- 1College of Fisheries and Life Science, Dalian Ocean University, Dalian, Liaoning, China
- 2Dalian Key Laboratory of Breeding, Reproduction and Aquaculture of Crustaceans, Dalian, Liaoning, China
A 50-day feeding trial was conducted to evaluate the effects of dietary supplementation with different levels of compound lactic acid bacteria on the growth performance, antioxidant capacity, intestinal microbiota composition, and immunity of the Strongylocentrotus intermedius. In this study, S. intermedius with an initial body weight of 26.47 ± 0.27 g was used as the experimental subject. Based on dietary supplementation with compound lactic acid bacteria (containing 56.15% Lentilactobacillus and 20.59% Acetobacter) at different levels, the subjects were categorized into four experimental groups: 0% (RC), 0.5% (RL), 1% (RM), and 2% (RH). The dietary supplementation with compound lactic acid bacteria significantly improved the growth performance of S. intermedius in the RL, RM, and RH groups, including final body weight (FBW), weight gain rate (WGR), specific growth rate (SGR), and gonadosomatic index (GSI). Furthermore, antioxidant and immune indicators such as total antioxidant capacity (T-AOC), superoxide dismutase (SOD), catalase (CAT), acid phosphatase (ACP), alkaline phosphatase (AKP), and lysozyme (LZM) were significantly enhanced. Notably, compound lactic acid bacteria improved digestive enzyme activities in the intestine and reduced the feed conversion ratio (FCR). Supplementation with compound lactic acid bacteria reduced Arcobacter and Vibrio colonization in the intestinal tract and enhanced the expression of genes related to antioxidant, stress, and immune responses in the RM and RH groups. Overall, 1% compound lactic acid bacteria supplementation in the diet significantly improved growth performance, digestive capacity, non-specific immune ability, and intestinal microbial stability in S. intermedius.
1 Introduction
Strongylocentrotus intermedius, a marine organism of significant value, belongs to the class Echinoidea within the phylum Echinodermata (Ning et al., 2022). Its gonads are known for their delicious taste and are rich in various amino acids and unsaturated fatty acids, making them highly favored by consumers, especially in certain Asian and European countries (Zuo et al., 2022). In recent years, S. intermedius has become an important economic aquaculture species in northern China, generating an economic benefit of estimated 14 million USD in 2023 (Di et al., 2023). However, with the rapid increase in consumer demand, the sea urchin farming industry has also grown swiftly. This rapid development, however, brings challenges, such as slow growth rates and frequent diseases, which severely hinder the sustainable development of the sea urchin farming industry (Zhang et al., 2025b).
The widespread use of antibiotics to control disease outbreaks and enhance crop yields has long been associated with the development of antibiotic resistance in many bacteria, along with the presence of drug residues in agricultural products, water sources, and soil (Hoseinifar et al., 2018). These residues pose adverse effects and potential threats to the environment and human health (Fečkaninová et al., 2017). Due to the restrictions on antibiotic use, some diseases and water quality deterioration in aquaculture cannot be effectively managed. This has become one of the limiting factors for the development of the aquaculture industry (Amenyogbe et al., 2024). Lactic acid bacteria offer advantages such as being environmentally friendly, safe, and non-polluting, and they do not induce antibiotic resistance (Li et al., 2024). Therefore, they can promote ecological health and sustainability. Numerous studies have demonstrated that the addition of lactic acid bacteria to feed can not only enhance the nutritional and growth performance of aquatic animals but also improve their immune activity and disease resistance (Giri et al., 2020). Therefore, regulating the immunity and disease resistance of aquaculture animals through lactic acid bacteria may represent a potential solution to these challenges.
Probiotics, an emerging field in nutritional immunology, offer significant bioregulatory functions that positively influence the host in various ways, including enhancing immune capacity, inhibiting the proliferation of harmful bacteria, maintaining intestinal microbiota stability, and promoting growth performance (Feng et al., 2020). Probiotics are widely used in aquaculture for disease prevention and treatment (Mohammadi et al., 2021). Additionally, probiotics offer a potential alternative to antibiotics due to their lack of drug residues in the environment, non-polluting characteristics, and ability to reduce antibiotic resistance. In aquaculture, the application of Lactobacillaceae probiotics is particularly extensive, with primary functions that include promoting digestion, enhancing immunity, and balancing intestine microbiota (Li et al., 2018). The intestinal microbiota micro-ecosystem serves as an indicator of the health status in aquatic animals, and the most effective strategy to maintain the balance of the intestinal microbiota is the auxiliary use of probiotics (Meng et al., 2021). Promoting the balance of intestinal microbiota through dietary supplementation of probiotics may directly stimulate the host digestive function (Chen et al., 2020), inhibit the colonization of pathogens in the gastrointestinal tract (Lee et al., 2024), and increase the relative abundance of beneficial bacteria in the intestine (Wang et al., 2022), which in turn improves the growth performance of aquatic animals. However, there is still a lack of adequate research on the changes in the intestinal microbiota of sea urchins caused by probiotics and how these changes affect growth performance, digestive capacity, and nonspecific immunity. Additionally, the changes induced by dietary supplements of different kinds of probiotics may differ.
Previous studies have shown that the addition of lactic acid bacteria can improve the growth performance, intestinal health, and upregulate the expression of immunity-related genes in crucian carp (Carassius auratus) (Zhang et al., 2022; Li et al., 2024). The addition of Bacillus in the diet of sea cucumbers (Apostichopus japonicus) has been shown to improve growth, enhance immunity, and modulate the intestine microbiota (Wang et al., 2022). Supplementing heat-killed Lactobacillus acidophilus in the diet significantly enhances the growth performance, antioxidant capacity, and intestinal barrier integrity and effectively mitigates inflammatory responses in large yellow croaker (Larimichthys crocea) (He et al., 2024). Appropriate supplementation with Bacillus subtilis in the diet has been found to improve growth performance, antioxidant capacity, and immune capacity in red seabream (Pagrus major) (Zaineldin et al., 2018). Additionally, several studies have demonstrated that the simultaneous addition of compound probiotics to the diet effectively promotes the growth performance, digestive capacity, and nonspecific immune function in various aquatic animals, such as rainbow trout (Oncorhynchus mykiss) (Pillinger et al., 2022), shrimp (Litopenaeus vannamei) (Wang et al., 2019), crucian carp (C. auratus) (Zhang et al., 2022), and sea cucumber (A. japonicus) (Liu et al., 2024).
Currently, most research has focused primarily on the application of single probiotics as dietary supplements in aquaculture, while studies on the synergistic effects of compound lactic acid bacteria on aquatic animals remain relatively scarce (Saravanan et al., 2021; Luo et al., 2023). To date, no studies have been reported on the supplementation of compound lactic acid bacteria in the diet of S. intermedius. Therefore, this study supplements varying levels of compound lactic acid bacteria into the diet of S. intermedius to evaluate their effects on growth performance, nonspecific immune function, intestine health, and the expression of related genes, with the aim of providing a scientific reference for the development and application of compound lactic acid bacteria in sea urchin aquaculture.
2 Materials and methods
2.1 Statement of ethics and legitimacy
Since sea urchins are invertebrates, the Ethics Committee of Dalian Ocean University did not require the study to undergo review or approval.
2.2 Preparation of experimental diets
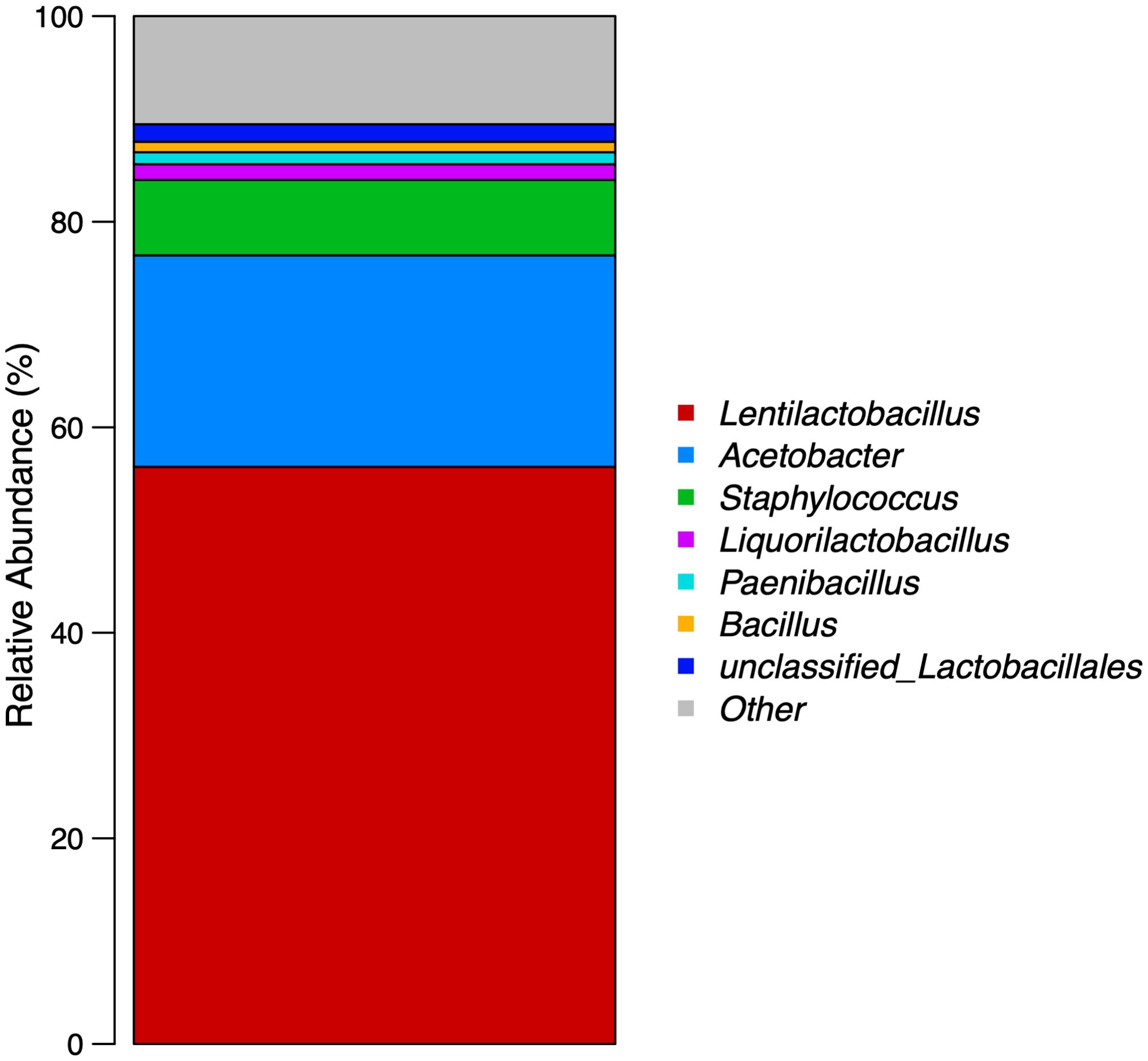
The compound lactic acid bacteria (production batch number Q/DLST 001-2021) used in this study were provided by Dalian Scitech Biotechnology Co., Ltd. (Dalian, China). The total probiotic count was approximately 5 × 108 CFU/mL measured using the dilution plate count method, and the species were identified via cluster analysis of the 16S rDNA sequence prior to the feeding trial. The relative abundance of microorganisms in the compound lactic acid bacteria is as follows: Lentilactobacillus accounted for 56.15%, Acetobacter 20.59%, Staphylococcus 7.33%, Liquorilactobacillus 1.53%, Paenibacillus 1.16%, Bacillus 1.02%, unclassified Lactobacillales 1.70%, and the remaining bacteria, each accounting for less than 1%, constituted “Other”. The relative abundance proportions are presented in Figure 1.
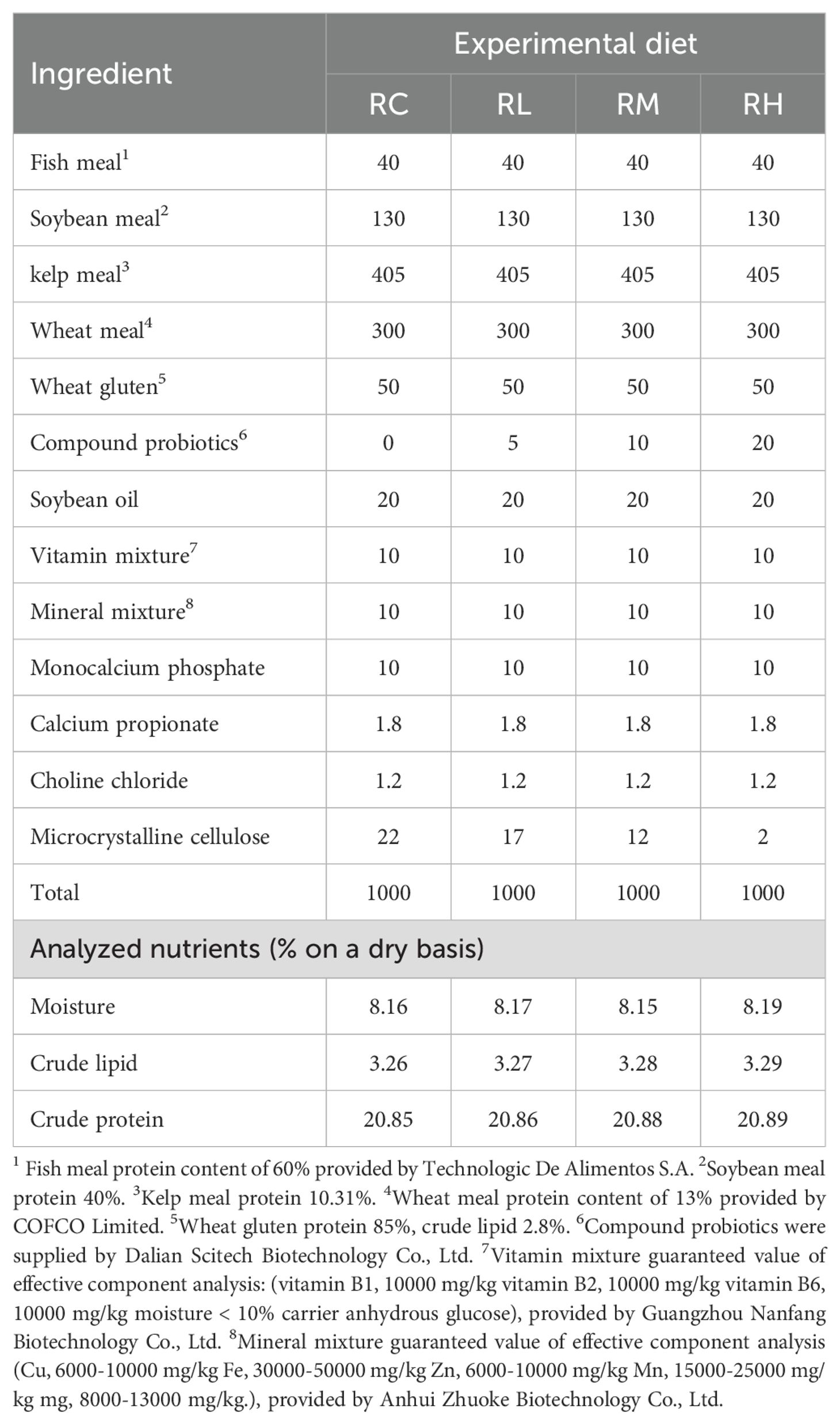
In this study, four isoproteic (21%) and isolipidic (3.3%) basal feeds were formulated. Compound lactic acid bacteria were then added to the basal feeds at levels of 0%, 0.5%, 1%, and 2%, and the groups were named RC, RL, RM, and RH, respectively (0, 2.5, 5, and 10 × 108 CFU/mL). The feed formula and general nutritional components are detailed in Table 1. The feed formula was based on previous studies (Lourenço et al., 2020; Ning et al., 2022; Di et al., 2023; Zhang et al., 2025a), which found that a protein content of 20-25% and a lipid content of 2-8% were sufficient to meet the nutritional requirements of sea urchins and enhance their growth performance. All solid ingredients were ground and passed through an 80-mesh sieve. After precise weighing, each feed powder and soybean oil were mixed uniformly using a stepwise amplification method, followed by the addition of approximately 35% water for thorough mixing. Finally, a tablet press (MT-40-I, Xingtai, China) was used to prepare the pellet feed (1 × 1 × 0.1 cm), air-dried, and stored at −20°C. To maintain lactic acid bacteria activity, the feed was prepared every seven days.
2.3 Feeding experiment
The S. intermedius was collected from Dalian Feikun Aquaculture Co., Ltd. (Dalian, China). Prior to the commencement of the study, S. intermedius were placed in a pool for 10 days of acclimation to adapt to the water quality and environment of the laboratory. Subsequently, 180 S. intermedius with an initial body weight of 26.47 ± 0.27 g were divided into three replicate groups by randomly assigning them to twelve 50 L tanks (60 × 40 × 23 cm), with each group divided into three replicate tanks and each tank containing 15 sea urchins. Each tank in the recirculating water system had an independent inlet and outlet. The used seawater was uniformly settled, disinfected, filtered, and wastewater was removed. The same amount of seawater was then replenished, chemically treated, and added back into each tank. The water flow rate was maintained at 10 L/min in a continuous circulation, and approximately one-third of the treated fresh seawater was replenished daily. The four different levels of compound lactic acid bacteria feed groups were fed daily at 10:00 AM, and uneaten feed and feces were collected using a siphon before each feeding. The collected wet feed was dried in an oven at 75°C until it reached the same dry weight as the feed provided at the time of feeding in order to measure the feed consumption. The feeding period lasted for 50 days. Air was continuously supplied to the tanks throughout the study. The water’s physicochemical properties during the study were: temperature (17 ± 1°C), pH (7.5 ± 0.5), salinity (30.0 ± 1.0‰), and dissolved oxygen (6.5 ± 0.5 mg/L).
2.4 Sample collection
After the feeding study, S. intermedius were fasted for 24 hours. All S. intermedius were then counted individually, and their wet weights were measured. Subsequently, seven S. intermedius were randomly selected from each tank, and coelomic fluid was extracted using a 1 mL syringe with a 27-gauge needle. The coelomic fluid samples from the seven S. intermedius in each tank were pooled to create a composite sample, resulting in three composite coelomic fluid samples per group. The coelomic fluid was centrifuged (1000 × g, 4°C, 10 min), and the supernatant was collected, snap-frozen in liquid nitrogen, and subsequently stored at −80°C in sterile, enzyme-free 1.5 mL cryovials. Three composite coelomic fluid samples per group were used for subsequent antioxidant capacity and enzyme activity analysis.
Following this, eight S. intermedius were randomly selected from each tank and disinfected with 70% ethanol. Using sterile instruments, the S. intermedius were dissected, and the midgut tissues along with intestinal contents from three randomly selected individuals in each tank were collected and pooled into a single sample for subsequent intestinal microbiota diversity analysis. The digestive tracts and gonads of the remaining five S. intermedius in each tank were separately weighed. The digestive tracts from these five individuals were combined into a composite sample, with three composite digestive tract samples per group. Each composite sample was then individually stored in sterile, enzyme-free 1.5 mL cryovials, snap-frozen in liquid nitrogen, and subsequently stored at -80°C. For subsequent analyses, including digestive enzyme activity assays and real-time quantitative detection of genes related to antioxidation, stress response, inflammation, and immunity, three composite digestive tract samples per group were measured. Before measuring digestive enzyme activity, the digestive tract tissue was accurately weighed. Phosphate-buffered saline (PBS, 0.1 mol/L, pH 7.4) was added at a ratio of 1:9 (weight in grams: volume in milliliters). The mixture was placed in sterile, enzyme-free tubes and mechanically homogenized in an ice-water bath. The homogenized samples were centrifuged (1000 × g, 4°C, 10 min), and the supernatant was collected and stored in sterile, enzyme-free tubes for subsequent digestive enzyme activity analysis.
2.5 Analysis of digestive enzyme activity, immune and antioxidant capacity
Using a commercial assay kit from Nanjing Jiancheng Bioengineering Institute, the digestive enzyme indicators in the digestive tracts and the immune and antioxidant enzyme indicators in the coelomic fluid of S. intermedius were measured. These enzyme activity indicators were measured using spectrophotometry. The kits, catalog numbers, and wavelengths used for each measurement were as follows: lipase (LPS), A054-1-1, 420 nm; amylase (AMS), C016-1-1, 660 nm; pepsin (PEP), A080-1-1, 660 nm; acid phosphatase (ACP), A060-2, 520 nm; alkaline phosphatase (AKP), A059-2, 520 nm; total antioxidant capacity (T-AOC), A015-2-1, 425 nm; superoxide dismutase (SOD), A001-3, 450 nm; malondialdehyde (MDA), A003-1, 532 nm; glutathione-S transferase (GSH-S), A004-1-1, 412 nm; glutathione peroxidase (GSH-PX), A005-1, 412 nm; catalase (CAT), A007-1-1, 405 nm; and lysozyme (LZM), A050-1-1, 530 nm. The measurements were conducted with a visible spectrophotometer (YA152010226, Shanghai Youke Instrument Co., Ltd., China) and a light absorption microplate reader (ReadMax 500F, Shanghai Shining Biotechnology Co., Ltd., China). All measurements adhered strictly to the manufacturer’s instructions to ensure precision and reliability of the results. All test kits were sourced from Nanjing Jiancheng Bioengineering Institute, China (http://www.njjcbio.com).
2.6 Microbial diversity analysis
Total microbial genomic DNA was extracted from S. intermedius intestines using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) according to manufacturer’s instructions. The quality and concentration of DNA were determined by 1.0% agarose gel electrophoresis and a NanoDrop2000 spectrophotometer (Thermo Scientific, United States) and kept at −80°C prior to further use. DNA samples were amplified by PCR using the primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) for the V3–V4 region of the 16S rRNA gene. The PCR was conducted using TransGen AP221-02 in a total volume of 20 μL with 4 μL 5 × FastPfu Buffer, 2 μL dNTPs (2.5 mM), 0.8 μL of each primer (5 μM), 0.4 μL FastPfu polymerase, 0.2 μL BSA, and 10 ng of template DNA. PCR was performed using GeneAmp® 9700 (Applied Biosystems, USA) and the PCR program was 95°C for 3 min, then 25 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, followed by a final extension at 72°C for 10 min and ended at 4°C. Purified amplicons were pooled in equimolar and paired-end sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Total reads represent the total number of DNA sequences generated during sequencing, indicating the dataset size. Operational Taxonomic Units (OTUs) were used to group similar microbial organisms based on 97% sequence similarity. Species richness was estimated using the Chao index, which accounts for undetected rare species, and the Ace estimator, which emphasizes rare species to better estimate community diversity. The Shannon index was employed to measure species diversity, considering both richness and evenness, with higher values indicating greater diversity. The Simpson index was also used, reflecting the probability that two randomly selected individuals belong to different species, where lower values suggest higher diversity. The coverage index was applied to assess the completeness of sampling, with values close to 1 indicating that most species were captured.
2.7 RNA extraction and quantitative real-time PCR
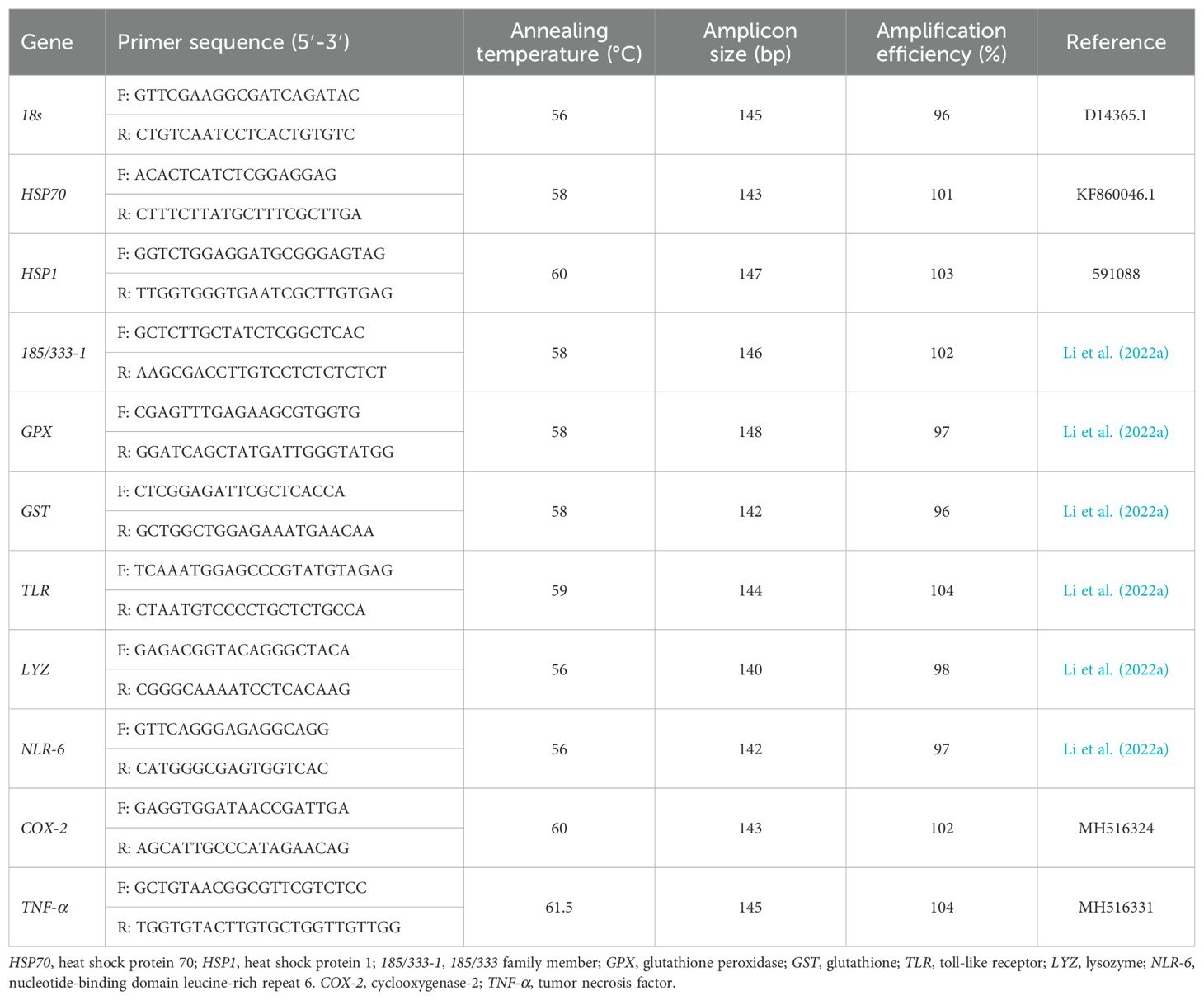
The method for extracting RNA from the S. intermedius digestive tract was based on the work of Zuo et al. (2017). The reference gene (18s) and the other target genes were all cited from relevant literature. The sequences of the specific primers are shown in Table 2. Briefly, total RNA was extracted from the intestinal tract of S. intermedius using TianGen’s RNA Easy Fast kit (DP451) and FastKing gDNA Dispelling RT SuperMix (KR118) (TianGen, Beijing, China). The RNA integrity was confirmed via agarose gel electrophoresis using 50× TAE Buffer (B548101) and Agarose, Regular (A620014) (Sangon Biotech, Shanghai, China). cDNA synthesis was performed under the following conditions: incubation at 37°C for 15 minutes, denaturation at 85°C for 5 seconds, and storage of the cDNA at −20°C.
The qPCR was performed by using the FastKing One Step RT-PCR Kit (KR123) (TianGen, Beijing, China). The qPCR employed SYBR Green I chemistry in a 20 μL reaction mixture containing 10 μL 2 × TransStart® Tip Green qPCR Super Mix, 0.8 μL each of forward and reverse primers (20μM), 2 μL of cDNA, and 6.4 μL of RNase-free water. PCR cycling involved an initial denaturation at 95°C for 2 min, followed by 45 cycles of 95°C for 5 s and annealing/extension at 60°C for 10 s, with a final melt curve analysis. Biological replicates were conducted three times, and a blank control group was set up. The relative expression levels of each gene were calculated using the 2-ΔΔCT method.
2.8 Formulas for calculation
The above parameters were established based on the formulas presented by Ning et al. (2022). Ni and Nf represent the initial and final numbers of S. intermedius, respectively. Wi and Wf are the initial and final average body weight of S. intermedius, respectively (both in grams, g); FM is the mean feed consumption (in grams, g) (based on the average feed amount minus the average uneaten feed); T is the feeding trial duration (days); and Wg and Wd are the final wet weights (in grams, g) of the gonads and digestive tracts of S. intermedius, respectively. The following abbreviations are used for body indicators and growth performance: SR, survival rate; IBW, initial body weight; FBW, final body weight; WGR, weight gain rate; SGR, specific growth rate; GWW, gonad wet weight; GSI, gonadosomatic index; DTW, digestive tract weight; DTI, digestive tract index; FCR, feed conversion ratio.
2.9 Data analysis
All data were checked for normality using the Shapiro-Wilk test and for homogeneity of variance using Levene’s test before being statistically analyzed using one-way analysis of variance (ANOVA) in SPSS 29 software. P < 0.05 was deemed statistically significant. Then, Duncan’s multiple range test was used to compare differences in the means between dietary treatments. Data are expressed as mean values ± standard deviation (SD).
3 Results
3.1 Survival and growth performance
During the 50-day study, no mortality was observed in any of the S. intermedius across all experimental groups. The growth performance results of S. intermedius are presented in Table 3. The RM group showed significantly higher values in final body weight (FBW), weight gain rate (WGR), specific growth rate (SGR), gonad wet weight (GWW), and gonadosomatic index (GSI) than the RC, RL, and RH groups (P < 0.05). The RC group (control) exhibited the lowest values for these indicators (P < 0.05). The digestive tract wet weight (DTW) in the RC group was significantly lower than in the other experimental groups (RL, RM, RH) (P < 0.05). No significant differences in digestive tract index (DTI) were observed among the groups (RC, RL, RM, RH) (P > 0.05). The RM group had a significantly lower feed conversion ratio (FCR) than the RC, RL, and RH groups (P < 0.05).
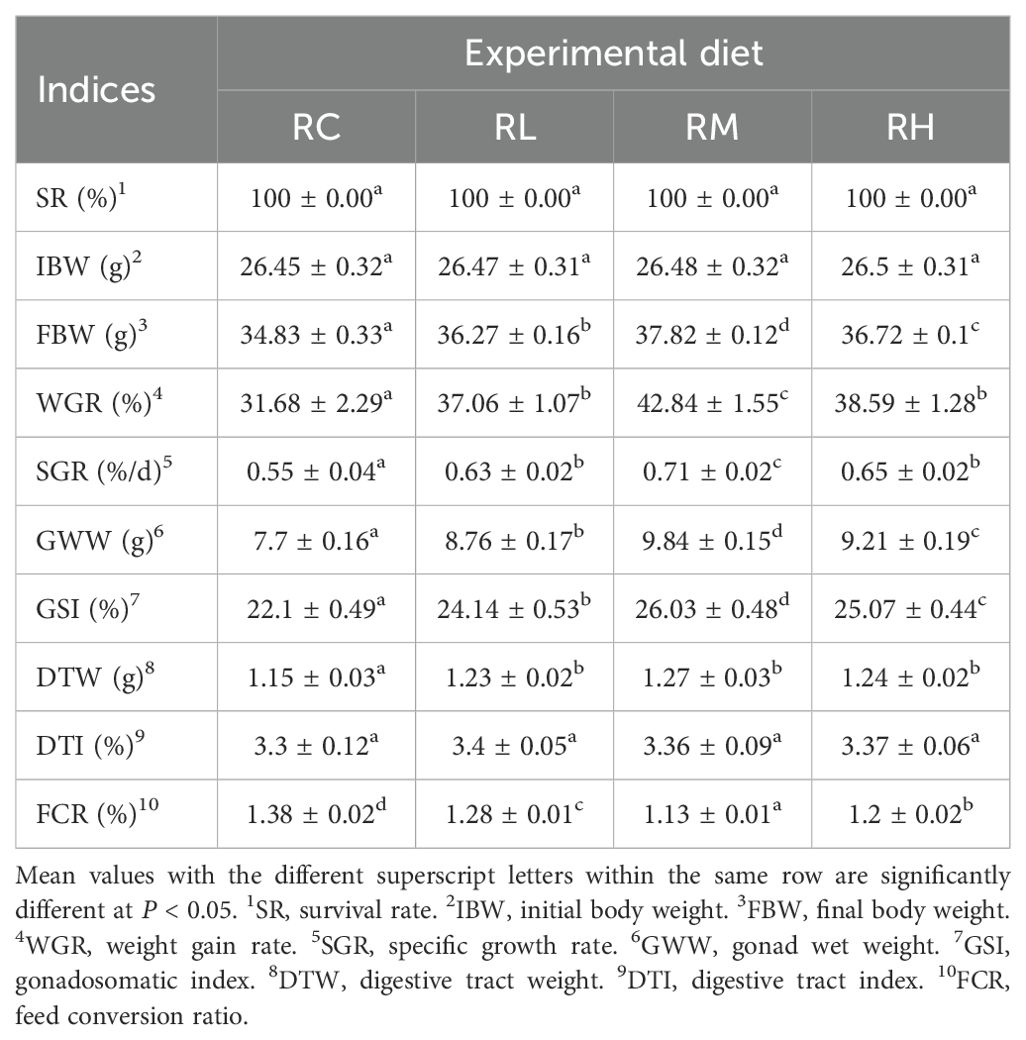
Table 3. Effect of experimental diets on the survival and growth performance of sea urchins (Strongylocentrotus intermedius) (mean ± SD, n = 3).
3.2 Digestive enzyme activity, immune indicator activity, and antioxidant capacity
In the intestines of S. intermedius from the RM group, the activities of pepsin, lipase, and amylase (except for amylase in the RH group) were significantly higher than those in the other three groups (RC, RL, and RH) (P < 0.05). No significant difference in lipase activity was observed between the RL and RH groups (P > 0.05), but both were significantly higher than in the RC group (P < 0.05). The specific parameters for each biochemical index of S. intermedius are shown in Table 4.
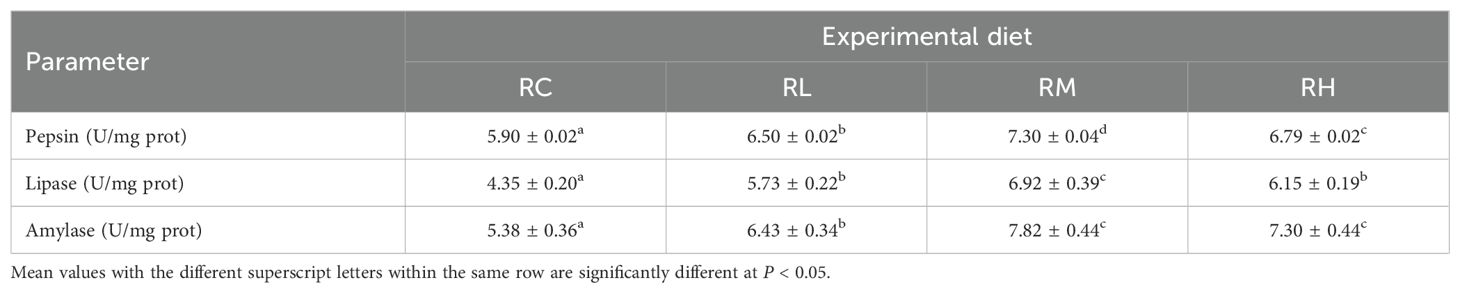
Table 4. Effect of experimental diets on the intestinal digestive enzyme activities of sea urchins (Strongylocentrotus intermedius) (mean ± SD, n = 3).
With the increased supplementation levels of compound lactic acid bacteria in the S. intermedius diet, the levels of T-AOC, SOD, CAT, GSH-PX, ACP, AKP, and LZM in the coelomic fluid of S. intermedius gradually increased, peaking in the RM group and then decreasing in the RH group. There was no significant difference in GSH-S levels between the RL and RH groups (P > 0.05), but both were significantly higher than those in the RC group (P < 0.05). The MDA level in the RM group was significantly lower than in the RC, RL, and RH groups (P < 0.05). Specific biochemical parameters for each indicator in S. intermedius are presented in Table 5.
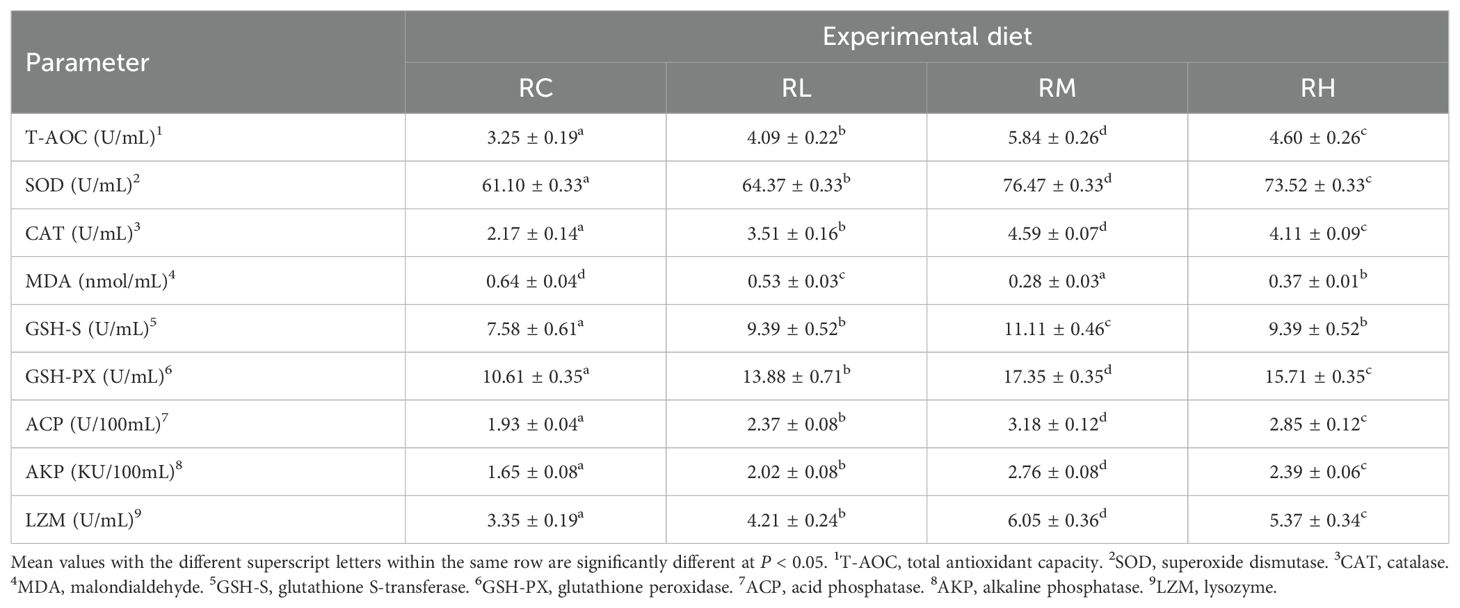
Table 5. Effect of experimental diets on the immune and antioxidant capacity in the coelomic fluid of sea urchins (Strongylocentrotus intermedius) (mean ± SD, n = 3).
3.3 Intestinal microbiota diversity and composition
As shown in Table 6, the coverage rate of intestinal samples in all S. intermedius experimental groups reached 0.999 or higher. The OTU count, Chao index, and Ace index exhibited a trend of initially decreasing and then increasing, with the lowest values observed in the RL group, followed by a gradual increase, reaching the highest values in the RH group. The Shannon index was highest in the RH group, followed by the RL, RC, and RM groups. The Simpson index was highest in the RC group, followed by the RM, RL, and RH groups. According to the Venn diagram in Figure 2, feeding S. intermedius different experimental diets had a varied impact on the number of OTUs in their intestines. The four groups shared 51 OTUs in common, with the RH group showing the highest abundance of unique OTUs (515 OTUs), followed by the RC group (86 OTUs), the RM group (76 OTUs), and the RL group (35 OTUs).
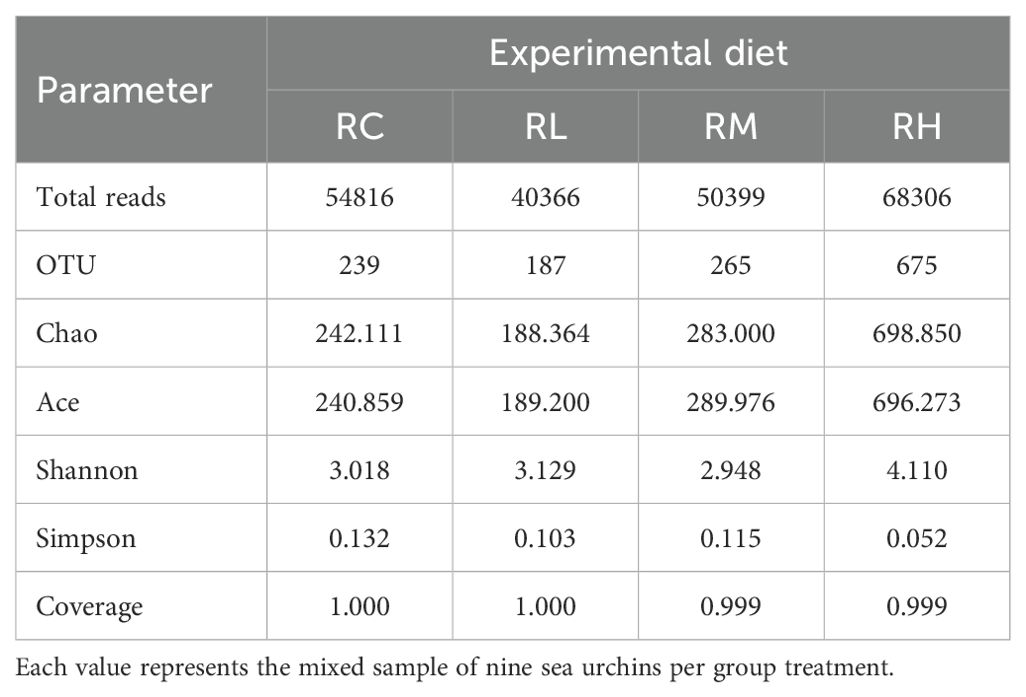
Table 6. Effect of experimental diets on overall sequences, operational taxonomic units (OTU), alpha diversity indices and richness of intestinal microbiota of sea urchins (Strongylocentrotus intermedius) (n = 1).
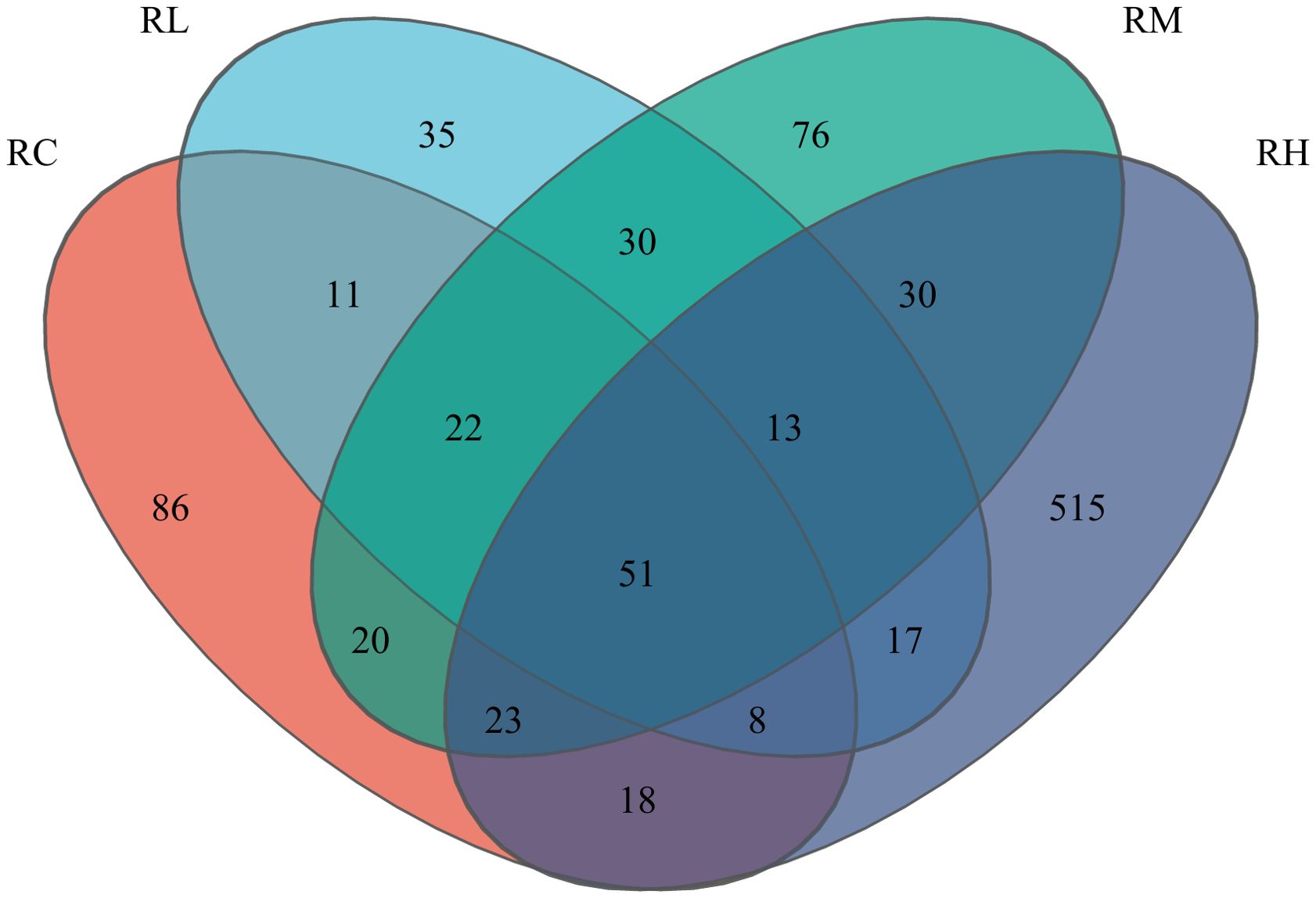
Figure 2. Venn diagram analysis of the intestine microbiota of sea urchin (Strongylocentrotus intermedius) intestines with experimental diets. In Image, different colors represent distinct groups. The numbers in overlapping sections indicate the number of species shared by multiple groups, and the numbers in non-overlapping sections denote the number of species unique to each respective group.
Figure 3A shows the relative abundance of intestinal microbiota at the phylum level for each experimental group. The results indicate that the S. intermedius intestinal microbiota was primarily composed of Proteobacteria, Epsilonbacteraeota, Tenericutes, and Firmicutes. Proteobacteria, as the dominant phylum in the intestine, had relative abundances of 51.64%, 91.40%, 59.21%, and 54.47% in the RC, RL, RM, and RH groups, respectively. Epsilonbacteraeota had the second highest relative abundance in the FC group, at 38.51%. Tenericutes had the second highest relative abundance in the RM group, at 23.34%. Firmicutes had the second highest relative abundance in the RH group, at 19.99%.
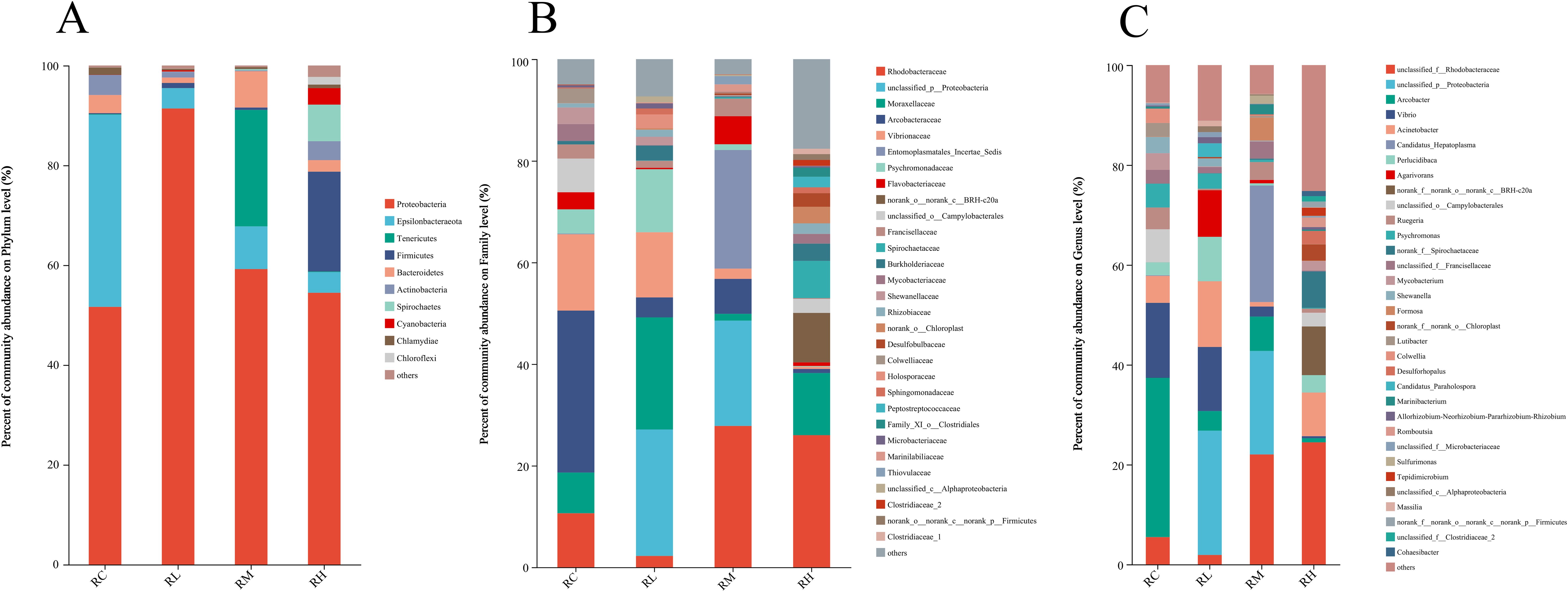
Figure 3. Effects of experimental diets on the relative abundance of intestinal microbiota at the phylum (A), family (B), and genus (C) levels in sea urchins (Strongylocentrotus intermedius).
Figure 3B shows the relative abundance of intestinal microbiota at the family level for each experimental group. The results indicate that the top five families in the intestinal microbiota of S. intermedius are Rhodobacteraceae, unclassified Proteobacteria, Moraxellaceae, Arcobacteraceae, and Vibrionaceae. With the increasing levels of compound lactic acid bacteria added to the diet, the relative abundance of Rhodobacteraceae in the RM and RH groups was significantly higher than that in the RC and RL groups. The relative abundances of Rhodobacteraceae in the RC, RL, RM, and RH groups were 10.63%, 2.23%, 27.82%, and 25.99%, respectively. The highest relative abundance of Moraxellaceae was found in the RL group at 22.06%. The relative abundances of Arcobacteraceae in the RC, RL, RM, and RH groups were 31.88%, 3.93%, 6.88%, and 0.81%, respectively. The relative abundances of Vibrionaceae in the RC, RL, RM, and RH groups were 15.02%, 12.83%, 2.02%, and 0.42%, respectively. With the increasing levels of compound lactic acid bacteria added to the diet, the relative abundances of Arcobacteraceae and Vibrionaceae in the intestinal microbiota of the RM and RH groups significantly decreased.
Figure 3C shows the relative abundance of intestinal microbiota at the genus level for each experimental group. The results indicate that the top five taxa in the intestinal microbiota of S. intermedius are unclassified Rhodobacteraceae, unclassified Proteobacteria, Arcobacter, Vibrio, and Acinetobacter. With the increasing levels of compound lactic acid bacteria added to the diet, the relative abundance of unclassified Rhodobacteraceae in the RM and RH groups was significantly higher than that in the RC and RL groups. The relative abundances of unclassified Rhodobacteraceae in the RC, RL, RM, and RH groups were 5.46%, 1.90%, 22.03%, and 24.45%, respectively. The relative abundances of unclassified Proteobacteria in the RC, RL, RM, and RH groups were 0.02%, 24.88%, 20.72%, and 0.01%, respectively. The relative abundances of Arcobacter in the RC, RL, RM, and RH groups were 31.88%, 3.93%, 6.88%, and 0.81%, respectively. The relative abundances of Vibrio in the RC, RL, RM, and RH groups were 15.02%, 12.83%, 2.02%, and 0.42%, respectively. The relative abundances of Acinetobacter in the RC, RL, RM, and RH groups were 5.40%, 13.17%, 0.90%, and 8.74%, respectively.
3.4 Expression of antioxidant and stress-related genes
As shown in Figure 4, the GPX gene expression in the digestive tract of S. intermedius increased with higher levels of compound lactic acid bacteria, peaking in the RM group before declining in the RH group. The expression of the GST gene in the digestive tract of S. intermedius showed an upward trend, reaching its peak in the RH group. Notably, there was no significant difference in the expression levels of the GPX and GST genes between the RM and RH groups (P > 0.05), but both were significantly higher than those in the RC and RL groups (P < 0.05).
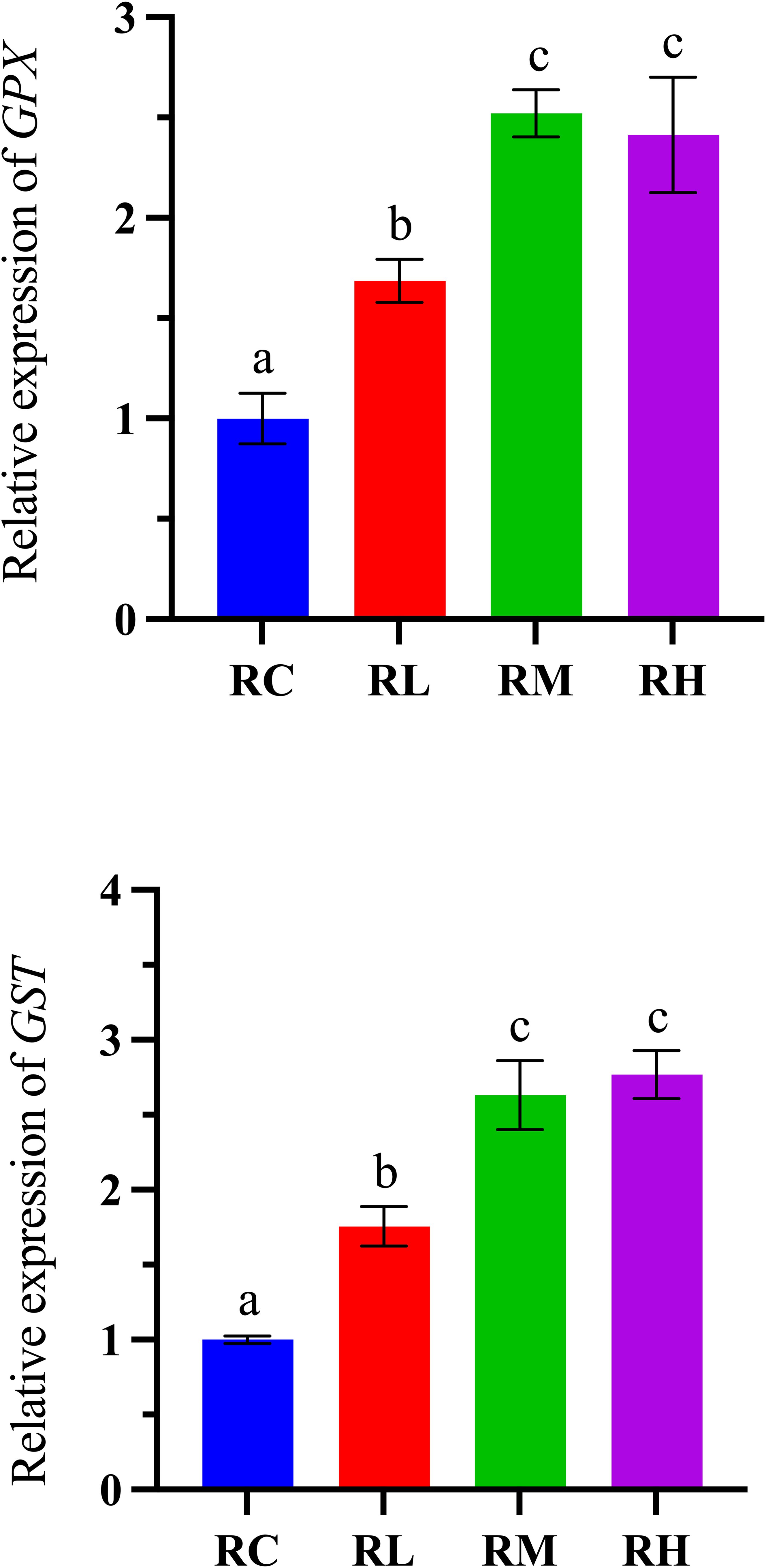
Figure 4. Effects of experimental diets on the relative expression of antioxidant-related genes in the intestines of sea urchins (Strongylocentrotus intermedius) (normalized to 18S rRNA) (mean ± SD, n = 3). Bars sharing letters are not significantly different (P < 0.05). GPX, glutathione peroxidase; GST, glutathione S-transferase.
As shown in Figure 5, with the increased supplementation levels of compound lactic acid bacteria in the diet, the expression level of HSP70 in the intestines of S. intermedius showed an upward trend, reaching a peak in the RH group. Notably, there was no significant difference between the RM and RH groups (P > 0.05), but both were significantly higher than the RC and RL groups (P < 0.05). The expression level of the HSP1 gene in the intestines of S. intermedius initially increased, peaked in the RM group, and then decreased in the RH group (P < 0.05).
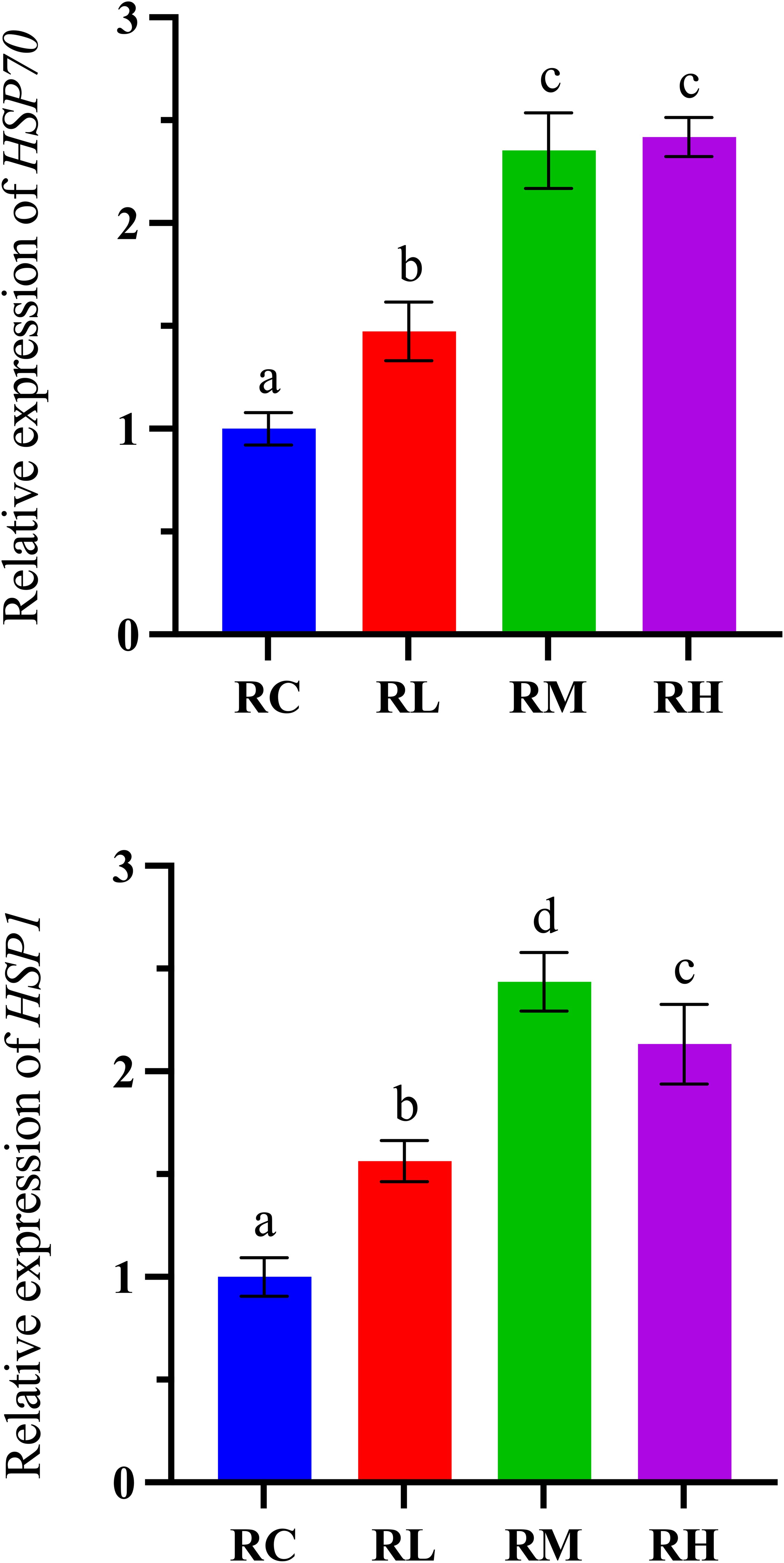
Figure 5. Effects of experimental diets on the relative expression of oxidative stress-related genes in the intestines of sea urchins (Strongylocentrotus intermedius) (normalized to 18S rRNA) (mean ± SD, n = 3). Bars sharing letters are not significantly different (P < 0.05). HSP70, heat shock protein; HSP1, heat shock protein.
3.5 Expression of immune-related genes
As shown in Figure 6, increasing dietary compound lactic acid bacteria supplementation led to an initial increase in the gene expression levels of LYZ, NLR-6, and 185/331-1 in the intestines of S. intermedius, which peaked in the RM group and declined in the RH group. Specifically, the expression levels of LYZ and 185/331-1 showed no significant difference between the RM and RH groups (P > 0.05), but both were significantly higher than those in the RC and RL groups (P < 0.05). The gene expression level of TLR in the intestines of S. intermedius showed an upward trend, reaching a peak in the RH group. Notably, there was no significant difference in TLR gene expression between the RM and RH groups (P > 0.05), but both were significantly higher than in the RC and RL groups (P < 0.05).
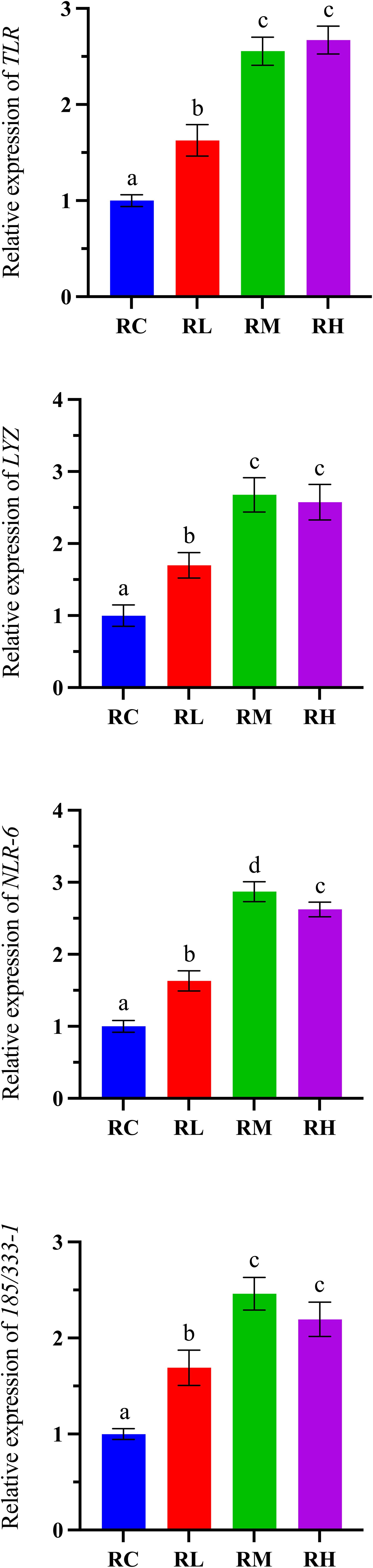
Figure 6. Effects of experimental diets on the relative expression of immune-related genes in the intestine of sea urchin (Strongylocentrotus intermedius) (standardized to 18S rRNA) (mean ± SD, n = 3). Bars sharing letters are not significantly different (P < 0.05). TLR, toll-like receptor; LYZ, lysozyme. NLR-6, nucleotide-binding domain leucine-rich repeat 6. 185/333-1: 185/333 family member.
3.6 Expression of inflammation-related genes
As shown in Figure 7, the expression level of the COX-2 gene in the intestines of S. intermedius exhibited a downward trend, reaching its lowest point in the RH group. Specifically, COX-2 gene expression in the RH group was significantly lower than in the RC and RL groups (P < 0.05). The expression level of the TNF-α gene showed a trend of initially decreasing and then increasing, reaching its lowest level in the RM group and rising in the RH group. Notably, there was no significant difference in TNF-α gene expression between the RM and RH groups (P > 0.05), but both were significantly lower than in the RC and RL groups (P < 0.05).
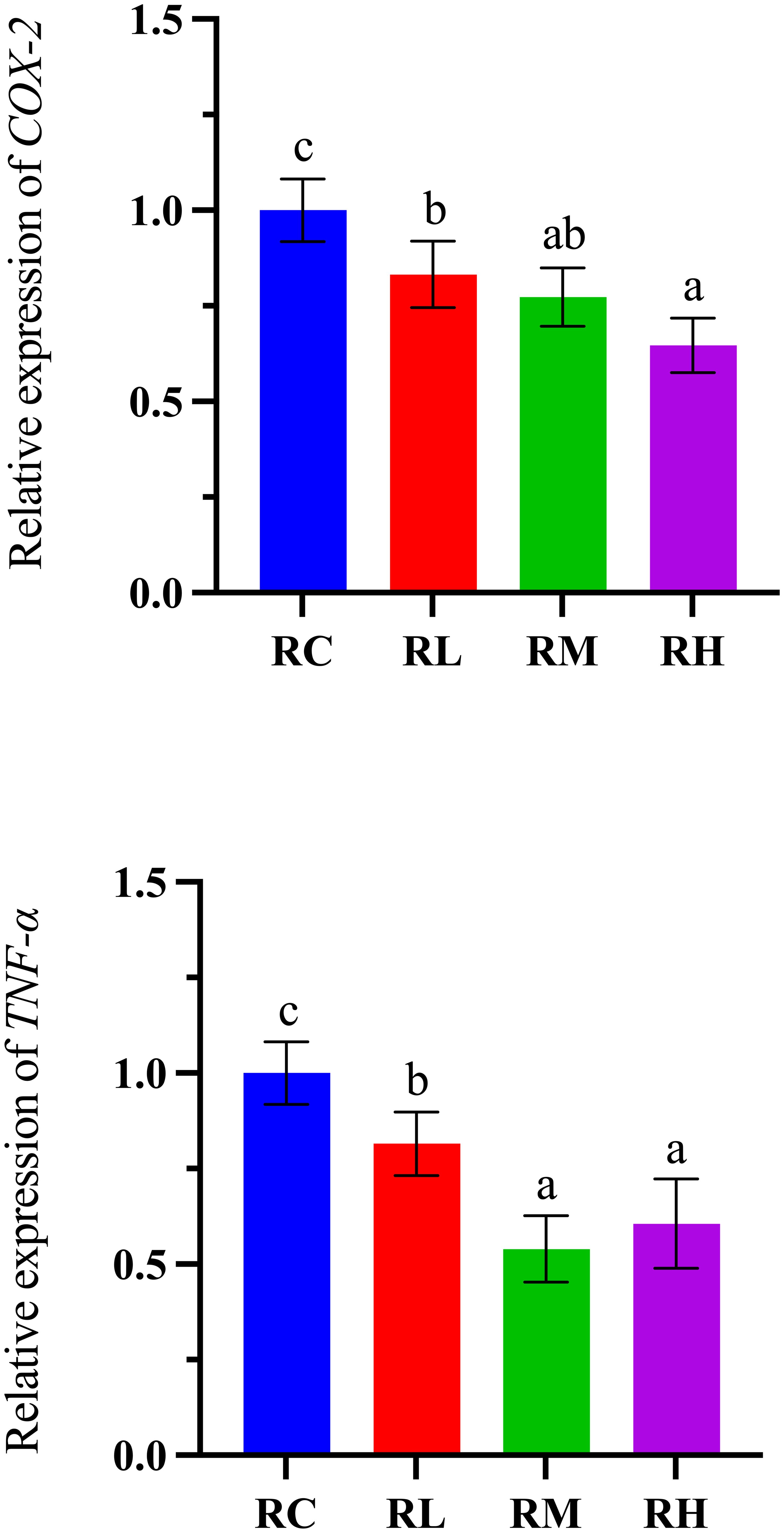
Figure 7. Effects of experimental diets on the relative expression of inflammation-related genes in the intestine of sea urchin (Strongylocentrotus intermedius) (standardized to 18S rRNA) (mean ± SD, n = 3). Bars sharing letters are not significantly different (P < 0.05). COX-2, cyclooxygenase-2; TNF-α, tumor necrosis factor.
4 Discussion
The deterioration of water quality and bacterial diseases in aquaculture have led to substantial economic losses, significantly hindering the industry’s development (Kuebutornye et al., 2019; Ringø et al., 2020). Probiotics, as eco-friendly, non-polluting nutritional additives, represent a potential alternative to antibiotics for controlling bacterial diseases and mitigating water quality issues (e.g., increased ammonia levels, spread of antibiotic-resistant bacteria) (Incharoen et al., 2019; El-Saadony et al., 2021). Previous studies have shown that supplementing the diet with different levels of composite lactic acid bacteria (including Acetobacter spp., Lactobacillus spp., and Pseudomonas spp.) for 60 days can significantly improve the growth performance metrics (FBW, SGR, BWG) of turbot (Scophthalmus maximus) and reduce the FCR (Li et al., 2019). Wang et al. (2022) pointed out in their study that supplementing the diet of sea cucumber (A. japonicus) with 107 CFU/g of probiotics (Bacillus subtilis D1-2) for 56 days significantly improved its growth performance (FBW, WGR) and promoted the colonization of beneficial bacteria (Lactobacillus) in the intestine. The results of this study suggest that supplementing the experimental diet with compound lactic acid bacteria had no effect on the survival rate of S. intermedius but significantly improved their growth performance. Specifically, compared to the control group (RC), the other three experimental groups (RL, RM, RH) showed significant increases in FBM, WGR, SGR, GWW, and GSI, with the RM group demonstrating the most pronounced improvement. This is closely linked to the abundant proteins, polysaccharides, vitamins, and other active substances found in compound lactic acid bacteria (Zhang et al., 2024). These micronutrients and active substances can improve the growth performance of aquatic animals, promote digestion and absorption, and enhance immune function (Chumpol et al., 2017). Additionally, we found that supplementing the diet with composite lactic acid bacteria can further improve growth performance (e.g., WGR, SGR, GWW, GSI). These findings suggest that appropriately supplementing the diet with compound probiotics can offer more abundant nutrients to S. intermedius. Therefore, appropriately supplementing composite probiotics in the diet can be considered one of the potential solutions to enhance the growth performance of S. intermedius.
Intestinal digestive enzymes play a crucial role in enhancing digestion and absorption in the intestine, which can further influence the growth and development of aquatic animals (Li et al., 2019). Previous studies have pointed out that supplementing the feed of sea cucumbers (A. japonicus) with 5 × 108 CFU/mL of compound lactic acid bacteria can significantly improve the activity of amylase, lipase, and pepsin in the intestine. Lee et al. (2024) pointed out that supplementing three different probiotics, all at 108 CFU/mL (Rummeliibacillus stabekisii PM227, Microbacterium oxydans NB29, and Microbacterium foliorum BZ24), in the diet of olive flounder (Paralichthys olivaceus) can significantly enhance growth performance and digestive enzyme activity (amylase, lipase, and protease). Ren et al. (2022) also reported that supplementing the diet of shrimp (L. vannamei) with heat-killed Lactobacillus plantarum (2 × 10¹¹ CFU/mL) and compound probiotics (107 CFU/mL) from Lachnospiraceae, Porphyromonadaceae, and Lactobacillus for 28 days significantly enhanced growth performance and digestive enzyme activity (e.g., lipase and amylase), while also reducing the FCR. Additionally, previous studies have shown that probiotics can enhance the digestive and absorptive capacity of the intestine by stimulating the host’s intestine to secrete digestive enzymes (Chumpol et al., 2017; Torres-Maravilla et al., 2024). In this study, the digestive enzyme activities (pepsin, lipase, and amylase) in the probiotic-treated groups (RL, RM, and RH) were significantly higher than those in the control group (RC). Furthermore, we found that this trend was consistent with the growth performance of S. intermedius, which also exhibited a trend of initially increasing and then decreasing. This indicates that digestive enzymes play a role in enhancing the host’s digestive and absorptive abilities, thereby indirectly improving the host’s growth performance (Wu et al., 2023). Different digestive enzymes have distinct functions. Specifically, pepsin (PEP) can break down proteins into peptides, amylase (AMS) can hydrolyze starch into glucose, and lipase (LPS) can break down fats into glycerol and fatty acids (Peng et al., 2022). The increased activity of these digestive enzymes (PEP, AMS, LPS) accelerates the digestion and absorption of nutrients (such as amino acids, fatty acids, and monosaccharides) in the host’s intestine, thereby improving its growth performance (Zhang et al., 2025b). Additionally, probiotics can digest and ferment non-digestible fibers and polysaccharides, leading to the production of various beneficial short-chain fatty acids (SCFAs). SCFAs play a crucial role in regulating lipid metabolism and maintaining intestinal health in animals (Ma et al., 2009; Dawood, 2021). Through the aforementioned mechanisms and the effects of probiotics, it is evident that an appropriate amount of compound lactic acid bacteria not only enhanced intestinal digestion but also reduced FCR, thereby lowering the economic costs of aquaculture.
Sea urchins primarily rely on their innate immune system (such as cells and coelomic fluid) to resist pathogenic microorganisms (Li et al., 2021). Their immunity is enforced through phagocytosis and the activity of nonspecific immune enzymes (e.g., SOD, CAT, MDA, LZM, AKP, ACP) (Torres-Maravilla et al., 2024). Antioxidants and oxidative free radicals exist in a delicate equilibrium, and any disruption to this balance can have significant impacts on overall health (Zhang et al., 2022). T-AOC reflects the overall antioxidant capacity of all antioxidant systems in the body. SOD can reduce oxidative damage to cells by eliminating superoxide free radicals (O2-) (He et al., 2024). CAT helps reduce the accumulation of hydrogen peroxide, preventing oxidative damage to cells (Wang et al., 2022). GSH-S and GSH-PX play crucial roles in clearing peroxides within cells, protecting them from oxidative damage, and maintaining normal cellular function and health (Wang et al., 2024). AKP, ACP, and LZM are essential in the immune system; increased levels of these indicators enhance immune capacity and defense against pathogens (Yi et al., 2018). AKP, ACP, and LZM contribute to breaking down pathogens, damaging bacterial cell walls, and phagocytizing and eliminating harmful foreign substances, thereby boosting immune strength and defense capacity (Wu et al., 2023). MDA is a primary product of mitochondrial peroxidation and generally reflects the state of oxidative stress and health in animals (Feng et al., 2020). The elevation of MDA results in alterations in the biochemical properties of biomolecules and accumulation in aging and disease, thus rendering it as the most dependable substance for assessing oxidative stress (Gao et al., 2022). The overall antioxidant capacity and antioxidant enzymes help protect cells and organisms from oxidative stress by scavenging reactive oxygen species (ROS) (Qi et al., 2024). Previous studies have demonstrated that adding probiotics to the diet can enhance host antioxidant indicators while reducing MDA levels, thereby decreasing oxidative stress and protecting the organism from damage (Zaineldin et al., 2018; Abdel-Latif et al., 2023). Wang et al. (2021) reported that supplementing common carp diets with 5×108 CFU/mL of Lactococcus lactis Z-2 significantly increased serum LZM and AKP activity, enhanced hepatopancreatic antioxidant indices (T-AOC, SOD, CAT, GSH-S, GSH-PX), and reduced MDA levels. Wu et al. (2023) found that supplementing the diet of koi fish (Cyprinus carpio haematopterus) with different levels of compound probiotics, including Lactobacillus plantarum, Lactobacillus acidophilus, and Pseudomonas aeruginosa, significantly increased the activity of AKP, ACP, CAT, and SOD. Similar results were observed in this study, where supplementing the diet with compound lactic acid bacteria enhanced antioxidant and immune indicators while reducing MDA levels. Improved antioxidant and immune capacities strengthen the organism’s defense mechanisms and resistance to pathogens, thereby decreasing disease incidence (Gobi et al., 2016). Enhanced antioxidant capacity not only helps aquatic animals resist external oxidative stress and prevent tissue damage from excessive responses, but also maintains a stable internal environment for the immune system (Tao et al., 2022). This allows aquatic animals to better adapt to environmental pressures and disease challenges (Cao et al., 2019). This synergistic effect is particularly beneficial in aquaculture, as it can reduce disease-related losses, improve survival rates and growth performance, and ultimately increase the yield of aquatic species (Vijayaram et al., 2024).
The intestinal microbiota is an essential barrier for intestinal health and plays a significant role in the host’s growth, metabolism, and digestion and absorption processes (El-Saadony et al., 2021). Factors influencing the composition of intestinal microbiota include age, diet, and environmental changes (Chen et al., 2024). Studies have reported that compound lactic acid bacteria not only promote digestion but also regulate intestinal microbial communities by colonizing the intestine or producing antimicrobial agents (Qi et al., 2024). In the intestinal microbiota of sea urchins, Proteobacteria often dominate (Li et al., 2022b). Proteobacteria encompass a wide variety of species, including harmful bacteria such as Arcobacter and Vibrio, which can cause digestive system inflammation, and beneficial bacteria such as unclassified Rhodobacteraceae, which enhance digestion and metabolism by producing bioactive substances (Luo et al., 2023). In the results of this study, at the genus level, the relative abundance of unclassified Rhodobacteraceae in the intestine was higher in the RM and RH groups compared to the RC (control) group. Previous research has indicated that Rhodobacteraceae, as beneficial bacteria, play a role in enhancing the stability of the intestinal microbiota community in sea urchins (Li et al., 2021). Additionally, during intestinal metabolism, compound lactic acid bacteria produce bioactive substances such as antimicrobial peptides, fatty acids, and small molecules with antibacterial properties (Zhang et al., 2025b). These compounds not only directly inhibit or kill pathogens but also promote the secretion of digestive enzymes in the intestinal lining (Tao et al., 2024). Compound lactic acid bacteria promote growth, aid digestion and nutrient absorption, improve intestinal microbiota, reduce the relative abundance of Arcobacter and Vibrio in the intestine, and enhance immune function. Ren et al. (2022) showed that compound lactic acid bacteria supplementation in the diet of shrimp (L. vannamei) increased microbial diversity in the intestine and indicated that compound lactic acid bacteria can promote the growth of beneficial bacteria, enhancing the organism’s capacity to adapt to bacterial diversity. Similar results were observed in this study, where supplementation with compound lactic acid bacteria in the experimental groups (RM, RH) increased the diversity of intestinal microbiota (e.g., OTUs, Chao, and Ace indices) and reduced the relative abundance of Arcobacter and Vibrio at the genus level. Previous studies have reported that beneficial bacteria, such as lactic acid bacteria and Rhodobacteraceae, can inhibit pathogens by producing short-chain fatty acids, diacetyl, hydrogen peroxide, and bactericidal proteins (Verschuere et al., 2000; Hai, 2015). Moreover, Rhodobacteraceae compete with pathogens for adhesion sites in the intestine, effectively preventing pathogen colonization and enhancing the host’s disease resistance. This competitive mechanism helps maintain intestinal microbial balance and reduces the invasion of harmful microorganisms (Chizhayeva et al., 2022). Results from this study indicate that as the proportion of compound lactic acid bacteria increased, the abundance of Arcobacter and Vibrio at the genus level in the RM and RH groups decreased noticeably compared to the control group (RC). We also found that the reduction in the abundance of Arcobacter and Vibrio corresponded with an increase in growth performance indicators (e.g., FBM, WGR, SGR) in the RM and RH groups. This finding further demonstrates that adding compound lactic acid bacteria can not only reduce the colonization of harmful bacteria (Arcobacter and Vibrio) in the host’s intestine but also enhance the host’s growth performance (Zan et al., 2023). Based on the findings of this study, it can be inferred that compound probiotics can alleviate the stress caused by harmful substances in the environment by reducing the colonization of Arcobacter and Vibrio in the intestine and increasing microbial diversity in the intestinal tract (e.g., OTU, Chao, and Ace indices). Additionally, probiotics can minimize the damage these harmful substances cause to the intestine (Hassaan et al., 2021; Saba et al., 2024).
Probiotics play a role in regulating the immune system by modulating the gene expression associated with oxidative stress, immunity, and inflammation, thereby enhancing both immune and antioxidant indicators (Yang et al., 2015; Zhang et al., 2025a). HSP70 and HSP1 are involved in recognizing and eliminating pathogens, and they protect cells from damage caused by oxidation or stress by regulating their expression levels (Ren et al., 2013). The GPX and GST genes can eliminate organic peroxides to protect the body from ROS damage and can help the body adapt to adverse conditions by enhancing antioxidant capacity (He et al., 2024). The TLR, LYZ, NLR-6, and 185/333-1 genes play important roles in the immune defense of sea urchins (Li et al., 2022a). TLR can recognize pathogen-associated molecules, activating inflammatory responses and the production of antimicrobial peptides (Nguyen et al., 2022). LYZ can lyse bacterial cell walls, directly killing bacteria (Feng et al., 2020). NLR-6 emits stress signals after recognizing pathogens, which can activate immune responses (Liu et al., 2023). The 185/333-1 gene encodes diversified proteins that enhance the recognition and defense capabilities against pathogens (Zhang et al., 2025b). TNF-α and COX-2 jointly regulate immune and inflammatory responses in sea urchins (Li et al., 2022a). TNF-α enhances the sea urchin’s resistance to pathogens by promoting inflammation and apoptosis (El-Kady et al., 2022). COX-2 is increased under inflammatory stimulation and catalyzes the production of prostaglandin E2 (PGE2), promoting vasodilation, leukocyte aggregation, and the release of inflammatory mediators, thereby enhancing local inflammatory responses and improving the organism’s defense (Li et al., 2022a). Together, these two factors enhance the immune defense capability of sea urchins, protecting them from pathogen invasion. In a previous study, Giri et al. (2020) found that supplementing the diet of carp (Cyprinus carpio) with heat-killed Pseudomonas aeruginosa strain VSG2 significantly increased the expression of antioxidant genes (SOD, GPX, CAT). In another study, Büyükdeveci et al. (2023) found that supplementing the diet of Nile tilapia (Oreochromis niloticus) with 1×108 CFU/g of probiotics (Bacillus mojavensis B191 and Bacillus subtilis MRS11) for 60 days significantly enhanced the expression of the HSP70 gene, while simultaneously downregulating the expression of the TNF-α gene. Tao et al. (2022) found that supplementing the diet of largemouth bass with compound probiotics (Bacillus subtilis, Lactobacillus plantarum, and Saccharomyces cerevisiae) for 8 weeks significantly increased the gene expression levels of GPX, GST, and SOD, while downregulating the expression of the TNF-α gene. This study found that supplementing the diet with compound lactic acid bacteria increased the upregulation of oxidative stress and immune gene expression in sea urchins. Specifically, the expression levels of HSP70, HSP1, GPX, GST, TLR, LYZ, NLR-6, and 185/333-1 genes in the RM and RH groups were significantly higher than those in the RC (control) and RL groups. We speculate that this may be due to the activation of the expression of these genes by compound lactic acid bacteria, thereby enhancing the activity of the immune system and improving the organism’s immunity and antioxidant stress resistance. This enables aquatic animals to better cope with external environmental pressures and protects them from pathogen invasion (Saba et al., 2024). Additionally, we observed in this study that the expression levels of COX-2 and TNF-α genes in the intestines of the RM and RH groups were significantly lower than those in the control group (RC group). We speculate that this may be due to the supplementation of compound lactic acid bacteria in the diet, which increases the relative abundance of beneficial microbes (such as Rhodobacteraceae) in the host’s intestine and reduces the colonization of harmful bacteria (such as Vibrio and Arcobacter). This, in turn, leads to a decrease in the expression levels of inflammation-related genes. This further demonstrates that the expression of related genes and the intestinal microbiota are interconnected. Therefore, appropriately supplementing the diet with compound lactic acid bacteria can improve the relative expression of genes related to oxidative stress, immunity, and inflammation in aquatic animals, thereby enhancing the immune capacity and health status of the intermediate sea urchin.
5 Conclusion
This study demonstrates that supplementing the diet of S. intermedius with 1% compound lactic acid bacteria optimally enhances its growth performance, antioxidant capacity, and immune function. At this level, intestinal microbiota richness and stability improved significantly, alongside upregulated expression of genes related to antioxidants, stress response, and immunity. The supplementation also lowered inflammation-related gene expression and reduced FCR, decreasing aquaculture costs. Thus, incorporating 1% compound lactic acid bacteria in S. intermedius diets strengthens immune defenses, boosts overall health, and enhances resistance to environmental pathogens, ultimately benefiting sea urchin aquaculture productivity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Since sea urchins are invertebrates, the ethics committee of Dalian Ocean University did not require the study to undergo review or approval. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YZ: Conceptualization, Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. RZ: Formal analysis, Investigation, Writing – review & editing. YC: Formal analysis, Investigation, Writing – review & editing. ZG: Formal analysis, Writing – review & editing. XM: Formal analysis, Writing – review & editing. YH: Funding acquisition, Project administration, Writing – review & editing. XZ: Funding acquisition, Project administration, Writing – review & editing. TR: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key Research and Development Program of China (No. 2022YFE0117900) and the Project of the Educational Department of Liaoning Province (No. LJKMZ20221121).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Latif H. M. R., Chaklader M. R., Shukry M., Ahmed H. A., Khallaf M. A. (2023). A multispecies probiotic modulates growth, digestive enzymes, immunity, hepatic antioxidant activity, and disease resistance of Pangasianodon hypophthalmus fingerlings. Aquaculture 563, 738948. doi: 10.1016/j.aquaculture.2022.738948
Amenyogbe E., Droepenu E. K., Ayisi C. L., Boamah G. A., Duker R. Q., Abarike E. D., et al. (2024). Impact of probiotics, prebiotics, and synbiotics on digestive enzymes, oxidative stress, and antioxidant defense in fish farming: current insights and future perspectives. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1368436
Büyükdeveci M. E., Cengizler İ., Balcázar J. L., Demirkale İ. (2023). Effects of two host-associated probiotics Bacillus mojavensis B191 and Bacillus subtilis MRS11 on growth performance, intestinal morphology, expression of immune-related genes and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus iniae. Dev. Comp. Immunol. 138, 104553. doi: 10.1016/j.dci.2022.104553
Cao H., Yu R., Zhang Y., Hu B., Jian S., Wen C., et al. (2019). Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze) Aquac. 508, 106–112. doi: 10.1016/j.aquaculture.2019.04.064
Chen Y., Cui S., Wu L., Han Y., Zhao X., Ren T. (2024). Dietary silicate minerals relieving cadmium or lead poisoning in juvenile sea cucumber, Apostichopus japonicus. Mar. Environ. Res. 202, 106795. doi: 10.1016/j.marenvres.2024.106795
Chen D., Jin D., Huang S., Wu J., Xu M., Liu T., et al. (2020). Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 469, 456–467. doi: 10.1016/j.canlet.2019.11.019
Chizhayeva A., Amangeldi A., Oleinikova Y., Alybaeva A., Sadanov A. (2022). Lactic acid bacteria as probiotics in sustainable development of aquaculture. Aquat. Living Resour. 35, 10. doi: 10.1051/alr/2022011
Chumpol S., Kantachote D., Nitoda T., Kanzaki H. (2017). The roles of probiotic purple nonsulfur bacteria to control water quality and prevent acute hepatopancreatic necrosis disease (AHPND) for enhancement growth with higher survival in white shrimp (Litopenaeus vannamei) during cultivation. Aquaculture 473, 327–336. doi: 10.1016/j.aquaculture.2017.02.033
Dawood M. A. O. (2021). Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev. Aquac. 13, 642–663. doi: 10.1111/raq.12492
Di W., Heqiu Y., Gou D., Gong P., Ding J., Chang Y., et al. (2023). Effects of supplementary kelp feeding on the growth, gonad yield, and nutritional and organoleptic quality of subadult sea urchin (Strongylocentrotus intermedius) with soya lecithin intake history. Aquac. Nutr. 2023, 8894923. doi: 10.1155/2023/8894923
El-Kady A. A., Magouz F. I., Mahmoud S. A., Abdel-Rahim M. M. (2022). The effects of some commercial probiotics as water additive on water quality, fish performance, blood biochemical parameters, expression of growth and immune-related genes, and histology of Nile tilapia (Oreochromis niloticus). Aquaculture 546, 737249. doi: 10.1016/j.aquaculture.2021.737249
El-Saadony M. T., Alagawany M., Patra A. K., Kar I., Tiwari R., Dawood M. A. O., et al. (2021). The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 117, 36–52. doi: 10.1016/j.fsi.2021.07.007
Fečkaninová A., Koščová J., Mudroňová D., Popelka P., Toropilová J. (2017). The use of probiotic bacteria against Aeromonas infections in salmonid aquaculture. Aquaculture. 469, 1–8. doi: 10.1016/j.aquaculture.2016.11.042
Feng Z., Song X., Zhao L., Zhu W. (2020). Isolation of probiotics and their effects on growth, antioxidant and non-specific immunity of sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 106, 1087–1094. doi: 10.1016/j.fsi.2020.08.049
Gao Q., Liu B., Shan F., Liu B., Gu Z., Song C., et al. (2022). Effects of oxidized fish oil on digestive enzyme activity and antioxidant system in Macrobrachium rosenbergii post-larvae. Aquac. Rep. 23, 101062. doi: 10.1016/j.aqrep.2022.101062
Giri S. S., Jun J. W., Yun S., Kim H. J., Kim S. G., Kim S. W., et al. (2020). Effects of dietary heat-killed Pseudomonas aeruginosa strain VSG2 on immune functions, antioxidant efficacy, and disease resistance in Cyprinus carpio. Aquaculture 514, 734489. doi: 10.1016/j.aquaculture.2019.734489
Gobi N., Malaikozhundan B., Sekar V., Shanthi S., Vaseeharan B., Jayakumar R., et al. (2016). GFP tagged Vibrio parahaemolyticus Dahv2 infection and the protective effects of the probiotic Bacillus licheniformis Dahb1 on the growth, immune and antioxidant responses in Pangasius hypophthalmus. Fish Shellfish Immunol. 52, 230–238. doi: 10.1016/j.fsi.2016.03.006
Hai N. V. (2015). The use of probiotics in aquaculture. J. Appl. Microbiol. 119, 917–935. doi: 10.1111/jam.12886
Hassaan M. S., El-Sayed A. M. I., Mohammady E. Y., Zaki M. A. A., Elkhyat M. M., Jarmołowicz S., et al. (2021). Eubiotic effect of a dietary potassium diformate (KDF) and probiotic (Lactobacillus acidophilus) on growth, hemato-biochemical indices, antioxidant status and intestinal functional topography of cultured Nile tilapia Oreochromis niloticus fed diet free fishmeal. Aquaculture 533, 736147. doi: 10.1016/j.aquaculture.2020.736147
He W., Liu Y., Zhang W., Zhao Z., Bu X., Sui C., et al. (2024). Effects of dietary supplementation with heat-killed Lactobacillus acidophilus on growth performance, digestive enzyme activity, antioxidant capacity, and inflammatory response of juvenile large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 151, 109651. doi: 10.1016/j.fsi.2024.109651
Hoseinifar S. H., Sun Y.-Z., Wang A., Zhou Z. (2018). Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02429
Incharoen T., Charoensook R., Onoda S., Tatrakoon W., Numthuam S., Pechkong T. (2019). The effects of heat-killed Lactobacillus plantarum L-137 supplementation on growth performance, intestinal morphology, and immune-related gene expression in broiler chickens. Anim. Feed Sci. Technol. 257, 114272. doi: 10.1016/j.anifeedsci.2019.114272
Kuebutornye F. K. A., Abarike E. D., Lu Y. (2019). A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 87, 820–828. doi: 10.1016/j.fsi.2019.02.010
Lee S. J., Lee Y. S., Noh D.-I., Hasan M. T., Hur S. W., Lee S., et al. (2024). Dietary supplementation with host-associated low-temperature potential probiotics improves the growth, immunity, digestive enzyme activity, and intestinal microbial population of olive flounder (Paralichthys olivaceus). Aquac. Rep. 36, 102128. doi: 10.1016/j.aqrep.2024.102128
Li Z., Bao N., Ren T., Han Y., Jiang Z., Bai Z., et al. (2019). The effect of a multi-strain probiotic on growth performance, non-specific immune response, and intestinal health of juvenile turbot, Scophthalmus maximus L. Fish Physiol. Biochem. 45, 1393–1407. doi: 10.1007/s10695-019-00635-4
Li C., Ren Y., Jiang S., Zhou S., Zhao J., Wang R., et al. (2018). Effects of dietary supplementation of four strains of lactic acid bacteria on growth, immune-related response and genes expression of the juvenile sea cucumber Apostichopus japonicus Selenka. Fish Shellfish Immunol. 74, 69–75. doi: 10.1016/j.fsi.2017.12.037
Li M., Tang L., Heqiu Y., Lv D., Ding J., Chang Y., et al. (2022a). Effects of oxidized fish oil on the growth, immune and antioxidant capacity, inflammation-related gene expression, and intestinal microbiota composition of juvenile sea urchin (Strongylocentrotus intermedius). Aquac. Nutr. 2022, 2340308. doi: 10.1155/2022/2340308
Li M., Zhang F., Ding J., Zuo R., Chang Y. (2021). Effects of lipid sources on the growth performance, gonad development, fatty acid profile and transcription of related genes in juvenile sea urchin (Strongylocentrotus intermedius). Aquac. Nutr. 27, 28–38. doi: 10.1111/anu.13162
Li R., Zhang M., Zhou Y., Wei D., Yang Y., Gao D., et al. (2024). Fish-derived lactic acid bacteria supplementation enhanced the immunity and resistance in Crucian carp (Carassius auratus). Aquac. Rep. 36, 102037. doi: 10.1016/j.aqrep.2024.102037
Li Y., Zhao Y., Liu X., Yuan L., Liu X., Wang L., et al. (2022b). Effects of endogenous potential probiotic Lactobacillus rhamnosus M2-4 on intestinal microflora and metabonomics in juvenile sea cucumber Apostichopus japonicus. Aquaculture 555, 738247. doi: 10.1016/j.aquaculture.2022.738247
Liu L., Liu Y., Qin G., Wei C., Li Y., Cui L., et al. (2024). Evaluation of regulatory capacity of three lactic acid bacteria on the growth performance, non-specific immunity, and intestinal microbiota of the sea cucumber Apostichopus japonicus. Aquaculture 579, 740156. doi: 10.1016/j.aquaculture.2023.740156
Liu Y., Sheng X., Tang X., Xing J., Chi H., Zhan W. (2023). Genome-wide identification, phylogenetic relationships and expression patterns of the NOD-like receptor (NLR) gene family in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 141, 109083. doi: 10.1016/j.fsi.2023.109083
Lourenço S., José R., Andrade C., Valente L. M. P. (2020). Growth performance and gonad yield of sea urchin Paracentrotus lividus (Lamarck 1816) fed with diets of increasing protein: energy ratios. Anim. Feed Sci. Technol. 270, 114690. doi: 10.1016/j.anifeedsci.2020.114690
Luo K., Liu Y., Qin G., Wang S., Wei C., Pan M., et al. (2023). A comparative study on effects of dietary three strains of lactic acid bacteria on the growth performance, immune responses, disease resistance and intestinal microbiota of Pacific white shrimp, Penaeus vannamei. Fish Shellfish Immunol. 136, 108707. doi: 10.1016/j.fsi.2023.108707
Ma C.-W., Cho Y.-S., Oh K.-H. (2009). Removal of pathogenic bacteria and nitrogens by Lactobacillus spp. JK-8 and JK-11. Aquaculture 287, 266–270. doi: 10.1016/j.aquaculture.2008.10.061
Meng X., Wu S., Hu W., Zhu Z., Yang G., Zhang Y., et al. (2021). Clostridium butyricum improves immune responses and remodels the intestinal microbiota of common carp (Cyprinus carpio L.). Aquaculture 530, 735753. doi: 10.1016/j.aquaculture.2020.735753
Mohammadi G., Rafiee G., Tavabe K. R., Abdel-Latif H. M. R., Dawood M. A. O. (2021). The enrichment of diet with beneficial bacteria (single- or multi- strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 539, 736640. doi: 10.1016/j.aquaculture.2021.736640
Nguyen T. P., Nguyen B. T., Nan F.-H., Lee M.-C., Lee P.-T. (2022). TLR23, a fish-specific TLR, recruits MyD88 and TRIF to activate expression of a range of effectors in melanomacrophages in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 126, 34–46. doi: 10.1016/j.fsi.2022.05.032
Ning Y., Zhang F., Tang L., Song J., Ding J., Chang Y., et al. (2022). Effects of dietary lipid sources on the growth, gonad development, nutritional and organoleptic quality, transcription of fatty acid synthesis related genes and antioxidant capacity during cold storage in adult sea urchin (Strongylocentrotus intermedius). Aquaculture 548, 737688. doi: 10.1016/j.aquaculture.2021.737688
Peng Z., Yan L., Wei L., Gao X., Shi L., Ren T., et al. (2022). Effect of dietary chicken gut meal levels on growth performance, plasma biochemical parameters, digestive ability and fillet quality of Cyprinus carpio. Aquac. Rep. 24, 101183. doi: 10.1016/j.aqrep.2022.101183
Pillinger M., Weber B., Standen B., Schmid M. C., Kesselring J. C. (2022). Multi-strain probiotics show increased protection of intestinal epithelial cells against pathogens in rainbow trout (Oncorhynchus mykiss). Aquaculture 560, 738487. doi: 10.1016/j.aquaculture.2022.738487
Qi X., Xue M., Shi K., Wang G., Ling F. (2024). Evaluating Pseudomonas monteilii JK-1 as an in-feed probiotic: Enhancing growth, immune-antioxidant, disease resistance and modulating gut microflora composition in grass carp (Ctenopharyngodon idella). Aquaculture 585, 740715. doi: 10.1016/j.aquaculture.2024.740715
Ren X., Han Y., Zeng F., Rabbi M. H., Li Z., Cui S., et al. (2022). Effects of dietary heat-killed Lactobacillus plantarum and compound probiotics on the growth performance, antioxidative capacity, intestinal morphology and microbiota of Pacific white shrimp Litopenaeus vannamei. Aquac. Res. 53, 3516–3530. doi: 10.1111/are.15857
Ren P., Xu L., Yang Y., He S., Liu W., Ringø E., et al. (2013). Lactobacillus planarum subsp. plantarum JCM 1149 vs. Aeromonas hydrophila NJ-1 in the anterior intestine and posterior intestine of hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂: An ex vivo study. Fish Shellfish Immunol. 35, 146–153. doi: 10.1016/j.fsi.2013.04.023
Ringø E., Van Doan H., Lee S. H., Soltani M., Hoseinifar S. H., Harikrishnan R., et al. (2020). Probiotics, lactic acid bacteria and bacilli: interesting supplementation for aquaculture. J. Appl. Microbiol. 129, 116–136. doi: 10.1111/jam.14628
Saba A. O., Yasin I. S. M., Azmai M. N. A. (2024). Meta-analyses indicate that dietary probiotics significantly improve growth, immune response, and disease resistance in tilapia. Aquac. Int. 32, 4841–4867. doi: 10.1007/s10499-024-01404-8
Saravanan K., Sivaramakrishnan T., Praveenraj J., Kiruba-Sankar R., Haridas H., Kumar S., et al. (2021). Effects of single and multi-strain probiotics on the growth, hemato-immunological, enzymatic activity, gut morphology and disease resistance in Rohu, Labeo rohita. Aquaculture 540, 736749. doi: 10.1016/j.aquaculture.2021.736749
Tao L.-t., Lu H., Xiong J., Zhang L., Sun W.-W., Shan X.-F. (2024). The application and potential of postbiotics as sustainable feed additives in aquaculture. Aquaculture 592, 741237. doi: 10.1016/j.aquaculture.2024.741237
Tao J., Wang S., Qiu H., Xie R., Zhang H., Chen N., et al. (2022). Modulation of growth performance, antioxidant capacity, non-specific immunity and disease resistance in largemouth bass (Micropterus salmoides) upon compound probiotic cultures inclusion. Fish Shellfish Immunol. 127, 804–812. doi: 10.1016/j.fsi.2022.07.031
Torres-Maravilla E., Parra M., Maisey K., Vargas R. A., Cabezas-Cruz A., Gonzalez A., et al. (2024). Importance of probiotics in fish aquaculture: towards the identification and design of novel probiotics. Microorganisms 12, 626. doi: 10.3390/microorganisms12030626
Verschuere L., Rombaut G., Sorgeloos P., Verstraete W. (2000). Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 64, 655–671. doi: 10.1128/mmbr.64.4.655-671.2000
Vijayaram S., Chou C.-C., Razafindralambo H., Ghafarifarsani H., Divsalar E., Van Doan H. (2024). Bacillus sp. as potential probiotics for use in tilapia fish farming aquaculture - A review. Ann. Anim. Sci. 24 (4), 995–1006. doi: 10.2478/aoas-2024-0031
Wang J., Feng J., Liu S., Cai Z., Song D., Yang L., et al. (2021). The probiotic properties of different preparations using Lactococcus lactis Z-2 on intestinal tract, blood and hepatopancreas in Cyprinus carpio. Aquaculture 543, 736911. doi: 10.1016/j.aquaculture.2021.736911
Wang Y.-C., Hu S.-Y., Chiu C.-S., Liu C.-H. (2019). Multiple-strain probiotics appear to be more effective in improving the growth performance and health status of white shrimp, Litopenaeus vannamei, than single probiotic strains. Fish Shellfish Immunol. 84, 1050–1058. doi: 10.1016/j.fsi.2018.11.017
Wang M., Lv C., Chen Y., Bi X., Yang D., Zhao J. (2022). Effects of the potential probiotic Bacillus subtilis D1-2 on growth, digestion, immunity and intestinal flora in juvenile sea cucumber, Apostichopus japonicus. Fish Shellfish Immunol. 124, 12–20. doi: 10.1016/j.fsi.2022.03.043
Wang Y., Peng Z., Yan L., Gao X., Wu L., Cui S., et al. (2024). Effects of dietary glutamine supplementation on growth performance, intestinal digestive ability, antioxidant status and hepatic lipid accumulation in Xenocypris davidi (Bleeker 1871). Aquac. Int. 32, 725–743. doi: 10.21203/rs.3.rs-2975524/v1
Wu L., Wang L., Cui S., Peng Z., Liu Z., Li M., et al. (2023). Effects of dietary compound probiotics and heat-killed compound probiotics on antioxidative capacity, plasma biochemical parameters, intestinal morphology, and microbiota of Cyprinus carpio haematopterus. Aquac. Int. 31, 2199–2219. doi: 10.1007/s10499-023-01080-0
Yang G., Tian X., Dong S., Peng M., Wang D. (2015). Effects of dietary Bacillus cereus G19, B. cereus BC-01, and Paracoccus marcusii DB11 supplementation on the growth, immune response, and expression of immune-related genes in coelomocytes and intestine of the sea cucumber (Apostichopus japonicus Selenka). Fish Shellfish Immunol. 45, 800–807. doi: 10.1016/j.fsi.2015.05.032
Yi Y., Zhang Z., Zhao F., Liu H., Yu L., Zha J., et al. (2018). Probiotic potential of Bacillus velezensis JW: Antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish Shellfish Immunol. 78, 322–330. doi: 10.1016/j.fsi.2018.04.055
Zaineldin A. I., Hegazi S., Koshio S., Ishikawa M., Bakr A., El-Keredy A. M. S., et al. (2018). Bacillus subtilis as probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol. 79, 303–312. doi: 10.1016/j.fsi.2018.05.035
Zan Z., Chen K., Wang H., Han Z., Sun J. (2023). Effects of a multistrain probiotic on the growth, immune function and intestinal microbiota of the tongue sole Cynoglossus semilaevis. Aquaculture 575, 739813. doi: 10.1016/j.aquaculture.2023.739813
Zhang Y., Chen Y., Guo Z., Zhang R., Guo J., Wang F., et al. (2025a). Effects of a dietary probiotic on growth, gonadal development and quality, antioxidant capacity, intestinal health, and non-specific immunity of a sea urchin (Strongylocentrotus intermedius). Anim. Feed Sci. Technol. 319, 116184. doi: 10.1016/j.anifeedsci.2024.116184
Zhang Y., Ji T., Jiang Y., Zheng C., Yang H., Liu Q. (2022). Long-term effects of three compound probiotics on water quality, growth performances, microbiota distributions and resistance to Aeromonas veronii in crucian carp Carassius auratus gibelio. Fish Shellfish Immunol. 120, 233–241. doi: 10.1016/j.fsi.2021.11.036
Zhang R., Zhang Y., Chen Y., Zhang Y., Guo J., Zhao X., et al. (2024). Effects of composite lactic acid bacteria on the growth, intestinal physiology, and non-specific immunity of sea cucumber (Apostichopus japonicus). Aquac. Int. 33, 52. doi: 10.1007/s10499-024-01681-3
Zhang Y., Zhang R., Guo Z., Chen Y., Meng X., Han Y., et al. (2025b). Effects of Rhodopseudomonas palustris and composite probiotics on growth performance, intestinal health, and non-specific immunity of sea urchin (Strongylocentrotus intermedius). Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 276, 111058. doi: 10.1016/j.cbpb.2024.111058
Zuo R., Hou S., Wu F., Song J., Zhang W., Zhao C., et al. (2017). Higher dietary protein increases growth performance, anti-oxidative enzymes activity, and transcription of heat shock protein 70 in the juvenile sea urchin (Strongylocentrotus intermedius) under heat stress. Aquaculture and Fisheries. 2 (1), 18–23. doi: 10.1016/j.aaf.2017.01.005
Zuo R., Ning Y., Di W., Heqiu Y., Song J., Ding J., et al. (2022). Effects of dietary lipid sources on the growth, gonad development, fatty acid composition and spawning performance of broodstock, and early larvae quality of sea urchin (Strongylocentrotus intermedius). Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.927116
Keywords: echinoid, Strongylocentrotus intermedius, probiotics, intestinal microbiota, immunity
Citation: Zhang Y, Zhang R, Chen Y, Guo Z, Meng X, Han Y, Zhao X and Ren T (2025) Growth performance, intestinal health, and non-specific immunity were significantly affected by feeding different compound lactic acid bacteria supplementation in sea urchin (Strongylocentrotus intermedius). Front. Mar. Sci. 12:1525330. doi: 10.3389/fmars.2025.1525330
Received: 09 November 2024; Accepted: 03 January 2025;
Published: 20 January 2025.
Edited by:
Jin Niu, Sun Yat-sen University, ChinaReviewed by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaYun Wang, Chinese Academy of Fishery Sciences (CAFS), China
Copyright © 2025 Zhang, Zhang, Chen, Guo, Meng, Han, Zhao and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tongjun Ren, dG9uZ2p1bnJlbkBkbG91LmVkdS5jbg==
 Yuntian Zhang
Yuntian Zhang Rongwei Zhang1,2
Rongwei Zhang1,2 Yi Chen
Yi Chen Yuzhe Han
Yuzhe Han Tongjun Ren
Tongjun Ren