging contaminants by persulfate oxidation technology. Water Air Soil Poll. 234, 754. doi: 10.1007/s11270-023-06770-2
Tang J., Wang M., Wang F., Sun Q., Zhou Q. (2011). Eco-toxicity of petroleum hydrocarbon contaminated soil. J. Environ. Sci. 23, 845–851. doi: 10.1016/S1001-0742(10)60517-7
Torres C. M., Taboada M. E., Graber T. A., Herreros O. O., Ghorbani Y., Watling H. R. (2015). The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 71, 139–145. doi: 10.1016/j.mineng.2014.11.008
Unda-Calvo J., Martinez-Santos M., Ruiz-Romera E. (2017). Chemical and physiological metal bioaccessibility assessment in surface bottom sediments from the Deba River urban catchment: Harmonization of PBET, TCLP and BCR sequential extraction methods. Ecotox. Environ. Safe. 138, 260–270. doi: 10.1016/j.ecoenv.2016.12.029
Urbancl D., Goricanec D., Simonic M. (2023). Zero-waste approach for heavy metals’ removal from water with an enhanced multi-stage hybrid treatment system. Materials 16, 1816. doi: 10.3390/ma16051816
Wang Y., Tang L., Liu L. (2021). Removal behaviors of Cu and Pb from heavy metal contaminated silts flushed by citric acid. Geomech. Eng. 26, 489–497. doi: 10.12989/gae.2021.26.5.489
Wang J., Wang S. (2018). Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 334, 1502–1517. doi: 10.1016/j.cej.2017.11.059
Wang S., Xu W., Yi P., Yuan J., Li M., Su F., et al. (2022). Transformation of heavy metals via sludge composite conditioning. ScienceAsia 48, 782–787. doi: 10.2306/scienceasia1513-1874.2022.120
Xiao R., Ali A., Wang P., Li R., Tian X., Zhang Z. (2019). Comparison of the feasibility of different washing solutions for combined soil washing and phytoremediation for the detoxification of cadmium (Cd) and zinc (Zn) in contaminated soil. Chemosphere 230, 510–518. doi: 10.1016/j.chemosphere.2019.05.121
Xu Q., Wu B. (2023). Recent progress on ex situ remediation technology and resource utilization for heavy metal contaminated sediment. Toxics 11, 207. doi: 10.3390/toxics11030207
Yan N., Zhong H., Brusseau M. L. (2019). The natural activation ability of subsurface media to promote in-situ chemical oxidation of 1,4-dioxane. Water Res. 149, 386–393. doi: 10.1016/j.watres.2018.11.028
Yen C. H., Chen K. F., Kao C. M., Liang S. H., Chen T. Y. (2011). Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: feasibility and comparison with common oxidants. J. Hazard. Mater. 186, 2097–2102. doi: 10.1016/j.jhazmat.2010.12.129
Yoo J., Jeon P., Tsang D. C. W., Kwon E. E., Baek K. (2018). Ferric-enhanced chemical remediation of dredged marine sediment contaminated by metals and petroleum hydrocarbons. Environ. pollut. 243, 87–93. doi: 10.1016/j.envpol.2018.08.044
Yoo J. C., Lee C., Lee J. S., Baek K. (2017). Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil Co-contaminated with petroleum and heavy metals. J. Environ. Manage. 186, 314–319. doi: 10.1016/j.jenvman.2016.03.016
Yoo J. C., Lee C. D., Yang J. S., Baek K. (2013). Extraction characteristics of heavy metals from marine sediments. Chem. Eng. J. 228, 688–699. doi: 10.1016/j.cej.2013.05.029
Yoo J. C., Shin Y. J., Kim E. J., Yang J. S., Baek K. (2016). Extraction mechanism of lead from shooting range soil by ferric salts. Process Saf. Environ. 103, 174–182. doi: 10.1016/j.psep.2016.07.002
Yoon N. K., Choi J., Shin W. S. (2018). A continuous process of persulfate oxidation and citric acid washing for the treatment of complex-contaminated soil containing total recoverable petroleum hydrocarbons and heavy metals. J. Environ. Sci. Int. 27, 1–10. doi: 10.5322/JESI.2018.27.1.1
Zhang Y., Labianca C., Chen L., Gisi S. D., Notarnicola M., Guo B., et al. (2021). Sustainable ex-situ remediation of contaminated sediment: A review. Environ. pollut. 287, 117333. doi: 10.1016/j.envpol.2021.117333
Zhang L., Liu Y., Wang J. (2023). Selective and effective oxidation of ammonium to dinitrogen in MgO/Na2SO3/K2S2O8 system. Chemosphere 325, 138401. doi: 10.1016/j.chemosphere.2023.138401
Zhang W., Lo I. (2006). EDTA-enhanced washing for remediation of Pb- and/or Zn-contaminated soils. J. Environ. Eng. 10, 1282–1288. doi: 10.1061/(ASCE)0733-9372(2006)132:10(1282)
Zhou Z., Liu X., Sun K., Lin C., Ma J., He M., et al. (2019). Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 372, 836–851. doi: 10.1016/j.cej.2019.04.213
Keywords: marine dredged sediment, co-contamination, chemical extraction, chemical oxidation, barley growth
Citation: Yang W, Liu J, Sun Y, Wang Z, Han C and Han J (2025) Evaluation of extraction-oxidation synergistic remediation of contaminated dredged sediment and plant suitability effects. Front. Mar. Sci. 12:1550274. doi: 10.3389/fmars.2025.1550274
Received: 23 December 2024; Accepted: 23 January 2025;
Published: 14 February 2025.
Edited by:
Lingshi Yin, Hunan Agricultural University, ChinaCopyright © 2025 Yang, Liu, Sun, Wang, Han and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenchao Yang, d2N5YW5nQG5tZW1jLm9yZy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.