
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Mar. Sci., 18 February 2025
Sec. Marine Megafauna
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1508097
Plastination represents the most advanced technique for preserving biological specimens, widely adopted in education, scientific research, and popular science. This study employed the technique of plastination to explore an innovative method for preserving and exhibiting minke whales, addressing challenges to optimize the use of rare marine mammal specimens and the multi-layered understanding of cetacean anatomy. A 6.5-meter-long adult female minke whale cadaver, weighing 3,368 kg, was dissected, plastinated with polymers, and assembled with internal support frames. Subsequently, skin, muscle, bone, and viscera specimens were prepared separately. The four specimens of the minke whale can be displayed together or individually, and their internal and external structures have been mostly well preserved, with superb surface textures and fine details. This marks the first application of plastination technique to create four distinct presentations from a single minke whale cadaver globally, which significantly conserves biological materials by maximizing the use of a single rare animal body. The innovative plastination technique not only broadens our understanding of cetacean biology but also establishes a sustainable model for preserving rare marine specimens, offering insights into the relationship between structures and functions, as well as environmental adaptations. This study will provide valuable tools for education, research, and popular science education.
The minke whale (Balaenoptera acutorostrata) is a relatively small baleen whale belonging to the mammalian order Cetacea, family Balaenopteridae, and genus Balaenoptera. Adult minke whales typically reach lengths of 6.5 to 8.8 meters and weigh up to 9.2 tons (Perrin et al., 2018). In recent years, commercial whaling and climate change have severely impacted the habitats and ecosystems of baleen whales, leading to a marked decline in their populations (Glover et al., 2010; Brown et al., 2014; Tulloch et al., 2019; Pitcher et al., 2022). Therefore, public engagement through popular science education, particularly in ocean sciences, is essential for raising awareness and fostering a sense of responsibility toward ocean conservation.
Current research on minke whales focuses mainly on their echolocation, foraging behaviors, and migratory patterns, aiming to enhance our understanding of their ecological interactions (Eerkes-Medrano et al., 2021; Rossi-Santos et al., 2022; Ten et al., 2022; Cade et al., 2023). This knowledge is essential for developing effective conservation policies for the species (Risch et al., 2019). Some studies have explored the evolutionary processes of minke whales by examining specific systems or structures, such as the olfactory or reproductive systems, skull, and temporomandibular joint (Godfrey et al., 2013; Sasaki et al., 2013; Nakamura and Kato, 2014; Werth et al., 2020). However, the large size and long growth cycles of whales pose significant challenges in accurately reconstructing their overall anatomical structures. Moreover, the scarcity of sufficient samples and technological limitations complicate research on the ecological conservation and evolutionary development of whales. This suggests that effective conservation of whale cadavers is crucial for advancing our understanding of marine ecosystems and evolutionary processes.
With the advent of plastination technology, the long-term preservation and display of large specimens have become feasible (Nader et al., 2019). For instance, previous studies have successfully plastinated a sperm whale, which is now the largest plastinated marine specimen in the world (Jiang et al., 2021). Traditional biological preservation methods primarily focus on maintaining the appearance and skeletal structure of whales by preserving their skin and bones. Fortunately, the introduction of plastination techniques allows for the preservation of soft tissues, including muscles and internal organs (von Hagens, 1979; von Hagens et al., 1987; Yu et al., 2015; Samuel and Sui, 2019; Sora et al., 2019). These techniques enhance the visualization of anatomical structures and provide more comprehensive information for scientific education and public outreach (Sora et al., 2019). By preserving and studying large whales, researchers can gain deeper insights into their ecological habits, behavioral characteristics, and evolutionary processes (Nader et al., 2019; Jiang et al., 2021). Most importantly, this approach offers a robust scientific foundation for the study and conservation of these invaluable marine organisms (Nader et al., 2019; Jiang et al., 2021).
Currently, the plastination technique is limited to isolating one or two independent specimens from a single large vertebrate (Yu et al., 2015; Jiang et al., 2021). This method hinders the ability to fully understand the detailed relationships between hierarchical structures and restricts the representation of specific living states in vertebrates. Moreover, this splitting approach often requires more raw materials to create multiple specimens, making it unsuitable for rare specimens.
In response to these challenges, we planned a study to address the high demand for raw materials and the limitations in anatomical accuracy presented by biological plastination methods. This study transformed traditional specimen exhibition by offering a multi-angle view of the internal structures of a minke whale. It helped clarify the adaptive relationships between structure and function, as well as structure and environment, in whales during cetacean evolution, thereby deepening our understanding of their evolutionary history and ecosystems.
The specimen used in this study was a 6.5-meter-long adult female minke whale, weighing approximately 3368 kg. The minke whale became stranded on the beach in Changhai County, Dalian City, Liaoning Province, and showed no signs of life. This specimen was successfully collected under permission from the Chinese Authorities for Animal Protection. After discussions between the Dalian municipal government and experts, the minke whale was transported to Dalian Hoffen Biotechnique Institute, Dalian City, Liaoning Province, for preservation by plastination.
The adult mink whale was dissected according to Figure 1.
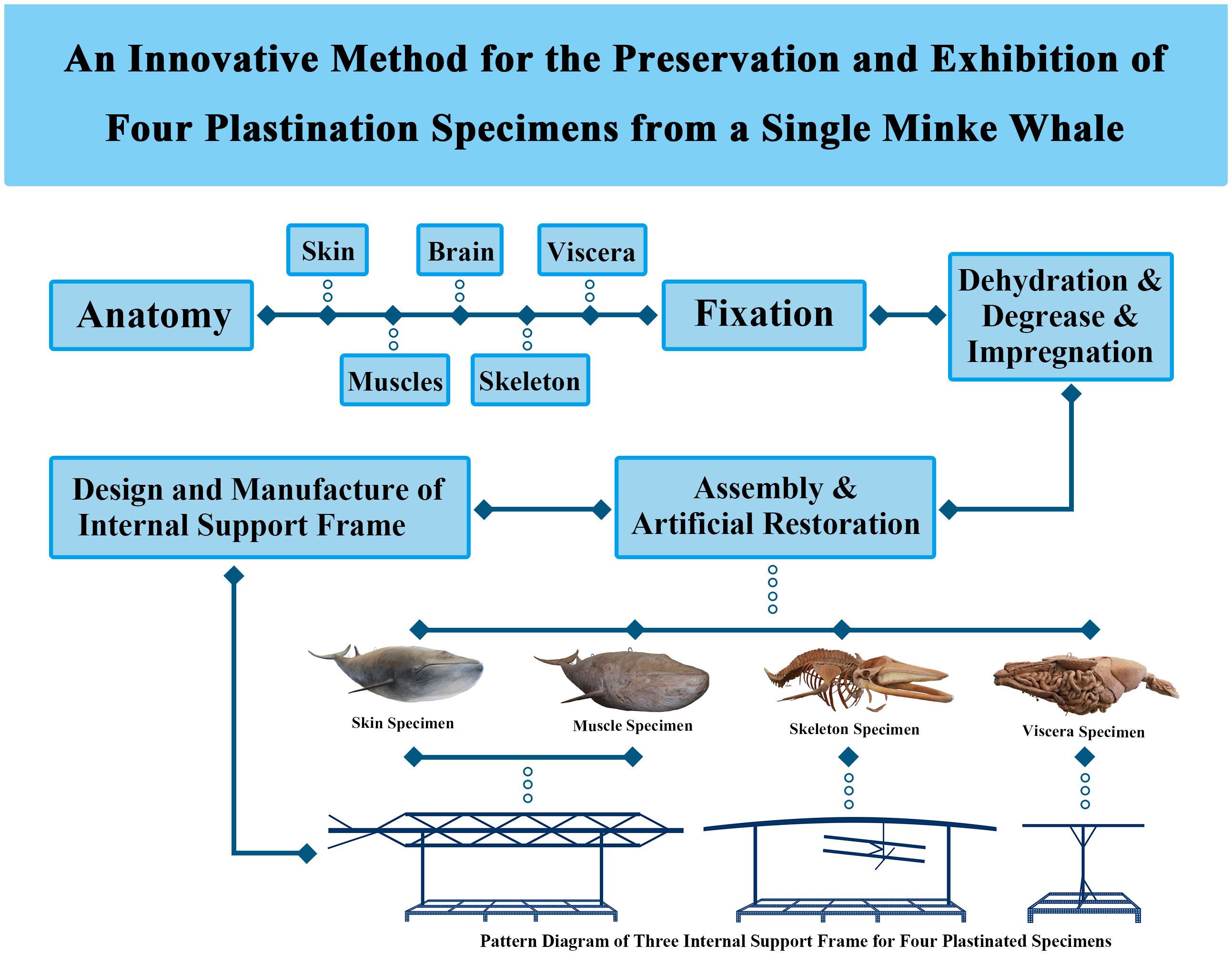
Figure 1. Flowchart of the study and pattern diagram of three internal support frames for four plastinated specimens.
Firstly, the skin of the head and trunk was carefully stripped along the median sagittal axis. The incisions along each fin followed the edges toward the distal ends. Skin was separated along these incision lines toward the dorsal side until it was completely removed. The dimensions of the peeled skin were measured and recorded to ensure accurate positioning during reassembly. The minke whale, after peeling off the skin, was dissected to show the superficial structures, including muscles, nerves, blood vessels, and glands.
Secondly, muscles were carefully peeled from the bone surface layer by layer, from superficial to deep, maintaining clear connections between them. Special care was taken with smaller, deeper muscles to avoid damaging the bone or ligaments. Blood vessels and nerves were cut at their distal ends but left attached to the muscles. All structures were meticulously dissected while preserving their original anatomy and integrity.
Thirdly, the visceral organs were dissected and removed as a whole from the mink whale. The visceral organs were completely removed from the thoracic and abdominal cavities and dissected to display the structures of each system and their adjacent relationships. Then, the skeleton and its connecting ligaments were dissected to better display the skeletal landmarks and the ligaments attached to the skeleton.
Finally, the skull was cut open and brain tissue was dissected and collected. The skull was cut along the median sagittal line, and the brain, along with its cranial nerves, was fully removed, leaving about 0.5 cm of nerve roots intact. The skull was then restored to its original state.
The dissected body parts of the specimen were fixed for several months using a combination of vascular perfusion and immersion in a 10% formalin solution. After selecting a suitable container for the samples, the anatomical specimens, including skin, muscles, bone ligaments, internal organs, and brain, were dehydrated, degreased, and impregnated with polymer using Hoffen-S10 technology (developed by Hoffen independently). Firstly, the anatomical specimens were dehydrated and defatted by immersing them in gradient acetone. This process was strictly monitored. Dehydration was performed at -25°C with the concentration of acetone increasing from 85% to 98%. Acetone levels were checked every 2 to 3 days and replaced with fresh acetone approximately six times to maintain the desired concentrations. Defatting was performed with the concentration of acetone increasing from 98% to over 99.95%, lasting for more than 60 days. The process was considered complete when the color of acetone was clear and not yellowed. Then, the dissected specimens were removed from the acetone bath and separately transferred to specially designed tanks containing silicone rubber for forced impregnation. The impregnation process was carried out in an environment of -25°C and -0.1 MPa, taking approximately 50 days.
This method was based on previous literature with minor adjustments (Jiang et al., 2021). The internal support frame, composed of stainless steel, iron, copper, or high-strength plastic rods, was designed using skeletal and muscle data to replicate the anatomy of the minke whale. Key structural regions, joint connections, and movement patterns were carefully recreated, with special attention to muscle layers, craniofacial contours, and nerve and vessel course. Three types of internal support frames were designed for four specimens, and their schematic diagrams are shown in Figure 1. Among them, the internal support frames of the skin specimen and the muscle specimen were similar.
The assembly process involved positioning the skin, muscles, skeleton, and internal organs, including the brain, on internal support frames to accurately recreate the natural anatomical structures of the minke whale. Careful adjustments were made to restore the appearance of muscles, nerves, bones, ligaments, and brain features.
The structural damage caused during the process of dissection and other processes was repaired by professionals to restore the original anatomical structure of the minke whale. As the brain tissue was severely damaged during collection, the sulci and gyri on its surface were artificially reconstructed. After artificial restoration, the specimens were placed in a closed chamber at 45°C and cured with the curing agent (Hoffen S6, developed by Hoffen independently) over one month. Hoffen S6 was vaporized using a small peristaltic pump, facilitating a cross-linking reaction of the silicone polymer within the tissues, which achieved the purpose of effect. Finally, the specimens were colored using a proprietary brand of water-based paint and a soft-tissue brush.
An adult female minke whale cadaver, measuring 6.5 meters in length and weighing approximately 3,368 kg, underwent dissection, polymer impregnation, and internal support frame construction (Figure 1). It was then reassembled into its correct anatomical position, resulting in four specimens: skin, muscle, bone, and internal organs, which could be displayed either together or separately. Macroscopically, the external and internal structures of the plastinated minke whale were well-preserved (Figure 2). Each specimen highlighted specific anatomical features and the morphological characteristics associated with various activities of daily living. Additionally, the plastinated specimens were dry, non-toxic, and required no additional storage medium, enhancing their value for scientific collections in museums.
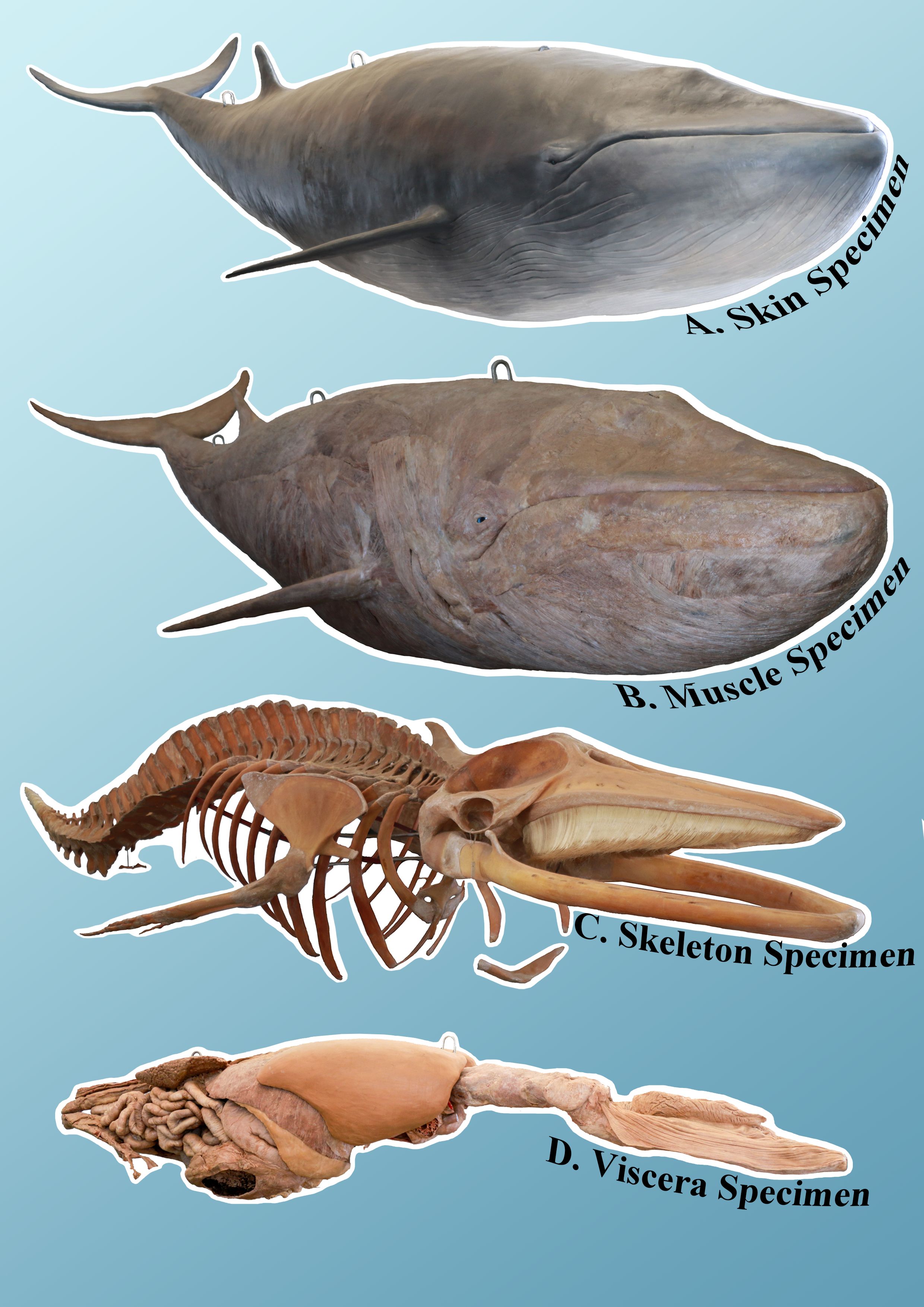
Figure 2. Overall presentation of the four plastinated specimens of the fully assembled minke whale: skin specimen (A) muscle specimen (B) bone specimen (C) and viscera specimen (D).
First, as shown in Figure 3A, the minke whale skin specimen revealed a thick, smooth outer epidermis (Figure 3a1), which was nearly hairless.
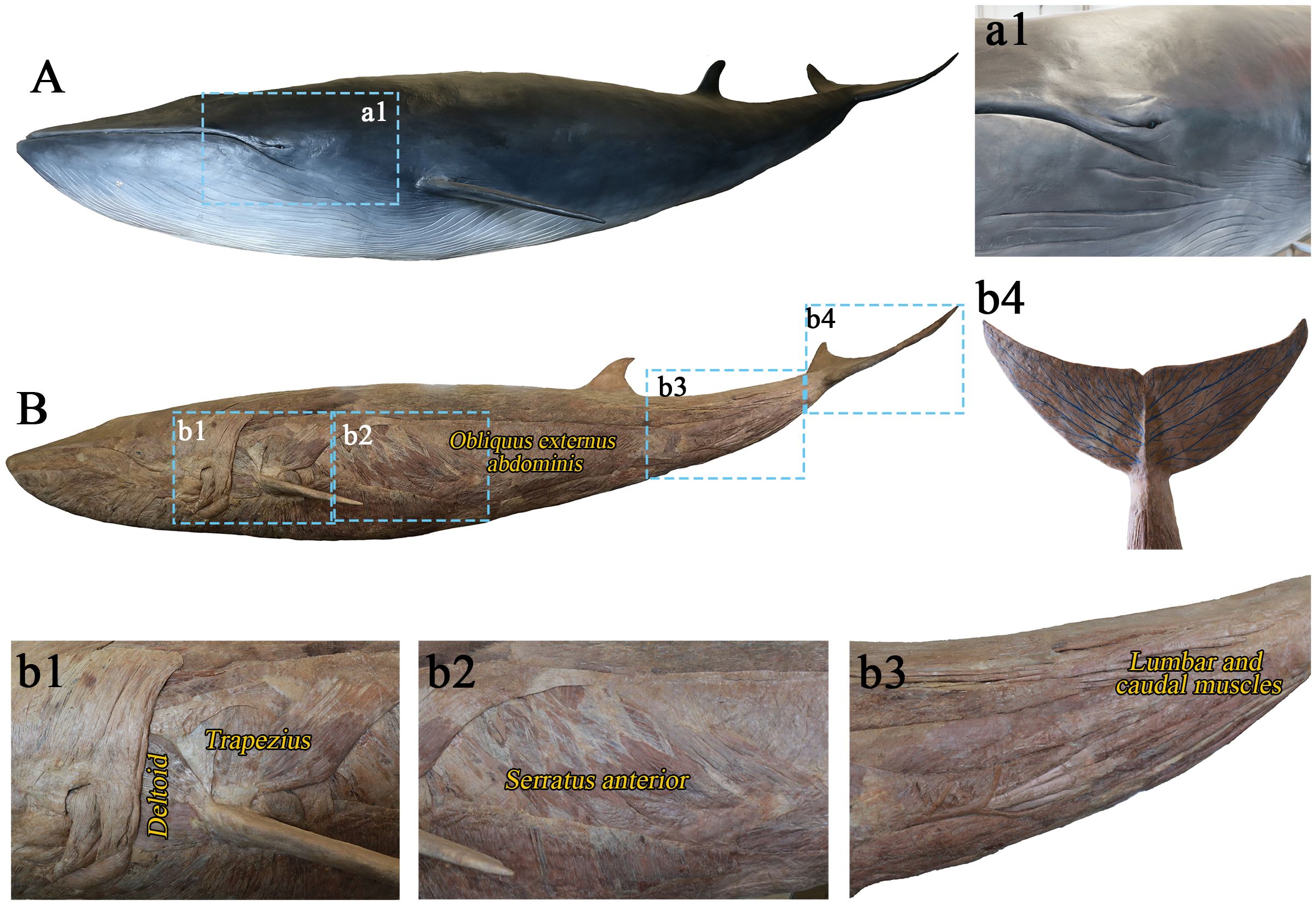
Figure 3. Skin plastination specimen (A) and muscle plastination specimen (B). (a1) Thick and smooth skin; (b1) Deltoid and trapezius muscles around the glenohumeral joint; (b2) Strong ventral lateral muscles (M. serratus anterior, M. obliquus externus abdominis, etc.); (b3) The lumbar and caudal muscles; (b4) Artificially drawn veins on the caudal fin.
Secondly, the muscle specimen (Figure 3B) showed a concentration of muscle tissues in the forelimbs, particularly the thick deltoid and trapezius muscles surrounding the glenohumeral joints (Figure 3b1). The triceps brachii complex exhibited atrophy, and the flippers lacked muscle tissue (Figures 3b1-2). Strong muscles, such as obliquus externus abdominis and serratus anterior muscle, were present along the belly of the whale (Figure 3b2). The caudal fin was composed of strong muscles (Figure 3b3), and the caudal fin veins have been artificially drawn (Figure 3b4).
Thirdly, well-preserved connecting structures such as vertebra (Figures 4C–E, G) ligaments, intervertebral discs, joint capsules, and cartilage were clearly shown in the skeletal specimen (Figure 4J). The glenohumeral joint was polyaxial (Figure 4B), while the digits showed signs of supernumerary phalanges and joints (hyperphalangy) and were reinforced by dense connective tissues (Figure 4F). The hind limbs were degenerated, leaving only bone fragments (Figure 4I). The baleen plates in the whale’s mouth, resembling human gums, were covered with baleen (Figure 4A). Additionally, hyoid bone also can be seen (Figure 4H).
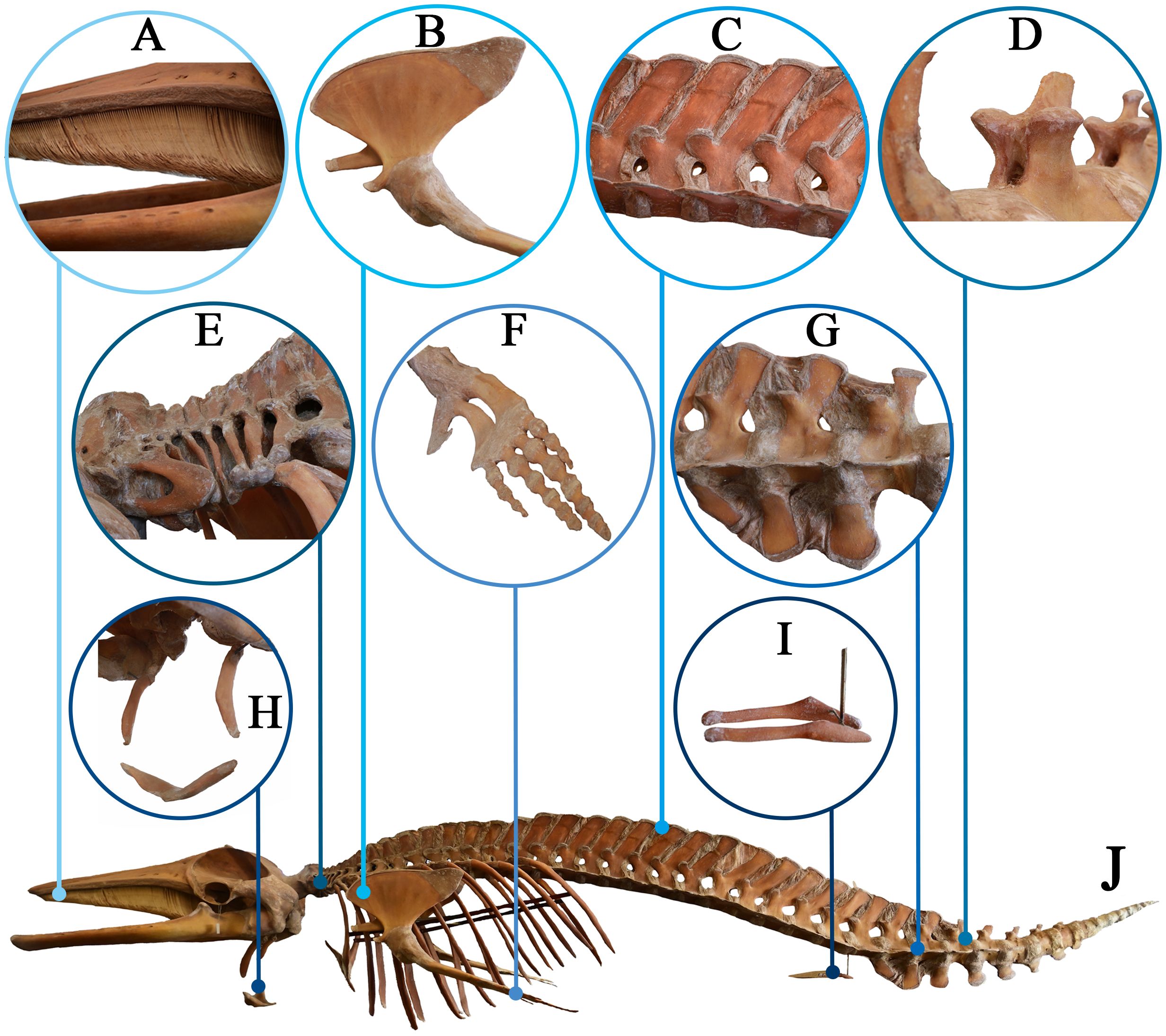
Figure 4. Skeleton plastination specimen of the minke whale (J) Lateral view of the skeleton specimen). (A) Whale baleen; (B) Glenohumeral joint; (C) Lumbar vertebra; (D) Transverse and spinous processes of the vertebrae; (E) Cervical vertebra; (F) A flipper; (G) Sacral vertebra; (H) hyoid bone; (I) Degenerated hind limb bones.
Finally, the viscera specimen (Figure 5) clearly displayed the sulci and gyri on the surface of the brain (Figures 5A, D), lung tissues (Figure 5E), kidney tissues (Figure 5F), heart structure (Figure 5B), and the large arteries that originate from the heart (Figure 5B). The kidney was a compound structure, consisting of multiple independent renal lobules (Figure 5F). Incompletely digested krill were still present in the stomach (Figure 5H), and the intestines were approximately the diameter of a human arm (Figure 5G). Additionally, the whale had a thick diaphragm (Figure 5C).

Figure 5. Viscera plastination specimen of the minke whale (A) Front view of the specimen; (G) Lateral view of the specimen). (B) Heart; (C) Adjacent diaphragm, liver and stomach in situ; (D) Surface view of brain; (E) Lung; (F) Kidney; (H) Stomach contains undigested krill.
Cetaceans are unique mammals that spend their entire lives in water and, over millions of years of evolution, have become integral to marine ecosystems. To adapt to a fully aquatic environment, whales have undergone extensive morphological, anatomical, physiological changes, and behavioral adaptations (Arnason et al., 2004; Slater et al., 2010; Gatesy et al., 2013). Therefore, conducting anatomical studies on stranded or accidentally deceased whales provides valuable insights into their physiological structure, biological characteristics, and ecological roles. This comprehensive knowledge is crucial for protecting marine animals and their complex ecological relationships (Chenoweth et al., 2022). In addition, their survival is increasingly threatened by factors such as overhunting, environmental pollution, and climate change (Gissi et al., 2021). Therefore, enhancing the understanding of cetaceans and improving the public’s scientific literacy has also become extremely important.
Traditional anatomical methods have been employed to study cetacean anatomy (Jiang et al., 2021). With the development of technology, plastination has become an advanced technique for the preservation and study of large cetaceans (von Hagens, 1979; von Hagens et al., 1987; Nader et al., 2019; Jiang et al., 2021). Plastinated specimens are durable, free of hazardous chemicals, odorless, capable of displaying intricate anatomical details, and require no additional storage medium, making them ideal for museum exhibitions. High-quality plastination specimens can last for decades and have been used to preserve large marine mammals (Ottone et al., 2015; Nader et al., 2019).
However, current methods typically allow for the extraction of only 1-2 anatomical layers, limiting the ability to fully demonstrate its morphological and behavioral features. This approach fails to capture the complexity of anatomical structures in a single individual. More detailed layers and features typically require multiple specimens of similar size (Yu et al., 2015; Nader et al., 2019; Jiang et al., 2021). Fortunately, the method used in this study—creating four plastinated specimens from a single minke whale—maximizes the use of a rare specimen. This approach effectively preserves biological materials and addresses the challenges associated with specimen scarcity in marine mammal research.
This method conserves raw materials and simplifies storage. For instance, the skin specimen overcomes issues like deformation and decay. Importantly, this method allows for a comprehensive presentation of anatomical structures from different perspectives and levels of detail. The skin specimen of minke whale shows a thick and smooth outer epidermis, almost hairless. Meanwhile, its skin lacks sweat and sebaceous glands and has a thick layer of whale blubber that provides insulation and mechanical protection (Jablonski, 2021). The muscle specimen, supported by stainless steel bars replicating the whale’s bone morphology and muscle attachment points, provides detailed views of muscles, nerves, blood vessels, and ligaments. Their forelimbs contain the most muscle tissues, allowing their flippers to act as stabilizers (Fish, 2002). The caudal fin, composed of strong muscles, propels the whale by swinging up and down (Woodward et al., 2006).
Moreover, the bone and viscera specimens were assembled based on normal physiological morphology, preserving rare specimens for in-depth anatomical studies, facilitating comparisons with other species, and offering insights into better assessing their environmental adaptability. The baleen plates of the minke whale, similar to human gums, are covered with whalebone which is used to filter small food particles. The degenerated hind limbs of the minke whales, now reduced to residual bone fragments, help reduce resistance in water during movement. Their digits showed signs of hyperphalangy and were reinforced by dense connective tissues, limiting the mobility of the metacarpophalangeal joint and interphalangeal joint. The presence of dense connective tissue surrounding these digits results in the absence or reduction of muscles that allow them to bend and extend (Cooper et al., 2007). Their flippers and digits with hyperphalangy effectively increase the surface area of the cetacean manus, which allows the limb to act as a control surface and may assist in aquatic locomotion (Cooper et al., 2017). The glenohumeral joints are polyaxial and primarily located within the body wall, with the surrounding muscles responsible for the movement of the flippers in cetaceans (Cooper et al., 2017). This contrasts with other aquatic species, such as penguins, seals, and sea lions, which retain flexibility in their glenohumeral joints, though the mobility of their metacarpophalangeal and interphalangeal joints varies (Fish, 2004). Consequently, this method of dissecting and plastinating multiple specimens from one same minke whale allows for a comprehensive visualization of the internal and external structures of the minke whale, offering insights into the relationships between structure and function, as well as environmental adaptations.
This study marks the first application of the plastination technique to create four plastinated specimens with different structural levels from one minke whale, effectively addressing the complexity of hierarchical structures and the diversity of living forms in plastinated vertebrate specimens. By presenting specimens that highlight various anatomical features in an accessible and durable format, observing these structures in more detail enhances our understanding of their functions, and analyzing these functions deepens our knowledge of the structures. In addition, the plastinated specimens are non-toxic, odorless, and require no additional storage medium, making them an ideal choice for museum exhibitions. By describing the evolutionary process of structures and how they adapt to the marine environment, it will help to stimulate the public interest in marine life and raise awareness of environmental and animal protection. This innovative plastination technique will provide valuable tools for marine biology, anatomical education, preservation science, and public engagement.
Despite the great effort we have made in this work, there is no guarantee that this work was carried out perfectly. In terms of anatomy, design, and display, we can choose asymmetric methods. For example, muscle specimens can be displayed with superficial muscles on one side and deep muscles on the other. Alternatively, different layers of muscle distribution can be presented through windowing techniques. This selective dissection is based on the needs of the display. In the future, this technology will be applied to more vertebrates, not just marine organisms.
This study presents a groundbreaking application of plastination, demonstrating an innovative method for the preservation and exhibition of four specimens derived from a single minke whale cadaver. The innovation of this technology lies not only in promoting the effective preservation of rare species such as cetaceans, but also in providing novel exhibition methods for museums around the world. Importantly, it offers scientists a valuable opportunity to study the ecology and evolution of whales, thereby significantly advancing the popularization of marine education and conservation efforts.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal was collected under permission from the Chinese Authorities for Animal Protection. After discussions between the Dalian municipal government and experts, The animal was transported to Dalian Hoffen Biotechnique Institute, Dalian City, Liaoning Province, for preservation by plastination. The study was conducted in accordance with the local legislation and institutional requirements.
W-BJ: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. XS: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MS: Writing – review & editing. JH: Data curation, Methodology, Resources, Writing – original draft. X-WM: Data curation, Methodology, Resources, Writing – original draft. H-JS: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank Professor Sheng-Bo Yu of Dalian Medical University for guidance on anatomy, Large Language Models ChatGPT 4o model (https://chatgpt.com/) for the revision of this manuscript language, Dr. Campbell Gilmore of St. George’s University of London for proofreading the manuscript and Rui-Ze Geng for assistance with photography.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation and revision process of this manuscript, ChatGPT 4o model (https://chatgpt.com/) was used for assistance in improving the quality of language and the clarity of the text. The use of AI was limited to language improvements and did not affect the scientific integrity or originality of the research.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arnason U., Gullberg A., Janke A. (2004). Mitogenomic analyses provide new insights into cetacean origin and evolution. Gene 333, 27–34. doi: 10.1016/j.gene.2004.02.010
Brown S., Reid D., Rogan E. (2014). Characteristics of fishing operations, environment and life history contributing to small cetacean bycatch in the Northeast Atlantic. PloS One 9, e104468. doi: 10.1371/journal.pone.0104468
Cade D. E., Kahane-Rapport S. R., Gough W. T., Bierlich K. C., Linsky J. M. J., Calambokidis J., et al. (2023). Minke whale feeding rate limitations suggest constraints on the minimum body size for engulfment filtration feeding. Nat. Ecol. Evol. 7, 535–546. doi: 10.1038/s41559-023-01993-2
Chenoweth E. M., Houston J., Burek Huntington K., Straley J. M. (2022). A virtual necropsy: applications of 3D scanning for marine mammal pathology and education. Animals 12, 527. doi: 10.3390/ani12040527
Cooper L. N., Dawson S. D., Reidenberg J. S., Berta A. (2007). Neuromuscular anatomy and evolution of the cetacean forelimb. Anatom. Rec. 290, 1121–1137. doi: 10.1002/ar.20571
Cooper L. N., Sears K. E., Armfield B. A., Kala B., Hubler M., Thewissen J. G. M. (2017). Review and experimental evaluation of the embryonic development and evolutionary history of flipper development and hyperphalangy in dolphins (Cetacea: Mammalia). Genesis 56 (1). doi: 10.1002/dvg.23076
Eerkes-Medrano D., Aldridge D. C., Blix A. S. (2021). North Atlantic minke whale (Balaenoptera acutorostrata) feeding habits and migrations evaluated by stable isotope analysis of baleen. Ecol. Evol. 11, 16344–16353. doi: 10.1002/ece3.8224
Fish F. E. (2002). Balancing requirements for stability and maneuverability in cetaceans1. Integr. Comp. Biol. 42, 85–93. doi: 10.1093/icb/42.1.85
Fish F. E. (2004). Structure and mechanics of nonpiscine control surfaces. IEEE J. Ocean. Eng. 29, 605–621. doi: 10.1109/JOE.2004.833213
Gatesy J., Geisler J. H., Chang J., Buell C., Berta A., Meredith R. W., et al. (2013). A phylogenetic blueprint for a modern whale. Mol. Phylogenet. Evol. 66, 479–506. doi: 10.1016/j.ympev.2012.10.012
Gissi E., Manea E., Mazaris A. D., Fraschetti S., Almpanidou V., Bevilacqua S., et al. (2021). A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 755, 142564. doi: 10.1016/j.scitotenv.2020.142564
Glover K. A., Kanda N., Haug T., Pastene L. A., Øien N., Goto M., et al. (2010). Migration of Antarctic minke whales to the Arctic. PloS One 5, e15197. doi: 10.1371/journal.pone.0015197
Godfrey S. J., Geisler J., Fitzgerald E. M. G. (2013). On the olfactory anatomy in an archaic whale (Protocetidae, cetacea) and the minke whale balaenoptera acutorostrata (Balaenopteridae, cetacea). Anatom. Rec. 296, 257–272. doi: 10.1002/ar.22637
Jablonski N. G. (2021). Evolution: How to evolve a thick skin. Curr. Biol. 31, R483–R4R6. doi: 10.1016/j.cub.2021.03.077
Jiang W. B., Han J., Ma X. W., Liu H., Yu S. B., Sui H. J. (2021). Plastination of a sperm whale. J. Anatom. 240, 669–677. doi: 10.1111/joa.13581
Nader P. B., Henry R. W., Sui H. J. (2019). Plastination of larger and massive specimens—With Silicone. Anatom. Histol. Embryol. 48, 547–551. doi: 10.1111/ahe.12481
Nakamura G., Kato H. (2014). Developmental changes in the skull morphology of common minke whales Balaenoptera acutorostrata. J. Morphol. 275, 1113–1121. doi: 10.1002/jmor.20288
Ottone N. E., Cirigliano V., Bianchi H. F., Medan C. D., Algieri R. D., Borges Brum G., et al. (2015). New contributions to the development of a plastination technique at room temperature with silicone. Anatom. Sci. Int. 90, 126–135. doi: 10.1007/s12565-014-0258-6
Perrin W. F., Mallette S. D., Brownell R. L. (2018). “Minke Whales: Balaenoptera acutorostrata and B. bonaerensis,” in Encyclopedia of Marine Mammals, 3rd ed. Eds. Würsig B., Thewissen J. G. M., Kovacs K. M. (London, U.K: Academic Press), 608–613.
Pitcher C. R., Hiddink J. G., Jennings S., Collie J., Parma A. M., Amoroso R., et al. (2022). Trawl impacts on the relative status of biotic communities of seabed sedimentary habitats in 24 regions worldwide. Proc. Natl. Acad. Sci. 119, e2109449119. doi: 10.1073/pnas.2109449119
Risch D., Norris T., Curnock M., Friedlaender A. (2019). Common and Antarctic minke whales: conservation status and future research directions. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00247
Rossi-Santos M. R., Filun D., Soares-Filho W., Paro A. D., Wedekin L. L. (2022). Playing the beat”: Occurrence of Bio-duck calls in Santos Basin (Brazil) reveals a complex acoustic behaviour for the Antarctic minke whale (Balaenoptera bonaerensis). PloS One 17, e0255868. doi: 10.1371/journal.pone.0255868
Samuel O. C., Sui H.-J. (2019). Updated protocol for the hoffen P45 sheet plastination technique. J. Plastin. 31, 22–25. doi: 10.56507/KDAS3395
Sasaki M., Amano Y., Hayakawa D., Tsubota T., Ishikawa H., Mogoe T., et al. (2013). Structure and steroidogenesis of the placenta in the Antarctic minke whale (Balaenoptera bonaerensis). J. Reprod. Dev. 59, 159–167. doi: 10.1262/jrd.2012-132
Slater G. J., Price S. A., Santini F., Alfaro M. E. (2010). Diversity versus disparity and the radiation of modern cetaceans. Proc. R. Soc. B: Biol. Sci. 277, 3097–3104. doi: 10.1098/rspb.2010.0408
Sora M.-C., Latorre R., Baptista C., López-Albors O. (2019). Plastination—A scientific method for teaching and research. Anatom. Histol. Embryol. 48, 526–531. doi: 10.1111/ahe.12493
Ten S., Konishi K., Raga J. A., Pastene L. A., Aznar F. J. (2022). Epibiotic fauna of the Antarctic minke whale as a reliable indicator of seasonal movements. Sci. Rep. 12, 22214. doi: 10.1038/s41598-022-25929-1
Tulloch V. J. D., Plagányi É. E., Brown C., Richardson A. J., Matear R. (2019). Future recovery of baleen whales is imperiled by climate change. Global Change Biol. 25, 1263–1281. doi: 10.1111/gcb.14573
von Hagens G. (1979). Impregnation of soft biological specimens with thermosetting resins and elastomers. Anatom. Rec. 194, 247–255. doi: 10.1002/ar.1091940206
von Hagens G., Tiedemann K., Kriz W. (1987). The current potential of plastination. Anat Embryol. (Berl). 175, 411–421. doi: 10.1007/BF00309677
Werth A. J., Ito H., Ueda K. (2020). Multiaxial movements at the minke whale temporomandibular joint. J. morphol. 281, 402–412. doi: 10.1002/jmor.21107
Woodward B. L., Winn J. P., Fish F. E. (2006). Morphological specializations of baleen whales associated with hydrodynamic performance and ecological niche. J. Morphol. 267, 1284–1294. doi: 10.1002/jmor.10474
Keywords: plastination technique, minke whale, anatomy, marine mammals, popular science education
Citation: Jiang W-B, Song X, Shah MAA, Han J, Ma X-W and Sui H-J (2025) An innovative method for the preservation and exhibition of four plastination specimens from a single minke whale (Balaenoptera acutorostrata). Front. Mar. Sci. 12:1508097. doi: 10.3389/fmars.2025.1508097
Received: 09 October 2024; Accepted: 21 January 2025;
Published: 18 February 2025.
Edited by:
Philip Adds, St George’s, University of London, United KingdomReviewed by:
Carlos Baptista, University of Toledo, United StatesCopyright © 2025 Jiang, Song, Shah, Han, Ma and Sui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Jin Sui, c3VpaGpAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.