- 1Saving the Blue, Davie, FL, United States
- 2Institute of Environment, Department of Biological Sciences, Florida International University, North Miami, FL, United States
- 3National Oceanic and Atmospheric Administration (NOAA) Fisheries, Office of International Affairs, Trade, and Commerce, Silver Spring, MD, United States
In light of global declines of upper-level marine predators, such as the great hammerhead, (Sphyrna mokarran) a thorough understanding of their behavioral ecology is needed for designing effective management strategies to preserve their key role in maintaining ecosystem functioning, stability, and resilience. Within the northwestern Atlantic, great hammerheads display regional connectivity between the U.S. East Coast and the western edge of The Bahamas, but despite the suggested importance of the Bahamian shark sanctuary towards regional population recovery strategies, relatively few data exist from other areas of The Bahamas. This study used fisheries-independent drumline captures, satellite telemetry, and bulk stable isotope analysis to advance our understanding of the residency, space use, and trophic role of great hammerheads in Andros, the largest island in The Bahamas. We examined movement behaviors and thermal range within the Bahamian Exclusive Economic Zone, and constructed Bayesian mixing models based on carbon, nitrogen, and sulfur isotope ratios to estimate the importance of prey species in the diet of great hammerheads. Our data revealed year-round residency of Andros-caught great hammerheads in Bahamian waters with site-fidelity to and high use of habitats along the reef-drop off and flats of Andros. Great hammerheads predominantly fed on barracuda and small-bodied elasmobranchs in Andros connecting food webs from the pelagic zone to the shoreline. This study expands our knowledge of the ecology of great hammerheads in the northwestern Atlantic and shows that, despite their highly-mobile nature, some individuals reside in the Bahamas year round. These findings suggest the Bahamian shark sanctuary could be more than just a seasonal refuge for this species as previously proposed, and merit further research to assess the conservation value of the sanctuary towards regional rebuilding goals for greathammerheads.
1 Introduction
The great hammerhead (Sphyrna mokarran) is a large-bodied shark primarily found in coastal-pelagic waters across its circumtropical range (Ebert et al., 2021). The species uses deep waters, coral reefs, and shallow coastlines and is considered highly mobile, with long-distance return migrations of 1500 to 3000 km documented in the south Pacific and northwestern Atlantic (NWA), respectively (Guttridge et al., 2017; Lubitz et al., 2023). Adult great hammerheads are apex predators, mainly feeding on rays and other sharks (Cliff, 1995; Chapman and Gruber, 2002; Mourier et al., 2013; Raoult et al., 2019) and likely play a key ecological role in ecosystem maintenance, stability, and function (Roff et al., 2016). Like other large coastal sharks, great hammerheads have conservative life history traits, including slow growth, late maturation [7-10 years], biennial reproduction, and a long gestation period [~11 months] with low fecundity ([average litter size 15]; Piercy et al., 2010; Harry et al., 2011; Miller et al., 2014; SEDAR, 2024). They are the largest species in the hammerhead family, Sphyrnidae, reaching a maximum of 6.1m in length with a distinctive tall, sickle-shaped dorsal fin (Compagno, 1984). Their fins are highly valued in the shark fin trade, and they are a target or bycatch species in multiple fisheries around the world (Cardeñosa et al., 2018). As a result, dramatic population declines have occurred in many regions, and these have contributed to the species being assessed as globally critically endangered on IUCN Red List (Rigby et al., 2019; Sherley et al., 2020) and listed on Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals (CMS, 2019). Global population reduction was recently estimated at >80% over the last 3 generations (Sherley et al., 2020) and genomic analysis of great hammerheads revealed low genetic variation and inbreeding (Stanhope et al., 2023). As such, understanding the distribution and movement patterns of great hammerheads is important for improving conservation and management efforts in order to assess current and future risk.
Our knowledge of great hammerheads’ movement ecology has expanded considerably across the last decade with studies documenting philopatric behavior, namely site fidelity to discrete reefs, atolls, and shallow coastlines (Guttridge et al., 2017; Heim et al., 2021a; Boube et al., 2023; Casselberry et al., 2024). In the Tuamotu archipelago of French Polynesia, 32 individuals returned to the same atoll for up to 12 years between the first and last sighting, with limited movements to other atolls in the region (Boube et al., 2023). Great hammerheads tracked in the Great Barrier Reef Marine Park in Australia showed comparatively small home ranges, hypothesized to be a result of the area’s productive coastal habitats and year-round availability of batoid prey (Lubitz et al., 2023). Similarly, great hammerheads monitored in the Florida Keys (FL, U.S.A.) exhibited “stopover” behavior, in response to predictable, seasonal spawning aggregations of Atlantic tarpon (Megalops atlanticus) and permit (Trachinotus falcatus) (Lowerre-Barbieri et al., 2021; Griffin et al., 2022; Casselberry et al., 2024). However, this behavior was preceded or followed by sharks transiting through migratory corridors at reef tracts and seamounts along the U.S. East Coast or Gulf of Mexico (GOM), highlighting their transiting nature in some regions (Lowerre-Barbieri et al., 2021). Taken together these findings highlight the complexity and variability of great hammerheads’ movement behavior across their range and long-term monitoring of individuals tagged in diverse habitats and regions.
Among the most well studied populations of great hammerheads, movement data from the NWA shows connectivity between the western Bahamas and U.S. East Coast (Graham et al., 2016; Guttridge et al., 2017, 2022) suggesting that the species should be managed as one stock across the U.S. Atlantic, GOM, and nearby waters (SEDAR, 2024). However, information from the Bahamas is limited to select tagging sites, predominantly Bimini, the most western island in the Bahamas archipelago, which lies <100 km from Florida and is separated by the Gulf Stream (Graham et al., 2016; Guttridge et al., 2017). Adult great hammerheads of both sexes overwinter in the Bimini Islands, with some having been recorded for 10 consecutive years (Guttridge et al., 2017; Heim et al., 2024). Pregnant females migrated from Bimini to South Carolina (U.S.A.), and north of Tampa (FL, U.S.A.), in late spring, providing evidence of regional connectivity and likely parturition off the U.S. East Coast and GOM (Heim et al., 2021b; Guttridge et al., 2022). Indeed, juveniles or young-of-the-year great hammerheads have been documented in Florida (Hueter and Tyminski, 2007; Macdonald et al., 2021; SEDAR, 2024) and South Carolina (Barker et al., 2017), as well as other locations across the GOM (McCandless et al., 2002). Comparatively, there is limited information available on great hammerheads during the summer months in the Bahamas. One female and one male were detected on acoustic receivers sporadically throughout the wet season (May-October) in Bimini (van Zinnicq Bergmann et al., 2022; Heim et al., 2024), however dedicated great hammerhead shark dive operations close during this time due to the general absence of great hammerheads from the local tourism-related feeding site (Heim et al., 2021a, 2024). It is unknown whether great hammerheads’ seeming absence from Bimini’s waters in the wet season is representative of the rest of the Bahamas, or if there are island-specific differences in behavior among great hammerheads. All sharks are protected by the Bahamas shark sanctuary (Haas et al., 2017), thus understanding great hammerheads’ propensity to leave the Bahamas is consequential for their conservation and management in the region.
Here we expand on our understanding of great hammerheads’ space and resource use in The Bahamas by using opportunistic encounter records and captures in drumlines alongside satellite telemetry to investigate residency, site fidelity, and horizontal movements, and bulk stable isotope analysis to explore trophic links to prey species. Our objectives were to 1) assess the movement of tagged sharks between the United States and The Bahamas, 2) quantify the residency of sharks within the Bahamian EEZ and subsequently, the shark sanctuary, and 3) understand the functional role of great hammerheads in coastal food webs. These data collectively advance our understanding of the spatiotemporal ecology and resource use of great hammerheads with important implications for the conservation and management of the species.
2 Materials and methods
2.1 Research permits
Research conducted for this study in Andros Island, The Bahamas, was carried out under permits issued by the Department of Marine Resources and the Department of Environmental Planning and Protection of The Bahamas. CITES export permits were issued by the Department of Agriculture, The Bahamas (See Supplementary Table S1).
2.2 Study site
Andros Island is the largest island (5,957 km2) in The Bahamas; it supports vast pristine ecosystems (Casola et al., 2021), including extensive shallow mud and seagrass flats connected to a matrix of tidal creeks bordered by mangroves. Along the eastern edge of the island is a fringing coral reef sloping to a deep pelagic zone referred to as the Tongue of the Ocean (Figure 1). The island’s habitats host biodiverse and productive populations of game fish (e.g. Atlantic Tarpon [M. atlanticus] and Permit [T. falcatus]; Smith et al., 2023), sharks and rays (e.g. lemon [Negaprion brevirostris]; Postaire et al., 2022; Caribbean reef [Carcharhinus perezi]; Talwar et al., 2022; and silky [C. falciformis]; Shipley et al., 2023), turtles (e.g. green [Chelonia mydas] and loggerhead [Caretta caretta]; Carr et al., 1982) as well as other large marine mammals (e.g. beaked whales [Mesoplodon densirostris]; Hazen et al., 2011). Little published information is available regarding great hammerheads’ use of Andros’ waters, however one was observed attempting to predate a N. brevirostris in South Andros (Roemer et al., 2016) and another was noted to have swam from Jupiter, Florida to the eastern shoreline of central Andros (Guttridge et al., 2017).
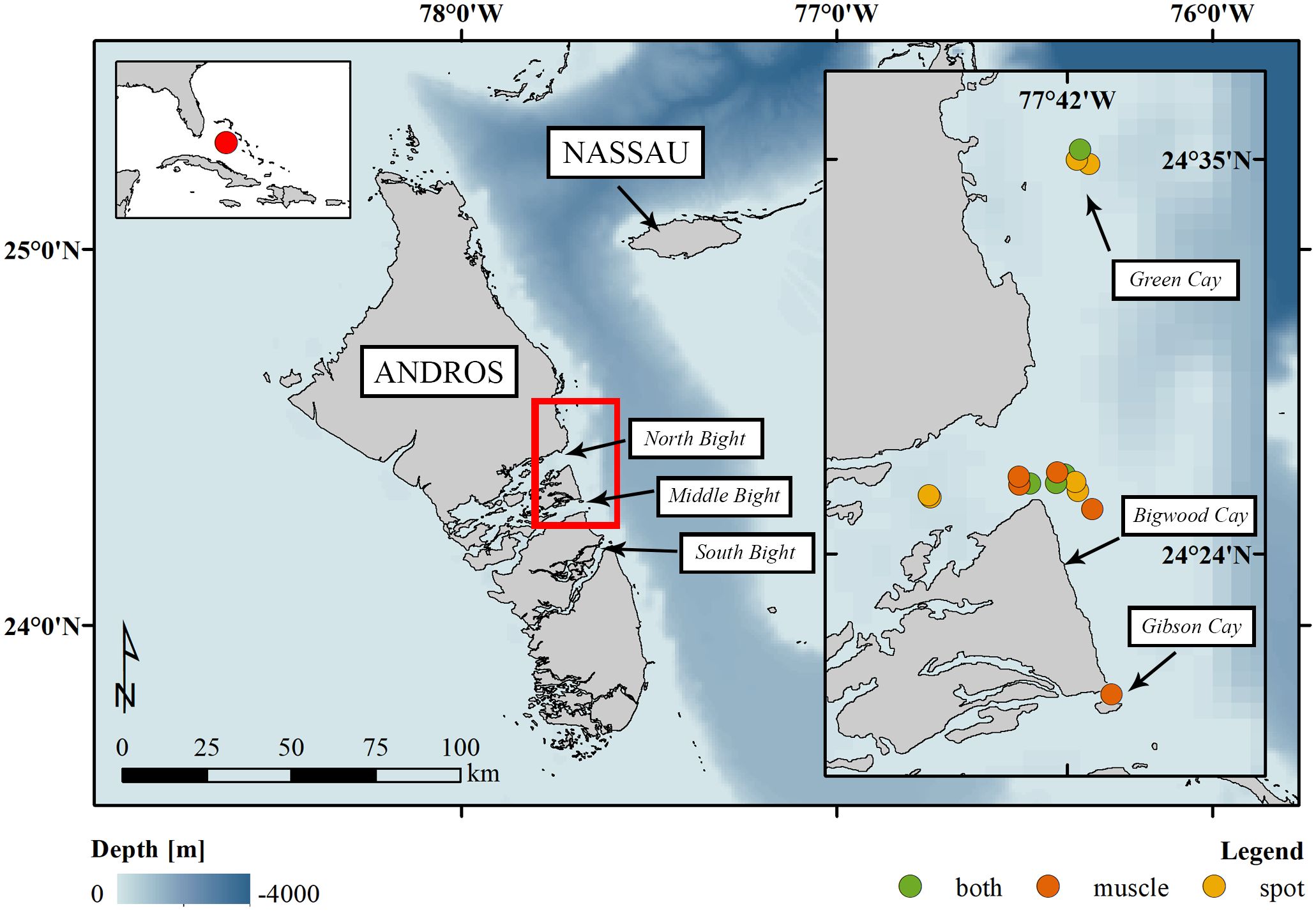
Figure 1. Study site - Andros Island. The small inset in the top-left corner shows the location of Andros within the northwestern Atlantic (NWA). The far-right inset details the area of the highlighted rectangle, which contains the locations where great hammerheads, Sphyrna mokarran, were tagged with Smart Position and Temperature (SPOT) tags and/or sampled for white muscle tissue. Cays and geographical features that are mentioned in the text are labeled. Bathymetry map from Ryan et al. (2009).
2.3 Sightings
This study incorporates opportunistic great hammerhead sightings recorded by scientists and members of the public. For each encounter, reporters were asked to provide details including date, exact location (GPS coordinates), water depth (nearest meter) and any other environmental information (habitat variables were estimated during public encounters and may be susceptible to error). Where possible, photographic evidence or video footage was provided for species confirmation, individual identification (via fin notches, ventral patterning: see Guttridge et al., 2017) and in some cases sex determination and size estimates (nearest 0.5 m).
2.4 Captures and satellite tracking
Great hammerheads were captured via single-hook drumlines between 13 March 2020 and 11 June 2024 (Supplementary Figure S1A). Based on expert knowledge of the study site, sampling locations were selected along the drop-off and flats of Eastern Andros to represent the diversity of nearshore habitats available to sharks in the area, including great hammerheads. Each rig consisted of a 20kg cement block connected to a surface buoy, to which a ca. 5m, 3.5mm monofilament leader was connected. The monofilament leader terminated with a single 18/0 non-offset circle hook baited with Atlantic bonito (Sarda sarda) or great barracuda (Sphyraena barracuda) for 45 - 60 mins undisturbed, unless there was indication of a capture (buoy movement), and re-baited as necessary. One individual S. mokarran was captured via polyball float fishing in 130 m (see Guttridge et al., 2017 for method).
Great hammerheads are recognized for their sensitivity to capture-related stress resulting in high at-vessel and post-release mortality (Morgan and Burgess, 2007; Gallagher et al., 2014). Individuals were therefore processed immediately upon capture. Sharks were temporarily restrained alongside the research vessel, sexed based on the absence or presence of claspers (i.e. external reproductive organs of males), and measured to the nearest cm (pre-caudal (LPC), fork (LF) and stretch total length (LSTL). A small (5mm) muscle tissue biopsy was collected below the base of the first dorsal fin for stable isotope analysis (see below). Muscle samples were kept on ice until frozen at -20°C upon return to shore.
We used visual indicators such as fighting intensity, reflexes, and changes in skin coloration (Charbeneau, 2004) to decide if caught individuals were in suitably good condition to be tagged; if so, they were tagged with fin-mounted smart position and temperature satellite tags (SPOT 6 Wildlife Computers, Redmond, WA, USA; Models SPOT 380B) with estimated battery life of 180 days, a 30 s repetition rate, and 250 daily location uplink limit. Sharks deemed unfit for tagging were released. SPOT tags were affixed to the leading edge of the first dorsal fin via two polyurethane bolts secured using rubber and stainless steel washers and stainless steel screws (see Heim et al., 2022, 2023 for attachment details). We enabled the time-at-temperature (TAT) histograms on the SPOT tags, so that they recorded the relative amount of time each shark spent within predefined temperature bins during the tracking period (see Supplementary Material). We adhered to a < 15 minute time limit from bite to release in order to minimize risk of post-release mortality.
SPOT tags attempt direct communication with orbiting Argos satellites each time the first dorsal fin of a great hammerhead, and thus the affixed tag, emerges from the water. Successful transmissions to the satellite are processed and used to calculate location estimates via the Doppler Effect on the frequency between two subsequent transmissions using a Kalman filtering algorithm (CLS, 2016). The accuracy of location estimates is represented by the radius, the length of the semi major and minor axes, and the orientation of the position error ellipse. In addition, location classes (LCs) are produced that are based on the number of transmissions the satellite received and represent an estimated error ranging from < 250 m (LC 3), 250 - 500 m (LC2), 500 - 1500 m (LC1) and > 1500 m (LC 0). LCs A and B represent locations without associated error estimations and LC Z represent invalid locations1. Prior to deployment all SPOT tags were painted with an anti-biofouling paint (Propspeed® Clear Coat, Propspeed International, Auckland, New Zealand) and were initiated on land no more than 4 days prior to deployment to guarantee location accuracy.
2.5 Stable isotope analysis
Collected muscle samples were analyzed for carbon, nitrogen and sulfur isotope ratios to examine the trophic ecology of great hammerheads caught in Andros. Samples were kept frozen until dried at 60°C, homogenized, and urea extracted following Li et al. (2016). Samples were analyzed for δ13C, δ15N, and δ34S with industry standards (ATM-N, VPDB, CVDT) at the Stable Isotope Core Laboratory of Washington State University, with precision ≤ 0.25‰ for δ13C and δ15N, and ≤ 0.50‰ for δ34S.
Because of the wide-ranging nature of great hammerheads and their propensity to use both shallow and deep nearshore habitats, we sampled potential prey species in seagrass beds, coral reefs, and pelagic waters of central eastern Andros (Supplementary Figure S1B). Muscle tissue was collected from blacknose sharks (Carcharhinus acronotus; n = 4), blacktip sharks (C. limbatus; n = 2), Caribbean reef sharks (n = 5), lemon sharks (n = 5) and silky sharks (n = 5). These were caught via the drumline method described above or polyball float fishing (described elsewhere; Shipley et al., 2023). Barracuda (S. barracuda; n = 5) were sampled from local fishers and southern stingrays (Hypanus americanus; n = 4) via dipnet captures in the shallow (<1 m) sand flats (see Schwanck et al., 2020 for capture description). All sharks considered potential prey were < 150 cm TL. The sampled barracuda and southern stingrays were < 90 cm TL and < 100 cm disc width, respectively. Prey muscle samples were processed as described for great hammerheads above.
2.6 Data analysis
All analyses were completed using the R statistical environment (version 4.2.2.; R Core Team, 2022). We chose standard deviation (SD) as our measure of variance and averaged values are written as mean ± 1 SD. Where described, seasonal comparisons were performed between the wet (May to October) and dry (November to April) season.
2.6.1 Captures
Capture data from drumlines were quantified as the number of great hammerheads caught per hook-hour for months in which at least three days of standardized sampling was conducted, with effort (soak time) adjusted for empty hooks (half effort to account for bait loss; Heithaus et al., 2007).
2.6.2 Satellite tracking
2.6.2.1 Raw data processing and filtering
We downloaded the raw movement data from deployed SPOT tags directly from the online data portal of the manufacturer. All accompanying code is available online (https://github.com/SimonDedman/SavingTheBlue/blob/main/R/). While two SPOT tags were actively transmitting data at the time of writing this manuscript, we only included movement data until 15 September 2024 in our analyses. Following the removal of all invalid (‘LC Z’) location estimates (i.e. locations that do not pass at least two of Argos’ plausibility tests: minimum residual error, transmission frequency continuity (CLS, 2016) we used the argosfilter R package (version 0.70; Freitas et al., 2008) to apply a speed-distance angle filter to discard biologically unrealistic location estimates. Location estimates that were > 5 km apart and would have required an average swimming speed of > 2.1 m/s were removed, same as any location estimates that were > 5 and > 8 km apart and presented internal turning angles of < 15 and < 25° degree, respectively as suggested by Vaudo et al. (2017). We accounted for the location error of Kalman-filtered raw data (Boyd and Brightsmith, 2013) and calculated the most probable locations at the initial observation times by fitting a continuous-time correlated random walk (CTCRW) model within a state-space model to the filtered movement data using the aniMotum R package (version 1.1-06; Jonsen et al., 2023). Additionally, and to avoid the introduction of a spatiotemporal bias in later analyses (see below) due to the SPOT tags’ characteristic to transmit data whenever they are exposed to air (i.e. when sharks surface; Lea et al., 2015), we normalized movement tracks at 12 hour time intervals (~80% of time gaps between two subsequent locations across all tagged individuals <= 12 h) by predicting the two most probable locations per day using the CTCRW model. Extended periods of time between location estimates and short tracking durations can diminish the accuracy of location predictions in CTCRW models (Logan et al., 2020; Vaudo et al., 2017). Consequently, we split individual movement tracks into segments if they contained subsequent location estimates that were > 20 days apart followed by the removal of track segments of < 20 days or less than 12 total location estimates prior to predicting location estimates at fixed time intervals. We re-routed fitted and predicted CTCRW model outputs to prevent movement across land and for each location estimate we calculated a 95% confidence interval. Finally, segments were combined per individual for later analyses (see below).
To understand the importance of the Bahamas Shark Sanctuary, i.e. the Exclusive Economic Zone (EEZ) of The Bahamas to tagged great hammerheads, we used the most probable locations fitted at original observation times to calculate the percentage of days, (i.e. the nr. days within the EEZ boundaries divided by the total nr. of days with location estimates) each shark spent within and outside the EEZ boundaries during the wet and dry season, and throughout the year, using indexing after converting fitted locations to spatial data using st_as_sf from the sf R package (version 1.0-14; Pebesma and Bivand, 2023; Pebesma, 2018) followed by conversion to percentages.
2.6.2.2 Movement states
We identified different movement states, i.e. behaviors, along the movement tracks via a clustering analysis using the k-means procedure based on step length and turning angles between the predicted locations using code by van Moorter et al. (2010), customized by co-author SD. Step lengths, i.e. the distance between two consecutive locations, and turning angles in relative degrees (0 - 180) were calculated using the amt R package (version 0.2.1.0; Signer et al., 2019). Following conversion from km to number of body lengths using LSTL and log-transformation to eliminate skewness and kurtosis, step lengths were standardized on their range (Steinley, 2004, 2006). Similarly, the absolute values of the relative degrees of the turning angles were log-transformed and standardized on their range as well. The number of clusters was calculated for each shark and we assessed the best fitting number of clusters using the gap statistic with tolerance levels 1 and 2 (Tibshirani et al., 2001; van Moorter et al., 2010). Once known, the spatiotemporal patterns of these clusters, i.e. movement behaviors, were assessed by shark, and overall, by summarizing the proportion of displayed behaviors within 0.5*0.5° cells, to look for grids with increased likelihood of residency and transiting behavior by season. Conversion to geographic coordinates resulted in 1.21 km2 (0.0001°2) pixels in the output plots.
2.6.2.3 Space use estimates
We fitted dynamic Brownian Bridge movement models (dBBMMs; Kranstauber et al., 2012) to the predicted locations to explore the space use of tagged great hammerheads. The dBBMMs were fitted with a sliding window size of 23 within a 1 km raster resolution, and the time threshold of relocation positions above which to break a track into separate tracks, was set to 13 hours to retain the segmented track data structure across an individual track. Location errors were calculated using the moveLocError function from the movegroup R package (version 24.03.05; Dedman and van Zinnicq Bergmann, 2024). Following van Zinnicq Bergmann et al. (2022) the resulting individual-level utilization distributions (UDs; the spatiotemporal probability of an animals presence within an area; van Winkle, 1975) were scaled, summed, and re-scaled to produce group-level UDs. Individual- and group-level general and core space use areas were estimated by calculating the 95% and 50% UDs, respectively. All UD calculations were performed using the movegroup R package (version 24.03.05; Dedman and van Zinnicq Bergmann, 2024).
2.6.2.4 Thermal range
Temperature ranges of tagged great hammerheads were explored by averaging the time spent in each temperature bin of TAT histograms across all individuals during the wet and dry season, then histograms were compared qualitatively.
2.6.3 Analysis of stable isotope ratios
Bayesian mixing models were used to assess the importance of potential prey species in the diet of each great hammerhead and collectively using the MixSIAR R package (version 3.1.12; Stock et al., 2018). Uninformative (i.e. default) priors were employed, and discrimination values of Δ13C = 0.895‰, Δ15N = 2.250‰, and Δ34S = 0.641‰ were used (Hesslein et al., 1993; McCutchan et al., 2003; Hussey et al., 2010).
3 Results
3.1 Encounters and captures
From June 2018 - June 2024, 78 great hammerheads were encountered (n = 56) or captured (n = 22) in the study area (Supplementary Figures S1, S2; Supplementary Table S2) ranging from ca. 1.25 m LSTL to ca. 4.75 m LSTL (Supplementary Figure S3). Most great hammerheads were measured or estimated to be 2.5-3.5 m LSTL (86%) and encountered in Jan-Mar (51%). Although there were a notable number of sharks encountered in June and July (25%). Two individuals were re-sighted using fin notches and markings (see Guttridge et al., 2017 for methodology), one female was encountered across a 30-day period in the same location and another male was sighted two years apart (April 2019 - February 2021) in the same location. One encounter on 30 July 2023 was of two individuals (~3 m and ~4 m length, sex unknown) observed slow swimming on their sides, in close contact with each other for ~ 5 mins. They were moving at 12 m depth along patch reef close to the substrate.
Among great hammerheads that were captured via drumlines (n = 21, 10 females, 7 males, 4 unknown sex), catch per unit effort (CPUE; sharks per hook hour) was greatest in February (0.034 sharks hook hr-1) and May (0.031 sharks hook hr-1), and lowest in January (0.005 sharks hook hr-1) and July (0.007 sharks hook hr-1), with considerable variability across months and years (Supplementary Figure S4). During 10 of the 21 captures, great hammerheads were equipped with fin-mounted SPOT tags (8 individuals, 6 females, 2 males, Table 1) and 9 individuals were sampled for muscle tissue (Table 1). Two individuals were tagged twice (PTTs: 200369/244607; 200368/222133, Table 1) upon re-capture 1205 and 671 days later, respectively. In both sharks the first SPOT tag was completely shed from the dorsal fin (Heim et al., 2023). One of the male sharks (PTT: 183623) was tagged with a SPOT tag March 2020 and recaptured almost 4 years to the day in the same location.
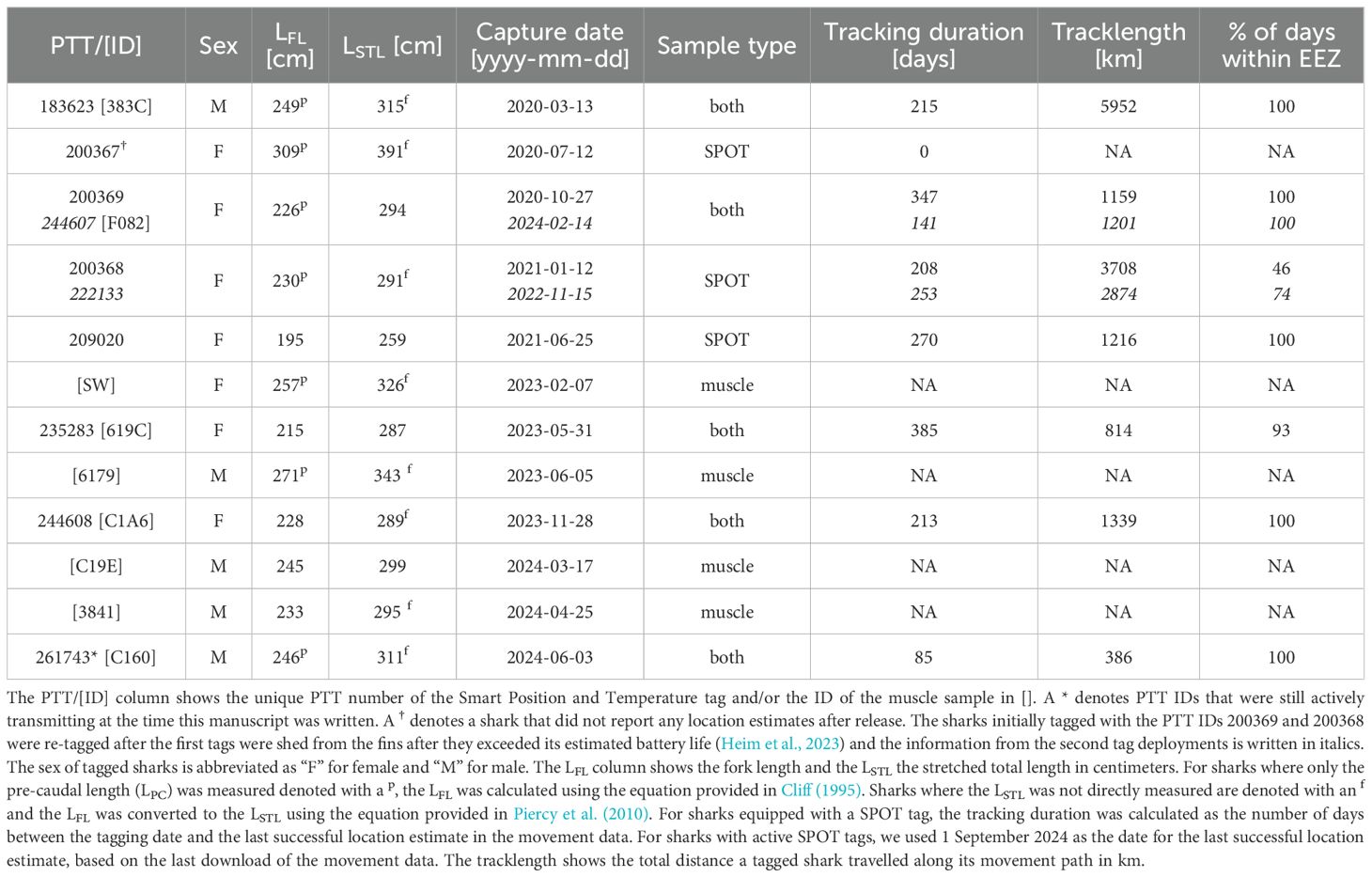
Table 1. Metadata of great hammerheads tagged with fin-mounted Smart Position and Temperature (SPOT) tags and/or sampled for muscle tissue.
3.2 Satellite tracking
All but one (PTT: 200367, Table 1) reported location estimates, yielding data from 9 SPOT tags from 7 sharks (two recaptures, PTTs: 200369/244607; 200368/222133, Table 1). The mean length of tagged individuals was 302 ± 35 cm LSTL (mean ± SD, range: 259 - 391 cm, Table 1) and based on published size-at-maturity values (Piercy et al., 2010), all individuals but one (PTT: 209020, Table 1) were sexually mature. Individuals that reported location estimates were tracked for 235 ± 93 days (range: 85 – 385 days, Table 1) and across traveled distances ranged from 853 - 3729 km (1730 ± 1120 km) in females, and 386 - 5902 km (3144 ± 3900 km) in males (Table 1).
Following the removal of invalid locations and the application of the speed-angle-distance filter (Freitas et al., 2008), we used 3128 locations (Figure 2) to fit the CTCRW models predicting 2938 bi-daily locations (Supplementary Figure S5). Movement tracks were generally restricted towards the east side of Andros and adjacent deep waters as well as towards neighboring islands in The Bahamas (e.g. Abaco [PTT: 183623] and Great Exuma [PTT: 244607], Figure 2). One individual (PTT: 200368/222133) displayed repeated movement into the GOM by departing from Andros in April during both tracking periods, subsequently crossing the Gulf Stream and the Florida Keys and moving North along Florida’s West Coast towards Apalachee Bay (FL, U.S.A.) in June and August, respectively. One individual (PTT: 235283) traveled North into offshore habitats of Florida’s East Coast (Figure 2).
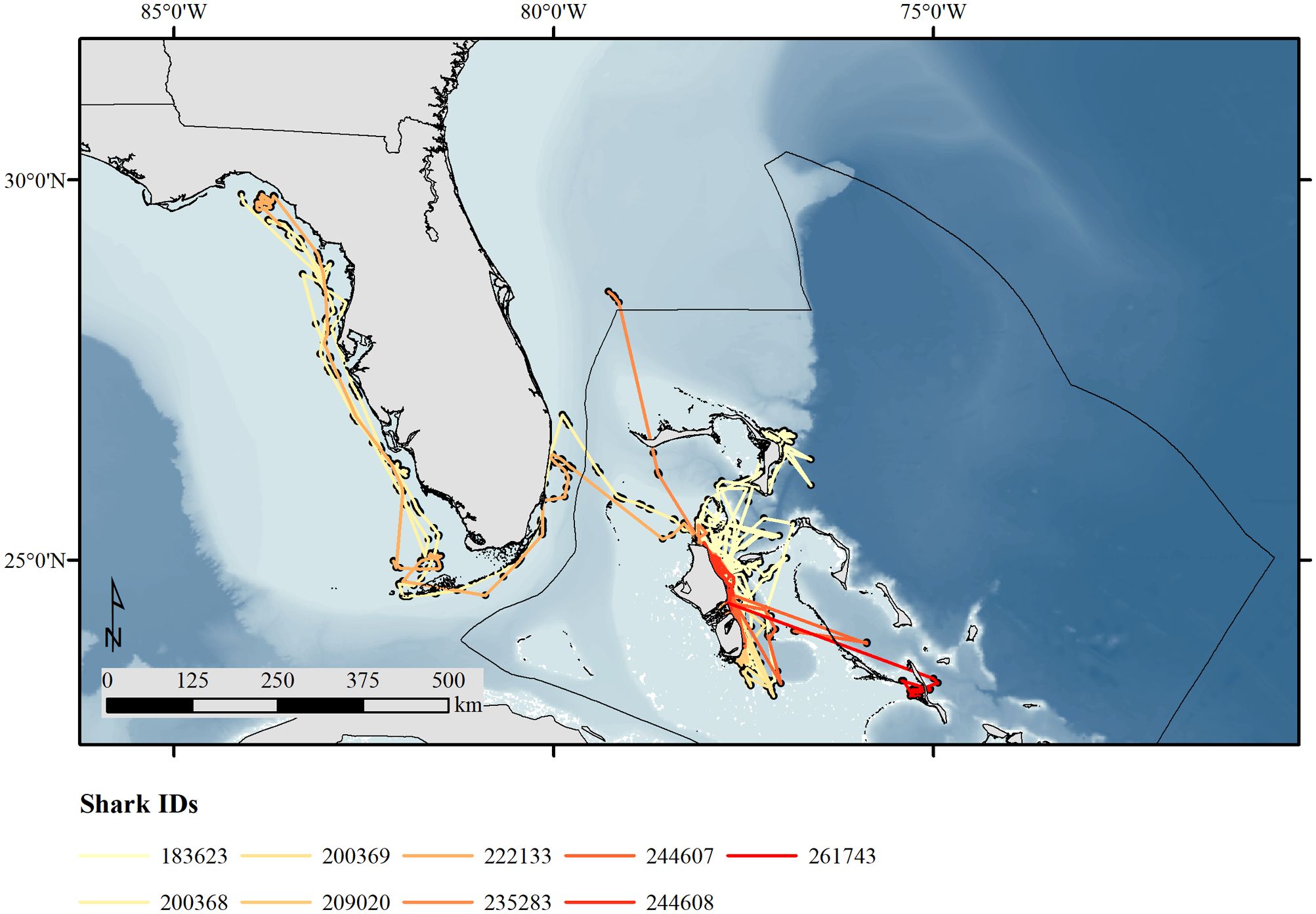
Figure 2. Most probable locations of great hammerheads, Sphyrna mokarran, tagged with Smart Position and Temperature (SPOT) tags. The corresponding movement paths are colored by the unique PTT, i.e. Shark ID, of each tag. The Bahamas Exclusive Economy Zone (EEZ) is shown as black line. Bathymetry map from Ryan et al. (2009).
Correspondingly, only two individuals (PTT: 200368/222133 and 235283) across three occasions left the Bahamian EEZ (Supplementary Figure S6) whereas all other sharks spent 100% of days across their tracking durations of 85 - 347 days within the EEZ boundaries (Table 1). On average, sharks spent 75.6 ± 40.9% and 96.5 ± 8.3% of their days within the Bahamian EEZ during the wet and dry season, respectively (Supplementary Table S3). One of the sharks (PTT: 200368/222133) that was tagged on two occasions, spent both wet seasons during her tracking periods entirely outside of the EEZ boundaries (Supplementary Table S3).
3.2.1 Movement states
Analysis of k-means clusters showed that two, one or three movement behavior clusters best fit the data, with two outperforming one and three (Supplementary Figure S7). This is the typical result for spatial and tracking data, where resident behavior is characterized by shorter step lengths and greater turning angles, and transiting behavior is characterized by longer step lengths and narrower turning angles (Table 2; Supplementary Figure S8).
Generally, we identified both movement states for all sharks along the east coast of Andros, where resident and transiting behaviors were displayed equally between north and central Andros (Supplementary Figures S9, S10). However, once sharks moved south or past the northern tip of Andros, transiting movement behaviors became more frequent. We found a pattern of resident movement behaviors being displayed less frequently in the deep waters to the east of Andros but an increase in these behaviors once sharks moved to a neighboring island and/or shallower waters. Shark PTT ID 200368/222133 that left the Bahamas EEZ displayed mainly transiting movement behaviors on her path across the Gulf Stream and along Florida’s west coast with a temporary increase in resident behaviors in the Florida Keys and off Crystal River and Steinhatchee (FL, U.S.A., Supplementary Figures S9, S10B, E).
3.2.2 Space use estimates
Group-level UDs across the entire study period revealed core use (50%) UD areas of 400 km2 exclusively within Bahamian waters east of north and central Andros (Figure 3A). Much of the total general use (95%) UD area (6300 km2) surrounds the core area (Figures 3A–C). However, there are several small pockets of increased space use, proliferating off the southern tip of Andros, Marsh Harbor (Great Abaco, The Bahamas) and west of Crystal River (FL, U.S.A.) in the GOM (Figure 3A). Seasonally, we found that group-level UDs during the dry season (general space use area: 6300 km2; core space use area: 400 km2) were limited to Bahamian waters with very limited space use in areas other than along the east coast of Andros (Figure 3C), while the group-level UDs during the wet season (general space use area: 6900 km2; core space use area: 400 km2) included some areas outside of the Bahamian EEZ in Florida waters (Figure 3B). The tight area of core use suggests that – while locations may be somewhat biased by this being the site of our tagging operations – these large species, capable of long-distance migration, are genuinely residing within this small area of tropical island coastal shallows.
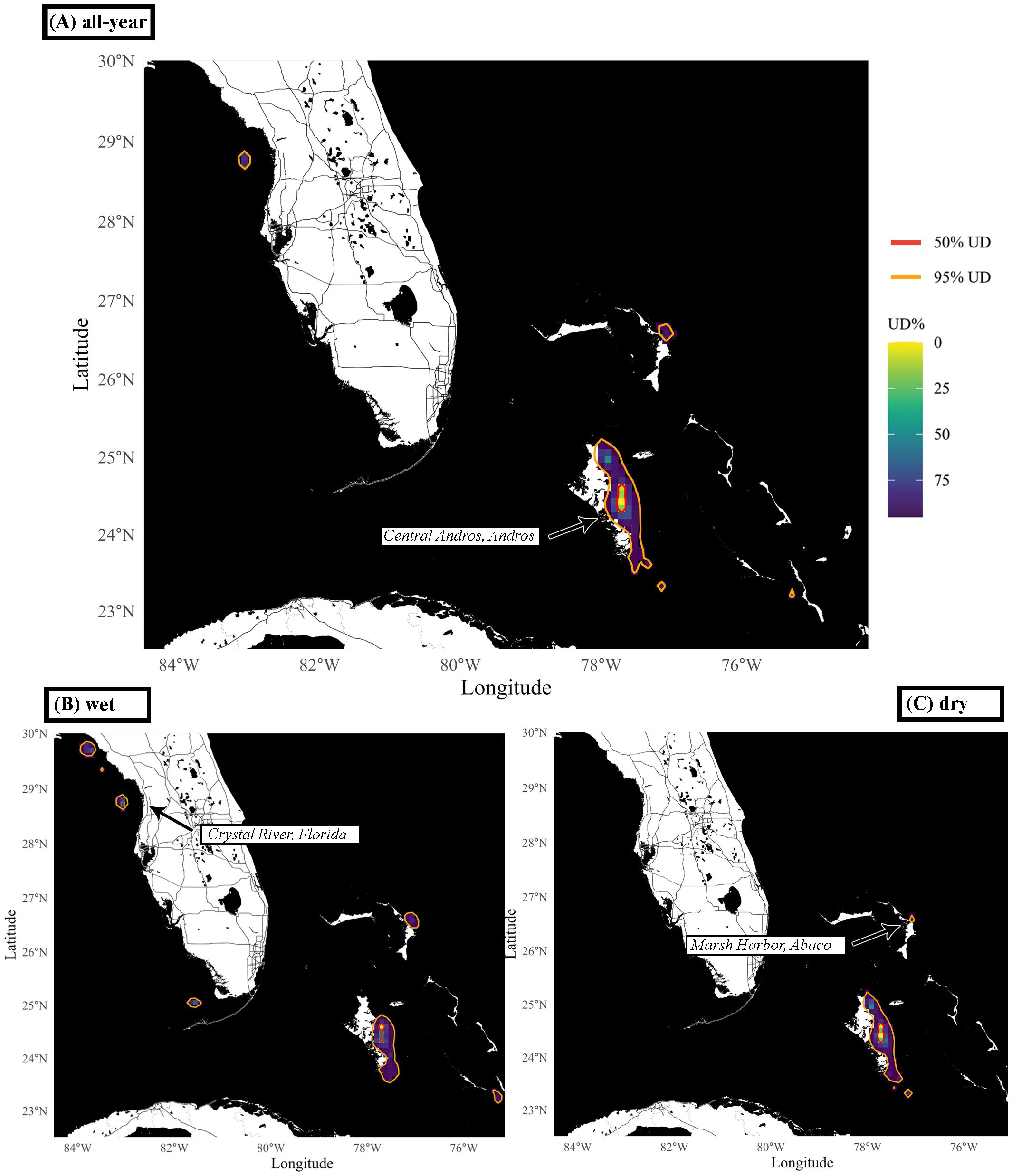
Figure 3. Group-level utilization distributions (UDs) of great hammerheads, Sphyrna mokarran, across the (A) entire study period, (B) wet and (C) dry season. UDs were calculated using dynamic Brownian bridge movement models (dBBMM) and the UD surface is displayed on a continuous scale. The contours of the 95% (general use, orange) and 50% (core use, red) UDs are shown. Cities and/or areas referenced in the Results are labeled within the figure panels.
Individual sharks have differential space use (Supplementary Figure S11; Supplementary Table S4), although during the dry season all individuals displayed general space use areas along the entire or parts of the eastern coast of Andros, including waters off central Andros (Supplementary Figure S11). But we also found additional pockets of general space use areas in The Bahamas off Great Guana Cay, east of Abaco and at the southwestern tip and along the western drop off of the Tongue of the Ocean during the dry season. The general space use area sizes of individual-level UDs during the dry season ranged from 1100 - 11 100 km2 (5052 ± 3793 km2, Supplementary Table S4). The core use of individuals during the dry season was mostly focused towards the eastern entrance of the north and middle bights, a direct passage connecting the west and east side of Andros, with additional core space use areas off the northeastern and southern tips of Andros and off Abaco (Supplementary Figure S11). The core space use area sizes of individual-level UDs during the dry season ranged from 100 - 800 km2 (350 ± 239 km2, Supplementary Table S4). During the wet season individual-level UDs showed a change of general and core space use areas into waters of the GOM (PTT: 200368/222133), north of the Florida Keys, as far as the waters in proximity of Crystal River and Steinhatchee (Supplementary Figures S11B, E). Individuals remaining within the Bahamian EEZ during the wet season displayed similar general and core space use patterns as during dry season with individuals spending much of their time in east Andros, but with core space use areas being less focused on the west entrance of the north and middle bights (Supplementary Figures S11A, C, D, F–I). The general space use of individual-level UDs during the wet season ranged from 900 - 14 500 km2 (5186 ± 5193 km2, Supplementary Table S4). The core space use of individual-level UDs during the wet season ranged from 100 - 600 km2 (329 ± 214 km2, Supplementary Table S4), Other than north bight (central Andros), the individuals do not share areas of high space use.
3.2.3 Thermal range
When assessed across all individuals throughout the year we found sharks generally occupied habitats with temperatures between 25 - 26°C (42.4 ± 34.3%, Figure 4; Supplementary Table S5), but experienced warmer temperatures between 28 - 30°C (45.4 ± 35.3%, Figure 4, Supplementary Table S5) during the wet season. Overall sharks spent more time in temperatures from 30 - 34°C (33.0 ± 36.7%, Figure 4; Supplementary Table S5) during the wet season compared to the dry season which is reflective of increased water temperature of the same habitats across time (0.1 ± 1.8%, Figure 4; Supplementary Table S5).
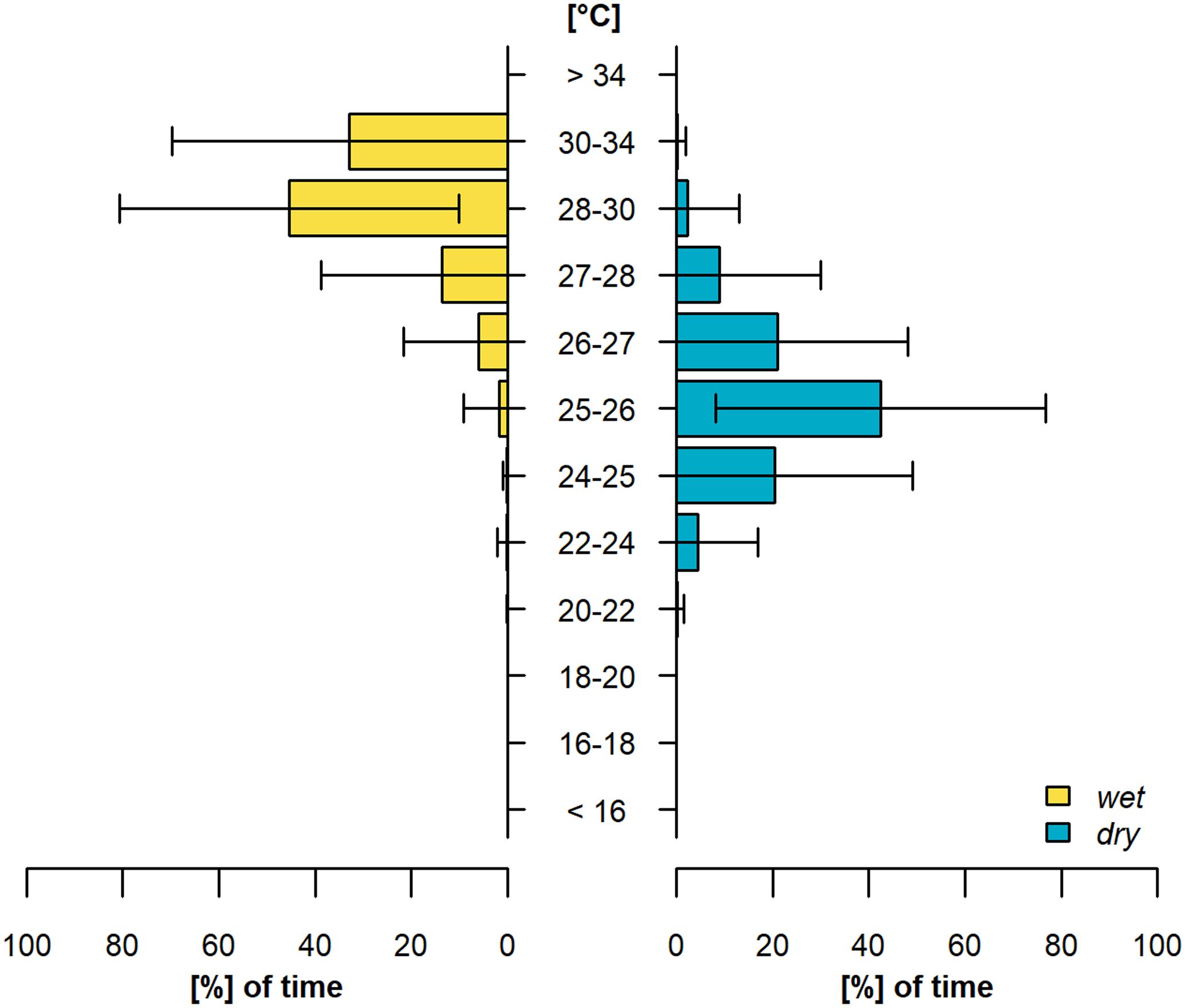
Figure 4. Seasonal time-at-temperature histograms across all tagged great hammerheads, Sphyrna mokarran, for the wet (left side) and dry (right side) season. The horizontal lines represent the standard deviation within each temperature bin.
When assessed at the individual-level we found a similar pattern of temperatures between 25 - 26°C during the dry season (Supplementary Figure S12; Supplementary Table S5) and between 27 - 30°C during the wet season. Additionally, we found that some sharks showed an even more pronounced shift towards higher temperatures during the wet season than when assessed across all individuals. For example, shark ID 235283 spent 47.2 ± 36.3% of its time (Supplementary Figure S12F; Supplementary Table S5) between 30 - 34°C during the wet season. Shark ID 261743 also spent 59.7 ± 33.1% of its time (Supplementary Figure S12I; Supplementary Table S5) within this thermal range during the same season. Interestingly, the shark that ventured into US waters twice, did not show such a stark shift towards higher temperatures during the first tracking period when no time was spent above 28°C during the wet season (Supplementary Figure S12B; Supplementary Table S5). This pattern was not apparent during the second tracking period where thermal ranges during the wet and dry season were broader and the shark spent 4.8 ± 16.7%, between 28 - 30°C during the wet season (Supplementary Figure S12E; Supplementary Table S5).
3.3 Stable isotope analysis
Collectively, great hammerheads in Andros were estimated to feed on barracuda (mean estimate = 34.6% of diet), southern stingrays (30.3%), Caribbean reef sharks (11.5%), silky sharks (6.8%), blacknose sharks (6.6%), blacktip sharks (5.5%), and lemon sharks (4.7%) based on δ13C, δ15N, and δ34S values. As expected, the predicted diets of great hammerheads varied across individuals (Figure 5). The diets of many hammerheads (n = 9, Table 1) were largely comprised of southern stingrays (mean estimate = 28 - 40% of diet), with some individuals feeding more on barracuda (F082 & C160; Table 1; Figure 5C) and others more on blacktip sharks (SW, 383C & C19E; Table 1; Figure 5C). Silky sharks were a small part of the estimated diets of these sharks. Yet, the mixing models indicated that silky sharks were important prey species for some great hammerheads, including shark 3841 (Table 1; Figure 5C) that was estimated to predominantly feed upon silky sharks (mean estimate = 59% of diet), with only a small portion of its estimated diet attributed to southern stingrays (5%). Shark 6179 (Table 1; Figure 5C) was also estimated to have fed less on southern stingrays (15% of diet), with Caribbean reef sharks as the most important prey species (19% of diet).
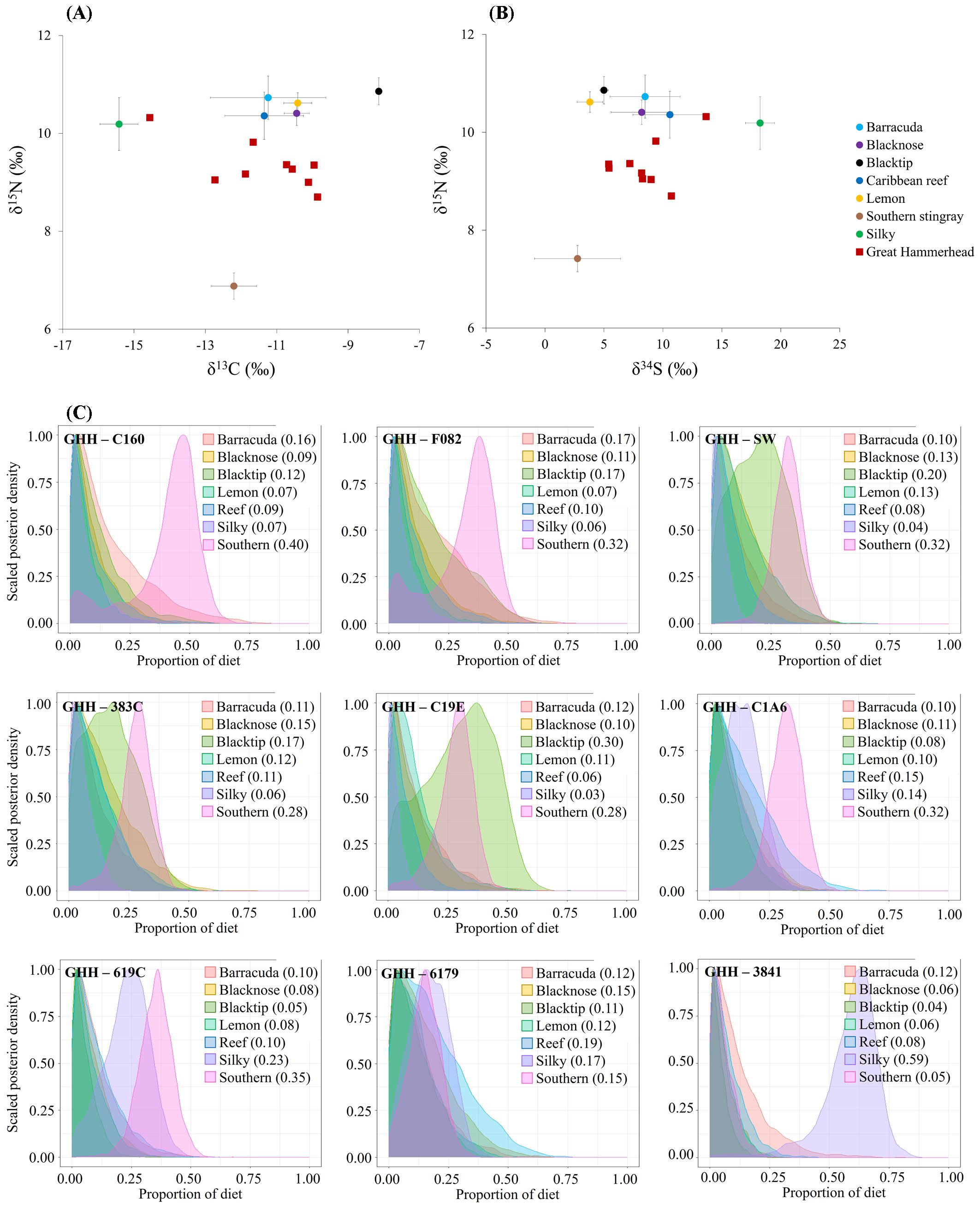
Figure 5. Isotopic (A) δ13C- δ15N and (B) δ34S- δ15N biplots of great hammerheads, Sphyrna mokarran, and potential prey species as well as (C) posterior plots resultant from Bayesian mixing models illustrating the probabilities of potential prey species in the diet of each shark. Data points, horizontal and vertical bars in the biplots show the mean ± SD values. Great hammerhead values in (A, B) are corrected for diet tissue discrimination factors (Δ13C = 0.895‰, Δ15N = 2.250‰, and Δ34S = 0.641‰).
4 Discussion
This study examined the space and resource use of great hammerheads off Andros Island, The Bahamas. Adult great hammerheads of both sexes were documented year-round. We found evidence of site fidelity, with some individuals re-sighted or recaptured in the same locations (< 1 km) up to four years apart. Satellite tracking in both the wet and dry season revealed high-use areas along the reef drop-off and flats that border eastern Andros, particularly at cuts in the fringing reef that access the northern bight of the island. Stable isotope analysis of great hammerhead muscle samples and their potential prey revealed great hammerheads are important predators in Andros nearshore food webs, with a diet including smaller-bodied sharks, rays, and large-bodied bony fishes. Individual variation in diet was apparent, with diverse resource use by great hammerheads connecting food webs from pelagic waters to the shoreline. Productive habitats and abundant prey coupled with trophic flexibility might enable great hammerheads to remain in or nearby Andros waters year-round considering the thermal refuge the deep waters the Tongue of the Ocean provides during the warmer wet season. This explanation seems likely as the sharks use the same habitats throughout the year, despite the increases in water temperature. In addition, movement states estimated from satellite tracks revealed resident behavior within shallow coastal waters, with more transiting movement states identified when great hammerheads used deeper water, or during regional movements. Although only two of eight individuals departed the Bahamas EEZ, these movements to the United States east coast and GOM suggest that great hammerheads in Andros Island are part of the U.S. Atlantic stock. Importantly those that reside in the Bahamas year-round never leave the Shark Sanctuary, which merits further research into the efficacy of the sanctuary acting as a refuge from fishing pressures.
4.1 Site fidelity & year-round residency
This study revealed that great hammerheads were found year-round in Andros waters. These findings were surprising as Guttridge et al. (2017) found great hammerheads in the Bimini Islands migrated in late spring (start of the wet season). Indeed, year-round residency is not only corroborated by our sighting, capture and tracking data, but also the thermal analysis, which indicated individuals persisted in the same habitats throughout the warmer months of the wet season. The lack of migration indicates the Andros-caught individuals may not need to adapt horizontal space use patterns in the face of seasonal abiotic variation. We suspect this could be due to the alimentary benefits of year-round residency in Andros, which is discussed in greater detail below.
Life stages were predominantly large juvenile to adult, however young great hammerheads (1.3 m LSTL) were encountered on two occasions, corroborating the only previous capture of a similar age class in the region (Guttridge et al., 2017). During the wet season (June), one female, estimated to be 4.75 m (LSTL), was caught with fresh mating scars above her gills, and two large great hammerheads were observed swimming slowly, in close contact with each other for 120-180 seconds, displaying what is likely pre- or post-copulatory behavior (Pratt and Carrier, 2011). These findings are unique for the Bahamas archipelago and indicate that Andros Island may serve an important reproductive function for great hammerheads in the region. Future work should focus more on the wet season when gravid females are more frequently encountered in Bahamian waters and or on targeting smaller individuals using lighter gear (Smukall et al., 2021).
Similar to adult great hammerheads monitored across their range in the NWA, GOM (Guttridge et al., 2017; Casselberry et al., 2024; Heim et al., 2024), and South Pacific (Boube et al., 2023; Lubitz et al., 2023), we found evidence of site fidelity to Andros Island across multiple years. Three individuals (2 females and 1 male) were recaptured and one male was re-sighted via photo ID, all < 1 km from their initial tagging or observation sites, ranging from 18 to 40 months at liberty. Core use areas (50% UD) for great hammerheads during the wet and dry season were identified along the eastern shoreline of central and northern Andros in back-reef habitat, centered at the entryway to large cuts in the fringing reef adjacent to Bigwood and Gibson Cays. Other than these locations, individuals did not share areas of high space use. These edge-habitat sites are characterized by shallow water (< 3 – 12 m), strong tidal flow, and proximity to productive reef drop-off (25 m), mangrove, and flats habitat. These findings are well represented in the literature for other species as predators in marine and terrestrial environments are often associated with edge habitats (Rogers et al., 2015; Hansen et al., 2019). Indeed, crustaceans and polychaetes have been recorded in higher abundances along the edge of seagrass habitats (Bowden et al., 2001; Bologna and Heck, 2002; Sambrook et al., 2016), and carnivorous coral reef fishes along reef edges. These species are common prey items for southern stingrays (e.g. O’Shea et al., 2020) and other mesopredatory sharks and bony fishes (Bond et al., 2018; Shipley et al., 2023), which are prey for great hammerheads according to previous studies (Chapman and Gruber, 2002; Roemer et al., 2016; Doan and Kajiura, 2020) and our results.
The reef drop-off zone has been highlighted as a key habitat and movement corridor for great hammerheads in the Bimini Islands (van Zinnicq Bergmann et al., 2024; Heim et al., 2024) and Florida Keys (Lowerre-Barbieri et al., 2021; Griffin et al., 2022). However, when exploring great hammerheads’ movement states via step length and turning angles between predicted locations, our data in northern and central Andros revealed both resident and transiting movement behaviors equally, even within core use areas. This is likely a result of SPOT tags’ relatively coarse location estimates (0.08 to 600 km) and the highly mobile nature of great hammerheads patrolling the reef drop-off, regularly moving large distances within a day (e.g. PTT ID 183623 moved 30.0 ± 22.3 km per day). Future approaches would benefit from additional measures (e.g. acceleration or depth) and or other technologies at different scales or resolution to differentiate behaviors (Bullock et al., 2024). As expected, transiting movement behaviors were identified more frequently when sharks traveled between islands or across jurisdictional boundaries (e.g. PTT 200368/222133 that migrated to the GOM), with a decrease in residency behavior when they moved offshore. Direct movements are often associated with dispersal and are consistent with previous tracks of great hammerheads and other large migratory sharks (Lea et al., 2015; Skomal et al., 2017; Guttridge et al., 2022).
Despite great hammerheads’ high use of edge habitats along the shelf of eastern Andros, most individuals frequently visited shallow water (< 2 m) flats, up to 60 km from the reef, as well as similar habitat off northern Abaco and Long Island. The Bahamas archipelago supports islands with mangrove-fringed creeks, tidal flats and extensive seagrass beds providing critical habitat for abundant great hammerhead prey species such as smaller sharks, rays, and bony fishes (Newman et al., 2007; Jennings et al., 2012; Shipley et al., 2023; van Zinnicq Bergmann et al., 2024). Great hammerheads have regularly been observed foraging in extreme shallow water across their range, including on blacktip sharks off southern Florida (Doan and Kajiura, 2020), cowtail rays (Pastinachus sephen) in Queensland, Australia (Lubitz et al., 2023), and other small sharks and rays in the Bahamas (Chapman and Gruber, 2002; Roemer et al., 2016). Movement state analysis provided further support, as residency behavior increased when individuals arrived at neighboring islands and when using shallow water. More tortuous movements are typically associated with foraging behavior (Sims, 2010; Papastamatiou et al., 2011; Lea et al., 2015) suggesting the importance of shallow, nearshore food webs for great hammerheads.
4.2 Resource use – food web role
Using bulk stable isotopes great hammerheads caught in Andros waters were estimated to feed on small sharks (35%), barracuda (34.6%), and stingrays (30.4%). Published reports of great hammerheads’ trophic interactions in the Bahamas are limited, but our data support these predictions based on observations of 2 - 4 meter great hammerheads feeding on a southern stingray (Strong et al., 1990), two eagle rays (Aetobatus nari nari; Chapman and Gruber, 2002; Roemer et al., 2016), and a lemon shark (Roemer et al., 2016). These prey species are found in diverse habitats, including the fore reef, back reef, and flats of Andros, and our results show that great hammerheads connect food webs from pelagic waters to the shoreline as previously described for a variety of other shark species in Bahamian waters (Shipley et al., 2023).
However, we also found considerable variation between the estimated diets of the nine sampled individuals, with some (ID 3841) showing a preference for silky sharks (59% of estimated diet), and others relying more on barracuda and/or stingray prey (e.g. ID C160 and ID F082). The estimated importance of different shark species in the diet of great hammerheads also varied among individuals; for example, blacktip sharks comprised 30% of the estimated diet of ID C19E, compared to < 5% of the estimated diet of ID 3841. ID 3841 was caught in notably deeper waters (> 130 m) compared to the other great hammerheads, which were sampled at depths of 2 - 8 m, suggesting some great hammerheads may preferentially use deeper habitats and thus serve a different regulatory role in nearshore food webs and/or connecting ecosystems at broader spatial scales. Variation in individual resource use is understudied, but increasingly thought to be a crucial component in the ecological roles and importance of apex predatory sharks (Dedman and van Zinnicq Bergmann, 2024; Dedman et al., 2024). Although sampling was almost exclusively in waters < 10 m, therefore great hammerheads’ reliance on pelagic prey (i.e. silky sharks) may be underestimated in our study. Additional sampling efforts of great hammerheads across different habitats are required to explore this individual variation in resource use.
Globally, great hammerhead diets vary across regions, but this variability is more attributed to prey availability than differences in the taxonomic groups of great hammerheads’ prey choice. Both stomach contents and bulk stable isotopes indicate sharks and batoids are key prey groups in Australia (Stevens and Lyle, 1989; Raoult et al., 2019; de Bruyn et al., 2021), Florida (Clark and von Schmidt, 1965), and South Africa (Cliff, 1995; Smale and Cliff, 1998; Dudley and Cliff, 2010), comprising up to > 90% of mature great hammerheads’ diets. The Bahamas supports over 40 species of sharks (Shipley et al., 2023), and among the large-bodied species that can fill an apex predatory role, great hammerheads are the most abundant in east Andros, and more frequently encountered than large bull sharks (C. leucas) and tiger sharks (Galeocerdo cuvier; Guttridge et al., unpublished data). As such, they may be the most important top predator in these food webs, and the abundance of potential prey across vast seascapes coupled with the trophic flexibility of great hammerheads (e.g. Clark and von Schmidt, 1965; Stevens and Lyle, 1989; Cliff, 1995) enables these sharks to remain in Bahamas waters year-round.
4.3 Regional movements
Only two great hammerheads (both females) of eight were tracked outside of the Bahamas EEZ with all others predominantly using the eastern shoreline of Andros Island. Two males spent much of the months of the wet season at other islands in the Bahamas, one moved to northern Abaco (ID 183623), and another used the shallow bank west of Long Island (ID 261743). Such patterns of partial migration, where some individuals stay within certain areas while others move away, as a consequence of a variety of biotic and abiotic drivers, are well described among marine vertebrates, including sharks (Brodersen et al., 2011; Chapman et al., 2012). Yet, evidence of residency to the Bahamas during the wet season is unique to great hammerheads tagged off Andros, as those tracked in the Bimini Islands migrated to the U.S. eastern shoreline or GOM in late spring, returning in November to overwinter (Guttridge et al., 2017; 2022). With food availability being an important driver of movements (Gunn et al., 2022), our results suggest that the year-round residency of individuals in Andros could be a consequence of Andros offering year-round feeding opportunities due to its vast, pristine habitats, including nursery habitat for many coastal sharks and rays, as well as bony fishes (Postaire et al., 2022; Talwar et al., 2022; Shipley et al., 2023) shown here to be prey for great hammerheads. Movement from the U.S. to Andros, even during the dry season, seems uncommon for great hammerheads, with only one female acoustically tagged in Jupiter, Florida documented to travel to Andros Island, returning to Jupiter in early April of 2015 (Guttridge et al., 2017). Estimated core habitat use areas for 18 great hammerheads satellite-tagged off Florida revealed only 8.43% of their time was spent within the Bahamas EEZ, with location estimates in the most western part of the Bahamas Bank or near Cay Sal Bank, The Bahamas (Graham et al., 2016). In addition, a further 12 great hammerheads satellite-tagged off Jupiter (2023 – 2024) did not move into the Bahamas EEZ across 9-12 months of tracking (Guttridge et al., unpublished data).
One female was tagged on two occasions in the same location two years apart (PTT 200368/222133). She spent the dry season off Andros using drop-off habitat along the eastern shoreline until mid-April, when she departed via Bimini to the east coast of the U.S. On both occasions she traveled south past the Florida Keys and moved as far north as Cedar Key (FL, U.S.A.) in the GOM. Although we did not track her returning to Andros, her recapture suggests this is an annual migration, much like other great hammerheads that were previously tracked in Bimini and migrated to the U.S. during the wet season for parturition (Guttridge et al., 2017; 2022). Indeed, an almost-full-term female, confirmed via ultrasonic exam, tagged in Bimini, undertook a very similar migration at the same time of year, staying for four weeks from June to July off Crystal River, presumably to pup (Heim et al., 2021b). Young-of-the-year great hammerheads have been captured in this region (Hueter and Tyminski, 2007), providing supporting evidence for this being an important pupping ground for the species. This female’s behavior provides further evidence that great hammerheads, like other large coastal sharks, display reproductive philopatry, returning to specific regions for pupping and/or mating (Chapman et al., 2015; Guttridge et al., 2017; Rider et al., 2021).
4.4 Drivers of space use: thermal range
Water temperature is an important abiotic factor that influences key metabolic and physiological processes for sharks (Schlaff et al., 2014). Seasonal changes in water temperatures can represent an important driver of shark habitat use (Kessel et al., 2014; Matich et al., 2024) as they must balance the demands for thermoregulation with food acquisition within a 3-dimensional marine environment (Arrowsmith et al., 2021; Spurgeon et al., 2024). Great hammerheads tagged off the Bimini Islands typically made northward latitudinal movements from March to July (Guttridge et al., 2022) indicative of expansion in habitat use during the wet season and showing a narrow temperature range, with 89% of records between 23 and 28°C. In this study great hammerheads occupied habitats with temperatures between 25 and 26°C across the year with the sharks occupying warmer waters during the wet season (e.g. PTT 235283 spent 30% of its time in waters 30 - 34°C). Unlike other research which documented the seasonal effects of water temperatures on shark habitat use (Schlaff et al., 2014), we find that some individuals maintained horizontal space use patterns despite increased water temperatures. Similarly, two great hammerheads that were monitored across the wet season by Guttridge et al. (2022) also showed warm water use. One female ceased diving behavior > 30 m, contracted her depth use across the wet season, and experienced a temperature range of 7.1°C (23.7 - 30.9°C; Guttridge et al., 2022). It was hypothesized that this use of warm and shallow waters might reflect a seasonal shift in foraging strategy or habitat selection, as some great hammerheads use seasonally-available pulses of prey resources (e.g. spawning tarpon in Bahia Honda [FL, U.S.A.], April-August; Casselberry et al., 2024). While the female great hammerhead that ventured into the GOM did not show a distinct use of warmer waters during the wet season, the observed switch in great hammerheads that remained in Andros and its environments could be a result of continued foraging behaviors in shallow waters even during this warmer period of the year. We conclude that the use of warmer waters throughout the wet season is likely not due to a change in habitat use patterns, which is supported by our tracking data, but rather the continued use of the same areas despite increases in water temperature. Given the proximity of Andros’ east side to deep shelf waters, in which an individual could use the cooler waters for rest after expending energy on the flats, Andros not only offers year-round resource availability but also access to great hammerheads’ preferred thermal habitat during the wet and dry season.
4.5 Management implications
Most of the sharks tagged in this study remained exclusively within the boundaries of the Bahamian EEZ, where commercial longline fishing has been banned since 1993 (Sherman et al., 2018), and where various prohibitions exist regarding the possession, sale, or trade of sharks due to the 2011-implemented sanctuary (Ward-Paige, 2017). From a global standpoint, unsustainable mortality resulting from targeted and incidental catch is the major threat to shark populations (Davidson et al., 2016; Dulvy et al., 2021; Pacoureau et al., 2021). While preliminary results from the most recent stock assessment for great hammerheads in the NWA indicated that they remain overfished, improved management has resulted in initial signs of recovery in this region (SEDAR, 2024). Nevertheless, great hammerheads’ low tolerance to stress (Prohaska et al., 2021) and corresponding high capture related and post-release delayed mortality rates across a variety of fishing methods (Morgan and Burgess, 2007; Gallagher et al., 2014; Ellis et al., 2017) indicates that reducing fishing-related mortality remains a priority to further support the stock’s recovery. Protected areas such as the Bahamian shark sanctuary can offer a different approach for effective management, as they minimize the interaction risk between great hammerheads and fishing gear (Davidson and Dulvy, 2017; Grorud-Colvert et al., 2021; MacKeracher et al., 2019). It was previously suggested that the Bahamian EEZ provides such a refuge for great hammerheads within the NWA at least during the months of the dry season, with sharks being at risk of directed fishing related mortality once they moved into U.S. waters during the wet season (Guttridge et al., 2017; Heim et al., 2024).
However, while our study supports the previously documented regional connectivity of great hammerheads tagged at the western edge of the Bahamas (Guttridge et al., 2017; 2022; Graham et al., 2016) into U.S. waters, it provides new evidence that some individuals benefit from the protective regulations within the Bahamian EEZ year-round. This could prove an important factor when assessing regional rebuilding goals, as great hammerheads within the U.S. Atlantic, GOM, and nearby waters such as The Bahamas comprise a single management stock (SEDAR, 2024). However, to fully understand to what extent individuals that remain within the Bahamian EEZ year-round contribute to the stock and corresponding management strategies for the species at a regional scale, additional tagging efforts and genetic analyses across different life stages and both sexes are needed. The analysis of genetic samples from Andros and other Bahamian islands further to the east will be vital to adequately assess stock identity of great hammerheads that display year-round residency to the central Bahamas. This is required if we wish to assess the significance of the efficacy of Bahamian shark sanctuary towards rebuilding goals for great hammerheads within the NWA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Department of Environmental Planning and Protection (DEPP) and Department of Marine Resources (DMR), The Bahamas. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TG: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, Formal analysis. VH: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SD: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization. AG: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing. SB: Investigation, Writing – review & editing. BK: Investigation, Writing – original draft, Writing – review & editing. PM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this work was provided by Saving the Blue, Warner Bros. Discovery, Fahlo, Storm Story and the David and Carol Lackland Family Foundation. All funders supported either the running of research expeditions or satellite tags.
Acknowledgments
We thank Saving the Blue board of Directors, Advisors, Science Committee and team for continued support, guidance, and expertise. Special mention to Boat Captain’s Cole McVay, Stevano Smith and Marvin Sargant, and the Andros Island Bonefish Club in Cargill Creek. We thank Saving the Blue trip leaders, field technicians, and volunteers for joining us on expeditions to Andros and assisting with shark tagging and captures. We thank the water users who reported sightings of great hammerheads and Mike Lott from Washington State University for support of the Stable Isotope Analysis. The scientific results and conclusions, as well as any view or opinions expressed herein, are those of the author(s) and do not necessarily reflect those of NOAA or the Department of Commerce. Funding for this work was provided by Saving the Blue, Warner Bros. Discovery, Fahlo, Storm Story and the David and Carol Lackland Family Foundation. All funders supported either the running of research expeditions or satellite tags. We also thank Warner Bros. Discovery (Shark Week), Hazmat Productions and Bigwave Productions for providing the platform to showcase our great hammerhead research to a global audience. We thank the Shark Biology and Fisheries Science Lab at Texas A & M Galveston for logistical support. We are grateful to the Department of Environmental Planning and Protection (DEPP) particularly Dr. Rhianna Neely and Dr. Lester Gittens of the Department of Marine Resources (DMR) in The Bahamas for continued support and for issuing research permits.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1544482/full#supplementary-material
Footnotes
- ^ [1] https://www.argos-system.org/, accessed August 20th, 2024
References
Arrowsmith L., Sequeira A., Pattiaratchi C., Meekan M. (2021). Water temperature is a key driver of horizontal and vertical movements of an ocean giant, the whale shark Rhincodon typus. Mar. Ecol. Prog. Ser. 679, 101–114. doi: 10.3354/meps13899
Barker A. M., Frazier B. S., Bethea D. M., Gold J. R., Portnoy D. S. (2017). Identification of young-of-the-year great hammerhead shark Sphyrna mokarran in northern Florida and South Carolina. J. Fish Biol. 91, 664–668. doi: 10.1111/jfb.13356
Bologna P. A. X., Heck K. L. (2002). Impact of habitat edges on density and secondary production of seagrass-associated fauna. Estuaries 25, 1033–1044. doi: 10.1007/BF02691350
Bond M. E., Valentin-Albanese J., Babcock E. A., Hussey N. E., Heithaus M. R., Chapman D. D. (2018). The trophic ecology of Caribbean reef sharks (Carcharhinus perezi) relative to other large teleost predators on an isolated coral atoll. Mar. Biol. 165, 67. doi: 10.1007/s00227-018-3322-2
Boube T., Azam C.-S., Guilbert A., Huveneers C., Papastamatiou Y. P., Mourier J., et al. (2023). First insights into the population characteristics and seasonal occurrence of the great hammerhead shark, Sphyrna mokarran (Rüppell 1837) in the Western Tuamotu archipelago, French Polynesia. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1234059
Bowden D. A., Rowden A. A., Attrill M. J. (2001). Effect of patch size and in-patch location on the infaunal macroinvertebrate assemblages of Zostera marina seagrass beds. J. Exp. Mar. Biol. Ecol. 259, 133–154. doi: 10.1016/S0022-0981(01)00236-2
Boyd J. D., Brightsmith D. J. (2013). Error properties of argos satellite telemetry locations using least squares and kalman filtering. PloS One 8, e63051. doi: 10.1371/journal.pone.0063051
Bullock R. W., Dedman S. L., van Zinnicq Bergmann M. P. M., Grimmel H. M. V., Cowx I. G., et al. (2024). A day in the life: quantifying nursery habitat use in a coastal shark species. Animal Behaviour 213, 219–234. doi: 10.1016/j.anbehav.2024.04.008
Brodersen J., Nicolle A., Nilsson P. A., Skov C., Brönmark C., Hansson L. (2011). Interplay between temperature, fish partial migration and trophic dynamics. Oikos 120, 1838–1846. doi: 10.1111/j.1600-0706.2011.19433.x
Cardeñosa D., Fields A. T., Babcock E. A., Zhang H., Feldheim K., Shea S. K. H., et al. (2018). CITES-listed sharks remain among the top species in the contemporary fin trade. Conserv. Lett. 11, e12457. doi: 10.1111/conl.12457
Carr A., Meylan A., Mortimer J., Bjorndal K., Carr T. (1982). Surveys of sea turtle populations and habitats in the western North Atlantic. NOAA Tech. Memorandum NMFS_SEFC-91, 1–96.
Casola W. R., Oren J., Register M. L., Littlejohn J., Peterson M. N., Langerhans R. B. (2021). Modernization of artisanal fishing communities on Andros Island, The Bahamas, as a treadmill of production. Ocean Coast. Manage. 201, 105487. doi: 10.1016/j.ocecoaman.2020.105487
Casselberry G. A., Skomal G. B., Griffin L. P., Brownscombe J. W., Filous A., Holder P. E., et al. (2024). Depredation rates and spatial overlap between Great Hammerheads and Tarpon in a recreational fishing hot spot. Mar. Coast. Fisheries 16, e10277. doi: 10.1002/mcf2.10277
Chapman D. D., Feldheim K. A., Papastamatiou Y. P., Hueter R. E. (2015). There and back again: A review of residency and return migrations in sharks, with implications for population structure and management. Annu. Rev. Mar. Sci. 7, 547–570. doi: 10.1146/annurev-marine-010814-015730
Chapman D. D., Gruber S. H. (2002). A further observation of the prey-handling behavior of the great hammerhead shark, Sphyrna mokarran: Predation upon the spotted eagle ray, Aetobatus narinari. Bull. Mar. Sci. 70, 947–952.
Chapman B. B., Hulthén K., Brodersen J., Nilsson P. A., Skov C., Hansson L.-A., et al. (2012). Partial migration in fishes: causes and consequences. J. Fish Biol. 81, 456–478. doi: 10.1111/j.1095-8649.2012.03342.x
Charbeneau G. (2004). “Physiological and Behavioral Changes to Elasmobranchs in Controlled Environments,” in The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and their Relatives. Eds. Smith M., Warmolts D., Thoney D., Editors R. H., Hueter R. (Columbus, Ohio: Ohio Biological Survey, Inc), 261–270.
Clark E., von Schmidt K. (1965). Sharks of the central gulf coast of Florida. Bull. Mar. Sci. 15, 13–83.
Cliff G. (1995). Sharks caught in the protective gill nets off KwaZulu-Natal, South Africa. 8. The Great hammerhead shark Sphyrna mokarran (Rüppell). South Afr. J. Mar. Sci. 15, 105–114. doi: 10.2989/025776195784156331
CMS (2019). “Appendices I and II of the convention on the conservation of migratory species of wild animals (CMS),” in As Amended by the Conference of the Parties in 1985, 1988, 1991., 2002, 2005, 2008. and 2020, May 22, 2020.
Compagno L. J. V. (1984). FAO species catalogue. Vol. 4: Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 2: Carcharhiniformes (Rome), Vol. 4.
Davidson L. N. K., Dulvy N. K. (2017). Global marine protected areas to prevent extinctions. Nat. Ecol. Evol. 1, 40. doi: 10.1038/s41559-016-0040
Davidson L. N. K., Krawchuk M. A., Dulvy N. K. (2016). Why have global shark and ray landings declined: improved management or overfishing? Fish Fisheries 17, 438–458. doi: 10.1111/faf.12119
de Bruyn M., Barbato M., DiBattista J. D., Broadhurst M. K. (2021). Secondary predation constrains DNA-based diet reconstruction in two threatened shark species. Sci. Rep. 11, 18350. doi: 10.1038/s41598-021-96856-w
Dedman S., van Zinnicq Bergmann M. (2024). “movegroup: Visualizing and Quantifying Space Use Data for Groups of Animals,” in CRAN: Contributed Packages. doi: 10.32614/CRAN.package.movegroup
Dedman S., Moxley J. H., Papastamatiou Y. P., Braccini M., Caselle J. E., Chapman D. D., et al. (2024). Ecological roles and importance of sharks in the Anthropocene Ocean. Science 385 (6708), adl2362.
Doan M. D., Kajiura S. M. (2020). Adult blacktip sharks (Carcharhinus limbatus) use shallow water as a refuge from great hammerheads (Sphyrna mokarran). J. Fish Biol. 96, 1530–1533. doi: 10.1111/jfb.14342
Dudley S. F. J., Cliff G. (2010). Influence of the annual sardine run on catches of large sharks in the protective gillnets off KwaZulu-Natal, South Africa, and the occurrence of sardine in shark diet. Afr. J. Mar. Sci. 32, 383–397. doi: 10.2989/1814232X.2010.502641
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 31, 4773–4787. doi: 10.1016/j.cub.2021.08.062
Ebert D. A., Fowler S., Dando M. (2021). Sharks of the World: A complete guide (Woodstock, Oxfordhsire, UK: Princeton University Press).
Ellis J. R., McCully Phillips S. R., Poisson F. (2017). A review of capture and post-release mortality of elasmobranchs. J. Fish Biol. 90, 653–722. doi: 10.1111/jfb.13197
Freitas C., Lydersen C., Fedak M. A., Kovacs K. M. (2008). A simple new algorithm to filter marine mammal Argos locations. Mar. Mammal Sci. 24, 315–325. doi: 10.1111/j.1748-7692.2007.00180.x
Gallagher A. J., Serafy J., Cooke S., Hammerschlag N. (2014). Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Mar. Ecol. Prog. Ser. 496, 207–218. doi: 10.3354/meps10490
Graham F., Rynne P., Estevanez M., Luo J., Ault J. S., Hammerschlag N. (2016). Use of marine protected areas and exclusive economic zones in the subtropical western North Atlantic Ocean by large highly mobile sharks. Diversity Distributions 22, 534–546. doi: 10.1111/ddi.12425
Griffin L. P., Casselberry G. A., Lowerre-Barbieri S. K., Acosta A., Adams A. J., Cooke S. J., et al. (2022). Predator–prey landscapes of large sharks and game fishes in the Florida Keys. Ecol. Appl. 32, e2584. doi: 10.1002/eap.2584
Grorud-Colvert K., Sullivan-Stack J., Roberts C., Constant V., Horta e Costa B., Pike E. P., et al. (2021). The MPA Guide: A framework to achieve global goals for the ocean. Science 373, eabf0861. doi: 10.1126/science.abf0861
Gunn R. L., Hartley I. R., Algar A. C., Nadiarti N., Keith S. A. (2022). Variation in the behaviour of an obligate corallivore is influenced by resource availability. Behav. Ecol. Sociobiology 76, 24. doi: 10.1007/s00265-022-03132-6
Guttridge T. L., Müller L., Keller B. A., Bond M. E., Grubbs R. D., Winram W., et al. (2022). Vertical space use and thermal range of the great hammerhead (Sphyrna mokarran), (Rüppell 1837) in the western North Atlantic. J. Fish Biol. 101, 797–810. doi: 10.1111/jfb.15185
Guttridge T. L., Van Zinnicq Bergmann M. P. M., Bolte C., Howey L. A., Finger J. S., Kessel S. T., et al. (2017). Philopatry and regional connectivity of the great hammerhead shark, sphyrna mokarran in the U.S. and Bahamas. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00003
Haas A. R., Fedler T., Brooks E. J. (2017). The contemporary economic value of elasmobranchs in The Bahamas: Reaping the rewards of 25 years of stewardship and conservation. Biol. Conserv. 207, 55–63. doi: 10.1016/j.biocon.2017.01.007
Hansen N. A., Sato C. F., Michael D. R., Lindenmayer D. B., Driscoll D. A. (2019). Predation risk for reptiles is highest at remnant edges in agricultural landscapes. J. Appl. Ecol. 56, 31–43. doi: 10.1111/1365-2664.13269
Harry A. V., Macbeth W. G., Gutteridge A. N., Simpfendorfer C. A. (2011). The life histories of endangered hammerhead sharks (Carcharhiniformes, Sphyrnidae) from the east coast of Australia. J. Fish Biol. 78, 2026–2051. doi: 10.1111/j.1095-8649.2011.02992.x
Hazen E. L., Nowacek D. P., St. Laurent L., Halpin P. N., Moretti D. J. (2011). The relationship among oceanography, prey fields, and beaked whale foraging habitat in the tongue of the ocean. PloS One 6, e19269. doi: 10.1371/journal.pone.0019269
Heim V., Dhellemmes F., Smukall M. J., Gruber S. H., Guttridge T. L. (2021a). Effects of food provisioning on the daily ration and dive site use of great hammerhead sharks, sphyrna mokarran. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.628469
Heim V., Grubbs R. D., Frazier B. S., Smukall M. J., Guttridge T. L. (2021b). Regional movements of great, Sphyrna mokarran, and scalloped, Sphyrna lewini, hammerhead sharks in the US Atlantic, Gulf of Mexico and the 2 Bahamas: preliminary results. SEDAR77- SID01 (North Charleston, SC.: SEDAR), 8.
Heim V., Grubbs R. D., Smukall M. J., Frazier B. S., Carlson J. K., Guttridge T. L. (2023). Observations of fin injury closure in Great Hammerheads and implications for the use of fin-mounted geolocators. J. Aquat. Anim. Health 35, 53–63. doi: 10.1002/aah.10178
Heim V., Lüscher D., Hottinger J., Ebert D. (2022). Development and validation of a drill attachment for faster and safer deployments of fin-mounted geolocators in large-bodied sharks. Anim. Biotelemetry 10, 33. doi: 10.1186/s40317-022-00304-z
Heim V., van Zinnicq Bergmann M. P. M., Smukall M. J., Guttridge T. L. (2024). Multiyear tourism-related feeding reduces short- and long-term local space use in a marine apex predator. Anim. Behav. 217, 81–107. doi: 10.1016/j.anbehav.2024.08.012
Heithaus M. R., Burkholder D., Hueter R. E., Heithaus L. I., Pratt H. L. Jr., Carrier J. C. (2007). Spatial and temporal variation in shark communities of the lower Florida Keys and evidence for historical population declines. Can. J. Fisheries Aquat. Sci. 64, 1302–1313. doi: 10.1139/f07-098
Hesslein R. H., Hallard K. A., Ramlal P. (1993). Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can. J. Fisheries Aquat. Sci. 50, 2071–2076. doi: 10.1139/f93-230
Hueter R. E., Tyminski J. P. (2007). Species-specific distribution and habitat characteristics of shark nurseries in Gulf of Mexico waters off peninsular Florida and Texas. Am. Fisheries Soc. Symposium 50, 193–223. doi: 10.1007/BF00842902
Hussey N. E., MacNeil M. A., Fisk A. T. (2010). The requirement for accurate diet-tissue discrimination factors for interpreting stable isotopes in sharks. Hydrobiologia 654, 1–5. doi: 10.1007/s10750-010-0361-1
Jennings D. E., DiBattista J. D., Stump K. L., Hussey N. E., Franks B. R., Grubbs R. D., et al. (2012). Assessment of the aquatic biodiversity of a threatened coastal lagoon at Bimini, Bahamas. J. Coast. Conserv. 16, 405–428. doi: 10.1007/s11852-012-0211-6
Jonsen I. D., Grecian W. J., Phillips L., Carroll G., McMahon C., Harcourt R. G., et al. (2023). aniMotum, an R package for animal movement data: Rapid quality control, behavioural estimation and simulation. Methods Ecol. Evol. 14, 806–816. doi: 10.1111/2041-210X.14060
Kessel S., Chapman D., Franks B., Gedamke T., Gruber S., Newman J., et al. (2014). Predictable temperature-regulated residency, movement and migration in a large, highly mobile marine predator (Negaprion brevirostris). Mar. Ecol. Prog. Ser. 514, 175–190. doi: 10.3354/meps10966
Kranstauber B., Kays R., LaPoint S. D., Wikelski M., Safi K. (2012). A dynamic Brownian bridge movement model to estimate utilization distributions for heterogeneous animal movement. J. Anim. Ecol. 81, 738–746. doi: 10.1111/j.1365-2656.2012.01955.x
Lea J. S. E., Wetherbee B. M., Queiroz N., Burnie N., Aming C., Sousa L. L., et al. (2015). Repeated, long-distance migrations by a philopatric predator targeting highly contrasting ecosystems. Sci. Rep. 5, 11202. doi: 10.1038/srep11202
Li Y., Zhang Y., Hussey N. E., Dai X. (2016). Urea and lipid extraction treatment effects on δ15N and δ13C values in pelagic sharks. Rapid Commun. Mass Spectrometry 30, 1–8. doi: 10.1002/rcm.7396
Logan R. K., Vaudo J. J., Sousa L. L., Sampson M., Wetherbee B. M., Shivji M. S. (2020). Seasonal movements and habitat use of juvenile smooth hammerhead sharks in the Western North Atlantic Ocean and significance for management. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.566364
Lowerre-Barbieri S. K., Friess C., Griffin L. P., Morley D., Skomal G. B., Bickford J. W., et al. (2021). Movescapes and eco-evolutionary movement strategies in marine fish: Assessing a connectivity hotspot. Fish Fisheries 22, 1321–1344. doi: 10.1111/faf.12589
Lubitz N., Abrantes K., Crook K., Currey-Randall L. M., Chin A., Sheaves M., et al. (2023). Trophic ecology shapes spatial ecology of two sympatric predators, the great hammerhead shark (Sphyrna mokarran) and bull shark (Carcharhinus leucas). Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1274275
Macdonald C., Jerome J., Pankow C., Perni N., Black K., Shiffman D., et al. (2021). First identification of probable nursery habitat for critically endangered great hammerhead Sphyrna mokarran on the Atlantic Coast of the United States. Conserv. Sci. Pract. 3, e418. doi: 10.1111/csp2.418
MacKeracher T., Diedrich A., Simpfendorfer C. A. (2019). Sharks, rays and marine protected areas: A critical evaluation of current perspectives. Fish Fisheries 20, 255–267. doi: 10.1111/faf.12337
Matich P., Plumlee J. D., Bubley W., Curtis T. H., Drymon J. M., Mullins L. L., et al. (2024). Long-term effects of climate change on juvenile bull shark migratory patterns. J. Anim. Ecol. 93, 1445–1461. doi: 10.1111/1365-2656.14140
McCandless C. T., Pratt H. L. Jr., Kohler N. E. (2002). Shark nursery grounds of the Gulf of Mexico and the East Coast waters of the United States: an overview. An interal report to NOAA’s Highly Migratory Species Office. NOAA Fisheries Narragansett Lab, 28 Tarzwell Drive, Narragansett, RI 02882. (Narragansett, RI).
McCutchan J. H., Lewis W. M., Kendall C., McGrath C. C. (2003). Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390. doi: 10.1034/j.1600-0706.2003.12098.x
Miller M. H., Carlson J. K., Hogan L., Kobayashi D. (2014). Status review report: great hammerhead shark (Sphyrna mokarran). Final Rep. to Natl. Mar. Fisheries Service Office Protected Resources., 116.
Morgan A., Burgess G. H. (2007). At-vessel fishing mortality for six species of sharks caught in the northwest atlantic and gulf of Mexico. Gulf Caribbean Res. 19, 123–129. doi: 10.18785/gcr.1902.15
Mourier J., Planes S., Buray N. (2013). Trophic interactions at the top of the coral reef food chain. Coral Reefs 32, 285. doi: 10.1007/s00338-012-0976-y
Newman S. P., Handy R. D., Gruber S. H. (2007). Spatial and temporal variations in mangrove and seagrass faunal communities at Bimini, Bahamas. Bull. Mar. Sci. 80, 529–553.
O’Shea O. R., Meadows M. H., Wrigglesworth E. E., Newton J., Hawkes L. A. (2020). Novel insights into the diet of southern stingrays and Caribbean whiptail rays. Mar. Ecol. Prog. Ser. 655, 157–170. doi: 10.3354/meps13529
Pacoureau N., Rigby C. L., Kyne P. M., Sherley R. B., Winker H., Carlson J. K., et al. (2021). Half a century of global decline in oceanic sharks and rays. Nature 589, 567–571. doi: 10.1038/s41586-020-03173-9
Papastamatiou Y. P., Cartamil D. P., Lowe C. G., Meyer C. G., Wetherbee B. M., Holland K. N. (2011). Scales of orientation, directed walks and movement path structure in sharks. J. Anim. Ecol. 80, 864–874. doi: 10.1111/j.1365-2656.2011.01815.x
Pebesma E. (2018). Simple features for R: standardized support for spatial vector data. R J. 10, 439–446. doi: 10.32614/RJ-2018-009
Pebesma E., Bivand R. (2023). Spatial Data Science: With Applications in R (New York: Chapman and Hall/CRC). doi: 10.1201/9780429459016
Piercy A. N., Carlson J. K., Passerotti M. S. (2010). Age and growth of the great hammerhead shark, Sphyrna mokarran, in the north-western Atlantic Ocean and Gulf of Mexico. Mar. Freshw. Res. 61, 992–998. doi: 10.1071/MF09227
Postaire B. D., Feldheim K. A., Clementi G. M., Quinlan J., van Zinnicq Bergmann M. P. M., Brooks E. J., et al. (2022). Small localized breeding populations in a widely distributed coastal shark species. Conserv. Genet. 23, 51–61. doi: 10.1007/s10592-021-01398-3
Pratt H. L. Jr., Carrier J. C. (2011). “Elasmobranch courtship and mating behavior,” in Reproductive biology and phylogeny of Chondrichthyes (Boca Raton: CRC Press), 139–175.
Prohaska B. K., Marshall H. R., Grubbs R. D., Frazier B. S., Morris J. J., Andres A., et al. (2021). Stress physiology of scalloped and great hammerhead sharks from a bottom longline fishery. SEDAR77-DW09 (North Charleston, SC: SEDAR), 34.
Raoult V., Broadhurst M. K., Peddemors V. M., Williamson J. E., Gaston T. F. (2019). Resource use of great hammerhead sharks (Sphyrna mokarran) off eastern Australia. J. Fish Biol. 95, 1430–1440. doi: 10.1111/jfb.14160
R Core Team (2022). R: A Language and Environment for Statistical Computing (version 4.2.2.) (R Foundation for Statistical Computing). Available at: https://www.r-project.org/ (Accessed January 30, 2025).
Rider M. J., McDonnell L. H., Hammerschlag N. (2021). Multi-year movements of adult and subadult bull sharks (Carcharhinus leucas): philopatry, connectivity, and environmental influences. Aquat. Ecol. 55, 559–577. doi: 10.1007/s10452-021-09845-6
Rigby C. L., Barreto R., Carlson J., Fernando D., Fordham S., Francis M. P., et al. (2019). Sphyrna mokarran. IUCN Red List Threatened Species, e.T39386A2920499. doi: 10.2305/IUCN.UK.2019-3.RLTS.T39386A2920499.en
Roemer R. P., Gallagher A. J., Hammerschlag N. (2016). Shallow water tidal flat use and associated specialized foraging behavior of the great hammerhead shark (Sphyrna mokarran). Mar. Freshw. Behav. Physiol. 49, 235–249. doi: 10.1080/10236244.2016.1168089
Roff G., Doropoulos C., Rogers A., Bozec Y.-M., Krueck N. C., Aurellado E., et al. (2016). The ecological role of sharks on coral reefs. Trends Ecol. Evol. 31, 395–407. doi: 10.1016/j.tree.2016.02.014
Rogers P. J., Huveneers C., Page B., Goldsworthy S. D., Coyne M., Lowther A. D., et al. (2015). Living on the continental shelf edge: habitat use of juvenile shortfin makos Isurus oxyrinchus in the Great Australian Bight, southern Australia. Fisheries Oceanography 24, 205–218. doi: 10.1111/fog.12103
Ryan W. B. F., Carbotte S. M., Coplan J. O., O’Hara S., Melkonian A., Arko R., et al. (2009). Global multi-resolution topography synthesis. Geochemistry Geophysics Geosystems 10, Q03014. doi: 10.1029/2008GC002332
Sambrook K., Jones G., Bonin M. (2016). Life on the edge: Coral reef fishes exhibit strong responses to a habitat boundary. Mar. Ecol. Prog. Ser. 561, 203–215. doi: 10.3354/meps11940
Schlaff A. M., Heupel M. R., Simpfendorfer C. A. (2014). Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev. Fish Biol. Fisheries 24, 1089–1103. doi: 10.1007/s11160-014-9364-8
Schwanck T. N., Schweinsberg M., Lampert K. P., Guttridge T. L., Tollrian R., O’Shea O. (2020). Linking local movement and molecular analysis to explore philopatry and population connectivity of the southern stingray Hypanus americanus. J. Fish Biol. 96, 1475–1488. doi: 10.1111/jfb.14325
SEDAR (2024). Southeast Data, Assessment & Review (SEDAR) 77 HMS Hammerhead Sharks (SEDAR North Charleston, SC). Available at: https://sedarweb.org/assessments/sedar-77/ (Accessed January 30, 2025).
Sherley R. B., Winker H., Rigby C. L., Kyne P. M., Pollom R., Pacoureau N., et al. (2020). Estimating IUCN Red List population reduction: JARA — A decision-support tool applied to pelagic sharks. Conserv. Lett. 13, e12688. doi: 10.1111/conl.12688
Sherman K. D., Shultz A. D., Dahlgren C. P., Thomas C., Brooks E., Brooks A., et al. (2018). Contemporary and emerging fisheries in The Bahamas—Conservation and management challenges, achievements and future directions. Fisheries Manage. Ecol. 25, 319–331. doi: 10.1111/fme.12299
Shipley O. N., Matich P., Hussey N. E., Brooks A. M. L., Chapman D., Frisk M. G., et al. (2023). Energetic connectivity of diverse elasmobranch populations – implications for ecological resilience. Proc. R. Soc. B: Biol. Sci. 290, 20230262. doi: 10.1098/rspb.2023.0262
Signer J., Fieberg J., Avgar T. (2019). Animal movement tools (amt): R package for managing tracking data and conducting habitat selection analyses. Ecol. Evol. 9, 880–890. doi: 10.1002/ece3.4823
Sims D. W. (2010). “Tracking and analysis techniques for understanding free-ranging shark movements and behavior,” in Sharks and their relatives II. Eds. Carrier J. C., Musick J. A., Heithaus M. R. (Boca Raton, FL: CRC Press), 367–408. doi: 10.1201/9781420080483
Skomal G., Braun C., Chisholm J., Thorrold S. (2017). Movements of the white shark Carcharodon carcharias in the North Atlantic Ocean. Mar. Ecol. Prog. Ser. 580, 1–16. doi: 10.3354/meps12306
Smale M. J., Cliff G. (1998). Cephalopods in the diet of four shark species (Galeocerdo cuvier, Sphyrna lewini, S. zygaena, and S. mokarran) from Kwazulu-Natal, South Africa. Afr. J. Mar. Sci. 20, 241–253. doi: 10.2989/025776198784126610
Smith M., Fedler A. J., Adams A. J. (2023). Economic assessments of recreational flats fisheries provide leverage for conservation. Environ. Biol. Fishes 106, 131–145. doi: 10.1007/s10641-022-01375-w
Smukall M. J., Guttridge T. L., Dhellemmes F., Seitz A. C., Gruber S. H. (2021). Effects of leader type and gear strength on catches of coastal sharks in a longline survey around Bimini, The Bahamas. Fisheries Res. 240, 105989. doi: 10.1016/j.fishres.2021.105989
Spurgeon E., Thompson M. L., Alexander M. D., Anderson J. M., Rex P. T., Stirling B., et al. (2024). The influence of micro-scale thermal habitat on the movements of juvenile white sharks in their Southern California aggregation sites. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1290769
Stanhope M. J., Ceres K. M., Sun Q., Wang M., Zehr J. D., Marra N. J., et al. (2023). Genomes of endangered great hammerhead and shortfin mako sharks reveal historic population declines and high levels of inbreeding in great hammerhead. IScience 26, 105815. doi: 10.1016/j.isci.2022.105815
Steinley D. (2004). “Standardizing Variables in K-means Clustering,” in Classification, Clustering, and Data Mining Applications: Proceedings of the Meeting of the International Federation of Classification Societies (IFCS), Illinois Institute of Technology, Chicago, 15–18 July 2004 (Berlin, Heidelberg: Springer Berlin Heidelberg). doi: 10.1007/978-3-642-17103-1_6
Steinley D. (2006). K-means clustering: A half-century synthesis. Br. J. Math. Stat. Psychol. 59, 1–34. doi: 10.1348/000711005X48266
Stevens J., Lyle J. (1989). Biology of three hammerhead sharks (Eusphyra blochii, Sphyrna mokarran and S. lewini) from Northern Australia. Mar. Freshw. Res. 40, 129–146. doi: 10.1071/MF9890129
Stock B. C., Jackson A. L., Ward E. J., Parnell A. C., Phillips D. L., Semmens B. X. (2018). Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6, e5096. doi: 10.7717/peerj.5096
Strong W. R., Snelson F. F., Gruber S. H. (1990). Hammerhead shark predation on stingrays: an observation of prey handling by sphyrna mokarran. Copeia 1990, 836–840. doi: 10.2307/1446449
Talwar B. S., Bradley D., Berry C., Bond M. E., Bouyoucos I. A., Brooks A. M. L., et al. (2022). Estimated life-history traits and movements of the Caribbean reef shark (Carcharhinus perezi) in The Bahamas based on tag-recapture data. Mar. Biol. 169, 55. doi: 10.1007/s00227-022-04044-9
Tibshirani R., Walther G., Hastie T. (2001). Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B: Stat. Method. 63, 411–423. doi: 10.1111/1467-9868.00293
van Moorter B., Visscher D. R., Jerde C. L., Frair J. L., Merrill E. H. (2010). Identifying movement states from location data using cluster analysis. J. Wildlife Manage. 74, 588–594. doi: 10.2193/2009-155
van Winkle W. (1975). Comparison of several probabilistic home-range models. J. Wildlife Manage. 39, 118–123. doi: 10.2307/3800474
van Zinnicq Bergm`ann M. P. M., Griffin L. P., Bodey T. W., Guttridge T. L., Aarts G., Heithaus M. R., et al. (2024). Intraguild processes drive space-use patterns in a large-bodied marine predator community. J. Anim. Ecol. 93, 876–890. doi: 10.1111/1365-2656.14108
van Zinnicq Bergmann M. P. M., Guttridge T. L., Smukall M. J., Adams V. M., Bond M. E., Burke P. J., et al. (2022). Using movement models and systematic conservation planning to inform marine protected area design for a multi-species predator community. Biol. Conserv. 266, 109469. doi: 10.1016/j.biocon.2022.109469
Vaudo J. J., Byrne M. E., Wetherbee B. M., Harvey G. M., Shivji M. S. (2017). Long-term satellite tracking reveals region-specific movements of a large pelagic predator, the shortfin mako shark, in the western North Atlantic Ocean. J. Appl. Ecol. 54, 1765–1775. doi: 10.1111/1365-2664.12852
Keywords: elasmobranch, smart position and temperature tags, management stock, trophic flexibility, dynamic brownian bridge movement model, K-means
Citation: Guttridge TL, Heim V, Dedman S, Guttridge AE, Bain SA, Keller BA and Matich P (2025) Stay or go? Space and resource use of the great hammerhead (Sphyrna mokarran) off Andros Island, The Bahamas. Front. Mar. Sci. 12:1544482. doi: 10.3389/fmars.2025.1544482
Received: 12 December 2024; Accepted: 21 January 2025;
Published: 21 March 2025.
Edited by:
David M. P. Jacoby, Lancaster University, United KingdomReviewed by:
Javier Tovar-Ávila, Instituto Nacional de Pesca y Acuacultura (INAPESCA), MexicoNeil Hammerschlag, Shark Research Foundation Inc, United States
Copyright © 2025 Guttridge, Heim, Dedman, Guttridge, Bain, Keller and Matich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. L. Guttridge, dHJpc3Rhbmd1dHRyaWRnZUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID: T. L. Guttridge, orcid.org/0000-0002-6706-1452
V. Heim, orcid.org/0000-0002-8492-9196
S. Dedman, orcid.org/0000-0002-9108-972X
A. E. Guttridge, orcid.org/0000-0002-0385-4832
B. A. Keller, orcid.org/0000-0001-6927-2993
P. Matich, orcid.org/0000-0003-4327-7109
S. A. Bain, orcid.org/0000-0003-1781-9891
 T. L. Guttridge
T. L. Guttridge V. Heim
V. Heim S. Dedman
S. Dedman A. E. Guttridge1‡
A. E. Guttridge1‡ B. A. Keller
B. A. Keller