- 1Division of Ecology and the Environment, Maine Department of Marine Resources, West Boothbay Harbor, ME, United States
- 2Atlantic White Shark Conservancy, Chatham, MA, United States
- 3School of Marine and Environmental Programs, University of New England, Biddeford, ME, United States
- 4Division Newport, Naval Undersea Warfare Center, Newport, RI, United States
- 5Coastal Oregon Marine Experiment Station, Oregon State University, Newport, OR, United States
- 6Atlantic Highly Migratory Species Management Division, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, Gloucester, MA, United States
- 7Outcast Sport Fishing, Hilton Head, SC, United States
- 8Massachusetts Division of Marine Fisheries, New Bedford, MA, United States
While significant progress has been made to characterize life history patterns, movement ecology, and regional estimates of abundance of white sharks (Carcharodon carcharias) in the Western North Atlantic (WNA), patterns of spatial distribution remain relatively unknown in the northern Gulf of Maine. In this study, we utilize data collected from multiple acoustic telemetry projects from 2012-2023 to assess the spatiotemporal distribution of white sharks along sections of the Maine coastline and regional offshore waters. Acoustic receivers were deployed each year from 2012-2019 (mean number of receivers ± SD: 11 ± 4), and effort increased following the first-ever white shark related fatality in Maine in 2020 (2020-2023: 40 ± 15). In total, 107 white sharks tagged by researchers in the WNA were detected, with the majority (n = 90) detected in shallow (<50 m depth) waters post-2019. Reflective of the tagged population at-large, total length of individuals ranged from 2.1 to 4.9 m, with most individuals estimated to be in the juvenile or subadult life stages. White sharks were detected between the months of May-December, with peaks between July and September, and were observed in close proximity to several of Maine’s western beaches and islands/outcroppings, with higher numbers observed at several sites in eastern Casco Bay. Although the overall quantity of detections was relatively low when compared to white shark aggregation sites in other regions, this study provides baseline information on the presence of this species in the northern Gulf of Maine. While future research should include expanded receiver coverage in eastern Maine and the use of additional tagging technologies, this study contributes early insights for informing marine spatial planning, fisheries management, and conservation strategies for white sharks in the region.
1 Introduction
The Gulf of Maine is a marginal sea of the North Atlantic Ocean and is traditionally characterized as a distinct combination of temperate and subarctic conditions. The region has historically been associated with plentiful fisheries and economic opportunity, providing rich habitat for a variety of marine species, including those with commercial (e.g., American lobster Homarus americanus), recreational (e.g., striped bass Morone saxatilis), and ecological value (e.g., Calinus finmarchicus) (Bigelow and Schroeder, 1953; Fish, 1936). However, marine biomes around the world remain in a near-constant state of flux from anthropogenic activities (Wright and Kyhn, 2015), which can lead to physical, chemical, and biological changes. In the Gulf of Maine, where scientists have recently estimated the sea surface temperature (SST) to be warming faster than 99% of the global ocean (Pershing et al., 2015; Saba et al., 2016), it is estimated that under the high CO2 emission model (RCP8.5), salinity will decrease, water temperatures will increase, and subarctic species including, but not limited to, trophically-important zooplankton (Pershing et al., 2021), demersal mesopredators (Grieve et al., 2021), important fishery species (Pinsky et al., 2013; Pershing et al., 2015), and marine mammals (Ross et al., 2021) could decline, followed by an increase in temperate species. The effects of climate change on the Gulf of Maine are exacerbated by ongoing concurrent challenges with sustainable fisheries management (Brodziak et al., 2004), offshore energy development (Davis and Kneebone, 2023), and related ecosystem-based management (Haugen et al., 2024). Such large-scale changes have dire implications for human communities who depend on these marine ecosystems for sources of food, livelihood, culture, and heritage (Pershing et al., 2021).
When considering the implications of change on ecological systems, one of the key areas of scientific investigation is the characterization of species-specific spatiotemporal distributions (Pinsky et al., 2013; Hammerschlag et al., 2022). Predicting the timing and use of habitat by highly mobile predators, such as sharks, is especially useful when assessing the overall impacts of fluctuating conditions on an environment (Bangley et al., 2018; Hammerschlag et al., 2022; Braun et al., 2023; Crear et al., 2023), as these taxa influence local ecosystem function and connectivity with high trophic placement and are relatively vulnerable to extinction risk compared with that of many other marine fishes (Myers and Ottensmeyer, 2005; Rosenblatt et al., 2017; Bastille-Rousseau et al., 2018; Hammerschlag et al., 2019). Furthermore, range expansion or re-establishment of sharks into areas where populations have been previously depleted or absent can have a variety of ramifications. Despite the importance of this information to fisheries and ecosystem managers, patterns of distribution and habitat use by a number of shark species in the northern Gulf of Maine (NGOM; see Methods section for boundaries) remain relatively understudied, including for the region’s largest predatory marine fish, the white shark (Carcharodon carcharias).
White sharks are a large-bodied cartilaginous fish found throughout the world’s temperate and subtropical oceans, with limited observations occurring in tropical waters (Briggs, 1960; Bruce, 2008; Compagno, 2001; Tirard et al., 2010; Guttridge et al., 2024). Despite its wide distribution, the species is globally considered Vulnerable by the International Union for the Conservation of Nature Red List (Rigby et al., 2022). As an apex predator, white sharks exert top-down pressure throughout pelagic and coastal shelf regions and exhibit ontogenetic changes in diet and distribution as they mature from juveniles to adults (Estrada et al., 2006; Hussey et al., 2012; Kim et al., 2012; Grainger et al., 2020; Machovsky-Capuska and Raubenheimer, 2020). In the WNA, biotelemetry studies have shown the majority of white sharks to engage in large scale seasonal migrations along the US east coast, traveling from Atlantic Canada and New England waters in the summer to the Carolinas, Florida, the Bahamas, and the Gulf of Mexico during winter months (Curtis et al., 2014; Skomal et al., 2017; Guttridge et al., 2024). Juvenile WNA white sharks consume predominantly bony fish, squid, and elasmobranchs before transitioning to include more lipid-dense animals such as marine mammals in adulthood (Estrada et al., 2006; Casey and Pratt, 1985), which is hypothesized to support greater migratory capacity and the increased energetic demands of their endothermic metabolism, as well as reproduction and active foraging in colder waters (Klimley, 1985; Watanabe et al., 2015; Grainger et al., 2020; Machovsky-Capuska et al., 2016; Skomal et al., 2017; Franks et al., 2021). In particular, gray seal (Halichorerus grypus) colonies are thought to provide a valuable source of energy to foraging subadult and adult white sharks along the northeast continental shelf of the North American continent (Skomal et al., 2012, Skomal et al., 2017; Grainger et al., 2020; Moxley et al., 2020; Franks et al., 2021; Winton et al., 2021).
While significant progress has been made towards understanding the movement patterns and habitat use of juvenile and adult white sharks in the WNA, particularly near aggregation sites at Cape Cod, Massachusetts (Winton et al., 2021), and Nova Scotia, Canada (Bowlby et al., 2022), substantial knowledge gaps regarding white shark distribution and habitat use within parts of their range remain (Curtis et al., 2014). One such location includes the waters surrounding the State of Maine, which has a coastline that extends 367 km (5,597 km including tidal coastline) and is located between three of the largest gray seal colonies in North America (Wood et al., 2020; Den Heyer et al., 2021). Recent surveys of pinniped abundance in Maine estimate the harbor seal population to have exceeded 61,000 as of 2018 (Sigourney et al., 2021), and pupping surveys have indicated a growing gray seals population in the region more broadly (Wood et al., 2020). Though white sharks have historically been documented in Maine waters (Bigelow and Schroeder, 1936, Bigelow and Schroeder, 1953), fewer than 20 records of sightings, strandings, or fishery encounters were reported between 1880 and 2000 (Casey and Pratt, 1985; Mollomo, 1998; Curtis et al., 2014). However, there are signs that the WNA white shark population is recovering from historical overfishing (Curtis et al., 2014; Winton et al., 2023), and their presence in the region may be increasing.
The application of acoustic telemetry systems provides the opportunity to assess the white shark’s spatiotemporal distribution that can inform fisheries and ecosystem management. Given the rare but potential dangers posed to humans by the presence of sharks in coastal waters (Ferretti et al., 2015; Winton et al., 2021), the information garnered through these endeavors has the added benefit of being used to inform beach management personnel on behavioral patterns and trends of nearshore shark activity. This is particularly useful in locations where human-shark encounters have historically been rare, such as in coastal Maine, which experienced its first ever shark-related fatality attributed to a white shark in 2020 when a woman was attacked while swimming off Bailey’s Island in Casco Bay. Herein, we report on the patterns of seasonal and spatial movement of white sharks along the western Maine coastline, and broadly summarize data from eastern nearshore and offshore waters. This exploratory study provides the first summary of white shark activity in the coastal waters between aggregation sites off Massachusetts and Nova Scotia, and offers baseline data from which future regional projects can build and ecosystem- and/or fisheries-based management decisions can be considered.
2 Methods
2.1 Sampling methods
2.1.1 Tagging
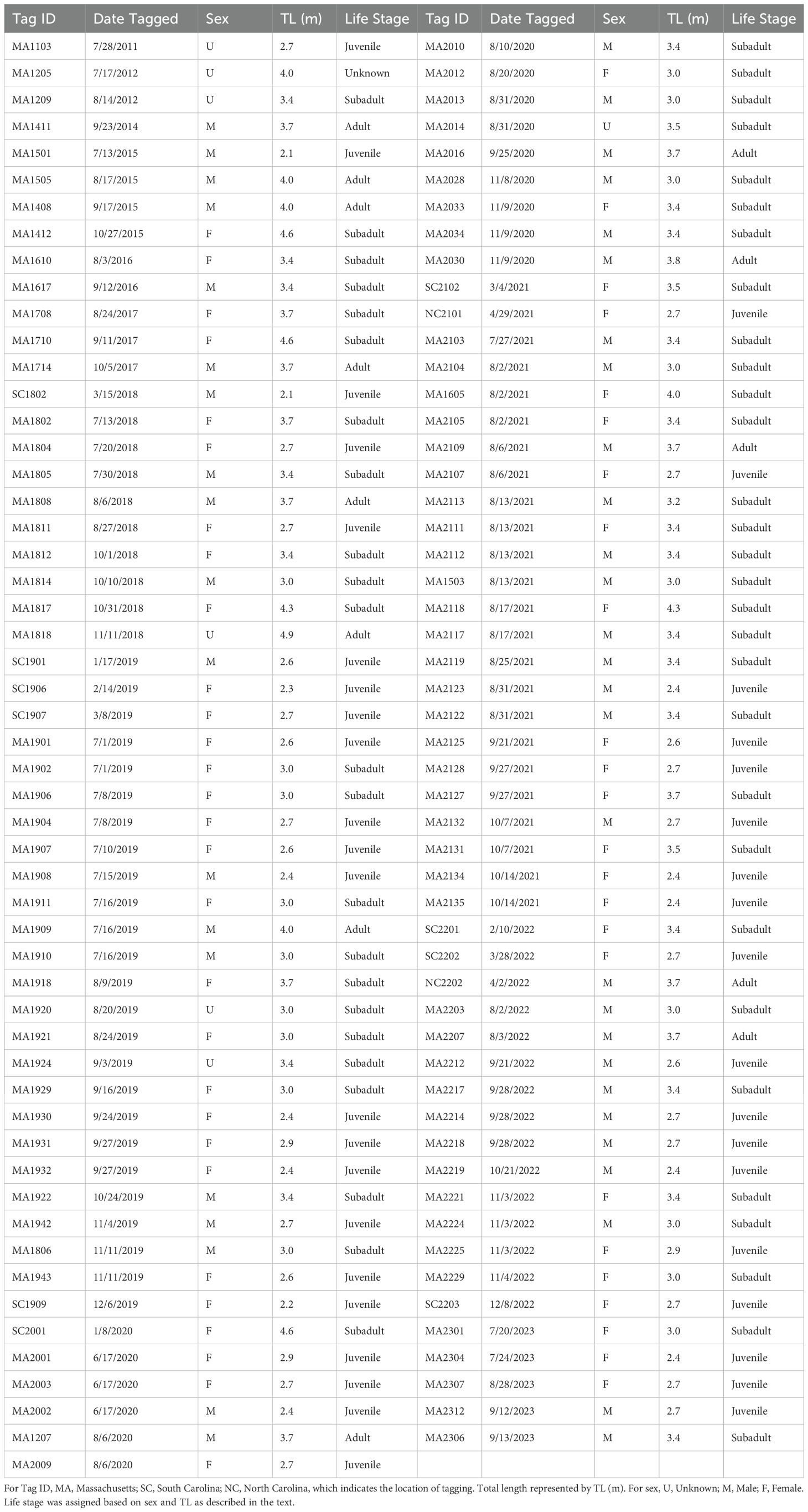
Researchers with the Massachusetts Division of Marine Fisheries (DMF) and the Atlantic White Shark Conservancy (AWSC) deployed 326 acoustic transmitters on white sharks off the coast of Massachusetts during the summer and fall seasons of 2010-2023 (Models V16-4x, V16-6x, V16AT-6x, V16P-6x, or V16TP-6x; Innovasea Systems Inc., Bedford, NS, Canada). An additional 35 transmitters were deployed off the coasts of South Carolina (n = 29), North Carolina (n = 3), Florida (n = 1), New Jersey (n = 1), and New Brunswick, Canada (n = 1), from 2013-2022. Most of the transmitters deployed were tethered to an intramuscular dart that was inserted into the dorsal musculature of free-swimming (n = 289) or baited in and/or captured (n = 34) sharks using a tagging pole (Chaprales et al., 1998; Skomal et al., 2017). Transmitters were implanted internally in five individuals in 2012 and 2013; these sharks were captured on handlines and tagged following the methods described by Domeier and Nasby-Lucas (2012). Of the 293 individual sharks tagged off Massachusetts, 27 were retagged (5 more than once) following battery expiration, the loss of external transmitters, or as part of a double tagging experiment to estimate tag shedding rates. For time periods when individuals had more than one functioning tag, transmissions from a single tag were used for analysis to avoid bias related to multiple tag transmissions. All tagging operations were conducted under Exempted Fishing Permits (SHK-EFP-11-04, SHK-EFP-12-08, SHK-EFP-13-01, SHK-EFP-14-03) issued by the National Marine Fisheries Service Highly Migratory Species Management Division and permits issued by DMF.
The battery life of transmitters used in this study varied from an estimated 1,365 to 3,321 days, with an average time of 2,959 ± 540 (SD) days. Each transmitter had a unique ID, emitting a signal either every 40-80 or 60-120 seconds. All transmitters deployed were programmed to transmit at high power (158 dB re 1 µPa at 1m), but nominal transmission intervals varied among tag batches. The majority of transmitters deployed (n = 299) had random transmission intervals between 60 and 100/120 seconds. A subset of transmitters deployed near the beginning of the tagging period from 2010-2014 (n = 32), in 2019 (n = 10), and in 2022-2023 (n = 20) had random transmission intervals of 30-90, 80-160, and 33-57 seconds, respectively.
The total length (TL) of each free-swimming shark tagged off Massachusetts was visually estimated to the nearest 0.3 m via expert consensus using the tagging vessel’s pulpit length (320 cm) for scale (Skomal et al., 2017). In most cases (n = 268 individuals), underwater video or photographs were also captured of free-swimming sharks, allowing for sex determination by the presence (male) or absence (female) of claspers, and documentation of identifying characteristics (e.g. pigmentation patterns or scars; Domeier and Nasby-Lucas, 2007; Winton et al., 2023). For captured sharks, TL was measured directly and the sex recorded. With the understanding that age and length estimations have variable accuracy when applied to free-swimming tagged fish, three sex-specific maturity stages were assigned as defined by Bruce and Bradford (2012), based on previously published estimates of size-at-maturity (Francis, 1996; Pratt, 1996; Castro, 2011): “juvenile” (≤ 3.0 m); “subadult” (males 3.1-3.5 m; females 3.1-4.7 m); and “adult” (males > 3.5 m; females > 4.8 m). In cases where the sex of tagged individuals could not be determined, maturity stage was only assigned if the estimated TL was ≤ 3.6 m or > 4.8 m. Due to the variable time between tagging event and subsequent detection(s) in this study, estimated changes in TL for individuals were not considered.
2.1.2 Study site and telemetry
The Gulf of Maine is a semi-enclosed region historically defined as being a subarctic sea, characterized by waterflow from the Newfoundland and Labrador Shelf areas through the Gulf of St. Lawrence and Scotian Shelf, conjoined with variable inflow from the northeast-moving Gulf Stream (Loder et al., 1998; Pershing et al., 2021). For this research, we restricted our study area to the northern Gulf of Maine (NGOM), which we define as the coast of Maine from the southwest border with New Hampshire (43.00° N) northeast to the US-Canadian border (44.80° N), and extending into adjacent federal waters (Figure 1). The marine waters of this region feature a complex variety of benthic substrates including, but not limited to, mud, silt, non-vegetative sand, scoured ledge, gravel, and rock/boulder fields, with coastal vegetation including beds of eelgrass (Zostera marina), subtidal red algae (Phyllophora spp.), and a variety of brown algal species (e.g., Laminaria saccharina) (Stevenson et al., 2014). To detect animals carrying acoustic transmitters, passive acoustic telemetry receivers (models VR2W, VR2Tx, and VR2AR; Innovasea Systems, Inc) were deployed at fixed sites in the study area for variable periods from 2012-2023.
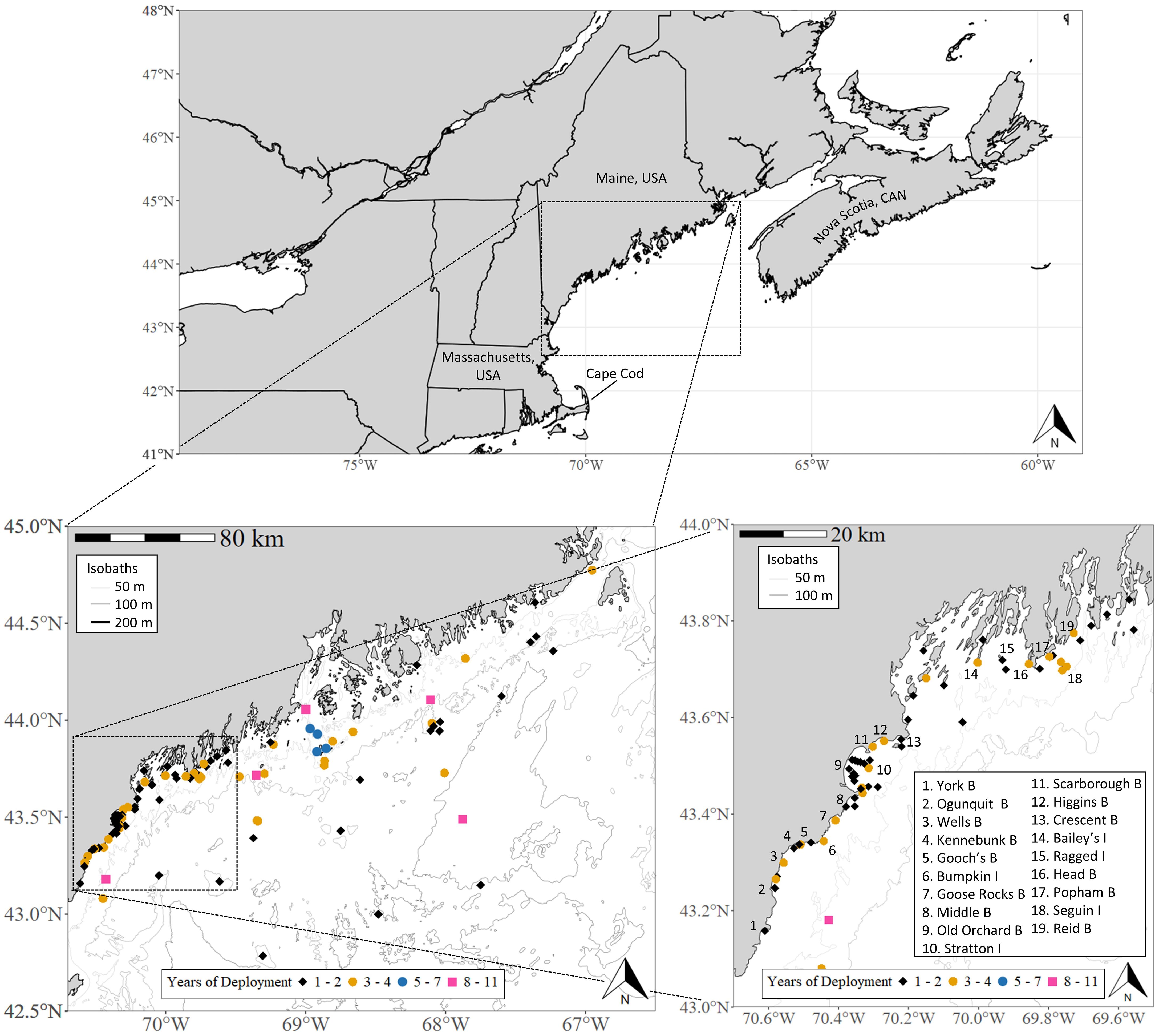
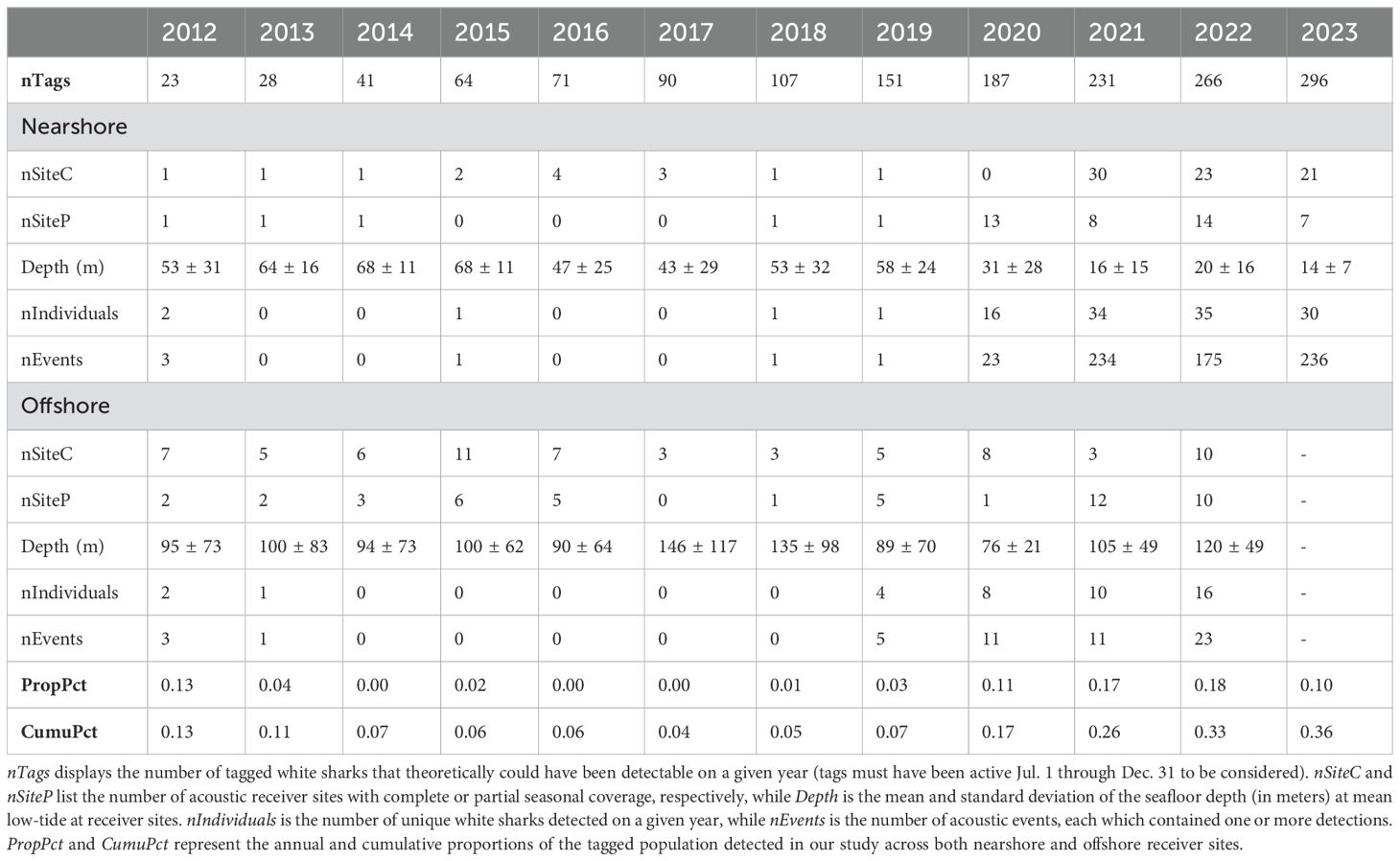
Figure 1. Locations of fixed-position receiver sites in the northern Gulf of Maine study region. The deployment of each receiver varied by year, location, and organization (see Supplementary Figure 1 for annual deployments). Due to the high density of receiver sites in western Maine, a magnified map is provided, alongside a non-exhaustive list of beaches B and islands I adjacent to receiver sites. Bathymetry in the bottom panels is displayed at 1 arc-second resolution (Digital Elevation Models Global Mosaic, NOAA National Centers for Environmental Information). To view receiver-owner organizations, see Supplementary Table 1.
Receiver data were collected by several research programs (see Supplementary Table 1 for list) and shared through the Atlantic Cooperative Telemetry Network (theactnetwork.com) or direct communication. Receiver sites were classified as either nearshore (state waters < 3 nautical miles from the continental US) or offshore (federal waters > 3 nautical miles). Nearshore sites were further categorized by geographic characteristics (nearby beach or island/rock outcropping), and proximity to land was estimated using Euclidean distances from satellite imagery. Offshore sites were not categorized further due to a lack of prominent bathymetry or surface-level landmarks. Nearshore receivers west of 69.00°W were moored near the seafloor (2012-2019) or surface (2020-2023) at mean low-tide depths of 5-60 m (16.9 ± 10.9 m; mean ± SD), while ‘Downeast’ sites (east of 69.00°W) were moored near the seafloor at depths of 30-75 m (67.8 ± 16.1 m). From 2021-2023, two of the western nearshore receivers were real-time acoustic telemetry systems (model Rx-LIVE) housed in custom surface buoys (model NexSens CB-150 Data Buoy; Fondriest Environmental, Inc.). Unlike other models, the Rx-LIVE systems relay transmitter detections near-instantaneously via email/text. During western nearshore deployments, crewmembers documented basic benthic substrate characteristics using standardized definitions from Stevenson et al. (2014) and classified habitat structure as soft (presence of sand, silt, and/or mud), hard (presence of gravel and/or rocks/boulders), or mixed. Observations were conducted using a vessel-mounted echo sounder and anchor sampling, occasionally supplemented by dive operations. Offshore receivers were moored near the seafloor in depths of 35-280 m (100.6 ± 70.3 m). Range tests on a subsample of western nearshore receivers (2021-2022) indicated consistent detection of V16 transmitters at 400-500 m. Detection distances can vary due to factors such as temperature (Gjelland and Hedger, 2013), turbidity (Heupel et al., 2006), depth (Singh et al., 2009), ambient noise (Simpfendorfer et al., 2002), and biofouling (Heupel et al., 2008). Kessel et al. (2014) estimated that approximately 50% of tag transmissions are recorded at 500-600 m. As range tests were not conducted for all sites in this study and detection ranges can fluctuate, no definitive distance threshold for 50% detection was set.
The number of receivers deployed and site locations varied annually, with notable increases in western nearshore waters beginning in 2020 (Figure 1; see Supplementary Figure 1 for annual deployments). This effort was initiated by state authorities to gain a more thorough understanding of shark activity following the white shark-related fatality at Bailey’s Island in July of 2020. That year, receivers were deployed in the western nearshore region from August to November, with sites selected near beaches with high human water use to monitor white shark presence in those areas. From 2021-2023, receivers at nearshore sites (excluding Downeast and year-round sites near Seguin Island and Popham Beach focused on bony fish monitoring) were deployed from May through December to February to avoid potential equipment loss during winter storms. Furthermore, white shark activity in New England waters during winter is low (Skomal et al., 2017). Given the variable timing of receiver deployments, retrievals, and intermittent losses that occurred over this study, for each year site seasonality was categorized as either complete (i.e., deployment for the full term from June 1 - November 30) or partial (≥30 days missed during this period) (see Supplementary Figure 1). New site locations were selected based on historical or anecdotal white shark sightings and areas of observed marine mammal activity (i.e., seal haul-out sites).
2.2 Data analysis
Receiver data were either manually downloaded via the VUE platform (Innovasea Systems, Inc.) or received from collaborating entities and imported into R (R Core Team, 2022). Following the methods outlined by Pincock (2012), data were filtered to remove false detections by identifying the minimum time interval between successive detections using R-package glatos (Holbrook et al., 2024). Remaining data were grouped into ‘events’ for temporal residency analysis following the methodology of Bowlby et al. (2022), whereby an acoustic event was defined as one or more detections received from a shark at a given receiver, followed by >60 minutes of no transmissions from that shark at the same individual receiver. The duration of each event was recorded as the time between first and last detection, calculated in 60 second intervals. To visualize the spatiotemporal movement of detected sharks, events were cataloged by transmitter ID and ordered by date, then mapped using R package ggplot2 (Wickham, 2016).
To characterize the thermal habitat use of white sharks at time of detection, daily satellite 0.25° SST estimates were collected from NOAA’s 1/4 degree daily Optimum Interpolation SST (Huang et al., 2020) and matched to receiver sites by minimum distance and date using R package rerddap (Chamberlain, 2023). Using a limited number of VR2Tx/Rx-LIVE devices from 2021-2023 (n = 6, 4, 3, respectively), we performed a validation test between receiver-recorded temperatures and matched SST data, and we estimated and applied a correction factor of -0.4°C. We employed Kendall’s Tau tests to investigate potential correlations between temporal residency (event duration) and estimated SST, and receiver site depth. A Mann-Whitney U Test was used to test for estimated SST differences between nearshore and offshore receiver sites. Long-term trends in spatial distribution or regional presence of white sharks were not evaluated due to annual variability in receiver deployments and quantity of active transmitters.
The demographics (life stage, sex) of detected white sharks were compared to the larger tagged population using Fisher’s Exact Tests to examine potential regional distribution differences. Time at-liberty between initial tagging and detection was also recorded. Spatial distribution patterns amongst nearshore sites were characterized by a series of metrics including number of sharks observed, acoustic events, site revisits, average days of activity, and receiver-unit effort (e.g., number of area-specific receiver sites multiplied by deployment years). Receiver-unit effort was used to estimate acoustic events per receiver-unit effort (EPUE) and sharks per receiver-unit effort (SPUE). When calculating nearshore site-specific metrics, sites were treated as though each year of deployment was complete. Given that several sites experienced only partial coverage some years due to gear loss or delayed deployment, our metric estimates are likely conservative. To assess differences in event duration between beach- and island-adjacent sites as well as between benthic habitat, we conducted a series of non-parametric Mann-Whitney U Tests with rank-biserial correlations post-hoc.
Seasonal detection patterns were investigated for both life stage and sex. Events were categorized by calendar week, with each individual shark’s presence considered only once per week to avoid oversampling. For life stage and sex, respectively, data were analyzed using Kruskal-Wallis rank sum tests, and post-hoc epsilon square tests were performed to measure effect size; in the case of life stage, we performed a Dunn’s test with Holm-correction for pairwise comparisons. Diel activity patterns were assessed using acoustic events categorized by time of day using R package suncalc (categories: daylight, nautical twilight, or night; Thieurmel and Elmarhraoui, 2022). Following a similar methodology to our seasonal analyses, each individual shark’s presence was considered only once per diel period per day to avoid oversampling. Tests for independence between sex and diel period were estimated using Pearson’s chi-square test, while we employed Fisher’s Exact Test when assessing life stage due to the relatively low number of acoustic events from the adult class. Correlations between event duration and diel period at time of event origin were tested using Mann-Whitney U, while event durations during night and nautical twilight were analyzed by lunar cycle via Kruskal-Wallis and Dunn’s tests. Lunar stages were assigned to events via R package suncalc (categories: new moon [phase >0.875 or <0.125 lunar illumination], waxing moon [phase >0.125 and <0.375], full moon [phase >0.375 and <0.625], and waning moon [phase >0.625 and <0.875]; Thieurmel and Elmarhraoui, 2022). Finally, we applied a chi-square goodness-of-fit to investigate the number of sharks present and quantity of events between day and night periods at beach-adjacent receiver sites to assess potential overlap between white sharks and ocean recreators.
3 Results
A total of 6,317 detections were attributed to white sharks; of these, 0.49% were identified as being questionably false, resulting in a total of 6,287 confirmed detections across 728 separate acoustic events. Most events occurred post-2019 (n = 713, 97.9%) and at nearshore sites (n = 674, 92.6%, Table 1). Between 2012-2023, 94 individuals were detected across 239 dates at nearshore sites and 37 individuals across 47 dates at offshore sites. In total, 107 unique individuals were detected across 276 unique dates. The sample demographic comprised 53 female (26 juvenile, 27 subadult) and 47 male (13 juvenile, 22 subadult, 12 adult) individuals; for the remaining seven, sex could not be determined (Table 2). Sharks were detected in Maine and adjacent NGOM waters from 5 to 2,539 days after initial tag deployment (548 ± 526). The TL of detected sharks ranged from 2.13 m to 4.88 m (3.30 ± 0.58 m) (Figure 2A). This aligned closely with the broader tagged population (3.39 ± 0.66 m), with a slight difference between life stage composition (Figure 2B), though it was not statistically significant (Fisher’s Exact Test, p = 0.107). Likewise, sex demographics were not significantly different between the greater population and the subsample detected here (p = 0.736).
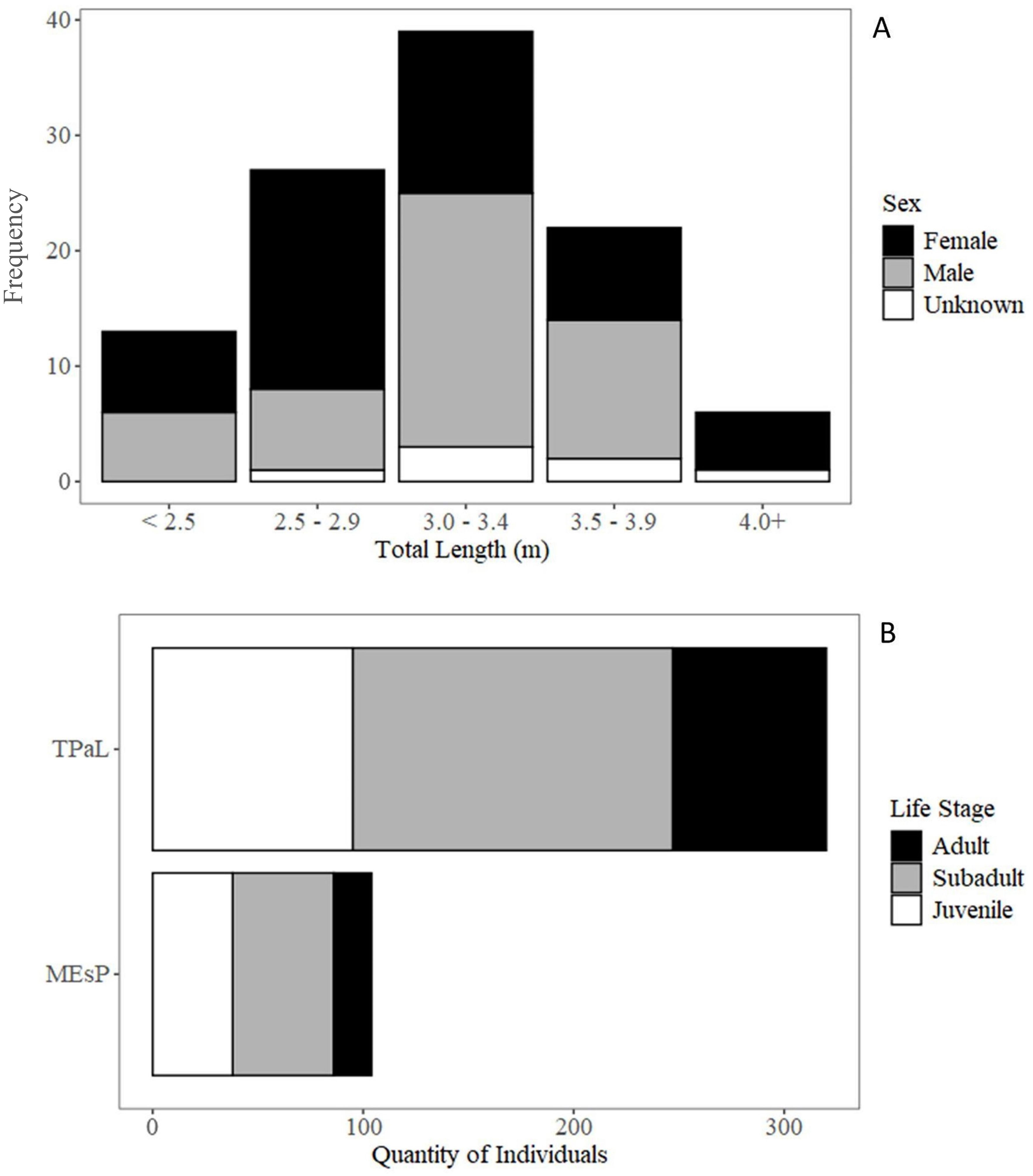
Figure 2. Total length of white sharks detected on acoustic telemetry receivers in the NGOM (A) and the demographics of the tagged population at large (TPaL) and the subpopulation detected in the NGOM (MEsP) (B). Note that one individual (MA1205) was omitted from plot (B) because its sex could not be determined and life stage could not be assigned.
3.1 Spatial observations
Presence of tagged white sharks was widespread across the NGOM region. Sharks were detected in ocean depths ranging from 4.9 to 171.0 m, with the predominance of acoustic events (n = 491, 67.5%) occurring at receiver sites within 1-km of the shoreline (30.7% of total sites fell in this range). It is also noteworthy that sharks were detected at receiver sites located in depths of <20 m at mean low-tide (n = 431 events, 59.2%). Sea surface temperature estimates at time of event origin ranged from 8.4 to 21.3°C (15.9 ± 2.3°C; n = 657 events), and while no VR2Tx model receivers were deployed at offshore sites for validation, daily SST values associated with acoustic events at offshore sites were lower than nearshore sites (mean = 14.0°C offshore vs. 15.9°C nearshore; p < 0.001). While we observed a statistically significant relationship between event duration and estimated SST (p = 0.048), the association was minimal (τ = 0.050, z-score = 1.981), and in practical terms may not have been a primary driver of site-specific temporal residency in our study.
White sharks were detected at receiver sites adjacent to several of Maine’s prominent sandy beaches used for human recreation, including Ogunquit, Kennebunk, Higgins, Popham, and others (Figure 3A; Table 3). Notably, the receiver site near Head Beach on Hermit Island, situated at the southwest tip of the Phippsburg peninsula, recorded a greater number of unique white sharks (n = 40) and sharks per receiver-unit effort (SPUE = 13.3) than any other in this study. Moreover, the presence of tagged sharks at the Head Beach site was recorded on 53 unique dates across three years of surveying, and the number of acoustic events per receiver-unit effort surpassed that of the other NGOM beach sites by more than three standard deviations (EPUE = 18.7; beach mean EPUE = 4.4 ± 4.2). Interestingly, however, EPUE of the nearby receiver site adjacent to Ragged Island in eastern Casco Bay was more than double (37.5) that of Head Beach’s site (Figure 3B), and although the site observed less sharks overall (n = 23), SPUE was relatively similar (11.5). Additional areas with high EPUE included Seguin Island off the SE coast of Popham Beach, which between its three surrounding receiver sites detected 37 unique white sharks (SPUE = 4.1) with a mean EPUE of 13.3, and the receiver site situated near Bailey’s Island in Casco Bay, which detected 17 white sharks (SPUE = 4.3) over 45 separate acoustic events since August of 2020 (EPUE = 10.5). Excluding one receiver site approximately three miles south of Petit Manan, Downeast and offshore NGOM receivers sites detected relatively low numbers of tagged individuals (ranging from one to seven). While the offshore site adjacent to Petit Manan observed comparatively high EPUE (7.0) to others in the sub-region, the number of tagged sharks recorded (n = 16; SPUE = 5.3) was similar to several of the western nearshore NGOM sites (Table 3).
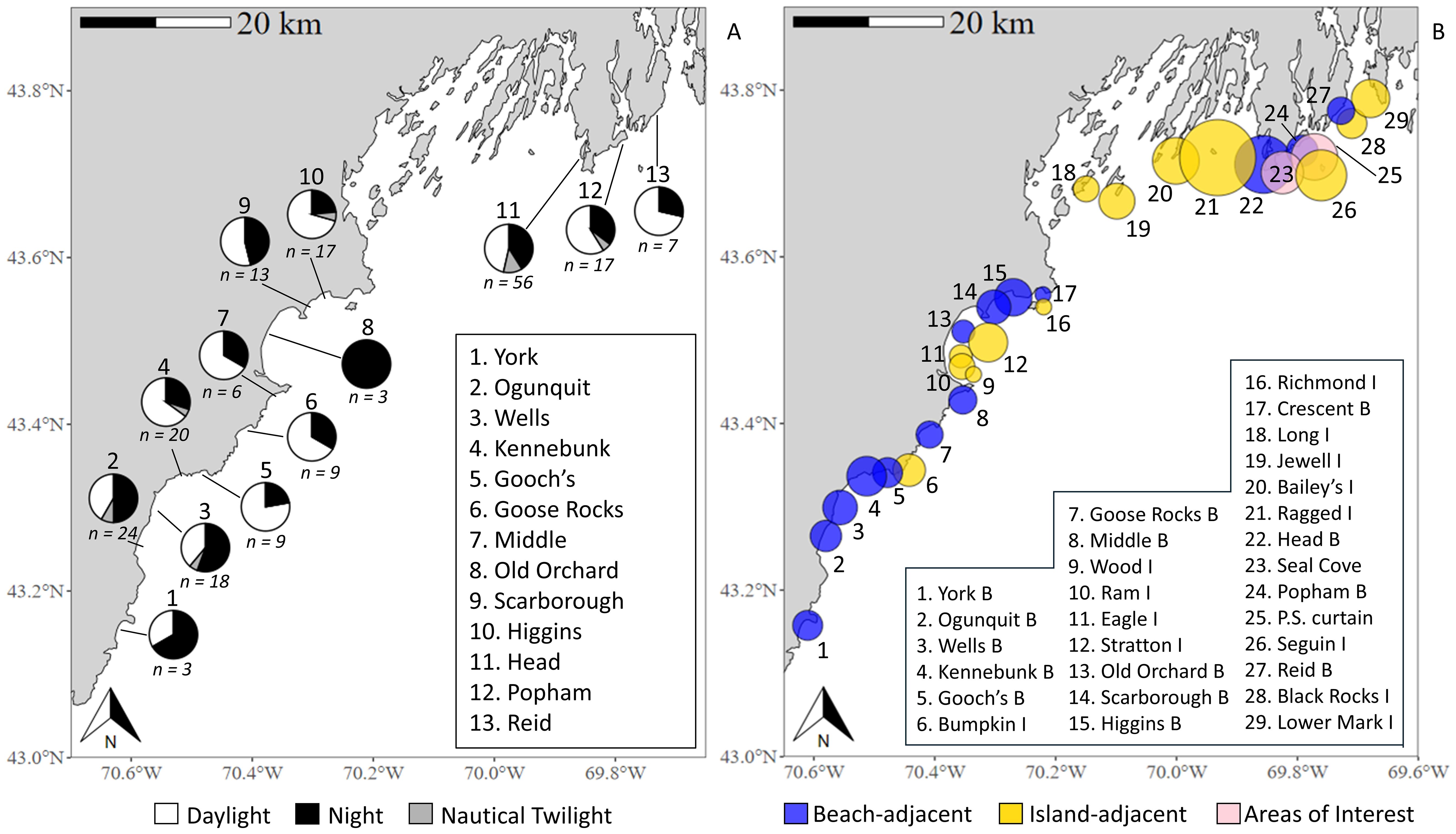
Figure 3. Proportion of acoustic events by daylight cycle from beach-adjacent receiver sites, sans Crescent Beach (n = 1 event; nautical twilight) (A). Number of acoustic events (n) is displayed below each pie chart. Plot (B) displays the relative number of events per receiver-unit effort (EPUE) at western nearshore receiver sites. Western nearshore sites were categorized as being either adjacent to beaches (blue) or islands (gold). Sites that did not fit either description were characterized as additional Areas of Interest (pink).
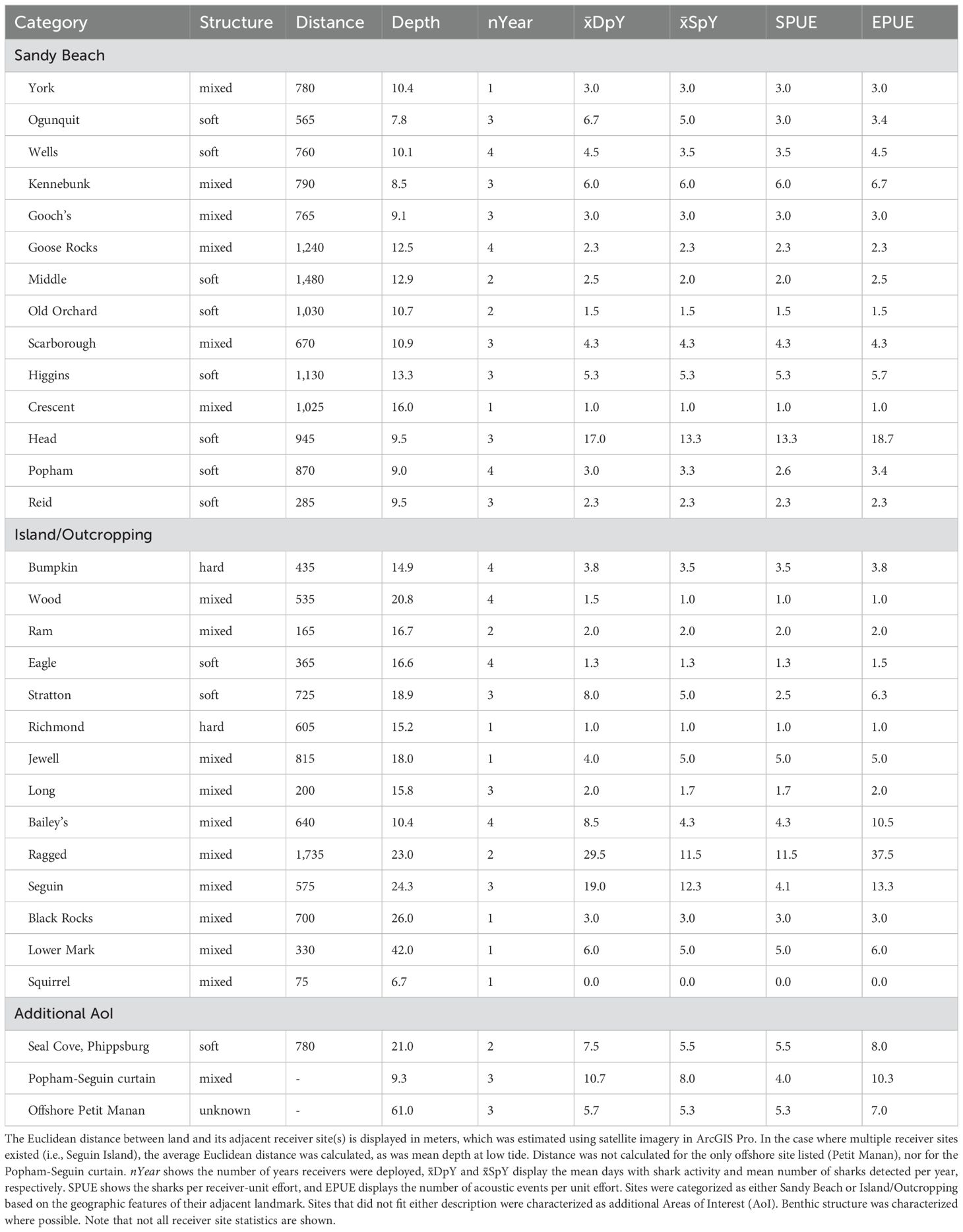
Table 3. Summary of detections of tagged white sharks at receiver sites (ordered south to north by category) in the northern Gulf of Maine.
Of the sharks detected, most were only detected in one calendar year (n = 76, 71.03%). Seventy-two individuals were detected on two or more dates (mean = 3.24 ± 4.06 days) and 79 at two or more sites (4.83 ± 4.43 sites). Thirty-four sharks were detected at >3 sites within a 7-day period, and twenty-two within a 24-hr period. Sharks of all sizes and sexes were detected at both nearshore and offshore receiver sites, displaying variability in space use within. For example, shark MA2217 was detected by a multitude of nearshore receiver sites for >30 hours over seven days (Figure 4A), while MA2134 was detected at four offshore receiver sites for a combined total of <60 min across 112 days (Figure 4B). Among sharks detected over multiple years (2-years n = 22 sharks, 3-years n = 8, 4-years n = 1), all but seven returned consecutively, and nine returned to one or more previous sites. Of those, five returned to the site at Head Beach; no other site showed such inter-annual revisitation. On shorter time scales, 14 sharks revisited the same site within a 24-hr period. These revisits comprised 88 acoustic events - most involving receiver sites adjacent to Ragged Island (n = 24), Seguin Island (n = 19), or Bailey’s Island (n = 16). However, intra-annual revisitation rates were generally low across all nearshore receiver sites (mean = 12.71%). While no sharks were detected concurrently at one site, we observed 43 cases of multiple sharks detected within 24-hrs at the same site, with no clear location of increased frequency.
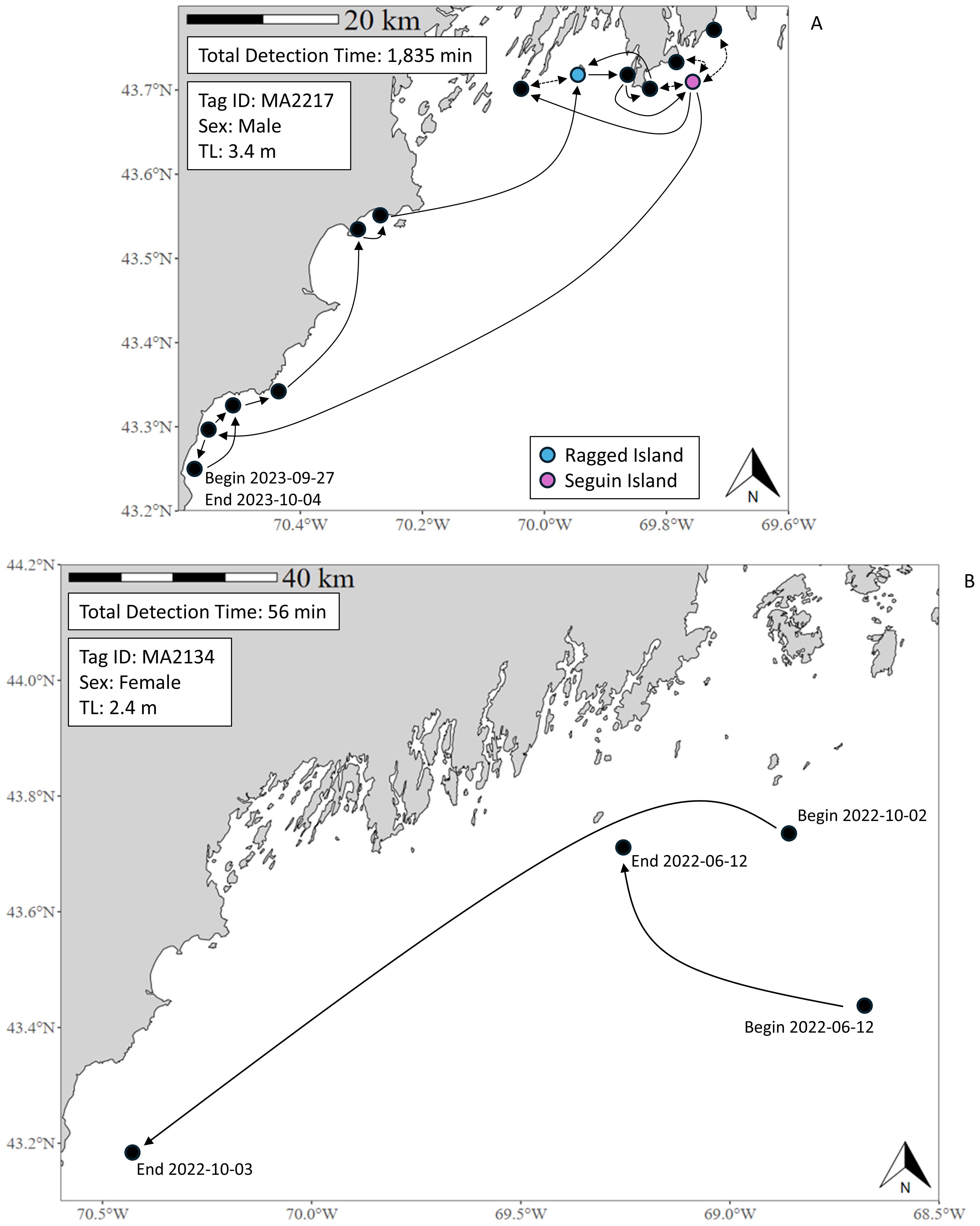
Figure 4. Spatial movement of two individuals. MA2217 (A) was within receiver detection range for more than 30 hours across a 7-day period, spending most of that time around receiver sites adjacent to Ragged Island and Seguin Island. MA2134 (B) was within receiver detection range for less than one hour across a 112-day period, with no apparent preference for any given site or area.
3.2 Seasonality and temporal residency
White sharks were detected during 23-25 weeks of the calendar year when nearshore receiver effort was greatest (2021-2023). Seasonal timing of activity varied little between calendar years, with the earliest detection occurring in week 21 (May 26th, 2021) and the latest in week 49 (December 7th, 2021). Across years, most events occurred during summer and early fall (June-October; Figure 5A) when SST generally exceeded 13°C. When assessing for differences in activity between life stages by week, we observed near-significant differences between groups (x2 = 5.881[2], p = 0.053, ϵ2 = 0.028), with the greatest differences between juveniles and subadults (z = 1.867) and adults (z = -2.173), though neither were statistically significant (Holm-adjusted p = 0.124 and 0.089, respectively) (Figure 5B). Conversely, sex was determined to have a statistically significant effect (x2 = 5.530[1], p = 0.019), with females arriving earlier but largely overlapping with males (ϵ2 = 0.028) (Figure 5C). When investigating how these demographic characteristics related to diel activity, neither life stage (p = 0.155) nor sex (x2 = 1.644[2], p = 0.440) showed significant effect. However, when assessing diel patterns of activity at beach-adjacent receiver sites, event quantity was greater during daylight periods (54.8%, n = 115 events) than at both night (39.0%, n = 82) and the relatively short period of nautical twilight (6.2%, n = 13) (Figure 3A). However, when we assessed shark activity by presence and not by quantity of events (i.e., we only considered the first event from each transmitter ID “x” within diel period “y” on date “z”), there was no significant difference between daylight and night periods (x2 = 0.374[1], p = 0.541), nor event durations between them (p = 0.856). Amongst night and nautical twilight periods, lunar phase showed significance (x2 = 8.589[3], p = 0.035), with longer event durations during full moon phases (mean = 20:24; mm:ss) than new moon phases (mean = 09:56; z = 2.862, Holm-adjusted p = 0.013), though other lunar phases did not significantly differ (p-range = 0.084-0.430).
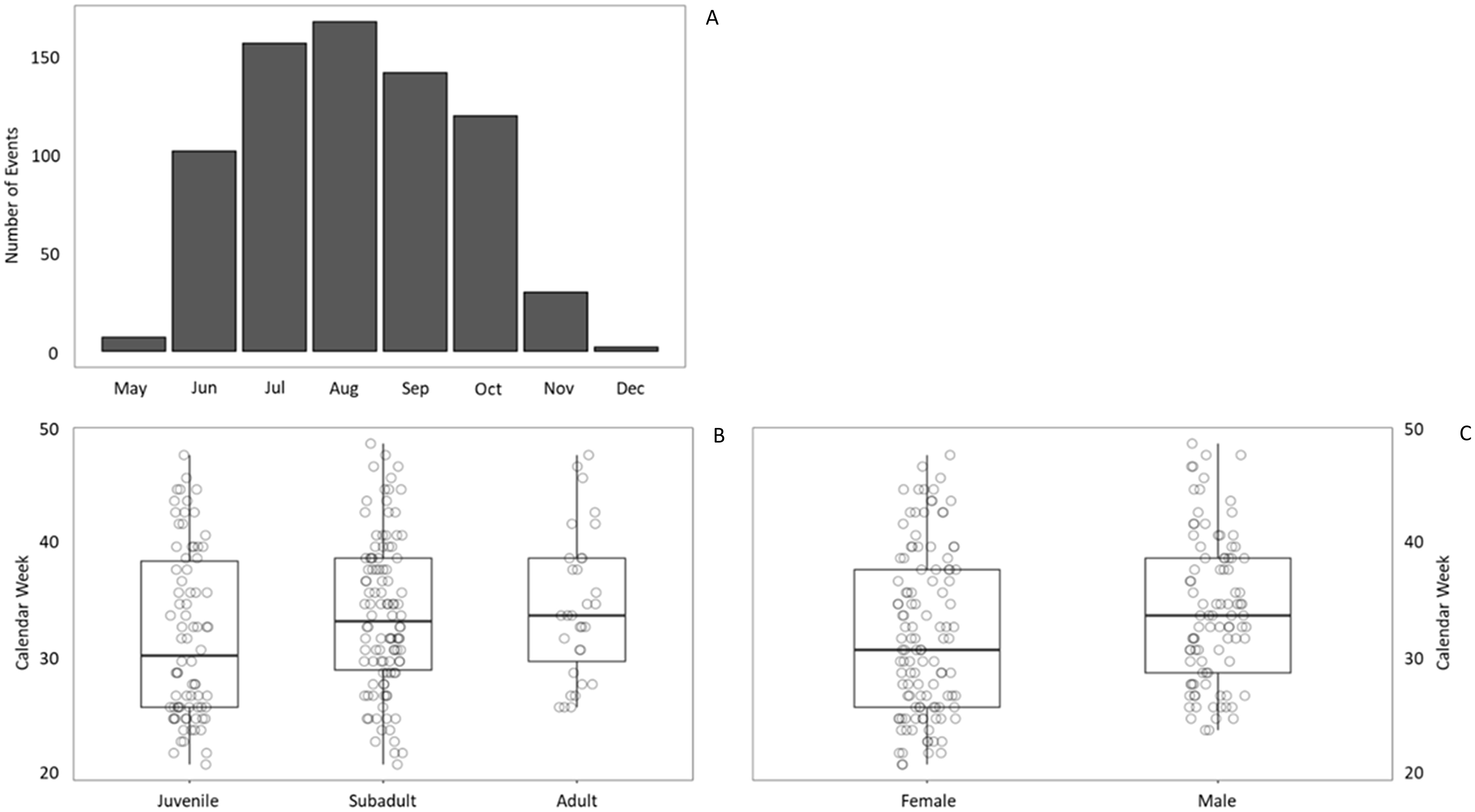
Figure 5. Annual timing of white shark acoustic events. Events are displayed across months (A) and are categorized across weeks by life stage (B) and sex (C) from 2012 - 2023. Each dot in plots (B, C) represents an event, with each individual shark’s presence considered only once per week to avoid oversampling. Plots (B, C) display the mean, first and third quartile, and whiskers extending up to 150% of the first and third quartile. Events associated with one and seven sharks were omitted from plots (B, C), respectively, due to their unknown sex.
The duration of acoustic events ranged from 1 to 166 minutes (nearshore site mean = 14:11, offshore site mean = 08:07; mm:ss). Among nearshore sites, results indicated event duration was not correlated with depth (τ = 0.036, z = 1.337, p = 0.183) or benthic substrate (p = 0.534), but that white sharks were on average detected for longer durations when near island-adjacent sites than beach-adjacent sites (p = 0.004). However, this effect was small (rank-biserial correlation = 0.099). Across sites, the majority of acoustic events were recorded as being less than 10 minutes in length (n = 404), and with fewer than 10% surpassing 30 minutes (n = 59). The predominance of detections (n = 5,419, 86.2%) and events (n = 548, 75.3%) were recorded at 15 locations monitored over the study. All but one of those areas were located in nearshore habitat and had similar estimated event lengths, with a number of events lasting between 10 and 20 minutes, and occasionally exceeding 60 minutes (Figure 6).
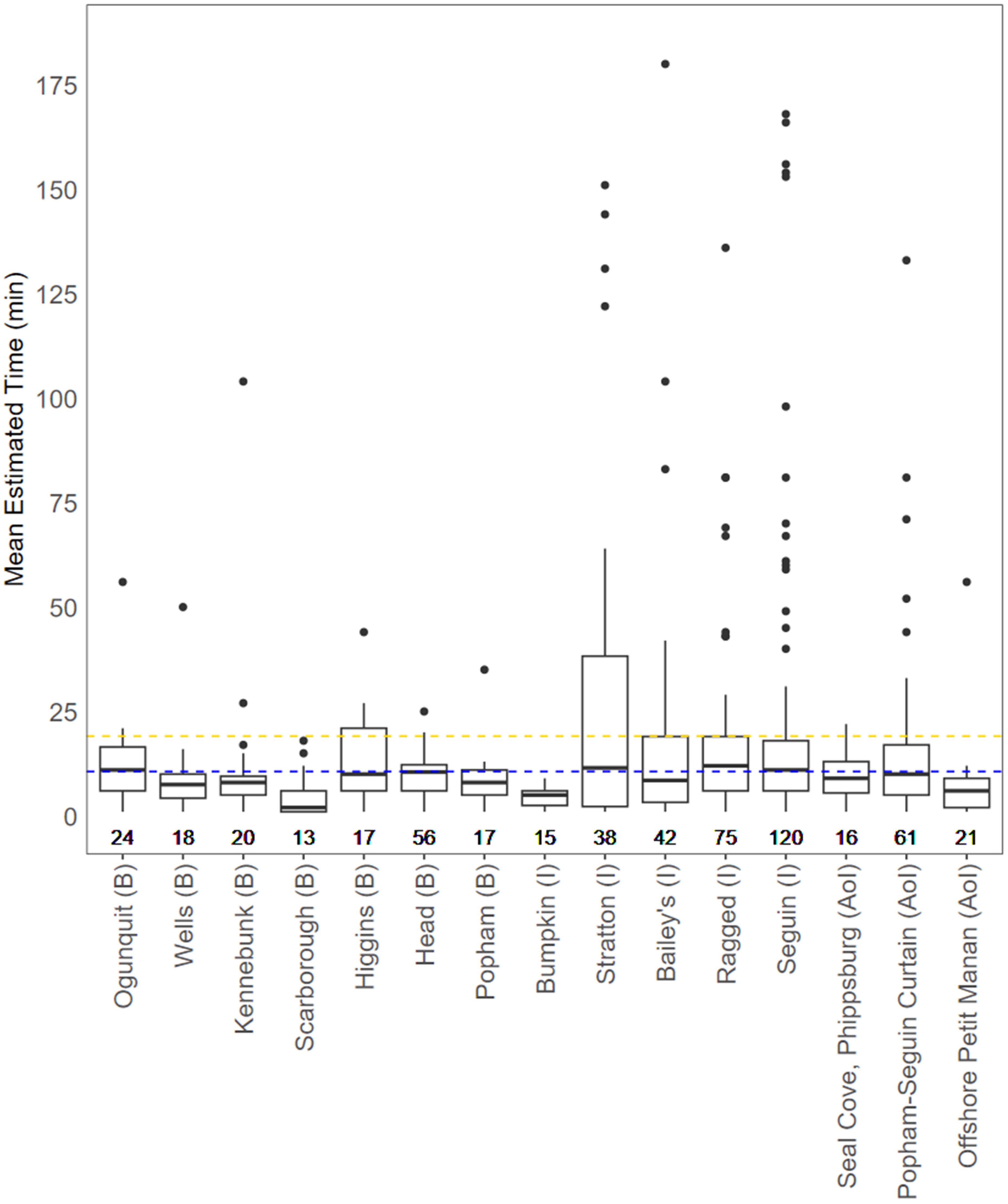
Figure 6. Average estimated event duration, in minutes, for the 15 locations which logged the greatest number of white shark detections. Each boxplot displays the median, first and third quartile, and whiskers extending up to 150% of the first and third quartile. The weighted average duration time at beach sites (blue dashed line) and island sites (gold dashed line) are also shown. Sites are ordered from west to east and grouped by category, identified as beach-adjacent B, island-adjacent I, or as an area of interest AoI. The number of events per site, uncorrected for unit-effort, are displayed below each plot. Sites that did not record ≥ 10 acoustic events were excluded as were data points >180 minutes to maintain visual resolution. This includes three events from Bailey’s Island and two from Seguin Island. The receiver site labeled as ‘Offshore Petit Manan’ was the only offshore site to record ≥ 10 events.
4 Discussion
While white sharks have long been known to occur along the Maine coast (Casey and Pratt, 1985; Mollomo, 1998), this is the first study to characterize the seasonal and interannual spatiotemporal distribution patterns of their occurrence. Although the number of white shark detections in this study was relatively low when compared to that observed in studies of aggregation sites (e.g., Winton et al., 2021), habitats adjoining aggregation sites (e.g., Harasti et al., 2017), young-of-year (YOY) habitats (e.g., Anderson et al., 2021a), or large regional arrays (e.g., McAuley et al., 2017), this outcome can likely be attributed to the exploratory nature of our array. Receivers were distributed across a broad section of the coastline, and not all were specifically placed to target expected white shark habitat, particularly from 2012-2019, leading to a less concentrated effort in areas that may experience peak activity. Furthermore, the partial seasonality of many receivers is likely to have led to lower counts than if receivers had been deployed for the entirety of each season, particularly at nearshore sites. Nonetheless, this study reflects the largest collaborative white shark acoustic tag monitoring effort in the NGOM, providing new insights into this important predator’s distribution and site-specific residency in an understudied region. As white sharks continue to inhabit Maine waters, these results will be valuable to marine resource managers as they balance the conservation, fisheries management, and potential human-wildlife conflict challenges presented by this species.
4.1 Broad patterns of spatiotemporal distribution
Despite the current constraints of acoustic technologies and their fluctuating range capacities (Kessel et al., 2014) as well as the limited receiver coverage along much of Maine’s coastline, NGOM receivers detected a substantial proportion of the tagged white shark population at large, particularly between 2020-2023 when receiver effort and the number of tagged sharks were highest (Table 1). Furthermore, shark demographics in the NGOM were reflective of the broader tagged population, indicating that a considerable portion of white sharks observed in nearshore Cape Cod waters, where most of these sharks were tagged, also use western Maine’s nearshore waters, suggesting evidence of ecosystem connectivity and gene flow across the region. This was further supported by observations where one or more sharks were detected in both areas in close succession. For example, shark MA2212 was detected at Newcomb Hollow Beach in Cape Cod and at Kennebunk Beach (approximately 177-km northeast) the following day, and shark MA2217 was observed moving back and forth between Cape Cod and NGOM sites from September to October 2023. Several sharks were also detected at NGOM receivers soon after being tagged (e.g., MA1605, MA2306, MA2107, MA1205 within 7-days following tagging in Cape Cod waters). While only 37 acoustic events from this study occurred in the Downeast region, additional evidence from marine mammal bite wound reports (Rosemary Seton, Allied Whale, unpublished data) supports regional white shark activity. Previous studies using satellite-linked tags (Skomal et al., 2017; Franks et al., 2021; Winton et al., 2021; Bowlby et al., 2022) have also documented white sharks moving through Downeast Maine and the Bay of Fundy, suggesting that use of the Downeast region may currently be underrepresented in our data due to limited receiver coverage.
Data from this study provide evidence that white sharks moving into the NGOM regularly inhabit shallow coastal areas, as the highest levels of EPUE occurred at nearshore receiver sites in depths <30 m and within 1-km of shore. Although white sharks typically undergo ontogenetic shifts in feeding and migratory behavior, with juveniles preferring shallower nearshore habitats (Shaw et al., 2021) and gradually expanding to include deeper offshore waters with size (Skomal et al., 2017), the convergence of all life stages on the northeast continental shelf’s nearshore waters during summer and fall is likely driven by abundant regional foraging opportunities, which provide key sources of energy acquisition (Casey and Pratt, 1985; Skomal et al., 2012; Curtis et al., 2014, Curtis et al., 2018; Franks et al., 2021; Winton et al., 2021, Winton et al., 2023). Although YOY sharks were not detected, individuals tagged as YOY in the New York Bight (Curtis et al., 2018; Shaw et al., 2021) have been detected in Maine waters in subsequent years of life (i.e., at least age-1; T.H. Curtis, unpublished data), and while broad temporal overlap between sexes was observed (Figure 5C), our results indicate that female white sharks arrive in the NGOM earlier than their counterparts. Future research would benefit from the implementation of equipment which records abiotic conditions at higher-resolutions (e.g., satellite-linked archival transmitters) to investigate potential drivers of sex- and size-specific habitat use patterns in the NGOM.
Here, we observed a weak but statistically significant relationship between white shark event durations and estimated SST. Given the broad temporal and spatial scale from which temperature data were derived (0.25° surface daily estimates) and the limited number of temperature-logging receivers available for validation, our ability to precisely monitor temperatures experienced by white sharks was constrained. This limitation is further compounded by the vertical movement of white sharks, as they often spend time at depths below the surface layer (Bonfil et al., 2005; Weng et al., 2007; Nasby-Lucas et al., 2009; Skomal et al., 2017; Winton et al., 2021; Franks et al., 2021; Spaet et al., 2022), thus experiencing different thermal regimes. Given the endothermic capabilities of lamnid species (Goldman, 1997; Dolton et al., 2023) in conjunction with their relatively large body mass, white sharks can inhabit a wide range of water temperatures in the WNA (observed range:-0.9-30.5°C; Franks et al., 2021). However, remaining within specific thermal ranges optimizes bioenergetic processes (Watanabe et al., 2015), and previous research has suggested that water temperature is one of several migratory cues for WNA white sharks (Casey and Pratt, 1985; Cliff et al., 1989; Curtis et al., 2014; Skomal et al., 2017; Franks et al., 2021). In our study, most events (90%) coincided with SST estimates between 13-20°C, a range similar to that observed in prior studies that considered regional SST preference (Casey and Pratt, 1985; Curtis et al., 2014), albeit with a truncated upper thermal range similar to that inhabited by white sharks at the Cape Cod aggregation site (Winton et al., 2021) and in Atlantic Canada (Bowlby et al., 2022). Generally, departure from New England to overwintering grounds in the southeastern US and Gulf of Mexico occurs in the late fall/early winter when water temperatures drop below 12°C (Casey and Pratt, 1985; Curtis et al., 2014; Skomal et al., 2017; Bowlby et al., 2022), and is less related to prey availability (Hammill et al., 2017; Franks et al., 2021). With the Gulf of Maine reportedly warming faster than most of the world’s oceans (Pershing et al., 2015; Saba et al., 2016), it is possible that the seasonal occurrence of white sharks (and their prey species) in coastal New England and Atlantic Canada will change with shifting temperature regimes, as has been observed in other highly migratory predators (Crear et al., 2023). Thus, continued monitoring is needed, particularly since resulting range shifts by highly migratory predators can have a series of cascading effects, including but not limited to increased or decreased chances of human-wildlife conflict (Chapman and McPhee, 2016; Tanaka et al., 2021) and changes in trophic dynamics (Rosenblatt et al., 2017; Bastille-Rousseau et al., 2018; Hammerschlag et al., 2019, Hammerschlag et al., 2022).
4.2 Regional nearshore movement
Based on movement data from tagged sharks, our results suggest that western Maine’s coastal waters may serve as a migratory corridor for some individuals. In multiple instances (n = 13 individuals), sharks were observed traveling along the coastline in a stepwise pattern (>4 sites) over a period of <24-hr. For example, in July of 2022, shark MA2009 was detected near Kennebunk Beach, then traveled north along eight sites to Seguin Island, covering over 90 km in a 24-hr period. A similar pattern was observed in other individuals over multiple time periods, including shark MA1911 who was detected up and down the coast between sites near Ogunquit and Saco Bay in both July and October of 2021. Comparable latitudinal and longitudinal movements have been observed from satellite-tagged white sharks at WNA aggregation sites (Skomal et al., 2017; Curtis et al., 2018; Bowlby et al., 2022), suggesting that western Maine’s coastline may connect areas of biological importance, such as Cape Cod and Nova Scotia. Such behavior has been observed elsewhere in this species (i.e., Jorgensen et al., 2009).
Several sharks were detected at multiple receiver sites in close proximity before exiting an area or were observed returning to one or more site(s) within a 24-hr period, particularly near Stratton, Bailey’s, Ragged, and Seguin islands. Receiver sites at these locations also recorded a number of events with durations significantly longer than the mean (Figure 6), suggesting the presence of one or more factors sharks found beneficial. Although acoustic telemetry data alone cannot explain these events, the consistent presence of local pinniped activity (M.D., B.J., and J.I., unpublished) could indicate that some of these events may have captured active foraging or patrolling behaviors from white sharks. This movement pattern is typical of the summer residency phase of WNA white sharks, wherein sharks patrol relatively small areas while foraging at aggregation sites, and are often observed near gray seal haul-outs (Skomal et al., 2012, Skomal et al., 2017; Moxley et al., 2020; Franks et al., 2021; Winton et al., 2021, Winton et al., 2023). Unfortunately, reports of coastal Maine pinniped abundance do not provide spatial-linked population data, and despite past seal monitoring efforts at these and other Maine islands (Sigourney et al., 2021), the ability to perform more robust comparisons of habitat use in relation to NGOM pinniped prey abundance remains limited.
While most sites with multi-year visitation were only linked to one or two sharks, the site near Head Beach recorded five sharks re-visiting in consecutive years, with four also returning to sites near to Popham Beach, Seguin Island, or Reid Beach. Intra-annual revisitation rates were relatively high at sites near Seguin and Ragged islands compared to other nearshore NGOM sites, with more than one-in-four sharks returning. This pattern of intra- and inter-annual visitation, while certainly not unique to Maine’s coastline (Anderson et al., 2011; Winton et al., 2023), indicates that eastern Casco Bay and the waters surrounding the Phippsburg peninsula may act as foraging grounds or function as a migratory corridor for coastal white shark movement while in the NGOM. This is further supported by the comparatively high number of days with shark activity per year at sites near Ragged Island, Head Beach, and Seguin Island relative to other NGOM sites (Table 1). Future research would benefit from increased receiver densities to capture higher resolution data on habitat use and differences between proximal NGOM sites, as well as a more thorough understanding of regional prey distribution.
Although patterns of vertical and fine-scale habitat use have been observed in the Atlantic and elsewhere (Weng et al., 2007; Bradford et al., 2020; Winton et al., 2021), such patterns were not evident in this study and are likely the result of the exploratory placement of receivers, relatively low regional receiver coverage, and limited-resolution habitat data. Furthermore, acoustic telemetry analyses are complicated by variable depths and potential variation in substrate/habitat types within each receiver’s detection radius. Regarding events which occurred at night and nautical twilight, those which occurred during the full moon phase were observed to be significantly longer than during the new moon phase, and while more work is needed to understand the relationship between lunar phase and white shark behavior in the NGOM, sharks have exhibited different behaviors with lunar cycle (Weng et al., 2007; Fallows et al., 2016; Winton et al., 2021). For example, research in the eastern Pacific suggests that juvenile white sharks may forage at night during the full moon phase (Weng et al., 2007), and that in South Africa, white sharks may benefit from increased lunar illumination that occurs during a full moon while hunting (Fallows et al., 2016).
4.3 White sharks and public safety in Maine
Results from this study highlight the broad use of coastal NGOM waters by white sharks, with activity occurring in waters adjacent to observed seal haul-outs (i.e., Ragged Island) as well as several of Maine’s popular beaches for recreation. However, though nearly half of detection activity at beach-adjacent sites occurred during daylight hours (when human use of beaches is highest), the degree to which white sharks were detected near Maine beaches was very low when compared to known aggregation sites (Anderson et al., 2021b; Rex et al., 2023; Winton et al., 2023; Bowlby et al., 2023), and event durations were generally short. Notably, apart from Head Beach, every beach-adjacent receiver site in this study logged, on average, less than seven days of presence from tagged white sharks per year (Table 3). However, it is important to remember that receiver coverage along beaches was typically limited to only one site per year in the NGOM. As a result, some tagged animals may have gone undetected for one of the following reasons: 1) they were outside the receiver’s detection range, 2) a physical barrier was present, or 3) seasonal receiver coverage was not complete. Additionally, there are an unknown number of untagged white sharks in the population, which may have similar movement patterns near beach areas, but remain undetected. Thus, acoustic telemetry is a valuable tool to characterize the presence of sharks at sites of interest, but it will always benefit from increased receiver coverage and deployment of tags on individuals throughout the species’ range.
While white sharks can pose potential risk to humans, spatiotemporal overlap does not necessarily result in conflict (Rex et al., 2023), and even at Cape Cod and other aggregation sites, negative interactions between humans and white sharks remain rare (Curtis et al., 2012; Ferretti et al., 2015; Winton et al., 2021). Furthermore, the presence of white sharks in nearshore Maine waters has been historically documented (Casey and Pratt, 1985; Mollomo, 1998), yet no attacks had been attributed to the species in these waters before 2020 (Florida Museum of Natural History, 2024). Therefore, despite the presence of white sharks off Maine, there is no reason to expect significant overall risk to water users, though risk may be higher when abundant prey species are in close proximity. Given the limited interaction between ocean users and white sharks in Maine waters, along with the protections afforded to the species under various international conservation and management bodies, there is no justification for implementing regional anti-shark measures, such as shark nets. Nonetheless, it is important that beach management be ready to respond should an unwanted shark encounter occurs. With water temperatures rising in the Gulf of Maine due to the effects of climate change (Pershing et al., 2015), there is a strong possibility that the seasonality of white sharks in regional waters could extend beyond current trends in the fall, as well as increased beach attendance by swimmers, leading to prolonged periods of spatial overlap between white sharks and ocean recreators in this region.
4.4 Conclusions and future directions
By assessing acoustic transmitter data from over three hundred individuals, our findings provide evidence that a sizable portion of the WNA white shark population that visit the Cape Cod aggregation site also uses the waters of western nearshore Maine, particularly around the eastern Casco Bay area. However, due to limited receiver coverage, this study was not able to quantify habitat use patterns that may exist in parts of Downeast Maine and in offshore NGOM waters. At this time, the overall low number of detections in this study would suggest that shark activity is more diffuse along Maine’s coastline than at the nearby Cape Cod aggregation site, and more focused work is needed to identify any potential areas of unrealized activity. In addition to the implementation of higher receiver densities, the inclusion of focused tagging operations and other sample collections (e.g., genetic material) in Maine waters alongside that performed in Massachusetts, Canada, and elsewhere will support federal and international research initiatives around conservation management of the species in this historically understudied region. These early findings merit further study of white shark distribution throughout this region to improve the resolution of data available to fisheries and marine resource management, and results from this and future regional studies can be used to inform the boundaries of Essential Fish Habitat for white sharks in NOAA’s Atlantic Highly Migratory Species Fishery Management Plan, which currently retains a northerly nearshore limit at latitude 42.657° N, near Cape Ann, Massachusetts (NOAA Fisheries, 2017).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements. All tagging operations were conducted under Exempted Fishing Permits (SHK-EFP-11-04, SHK-EFP-12-08, SHK-EFP-13-01, SHK-EFP-14-03) issued by the NMFS Highly Migratory Species Management Division and permits issued by the Massachusetts Division of Marine Fisheries.
Author contributions
MD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Visualization. MW: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. JM: Data curation, Funding acquisition, Investigation, Resources, Writing – review & editing. JI: Data curation, Investigation, Resources, Writing – review & editing. JS: Conceptualization, Resources, Writing – review & editing. BJ: Conceptualization, Investigation, Resources, Writing – review & editing. AN: Data curation, Resources, Validation, Writing – review & editing. VM: Data curation, Resources, Validation, Writing – review & editing. TC: Writing – review & editing. CM: Methodology, Resources, Writing – review & editing. GS: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this work was provided by the State of Maine, and funding for two Rx-LIVE receiver systems included in this research was supported through the Maine Outdoor Heritage Fund (MOHF grants #211-04-02 and #212-04-01).
Acknowledgments
Receiver data was courteously shared by several parties: the NOAA Northeast Fisheries Science Center, including the Gulf of Maine Coastal Telemetry Network; Bill DeVoe of the Maine DMR Atlantic Halibut Tagging Program; and Kevin Staples and Cathryn Wood of the Maine DMR Passive Acoustic Monitoring Program. Deployment of Maine DMR receivers were made possible in part by Maine Marine Patrol, Justin Papkee of the F/V Pull n’ Pray, Ed Hutchins and Riley Austin of the F/V Christina Mae II, and Joshua Audet of the F/V Karamel. The authors would like to thank the many scientists, citizen scientists, and fishermen who have provided support to our monitoring efforts more broadly. In particular, we thank AWSC and Massachusetts DMF for their generous contribution of receivers to the Maine DMR; Frank McNeilly from Naval Undersea Warfare Center, Division Newport and Jason Krumholz from McLaughlin Research Corporation for extensive field support of the array off Popham Beach; John Chisholm, Rosie Seton, and the support staff, volunteers, and students of Allied Whale and College of the Atlantic for their time and expertise in identifying shark predation events; Jon Dodd of the Atlantic Shark Institute and members of the New England White Shark Research Consortium for their continued support of the Maine DMR research program both in written works and in guidance; and to Arthur Howe, former head of Safety in the town of Harpswell, Gary Best of the Maine Bureau of Parks and Lands, and Sean Vaillancourt of Popham Beach State Park for their engagement as beach and emergency response officials. We also thank the reviewers for their insightful recommendations and constructive feedback, which greatly improved the quality of this manuscript.
Conflict of interest
Author CM was employed by Outcast Sport Fishing.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1535123/full#supplementary-material
References
Anderson J. M., Burns E. S., Meese E. N., Farrugia T. J., Stirling B. S., White C. F., et al. (2021a). Interannual nearshore habitat use of young of the year white sharks off southern california. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.645142
Anderson S. D., Chapple T. K., Jorgensen S. J., Klimley A. P., Block B. A. (2011). Long-term individual identification and site fidelity of white sharks, Carcharodon carcharias, off California using dorsal fins. Mar. Biol. 158, 1233–1237. doi: 10.1007/s00227-011-1643-5
Anderson J. M., Clevenstine A. J., Stirling B. S., Burns E. S., Meese E. N., White C. F., et al. (2021b). Non-random Co-occurrence of Juvenile White Sharks (Carcharodon carcharias) at Seasonal Aggregation Sites in Southern California. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.688505
Bangley C. W., Paramore L., Shiffman D. S., Rulifson R. A. (2018). Increased abundance and nursery habitat use of the bull shark (Carcharhinus leucas) in response to a changing environment in a warm-temperate estuary. Sci. Rep. 8, 1–10. doi: 10.1038/s41598-018-24510-z
Bastille-Rousseau G., Schaefer J. A., Peers M. J. L., Ellington E. H., Mumma M. A., Rayl D., et al. (2018). Climate change can alter predator–prey dynamics and population viability of prey. Oecologia 186, 141–150. doi: 10.1007/s00442-017-4017-y
Bigelow H. B., Schroeder W. C. (1936). Supplemental notes on fishes of the Gulf of Maine. Bull. Bureau Fisheries 48, 319–343.
Bigelow H. B., Schroeder W. C. (1953). Fishes of the gulf of maine (Washington, D.C.: U.S. GovernmentPrinting Office).
Bonfil R., Mey_x0308;er M., Scholl M. C., Johnson R., O’Brien S., Oosthuizen H., et al. (2005). Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science 310, 100–103. doi: 10.1126/science.1114898
Bowlby H. D., Dicken M. L., Towner A. V., Waries S., Rogers T., Kock A. (2023). Decline or shifting distribution? A first regional trend assessment for white sharks (Carcharodon carcharias) in South Africa. Ecol. Indic. 154, 110720. doi: 10.1016/j.ecolind.2023.110720
Bowlby H. D., Joyce W. N., Winton M. V., Coates P. J., Skomal G. B. (2022). Conservationimplications of white shark (Carcharodon carcharias) behaviour at the northern extent of their range in the Northwest Atlantic. Can. J. Fisheries Aquat. Sci. 79, 1843–1859. doi: 10.1139/cjfas-2021-0313
Bradford R., Patterson T. A., Rogers P. J., McAuley R., Mountford S., Huveneers C., et al. (2020). Evidence of diverse movement strategies and habitat use by white sharks, Carcharodon carcharias, off southern Australia. Mar. Biol. 167, 96. doi: 10.1007/s00227-020-03712-y
Braun C. D., Lezama-Ochoa N., FarChadi N., Arostegui M. C., Alexander M., Allyn A., et al. (2023). Widespread habitat loss and redistribution of marine top predators in a changing ocean. Sci. Adv. 9, eadi2718. doi: 10.1126/sciadv.adi2718
Briggs J. C. (1960). Fishes of worldwide (circumtropical) distribution. Copeia 1960, 171–180. doi: 10.2307/1439652
Brodziak J. K. T., Mace P. M., Overholtz W. J., Rago P. J. (2004). Ecosystem trade-offs in managing new england fisheries. Bull. Mar. Sci. 74, 529–548.
Bruce B. D. (2008). “The biology and ecology of the white shark, carcharodon carcharias,” in Sharks of the open ocean, 1st ed. Eds. Camhi M. D., Pikitch E. K., Babcock E. A. (Hoboken, NJ: Wiley), 69–81. doi: 10.1002/9781444302516.ch5
Bruce B. D., Bradford R. W. (2012). Habitat use and spatial dynamics of juvenile white sharks, carcharodon carcharias, in eastern Australia, in Global perspectives on the biology and life history of the white shark (CRC Press, Boca Raton, FL).
Casey J. G., Pratt H. L. (1985). Distribution of the white shark, Carcharodon carcharias, in the western North Atlantic. S Cal Acad. Sci Mem 9, 2–14.
Chamberlain S. (2023). rerddap: general purpose client for ‘ERDDAP’ Servers (R package Version 1.0.2). Available at: https://CRAN.R-project.org/package=rerddap.
Chapman B. K., McPhee D. (2016). Global shark attack hotspots: Identifying underlying factors behind increased unprovoked shark bite incidence. Ocean & Coastal Management. 133, 72–84. doi: 10.1016/j.ocecoaman.2016.09.010
Chaprales W., Lutcavage M., Brill R., Chase B., Skomal G. (1998). Harpoon method for attaching ultrasonic and “popup” satellite tags to giant bluefin tuna and large pelagic fishes. Mar. Technol. Soc J. 32, 104–105.
Cliff G., Dudley S. F. J., Davis B. (1989). Sharks caught in the protective gill nets off Natal, South Africa. 2. The great white shark Carcharodon carcharias (Linnaeus). South Afr. J. Mar. Sci. 8, 131–144. doi: 10.2989/02577618909504556
Compagno L. J. V. (2001). “Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Volume 2. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes),” in FAO species catalogue for fishery purposes (FAO, Rome: Food and Agriculture Organization of the United Nations (FAO)).
Crear D. P., Curtis T. H., Hutt C. P., Lee Y. (2023). Climate-influenced shifts in a highly migratory species recreational fishery. Fisheries Oceanography 32, 327–340. doi: 10.1111/fog.12632
Curtis T. H., Bruce B. D., Cliff G., Dudley S. F., Klimely A. P., Kock A., et al. (2012). “Responding to the risk of White Shark attack,” in Global perspectives on the biology and life history of the white shark (Boca Raton, FL: CRC Press), 477–510.
Curtis T. H., McCandless C. T., Carlson J. K., Skomal G. B., Kohler N. E., Natanson L. J., et al. (2014). Seasonal distribution and historic trends in abundance of white sharks, carcharodon carcharias, in the western north atlantic ocean. PloS One 9, e99240. doi: 10.1371/journal.pone.0099240
Curtis T. H., Metzger G., Fischer C., McBride B., McCallister M., Winn L. J., et al. (2018). First insights into the movements of young-of-the-year white sharks (Carcharodon carcharias) in the western North Atlantic Ocean. Sci. Rep. 8, 10794. doi: 10.1038/s41598-018-29180-5
Davis M. D., Kneebone J. (2023). Characterization of fishing efforts for highly migratory species in the Gulf of Maine and how this relates to areas considered for offshore wind development. Available online at: https://www.maine.gov/dmr/sites/maine.gov.dmr/files/inline-files/Report%20to%20the%20Gulf%20of%20Maine%20Mapping%20Project%20for%20Highly%20Migratory%0Species%20-%20Final%20Draft_4.pdf (Accessed August, 2024).
Den Heyer C. E., Bowen W. D., Dale J., Gosselin J., Hammill M. O., Johnston D. W., et al. (2021). Contrasting trends in gray seal (Halichoerus grypus) pup production throughout the increasing northwest Atlantic metapopulation. Mar. Mammal Sci. 37, 611–630. doi: 10.1111/mms.12773
Dolton H., Jackson A., Deaville R., Hall J., Hall G., McManus G., et al. (2023). Regionally endothermic traits in planktivorous basking sharks Cetorhinus maximus. Endangered Species Res. 51, 227–232. doi: 10.3354/esr01257
Domeier M. L., Nasby-Lucas N. (2007). Annual re-sightings of photographically identified white sharks (Carcharodon carcharias) at an eastern Pacific aggregation site (Guadalupe Island, Mexico). Mar. Biol. 150, 977–984. doi: 10.1007/s00227-006-0380-7
Domeier M. L., Nasby-Lucas N. (2012). “Chapter 11: sex-specific migration patterns and sexual segregation of adult white sharks, carcharodon carcharias, in the northeastern pacific,” in Global perspectives on the biology and life history of the white shark (CRC Press, Boca Raton, FL).
Estrada J. A., Rice A. N., Natanson L. J., Skomal G. B. (2006). Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology 87, 829–834. doi: 10.1890/0012-9658(2006)87[829:UOIAOV]2.0.CO;2
Fallows C., Fallows M., Hammerschlag N. (2016). Effects of lunar phase on predator-prey interactions between white shark (Carcharodon carcharias) and Cape fur seals (Arctocephalus pusillus pusillus). Environ. Biol. Fishes 99, 805–812. doi: 10.1007/s10641-016-0515-8
Ferretti F., Jorgensen S., Chapple T. K., De Leo G., Micheli F. (2015). Reconciling predator conservation with public safety. Front. Ecol. Environ. 13, 412–417. doi: 10.1890/150109
Fish C. J. (1936). The biology of Calanus finmarchicus in the Gulf of Maine and Bay of Fundy. Biol. Bull. 70, 118–141. doi: 10.2307/1537318
Florida Museum of Natural History (2024). International shark attack file. Available online at: https://www.floridamuseum.ufl.edu/shark-attacks (Accessed October 2024).
Francis M. (1996). Observation on A pregnant white shark with A review of reproductive biology, in Great white sharks: the biology of carcharodon carcharias (Academic Press, San Diego, CA).
Franks B. R., Tyminski J. P., Hussey N. E., Braun C. D., Newton A. L., Thorrold S. R., et al. (2021). Spatio-temporal variability in white shark (Carcharodon carcharias) movement ecology during residency and migration phases in the western north atlantic. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.744202
Gjelland K.Ø., Hedger R. D. (2013). Environmental influence on transmitter detection probability in biotelemetry: developing a general model of acoustic transmission. Methods Ecol. Evol. 4, 665–674. doi: 10.1111/2041-210X.12057
Goldman K. J. (1997). Regulation of body temperature in the white shark, carcharodon carcharias. J. Com Physiol. B 167, 423–429. doi: 10.1007/s003600050092
Grainger R., Peddemors V. M., Raubenheimer D., Machovsky-Capuska G. E. (2020). Diet composition and nutritional niche breadth variability in juvenile white sharks(Carcharodon carcharias). Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00422
Grieve B. D., Hare J. A., McElroy W. D. (2021). Modeling the impacts of climate change on thorny skate (Amblyraja radiata) on the Northeast US shelf using trawl and longline surveys. Fisheries Oceanography 30, 300–314. doi: 10.1111/fog.12520
Guttridge T. L., Matich P., Guttridge A. E., Winton M., Dedman S., Skomal G. (2024). First evidence of white sharks, Carcharodon carcharias, in the tongue of the ocean, central Bahamas. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1451808
Hammerschlag N., McDonnell L. H., Rider M. J., Street G. M., Hazen E. L., Natanson L. J., et al. (2022). Ocean warming alters the distributional range, migratory timing, and spatial protections of an apex predator, the tiger shark (Galeocerdo cuvier). Global Change Biol. 28, 1990–2005. doi: 10.1111/gcb.16045
Hammerschlag N., Schmitz O. J., Flecker A. S., Lafferty K. D., Sih A., Atwood T. B., et al. (2019). Ecosystem function and services of aquatic predators in the anthropocene. Trends Ecol. Evol. 34, 369–383. doi: 10.1016/j.tree.2019.01.005
Hammill M. O., den Heyer C. E., Bowen W. D., Lang S. L. C. (2017). Grey seal population trends in canadian water[amp]]ndash;2016 and harvest advice. DFO Can. Sci. Advis. Sec. Res. Doc. Available at: https://www.frontiersin.org/articles/10.3389/fmars.2021.744202/fullB78.
Harasti D., Lee K., Bruce B., Gallen C., Bradford R. (2017). Juvenile white sharks Carcharodon carcharias use estuarine environments in south-eastern Australia. Mar. Biol. 164, 58. doi: 10.1007/s00227-017-3087-z
Haugen J. B., Link J. S., Cribari K., Bundy A., Dickey-Collas M., Leslie H. M., et al. (2024). Marine ecosystem-based management: challenges remain, yet solutions exist, and progress is occurring. NPJ Ocean Sustainability 3, 1–11. doi: 10.1038/s44183-024-00041-1
Heupel M. R., Reiss K. L., Yeiser B. G., Simpfendorfer C. A. (2008). Effects of biofouling on performance of moored data logging acoustic receivers. Limnology Oceanography: Methods 6, 327–335. doi: 10.4319/lom.2008.6.3274
Heupel M. R., Semmens J. M., Hobday A. J. (2006). Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar. Freshw. Res. 57, 1–13. doi: 10.1071/MF05091
Holbrook C., Hayden T., Binder T., Pye J. (2024). glatos: A package for the Great Lakes Acoustic Telemetry Observation System. R package version 0.8.0. Available online at: https://github.com/ocean-tracking-network/glatos.
Huang B., Liu C., Banzon V. F., Freeman E., Graham G., Hankins W., et al. (2020). NOAA 0.25-degree daily optimum interpolation sea surface temperature (OISST), version 2.1 (NOAA National Centers for Environmental Information). doi: 10.25921/RE9P-PT57
Hussey N., McCann H., Cliff G., Dudley S., Wintner S., Fisk A. (2012). Size-based analysis of diet and trophic position of the white shark, carcharodon carcharias, in South African waters, in Global perspectives on the biology and life history of the white shark (Boca Raton, FL: CRC Press), 27–50.
Jorgensen S. J., Reeb C. A., Chapple T. K., Anderson S., Perle C., Van Sommeran S. R., et al. (2009). Philopatry and migration of Pacific white sharks. Proc. R. Soc. B: Biol. Sci. 277 (1682), 679–688. doi: 10.1098/rspb.2009.1155
Kessel S. T., Cooke S. J., Heupel M. R., Hussey N. E., Simpfendorfer C. A., Vagle S., et al. (2014). A review of detection range testing in aquatic passive acoustic telemetry studies. Rev. Fish Biol. Fisheries 24, 199–218. doi: 10.1007/s11160-013-9328-4
Kim S. L., Tinker M. T., Estes J. A., Koch P. L. (2012). Ontogenetic and among-individual variation in foraging strategies of northeast pacific white sharks based on stabl 4 isotope analysis. PloS One 7, e45068. doi: 10.1371/journal.pone.0045068
Klimley A. P. (1985). The areal distribution and autoecology of the white shark, Carcharodon carcharias, off the West Coast of North America. Memoirs. South. Cal. Acad. Sci. 9, 15–40.
Loder J. W., Petrie B., Gawarkiewicz G. (1998). “Chapter 5: the coastal ocean off northeastern north america: A large-scale view,” in The global coastal ocean: regional studies and synthesis (New York, NY: Wiley & Sons).
Machovsky-Capuska G. E., Raubenheimer D. (2020). The nutritional ecology of marine apex predators. Annu. Rev. Mar. Sci. 12, 361–387. doi: 10.1146/annurev-marine-010318-095411
Machovsky-Capuska G. E., Senior A. M., Simpson S. J., Raubenheimer D. (2016). The multidimensional nutritional niche. Trends Ecol. Evol. 31, 355–365. doi: 10.1016/j.tree.2016.02.009
McAuley R. B., Bruce B. D., Keay I. S., Mountford S., Pinnell T., Whoriskey F. G. (2017). Broad-scale coastal movements of white sharks off Western Australia described by passive acoustic telemetry data. Mar. Freshw. Res. 68, 1518–1531. doi: 10.1071/MF16222
Mollomo P. (1998). The white shark in Maine and Canadian waters. Northeast Nat. 5, 207–214. doi: 10.2307/3858620
Moxley J. H., Skomal G., Chisholm J., Halpin P., Johnston D. W. (2020). Daily and seasonal movements of Cape Cod gray seals vary with predation risk. Mar. Ecol. Prog. Ser. 644, 215–228. doi: 10.3354/meps13346
Myers R. A., Ottensmeyer C. A. (2005). “Extinction risk in marine species,” in Marine conservation biology: The science of maintaining the sea’s biodiversity. Eds. Norse E. A., Crowder L. B. (Washington, D.C.: Island Press), 58–79. doi: 10.1111/gcb.16045
Nasby-Lucas N., Dewar H., Lam C. H., Goldman K. J., Domeier M. L. (2009). White shark offshore habitat: A behavioral and environmental characterization of the eastern pacific shared offshore foraging area. PloS One 4, e8163. doi: 10.1371/journal.pone.0008163
NOAA Fisheries (2017). Amendment 10 to the 2006 consolidates atlantic highly migratory species fishery management plan: essential fish habitat (US Department of Commerce, NOAA Fisheries). Available at: https://www.habitat.noaa.gov/application/efhinventory/docs/a10_hms_efh.pdf (Accessed November 2024).
Pershing A. J., Alexander M. A., Brady D. C., Brickman D., Curchitser E. N., Diamond A. W., et al. (2021). Climate impacts on the Gulf of Maine ecosystem. Elementa: Sci. Anthropocene 9, 76. doi: 10.1525/elementa.2020.00076
Pershing A. J., Alexander M. A., Hernandez C. M., Kerr L. A., Le Bris A., Mills K. E., et al. (2015). Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery. Science 350, 809–812. doi: 10.1126/science.aac9819
Pincock D. G. (2012). “False detections: what they are and how to remove them from detection data,” in VEMCO whitepaper document DOC-004691 (Amirix Systems Inc., Halifax, NS, Canada).
Pinsky M. L., Worm B., Fogarty M. J., Sarmiento J. L., Levin S. A. (2013). Marine taxa track local climate velocities. Science 341, 1239–1242. doi: 10.1126/science.1239352
Pratt H. L. (1996). Reproduction in the male white shark, in Great white sharks: the biology of carcharodon carcharias (Academic Press, San Diego, CA).
R Core Team (2022). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.r-project.org/.
Rex P. T., Iii J. H. M., Pierce E. K., Lowe C. G. (2023). Patterns of overlapping habitat use of juvenile white shark and human recreational water users along southern California beaches. PloS One 18, e0286575. doi: 10.1371/journal.pone.0286575
Rigby C. L., Barreto R., Carlson J., Fernando D., Fordham S., Francis M. P., et al. (2022). “Carcharodon carcharias (amended version of 2019 assessment),” in The IUCN red list of threatened species 2022. doi: 10.2305/IUCN.UK.2022-1.RLTS.T3855A212629880.en
Rosenblatt A. E., Smith-Ramesh L. M., Schmitz O. J. (2017). Interactive effects of multiple climate change variables on food web dynamics: Modeling the effects of changing temperature, CO2, and water availability on a tri-trophic food web. Food Webs 13, 98–108. doi: 10.1016/j.fooweb.2016.10.002
Ross C. H., Pendleton D. E., Tupper B., Brickman D., Zani M. A., Mayo C. A., et al. (2021). Projecting regions of North Atlantic right whale, Eubalaena glacialis, habitat suitability in the Gulf of Maine for the year 2050. Elementa: Sci. Anthropocene 9, 58. doi: 10.1525/elementa.2020.20.00058
Saba V. S., Griffies S. M., Anderson W. G., Winton M., Alexander M. A., Delworth T. L., et al. (2016). Enhanced warming of the Northwest Atlantic Ocean under climate change. J. Geophysica lResearch: Oceans 121, 118–132. doi: 10.1002/2015JC011346
Shaw R. L., Curtis T. H., Metzger G., McCallister M. P., Newton A., Fischer G. C., et al. (2021). Three-dimensional movements and habitat selection of young white sharks (Carcharodon carcharias) across a temperate continental shelf ecosystem. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.643831
Sigourney D. B., Murray K. T., Gilbert J. R., Ver Hoef J. M., Josephson E., DiGiovanni R. A. (2021). Application of a Bayesian hierarchical model to estimate trends in Atlantic harbor seal (Phoca vitulina vitulina) abundance in Maine, U.S.A[amp]]ndash;2018. Mar. Mammal Sci. 38, 500–516. doi: 10.1111/mms.12873
Simpfendorfer C. A., Heupel M. R., Hueter R. E. (2002). Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can. J. Fisheries Aquat. Sci. 59, 23–32. doi: 10.1139/f01-191
Singh L., Downey N. J., Roberts M. J., Webber D. M., Smale M. J., Van Den Berg M. A., et al. (2009). Design and calibration of an acoustic telemetry system subject to upwelling events. Afr. J. Mar. Sci. 31, 355–364. doi: 10.2989/AJMS.2009.31.3.8.996
Skomal G., Braun C., Chisholm J., Thorrold S. (2017). Movements of the white shark Carcharodon carcharias in the North Atlantic Ocean. Mar. Ecol. Prog. Ser. 580, 1–16. doi: 10.3354/meps12306
Skomal G., Chisholm J., Correia S. (2012). “Implications of increasing pinniped populations on the dier and abundance of white sharks off the coast of massachusetts,” in Global perspectives on the biology and life history of the white shark (CRC Press, Boca Raton, FL).
Spaet J. L. Y., Butcher P. A., Manica A., Lam C. H. (2022). Spatial dynamics and fine-scale vertical behaviour of immature eastern australasian white sharks (Carcharodon carcharias). Biology 11, 1689. doi: 10.3390/biology11121689
Stevenson D. K., Tuxbury S., Johnson M. R., Boelke C. (2014). Shallow water benthic habitats in the gulf of maine: A summary of habitat use by common shellfish species in the gulf of maine. Greater atlantic region policy series 14-01 (NOAA Fisheries Greater Atlantic Regional Fisheries Office), 77. Available at: www.greateratlantic.fisheries.noaa.gov/policyseries/ (Accessed December 2025).
Tanaka K. R., Van Houtan K. S., Mailander E., Dias B. S., Galginaitis C., O’Sullivan J., et al. (2021). North Pacific warming shifts the juvenile range of a marine apex predator. Sci. Rep. 11, 3373. doi: 10.1038/s41598-021-82424-9
Thieurmel B., Elmarhraoui A. (2022). suncalc: compute sun position, sunlight phases, moon position and lunar phase (R package version 0.5.1).
Tirard P., Manning M. J., Jollit I., Duffy C., Borsa P. (2010). Records of great white sharks (Carcharodon carcharias) in New Caledonian waters. Pac. Sci. 64, 567–576. doi: 10.2984/64.4.567
Watanabe Y. Y., Goldman K. J., Caselle J. E., Chapman D. D., Papastamatiou Y. P. (2015). Comparative analyses of animal-tracking data reveal ecological significance of endothermy in fishes. Proc. Natl. Acad. Sci. 112, 6104–6109. doi: 10.1073/pnas.1500316112
Weng K. C., O’Sullivan J. B., Lowe C. G., Winkler C. E., Dewar H., Block B. A. (2007). Movements, behavior and habitat preferences of juvenile white sharks Carcharodon carcharias in the eastern Pacific. Mar. Ecol. Prog. Ser. 338, 211–224. doi: 10.3354/meps338211
Wickham H. (2016). ggplot2: elegant graphics for data analysis (Springer-Verlag New York). Available at: https://ggplot2.tidyverse.org.
Winton M., Fay G., Skomal G. (2023). An open spatial capture-recapture framework for estimating the abundance and seasonal dynamics of white sharks at aggregation sites. Mar. Ecol. Prog. Ser. 715, 1–25. doi: 10.3354/meps14371
Winton M. V., Sulikowski J., Skomal G. B. (2021). Fine-scale vertical habitat use of white sharks at an emerging aggregation site and implications for public safety. Wildlife Res. 48, 345. doi: 10.1071/WR20029
Wood S. A., Murray K. T., Josephson E., Gilbert J. (2020). Rates of increase in gray seal (Halichoerus grypus atlantica) pupping at recolonized sites in the United State[amp]]ndash;2019. J. Mammalogy 101, 121–128. doi: 10.1093/jmammal/gyz184
Keywords: white shark distribution, fish movement ecology, acoustic telemetry, Gulf of Maine, Western North Atlantic
Citation: Davis MM, Winton MV, Mohan JA, Iafrate JD, Sulikowski JA, Jenner BP, Novak AJ, Migneco V, Curtis TH, Michalove C and Skomal GB (2025) Early insights into the seasonal and spatial distribution of white sharks (Carcharodon carcharias) along the Maine coastline. Front. Mar. Sci. 12:1535123. doi: 10.3389/fmars.2025.1535123
Received: 26 November 2024; Accepted: 11 February 2025;
Published: 03 March 2025.
Edited by:
Jeremy Kiszka, Florida International University, United StatesReviewed by:
Peter Gausmann, Ruhr University Bochum, GermanyChristopher G Lowe, California State University, Long Beach, United States
Copyright © 2025 Davis, Winton, Mohan, Iafrate, Sulikowski, Jenner, Novak, Migneco, Curtis, Michalove and Skomal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew M. Davis, TWF0dGhldy5NLkRhdmlzQE1haW5lLmdvdg==
 Matthew M. Davis
Matthew M. Davis Megan V. Winton2
Megan V. Winton2 John A. Mohan
John A. Mohan Joseph D. Iafrate
Joseph D. Iafrate James A. Sulikowski
James A. Sulikowski Blaise P. Jenner
Blaise P. Jenner Ashleigh J. Novak
Ashleigh J. Novak Victoria Migneco
Victoria Migneco Tobey H. Curtis
Tobey H. Curtis