
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 27 January 2025
Sec. Aquatic Physiology
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1522448
This article is part of the Research Topic Physiological Regulation in Species Infections: Investigating Pathogen-Host Dynamics and Stress Responses in Aquatic Organisms View all articles
As the main pathogen causing growth retardation, EHP is considered to be mainly parasitic in the hepatopancreas of shrimp. However, the intestines of shrimp infected with EHP frequently exhibit syndromes such as jejunum and white midgut. Therefore, the challenge experiment was carried out in this study to compare the differences in intestinal histology, digestion and absorption, immune defense and oxidative stress of P. vannamei between the control group and EHP infection group. Histological analysis showed that EHP infection significantly damaged the intestine of the shrimp, including intestinal villus rupture and outer membrane impairment. Concurrently, EHP infection can trigger intestinal immune response, and the expression of key immune genes like Toll, myeloid differentiation factor, anti-lipopolysaccharide factor, and Relish was significantly enhanced, while the expression of IMD and alkaline phosphatase was suppressed. Additionally, antioxidant genes manganese superoxide dismutase, catalase, glutathione peroxidase, glutathione-S-transferase, and nuclear factor E2-related factor 2 were up-regulated to varying extents in EHP infection group, and the contents of lipid peroxides and malondialdehyde were heavily accumulated. Moreover, the expression levels of key genes involved in nutrient absorption, transport and synthesis, such as glucose transporter 1, Na+-K+ATPase, fatty acid synthase, acetyl-CoA carboxylase, rapamycin kinase, mTOR regulation-related protein, eukaryotic translation initiation factor 4E binding protein, ribosomal protein S6 kinase, were significantly up-regulated. However, the activities of amylase, lipase, and trypsin were inhibited in EHP infection group throughout the experiment. In summary, EHP infection damaged the intestine of P. vannamei, accompanied by immune response and oxidative stress. At the same time, nutrient transport and synthesis pathways were activated, while digestive enzyme activities were inhibited, indicating that in order to maintain survival, shrimps must accelerate material transport. Unfortunately, it remains in a state of nutrient deficiency that ultimately affects growth.
Penaeus vannamei is the preferred species for shrimp culture because of it has the advantages of stress resistance and rapid growth. However, due to the lack of effective methods for disease prevention and control, infectious diseases have become the greatest constraint to the development of large-scale breeding (Thitamadee et al., 2016). Enterocytozoon hepatopenaei (EHP) belongs to the microsporidian family and proliferates by parasitizing in the host. Studies have shown that EHP has a wide range of vectors in nature, including dragonfly nymphs, polychaetes, and false mussels (Kumar Dewangan et al., 2023; Wan Sajiri et al., 2023). Furthermore, EHP has resulted in significant production losses of 0.77 million tons and economic losses of US$ 567.62 million in the India shrimp farming industry (Patil et al., 2021). Shinn et al. (2018) estimated that the economic losses due to EHP in Thailand were approximately US$ 232 million. This indicates that the prevention and control of EHP is imperative.
The intestine, known as the “second brain”, is an important hub for digesting and absorbing substances as well as immune defense. Importantly, the membrane structure of the intestine is the first line of defense against the invasion of pathogenic microorganisms. Once the pathogen successfully breaks through this barrier, it will subsequently trigger a severe inflammatory response in the host (Zhang et al., 2014).Consequently, the structural integrity of the membrane is of utmost importance for material absorption, immune defense, as well as the growth and development of the individual. The intestine of the shrimp possesses an innate immune defense mechanism. Empirical research has indicated that the expression of key immune genes in the shrimp intestine is markedly altered after WSSV infection (Hui et al., 2019). Similarly, anti-lipopolysaccharide factor (ALF) and crustin expression levels were observed to be significantly elevated in the foregut after infection with Vibrio harveyi in Penaeus monodon, and their expression was also significantly upregulated when the pathogen invaded the midgut, suggesting that these genes may contribute to the local immune response in the intestine (Soonthornchai et al., 2010).
Furthermore, the dense intestinal villi, diverse types of transporters and digestive enzymes present in the intestine exert a crucial role in facilitating the transport and digestion of substances, and the invasion of pathogens has the potential to disrupt the function of the intestine (Jiao et al., 2020; Wu et al., 2022). It has been reported that EHP induced bacterial colony imbalance in the intestine of shrimp, with a notable increase in pathogenic bacteria and a marked decrease in dominant bacteria (Shen et al., 2022; Subash et al., 2023; Li et al., 2024). For instance, the proliferation of Vibrio and rod-shaped bacteria in the intestine triggers a stress response and activates the immune system, thereby inducing an interaction between these pathogens that poses challenges to the shrimp (Alfiansah et al., 2020; Huang et al., 2020). These studies indicate that the normal operation of the intestine serves as a significant criterion in ascertaining whether an organism can keep in good health over the long term. It is agreed that the target organ of EHP is the hepatopancreas of shrimp, and as such, a considerable number of studies have focused on the hepatopancreas of shrimp (Aranguren et al., 2017; Santhoshkumar et al., 2017). However, EHP-infected shrimp often exhibit intestinal damage, such as jejunum and intestinal whiteness, and some researchers, including our group, have detected EHP spores in the white feces of EHP-infected shrimp (Cao et al., 2023; Subash et al., 2023). This suggests that the damage caused by EHP is extensive and not merely confined to the hepatopancreas. However, the direct effects of EHP on the intestine of shrimp have rarely been reported.
Accordingly, this study explored the physiological changes and immune responses in the intestine of shrimp infected with EHP, including intestinal histology, digestion and absorption, immune defense and oxidative stress, which will provide a new perspective for disease prevention and control of EHP.
P. vannamei (4.0 ± 0.5 g) were purchased from Qingdao, Shandong Province, and all were specific pathogen-free (SPF) shrimp. To acclimatize the shrimp to the laboratory environment, the shrimp were temporarily cultivated in glass tanks (L: 120 cm, W: 50 cm, H: 80 cm) for 10 d (days) and fed pelleted feed (Tongwei, Shandong) at 5% of body weight three times a day. During this period, shrimp were randomly sampled for pathogen detection using conventional PCR. The culture environment was set to a temperature of 26°C ± 0.3°C, a salinity of 19‰, a pH 7.5 ± 0.2, and half of the fresh seawater was replaced every 2 d while maintaining an adequate oxygen supply. All procedures were in accordance with the guidelines of the respective Animal Research and Ethics Committees of Ludong University and did not involve endangered or protected species.
For the challenge experiment, healthy shrimps were divided into the EHP infection group and control group (n = 30, each replicate group contained 30 shrimps). The EHP infection group was fed with fresh hepatopancreas of EHP-infected shrimp (EHP copies = 105 copies/ng DNA) for 3 d to keep the shrimp satiated. The control group was fed with fresh hepatopancreas of healthy shrimp for 3 d to keep the shrimp satiated. After 3 d, both the EHP group and control group were fed with normal commercial feed (TongWei, Shandong), and the growth status of the shrimp was monitored. The sampling timeline is shown in Figure 1A, and “-10d” represents the acclimation time. Specifically, samples were gathered from the EHP infection group and control group at the 0th (healthy), 5th, 10th and 20th days post-challenge (dpc). These samples were hepatopancreas and intestines of shrimp, which were then stored in an ultra-low temperature (-80°C) environment for subsequent experiments.
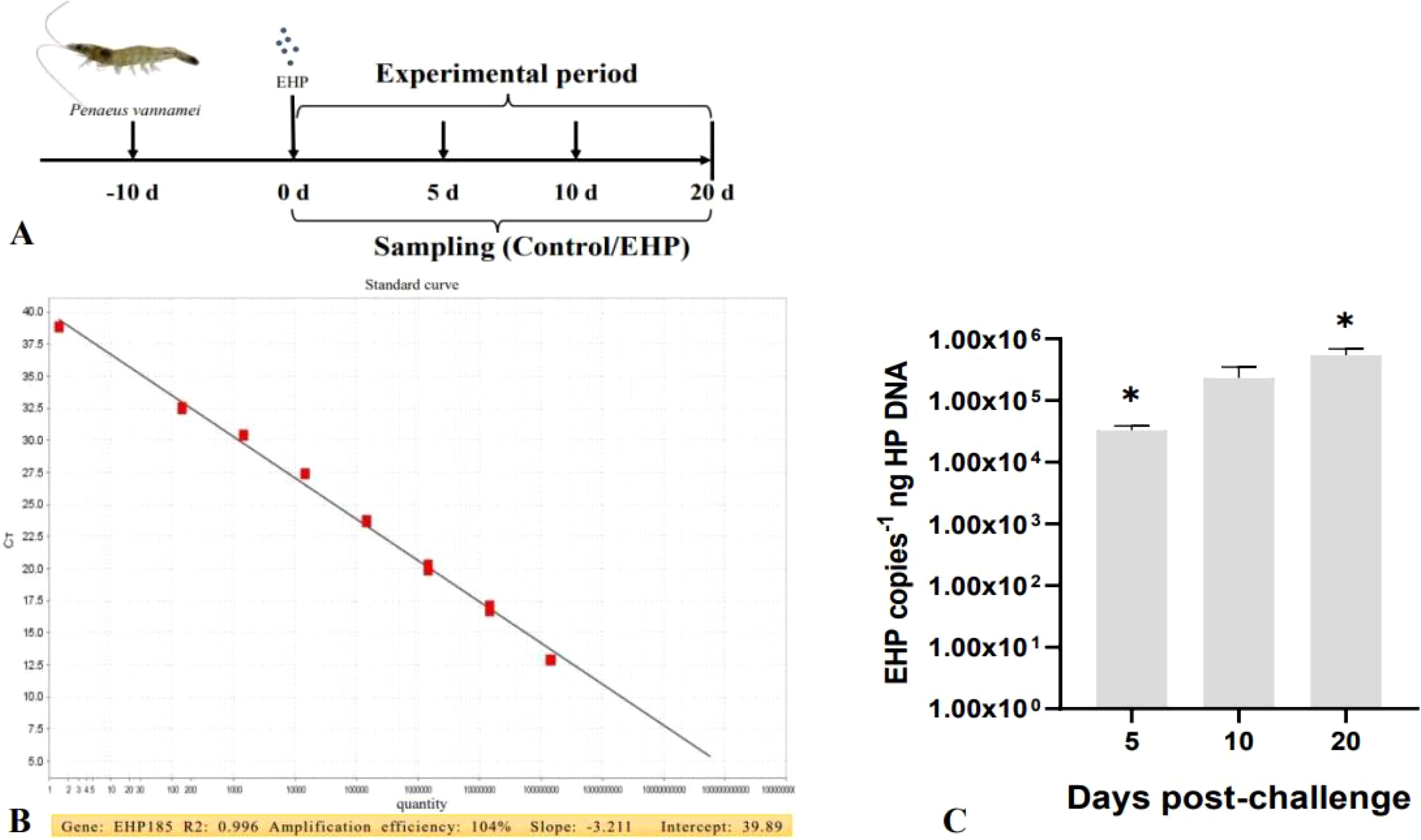
Figure 1. The challenge experiment of P. vannamei infected by EHP (A); The standard curve of EHP 185 (B); EHP load of shrimp in the EHP infection group on the 5th, 10th and 20th dpc (C). The superscript symbol * depicts the statistical significance at p < 0.05.
Total DNA from the hepatopancreas of shrimp was extracted in accordance with the instructions of the Marine Animal Genomic DNA Extraction Kit (DP324, TIANGEN, Beijing). The EHP load was quantified by qPCR. The specific primers EHP185 (F: 5’-GTAGCGGAACGGATAGGG-3’, R: 5’-CCAGCATTGTCGGCATAG-3’) and the reaction procedures used for qPCR were referred to Liu et al. (2016). Concurrently, the constructed recombinant plasmids were subjected to serial gradient dilutions, and standard curves were established.
The mid-intestine of shrimp with diverse infection time was rapidly isolated using a sterile scalpel and placed in Davidson's AFA fixative for 16 h. The tissue was dehydrated until transparent, and then the intestinal tissue was embedded vertically and sliced (4 μm). Ultimately, it was observed by an light microscope (Olympus, IX 73 SC).
The total RNA extraction and cDNA synthesis of the intestine were performed according to the previous work (Cao et al., 2023). The expression levels of related genes in the intestine of the EHP group and control group were analyzed by qPCR (ABI 7500 Applied Biosystems, USA), with 18S rRNA gene and β-actin gene being used as housekeeping genes. It mainly contained nine immune-related genes Toll, myeloid differentiation factor (Myd88), interleukin-16 (IL-16), ALF, Immune deficiency (IMD), Relish, Acid phosphatase (ACP), alkaline phosphatase (ALP) and Crustin A, and five antioxidant genes manganese superoxide dismutase (MnSOD), catalase (CAT), glutathione peroxidase (GPX), glutathione-S-transferases (GST) and nuclear factor E2-related factor 2 (Nrf2), as well as eight substance transport and synthesis related genes glucose transporter 1 (GLUT-1), Na+-K+ ATPase, fat synthesis key genes fat synthase (FAS), acetyl coenzyme carboxylase A (ACC) rapamycin target protein (TOR), Raptor, eukaryotic translation initiation factor 4E binding protein (4EBP) and ribosomal protein S6 kinase (S6K). The relative expression levels of the genes were analyzed by comparative Ct methods (2-ΔΔCT). The primers for the genes in the qPCR experiments were designed using the Primer 5.0 software, as shown in Table 1. The reaction procedure and reaction system for qPCR are shown in Table 2.
The contents of lipid peroxide (LPO) and malondialdehyde (MDA) in the intestines of the EHP group and control group were measured by specific kits (No. A160-1, No. A003-1, Jiancheng Bioengineering Nanjing). The determination procedures were performed according to the instructions (Cao et al., 2023).
The activities of amylase (AMS), trypsin, and lipase (LPS) in the intestines of the EHP group and control group were measured, respectively. The above indicators were detected by specific kits according to the instructions (C016-1-1, A080-2, A054-1-1, Jiancheng Bioengineering Nanjing).
Data analysis was performed by student’s t-test using SPSS17.0 to determine whether there were significant differences between the groups. p < 0.05 was considered statistically significant. Asterisk and the double asterisk represent the significant difference and the extremely significant difference, respectively, and “ns” indicates no significant difference. All data are presented as mean ± SE.
The PCR amplification product, which was 185 bp, was first obtained. Following gel recovery and sequencing comparison, the target product was cloned. Subsequently, the plasmid was extracted to prepare the recombinant plasmid DNA. The plasmid standard template was amplified well in the range of 1.44 × 100 ~ 1.44 × 108. The obtained standard curve had an R2 = 0.996, amplification efficiency = 104%, slope = -3.211, and intercept = 39.89, which fulfilled the quantitative requirements (Figure 1B). The qPCR results showed that all the control groups were negative. In contrast, the EHP load of shrimp in the EHP group was 3.30 × 104 ± 6.19 × 103copies-1 /ng DNA on the 5th dpc, then 2.34 × 105 ± 1.25 × 105 copies-1 ng DNA on the 10th dpc, and the peak value was reached on the 20th dpc at 5.42 × 105 ± 1.51 × 105 copies-1 ng DNA (Figure 1C).
The microscopic results disclosed that the intestinal structure of healthy shrimp was intact, presenting a regular circular cavity. Moreover, the intestinal villi were tightly arranged and firmly adhered to the muscular layer (Figure 2A). However, on the 5th dpc, there were noticeable breaks and detachment in the intestinal villi from the muscular layer. (Figure 2B). On the 10th and 20th dpc, the intestinal villi were even more severely detached from the muscular layer with adhesions, and the intestine present severe damage (Figures 2C, D).
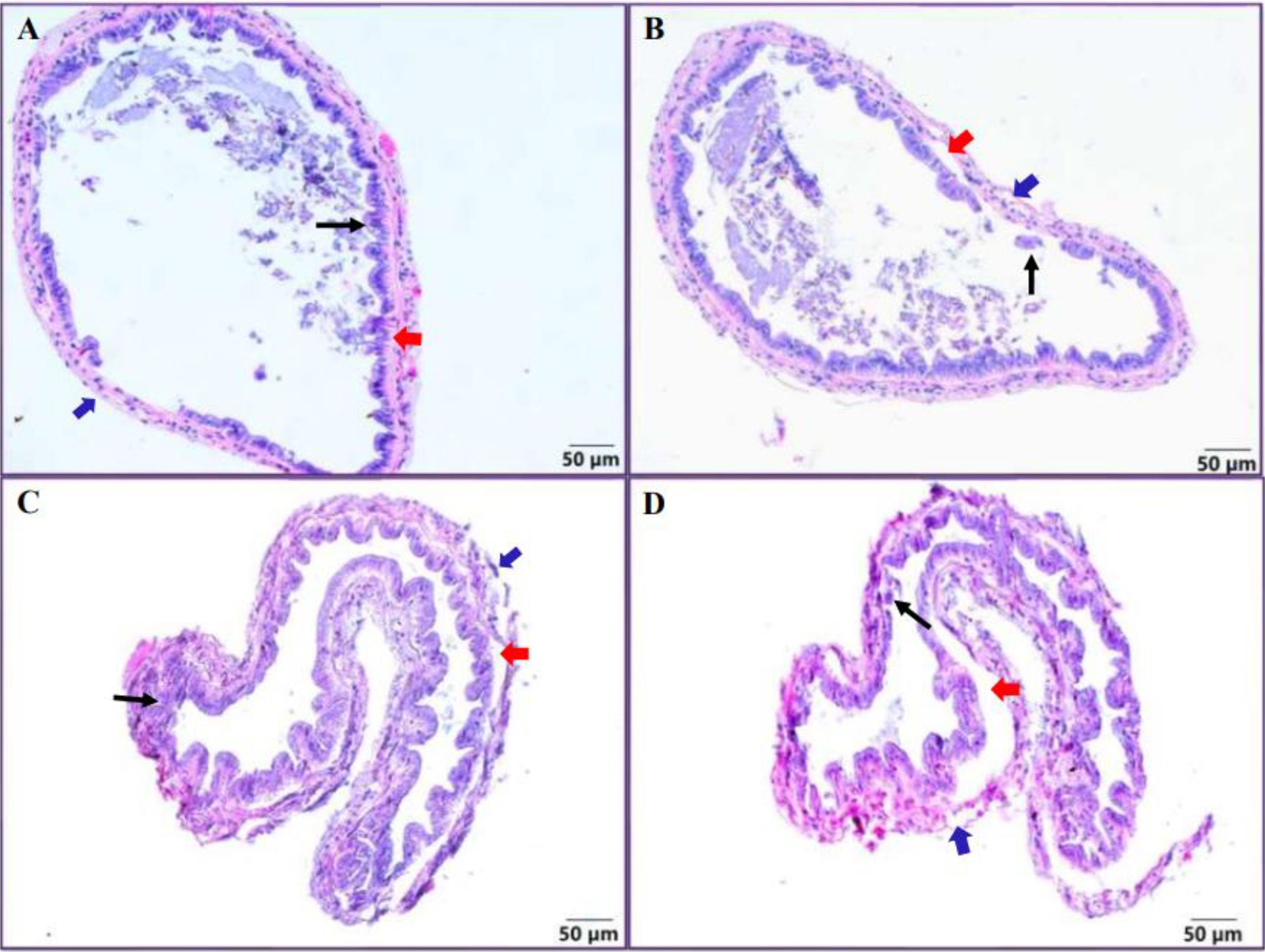
Figure 2. Histology of shrimp intestine. The intestine of the control group (A), intestinal structure of shrimp infected with EHP on the 5th (B), 10th (C) and 20th (D) dpc. The blue arrow indicates the outer membrane of the intestine, the red arrow indicates the broken muscularis mucosae, and the black arrow indicates the broken intestinal villi.
qPCR results showed that the expression levels of immune-related genes Toll, Myd88, ALF, and Relish were significantly increased at all sampling points compared with the control group (Figures 3A, B, D, F, p < 0.05). Furthermore, the expression levels of ACP and Crustin A had significant rises at 10th and 20th dpc (Figures 3G, I, p < 0.05). However, IL-16 showed a significant difference only on the 10th dpc, yet the expression level displayed an overall upward trend (Figure 3C). In contrast, the expression levels of IMD and ALP were notably repressed (Figures 3E, H, p < 0.05). In the field of oxidative stress, the expression level of the antioxidant transcription factor Nrf2 was significantly up-regulated with a large change (Figure 4A, p < 0.05), while SOD showed a significant increase on the 5th and 10th dpc, but presented a downward trend as a whole (Figure 4B, p < 0.05). The expression of GST was significantly suppressed in the early stage (Figure 4E, p < 0.05). GPX was significantly up-regulated on the 5th and 20th dpc (Figure 4C, p < 0.05), whereas CAT appeared to be significantly up-regulated only on the 20th dpc (Figure 4D, p < 0.05), with no significant changes in either on the 10th day.
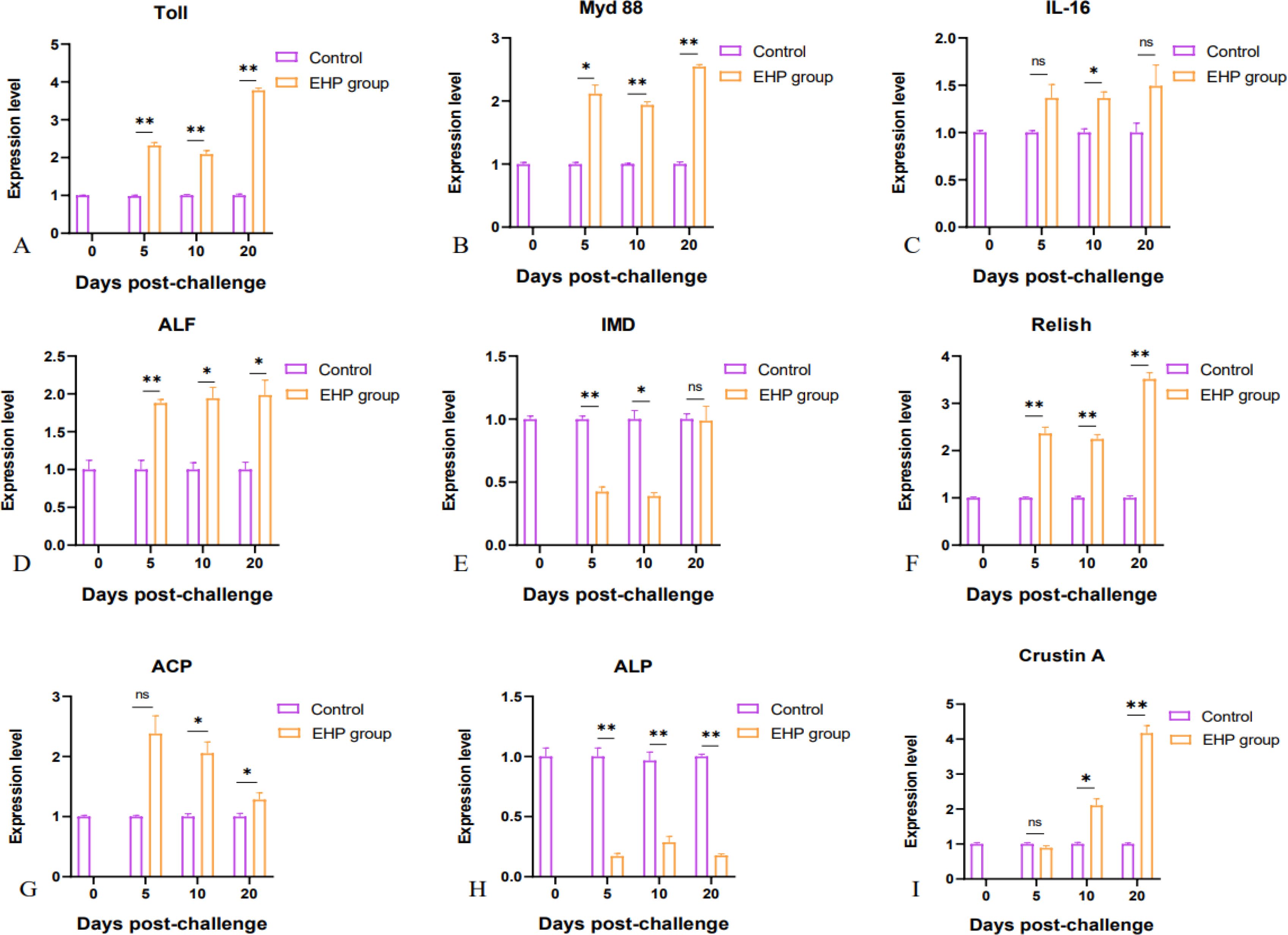
Figure 3. Relative expression levels of immune-related genes in the intestine of shrimp after EHP infection (mean ± SE, n = 6). Immune-related genes: Toll, Myd88, IL-16, ALF, IMD, Relish, ACP, ALP and Crustin A (A–I). The superscript symbol * depicts the statistical significance at p < 0.05, and ** depicts the statistical significance at p < 0.01. ns, not significant.
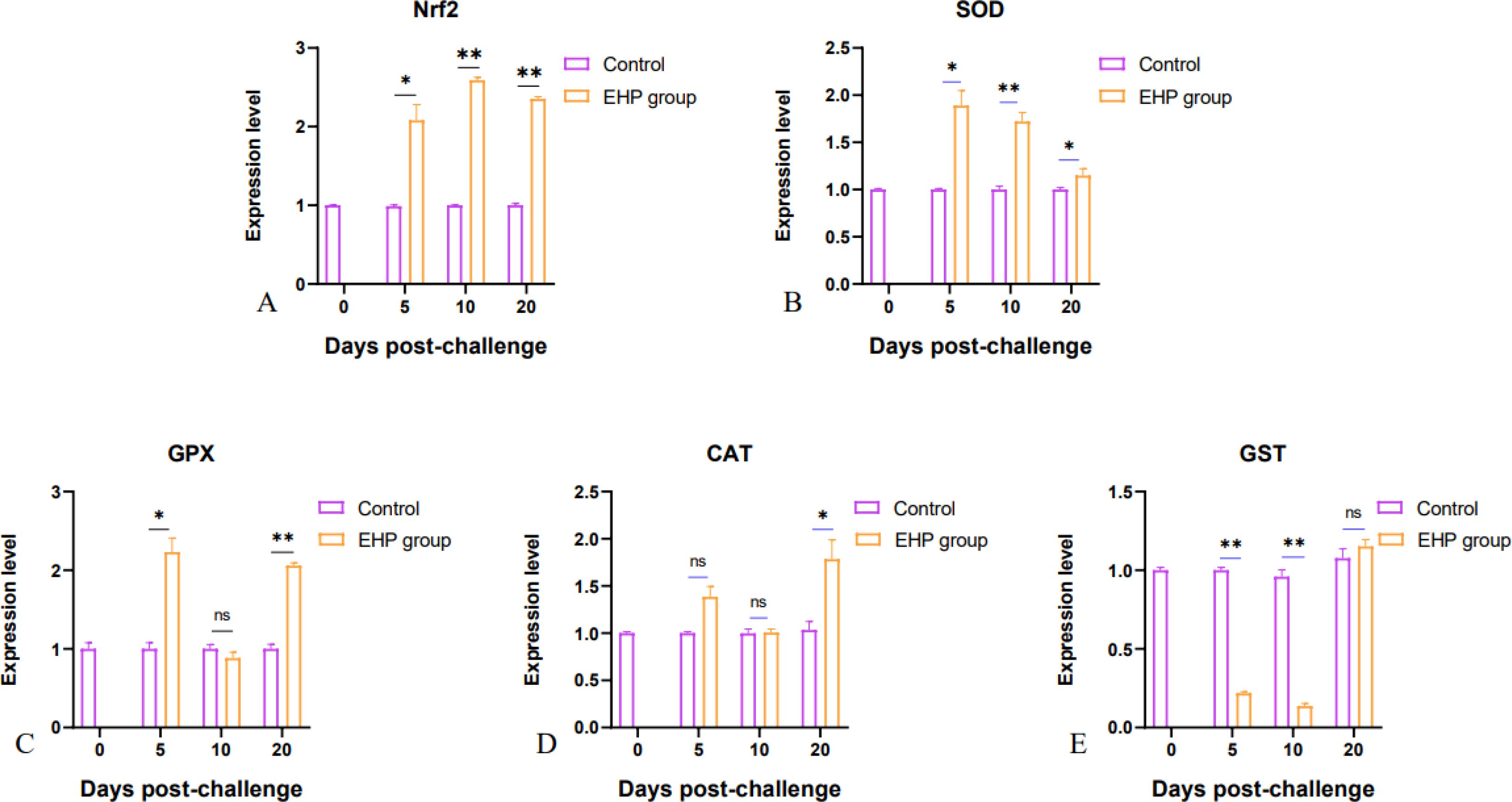
Figure 4. Relative expression levels of antioxidant genes Nrf2 (A), SOD (B), GPX (C), CAT (D) and GST (E) in the intestine of shrimp after EHP infection (mean ± SE, n = 6). The superscript symbol * depicts the statistical significance at p < 0.05, and ** depicts the statistical significance at p < 0.01. ns, not significant.
Meanwhile, qPCR results revealed that the expression levels of genes related to substance transport and synthesis exhibited an overall upward trend. The expression of GLUT-1 and Na+-K+ATPase in the intestine of EHP-infected shrimp was relatively elevated, but Na+-K+ATPase was significantly inhibited on the 20th dpc (Figures 5A, B, p < 0.05). Compared with the control group, the fat synthesis key genes FAS and ACC, and protein synthesis key genes TOR, Raptor, 4EBP and S6K, were significantly up-regulated and changed greatly, among which the expression of 4EBP and S6K was significantly up-regulated throughout the experiment (Figures 5C–H, p < 0.05).
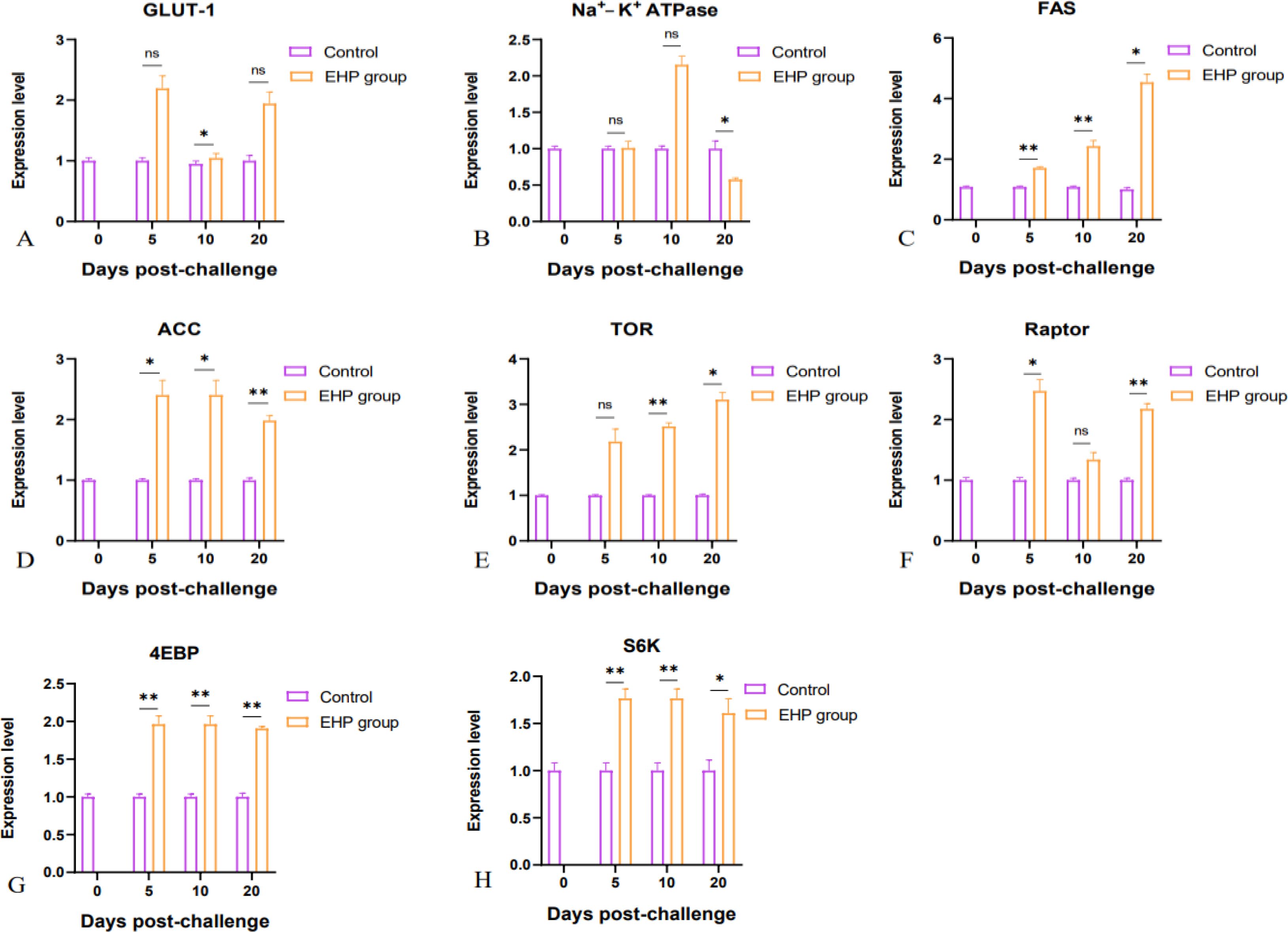
Figure 5. Relative expression levels of substance transport and synthesis related genes GLUT-1 (A), Na+-K+ATPase (B), FAS (C), ACC (D), TOR (E), Raptor (F), 4EBP (G) and S6K (H) in the intestine of shrimp after EHP infection (mean ± SE, n = 6). The superscript symbol * depicts the statistical significance at p < 0.05, and ** depicts the statistical significance at p < 0.01. ns, not significant.
The results of LPO and MDA content determination indicated that the LPO content in the intestine of the EHP group showed a gradual increase and the difference was significant at all sampling points (Figure 6A, p < 0.05). The content of MDA also increased with the extension of time, especially at 20dpc, but only has the remarkable difference on the 5th and 10th dpc (Figure 6B, p < 0.05).
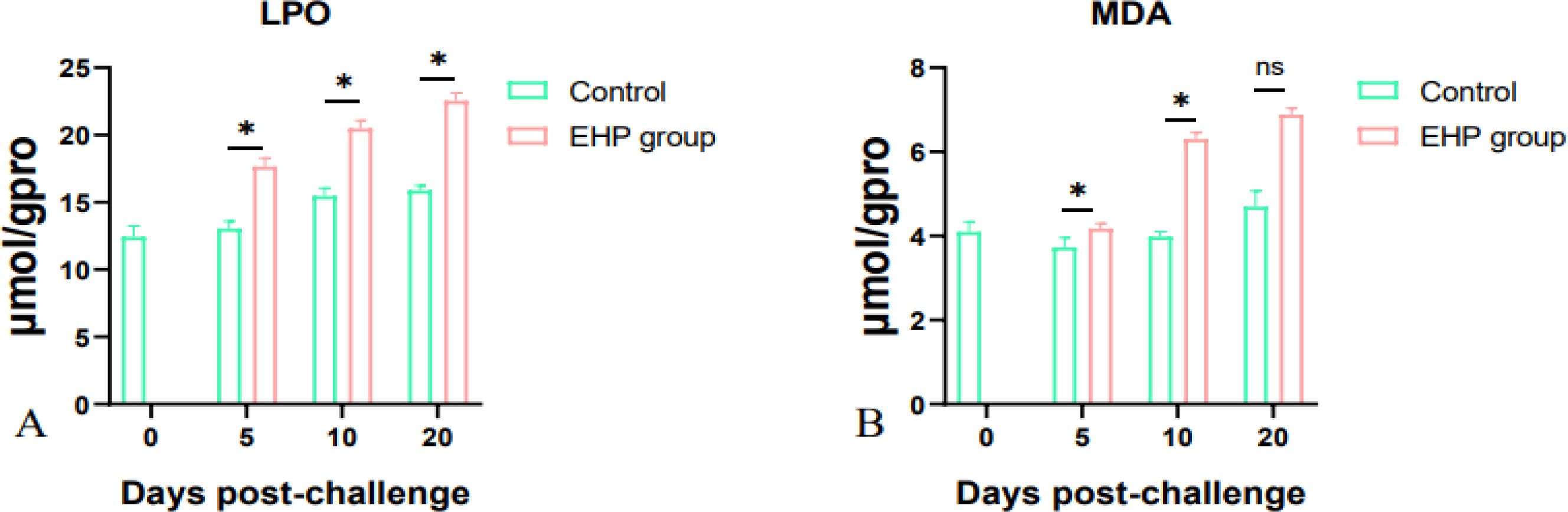
Figure 6. LPO (A) and MDA (B) content in the intestine of shrimp after EHP infection (mean ± SE, n = 6). The superscript symbol * depicts the statistical significance at p < 0.05. ns, not significant.
The results indicated that the activities of AMS, trypsin and LPS, which are the major digestive enzymes in the intestine, were inhibited after EHP infection. In the EHP group, the activity of trypsin was significantly different from that of the control group on the 10th and 20th dpc (Figure 7A, p < 0.05), the activity of AMS was significantly different between the EHP group and control group on the 5th and 20th dpc (Figure 7B, p < 0.05) However, LPS activity decreased significantly at all sampling time points (Figure 7C, p < 0.05).
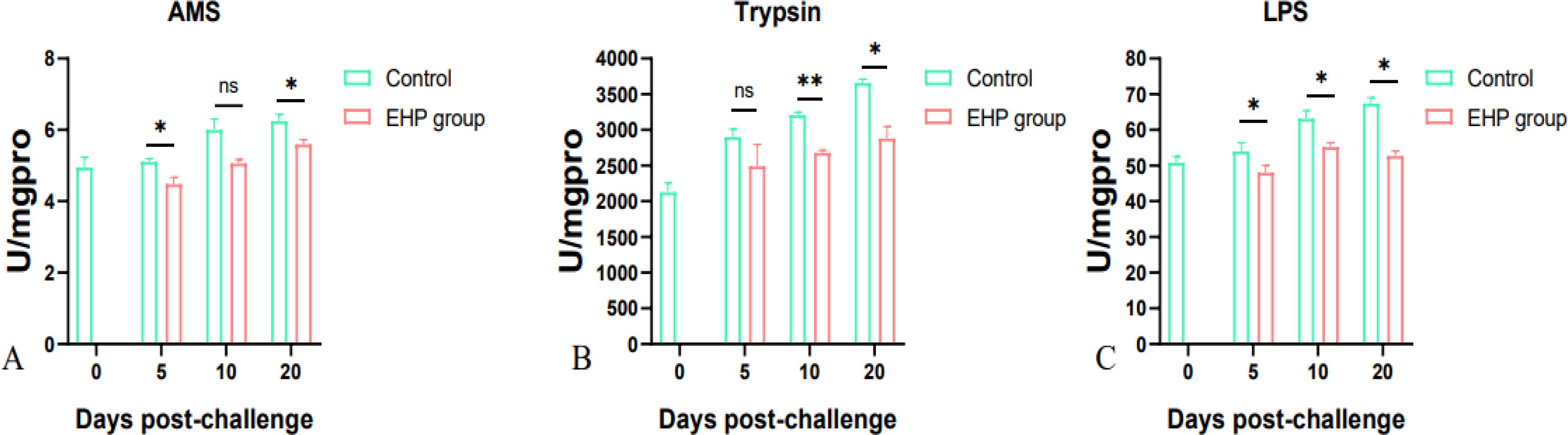
Figure 7. Changes in the activities of digestive enzymes AMS (A), trypsin (B) and LPS (C) in the intestine of P. vannamei infected with EHP (mean ± SE, n = 6). The superscript symbol * depicts the statistical significance at p < 0.05, and ** depicts the statistical significance at p < 0.01. ns, not significant.
The intestine has a large surface area with closely arranged villi, as well as various types of transport proteins associated with the absorption and transport of nutrients and ions, and is rich in digestive enzymes (Lankelma et al., 2015). Moreover, the intestinal mucosa is a crucial barrier against exogenous pathogenic bacteria to maintain the homeostasis of the internal environment and contribute to the healthy growth of shrimp (Zuhl et al., 2014). The structure of the intestine from inside to outside is mainly composed of the mucosa, the submucosa, the muscular layer and the outer membrane. Histology showed that EHP induced distinct inflammatory responses in the intestinal structures of shrimp. In the early stage of infection (5 d), shedding of intestinal villi and loosening of the muscular layer structure were observed. Severe damage occurred in the middle (10 d) and late (20 d) stages, mainly manifested as adhesions of the intestinal mucosa, stripping of intestinal villi from the muscularis propria, and even severe rupture of the tunica albuginea. This will inevitably trigger the immune response and affect metabolic processes of the body, providing an opportunity for pathogen invasion (Ogata et al., 2017). Toll-like genes are crucial in the invertebrate immune strategy. It mediates immune responses elicited by fungi, viruses, and gram-positive bacteria and establishes an immune defense front by inducing the expression of the downstream factors Myd88 and interleukin through a cascade amplification effect (Deepika et al., 2014; Wang et al., 2023). It was discovered that EHP significantly induced the transcription of Toll, Myd88 and IL-16, and the expression of the Toll and Myd88 was significantly increased. This appears to indicate that EHP can activate intestinal innate immunity through the Toll pathway. Similarly, the stimulation of Vibrio and WSSV resulted in significant changes in their transcript levels (Wang et al., 2012; Li and Xiang, 2013). Understandably, the target tissue of these two pathogens is originally the intestines of shrimp. Strangely, IMD and Relish showed completely opposite expression patterns. Generally, Relish receives signals from the IMD and self-activates, subsequently regulating the transcription of immune genes in response to pathogen invasion (Bai et al., 2020). However, the experimental results showed that EHP infection significantly inhibited IMD, while significantly induced Relish. It is hypothesized that the reason may be related to the priority recognition of gram-negative bacteria and viruses by IMD. Possibly, EHP interferes with the normal transmission of the IMD-Relish signaling pathway. Alternatively, Relish acts as a hub for many signal transduction, and its upregulation may play a role in other ways such as inflammatory response or apoptosis (Lemaitre and Hoffmann, 2007; Lan et al., 2013). ALF and Crustin A are crucial elements in the battle against pathogens. It has been reported that ALF is abundantly expressed in the shrimp intestine at the time of injury to promote tissue healing (Matos et al., 2018). In the present study, EHP caused intestinal damage and significantly activated ALF, also, Crustin A was able to respond rapidly in the face of EHP invasion (Li et al., 2018; Matos et al., 2018). Both ACP and ALP are phosphatases, which are crucial immune enzymes that safeguard cells from pathogen-induced damage (Long et al., 2019). Notably, the results indicated that the expression level of ALP was significantly repressed. We hypothesized that the cause was the imbalance of intestinal microflora influencing the expression pattern of ALP. Concurrently, the down-regulation of ALP augmented the likelihood of cell invasion by pathogens and exacerbated the inflammatory damage within the intestine.
In addition, oxidative stress is a crucial manifestation of antioxidant defense, which is characterized by increased peroxidation products and altered expression of antioxidant genes (Lan et al., 2013). The detection of lipid peroxidation products showed that LPO and MDA accumulated in the intestine with the extension of EHP infection time. LPO and MDA are regarded as the products of the interaction of active oxygen species with lipids and proteins. An appropriate amount of reactive oxygen species is necessary for the removal of pathogenic bacteria, but excessive amounts of reactive oxygen species interact with the membrane structure, mainly composed of lipids, leading to cell damage. This is reflected in the severe damage to the intestinal membrane structure that is clearly observed in histology (Lushchak, 2011). Meanwhile, a large number of antioxidant genes (Nrf2, SOD, GPX, CAT, and GST) participate in oxidative stress and cell damage repair. Nrf2 is a multifunctional transcription factor that plays an important role in the antioxidant process (Shen et al., 2019). The knockdown of its expression resulted in the successive down-regulation of the expression levels of antioxidant genes such as SOD, suggesting that it can regulate the expression of antioxidant genes (Wu et al., 2020). It has been reported that the expression patterns of Nrf2 and SOD were concordant (Jiang et al., 2013), which is consistent with the present study. SOD is expressed in all tissues of crustaceans, with high transcript levels in hepatopancreas and intestine. It can dissociate superoxide anion into oxygen and hydrogen peroxide, reducing toxicity to cells (Duan et al., 2016). In this experiment, EHP infection was found to significantly induce the expression of SOD in intestinal tissues. Conversely, SOD expression was down-regulated when shrimp were infected with WSSV and Vibrio alginolyticus, suggesting that its transcription level is influenced by the pathogen species (Tian et al., 2011). CAT and GPX act on the upstream catabolic hydrogen peroxide and cause its complete decomposition. GPX reacts rapidly, enabling its action even at trace concentrations of hydrogen peroxide. However, CAT requires high concentrations of hydrogen peroxide to stimulate its expression (Arockiaraj et al., 2012). The results demonstrated that EHP could induce the expression of GPX and CAT, but the expression levels were different. GPX was significantly increased on the 5th and 20th dpc, while CAT showed a significant difference only on the 20th dpc. Additionally, the peroxidation product content reached a peak on the 20th dpc, suggesting that CAT was induced under more stringent conditions. It was also found that the expression level of GST in the intestine was significantly reduced on the 5th and 10th dpc, assuming that GST may be influenced by individual development (Zhou et al., 2009). The above findings suggest that the damage caused by EHP to the intestine is significant.
The intestine plays a crucial role in the process of absorption, transport, and synthesis of substances. Absorption of nutrients from food is necessary and sufficient for maintaining growth and development. In addition, if digestive enzymes (AMS, trypsin and LPS) are inactivated, the intestinal digestion and absorption function will be impeded (Duan et al., 2017). Enzyme activity assays revealed that the activities of all the above-mentioned digestive enzymes were significantly decreased after EHP infection, suggesting that the digestive function was affected, thereby hindering the digestion process of carbohydrate, protein and fat, and affecting the growth and molting cycle (Van Wormhoudt and Favrel, 1988). The intestinal epithelium is an important site for glucose absorption and transport. GLUT-1 transports absorbed glucose to various organs to maintain glucose homeostasis (Verri et al., 2001; Martínez-Quintana et al., 2014). Compared with the control group, EHP infection up-regulated the transcription level of GLUT-1, suggesting that it could accelerate the rate of glucose transport. Na+-K+ATPase acts as a driver of substance transportation and can provide power for nutrient absorption (Conte et al., 1977). In this study, a relative increase in its transcription during the early stage of EHP infection was detected, which to a certain extent represents the activation of the substance transport system. Fatty acids are important raw materials for various types of membrane structures. ACC and FAS have a clear division of labor in the fatty acid synthesis pathway. ACC can convert acetyl-CoA into malonyl-CoA, providing raw materials for fatty acid synthesis, while FAS plays a rate-limiting role in the synthesis process (Sul and Wang, 1998; Montero et al., 2003). In the current study, EHP significantly activated the expression of ACC and FAS, which may be because intestinal injury requires large amounts of fatty acids to form new membrane structures (Hui et al., 2019). Additionally, it has been reported that FAS is closely related to the immune process. Toll-like receptors and IMD pathway can activate the expression of FAS, enabling it to participate in the immune process. By knocking down the expression of FAS, it was observed that the load of Vibrio parahaemolyticus in shrimp increased and the mortality rate rose, suggesting that FAS played a specific role in antibacterial defense (Zuo et al., 2017). The mTOR signaling pathway is involved in nutrient regulation and cell growth, and plays a vital role in growth and metabolic processes. It also balances protein synthesis and catabolism and maintains the homeostasis of the intracellular environment (Kakanj et al., 2016). TOR and S6K positively regulate the conduction direction of this pathway. When Raptor binds to TOR, it regulates the downstream ribosomal protein kinase S6K and the downstream transcription factor 4EBP to promote protein synthesis (Wullschleger et al., 2006). Nevertheless, a few studies have indicated that 4EBP is a negative regulator of the mTOR pathway and inhibits the translation initiation process (De la Parra et al., 2018). This is slightly different from the present study. It is possible that the increase in 4EBP transcription cannot be simply interpreted as an inhibition of translation initiation, and there may be other complex regulatory mechanisms in the organism that coordinate the collaboration between these pathways. Additionally, the mTOR pathway is also responsible for the synthesis of immune proteins and nutrient translocation (Cardenas et al., 1999). In this experiment, the pathway was activated after shrimp were infected with EHP, indicating an enhanced rate of protein synthesis. This may be due to two reasons: first, shrimp infected with EHP may not be able to obtain sufficient nutrition, resulting in a starvation state, so the synthesis rate of essential proteins must be accelerated to maintain normal life activities; second, this pathway is associated with immune protein synthesis, and it may be possible to synthesize the corresponding immune proteins through this pathway.
In conclusion, this experiment focused on the immune and physiological responses of the shrimp intestine, which further confirmed the comprehensive damage caused by EHP to the organism. This study concluded that EHP infection severely damaged the intestine and triggered innate immunity and oxidative stress, leading to the accumulation of peroxide products. In addition, EHP infection inhibited the activities of intestinal digestive enzymes, but activated nutrient transport and synthesis pathways, indicating that in order to survive, EHP-infected shrimp must accelerate substance transport, but the degree of supplementation is not enough to support their normal growth and development, thereby affecting the growth of shrimps. This can provide a new reference for the prevention and control of EHP.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
The animal study was approved by Animal Research and Ethics Committees of Ludong University. The study was conducted in accordance with the local legislation and institutional requirements.
ZC: Writing – original draft, Writing – review & editing, Conceptualization. CH: Writing – original draft, Software. ZY: Investigation, Writing – review & editing. CC: Writing – review & editing, Data curation. FL: Writing – review & editing, Funding acquisition, Supervision. DY: Funding acquisition, Supervision, Writing – review & editing, Formal analysis. TL: Writing – review & editing, Data curation. LC: Writing – review & editing, Methodology. LS: Writing – review & editing, Funding acquisition, Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Shandong Province (ZR2023QC050, ZR2024MC115), East-West Science and Technology Cooperation Project (24CXNA039), Innovation Project for graduate students of Ludong University (IPGS2024-095) and7nbsp;Start-up funding for scientific research of Ludong University (20220022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alfiansah Y. R., Peters S., Harder J., Hassenrück C., Gärdes A. (2020). Structure and co-occurrence patterns of bacterial communities associated with white faeces disease outbreaks in Pacific white-leg shrimp Penaeus vannamei aquaculture. Sci. Rep. 10, 11980. doi: 10.1038/s41598-020-68891-6
Aranguren L. F., Han J. E., Tang K. F. J. (2017). Enterocytozoon hepatopenaei (EHP) is a risk factor for acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN) in the Pacific white shrimp Penaeus vannamei. Aquaculture 471, 37–42. doi: 10.1016/j.aquaculture.2016.12.038
Arockiaraj J., Easwvaran S., Vanaraja P., Singh A., Othman R. Y., Bhassu S. (2012). Molecular cloning, characterization and gene expression of an antioxidant enzyme catalase (MrCat) from Macrobrachium rosenbergii. Fish shellf. Immunol. 32, 670–682. doi: 10.1016/j.fsi.2012.01.013
Bai L. W., Zhou K. M., Li H., Qin Y. K., Wang Q., Li W. W. (2020). Bacteria-induced IMD-Relish-AMPs pathway activation in Chinese mitten crab. Fish shellf. Immunol. 106, 866–875. doi: 10.1016/j.fsi.2020.08.046
Cao Z., Chen C. Y., Wang C. X., Li T., Chang L. R., Si L. J., et al. (2023). Enterocytozoon hepatopenaei (EHP) Infection Alters the Metabolic Processes and Induces Oxidative Stress in Penaeus vannamei. Animals 13, 3661. doi: 10.3390/ani13233661
Cardenas M. E., Cutler N. S., Lorenz M. C., Di Como C. J., Heitman J. (1999). The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13, 3271–3279. doi: 10.1101/gad.13.24.3271
Conte F. P., Droukas P. C., Ewing R. D. (1977). Development of sodium regulation and de novo synthesis of Na+K-activated ATPase in larval brine shrimp, Artemia salina. J. Exp. Zool. 202, 339–361. doi: 10.1002/jez.1402020306
Deepika A., Sreedharan K., Paria A., Makesh M., Rajendran K. V. (2014). Toll-pathway in tiger shrimp (Penaeus monodon) responds to white spot syndrome virus infection: evidence through molecular characterisation and expression profiles of MyD88, TRAF6 and TLR genes. Fish shellf. Immunol. 41, 441–454. doi: 10.1016/j.fsi.2014.09.026
De la Parra C., Walters B. A., Geter P., Schneider R. J. (2018). Translation initiation factors and their relevance in cancer. Curr. Opin. Genet. Dev. 48, 82–88. doi: 10.1016/j.gde.2017.11.001
Duan Y. F., Zhang Y., Dong H. B., Zhang J. S. (2016). Effect of desiccation on oxidative stress and antioxidant response of the black tiger shrimp Penaeus monodon. Fish shellf. Immunol. 58, 10–17. doi: 10.1016/j.fsi.2016.09.004
Duan Y. F., Zhang Y., Dong H. B., Zheng X. T., Wang Y., Li H., et al. (2017). Effect of dietary poly-β-hydroxybutyrate (PHB) on growth performance, intestinal health status and body composition of Pacific white shrimp Litopenaeus vannamei (Boone 1931). Fish shellf. Immunol. 60, 520–528. doi: 10.1016/j.fsi.2016.11.020
Huang Z. J., Zeng S. Z., Xiong J. B., Hou D. W., Zhou R. J., Xing C. G., et al. (2020). Microecological Koch's postulates reveal that intestinal microbiota dysbiosis contributes to shrimp white feces syndrome. Microbiome 8, 32. doi: 10.1186/s40168-020-00802-3
Hui K., Ren Q., Cao J. (2019). Insights into the intestine immune of Marsupenaeus japonicus under the white spot syndrome virus challenge using RNA sequencing. Vet. Immunol. Immunopathol. 208, 25–33. doi: 10.1016/j.vetimm.2018.12.001
Jiang W. D., Liu Y., Jiang J., Hu K., Li S. H., Feng L., et al. (2013). In vitro interceptive and reparative effects of myo-inositol against copper-induced oxidative damage and antioxidant system disturbance in primary cultured fish enterocytes. Aquat. Toxicol. 132, 100–110. doi: 10.1016/j.aquatox.2013.02.005
Jiao L. F., Dai T. M., Zhong S. Q., Jin M., Sun P., Zhou Q. C. (2020). Vibrio parahaemolyticus infection impaired intestinal barrier function and nutrient absorption in Litopenaeus vannamei. Fish shellf. Immunol. 99, 184–189. doi: 10.1016/j.fsi.2020.02.009
Kakanj P., Moussian B., Grönke S., Bustos V., Eming S. A., Partridge L., et al. (2016). Insulin and TOR signal in parallel through FOXO and S6K to promote epithelial wound healing. Nat. Commun. 7, 12972. doi: 10.1038/ncomms12972
Kumar Dewangan N., Pang J. H., Zhao C. Y., Cao C. S., Yin B., Weng S. P., et al. (2023). Host and transmission route of Enterocytozoon hepatopenaei (EHP) from dragonfly to shrimp. Aquaculture 574, 739642. doi: 10.1101/2023.02.17.528912
Lan J. F., Zhou J., Zhang X. W., Wang Z. H., Zhao X. F., Ren Q., et al. (2013). Characterization of an immune deficiency homolog (IMD) in shrimp (Fenneropenaeus chinensis) and crayfish (Procambarus clarkii). Dev. Comp. Immunol. 41, 608–617. doi: 10.1016/j.dci.2013.07.004
Lankelma J. M., Nieuwdorp M., de Vos W. M., Wiersinga W. J. (2015). The gut microbiota in internal medicine: implications for health and disease. NETH J. Med. 73, 61–68. Available at: http://www.njmonline.nl/getpdf.php?id=1539.
Lemaitre B., Hoffmann J. (2007). The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743. doi: 10.1146/annurev.immunol.25.022106.141615
Li F. H., Xiang J. H. (2013). Signaling pathways regulating innate immune responses in shrimp. Fish shellf. Immunol. 34, 973–980. doi: 10.1016/j.fsi.2012.08.023
Li M., Ma C., Li H., Peng J., Zeng D., Chen X., et al. (2018). Molecular cloning, expression, promoter analysis and functional characterization of a new Crustin from Litopenaeus vannamei. Fish shellf. Immunol. 73, 42–49. doi: 10.1016/j.fsi.2017.12.002
Li W. Y., Hua S. S., Du Z. W., Jiang H. Y., Jiang S. S., Yu M. M., et al. (2024). Interactions between the gut bacterial community of Exopalaemon carinicauda and infection by Enterocytozoon hepatopenaei. J. Invertebr. Pathol. 204, 108115. doi: 10.1016/j.jip.2024.108115
Liu Z., Zhang Q. L., Wan X. Y., Ma F., Huang J. (2016). Development of real-time PCR assay for detecting microsporidian Enterocytozoon hepatopenaei and the application in shrimp samples with different growth rates. Prog. Fish Sci. 37, 119–126. doi: 10.11758/yykxjz.20150512003
Long L., Zhang H., Ni Q., Liu H., Wu F., Wang X. (2019). Effects of stocking density on growth, stress, and immune responses of juvenile Chinese sturgeon (Acipenser sinensis) in a recirculating aquaculture system. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 219, 25–34. doi: 10.1016/j.cbpc.2019.02.002
Lushchak V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 101, 13–30. doi: 10.1016/j.aquatox.2010.10.006
Martínez-Quintana J. A., Peregrino-Uriarte A. B., Gollas-Galván T., Gómez-Jiménez S., Yepiz-Plascencia G. (2014). The glucose transporter 1 GLUT1 from the white shrimp Litopenaeus vannamei is up-regulated during hypoxia. Mol. Biol. Rep. 41, 7885–7898. doi: 10.1007/s11033-014-3682-8
Matos G. M., Schmitt P., Barreto C., Farias N. D., Toledo-Silva G., Guzmán F., et al. (2018). Massive gene expansion and sequence diversification is associated with diverse tissue distribution, regulation and antimicrobial properties of anti-lipopolysaccharide factors in shrimp. Mar. Drugs 16, 381. doi: 10.3390/md16100381
Montero D., Kalinowski T., Obach A., Robaina L., Tort L., Caballero M. J., et al. (2003). Vegetable lipid sources for gilthead seabream (Sparus aurata): effects on fish health. Aquaculture 225, 353–370. doi: 10.1016/S0044-8486(03)00301-6
Ogata S., Shimizu K., Tominaga S., Nakanishi K. (2017). Immunohistochemical study of mucins in human intestinal spirochetosis. Hum. Pathol. 62, 126–133. doi: 10.1016/j.humpath.2017.01.013
Patil P. K., Geetha R., Ravisankar T., Avunje S., Vijayan K. K. (2021). Economic loss due to diseases in Indian shrimp farming with special reference to Enterocytozoon hepatopenaei (EHP) and white spot syndrome virus (WSSV). Aquaculture 533, 736231. doi: 10.1016/j.aquaculture.2020.736231
Santhoshkumar S., Sivakumar S., Vimal S., Abdul Majeed S., Taju G., Haribabu P., et al. (2017). Biochemical changes and tissue distribution of Enterocytozoon hepatopenaei (EHP) in naturally and experimentally EHP-infected white leg shrimp, Litopenaeus vannamei (Boone 1931), in India. J. Fish Dis. 40, 529–539. doi: 10.1111/jfd.12530
Shen H., Dou Y. B., Li H. L., Qiao Y., Jiang G., Wan X. H., et al. (2022). Changes in the intestinal microbiota of Pacific white shrimp (Litopenaeus vannamei) with different severities of Enterocytozoon hepatopenaei infection. J. Invertebr. Pathol. 191, 107763. doi: 10.1016/j.jip.2022.107763
Shen Y. M., Liu X. J., Shi J. H., Wu X. (2019). Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int. J. Biol. Macromol. 125, 496–502. doi: 10.1016/j.ijbiomac.2018.11.190
Shinn A. P., Pratoomyot J., Griffiths D., Trong T. Q., Vu N. T., Jiravanichpaisal P., et al. (2018). Asian shrimp production and the economic costs of disease. Asian Fish. Sci. 31, 29–58. doi: 10.33997/j.afs.2018.31.s1.003
Soonthornchai W., Rungrassamee W., Karoonuthaisiri N., Jarayabhand P., Klinbunga S., Söderhäll K., et al. (2010). Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev. Comp. Immunol. 34, 19–28. doi: 10.1016/j.dci.2009.07.007
Subash P., Chrisolite B., Sivasankar P., Rosalind George M., Vijay Amirtharaj K. S., Padmavathy P., et al. (2023). White feces syndrome in Penaeus vannamei is potentially an Enterocytozoon hepatopenaei (EHP) associated pathobiome origin of Vibrio spp. J. Invertebr. Pathol. 198, 107932. doi: 10.1016/j.jip.2023.107932
Sul H. S., Wang D. (1998). Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu. Rev. Nutr. 18, 331–351. doi: 10.1146/annurev.nutr.18.1.331
Thitamadee S., Prachumwat A., Srisala J., Jaroenlak P., Salachan P. V., Sritunyalucksana K., et al. (2016). Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 452, 69–87. doi: 10.1016/j.aquaculture.2015.10.028
Tian J. X., Chen J., Jiang D., Liao S. A., Wang A. L. (2011). Transcriptional regulation of extracellular copper-zinc superoxide dismutase from white shrimp Litopenaeus vannamei following Vibrio alginolyticus and WSSV infection. Fish shellf. Immunol. 30, 234–240. doi: 10.1016/j.fsi.2010.10.013
Van Wormhoudt A., Favrel P. (1988). Electrophoretic characterization of Palaemon elegans (crustacea, decapoda) α amylase system: Study of amylase polymorphism during the intermolt cycle. Comp. Biochem. Physiol. B. 89, 201–207. doi: 10.1016/0305-0491(88)90211-8
Verri T., Mandal A., Zilli L., Bossa D., Mandal P. K., Ingrosso L., et al. (2001). D-glucose transport in decapod crustacean hepatopancreas. Comp. Biochem. Physiol. 130, 585–606. doi: 10.1016/s1095-6433(01)00434-2
Wang P. H., Liang J. P., Gu Z. H., Wan D. H., Weng S. P., Yu X. Q., et al. (2012). Molecular cloning, characterization and expression analysis of two novel Tolls (LvToll2 and LvToll3) and three putative Spätzle-like Toll ligands (LvSpz1-3) from Litopenaeus vannamei. Dev. Comp. Immunol. 36, 359–371. doi: 10.1016/j.dci.2011.07.007
Wang S., Li H. Y., Li Q. Y., Yin B., Li S. D., He J. G., et al. (2023). Signaling events induced by lipopolysaccharide-activated Toll in response to bacterial infection in shrimp. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1119879
Wan Sajiri W. M. H., Kua B. C., Borkhanuddin M. H. (2023). Detection of Enterocytozoon hepatopenaei (EHP) (microsporidia) in several species of potential macrofauna-carriers from shrimp (Penaeus vannamei) ponds in Malaysia. J. Invertebr. Pathol. 198, 107910. doi: 10.1016/j.jip.2023.107910
Wu J. L., Liu W. X., Wen C. G., Qian G. M., Hu B. Q., Jian S. Q., et al. (2020). Effect of microcystin on the expression of Nrf2 and its downstream antioxidant genes from Cristaria plicata. Aquat. Toxicol. 225, 105526. doi: 10.1016/j.aquatox.2020.105526
Wu J., Tian S. J., Luo K., Zhang Y. J., Pan H. T., Zhang W. B., et al. (2022). Dietary recombinant human lysozyme improves the growth, intestinal health, immunity and disease resistance of Pacific white shrimp Litopenaeus vanname. Fish shellf. Immunol. 121, 39–52. doi: 10.1016/j.fsi.2021.12.052
Wullschleger S., Loewith R., Hall M. N. (2006). TOR signaling in growth and metabolism. Cell 124, 471–484. doi: 10.1016/j.cell.2006.01.016
Zhang M. L., Sun Y. H., Chen K., Yu N., Zhou Z. G., Chen L. Q., et al. (2014). Characterization of the intestinal microbiota in Pacific white shrimp, Litopenaeus vannamei, fed diets with different lipid sources. Aquaculture 434, 449–455. doi: 10.1016/j.aquaculture.2014.09.008
Zhou J., Wang W. N., Wang A. L., He W. Y., Zhou Q. T., Liu Y., et al. (2009). Glutathione S-transferase in the white shrimp Litopenaeus vannamei: Characterization and regulation under pH stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 150, 224–230. doi: 10.1016/j.cbpc.2009.04.012
Zuhl M., Schneider S., Lanphere K., Conn C., Dokladny K., Moseley P. (2014). Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 48, 980–986. doi: 10.1136/bjsports-2012-091585
Keywords: Penaeus vannamei, microsporidia, Enterocytozoon hepatopenaei, intestinal immunity, intestinal physiology
Citation: Cao Z, He C, Yin Z, Chen C, Li F, Yan D, Li T, Chang L and Si L (2025) Effects of Enterocytozoon hepatopenaei infection on intestinal immune defense and physiological processes of Penaeus vannamei. Front. Mar. Sci. 12:1522448. doi: 10.3389/fmars.2025.1522448
Received: 04 November 2024; Accepted: 10 January 2025;
Published: 27 January 2025.
Edited by:
Mario Alberto Burgos-Aceves, Autonomous University of San Luis Potosí, MexicoReviewed by:
Basanta Kumar Das, Central Inland Fisheries Research Institute (ICAR), IndiaCopyright © 2025 Cao, He, Yin, Chen, Li, Yan, Li, Chang and Si. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingjun Si, c2lsaW5nanVuMTk5MUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.