- 1Southern Zhejiang Key Laboratory of Crop Breeding, Wenzhou Academy of Agricultural Sciences, Wenzhou, China
- 2Institute of Eco-Environmental Sciences, Wenzhou Academy of Agricultural Sciences, Wenzhou, China
- 3National and Local Joint Engineering Research Center of Ecological Treatment Technology for Urban Water Pollution, Wenzhou University, Wenzhou, China
- 4Zhejiang Provincial Key Lab for Subtropical Water Environment and Marine Biological Resources Protection, Wenzhou University, Wenzhou, China
- 5Department of Land, Air and Water Resources, University of California, Davis, Davis, CA, United States
- 6Key Laboratory of Marine Ecosystem Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China
Seaweed cultivation contributes to coastal carbon sequestration making it a compelling strategy to mitigate global climate change. Porphyra (commonly known as nori) is an economically important seaweed known to have high release rates for biogenic dissolved and particulate organic matter (DOM and POM). However, the impact of Porphyra cultivation on coastal organic matter dynamics remains unclear. To fill this knowledge gap, we conducted investigations examining the quantity and optical properties of DOM and POM, microbial community structures and relevant environmental factors along a continuum from a subtropical river through its adjacent coastal Porphyra cultivation zone during the cultivation and non-cultivation periods. Dissolved organic carbon (DOC) concentration was significantly elevated during the cultivation versus non-cultivation period, while particulate organic carbon (POC) concentration decreased, thereby resulting in a higher DOC/POC ratio in the water column. Endmember mixing analysis further suggested that autochthonous organic matter dominated in the coastal cultivation zone during both periods, with limited inputs of terrestrial organic carbon. Redundancy analysis revealed that more microbial modules mediated organic matter transformations during the cultivation period, leading to a 169% higher estuarine addition of microbially-sourced humic-like C3 compared to the non-cultivation period. Our findings demonstrate that Porphyra cultivation enhanced coastal carbon sequestration by promoting the autochthonous production and transformation of refractory DOM, which has important implications for the sustainable management and development of coastal blue carbon strategies.
1 Introduction
Atmospheric carbon dioxide (CO2) is steadily increasing due to intensive global anthropogenic activities, which is rapidly driving climate change (Cai et al., 2014; IPCC, 2022). Oceans acts as a significant carbon sink, with over 30% of global anthropogenic CO2 sequestrated by the oceans (Le Quéré et al., 2014). Consequently, the management and development of blue carbon systems offer valuable opportunities for mitigating climate change (Macreadie et al., 2021). In addition to traditional blue carbon systems (e.g., mangroves, saltmarshes and seagrass), recent studies have highlighted the potential of seaweed-dominated systems to enhance oceanic carbon sequestration owing to their high net primary production and biomass (Krause-Jensen and Duarte, 2016; Ross et al., 2023). If sustainable and extensive seaweed cultivation can be developed, the carbon sequestration potential for coastal systems may increase significantly (Zhang et al., 2017).
Compared with the positive effect of seaweed cultivation on atmospheric CO2 sequestration, knowledge concerning the role of seaweed ecosystems in ocean carbon cycling is limited. Approximately 43% of seaweed biomass is exported as DOM and POM to coastal waters, which could theoretically lead to an increase of DOC and POC within cultivation areas (Krause-Jensen and Duarte, 2016). Most seaweed-derived organic matter is abundant in bio-available components (e.g., protein and aliphatic compounds), which are subject to microbial degradation and partly outgassed back to the atmosphere as CO2 (Feng et al., 2022; Zhao et al., 2023a).
Moreover, some macroalgal organic matter is transformed into refractory DOM (RDOM), which can persist in the water column for hundreds or even thousands of years (Jiao et al., 2018; Li et al., 2022). Thus, the potential for carbon sequestration through seaweed cultivation depends on the degree to which seaweed-derived organic carbon can persist in oceans. It is noteworthy that the impact of seaweed cultivation on the coastal organic carbon pool is not only dependent on the chemical composition of seaweed-derived organic matter, but also on the biogeochemical conditions (e.g., microbial community structure, water temperature, terrestrial inputs) within the cultivation ecosystem (Huang et al., 2024; Qu et al., 2022). For instance, microbial composition and traits largely determine the utilization and transformation efficiency of coastal organic matter, which vary across different cultivation stages (Fontaine et al., 2003; Xu et al., 2022). The relatively cool cultivation environment also contrasts with the warmer conditions conducive to microbial carbon transformations (Qu et al., 2022). Further, the complex biogeochemical conditions associated with coastal seaweed cultivation systems greatly increases the uncertainty of seaweed-derived carbon transformations. However, the ultimate effects of variations in microbial assemblages and geochemical conditions on the turnover of DOM and POM, particularly the dynamics of RDOM, in coastal cultivation zones remain uncertain.
Previous studies have primarily focused on the effects of kelp cultivation on DOM cycling in coastal systems (Huang et al., 2024; Li et al., 2022). It has been reported that kelp cultivation introduces a variety of sulfur-containing DOM compounds into coastal waters, which eventually contributes to the RDOM pool after microbial transformation (Li et al., 2022). An incubation experiment further revealed that leachates from kelp-derived detritus contained refractory DOM components, which may directly increase coastal RDOM (Feng et al., 2022). Notably, there is a paucity of studies examining the impact of coastal carbon sequestration from other widely cultured seaweed species, preventing a comprehensive evaluation of their overall potential for carbon sequestration.
Porphyra is one of the major economically important seaweeds, characterized by high yields and a short growth period. The rapid carbon accumulation of Porphyra leads to a higher release rate of DOC compared to other cultivated seaweeds (e.g., kelp, Wakame, Gracilaria and Eucheuma) and natural seaweeds (e.g., Sargassum and Lactuca) (Chen, 2020). The release rate of POC from Porphyra is higher than cultivated seaweeds, but lower than that of Sargassum and Lactuca. Therefore, a comprehensive study of the impacts of Porphyra cultivation on coastal organic carbon dynamics is strongly warranted.
Herein, we investigated the quantity and optical composition of DOM and POM, microbial communities and relevant water quality parameters along a subtropical river and its adjacent coastal Porphyra cultivation zone during both non-cultivation and cultivation periods. The primary objectives of this study were to: (1) investigate the temporal variation and sources of DOM and POM in the coastal Porphyra cultivation zone; (2) assess the influence of Porphyra cultivation on the coastal RDOM pool; and (3) elucidate the key organic matter transformation mechanisms, with a particular focus on RDOM production in the Porphyra cultivation zone. Our study aims to provide fundamental data and new insights into how Porphyra cultivation affects coastal organic matter dynamics and carbon sequestration potential.
2 Materials and methods
2.1 Study area
Dayu Bay is a shallow (depth< 5 m), semi-enclosed bay located in southeast China (Figure 1). Porphyra cultivation area within Dayu Bay covers 382 hectares, with an annual production of 31,460 tons, making it one of the largest Porphyra cultivation zones in China. The cultivation period for Porphyra spans from late summer (September) to early spring (April) with harvest occurring approximately every 40 days after seeding. The Chi River watershed, a small river situated in southwestern Dayu Bay, has a catchment area of 70 km2. The Chi River watershed is a relatively undeveloped basin with a population density of 244 people per km² in 2020. Land use in the watershed is predominantly forest (80.6%) and cropland (15.2%) with limited urban area (3.1%). The Chi River discharges limited freshwater (multi-year average ~ 3.78×107 m3) into Dayu Bay due to its small catchment area (70 km2). Consequently, Dayu Bay is an ideal site to assess the impact of seaweed cultivation on coastal carbon dynamics due to the limited disturbance from terrestrial inputs.
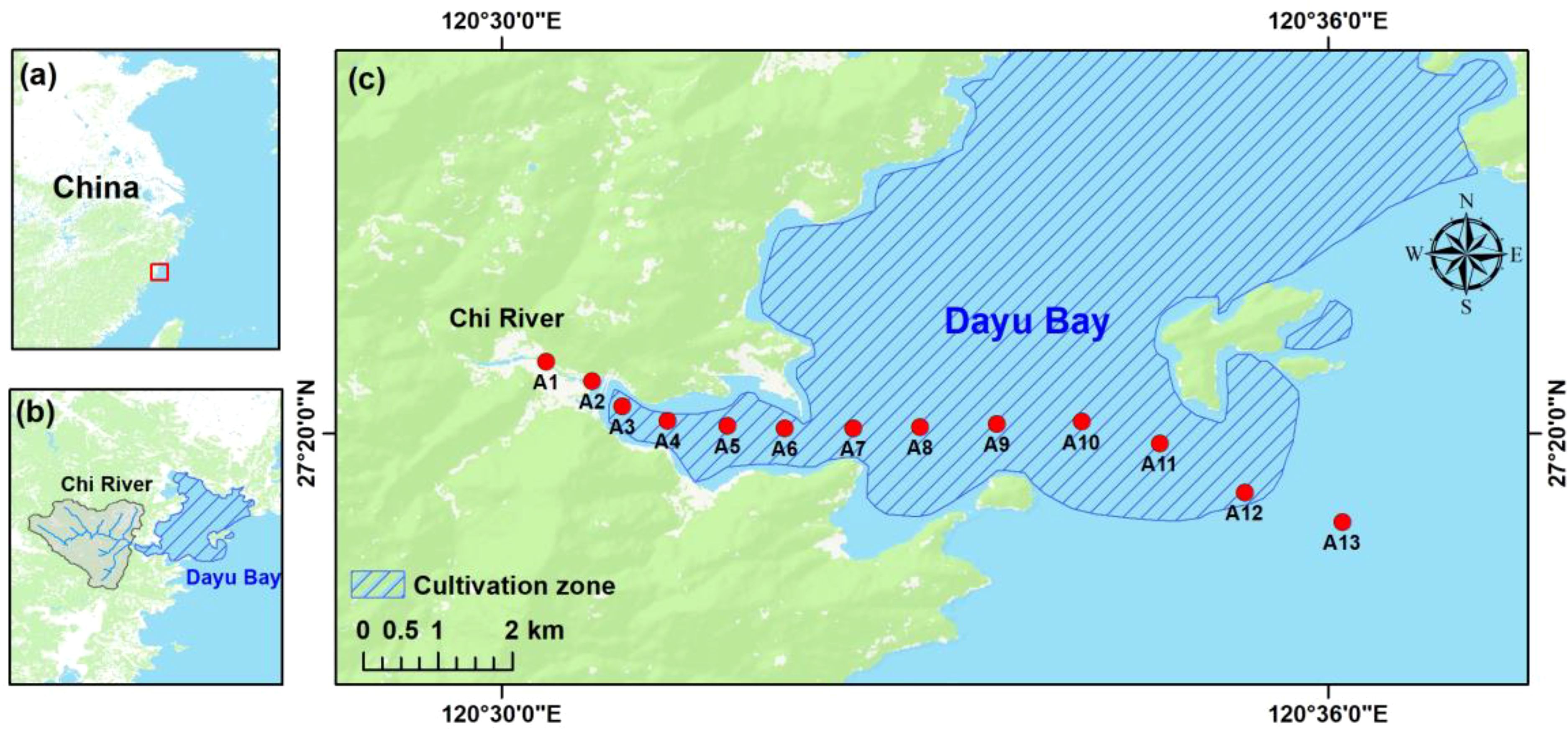
Figure 1. (A–C) Study area and sampling sites. Hatched area within Dayu Bay indicates Porphyra cultivation zone.
2.2 Sample collection and pretreatment
Field investigations were conducted in August (non-cultivation period) and October 2023 (cultivation period). To better capture the organic matter signal derived from Porphyra cultivation, the field investigation for the cultivation period was selected before the first harvest, during which time the seaweed was in its aging stage (Xiong et al., 2024). A 1.5 L surface water sample (0.5 m) was collected from the well-mixed water column using Niskin bottles at each station along the river (A1-A2) to coastal cultivation zone (A3-A13) (Figure 1). Sampling campaigns were conducted during flood tide to minimize the influence of terrestrial inputs on the cultivation zone. At each station, temperature (± 0.01°C), salinity (± 0.01), DO (± 0.1 mg/L) and turbidity (± 0.1 NTU) were measured using a calibrated EXO2 Multiparameter Water Quality Sonde (Xylem, USA).
Water samples were filtered immediately through 0.7 μm, pre-combusted (500°C, 5 h) GF/F filters (Whatman, UK) after returning to the laboratory. Unfiltered water samples for chemical oxygen demand (COD) and filtrates for DOC (acidified to pH = 2), FDOM and nutrient analyses were stored in the dark at 4°C and measured within 3 days of collection. Filters for POC, FPOM and Chl a were stored in the dark at -20°C before analysis. Microorganisms were collected by filtering 500-800 mL of seawater through 0.2 μm GTTP filters (Millipore, USA). The filters were then stored at -80°C before DNA extraction.
2.3 Analysis of nutrients, COD and Chl a
Ammonium (NH4-N), nitrite (NO2-N), nitrate (NO3-N) and soluble-reactive phosphate (SRP) concentrations were measured with a San++ Auto-Analyzer (Skalar, Netherlands) with duplicates (± 5%, analytical error). Dissolved inorganic nitrogen (DIN) was calculated as the sum of NH4-N, NO2-N and NO3-N. CODMn concentration was determined using the alkaline potassium permanganate method. Chl a concentration was analyzed using a 2300 UV-Visible spectrophotometer (Techcomp, China) after extraction in the dark with 90% acetone for 24 hours at 4°C. A simple eutrophication index (EI) was calculated following the method described by Lin et al. (2018):
Where a is a constant (a = 4500) based on the Chinese seawater background (Lin et al., 2018). EI values greater than 1 indicate an eutrophic status.
2.4 Measurement of DOC and POC
DOC concentration was measured by high-temperature catalytic oxidation using a TOC-L analyzer (Shimadzu, Japan), with triplicate measurements ensuring an analytical error less than ±2%. POC concentration was measured using an Elemental Analyzer (Elementar, Germany). Prior to POC analysis, filters were freeze-dried followed by acid fuming using concentrated HCl for 24 hours to remove any inorganic carbon (Qu et al., 2022).
2.5 DOM and POM optical spectroscopy and parallel factor analysis
FPOM filters were extracted using 0.1 M NaOH for 24 hours in dark at 4°C, then neutralized with 1 M HCl to pH ~7, and finally filtered through a 0.22 μm polyethersulfone filter for fluorescence analysis (Qu et al., 2022). The extraction efficiency for FPOM samples was 38.8 ± 6.6% (the ratio of extracted POC concentration to total POC concentration). This extraction efficiency is consistent with typical recovery rates (~38%) reported for estuaries and coastal zones in southeastern USA (Brym et al., 2014).
Excitation-emission matrices (EEMs) for FDOM and FPOM samples were scanned using an Aqualog fluorescence spectrometer (Horiba, Japan). The excitation wavelength (Ex) ranged from 240-450 nm in 5 nm intervals, and the emission wavelength (Em) ranged from 280-600 nm with 1 nm intervals, with an integration time of 1 s. Calibration for Raman and Rayleigh scattering, inner-filter effects and normalization to Raman units were conducted according to the Aqualog manual.
The corrected EEMs were modeled with parallel factor analysis (PARAFAC) in Matlab R2018 using DOMFluor toolbox (Stedmon and Bro, 2008; Qu et al., 2022). The number of PARAFAC components was determined by split-half validation. The fluorescence intensity of FPOM samples was corrected for the volumes of filtered water sample, extraction and neutralization solutions (Osburn et al., 2012). The relative abundance of each component to the total fluorescence intensity of all components was used to characterize FDOM and FPOM composition. The humification index (HIX) and biological index (BIX) of DOM and POM were calculated as described by Huguet et al. (2009). A higher HIX represents an increasing degree of humification, while a higher BIX reflects a greater contribution from autochthonous sources. The subscripts ‘d’ and ‘p’ added for each optical parameter represent DOM and POM, respectively.
Four PARAFAC components (C1-C4) were identified from FDOM and FPOM samples (Supplementary Figure S1). Component C1 (Ex=250 and 300 nm; Em=492 nm) is a humic-like component in the ultraviolet region, which was ascribed to typical terrestrial organic matter (Lei et al., 2021). Component C2, with Ex and Em maxima at 275 and 305 nm, was characterized as a tyrosine-like component (He D. et al., 2022). Component C3 (Ex=240 and 300 nm; Em=392 nm) matches the previously reported component C4 from a DOM microbial degradation experiment, which is related to a humic-like, microbially derived fluorophore (Catalán et al., 2021). Component C4 (Ex=255 nm; Em=274 nm) was also ascribed to a tyrosine-like component, however, the Ex and Em maxima for C4 are shorter than those of C2, which might be related to simpler aromatic proteins (Chen et al., 2003).
2.6 Calculating addition or removal of DOM and POM in the cultivation zone
To estimate the addition or removal of DOM and POM in the cultivation zone, we calculated the difference between the observed concentration of organic matter parameters in the freshwater sites (sites A1 and A2; sal = 0) and its effective concentration in the freshwater sites using a conservative mixing line (Qu et al., 2020; Watanabe and Kuwae, 2015). The conservative mixing line was based on organic matter concentration and salinity using the least-squares method and extrapolated to zero salinity to obtain the effective concentration. Higher effective concentrations than actual concentrations represent the addition of DOM and POM in the cultivation zone, whereas lower effective concentrations indicate removal.
2.7 Microbial community composition and co-occurrence network analysis
For microbial community analysis, total bacterial DNA was extracted from filters using an OMEGA Soil DNA Kit (Omega Bio-Tek, USA). PCR amplification of the bacterial 16S rRNA genes V3–V4 region was performed using the primer 338F (5’-ACTCCTACGGGAGGCAGCA-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). Thermal cycling of PCR was conducted as follows: denaturation at 98°C (5 min), 25 cycles at 98°C (30 s), annealing at 53°C (30 s), elongation at 72°C (45 s), and a final extension at 72°C (5 min). PCR amplicons were purified with Vazyme VAHTSTM DNA Clean Beads (Vazyme, China) and quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, USA). The purified amplicons were pooled in equal amounts and pair-end sequenced (2×250 bp) on an Illlumina NovaSeq platform at Shanghai Personal Biotechnology (Shanghai, China). Raw sequence data were then imported to QIIME 2 for quality control and taxonomic identification, including quality filtering, denoising, merging, chimera removal and clustering into operational taxonomic units (OTUs). After normalization, OTUs were rarefied to 14,513 based on the minimum sequence number. All sequences in this study are available from the NCBI Sequence Read Archive (SRA) under the accession number PRJNA1181770.
To simplify and determine interactions among bacterial communities and to better understand how key communities affect coastal DOM composition, a co-occurrence network of bacterial communities was constructed using SparCC implemented in the ‘SpiecEasi’ package of R software (Version 4.3.1). To reduce noise and complexity of the datasets, we retained OTUs with an occurrence frequency greater than 50% in all samples for network analysis. Bacterial communities with strong interactions were filtered based on a Spearman correlation coefficient (r) > 0.75 and p< 0.01. The co-occurrence network was modularized and visualized using Gephi. Standardized scores for each module were calculated based on relative abundance (Zhou et al., 2024).
2.8 Common data analysis and visualization
A t-test for all parameters was conducted after confirming normal distribution and equal variance assumptions using SPSS 22.0 (IBM, USA). Redundancy analysis (RDA) was performed using Origin 2024b (OriginLab, USA) after data standardization. All figures were generated using Origin 2024b and ArcGIS 10.2 (Esri, USA).
3 Results
3.1 Environmental factors during cultivation and non-cultivation periods
Water salinity sharply increased from freshwater sites (A1-A2: 0.03-0.17) to the first cultivation site (A3: 27.7) during the non-cultivation period (Supplementary Figure S2). However, tidal intrusion of seawater during the cultivation period resulted in the salinity at A2 reaching up to 30.9. The average salinity of the coastal cultivation zone was significantly higher during the cultivation versus non-cultivation period (p<0.05; Figure 2A). Overall, the average salinity during both periods was lower than the salinity boundary (<32) of the Taiwan Warm Current. Therefore, we assume that the water mass in Dayu Bay was primarily dominated by the Min-Zhe Coastal Current (Li et al., 2006; Qi et al., 2017). Turbidity, DIN, SRP, COD and EI all exhibited a decreasing trend along the river-to-coastal gradient during both periods. Chl a was higher in the coastal cultivation zone than in freshwater sites during the non-cultivation period, but this difference disappeared during the cultivation period.
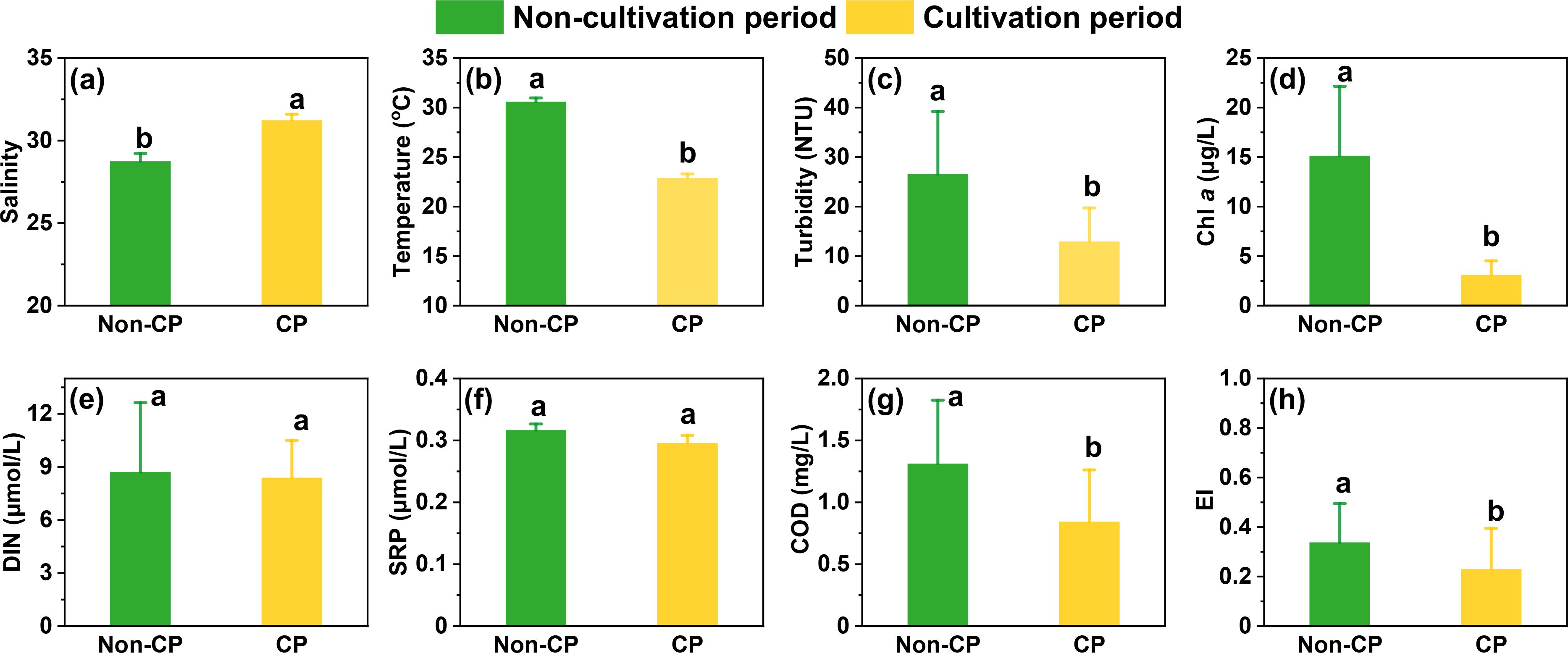
Figure 2. (A–H) Environmental factors (mean ± SD) in south Dayu Bay during non-cultivation (Non-CP) and cultivation (CP) periods. Data exclude the freshwater sites. Different lowercase letters above bars indicate significant differences at p<0.05.
In the coastal cultivation zone, average water temperature during the non-cultivation period was 30.6 ± 0.4 °C (Figure 2B), which was significantly higher than that during the cultivation period (22.9 ± 0.4 °C; p<0.05). Turbidity also showed higher values during the non-cultivation (26.6 ± 12.7 NTU; Figure 2C) versus cultivation period (12.9 ± 6.8 NTU; p<0.05). Average Chl a concentration for the cultivation period was 3.09 ± 1.43 μg/L, which was significantly lower than that in non-cultivation period (15.1 ± 7.02 μg/L, p<0.05; Figure 2D). Average DIN concentration was 8.71 ± 3.92 μmol/L and 8.39 ± 2.11 μmol/L (p>0.05) during the cultivation and non-cultivation periods, respectively (Figure 2E). SRP showed a significantly lower average concentration during the cultivation period (0.30 ± 0.01 μmol/L) than non-cultivation period (0.32 ± 0.01 μmol/L, p<0.05; Figure 2F). The temporal variation of COD was similar to SRP, which showed a significantly lower average concentration during cultivation (0.84 ± 0.42 mg/L) than the non-cultivation period (1.31 ± 0.51 mg/L, p<0.05; Figure 2G). The eutrophic index was below the threshold (EI ≥1 indicates eutrophic) during both periods, however, it was significantly higher in the non-cultivation period (0.34 ± 0.16) compared to the cultivation period (0.21 ± 0.13, p<0.05; Figure 2H).
3.2 DOM and POM concentrations and optical properties during cultivation and non-cultivation periods
DOC concentration was 97.7 ± 8.8 μmol/L during cultivation period, which was significantly higher than 83.9 ± 7.6 μmol/L during non-cultivation period (p<0.05; Figure 3A). For humic-like FDOM components, terrestrial-sourced C1d showed no significant difference between the two periods, while the microbial-sourced C3d exhibited a higher relative abundance during the cultivation (37 ± 7%) versus non-cultivation (30 ± 8%; p<0.05) period. For protein-like FDOM components, C2d showed a higher relative abundance during the non-cultivation period (26 ± 6%) than that in cultivation period (11 ± 4%), whereas C4d showed the opposite trend (p<0.05). HIXd exhibited a significantly higher value during the cultivation period (4.64 ± 0.69) than in non-cultivation period (3.27 ± 1.00; Figure 3B). BIXd values were 0.98 ± 0.08 and 1.00 ± 0.03 during the non-cultivation and cultivation periods, respectively, showing no statistical differences between the two periods (p>0.05). The HIXd values during the cultivation period were at a medium to low level (4-6), whereas the HIXd during non-cultivation period are quite low (<4). The BIXd values remained consistently high (>0.8) during both periods.
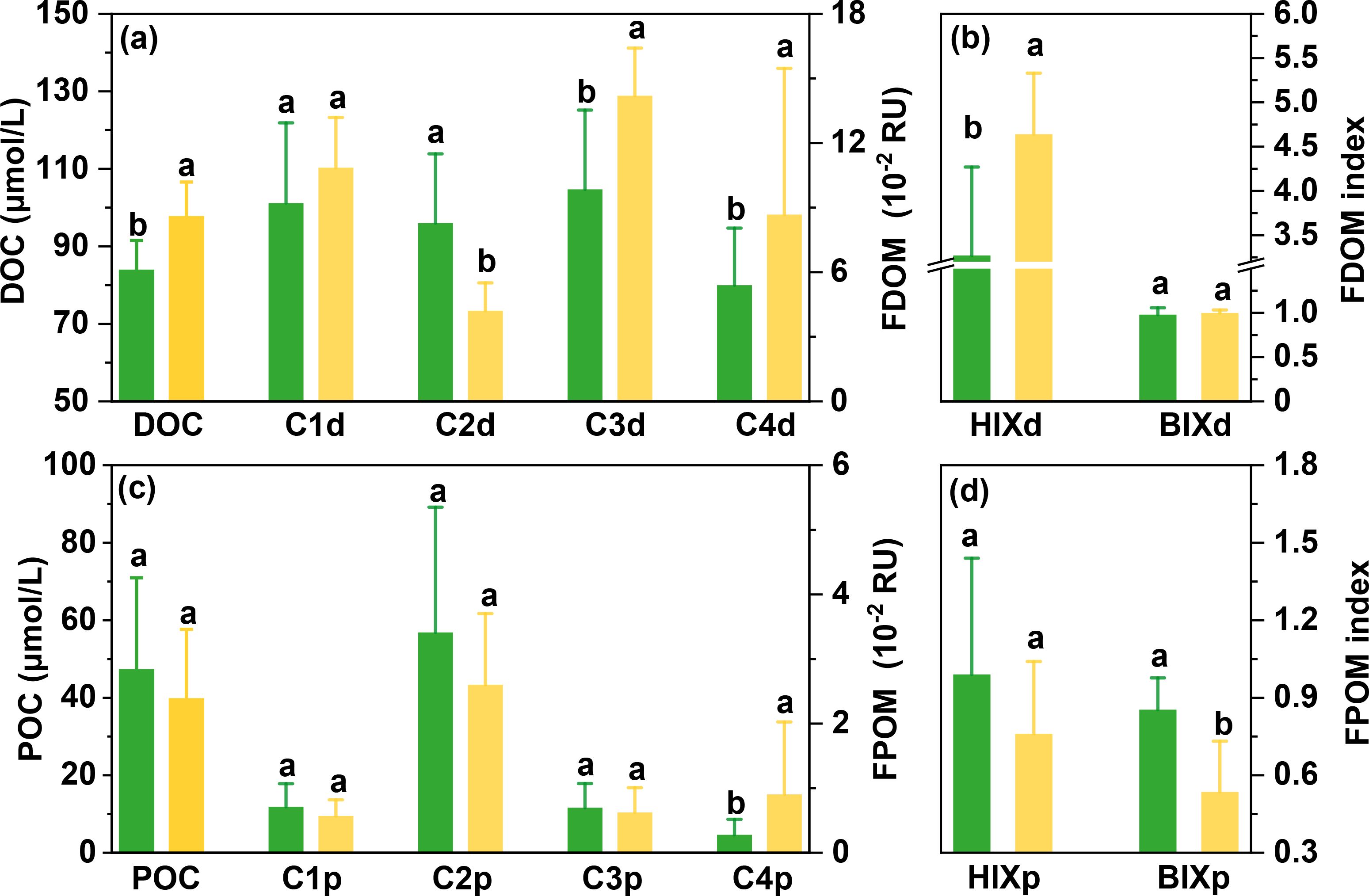
Figure 3. (A–D) DOM and POM parameters (mean ± SD) in Dayu Bay during the non-cultivation and cultivation periods. Data exclude the freshwater sites. Different lowercase letters above bars indicate significant differences at p<0.05.
POC concentration was 39.8 ± 17.8 μmol/L and 49.1 ± 21.3 μmol/L during the cultivation and non-cultivation periods, respectively (p>0.05; Figure 3C). Humic-like C1p, C3p and protein-like C2p showed similar levels during the two periods (p>0.05), whereas protein-like C4p exhibited a higher relative abundance during the cultivation (19 ± 17%) versus non-cultivation (6 ± 4%) period. HIXp values were 0.99 ± 0.45 and 0.76 ± 0.28 during the non-cultivation and cultivation periods, respectively, with no significant difference between the two periods (p>0.05; Figure 3D). Both periods exhibited low HIXp values (<4). BIXp showed a significantly higher value in the non-cultivation period (0.85 ± 0.12; >0.8) than that in the cultivation period (0.53 ± 0.20;<0.8; p<0.05).
The effective DOC concentration in the freshwater endmember was 411 ± 106 μmol/L and 766 ± 113 μmol/L during the non-cultivation and cultivation periods (Figure 4). The effective POC concentration during the non-cultivation period was 1159 ± 295 μmol/L; however, it was not possible to calculate during the cultivation period due to strong non-conservative mixing processes.
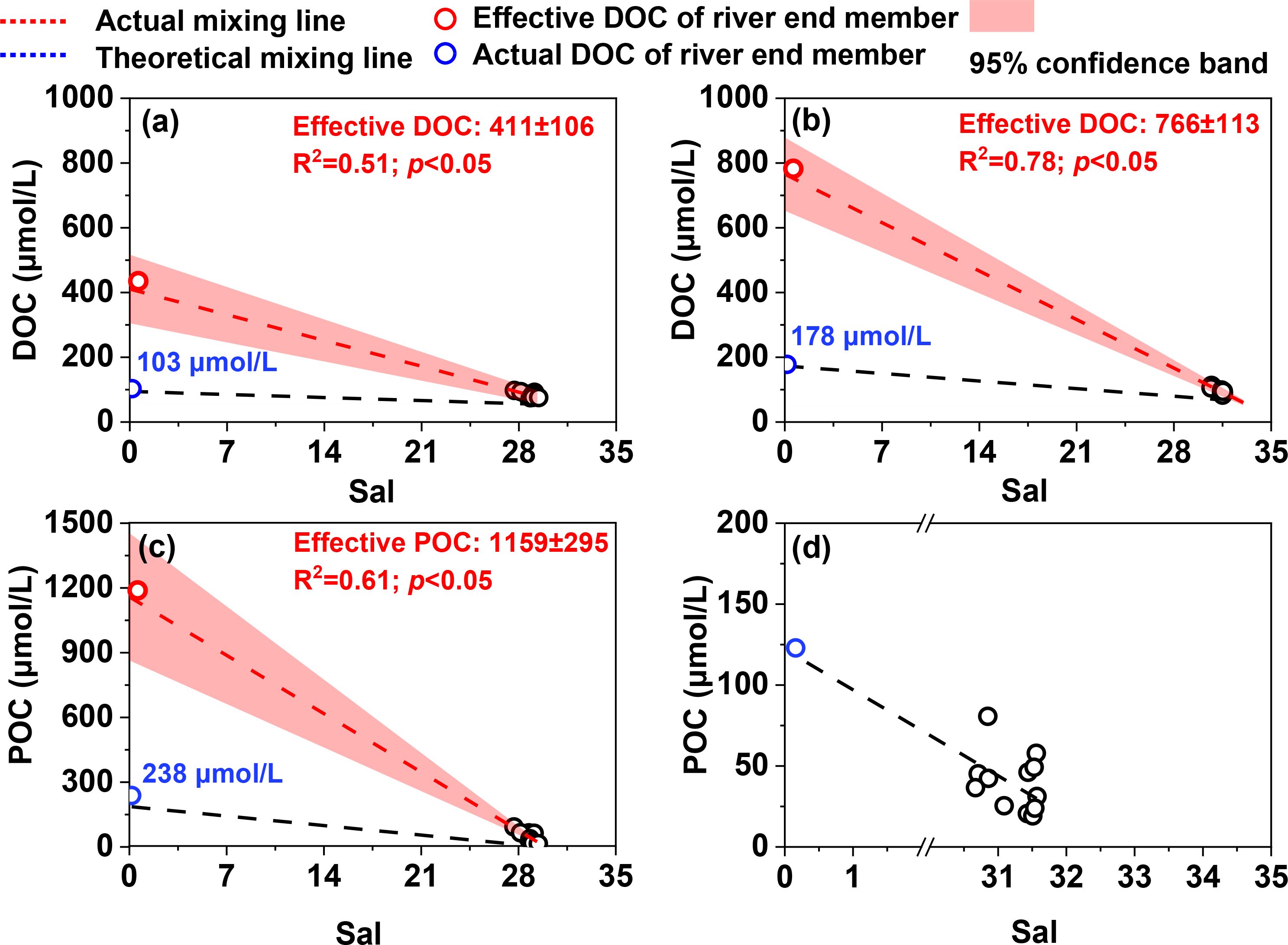
Figure 4. Mixing behavior of DOC and POC during the non-cultivation (A, C) and cultivation periods (B, D) along the Chi River to Dayu Bay continuum.
3.3 Microbial composition between non-cultivation and cultivation periods
The microbial community structure exhibited clear differences between the non-cultivation and cultivation periods (Figure 5). During the non-cultivation period, dominant phyla were Proteobacteria (32.3 ± 6.2%), Cyanobacteria (29.3 ± 9.9%), Actinobacteriota (18.6 ± 2.1%), and Bacteroidota (16.6 ± 4.1%). In contrast, during the cultivation period, the proportion of Proteobacteria (47.8 ± 15.2%) significantly increased, whereas Cyanobacteria (14.1 ± 7.5%), Actinobacteriota (19.7 ± 7.0%) and Bacteroidota (8.0 ± 2.4%) decreased. Proportions of low-abundance phyla such as Desulfobacterota, and Acidobacteriota significantly increased during the cultivation period (p<0.05). The Shannon index and Chao 1 indicated that alpha-diversity was higher during the cultivation period compared to the non-cultivation period (Figure 5B). PCoA demonstrated that the microbial communities were significantly distinct along PCo1 (Figure 5C). Our results are consistent with previous investigations in other Porphyra cultivation zones, which suggest that Porphyra cultivation significantly alters the microbial communities in Dayu Bay (Wang et al., 2020; Xu et al., 2022).
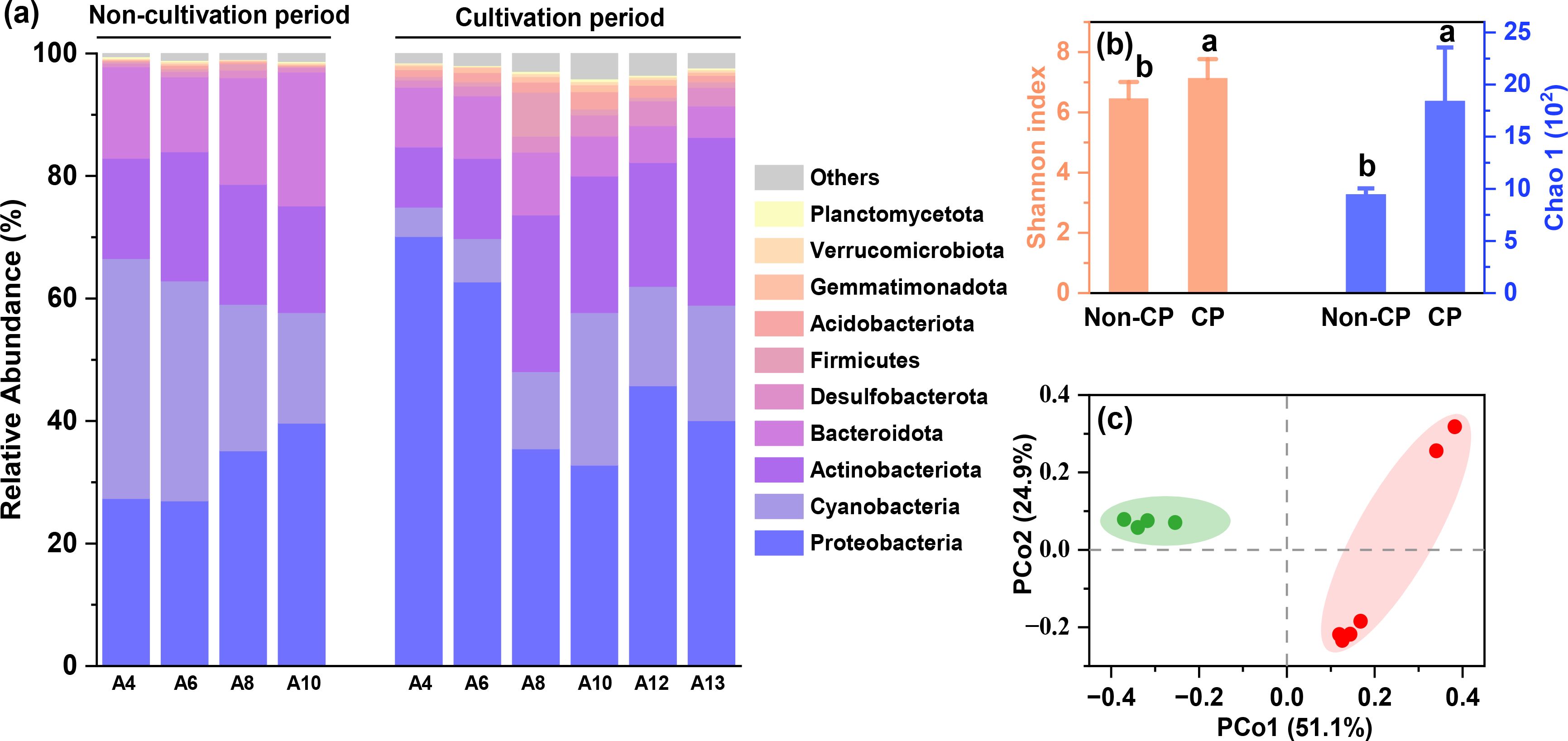
Figure 5. Relative abundance of the top 10 dominant phyla (A), alpha-diversity (B) and PCoA results (C) for microbial communities in Dayu Bay during the non-cultivation and cultivation periods.
The co-occurrence network was composed of 108 nodes (OTUs) and 274 edges (correlations) that clustered into seven main modules (Figure 6A). Bacteria with similar niches and functions clustered into the same module of the network. The relative abundance of modules 1 and 2 was significantly correlated (r=0.66), and were dominated by Cyanobacteria followed by Gammaproteobacteria and Actinobacteriota (Figure 6B). Alphaproteobacteria and Gammaproteobacteria were the major clades in module 3 and module 4, respectively. For correlated modules 5-7 and others (r=0.82-0.90), the keystone taxa were more diverse, including Actinobacteriota, Alphaproteobacteria, Bacteroidia, Gammaproteobacteria and Desulfobacterota.
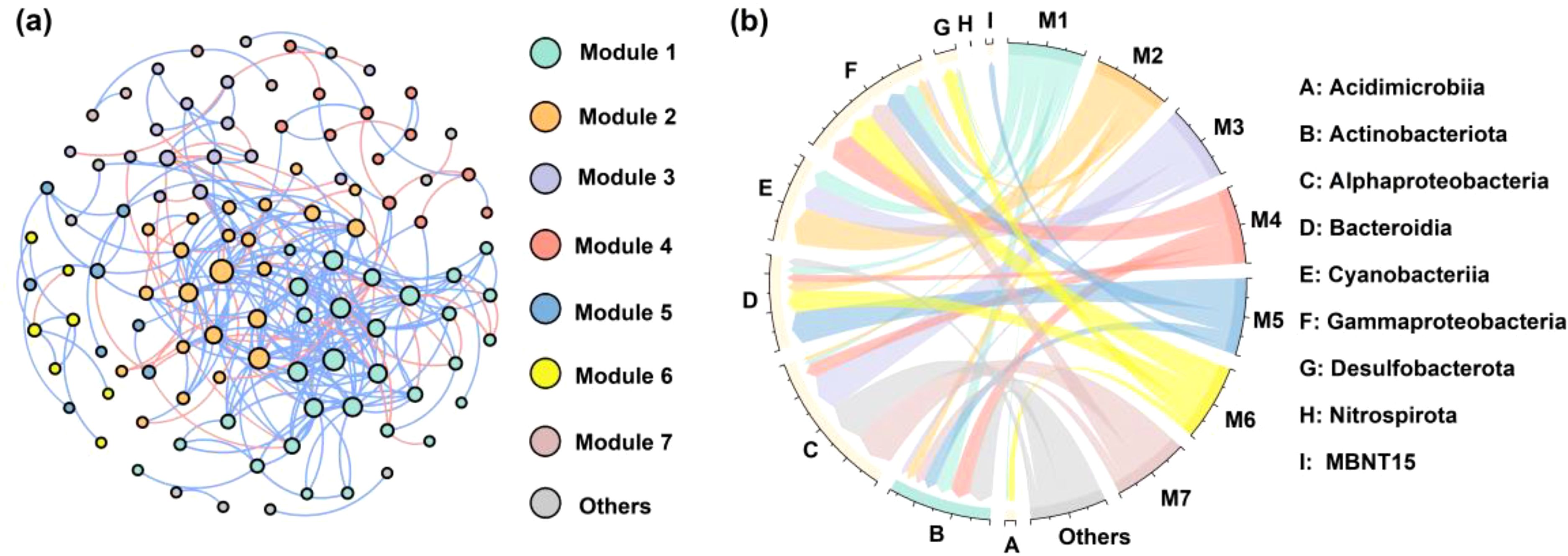
Figure 6. Co-occurrence network for bacterial communities with corresponding modules (A) and phyla or class distribution of various modules (B). The size of the points in (A) is proportional to the number of their connections. Blue lines represent positive correlations, while red lines represent negative correlations. The width of color bands in (B) represents the relative abundance of OTUs in each module.
4 Discussion
4.1 Porphyra cultivation changes the sources and proportion of coastal DOM and POM pools
Organic matter in coastal zones is typical sourced from terrestrial inputs, autochthonous sources and sediment resuspension (Watanabe and Kuwae, 2015; Kubo and Tanaka, 2023). In Dayu Bay, the HIXd value (<6) is far lower than that in some river-input-dominated estuaries (e.g., HIX ranges from 10 to 16 in the Gironde, Loire, and Seine estuaries, France), indicating a limited terrestrial contribution in this coastal bay with a small watershed area. The estimated effective riverine DOC concentrations are 4-4.3 times higher than actual riverine DOC concentrations suggesting that autochthonous sources or sediment additions are higher than the terrestrial loading from the watershed during both monitoring periods (Figure 4A, B). The high BIX values (0.98-1; values >0.8 indicate predominantly autochthonous contributions) indicate that the additional DOM in the cultivation zone is primarily sourced from freshly produced organic matter rather than sediment resuspension or release (Huguet et al., 2009). POC also showed a similar pattern of increasing concentrations during the non-cultivation period when terrestrial POM input only accounted for 20.5% of effective riverine POC (Figure 4C). This result is consistent with the low HIXp values (<4) observed during the non-cultivation period. We were unable to rigorously quantify the additional POC due to strong non-conservative mixing processes during the cultivation period (Figure 4D). The dispersed nature of samples distributed on both sides of the theoretical mixing line infer the occurrence of both addition and removal of POC (Watanabe and Kuwae, 2015).
The significant differences in DOC and POC concentration and their proportions between investigation periods further implies the influence of seaweed cultivation on the autochthonous organic carbon pool (Figure 3). Significantly higher EI and Chl a (>10 μg/L) during the non-cultivation period suggest that algal blooms likely dominated autochthonous contributions to the organic matter pool (Asmala et al., 2018). These results are further supported by the high relative abundance of Cyanobacteria during the non-cultivation period (Figure 5A; Bai et al., 2017). Conversely, the decrease in Chl a and Cyanobacteria during the cultivation period indicates that nutrient or light competition and inhibitory effects from large-scale cultivation of Porphyra attenuated algal blooms (Zhang et al., 2018). Similarly, the significantly higher DOC during the cultivation period reflects a shift and increased contribution of biogenic DOM from algal bloom sources to macroalgal Porphyra sources (Liu et al., 2022; Wada and Hama, 2013). Moreover, the higher salinity during the cultivation period indicates that there might be dilution of DOC due to intrusion of offshore non-cultivation water (Figure 2A). Therefore, the contribution of Porphyra cultivation on the coastal DOM pool could be even more significant, which is consistent with the higher DOC addition (Figure 4).
The aging of Porphyra during the cultivation period increased POC through the rupture and release of litter caused by wave crash, animal grazing and tissue decay (Canvin et al., 2024). However, POC concentrations did not show as significant of an elevation as DOC during the cultivation period (Figure 3A). We posit two possible explanations for this phenomenon. Firstly, the POC yield of Porphyra was significantly lower than DOC. Chen (2020) reported that the DOC release rate from Porphyra was 24.4-31.5 mg g-1 d-1 DW, whereas the POC release rate was only 1.7-5.9 mg g-1 d-1 DW. Secondly, the relatively large particle size of the Porphyra detritus allows rapid sinking and sedimentation (Zhang et al., 2012). Additionally, enhanced aggregation owing to transparent exopolymer particles sourced from the Porphyra might accelerate the sedimentation of biogenic POC (Li et al., 2023). Overall, cultivation of Porphyra resulted in a higher DOC/POC ratio (2.9 ± 1.1) in the water column than during the non-cultivation period (2.0 ± 0.8; p<0.05). Similar changes in the DOC/POC ratio were observed in other seaweed cultivation zones (Liu et al., 2022; Zhang et al., 2018), which indicates that seaweed cultivation could generally alter the proportion of dissolved and particulate organic matter in the water column of coastal ecosystems.
Results from our study further suggest that the cultivation of Porphyra altered the composition of autochthonous DOM and POM. FDOM and FPOM during the non-cultivation period were characterized by high tyrosine-like C2, which was ascribed to protein-like materials from phytoplankton production (He D. et al., 2022). However, a significant decrease in C2d along with an increase of C4d and C4p was observed during the cultivation period (Figures 3A, C), suggesting that Porphyra cultivation altered the organic matter composition away from tyrosine and tryptophan features toward blue-shift (shorter wavelengths) protein-like components (Coble, 2014). A similar phenomenon was also reported in a kelp cultivation zone (Sanggou Bay, China), where an increased degree of blue-shift protein-like C2 (Ex=210; Em=290 nm) was more significant than protein-like C1 (Ex=295; Em=340 nm) and C3 (Ex=275; Em=300 nm) (Li et al., 2022). The blue-shifted fluorescent signal might indicate that the Porphyra sourced organic matter has a higher fraction of low-molecular-weight, hydrophilic aliphatic carbon and nitrogen compounds (e.g., saccharides, carboxylic acids and amino acids) compared to algal bloom sources (Coble, 2014; Li et al., 2023). This is consistent with previous studies of other seaweeds, for example, Feng et al. (2022) observed a high content of polysaccharides and lipids in leachates extracted from fresh kelp detritus. However, differences in growth environments and physiological composition between kelp and Porphyra might lead to contrasting compositions of released organic matter, which warrants further exploration (Chen, 2020; Kubo and Tanaka, 2023).
4.2 Microbial-mediated autochthonous production of RDOM during Porphyra cultivation period
Seaweed cultivation has been shown to increase bio-labile DOM and POM in coastal waters (Krause-Jensen and Duarte, 2016; Liu et al., 2022). Previous incubation experiments reported a high propensity for this biogenic organic matter to undergo transformation to more refractory organic components (Feng et al., 2022; Li et al., 2022). The humic-like component is widely acknowledged as a proxy for RDOM (Yamashita and Tanoue, 2008). In Dayu Bay, we observed higher microbially sourced, humic-like C3d during the cultivation period (Figure 3A), suggesting either (1) degradation and transformation of Porphyra sourced bio-labile organic matter (Feng et al., 2022; Li et al., 2022); or (2) greater fluvial inputs from the Chi River (He D. et al., 2022). By comparison, C1d addition, a proxy for terrestrial DOM input (Lei et al., 2021), was only 34% higher during the cultivation period than that in non-cultivation period (Supplementary Figure S3). However, the addition of microbial C3d was much higher during the cultivation (169% higher) versus non-cultivation period. Therefore, we infer that a considerable proportion of Porphyra-sourced organic matter has been processed into humic-like C3d (Li et al., 2022; Watanabe and Kuwae, 2015) and the terrestrial input induced variation in C3d was relatively limited in comparison. As a result, the ratio of microbial humic-like C3d to terrestrial humic-like C1d increased from 0.71 in the river zone to 1.33 ± 0.12 in the cultivation zone.
Although these results are only based on the investigation from Dayu Bay, we posit that seaweed cultivation induced production of RDOM could be ubiquitous in coastal cultivation zones with similar climate, geomorphology and hydrology. Because seaweed cultivation is usually established in small to medium estuaries and the adjacent coastal zone (Fu et al., 2021), limited freshwater inputs and longer water residence time allow microorganism to extensively process bio-labile organic matter (Asmala et al., 2018; Qu et al., 2022). Moreover, the relatively low terrestrial DOM input from the watershed certainly contributes to the dominance of autochthonous-derived RDOM in these other coastal cultivation zones (Markager et al., 2011; Li P. et al., 2021).
Notably, algal blooms also contribute to biogenic organic matter production in coastal systems, and the higher water temperature during the non-cultivation period is expected to enhance the microbial production of RDOM (Qu et al., 2022). However, the addition of humic-like C3d during the non-cultivation period was only 37.2% of that during the cultivation period (Supplementary Figure S3). This difference was mainly attributed to the ability and preference of bacterial communities to consume and transform carbon substrates (Feng et al., 2022; Zhou et al., 2024). This hypothesis is supported by the positive correlations between C3d, HIX and modules 3-7 during cultivation, whereas C3d and HIX were negatively correlated with modules 1-2 (Figure 7). Specifically, keystone taxa such as Rhodobacterales (Alphaproteobacteria), Flavobacteriales (Bacteroidia), UBA10353 marine group (Gammaproteobacteria) and Desulfobacterales (Desulfobacterota) showed the highest correlations with C3d, highlighting their importance in the autochthonous production of RDOM (Supplementary Figure S4; Zhou et al., 2024; Shi et al., 2025).
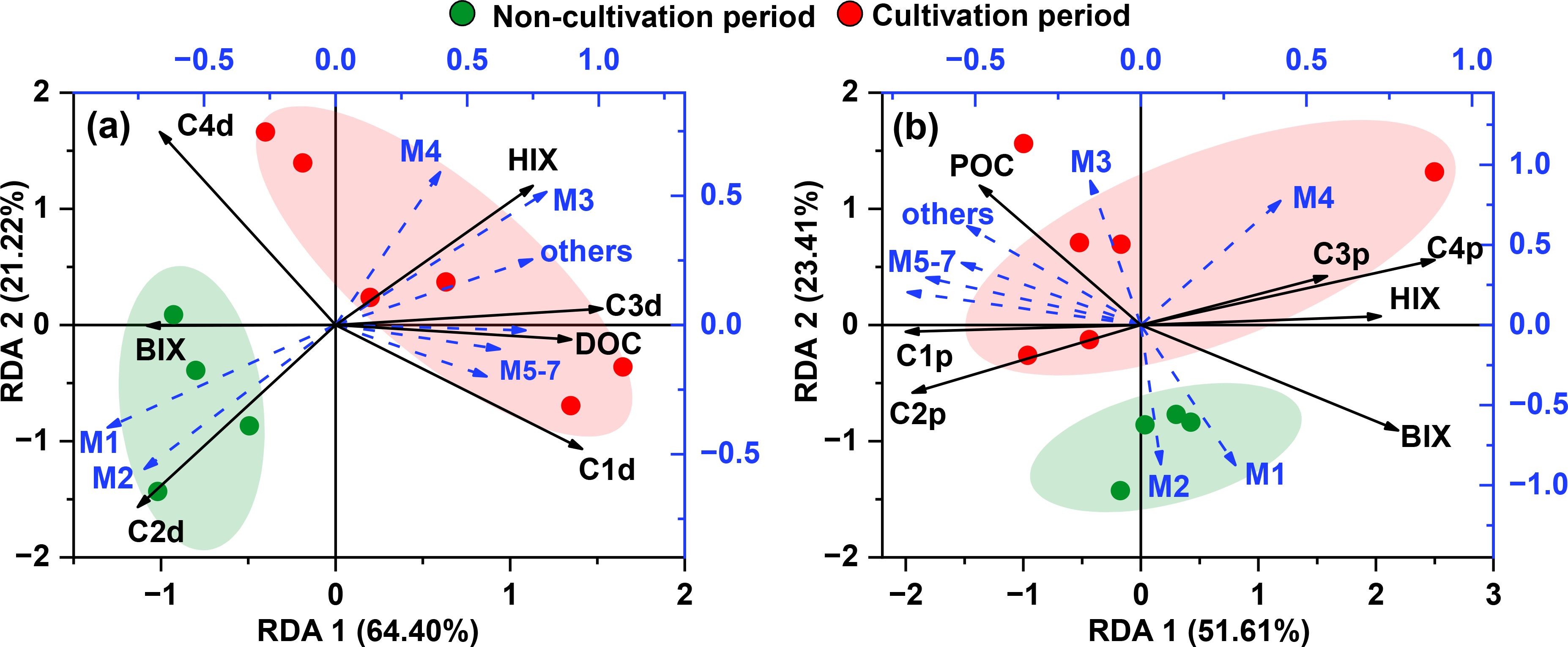
Figure 7. Redundancy analysis (RDA) plots for Dayu Bay samples based on DOM and POM parameters and microbial communities. (A) DOM model; (B) POM model.
It is well recognized that Alpha- and Gammaproteobacteria utilize bio-labile substrates and produce RDOM components (Li et al., 2023; Zhou et al., 2024). For instance, Rhodobacterales and UBA10353 (i.e., UBA868) are reported to have rapid transformation rates for low-molecular-weight, S- or N-containing DOM, which is abundant in seaweed cultivation zones (Baltar et al., 2023; Li et al., 2022; Lian et al., 2021). He C. et al. (2022) demonstrated that the central carbon metabolism of these bacteria transforms algal-derived bio-labile DOM into acetyl-CoA, providing substrates for the mevalonate pathway and generating precursors of RDOM. Similarly, Flavobacteria are highly efficient degraders with a preference for proteins and polysaccharides, thereby contributing to RDOM production (Varela et al., 2020). Further, Desulfobacterales are capable of degradation and transformation of Porphyra-derived sulfur-containing DOM compounds (i.e., sulfated polysaccharides) (Huang et al., 2024).
Modules 3 to 7 appeared to regulate the degradation and transformation of POM during the cultivation period (Figure 7B). Some specialized microbial taxa in these modules (e.g., Bacteroidota and Gammaproteobacteria) can secrete extracellular enzymes to break down seaweed derived POM (Feng et al., 2022). The microbial degradation of POM tends to release protein-like DOM, whereas humic-like components are retained (Lee et al., 2023). This is consistent with the positive correlations observed between module 3-7 with C1p and C2p (Figure 7B). The released bio-labile DOM derived from POM could contribute to the coastal RDOM pool following microbial transformations (Li et al., 2022).
During the non-cultivation period, DOM cycling in Dayu Bay was predominantly controlled by fewer microbial modules (namely modules 1 and 2), which were mainly composed of autotrophic Synechococcales (Cyanobacteria). The relative abundance of Synechococcales reached 40% in these two modules. In contrast, the proportion of some heterotrophic bio-labile DOM consumers, such as Alpha or Gammaproteobacteria and Bacteroidota, was relatively low (2.6-23%). The lower Shannon and Chao 1 index values also suggest reduced microbial community diversity during the non-cultivation period (Figure 5B). Previous studies showed that a higher alpha-diversity and broader range of metabolic niches are promoted by interactions between bacteria and DOM (Muscarella et al., 2019; Zhou et al., 2024). Thus, the lower microbial community diversity during the non-cultivation period may limit the microbial utilization and transformation of bio-labile DOM. This inference is supported by a study on three coastal bays, which found that protein-like components, rather than humic-like DOM, accumulated with increasing levels of eutrophication (Zhao et al., 2023b).
4.3 Implications of seaweed cultivation on coastal carbon cycling
Coastal systems have a disproportionately large effect on oceanic carbon sequestration, wherein they account for 8% of total ocean area but contribute 20% of the ocean carbon sink (Field et al., 1998). In particular, coastal bays with limited terrestrial inputs and longer water residence times function as a complex organic carbon “reactor” due to the enhanced autochthonous inputs and transformations (Asmala et al., 2018; Markager et al., 2011). Our study provides compelling evidence that microbial-mediated carbon transformations significantly increase the production of RDOM within the water column of Porphyra cultivation zones through utilization of autochthonous organic matter (Figure 8). Another common phenomenon in coastal bays is eutrophication, where elevated nutrient levels promote the autochthonous production of biogenic DOM and POM (Dai et al., 2023; Markager et al., 2011). However, current studies indicate that despite the availability of abundant organic substrates, increased RDOM concentrations in these eutrophic systems are not significant (Asmala et al., 2018; Zhao et al., 2023b), consistent with our observations in Dayu Bay. In contrast, the intense mineralization of autochthonous organic matter often causes eutrophic coastal bays to act as an appreciable CO2 source (Muthukumar et al., 2022; Sunda and Cai, 2012). Li B. et al. (2021) reported that the partial pressure of CO2 in the coastal Yellow Sea progressively increased from the beginning to the end of an algae bloom period. In contrast, seaweed cultivation zones usually serve as a CO2 sink during its fast-growth period, but gradually transform into a weak CO2 source during the aging period (Xiong et al., 2024). Although Porphyra cultivation and algae blooms might both result in coastal systems functioning as a dynamic CO2 source, the stronger RDOM production during seaweed cultivation might provide a more efficient carbon sequestration pathway.
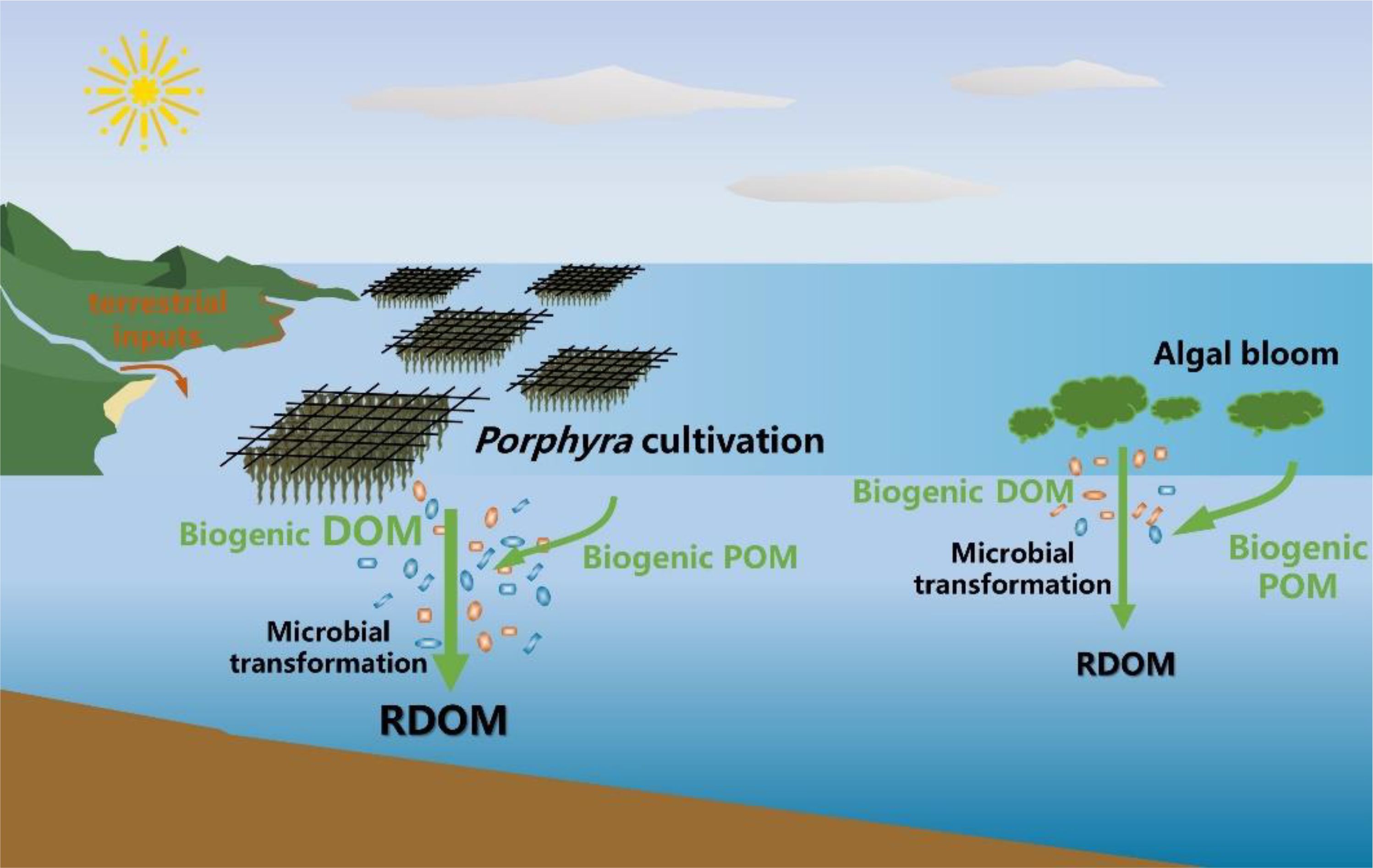
Figure 8. Conceptual diagram of organic matter sources and transformations in coastal Porphyra cultivation zone. Modified after Li et al. (2022).
Moreover, competitive effects between Porphyra and microalgae might mitigate some of the environmental issues caused by eutrophication, such as ocean deoxygenation and acidification (Zheng et al., 2019). Expanding the scale of Porphyra cultivation provides additional benefits, including job creation, contributions to global trade and greater marine diversity/habitat. Although the current global cultivation area for Porphyra is relatively limited (primarily in China), models suggest that North America, South America, and Australia have biophysical environments suitable for Porphyra growth. A predictive model indicates that the cultivation area for Porphyra could increase by 10.8-26.1% over the next 30 years under global initiatives, which may effectively contribute to the sustainable development of food production and blue carbon strategies (Arzeno-Soltero et al., 2023; Zhou, 2023).
However, we acknowledge that there are some limitations in our present study that we intend to address in future studies. For the cultivation period, we only performed a single investigation during the late-growth stage of Porphyra. The release rates of DOM and POM from macroalgae likely varies across different growth stages. For instance, kelp exhibits higher release rates of DOC and POC during its late-growth and aging periods compared to the early-growth period (Xiong et al., 2024; Zhang et al., 2012). Therefore, more rigorous temporal observations (e.g., FDOM sensor and sediment traps) are warranted to further constrain the impacts of Porphyra cultivation on coastal carbon budgets. Our study demonstrated that Porphyra cultivation enhances carbon sequestration through the microbial carbon pump, consistent with previous findings for other cultivated or natural seaweeds (Ou et al., 2024; Zhao et al., 2023a). However, the carbon sequestration potential of different seaweed species varies significantly. For example, Zhao et al. (2023a) reported that 2.71-3.55% of DOC derived from the macroalgal Sargassum horneri was subjected to long-term storage in seawater as compared to 1.27% for kelp (Feng et al., 2022). The 169% higher levels of additional C3d during the cultivation versus non-cultivation period highlights the substantial carbon sequestration potential of Porphyra cultivation. However, different carbon sequestration potentials for Porphyra compared to other seaweeds need further assessment by field and incubation experiments. We also emphasize the opportunity to employ advanced characterization techniques (e.g., Fourier transform ion cyclotron resonance mass spectrometry, FT-ICR-MS) to identify the molecular fingerprint of seaweed-derived organic carbon, which is essential to better characterize transformation and sequestration mechanisms among various seaweed species (Li et al., 2022).
A key statistic reported in a review by Krause-Jensen and Duarte (2016) indicated that more than 90% of biogenic POC from macroalgae rapidly sinks to the sediment layer or is exported to the deeper ocean. Hence, we speculate that carbon transformation processes may occur in the bottom waters or at the water column-sediment interface of cultivation zones due to the increased availability of carbon substrates. However, the feedback of rapidly deposited biogenic POM to the RDOM pool in the sediment environment remains unexplored. This large influx of biogenic POM could lead to (1) accelerated microbial production of RDOM; (2) degradation of previously produced RDOM in the sediment environment due to priming effects (Huang et al., 2024); or (3) limited decomposition of deposited organic matter due to predominantly anaerobic conditions (Pedersen et al., 2021). Therefore, several questions remain to be addressed that require integrated field investigations and incubation experiments to comprehensively assess the influence of seaweed cultivation on coastal sedimentary carbon dynamics.
5 Conclusions
This study investigated the quantity and optical composition of DOM, POM and microbial communities along a subtropical river through its adjacent coastal Porphyra cultivation zone during cultivation and non-cultivation periods to assess how cultivation affects coastal organic matter dynamics. Endmember mixing analysis from freshwater to coastal waters revealed that autochthonous processes dominated in the cultivation zone during both the cultivation and non-cultivation periods, with limited terrestrial organic carbon inputs. Porphyra cultivation increased the DOC concentration, but decreased the POC concentration of coastal waters, leading to a higher DOC/POC ratio during the cultivation period. In contrast, algal blooms were the primary autochthonous source of DOC and POC during the non-cultivation period. Coupling analysis of DOM, POM and microbial communities revealed that more microbial modules were involved in RDOM production during the cultivation period. In contrast, lower microbial diversity during the non-cultivation period may limit carbon transformations. Ultimately, enhanced transformation of biogenic DOM and POM during the cultivation period resulted in a 169% higher addition of humic-like C3 (a proxy for RDOM) compared to the non-cultivation period. Our study is the first to corroborate that Porphyra cultivation can significantly contribute to autochthonous RDOM production in coastal cultivation zones and provides fundamental information for assessing coastal carbon sequestration derived from seaweed cultivation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1181770, PRJNA1181770.
Author contributions
TW: Visualization, Writing – original draft, Writing – review & editing. JX: Formal analysis, Investigation, Writing – review & editing. RD: Writing – review & editing. QL: Writing – review & editing. YJ: Writing – review & editing. BC: Writing – review & editing. HX: Writing – review & editing. ZM: Conceptualization, Supervision, Writing – review & editing. LQ: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (42406037), Open Fund of State Key Laboratory of Satellite Ocean Environment Dynamics (MED202202), Science and Technology Project of Wenzhou Science and Technology Bureau (S20240009 and S20240022) and Scientific Research Fund of Zhejiang Provincial Education Department (Y202456606).
Acknowledgments
We would like to thank the reviewers for their valuable comments and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1529148/full#supplementary-material
References
Arzeno-Soltero I. B., Saenz B. T., Frieder C. A., Long M. C., DeAngelo J., Davis S. J., et al. (2023). Large global variations in the carbon dioxide removal potential of seaweed farming due to biophysical constraints. Commun. Earth Environ. 4, 185. doi: 10.1038/s43247-023-00833-2
Asmala E., Haraguchi L., Markager S., Massicotte P., Riemann B., Staehr P. A., et al. (2018). Eutrophication leads to accumulation of recalcitrant autochthonous organic matter in coastal environment. Global Biogeochem. Cy 32, 1673–1687. doi: 10.1029/2017GB005848
Bai L. L., Cao C. C., Wang C. H., Xu H. C., Zhang H., Slaveykova V. I., et al. (2017). Toward quantitative understanding of the bioavailability of dissolved organic matter in freshwater lake during cyanobacteria blooming. Environ. Sci. Technol. 51, 6018–6026. doi: 10.1021/acs.est.7b00826
Baltar F., Martínez-Pérez C., Amano C., Vial M., Robaina-Estévez S., Reinthaler T., et al. (2023). A ubiquitous gammaproteobacterial clade dominates expression of sulfur oxidation genes across the mesopelagic ocean. Nat. Microbiol. 8, 1137–1148. doi: 10.1038/s41564-023-01374-2
Brym A., Paerl H. W., Montgomery M. T., Handsel L. T., Ziervogel K., Osburn C. L. (2014). Optical and chemical characterization of base-extracted particulate organic matter in coastal marine environments. Mar. Chem. 162, 96–113. doi: 10.1016/j.marchem.2014.03.006
Cai W. J., Borlace S., Lengaigne M., van Rensch P., Collins M., Vecchi G., et al. (2014). Increasing frequency of extreme El Nino events due to greenhouse warming. Nat. Clim. Change 4, 111–116. doi: 10.1038/nclimate2100
Canvin M. C., Moore P. J., Smale D. A. (2024). Quantifying growth, erosion and dislodgement rates of farmed kelp (Saccharina latissima) to examine the carbon sequestration potential of temperate seaweed farming. J. Appl. Phycol. 36, 3091–3102. doi: 10.1007/s10811-024-03323-w
Catalán N., Pastor A., Borrego C. M., Casas-Ruiz J. P., Hawkes J. A., Gutiérrez C., et al. (2021). The relevance of environment vs. composition on dissolved organic matter degradation in freshwaters. Limnol. Oceanogr. 66, 306–320. doi: 10.1002/lno.11606
Chen S. W. (2020). Production of organic matter by cultivated macroalgae and effects on phytoplankton. Jimei University, Xiamen.
Chen W., Westerhoff P., Leenheer J. A., Booksh K. (2003). Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 37, 5701–5710. doi: 10.1021/es034354c
Coble P. G. (2014). Aquatic organic matter fluorescence (Cambridge: Cambridge University Press), 233–277.
Dai M., Zhao Y., Chai F., Chen M., Chen N., Chen Y., et al. (2023). Persistent eutrophication and hypoxia in the coastal ocean. Cambridge Prisms: Coast. Futures 1, e19. doi: 10.1017/cft.2023.7
Feng X. T., Li H. M., Zhang Z. H., Xiong T. Q., Shi X. Y., He C., et al. (2022). Microbial-mediated contribution of kelp detritus to different forms of oceanic carbon sequestration. Ecol. Indic 142, 109186. doi: 10.1016/j.ecolind.2022.109186
Field C. B., Behrenfeld M. J., Randerson J. T., Falkowski P. (1998). Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 281, 237–240. doi: 10.1126/science.281.5374.237
Fontaine S., Mariotti A., Abbadie L. (2003). The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 35, 837–843. doi: 10.1016/S0038-0717(03)00123-8
Fu Y. Y., Deng J. S., Wang H. Q., Comber A., Yang W., Wu W. Q., et al. (2021). A new satellite-derived dataset for marine aquaculture areas in China’s coastal region. Earth Syst. Sci. Data 13, 1829–1842. doi: 10.5194/essd-13-1829-2021
He C. F., Liu J. H., Wang R., Li Y. N., Zheng Q., Jiao F. L., et al. (2022). Metagenomic evidence for the microbial transformation of carboxyl-rich alicyclic molecules: A long-term macrocosm experiment. Water Res. 216, 118281. doi: 10.1016/j.watres.2022.118281
He D., Li P. H., He C., Wang Y. T., Shi Q. (2022). Eutrophication and watershed characteristics shape changes in dissolved organic matter chemistry along two river-estuarine transects. Water Res. 214, 118196. doi: 10.1016/j.watres.2022.118196
Huang H., Zan S., Shao K., Chen H., Fan J. (2024). Spatial distribution characteristics and interaction effects of DOM and microbial communities in kelp cultivation areas. Sci. Total Environ. 920, 170511. doi: 10.1016/j.scitotenv.2024.170511
Huguet A., Vacher L., Relexans S., Saubusse S., Froidefond J. M., Parlanti E. (2009). Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 40, 706–719. doi: 10.1016/j.orggeochem.2009.03.002
IPCC (2022). Climate Change 2022: Impacts, adaptation, and vulnerability. Contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change (Cambridge: Cambridge University Press).
Jiao N. Z., Cai R. H., Zheng Q., Tang K., Liu J. H., Jiao F. L. E., et al. (2018). Unveiling the enigma of refractory carbon in the ocean. Natl. Sci. Rev. 5, 459–463. doi: 10.1093/nsr/nwy020
Krause-Jensen D., Duarte C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 9, 737–742. doi: 10.1038/ngeo2790
Kubo A., Tanaka H. (2023). Recalcitrant dissolved organic carbon release and production from aquatic plants leachate. Mar. pollut. Bull. 189, 114742. doi: 10.1016/j.marpolbul.2023.114742
Lee H. S., Hur J., Shin H. S. (2023). Photochemical and microbial transformation of particulate organic matter depending on its source and size. Sci. Total Environ. 857, 159506. doi: 10.1016/j.scitotenv.2022.159506
Lei J. J., Yang L. Y., Zhu Z. Y. (2021). Testing the effects of coastal culture on particulate organic matter using absorption and fluorescence spectroscopy. J. Clean Prod. 325, 129203. doi: 10.1016/j.jclepro.2021.129203
Le Quéré C., Peters G. P., Andres R. J., Andrew R. M., Boden T. A., Ciais P., et al. (2014). Global carbon budget 2013. Earth Syst. Sci. Data 6, 235–263. doi: 10.5194/essd-6-235-2014
Li H. M., Feng X. T., Xiong T. Q., Shao W., Wu W. C., Zhang Y. Y. (2023). Particulate organic carbon released during macroalgal growth has significant carbon sequestration potential in the ocean. Environ. Sci. Technol. 57, 19723–19731. doi: 10.1021/acs.est.3c04959
Li G. X., Han X. B., Yue S. H., Wen G. Y., Yang R. M., Kusky T. M. (2006). Monthly variations of water masses in the East China Seas. Cont. Shelf Res. 26, 1954–1970. doi: 10.1016/j.csr.2006.06.008
Li B. H., Liu C. Y., Deng X., Wang K. K., Han L., Huang Y. H., et al. (2021). Responses of the marine carbonate system to a green tide: A case study of an Ulva prolifera bloom in Qingdao coastal waters. Harmful Algae 110, 102133. doi: 10.1016/j.hal.2021.102133
Li H. M., Zhang Z. H., Xiong T. Q., Tang K. X., He C., Shi Q., et al. (2022). Carbon sequestration in the form of recalcitrant dissolved organic carbon in a seaweed (Kelp) farming environment. Environ. Sci. Technol. 56, 9112–9122. doi: 10.1021/acs.est.2c01535
Li P. H., Zhao C., Liu K., Xiao X. T., Wang Y. J., Wang Y. T., et al. (2021). Anthropogenic influences on dissolved organic matter in three coastal bays, North China. Front. Earth Sci. 9, 697758. doi: 10.3389/feart.2021.697758
Lian J., Zheng X. X., Zhuo X. C., Chen Y. L., He C., Zheng Q., et al. (2021). Microbial transformation of distinct exogenous substrates into analogous composition of recalcitrant dissolved organic matter. Environ. Microbiol. 23, 2389–2403. doi: 10.1111/1462-2920.15426
Lin X. J., Zhang T. Y., Liu G. M. (2018). Research progress and application status of eutrophication evaluation method of seawater. Adv. Earth Sci. 33, 373–384. doi: 10.11867/j.issn.1001-8166.2018.04.0373
Liu Y., Zhang J. H., Wu W. G., Zhong Y., Li H. M., Wang X. M., et al. (2022). Effects of shellfish and macro-algae IMTA in North China on the environment, inorganic carbon system, organic carbon system, and sea-air CO2 fluxes. Front. Mar. Sci. 9, 864306. doi: 10.3389/fmars.2022.864306
Macreadie P. I., Costa M. D. P., Atwood T. B., Friess D. A., Kelleway J. J., Kennedy H., et al. (2021). Blue carbon as a natural climate solution. Nat. Rev. Earth Env. 2, 826–839. doi: 10.1038/s43017-021-00224-1
Markager S., Stedmon C. A., Sondergaard M. (2011). Seasonal dynamics and conservative mixing of dissolved organic matter in the temperate eutrophic estuary Horsens Fjord. Estuar. Coast. Shelf S 92, 376–388. doi: 10.1016/j.ecss.2011.01.014
Muscarella M. E., Boot C. M., Broeckling C. D., Lennon J. T. (2019). Resource heterogeneity structures aquatic bacterial communities. ISME J. 13, 2183–2195. doi: 10.1038/s41396-019-0427-7
Muthukumar C., Balasubramaniyan S., Garlapati D., Bharathi M. D., Kumar B. C., James R. A., et al. (2022). Impact of untreated sewage and thermal effluent discharges on the air-sea CO2 fluxes in a highly urbanized tropical coastal region. Mar. pollut. Bull. 175, 113166. doi: 10.1016/j.marpolbul.2021.113166
Osburn C. L., Handsel L. T., Mikan M. P., Paerl H. W., Montgomery M. T. (2012). Fluorescence tracking of dissolved and particulate organic matter quality in a river-dominated estuary. Environ. Sci. Technol. 46, 8628–8636. doi: 10.1021/es3007723
Ou X. L., Ou L. J., Yang Y. F. (2024). Bioavailability of dissolved organic matter (DOM) derived from seaweed Gracilaria lemaneiformis meditated by microorganisms. Mar. pollut. Bull. 209, 117243. doi: 10.1016/j.marpolbul.2024.117243
Pedersen M. F., Filbee-Dexter K., Frisk N. L., Sárossy Z., Wernberg T. (2021). Carbon sequestration potential increased by incomplete anaerobic decomposition of kelp detritus. Mar. Ecol. Prog. Ser. 660, 53–67. doi: 10.3354/meps13613
Qi J. F., Yin B. S., Zhang Q. L., Yang D. Z., Xu Z. H. (2017). Seasonal variation of the Taiwan Warm Current Water and its underlying mechanism. China J. Oceanol. Limn. 35, 1045–1060. doi: 10.1007/s00343-017-6018-4
Qu L. Y., He C., Wu Z. T., Dahlgren R. A., Ren M. X., Li P. H., et al. (2022). Hypolimnetic deoxygenation enhanced production and export of recalcitrant dissolved organic matter in a large stratified reservoir. Water Res. 219, 118537. doi: 10.1016/j.watres.2022.118537
Qu L. Y., Wu Y. F., Li Y., Stubbins A., Dahlgren R. A., Chen N. W., et al. (2020). El Niño-driven dry season flushing enhances dissolved organic matter export from a subtropical watershed. Geophys. Res. Lett. 47, e2020GL089877. doi: 10.1029/2020GL089877
Ross F. W. R., Boyd P. W., Filbee-Dexter K., Watanabe K., Ortega A., Krause-Jensen D., et al. (2023). Potential role of seaweeds in climate change mitigation. Sci. Total Environ. 885, 163699. doi: 10.1016/j.scitotenv.2023.163699
Shi X. J., Li W. Z., Wang B. L., Liu N., Liang X., Yang M. L., et al. (2025). Keystone taxa drive the synchronous production of methane and refractory dissolved organic matter in inland waters. Water Res. 269, 122821–122821. doi: 10.1016/j.watres.2024.122821
Stedmon C. A., Bro R. (2008). Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr-Meth 6, 572–579. doi: 10.4319/lom.2008.6.572
Sunda W. G., Cai W.-J. (2012). Eutrophication induced CO2-acidification of subsurface coastal waters: Interactive effects of temperature, salinity, and atmospheric pCO2. Environ. Sci. Technol. 46, 10651–10659. doi: 10.1021/es300626f
Varela M. M., Rodríguez-Ramos T., Guerrero-Feijóo E., Nieto-Cid M. (2020). Changes in activity and community composition shape bacterial responses to size-fractionated marine DOM. Front. Microbiol. 11, 586148. doi: 10.3389/fmicb.2020.586148
Wada S., Hama T. (2013). The contribution of macroalgae to the coastal dissolved organic matter pool. Estuar. Coast. Shelf S 129, 77–85. doi: 10.1016/j.ecss.2013.06.007
Wang W., Wu L., Xu K., Xu Y., Ji D., Chen C., et al. (2020). The cultivation of Pyropia haitanensis has important impacts on the seawater microbial community. J. Appl. Phycol. 32, 2561–2573. doi: 10.1007/s10811-020-02068-6
Watanabe K., Kuwae T. (2015). How organic carbon derived from multiple sources contributes to carbon sequestration processes in a shallow coastal system? Global Change Biol. 21, 2612–2623. doi: 10.1111/gcb.12924
Xiong T., Li H., Hu Y., Zhai W.-d., Zhang Z., Liu Y., et al. (2024). Seaweed farming environments do not always function as CO2 sink under synergistic influence of macroalgae and microorganisms. Agr. Ecosyst. Environ. 361, 108824. doi: 10.1016/j.agee.2023.108824
Xu N. N., Wang W. L., Xu K., Xu Y., Ji D. H., Chen C. S., et al. (2022). Cultivation of different seaweed species and seasonal changes cause divergence of the microbial community in coastal seawaters. Front. Microbiol. 13, 988743. doi: 10.3389/fmicb.2022.988743
Yamashita Y., Tanoue E. (2008). Production of bio-refractory fluorescent dissolved organic matter in the ocean interior. Nat. Geosci. 1, 579–582. doi: 10.1038/ngeo279
Zhang J. H., Fang J. G., Wang W., Du M. R., Gao Y. P., Zhang M. L. (2012). Growth and loss of mariculture kelp in Sungo Bay, China. J. Appl. Phycol. 24, 1209–1216. doi: 10.1007/s10811-011-9762-4
Zhang A. H., Wen X., Yan H. Y., He X. F., Su H., Tang H. Q., et al. (2018). Response of microalgae to large-seaweed cultivation as revealed by particulate organic matter from an integrated aquaculture off Nan’ao Island, South China. Mar. pollut. Bull. 133, 137–143. doi: 10.1016/j.marpolbul.2018.05.026
Zhang Y. Y., Zhang J. H., Liang Y. T., Li H. M., Li G., Chen X., et al. (2017). Carbon sequestration processes and mechanisms in coastal mariculture environments in China. Sci. China Earth Sci. 60, 2097–2107. doi: 10.1007/s11430-017-9148-7
Zhao C., Sun J., Shen Y., Xia Z., Hu M., Wu T., et al. (2023a). Removable carbon and storage carbon of golden tides. Mar. pollut. Bull. 191, 114974. doi: 10.1016/j.marpolbul.2023.114974
Zhao C., Zhang H. B., Li P. H., Yi Y. B., Zhou Y. P., Wang Y. T., et al. (2023b). Dissolved organic matter cycling revealed from the molecular level in three coastal bays of China. Sci. Total Environ. 904, 166843. doi: 10.1016/j.scitotenv.2023.166843
Zheng Y. H., Jin R. J., Zhang X. J., Wang Q. X., Wu J. P. (2019). The considerable environmental benefits of seaweed aquaculture in China. Stoch. Env. Res. Risk A 33, 1203–1221. doi: 10.1007/s00477-019-01685-z
Zhou W. Y. (2023). Effects of major environmental factors on the growth and development of laver. Zhejiang Ocean University, Xiamen.
Keywords: Porphyra, coastal cultivation zone, organic matter transformation, refractory dissolved organic matter, optical analysis, blue carbon strategies
Citation: Wang T, Xu J, Dahlgren RA, Liu Q, Jia Y, Chen B, Xu H, Ma Z and Qu L (2025) Seaweed (Porphyra) cultivation enhances production of autochthonous refractory dissolved organic matter in coastal ecosystems. Front. Mar. Sci. 12:1529148. doi: 10.3389/fmars.2025.1529148
Received: 16 November 2024; Accepted: 20 January 2025;
Published: 06 February 2025.
Edited by:
Liyang Yang, Fuzhou University, ChinaCopyright © 2025 Wang, Xu, Dahlgren, Liu, Jia, Chen, Xu, Ma and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyin Qu, bGl5aW5xdUB3enUuZWR1LmNu; Zengling Ma, bWF6ZW5nbGluZ0B3enUuZWR1LmNu
 Ting Wang1,2
Ting Wang1,2 Randy A. Dahlgren
Randy A. Dahlgren Qiang Liu
Qiang Liu Yang Jia
Yang Jia Binbin Chen
Binbin Chen Zengling Ma
Zengling Ma Liyin Qu
Liyin Qu