
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 30 January 2025
Sec. Coral Reef Research
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1512361
Polyp dimorphism, the presence of distinct autozooid (feeding) and siphonozooid (water circulation) polyps, has evolved multiple times within octocorals (class Octocorallia). Traditional anatomical descriptions have been limited to early hand-drawn publications. In precious corals (family Coralliidae), polyp dimorphism has been documented in the pacific species such as Corallium japonicum, Pleurocorallium elatius and Pleurocorallium konojoi, over the past century, yet in the Mediterranean red coral, Corallium rubrum, the literature has consistently referred to these structures generically as “polyps”, neglecting the putative dimorphism and their respective roles in reproduction, growth, and development. A key distinction between red coral species lies in their reproductive strategies: Pacific species are broadcast spawners, with gametes developing in siphonozooids, while C. rubrum is a larval brooder, with gametes maturing in autozooids. In this study, we utilized laboratory-cultured C. rubrum and a custom video imaging system to document colony growth over extended time periods. Through histological analyses and long-term observations, we demonstrated that siphonozooids, previously thought to have purely structural roles, are precursors to autozooids, suggesting a novel mechanism for colony growth in C. rubrum. This finding has important implications for understanding the extraordinary lifespan of precious coral colonies, contributing a broader knowledge to octocoral biology.
Corals are colonial organisms belonging to diverse branches of the phylum Cnidaria where polyps, or zooids, represent repeating organizational units. In some lineages, clonal multiplication can result in dimorphism between polyps, as observed in hydrozoans and various Octocorals (Hiebert et al., 2021; Hyman, 1923). In octocorals, polyp dimorphism, a historically underexplored trait, is characterized by the coexistence of two distinct zooid types: autozooids, the classical feeding polyps with eight pinnate tentacles, and siphonozooids, which lack tentacles and are thought to regulate water circulation within the colony (Hickson, 1883; Hyman, 1923; McFadden et al., 2022). This concept of polyp dimorphism was first introduced by Moseley (1881) and quickly adopted in major zoological textbooks, such as Delage and Hérouart’s treatise on zoology in the early 1900s (Delage and Hérouard, 1901). The systematics for the >3500 octocorallian species have recently been updated based on phylogenomics (McFadden et al., 2022). Based on this updated phylogeny, polyp dimorphism appears as a trait that has been gained and/or lost multiple times across octocorallians lineages (Figure 1), suggesting the absence of a unifying selection pressure.
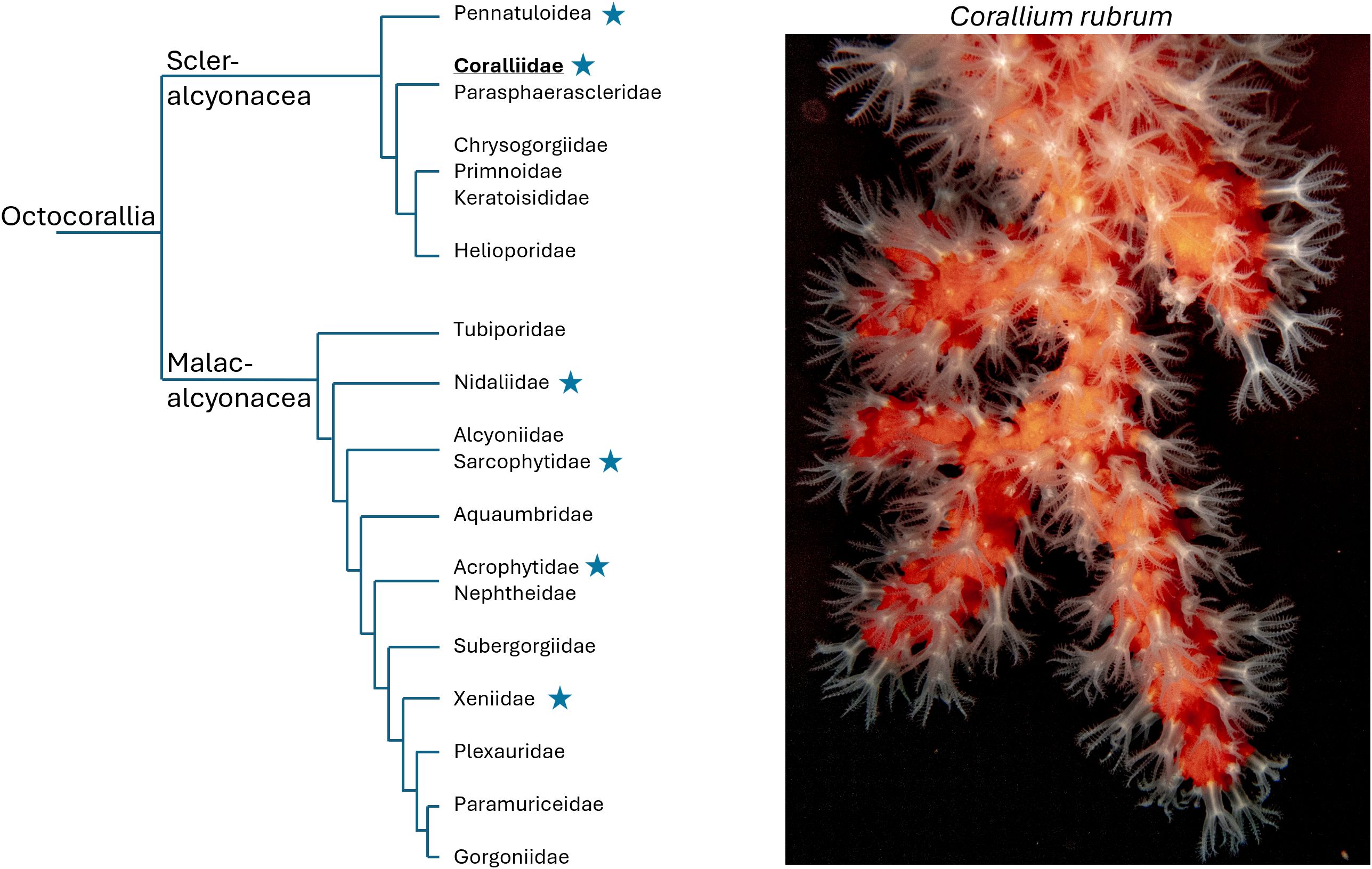
Figure 1. Scattered polyp dimorphism among octocorallians. Simplified phylogeny of Octocorallia (after McFadden et al., 2022), highlighting a selection of representative families. Star symbols indicate families that encompass species known to exhibit polyp dimorphism as described in (McFadden et al., 2022). Corallium rubrum belongs to the family Coralliidae (bolded and underlined). The photograph on the right is a representative C. rubrum colony cultivated in our laboratory (CSM).
Among octocorals, precious corals include the Mediterranean red coral Corallium rubrum and the Japanese species Corallium japonicum, Pleurocorallium elatius, and Pleurocorallium konojoi. They belong to a subset of the Coralliidae family, within the Phylum Cnidaria (subphylum Anthozoa, class Octocorallia, order Scleralcyonacea, Figure 1) (Kishinouye, 1903; Lacaze-Duthiers, 1864; McFadden et al., 2022; Vielzeuf et al., 2022). Sexually produced larvae, after settling on a substrate, transform into primary polyps, which eventually develop to colonial organisms (Giordano et al., 2023). Colony growth involves two processes, the production of a central axial skeleton to support the colony, and the multiplication of polyps connected by a common tissue called the coenenchyma. Polyps are interconnected by canals running through the coenenchyma. While canals ensure fluid circulation to maintain physiological homeostasis of the colony, polyps play a crucial role in prey capture for feeding, mouth opening and closing for water exchange, and reproduction (Lacaze-Duthiers, 1864; Hyman, 1923; Bayer, 1973; Vielzeuf et al., 2022). Consequently the long-term survival and reproduction of octocoral colonies, including the Mediterranean red coral, rely on the maintenance and replacement of these polyps -an area that remains poorly understood.
Early anatomical studies laid the groundwork for understanding polyp dimorphism in precious corals. The presence of two types of polyps in the Japanese precious corals was described by Kishinouye (1904) at the very beginning of the 20th century. This author observed that siphonozooids were not immature autozooids but a distinct type of zooid involved in reproduction, as they contain the gonads. In contrast, despite very detailed observations, Lacaze-Duthiers (1864) in his monography on the Mediterranean red coral, never mentioned such dimorphism, even if he noted the presence of two polyp morphologies (“younger ones resembling white pores” [(Lacaze-Duthiers, 1864) pp96-99_Planche VII], but attributed them to different growth stages. McIntosh (1910) later revisited C. rubrum and argued for dimorphism, observing that siphonozooids lacked reproductive organs. Despite this early work, subsequent studies on C. rubrum have overlooked the concept of polyp dimorphism, instead referring to all polyps generically without addressing potential differences in form and function.
Precious corals, despite a slow growth rate (millimeters linear extension and tenths of millimeters radial growth per year) (Garrabou and Harmelin, 2002; Grigg, 1984; Iwasaki and Suzuki, 2010; Le Goff et al., 2017; Marschal et al., 2004; Yamada et al., 2023) have a remarkable longevity. Colonies can live for centuries, possibly even millennia (Roark et al., 2009), while individual polyps generally only last about a decade (Benedetti et al., 2020; Vielzeuf et al., 2008). This discrepancy raises important questions about how colonies maintain their vitality over time. Specifically, how are polyps continuously replaced, and what mechanisms support the long-term survival of the colony?
In this study, we provided an extensive investigation of the macroscopic and microscopic anatomy of C. rubrum, focusing on the structural distinctions between siphonozooids and autozooids, the primary and secondary canal networks, and the locations of developing gametes. Our laboratory-based video analysis has revealed novel insights into polyp dimorphism and development, highlighting the potential for siphonozooids to transform into autozooids under the right conditions. This discovery opens up exciting new avenues for research, particularly regarding the long-term survival mechanisms of colonial corals and how these might inform conservation efforts for precious corals in the Mediterranean and beyond.
All references and suppliers for the products and materials used in this study are listed in the Supplementary Table 1.
For coral sampling, sustainable practices were followed, with small fragments of Corallium rubrum collected from larger, unharmed colonies, under permits issued by French authorities. At the coral culture facility of the Centre Scientifique de Monaco, aquariums are continuously supplied with seawater from the Mediterranean Sea, which is further filtered and maintained at controlled temperature of 15°C. C. rubrum colonies were maintained in darkness and fed daily with frozen plankton mixture (Ocean Nutrition). Two primary culturing methods for C. rubrum are employed: branches suspended on strings, and colonies growing laterally on glass slides. The preparation of C. rubrum colonies growing laterally on glass coverslips was previously described in (Le Goff et al., 2017). For time-lapse Video, a total of four laterally grown colonies, each about 2 cm2, were monitored for different period of time (Table 1).
Close-up photographs of laterally grown colonies were taken at 20-minute intervals using a Canon 5 DS (sensor: surface area 864 mm², 50.6 megapixels, 4.1µm pixel pitch) equipped with a Laowa 24mm probe lens (2:1 macro, f/40 aperture, waterproof, full-frame). Lighting was provided by a Flash Godox MS300 transmitted via four 0.8mm optical fibers necessary to compensate for the f/40 aperture.
The camera was set to ISO 100 and a 1/200 shutter speed, synchronized with an X2T-C radio transmitter, and controlled by a TC-80N3 remote for 20-minute intervals shooting. It was powered by an AC-E6N adapter with a daily reset at 12:01 AM to ensure continuous 24-hour operation.
A custom culture chamber for the coral colony was designed using Rhinoceros 8 and printed on an Elegoo Saturn 3D printer. Its design ensured stability during time-lapse acquisition, facilitated fine adjustments of the focal distance, and allowed removal, maintenance, and repositioning of the colony.
Photos were saved on a 512GB SDXC Extreme Pro UHS-1 card and processed in Adobe Lightroom (RAW to JPG formatting). The time-lapse videos were assembled and compressed in Adobe Premiere Pro and exported as MP4 files. See Supplementary Figure 1 for photographs of the system.
In order to investigate anatomy, a branch of C. rubrum was fixed overnight at 4°C in chilled artificial seawater/paraformaldehyde (PAF) fixation buffer [425 mM NaCl, 9 mM KCl, 9.3 mM CaCl2, 25.5 mM MgSO4, 23 mM MgCl2, 2 mM NaHCO3, 100 mM HEPES, pH 7.9, 1% Triton X-100, 4.5% PAF]. The sample was then decalcified in [100 mM HEPES, pH 7.9, 500 mM NaCl, 250 mM EDTA, pH 8.0, 0.4% PAF, 0.1% Tween 20] at 4°C until complete skeleton dissolution (5 days). After three rinses in Milli-Q water, the sample was post-fixed in ice-cold methanol for 3 hours. The sample was then gradually rehydrated with increasing dilutions of water/methanol with a final Milli-Q water bath. Next, the sample was immersed in a 2% potassium hydroxide (KOH) solution for 2 hours, then rinsed in water and buffered with [200 mM Tris, pH 7.8]. Finally, the sample was carefully incubated in 5% hydrogen peroxide (H2O2) for 1 hour and photographed with a Nikon SMZ1270 trinocular equipped with a DS-Fi3 camera.
In order to investigate histology using paraffin-embedded sections, a branch of C. rubrum was fixed and decalcified as above. Then, the sample was dehydrated with increasing concentrations of ethanol and embedded in paraffin. Seven- micrometer sections were cut on a Leica RM 2155 microtome and mounted on silane-coated glass slides. Paraffin was removed with baths of xylene substitute and further rehydrated in several decreasing concentrations of ethanol with a final Milli-Q water bath. Sections were post-fixed in Bouin’s solution for 1 hour at 56°C and rinsed in running tap water for 5-10 minutes to remove the yellow color. Slides were first stained in Weigert’s iron hematoxylin working solution for 10 minutes, rinsed under running water, then stained in Biebrich scarlet-acid fuchsin solution for 10-15 minutes, then rinsed and differentiated in phosphomolybdic-phosphotungstic acid solution for 10-15 minutes. Sections were transferred directly (without rinse) into aniline blue solution for 5-10 minutes, then rinsed briefly and differentiated in 1% acetic acid solution for 2-5 minutes. After a final rinsing step, slides were quickly dehydrated in ethyl alcohol, and mounted with Canada balsam resinous mounting medium. Samples were then imaged with a Leica microscope equipped with a CCD camera.
To observe very slow biological processes on visible time scales, we assembled a custom-made video imaging system capable of tracking growing C. rubrum colonies over extended periods of time (weeks to months, see Supplementary Figure 1 and the materials and methods section for a detailed description). It is important to emphasize to the reader, that in order to fully appreciate the implications from this study, one must refer to the Supplementary Video Files (listed Table 1) which illustrate the data that is discussed throughout the manuscript.
We captured the growth and development of a primary polyp, at the approximate age of two months old (Figure 2A; Supplementary Video 1). Notably, this primary polyp clearly exhibited the dimorphism of both an autozooid and a siphonozooid. By documenting this rarely observed event of the settled primary polyp, we were able to compare a side-by-side contrast of a primary polyp expressing only one siphonozooid and autozooid to a colony expressing multiple copies of this dimorphism (Figure 2B; Supplementary Video 2). Our imaging system also enabled documentation of simultaneous (coordinated) contractions and expansions of autozooids and siphonozooids. This behavior likely helps to maintain hydrostatic pressure within the colony’s water canal system.
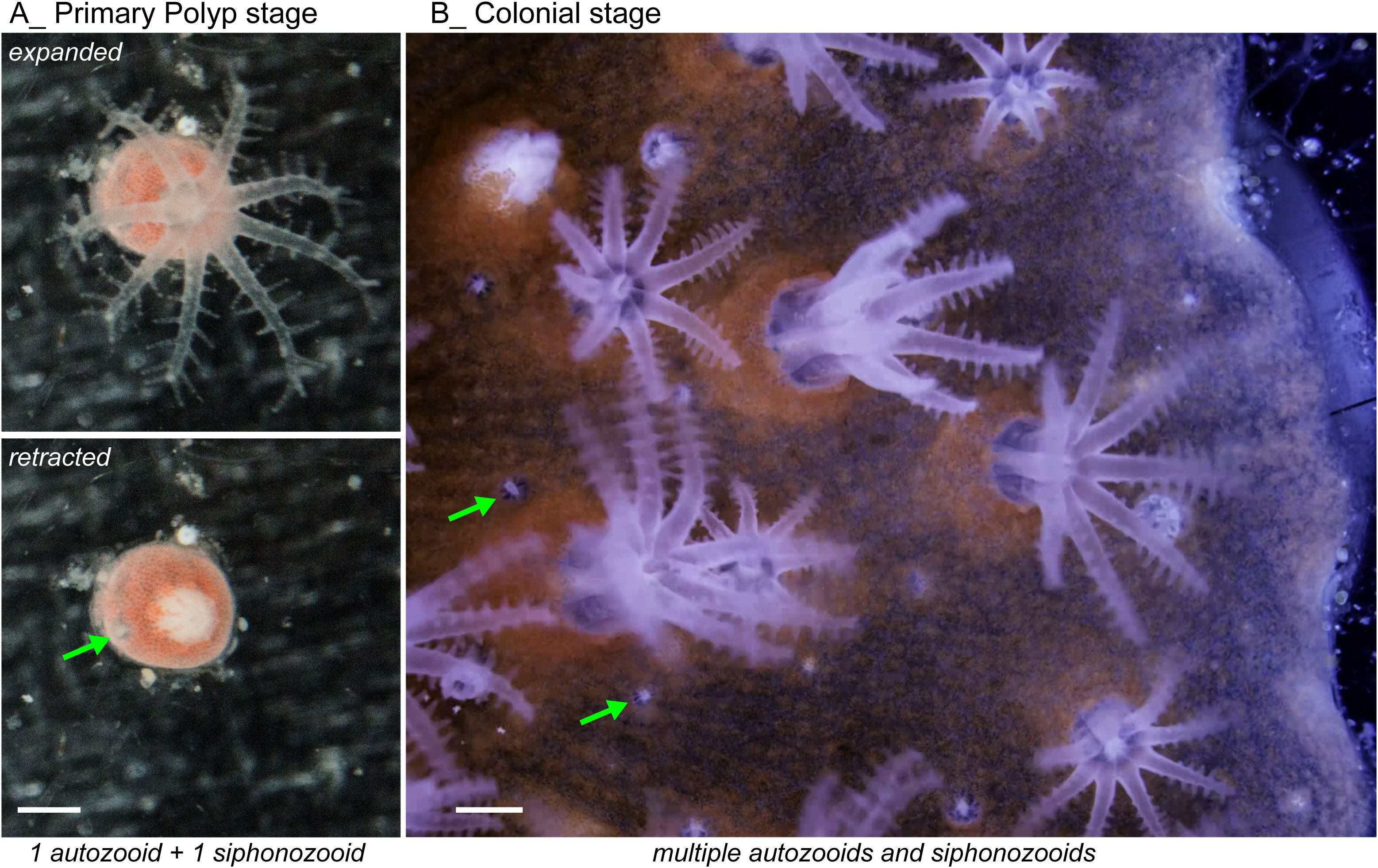
Figure 2. Live view of dimorphic polyps in Corallium rubrum. (A) Primary Polyp Stage (2 months old) of a coral born in our aquarium. This image shows expanded polyps (top) and retracted polyps (bottom). A green arrow indicates the siphonozooid, already present at this early developmental stage, beside a mature autozooid (see Supplementary Video 1 for time-lapse). These images are shown in both an expanded and retracted state for clarity. (B) Colonial Stage showing a laterally grown colony with multiple siphonozooids indicated by green arrows and autozooids (see Supplementary Video 2 for time-lapse). The underlying network of canals connecting the different polyps is visible. Scale bars = 0.5 mm.
A striking feature of C. rubrum is its intense red coloration due to a high concentration of carotenoid pigments in its biomineralized structures (Cvejic et al., 2007). However, this coloration complicates the observation of the anatomical arrangement of the C. rubrum branches. When examining the external features of a branch of live C. rubrum, the external arrangement of the polyp dimorphism is readily apparent with interspersed siphonozooids and autozooids spread across the tissue (Figures 3A, B). To better visualize the internal structure, we developed a fixation-depigmentation protocol (see materials and methods). We observed the large primary canals, located deeper in the tissue, which run parallel to the longitudinal axis of the branch, while smaller secondary canals, closer to the surface, connect to the primary canals. Also, this is the first clear visualization of siphonozooids and autozooids connecting to the canal system (Figures 3C, D).
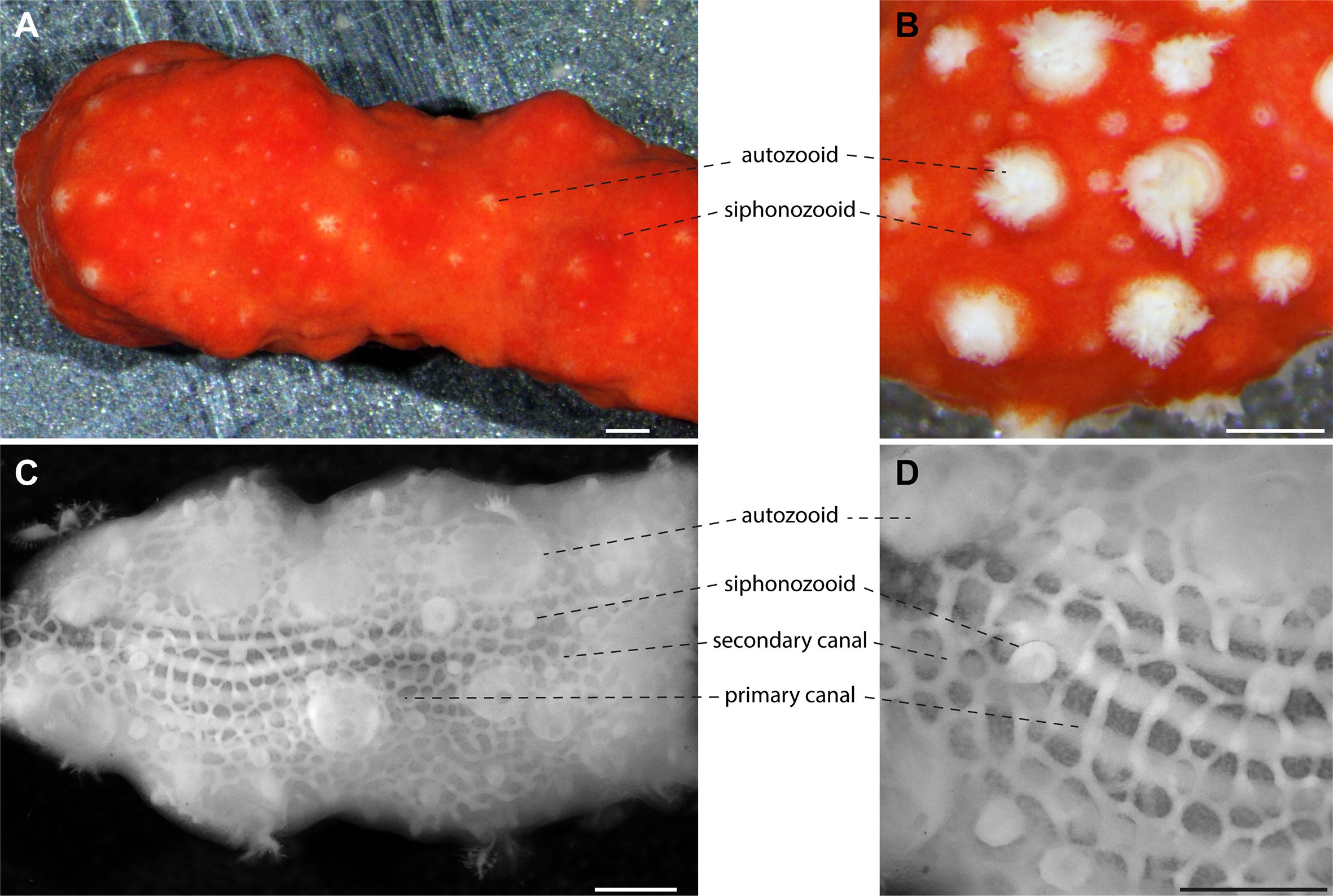
Figure 3. Histology of Corallium rubrum. Both (A, B) correspond to a live branch of C. rubrum. The colony displays retracted polyps in (A) and partially opened polyps in (B). The dimorphic polyps with autozooids and siphonozooids are more easily discriminated in (B) with the white appearance contrasting with the intense red coloration of the branch. (C, D) show a decalcified and de-pigmented colony of C. rubrum (polyps retracted) revealing the histological features with little interference from coloration and calcification. Numerous siphonozooids are easily distinguished from the larger autozooids. The primary canals (longitudinal to the axial skeleton) and the secondary canals network are visible. Note the continuity of the canal network, which is connected to both type of polyps. Scale bars = 1 mm.
Histological sections of C. rubrum stained with Masson’s trichrome revealed the detailed arrangement of siphonozooids, autozooids and the canals (Figure 4). Under microscopic examination, the retracted autozooids are distinguishable from siphonozooids, both of which connect to the canal network. A magnified view of the siphonozooid shows the asymmetrical ciliated siphonoglyph (Figure 4B) while the larger autozooid reveals a developing oocyte (Figure 4C) (see discussion section).
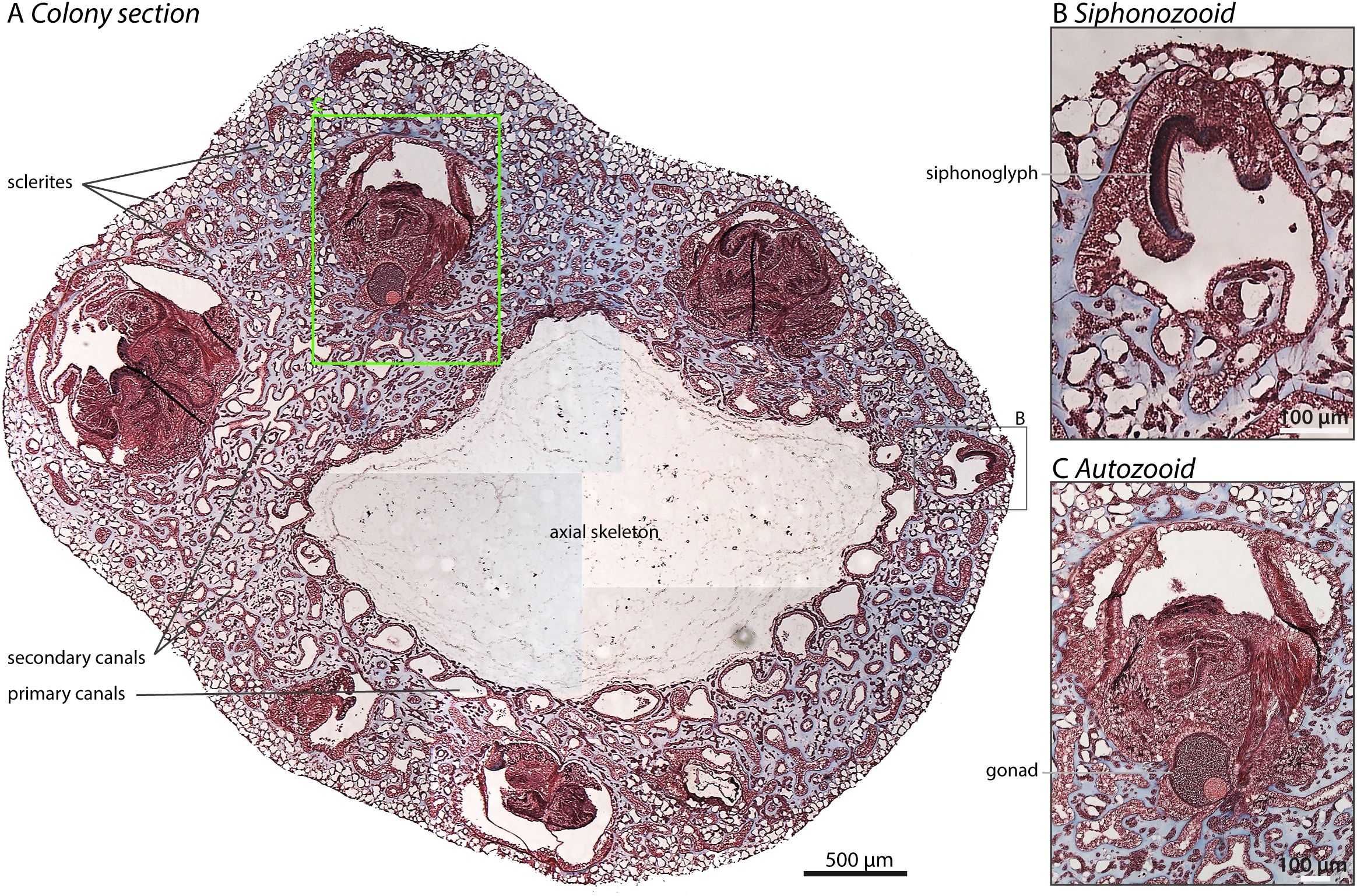
Figure 4. Cross-section through a demineralized branch of C. rubrum: Paraffin-embedded section of a colony stained with trichrome staining is shown. (A) Coral histology: The various components of the coral’s internal structure are evident with the demineralized central axial skeleton staining weakly compared to the surrounding tissues. Five retracted autozooids and one siphonozooid are readily identifiable. The remaining tissue is the coenenchyma, the connective tissue containing canals (including primary canals encircling the axial skeleton), scleroblasts (which produce sclerites but are not visible due to demineralization), the axial epithelium that forms the axial skeleton, and the oral epithelium facing the water surface. For further details, please refer to (Grillo et al., 1993). (B) This section shows an enlarged magnification of the siphonozooid (lacking tentacles) with the prominent cilia lining the siphonoglyph. (C) This section reveals a retracted autozooid at higher magnification with retracted polyp tentacles wrapped around each other, and the presence of a gonad containing an oocyte identified by the large Germinal Vesicle surrounded by a layer of cells. Scale bars are indicated on their respective images.
To date, work on Octocorallia has relied heavily on field observations and sampling, which provide an individual snap-shot of time with respect to the biology of these corals. We made a number of novel observations by imaging C. rubrum horizontal growth over extended periods of time in the laboratory. Firstly, we observed that damage or removal of the autozooid stalk including tentacles leads to regeneration [one of the biological hallmarks of cnidarians (Child, 1903; Henry and Hart, 2005)] of the complete autozooid within 2 weeks (Supplementary Video 3). During our extensive surveying of coral colonies over time, we did not observe that damaging or removing the autozooid stalks would stimulate de novo polyp synthesis in the surrounding area. Instead repair and regeneration of the damaged autozooid would occur following injury.
By tracking the same C. rubrum colony for 11 months, we were able to observe its growth, including lateral expansion through the formation of new tissue at the margins, and the development of new polyps (Supplementary Video 4). Notably, these laterally growing colonies also produce a calcified skeleton between themself and the glass (Le Goff et al., 2017), implying that lateral growth reproduces the typical growth pattern (i.e. calcification and tissue expansion) of a wild colony. Growth of the expanding C. rubrum colony over this extended period of observation can be visualized in the supplementary videos, which reveals the slow rate of expansion. During recording, we gathered novel information about connections within the polyp dimorphism. In particular, we demonstrated that a number of the siphonozooids are precursors to autozooids, as this transformation was observed multiple times over several months (Figure 5; Supplementary Videos 5, 6). Importantly, we also observed that new siphonozooids form in the colony and that not all siphonozooids that form will transform into autozooids (Figure 5; Supplementary Videos 5, 7). Furthermore, in some cases, autozooid formation can occur continuously without pausing at the siphonozooid stage (Supplementary Video 8). Thus, in C. rubrum, the siphonozooid is the precursor developmental stage to the autozooid. We were not able to determine whether all siphonozooids eventually transform into autozooids. Interestingly, our data also show that, contrary to the two-week period for autozooid regeneration (Supplementary Video 3), siphonozooid-to-autozooid differentiation takes approximately four weeks to complete (Supplementary Video 6). If the formation of a novel siphonozooid also takes about four weeks to complete (Supplementary Video 7), the formation of an autozooid without pausing at the siphonozooid stage required two months to complete (Supplementary Video 8). This suggests that, in addition to potential differences in the energetic requirements for de novo synthesis, the longer time-frame for differentiation favors autozooid repair following damage, to restore the colony’s functional physiology in the shortest time possible.
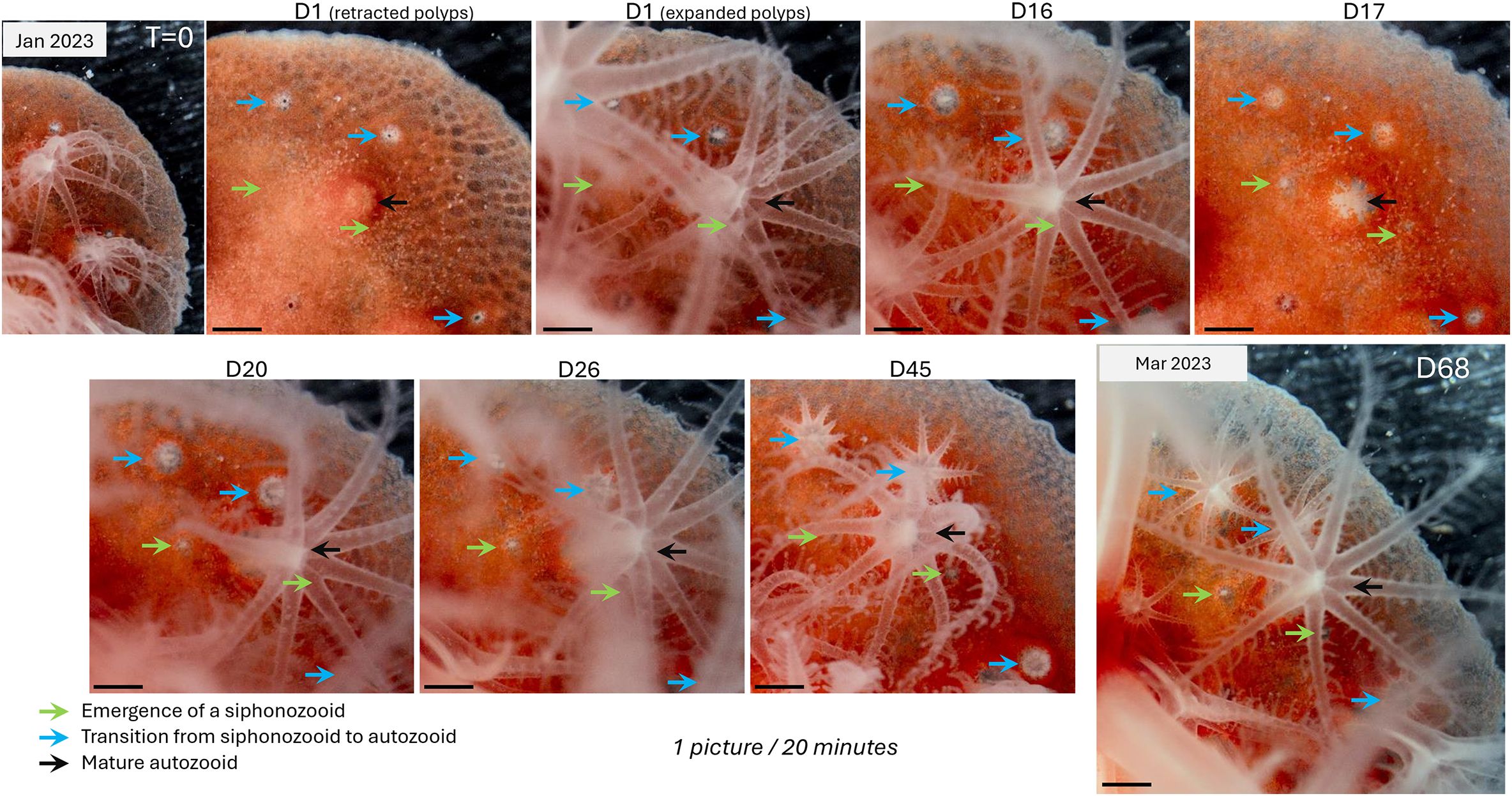
Figure 5. Siphonozooids are precursor to autozooids. This series of images captures the emergence and subsequent opening of siphonozooids (indicated by green arrows) and their transition into autozooids (identified by blue arrows), all surrounding a mature autozooid (black arrow). These images are stills from the time-lapse video provided in Supplementary Video 5. The entire process spans 68 days, from January to March 20. The timing (in days) is indicated for each picture. As shown, the emergence of a siphonozooid (D1-D26) and its transition to an autozooid (D16-D45) take approximately one month. For a closer look at these polyp emergence and the transition processes, refer to Supplementary Videos 6, 7, which provide high magnification details of these events.
Similar to other modular organisms (Hiebert et al., 2021; Jackson and Coates, 1986; Lartaud et al., 2016; Rosen, 1986), the Mediterranean red coral Corallium rubrum uses polyp budding to generate colonial organisms containing several hundred of polyp units. This process called blastogenesis (as opposed to oogenesis) refers to the development of a new “unit”, or “module”, or “zooid” (Huxley, 1851), from an existing unit, the first being the oozooid resulting from the development of the egg. Although this process has been described since the second half of the eighteenth century in various phyla such as Cnidarians, Tunicates and Ctenophores [see (Hiebert et al., 2021)], knowledge about its regulation remains limited (Levanoni et al., 2024).
The process by which octocoral colonies, including C. rubrum, sustain their growth involves both embryogenesis and blastogenesis. While embryogenesis follows a predictable development from germline tissues, blastogenesis involves the creation of new polyps from existing somatic tissue in the coenenchyme. This concept of blastogenesis in C. rubrum was first described by Lacaze-Duthiers (Lacaze-Duthiers, 1864), who noted that polyps could develop de novo from the coenenchyme, a process essential for colony expansion and regeneration. Later, Hickson (1894) extended the concept to other octocorals, observing that new polyps emerge as buds from the “coenosarcal canals”. However, the opaque nature of the tissues has historically made it difficult to study these internal structures and processes in detail. In C. rubrum, we here clearly demonstrate the emergence of new polyp (buds) at the surface of the coenenchyme (Supplementary Video 7) and their connection to the canal system (Figures 3, 4).
Our observations demonstrate that polyps were dimorphic in C. rubrum, with both autozooids and siphonozooids, as observed in other Coralliidae (McFadden et al., 2022), including Pacific precious corals (Kishinouye, 1904; Nonaka et al., 2012). Time-lapse observations have allowed us, for the first time, to witness the transformation of certain siphonozooids into autozooids in C. rubrum. Further studies are needed to determine whether the same processes occur in other dimorphic octocorals. However, based on molecular phylogeny, amongst the closest species to Corallium rubrum is Corallium japonicum (Ardila et al., 2012; Uda et al., 2013). It is therefore highly likely that similar processes occurs in closely related species, including the Japanese species C. japonicum, Pleurocorallium elatius, and Pleurocorallium konojoi. In C. rubrum, autozooids and siphonozooids show clear differences in their internal and external morphology, as well as in their roles in reproduction, feeding and fluid circulation. The fact that siphonozooids remain in this developmental stage for extended periods and that not all siphonozooids transform into autozooids supports the idea that siphonozooids represent an arrested stage of polyp development. However, we were unable to determine the exact proportion of siphonozooids that eventually transform into autozooids. Based on our data, and those from other precious coral species (Nonaka et al., 2015, 2012), it appears that the number of siphonozooids generally exceeds the number of autozooids. Given that individual autozooids have a lifespan of about a decade (Benedetti et al., 2020; Vielzeuf et al., 2008), it would be valuable to estimate the ratio of siphonozooids to autozooids in different parts of a colony, such as intensive and slow growth zones (such as branch apices versus branch columns), to better understand the dynamics of old autozooid replacement by transforming siphonozooids. By replacing individual modules (i.e., polyps), precious corals can delay senescence and achieve lifespans of over 1000 years (Roark et al., 2009).
While blastogenesis appears as the primary mechanism for new polyp formation in C. rubrum, the cellular and molecular underpinnings of this process are unknown across octocorallians. In contrast, studies on other cnidarians, such as hexacorallians and hydrozoans, have pointed to the recruitment and differentiation of Adult Stem Cells in tissue regeneration (Gierer et al., 1972; Gold and Jacobs, 2013). Furthermore, recent research on the scleractinian coral Stylophora pistillata suggested that similar cellular mechanisms may underlie polyps formation (Levanoni et al., 2024). Our ability to monitor polyp dimorphism differentiation in C. rubrum offers a new perspective on the studying of the role of blastogenesis in zooid formation, perpetuating the longevity of these coral colonies.
The classic evolutionary paradigm held that anthozoans exhibited radial symmetry. However, detailed analyses of their gastrovascular cavity have revealed evidence for bilateral symmetry (Berking, 2007; Boero et al., 2005; Finnerty, 2005; Malakhov, 2016; Martindale et al., 2002). In the gastrovascular cavity of octocorals, the orientation of the mesenteries (internal septa or walls) relative to their retractor muscle, along with the relative positioning of the siphonoglyph, defines sulcal and asulcal chambers (analogous to ventral and dorsal chambers), which establish the bilateral symmetry axis (Bayer, 1974; Beklemishev, 1969; Hyman, 1923; Sekida et al., 2016). In Hickson’s original description (1883) of various octocorallians, the siphonoglyph was identified as a ciliated groove on the ventral side of the stomodaeum (gastrovascular cavity), that extends long, strong cilia. He observed that while it is evenly present in all polyps of monomorphic species, the siphonoglyph was more strongly marked in siphonozooids than in autozooids of dimorphic species and may even be absent in some genera like Paragorgia, a non-precious Coralliidae (Hickson, 1883).
Our histological examination of C. rubrum also clearly demonstrates a non-radial symmetry with respect to the siphonoglyph’s location in the siphonozooid (see Figure 4). In C. japonicum, Sekida and colleagues found that the mesenteries orientation relative to the siphonoglyph was reversed in siphonozooids compared to what was described in autozooids, suggesting this as a distinguishing marker between the two polyp types (Sekida et al., 2016). On the other hand, there is no clear evidence for a siphonoglyph in either the autozooids of C. rubrum or other Corallium species suggesting a substantial reduction of the ciliate groove during the transition from siphonozooids to autozooids in Coralliidae. While we could not examine the finer details of its orientation in the gastrovascular cavity, the distinct asymmetry of the siphonoglyph’s localization observed in this study, as well as in other representative red corals such as Primnoa pacifica (Waller et al., 2014), provides compelling evidence that the siphonoglyph serves as a hallmark of bilateral symmetry in the gastrovascular cavity of Coralliidae polyps. This is true even though the siphonoglyph, marked during early development in siphonozooids, may be substantially reduced or entirely absent later in autozooids.
The strong development of the ciliated siphonoglyph in siphonozooids compared to autozooids has led to the proposal that siphonozooids are specialized for water propulsion, while autozooids are primarily involved in feeding and reproduction (Bayer, 1974; Beklemishev, 1969; Claus, 1884; Hickson, 1883; Hyman, 1923; Moseley, 1876). Our observations (Supplementary Video 1) reveal that even in young C. rubrum primary polyps, a siphonozooid is positioned near an autozooid, suggesting that coordination between these zooid types is essential for coral physiology. Furthermore, the synchronized opening and closing of both siphonozooids and autozooids in colonial forms (Supplementary Video 2) indicates coordination between these zooid types, suggesting that both respond to common environmental cues. However, the extent to which the relative proportion of each zooid type and their respective roles in fluid control and feeding influence the regulation of fluid and food dissemination throughout the colony remains to be determined.
Understanding the role of polyp dimorphism is also crucial because it directly influences reproductive strategies. In most gonochoristic octocorals with polyp dimorphism, gametes develop either in the autozooids or, more rarely, in siphonozooids (Hyman, 1923; McFadden et al., 2022). For the Mediterranean red coral, the development of gonads has been confirmed to occur in the feeding polyp (i.e. autozooid, Figure 4) (Santangelo et al., 2003; Tsounis et al., 2006; Torrents and Garrabou, 2011), contrary to Pacific precious corals, where siphonozooids host the gonads (Nonaka et al., 2012, 2015; Sekida et al., 2016). In addition, it was observed that in rare cases, some gametes were also observed in the autozooids of Japanese precious coral species (Nonaka et al., 2015). In light of our findings that siphonozooids can transform into autozooids in C. rubrum and potentially other precious corals, the observed gamete development in the autozooid of Japanese corals can now be attributed to a limited number of siphonozooids transforming into autozooids while still developing gonads (gamete viability was not assessed in Nonaka et al. (2015). Interestingly, this difference is associated with distinct reproductive strategies: the Mediterranean red coral is larval brooder, while Pacific species are broadcast spawners (Nonaka et al., 2015). This suggest that Pacific precious corals may have developed an adaptive trait involving siphonozooid-based reproduction (Figure 6). This division aligns with the idea that brooding, being more energetically demanding, benefits from the autozooid’s specialization in prey capture, which provides essential nutrients for developing larvae. Conversely, because siphonozooids outnumber autozooids, the total number of gametes produced by siphonozooids may be higher broadcast spawners, assuming an equivalent number of gametes per zooid. A critical question that arises from these observations is whether the mesenteries of siphonozooids that transform into autozooids are capable of germline maturation, or if this function is restricted solely to direct autozooid development. It remains undocumented whether siphonozooids in C. rubrum can develop gametes, a potential phenomenon that could have been overlooked in past research.
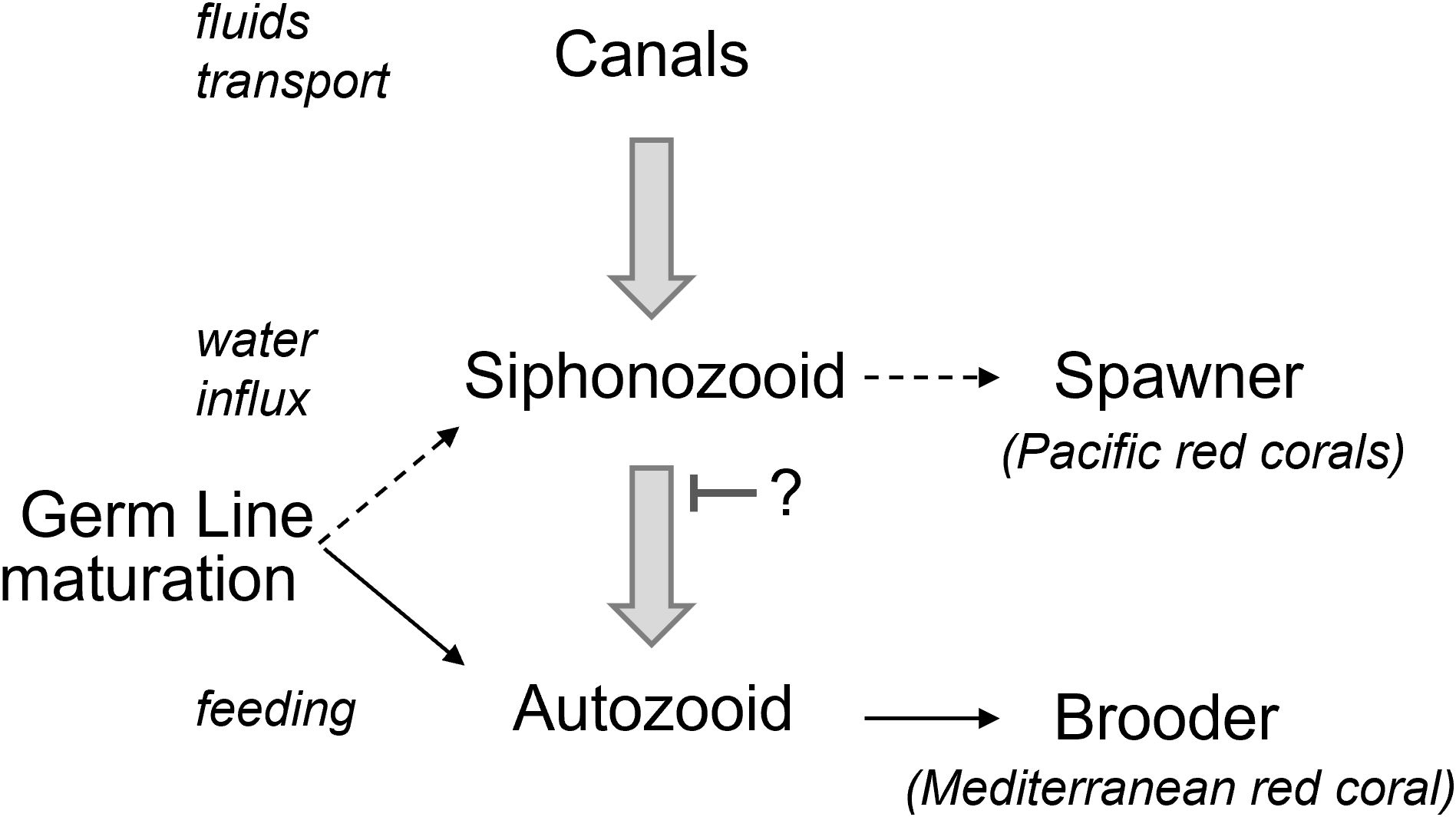
Figure 6. Schematic model for development of a polyp with implication of the oocyte development. Siphonozooids arise from the endothelial network of canals that transport fluids throughout the Corallium rubrum colony. These specialized polyps are connected to the canal network at their base and open to the surrounding seawater at the colony’s surface. Siphonozooids correspond to an arrested developmental stage. Some siphonozooids will resume their development and transform into autozooids. The mechanisms controlling the arrest and resumption of the siphonozooid-to-autozooid transition remain unknown. Siphonozooids, lacking tentacles, facilitate water flow through the canals. Autozooids, on the other hand, possess tentacles that enable them to also capture planktonic prey, making them the colony’s feeding polyps. In Corallium rubrum, gonads development occurs within autozooids, unlike in Pacific Corallium species where gonads develop within siphonozooids. This difference is correlated with Corallium rubrum releasing developed planula larvae (brooder), while Pacific Corallium species release their gametes directly into the environment (spawner) (Nonaka et al., 2015). This suggests that the reproductive strategy, whether brooding or spawning, varies depending on the metabolic environment in which the gonads develop.
Our current finding that siphonozooids are precursors to autozooids raises new questions about the tissue characteristics of each polyp types and the physiological and/or environmental factors that drive the divergent reproductive strategies between Pacific and Mediterranean precious corals. Given that one polyp derives from the other, it remains unclear at which stage -siphonozooid or autozooid- the germline is first established. If siphonozooids represent developmentally arrested polyps awaiting the resumption of their development to autozooids, we must ask to what extent this somatic developmental control influences germline progression (formation/migration/maturation). Investigating these developmental processes through gene expression analysis and cellular tracking would help clarify this issue. Moreover, future studies combined with feeding manipulations could shed light on how nutrient availability impacts polyp dimorphism and reproductive success. In other words, understanding the intricate relationship between zooid development and gonad maturation remains a major focus for future coral research.
Although recognized over 150 years ago in octocorals, the process of polyp budding -a fully differentiated unit- from the underlying canal, an endodermal tissue, remains poorly understood. The environmental, physiological, cellular, and molecular factors controlling this process are largely unknown. Our experimental setup, combining the culture of laterally growing Corallium rubrum colonies with long-term video monitoring, enables us to visualize this rare and slow process, which is otherwise hidden within the coral’s tissues. This new understanding -that autozooids develop from siphonozooid precursors- underscores the importance of recognizing polyp dimorphism in future studies on precious corals and, more broadly, octocorals. It is no longer sufficient to refer to these structures simply as ‘polyps’ without acknowledging their common origin, functional differences or determining whether pressures influencing siphonozooid or autozooid development were at play. A nuanced approach that accounts for the distinct roles of siphonozooids and autozooids is crucial for accurate biological and ecological interpretations.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
GL: Conceptualization, Investigation, Methodology, Resources, Visualization, Writing – review & editing. SP: Visualization, Writing – original draft, Writing – review & editing. DA: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition. ST: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition. PG: Conceptualization, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by CHANEL and the government of the Principality of Monaco.
We are grateful to the members of the Centre Scientifique de Monaco who participated in helpful discussions in the development of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. AI was only used for language details.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1512361/full#supplementary-material
Supplementary Figure 1 | Hardware setup for the time-Lapse acquisition apparatus. See Materials and Methods for a more detailed description of this setup. The numbers indicated on the images correspond to the following pieces of equipment/experimental setup 1 Canon 5DS camera; 2 X2T- C radio transmitter; 3 Laowa 24mm F14 lens; 4 Flash Godox MX300; 5 Fiber optics (8 mm); 6 Remote control TC-80N3; 7 Custom made culture chamber (Elegoo Saturn 3D printer); 8 location of the C. rubrum colony growing laterally. A laterally grown C. rubrum colony was placed within the custom chamber and submerged in an aquarium. The culture conditions mimicked those used fo other red coral colonies at the CSM. Images were captured at 20-minute intervals for varying durations (weeks to months, as detailed in Table 1). The captured images were then assembled and compressed into time-lapse videos.
Supplementary Video 1 | Primary_polyp.mp4 (complements Figure 2A): This clip shows a two-month-old primary polyp born at the CSM monitored for 9 days. The single autozooid opens and retracts. A green arrow indicates the siphonozooid.
Supplementary Video 2 | all_polyp_types.mp4 (complements Figure 2B): This clip shows a laterally growing colony monitored for 3 weeks. Siphonozooids (green arrows), growing autozooids (blue arrows), and mature autozooids (black arrows) can be seen synchronously opening and retracting. Note the clearly visible canals connecting the different polyps, which contract and dilate as the polyps open and retract, respectively. This illustrates that the transport of internal fluids likely occurs through successive hydrostatic pressure changes.
Supplementary Video 3 | regenerating_polyp.mp4: On a laterally grown colony, 2 autozooids were sectioned using microscissors and left to regenerate (2 weeks) under monitoring. The regenerating polyps (in the black squares) can be observed first emerging with a scarred stem that attains its mature size. Then, after 10 days, the rapid growth (8 days) of the tentacles is observed. A time counter at the bottom left counts the passing days (“D”) and weeks (“W”).
Supplementary Video 4 | Time_lapse_fullLength.mp4: This clip shows a laterally growing colony monitored for 11 months. Different phases of the lateral growth can be observed, such as a latency phase with retracted polyps due to the absence of maintenance for 3 months, followed by an expanding phase of the tissues on the cover slip coinciding with the restart of the maintenance. During tissue expansion, mature polyps stay open for long periods of time, and new polyps appear. Scale bars are indicated.
Supplementary Video 5 | zero-to-sipho_&_sipho-to-auto.mp4 (complements Figure 5): This 68-day clip from Supplementary Video 4 magnifies the emergence of 2 siphonozooids (green squares) alongside the transition of 2 siphonozooids becoming autozooids (blue squares). Note that the 2 siphonozooids that are becoming autozooids remained at the siphonozooid stage for several months before transitioning into autozooids (see Supplementary Video 4).
Supplementary Video 6 | sipho-to-autozoid_zoom.mp4: This clip shows a magnified version of the transition of a siphonozooid to an autozooid over a one-month period (selected from Supplementary Video 5).
Supplementary Video 7 | sypho_arise_zoom.mp4: This clip shows a magnified version of the emergence of the two siphonozooids over a one-month period (selected from Supplementary Video 5).
Supplementary Video 8 | zero-to-autozoid_No-stop.mp4: This 2-month clip from Supplementary Video 4 magnifies the parallel emergence of 2 siphonozooids (green arrows). The first one remains at this stage, while the second one continues its development into an autozooid (blue arrow) without an apparent pause, eventually becoming a mature autozooid (black arrow).
Ardila N. E., Giribet G., Sánchez J. A. (2012). A time-calibrated molecular phylogeny of the precious corals: reconciling discrepancies in the taxonomic classification and insights into their evolutionary history. BMC Evol. Biol. 12, 246. doi: 10.1186/1471-2148-12-246
Bayer F. M. (1973). “Colonial organization in octocorals,” in Animal Colonies, Animal Colonies Dowden, Hutchinson & Ross, Inc., Stroudsburg, PA., 69–93.
Bayer F. M. (1974). Studies on the anatomy and histology of Plexaura homomalla in Florida. Stud. Trop. Oceanogr. 12, 62–100.
Beklemishev. (1969). Principles of comparative anatomy of invertebrates promorphology. (Oliver & Boyd).
Benedetti M. C., Bramanti L., Priori C., Erra F., Iannelli M., Bulleri F., et al. (2020). Polyp longevity in a precious gorgonian coral: hints toward a demographic approach to polyp dynamics. Coral Reefs 39, 1125–1136. doi: 10.1007/s00338-020-01942-6
Berking S. (2007). Generation of bilateral symmetry in Anthozoa: A model. J. Theor. Biol. 246, 477–490. doi: 10.1016/j.jtbi.2007.01.008
Boero F., Bouillon J., Piraino S. (2005). The role of Cnidaria in evolution and ecology. Ital. J. Zool. 72, 65–71. doi: 10.1080/11250000509356654
Child C. M. (1903). Form regulation in cerianthus: I. The typical course of regeneration. Biol. Bull. 5, 239–260. doi: 10.2307/1535783
Claus C. (1884). Traité de Zoologie (Traduite sur la 4ème édition allemande par G. Moquin-Tandon). Ed. Savy F. (Paris: Deuxième édition française).
Cvejic J., Tambutté S., Lotto S., Mikov M., Slacanin I., Allemand D. (2007). Determination of canthaxanthin in the red coral (Corallium rubrum) from Marseille by HPLC combined with UV and MS detection. Mar. Biol. 152, 855–862. doi: 10.1007/s00227-007-0738-5
Delage Y., Hérouard E.J.É. (1901). “Traité de zoologie concrète,” in Librairie H. Le Soudier, Paris.
Finnerty J. R. (2005). Did internal transport, rather than directed locomotion, favor the evolution of bilateral symmetry in animals? BioEssays 27, 1174–1180. doi: 10.1002/bies.20299
Garrabou J., Harmelin J. G. (2002). A 20-year study on life-history traits of a harvested long-lived temperate coral in the NW Mediterranean: insights into conservation and management needs. J. Anim. Ecol. 71, 966–978. doi: 10.1046/j.1365-2656.2002.00661.x
Gierer A., Berking S., Bode H., David C. N., Flick K., Hansmann G., et al. (1972). Regeneration of hydra from reaggregated cells. Nature. New Biol. 239, 98–101. doi: 10.1038/newbio239098a0
Giordano B., Bramanti L., Perrin J., Kahramanoğulları O., Vielzeuf D. (2023). Early stages of development in Mediterranean red coral (Corallium rubrum): The key role of sclerites. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1052854
Gold D. A., Jacobs D. K. (2013). Stem cell dynamics in Cnidaria: are there unifying principles? Dev. Genes Evol. 223, 53–66. doi: 10.1007/s00427-012-0429-1
Grigg R. W. (1984). Resource management of precious corals: A review and application to shallow water reef building corals. Mar. Ecol. 5, 57–74. doi: 10.1111/j.1439-0485.1984.tb00307.x
Grillo M.-C., Goldberg W. M., Allemand D. (1993). Skeleton and sclerite formation in the precious red coral Corallium rubrum. Mar. Biol. 117, 119–128. doi: 10.1007/BF00346433
Henry L.-A., Hart M. (2005). Regeneration from injury and resource allocation in sponges and corals - a review. Int. Rev. Hydrobiol. 90, 125–158. doi: 10.1002/iroh.200410759
Hickson S. (1894). XIII_ A Revision of the Genera of the Alcyonaria Stolonifera, with a Description of One New Genus and Several New Species. Transactions of the Zoological Society of London, XIII, 325–349. Available online at: https://www.biodiversitylibrary.org/bibliography/45493.
Hickson S. J. (1883). On the ciliated groove (siphonoglyphe) in the stomodeum of the alcyonarians. Philos. Trans. Roy. Soc. (N.S.) 174, 693–705. doi: 10.1098/rstl.1883.0022
Hiebert L. S., Simpson C., Tiozzo S. (2021). Coloniality, clonality, and modularity in animals: The elephant in the room. J. Exp. Zoolog. B Mol. Dev. Evol. 336, 198–211. doi: 10.1002/jez.b.22944
Huxley T.H. (1851). XXIV. Observations upon the anatomy and physiology of salpa and pyrosoma. Philos. Trans. R. Soc. 141, 567–593. doi: 10.1098/rstl.1851.0027
Hyman L. H. (1923). “The-invertebrates, protozoa through ctenophora,” in Lankester’s Treatise On Zoology. Available online at: https://archive.org/details/in.ernet.dli.2015.461506/mode/1up.
Iwasaki N., Suzuki T. (2010). Biology of precious coral (Kanagawa: Biohistory Precious Coral Tokai Univ. Press), 3–25.
Jackson J. B. C., Coates A. G. (1986). Life cycles and evolution of clonal (modular) animals. Philos. Trans. R. Soc Lond. B Biol. Sci. 313, 7–22. doi: 10.1098/rstb.1986.0022
Kishinouye K. (1903). Preliminary note on the coralliidae of Japan. Zool. Anz. 26, 623–626. doi: 10.34435/zm000880
Lacaze-Duthiers H. (1864). Histoire naturelle du corail, organisation, reproduction, pêche en Algérie, industrie et commerce. J.B. Baillière et Fils, Paris. 372 pp. doi: 10.5962/bhl.title.11563
Lartaud F., Galli G., Raza A., Priori C., Benedetti M. C., Cau A., et al. (2016). “Growth patterns in long-lived coral species,” in Marine Animal Forests. Eds. Rossi S., Bramanti L., Gori A., Orejas C. (Springer International Publishing, Cham), 595–626. doi: 10.1007/978-3-319-21012-4_15
Le Goff C., Tambutté E., Venn A. A., Techer N., Allemand D., Tambutté S. (2017). In vivo pH measurement at the site of calcification in an octocoral. Sci. Rep. 7, 11210. doi: 10.1038/s41598-017-10348-4
Levanoni J., Rosner A., Lapidot Z., Paz G., Rinkevich B. (2024). Coral tissue regeneration and growth is associated with the presence of stem-like cells. Journal of Marine Science and Engineering. 12 (2), 343. doi: 10.3390/jmse12020343
Malakhov V. V. (2016). Symmetry and the tentacular apparatus in Cnidaria. Russ. J. Mar. Biol. 42, 287–298. doi: 10.1134/S1063074016040064
Marschal C., Garrabou J., Harmelin J. G., Pichon M. (2004). A new method for measuring growth and age in the precious red coral Corallium rubrum (L.). Coral Reefs 23, 423–432. doi: 10.1007/s00338-004-0398-6
Martindale M. Q., Finnerty J. R., Henry J. Q. (2002). The Radiata and the evolutionary origins of the bilaterian body plan. Mol. Phylogenet. Evol. 24, 358–365. doi: 10.1016/S1055-7903(02)00208-7
McFadden C. S., Van Ofwegen L. P., Quattrini A. M. (2022). Revisionary systematics of Octocorallia (Cnidaria: Anthozoa) guided by phylogenomics. Bull. Soc Syst. Biol. 1 (3), 8735. doi: 10.18061/bssb.v1i3.8735
Moseley H. N. (1881). REPORT on Certain Hydroid, Alcyonarian, and Madreporarian Corals Procured during the Voyage of H.M.S. Challenger, in the Years 1873-1876. In Challenger Expedition (1872-1876) (London: HMSO and sold by Longmans & Co.). Available online at: https://escholarship.org/uc/item/9zh4v40f.
Moseley H. N. (1876). On the Structure and Relation of the Alcyonarian Heliopora coerulea, with some Account of the Anatomy of a Species of Sarcophyton. Philosophical Transactions of the Royal Society of London. 165 (166), 91–129. doi: 10.1098/rstl.1876.0004
Nonaka M., Nakamura M., Muzik K. (2015). Sexual reproduction in precious corals (Coralliidae) collected in the Ryukyu archipelago. Pac. Sci. 69, 15–46. doi: 10.2984/69.1.2
Nonaka M., Nakamura M., Tsukahara M., Reimer J. D. (2012). Histological examination of precious corals from the Ryukyu archipelago. J. Mar. Biol. 2012, 1–14. doi: 10.1155/2012/519091
Roark E. B., Guilderson T. P., Dunbar R. B., Fallon S. J., Mucciarone D. A. (2009). Extreme longevity in proteinaceous deep-sea corals. Proc. Natl. Acad. Sci. 106, 5204–5208. doi: 10.1073/pnas.0810875106
Rosen B. R. (1986). Modular growth and form of corals: a matter of metamers? Philos. Trans. R. Soc Lond. B Biol. Sci. 313, 115–142. doi: 10.1098/rstb.1986.0029
Santangelo G., Carletti E., Maggi E., Bramanti L. (2003). Reproduction and population sexual structure of the overexploited Mediterranean red coral Corallium rubrum. Mar. Ecol. Prog. Ser. 248, 99–108. doi: 10.3354/meps248099
Sekida S., Iwasaki N., Okuda K. (2016). Gonadal Morphology and Gametogenesis in Japanese Red Coral Corallium japonicum (Octocorallia: Alcyonacea) Collected off Cape Ashizuri, Japan. Zoolog. Sci. 33, 320. doi: 10.2108/zs150140
Torrents O., Garrabou J. (2011). Fecundity of red coral Corallium rubrum (L.) populations inhabiting in contrasting environmental conditions in the NW Mediterranean. Mar. Biol. 158, 1019–1028. doi: 10.1007/s00227-011-1627-5
Tsounis G., Rossi S., Aranguren M., Gili J.-M., Arntz W. (2006). Effects of spatial variability and colony size on the reproductive output and gonadal development cycle of the Mediterranean red coral (Corallium rubrum L.). Mar. Biol. 148, 513–527. doi: 10.1007/s00227-005-0100-8
Uda K., Komeda Y., Fujita T., Iwasaki N., Bavestrello G., Giovine M., et al. (2013). Complete mitochondrial genomes of the Japanese pink coral (Corallium elatius) and the Mediterranean red coral (Corallium rubrum): a reevaluation of the phylogeny of the family Coralliidae based on molecular data. Comp. Biochem. Physiol. Part D Genomics Proteomics 8, 209–219. doi: 10.1016/j.cbd.2013.05.003
Vielzeuf D., Allemand D., Shick J. M., Arnaud V., Bodin S., Bramanti L. (2022). The biology and biomineralogy of the red coral: The Lacaze-Duthiers Legacy. Vie Milieu 72 (3-4), 63–127. doi: 10.57890/VIEMILIEU/2022.72.3/4:63-127
Vielzeuf D., Garrabou J., Baronnet A., Grauby O., Marschal C. (2008). Nano to macroscale biomineral architecture of red coral (Corallium rubrum). Am. Mineral. 93, 1799–1815. doi: 10.2138/am.2008.2923
Waller R. G., Stone R. P., Johnstone J., Mondragon J. (2014). Sexual reproduction and seasonality of the Alaskan red tree coral, Primnoa pacifica. PLoS One 9, e90893. doi: 10.1371/journal.pone.0090893
Keywords: precious corals, coral anatomy, coral reproduction, coral development, red corals, siphonoglyph, bilateral symmetry
Citation: Loentgen G, Parks SK, Allemand D, Tambutté S and Ganot P (2025) Polyp dimorphism in the Mediterranean Red Coral Corallium rubrum: siphonozooids are precursors to autozooids. Front. Mar. Sci. 12:1512361. doi: 10.3389/fmars.2025.1512361
Received: 16 October 2024; Accepted: 08 January 2025;
Published: 30 January 2025.
Edited by:
Charles Alan Jacoby, University of South Florida St. Petersburg, United StatesCopyright © 2025 Loentgen, Parks, Allemand, Tambutté and Ganot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philippe Ganot, cGdhbm90QGNlbnRyZXNjaWVudGlmaXF1ZS5tYw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.