
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 26 February 2025
Sec. Marine Conservation and Sustainability
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1499607
 Michael Dadole Ubagan1
Michael Dadole Ubagan1 Taekjun Lee1,2
Taekjun Lee1,2 Yongeun Kim3
Yongeun Kim3 Jeonghee Lee1
Jeonghee Lee1 Hoon Jeong2
Hoon Jeong2 Yun-Sik Lee4,5*
Yun-Sik Lee4,5* Sook Shin1,2*
Sook Shin1,2*Sessile invertebrates perform essential ecological functions in coastal ecosystems. This study aimed to provide an in-depth analysis of the status and distribution of sessile invertebrates along the peninsular coasts of South Korea, focusing on the potential ecological impacts of non-indigenous species. Fourteen sampling sites along the coastline of the Korean Peninsula were surveyed four times over a year, once in each season, to investigate the subtidal communities of sessile invertebrates. Based on the community data, this study identified indigenous and non-indigenous species and classified them into broadly present and regionally dominant species among geographically distinct coastal ecosystems in Korea. Effects of non-indigenous species on biodiversity within their dominance range were analyzed to identify species with potential significant ecological impacts. Results indicated that while some dominant non-indigenous species had no significant effects, others such as Amphibalanus amphitrite were associated with a loss of biodiversity in the Yellow Sea. This study highlights the importance of clearly distinguishing the range of dominant species and emphasizes the need for continuous monitoring to support early detection and inform management strategies for reducing negative impacts of non-indigenous species. This research provides new insights for assessing the influence of non-indigenous species within sessile invertebrate communities.
In coastal ecosystems, sessile invertebrates perform crucial ecological functions (Sarà, 1986). These communities can exhibit multiple stable states depending on environmental conditions and disturbances (Sutherland, 1990). Due to their inability to relocate once settled, they are often considered a stable source of biodiversity within specific marine ecosystems (Stachowicz et al., 1999). These organisms carry out various ecological roles from their fixed positions, contributing to forming physical structures and cycling nutrients within the ecosystem (Sarà, 1986). For example, sessile invertebrates such as sponges and corals can filter water and feed on organic particles, thereby supporting the health and survival of marine biological communities (Stachowicz et al., 2007). They also provide habitat and protection for other marine organisms, acting as key structural elements within the ecosystem (Stachowicz et al., 2007). As ecosystem engineers, sessile invertebrates can modify physical properties of their habitats, regulate resource availability, and facilitate interspecies interactions. These ecological functions are essential for maintaining the complexity and health of marine ecosystems (Stachowicz et al., 1999).
Sessile invertebrates tend to carefully select their habitats during the early larval settlement stage based on environmental factors (Ubagan et al., 2021). Additionally, specific surface structures and chemical signals play significant roles in helping larvae find suitable habitats (Whalan et al., 2015). Furthermore, larvae can avoid substrates with high mortality risks, demonstrating that their habitat choice during the larval stage can significantly impact their survival and reproduction after settlement (Grosberg, 1981). Due to these characteristics, extensive research has been conducted on the distribution and spread of sessile invertebrates (Bishop et al., 2015; Williams et al., 2016).
Increasingly, the introduction and spread of non-indigenous species have been threatening marine ecosystems. These species can enter marine ecosystems through various pathways, leading to reduced biodiversity and impaired ecosystem functions (Bishop et al., 2015). Non-indigenous species often possess high competitiveness and adaptability, allowing them to infringe upon indigenous species’ habitats and compete with them for resources. For example, several non-indigenous species, such as the green mussel Perna viridis, the purse oyster Isognomon bicolor, and the Pacific oyster Crassostrea gigas, exhibited higher tolerance to environmental stressors like low salinity, low oxygen, and high temperature compared to their native counterparts (Lenz et al., 2011). This ability to better withstand adverse environmental conditions may provide a significant advantage for non-indigenous species in establishing and spreading within new ecosystems. Beyond these ecological impacts, the spread of non-indigenous species can result in economic losses and social issues (Williams et al., 2016). Therefore, research and policy efforts are crucial to preventing and managing the introduction and spread of non-indigenous species (Lehtiniemi et al., 2015).
Recent research trends emphasize the need to distinguish whether non-indigenous species contribute to biodiversity loss in the ecosystems where they are introduced (Jeschike et al., 2014). This is because some non-indigenous species may have neutral or even positive interactions within ecosystems they invade (Vilà and Hulme, 2017; Guerin et al., 2018). To make this distinction, it is important to clearly delineate the scope of their potential impacts when assessing and managing effects of non-indigenous species on ecosystems. Accurately understanding whether non-indigenous species have positive, neutral, or negative impacts is crucial for assessing their influence on ecosystems.
Marine ecosystems of South Korea, encompassing those in the East Sea, Korea Strait, and Yellow Sea, provide a useful environment for investigating the complex dynamics of sessile invertebrate communities (Park et al., 2017). These three coastal areas exhibit different marine environmental characteristics, significantly influencing sessile invertebrates’ distribution and community structure (Ubagan et al., 2021). The East Sea is influenced by both warm and cold currents, while the Korea Strait is primarily affected by a warm current. The shallow, semi-enclosed Yellow Sea is highly influenced by freshwater inflow. This diversity of environmental conditions within the relatively narrow coastal regions allows for research across a variety of complex habitats (Chang et al., 2002; Choi et al., 2009). The diversity of habitats in coastal ecosystems of South Korea is related to high biodiversity (Chung et al., 2015). Local surveys and classifications of specific invertebrates have been continuously conducted for many years (Ryu et al., 2012; Park et al., 2014; Yu et al., 2021). However, differences in survey periods and techniques among studies have limited our ability to conduct comprehensive ecological analyses.
This study aimed to investigate the status and distribution of sessile invertebrates along the coasts of South Korea. By identifying dominant indigenous and non-indigenous species, the ecological impacts of non-indigenous species could be understood. To achieve our aim, we categorized species into two types based on their distribution patterns: (1) broadly present species that occur across all study regions and (2) regionally dominant species that are abundant only in specific regions. We then analyzed how these non-indigenous species affect local biodiversity to identify which species have significant ecological impacts. This study aligns with current ecological research trends and can serve as an example for examining the effects of non-indigenous species.
The coastal regions of South Korea are divided into three distinct areas: the East Sea, the Korea Strait, and the Yellow Sea (Figure 1). Given that ports and harbors serve as primary entry points for non-indigenous species via international maritime traffic, sampling sites were strategically selected to focus on major ports that receive international trading vessels and recreational boats (Bailey, 2015; Carlton and Ruiz, 2015). Therefore, all the names of sampling sites represent the most critical harbors in each sampling site. In the East Sea, five sampling sites (Sokcho, Donghae, Jukbyeon, Yangpo, and Ulsan) were selected. In the Korea Strait, five sites (Busan, Tongyeong, Gwangyang, Yeosu, and Wando) were selected. In the Yellow Sea, four sites (Mokpo, Bieung, Dangjin, and Incheon) were chosen. Thus, a total of 14 sampling sites were selected for our investigation. These sampling sites are characterized by:
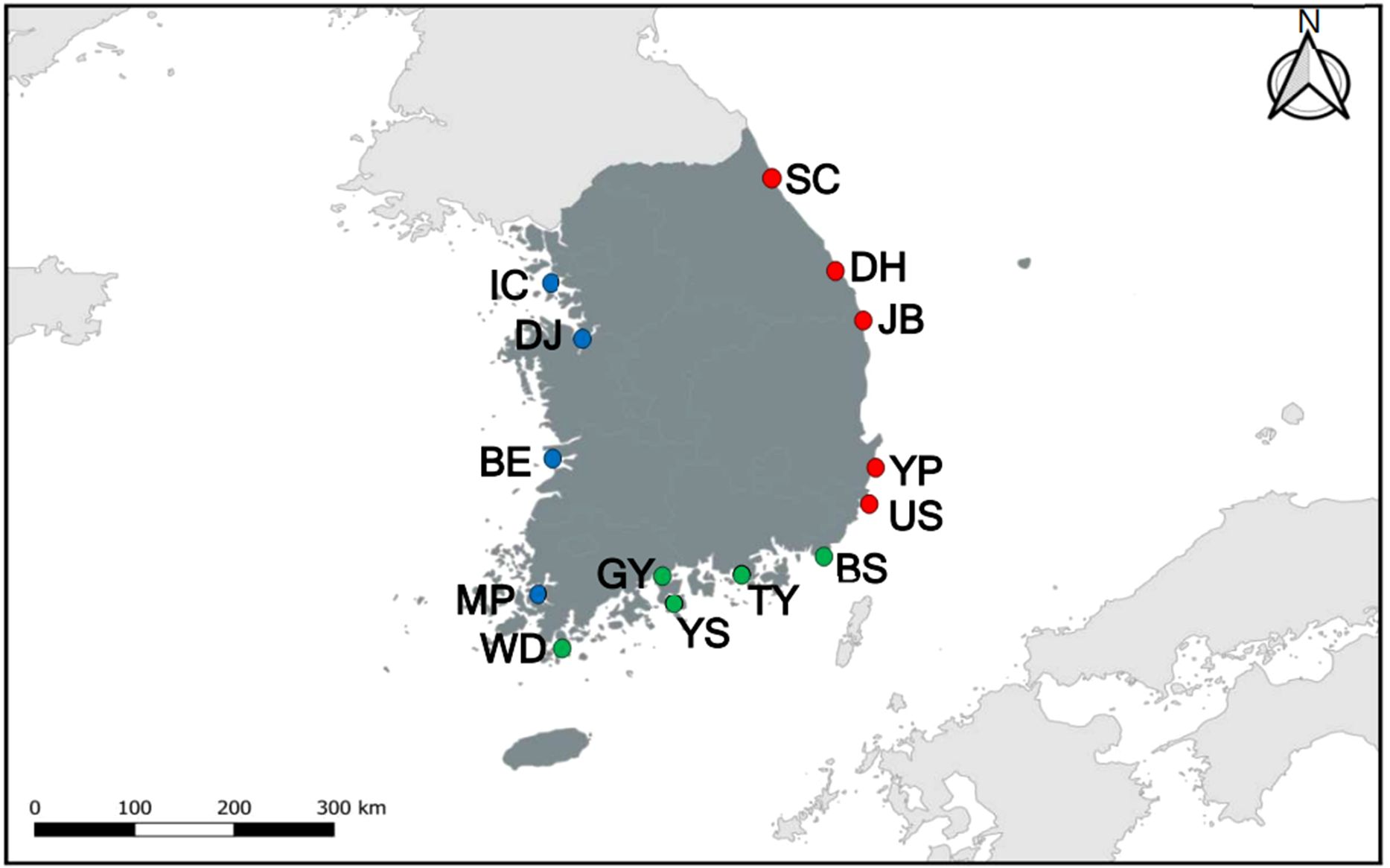
Figure 1. Map for coastal regions and sampling sites with abbreviation in parentheses. East Sea (red circles): Sokcho (SC), Donghae (DH), Jukbyeon (JB), Yangpo (YP), Ulsan (US); Korea Strait (green circles): Busan (BS), Tongyeong (TY), Gwangyang (GY), Yeosu (YS), Wando (WD); Yellow Sea (blue circles): Mokpo (MP), Bieung (BE), Dangjin (DJ), Incheon (IC).
1) Regular international traffic, including cargo ships and recreational vessels.
2) Diverse artificial structures such as port walls and anchors provide suitable settlement substrates for non-indigenous sessile invertebrates, which can also settle on natural substrates such as rocks and wood.
This site selection strategy allows for effective monitoring of the initial establishment and subsequent spread of non-indigenous species, which is crucial for early detection and management of potential invasive species in Korean coastal waters.
Sessile invertebrates at each sampling site were investigated following the method outlined in a previous study (Ubagan et al., 2021). At each sampling site, three points were selected at both ends and center of the harbor, maintaining a minimum spacing of 10 meters between points. At each point, ten acrylic attachment plates (30 cm x 30 cm, positioned with an interval of 20 cm; Supplementary Figure S1A) were connected in a single vertical line and installed at depths ranging from 1 m to 3 m from the sea level at low tide. The plates were installed with a vertical orientation to the water surface to allow for the colonization of sessile invertebrates (Supplementary Figure S1B). Given that these ten plates at each point were attached to a single line and thus not independent samples, data from all ten plates were combined to generate a single measurement for each point. Therefore, each sampling site yielded three independent replicate measurements (one from each point) per season.
Seasonal sampling can effectively capture community-level changes in invertebrates; monitoring was conducted quarterly to represent seasonal variations in community structure (Turner and Trexler, 1997). While some fouling organisms may complete their life cycles in shorter periods, our three-month intervals were designed to capture broader seasonal patterns (July, summer; October, fall; January, winter; April, spring) that characterize environmental conditions in South Korean coastal regions. Each attachment plate was installed during the first week of April, July, and October in 2017 and January in 2018. Monitoring of sessile invertebrates on attachment plates was carried out three months after installation (i.e., July 2017, October 2017, January 2018, and April 2018). Attachment plates were removed from under the seawater and placed on a flat surface for monitoring (Supplementary Figure S1C). Subsequently, data were collected by capturing images of the attachment plate surfaces using a vertically fixed digital camera (Olympus Tough TG-5; Supplementary Figure S1D). During each seasonal monitoring event, environmental parameters (water temperature and salinity) were measured once using a YSI Pro Plus meter (YSI, USA) at the time of plate monitoring (Supplementary Table S1).
Most of the observed sessile invertebrates were identified to the genus or species level based on identification manuals (Kim, 1998; Seo, 2005; Kim, 2011). Due to the three-dimensional nature of target species where organisms grow on top of each other, our observations and measurements were limited to the visible top layer of organisms when photographing the plates. Identification of target species was primarily conducted in the field and confirmed in the laboratory using image data. In instances where species identification posed difficulties, additional samples were collected from attachment plates for further examination. Through these extensive identification efforts, unidentified coverage area was less than 1% of the total attachment plate area. Monitoring image data of each attachment plate was processed using ImageJ software (Schneider et al., 2012) to calculate the area of each identified species. The percent cover was calculated as the ratio of the area occupied by each identified species to the total area of the attachment plate. This measure of percent cover was used as a proxy for species abundance. Species richness was determined as the total number of species present on each plate, while species diversity was calculated using the Shannon-Wiener index based on the presence and relative proportions of different species. These methods have been widely employed to measure the recruitment potential and community characteristics of sessile invertebrates (Fisk and Harriott, 1990; Guy-Haim et al., 2015; Ubagan et al., 2021; Lee et al., 2022).
The classification of species as indigenous or non-indigenous was based on published taxonomic and biogeographic literature (see Table 1 for complete references). This literature-based approach, while not able to determine the exact arrival dates of non-indigenous species, provides the most reliable scientific basis currently available for distinguishing between indigenous and non-indigenous species in coastal waters of South Korea (Lozano et al., 2017).
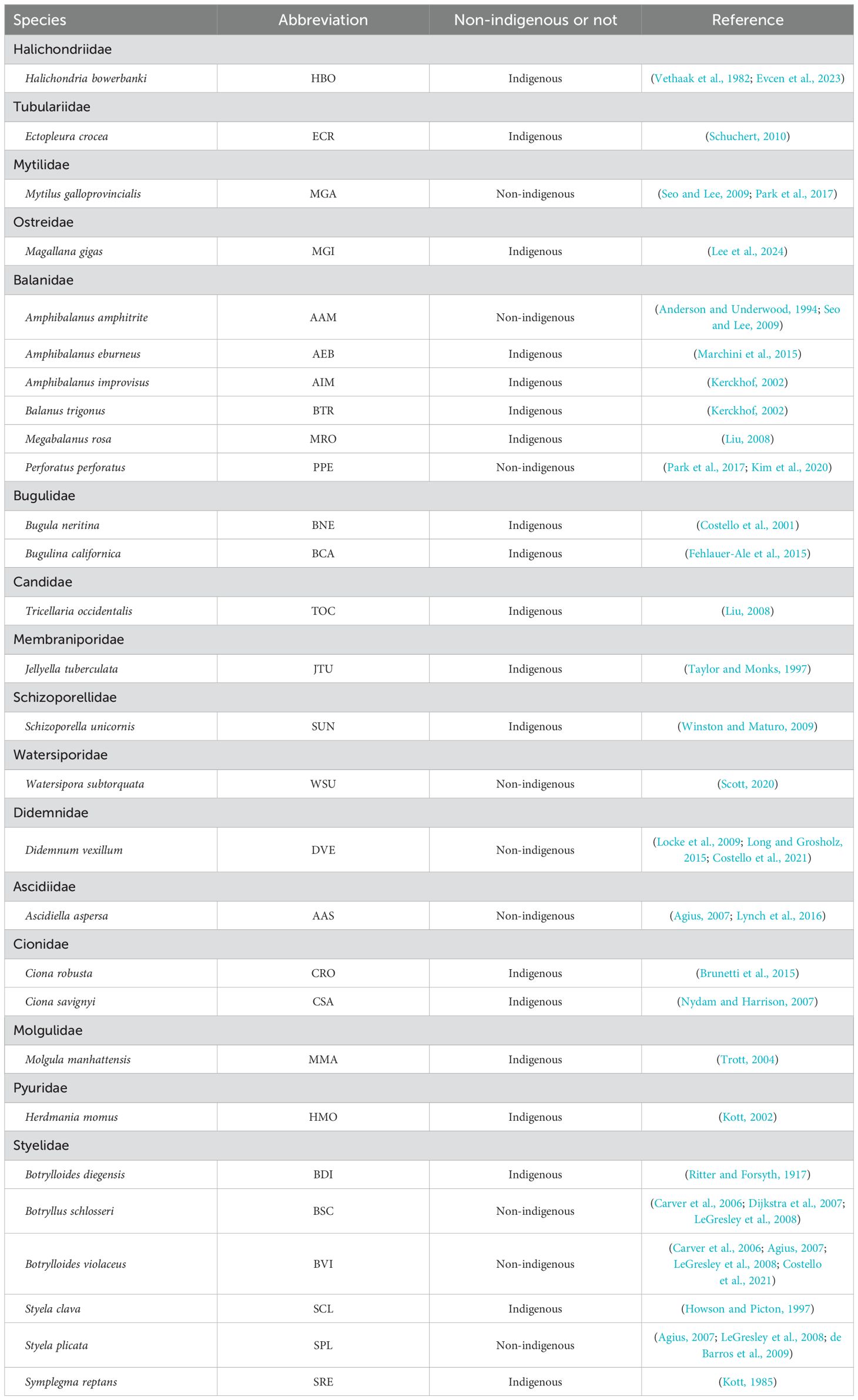
Table 1. List of observed species and their abbreviations (species identified as non-indigenous are specially indicated with references).
Data from all ten plates were pooled for each point within a site and season to generate a single measurement of percent cover, species richness, and diversity. Therefore, all statistical analyses were conducted using three true replicates per site (from the three separate points), with each replicate representing the combined data from ten plates.
In this study, the Shannon-Wiener Diversity index (H’) was used to evaluate species diversity of the invertebrate community at each sampling site. The Shannon-Wiener Diversity index is a comprehensive metric that considers species richness and evenness of species abundance within a community. This index is commonly used in ecological research. It was calculated using the following equation (Shannon, 1948):
where pi was the relative frequency of species i in the community and S was the number of species in that community. In this study, we designed our research to investigate the potential influence of four monitoring time points and three different coastal regions as primary factors affecting the clustering of sessile invertebrates. To assess the impact of these two factors, we conducted a two-way analysis of variance (ANOVA) considering environmental variables (sea surface temperature and salinity) and community characteristics (abundance, species richness, Shannon-Wiener diversity index).
To differentiate between broadly present species, which are significantly abundant across all regions, and regionally dominant species, which are significantly abundant in specific regions, we used Z-score and Indicator value (IndVal). The Z-score was used to identify broadly present species. A Z-score standardizes the frequency of each species, indicating standard deviations from the mean (Warner, 2016). This allowed us to evaluate whether the frequency of a particular species in each region was statistically significant. The mean and standard deviation of the frequency for each species were calculated and used to convert the frequency into a Z-score. The Z-score was calculated as follows:
where X was the percent cover of each species, μ was the mean percent cover of all detected species, and σ was the standard deviation of percent covers of all detected species. If a species has a Z-score of 1.96 or higher, the species is significantly more abundant than average at a 95% confidence interval. Therefore, species with a Z-score higher than 1.96 were classified as broadly present species.
For regionally dominant species, indicator value (IndVal) of species was determined following the approach outlined by Dufrêne and Legendre (1997). The maximum value of IndVal is 1.00 when all individuals of a species are exclusively found in a single treatment group of sites (indicating high specificity) and when the species is present in all sites within that group (indicating high fidelity). In essence, the IndVal takes into account both specificity and fidelity simultaneously, with the goal of identifying species that exhibit a significant preference for the analyzed site or treatment group.
Among non-indigenous species, broadly present species and regionally dominant species were selected. Although two-way ANOVA did not indicate significant differences in most community characteristics, species richness varied depending on season. Consequently, Min-Max normalization was employed to normalize the Shannon-Wiener index by season. This data transformation method involves organizing data based on the maximum and minimum values ratio, adjusting all values within a range of 0 to 1. In this study, the normalized Shannon-Wiener index was calculated using the following formula:
Linear regression analyses were performed to examine relationships between the percent cover (%) of selected non-indigenous and the Normalized Shannon - Wiener Index. If the estimated slope of the linear regression analysis showed a significant negative correlation, it indicated that an increase in the abundance of the non-indigenous species was associated with a decrease in the biodiversity of the sessile invertebrate community. To unravel this relationship, the null hypothesis that the regression slope was non-negative was tested using one-sided t-test.
Two-way ANOVA and linear regression analysis were conducted using statistical analysis software (SAS Institute, 2011). IndVal analyses were performed using R version 4.3.1. The ‘indval’ function in the labdsv package was employed. The R programming language was obtained from http://cran.r-project.org (R Core Team, 2013). All analyses were conducted at a significant level of 5%.
Sea surface temperature and salinity data in the three coastal regions from 2017 to 2018 were collected (Figure 2; Supplementary Table S1). The average sea surface temperature exhibited significant variations depending on the season, clearly indicating seasonal effects. The lowest average temperature was recorded in January 2018 at 10.93°C ± 0.49°C in the East Sea, while the highest average temperature of 23.63°C ± 0.86°C was observed in the Yellow Sea in July 2017. Similarly, other coastal regions displayed similar seasonal temperature trends. Salinity levels also varied with different patterns. In January 2018, the East Sea had the highest average salinity at 34.57 ± 0.53 psu, whereas the Korean Strait had the lowest average salinity in July at 30.02 ± 5.62. These observations were further emphasized through a two-way ANOVA (Supplementary Table S2). In the sea surface temperature analysis, the effect of season was highly significant (F3, 56 = 105.9, p < 0.001). However, the coastal region’s effect was insignificant (F2, 56 = 2.36, p = 0.11), indicating that seasonal variations primarily drove regional differences. Furthermore, both effects of monitoring time points and coastal regions on salinity were significant (F3, 56 = 3.74, p = 0.018 for sea surface temperature; F2, 56 = 6.3, p = 0.004 for salinity).
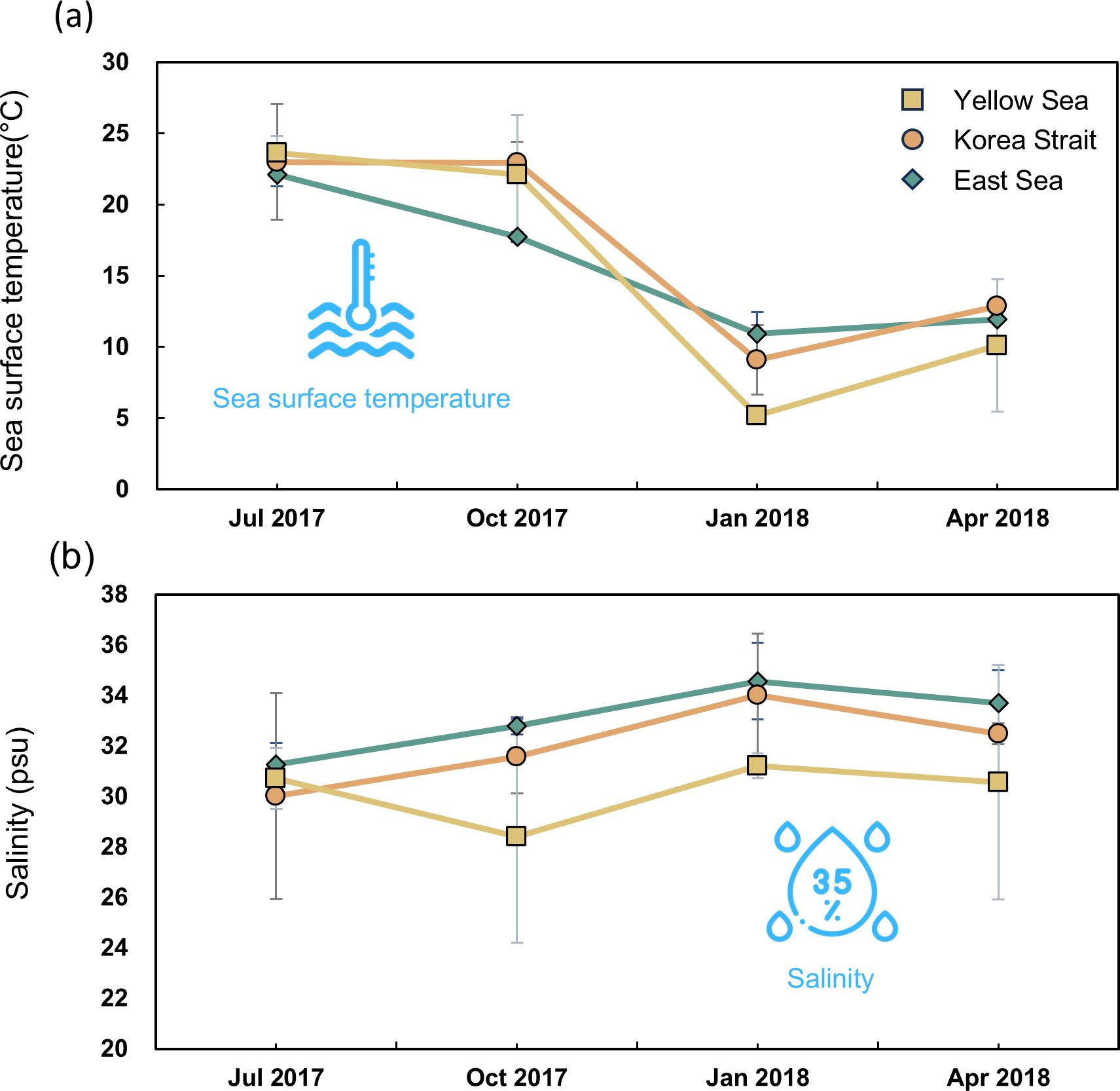
Figure 2. Temporal variations in sea surface temperature (°C; A) and salinity (psu; B) in coastal regions across sampling date. The accompanying error bar represents standard deviation.
During a year of monitoring, a total of 16 families and 28 species were identified (Table 1). Among them, nine species were classified as non-indigenous based on the literature (Table 1). These species include Mytilus galloprovincialis (MGA), Amphibalanus amphitrite (AAM), Perforatus perforatus (PPE), Watersipora subtorquata (WSU), Didemnum vexillum (DVE), Ascidiella aspersa (AAS), Botryllus schlosseri (BSC), Botrylloides violaceus (BVI), and Styela plicata (SPL) (Table 1).
Seasonal variations in dominant species and the presence of non-indigenous species were investigated based on percent cover of observed species on attachment plates at different sampling sites (Figure 3). In July 2017, the dominant species in the East Sea were B. schlosseri and W. subtorquata. These two non-indigenous species showed the highest percent cover of 4.3% at DH and 6.7% at YP, respectively. In the Korea Strait, the dominant species was P. perforatus at BS with an percent cover of 15.3% and A. aspersa at TY with an percent cover of 8.1%. In the Yellow Sea, dominant species included D. vexillum at BE with a percent cover of 13.6% and Tricellaria occidentalis at IC with a percent cover of 11.1%. In October 2017, the East Sea was dominated by M. galloprovincialis at YP with a percent cover of 6.7%. The Korea Strait was dominated by M. galloprovincialis at BS with a substantial percent cover of 44.6%. In the Yellow Sea, A. amphitrite, a non-indigenous species, was the dominant species at Incheon with a percent cover of 10.8%. The significant presence of M. galloprovincialis in both the East Sea and Korea Strait indicated its strong seasonal adaptation in October 2017. In January 2018, M. galloprovincialis dominated YP in the East Sea with a percent cover of 16.0%. The Korea Strait showed dominance by D. vexillum at YS with an percent cover of 11.3% and Amphibalanus eburneus at TY with a percent cover of 20.6%. In the Yellow Sea, M. galloprovincialis was highly prevalent at MP with a percent cover of 44.4%, while D. vexillum was dominant at BE with a percent cover of 22.2%. In April 2018, the dominant species in the East Sea was D. vexillum at YP and US with percent cover of 20.1% and 11.4%, respectively. The Korea Strait featured A. aspersa as a dominant species at TY with a percent cover of 17.6% and Amphibalanus improvisus at YS with a percent cover of 34.9%. The Yellow Sea’s dominant species included M. galloprovincialis at MP with a percent cover of 46.8% and D. vexillum at BE with an percent cover of 8.2%. Two-way ANOVA of total percent cover of non-indigenous species (grayscale bar in Figure 3; Supplementary Table S3) showed no significant differences among sampling dates (F3,55 = 1.45, p = 0.2423), but revealed significant differences among coastal regions (F2,55 = 4.05, p = 0.0244). Post-hoc Tukey test indicated that the Yellow Sea had significantly higher percent cover of non-indigenous species compared to the Korea Strait (p < 0.05).
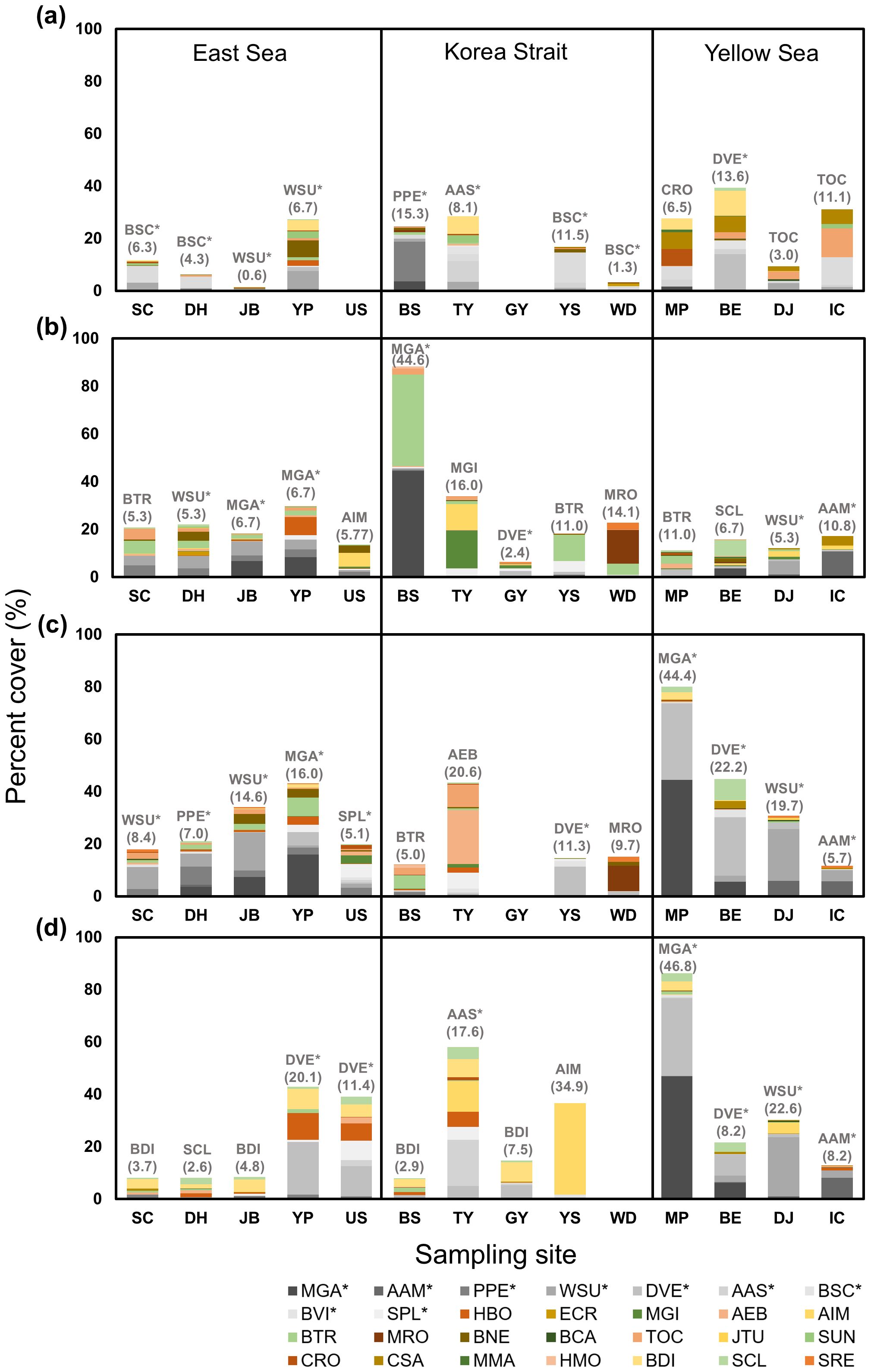
Figure 3. Percent cover (%) of observed species on attachment plates for each sampling site according to sampling date [(A) Summer, 2017-07; (B) Fall, 2017-10; (C) Winter, 2018-01; (D) Spring, 2018-04). Dominant species and their percent cover (in parentheses) are indicated above the bar for each sampling site. Species shown in grayscale and marked with an asterisk (*) indicate non-indigenous species.
Community characteristics of sessile invertebrates were analyzed based on data collected from three coastal regions (East Sea, Korea Strait, and Yellow Sea) at four different time points (July 2017, October 2017, January 2018, and April 2018). For all species combined, means and standard deviations of total abundance, species richness, and Shannon-Wiener Index for each sampling date and region were estimated and tested by two-way ANOVA to determine whether there was a significant difference according to sampling date and region (Figure 4; Supplementary Table S4, S5).
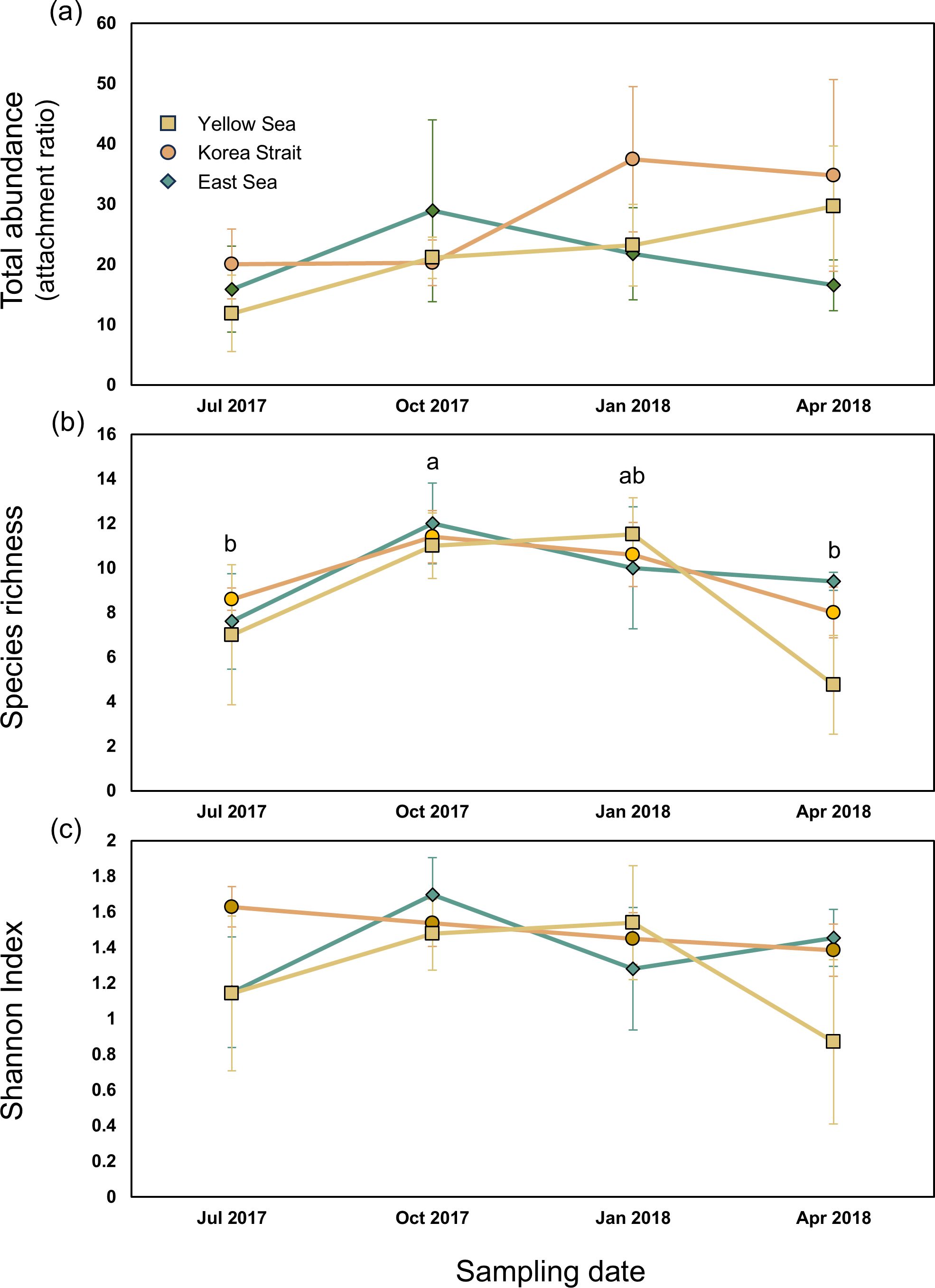
Figure 4. Means and standard errors of total abundance (A), species richness (B), and Shannon index (C) were calculated for each sampling date across the coastal region. A two-way ANOVA revealed a significant difference in species richness among sampling dates (Supplementary Table S3). Groups labelled with the same letter indicate that the group is statistically equivalent (Tukey’s test; p < 0.05).
The mean total abundance of invertebrates varied across sampling dates and regions. The East Sea showed the highest mean abundance (28.8 ± 15.7) in October 2017 and the lowest (16.5 ± 4.2) in April 2018. In the Korea Strait, the highest mean abundance (34.7 ± 15.9) was observed in April 2018 and the lowest (20.3 ± 3.7) was observed in October 2017. The Yellow Sea exhibited the highest mean abundance (29.7 ± 10.0) in April 2018 and the lowest (11.9 ± 6.3) in July 2017. A two-way ANOVA revealed no significant difference in total abundance across sampling dates (F3, 55 = 0.914, p = 0.442) or regions (F2, 55 = 0.769, p = 0.470). Species richness represented by the mean number of species also varied across sampling dates and regions. The East Sea showed the highest species richness (12.0 ± 1.8) in October 2017 and the lowest (9.4 ± 0.9) in April 2018. In the Korea Strait, the highest species richness was recorded at 11.4 ± 1.2 in October 2017 and the lowest one (8.0 ± 1.1) was found in April 2018. The Yellow Sea exhibited the highest species richness (11.5 ± 1.7) in January 2018 and the lowest (4.8 ± 2.2) in April 2018. A two-way ANOVA indicated significant differences in species richness across sampling dates (F3, 55 = 3.784, p = 0.017), while no significant difference was found across regions (F2, 55 = 0.507, p = 0.606). These results indicated that species richness was higher in October 2017, corresponding to the Fall season than in other sampling dates (Figure 4B). The Shannon-Wiener diversity index known to consider both species richness and evenness was analyzed. The East Sea had the highest mean Shannon index (1.5 ± 0.5) in October 2017 and the lowest (1.1 ± 0.3) in July 2017. In the Korea Strait, the highest Shannon index (1.628 ± 0.253) was observed in July 2017 and the lowest one (1.4 ± 0.3) was found in April 2018. The Yellow Sea showed the highest Shannon index (1.5 ± 0.3) in January 2018 and the lowest (0.9 ± 0.5) in April 2018. Two-way ANOVA results showed no significant difference in corrected Shannon index across sampling dates (F3, 55 = 0.862, p = 0.468) or regions (F2, 55 = 0.839, p = 0.439).
In summary, while total abundance and the Shannon-Wiener diversity index did not show significant variation across different sampling dates or regions, species richness was significantly higher in Fall than those from other sampling dates.
According to the season, the three broadly present species were significantly more dominant based on Z-score analysis (Table 2, Supplementary Table S6). The Fall sampling showed an average percent cover (Balanus trigonus) of 3.2% for indigenous species, with a Z-score of 2.15. In contrast, for non-indigenous species, B. schlosseri exhibited a percent cover of 4.8% (Z-score = 2.17) in the summer sampling and M. galloprovincialis showed a percent cover of 6.0% (Z-score = 1.96) in the winter sampling.
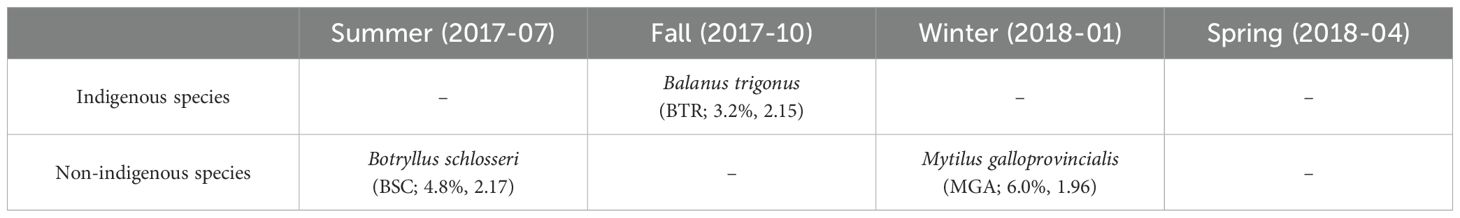
Table 2. Indigenous and non-indigenous species for broadly present species identified using Z-scores across different sampling periods (numbers in parentheses represent percent cover and the Z-score, respectively).
Indigenous and non-indigenous species of regionally dominant species were subjected to Indicator (IndVal) analysis (Table 3). In the East Sea, the indigenous species Halichondria bowerbanki demonstrated a significant IndVal of 0.495. The non-indigenous species P. perforatus showed a notable IndVal of 0.656. The Korea Strait’s data revealed no significant indigenous or non-indigenous species. The Yellow Sea showed significant indigenous species, including Bugulina californica (IndVal: 0.324), Ciona savignyi (IndVal: 0.835), and Molgula manhattensis (IndVal: 0.250). In terms of non-indigenous species, A. amphitrite and D. vexillum had IndVal of 0.480 and 0.420, respectively.
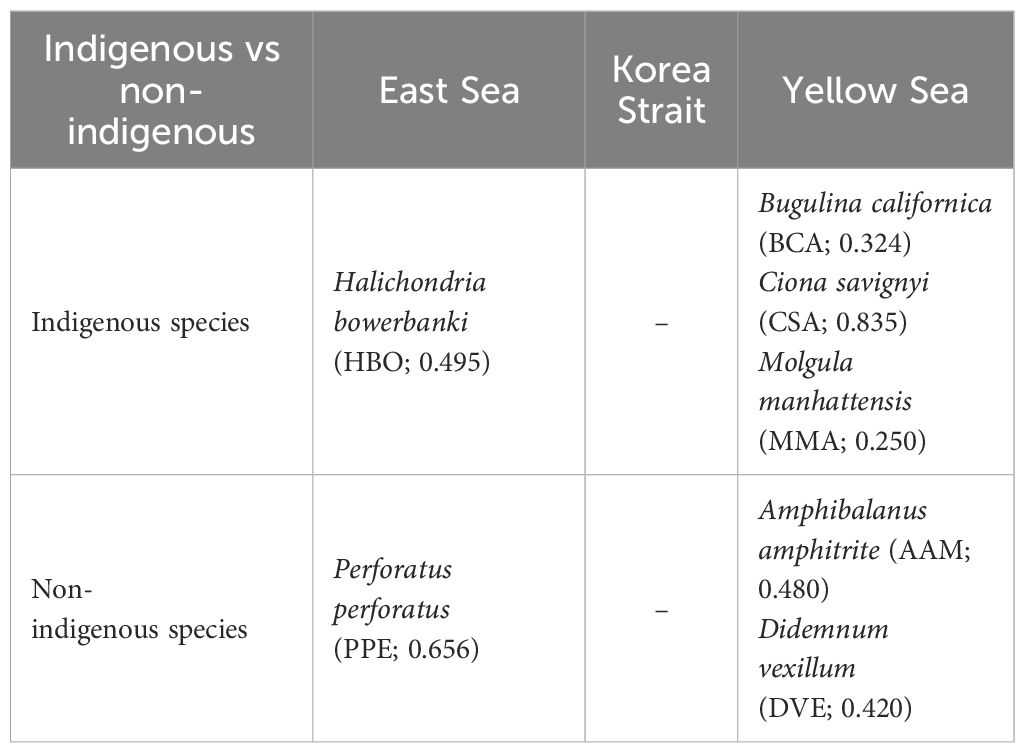
Table 3. Indigenous and non-indigenous species for regionally dominant species with statistically significant IndVal across different coastal regions (p < 0.05, numbers in parentheses represent IndVal values of respective species in each region).
Linear regression analyses were performed to examine the relationship between percent cover (%) of selected non-indigenous species (broadly present species and regionally dominant species) and normalized Shannon-Wiener index (H’) (Figure 5). The 95% confidence intervals for slopes and intercepts of each regression analysis are summarized in Supplementary Table S7.
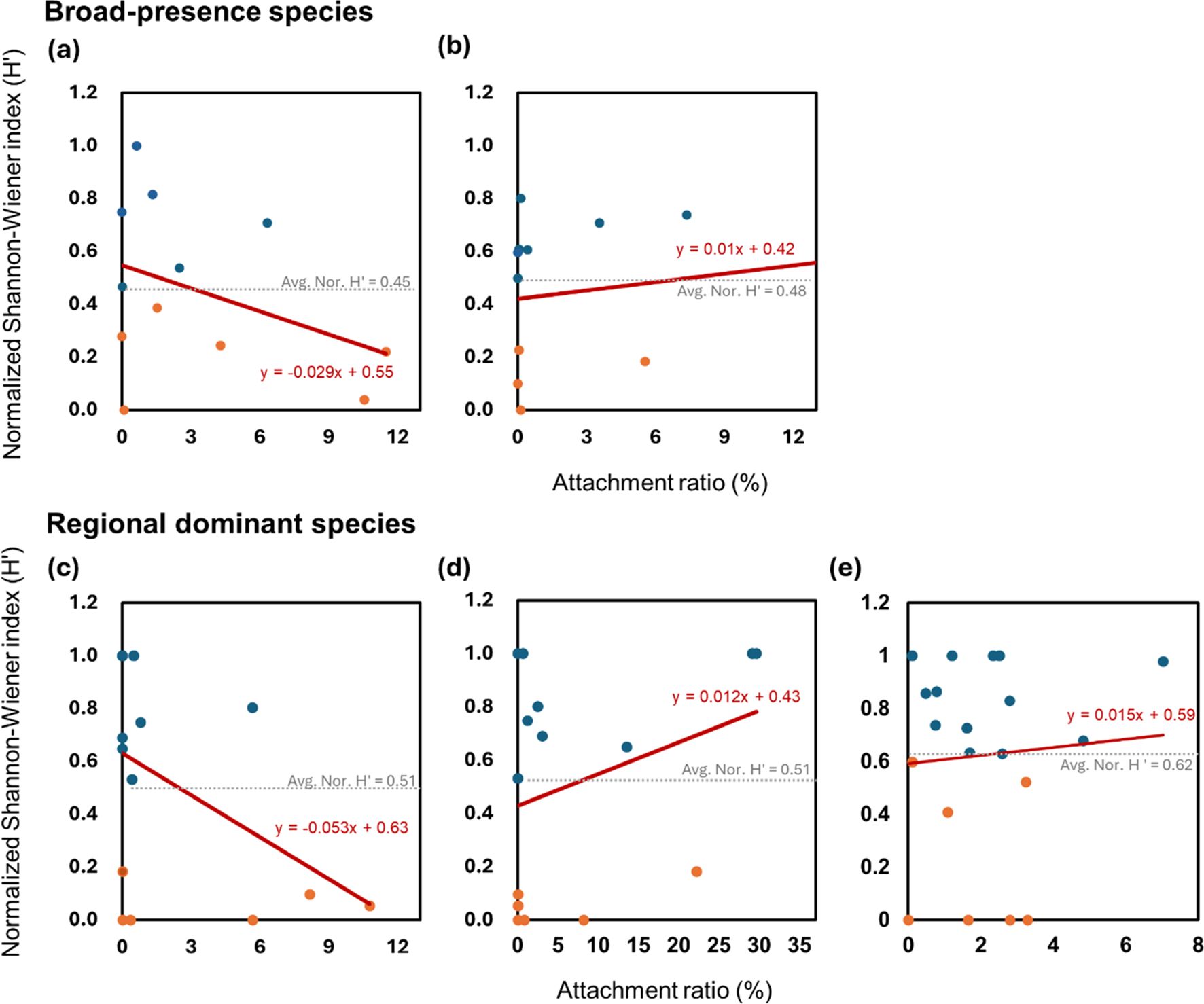
Figure 5. Linear regression analyses examining the relationship between selected non-indigenous species’ percent cover (%) and normalized Shannon-Wiener index (H’). Broadly present species: (A) Botryllus schlosseri (BSC), (B) Mytilus galloprovincialis (MGA); regionally dominant species: (B) Amphibalanus amphitrite (AAM), (D) Didemnum vexillum (DVE), and (E) Perforatus perforatus (PPE). Red lines represent regression lines with their respective equations shown. The horizontal dashed line indicates average of normalized Shannon-Wiener Index (Avg. Nor. H’). Blue and orange dots represent data points higher or lower than Avg. nor. H’, respectively. R² values, confidence intervals for the slope and intercept, and hypothesis test results for the slopes are provided. Detailed confidence intervals are summarized in Supplementary Table S5.
For broadly present species, regression analysis showed that B. schlosseri had a regression line of y = −0.029x + 0.55 with an R2 value of 0.1440 and that M. galloprovincialis had a regression line of y = 0.011x + 0.42 with an R2 value of 0.1830 (Figures 5A, B). B. schlosseri displayed a negative slope. However, statistical analysis indicated that this result was insignificant (t = -1.3005, p = 0.1113). The slope of M. galloprovincialis was not statistically significant either (t = 1.5698, p = 0.9276). For regionally dominant species, the regression analysis for A. amphitrite showed a significant negative correlation with a slope of -0.053 (t = -1.8439; p < 0,0441) (Figure 5C). The equation of the regression line was y = −0.053x+0.63 with an R² value of 0.2073. These results indicate that an increase in the percent cover of A. amphitrite is associated with a significant decrease in the normalized Shannon-Wiener index (Nor. H’). For D. vexillum, the regression analysis resulted in a positive slope of 0.012 (Figure 5D). The equation of the regression line was y = 0.012x + 0.43 with an R² value of 0.0987. Results of t-test revealed that the slope was not negative (t = 1.1930; p = 0.8729) and that the percent cover of D. vexillum was not associated with a decrease in Nor. H’. For P. perforatus, the regression analysis showed a positive slope of 0.015 (Figure 5E). The equation of the regression line was y = 0.015x + 0.59 with an R² value of 0.0053. Similar to D. vexillum, the slope for P. perforatus was not negative either (t = 0.3111; p = 0.6204), indicating no significant negative impact on Nor. H’ from this species.
Sessile invertebrates in coastal regions are often considered an important and stable source of biodiversity within specific marine ecosystems due to their limited mobility within their habitats (Sutherland, 1990). These organisms perform a variety of ecological functions and hold a crucial status within the food web as consumers of organic particles (Yang et al., 2019). Habitats of these sessile invertebrates are essential for completing their life cycles, suggesting that they experience more significant resource limitations than other biological groups, especially concerning non-indigenous species (Grosberg, 1981; Blythe and Pineda, 2009; Sellheim et al., 2010). While adverse impacts of non-indigenous species are widely recognized, recent studies have indicated that impacts of non-indigenous species are often challenging to assess since not all species play detrimental roles in their ecosystems (Charro et al., 2010; Milanović et al., 2020). These finding suggest that consistently monitoring non-native species is crucial to accurately identifying regions and seasons for negative impact of non-indigenous species.
Our study suggests the importance of distinguishing between broadly present species and regionally dominant species when considering the potential ecological impacts of non-indigenous species. Often, studies related to non-indigenous species focus on categorizing the level of risk or predicting their future spread over time (Leidenberger et al., 2015; Ojaveer et al., 2015; Ubagan et al., 2021). Although the three coastal regions of Korea are closely connected, they exhibit different environments (Park et al., 2017). Due to significantly different oceanographic characteristics among these three coastal regions, the Yellow Sea has the lowest salinity and the most significant annual variation in water temperature, while the East Sea shows an opposite trend (Chang et al., 2002; Choi et al., 2009). These trends can potentially result in certain sessile invertebrate species predominantly appearing in specific coastal regions. Despite the environmental differences among these regions, some species are dominant in all of them during particular seasons. Therefore, it is crucial to understand non-indigenous species’ habitats in the area and clearly define the extent of their potential influence to assess their impact in specific regions.
Our findings support several studies suggesting that sessile organisms in marine ecosystems can be relatively stable against environmental changes such as annual climate variation (Smale et al., 2015; Virta et al., 2020). Richness showed slight seasonal variations based on various indices presented in this study and the diversity index did not exhibit significant differences across seasons or regions throughout the year. For sessile organisms, relocation is impossible once they select a habitat, suggesting that they may invest substantial energy to maintain their ecological functions under varying environmental conditions. This energy investment is crucial for maintaining their stability and ensuring their survival. For instance, corals tend to concentrate energy on keeping their body tissues when resource availability is low to survive (Leuzinger et al., 2012). This characteristic of marine sessile organisms implies that they can maintain biodiversity and perform ecosystem functions despite relatively high levels of environmental changes. In this respect, due to their inability to relocate once settled, when non-indigenous species successfully establish and begin to dominate, it becomes extremely challenging to mitigate their negative impact on local biodiversity. For example, the non-indigenous sponge Diplosoma listerianum can negatively impact the survival of native species by occupying more space as the frequency and magnitude of disturbances increase, making ecosystem recovery challenging (Altman and Whitlatch, 2007). This suggests the importance of comprehensive monitoring of invasive species for several key reasons: (1) early detection of potentially harmful non-indigenous species before they become established and cause significant biodiversity loss (Lehtiniemi et al., 2015); (2) identification of areas susceptible to invasion, which is crucial for implementing targeted prevention and management strategies (Chainho et al., 2015; Zabin et al., 2014); (3) evaluation of invasion success rates and spread patterns to predict future invasions (Ubagan et al., 2021); and (4) assessment of management effectiveness through tracking temporal changes in species abundance and distribution (Chainho et al., 2015). Furthermore, as shown in studies like Ávila et al. (2018), without comprehensive monitoring it would be impossible to identify problematic species like A. amphitrite and implement interventions before significant ecological damage occurs. The remarkable success of some non-indigenous species in colonizing new areas, as evidenced by our findings, emphasizes why monitoring must be a cornerstone of invasion management strategies.
Generally, for a specific sessile organism to complete its life cycle, environmental factors affecting it must be within the range that allows for its survival and reproduction (Bates, 2005). This indicates that each species has a different range within which it can spread and influence a particular ecological community. In this study, the different ranges of temperature and salinity observed in the three coastal regions highlight their unique characteristics, leading to the conclusion that distinguishing broadly present species from regionally dominant species can be a highly effective research approach. In results of Z-score, among indigenous species, B. trigonus identified as a broadly present species in Fall is one of the best-known native barnacles. It is widely distributed across all coastal regions of Korea (Ubagan et al., 2021). Additionally, C. savignyi showing the highest IndVal is known to be a well-established indigenous species along the Korean coast (Yi and Kim, 2020). These analysis results suggest that both native species are dominantly present along coasts of Korea, with B. trigonus being widespread nationally and C. savignyi predominantly in the Yellow Sea. This indicates that our approaches can scientifically derive the dominance and main distribution of native species.
The distinction of dominance areas is even crucial for non-indigenous species, which always have the potential to cause negative impacts (Jeschike et al., 2014). In this study, the distinction of dominant areas is crucial for non-indigenous species, as our results showed that dominant non-indigenous species are associated with a significant reduction in biodiversity in specific regions. If their dominance leads to resource competition and a subsequent decrease in biodiversity in specific areas, it becomes essential to define areas where such decreases can occur. Additionally, one-year sampling results of this study revealed that the diversity index remained statistically stable both temporally and spatially. This result enables further analysis. This finding made it possible to examine the correlation between the density of each dominant non-indigenous species and the overall diversity index to determine whether these species are associated with the reduction in biodiversity. This study statistically identified two broadly present species and three regionally dominant species. However, the only species that contributed to a statistically significant decrease in biodiversity was A. amphitrite in the Yellow Sea. A. amphitrite is one of the major sessile marine organisms (barnacle) distributed worldwide. It is known for its high survival rate and rapid growth in various environmental conditions (Ávila et al., 2018). In addition, it exhibits a high filtration rate, which gives it a significant competitive advantage in resource competition (Nakai et al., 2018). Recent related studies have reported that A. amphitrite contributes to regional biodiversity declines in areas such as Mexico, Europe, and South Korea (Torres et al., 2012; Ávila et al., 2018; Rech et al., 2018; Ubagan et al., 2021).
This study provides a basis and approach for prioritizing the management of non-indigenous species. It suggests that A. amphitrite requires focused management due to its potential role in biodiversity decline along the Korean coast. Management efforts should prioritize high-risk areas, such as ports and harbors, which often serve as key entry points for non-indigenous species. Despite these results, there are current limitations in species-specific control methods. Available management approaches such as antifouling coatings (Jin et al., 2014), nitric oxide treatments (Zhang et al., 2015), and surface modifications (Chaw et al., 2011) show significant controlling A. amphitrite. However, these methods affect entire communities rather than targeting specific species, making it difficult to achieve control of individual species. This limitation makes the accumulation of continuous monitoring results even more important. Both evaluating current control methods and assessing future technological solutions require comprehensive long-term monitoring data. Marine ecosystems exhibit substantial heterogeneity in environmental conditions, community compositions, and ecological interactions across different regions and temporal scales. Systematic monitoring across these diverse environments serves multiple crucial purposes: (1) identifying how the impacts of non-indigenous species vary under different environmental conditions, (2) providing baseline data essential for evaluating both current and future control methods, and (3) revealing specific biological patterns and behavioral responses that could inform the development of species-specific management approaches. The effectiveness of any new control methods may vary depending on local environmental conditions and population characteristics.
There are certain limitations that warrant further study. First the three-month exposure period of our attachment plates may not have been sufficient to demonstrate complete succession and thus may not fully reflect competitive interactions regarding space occupancy. This limitation aligns with the findings of Sutherland and Karlson (1977), who emphasized the unpredictable nature of early community development due to variable larval recruitment patterns and the strong inhibitory effects of resident adults on subsequent colonization. They observed that a longer observation period, often exceeding one year, was necessary to capture equilibrium species composition and fully understand the dynamics of competitive interactions. These insights suggest that the three-month exposure may not adequately capture the temporal complexity of marine community succession. Moreover, Arnold and Steneck (2011) showed that substrate succession using attachment plates in coral nursery microhabitats typically progresses from early colonizers to late successional species over extended periods. Our short exposure period might have captured only the early to mid-successional stages of community development. However, even within our one-year sampling period, we found compelling evidence of the impact of A. amphitrite on biodiversity across multiple sampling sites. The dominance of A. amphitrite in the Yellow Sea was associated with significantly lower species diversity than in other regions, even in studies with relatively short exposure periods. Although the observation period was insufficient to capture equilibrium species composition, our findings indicate that the early establishment of A. amphitrite can cause short-term reductions in biodiversity. These results align with Ávila et al. (2018), who investigated A. amphitrite presence on native oyster species in the Gulf of Mexico and found significant spatiotemporal variation in its distribution and abundance patterns within a similar timeframe. They demonstrated that increases in A. amphitrite population density and competitive ability could threaten the stability of oyster populations and other organisms. Similarly, the significant negative correlation between A. amphitrite abundance and biodiversity underscores its potential to disrupt ecosystem stability as an invasive species. Long-term monitoring will be essential to validate these patterns further and provide insights into temporal changes, including the dynamics of early and late successional species. Consequently, additional research is necessary to understand the mechanisms of space occupancy and its role in shaping community structure.
This study provides an analysis of the current status and distribution of sessile invertebrates along coasts of South Korea, with a particular focus on ecological impacts of non-indigenous species. By identifying and classifying dominant species into broadly present and regionally dominant categories, it was possible to assess their influence on biodiversity within their respective dominance ranges. Findings of this study revealed that while some non-indigenous species might interact neutrally or positively with the local ecosystem, others such as A. amphitrite could pose significant threats to biodiversity, especially in regions like the Yellow Sea.
This study supports the idea that the community of sessile invertebrates is a stable source of biodiversity. In addition, the results emphasize the importance of continued monitoring of non-indigenous species to protect biodiversity and ecosystem functions. Understanding the dynamics of invasive species, such as A. amphitrite, and their interactions with native communities is crucial for predicting potential ecosystem disturbances. Although this study focused on the effects of a relatively short exposure period, the findings provide valuable insights into priority species and regions that require attention, further underscoring the need for long-term monitoring. Given the rapid changes occurring in marine environments, proactive biodiversity monitoring is essential for effective responses.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The manuscript presents research on animals that do not require ethical approval for their study.
MU Conceptualization, Investigation, Writing – review & editing. TL: Investigation, Methodology, Writing – review & editing. YK: Formal analysis, Methodology, Writing – review & editing. JL: Formal analysis, Writing – review & editing. HJ: Conceptualization, Validation, Writing – review & editing. Y-SL: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – original draft. SS: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the 'Improvement of management strategies on marine ecosystem disturbing and harmful organisms (No. 20190518)' and 'Monitoring survey on the distribution of disturbing and harmful benthos in the marine ecosystem (2022)' funded by the Ministry of Oceans and Fisheries. Additionally, this research was supported by the Global - Learning & Academic research institution for Master’s·PhD students, and Postdocs (LAMP) Program of the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (No. RS-2023-00301938).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1499607/full#supplementary-material
Agius B. P. (2007). Spatial and temporal effects of pre-seeding plates with invasive ascidians: growth, recruitment and community composition. J. Exp. Mar. Biol. Ecol. 342, 30–39. doi: 10.1016/j.jembe.2006.10.012
Altman S., Whitlatch R. B. (2007). Effects of small-scale disturbance on invasion success in marine communities. J. Exp. Mar. Biol. Ecol. 342, 15–29. doi: 10.1016/j.jembe.2006.10.011
Anderson M., Underwood A. (1994). Effects of substratum on the recruitment and development of an intertidal estuarine fouling assemblage. J. Exp. Mar. Biol. Ecol. 184, 217–236. doi: 10.1016/0022-0981(94)90006-X
Arnold S. N., Steneck R. S. (2011). Settling into an increasingly hostile world: the rapidly closing “recruitment window” for corals. PloS One 6, e28681. doi: 10.1371/journal.pone.0028681
Ávila E., Araujo-Leyva O. R., Rodríguez-Santiago M. A., López-Rosas H. (2018). Alien barnacle Amphibalanus amphitrite epizoic on two native oyster species in the southern Gulf of Mexico: spatio-temporal variability and current status of its epibiosis. Mar. Biol. Res. 14, 581–589. doi: 10.1080/17451000.2018.1459723
Bailey S. A. (2015). An overview of thirty years of research on ballast water as a vector for aquatic invasive species to freshwater and marine environments. Aquat. Ecosyst. Health Manage. 18, 261–268.
Bates W. R. (2005). Environmental factors affecting reproduction and development in ascidians and other protochordates. Can. J. Zool. 83, 51–61. doi: 10.1139/z04-164
Bishop J. D., Wood C. A., Yunnie A. L., Griffiths C. A. (2015). Unheralded arrivals: non-native sessile invertebrates in marinas on the English coast. Aquat. Invasions 10, 249–264. doi: 10.3391/ai.2015.10.3.01
Blythe J. N., Pineda J. (2009). Habitat selection at settlement endures in recruitment time series. Mar. Ecol.-Prog. Ser. 396, 77–84. doi: 10.3354/meps08309
Brunetti R., Gissi C., Pennati R., Caicci F., Gasparini F., Manni L. (2015). Morphological evidence that the molecularly determined Ciona intestinalis type A and type B are different species: Ciona robusta and Ciona intestinalis. J. Zool. Syst. Evol. Res. 53, 186–1193. doi: 10.1111/jzs.12101
Carlton J. T., Ruiz G. M. (2015). “Anthropogenic vectors of marine and estuarine invasions: an overview framework,” in Biological Invasions in Changing Ecosystems: Vectors, Ecological Impacts, Management and Predictions. Ed. Canning-Clode J. (De Gruyter Open Ltd, Warsaw; Berlin), 24–36.
Carver C. E., Mallet A. L., Vercaemer B. (2006). Biological synopsis of the colonial tunicates (Botryllus schlosseri and Botrylloides violaceus) (Nova Scotia, Canada: Bedford Institute of Oceanography Dartmouth).
Chainho P., Fernandes A., Amorim A., Ávila S. P., Canning-Clode J., Castro J. J., et al. (2015). Non-indigenous species in Portuguese coastal areas, coastal lagoons, estuaries and islands. Estuarine Coast. Shelf Sci. 167, 199–211.
Chang K. I., Hogg N. G., Suk M. S., Byun S. K., Kim Y. G., Kim K. (2002). Mean flow and variability in the southwestern East Sea. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 49, 2261–2279. doi: 10.1016/S0967-0637(02)00120-6
Charro E., Gallardo J. F., Moyano A. (2010). Degradability of soils under oak and pine in Central Spain. Eur. J. For. Res. 129, 83–91. doi: 10.1007/s10342-009-0320-4
Chaw K. C., Dickinson G. H., Ang K. Y., Deng J., Birch W. R. (2011). Surface exploration of Amphibalanus amphitrite cyprids on microtextured surfaces. Biofouling 27, 413–422.
Choi B. J., Haidvogel D. B., Cho Y. K. (2009). Interannual variation of the Polar Front in the Japan/East Sea from summertime hydrography and sea level data. J. Mar. Syst. 78, 351–362. doi: 10.1016/j.jmarsys.2008.11.021
Chung M. G., Kang H., Choi S. U. (2015). Assessment of coastal ecosystem services for conservation strategies in South Korea. PloS One 10, e0133856.
Costello M. J., Emblow C., White R. (2001). European register of marine species: a check-list of marine species in Europe and a bibliography of identification guides. Collect. Patrimoines Nat. 50, 1–463.
Costello K. E., Lynch S. A., McAllen R., O’Riordan R. M., Culloty S. C. (2021). The role of invasive tunicates as reservoirs of molluscan pathogens. Biol. Invasions 23, 641–655. doi: 10.1007/s10530-020-02392-5
de Barros R. C., da Rocha R. M., Pie M. R. (2009). Human-mediated global dispersion of Styela plicata (Tunicata, Ascidiacea). Aquat. Invasions 4, 45–57. doi: 10.3391/ai.2009.4.1.4
Dijkstra J., Harris L. G., Westerman E. (2007). Distribution and long-term temporal patterns of four invasive colonial ascidians in the Gulf of Maine. J. Exp. Mar. Biol. Ecol. 342, 61–68. doi: 10.1016/j.jembe.2006.10.015
Dufrêne M. P., Legendre P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67, 345–366. doi: 10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Evcen A., Gözcelioğlu B., Çinar M. E. (2023). New sponge records (Porifera) from the Black Sea. Reg. Stud. Mar. Sci. 65, 103043. doi: 10.1016/j.rsma.2023.103043
Fehlauer-Ale K. H., Winston J. E., Tilbrook K. J., Nascimento K. B., Vieira L. M. (2015). Identifying monophyletic groups within Bugula sensu lato (Bryozoa, Buguloidea). Zool. Scr. 44, 334–347. doi: 10.1111/zsc.12103
Fisk D., Harriott V. (1990). Spatial and temporal variation in coral recruitment on the Great Barrier Reef: implications for dispersal hypotheses. Mar. Biol. 107, 485–490. doi: 10.1007/BF01313433
Grosberg R. K. (1981). Competitive ability influences habitat choice in marine invertebrates. Nature 290, 700–702. doi: 10.1038/290700a0
Guerin G. R., Martín-Forés I., Sparrow B., Lowe A. J. (2018). The biodiversity impacts of non-native species should not be extrapolated from biased single-species studies. Biodivers. Conserv. 27, 785–790. doi: 10.1007/s10531-017-1439-0
Guy-Haim T., Rilov G., Achituv Y. (2015). Different settlement strategies explain intertidal zonation of barnacles in the Eastern Mediterranean. J. Exp. Mar. Biol. Ecol. 463, 125–134. doi: 10.1016/j.jembe.2014.11.010
Costello M. J., Emblow C, White R. (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collect. Patrimoines Natur. 50, 1–463.
Howson C. M., Picton B. E. (1997). The species directory of the marine fauna and flora of the British Isles and surrounding seas Vol. 276 (Belfast, Northern Ireland: Ulster Museum Publication).
Jeschike J. M., Bacher S., Blackburn T. M., Dick J. T. A., Essl F., Evans T., et al. (2014). Defining the impact of non-native species. Conserv. Biol. 28, 1188–1194. doi: 10.1111/cobi.12299
Jin C., Qiu J., Miao L., Feng K., Zhou X. (2014). Antifouling activities of anti-histamine compounds against the barnacle Amphibalanus (= Balanus) amphitrite. J. Exp. Mar. Biol. Ecol. 452, 47–53.
Kerckhof F. (2002). Barnacles (Cirripedia, Balanomorpha) in Belgian waters, an overview of the species and recent evolutions, with emphasis on exotic species. Bull. Kon. Belg. Inst. Natuurwet. Biol. 72, 93–104.
Kim I. (1998). “Illustrated Encyclopedia of Fauna and Flora of Korea,” in Cirripedia, Symbiotic Copepoda, Pycnogonida, vol. 38. (Ministry of Education, Seoul, Korea).
Kim I. (2011). Invertebrate Fauna of Korea: Barnacles (Seoul, Korea: National Institute of Biological Resources, Ministry of Environment).
Kim H. K., Chan B. K., Lee S. K., Kim W. (2020). Biogeography of intertidal and subtidal native and invasive barnacles in Korea in relation to oceanographic current ecoregions and global climatic changes. J. Mar. Biol. Assoc. U.K. 100, 1079–1091. doi: 10.1017/S0025315420001009
Kott P. (1985). The Australian ascidiacea part 1, phlebobranchia and stolidobranchia. Mem Qd Mus. 23, 1–440.
Kott P. (2002). The genus Herdmania Lahille 1888 (Tunicata, Ascidiacea) in Australian waters. Zool. J. Linn. Soc 134, 359–374. doi: 10.1046/j.1096-3642.2002.00009.x
Lee Y. J., Lee T., Kim J., Kim D. G., Shin S. (2022). Community structure of marine benthic invertebrates recruited on artificial substrates in the Korean coast. Korean J. Environ. Biol. 40, 87–98. doi: 10.11626/KJEB.2022.40.1.87
Lee H. G., Yu O. H., Kim S. L., Kang J. H., Shin K. S. (2024). Species composition and distribution of hull-fouling macroinvertebrates differ according to the areas of research vessel operation. J. Mar. Sci. Eng. 12, 613. doi: 10.3390/jmse12040613
LeGresley M. M., Martin J. L., McCurdy P., Thorpe B., Chang B. D. (2008). Non-indigenous tunicate species in the Bay of Fundy, eastern Canada. ICES J. Mar. Sci. 65, 770–774. doi: 10.1093/icesjms/fsn020
Lehtiniemi M., Ojaveer H., David M., Galil B., Gollasch S., McKenzie C., et al. (2015). Dose of truth—monitoring marine non-indigenous species to serve legislative requirements. Mar. Pol. 54, 26–35. doi: 10.1016/j.marpol.2014.12.015
Leidenberger S., Obst M., Kulawik R., Stelzer K., Heyer K., Hardisty A., et al. (2015). Evaluating the potential of ecological niche modelling as a component in marine non-indigenous species risk assessments. Mar. pollut. Bull. 97, 470–487. doi: 10.1016/j.marpolbul.2015.04.033
Lenz M., da Gama B. A., Gerner N. V., Gobin J., Gröner F., Harry A., et al. (2011). Non-native marine invertebrates are more tolerant towards environmental stress than taxonomically related native species: results from a globally replicated study. Environ. Res. 111, 943–952. doi: 10.1016/j.envres.2011.05.001
Leuzinger S., Willis B. L., Anthony K. R. N. (2012). Energy allocation in a reef coral under varying resource availability. Mar. Biol. 159, 177–186. doi: 10.1007/s00227-011-1797-1
Liu J. Y. (2008). Checklist of marine biota of China seas (Beijing, China: China Science Press), 1267.
Locke A., Carman M., Lambert G. (2009). Adventures of a sea squirt sleuth: unraveling the identity of Didemnum vexillum, a global ascidian invader. Aquat. Invasions 4, 5–28.
Long H. A., Grosholz E. D. (2015). Overgrowth of eelgrass by the invasive colonial tunicate Didemnum vexillum: consequences for tunicate and eelgrass growth and epifauna abundance. J. Exp. Mar. Biol. Ecol. 473, 188–194. doi: 10.1016/j.jembe.2015.08.014
Lozano V., Chapman D. S., Brundu G. (2017). Native and non-native aquatic plants of South America: comparing and integrating GBIF records with literature data. Manage. Biol. Invasion 8, 443–454. doi: 10.3391/mbi.2017.8.3.18
Lynch S. A., Darmody G., O’Dwyer K., Gallagher M. C., Nolan S., McAllen R., et al. (2016). Biology of the invasive ascidian Ascidiella aspersa in its native habitat: Reproductive patterns and parasite load. Estuar. Coast. Shelf Sci. 181, 249–255. doi: 10.1016/j.ecss.2016.08.048
Marchini A., Ferrario J., Sfriso A., Occhipinti-Ambrogi A. (2015). Current status and trends of biological invasions in the Lagoon of Venice, a hotspot of marine NIS introductions in the Mediterranean Sea. Biol. Invasions 17, 2943–2962. doi: 10.1007/s10530-015-0922-3
Milanović M., Knapp S., Pyšek P., Kühn I. (2020). Linking traits of invasive plants with ecosystem services and disservices. Ecosyst. Serv. 42, 101072. doi: 10.1016/j.ecoser.2020.101072
Nakai S., Shibata J. Y., Umehara A., Okuda T., Nishijima W. (2018). Filtration rate of the ascidian Ciona savignyi and its possible impact. Thalassas 34, 271–277. doi: 10.1007/s41208-017-0061-y
Nydam M. L., Harrison R. G. (2007). Genealogical relationships within and among shallow-water Ciona species (Ascidiacea). Mar. Biol. 151, 1839–1847. doi: 10.1007/s00227-007-0617-0
Ojaveer H., Galil B. S., Campbell M. L., Carlton J. T., Canning-Clode J., Cook E. J., et al. (2015). Classification of non-indigenous species based on their impacts: considerations for application in marine management. PLoS. Biol. 13, e1002130. doi: 10.1371/journal.pbio.1002130
Park C., Kim S. T., Hong J. S., Choi K. H. (2017). A rapid assessment survey of invasive species of macrobenthic invertebrates in Korean waters. Ocean Sci. J. 52, 387–395. doi: 10.1007/s12601-017-0024-5
Park J., Song S. J., Ryu J., Kwon B. O., Hong S., Bae H., et al. (2014). Macrozoobenthos of Korean tidal flats: A review on species assemblages and distribution. Ocean Coast. Manage. 102, 483–492. doi: 10.1016/j.ocecoaman.2014.07.019
R Core Team. (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/.
Rech S., Salmina S., Pichs Y. J. B., García-Vazquez E. (2018). Dispersal of alien invasive species on anthropogenic litter from European mariculture areas. Mar. pollut. Bull. 131, 10–16. doi: 10.1016/j.marpolbul.2018.03.038
Ritter W. E., Forsyth R. H. (1917). Ascidian of the littoral zone of southern California. Univ. California Publ. Zool. 16, 439–512.
Ryu S.-H., Jang K.-H., Choi E.-H., Kim S.-K., Song S.-J., Cho H.-J., et al. (2012). Biodiversity of marine invertebrates on rocky shores of Dokdo, Korea. Zool. Stud. 51, 710–726.
Sarà M. (1986). Sessile macrofauna and marine ecosystem. Ital. J. Zool. 53, 329–337. doi: 10.1080/11250008609355518
Schneider C. A., Rasband W. S., Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Schuchert P. (2010). The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Capitata part 2. Rev. suisse zool. 117, 337–555. doi: 10.5962/bhl.part.117793
Scott Z. R. (2020). The effect of an invasive foundation species on diversity is due to increased, habitat availability. J. Exp. Mar. Biol. Ecol. 528, 151384. doi: 10.1016/j.jembe.2020.151384
Sellheim K., Stachowicz J. J., Coates R. C. (2010). Effects of a nonnative habitat-forming species on mobile and sessile epifaunal communities. Mar. Ecol.-Prog. Ser. 398, 69–80. doi: 10.3354/meps08341
Seo J. E. (2005). Illustrated Encyclopedia of Fauna and Flora of Korea Vol. 40 (Seoul: Ministry of Education and Human Resources Development), 330–331.
Seo K. S., Lee Y. (2009). “A First Assessment of Invasive Marine Species on Chinese and Korean Coasts,” in Biological Invasions in Marine Ecosystems. Ecological Studies, vol. 204 . Eds. Rilov G., Crooks J. A. (Berlin, Heidelberg, Springer). doi: 10.1007/978-3-540-79236-9_32
Shannon C. E. (1948). A mathematical theory of communication. Bell Syst. Tech. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Smale D. A., Yunnie A. L., Vance T., Widdicombe S. (2015). Disentangling the impacts of heat wave magnitude, duration and timing on the structure and diversity of sessile marine assemblages. PeerJ 3, e863. doi: 10.7717/peerj.863
Stachowicz J. J., Bruno J. F., Duffy J. E. (2007). Understanding the effects of marine biodiversity on communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 38, 739–766. doi: 10.1146/annurev.ecolsys.38.091206.095659
Stachowicz J. J., Whitlatch R. B., Osman R. W. (1999). Species diversity and invasion resistance in a marine ecosystem. Science 286, 1577–1579. doi: 10.1126/science.286.5444.1577
Sutherland J. P. (1990). Perturbations, resistance, and alternative views of the existence of multiple stable points in nature. Am. Nat. 136, 270–275. doi: 10.1086/285097
Sutherland J. P., Karlson R. H. (1977). Development and stability of the fouling community at Beaufort, North Carolina. Ecol. Monogr. 47, 425–446.
Taylor P. D., Monks N. (1997). A new cheilostome bryozoan genus pseudoplanktonic on molluscs and algae. Invertebr. Biol. 116, 39–51. doi: 10.2307/3226923
Torres P., Costa A. C., Dionísio M. A. (2012). New alien barnacles in the Azores and some remarks on the invasive potential of Balanidae. Helgoland Mar. Res. 66, 513–522. doi: 10.1007/s10152-011-0287-7
Trott T. J. (2004). Cobscook Bay inventory: a historical checklist of marine invertebrates spanning 162 years. Northeast. Nat. 11, 261–324. doi: 10.1656/1092-6194(2004)11[261:CBIAHC]2.0.CO;2
Turner A. M., Trexler J. C. (1997). Sampling aquatic invertebrates from marshes: evaluating the options. J. N. Am. Benthol. Soc. 16, 694–709. doi: 10.2307/1468154
Ubagan M. D., Lee Y. S., Lee T., Hong J., Kim I. H., Shin S. (2021). Settlement and recruitment potential of four invasive and one indigenous barnacles in South Korea and their future. Sustainability 13, 634. doi: 10.3390/su13020634
Vethaak A. D., Cronie R. J. A., van Soest R. (1982). Ecology and distribution of two sympatric, closely related sponge species, Halichondria panicea (Pallas 1766) and H. bowerbanki Burton 1930 (Porifera, Demospongiae), with remarks on their speciation. Bijdragen tot dierkunde 52, 82–102. doi: 10.1163/26660644-05202002
Vilà M., Hulme P. E. (2017). “Non-native Species, Ecosystem Services, and Human Well-Being,” in Impact of Biological Invasions on Ecosystem Services. Invading Nature - Springer Series in Invasion Ecology, vol. 12 . Eds. Vilà M., Hulme P. (Springer, Cham). doi: 10.1007/978-3-319-45121-3_1
Virta L., Soininen J., Norkko A. (2020). Stable seasonal and annual alpha diversity of benthic diatom communities despite changing community composition. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00088
Warner R. A. (2016). Chapter 2 - Using Z Scores for the Display and Analysis of Data. Optimizing the Display and Interpretation of Data, 7–51. Boston: Elsevier. doi: 10.1016/B978-0-12-804513-8.00002-X
Whalan S., Abdul Wahab M. A., Sprungala S., Poole A. J., de Nys R. (2015). Larval settlement: the role of surface topography for sessile coral reef invertebrates. PloS One 10, e0117675. doi: 10.1371/journal.pone.0117675
Williams L., Matthee C. A., Simon C. A. (2016). Dispersal and genetic structure of Boccardia polybranchia and Polydora hoplura (Annelida: Spionidae) in South Africa and their implications for aquaculture. Aquaculture 465, 235–244. doi: 10.1016/j.aquaculture.2016.09.001
Winston J. E., Maturo F. J. Jr. (2009). “Bryozoans (Ectoprocta) of the Gulf of MEXICO,” in Gulf of Mexico: Its origins, waters, and biota, vol. 68 . Eds. Felder D. L., Camp D. K. (Texas A&M University Press, College Station, TX), 1147–1164.
Yang Q., Zhang W., Franco C. M. M. (2019). “Response of Sponge Microbiomes to Environmental Variations,” in Symbiotic Microbiomes of Coral Reefs Sponges and Corals. Ed. Li Z. (Springer, Dordrecht). doi: 10.1007/978-94-024-1612-1_11
Yi C. H., Kim W. (2020). Assessing cryptic Invasion state: fine-scale genetic analysis of Ciona savignyi population in putative native habitat of the Korean coast. Ocean Sci. J. 55, 99–113. doi: 10.1007/s12601-019-0041-7
Yu C., Kim S., Hong J. S., Choi K. H. (2021). The occurrence of two non-indigenous Conopeum (Bryozoa: Cheilostomata) species in the coastal waters of South Korea. Aquat. Invasions 16, 281–296. doi: 10.3391/ai.2021.16.2.05
Zabin C. J., Ashton G. V., Brown C. W., Davidson I. C., Sytsma M. D., Ruiz G. M. (2014). Small boats provide connectivity for nonindigenous marine species between a highly invaded international port and nearby coastal harbors. Manage. Biol. Invasions 5, 97–112.
Keywords: biodiversity, invasive species, Korean coastal region, species richness, community characteristics
Citation: Ubagan MD, Lee T, Kim Y, Lee J, Jeong H, Lee Y-S and Shin S (2025) Evaluating the ecological impacts of dominant non-indigenous sessile invertebrates in peninsular coastal ecosystems. Front. Mar. Sci. 12:1499607. doi: 10.3389/fmars.2025.1499607
Received: 21 September 2024; Accepted: 06 February 2025;
Published: 26 February 2025.
Edited by:
James Scott Maki, Marquette University, United StatesReviewed by:
Daniel Rittschof, Duke University, United StatesCopyright © 2025 Ubagan, Lee, Kim, Lee, Jeong, Lee and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Sik Lee, eXVuc2lrbGVlQHB1c2FuLmFjLmty; Sook Shin, c2hpbnNAc3l1LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.