- 1Harbor Branch Oceanographic Institute, Florida Atlantic University, Fort Pierce, FL, United States
- 2Fisheries and Oceans Canada, Winnipeg, MB, Canada
- 3Independent Researcher, Irvine, CA, United States
- 4World Wildlife Fund – Canada, Iqaluit, NU, Canada
- 5Wildlife Conservation Society Canada, Whitehorse, YT, Canada
- 6Department of Biology, University of Victoria, Victoria, BC, Canada
- 7Department of Biological Sciences, University of Manitoba, Winnipeg, MB, Canada
Despite the universal fascination with the tusk of the narwhal, the function of this long, spiraled tooth is still debated, primarily because few people have observed how narwhals (Monodon monoceros) use their tusks in the wild. Using an unmanned aerial vehicle (UAV), we recorded previously unreported interactions between multiple narwhals, Arctic char (Salvelinus alpinus) and glaucous gulls (Larus hyperboreus) in Canada’s High Arctic. Narwhals were recorded chasing char and using their tusks to hit, manipulate and influence the behavior of fish. Differences in tusk use likely reflected differences in behavioral intent with some actions associated with prey capture and others with exploration and likely play. Kleptoparasitic behavior by gulls when narwhals pursued char near the surface substantially reduced prey capture for narwhals. Associative and interactive behaviors among narwhals were linked to the ecological context including fish density and gull behavior. Some interactions appeared competitive in nature while others may have been communicative and affiliative. This study revealed that narwhals can use their tusks to investigate and manipulate objects, including prey, and deliver sufficient force with their tusks to stun and possibly kill fish. The speed and agility of char combined with kleptoparasitic behavior of gulls indicate that char may be a challenging species to predate while aspects of the narwhals’ actions may include social learning and exploration of a novel prey species, and are the first reported evidence of likely play, specifically exploratory-object play, in narwhals.
1 Introduction
The tusk of the narwhal is one of the most fascinating traits in nature. The inspiration for myths including origin stories for the unicorn, the function of the long, spiraled tooth of the narwhal, which protrudes from the upper lip and can attain lengths of 3m, remains a matter of some debate (Gerson and Hickie, 1985; Nweeia et al., 2014; Graham et al., 2020). This is primarily because few studies have observed how narwhals use their tusks in the wild.
The tusk, which is predominantly present in males, is likely a sexually selected trait used in contest or display competition by males to gain access to mates (Graham et al., 2020). It is important to note, however, that some females also grow tusks (Hay, 1984; Garde and Heide-Jørgensen, 2022). Narwhals are known for the behavior of ‘tusking’, where two or more narwhals simultaneously raise their tusks almost vertically out of the water, crossing them in what may be a ritualistic behavior to assess a potential opponent’s qualities or to display those qualities to potential mates (Graham et al., 2020). A higher incidence of head scarring in males, including reports of broken tips of tusks embedded in the jaws of male narwhals, indicates that tusks may also be used in aggressive contests for females (Silverman and Dunbar, 1980; Gerson and Hickie, 1985).
Narwhal tusks may have other uses. The elongated tooth is both porous and highly innervated (Nweeia et al., 2014). As such it may function as an environmental sensor capable of detecting changes in water temperature and salinity. This could potentially aid male narwhals to detect differences in sea ice conditions that could, in turn, assist males in optimizing foraging effort (Nweeia et al., 2014). The tusk may also be used more directly in food capture. In 2016, unmanned aerial vehicle (UAV) footage recorded narwhals near the water surface striking and stunning small fish (Arctic cod, Boreogadus saida) prior to consuming them (M. Marcoux, pers. comm.).
That such uses would be predominantly restricted to males, however, requires explanation. Sex differences have been found in narwhal diet (Watt et al., 2013) and dive behavior (Watt et al., 2015) that indicate differences in foraging strategies between male and female narwhals. How tusk use might contribute to such differences, however, remains unclear, and further highlights the need for more prolonged observations of how narwhals use their tusks in nature.
Little is known about many other aspects of the behavior of this highly gregarious Arctic whale, including social and reproductive behaviors, how narwhals explore novel situations and adapt their behaviors to changing environmental conditions, if there are complex social hierarchies, or whether narwhals engage in behaviors that are not linked directly to fitness, like play. To determine how narwhals are being impacted by, and adapting to, a changing Arctic, more field studies using new non-invasive tools, including UAVs (drones), are needed to investigate narwhal behavior.
Here we report on two encounters, recorded using a UAV, between narwhals, fish and in one case glaucous gulls in Creswell Bay, Nunavut that consist of a number of distinct behaviors, including how narwhals use their tusks, that have not been reported on before. The fish were identified as Arctic char, a species that has not been widely recorded in the diet of this narwhal population to date (Heide-Jørgensen et al., 1994; Watt et al., 2013; Watt and Ferguson, 2015). However, there was a single observation of small juvenile Arctic char (along with Arctic cod and shrimp) in the stomach of a narwhal sampled in August 1991 in Creswell Bay (DFO unpublished data). Our findings provide new insight into tusk use, the tactics used in the targeting and tracking of potential prey, social behavior, and the first reports of attempted kleptoparasitism on narwhals, and likely exploratory behavior and play in narwhals.
2 Materials and methods
2.1 Field study
A field study was conducted on narwhal behavior in Creswell Bay, Somerset Island in the Canadian High Arctic, Nunavut in the summer of 2022 (Figure 1). We used UAVs to record behaviors at the western end of the Bay, termed the inner bay hereafter. On August 4 a small UAV (DJI Phantom 4 PRO+V2.0) was launched from shore and flown at various altitudes (all ≥ 20m) over a dispersed grouping of narwhals that had entered the inner bay. On August 6 the UAV was flown over a more compact grouping of narwhals off the mouth of the Kuksik River in the inner bay.

Figure 1. Map indicating Creswell Bay on Somerset Island in Nunavut in the Canadian High Arctic where UAV video of narwhal behavior was recorded. The symbols indicate the locations where the two observations were made near the Union (light grey) and Kuksik (dark grey) rivers.
2.2 Video analysis and ethogram development
Video footage was initially viewed using the video analysis software DaVinci Resolve (v. 18.6) to identify and describe discrete behaviors that would subsequently be used in developing an ethogram. The video editor programs CapCut and Movavi were also used to view footage and highlight key aspects of behavioral sequences. Video sequences were slowed to 0.1 X to 0.5 X speed and zoomed in to assist with the detection, monitoring and description of discrete actions for ethogram construction. Behaviors were defined as either state event behaviors which had a time duration (e.g., >5 seconds) or point event behaviors that were brief and had little or no duration (e.g., ≤5 seconds).
Point and state behaviors were coded by one of us (GOCC). Only behaviors that were distinct and easily recognizable across animals and observations were coded. Trials were run where video sequences were analyzed and scored for the coded behaviors by two independent observers. Each observer was provided with the descriptions of each behavior. Behaviors that were correctly scored (i.e., timing, duration, frequency) across trials at rates at ≥90% were adjudged to have high detection reliability, and were the ones used in subsequent analysis. We then used the event-logging software Boris (v. 8.22, Friard and Gamba, 2016) to construct the ethograms of each individual (whale, fish, gull), and analyze the patterns of observed behaviors, including time budgets. Statistical analyses were conducted in R 4.4.2. Horizontal distances mentioned in the results are estimated distances relative to an estimated length of the first whale in observation #1 (W1, see Results) of 4.5m. This was an adult animal with a tusk length about 50% that of its body length. Adult male narwhals with similar tusk-length to body-length ratios for this population average 4.5m (Dietz et al., 2008).
3 Results
On most days, small, dispersed groups of narwhals comprising both sexes and different age classes entered the inner bay. Grouping patterns were characterized by fission-fusion dynamics where individual whales or small groups of 2-6 whales regularly coalesced into larger groupings of up to 17 individuals only to split again. Social behaviors and slow movements that likely indicated resting behavior predominated. Whales spent considerable time at or near the surface. This, combined with calm waters, 24 hours of daylight, and visibility regularly to depths of 5m, facilitated near-continuous observations via UAV of whale behaviors comprising successive 20-minute flights for periods of up to 2 hours. The Kuksik River enters the bay from the north and char were present in the river’s mouth and close to shore during our observations. The Union River, which enters the inner bay from the west, typically has a summer run of Arctic char. This run had not yet occurred during the observations reported here.
3.1 Behavior sequence
3.1.1 Observation #1
On August 4, we observed an adult narwhal (labelled W1) with a long tusk near the Union River (Figure 1) swimming at the surface in water depths estimated to be in excess of 10m (0min, 0sec). W1 executed a slow, tight turn and actively pursued a single Arctic char (Figure 2, Supplementary Video 1). The fish was 30 cm in front of the tip of the whale’s tusk. The fish regularly made rapid, evasive changes in direction. W1 made similarly quick course corrections such that the tip of its tusk tracked the movements of the fish very closely. The whale and fish movements were so closely mirrored, it was unclear at times which animal was the primary actor and who was the responder. Slowing of the video revealed sequences where the fish’s reaction to the whale elicited a subsequent reaction from the whale less than a second later (see below). W1 was joined by a second, smaller whale (W2, ~ 0.8 total length of W1) with a shorter tusk almost immediately (Figure 2A). It was not clear if this second whale had been attracted to W1’s behavior or had independently detected the fish. Both then pursued the fish, although W1 maintained its position closest to the fish almost all of the time. A third whale (W3), similar in size to the first and also with a long tusk, then joined the pair (+2 min, 24 sec) (Figure 2C). It too joined in the pursuit of the fish. W3 briefly broke off from the group (+3 min 39 sec) only to rejoin the pair once more (+4 min 28sec) and continue in the pursuit of the fish. The whales kept pace with the fish and regularly adjusted their speed to keep the fish just anterior to the tip of their tusks. This sometimes necessitated slowing down their speed of forward travel. The fish eventually escaped by making a rapid, tight 180° turn that elicited an immediate response from W1 and W3 which churned up the water. This appeared to facilitate the fish’s escape (+4 min 54 sec). The narwals then made rapid side-to-side movements with their heads scanning the area continuously and circled the area for 30 seconds afterwards. W1 and W2 then separated from W3 and traveled together for a further 1 min 35 seconds, sweeping their tusks from side-to-side scanning frequently.
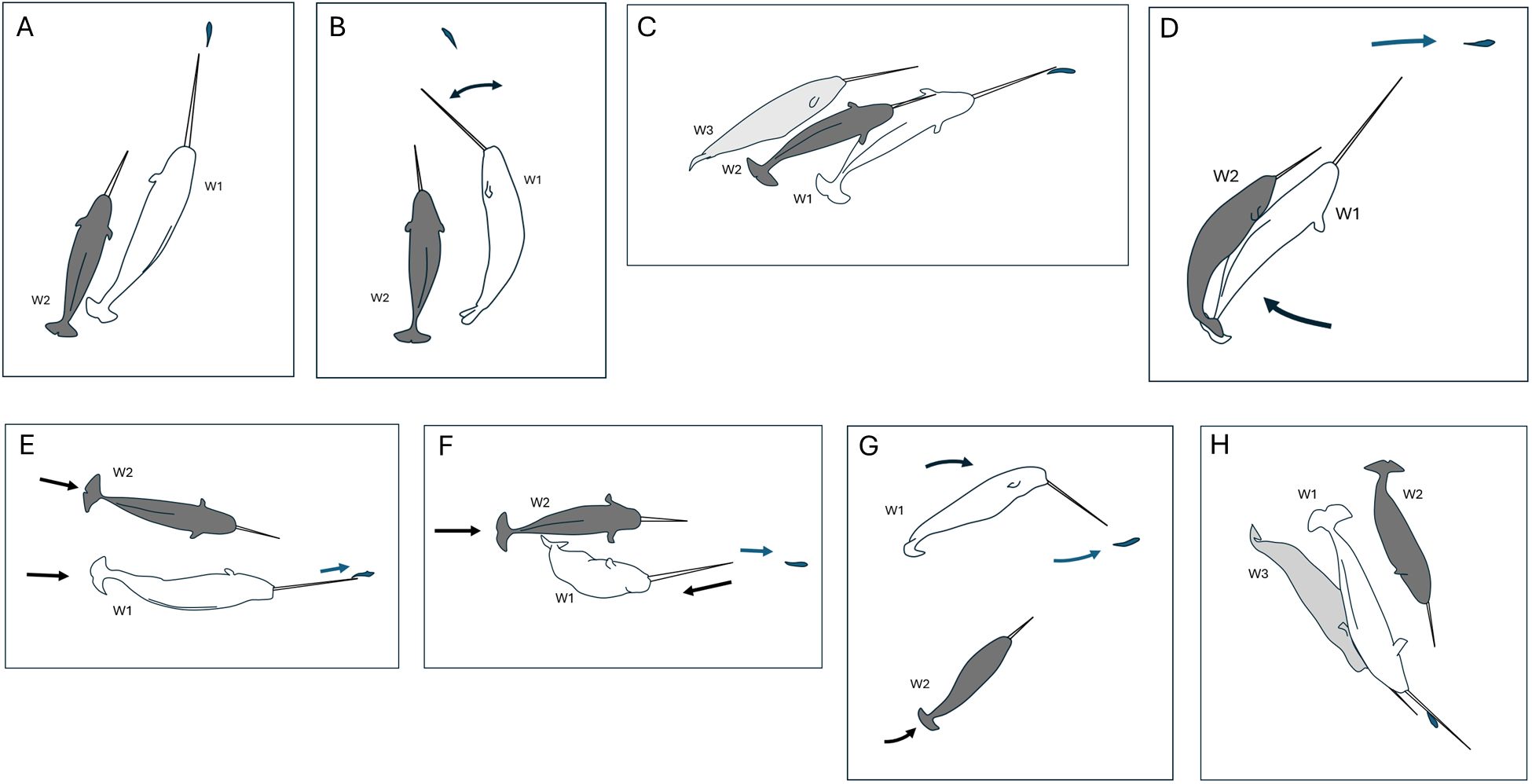
Figure 2. (A-H) A series of narwhal and fish behaviors recorded during the course of an extended interaction between three narwhal and a single Arctic char in Creswell Bay, Somerset Island, Nunavut. The two larger, adult whales (W1, W3) had a similar mottled pattern that was lighter in overall color than the mottled pattern of the smaller subadult whale (W2). However, each whale is denoted by a label and distinct color for ease of continuity across the panel of diagrams. The fish is shaded in blue. Arrows denote movements of individual animals.
3.1.2 Observation # 2
On August 6, we observed a group of 17 narwhal cruising eastward near the Kuksik River (Figure 1). Char were observed at several locations closer to shore, while glaucous gulls frequently flew overhead. The char varied in size with some substantially smaller than the adult char in observation #1. After 35 minutes, we observed three tusked narwhals in close association near shore just east of the river mouth (0min 0sec) (Figure 3; Supplementary Video 2) (note that the three videos in Supplementary Material for observation #2 cover parts of this behavioral sequence). All three were identified as young adult males based on the relative lengths of their tusks. One individual (labelled W2) was slightly smaller than the other two (labelled W1 and W3) (Figure 3A). The narwhals executed tight turns at the surface and rapid side-to-side movements of their heads and tusks, churning up the water. Soon 6 glaucous gulls converged on the 3 whales. A fish was sighted at the surface just in front of the tusk of W3 (Figure 3A; Supplementary Video 2). The fish recorded in this observation were more difficult to identify than in observation #1 due to their position in the water. Their size, color, behavior, and location contemporaneous with confirmed char sightings identified them as most likely char. A moment later a gull dove on this fish, landing on the water with a splash. The gull took off almost immediately, the fish was lost from view and appeared to have escaped (+0min 6sec). All three whales dispersed, and appeared to act independently of each other, moving in different directions and exhibiting no obvious intraspecific interactions even when close together. Much of their behavior involved regular scanning of their heads from side-to-side and the completion of tight turns, some up to 360°. Soon W3 pursued a fish, was followed by W1, and a gull flew in overhead (+0min 29sec). The gull dove on the fish, the fish escaped (+0min 32sec) (Figure 3B; Supplementary Video 3). W3 then closed in on the gull and the fish. The gull dove on the fish again, and the fish escaped once more (+0min 35sec) (Supplementary Video 3). W3 stayed in pursuit of the escaping fish which had now rapidly increased its swim speed. W1 closed in and joined in the pursuit of the fleeing char (+0min 51sec). W3 made a tight turn to keep the fish just in front of its tusk tip, then hit the fish 5 times in rapid succession; twice with the tip and three times with the shaft of its tusk (+0min 54 sec) (Figure 3C; Supplementary Video 4). The fish was knocked over and momentarily stunned but escaped by swimming aft along W3’s body (Figure 3D; Supplementary Video 4). W1 remained focused on the escaping target and continued its pursuit. All these actions churned the surface of the water. Several gulls converged on the disturbance, and one of the gulls dove on the fish. The fish escaped (+0min 59 sec) (Supplementary Video 2). The gull quickly took off and dove on the fish once more. Again, the fish escaped (+1min 02 sec). Over the next 1 min 20 seconds W1 and W3 moved in and out of the video frame where they are observed conducting slow movements and frequent scanning behaviors. While W1 and W3 were pursuing the above-mentioned fish, the other narwhal, W2, independently pursued a different fish at the same time (+0min 58 sec) (Supplementary Video 2). This whale and fish were not as close to the surface making some behaviors difficult to discern. W2 was observed to hit the fish with its tusk at least once (Figure 3F), then continue its pursuit, and execute multiple side-to-side slashing actions with its tusk including two occasions when the fish was observed being hit by the side of the tusk (+1min 03sec) (Figure 3E). The last sighting of this fish was close to W2’s head at which time the whale ceased the thrashing behavior, became stationary for several seconds, and appeared to consume the fish (+1min 07sec). W2 then began to move forward once more scanning from side-to-side before exiting the video frame.

Figure 3. (A-F)A series of narwhal, fish and seagull behaviors recorded during the course of an extended interaction between three narwhal, multiple Arctic char and multiple glaucous gulls in Creswell Bay, Somerset Island, Nunavut. The three young adult whales had distinctive mottled patterns and coloring ranging from light mottling and overall color in W1 to darker coloration in W2. Each whale is denoted by a label and distinct color for ease of continuity across the panel of diagrams. Fish are shaded in blue, seagulls in white. Arrows denote movements of individual animals with the arrow colors matching the species colors.
In both observations the whales executed some very rapid, tight turning angles in pursuit of the fish, exhibiting remarkable dexterity, precision, and speed of movement of the tusk. This included near instantaneous turns up to 360°, completed in under 3 seconds which were achieved by rotating the body on its side and moving the head downwards towards its tail rather than maintaining a dorso-ventral position in the water and turning the head and body sideways through a horizontal plane. This lateral body position in the water appeared to facilitate precise tracking of the fish with the tip of the tusk (Figure 2G).
3.2 Ethogram
Seventeen distinct behaviors were identified and described (Table 1). Six were state event behaviors and eleven were point event behaviors. A time diagram of the recorded behaviors for each individual in each observation are presented in Figures 4, 5. The number of point event behaviors and the total duration of state event behaviors are summarized for each individual in Figures 6, 7, respectively. Inter- and intra-specific interactions differed markedly among individual narwhal both within observations and also among observations.
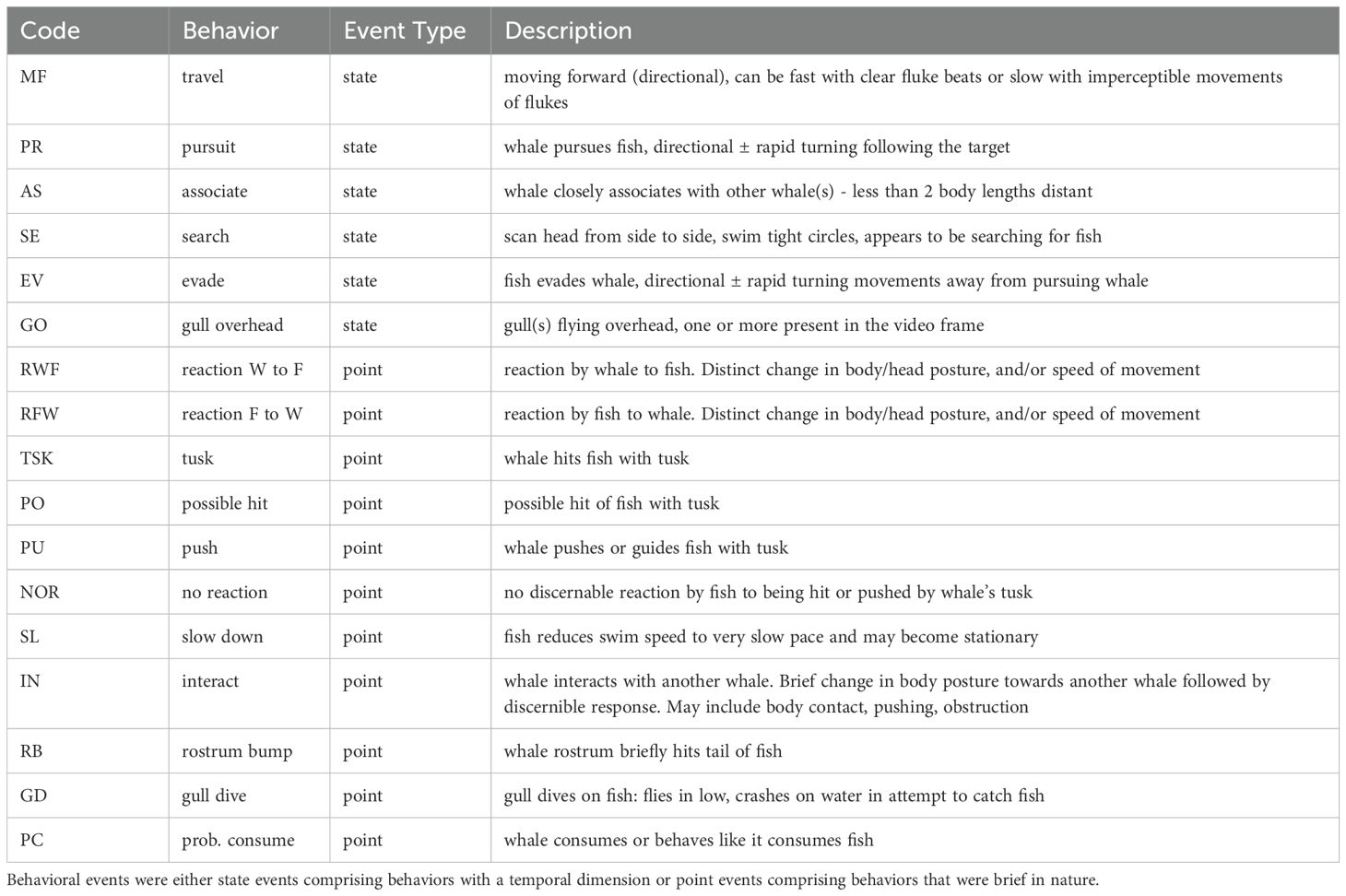
Table 1. Ethogram of distinct behaviors identified during observations of narwhals interacting with Arctic char and glaucous gulls in Creswell Bay, In the Canadian High Arctic.
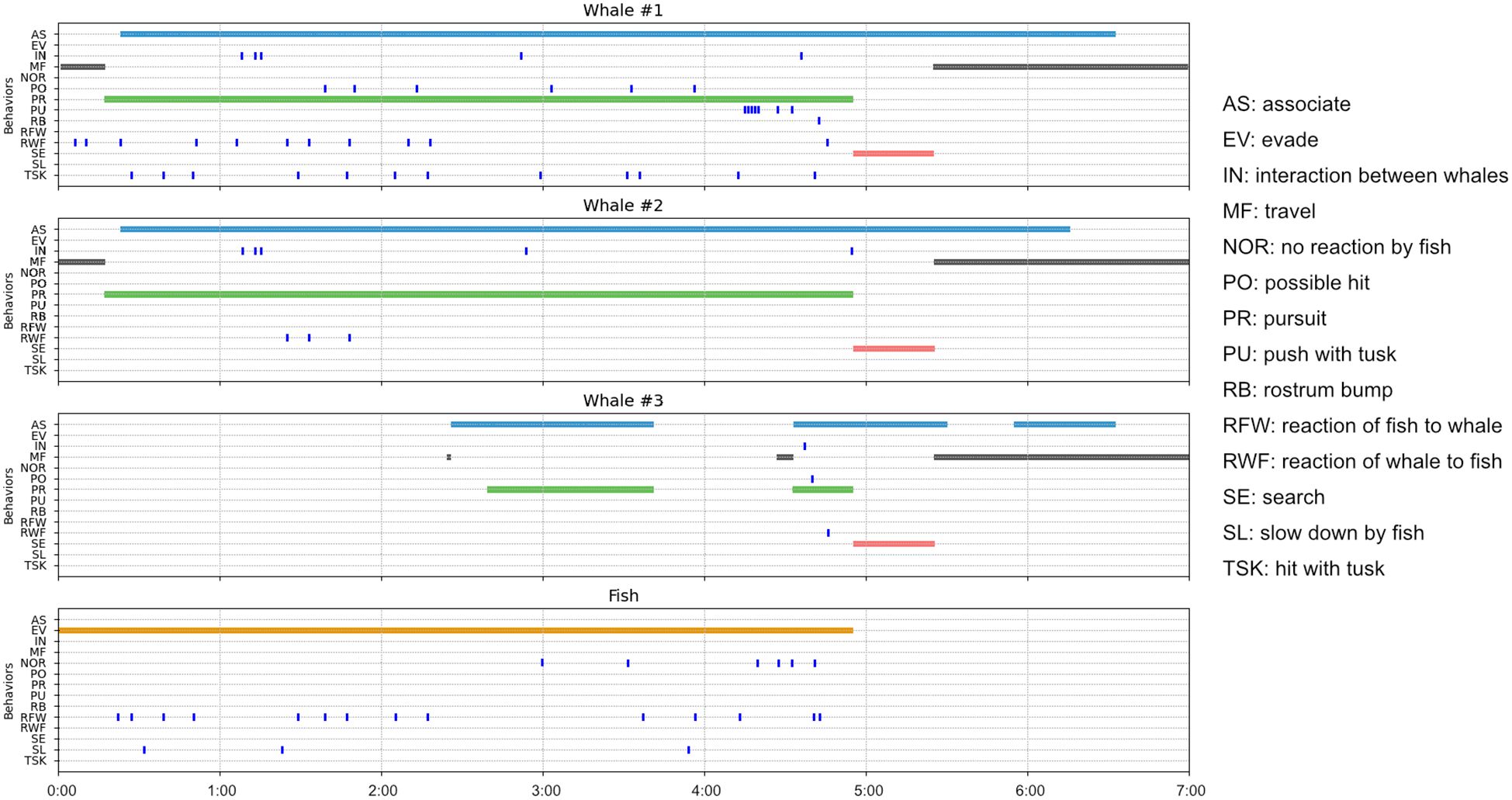
Figure 4. Time series plots of narwhal and char behaviors during observation #1 recorded in Creswell Bay, Somerset Island, Nunavut in the summer of 2022. Point event behaviors are denoted by tick marks while the duration of each state behavior is denoted by different colored strips. See Table 1 for the definitions of each behavior and the text for details.
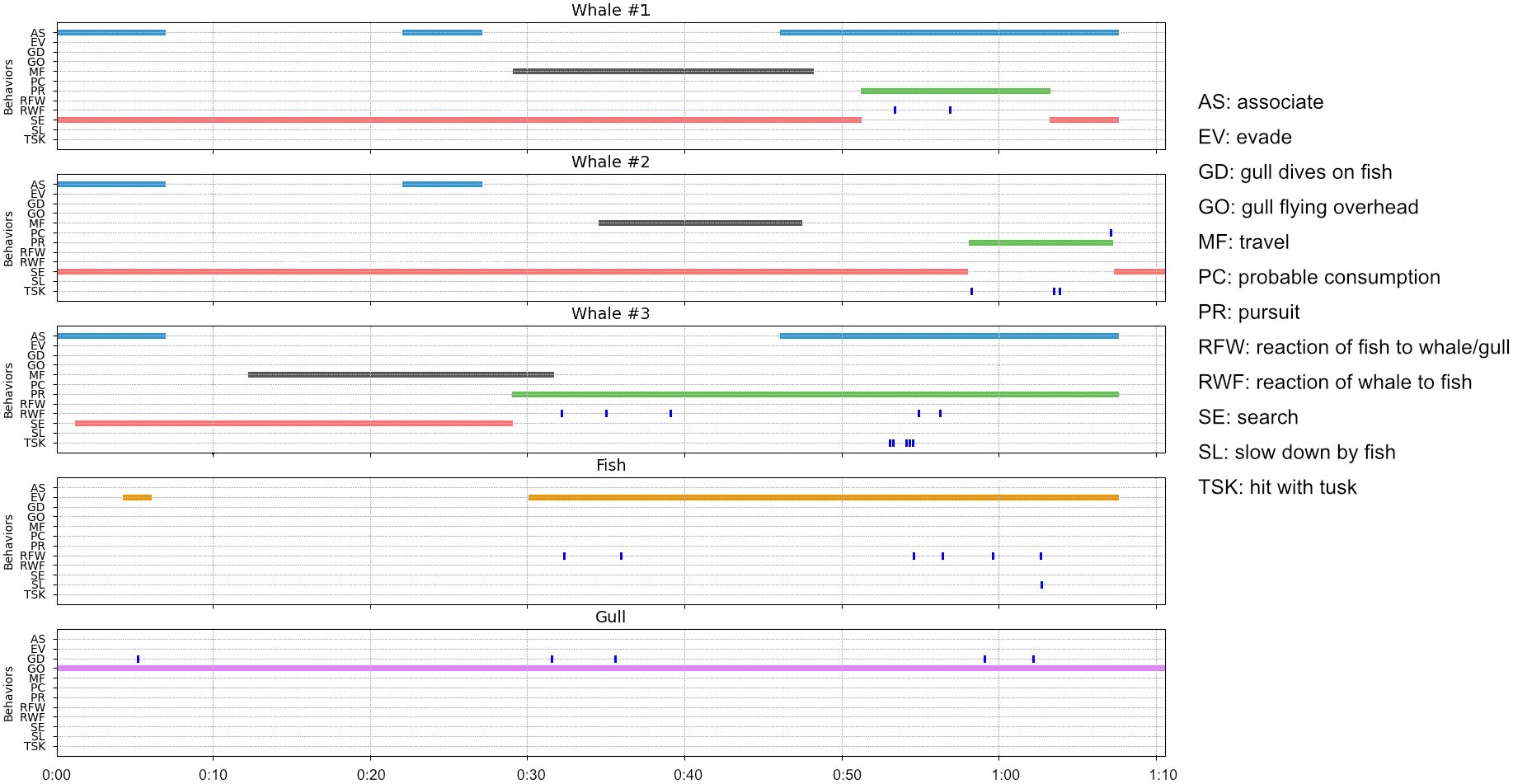
Figure 5. Time series plots of narwhal, char and glaucous gull behaviors during observation #2 recorded in Creswell Bay, Somerset Island, Nunavut in the summer of 2022. Point event behaviors are denoted by tick marks while the duration of each state behavior is denoted by different colored strips. See Table 1 for the definitions of each behavior and the text for details.
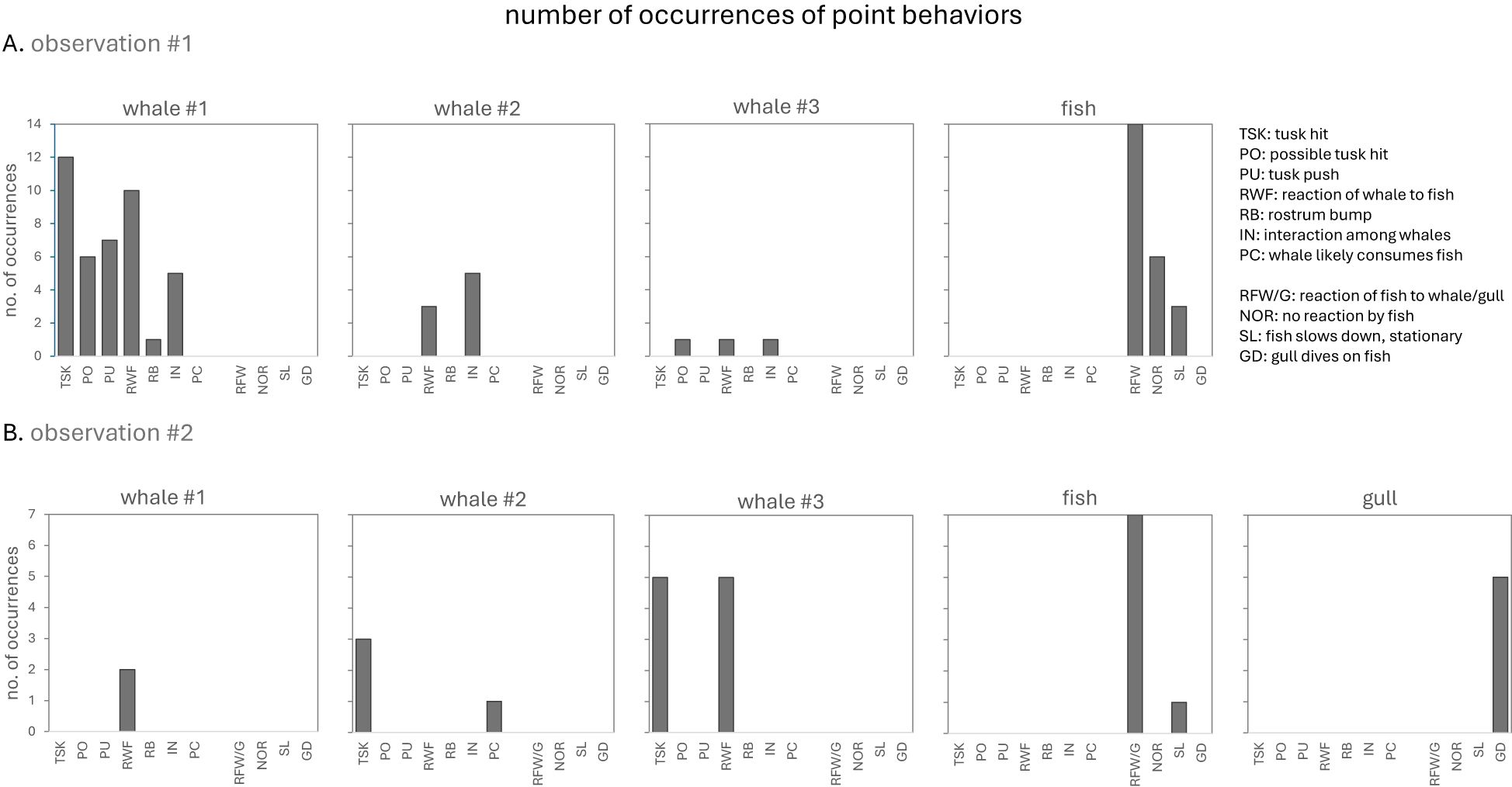
Figure 6. (A, B) Summary of the number of point event behaviors for each subject in two separate behavioral sequences involving multiple narwhals, Arctic char and glaucous gulls in the summer of 2022 in Creswell Bay, Nunavut. The fish category comprised 3 individuals while the gull category comprised 6 individuals. See Table 1 for the descriptions of each behavior.
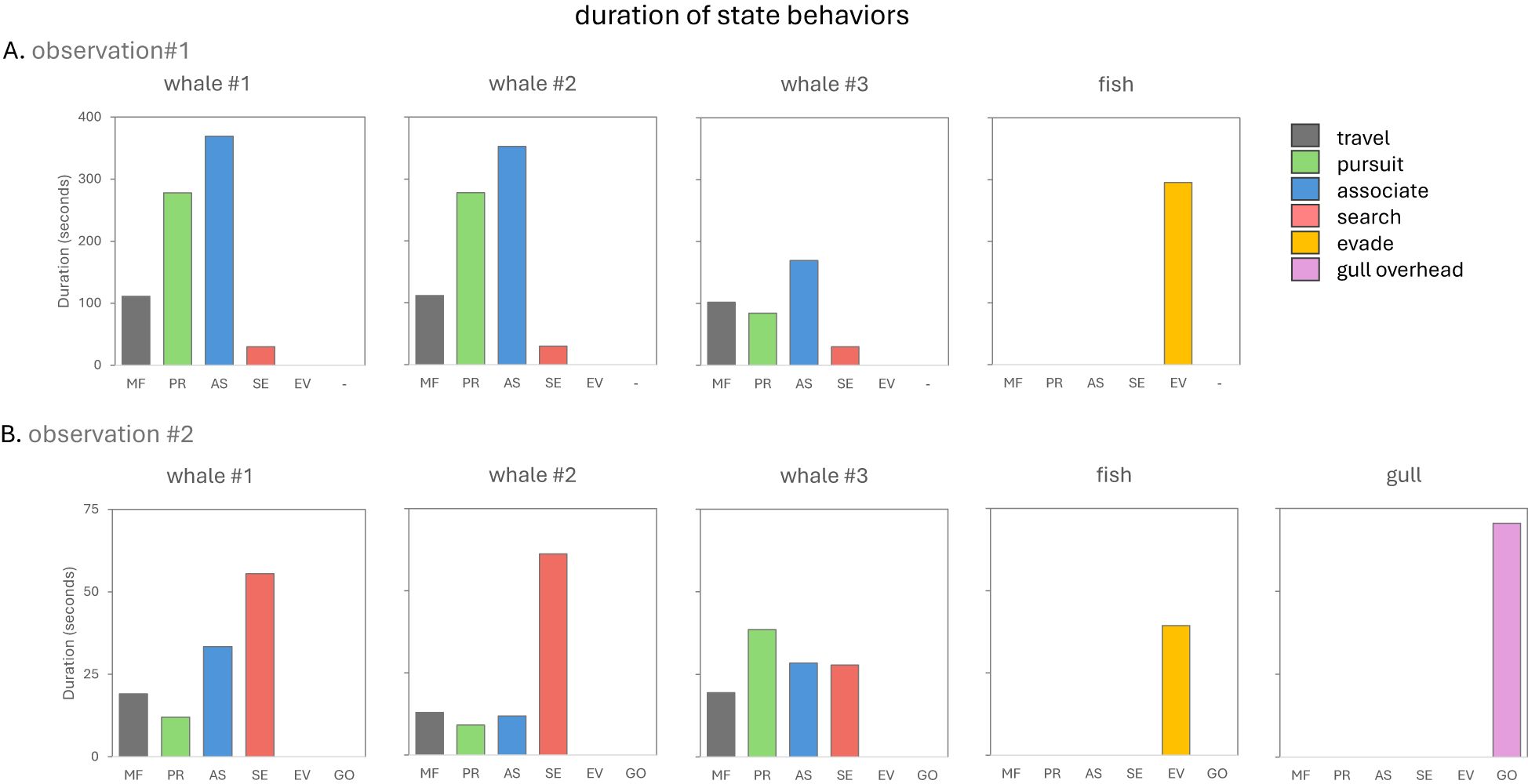
Figure 7. (A, B) Summary of the duration of state event behaviors for each subject in two separate behavioral sequences involving multiple narwhals, Arctic char and glaucous gulls in the summer of 2022 in Creswell Bay, Nunavut. The fish category comprised 3 individuals while the gull category comprised 6 individuals See Table 1 for the descriptions of each behavior.
3.2.1 Observation #1
During this behavioral sequence W1 briefly hit the fish with the tip of its tusk on 12 occasions (Figures 2E, 4, 6A). On a further 6 occasions a possible or near hit by W1 was recorded. Hits were executed with precision and were not forceful. Some involved a brief tap, others a slow push or downward pressure applied to the fish. Two comprised a narrow rotation of the tusk tip around the fish which briefly flipped the fish on its side. In most instances (n=9/12) the hit briefly knocked the fish off-course, but the fish soon resumed its evasive travel. Towards the end of the sequence W1 advanced forward such that the fish was parallel to the tusk and over a period of 17 seconds pushed or guided the fish sideways using its tusk (Figure 2H). Contact was prolonged and did not involve force. As with W1, the other adult, W3 regularly approached very close to the fish (Figures 2H, 4) and on at least one occasion completed a possible hit. By contrast, the sub-adult W2 never approached within touching distance of the fish and made no attempt to hit, push or guide the fish (e.g., Figures 2A-H, 4). On one occasion when there were only two whales involved in the pursuit W2 advanced ahead of W1, came within 1m of the fish, but hesitated to close in on the fish. W1, by contrast, soon moved forward to resume its pole position with the tusk tip centimeters behind the fish. No attempt was made by any whale to capture or consume the fish. On one occasion the fish came within touching distance of a whale’s mouth and its tail briefly made contact with W1’s rostrum. This caused the fish to rapidly increase its swim speed and make its final, successful, evasive move. On another occasion, immediately after W1 made a hit (Figure 2E), the fish reacted with a short flurry of rapid tail and body movements. This elicited an immediate recoil response from W1 (Figure 2F).
In general, the fish in observation #1 did not swim at top speed and executed evasive maneuvers by briefly increasing speed and regularly changing course. On three occasions it slowed down to a stationary or near-stationary position (Figures 4, 6A). The fish reacted demonstratively to most but not all hits. Towards the later end of the sequence the frequency of obvious reactions to tusk hits/pushes declined (Figures 4, 6A). The fish did not appear to incur any injury and was capable of escaping by rapid turns and high speed at the end.
Much of the time, whales were closely associating with one or more of the other narwhals (Figure 4). There was near continuous adjustments of body posture and position by all three whales in order to maintain their pursuit of the moving target, the fish, that required some level of interaction (Figure 6A). On five occasions there were clear behavioral interactions among the whales based on changes in posture and body contact. In three cases, W1 maneuvered to block W2’s advance on the fish (e.g., Figure 2D). In another, W1 maneuvered to block W3’s advance (Figure 2H). All four of these encounters involved brief body contact but did not appear overtly aggressive. In the fifth instance, W1 diverted from its linear path of following the fish to briefly turn towards W2 before returning to its original path (Figure 2B). This elicited a momentary shift in posture by W2. In contrast to whale-whale body contact, and tusk-fish contact, no clear observations of tusk-tusk contact were made.
3.2.2 Observation #2
The elements of this behavioral sequence were markedly different to that of observation #1. There were multiple fish involved as well as a third species, the glaucous gull. The fish were dispersed and appeared smaller than the fish in observation #1, and the gulls interacted directly with both the narwhals and fish. The narwhals in this observation spent significantly more time searching for prey and less time in pursuit of a target fish compared to observation #1 (χ2 = 327.6, p< 0.00001) (Figures 5, 7). They also spent less time associating with each other than in the earlier behavioral sequence (χ2 = 47.9, p< 0.00001) (Figures 5, 7). W2 and W3 were recorded hitting fish with their tusks. In contrast to observation #1, hits were often forceful and occurred in rapid succession. Hits sometimes involved side-to-side slashing of the tusk and resulted in stunning the fish (Figures 3C, E, F). On five occasions a narwhal was closing in on a fish when a gull flew in and dove on the fish before the whale could reach the fish (Figures 3A, B, 5, 6B). The gulls appeared to have been tracking the narwhal’s behavior prior to spotting the fish. In most cases (n=4/5), the fish escaped. On one occasion W3 pursued a fish in a wide arc, eventually caught up with the fleeing fish, and delivered a series of rapid blows with the side of its tusk (Figure 3C), but the fish escaped (Figure 3D). This fish had also successfully escaped from a diving gull on four occasions. W2 pursued a fish somewhat below the surface, hit it several times (Figures 3E and 3F), and appeared to successfully consume it.
In contrast to observation #1, fish tended to swim at high speed and execute evasive maneuvers by rapid course changes, and much of the time whales did not closely associate with each other and no obvious intraspecific interactive behaviors were recorded (Figures 5, 6B). Overall, actions were more rapid and behavioral transitions from one event type to another were more frequent in the whales in observation #2 (no. of behaviors/minute = 27.2) compared to observation #1 (no. of behaviors/minute = 10.65, p=0.069).
4 Discussion
These observations provide clear evidence of narwhals chasing fish and using their tusks to interact directly with the fish and to influence the fish’s behavior. In the first behavioral sequence this included occasional hits and rotations of the fish with the tusk tip and pushes using the side of the tusk, none with any apparent degree of force that could incapacitate or kill the fish. By contrast, in the second sequence, tusk use included multiple hits, often in quick succession and of sufficient force to incapacitate, stun, and possibly kill the fish. This study documented patterns of associative and independent behaviors, as well as various types of social interactions, among narwhals that were likely linked to the number, size and behavior of fish present. Some of the interactions appeared competitive in nature with one whale blocking or trying to block another whale’s access to the same target fish, while others may have been more subtle, possibly communicative and even affiliative. None appeared overtly aggressive. The study also documented interactions among narwhals and glaucous gulls, aspects of which indicate interspecific competition and kleptoparasitism.
There are a number of striking elements of this study’s findings that have a bearing on narwhal tusk use, behavioral intent, social interactions and foraging ecology, and on interspecific interactions between a cetacean, it’s prey and an avian competitor.
4.1 Tusk use
This study revealed that narwhals can use their tusks to investigate, manipulate and forcefully hit objects, including potential prey. The narwhals exhibited remarkable dexterity, precision and speed of movement of the tusk, and regularly made adjustments to track the moving target. The tusk, especially the tip of the tusk, was used to interrogate and manipulate the target by brief contacts which typically elicited a response from the fish. Rapid lateral movements of the tusk were used to stun and potentially kill fish. Narwhals are unusual among odontocetes in that they are the only known extant toothed whale species that have no teeth in their mouths (Hay and Mansfield, 1989). Prey capture likely comprises a sucking motion where even large prey are swallowed whole and occurs primarily at depth in the aphotic zone and under the ice (Laidre and Heide-Jørgensen, 2005). The tusk use reported here may assist tusk-bearing narwhals to capture fast, agile prey, especially in open water and in the photic zone near the surface.
Future research should investigate how the tusk interacts with echolocation in identifying, tracking and manipulating a target including potential prey. While sexual selection may have been the primary selective force behind the evolution of tusks in narwhals (Graham et al., 2020), narwhal tusks may serve many purposes (e.g., Nweeia et al., 2014). This study detailed a previously undocumented one. There are other species that possess large tusks, including elephants (Loxodonta Africana), and walrus (Odobenus rosmarus), that also use their enlarged teeth in multiple ways (Miller, 1975; Whyte and Hall-Martin, 2018). The multiple ways narwhals use their tusks appears to indicate selection for secondary functions of a sexually selected characteristic.
4.2 Foraging behavior
In the first behavioral sequence the fish was a target of intense interest, but no attempt was made to catch and consume it. Nor did it appear that attempts were made to hit it so hard as to stun or kill it. By contrast, in the second observation the whales appeared to be actively foraging. They spent considerably more time in search mode and less time in pursuit of a target fish compared to observation #1, after which fish were hit repeatedly, and in at least one instance likely consumed. It should be noted that such behaviors could only be confirmed when both whale and fish were at or near the surface and on a number of occasions whale behaviors were observed at somewhat deeper depths that were also consistent with active foraging (i.e., rapid lateral movements of tusks and changes in swim direction and speed).
These differences between the two behavioral sequences may indicate differences in foraging strategy based on fish density, behavior, and size. While we only observed a single fish in the area of observation #1, we observed schools of char on multiple occasions in the area of observation #2 over the preceding 3 hours, and in some instances the char formed tight groups near the surface. Narwhals have been observed using their tusks to hit and stun Arctic cod at the surface before consuming them (M. Marcoux, pers. comm.). The cod were much smaller and more plentiful than the adult char in observation #1. It may be more profitable for narwhals to actively hunt with their tusks when fish are below a certain size and are concentrated in dense schools. Significantly, perhaps, the char in the second observation appeared to be smaller as well as more abundant than the char in observation #1.
There is limited evidence as yet that char are an important prey species for this narwhal population. Preliminary stable isotope (SI) data, for example, did not identify char in the diet of whales sampled between 2002-2009 (C. Watt, unpubl.). However, sample sizes were small, and the SI signal, due to time lags in turnover rate, may not have reflected summer feeding patterns. Char may be costly prey to hunt. We observed that Arctic char are fast, agile prey. Even when they are present in schools, the narwhals pursued individual fish, sometimes for extended periods and often without catching them. Further research is needed to investigate whether a changing Arctic elicits behavioral ecological shifts in narwhals that includes increased predation on Arctic char.
4.3 Interspecific kleptoparasitism
Pursuing char near the surface has other potential costs for narwhals. Gull species regularly exhibit kleptoparasitism. As sight predators they observe feeding behaviors of other individuals, including individuals of other species, and regularly steal prey (Khatchikian et al., 2002; Spencer et al., 2017). In the current study glaucous gulls were frequently present, often seen patrolling the nearshore zone. This large and opportunistic gull species feeds on a variety of prey, obtained from hunting, scavenging and kleptoparasitism (Stempniewicz and lliszko, 2010; Varpe, 2010). They were a continuous presence during observation #2 and attempted several prey thefts from narwhals. While the majority were unsuccessful, they effectively reduced narwhal predation success, as the fish escaped. Significantly, perhaps, in the one instance of a likely successful capture of a fish by a narwhal the pursuit and capture occurred below the surface. It is worth noting that gulls were also present overhead during much of observation #1 but unlike in observation #2 they flew at altitudes >20m and never swooped low or dove on the large adult char. Prey size can be a significant determinant of kleptoparasitism rate in gulls (Spencer et al., 2017) which could further reduce narwhal predation success on smaller char near the surface.
4.4 Exploratory behavior
The behavior of the three narwhals in observation #1 raises the question of whether the whales were engaged in active foraging or whether they were just inquisitive. Many of the narwhals’ actions may have been exploratory behavior, perhaps of a prey species that was novel to them. The apparent absence of intent to harm, kill, or consume the fish, even when it bumped W1’s rostrum, the hesitancy of W2 to get closer to the fish when it had the opportunity, and the recoil by W1 when the fish made a particularly demonstrative reaction may reflect ignorance of and curiosity in the fish. Similarly, the behavior of the fish may also reflect an animal experiencing a novel encounter. At times it slowed to a near stationary position and rarely exhibited swim speeds it proved capable of during its final flight. Changing environmental conditions in the Arctic related to climate change will likely increase the number and type of novel situations Arctic species will experience (Moore and Huntington, 2008). This will include encountering new potential prey species for upper trophic levels species like narwhals and beluga whales. Essential to understanding behavioral and ecological adaptation to a changing Arctic are field studies like this one where new inter-specific encounters are going to occur.
4.5 Social behavior
The interactions among the whales, as well as their individual interactions with fish and birds provide insights into the social lives and behavioral development of narwhals. In observation #1, W1 was a large adult and was bold in its investigation and pursuit of the fish. It was the only whale to hit, push, and manipulate the fish and made several maneuvers to block the other whales from getting close to the fish. There was at least one moment, however, when it appeared to move aside providing an opportunity for W2 to close in on the fish. W2, by contrast was a younger whale that remained close to W1 almost all the time, was not always focused on the fish, and was hesitant to get close enough to the fish to touch it when the opportunity arose. The third whale, W3 was a more transient actor, and as with W1 was bold in getting close to and interacting with the fish. It appeared more enthusiastic and competitive in its pursuit, which is what ultimately precipitated the final flight of the fish.
Aspects of these behaviors may include experiential learning, social learning, and possibly social instruction and personality differences. In both observations, whales appeared to observe what other whales were doing, sometimes before taking decisive action themselves. In observation #2, for example, W1 tracked the actions of W3, who was in pursuit of a fish that it eventually caught up with and hit multiple times, before W1 too closed-in on the fish (Figures 3C, D). Such tracking of conspecifics followed by enacting similar behaviors to the individual(s) being tracked may indicate opportunism but also social learning. In observation #1 the behaviors of the young whale (W2) and the adult that it closely followed (W1) were more interactive and may also have involved learning through observation. It would be interesting to determine if these types of interactions in narwhals also involve instruction and scaffolding behavior by older, more experienced adults that provide learning environments for immature individuals (Thornton and McAuliffe, 2006; Whiten, 2019).
In the context of behavioral and ecological adaptations to a changing Arctic, how animals respond to and, where possible, exploit new situations is central to maximizing fitness and ensuring population viability (Stewart et al., 2012). The emergence and spread of new adaptive behaviors through social processes could fast track adaptation in narwhals, beluga whales, and other social Arctic species.
4.6 Play
Finally, aspects of the behavioral sequence in observation #1 may be the first recorded evidence of play, specifically exploratory-object play, in narwhals. Play has been documented in a few cetacean species (Janik, 2015; Hill et al., 2017) and notably in a number of other social species, including elephants (Weber and Lee, 2020) and chimpanzees (Pan troglodytes, Sabbi et al., 2024). The low frequency of play behaviors observed in the wild has been ascribed to the limited time most individuals have to spare when not pursuing activities essential to maintaining life and maximizing fitness (e.g., travel, foraging, mating, defense) (Weber and Lee, 2020; Sabbi et al., 2024). It may be for these same reasons that play is not frequently observed in adult animals (Sabbi et al., 2024). Play has been defined by five criteria: (1) lack of obvious functionality; (2) spontaneous, pleasurable or voluntary, comprising (3) exaggerated and (4) repeated actions, and (5) occurring in the absence of stress (Burghardt, 2005; Graham and Burghardt, 2010). The whale interactions with the fish in observation #1 appear to have elements of all five criteria. Our observations over the course of the field study in the summer of 2022 suggest that the narwhals were not intensely engaged in activities relating to foraging, predator evasion, or mating when in the inner bay. Movements were slow and animals spent considerable time at the surface interacting with other whales.
Whether Arctic char are an important prey species to the narwhal or not, the whales may simply not have been hungry. If prey is patchily distributed and/or can be super-abundant at times, narwhals may experience periods where little effort in foraging is required. Although narwhals are known to forage on the summering grounds, they are thought to meet most of their energetic needs in the winter foraging intensively on Gonatus squid and Greenland halibut (Laidre and Heide-Jørgensen 2005; Chambault et al., 2023). Furthermore, courtship, competition for mates, and reproduction is thought to be seasonal (Best and Fisher, 1974), and there may be locations and times of year where predator pressure is low and thus the need for vigilance is also low. Similarly, migratory species like narwhals may have periods of low activity to recuperate from long-distance travel. Opportunities for exploratory-object play and also social play may arise at these times.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Animal Care Committee/Fisheries and Oceans Canada. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GO: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MGh: Data curation, Investigation, Methodology, Writing – review & editing. MGi: Data curation, Investigation, Methodology, Writing – review & editing. PG: Data curation, Investigation, Methodology, Writing – review & editing. JH: Data curation, Investigation, Methodology, Writing – review & editing. LS: Data curation, Investigation, Methodology, Writing – review & editing. CW: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Fisheries and Oceans Canada - field studies, logistics, salaries, administrative support. Harbor Branch Oceanographic Institute at Florida Atlantic University - salaries, administrative support. The National Geographic Society (NGS-62171R-19) - field studies and logistics. The World Wildlife Fund, Canada - field studies and logistics. The Nunavut Wildlife Management Board - field studies and logistics Natural Resources Canada’s Polar Continental Shelf Program - logistics, accommodation.
Acknowledgments
Funding, logistical and administrative support for this research was provided by Fisheries and Oceans Canada, Harbor Branch Oceanographic Institute at Florida Atlantic University, the World Wildlife Fund, the Nunavut Wildlife Management Board, the National Geographic Society, and Natural Resources Canada’s Polar Continental Shelf Program. Thanks to the Resolute Bay Hunters and Trappers and Taloyoak Umaruliririgut Associations for their support for this field work. Thanks to Kevin Hedges and Tracey Loewen from DFO for assistance with fish identification. Thanks also to Tatiana Ferrer and Laura Lesyna for assistance with coding trials and figures. All research was conducted under permit A-22/23-002-NU and animal care protocol OPA-ACC-2022-25 both issued by Fisheries and Oceans Canada.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1518605/full#supplementary-material
Supplementary Video 1 | Behavioral sequence of interactions among three adult male narwhals and an Arctic char in Creswell Bay, Somerset Island, Nunavut in the summer of 2022.
Supplementary Video 2 | Behavioral sequence of interactions among three young adult male narwhals, and multiple Arctic char and glaucous gulls in Creswell Bay, Somerset Island, Nunavut in the summer of 2022.
Supplementary Video 3 | Behavioral sequence, slowed down to 0.3 X, of narwhals pursuing a fish, identified as an Arctic char, and of a glaucous gull attempting to catch the fish before the lead narwhal reaches it. Yellow circles are used to help track the movement of the fish, which escapes two attempts by the gull. The narwhal continues to track the fish.
Supplementary Video 4 | Behavioral sequence, slowed down to 0.3 X, of narwhals pursuing a fish, identified as an Arctic char, and the lead narwhal hitting the fish a number of times with its tusk. Yellow circles are used to help track the movement of the fish which escapes by swimming back along the lead narwhal’s flank.
References
Best R. C., Fisher H. C. (1974). Seasonal breeding of the narwhal (Monodon monoceros L.). Can. J. Zool. 52, 429–431. doi: 10.1139/z74-052
Burghardt G. M. (2005). The Genesis of Animal Play: Testing the Limits (Cambridge, MA, USA: MIT Press).
Chambault P., Blackwell S. B., Heide-Jørgensen M. P. (2023). Extremely low seasonal prey capture efficiency in a deep-diving whale, the narwhal. Biol. Lett. 19, 20220423. doi: 10.1098/rsbl.2022.0423
Dietz R., Heide-Jørgensen M. P., Richard P., Orr J., Laidre K., Schmidt H. C. (2008). Movements of narwhals (Monodon monoceros) from Admiralty Inlet monitored by satellite telemetry. Polar Biol. 31, 1295–1306. doi: 10.1007/s00300-008-0466-4
Friard O., Gamba M. (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Garde E., Heide-Jørgensen M. P. (2022). Tusk anomalies in narwhals (Monodon monoceros) from Greenland. Polar Res. 41, 8343. doi: 10.33265/polar.v41.8343
Gerson H. B., Hickie J. P. (1985). Head scarring on male narwhals (Monodon monoceros): evidence for aggressive tusk use. Can. J. Zool. 63, 2083–2087. doi: 10.1139/z85-306
Graham K. L., Burghardt G. M. (2010). Current perspectives on the biological study of play: Signs of progress. Q. Rev. Biol. 85, 393–418. doi: 10.1086/656903
Graham Z. A., Garde E., Heide-Jørgensen M. P., Palaoro A. V. (2020). The longer the better: evidence that narwhal tusks are sexually selected. Biol. Lett. 16, 20190950. doi: 10.1098/rsbl.2019.0950
Hay K. A. (1984). The life history of the narwhal (Monodon monoceros L.) in the eastern Canadian Arctic (Montreal, Canada: McGill University).
Hay K. A., Mansfield A. W. (1989). “Narwhal Monodon monoceros Linnaeus 1758,” in River Dolphins and the Larger Toothed Whales. Handbook of Marine Mammals, vol. 4 . Eds. Ridgeway H., Harrison R. (United Kingdom London: Academic Press), 145–176.
Heide-Jørgensen M. P., Dietz R., Leatherwood S. (1994). A note on the diet of narwhals (Monodon monoceros) in Inglefield Bredning (NW Greenland). Meddelelser om Grønland Bioscience 39, 213–216. doi: 10.7146/mogbiosci.v39.142551
Hill H. M., Dietrich S., Cappiello B. (2017). Learning to play: A review and theoretical investigation of the developmental mechanisms and functions of cetacean play. Learn. Behav. 45, 335–354. doi: 10.3758/s13420-017-0291-0
Khatchikian C. E., Favero M., Vassallo A. I. (2002). Kleptoparasitism by brown-hooded gulls and grey-hooded gulls on American Oystercatchers. Waterbirds 25, 137–264. doi: 10.1675/1524-4695(2002)025[0137:KBBGAG]2.0.CO;2
Laidre K., Heide-Jørgensen M. P. (2005). Winter feeding intensity of narwhals (Monodon monoceros). Mar. Mamm. Sci. 21, 45–57. doi: 10.1111/j.1748-7692.2005.tb01207.x
Miller E. H. (1975). Walrus ethology. I. The social role of tusks and applications of multidimensional scaling. Can. J. Zool. 53, 590–613. doi: 10.1139/z75-073
Moore S. E., Huntington H. P. (2008). Arctic marine mammals and climate change: impacts and resilience. Ecol. Appl. 18, S157–S165. doi: 10.1890/06-0571.1
Nweeia M. T., Eichmiller F. C., Hauschka P. V., Donahue G. A., Orr J. R., Ferguson S. H., et al. (2014). Sensory ability in the narwhal tooth organ system. Anat. Rec. 297, 599–617. doi: 10.1002/ar.22886
Sabbi K. H., Kurilla S. E., Monroe I. G., Zhang Y., Menante A., et al. (2024). Ecological variation in adult social play reveals a hidden cost of motherhood for wild chimpanzees. Curr. Biol. 34, 1364–1369. doi: 10.1016/j.cub.2024.02.025
Silverman H. B., Dunbar M. J. (1980). Aggressive tusk use by the narwhal (Monodon monoceros L.). Nature 284, 57–58. doi: 10.1038/284057a0
Spencer R., Russell Y. I., Dickins B. J. A., Dickins T. E. (2017). Kleptoparasitism in gulls Laridae at an urban and a coastal foraging environment: an assessment of ecological predictors. Bird Study 64, 12–19. doi: 10.1080/00063657.2016.1249821
Stempniewicz L., lliszko L. (2010). Glaucous gulls kleptoparasiting arctic foxes in Magdalenefjorden, NW Spitsbergen. Arctic 63, 107–111. doi: 10.14430/arctic651
Stewart A. J., Parsons T. L., Plotkin J. B. (2012). Environmental robustness and the adaptability of populations. Evolution 66, 1598–1612. doi: 10.1111/j.1558-5646.2011.01526.x
Thornton A., McAuliffe K. (2006). Teaching in wild meerkats. Science 313, 227–229. doi: 10.1126/science.1128727
Varpe Ø. (2010). Stealing bivalves from common eiders: kleptoparasitism by glaucous gulls in spring. Polar Biol. 33, 359–365. doi: 10.1007/s00300-009-0712-4
Watt C. A., Ferguson S. H. (2015). Fatty acids and stable isotopes (d13C and d15N) reveal temporal changes in narwhal (Monodon monoceros) diet linked to migration patterns. Mar. Mamm. Sci. 31, 21–44. doi: 10.1111/mms.2014.31.issue-1
Watt C. A., Heide-Jørgensen M. P., Ferguson S. H. (2013). How adaptable are narwhal? A comparison of foraging patterns among the world’s three narwhal populations. Ecosphere 4, 71. doi: 10.1890/ES13-00137.1
Watt C. A., Orr J. R., Heide-Jørgensen M. P., Nielsen N. H., Ferguson S. H. (2015). Differences in dive behaviour among the world’s three narwhal Monodon monoceros populations correspond with dietary differences. Mar. Ecol. Prog. Ser. 525, 273–285. doi: 10.3354/meps11202
Weber C. E., Lee P. C. (2020). Play in Elephants: Wellbeing, welfare or distraction? Animals 10, 305. doi: 10.3390/ani10020305
Whiten A. (2019). Wild chimpanzees scaffold youngsters’ learning in a high-tech community. PNAS 117, 802–804.
Keywords: narwhal, tusk use, foraging, play, kleptoparasitism, arctic char, glaucous gull
Citation: O’Corry-Crowe G, Ghazal M, Gillespie M, Galvin P, Harasimo J, Storrie L and Watt CA (2025) Use of tusks by narwhals, Monodon monoceros, in foraging, exploratory, and play behavior. Front. Mar. Sci. 12:1518605. doi: 10.3389/fmars.2025.1518605
Received: 28 October 2024; Accepted: 03 February 2025;
Published: 28 February 2025.
Edited by:
K. David Hyrenbach, Hawaii Pacific University, United StatesReviewed by:
Greg Breed, University of Alaska Fairbanks, United StatesAudra Ames, Fundación Oceanográfica, Spain
Copyright © 2025 O’Corry-Crowe, Ghazal, Gillespie, Galvin, Harasimo, Storrie and Watt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Greg O’Corry-Crowe, Z29jb3JyeWNAZmF1LmVkdQ==
 Greg O’Corry-Crowe
Greg O’Corry-Crowe Maha Ghazal
Maha Ghazal Mark Gillespie
Mark Gillespie Paul Galvin3
Paul Galvin3 Luke Storrie
Luke Storrie Cortney A. Watt
Cortney A. Watt