
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci., 24 February 2025
Sec. Coral Reef Research
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1518701
Rising sea levels are threatening the Reef Islands, which have restricted areas that too just a few meters above sea level, besides the global and local anthropogenic strains including devastative methods used for fishing and pollution that impact the majority of the tropical coastal areas as well as the encircling reef ecosystems that are the only sediment sources required for sustenance of these islands. The carbonate skeletal of the sediments is potentially changed due to these strains jeopardizing the physical existence of reef islands through enhanced coral mortality and producing a shift of macro algal supremacy over the corals. Further decline of these vital ecosystems can be stopped by addressing the primary causes of the destructing, enforcing the potential therapeutic and conservative measures, and promoting the stakeholder’s cooperation.
Coral reefs are vital ecosystems teeming with life, providing essential services to millions of people. These underwater wonders serve as food sources and support livelihoods for coastal communities. They also contribute to mitigating climate change by sequestering carbon and acting as a buffer against extreme weather events (Woodhead et al., 2021). Less than 1% of the benthic environment is occupied by approximately 250,000 km2 of seafloor area (Woodroffe and Webster, 2014; Spalding et al., 2014). The existence of a minimum of 835 species of reef assembling corals and biodiversity estimates of reefs in the range of 1-9 million have been reported by Veron (1995). These robust underwater cities support more than 800 species of hard corals and are home to 25% or more marine life.
Corals are one of the main component organisms in the coral reef ecosystem, which are oceanic invertebrates in the class Anthozoa of phylum Cnidaria (Liao et al., 2019). Coral reefs start establishing when the Planulae, the free-swimming larvae of corals, adhere to the submerged continents or island edges like scleractinians are colonial organisms, comprising individuals from a few hundred to hundreds of thousands, known as polyps (Lough, 1989). Corals are considered one of the three vital characteristic structures that thrive and propagate, according to the National Oceanic and Atmospheric Administrations (NOAA, US Department of Commerce), and can be adjacent, hurdle, or cay. In their biogeographic profiles, all three coral reef types exhibit similarities. Depth, current strength and waves, bottom topography, temperature, suspended sediments, and light all create characteristic vertical and horizontal zones of algae, corals, and other contributing species. These zones may vary at different locations and in various types of coral reefs, and this is the vital differentiation commonly observed in most reefs. While moving from shore to seaward the reefs are flat, algal ridge or reef crest, buttress regions, and seaward inclination (NOAA).
Coral reefs perform a variety of ecosystem services despite being a small fraction of the ecosystem and all these services are vital for the well-being of humans and also for our present way of living along the coastal areas. They are highly valuable and biologically most diverse ecosystems of the planet Earth. Coral reefs underpin a range of ecosystem goods and services that contribute to the wellbeing of millions of people (Woodhead et al., 2019). Over 1 billion humans are supported by these thriving ecosystems through priceless services of these ecosystems present throughout the tropics. The important services rendered by these coral reefs are i) services in connection with the coral reef physical structures like protection of shoreline and erosive processes regulation; ii) biogeochemical services, including fixation of nitrogen and control of CO2/Ca; iii) replenishing resources, like fisheries; iv) biodiversity preservation being home to almost one-third of the total marine life; and v) culture and information services, like leisure and tourism prospects (Moberg and Folke, 1999; Principe et al., 2021). In the marine environment, coral reefs support the highest number of species such as 800 hard coral species and 4000 different fishes. According to an estimate almost 8 million species of undiscovered animals and plants live near or in coral reefs.
It has been almost 100 years that work on coral reefs has started however, the interest in the coral reef state is quite recent, mostly exhibited during four decades in the recent past. It is one of the most threatened ecosystems, resulting from extended periods of anthropogenic exploitation and degradation (Hoegh-Guldberg et al., 2007) and disproportionate proneness to the emerging impacts of climatic changes across the globe. There is a decline in the coral reefs in the world including U.S. as well. It has been reported that almost a quarter of the species will go extinct until the pressures induced on the environment by humans is reversed back immediately (Hughes et al., 2017; Rull, 2022). Many scientists believe the coral reefs’ existence will be in jeopardy unless and until we consolidate our efforts to protect these (Frieler et al., 2013). Several stresses are affecting the coral reefs across the globe including local as well as global impacts (Burke et al., 2011; Wolff et al., 2018; Abelson, 2020; Knowlton et al., 2021). Destructive and overfishing (Burke et al., 2011; Abelson, 2020; Karasik et al., 2019), nutrient and sediment expulsion from catchments uprising from deforestation, agriculture, and urbanization (Burke et al., 2011; Abelson, 2020), Cyclones of tropics (Cheal et al., 2017), and outbursts of the crown of thorns starfish (Brodie et al., 2005) are historically are the main causes. Enhanced interest and concern are because of the climate change pressures that is primarily exhibited as heat waves which leads to the bleaching episodes of corals (Burke et al., 2011; Pratchett et al., 2021). The effect of these hazards disproportionately impacts the lower class such as Indigenous, low-income, and minority population groups that depend on these coral reefs for food security (food from fisheries on a small scale) and natural infrastructure protection (coastal safeguard) against ever-increasing strong storms (Kumar and Taylor, 2015; Neumann et al., 2015; Reguero et al., 2015).
Losses in ranges of 30-60% have been documented in the diversity of coral species on reefs by Edinger and colleagues as a result of human activities, out of which generic diversity losses were 25% over mere 15 years on only two of these reefs. Hence, in the context of economic growth and the increasing population of humans, the collective efforts to safeguard the habitat of the coral reef may result in slowing down the progressive decline, but it seems to be difficult to get optimistic in connection with the reef health over a short period across the globe (Wilkinson, 2004). The present review is an attempt to have a deep insight into the primary drivers responsible for the degradation of the tropical coral reef ecosystems, the spurting consequences, and the effective strategies required for conservation and restoration. During the last century, substantial changes have been explored in the structure as well as the function of reef ecosystems, and also in the range of services it provide to the human populations. The quite recent changes in the ecology are inexplicably ingrained within the social contexture, being both driven by as well as driven by the behavior of humans. Reefs have been changed increasingly along with a decline in the coral reef ecosystem cover with the increase in the per capita GDP and human population across the globe. They are not directly linked however there exists an undeniable, inevitable thread of cause; humans are fluctuating the coral reefs (Pandolfi et al., 2003; Williams et al., 2019), and the quick and biggest changes in the reef ecosystem structure happened in the present geological period.
It is produced on the land but ultimately ends up in the waters of coastal regions. There are various types of pollution originating from different sources as a result of activities on the land, for example, Sedimentation resulting from shore development, runoff from urban storm waters, agriculture, and forestry (Figure 1). The sediment loads produce a pressure and cover that may result in the smothering of corals thereby damaging the soft tissues and ultimately leading to the death of corals (Erftemeijer et al., 2012; Yeemin et al., 2013; Lamb et al., 2018). The primary stress for the recovery and existence of the coral species and their habitats is sedimentation. The deposited sediments onto the coral reefs result in the smothering of corals interfering with the food intake, growth, and reproduction potential. Pollution of nutrients damages the coral reefs. Nitrogen and phosphorus from fertilizer use in agriculture and residential use, animal wastes, and sewage unloads such as septic systems and plants for treatment of wastewater are adversely impacting the corals which are habituated to low levels of nutrients. Enhancement of the nutrients results in an imbalance in the nutrient exchange between the corals and the zooxanthellae, it also promotes the growth of the phytoplankton that hinders the penetration of light through the coral reefs and the most damaging aspect among all is the stimulation and expansion in the growth of seaweeds that may outgrow very quickly smothering the corals and ultimately replacing the corals which usually have a slow growth rate, in typical tropical ocean environments with low concentrations of nutrients. These alterations result in an imbalance that adversely affects the coral reef ecosystems. Microbe growth is also promoted in an environment rich in nutrients such as fungi and bacteria that may infect corals.
Corals are affected badly by toxic substances such as organic chemicals, metals, industrial toxic discharges, agriculture and urban runoff, sunscreens, landfills, and mining activities. The growth, physiological pathways and reproduction of corals are adversely affected by the pesticides. Symbiotic algae are in particular affected by herbicides. The interaction of these algae with corals is damaged resulting in bleaching. Organic chemicals like dioxin, oxybenzone PCBs (polychlorobiphenyls), and metals including lead and mercury are also supposed to impact the feeding, growth rate, defense reactions, and reproduction of corals. There is also a direct hazardous impact of trash like fishing gear, and plastic debris on coral reefs and various benthic communities through killing or damage to benthic organisms and stony corals, entanglement, and introduction of potential pathogens and parasites (Chiappone et al., 2005; Dameron et al., 2007; Abu-Hilal and Al-Najjar, 2009; Gilardi et al., 2010; Niaounakis, 2017; Sheehan et al., 2021; Lamb et al., 2018). Therefore, the land-generated pollution of plastics, enrichments of nutrients, and contaminations of chemicals degrade the water quality thus resulting in blooms of algae, dysfunction of ecosystems, and diseases in corals.
Coral decline also results by the disease outbreak caused by abiotic or biotic factors or both (Woodley et al., 2008). Significant alterations occur as a result of destructive diseases in the growth, and reproduction of corals which eventually modify the structure of the community, the abundance of organisms related to reef as well as the diversity of various species (Table 1) (Mulya et al., 2023). Furthermore, diseases caused by biotic factors including viruses, bacteria, protozoa, and fungi produce bands or lesions due to tissue loss on colonies of corals and ultimately impact the ecosystems of the reef as a whole (Sokolow et al., 2009). Increased temperature also impacts the factors controlling the virulence of coral-related microbes that disrupt photosynthesis and result in the disintegration of algae having symbiotic relationships (Ben-Haim et al., 2003; Rosenberg and Falkovitz, 2004) leading to the bleaching of corals. Biotic factors present in insufficient treated sewages, stormwater, and livestock pens runoff are adversely affecting the corals. Though very rare but parasites and bacteria present in the fecal contaminations produce disease in the corals specifically if there are stress conditions from other environmental factors. Diseases of corals may occur in even healthy ecosystems but the intensity and frequency of outbreaks are exacerbated in the presence of pathogens-borne pollution (Figure 1).
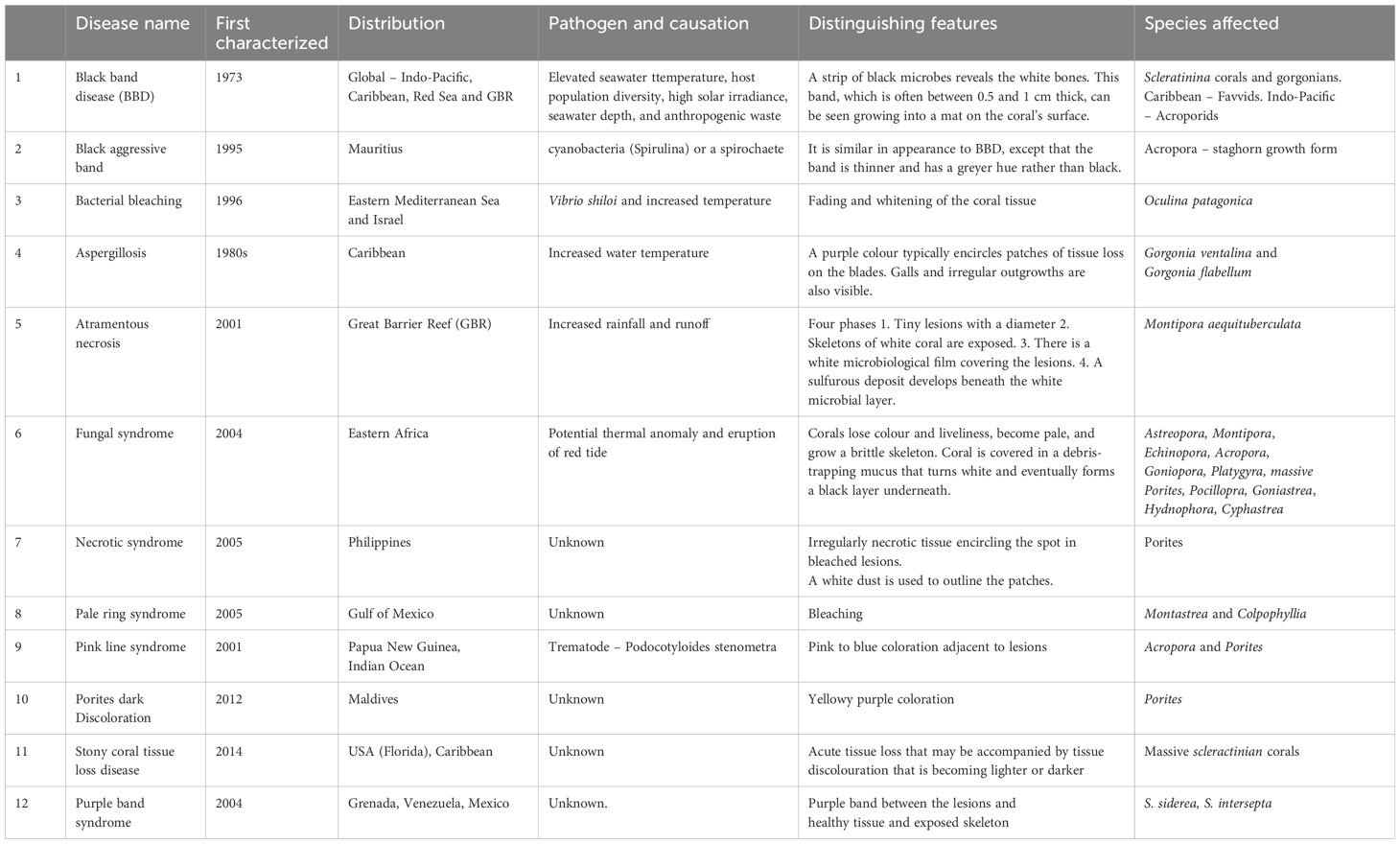
Table 1. List of coral diseases found in the literature, their distribution, and distinguishing features.
Harvesting of corals for trades of aquariums, curios, and jewelry leads to the extensive hunting of the specified species, biodiversity reduction, and reef habitat devastation. Coral reefs are destroyed at many locations when brightly colored reef fishes and coral heads are harvested for jewelry and aquarium trade. Untrained and careless divers stomp the delicate corals whereas various techniques used for fishing cause destruction. Heavy explosives or dynamites are used in blast fishing to startle fish out of the stashing places which kills a lot of organisms indiscriminately and also produce stress and cracks in corals to a level where zooxanthellae are expelled destroying large zones of reefs (Figure 1). Dumping or spraying of cyanide, in cyanide fishing, on coral reefs for capturing and stunning the live fish also damages polyps besides degrading the habitat of reefs. More than 15 countries are impacted by cyanide fishing and approximately 40 countries are reported to be influenced by blast fishing activities; (Hoegh-Guldberg et al., 2007).
The physical structure and function of the reef ecosystem are being jeopardized by the extensive loss of corals and their associated alterations of coral reef habitats (Hughes et al., 2003). In a few cases, corals are replaced by fleshy macroalgae (Seaweeds) as the predominant community thereby resulting in hurdles on the reclamation and replenishment capacity of corals assembly (Hughes et al., 2007, 2010). As an alternate, various sessile biota like soft corals, sponges, and ascidians flourish after the serious damage of hard corals (Scleractinian) (Norström et al., 2009; Tebbett and Bellwood, 2019). This shifting is further supported by variations in the ecological processes that may enhance the existence of non-coral biota and further restrict corals’ recruitment, survival, and growth (Hughes et al., 2010). Among all these non-coral biota, Algal turf is the widely distributed species. An algal turf ubiquitous growth found in most of the low-lying coral reef ecosystems exhibits a pertinacious changed state (Jouffray et al., 2015; Smith et al., 2016; Bellwood et al., 2018) strengthened by hazardous impacts of sediments captured in the turfs on the disposition of the coral juveniles (Ricardo et al., 2017; Speare et al., 2019). This settlement is further substantiated by the extensive fishing or the assemblage of terrestrial sediments that primarily change the function and behavior of the grazing fishes in the reefs (Goatley et al., 2016; Tebbett et al., 2017).
Deep water trawling is another destructive fishing method in which the fishing net is dragged along the bottom of the sea besides the muro-ami netting technique where coral reefs are stomped with heavy bags for startlement of fishes out of the hides. Among the serious effects are those in connection with the application of fishing gears which, even when lost or abandoned, continue to work uncontrolled and passively and contribute to the phenomenon called “ghost fishing”. (Al-Masroori et al., 2009; Gilardi et al., 2010; Gilman, 2015; Uhlmann and Broadhurst, 2015) whereas the abandoned equipment is known as “derelict fishing gear” (Morishige and McElwee, 2012; Edyvane and Penny, 2017), or “ghost nets” (Baeta et al., 2009; Butler et al., 2013; Wilcox et al., 2015), “fishery debris” (Ryan et al., 2009). The lost fishing gear is often called marine litter or debris (Gall and Thompson, 2015; Kühn et al., 2015; de Carvalho-Souza et al., 2018; Naranjo-Elizondo and Cortés, 2018) and is believed to be a vital contributor to sea plastic destroying corals and various sea animals (Lamb et al., 2018). In the Great Pacific Garbage Patch, almost 46% is the ghost nets (Lebreton et al., 2018). Marine life is endangered by the discarded gear and can kill or trap indiscriminately ocean animals, including those that belong to the endangered species or which are economically important. This hazard is quite prevalent in higher animals and hence the study of the impact of fishing gear and different ocean debris on vertebrates is given prime importance (Wilcox et al., 2013; Thiel et al., 2018). Food web structure can be altered by extensive fishing and may result in cascading impacts like a decline in the grazing fish number that is responsible for the cleaning of corals from overgrowth of algae.
During the recent past, climatic change has evolved as a viable threat endangering the coral reefs. Elevating sea temperatures are believed to be the cause of mass bleaching of corals during the last decade and this ocean warming may accelerate the severity and frequency of bleaching of corals in the decades to come (Donner et al., 2005). Prolonged or severe bleaching may kill colonies of corals or expose them to various other threats including diseases caused by biotic agents. It is a source of deliberation among the scientific community regarding the potential of corals and their symbionts to adjust to the changing climatic conditions as well as the chemistry of the ocean (Baker, 2004). The changing climate poses a precarious threat to the survival and functioning of coral reef ecosystems across the globe. Emissions of greenhouse gases are the predominant constituent during the present climatic change across the world (Cheng et al., 2019). The ocean is a big sink for the absorption of carbon dioxide, enhancing ocean acidification (Cornwall et al., 2021). Bleaching of corals and extensive damage to the ecosystem of coral reefs has become quite common resulting from the thermal strain due to the increased temperature of the ocean (Büscher et al., 2017). Shortage of nutrition required for the sustenance of the ecosystems of coral reefs and disturbance in the dispersal of larvae are both attributed to modified currents, upsurge, and/or vertical mixing due to changing winds and currents (Table 2) (Goreau, 2003). Cyclones and storms are the “agents of mortality” in the coral reef ecosystems and it may have a direct effect on the local distribution and structure of coral reef assemblages, predominantly by large waves produced by them (Dietzel et al., 2021). Declined salinity resulting from increased run-off to adjacent shore reefs and heavy rainfall during storms, or cyclones results in blooming algal growth besides other damaging outcomes (Baird et al., 2021; Kjerfve, 2021). The rising sea levels, resulting due to the expansion by increased temperature and the ice melting on land, exhibited variated trends in different parts of the world during the last century, showing an average approx. 20 cm rise (Tay et al., 2022).
The crucial physiological reef processes are jeopardized and intensified as a result of potential sedimentary processes activated by the increasing ocean levels like feeding, photosynthesis, and replenishment eventually inducing serious hazards to the coral reefs and their ecosystems like mangrove forests and seagrass meadows (Field et al., 2011; Woodroffe and Webster, 2014). This hazard along with the enhanced CO2 (carbon dioxide) release, adversely impacts the important ecosystems. In the absence of efficient strategies at the local level and without collaborative efforts to reduce CO2 emissions, these hazardous impacts are further intensified leading to the devastation of the ecological balance and biodiversity of the marine environment, which is unprecedented (Godoy and Lacerda, 2015).
Unplanned coastal development is not only a serious threat to coral reefs; it also leads to long-term socioeconomic loss. With the worldwide coastal population expected to double by 2050, coral reefs will be facing increased pressure from unmanaged development along the coasts. Building near the coast may smother corals through pollutants and sedimentation. According to Erftemeijer et al. (2012) and Cunning et al. (2019), recent studies have shown compelling evidence that dredging operations and port activities pose a serious threat to corals due to exacerbated sedimentation. This has resulted in mass coral mortality that ranges from thousands to millions of coral colonies. According to a recent finding from the Lakshadweep islands (Ravindran et al., 2014), even little coastal development may have a major negative impact on coral mortality, habitat degradation, and vulnerability. Furthermore, unless environmental sustainability policies are truly enforced, the ecological cost of these coastal development projects may significantly outweigh the economic value realized (Hussain et al., 2016).
The degradation of reef islands results in the loss of biodiversity. Coral reefs, also known as the “rainforests of the sea,” support a surprising range of marine life. These complex reef ecosystems sustain intricate interactions among animals, plants, and microbes. Degradation or loss of habitat is the major reasons of decline of the biodiversity (Hoekstra et al., 2005) destroying the crucial ecosystem structures and services. Scleractinian corals on coral reef ecosystems are important benefactors to both physical and biological structures of habitats, and evidence are there suggesting a sustained loss of corals in numerous geographical areas (Alvarez-Filip, 2019; Cinner et al., 2016) resulting in a decline of biodiversity and decay of crucial services being offered by the reef ecosystems (Hicks et al., 2019; Woodhead et al., 2019).
The fragile balance is damaged through the destruction of reef habitats by factors like pollution, climate change, and extensive fishing. Bleaching of corals resulting from rise of ocean temperatures led to the expulsion of algal symbionts and hence the death of the corals. Apart from the sustained loss of corals, the drifting of the composition of biota in the benthic habitats and reduction in topographic intricacy are playing role in the degradation of coral reef habitats (Eddy et al., 2018; Jackson et al., 2001). These alterations also accompany a reduction in the plethora of various corals reef related organisms, particularly non-coral invertebrates (Ferrario et al., 2014) and coral reef fishes (Worm et al., 2006; Sheppard et al., 2005). Alterations in the biological and physical structures of reef ecosystems may have intense intimations for the reef-related motile invertebrates and fishes and subsequently to the contributing ecological processes (Wilson et al., 2006; Pratchett et al., 2008; Stella et al., 2011). The most conspicuous severe loss to the corals is frequently related to the reduction in the diversity and overall abundance of other motile species primarily fishes (Wilson et al., 2006; Munday et al., 2008; Pratchett et al., 2018), although these reductions are usually manifested over many years (Graham et al., 2007; Pratchett et al., 2018).
Interspecific changes are there in the susceptibility of coral to loss in various reef-related fish species (Bellwood et al., 2006; Wilson et al., 2008), which depends on the specificity and nature of their territorial associations and dependence on corals. Responses to the behavior of some fishes also arbitrate the susceptibility of the loss of corals (Pratchett et al., 2018) primarily in areas where there are moderate disturbances and few of the coral species persist. This territorial damage affects directly a large number of species ranging from minute invertebrates to bigger predators. Furthermore, the damage to the vital species including herbivorous fishes that modulate the growth of algae may induce cascading impacts all throughout the reef ecosystems (Wismer et al., 2019). Algal growth proliferates with declined grazing stress resulting in the smothering of corals and outperforming numerous ocean organisms. This switching in the composition of the community may result in a decline in the resilience of ecosystem and the diversity of various species. The biodiversity loss has extensive consequences. Coral reefs are the source of numerous vital services to the ecosystem such as the production of fisheries, recreation, and protection of coastal areas. Damage to biodiversity hurts the offered services thereby impacting both global economies as well as local communities.
The structure and intricacy of coral reef habitats is primarily a characteristic of plenitude or Scleractinian cover (Graham et al., 2013). Nevertheless, the coral reef cover and complexity of structure can vary unaided if either the erect coral skeleton structural integrity is maintained after increased mortality of corals due to bleaching or predators of corals (Wilson et al., 2006) assemblage of corals gets dominated through growth forms having less complexity of structure (Alvarez-Filip et al., 2011) or geomorphic attributes may provide the basal complexities, instead of contemporary growth of corals (Graham et al., 2006). Declined covers of corals hinder the rates of calcification thereby impacting the stability and altogether the structure of the reefs. Reduced grazing by algal herbivorous fishes permits the proliferation of algae, corals smothering, and hurdling recovery of reefs.
Human populations have settled on the coastal areas of the tropics and coral reef islands as these reefs act as a natural efficient barrier against waves in coastal areas (Ferrario et al., 2014; Monismith et al., 2015). The most important threat predicted for the future is the rise in the sea levels especially for shallow areas (Dickinson, 2009). Nevertheless, on coral reefs, the interaction between the wave climate and the increased sea levels is supposed to be a crucial aspect in controlling the inundation of coastal areas and coastline erosions of the tropics in years to come (Storlazzi et al., 2015; Quataert et al., 2015). In live communities of corals on the coral reefs the three-dimensional structure (structure complexity) has an important role in squandering the energy of waves and hence safeguarding the coastal lines in tropical areas (Rogers et al., 2016). The enhanced complexity of structures in coral reefs develops in increased hydraulic harshness and enhanced frictional squandering of waves compared to other settings of the coastal areas. The increased squandering due to friction on coral reefs along with the breaking of waves on the rim of the reef produces increased wave energy squandering rates over comparatively small distances compared to different systems of coastal areas (Lowe et al., 2005).
Compared to various marine ecosystems the level of protection services in the coastal areas furnished by the coral reefs is much greater (Ferrario et al., 2014). Nevertheless, the potential of stressed and damaged coral reefs to manage the protection of coastal areas against waves and increasing levels of seas is becoming a great concern (Bellwood et al., 2004; Hoegh-Guldberg et al., 2007; Kennedy, 2013; Albert et al., 2016). The extensive damage to reef structuring corals will impact the efficacy of the coral reefs in squandering the energy of waves (Quataert et al., 2015) and grow vertically in reaction to the increasing levels of the sea (Woodroffe and Webster, 2014; Perry et al., 2015; Graham et al., 2015). A crucial loss of live corals resulted in a decline in the complexity of the structure of coral reefs (Alvarez-Filip et al., 2013) and frictional squandering of wave energy (Sheppard et al., 2005). Low squandering due to friction leads to increased heights of the waves at the backward reef environs and the destruction of the proximal zones of coastal areas in tropical island reefs and shores (Baldock et al., 2014). Moreover, if the accumulation of vertical reef is not able to pace up with the anticipated rate of rise in the sea levels then the decline in the breaker squandering of the crest of the reef will also permit the bigger waves to generate into the reef back environment (Storlazzi et al., 2015).
Degradation of the reef has ensuing effects on the abundance and distribution of the fish communities associated with it (Alvarez-Filip et al., 2015; Arias-Godínez et al., 2021; Pratchett et al., 2014), with intimations for communities of humans that are reef resource dependent for the livelihood and stipulation of various services of the ecosystems (Eddy et al., 2021). It has been reported that around 6 million reef fishers across the globe and fisheries of the coral reef are valued at 6 billion $ (Pratchett et al., 2014). Reefs and the associated fisheries of open water primarily furnished the vital source of proteins of animal origin for the people residing in most of the islands and the base for most of the cultural activities (Figure 2). This reduction in the supply of seafood results in a decreased level of income for fishers, affecting their potential to assist families and support the local economy (McClanahan, 2022). It’s possible that reef fish communities depend more on their underlying ecosystem than previously thought. Evidence points to habitat-limited recruitment as the cause of this dependency, however decreased food and shelter may also play a significant role in adult mortality. Reef fish families that depend less on coral, like the Lethrinidae and Lutjanidae, may experience less severe effects. This can only be verified, though, until we have a better understanding of these groups’ preferences for settlement sites. It might have an even more disastrous effect on small specialized families (like Gobiidae and Carancanthidae). Certain coral-dwelling gobies with limited geographic distributions may be at danger of going extinct (Bean et al., 2002). Furthermore, enhanced shortage results in price hikes of seafood, influencing consumers and the overall activity of the economy.
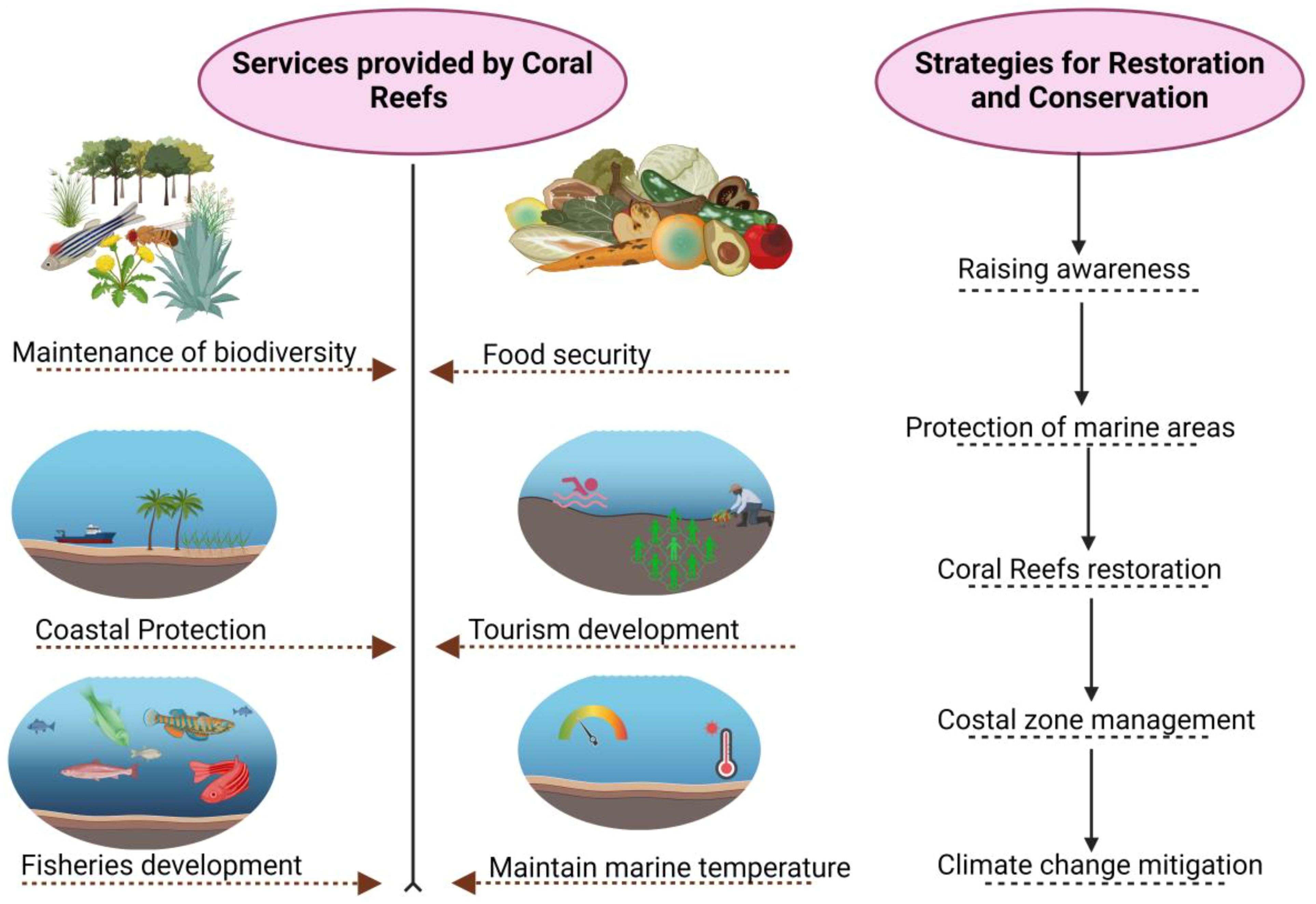
Figure 2. Ecosystem and socioeconomic services provided by coral reefs and strategies for the restoration and conservation of Coral reefs.
Ecotourism is a rapidly evolving field and these coral reefs are a vital source of attraction particularly for international tourists. In addition to destination changes and a decreased desire to pay for coral reefs, coral reef degradation may result in a drop in tourists and dives. The welfare of tourists accounts for a sizable amount of the economic losses, even though businesses and employees in the tourism industry also lose money. Although the extent varies by location, many tourists are aware of the deterioration of coral reefs. This awareness is typically linked to lower visitor pleasure, which lowers willingness to pay for coral reefs and lowers visitor welfare. Reefs lose their appeal to tourists as they deteriorate. Visitors are deterred by bleached, dead, or injured corals because they lose their natural beauty and vibrancy. An industry worth $6.4 billion annually in Australia alone is at risk due to the Great Barrier Reef’s degradation, which studies show has already resulted in a drop in tourists. In Indonesia, the South Pacific, and the Caribbean, the effect on commercial and subsistence tourism and fisheries already clearly demonstrated the impact of declining and destroyed coral reefs despite the presence of marine protected belts (Wabnitz, 2018; Siegel et al., 2019) which of course cannot give protection against climatic changes and endure the lack or absence of enforcement (Edgar and Stuart-Smith, 2014) as well as staff capacity of the protected areas.
Degradation of the ecosystems of Coral Reef Island greatly affects the nutritious and safe food availability i.e. compromising food security. Reefs are often termed as the “Sea Supermarkets” ensuring the availability of crucial proteins and livelihoods for communities residing in the coastal areas. Extensive fishing, destruction of habitats, and environmental pollution greatly reduce the population of fish thereby declining the seafood availability as a source of food, especially in areas where the primary protein source is fish (Hughes et al., 2012). Communities have to rely on other alternative food sources with a progressive decline in the stocks of fish which is often a poorly nutritious food option. In SIDS (small island developing states) fish is the most important food source (Cruz-Trinidad et al., 2014) encompassing 50 – 90% of the total dietary proteins from animal sources in Pacific islands and their territories and 37% in Southeast Asia (Lam et al., 2020; Teh et al., 2011). The amazing diversity of fish, invertebrates, and molluscs supported by coral reefs offers significant nutritional advantages. Bioavailable micronutrients like calcium, iron, and vitamin A, which are frequently deficient in diets throughout the tropics, are abundant in fish and other animal-based meals like beef, pork, and chicken. However, reef-associated fishes are especially high in vitamin A and omega 3 compared to other animal-source meals, and they also have similar amounts of other elements that are frequently deficient in diets. Compared to 134 g of chicken or 74 g of beef, a 90 g serving of an average reef fish would give a child an average of 33% of their minimum daily requirements across six micronutrients. Additionally, compared to other foods derived from animals, fish is typically more accessible and less expensive locally. Therefore, if sustainable, multi-species fisheries, like those from coral reefs, can sustain a variety of diets and provide the micronutrients required to maintain human health in the tropics. Insecurity of food may lead to malnutrition, especially in populations that are vulnerable like pregnant women and children.
Millions of people, mostly in developing nations, depend on coral reefs for their livelihoods. Degradation of coral reefs could pose serious socioeconomic risks to economies and people worldwide because human livelihoods are intimately linked to the oceans. There is a direct impact on the economy due to the destruction of the coral reefs in developing parts of the world by a decline in tourism especially diving tourists and reef fisheries. Millions of people worldwide depend heavily on coral reefs for their livelihoods. The three primary roles of coral reefs—shoreline protection, tourism, and fisheries production—realize over $30 billion in net benefits annually. The net benefits, when accrued over 50 years, will come to about $800 billion in net present value (Cesar et al., 2003). Also, there is an indirect cost involved if the shielding functions of reefs are taken away resulting in increased fragile coastline erosion and infrastructure destruction such as the cost of building concrete barriers in the sandy coastline of Maldives, and Male to safeguard the coral reefs is roughly 10 million US$ for each kilometer. Compared to fewer than 2% in high-income countries, tourism, and other ocean-related industries account for more than 20% of the GDP in several low-income countries and small island governments (Chaijaroen, 2022). There are typically few other options for employment and income in these impoverished states. The nine nations most at risk of coral reef deterioration are Kiribati, Fiji, Indonesia, Comoros, Vanuatu, Haiti, Grenada, the Philippines, and Indonesia. These nations rely heavily on reefs, which have a limited capacity for adaptability but are under a lot of threat (Burke et al., 2011).
Increasing marine temperatures particularly impact tropical corals due to their reduced tolerance (Berkelmans and Willis, 1999). Coastal erosion and flooding can be substantially reduced due to the presence of coral reefs by squandering almost 97% of the incident wave thermal energy (Table 3) (Ferrario et al., 2014). Coral reefs act like shallow crest water breaks with hydro dynamic conduct well characterized by various engineering coastal models (Hoeke et al., 2011; Taebi and Pattiaratchi, 2014; Quataert et al., 2015; Reguero et al., 2018a). By breaking waves and reducing their energy, coral reefs act as low-crested, submerged natural breakwaters that help prevent flooding. Reef depth and rugosity have a secondary role in these processes. Compared to conventional methods, coral reefs and other coastal habitats are expected to have significant and even cost-effective flood control effects (Reguero, et al., 2018b). There is a continuous loss of coral reefs across the globe and approximately 75% of the global coral reefs are considered threatened reefs (Hoegh-Guldberg et al., 2011; Mumby et al., 2007). Until the precise economic value of coral reefs is determined and factored into policy and management decisions, the damage to these ecosystems and the protection they provide will persist.
An appreciable role is played by the coral reefs in protecting coastal lines from destruction by storms and floods as well as against erosion. In more than 100 countries across the globe almost 150,000km of coastal line is protected (Burke et al., 2011). Meta-analyses of more than 200 studies suggest that coral reefs reduce the wave energy on average by roughly 97% and hence protect the coastal lines against storms and winds. A large part of this protection (86%) is from the crests of the coral reefs (Ferrario et al., 2014). This coral reef coastal line expectedly protects more than 100 million humans and therefore lowers the flooding risks and damages caused by storms. Several important trends were also identified by the literature review: First, coral reefs continuously lessen shoreline alteration, even when waves are 10 m or more. Second, both reef-protected and unprotected beaches usually completely recover in a year, with reef-protected locations frequently recovering more quickly. Third, additional storm-related factors also affect how much coastal erosion occurs. Storms with more erosive impact were those with longer durations, larger waves, and paths that caused wind reversals as they passed close to a beach. The properties of the storm and the reef will therefore affect the beach reaction, even if reefs lessen erosion from storm events (Cuttler et al., 2018).
Shifting the residents from the damaged islands or shoring up the shorelines of the ocean against elevating levels of the sea also results in additional impacts on the economies of the world (Wilkinson and Buddemeier, 1994). An economic “Rationalist” analysis revealed that it is better to surrender the cultures of islands rather than face the very high costs of managing the CO2 output across the globe (Adams, 1993). For example, Melanesian Indigenous tribes have a different perspective on coral reefs than do scientists because they usually see them through a spiritual lens and have a personal relationship to the ecology. The sea has enormous spiritual significance in Indigenous Melanesian culture. According to legend, “place spirits” reside on reefs and take the form of particular animals like rays, sharks, and dolphins. Sea burials, which are revered rituals that may result in the closure of entire reef areas to the public, are also frequent in coral reef zones (Foale, 2008). Shallow islands of coral will be abandoned with a mere 0.5 m rise of sea levels or even less due to the contamination of waters in the ground and erosion of coastal lines.
The present global destruction of coral reefs is an international issue that requires immediate consideration. By comprehending the extensive consequences of the destruction of Reef Island, it is clear that the issue needs a detailed and thoroughly designed strategy that will consider not only the socioeconomic factors but also the ecological factors into consideration. Many restorative approaches are being applied to counter the damages caused to the reefs.
Several reports revealed awareness of the significant damages to the reefs as a result of fishing and mining by employing destructive techniques besides those that are directly related to these activities in the reef islands (Perera et al., 2002). Nevertheless, the damaging impacts of these techniques are impacting other populations like tourists fellow fishermen, etc. Hence, increasing awareness among the broader group of people is a must to help support the enforcement of legal and protective measures. For coral reefs to survive, awareness-building regarding restoration is essential. Educating the public through workshops and social media campaigns, including communities in volunteer programs and citizen science initiatives, pushing for legislative reforms that save coral reefs, and motivating people via artistic expression and storytelling are all examples of successful tactics (Hesley et al., 2017). It seems especially vital to enhance the awareness and will of the people involved in political circles.
Indirect or passive strategies like launching Marine Protected Areas must target the decline of pressures on the coral reef islands supporting these to recover naturally. Natural recovery with no human involvement (Passive recovery) seems to be progressively insufficient (Ortiz-Lozano et al., 2018) as the bleaching of corals and their mortality incidences are getting more and more serious and frequent, thereby increasing the local anthropogenic stressors effects like pollution and extensive fishing (Hughes et al., 2018). Although MPAs protect about 18.7% of the world’s 527,072 km2 of coral reefs, less than 0.01% of them are low-risk, no-take MPAs that do not allow poaching (Mora et al., 2006). MPAs (Marine Protected Areas) are categorized as the most efficient techniques to recover coral reefs and their associated ocean systems (McLeod et al., 2009), nevertheless, these do not inevitably furnish safety against thermal impacts (Graham et al., 2007). Hence, proper investigation is necessary to help select the reef islands prone to destruction due to increasing temperatures (Obura et al., 2017; McClanahan et al., 2005).
Viable reclamation strategies like transplantation of corals and fragmentation at the micro level may enhance the recovery of reefs in the affected areas. Direct or active mediation like the transplantation of corals or reef artificial deployment targets reef rehabilitation by replacing the functional or structural features, at least, of the reef ecosystems that are lost or declined (Gomez et al., 2010). Direct transplantation of coral fragments from a donor reef to a recipient reef is the oldest and most popular technique of coral regeneration. The review database contains 94 descriptions of direct transplantation, which accounts for 20% of all records. Programs designed to save corals from planned construction projects that would otherwise destroy or disturb the colonies are the ones that use this technique the most. According to direct transplantation studies, transplanted corals had an average survival rate of 64% overall, with 20% having a survival rate of >90% (interactive database). Fast-growing corals have been the main focus of direct transplanting; branching coral morphologies have been used in over three-quarters of case studies (Young et al., 2012). Direct reef retrieval may be successful in the long term provided it also involves (a) Novel inventions like abetted recruitment and fertilization (Nakamura et al., 2011; dela Cruz et al., 2015); (b) conducts that increase the pace of adaptation or natural selection involving supported gene flow and orchestrated shifting (Aitken and Whitlock, 2013; Van Oppen et al., 2017) along with the feasible manifestation of abetted evolution and adaptation (Van Oppen et al., 2017); and (c) concentration of the preservation and increase in the diversity of various species and genetic variation in the populations of reef and orchestrated assembly of various species to fully augment the species consortia to its best (Zoccola et al., 2020).
Execution of these strategies will depend primarily on the direct reclamation in the areas where extensive fishing, pollution, and various human-triggered stressors onto the reefs are managed well. Direct restoration nonetheless aims at a very limited number of species and is also restricted to little spatial levels: even efforts at large scale stumble to reclaim a single hectare every year. The efficacy of the direct restoration hence is dependent on efforts to harvest population connectivity of corals to speed up the establishment of the thermal tolerant corals i.e. concentration reclamation on coral reefs where dispersal of larvae and their replenishment may trigger year-to-year dispersal and establishment over extended areas (Hock et al., 2017; Walsworth et al., 2019). In a few areas, restoration of structure is also required to improve the ecological reclamation of coastal lines (Beck et al., 2018) and the production of fishes (Rogers et al., 2018).
Execution of a comprehensive scheme encompassing land use management, management of water quality, and coastal safety ensures the health preservation of the coral reef ecosystem, however, approaches including traditional passive practices like enhancing the quality of water in catchments and the employment of marine no-take zones for the protection of coral reefs from immediate damaging acts have failed to a great extent in combating climate change affects (Suggett et al., 2023). The association of several regulatory bodies that supervise marine development with stakeholders in the private sector is pivotal to the fruitful ICZM implementation. The task demands unwavering government dedication to a coordinating system, like an interministerial commission or council comprising representation from all the private and public sectors. Besides, strategies ensuring appropriate execution are necessary, for instance, authoritative clarification, economic incentives, and accountable lead agencies such as retaining the funding for infrastructure till the project is implemented or completed. Consequently, efforts are made increasingly on active strategies of enhancing the coral reef persistence.
It is essential to decline the emissions of greenhouse gases and implement adaptive strategies to safeguard the coral reef ecosystems protecting these against hazards of climatic changes. Huge losses of the coral reefs and structure of reefs are anticipated if global warming increases by 1.5°C over the average pre-industrial temperatures (Hoegh-Guldberg et al., 2018), and the most efficient strategy to decrease the coral reefs decline would be an effective and quick alleviation of emissions of greenhouse gases (Hoegh-Guldberg et al., 2019). Besides alleviating greenhouse gas emissions, SRM (solar radiation modification) is another efficient means. Solar radiation modification comprises hypothetical approaches to divert sunlight to space like stratospheric aerosol injections which may act at a comprehensive scale, brightening the ocean cloud at the local scales. SRM is not condoned as it can’t decrease the acidification of the ocean and also it offers important risks and suspicions (Gattuso et al., 2018).
Tropical reef island ecosystems face unprecedented threats from local and global stressors, including pollution, invasive species, coastal development, climate change, and overexploitation. These factors are leading to widespread degradation and loss of biodiversity. The degradation of reefs has far-reaching impacts on the coastal economy, communities, and cultural heritage. Effective conservation and restoration measures are essential to preserve and restore these invaluable ecosystems. Coral reef restoration recommendations place a high priority on mitigating the effects of climate change by lowering greenhouse gas emissions, encouraging renewable energy sources, and enhancing energy efficiency. Sustainable development, pollution prevention, and overfishing control are all essential components of effective coastal zone management. For instance, the implementation of Marine Protected Areas (MPAs) has proven successful in reducing human impact and promoting reef recovery. Additionally, active restoration techniques such as coral gardening, where healthy coral fragments are cultivated and transplanted back to degraded reefs, have shown promising results. Community-based management initiatives, involving local stakeholders in the planning and execution of conservation efforts, have also been effective in ensuring sustainable practices and enhancing resilience. Lastly, to modify and enhance restoration tactics, ongoing study, observation, and assessment are required. By addressing the root causes of deterioration, implementing integrated management plans, and fostering community involvement, we can ensure that future generations benefit from healthy tropical reef island ecosystems.
SN: Conceptualization, Writing – original draft. RK: Software, Writing – review & editing. XD: Writing – review & editing. CW: Funding acquisition, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Financial Fund of the Ministry of Agriculture and Rural Affairs, P. R. of China (NFZX2024).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abelson A. (2020). Are we sacrificing the future of coral reefs on the altar of the “climate change” narrative?. ICES J. Mar. Sci. 77 (1), 40–45.
Abu-Hilal A., Al-Najjar T. (2009). Marine litter in coral reef areas along the Jordan Gulf of Aqaba, Red Sea. J. Environ. Manage. 90, 1043–1049. doi: 10.1016/j.jenvman.2008.03.014
Adams A. J. (1993). Dynamics of fish assemblages associated with an offshore artificial reef in the southern Mid-Atlantic Bight. Dissertations, Theses, and Masters Projects. William & Mary. Paper 1539617663. doi: 10.25773/v5-81y6-m990
Aitken S. N., Whitlock M. C. (2013). Assisted gene flow to facilitate local adaptation to climate change. Annu. Rev. Ecology Evolution Systematics 44, 367–388. doi: 10.1146/annurev-ecolsys-110512-135747
Albert S., Leon J. X., Grinham A. R., Church J. A., Gibbes B. R., Woodroffe C. D. (2016). Interactions between sea-level rise and wave exposure on reef island dynamics in the Solomon Islands. Environ. Res. Lett. 11, 54011. doi: 10.1088/1748-9326/11/5/054011
Al-Masroori H., Al-Oufi H., McShane P. (2009). Causes and mitigations on trap ghost fishing in Oman: scientific approach to local fishers’ perception. J. Fisheries Aquat. Sci. 4, 129–135. doi: 10.3923/jfas.2009.129.135
Alvarez-Filip L., Carricart-Ganivet J. P., Horta-Puga G., Iglesias-Prieto R. (2013). Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci. Rep. 3, 3486. doi: 10.1038/srep03486
Alvarez-Filip L., Gill J. A., Dulvy N. K., Perry A. L., Watkinson A. R., Côté I. M. (2011). Drivers of region-wide declines in architectural complexity on Caribbean reefs. Coral Reefs 30, 1051–1060. doi: 10.1007/s00338-011-0795-6
Alvarez-Filip L., Paddack M. J., Collen B., Robertson D. R., Côté I. M. (2015). Simplification of Caribbean reef-fish assemblages over decades of coral reef degradation. PloS One 10, e0126004. doi: 10.1371/journal.pone.0126004
Alvarez-Filip L., Estrada-Saldívar N., Pérez-Cervantes E., Molina-Hernández A., González-Barrios F. J. (2019). A rapid spread of the stony coral tissue loss disease outbreak in the mexican caribbean. PeerJ 7, e8069. doi: 10.7717/peerj.8069
Arias-Godínez G., Jiménez C., Gamboa C., Cortés J., Espinoza M., Beita-Jiménez A., et al. (2021). The effect of coral reef degradation on the trophic structure of reef fishes from Bahía Culebra, North Pacific coast of Costa Rica. J. Coast. Conserv. 25, 8. doi: 10.1007/s11852-021-00802-x
Baeta F., Costa M. J., Cabral H. (2009). Trammel nets’ ghost fishing off the Portuguese central coast. Fisheries Res. 98, 33–39. doi: 10.1016/j.fishres.2009.03.009
Baird M. E., Mongin M., Rizwi F., Bay L. K., Cantin N. E., Morris L. A., et al. (2021). The effect of natural and anthropogenic nutrient and sediment loads on coral oxidative stress on runoff-exposed reefs. Mar. pollut. Bull. 168, 112409. doi: 10.1016/j.marpolbul.2021.112409
Baker A. C. (2004). “Symbiont diversity on coral reefs and its relationship to bleaching resistance and resilience,” in Coral health and disease (Springer Berlin Heidelberg, Berlin, Heidelberg), 177–194.
Baldock T. E., Golshani A., Callaghan D. P., Saunders M. I., Mumby P. J. (2014). Impact of sea-level rise and coral mortality on the wave dynamics and wave forces on barrier reefs. Mar. pollut. Bull. 83, 155–164. doi: 10.1016/j.marpolbul.2014.03.058
Bean K., Jones G. P., Caley M. J. (2002). Relationships among distribution, abundance and microhabitat specialisation in a guild of coral reef triggerfish (family balistidae). Mar. Ecol. Prog. Ser. 233, 263–272. doi: 10.3354/meps233263
Beck M. W., Losada I. J., Menéndez P., Reguer B. G., Díaz-Simal P., Fernández F. (2018). The global flood protection savings provided by coral reefs. Nat. Commun. 9, 2186. doi: 10.1038/s41467-018-04568-z
Bellwood D. R., Hoey A. S., Ackerman J. L., Depczynski M. (2006). Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Global Change Biol. 12, 1587–1594. doi: 10.1111/j.1365-2486.2006.01204.x
Bellwood D. R., Hughes T. P., Folke C., Nyström M. (2004). Confronting the coral reef crisis. Nature 429, 827–833. doi: 10.1038/nature02691
Bellwood D. R., Tebbett S. B., Bellwood O., Mihalitsis M., Morais R. A., Streit R. P., et al. (2018). The role of the reef flat in coral reef trophodynamics: Past, present, and future. Ecol. Evol. 8, 4108–4119. doi: 10.1002/ece3.3967
Ben-Haim Y., Thompson F. L., Thompson C. C., Cnockaert M. C., Hoste B., Swings J., et al. (2003). Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Systematic Evolutionary Microbiol. 53, 309–315. doi: 10.1099/ijs.0.02402-0
Berkelmans R., Willis B. L. (1999). Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18, 219–228. doi: 10.1007/s003380050186
Brodie J., Fabricius K., De’ath G., Okaji K. (2005). Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. pollut. Bull. 51, 266–278. doi: 10.1016/j.marpolbul.2004.10.035
Burke L., Reytar K., Spalding M., Perry A. (2011). Reefs at risk revisited (Washington, DC: World Resources Institute (WRI). Available at: https://bvearmb.do/handle/123456789/1787.
Büscher J. V., Form A. U., Riebesell U. (2017). Interactive effects of ocean acidification and warming on growth, fitness and survival of the cold-water coral Lophelia pertusa under different food availabilities. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00101
Butler J. R., Gunn R., Berry H. L., Wagey G. A., Hardesty B. D., Wilcox C. (2013). A value chain analysis of ghost nets in the arafura sea: identifying trans-boundary stakeholders, intervention points and livelihood trade-offs. J. Environ. Manage. 123, 14–25. doi: 10.1016/j.jenvman.2013.03.008
Chaijaroen P. (2022). “Coral reef deterioration and livelihoods of coastal communities: an economics perspective,” in Corals-Habitat Formers in the Anthropocene (Rayong, Thailand: IntechOpen, Vidyasirimedhi Institute of Science and Technology). doi: 10.5772/intechopen.105355
Cheal A. J., MacNeil M. A., Emslie M. J., Sweatman H. (2017). The threat to coral reefs from more intense cyclones under climate change. Global Change Biol. 23, 1511–1524. doi: 10.1111/gcb.13593
Cheng F., Su F., Chen M., Wang Q., Jiang H., Wang X. (2019). An evolving assessment model for environmental carrying capacity: A case study of coral reef islands. J. Environ. Manage. 233, 543–552. doi: 10.1016/j.jenvman.2018.12.047
Chiappone M., Dienes H., Swanson D. W., Miller S. L. (2005). Impacts of lost fishing gear on coral reef sessile invertebrates in the Florida Keys National Marine Sanctuary. Biol. Conserv. 121, 221–230. doi: 10.1016/j.biocon.2004.04.023
Cinner J. E., Huchery C., MacNeil M. A., Graham N. A., McClanahan T. R., Maina J., et al. (2016). Bright spots among the world’s coral reefs. Nature 535, 416–419. doi: 10.1038/nature18607
Cornwall C. E., Comeau S., Kornder N. A., Perry C. T., van Hooidonk R., DeCarlo T. M., et al. (2021). Global declines in coral reef calcium carbonate production under ocean acidification and warming. Proc. Natl. Acad. Sci. 118, e2015265118. doi: 10.1073/pnas.2015265118
Cruz-Trinidad A., Aliño P. M., Geronimo R. C., Cabral R. B. (2014). Linking food security with coral reefs and fisheries in the coral triangle. Coast. Manage. 42, 160–182. doi: 10.1080/08920753.2014.877761
Cunning R., Silverstein R. N., Barnes B. B., Baker A. C. (2019). Extensive coral mortality and critical habitat loss following dredging and their association with remotely-sensed sediment plumes. Mar. pollut. Bull. 145, 185–199. doi: 10.1016/j.marpolbul.2019.05.027
Cuttler M. V. W., Hansen J. E., Lowe R. J., Drost E. J. F. (2018). Response of a fringing reef coastline to the direct impact of a tropical cyclone. Limnology Oceanography Lett. 3, 31–38. doi: 10.1002/lol2.10067
Dameron O. J., Parke M., Albins M. A., Brainard R. (2007). Marine debris accumulation in the Northwestern Hawaiian Islands: an examination of rates and processes. Mar. pollut. Bull. 54, 423–433. doi: 10.1016/j.marpolbul.2006.11.019
de Carvalho-Souza G. F., Llope M., Tinôco M. S., Medeiros D. V., Maia-Nogueira R., Sampaio C. L. (2018). Marine litter disrupts ecological processes in reef systems. Mar. pollut. Bull. 133, 464–471. doi: 10.1016/j.marpolbul.2018.05.049
dela Cruz D. W., Rinkevich B., Gomez Ed Yap H. T. (2015). Assessing an abridged nursery phase for slow growing corals used in coral restoration. Ecol. Eng. 84, 408–415. doi: 10.1016/j.ecoleng.2015.09.042
Dickinson W. R. (2009). Pacific atoll living: How long already and until when. GSA Today 19, 4–10. doi: 10.1130/GSATG35A.1
Dietzel A., Bode M., Connolly S. R., Hughes T. P. (2021). The population sizes and global extinction risk of reef-building coral species at biogeographic scales. Nat. Ecol. Evol. 5, 663–669. doi: 10.1038/s41559-021-01393-4
Donner S. D., Skirving W. J., Little C. M., Oppenheimer M., Hoegh-Guldberg O. V. E. (2005). Global assessment of coral bleaching and required rates of adaptation under climate change. Global Change Biol. 11, 2251–2265. doi: 10.1111/j.1365-2486.2005.01073.x
Eddy T. D., Cheung W. W., Bruno J. F. (2018). Historical baselines of coral cover on tropical reefs as estimated by expert opinion. PeerJ 6, e4308. doi: 10.7717/peerj.4308
Eddy T. D., Lam V. W., Reygondeau G., Cisneros-Montemayor A. M., Greer K., Palomares M. L. D., et al. (2021). Global decline in capacity of coral reefs to provide ecosystem services. One Earth 4, 1278–1285. doi: 10.1016/j.oneear.2021.08.016
Edgar G., Stuart-Smith R. (2014). Systematic global assessment of reef fish communities by the reef life survey program. Sci. Data 1, 140007. doi: 10.1038/sdata.2014.7
Edyvane K. S., Penny S. S. (2017). Trends in derelict fishing nets and fishing activity in northern Australia: Implications for trans-boundary fisheries management in the shared Arafura and Timor Seas. Fisheries Res. 188, 23–37. doi: 10.1016/j.fishres.2016.11.021
Erftemeijer P. L., Riegl B., Hoeksema B. W., Todd P. A. (2012). Environmental impacts of dredging and other sediment disturbances on corals: a review. Mar. pollut. Bull. 64, 1737–1765. doi: 10.1016/j.marpolbul.2012.05.008
Ferrario F., Beck M. W., Storlazzi C. D., Micheli F., Shepard C. C., Airoldi L. (2014). The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat. Commun. 5, 1–9. doi: 10.1038/ncomms4794
Field M. E., Ogston A. S., Storlazzi C. D. (2011). Rising sea level may cause decline of fringing coral reefs. Eos Trans. Am. Geophysical Union 92, 273–274. doi: 10.1029/2011EO330001
Foale S. J. (2008). Conserving Melanesia’s coral reef heritage in the face of climate change. Historic Environment 21(1), 30–36. doi: 10.3316/ielapa.485808643249656
Frieler K., Meinshausen M., Golly A., Mengel M., Lebek K., Donner S. D., et al. (2013). Limiting global warming to 2 C is unlikely to save most coral reefs. Nat. Climate Change 3, 165–170. doi: 10.1038/nclimate1674
Gall S. C., Thompson R. C. (2015). The impact of debris on marine life. Mar. pollut. Bull. 92, 170–179. doi: 10.1016/j.marpolbul.2014.12.041
Gattuso J. P., Magnan A. K., Bopp L., Cheung W. W., Duarte C. M., Hinkel J., et al. (2018). Ocean solutions to address climate change and its effects on marine ecosystems. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00337
Gilardi K. V., Carlson-Bremer D., June J. A., Antonelis K., Broadhurst G., Cowan T. (2010). Marine species mortality in derelict fishing nets in Puget Sound, WA and the cost/benefits of derelict net removal. Mar. pollut. Bull. 60, 376–382. doi: 10.1016/j.marpolbul.2009.10.016
Gilman E. (2015). Status of international monitoring and management of abandoned, lost and discarded fishing gear and ghost fishing. Mar. Policy 60, 225–239. doi: 10.1016/j.marpol.2015.06.016
Goatley C. H., Bonaldo R. M., Fox R. J., Bellwood D. R. (2016). Sediments and herbivory as sensitive indicators of coral reef degradation. Ecol. Soc. 21. doi: 10.5751/ES-08334-210129
Godoy M. D., Lacerda L. D. D. (2015). Mangroves response to climate change: a review of recent findings on mangrove extension and distribution. Anais da Academia Bras. Ciências 87, 651–667. doi: 10.1590/0001-3765201520150055
Goreau T. J., Hilbertz W., Azeez A., Hakeem A., Dodge R., Despaigne G., et al. (2003). “Restoring coral reefs, oyster banks, and fisheries by seawater electrolysis: coastal zone management and tourism applications,” in Oceans 2003. celebrating the past... teaming toward the future (IEEE cat. no. 03CH37492), vol. 2. (IEEE), 755–. doi: 10.1109/OCEANS.2003.178407
Graham N. A., Bellwood D. R., Cinner J. E., Hughes T. P., Norström A. V., Nyström M. (2013). Managing resilience to reverse phase shifts in coral reefs. Front. Ecol. Environ. 11, 541–548. doi: 10.1890/120305
Graham N. A., Jennings S., MacNeil M. A., Mouillot D., Wilson S. K. (2015). Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97. doi: 10.1038/nature14140
Graham N. A., Wilson S. K., Jennings S., Polunin N. V., Bijoux J. P., Robinson J. (2006). Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl. Acad. Sci. 103, 8425–8429. doi: 10.1073/pnas.0600693103
Graham N. A., Wilson S. K., Jennings S., Polunin N. V., Robinson J. A. N., Bijoux J. P., et al. (2007). Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 21, 1291–1300. doi: 10.1111/j.1523-1739.2007.00754.x
Hesley D., Burdeno D., Drury C., Schopmeyer S., Lirman D. (2017). Citizen science benefits coral reef restoration activities. J. Nat. Conserv. 40, 94–99. doi: 10.1016/j.jnc.2017.09.001
Hicks C. C., Cohen P. J., Graham N. A., Nash K. L., Allison E. H., D’Lima C., et al. (2019). Harnessing global fisheries to tackle micronutrient deficiencies. Nature 574, 95–98. doi: 10.1038/s41586-019-1592-6
Hock K., Wolff N. H., Ortiz J. C., Condie S. A., Anthony K. R., Blackwell P. G., et al. (2017). Connectivity and systemic resilience of the Great Barrier Reef. PloS Biol. 15, e2003355. doi: 10.1371/journal.pbio.2003355
Hoegh-Guldberg O., Kennedy E. V., Beyer H. L., McClennen C., Possingham H. P. (2018). Securing a long-term future for coral reefs. Trends Ecol. Evol. 33, 936–944. doi: 10.1016/j.tree.2018.09.006
Hoegh-Guldberg O., Mumby P. J., Hooten A. J., Steneck R. S., Greenfield P., Gomez E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Hoegh-Guldberg O., Ortiz J. C., Dove S. (2011). The future of coral reefs. Science 334, 1494–1495. doi: 10.1126/science.334.6062.1494-b
Hoegh-Guldberg O., Pendleton L., Kaup A. (2019). People and the changing nature of coral reefs. Regional Stud. Mar. Sci. 30, 100699. doi: 10.1016/j.rsma.2019.100699
Hoeke R., Storlazzi C., Ridd P. (2011). Hydrodynamics of a bathymetrically complex fringing coral reef embayment: Wave climate, in situ observations, and wave prediction. J. Geophysical Research: Oceans 116. doi: 10.1029/2010JC006170
Hoekstra J. M., Boucher T. M., Ricketts T. H., Roberts C. (2005). Confronting a biome crisis: global disparities of habitat loss and protection. Ecol. Lett. 8, 23–29. doi: 10.1111/j.1461-0248.2004.00686.x
Hughes T. P., Baird A. H., Bellwood D. R., Card M., Connolly S. R., Folke C., et al. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. doi: 10.1126/science.1085046
Hughes T. P., Graham N. A., Jackson J. B., Mumby P. J., Steneck R. S. (2010). Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642. doi: 10.1016/j.tree.2010.07.011
Hughes T. P., Kerry J. T., Álvarez-Noriega M., Álvarez-Romero J. G., Anderson K. D., Baird A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Hughes T. P., Kerry J. T., Baird A. H., Connolly S. R., Dietzel A., Eakin C. M., et al. (2018). Global warming transforms coral reef assemblages. Nature 556, 492–496. doi: 10.1038/s41586-018-0041-2
Hughes T. P., Rodrigues M. J., Bellwood D. R., Ceccarelli D., Hoegh-Guldberg O., McCook L., et al. (2007). Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 17, 360–365. doi: 10.1016/j.cub.2006.12.049
Hughes S., Yau A., Max L., Petrovic N., Davenport F., Marshall M., et al. (2012). A framework to assess national level vulnerability from the perspective of food security: The case of coral reef fisheries. Environ. Sci. Policy 23, 95–108. doi: 10.1016/j.envsci.2012.07.012
Hussain A., De K., Thomas L., Nagesh R., Mote S., Ingole B. (2016). Prevalence of skeletal tissue growth anomalies in a scleractinian coral: Turbinaria mesenterina of Malvan Marine Sanctuary, eastern Arabian Sea. Dis. Aquat. Organisms 121, 79–83. doi: 10.3354/dao03038
Jackson J. B. C., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Jouffray J. B., Nyström M., Norström A. V., Williams I. D., Wedding L. M., Kittinger J. N., et al. (2015). Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Philos. Trans. R. Soc. B: Biol. Sci. 370, 20130268. doi: 10.1098/rstb.2013.0268
Karasik R., Pickle A., Roady S. A., Vegh T., Virdin J.. (2019). Analysis of policies related to the protection of coral reefs: analysis of global and regional policy instruments and governance mechanisms related to the protection and sustainable management of coral reefs. United Nations Environment Programme (UNEP). Recuperado de. Available online at: https://bvearmb.do/handle/123456789/3196.
Kennedy E. V. (2013). Climate change impacts on Caribbean coral reefs: reef accretion and scope for acclimation through symbiont genetic diversity. (Townsville, Australia: Australian Institute of Marine Sciences).
Kjerfve B., McField M., Thattai D., Giró A. (2021). Coral reef health in the gulf of honduras in relation to fluvial runoff, hurricanes, and fishing pressure. Mar. pollut. Bull. 172, 112865. doi: 10.1016/j.marpolbul.2021.112865
Knowlton N., Corcoran E., Felis T., de Goeij J., Grottoli A., Harding S., et al. (2021). Rebuilding coral reefs: a decadal grand challenge. doi: 10.53642/NRKY9386
Kühn S., Bravo Rebolledo E. L., Van Franeker J. A. (2015). “Deleterious effects of litter on marine life,” in Marine anthropogenic litter, 75–116.
Kumar L., Taylor S. (2015). Exposure of coastal built assets in the South Pacific to climate risks. Nat. Clim Change 5, 992–996. doi: 10.1038/nclimate2702
Lam V. Y., Doropoulos C., Bozec Y. M., Mumby P. J. (2020). Resilience concepts and their application to coral reefs. Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.00049
Lamb J. B., Willis B. L., Fiorenza E. A., Couch C. S., Howard R., Rader D. N., et al. (2018). Plastic waste associated with disease on coral reefs. Science 359, 460–462. doi: 10.1126/science.aar3320
Lebreton L., Slat B., Ferrari F., Sainte-Rose B., Aitken J., Marthouse R., et al. (2018). Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 8, 1–15. doi: 10.1038/s41598-018-22939-w
Liao B., Xiao B., Li Z. (2019). “Coral reef ecosystem,” in Symbiotic Microbiomes of Coral Reefs Sponges and Corals. Ed. Li Z. (Springer, Dordrecht). doi: 10.1007/978-94-024-1612-1_1
Lough J. M., Barnes D. J. (1989). Possible relationships between environmental variables and skeletal density in a coral colony from the central great barrier reef. J. Exp. Mar. Biol. Ecol. 134 (3), 221–241. doi: 10.1016/0022-0981(89)90071-3
Lowe R. J., Falter J. L., Bandet M. D., Pawlak G., Atkinson M. J., Monismith S. G., et al. (2005). Spectral wave dissipation over a barrier reef. J. Geophysical Research: Oceans 110. doi: 10.1029/2004JC002711
McClanahan T. R. (2022). Fisheries yields and species declines in coral reefs. Environ. Res. Lett. 17, 044023. doi: 10.1088/1748-9326/ac5bb4
McClanahan T., Davies J., Maina J. (2005) Factors influencing resource users and managers’ perceptions towards marine protected area management in Kenya. Environmental Conserv. 32 (1), 42–49. doi: 10.1017/S0376892904001791
McLeod E., Salm R., Green A., Almany J. (2009). Designing marine protected area networks to address the impacts of climate change. Front. Ecol. Environ. 7, 362–370. doi: 10.1890/070211
Moberg F., Folke C. (1999). Ecological goods and services of coral reef ecosystems. Ecol. economics 29 (2), 215–233. doi: 10.1016/S0921-8009(99)00009-9
Monismith S. G., Rogers J. S., Koweek D., Dunbar R. B. (2015). Frictional wave dissipation on a remarkably rough reef. Geophysical Res. Lett. 42, 4063–4071. doi: 10.1002/2015GL063804
Mora C., Andréfouët S., Costello M. J., Kranenburg C., Rollo A., Veron J., et al. (2006). Coral reefs and the global network of marine protected areas. Science 312 (5781), 1750–1751. doi: 10.1126/science.1125295
Morishige C., McElwee K. (2012). At-sea detection of derelict fishing gear in the North Pacific: an overview. Mar. pollut. Bull. 65, 1–6. doi: 10.1016/j.marpolbul.2011.05.017
Mulya M. B., Aldyza N., Afkar A. (2023). The prevalence of coral health issues in the conservation area of Benteng, Weh Island, Sabang, Indonesia. Available online at: http://www.bioflux.com.ro/docs/2023.1166-1176.pdf.
Mumby P. J., Hastings A., Edwards H. J. (2007). Thresholds and the resilience of Caribbean coral reefs. Nature 450, 98–101. doi: 10.1038/nature06252
Munday P. L., Jones G. P., Pratchett M. S., Williams A. J. (2008). Climate change and the future for coral reef fishes. Fish Fisheries 9, 261–285. doi: 10.1111/j.1467-2979.2008.00281.x
Nakamura M., Ohki S., Suzuki A., Sakai K. (2011). Coral larvae under ocean acidification: survival, metabolism, and metamorphosis. PloS One 6, e14521. doi: 10.1371/journal.pone.0014521
Naranjo-Elizondo B., Cortés J. (2018). Observations of litter deposited in the deep waters of Isla del Coco National Park, Eastern Tropical Pacific. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00091
Neumann B., Vafeidis A. T., Zimmermann J., Nicholls R. J. (2015). Future coastal population growth and exposure to sea-level rise and coastal flooding-a global assessment. PloS One 10, e0118571. doi: 10.1371/journal.pone.0118571
Niaounakis M. (2017). The problem of marine plastic debris. In: Ebnesajjad S (ed) Management of marine plastic debris: prevention, recycling, and waste management. (Chadds Ford: Elsevier), pp 1–55.
Norström A. V., Nyström M., Lokrantz J., Folke C. (2009). Alternative states on coral reefs: beyond coral–macroalgal phase shifts. Mar. Ecol. Prog. Ser. 376, 295–306. doi: 10.3354/meps07815
Obura D., Gudka M., Rabi F. A., Gian S. B., Bijoux J., Freed S., et al. (2017). “Coral reef status report for the Western Indian Ocea,” in Nairobi convention (Global Coral Reef Monitoring Network (GCRMN)/International Coral Reef Initiative (ICRI). (Mauritius), pp 144.
Ortiz-Lozano L., Colmenares-Campos C., Gutiérrez-Velázquez A. (2018). Submerged Coral Reefs in the Veracruz Reef System, Mexico, and its implications for marine protected area management. Ocean Coast. Manage. 158, 11–23. doi: 10.1016/j.ocecoaman.2018.03.012
Pandolfi J. M., Bradbury R. H., Sala E., Hughes T. P., Bjorndal K. A., Cooke R. G., et al. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958. doi: 10.1126/science.1085706
Perera C., Wilhelmsson D., Rajasuriya A. (2002). “Reef fisheries and coral reef degradation in Sri Lanka,” in Coral Reef Degradation in the Indian Ocean, vol. 149.
Perry C. T., Murphy G. N., Graham N. A., Wilson S. K., Januchowski-Hartley F. A., East H. K. (2015). Remote coral reefs can sustain high growth potential and may match future sea-level trends. Sci. Rep. 5, 18289. doi: 10.1038/srep18289
Pratchett M. S., Hoey A. S., Wilson S. K. (2014). Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Curr. Opin. Environ. Sustainability 7, 37–43. doi: 10.1016/j.cosust.2013.11.022
Pratchett M. S., Sf H., Mellin C., Cumming G. S. (2021). “Recurrent mass-bleaching and the potential for ecosystem collapse on Australia’s great barrier reef,” in Ecosystem Collapse and Climate Change, vol. 241 . Eds. Canadell J. G., Jackson R. B. (Springer, Cham). doi: 10.1007/978-3-030-71330-0_10
Pratchett M. S., Thompson C. A., Hoey A. S., Cowman P. F., Wilson S. K. (2018). Effects of coral bleaching and coral loss on the structure and function of reef fish assemblages. . In: van Oppen M., Lough J. (eds) Coral Bleaching. Ecological Studies, vol 233. Springer, Cham. doi: 10.1007/978-3-319-75393-5_11
Pratchett M. S., Munday P. L., Wilson S. K., Graham N. A., Cinner J. E., Bellwood D. R., et al. (2008). “Effects of climate-induced coral bleaching on coral-reef fishes–ecological and economic consequences,” in Oceanography and marine biology (CRC Press), 257–302.
Principe S. C., Acosta A. L., Andrade J. E., Lotufo T. M. (2021). Predicted shifts in the distributions of Atlantic reef-building corals in the face of climate change. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.673086
Quataert E., Storlazzi C., Van Rooijen A., Cheriton O., Van Dongeren A. (2015). The influence of coral reefs and climate change on wave-driven flooding of tropical coastlines. Geophysical Res. Lett. 42, 6407–6415. doi: 10.1002/2015GL064861
Ravindran J., Manikandan B., Venkatesh M., ManiMurali R., Marimuthu N., Wafar M. V. M. (2014). Repercussions of embarkation wharves in Lakshadweep Islands on coral communities and their ecology. Indian J. Geo-Mar. Sci. Available online at: http://drs.nio.org/drs/handle/2264/7633.
Reguero B. G., Losada I. J., Diaz-Simal P., Mendez F. J., Beck M. W. (2015). Effects of climate change on exposure to coastal flooding in latin america and the caribbean. PloS One 10 (7), e0133409. doi: 10.1371/journal.pone.0133409
Reguero B. G., Beck M. W., Agostini V. N., Kramer P., Hancock B. (2018a). Coral reefs for coastal protection: A new methodological approach and engineering case study in Grenada. J. Environ. Manage. 210, 146–161. doi: 10.1016/j.jenvman.2018.01.024
Reguero B. G., Beck M. W., Bresch D., Calil J., Meliane I. (2018b). Comparing the cost effectiveness of nature-based and artificial coastal adaptation: a case study from the Gulf coast of the United States. PloS One 13, e0192132. doi: 10.1371/journal.pone.0192132
Ricardo G. F., Jones R. J., Nordborg M., Negri A. P. (2017). Settlement patterns of the coral Acropora millepora on sediment-laden surfaces. Sci. Total Environ. 609, 277–288. doi: 10.1016/j.scitotenv.2017.07.153
Rogers A., Blanchard J. L., Mumby P. J. (2018). Fisheries productivity under progressive coral reef degradation. J. Appl. Ecol. 55, 1041–1049. doi: 10.1111/1365-2664.13051
Rogers J. S., Monismith S. G., Koweek D. A., Torres W. I., Dunbar R. B. (2016). Thermodynamics and hydrodynamics in an atoll reef system and their influence on coral cover. Limnology Oceanography 61, 2191–2206. doi: 10.1002/lno.10365
Rosenberg E., Falkovitz L. (2004). The Vibrio shiloi/Oculina patagonica model system of coral bleaching. Annu. Rev. Microbiol. 58, 143–159. doi: 10.1146/annurev.micro.58.030603.123610
Rull V. (2022). Inductive prediction in biology: Are long-term ecological and evolutionary processes predictable? EMBO Rep. 23, e54846. doi: 10.15252/embr.202254846
Ryan K., Morison A. K., Conron S. (2009). Evaluating methods of obtaining total catch estimates for individual Victorian bay and inlet recreational fisheries (Queenscliff, VIC: Department of Primary Industries), 114.
Sheehan E. V., Holmes L. A., Davies B. F. R., Cartwright A., Rees A., Attrill M. J. (2021). Rewilding of protected areas enhances resilience of marine ecosystems to extreme climatic events. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.671427
Sheppard C., Dixon D. J., Gourlay M., Sheppard A., Payet R. (2005). Coral mortality increases wave energy reaching shores protected by reef flats: examples from the Seychelles. Estuarine Coast. Shelf Sci. 64, 223–234. doi: 10.1016/j.ecss.2005.02.016
Siegel K. J., Cabral R. B., McHenry J., Ojea E., Owashi B., Lester S. E. (2019). Sovereign states in the Caribbean have lower social-ecological vulnerability to coral bleaching than overseas territories. Proc. R. Soc. B 286, 20182365. doi: 10.1098/rspb.2018.2365
Smith J. E., Brainard R., Carter A., Grillo S., Edwards C., Harris J., et al. (2016). Re-evaluating the health of coral reef communities: baselines and evidence for human impacts across the central Pacific. Proc. R. Soc. B: Biol. Sci. 283, 20151985. doi: 10.1098/rspb.2015.1985
Sokolow S. H., Foley P., Foley J. E., Hastings A., Richardson L. L. (2009). Editor’s choice: Disease dynamics in marine metapopulations: modelling infectious diseases on coral reefs. J. Appl. Ecol. 46, 621–631. doi: 10.1111/j.1365-2664.2009.01649.x
Spalding M. D., Ruffo S., Lacambra C., Meliane I., Hale L. Z., Shepard C. C., et al. (2014). The role of ecosystems in coastal protection: Adapting to climate change and coastal hazards. Ocean Coast. Manage. 90, 50–57. doi: 10.1016/j.ocecoaman.2013.09.007
Speare K. E., Duran A., Miller M. W., Burkepile D. E. (2019). Sediment associated with algal turfs inhibits the settlement of two endangered coral species. Mar. pollut. Bull. 144, 189–195. doi: 10.1016/j.marpolbul.2019.04.066
Stella J. S., Munday P. L., Jones G. P. (2011). Effects of coral bleaching on the obligate coral-dwelling crab Trapezia cymodoce. Coral Reefs 30, 719–727. doi: 10.1007/s00338-011-0748-0
Storlazzi C. D., Norris B. K., Rosenberger K. J. (2015). The influence of grain size, grain color, and suspended-sediment concentration on light attenuation: Why fine-grained terrestrial sediment is bad for coral reef ecosystems. Coral Reefs 34, 967–975. doi: 10.1007/s00338-015-1268-0
Suggett D. J., Edwards M., Cotton D., Hein M., Camp E. F. (2023). An integrative framework for sustainable coral reef restoration. One Earth 6, 666–681 doi: 10.1016/j.oneear.2023.05.007
Taebi S., Pattiaratchi C. (2014). Hydrodynamic response of a fringing coral reef to a rise in mean sea level. Ocean Dynamics 64, 975–987. doi: 10.1007/s10236-014-0734-5
Tay C., Lindsey E. O., Chin S. T., McCaughey J. W., Bekaert D., Nguyen M., et al. (2022). Sea-level rise from land subsidence in major coastal cities. Nat. Sustainability 5, 1049–1057. doi: 10.1038/s41893-022-00947-z
Tebbett S. B., Bellwood D. R. (2019). Algal turf sediments on coral reefs: what’s known and what’s next. Mar. pollut. Bull. 149, 110542. doi: 10.1016/j.marpolbul.2019.110542
Tebbett S. B., Goatley C. H., Bellwood D. R. (2017). Fine sediments suppress detritivory on coral reefs. Mar. pollut. Bull. 114, 934–940. doi: 10.1016/j.marpolbul.2016.11.016
Teh L. S., Teh L. C., Sumaila U. R. (2011). Quantifying the overlooked socio-economic contribution of small-scale fisheries in sabah, malaysia. Fisheries Res. 110 (3), 450–458. doi: 10.1016/j.fishres.2011.06.001
Thiel M., Luna-Jorquera G., Álvarez-Varas R., Gallardo C., Hinojosa I. A. (2018). Impacts of marine plastic pollution from continental coasts to subtropical gyres—fish, seabirds, and other vertebrates in the SE Pacific. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00238
Uhlmann S. S., Broadhurst M. K. (2015). Mitigating unaccounted fishing mortality from gillnets and traps. Fish Fisheries 16, 183–229. doi: 10.1111/faf.12049
Van Oppen M. J., Gates R. D., Blackall L. L., Cantin N., Chakravarti L. J. (2017). Shifting paradigms in restoration of the world’s coral reefs. Global Change Biol. 23, 3437–3448. doi: 10.1111/gcb.13647
Veron J. E. N. (1995). Corals in space and time: the biogeography and evolution of the scleractinia (Cornell University Press).
Wabnitz C. C., Cisneros-Montemayor A. M., Hanich Q., Ota Y. (2018). Ecotourism, climate change and reef fish consumption in palau: Benefits, trade-offs and adaptation strategies. Mar. Policy 88, 323–332. doi: 10.1016/j.m
Walsworth T. E., Schindler D. E., Colton M. A., Webster M. S., Palumbi S. R., Mumby P. J., et al. (2019). Management for network diversity speeds evolutionary adaptation to climate change. Nat. Climate Change 9, 632–636. doi: 10.1038/s41558-019-0518-5
Wilcox C., Hardesty B. D., Sharples R., Griffin D. A., Lawson T. J., Gunn R. (2013). Ghostnet impacts on globally threatened turtles, a spatial risk analysis for northern Australia. Conserv. Lett. 6, 247–254. doi: 10.1111/conl.12001
Wilcox C., Heathcote G., Goldberg J., Gunn R., Peel D., Hardesty B. D. (2015). Understanding the sources and effects of abandoned, lost, and discarded fishing gear on marine turtles in northern Australia. Conserv. Biol. 29, 198–206. doi: 10.1111/cobi.12355
Wilkinson C. C. (2004). Status of coral reefs of the world: 2004 (Australia: Australian Institute of Marine Science (AIMS).
Wilkinson C. R., Buddemeier R. W. (1994). Global climate change and coral reefs: implications for people and reefs: report of the UNEP-IOC-ASPEI-IUCN global task team on the implications of climate change on coral reefs (Australia: IUCN).
Williams G. J., Graham N. A., Jouffray J. B., Norström A. V., Nyström M., Gove J. M., et al. (2019). Coral reef ecology in the Anthropocene. Funct. Ecol. 33, 1014–1022. doi: 10.1111/1365-2435.13290
Wilson S. K., Fisher R., Pratchett M. S., Graham N. A. J., Dulvy N. K., Turner R. A., et al. (2008). Exploitation and habitat degradation as agents of change within coral reef fish communities. Global Change Biol. 14, 2796–2809. doi: 10.1111/j.1365-2486.2008.01696.x
Wilson S. K., Graham N. A., Pratchett M. S., Jones G. P., Polunin N. V. (2006). Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Global Change Biol. 12, 2220–2234. doi: 10.1111/j.1365-2486.2006.01252.x
Wismer S., Tebbett S. B., Streit R. P., Bellwood D. R. (2019). Young fishes persist despite coral loss on the Great Barrier Reef. Commun. Biol. 2, 456. doi: 10.1038/s42003-019-0703-0
Wolff N. H., Mumby P. J., Devlin M., Anthony K. R. (2018). Vulnerability of the great barrier reef to climate change and local pressures. Global Change Biol. 24 (5), 1978–1991. doi: 10.1111/gcb.14043
Woodhead A. J., Graham N. A., Robinson J. P., Norström A. V., Bodin N., Marie S., et al. (2021). Fishers perceptions of ecosystem service change associated with climate-disturbed coral reefs. People Nat. 3, 639–657. doi: 10.1002/pan3.10220
Woodhead A. J., Hicks C. C., Norström A. V., Williams G. J., Graham N. A. (2019). Coral reef ecosystem services in the Anthropocene. Funct. Ecol. 33, 1023–1034. doi: 10.1111/1365-2435.13331
Woodley C. M., Bruckner A. W., McLenon A. L., Higgins J. L., Galloway S. B., Nicholson J. H. (2008). Field manual for investigating coral disease outbreaks.
Woodroffe C. D., Webster J. M. (2014). Coral reefs and sea-level change. Mar. Geology 352, 248–267. doi: 10.1016/j.margeo.2013.12.006
Worm B., Barbier E. B., Beaumont N., Duffy J. E., Folke C., Halpern B. S., et al. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790. doi: 10.1126/science.1132294
Yeemin T., Pengsakun S., Yucharoen M., Klinthong W., Sangmanee K., Sutthacheep M. (2013). Long-term changes in coral communities under stress from sediment. Deep Sea Res. Part II: Topical Stud. Oceanography 96, 32–40. doi: 10.1016/j.dsr2.2013.04.019
Young C. N., Schopmeyer S. A., Lirman D. (2012). A review of reef restoration and coral propagation using the threatened genus Acropora in the Caribbean and Western Atlantic (88(4), pp.1075-1098: Bulletin of marine science).
Keywords: tropical, marine, plant, ecosystem, biodiversity
Citation: Najeeb S, Khan RAA, Deng X and Wu C (2025) Drivers and consequences of degradation in tropical reef island ecosystems: strategies for restoration and conservation. Front. Mar. Sci. 12:1518701. doi: 10.3389/fmars.2025.1518701
Received: 28 October 2024; Accepted: 31 January 2025;
Published: 24 February 2025.
Edited by:
Hajime Kayanne, The University of Tokyo, JapanReviewed by:
Thamasak Yeemin, Ramkhamhaeng University, ThailandCopyright © 2025 Najeeb, Khan, Deng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Deng, ZHgwOTI4QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.