
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 14 February 2025
Sec. Marine Biogeochemistry
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1500594
Although policymakers and stakeholders are beginning to acknowledge the importance of the marine biosphere in blue carbon services, the role of large marine vertebrates in the marine carbon and nitrogen cycle and especially in carbon sequestration has not yet been fully understood. Large marine vertebrates store only a small percentage of total oceanic carbon in their bodies, but they can provide important and lasting contributions to the oceanic carbon flux. The Península Valdés Biosphere Reserve in southwestern Argentina was partially established to conserve these large marine vertebrates, including the South American sea lion (Otaria flavescens) and southern right whale (Eubalaena australis). Three locations in Peninsula Valdés were sampling for the proximity of marine vertebrate populations in the area and the presence of salt marshes to assess the organic carbon (OC) and total nitrogen (TN) stocks in the top 1 m of sediment. Our work provides the first quantitative data on the OC and TN sequestered in the coastal sediments of Península Valdés and shows that this protected area contributes significantly to blue carbon by storing relevant quantities of OC (140 to 317 Mg OC ha-1) and nitrogen (7.3 to 22.9 Mg TN ha-1). Specifically, we found that salt marshes and terrestrial plants were the main C sources in each sediment core, but a non-negligible proportion (from 0.8 to 6.8% dry weight) of the OC stocks showed an isotopic signal from the large vertebrates that usually inhabit the area. Therefore, our results provide novel hypotheses about the potential contribution of large marine vertebrates as an OC vector in coastal systems and may serve as a basis for further investigation about their role into coastal blue carbon.
In the last decade, the ocean has absorbed a quarter of the Earth’s greenhouse gas emissions in the form of carbon (C) buried in marine sediments (Friedlingstein et al., 2022) or C transformed into recalcitrant dissolved molecules (i.e., resistant to rapid biological decomposition; Baltar et al., 2021; Jiménez-Ramos et al., 2022). In either case, the C becomes “locked” in the ocean for thousands of years. This type of carbon sequestration can therefore serve as a relevant nature-based solution (NbS) for climate change mitigation (IUCN, 2020; Macreadie et al., 2021). However, NbS have historically been rooted almost exclusively in terrestrial ecosystems (referred to as ‘green carbon’), while ocean-based solutions (referred to as ‘blue carbon’) have lagged behind (Macreadie et al., 2021). Blue carbon habitats, which have been widely recognized for their ability to store and sequester autochthonous and allochthonous C, include mangrove forests, seagrass meadows, and salt marshes (Nellemann et al., 2009; Hilmi et al., 2021). However, the C cycle is much more complex in marine habitats relative to terrestrial habitats. For example, the marine C cycle encompasses both organic and inorganic forms of C in both dissolved and particulate states as they pass through other media, including water columns and living organisms in the sea (Ciais et al., 2013; Turrell et al., 2023). Furthermore, many natural processes and ecosystem components contribute to C sequestration and burial (Howard et al., 2017). Indeed, the ‘blue biosphere’ is beginning to be acknowledged as an important NbS in the policy and management arena (Krabbe et al., 2022; Pérez et al., 2024). In this regard, recent publications have specifically focused on the role of large marine vertebrates for the oceanic C cycle and C sequestration (Roman et al., 2014; Chami et al., 2020; Mariani et al., 2020; Martin et al., 2021; Pearson et al., 2023).
Marine vertebrates play an important role transporting marine nutrients, including organic C, when they move throughout the marine environment. For example, salmon (Field and Reynolds, 2011), sea turtles (Bouchard and Bjorndal, 2000) and penguins (Erskine et al., 1998) congregate in defined areas of the ocean at specific times of year for feeding or breeding. These natural congregations trigger the accumulation of feces, exuviae (i.e., sloughed skin), and carcasses, all of which are rich in organic C. Likewise, buoyant fecal plumes released by whales can provide limiting nutrient concentrations up to seven orders of magnitude higher than background seawater concentrations, thereby boosting phytoplankton productivity (Roman and McCarthy, 2010; Ratnarajah et al., 2016). Other large marine mammals like pinnipeds (e.g., Otaria flavescens) that typically feed offshore, reproduce and rest on the coast, where their excrement, molted skin, placenta, and carcasses accumulate (Crespo et al., 2021). Therefore, the large variety of ecological interactions between marine vertebrates and their environment, which include feeding and reproductive habits, vast migrations across oceans, and death, all contribute to multiple exchanges within the C cycle, many still disregarded (Martin et al., 2021; Roldán et al., 2022).
Coastal areas that are known for harboring high concentrations of large marine vertebrates can serve as local hotspots for marine carbon exchange. As noted above, the vast amount of fecal material, exuviae, prey scraps, and carcasses produced in these locations may introduce considerable pulses of C (Manno et al., 2020; Pearson et al., 2023), some of which can be accumulated and stored in marine sediments. However, previous studies in coastal areas with a high density of whales (Pershing et al., 2010; Pearson et al., 2023) have focused on the pulses of C to deep waters rather than the C deposited within the coastal system. This focus is probably because the lack of natural systems in those areas with a high ability to trap C from the water column –such as vegetated coastal ecosystems. However, in high-tide and sheltered coastal systems, wave and tidal action can promote the deposit of marine organic C on shores (Orr et al., 2005; Heiss, 2020) in the form of algae, small crustaceans, or drift macrophytes. In these protected coastal systems, organic matter is degraded and buried as the coast profile cycles through accretionary and depositional stages (Ford et al., 1999; Dugan et al., 2011). Likewise, high-tide and sheltered coastal systems that are dominated by high densities of large vertebrates may represent important areas of vertebrate-derived C deposition. We therefore hypothesized that the C from large marine vertebrates could accumulate in the sediments of protected coastal areas where these species are densely populated.
Península Valdés Biosphere Reserve was identified as a World Natural Heritage Site by UNESCO in 1999. The coastal system in this area mainly features shallow bays with extensive mudflats and some areas covered with salt marshes (Álvarez et al., 2016; Ríos et al., 2018). This Biosphere Reserve is considered a transition area between the Argentina and Magallanica marine biogeographic regions, resulting in a high diversity of marshes with muddy and rocky bottoms (Bortolus et al., 2009). Furthermore, Península Valdés is one of the most important calving grounds for the southwestern Atlantic population of the southern right whale (Eubalaena australis) (D’Agostino et al., 2023), which numbers approximately 1,707 individuals (Crespo et al., 2019; Romero et al., 2022). Relevant mortality events of adults and calves have been documented in the area (Rowntree et al., 2013; Wilson et al., 2016). Another abundant marine mammal in the Península Valdés area is the South American sea lion (Otaria flavescens), which remains in the area year-round in high-density and stable population colonies (Grandi et al., 2008) estimating a total of 36,500 individuals in 34 colonies (Grandi et al., 2020). These features make the Península Valdés Biosphere Reserve an excellent location to assess the contribution of large marine mammals to C sequestration in coastal systems. Our specific goal was to quantify the contribution of high-density populations of large marine mammals to soil C stocks in sheltered coastal systems. We specifically hypothesized that (i) South American sea lions and southern right whales, the two dominant marine mammals in this reserve, transfer a significant amount of offshore C to coastal systems; and (ii) the blue carbon in vegetated coastal communities within these systems contain significant contributions from large marine vertebrate species.
The study was performed in the Península Valdés Biosphere Reserve, Argentina (hereafter referred to as ‘Península Valdés’) in February 2022. The peninsula itself is surrounded by Golfo Nuevo (at the southern margin) and Golfo San José (at the northern margin), two protected gulfs of 2,500 and 817 km2 in size, respectively. Both gulfs have a semidiurnal tidal regime; with average amplitude of 1.9 m in Golfo Nuevo and mean amplitude between 7.01 and 4.57 m in Golfo San José (D’Agostino et al., 2018). The entire Península Valdés area was designated as a World Heritage Site and Biosphere Reserve by UNESCO due to its significance for the conservation of large marine vertebrate populations. In general, this reserve provides a natural laboratory for studying the effects of marine mammals on C sequestration because it is a dynamic coastal zone the contains active spits, bays that provide critical marine mammal habitat, coastal lagoons, numerous marshes and muddy bottoms (Ríos et al., 2018). To test the hypothesis raised that the C from large marine vertebrates could accumulate in the sediments of protected coastal areas where these species are densely populated, we select three locations in Península Valdés based on its proximity to high concentration of marine vertebrate populations in the area and the presence or absence of coastal vegetated communities. In addition, the three study sites are protected from human activities, where tourism and small-scale economic activities are strictly regulated. In this way, these sites were representative of the Península Valdés Biosphere Reserve. Particularly, the three study sites were: i) Riacho San José, a saltmarsh dominated by Spartina spp. on the west coast of the Golfo San José; ii) Caleta Valdés, a semi-diurnal coastal lagoon dominated by Sarcocornia spp. located at the eastern and external side of Península Valdés; and iii) Punta Loma, a protected area with restricted access in Golfo Nuevo, with sandy and largely unvegetated cliffs located 15.5 km from the city of Puerto Madryn (Figure 1).
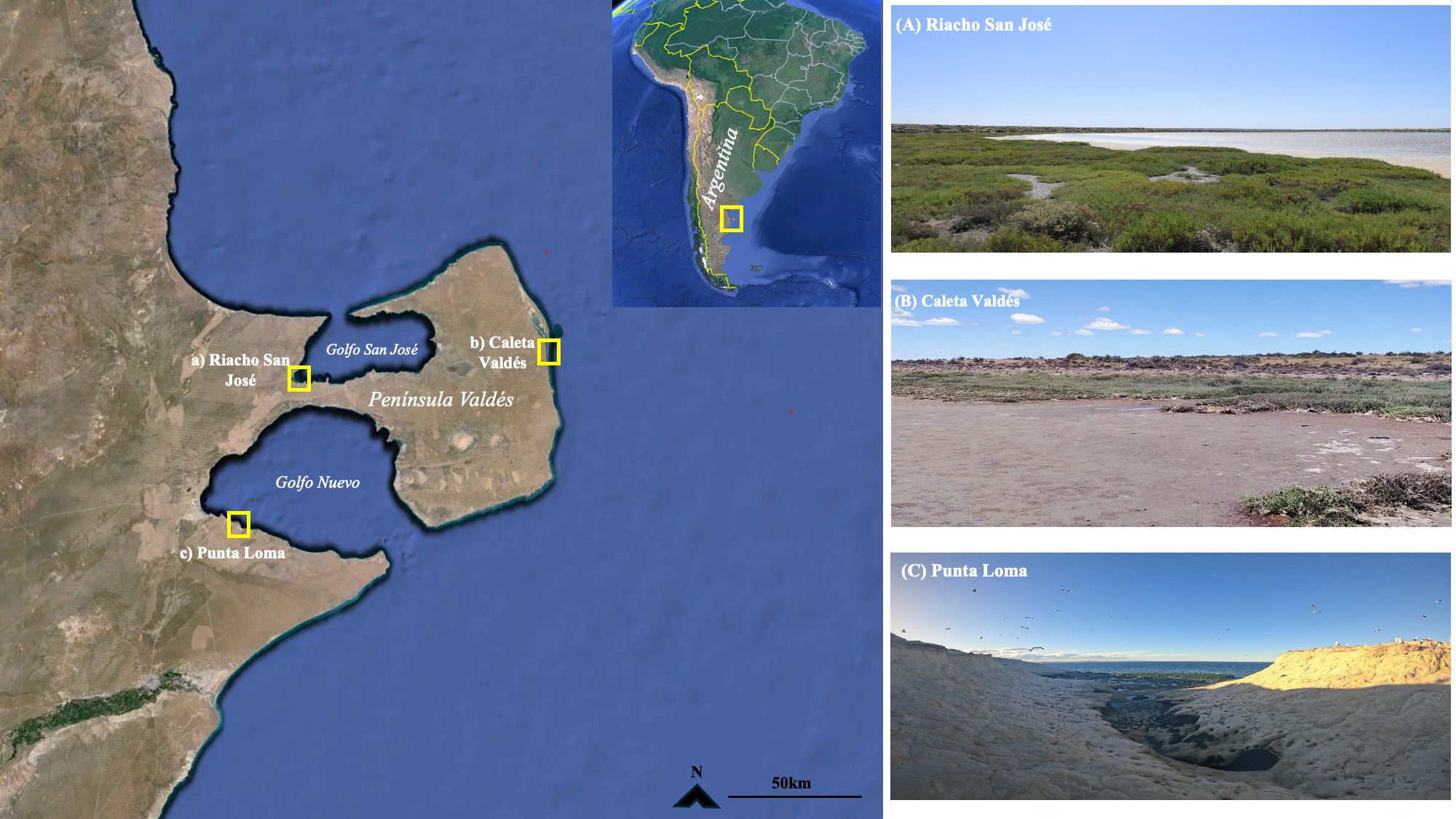
Figure 1. Map of the study area (Península Valdés Biosphere Reserve; Chubut, Argentina) and sampling locations in (A) Riacho San José, (B) Caleta Valdés and (C) Punta Loma.
Our first site, Riacho San José, is a restricted-entrance embayment characterized by extensive Spartina alterniflora Loesel meadows as well as spotted salt marsh areas dominated by Sarcocornia perennis (P. Mill.) (Bortolus et al., 2009). The area was included in the RAMSAR convention list for conservancy in 2012 (Committee on Characterization of Wetlands, 1995). Our second site, Caleta Valdés, is a coastal lagoon 30 km long. At its mouth on the southern end, water exchange and a gravel bank terminate the lagoon and generate a 200 m wide channel with a mean depth of 5 m (Kokot et al., 2005). We chose this study area because of its extensive Sarcocornia-dominated marsh and muddy sediments. This area is used by Magellanic penguins (Spheniscus magellanicus), South American sea lions (O. flavescens), and orcas (Orcinus orca) for resting, breeding, and hunting during the high tidal period (Table 1). Our last study area, Punta Loma, is a Natural Protected Area with restricted access in which a South American sea lion (O. flavescens) rookery is established year-round (Grandi et al., 2008). Punta Loma is formed by a rocky platform in the intertidal zone and an active cliff of sedimentary clay where can be observed colonies of the South American tern (Faria et al., 2010) and the black-necked cormorant (Sapoznikow and Quintana, 2008). Sea lion populations use the sedimentary clay canals in this area for resting and breeding (Dans et al., 2004). The area is also characterized mixed spots of bushy vegetation dominated by the perennial grass Pappostipa speciosa (Trin. & Rupr.) Romasch and the evergreen perennial shrub Chuquiraga avellanedae Lorentz (Bisigato et al., 2016).
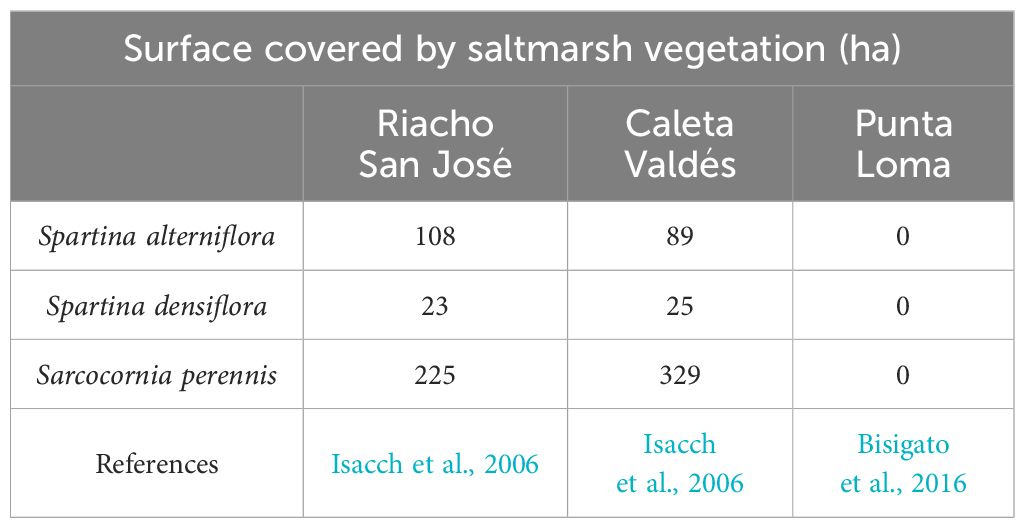
Table 1. Salt marsh vegetation cover (ha) and population abundance of marine mammals (in living biomass) and whale carcasses registered in the three sampling locations.
To ensure the representativity of sampling sites, the most dominant salt marsh species in Riacho San José (Spartina alterniflora) and Caleta Valdés (Sarcocornia perennis) were located. Thus, at Riacho San José, three sampling points dominated by Spartina alterniflora were sampled in zones where Eubalaena australis strandings are frequently found. The magnitude of the whale strandings in the study area up to 928 dead whales since 2003. The annual number of dead whales for the last study period (2022) was 73 (Sironi et al., 2022). Regarding Caleta Valdés and Punta Loma, the sampling points were taken in sedimentary areas where colonies of Otaria flavensis are found resting and breeding (Dans et al., 2004; Grandi et al., 2020). Three sampling points dominated by S. perennis saltmarsh were tested in Caleta Valdés; while the three sampling points in Punta Loma where characterized by bare sediment. To ensure the independence and representativeness of the samples, the sampling points in each location were spaced a minimum distance of 10 m apart and, in salt marsh areas, ensuring that the main vegetated species dominated at least 3 meters around the selected point. In this way the sampling strategy assured sampling in the most represented vegetated species, however, since we do not have historical data to be sure that the sediment core throughout the depth profile corresponds to the present species and the proximity of other vegetated species (Table 1), we use the cores as representative samples of the entire habitat (i.e., the salt marsh in the area) rather than representative of a specific saltmarsh species. At each sampling point, we collected a sediment core by manually hammering PVC pipes (150 cm length, 60 mm internal diameter) to a maximum penetration depth of 99 cm (Supplementary Table 1 in the Supplementary Material). We considered sediment compaction during coring to be a linear process and measured the degree of compaction based on the total length of the corer, the empty space inside the corer after the sediment sample had been cored but not retrieved, and the final length of sediment retrieved (Glew et al., 2001). In general, compaction ranged from 9 to 24% (Supplementary Table 1 in the Supplementary Material). We therefore report all variables for uncompressed depths. After collection, the cores were sealed at both ends and transported to our laboratory in vertical position, where they were then frozen until processed. The contributions of the main OM source to the sedimentary organic matter pool were identified at each location. As a result, we collected aboveground and belowground biomass samples from S. alterniflora and S. perennis (Riacho San José), S. perennis (Caleta Valdés), and P. speciosa and C. avellanedae (Punta Loma) for isotopic analysis (n = 6 in total). Samples of macroalgae species were taken in each sampling locations (e.g. Macrocystis sp.) as an allochthonous source. The samples of macrophytes were then transported to the laboratory under cool and dark conditions. The macrophytes biomass were gently cleaned with distilled water in the laboratory and oven-dried (60°C, 72h). Dry samples of macrophytes biomass were homogenized in a ball mill for later analysis. Moreover, stable isotope signatures of particulate organic matter (POM) on tidal-influenced areas were also considered as allochthonous OM. We collected water samples near the subtidal sampling points in Riacho San José (n = 6) and Caleta Valdés (n = 6) during low tide. These samples were filtered with a syringe (GF/F 47 mm) to obtain suspended particulate organic matter (POM) and oven-dried (60 °C, 72h). Stable isotope signatures of marine mammals were obtained from the literature (see Supplementary Table 2 in the Supplementary Material). Only species with a record of strandings in the sampling locations were considered (such as E. australis and L. obscurus in Riacho San José), or if they use directly the sampling area for resting or breeding (for example, Caleta Valdés and Punta Loma by Otaria flavensis). The recorded isotopic signals of fish and plankton (Supplementary Table 2 in the Supplementary Material) were considered in these sampling areas being potentially linked to the sea lion’s diet.
For analysis, the sediment cores were defrosted and opened lengthwise with an angle grinder. All geochemical analyses for the cores from Riacho San José and Caleta Valdés (n = 6) cores were conducted at 1 cm along the upper 30 cm. However, for Punta Loma cores, samples were extracted every 0.5 cm along the upper 10 cm, every 1 cm between 10 to 30 cm, and every 2 cm for every depth after 30 cm. The sediment samples were extracted from each core slice with a plastic syringe, lyophilized for 24 h, and weighed (dry weight, DW, ± 0.0001 g) to determine dry bulk density (DBD, g DW cm-3). From one half of core sediment samples grain size was classified into five different granulometry fractions using laser beam diffraction (Partica LA-950; HORIBA Instruments, Inc.) performed at Servizos de Apoio á Investigación (SAI) of the University of A Coruña: clay (< 6 μm), silt (6-60 μm), fine sand (60-200 μm), medium sand (200-600 μm) and coarse sand (> 600 μm). Sediment samples were then ground into a fine powder and subdivided into two subsamples for organic and inorganic carbon analyses, respectively. All carbon measurements were performed at the Institute of Marine Research (INMAR) at the University of Cádiz (Spain) following the procedures described by Howard et al. (2014). In brief, total carbon (and nitrogen) content (%) was measured in ca. 0.15 g dry weight of sample through an automated elemental analyzer [LECO CNS928; detection limits 0.002, Standard LECO 502-697 Lot. 1002, Soil (3.82% ± 0.05% C, 0.323% ± 0.031% N, 0.045% ± 0.008% S)]. Inorganic carbon content (%) was similarly determined from ca. 1 g of dry weight using the acidification method with HCL 1 N (Howard et al., 2014). We calculated organic carbon content (OC, %) as the difference between the total and inorganic carbon (Howard et al., 2014).
We further calculated the sediment, organic carbon, and nitrogen densities (g cm-3) based on the total dry weight of the sediment samples. This total dry weight included belowground biomass fragments, stones, and shells, all of which constitute an albeit minor part of the sediment volume. For our isotopic analysis, we used the samples that we retained after the inorganic carbon had been removed by the addition of 1 N HCl (Kennedy et al., 2005). In this case, subsamples of 10 mg dry weight for stable isotope ratios measurements were performed at Servizos de Apoio á Investigación (SAI) of the University of A Coruña using a continuous-flow isotope-ratio mass spectrometer MAT253 (Thermo Finnigan) coupled to an elemental analyser FlashEA1112 (ThermoFinnigan) through a Conflo III interface (ThermoFinnigan) with detection limits of 5 µg. Tin encapsulated samples were combusted at 1020°C in a quartz column containing chromium oxide and silvered colbatous/cobaltic oxide. Following combustion, excess oxygen and oxides of nitrogen were reduced in a reduction column (reduced copper at 650°C). N2 and CO2 were separated on a GC column before introduction to the IRMS. During analysis a set of international reference materials for δ15N (IAEA-N-1, IAEA-N-2, USGS25) and δ13C (NBS 22, IAEA-CH-6, USGS24) were analyzed for δ15N and δ13C calibration. An analytical measurement error of ± 0.15 ‰ was calculated for δ13C and δ15N; the error estimate was obtained from replicate assays of the laboratory standard acetanilide interspersed between sample analysis. All results are reported in δ notation in per mil units (‰) using PeeDee Belemnite and atmospheric N2 as internationally accepted standards for δ13C and δ15N, respectively. In other words, δX = (Rsample/Rstandard - 1), where R represents the relationship between heavy and light isotope (13C/12C or 15N/14N) in the samples and standards.
To estimate the proportional contribution of potential organic matter sources (i.e., endmembers) to each sedimentary profile, we used Bayesian Stable Isotope Mixing Models in R (SIMMR, version 0.3; Parnell and Inger, 2019) in a manner consistent with previous studies of coastal sediments (e.g., Dubois et al., 2012; Egea et al., 2023). All mixing models were run using the δ13C and δ15N values for each sediment core section as well as those from the endmembers of the core. The potential sources for the model included stable isotope values from (i) plant samples and suspended particulate matter (filtered through a GF/F Whatman filter) that we collected in the area and (ii) marine mammals described in the literature (Drago et al., 2009; Valenzuela et al., 2009; Grandi et al., 2021; Loizaga et al., 2023). Endmember δ13C and δ15N values are reported in Supplementary Material (Supplementary Table 2 in the Supplementary Material). All mixing models were run using Just Another Gibbs Sampler (JAGS) for each site and the default Markov chain Monte Carlo (MCMC) settings in SIMMR (iterations: 10000, size of burn-in: 1000, amount of thinning: 10, and chains: 4). We confirmed that all models satisfied the recommended convergence criteria (Gelman diagnostic values were ca. 1), and we present the results as the predicted proportional contribution (% dry weight) of each endmember to each sediment section of cores.
We present the depth profiles of DBD (g cm-3), OC (%), and TN (%) as the mean and standard error of the triplicate sediment cores collected at each of the three locations. Depth profiles were estimated to a standardized depth of 1 m from the deepest values reached in each location (97, 99, and 65 cm at Riacho San José, Caleta Valdés, and Punta Loma, respectively). This standardization was achieved by extrapolating the integrated values of each variable through the predictions of generalized linear models (GLMs) fitted to the obtained data. For each variable, we chose the error structure and link function that best achieved the assumptions of linearity, homogeneity of variances, and the absence of overdispersion, which we evaluated by visual inspection of residuals and Q–Q plots (Harrison et al., 2018). When several model structures met these assumptions, we chose the best-fit model using the Akaike Information Criteria (AIC) (Barton, 2019). Based on this approach, DBD was modeled using a Gaussian distribution with an ‘identity’ link, whereas OC and TN were modeled using Gaussian distribution with a ‘log’ link. The sediment stocks of OC and TN (megagram of OC or TN per hectare; Mg ha-1) were estimated by multiplying OC or TN content (% DW) by DBD (g cm-3) and then integrating the results using the trapezoidal rule –i.e., the sum of the areas of all trapezes conformed through the values in OC and TN plots along 1 m sediment depth (https://www.intmath.com/integration/5-trapezoidal-rule.php). Finally, the contribution (% dry weight) of large vertebrate endmembers (i.e., E. australis or O. flavescens) to the C and N fractions in each sediment section at each location were estimated as the 2.5 percentile of the credible interval obtained within the Bayesian Stable Isotope Mixing Model performed (van de Schoot et al., 2014). All statistical analyses were performed using R 4.4.1 (R Development Core Team, 2024).
In general, OC and TN content declined with sediment depth at each site, following a logarithmic regression (Figure 2). Conversely, DBD increased linearly along the sediment depth profile at each site. The OC content of the sediment profiles was consistently an order of magnitude higher than the TN content, ranging from 9.45 to 0.51% for OC compared to 0.73 to 0.01% for TN. Caleta Valdés had a 1.3-fold higher mean and maximum OC content than the average of the other two locations, which were otherwise similar. In contrast, Punta Loma had a 2.7-fold higher mean and 3.5-fold higher maximum TN than the average of the other two locations.
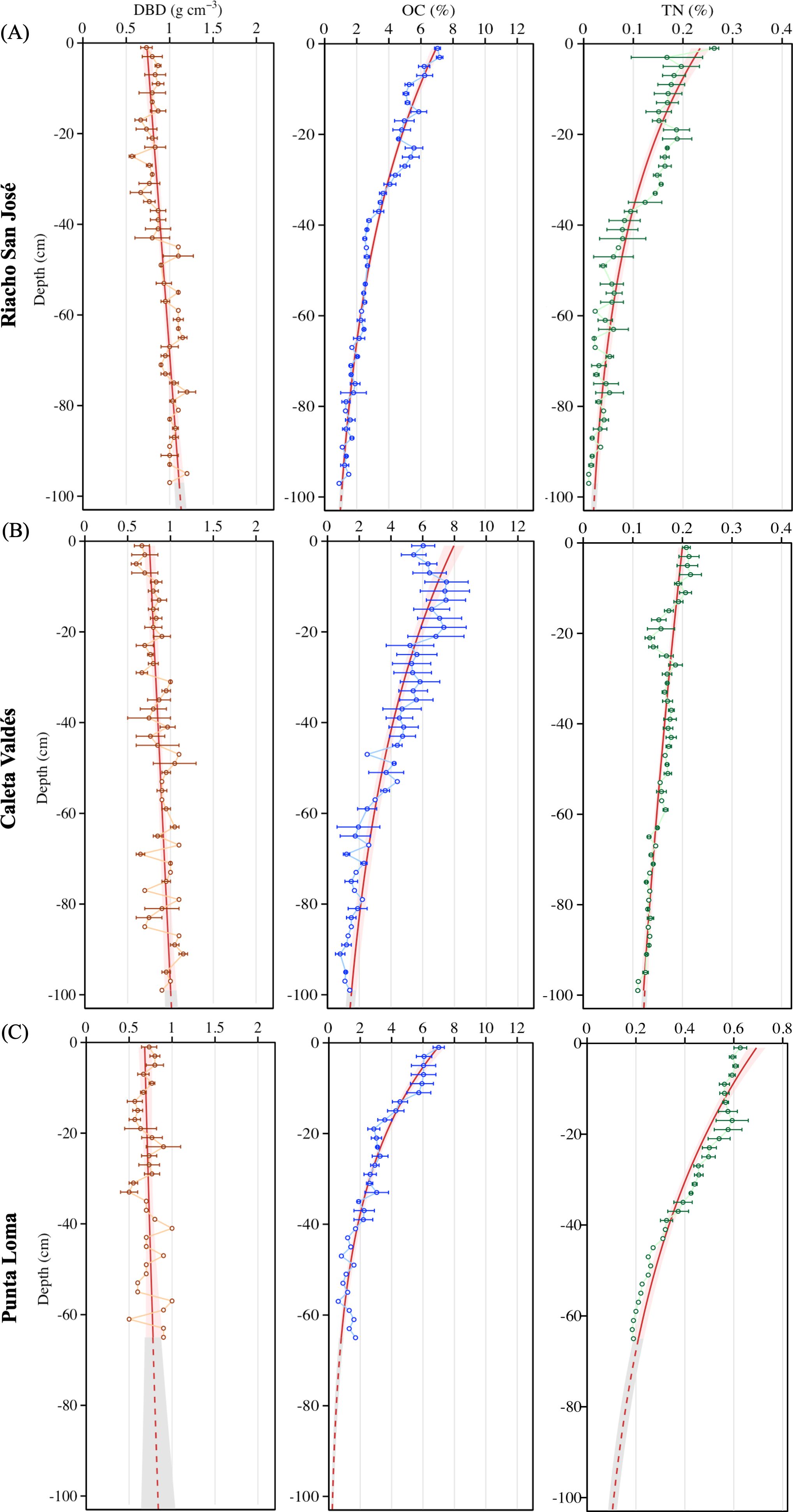
Figure 2. Sediment depth profiles of dry bulk density (DBD; g DW cm-3), organic carbon (OC, % DW), and total nitrogen (TN, % DW) contents in (A) Riacho San José, (B) Caleta Valdés, and (C) Punta Loma. Points and error bars represent the mean ± SE of three replicate sediment cores collected at each location. Solid red lines show the GLM model fitted to points, dashed red lines illustrate the model prediction over the depth gradient, and shaded areas indicate the 95% confidence intervals.
The largest OC stock in the upper 1 m sediment layer was observed in Caleta Valdés, followed by Riacho San José and Punta Loma (317, 270.2, and 140.1 Mg OC ha-1, respectively) (Figure 3). The TN stocks followed a different pattern, decreasing from Punta Loma to Caleta Valdés to Riacho San José (22.9, 13.6, and 7.3 Mg TN ha-1, respectively). Half the total OC stock was reached by ca. 31 cm depth at Riacho San José and Caleta Valdés, whereas in Punta Loma the halfway point was reached by ca. 19 cm depth. The depth at which half of the total TN stock was reached was deeper than for OC stocks, with a decreasing trend from Caleta Valdés to Punta Loma and Riacho San José (ca. 45, 33, 25 cm depth, respectively).
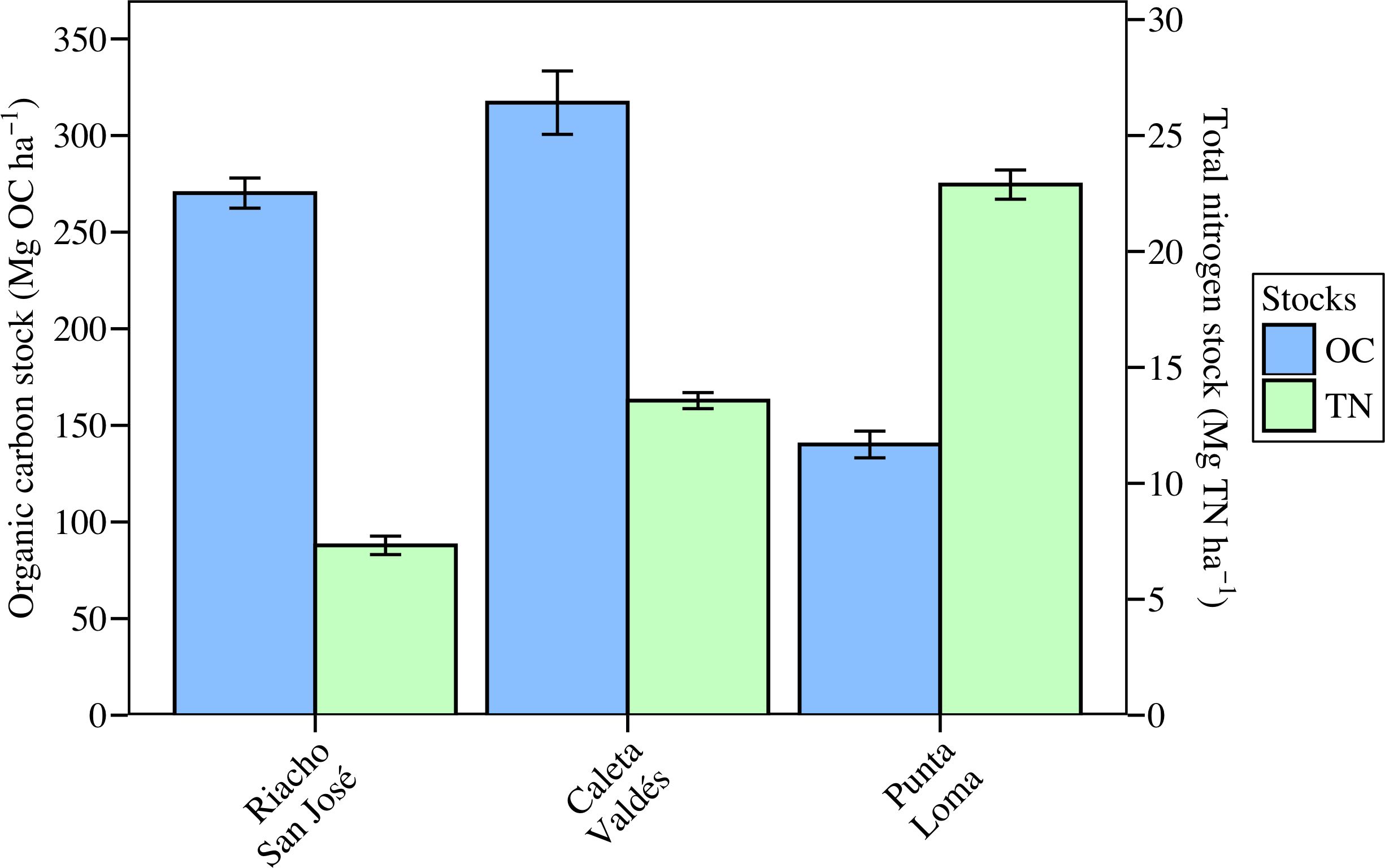
Figure 3. Stocks of organic carbon (Mg OC ha-1) and total nitrogen (Mg TN ha-1) shown to a standardized sediment depth of 1 meter.
The δ¹³C and δ15N isotopic composition of the sediment cores varied significantly with depth at all three locations, so we subdivided the sediment cores into different sections based on their isotopic ratios (Figure 4). In Riacho San José, we observed four sediment sections: 0-15 cm (average -14.29 ± 0.68 and 7.24 ± 0.59 for δ13C and δ15N, respectively), 15-55 cm (average -16.15 ± 0.29 and 8.38 ± 0.34), 55-77 cm (average -18.70 ± 0.54 and 7.78 ± 0.35), and 55-97 cm (average -20.68 ± 0.21 and 11.38 ± 0.78). Caleta Valdés showed only three sediment sections: 0-15 cm (average -21.79 ± 0.89 and 13.66 ± 0.76 for δ13C and δ15N, respectively), 15-53 cm (average -18.87 ± 0.55 and 16.78 ± 0.44), and 53-99 cm (average -23.46 ± 0.41 and 11.90 ± 0.50). Punta Loma also divided into three sediment sections: 0-19 cm (average -17.95 ± 1.18 and 15.80 ± 1.16 for δ13C and δ15N, respectively), 19-43 cm (average -22.81 ± 1.12 and 10.83 ± 1.04), and 43-65 cm (average -16.30 ± 1.05 and 18.16 ± 0.25).
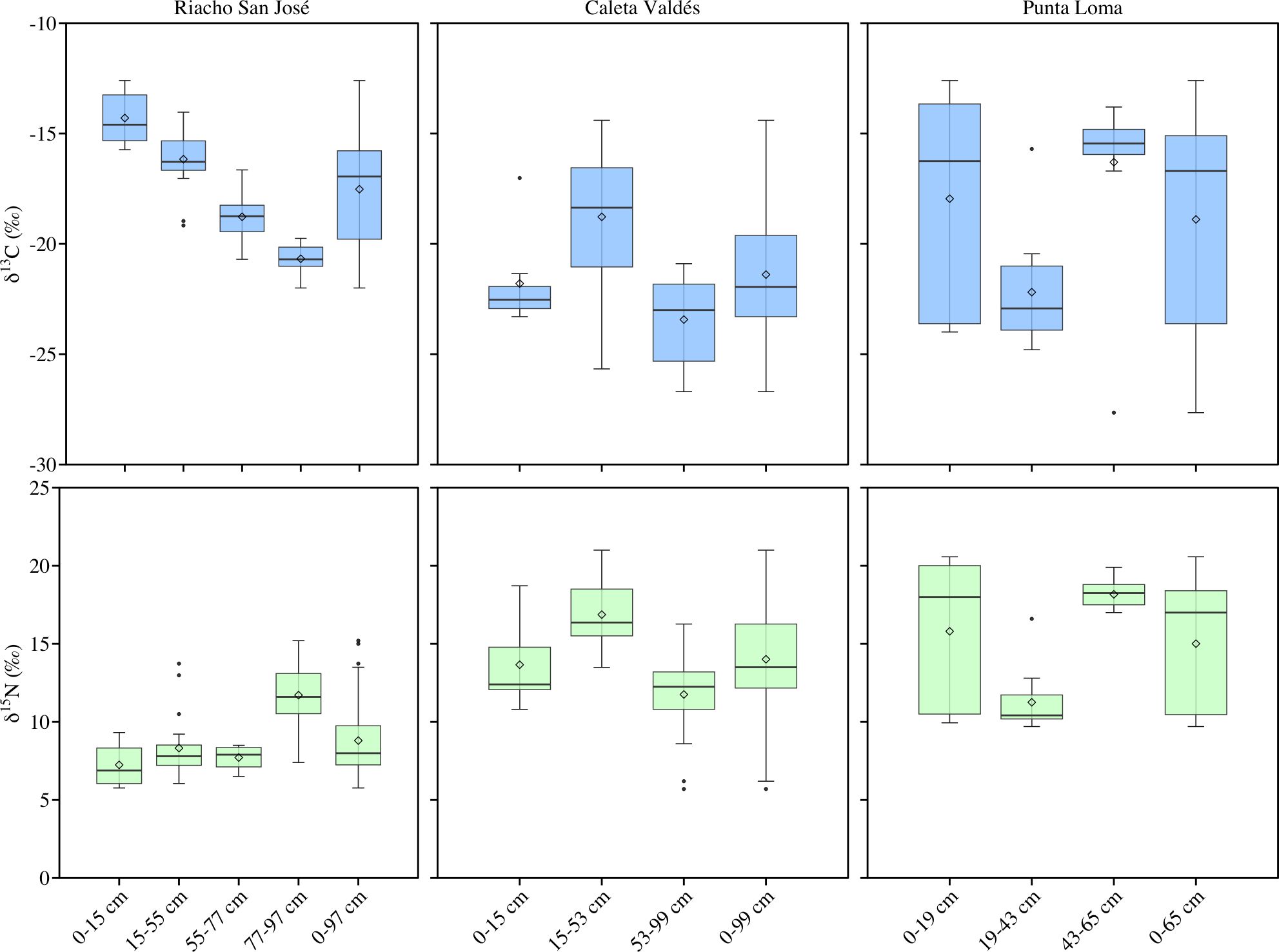
Figure 4. Box plots of δ13C and δ15N isotopic values for each sediment section in the three study locations. Each box plot represents the average of three sediment cores per location. The thick center line indicates the median, hinges represent the 25th and 75th quantiles, and whiskers represent the 5th and 95th quantiles. The center rhombus indicates the mean, and outliers are plotted as dots.
Although the δ¹³C and δ15N values in the sediment samples varied among habitats, most of values fell within the mixing polygon defined by the potential OM sources (Figure 5). In Riacho San José, the mixing model indicated that the main source in the upper surface layer (i.e., the first 15 cm) was the saltmarsh plant S. alterniflora. However, the contribution of S. alterniflora decreased with the sediment depth in favor of other sources. For example, allochthonous POM and E. australis were also important contributors in the 55-77 cm section, and in the deepest sediment section (77-97 cm), Macrocystis sp., E. australis, macroalgae spp. and S. perennis were the main contributors to sedimentary OM. In Caleta Valdés, the upper sediment section had a variety of sources in its sedimentary OM, with S. perennis and suspended POM as the main contributors. However, their relative contribution dwindled from 15 to 53 cm sediment depth in favor of O. flavescens, but then they became dominant again in the deepest sediment section (i.e., from 53 to 99 cm depth). In Punta Loma, the first 19 cm had mixed sources to its sedimentary OM, with fish and O. flavescens being the higher contributors. However, the terrestrial plants P. speciosa and C. avellanedae dominated in the 19-43 cm section, and O. favescens became the main contributor in the deepest sediment section (i.e., 43-65 cm).
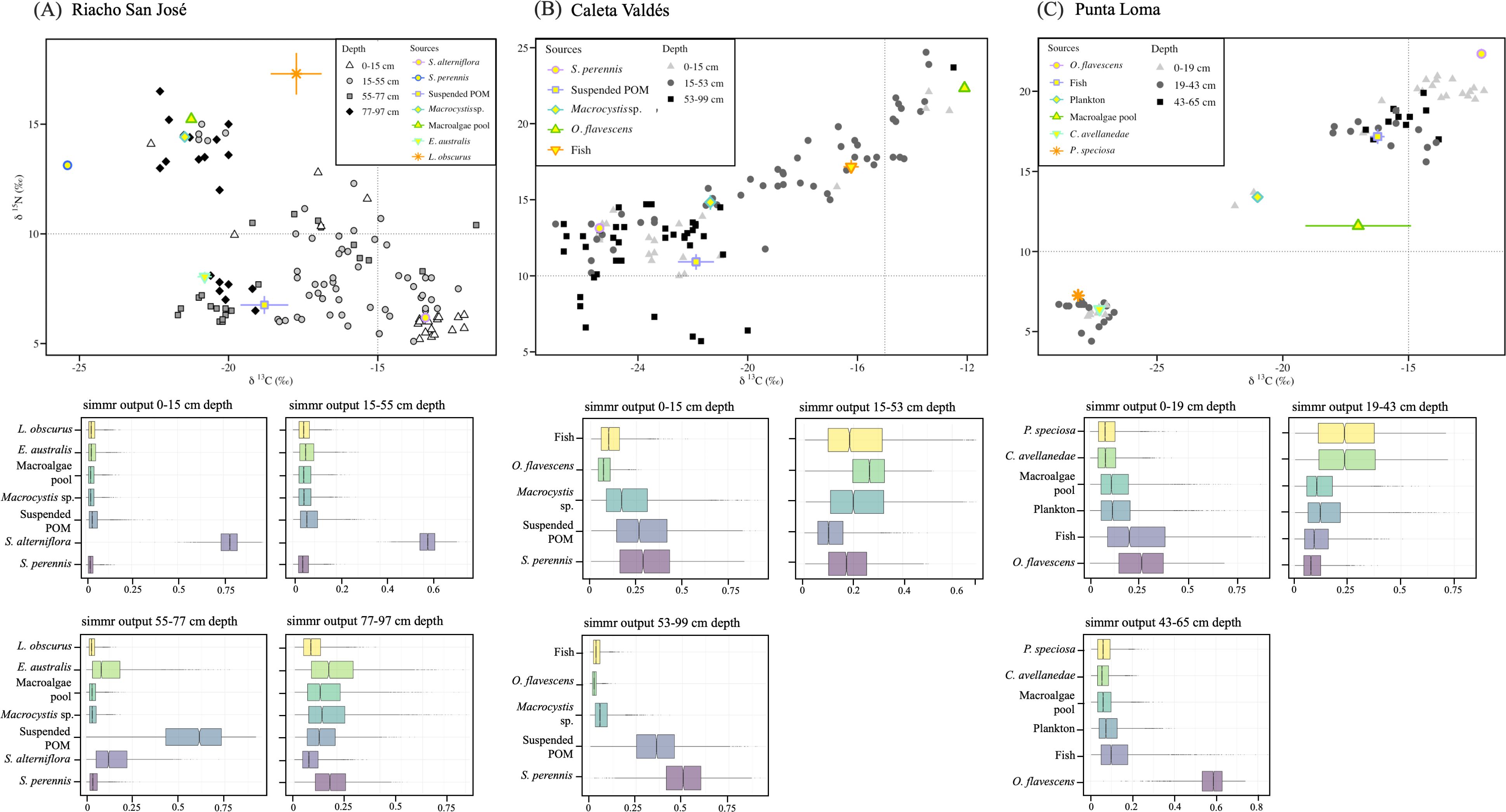
Figure 5. Scatter plot of the δ15N and δ13C isotopic signatures of sedimentary organic matter (OM) (above) and horizontal violin plots showing the predicted proportions of each source (bottom) on (A) Riacho San José, (B) Caleta Valdés, and (C) Punta Loma. We classified sedimentary OM according to the sediment core section defined in Figure 4. Note that the axis in the scatter plot varies among locations. Dotted lines were added to facilitate comparison of scatter plots. Shaded boxes in the horizontal violin plots represent the 0.25, 0.5 and 0.75 credibility quantiles.
We report, for the first time, that coastal sediments in the Península Valdés Biosphere Reserve contributes significantly to the sequestration of organic carbon (OC) and total nitrogen (TN). In general, we found higher OC stocks in Riacho San José and Caleta Valdés, the two sites dominated by salt marsh vegetation. The top 1 m of sediment at these two sites contained 317 and 270 Mg OC ha-1, respectively, which is within the global average estimate for soil OC buried in the top 1 m of tidal salt marshes (231 ± 134 Mg OC ha-1; Maxwell et al., 2023). We found that 50% of the total OC stock in the sediment columns was reached by 31 cm depth in both locations. Fewer studies have considered sedimentary N stocks in coastal vegetated systems (Arriola and Cable, 2017; Santos et al., 2019; de los Santos et al., 2023). Even so, the sedimentary TN stocks at our study sites (0.22 and 0.25% in the top surface sediment layer in Caleta Valdés and Riacho San José, respectively) were within the medium-to-high range of the few available data points in the literature (e.g., 0.04 to 0.79%; Arriola and Cable, 2017). Specifically, the TN content was 13.6 and 7.3 Mg TN ha-1 in Caleta Valdés and Riacho San José, respectively, and the 50% of TN stock was held within the first 45 and 25 cm of sediment depth at each site, respectively. We therefore suggest that C and N sequestration by coastal vegetated areas is a key ecosystem service provided by Península Valdés.
The study site at Punta Loma lacked any saltmarsh vegetation but harbored a stable year-round colony of South American sea lions, Otaria flavescens (Dans et al., 2004), which may also contribute to C and N deposition. Indeed, despite the absence of saltmarsh communities at Punta Loma, we found relatively high OC and TN stocks: we estimated OC and TN stocks for the top 1 m of sediments at 140 Mg OC ha-1 and 22.9 Mg TN ha-1, and 50% of the total OC and TN stocks were held within the top 19 and 33 cm of sediment, respectively. Instead of saltmarsh vegetation, Punta Loma includes terrestrial low-plants dominated by Pappostipa speciosa and Chuquiraga avellanedae on the top of the sedimentary tidal canals, although the canopy remains sparse (Bisigato et al., 2016). Previous studies have observed a positive correlation whereby terrestrial and vegetated coastal areas with low aboveground plant biomass density tend to be environments with soil OC loss (e.g., Achat et al., 2015; Santini et al., 2019; Macreadie et al., 2013; Song et al., 2023; Jiménez-Ramos et al., 2024). The relatively high OC stock at this location was therefore unexpected. We attribute the high OC stock to the nearby presence of terrestrial low-plants, location-specific sediment features such as grain size, and the large colony of O. flavescens. The sea lion populations (O. flavescens) in Punta Loma use the rocky zones and sedimentary tidal channels not only for resting but also for breeding, birthing, and defecation. We also observed a significant number of carcasses in these tidal channels, and we accordingly expected that the organic matter composition at Punta Loma would show a relevant fraction of seston derived from this species. Indeed, we detected the isotopic signatures O. flavescens in the sediment cores from Punta Loma (Figure 5), indicating that the high seal population density contributed to the transfer of offshore C (from feeding) to coastal systems (as sites of breeding, rest, and death). This result aligns with studies on other marine vertebrate species, highlighting their role as significant contributors of marine carbon to terrestrial environments, particularly through activities such as spawning, nesting, or nursing on land, as seen in salmon (Field and Reynolds, 2011), sea turtles (Bouchard and Bjorndal, 2000), and seals (Quaggiotto et al., 2018).
On the other hand, the sediments in Punta Loma tended to have a fine grain size rich in clays and silts (Supplementary Figure 1 in the Supplementary Material), which can reduce the vulnerability of organic matter to decomposition (Mayer et al., 1985; Chen et al., 2022). Organic matter can be further protected through physicochemical associations with minerals and cations in the sediment; indeed, organo-mineral associations are one of the main mechanisms contributing to the environmental persistence of sedimentary OC. For example, Moore et al. (2023) demonstrated that sediment cations (including Fe and Mn) and minerals catalyze the transformation of simple organic molecules (i.e., glucose and glycine) into recalcitrant forms, such as complex aromatics, via the Maillard reaction (i.e., polycondensation). The high metal concentrations that have been found in the feaces of South American sea lion populations in Argentina (Gerpe et al., 2006) indeed suggest that a high abundance of cations and minerals should be present in the surrounding sediments, thus contributing to the long-term stabilization and sequestration of organic material in the area. Another mechanism contributing to organic matter sequestration is that the hydrodynamic environment of our sampling environment may promote sedimentation (Cuitiño et al., 2023), which can be further boosted by the movement of sea lions over the sediment along the sampled tidal canals. To move onshore, pinnipeds either ¨walk¨ or ¨crawl¨ depending on their specific taxonomy. Phocids perform ‘ventral movements’ by pivoting on their belly, thereby “crawling” their heavy bodies (male weight often exceeds 4 tons) across the ground, whereas sea lions (male weight >350 kg) can rotate their hind flippers to “walk” on land. Either of these movements across the land could lead to soil compaction, which changes soil aggregate structures and reduces porewater O2. Soil compaction, in turn, can protect OC from degradation, as has been reported in studies of the ‘trampling movements’ of large terrestrial mammals in terrestrial ecosystems (e.g., elephants; Sandhage-Hofmann et al., 2021; Berzaghi et al., 2023). Overall, our study offers new insights into how sea lions in coastal systems may facilitate the accumulation of soil carbon through the horizontal transport of carbon from the open ocean (where they feed) to coastal areas (where they defecate, breed, and die).
We found that the main C source varied with sediment depth at each location, to the point where sediment cores could be subdivided based on their isotopic composition and presumed C sources. We specifically observed four clear sections in Riacho San José and three sections in Caleta Valdés and Punta Loma (Figures 4, 5). In Riacho San José and Caleta Valdés, the salt marsh plants S. alterniflora and S. perennis were the main C sources in the top sediment layers, which was expected because these species are present and abundant at these sites. In general, southwest Atlantic salt marsh plants are highly productive and effectively trap and retain carbon (Martinetto et al., 2023), thus favoring OM accumulation in the top sediment layers. However, the contribution of salt marsh plants decreased with sediment depth and was replaced by other sources. At Riacho San José, allochthonous POM and E. australis were important contributors of OM at 55-77 cm depth, whereas macroalgae and E. australis were the main contributors in the deepest sediment section (i.e., 77-97 cm). According to our Bayesian mixing models, the contribution of E. australis in each sediment section at Riacho San José adds up to a total of at least 2.25 Mg C ha-1 (Supplementary Table 3 in the Supplementary Material), which represents 0.8% of the total OC stock at this site. Península Valdés is considered a sanctuary for E. australis, which uses the area as for feeding and breeding (D’Agostino et al., 2023, 2024). Each individual can weigh 50-60 tons (Curry and Brownell, 2014), and our sampling area at Riacho San José encompassed a region where whale carcasses from massive die-off episodes have previously been encountered (Rowntree et al., 2013; Wilson et al., 2016), suggesting a potential pathway for E. australis-derived carbon storage in coastal sediments. At Caleta Valdés, the organic material in the sediment section from 15-53 cm depth was largely derived from O. flavescens (Figure 5B). The total contribution of O. flavescens across all sediment sections at Caleta Valdés was at least 11 Mg C ha-1 (Supplementary Table 3 in the Supplementary Material), representing 3.5% of the total OC stock in this location.
At Punta Loma, O. flavescens was also the main C source in the top-layer sediments, but the organic material in the mid-depth sediments (19-43 cm) favored the terrestrial plants P. speciosa and C. avellanedae, which are the most abundant plant species in the surrounding terrestrial area (Bisigato et al., 2016). This reduction of O. flavescens and increase of terrestrial plants in the origin of organic matter with depth was attributed to the different rates of degradation of organic matter – which is usually slower for plants (Raza et al., 2023). Changes in the number of individuals in the colony may also explain changes in the weight of O. flavescens contribution. Historical records in the area show variability of individuals from a steep reduction (up to 90%) decades ago because hunting to a continued recover since 1990 (Koen-Alonso and Yodzis, 2005; Romero et al., 2017; Grandi et al., 2020). The increase in O. flavescens-derived organic material in the deepest sediments at Punta Loma (43-65 cm; Figure 5C) corresponds with a high accumulation of pinnipeds found at 40-70 cm depth in a nearby location (Serrán et al., 2008), which was attributed to a natural die-off event. However, we noted that dating the sediment core would be necessary for better inference. Overall, the total contribution of O. flavescens across all sediment sections at Punta Loma was at least 9.5 Mg C ha-1 (Supplementary Table 3 in the Supplementary Material), which represents the 6.8% of total OC stock in this location. On the other hand, we correspondingly observed a marked TN reported in Punta Loma, which was higher than those found in the other two assessed sites (Figure 2). A possible reason for this marked increase is because the area is a breeding area for the South American tern (Faria et al., 2010) and the black-necked cormorant (Sapoznikow and Quintana, 2008) since sediments receiving seabird droppings often show high TN content and high δ15N values (García et al., 2002; Laguna et al., 2021; Wu et al., 2023). The OC/TN ratio indicated a high contribution of organic matter from marine origin in Punta Loma, whereas evidenced a high contribution of organic matter from salt marshes in Caleta Valdés and Riacho San José (Supplementary Figure 2 in the Supplementary Material). A recent study in a coastal whale sanctuary in San Francisco (Hutto et al., 2021) suggested that whale falls may represent up to 60% of the annual carbon sequestration in its marine sediments. The significantly lower projected contribution in our study could be due to differences in the number of individuals, number of mortalities, and in some of the other assumptions or data from previous work (e.g., the assumption that 50% of the whale carcasses reach the sea bottom (Smith and Baco, 2003) or previous reports of the fluxes of carbon exported by whale species (Pershing et al., 2010). Nevertheless, our results indicate that coastal systems with a high density of large marine vertebrate populations, such as the southern right whale E. australis and the South American sea lion O. flavescens, may derive a non-negligible proportion of their sediment organic matter from these species.
We note that stable isotope analysis is intended to provide a probabilistic indication of likely carbon sources, however, we acknowledge this technique is non-exempted of limitations. Despite δ13C and δ15N isotopes method are widely used as indicators of sources of organic matter in coastal wetland research (e.g., Bulmer et al., 2020; de los Santos et al., 2023), it shows limitations in discerning multiple sources, particularly to discern between sources with similar isotopic signature (e.g., between species of macrophytes; Geraldi et al., 2019). To reduce uncertainty when determining the sources of OC, recent studies recommend more specific markers such as compound-specific isotopes on fatty-acid (δ13C-FA) or environmental DNA (eDNA) techniques (Macreadie et al., 2019). Fatty-acids are the major constituents of lipids and some of them are synthesized only by specific groups of organisms or in particular proportions (Bergé and Barnathan, 2005). Moreover, some of them present higher resistance to bacterial degradation in comparison to other classes of organic compounds, which makes them suitable molecular biomarkers to identify sources of OM in sediment samples (Gardade et al., 2021; Chielle et al., 2024). Similarly, eDNA can provide finer taxonomic-scale data about which species or families of organisms are present in the sediment sample (Nguyen et al., 2023), and because DNA is specific to a species, values do not overlap. However, besides their elevated prizes, these techniques display other limitations, partly due to its still limited use compared with isotopes method (Geraldi et al., 2019). For instance, the robustness of δ13C-FA depends on the specificity and conservativeness of the δ13C-FA fingerprint (Upadhayay et al., 2017). This technique may underestimate short-chain FAs (typical from animals) in favor of long-chain FAs (>20 carbon atoms; typical from plants). In addition, it is still unknown how δ13C-FA fingerprint may be transformed into soils (Geraldi et al., 2019), which can trigger important artifacts in our experimental design. Regarding eDNA, this biomarker may degrade at a faster rate than other OC components and, thereby, may underestimate allochthonous OC and species with relatively less resistant cellular structure (e.g., those from large vertebrates or algae vs. vascular plants). In addition, primers need to be tested to ensure that the species thought to contribute to the soil OC are amplified because primer amplification is imperfect (Deagle et al., 2014). Since the complexity of our experimental design (i.e., logistically and economically) and because our main objective was to differentiate sources with isotopic signatures that were not close (i.e., among saltmarsh plants, large vertebrates in the area and algae), we opt the isotopes method. To enhance the robustness of our results, we produced a conservative estimate from large vertebrates by using the 2.5 percentile of the credible interval of the sources provided by the model. Therefore, our results are not intended to be conclusive, but the empirical data presented in this study serve as a basis for future studies which may indicate more rigorously values of contributions by combining some of previous techniques, such as isotopes and eDNA.
The present study provides the first quantitative data on the OC and TN sequestered in the coastal sediments of Península Valdés. Although higher OC stocks were typically found in areas dominated by salt marsh vegetation, the unvegetated area in Punta Loma also contained relatively high OC stocks, which we attributed to the high local density of South American sea lion (O. flavescens) colonies and the physicochemical characteristics of the sediments. Overall, we consider that our estimation ranged from 140 to 317 Mg OC ha-1 and from 7.3 to 22.9 Mg TN ha-1 is significant at the regional level and highlight this system as potential contributor to blue carbon. Therefore, considering the worldwide objective of promoting and protecting ecosystems with a high carbon sequestration capacity as nature-based solution (NbS) for climate change mitigation (IUCN, 2020; Macreadie et al., 2021), our results evidenced that conservation and management practices in the Península Valdés should not only limited to vegetated coastal meadows, but also unvegetated areas with stable year-round colonies of large vertebrates such as O. flavescens. Although the estimations presented here were obtained at three locations that covered the most representative habitats in the system, do not consider all the spatial variability in stocks within the habitats, which has been observed in many other coastal areas, especially those dominated by salt marshes (Li et al., 2022; Shen et al., 2024). Therefore, this study can also lead futures investigation to obtain more precise estimates using this methodology and combined it with other techniques such as eDNA of fatty acids. In summary, our results contribute to raise the ecological value of Península Valdés Biosphere Reserve as an important marine protected area which, in addition to exert as a crucial area to the conservation of marine biodiversity (e.g., Crespo et al., 2019; D’Agostino et al., 2023), represent an important sink of OC and TN, which should be considered in management practices.
On the other hand, the broad range of δ13C and δ15N values among sediment cores and depths indicated that the Península Valdés sediments receive organic matter contributions from multiple carbon sources. Beyond the contribution of salt marshes and terrestrial plants as main C sources in sediments, we found a non-negligible proportion (from 0.8 to 6.8% dry weight) of the OC stocks showing an isotopic signal from the large vertebrates that usually inhabit the area. Therefore, our findings offer new insights into the potential role of large vertebrates as an organic carbon source for coastal carbon sequestration in coastal systems with high density of these animals. Although the large marine vertebrates studies to the ocean carbon cycle and as a feasible nature-based solution (NbS) for climate change mitigation is still at an early stage of research (Meynecke et al., 2023; Duvall et al., 2024), our results lay the groundwork for future studies on the role of marine mammals may play as potential vectors of coastal “blue carbon” sequestration. This role may depend on the time scale being considered, the life cycles of different species, and the forms of population management implemented in marine conservation plans.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RJ-R: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. LE: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. VD: Conceptualization, Investigation, Resources, Writing – review & editing. MD: Conceptualization, Funding acquisition, Investigation, Resources, Writing – review & editing. RL: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Spanish National Project RECOUNT (PID2020-120237RJ-I00), financed by MCIN/AEI/10.13039/501100011033 (PI: RJ-R). Also was funded by the Argentine National Research Council project PIP 2021-2024 (PI: RL), ANPCyT PICT 2019-2006, 2021-0172 (PI: MD) and SER-CADY project (FEDER-UCA18-107451; PI: LE), co-financed by the European Union under the 2014–2020 ERDF Operational Program and by the Department of Economic Transformation, Industry, Knowledge, and Universities of the Regional Government of Andalusia. The open access fee was co-funded by the Plan Propio – UCA, 2024-2025 and by the QUALIFICA Project (QUAL21-0019, Junta de Andalucia).
Permits were granted by Subsecretaría de Conservación y Áreas Protegidas of Chubut Province, Argentina. We would like to express our sincere gratitude to Juan Ángel Allieri Nautical and Submarine Services for his valuable support in designing the PVC cores. This study is part of the thematic area “Trophic ecology and conservation of marine mammals” of the University Marine Research Institute INMAR (Spain). Thanks to the Integration and Application Network for courtesy in supplying vector symbols (ian. umces.edu/symbols/).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1500594/full#supplementary-material
Achat D. L., Fortin M., Landmann G., Ringeval B., Augusto L. (2015). Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 5, 15991. doi: 10.1038/srep15991
Álvarez M., del P., Carol E., Bouza P. J. (2016). Precipitation/dissolution of marine evaporites as determinants in groundwater chemistry in a salt marsh (Península Valdés, Argentina). Mar. Chem. 187, 35–42. doi: 10.1016/j.marchem.2016.10.005
Arriola J. M., Cable J. E. (2017). Variations in carbon burial and sediment accretion along a tidal creek in a Florida salt marsh. Limnol. Oceanogr. 62, S15–S28. doi: 10.1002/lno.10652
Baltar F., Alvarez-Salgado X. A., Arístegui J., Benner R., Hansell D. A., Herndl G. J., et al. (2021). What is refractory organic matter in the ocean? Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.642637
Barton K. (2019). Multi-model inference (R package 1.43.15). Available online at: https://CRAN.R-project.org/package=MuMIn (Accessed February 10, 2020).
Bergé J.-P., Barnathan G. (2005). Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv. Biochem. Eng. Biotechnol. 96, 49–125. doi: 10.1007/b135782
Berzaghi F., Bretagnolle F., Durand-Bessart C., Blake S. (2023). Megaherbivores modify forest structure and increase carbon stocks through multiple pathways. Proc. Natl. Acad. Sci. 120, e2201832120. doi: 10.1073/pnas.2201832120
Bisigato A. J., Hardtke L. A., del Valle H. F., Bouza P. J., Palacio R. G. (2016). Regional-scale vegetation heterogeneity in northeastern Patagonia: Environmental and spatial components. Community Ecol. 17, 8–16. doi: 10.1556/168.2016.17.1.2
Bortolus A., Schwindt E., Bouza P. J., Idaszkin Y. L. (2009). A characterization of Patagonian salt marshes. Wetlands 29, 772–780. doi: 10.1672/07-195.1
Bouchard S. S., Bjorndal K. A. (2000). Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 81, 2305. doi: 10.2307/177116
Bulmer R. H., Stephenson F., Jones H. F. E., Townsend M., Hillman J. R., Schwendenmann L., et al. (2020). Blue carbon stocks and cross-habitat subsidies. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00380
Chami R., Fullenkamp C., Cosimano T., Berzaghi F., Español-Jiménez S., Marcondes M., et al. (2020). On valuing nature-based solutions to climate change: A framework with application to elephants and whales. Available online at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3686168 (Accessed September 25, 2024).
Chen Z., Nie T., Zhao X., Li J., Yang B., Cui D., et al. (2022). Organic carbon remineralization rate in global marine sediments: A review. Reg. Stud. Mar. Sci. 49, 102112. doi: 10.1016/j.rsma.2021.102112
Chielle R., Meziane T., Rezende C. E., Cotovicz L. C. Jr., Abril G., Marins R. V. (2024). Fatty acids and stable isotopes distribution in the mangrove dominated Parnaíba River Delta. Estuar. Coast. Shelf Sci. 308, 108934. doi: 10.1016/j.ecss.2024.108934
Ciais P., Sabine C., Bala G., Bopp L., Brovkin V., Canadell J., et al. (2013). Carbon and other biogeochemical cycles. Climate Change: The Physical Science Basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge and New York: Cambridge University Press).
Committee on Characterization of Wetlands (1995). Wetlands: characteristics and boundaries (USA: National Academies Press).
Crespo E. A., de Oliveira L. R., Sepúlveda M. (2021). Ecology and conservation of pinnipeds in Latin America. Eds. Heckel G., Schramm Y. (Springer International). doi: 10.1007/978-3-030-63177-2
Crespo E. A., Pedraza S. N., Dans S. L., Svendsen G. M., Degrati M., Coscarella M. A. (2019). The southwestern Atlantic southern right whale, Eubalaena australis, population is growing but at a decelerated rate. Mar. Mammal Sci. 35, 93–107. doi: 10.1111/mms.12526
Cuitiño J. I., Bilmes A., Buono M. R., Bordese S., Herazo L., Scasso R. A. (2023). Stratigraphy, provenance, and timing of Neogene sedimentation in the western Valdés Basin, Patagonia. Accurate paleogeographic reconstructions as a key piece for andean-passive margin integration. J. South Am. Earth Sci. 124, 104278. doi: 10.1016/j.jsames.2023.104278
Curry B. E., Brownell R. L. Jr. (2014). Handbook of the mammals of the world Vol. 4. Eds. Wilson D. E., Mittermeier R. A. (Sea Mammals, Lynx Edicions), 186–214.
D’Agostino V. C., Degrati M., Santinelly N., Sastre V., Dans S. L., Hoffmeyer M. S. (2018). The seasonal dynamics of plankton communities relative to the foraging of the southern right whale (Eubalaena australis) in northern Patagonian gulfs, Península Valdés, Argentina. Continental Shelf Res. 164, 45–57. doi: 10.1016/j.csr.2018.06.003
D’Agostino V. C., Heredia F. M., Crespo E. A., Fioramonti A., Fioramonti P., Vélez Á., et al. (2023). Long-term monitoring of southern right whale feeding behavior indicates that Península Valdés is more than a calving ground. Mar. Biol. 170, 43. doi: 10.1007/s00227-023-04181-9
D’Agostino V. C., Nocera A. C., Abernathy K., Wilson A. M., Coscarella M. A., Degrati M. (2024). Foraging dives of southern right whales (Eubalaena australis) in relation to larger zooplankton size prey availability in Golfo Nuevo, Península Valdés, Argentina. Sci. Rep. 14, 14211. doi: 10.1038/s41598-024-63879-y
Dans S. L., Crespo E. A., Pedraza S. N., Alonso M. K. (2004). Recovery of the South American sea lion (Otaria flavescens) population in northern Patagonia. Can. J. Fish. Aquat. Sci. 61, 1681–1690. doi: 10.1139/f04-105
Deagle B. E., Jarman S. N., Coissac E., Pompanon F., Taberlet P. (2014). DNA metabarcoding and the cytochrome c oxidase subunit I marker: not a perfect match. Biol. Lett. 10, 20140562. doi: 10.1098/rsbl.2014.0562
de los Santos C. B., Egea L. G., Martins M., Santos R., Masqué P., Peralta G., et al. (2023). Sedimentary organic carbon and nitrogen sequestration across a vertical gradient on a temperate wetland seascape including salt marshes, seagrass meadows and rhizophytic macroalgae beds. Ecosystems 26, 826–842. doi: 10.1007/s10021-022-00801-5
Drago M., Crespo E., Aguilar A., Cardona L., García N., Dans S., et al. (2009). Historic diet change of the South American sea lion in Patagonia as revealed by isotopic analysis. Mar. Ecol. Prog. Ser. 384, 273–286. doi: 10.3354/meps08017
Dubois S., Savoye N., Grémare A., Plus M., Charlier K., Beltoise A., et al. (2012). Origin and composition of sediment organic matter in a coastal semi-enclosed ecosystem: An elemental and isotopic study at the ecosystem space scale. J. Mar. Syst. 94, 64–73. doi: 10.1016/j.jmarsys.2011.10.009
Dugan J. E., Hubbard D. M., Page H. M., Schimel J. P. (2011). Marine macrophyte wrack inputs and dissolved nutrients in beach sands. Estuaries Coasts 34, 839–850. doi: 10.1007/s12237-011-9375-9
Duvall E. S., le Roux E., Pearson H. C., Roman J., Malhi Y., Abraham A. J. (2024). Resisting the carbonization of animals as climate solutions. Nat. Clim. Change 14, 892–895. doi: 10.1038/s41558-024-02106-y
Egea L. G., Infantes E., Jiménez-Ramos R. (2023). Loss of POC and DOC on seagrass sediments by hydrodynamics. Sci. Total Environ. 901, 165976. doi: 10.1016/j.scitotenv.2023.165976
Erskine P. D., Bergstrom D. M., Schmidt S., Stewart G. R., Tweedie C. E., Shaw J. D. (1998). Subantarctic Macquarie Island - a model ecosystem for studying animal-derived nitrogen sources using 15 N natural abundance. Oecologia 117, 187–193. doi: 10.1007/s004420050647
Faria P. J., Campos F. P., Branco J. O., Musso C. M., Morgante J. S., Bruford M. W. (2010). Population structure in the South American tern Sterna hirundinacea in the South Atlantic: two populations with distinct breeding phenologies. J. Avian Biol. 41, 378–387. doi: 10.1111/j.1600-048X.2009.04902.x
Field R. D., Reynolds J. D. (2011). Sea to sky: impacts of residual salmon-derived nutrients on estuarine breeding bird communities. Proc. R. Soc B Biol. Sci. 278, 3081–3088. doi: 10.1098/rspb.2010.2731
Ford R., Thrush S., Probert P. (1999). Macrobenthic colonisation of disturbances on an intertidal sandflat:the influence of season and buried algae. Mar. Ecol. Prog. Ser. 191, 163–174. doi: 10.3354/meps191163
Friedlingstein P., O’Sullivan M., Jones M. W., Andrew R. M., Gregor L., Hauck J., et al. (2022). Global carbon budget 2022. Earth Syst. Sci. Data 14, 4811–4900. doi: 10.5194/essd-14-4811-2022
García L. V., Marañón T., Ojeda F., Clemente L., Redondo R. (2002). Seagull influence on soil properties, chenopod shrub distribution, and leaf nutrient status in semi-arid Mediterranean islands. Oikos 98, 75–86. doi: 10.1034/j.1600-0706.2002.980108.x
Gardade L., Khandeparker L., Desai D. V., Atchuthan P., Anil A. C. (2021). Fatty acids as indicators of sediment organic matter dynamics in a monsoon-influenced tropical estuary. Ecol. Indic. 130, 108014. doi: 10.1016/j.ecolind.2021.108014
Geraldi N. R., Ortega A., Serrano O., Macreadie P. I., Lovelock C. E., Krause-Jensen D., et al. (2019). Fingerprinting blue carbon: rationale and tools to determine the source of organic carbon in marine depositional environments. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00263
Gerpe M., Rodriguez D., Moreno J., Bastida R., Aizpun J. (2006). “Heavy metal distribution in southern Sea Lions (Otaria flavescens) from Argentina,” in Sea Lions of the World (University of Alaska Fairbanks, Alaska Sea Grant), 45–56. doi: 10.4027/slw.2006.04
Glew J. R., Smol J. P., Last W. M. (2001). “Sediment core collection and extrusion,” in Tracking environmental change using lake sediments, vol. 1 . Eds. Last W. M., Smol J. P. (Springer, New York), 73–105.
Grandi M. F., Dans S. L., Crespo E. A. (2008). Social composition and spatial distribution of colonies in an expanding population of South American sea lions. J. Mammal. 89, 1218–1228. doi: 10.1644/08-MAMM-A-088.1
Grandi M. F., Vales D. G., Crespo E. A., Loizaga R. (2021). Variation in trophic resources in female South American sea lions at a small geographic scale. Mar. Mammal Sci. 37, 314–327. doi: 10.1111/mms.12746
Grandi M. F., Vales D. G., Heredia F., Sosa D. A., D’Agostino V., Milano V., et al. (2020). Informe de relevamiento de los apostaderos de lobos marinos comunies, Otaria flavescenses, del norte de Chubut durante enero 2020. Technical Report (Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Available online at: https://www.conicet.gov.ar/new_scp/detalle.php?keywords=&id=25625&inst=yes&inf_tecnico=yes&detalles=yes&inf_tecnico_id=9788933 (Accessed January 01, 2025).
Harrison X. A., Donaldson L., Correa-Cano M. E., Evans J., Fisher D. N., Goodwin C. E. D., et al. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6, e4794. doi: 10.7717/peerj.4794
Heiss J. W. (2020). Whale burial and organic matter impacts on biogeochemical cycling in beach aquifers and leachate fluxes to the nearshore zone. J. Contam. Hydrol. 233, 103656. doi: 10.1016/j.jconhyd.2020.103656
Hilmi N., Chami R., Sutherland M. D., Hall-Spencer J. M., Lebleu L., Benitez M. B., et al. (2021). The role of blue carbon in climate change mitigation and carbon stock conservation. Front. Clim. 3. doi: 10.3389/fclim.2021.710546
Howard J., Hoyt S., Isensee K., Pidgeon E., Telszewski M. (2014). Coastal blue carbon: methods for assessing carbon stocks and emissions factors in mangroves, tidal salt marshes, and seagrass meadows (Arlington, Virginia, USA: Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature).
Howard J., Sutton-Grier A., Herr D., Kleypas J., Landis E., Mcleod E., et al. (2017). Clarifying the role of coastal and marine systems in climate mitigation. Front. Ecol. Environ. 15, 42–50. doi: 10.1002/fee.1451
Hutto S. H., Brown M., Francis E. (2021). Blue carbon in marine protected areas: part 2; a blue carbon assessment of greater farallones national marine sanctuary. national marine sanctuaries conservation science series ONMS-21-07 (Washington, DC: U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries).
Isacch J. P., Costa C. S. B., Rodríguez-Gallego L., Conde D., Escapa M., Gagliardini D. A., et al. (2006). Distribution of saltmarsh plant communities associated with environmental factors along a latitudinal gradient on the south-west Atlantic coast. J. Biogeogr. 33, 888–900. doi: 10.1111/j.1365-2699.2006.01461.x
IUCN (2020). Guidance for Using the IUCN Global Standard for Nature-Based Solutions. A User-Friendly Framework for the Verification, Design and Scaling Up of Nature-Based Solutions. 1st ed. (Gland, Switzerland: IUCN).
Jiménez-Ramos R., Brun F. G., Vergara J. J., Hernández I., Pérez-Lloréns J. L., Egea L. G. (2024). Nutrient enrichment and herbivory alter carbon balance in temperate seagrass communities. Mar. pollut. Bull. 206, 116784. doi: 10.1016/j.marpolbul.2024.116784
Jiménez-Ramos R., Tomas F., Reynés X., Romera-Castillo C., Pérez-Lloréns J. L., Egea L. G. (2022). Carbon metabolism and bioavailability of dissolved organic carbon (DOC) fluxes in seagrass communities are altered under the presence of the tropical invasive alga Halimeda incrassata. Sci. Total Environ. 839, 156325. doi: 10.1016/j.scitotenv.2022.156325
Kennedy P., Kennedy H., Papadimitriou S. (2005). The effect of acidification on the determination of organic carbon, total nitrogen and their stable isotopic composition in algae and marine sediment. Rapid Commun. Mass Spectrom. 19, 1063–1068. doi: 10.1002/rcm.1889
Koen-Alonso M., Yodzis P. (2005). Multispecies modelling of some components of the northern and central Patagonia marine community, Argentina. Can. J. Fish Aquat Sci. 62, 1490–1512. doi: 10.1139/f05-087
Kokot R. R., Monti A. A. J., Codignotto J. O. (2005). Morphology and short-term changes of the caleta valdés barrier spit, Argentina. J. Coast. Res. 215, 1021–1030. doi: 10.2112/03-703A.1
Krabbe N., Langlet D., Belgrano A., Villasante S. (2022). Reforming international fisheries law can increase blue carbon sequestration. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.800972
Laguna C., López-Perea J. J., Feliu J., Jiménez-Moreno M., Rodríguez-Martín-Doimeadios R. C., Florín M., et al. (2021). Nutrient enrichment and trace element accumulation in sediments caused by waterbird colonies at a Mediterranean semiarid floodplain. Sci. Total Environ. 777, 145748. doi: 10.1016/j.scitotenv.2021.145748
Li J., Yan D., Yao X., Liu Y., Xie S., Sheng Y., et al. (2022). Dynamics of carbon storage in saltmarshes across China’s eastern coastal wetlands from 1987 to 2020. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.915727
Loizaga R., García N. A., Durante C. A., Vales D. G., Crespo E. A. (2023). Killer whales at northern Patagonia, Argentina: Evidence of different foraging groups from stable isotopes. Mar. Mammal Sci. 39, 1121–1135. doi: 10.1111/mms.13048
Macreadie P. I., Anton A., Raven J. A., Beaumont N., Connolly R. M., Friess D. A., et al. (2019). The future of Blue Carbon science. Nat. Commun. 10, 3998. doi: 10.1038/s41467-019-11693-w
Macreadie P. I., Costa M. D. P., Atwood T. B., Friess D. A., Kelleway J. J., Kennedy H., et al. (2021). Blue carbon as a natural climate solution. Nat. Rev. Earth Environ. 2, 826–839. doi: 10.1038/s43017-021-00224-1
Macreadie P. I., Hughes A. R., Kimbro D. L. (2013). Loss of ‘Blue carbon’ from coastal salt marshes following habitat disturbance. PloS One 8, e69244. doi: 10.1371/journal.pone.0069244
Manno C., Fielding S., Stowasser G., Murphy E. J., Thorpe S. E., Tarling G. A. (2020). Continuous moulting by Antarctic krill drives major pulses of carbon export in the north Scotia Sea, Southern Ocean. Nat. Commun. 11, 6051. doi: 10.1038/s41467-020-19956-7
Mariani G., Cheung W. W. L., Lyet A., Sala E., Mayorga J., Velez L., et al. (2020). Let more big fish sink: Fisheries prevent blue carbon sequestration—half in unprofitable areas. Sci. Adv. 6, eabb4848. doi: 10.1126/sciadv.abb4848
Martin A. H., Pearson H. C., Saba G. K., Olsen E. M. (2021). Integral functions of marine vertebrates in the ocean carbon cycle and climate change mitigation. One Earth 4, 680–693. doi: 10.1016/j.oneear.2021.04.019
Martinetto P., Alberti J., Becherucci M. E., Cebrian J., Iribarne O., Marbà N., et al. (2023). The blue carbon of southern southwest Atlantic salt marshes and their biotic and abiotic drivers. Nat. Commun. 14, 8500. doi: 10.1038/s41467-023-44196-w
Maxwell T. L., Rovai A. S., Adame M. F., Adams J. B., Álvarez-Rogel J., Austin W. E. N., et al. (2023). Global dataset of soil organic carbon in tidal marshes. Sci. Data 10, 797. doi: 10.1038/s41597-023-02633-x
Mayer L. M., Rahaim P. T., Guerin W., Macko S. A., Watling L., Anderson F. E. (1985). Biological and granulometric controls on sedimentary organic matter of an intertidal mudflat. Estuar. Coast. Shelf Sci. 20, 491–503. doi: 10.1016/0272-7714(85)90091-5
Meynecke J.-O., Samanta S., de Bie J., Seyboth E., Prakash Dey S., Fearon G., et al. (2023). Do whales really increase the oceanic removal of atmospheric carbon? Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1117409
Moore O. W., Curti L., Woulds C., Bradley J. A., Babakhani P., Mills B. J. W., et al. (2023). Long-term organic carbon preservation enhanced by iron and manganese. Nature 621, 312–317. doi: 10.1038/s41586-023-06325-9
Nellemann C., Corcoran E., Duarte C. M., Valdés L., De Young C., Fonseca L., et al. (2009). Blue Carbon: A Rapid Response Assessment (United Nations Environment Pro- gramme. GRID-Arendal).
Nguyen N.-L., Devendra D., Szymańska N., Greco M., Angeles I. B., Weiner A. K. M., et al. (2023). Sedimentary ancient DNA: a new paleogenomic tool for reconstructing the history of marine ecosystems. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1185435
Orr M., Zimmer M., Jelinski D. E., Mews M. (2005). Wrack deposition on different beach types: spatial and temporal variation in the pattern of subsidy. Ecology 86, 1496–1507. doi: 10.1890/04-1486
Parnell A., Inger R. (2019). Stable isotope mixing models in R with simmr. Available online at: https://cran.r-project.org/web/packages/simmr/vignettes/simmr.html (Accessed September 25, 2024).
Pearson H. C., Savoca M. S., Costa D. P., Lomas M. W., Molina R., Pershing A. J., et al. (2023). Whales in the carbon cycle: can recovery remove carbon dioxide? Trends Ecol. Evol. 38, 238–249. doi: 10.1016/j.tree.2022.10.012
Pérez G., O’Leary B. C., Allegri E., Casal G., Cornet C. C., de Juan S., et al. (2024). A conceptual framework to help choose appropriate blue nature-based solutions. J. Environ. Manage. 352, 119936. doi: 10.1016/j.jenvman.2023.119936
Pershing A. J., Christensen L. B., Record N. R., Sherwood G. D., Stetson P. B. (2010). The impact of whaling on the ocean carbon cycle: why bigger was better. PloS One 5, e12444. doi: 10.1371/journal.pone.0012444
Quaggiotto M.-M., Barton P. S., Morris C. D., Moss S. E. W., Pomeroy P. P., McCafferty D. J., et al. (2018). Seal carrion is a predictable resource for coastal ecosystems. Acta Oecologica 88, 41–51. doi: 10.1016/j.actao.2018.02.010
Ratnarajah L., Melbourne-Thomas J., Marzloff M. P., Lannuzel D., Meiners K. M., Chever F., et al. (2016). A preliminary model of iron fertilisation by baleen whales and Antarctic krill in the Southern Ocean: Sensitivity of primary productivity estimates to parameter uncertainty. Ecol. Modell. 320, 203–212. doi: 10.1016/j.ecolmodel.2015.10.007
Raza T., Qadir M. F., Khan K. S., Eash N. S., Yousuf M., Chatterjee S., et al. (2023). Unrevealing the potential of microbes in decomposition of organic matter and release of carbon in the ecosystem. J. Environ. Manage. 344, 118529. doi: 10.1016/j.jenvman.2023.118529
R Development Core Team. (2024). R: A Language and Environment for Statistical Computing. (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Ríos I., Bouza P. J., Bortolus A., del Pilar Alvarez M. (2018). Soil-geomorphology relationships and landscape evolution in a southwestern Atlantic tidal salt marsh in Patagonia, Argentina. J. South Am. Earth Sci. 84, 385–398. doi: 10.1016/j.jsames.2018.04.015
Roldán V. A., Camino M., Argoitia A., Campos C. M., Caruso N., Eder E. B., et al. (2022). Potential contributions of mammals to human well-being in Argentina. Mastozoología Neotrop. 29, 001–0814. doi: 10.31687/saremMN.22.29.2.07.e0650
Roman J., Estes J. A., Morissette L., Smith C., Costa D., McCarthy J., et al. (2014). Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385. doi: 10.1890/130220
Roman J., McCarthy J. J. (2010). The whale pump: marine mammals enhance primary productivity in a coastal basin. PloS One 5, e13255. doi: 10.1371/journal.pone.0013255
Romero M. A., Coscarella M. A., Adams G. D., Pedraza J. C., González R. A., Crespo E. A. (2022). Historical reconstruction of the population dynamics of southern right whales in the southwestern Atlantic Ocean. Sci. Rep. 12, 3324. doi: 10.1038/s41598-022-07370-6
Romero M. A., Grandi M. F., Koen-Alonso M., Svendsen G., Ocampo Reinaldo M., García N. A., et al. (2017). Analysing the natural population growth of a large marine mammal after a depletive harvest. Sci. Rep. 7, 5271. doi: 10.1038/s41598-017-05577-6
Rowntree V., Uhart M., Sironi M., Chirife A., Di Martino M., La Sala L., et al. (2013). Unexplained recurring high mortality of southern right whale Eubalaena australis calves at Península Valdés, Argentina. Mar. Ecol. Prog. Ser. 493, 275–289. doi: 10.3354/meps10506
Sandhage-Hofmann A., Linstädter A., Kindermann L., Angombe S., Amelung W. (2021). Conservation with elevated elephant densities sequesters carbon in soils despite losses of woody biomass. Glob. Change Biol. 27, 4601–4614. doi: 10.1111/gcb.15779
Santini N. S., Adame M. F., Nolan R. H., Miquelajauregui Y., Piñero D., Mastretta-Yanes A., et al. (2019). Storage of organic carbon in the soils of Mexican temperate forests. For. Ecol. Manage. 446, 115–125. doi: 10.1016/j.foreco.2019.05.029
Santos R., Duque-Núñez N., de los Santos C. B., Martins M., Carrasco A. R., Veiga-Pires C. (2019). Superficial sedimentary stocks and sources of carbon and nitrogen in coastal vegetated assemblages along a flow gradient. Sci. Rep. 9, 610. doi: 10.1038/s41598-018-37031-6
Sapoznikow A., Quintana F. (2008). Colony and nest site fidelity of the rock shag (Phalacrocorax magellanicus). J. Ornithol. 149, 639–642. doi: 10.1007/s10336-008-0310-1
Serrán M., Centeno N., Weiler N., Otero J. G. (2008). Massive death of pinnipeds 1200 years ago: Taphonomic history of the “Lobos site” (Golfo Nuevo, Patagonia, Argentina). Quat. Int. 183, 135–142. doi: 10.1016/j.quaint.2006.12.001
Shen X., Zhang Y., Hong Y., Zeng X., Zhou W., Yue W., et al. (2024). Spatial variability in blue carbon storage and sequestration of seagrass meadows in southern China. Sci. Total Environ. 951, 175884. doi: 10.1016/j.scitotenv.2024.175884
Sironi M., Donini A., Rowntree V. J., Di Martino M., Fernández S., Ricciardi M., et al. (2022). Southern right whale mortalities at Península Valdés, Argentina: updated information for 2018-2022 (Instituto de Conservación de Ballenas/Ocean Alliance). Available at: https://ballenas.org.ar (Accessed September 25, 2024).
Smith C. R., Baco A. R. (2003). Ecology of whale falls at the deep-Sea floor. Oceanogr. Mar. Biol. an Annu. Rev. 41, 311–354. doi: 10.1201/9780203180570-33
Song S., Ding Y., Li W., Meng Y., Zhou J., Gou R., et al. (2023). Mangrove reforestation provides greater blue carbon benefit than afforestation for mitigating global climate change. Nat. Commun. 14, 756. doi: 10.1038/s41467-023-36477-1
Turrell W. R., Austin W. E. N., Philbrick S. P., Tilbrook C., Kennedy H. (2023). Clarifying the role of inorganic carbon in blue carbon policy and practice. Mar. Policy 157, 105873. doi: 10.1016/j.marpol.2023.105873
Upadhayay H. R., Bodé S., Griepentrog M., Huygens D., Bajracharya R. M., Blake W. H., et al. (2017). Methodological perspectives on the application of compound-specific stable isotope fingerprinting for sediment source apportionment. J. Soils Sediments 17, 1537–1553. doi: 10.1007/s11368-017-1706-4
Valenzuela L. O., Sironi M., Rowntree V. J., Seger J. (2009). Isotopic and genetic evidence for culturally inherited site fidelity to feeding grounds in southern right whales (Eubalaena australis). Mol. Ecol. 18, 782–791. doi: 10.1111/j.1365-294X.2008.04069.x
van de Schoot R., Kaplan D., Denissen J., Asendorpf J. B., Neyer F. J., van Aken M. A. G. (2014). A gentle introduction to Bayesian analysis: applications to developmental research. Child Dev. 85, 842–860. doi: 10.1111/cdev.12169
Wilson C., Sastre A. V., Hoffmeyer M., Rowntree V. J., Fire S. E., Santinelli N. H., et al. (2016). Southern right whale (Eubalaena australis) calf mortality at Península Valdés, Argentina: Are harmful algal blooms to blame? Mar. Mammal Sci. 32, 423–451. doi: 10.1111/mms.12263
Keywords: blue carbon, Eubalaena australis, nitrogen sequestration, Otaria flavescens, salt marshes, southern right whales, South American sea lions, sediment organic carbon
Citation: Jiménez-Ramos R, Egea LG, D’Agostino VC, Degrati M and Loizaga R (2025) Carbon and nitrogen stocks in sediment at Península Valdés Biosphere Reserve: novel insights into the potential contribution of large marine vertebrates to carbon sequestration. Front. Mar. Sci. 12:1500594. doi: 10.3389/fmars.2025.1500594
Received: 25 September 2024; Accepted: 27 January 2025;
Published: 14 February 2025.
Edited by:
Renato S. Carreira, Pontifical Catholic University of Rio de Janeiro, BrazilReviewed by:
Songlin Liu, Chinese Academy of Sciences (CAS), ChinaCopyright © 2025 Jiménez-Ramos, Egea, D’Agostino, Degrati and Loizaga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis G. Egea, Z29uemFsby5lZ2VhQHVjYS5lcw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.