
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 03 March 2025
Sec. Marine Biogeochemistry
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1533654
This article is part of the Research TopicBiogeochemical Cycling and Depositional Processes of Critical Metals in the Deep Sea and Their Constraints on Global ChangesView all 8 articles
 Xinyi He1,2,3,4
Xinyi He1,2,3,4 Qian Liu5
Qian Liu5 Xiaohu Li1,2,3*
Xiaohu Li1,2,3* Zhenggang Li1,2
Zhenggang Li1,2 Hao Wang1,2
Hao Wang1,2 Zhimin Zhu1,2
Zhimin Zhu1,2 Yanhui Dong1,2
Yanhui Dong1,2 Jie Li1,2
Jie Li1,2 Huaiming Li1,2
Huaiming Li1,2Polymetallic nodules, widely distributed in the deep seafloor of the Pacific Ocean, are characterized by their abundance of diverse metal elements and considerable economic value. Previous studies have suggested a partial biogenic origin of these nodules. This study investigated the role of microorganisms in nodule formation by examining biological-like structures and bacterial communities within nodules and sediments. Scanning electron microscopy revealed bacteria-like microspheres, skeleton-like structures and extracellular polymeric substances-like structures in the nodules. Energy dispersive spectroscopy showed that these biological-like structures facilitated metal enrichment, enabling subsequent mineral precipitation. Shewanella, Colwellia, Leptospirillum, Sulfitobacter, and other bacteria may possess mineralization potential due to their Mn or Fe oxidation capabilities. Differences in internal structures and bacterial community composition between nodules from the western and eastern Pacific Ocean could potentially suggest that growth environment factors may contribute to nodule formation variation. These findings highlight the involvement of microorganisms in nodule formation and contribute to a better understanding of the biogenic mineralization process.
Polymetallic nodules, also known as ferromanganese nodules, are widespread mineral resources in the deep seafloor. They are rich in Fe, Mn, Ni, Co, Cu, rare earth elements (REEs), and other metal elements, such as Ti, Mo, Zr, Li, and Y (Hein et al., 2020). These elements occupy crucial positions in various economic sectors, including the automotive, aerospace, and renewable energy industries (Hein et al., 2013). The substantial reserves of nodules are found primarily in the Clarion-Clipperton Fracture Zone (CCZ) in the eastern Pacific Ocean (Hein et al., 2013).
Initially, it was widely believed that nodule formation was primarily driven by physicochemical interactions (Takematsu et al., 1989; Roy, 1992). This process was thought to be dominated by water-rock interactions and precipitation dynamics, with iron and manganese oxides precipitating under redox gradient conditions as the main mineralization driver (Goldberg and G.O.S, 1958; Crerar and Barnes, 1974).
Nodules that form through the continuous precipitation of Fe and Mn minerals from oxygen-rich near-bottom waters are known as “hydrogenetic nodules.” These nodules typically exhibit a Fe/Mn ratio of approximately 1 in bulk analyses and are enriched in elements such as Fe and Co (Hein et al., 2013). In contrast, nodules that precipitate from oxygenated or suboxic sediment pore waters are referred to as “diagenetic nodules,” which typically have an Mn/Fe ratio > 5 and are enriched in elements such as Mn and Cu (Halbach et al., 1988). Additionally, nodules that exhibit a mixture of diagenetic and hydrogenetic origin are called “mixed-type nodules” (Halbach et al., 1981).
Since Graham and Cooper (1959) suggested that manganese-rich deposits on the seafloor may result from biological processes, research into the role of microorganisms in nodule mineralization has steadily gained attention. Subsequent studies employing techniques such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM) have further highlighted the independent relationships between microorganisms and the metals and minerals within the nodules (Burnett and Nealson, 1983; Hu et al., 2000; Wang and Müller, 2009; Nayak et al., 2013). The involvement of microorganisms in nodule formation has become an increasingly acknowledged and explored topic within the field.
Nodules exhibit distinct distribution patterns in various marine regions and are significantly influenced by the underlying environments (Glasby, 1976; Cochonat et al., 1992; Molari et al., 2020). The western Pacific contains one of the oldest oceanic crusts (Glasby et al., 1982) and the seafloor is coated with red clay (Glasby et al., 1987). The CCZ mainly develops on the smooth relief of abyssal hills (Halbach et al., 1981), and the seafloor is covered by rather thick, extensive layers of siliceous oozes (Margolis and Burns, 1976). Furthermore, the western Pacific mainly produces hydrogenetic nodules (Jiang et al., 2020b), while nodules from the CCZ are mostly of diagenetic or mixed-type origin (Wegorzewski and Kuhn, 2014; Hollingsworth et al., 2021).
In recent years, microorganisms within nodules have been acknowledged to significantly contribute to the process of nodule formation (Jiang et al., 2020a). Microorganisms significantly contribute to the cycling and mineralization of metals, utilizing their metabolic activities to influence nucleation and crystallization processes (Banfield and Nealson, 2018). By altering microenvironments such as pH and redox conditions, they facilitate mineral precipitation and shape mineral compositions (Konhauser, 1997). The deposition of minerals is crucial for the formation and growth of nodules, and microorganisms may exhibit a promoting effect on nodule formation due to their ability to mediate mineral formation and deposition (Hoffmann et al., 2021). Moreover, microbial mineralization activities are also associated with the rough surface structure and the growth of internal laminae in the nodules (Akai et al., 2013; Jiang et al., 2017).
However, the current understanding of microbial communities and their biomineralization functions in nodules in different regions of the Pacific Ocean remains limited. Comprehensive assessments of the variations in environmental conditions, nodule types, and abundance, as well as benthic organism activities across different regions, are challenging. Further research is needed to provide a more in-depth and comprehensive understanding.
In this study, we analyzed elemental composition of nodules and applied scanning electron microscopy coupled with energy dispersive X-ray spectroscopy (SEM-EDS) to analyze biological structures and surface elemental composition in nodules from the western and eastern Pacific. Furthermore, full-length 16S rRNA gene analysis was conducted to examine bacterial community composition within the nodules and underlying sediments, aiming to uncover potential bacterial mineralization processes and their role in the formation of mineralized structures in nodules.
Samples of nodules and underlying sediments were collected using a multicorer at depths of 5302-5562 m from zone M2 (MC02, BC38A, and BC78) in the western Pacific during August and September 2022 aboard the research vessel Dayangyihao on voyage 75 (Figure 1, Table 1). The M2 area in the northern Magellan Seamounts of the western Pacific is a geologically stable region with limited volcanic and tectonic activity (Hein et al., 1997). The surface sediments are mainly composed of brownish-yellow clay. The study area represents a typical tropical oligotrophic environment, with low surface productivity (Jiang et al., 2020a), moderately high bottom water oxygen concentrations (175–200 mmol/m³) (Dutkiewicz et al., 2020; Ren et al., 2022), and a bottom seawater temperature of approximately 1.5°C and salinity of around 34.6. The nodules in this area are primarily formed through hydrogenic processes, characterized by Co enrichment and a spheroidal morphology.
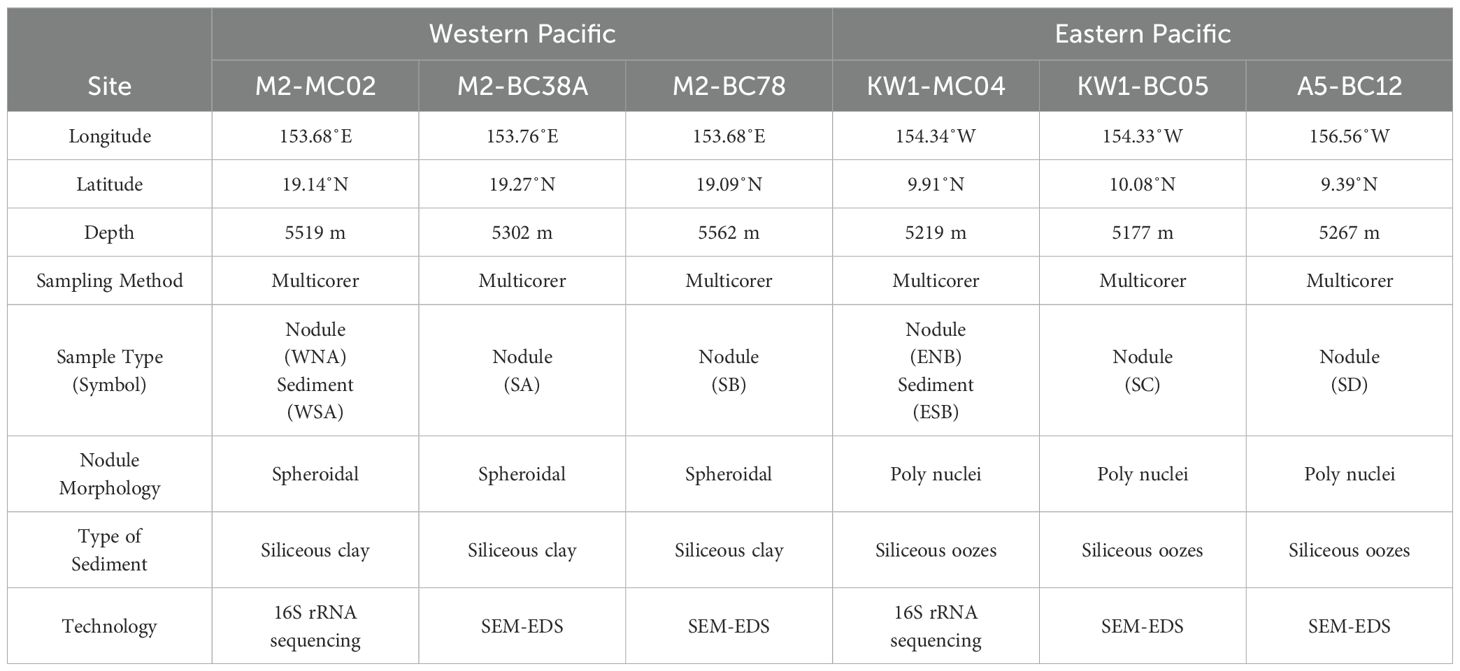
Table 1. Description of the polymetallic nodules and surrounding sediments sampled at six sites in the Pacific Ocean.
Samples were also collected using a multicorer at depths of 5177-5267 m from zone KW1 (MC04 and BC05) and zone A5 (BC12) in the eastern Pacific during voyage 73 of the research vessel Dayanghao within the same timeframe (Figure 1, Table 1). KW1 and A5 are adjacent blocks, both located in the western part of the CCZ in the eastern Pacific. The feature surface sediments with a brown hue, is primarily consisting of siliceous oozes. The dissolved oxygen concentration in the bottom seawater is approximately 150 mmol/m³ (Dutkiewicz et al., 2020), with a temperature of 1.5°C and salinity around 34.7 (Washburn et al., 2021). The nodules are predominantly poly-nodules and have a hydrogenetic or mixed-type origin (Reyss et al., 1985).
The collected nodules and sediments were stored at −80°C and transported on dry ice to the laboratory.
The polymetallic nodules were dried, crushed, weighed (5 g) and then ground into a homogeneous powder of 200 mesh size using an agate pestle and mortar. Elemental analyses were conducted at the Key Laboratory of Submarine Geosciences, Second Institute of Oceanography, Ministry of Natural Resources. The major elements in the nodules and sediments were determined using X-ray fluorescence spectroscopy (XRF, AxiosMAX, PANalytical, Netherlands). The trace elements in the nodules and sediments were determined using inductively coupled plasma-mass spectrometry(ICP-MS, Elan DRC-e, Perkin Elmer). All measured concentrations exceeded detection limits, with a relative standard deviation of laboratory precision less than 10%.
The nodules were mounted to epoxy and then cut in along the maximum axis to make thin sections. SEM images were performed using a TESCAN MIRA3 field-emission scanning electron microprobe (FE-SEM) at the Testing Center, Tuoyan Analytical Technology Co. Ltd. (Guangzhou, China). After the samples were carbon-coated, SEM images were acquired under an acceleration voltage of 20 kV, a beam current of 15 nA and a magnification of 300–500×. The surface elemental composition of carbon coated sample was characterized by energy dispersive X-ray analysis (SEM-EDAX) EDS detector (EDAX Element EDS detector) device attached to an SEM operating at 20 kV. Observing SEM images provides information about the internal structures within nodules, and further analysis can be conducted by combining the corresponding EDS results.
Under sterile conditions, the surface deposits adhering to the nodules were scraped away. The nodules were then fragmented using a chisel and then ground with a mortar. The nodules and surrounding sediments were individually weighed to 0.5 g. DNA was extracted using an Advanced Soil DNA Kit (MOBIO, Solana Beach, USA) according to the instructions.
The extracted DNA was used as a template for amplifying the V1-V9 regions with the primer set 27 F (5’-AGRGTTYGATYMTGGCTCAG-3’) and 1492 R (5’-RGYTACCTTGTTACGACTT-3’). Polymerase chain reaction (PCR) was conducted using the BioRad (S1000, Bio-Rad Laboratories, USA). Each sample was run in triplicate, and the PCR products from the same sample were pooled. Library preparation followed the 16S Amplification SMRTbell® Library Preparation protocol, and sequencing of the amplicon library was performed by Guangzhou Meige Biotechnology Co., Ltd., utilizing the PacBio Sequel II platform (PacBio, USA).
Fastp (v0.14.1) removed sequences over 2000 bp, and Cutadapt (v1.14) eliminated primers, resulting in valid fragments. Uparse defined operational taxonomic units (OTUs) at a 97% similarity threshold, with a confidence level of 0.8. Usearch-sintax (v10.0.240) aligned OTU representative sequences against the Silva v132 database.
Alpha diversity indices, including abundance-based coverage estimator (ACE) and Shannon, were calculated using usearch-alpha div (v10) based on the relative abundances of OTUs in each sample. Rarefaction curves were drawn by usearch-alpha div rare (v10) based on a richness index to estimate the sampling efforts. Community compositions were performed using R software.
Disparities were observed in the external morphological characteristics and internal structures of polymetallic nodules from the western and eastern Pacific (Figure 2). The nodules from the western Pacific were near-spherical (Figure 2A), with a homogeneous texture and no distinct core (Figure 2B). In contrast, nodules from the eastern Pacific were primarily poly-nodules containing more than three nuclei, exhibiting rough surfaces (Figure 2C) and distinct cores, which were encased by surrounding conduits (Figure 2D).

Figure 2. Photographs of hand-specimen and thin sections of representative nodules from the Pacific Ocean, captured by digital camera: (A) A near-spherical nodule from the western Pacific; (B) A thin section of a nodule from the western Pacific; (C) A typical coalesced nodule from the eastern Pacific; (D) A thin section of a nodule from the eastern Pacific.
Nodules exhibited a significant enrichment of metal elements such as Mn, Fe, Ti, Co, Cu and Ni compared to the sediments (Table 2). Mn, Cu and Ni were generally more enriched in nodules from the eastern Pacific, while Fe, Ti and Co were more enriched in nodules from the western Pacific. Based on the Mn/Fe ratio, nodules from the western Pacific were indicative of a pronounced hydrogenetic origin (Mn/Fe ≈ 1) (Verlaan et al., 2004; Hein and Koschinsky, 2014), whereas nodules from eastern Pacific generally show a mixture of diagenetic and hydrogenetic origin, with a predominantly diagenetic input (2.09-2.77) (Wegorzewski and Kuhn, 2014; Hein et al., 2020).
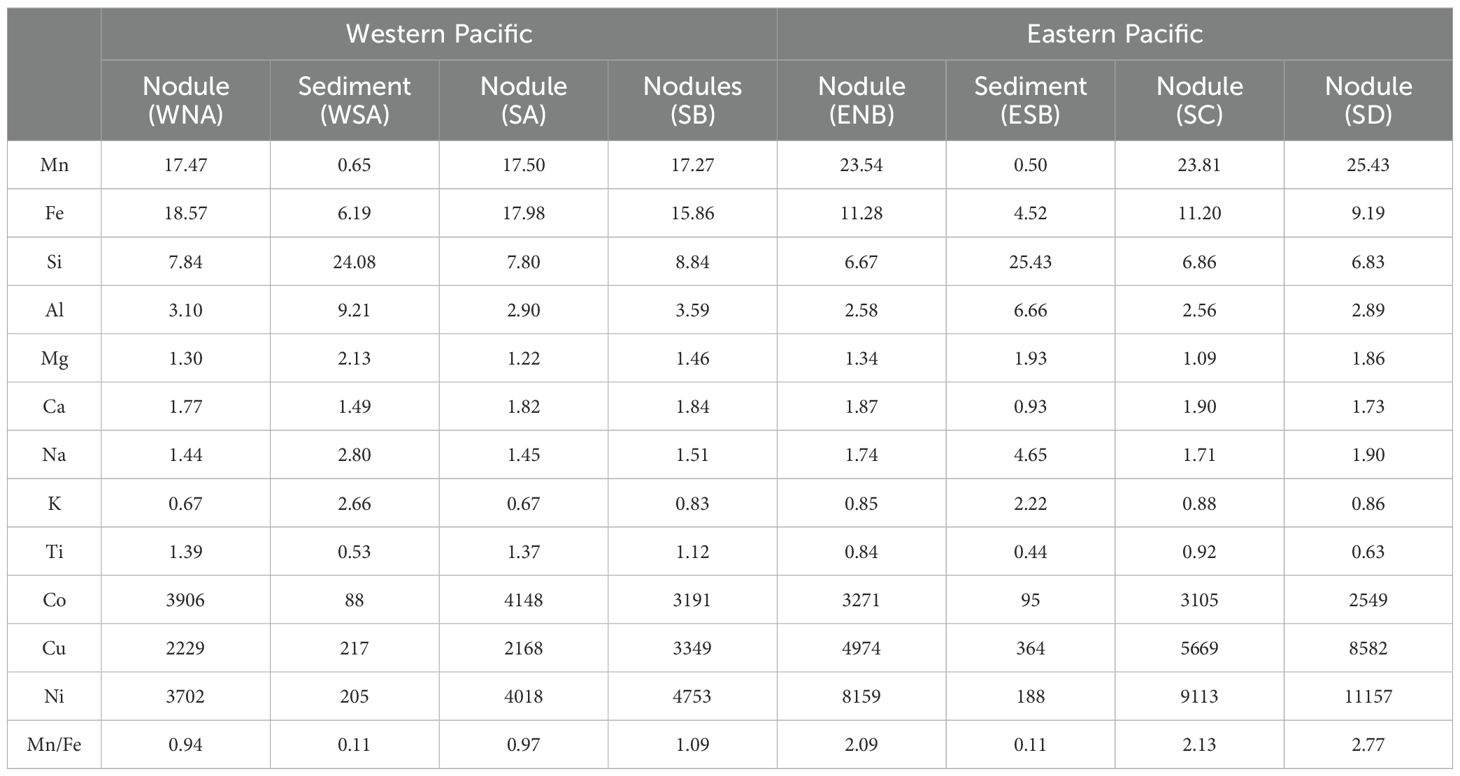
Table 2. Partial major and trace element compositions of the nodules and sediments. Co, Cu, and Ni in ppm; Mn/Fe dimensionless; others in wt.%.
Both western and eastern Pacific nodules exhibit regularly arranged laminated structures (Figure 3). The nodules from the western Pacific are characterized by laminated and concentric rims (He et al., in press), where compact layers (brighter) and loose layers (darker) alternate in a ring-like pattern, forming a rhythmic structure (Figures 3A–C). In contrast, nodules from the eastern Pacific predominantly display stromatolite-like rims with a loose texture and abundant pores (darker areas) distributed irregularly (Figures 3D–F). The compact structures in both regions show clear Mn enrichment on their surfaces, identified from the Mn elemental mapping, where lighter purple areas indicate higher Mn concentrations (Figures 3C, F). This is based on qualitative EDS analysis. The Mn distribution follows a rhythmic pattern, corresponding to concentric or stromatolite-like laminations.
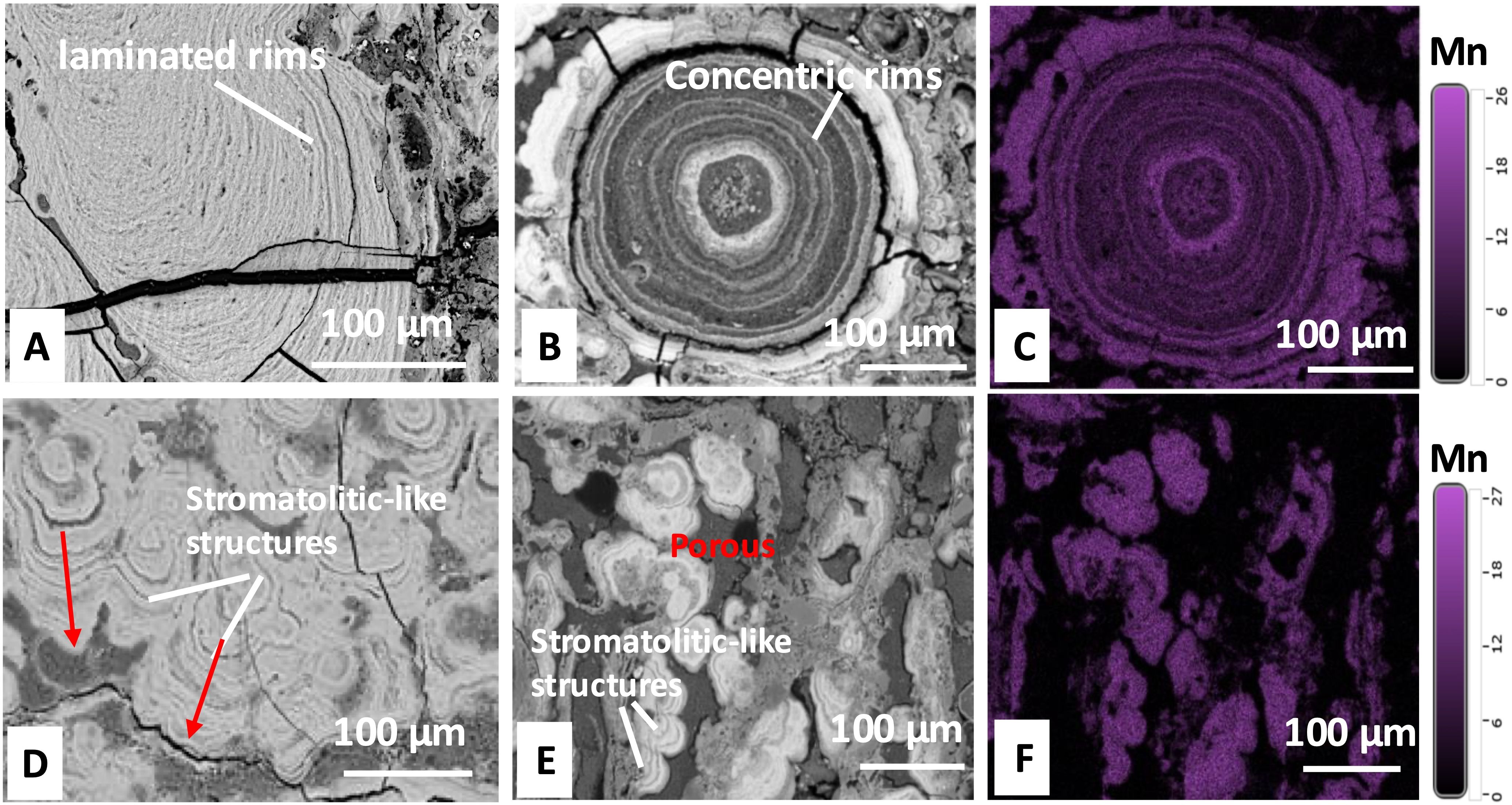
Figure 3. Images of internal structures in thin sections captured by SEM (A, B, D, E) and Mn distribution by EDS (C, F): (A, B) Internal structures of nodules from the western Pacific, with (A) densely distributed laminated rims and (B) Concentric rims growing around a micro-core; (C) Mn distribution map of the structure shown in (B); (D, E) Stromatolitic-like structures in nodules from the eastern Pacific; (F) Mn distribution map of the structure shown in (E). Lighter purple areas indicate higher Mn concentrations.
Furthermore, many biological-like structures were observed from the fissures and cavities in the thin sections. For example, some bacteria-like microspheres (Figures 4A) exhibited a ring-like arrangement, with morphologies similar to those considered bacteria by Jiang et al. (2019) and Reykhard and Shulga (2019). Additionally, another type of bacteria-like microsphere, characterized by a flattened, centrally concave shape, was densely distributed within the channels (Figure 4B). Larger biological-like structures (>20 μm), including porous spheroidal (Figure 4C) and annular (Figures 4D, E) structures were also observed. Furthermore, a transparent and highly reflective extracellular polymeric substance (EPS)-like structure (Figure 4F) (Ren and Jones, 2021).
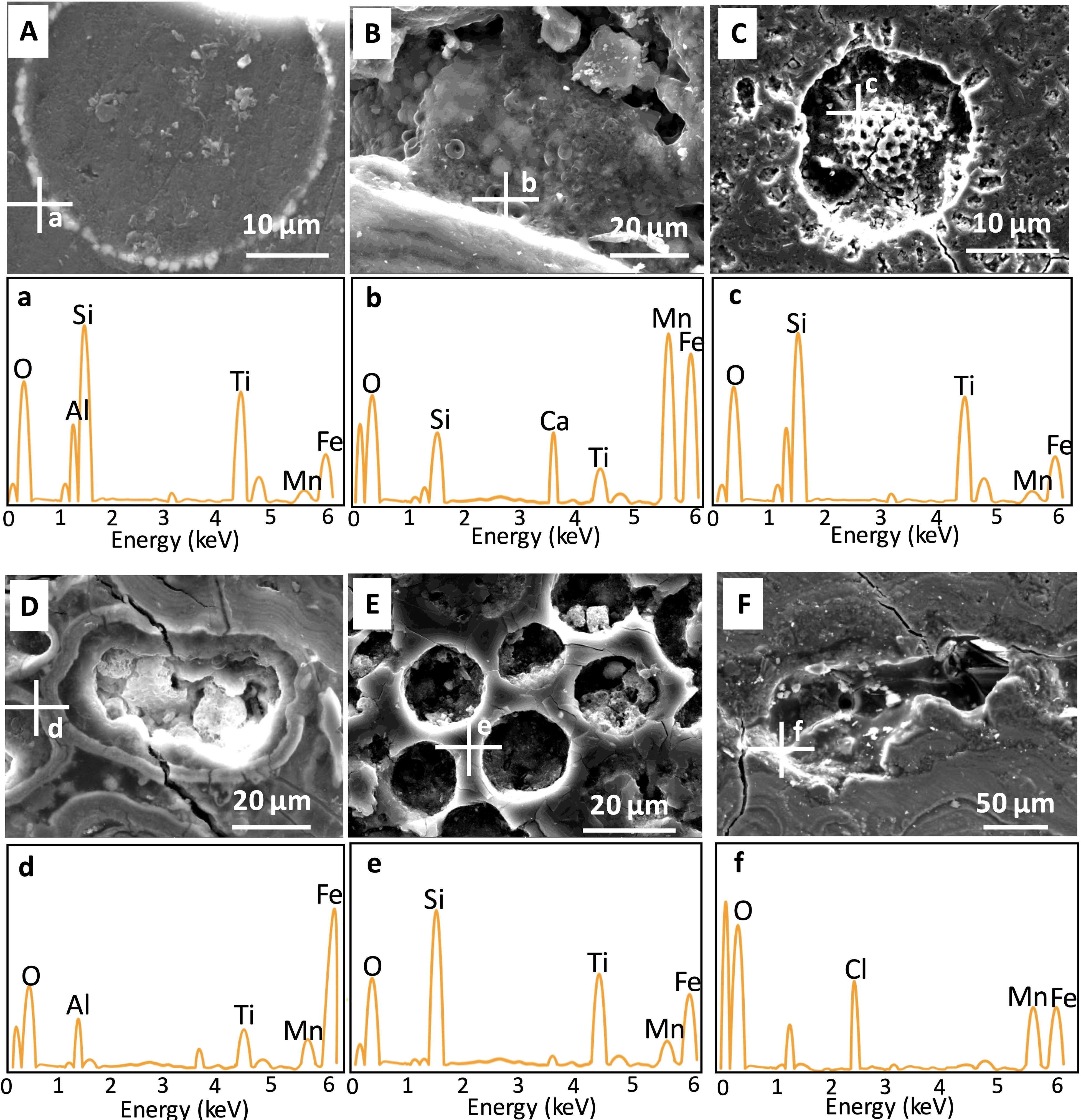
Figure 4. Images of biological-like structures in nodules from the western Pacific (A-C) and the eastern Pacific (D-F) captured using SEM-EDS: (A) Microspheres arranged in a ring on a platform; (B) Multiple flattened microspheroidal structures with central concavities; (C) A porous spheroidal structure; (D, F) Annular structures of varying shapes; (E, F) EPS-like structures coating tunnel surfaces. White crosses indicate analysis points. a-f indicate the regions in the images (A–F) where elemental analysis was performed using EDS.
To further elucidate the elemental composition of these structures, qualitative EDS analysis was performed. Higher peak intensities indicate greater element enrichment (Figure 4). Mn, Fe, and Ti are the primary metals that exhibit substantial enrichment within the nodules, and due to their considerable economic value, they have been the subject of extensive research (Hein et al., 2020). Furthermore, Mn and Fe play pivotal roles in cellular processes such as development, metabolism, and enzymatic activity (Bruins et al., 2000; Helmann, 2014), and are also known to facilitate the formation of bacterial biofilms (Avidan et al., 2010; Mhatre et al., 2016). Of particular interest, Mn, Fe, and Ti are also found to exhibit markedly high concentrations on the microstructural surfaces, which warrants further attention. The surfaces of microspheres exhibited the ability to enrich metals (Mn, Fe, Ti), particularly Mn (Figures 4A, B). Surfaces of other types of structures have similar elemental enrichments (Fe, Mn, Ti), particularly Fe (Figures 4C–E). EPS-like structure displayed comparable levels of enrichment for both Mn and Fe (Figure 4F).
The sequencing depth was sufficient, capturing most of the community diversity with a rich variety of bacterial taxa (Supplementary Figure S1; Figure 5) (He et al., in press). Proteobacteria (38.2%-46.9%) was predominant across the samples, with the dominance of the class Gammaproteobacteria (18.1% to 32.3%). Woeseia exhibited dominance in WNA (12.26%) and WSA (9.56%), while Arcicella was the dominant genus in ENB (28.02%) and ESB (22.22%).
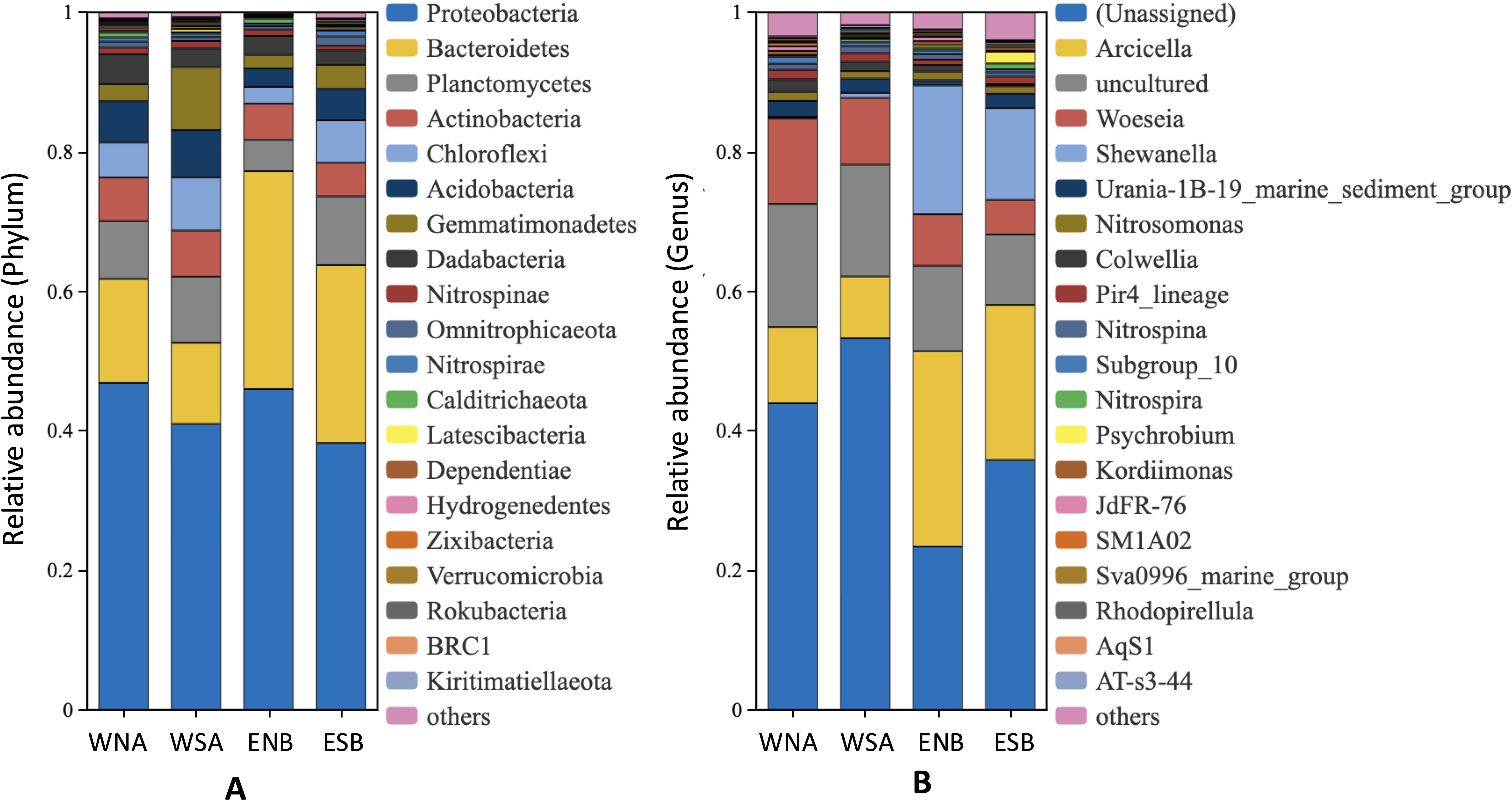
Figure 5. Bacterial community compositions in the nodules and underlying sediments (He et al., in press): (A) The relative abundance of bacteria at the phylum level (top 20); (B) The relative abundance of bacteria at the genus level (top 20). WNA and WSA represent the western Pacific samples, with WNA referring to the nodule and WSA referring to the sediment; ENB and ESB represent the eastern Pacific samples, with ENB referring to the nodule and ESB referring to the sediment.
The experiment identified some unique genera that appeared to be exclusive to specific samples (Table 3; Supplementary Table S1). For example, Leptospirillum and Lentisphaera were found only in western Pacific samples (WNA, WSA), while Psychrobium and Sulfitobacter were detected exclusively in eastern Pacific samples (ENB, ESB). Halomonas was enriched in ENB but was not detected in WNA. Similarly, wb1-A12, Blastocatella, and IS-44 were enriched in sediment samples (WSA, ESB) and were not identified in WNA. However, reliance on relative abundances alone was insufficient to definitively confirm the presence or absence of these genera, though it suggests the possibility of such patterns. It is important to note that these results are based on sequencing data from a single sample, highlighting the need for further replicates to robustly assess microbial enrichment preferences across different sample types.
The 16S full-length sequencing technique enabled species identification 29 species were detected from the nodule samples. Excluding the unsigned and uncultured species, 9 species could be identified (Table 4). These species, belonging to the Gamma or Alpha classes of Proteobacteria, were from various genera (Supplementary Table S2), indicating potential variations in their physiological characteristics and functions. It is also noteworthy that the occurrence of these species may be influenced by experimental factors, which may hinder the definitive determination of their exclusivity to a specific sample. Consequently, the zero abundances observed (Table 4) cannot be conclusively interpreted as the absence of these species but instead represent the potential for these species to be unique to certain samples.
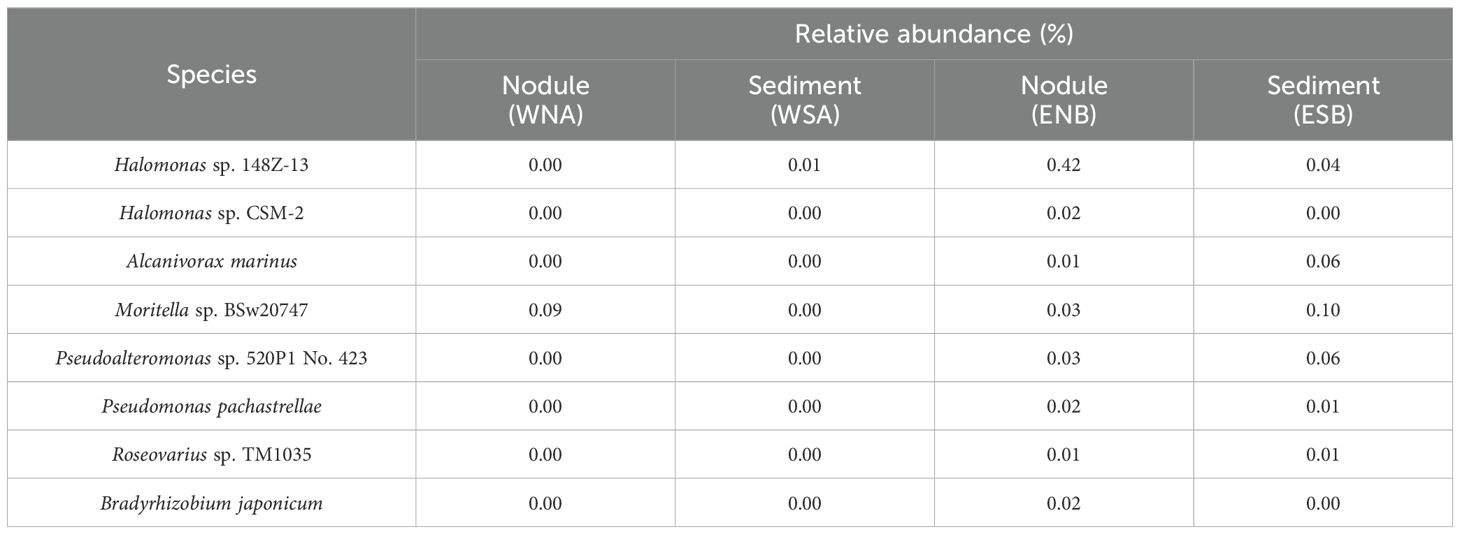
Table 4. Specific species in nodules and the surrounding sediments (removing unsigned and uncultured species).
Previous studies utilizing SEM-EDS have identified manganese-oxidizing bacteria (MOB) and biological structures involved in nodule mineralization (Wang and Müller, 2009; Jiang et al., 2020a; Shulga et al., 2022). In this study, metal-enriched biological-like structures were observed, including bacteria-like structures (Figures 4A, B) and other biomophs (Figures 4C–F), which are also likely involved in the mineralization process. SEM-EDS may have resolution limitations in analyzing biological structures, which can hinder accurate characterization of fine biological features and trace element distributions, making these findings speculative in the absence of direct evidence for their biological origin. However, careful comparisons with similar structures reported in the literature were made during the SEM-EDS analysis.
In addition to the microspheres potentially representing mineralizing bacteria (Figures 4A, B), other biomorphs that may contribute to the mineralization process include porous structures, likely remnants of radiolarian skeletons, characterized by high Si content and features such as spines or spine-like openings (Figure 4C) (Jiang et al., 2019);annular, skeleton-like structures resembling the solid shells produced by coccolithophores (Figures 4D, E), as proposed by Wang et al. (2012); and transparent, highly reflective structures capable of enriching Mn and Fe, similar to extracellular polymeric substances (EPS) (Figure 4F) (Ren and Jones, 2021).The surfaces of these structures seem to serve as sites for the precipitation of metal oxides, resulting in the accumulation of Fe and Mn minerals (Jiang et al., 2019), which fall under the category of biologically induced mineralization (BIM). BIM occurs when organisms secrete metabolites that react with ions or compounds in the environment, leading to the growth and deposition of mineral particles (Frankel and Bazylinski, 2003). Jiang et al. (2019) suggested that these biomorphs can induce mineralization processes through surface enrichment and proposed a mineralization process of mixed colloids of Mn- and Fe-oxide-hydroxides through biological-induced oxidation of Mn(II) and Fe(II) on the surfaces of biological structures. The significant enrichment of metals observed on the surfaces of biological structures in the present study provides further evidence for this idea (Figures 4).
However, it is crucial to acknowledge that the proposed biological structures are primarily qualitative interpretations based on the literature, rather than quantitative determinations. As such, their identification remains speculative, with EDS serving only as a supplementary tool. Consequently, the possibility of non-biological processes contributing to the observed morphological features cannot be excluded. For example, abiotic processes such as mineral precipitation or physical deposition could produce structures that resemble those associated with biological mineralization, complicating the interpretation of the results (Ren and Jones, 2021; Zhang et al., 2024). Moreover, the relationship between biological forms and mineral types in BIM remains unclear. Therefore, further research should aim to establish a more definitive link between specific biological structures and the minerals they may induce, with a focus on acquiring direct evidence of biological processes, while also critically considering the potential role of abiotic mechanisms in shaping these structures.
Previous studies have suggested that mineralizing bacteria, especially manganese-mineralizing bacteria, are present in nodules and actively contribute to the mineralization process (Blöthe et al., 2015; Reykhard and Shulga, 2019; Jiang et al., 2020b). SEM-EDS analysis has indicated the potential involvement of biological components, including bacteria, in mineralization (He et al., in press). Advances in sequencing technologies could further elucidate the mineralization functions of different bacterial species.
For example, members of the Magnetospiraceae and Hyphomicrobiaceae have been identified as playing significant roles in the iron cycle (Matsunaga et al., 1991; Shulga et al., 2022). The Magnetospiraceae group, characterized by magnetotaxis, also exhibits a unique iron-capturing ability that influences the distribution and migration of iron in the environment (Molari et al., 2020). Meanwhile, the Hyphomicrobiaceae group, as iron-oxidizing bacteria, alters the chemical form and reactivity of iron, thereby affecting its solubility, mobility, and bioavailability. This group may also indirectly impact the cycling of other metal elements (Molari et al., 2020). Additionally, Shewanella and Colwellia are known to participate in manganese reduction processes (Blöthe et al., 2015). Several Shewanella strains are also capable of oxidizing manganese to form manganese oxide nanoparticles (Omoike and Chorover, 2006; Bräuer et al., 2011; Wright et al., 2016). In addition, Shewanella may also participate in the Fe cycle, exemplified by Shewanella oneidensis MR-1, which is considered a model strain for dissimilatory iron reduction and is capable of mediating metal reduction through the production of EPS (Yan et al., 2021). EPS, composed of polysaccharides, proteins, and lipids, are biopolymers that serve as a prerequisite for biofilm formation (Liu et al., 2020) and act as carriers for biogeochemical processes such as cell adhesion, mineralization, and microbial metal redox cycling (Zheng et al., 2019). It should be noted that 16S rRNA sequencing has limitations in resolution and sensitivity, which may hinder accurate representation of the actual bacterial community composition (Poretsky et al., 2014). Future studies could employ metagenomics for a more comprehensive characterization of microbial community structure and functional traits (Arıkan and Muth, 2023; Satya et al., 2024).
Several genera, which were temporarily detected only in specific samples, also exhibit mineralization potential. For instance, Leptospirillum, which was detected exclusively in western Pacific nodule samples, possesses the ability for obligate Fe(II) oxidation and is widely distributed in metal-rich environments, suggesting its potential role in mineralization (Barrie Johnson, 2015). Lentisphaera, which was detected exclusively in western Pacific samples, has multiple strains capable of secreting transparent exopolymers, potentially facilitating metal adsorption on the bacterial surface and subsequent precipitation (Choi et al., 2013).
Similarly, several genera, including Sulfitobacter (Templeton et al., 2005), Halomonas (Templeton et al., 2005), Marinobacter (Handley and Lloyd, 2013), Pseudoalteromonas (Wu et al., 2013), and Marinomonas (Xuezheng et al., 2008), have been temporarily detected exclusively in nodules from the eastern Pacific (Supplementary Table S1) (He et al., in press). Strains of these manganese-oxidizing bacteria have been successfully isolated and cultured under laboratory conditions, demonstrating their ability to oxidize Mn(II) (Templeton et al., 2005; Zhang et al., 2019). Among these, Pseudoalteromona has received considerable attention for its production of extracellular organic compounds (Bowman, 2007), with EPS serving as effective carriers for inducing or controlling mineralization processes. For example, the strain Pseudoalteromonas TG12 has been reported to produce EPS capable of binding metals in sediments (Gutierrez et al., 2008). In addition, Marinobacter and Alcanivorax, both exclusively detected in eastern Pacific samples, have shown the ability to oxidize Fe(II) (Handley et al., 2009; Sudek et al., 2009; Smith et al., 2011). Beyond iron and manganese, some bacteria from eastern samples can also facilitate the oxidation of other metals. For instance, Acinetobacter has been found to oxidize Cr (Zakaria et al., 2007), while Marinobacter can oxidize As (Handley et al., 2009). While this does not diminish the discussion on bacterial roles in mineralization, the limitations of sequencing methods necessitate further evidence to definitively confirm the presence or absence of bacterial taxa across samples (Mainali et al., 2017).
Moreover, by utilizing the BIOCYC database (Caspi et al., 2015, 2016), several species with the potential for Mn(II)-oxidizing have been identified among species detected (Table 4): Roseovarius sp. TM1035, Pseudoalteromonas sp. 520P1 No. 423, and Bradyrhizobium japonicum were all found exclusively in nodules from the eastern Pacific. The enrichment of these species with Mn(II) oxidation capabilities may correlate with the higher Mn content in the nodule from the eastern Pacific (Table 2). However, the specific oxidation pathways and enzymes involved in the Mn oxidation process of these species still require further clarification.
Besides, Roseovarius sp. TM1035 can produce biofilm to adapt to changes in the underlying environment (Kent et al., 2018). Biofilm, an EPS containing embedded bacteria, aids in bacterial resistance against adverse environmental stresses and also provides sites on its surface for metal oxidation, mineral aggregation, and precipitation, potentially facilitating the mineralization process (Wang and Müller, 2009; Decho, 2010). Some strains of Pseudoalteromonas can also generate biofilms for the removal of Pb(II), Cr(VI), and Cd(II) (Priyadarshanee and Das, 2021), and some strains of Bradyrhizobium has been found to employ an EPS-mediated defense mechanism for metal precipitation (Santamaría et al., 2003). However, given our experimental results, more precise functional analysis or prediction of key genes may be required to further enhance our understanding of the mineralization functions of the aforementioned bacteria. It’s also important to recognize that the relative abundance data alone may not conclusively indicate the exclusive presence of certain bacterial taxa in specific samples, given potential variations arising from factors like limited sequencing depth and methodological biases.
The growth environments of polymetallic nodules in the western and eastern Pacific may differ. In the western Pacific, polymetallic nodules are thought to be primarily hydrogenetic (Mn/Fe ~ 1), with metals precipitating from oxygen-rich near-bottom seawater, enriching Fe and Co, while nodules from the eastern Pacific appear to be more significantly influenced by diagenetic processes, with metal precipitation occurring in sediment pore spaces, enriching Mn, Cu, and Ni (Halbach et al., 1981, 1988). The elemental composition of nodules is likely influenced by factors such as surface primary productivity, organic carbon content in the seafloor, and redox conditions. In the western Pacific, low primary productivity and high dissolved oxygen in bottom waters may contribute to the rapid oxidation of Fe and Co to stable +3 oxidation states (Liu and Millero, 2002; Dutkiewicz et al., 2020; Jiang et al., 2020a). In contrast, in the eastern Pacific, higher primary productivity and organic carbon content in sediments may promote the formation of metal-organic complexes, potentially enriching Mn, Cu, and Ni in the sediments (Verlaan et al., 2004). As organic matter degrades, reducing conditions at the seafloor may release Mn and other elements in their 2+ states into pore waters, which could further contribute to the enrichment of these elements in the nodules (Jorgensen, 2001; Arndt et al., 2013).
The differing genesis of polymetallic nodules may also suggest variations in the hydrodynamic and geological conditions under which the nodules form. Nodules of hydrogenetic origin in the western Pacific typically develop in relatively stable aquatic and geological environments, facilitating the growth of near-spherical nodules characterized by rhythmic, dense, and uniform laminae (Figure 2A, Figures 3A, B) (Li et al., 2020). In contrast, nodules from the eastern Pacific are predominantly influenced by diagenetic processes, displaying stromatolitic-like growth structures with broader fissures and pores (Figure 2D, Figures 3D, E). These features indicate that nodule growth in this region was influenced by ongoing environmental modifications, including seafloor bioturbation and bottom currents (Veillette et al., 2007; Wegorzewski and Kuhn, 2014).
Furthermore, the differences in microbial community composition across nodule fields in different marine regions may also reflect variations in the environmental conditions. For example, notable distinctions were observed when comparing the bacterial communities identified in this study with those from other Mn nodule fields, Such as the Korea Deep Ocean Study (KODOS) (Cho et al., 2018) and the German license area (Blöthe et al., 2015) in the Clarion-Clipperton Fracture Zone (CCFZ), as well as the Kara Sea (Vereshchagin et al., 2019)and the South Pacific Gyre (Shiraishi et al., 2016). For instance, Marinobacter and Idiomarina, two genera that are mostly strictly aerobic, were abundant in the KODOS area (Ivanova et al., 2000; Green et al., 2006). However, they were not detected in samples from the western Pacific in this study, and their abundances in samples from the eastern Pacific were extremely low (<0.2%), potentially linked to variations in environmental oxygen levels. Moreover, Shewanella and Colwellia, as potentially important genera in the nodule formation in this study, were not detected in the KODOS area and were only found in nodules from the German license area. However, Colwellia exhibited widespread enrichment in sediments from the Kara Sea (2%) and the South Pacific Gyre (>50%). Both of these two regions are characterized by extremely low surface primary productivity (D’Hondt et al., 2009; Demidov et al., 2017), suggesting that Colwellia may accumulate in environments with exceptionally low carbon content due to its capability to degrade recalcitrant substances (Tully and Heidelberg, 2013). The acquisition of more detailed environmental data in future studies may provide further empirical support for our hypothesis.
This study provides valuable insights into the relationship between microorganisms and polymetallic nodule formation in the Pacific Ocean. Through SEM-EDS analysis, we discovered various biological-like structures capable of enriching metal elements on their surfaces, indicating their potential role in promoting nodule formation and growth. Several bacteria-like microspheres may suggest the presence of mineralizing bacteria within the nodules. Furthermore, 16S rRNA gene sequencing identified several bacteria with mineralization potential. These bacteria were found to be involved in metal redox cycles through physiological metabolism or by facilitating mineral deposition through the production of biofilms or EPS. Additionally, differences in elemental compositions, internal structures, and bacterial community compositions may indicate variations in the growth environments of nodules across different regions. In the results and discussion, we drew upon literature to clarify the bio-analogous types and the functions of bacteria (especially potential mineralization functions). Further research is needed to explore the specific mechanisms of interaction between microorganisms and minerals, and to investigate the potential applications of these findings in areas such as deep-sea mining and environmental management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
XH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. QL: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – review & editing. XL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. ZL: Data curation, Methodology, Resources, Writing – review & editing. HW: Methodology, Resources, Writing – review & editing. ZZ: Methodology, Resources, Writing – review & editing. YD: Methodology, Resources, Writing – review & editing. JL: Methodology, Resources, Writing – review & editing. HL: Methodology, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Key R&D Program of China (Grant No. 2023YFC2811305, 2023YFC2811205-01, 2022YFC2806601 and 2022YFC2806602), the National Natural Science Foundation of China (U2244222).
We thank the captain, crew, and scientists for their assistance during the Dayangyihao and Dayanghao research cruise. We would like to express our appreciation to the technical staff, especially Zedong Fan, Yurong Qian, Mingyan Lai and Ziang Li for their helpful advice and suggestions with experimental operations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1533654/full#supplementary-material
Akai J., Akiyama S., Tsuchiyama A., Akai K. (2013). Ocean manganese nodules as stromatolite with a fractal like-signature. Phys. Chem. Earth Parts A/B/C 58-60, 42–48. doi: 10.1016/j.pce.2013.04.004
Arıkan M., Muth T. (2023). Integrated multi-omics analyses of microbial communities: a review of the current state and future directions. Mol. Omics. doi: 10.1039/D3MO00089C
Arndt S., Jørgensen B. B., LaRowe D. E., Middelburg J. J., Pancost R. D., Regnier P. (2013). Quantifying the degradation of organic matter in marine sediments: A review and synthesis. Earth-Science Rev. 123, 53–86. doi: 10.1016/j.earscirev.2013.02.008
Avidan O., Satanower S., Banin E. (2010). “Iron and bacterial biofilm development,” in Microbial Mats: Modern and Ancient Microorganisms in Stratified Systems (Dordrecht: Springer), 359–383.
Banfield J. F., Nealson K. H. (2018). Geomicrobiology: Interactions between microbes and minerals (Berlin: Walter de Gruyter GmbH & Co KG).
Barrie Johnson D. (2015). “Leptospirillum,” in Bergey’s Manual of Systematics of Archaea and Bacteria (Wiley, Hoboken, NJ), 1–8.
Blöthe M., Wegorzewski A., Muller C., Simon F., Kuhn T., Schippers A. (2015). Manganese-cycling microbial communities inside deep-dea manganese nodules. Environ. Sci. Technol. 49, 7692–7700. doi: 10.1021/es504930v
Bowman J. P. (2007). Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs 5, 220–241. doi: 10.3390/md504220
Bräuer S. L., Adams C., Kranzler K., Murphy D., Xu M., Zuber P., et al. (2011). Culturable Rhodobacter and Shewanella species are abundant in estuarine turbidity maxima of the Columbia river. Environ. Microbiol. 13, 589–603. doi: 10.1111/j.1462-2920.2010.02360.x
Bruins M. R., Kapil S., Oehme F. W. (2000). Microbial resistance to metals in the environment. Ecotoxicology Environ. Saf. 45, 198–207. doi: 10.1006/eesa.1999.1860
Burnett B. R., Nealson K. H. (1983). Energy dispersive X-ray analysis of the surface of a deep-sea ferromanganese nodule. Mar. Geology 53, 313–329. doi: 10.1016/0025-3227(83)90048-8
Caspi R., Billington R., Ferrer L., Foerster H., Fulcher C. A., Keseler I. M., et al. (2015). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 44, D471–D480. doi: 10.1093/nar/gkv1164
Caspi R., Billington R., Foerster H., Fulcher C. A., Keseler I., Kothari A., et al. (2016). BioCyc: online resource for genome and metabolic pathway analysis. FASEB J. 30, lb192–lb192. doi: 10.1096/fasebj.30.1_supplement.lb192
Cho H., Kim K.-H., Son S. K., Hyun J.-H. (2018). Fine-scale microbial communities associated with manganese nodules in deep-sea sediment of the Korea deep ocean study area in the northeast equatorial pacific. Ocean Sci. J. 53, 337–353. doi: 10.1007/s12601-018-0032-0
Choi A., Yang S.-J., Rhee K.-H., Cho J.-C. (2013). Lentisphaera marina sp. nov., and emended description of the genus Lentisphaera. Int. J. Systematic Evolutionary Microbiol. 63, 1540–1544. doi: 10.1099/ijs.0.046433-0
Cochonat P., Le Suavé R., Charles C., Greger B., Hoffert M., Lenoble J. P., et al. (1992). First in situ studies of nodule distribution and geotechnical measurements of associated deep-sea clay (Northeastern pacific ocean). Mar. Geology 103 (1), 373–380. doi: 10.1016/0025-3227(92)90027-F
Crerar D. A., Barnes H. L. (1974). Deposition of deep-sea manganese nodules. Geochimica Cosmochimica Acta 38, 279–300. doi: 10.1016/0016-7037(74)90111-2
D’Hondt S., Spivack A. J., Pockalny R., Ferdelman T. G., Fischer J. P., Kallmeyer J., et al. (2009). Subseafloor sedimentary life in the South Pacific Gyre. Proc. Natl. Acad. Sci. 106, 11651–11656. doi: 10.1073/pnas.0811793106
Decho A. W. (2010). Overview of biopolymer-induced mineralization: What goes on in biofilms? Ecol. Eng. 36, 137–144. doi: 10.1016/j.ecoleng.2009.01.003
Demidov A. B., Kopelevich O. V., Mosharov S. A., Sheberstov S. V., Vazyulya S. V. (2017). Modelling Kara Sea phytoplankton primary production: Development and skill assessment of regional algorithms. J. Sea Res. 125, 1–17. doi: 10.1016/j.seares.2017.05.004
Dutkiewicz A., Judge A., Müller R. D. (2020). Environmental predictors of deep-sea polymetallic nodule occurrence in the global ocean. Geology 48, 293–297. doi: 10.1130/g46836.1
Frankel R. B., Bazylinski D. A. (2003). Biologically induced mineralization by bacteria. Rev. Mineralogy Geochemistry 54, 95–114. doi: 10.2113/0540095
Glasby G. P. (1976). Manganese nodules in the south pacific: A review. New Z. J. Geology Geophysics 19 (5), 707–736. doi: 10.1080/00288306.1976.10426315
Glasby G. P., Gwozdz R., Kunzendorf H., Friedrich G., Thijssen T. (1987). The distribution of rare earth and minor elements in manganese nodules and sediments from the equatorial and S.W. pacific. Lithos 20 (2), 97–113. doi: 10.1016/0024-4937(87)90001-6
Glasby G. P., Stoffers P., Sioulas A., Thijssen T., Friedrich G. (1982). Manganese nodule formation in the pacific ocean: a general theory. Geo-Marine Lett. 2 (1), 47–53. doi: 10.1007/BF02462799
Goldberg E. D., G.O.S A. (1958). Chemistry of Pacific pelagic sediments. Geochimica cosmochimica Acta 13, 153–212. doi: 10.1016/0016-7037(58)90046-2
Graham J. W., Cooper S. C. (1959). Biological origin of manganese-rich deposits of the sea floor. Nature 183, 1050–1051. doi: 10.1038/1831050a0
Green D. H., Bowman J. P., Smith E. A., Gutierrez T., Bolch C. J. S. (2006). Marinobacter algicola sp. nov., isolated from laboratory cultures of paralytic shellfish toxin-producing dinoflagellates. Int. J. Systematic Evolutionary Microbiol. 56, 523–527. doi: 10.1099/ijs.0.63447-0
Gutierrez T., Shimmield T., Haidon C., Black K., Green D. H. (2008). Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by Pseudoalteromonas sp. strain TG12. Appl. Environ. Microbiol. 74, 4867–4876. doi: 10.1128/AEM.00316-08
Halbach P., Friedrich G., von Stackelberg U. (1988). The manganese nodule belt of the Pacific Ocean: geological environment, nodule formation, and mining aspects (Germany: F. Enke Stuttgart).
Halbach P., Scherhag C., Hebisch U., Marchig V. (1981). Geochemical and mineralogical control of different genetic types of deep-sea nodules from the Pacific Ocean. Mineralium Deposita 16, 59–84. doi: 10.1007/BF00206455
Handley K. M., Héry M., Lloyd J. R. (2009). Redox cycling of arsenic by the hydrothermal marine bacterium Marinobacter santoriniensis. Environ. Microbiol. 11, 1601–1611. doi: 10.1111/j.1462-2920.2009.01890.x
Handley K., Lloyd J. (2013). Biogeochemical implications of the ubiquitous colonization of marine habitats and redox gradients by Marinobacter species. Front. Microbiol. 4. doi: 10.3389/fmicb.2013.00136
He X., Liu Q., Li X., Li Z., Wang H., Zhu Z., et al. (in press). Preliminary study on microbial community structure and function in deep-sea polymetallic nodules and surrounding sediments. J. Mar. Sci. 43 (1).
Hein J. R., Koschinsky A. (2014). “Deep-ocean ferromanganese crusts and nodules,” in Treatise on Geochemistry, 2nd ed. (Elsevier, Newnes), 273–291.
Hein J. R., Koschinsky A., Halbach P., Manheim F. T., Bau M., Kang J.-K., et al. (1997). Iron and manganese oxide mineralization in the Pacific. Geological Society London Special Publications 119, 123–138. doi: 10.1144/GSL.SP.1997.119.01.09
Hein J. R., Koschinsky A., Kuhn T. (2020). Deep-ocean polymetallic nodules as a resource for critical materials. Nat. Rev. Earth Environ. 1, 158–169. doi: 10.1038/s43017-020-0027-0
Hein J. R., Mizell K., Koschinsky A., Conrad T. A. (2013). Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: comparison with land-based resources. Ore Geology Rev. 51, 1–14. doi: 10.1016/j.oregeorev.2012.12.001
Helmann J. D. (2014). Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. J. Biol. Chem. 289, 28112–28120. doi: 10.1074/jbc.R114.587071
Hoffmann T. D., Reeksting B. J., Gebhard S. (2021). Bacteria-induced mineral precipitation: a mechanistic review. Microbiology 167 (4), 1–13. doi: 10.1099/mic.0.001049
Hollingsworth A. L., Jones D. O. B., Young C. R. (2021). Spatial variability of abyssal nitrifying microbes in the north-eastern clarion-clipperton zone. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.663420
Hu W., Zhou H., Gu L., Zhang W., Lu X., Fu Q., et al. (2000). New evidence of microbe origin for ferromanganese nodules from the East Pacific deep sea floor. Sci. China Ser. D: Earth Sci. 43, 187–192. doi: 10.1007/bf02878148
Ivanova E. P., Romanenko L. A., Chun J., Matte M. H., Matte G. R., Mikhailov V. V., et al. (2000). Idiomarina gen. nov., comprising novel indigenous deep-sea bacteria from the Pacific Ocean, including descriptions of two species, Idiomarina abyssalis sp. nov. and Idiomarina zobellii sp. nov. Int. J. Systematic Evolutionary Microbiol. 50, 901–907. doi: 10.1099/00207713-50-2-901
Jiang X.-D., Gong J.-L., Ren J.-B., Liu Q.-S., Zhang J., Chou Y.-M. (2020a). An interdependent relationship between microbial ecosystems and ferromanganese nodules from the Western Pacific Ocean. Sedimentary Geology 398, 1–18. doi: 10.1016/j.sedgeo.2019.105588
Jiang X. D., Sun X. M., Guan Y. (2019). Biogenic mineralization in the ferromanganese nodules and crusts from the South China Sea. J. Asian Earth Sci. 171, 46–59. doi: 10.1016/j.jseaes.2017.07.050
Jiang X.-D., Sun X.-M., Guan Y., Gong J.-L., Lu Y., Lu R.-F., et al. (2017). Biomineralisation of the ferromanganese crusts in the Western Pacific Ocean. J. Asian Earth Sci. 136, 58–67. doi: 10.1016/j.jseaes.2017.01.025
Jiang X. D., Zhao X., Chou Y. M., Liu Q. S., Roberts A. P., Ren J. B., et al. (2020b). Characterization and quantification of magnetofossils within abyssal manganese nodules from the western Pacific ocean and implications for nodule formation. Geochemistry Geophysics Geosystems 21, e2019GC008811. doi: 10.1029/2019GC008811
Jorgensen B. (2001). “Diagenesis and sediment-water exchange,” in The Benthic Boundary Layer. Transport Processes and Biogeochemistry (New York: Oxford University Press), 211–244.
Kent A. G., Garcia C. A., Martiny A. C. (2018). Increased biofilm formation due to high-temperature adaptation in marine Roseobacter. Nat. Microbiol. 3, 989–995. doi: 10.1038/s41564-018-0213-8
Konhauser K. O. (1997). Bacterial iron biomineralisation in nature. FEMS Microbiol. Rev. 20, 315–326. doi: 10.1111/j.1574-6976.1997.tb00317.x
Li D., Fu Y., Sun X., Wei Z. (2020). Critical metal enrichment mechanism of deep-sea hydrogenetic nodules: Insights from mineralogy and element mobility. Ore Geology Rev. 118, 103371. doi: 10.1016/j.oregeorev.2020.103371
Liu H., Li P., Wang H., Qing C., Tan T., Shi B., et al. (2020). Arsenic mobilization affected by extracellular polymeric substances (EPS) of the dissimilatory iron reducing bacteria isolated from high arsenic groundwater. Sci. Total Environ. 735, 139501. doi: 10.1016/j.scitotenv.2020.139501
Liu X., Millero F. J. (2002). The solubility of iron in seawater. Mar. Chem. 77, 43–54. doi: 10.1016/S0304-4203(01)00074-3
Mainali K. P., Bewick S., Thielen P., Mehoke T., Breitwieser F. P., Paudel S., et al. (2017). Statistical analysis of co-occurrence patterns in microbial presence-absence datasets. PloS One 12, e0187132. doi: 10.1371/journal.pone.0187132
Margolis S. V., Burns R. G. (1976). Pacific deep-sea maganese nodules-their distribution, composition, and origin. Annu. Rev. Earth Planetary Sci. 4, 229–263.
Matsunaga T., Sakaguchi T., Tadokoro F. (1991). Magnetite formation by a magnetic bacterium capable of growing aerobically. doi: 10.1007/BF00169632
Mhatre E., Troszok A., Gallegos-Monterrosa R., Lindstädt S., Hölscher T., Kuipers O. P., et al. (2016). The impact of manganese on biofilm development of Bacillus subtilis. Microbiology 162, 1468–1478. doi: 10.1099/mic.0.000320
Molari M., Janssen F., Vonnahme T. R., Wenzhöfer F., Boetius A. (2020). The contribution of microbial communities in polymetallic nodules to the diversity of the deep-sea microbiome of the Peru Basin, (4130–4198 m depth). Biogeosciences 17, 3203–3222. doi: 10.5194/bg-17-3203-2020
Nayak B., Das S. K., Munda P. (2013). Biogenic signature and ultra microfossils in ferromanganese nodules of the Central Indian Ocean Basin. J. Asian Earth Sci. 73, 296–305. doi: 10.1016/j.jseaes.2013.03.032
Omoike A., Chorover J. (2006). Adsorption to goethite of extracellular polymeric substances from Bacillus subtilis. Geochimica Cosmochimica Acta 70, 827–838. doi: 10.1016/j.gca.2005.10.012
Poretsky R., Rodriguez-R L. M., Luo C., Tsementzi D., Konstantinidis K. T. (2014). Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PloS One 9, e93827. doi: 10.1371/journal.pone.0093827
Priyadarshanee M., Das S. (2021). Bioremediation potential of biofilm forming multi-metal resistant marine bacterium Pseudomonas chengduensis PPSS-4 isolated from contaminated site of Paradip Port, Odisha. J. Earth System Sci. 130, 125. doi: 10.1007/s12040-021-01627-w
Ren J., He G., Deng X., Deng X., Yang Y., Yao H., et al. (2022). Metallogenesis of Co-rich ferromanganese nodules in the northwestern Pacific: Selective enrichment of metallic elements from seawater. Ore Geology Rev. 143, 104778. doi: 10.1016/j.oregeorev.2022.104778
Ren M., Jones B. (2021). Modern authigenic amorphous and crystalline iron oxyhydroxides in subsurface Ordovician dolostones (Jinan, North China Block): Biomineralization and crystal morphology. Sedimentary Geology 426, 106044. doi: 10.1016/j.sedgeo.2021.106044
Reykhard L. Y., Shulga N. A. (2019). Fe-Mn nodule morphotypes from the NE Clarion-Clipperton Fracture zone, Pacific ocean: comparison of mineralogy, geochemistry and genesis. Ore Geology Rev. 110, 1–15. doi: 10.1016/j.oregeorev.2019.102933
Reyss J., Lemaitre N., Ku T., Marchig V., Southon J., Nelson D., et al. (1985). Growth of a manganese nodule from Peru Basin: A radiochemical anatomy. Geochimica Cosmochimica Acta 49, 2401–2408. doi: 10.1016/0016-7037(85)90240-6
Roy S. (1992). Environments and processes of manganese deposition. Economic Geology 87, 1218–1236. doi: 10.2113/gsecongeo.87.5.1218
Santamaría M., Díaz-Marrero A. R., Hernández J., Gutiérrez-Navarro A. M., Corzo J. (2003). Effect of thorium on the growth and capsule morphology of Bradyrhizobium. Environ. Microbiol. 5, 916–924. doi: 10.1046/j.1462-2920.2003.00487.x
Satya S., Sharma S., Choudhary G., Kaushik G. (2024). “Advances in environmental microbiology: A multi-omic perspective,” in Microbial Omics in Environment and Health. Eds. Kesheri M., Kanchan S., Salisbury T. B., Sinha R. P. (Springer Nature Singapore, Singapore), 175–204.
Shiraishi F., Mitsunobu S., Suzuki K., Hoshino T., Morono Y., Inagaki F. (2016). Dense microbial community on a ferromanganese nodule from the ultra-oligotrophic South Pacific Gyre: Implications for biogeochemical cycles. Earth Planetary Sci. Lett. 447, 10–20. doi: 10.1016/j.epsl.2016.04.021
Shulga N., Abramov S., Klyukina A., Ryazantsev K., Gavrilov S. (2022). Fast-growing Arctic Fe–Mn deposits from the Kara Sea as the refuges for cosmopolitan marine microorganisms. Sci. Rep. 12, 21967. doi: 10.1038/s41598-022-23449-6
Smith A., Popa R., Fisk M., Nielsen M., Wheat C. G., Jannasch H. W., et al. (2011). In situ enrichment of ocean crust microbes on igneous minerals and glasses using an osmotic flow-through device. Geochemistry Geophysics Geosystems 12, Q06007. doi: 10.1029/2010GC003424
Sudek L. A., Templeton A. S., Tebo B. M., Staudigel H. (2009). Microbial ecology of Fe (hydr)oxide mats and basaltic rock from Vailulu’u seamount, American Samoa. Geomicrobiology J. 26, 581–596. doi: 10.1080/01490450903263400
Takematsu N., Sato Y., Okabe S. (1989). Factors controlling the chemical composition of marine manganese nodules and crusts: a review and synthesis. Mar. Chem. 26, 41–56. doi: 10.1016/0304-4203(89)90063-7
Templeton A. S., Staudigel H., Tebo B. M. (2005). Diverse Mn(II)-oxidizing bacteria isolated from submarine basalts at Loihi seamount. Geomicrobiology J. 22, 127–139. doi: 10.1080/01490450590945951
Tully B. J., Heidelberg J. F. (2013). Microbial communities associated with ferromanganese nodules and the surrounding sediments. Front. Microbiol. 4. doi: 10.3389/fmicb.2013.00161
Veillette J., Sarrazin J., Gooday A. J., Galéron J., Caprais J.-C., Vangriesheim A., et al. (2007). Ferromanganese nodule fauna in the Tropical North Pacific Ocean: Species richness, faunal cover and spatial distribution. Deep Sea Res. Part I: Oceanographic Res. Papers 54, 1912–1935. doi: 10.1016/j.dsr.2007.06.011
Vereshchagin O. S., Perova E. N., Brusnitsyn A. I., Ershova V. B., Khudoley A. K., Shilovskikh V. V., et al. (2019). Ferro-manganese nodules from the Kara Sea: Mineralogy, geochemistry and genesis. Ore Geology Rev. 106, 192–204. doi: 10.1016/j.oregeorev.2019.01.023
Verlaan P. A., Cronan D. S., Morgan C. L. (2004). A comparative analysis of compositional variations in and between marine ferromanganese nodules and crusts in the South Pacific and their environmental controls. Prog. Oceanography 63, 125–158. doi: 10.1016/j.pocean.2004.11.001
Wang X., Müller W. E. (2009). Marine biominerals: perspectives and challenges for polymetallic nodules and crusts. Trends Biotechnol. 27, 375–383. doi: 10.1016/j.tibtech.2009.03.004
Wang X.-H., Schloßmacher U., Wang S.-F., Schröder H. C., Wiens M., Batel R., et al. (2012). From nanoparticles via microtemplates and milliparticles to deep-sea nodules: biogenically driven mineral formation. Front. Materials Sci. 6, 97–115. doi: 10.1007/s11706-012-0164-6
Washburn T. W., Jones D. O. B., Wei C.-L., Smith C. R. (2021). Environmental heterogeneity throughout the clarion-clipperton zone and the potential representativity of the APEI network. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.661685
Wegorzewski A. V., Kuhn T. (2014). The influence of suboxic diagenesis on the formation of manganese nodules in the Clarion Clipperton nodule belt of the Pacific Ocean. Mar. Geology 357, 123–138. doi: 10.1016/j.margeo.2014.07.004
Wright M. H., Farooqui S. M., White A. R., Greene A. C. (2016). Production of manganese oxide nanoparticles by Shewanella species. Appl. Environ. Microbiol. 82, 5402–5409. doi: 10.1128/AEM.00663-16
Wu Y.-H., Liao L., Wang C.-S., Ma W.-L., Meng F.-X., Wu M., et al. (2013). A comparison of microbial communities in deep-sea polymetallic nodules and the surrounding sediments in the Pacific Ocean. Deep Sea Res. Part I: Oceanographic Res. Papers 79, 40–49. doi: 10.1016/j.dsr.2013.05.004
Xuezheng L., Aiguo G., Haowen C. (2008). Isolation and phylogenetic analysis of cultivable manganese bacteria in sediments from the Arctic Ocean. Acta Ecologica Sin. 28, 6364–6370. doi: 10.1016/S1872-2032(09)60017-2
Yan W., Guo W., Wang L., Jing C. (2021). Extracellular polymeric substances from Shewanella oneidensis MR-1 biofilms mediate the transformation of Ferrihydrite. Sci. Total Environ. 784, 147245. doi: 10.1016/j.scitotenv.2021.147245
Zakaria Z. A., Zakaria Z., Surif S., Ahmad W. A. (2007). Hexavalent chromium reduction by Acinetobacter haemolyticus isolated from heavy-metal contaminated wastewater. J. Hazardous Materials 146, 30–38. doi: 10.1016/j.jhazmat.2006.11.052
Zhang J., Wang C., Han J.-R., Chen G.-J., Du Z.-J. (2019). Alteromonas flava sp. nov. and Alteromonas facilis sp. nov., two novel copper tolerating bacteria isolated from a sea cucumber culture pond in China. Systematic Appl. Microbiol. 42, 217–222. doi: 10.1016/j.syapm.2018.11.006
Zhang C., Yang K., Li F., Zhou J. (2024). The roles of amorphous phase on Ca–Mg carbonate mineralization under the action of bacteria and EPS. J. Crystal Growth 644, 127841. doi: 10.1016/j.jcrysgro.2024.127841
Keywords: polymetallic nodules, sediment, bacterial composition, biomineralization, scanning electron microscope, Pacific Ocean
Citation: He X, Liu Q, Li X, Li Z, Wang H, Zhu Z, Dong Y, Li J and Li H (2025) Bacterial contributions to the formation of polymetallic nodules in the Pacific Ocean. Front. Mar. Sci. 12:1533654. doi: 10.3389/fmars.2025.1533654
Received: 24 November 2024; Accepted: 03 February 2025;
Published: 03 March 2025.
Edited by:
Xiaodong Jiang, Guangdong University of Technology, ChinaReviewed by:
Ruiyong Zhang, Chinese Academy of Sciences (CAS), ChinaCopyright © 2025 He, Liu, Li, Li, Wang, Zhu, Dong, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohu Li, eGhsaUBzaW8ub3JnLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.