- 1Frontiers Science Center for Deep Ocean Multispheres and Earth System and Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao, China
- 2Laboratory for Marine Ecology and Environmental Science, Qingdao Marine Science and Technology Center, Qingdao, China
Antibiotic resistance genes (ARGs) may have significant impacts on human health and ecosystems. Airborne ARGs are reportedly widely distributed across inland cities, but little is known about their abundance in marine atmospheres. Here, we report observations of ambient ARGs during a cruise over the marginal seas of the Bohai Sea and Yellow Sea and compare them with ARGs in the coastal atmosphere. We characterized the ARGs in terms of their abundance, occurrence, degradation, and risk in the marine and coastal atmospheres. Using Na+ and Ca2+ as indicators of marine and continental aerosol sources, respectively, we quantified the mutual transport of airborne ARGs. Our results revealed that the airborne ARG abundances and the number of ARG types increased concomitantly with the mass concentrations of particulate matter because of the accumulation effect, but the ratios of ARG abundance/particulate matter concentration gradually decreased. The inconsistent trend suggested that the reduction in airborne ARGs was greater than their increase with bacterial reproduction during their accumulation and transport in the atmosphere. In addition, the number of ARG types in marine aerosols was greater than that in coastal aerosols. However, the airborne ARG abundance in marine aerosols was greater than that in clean coastal aerosols but not in polluted coastal aerosols. Some ARG types detected in marine aerosols were significantly and positively correlated with wind speed and relative humidity, implying that they may be derived from marine emissions, whereas the other ARGs are likely derived from long-range continental transport. Sea-derived airborne ARGs serve as important sources in coastal aerosols, but their contributions decrease with increasing air pollution levels. Our findings highlight the complex role of marine aerosols as both potential sources and reservoirs of airborne ARGs and highlight the critical importance of investigating the transport dynamics and variation mechanism during the long-range transport of ARGs.
1 Introduction
The widespread presence of antibiotic resistance genes (ARGs) in various environments has emerged as a critical issue in global public health (Zheng et al., 2021; Hernando-Amado et al., 2019; Martínez et al., 2015; Zainab et al., 2020). Approximately 0.7 million people reportedly die due to antibiotic resistance every year worldwide, and the number is projected to reach 10 million by 2050 (Willyard, 2017). ARG pollution caused by the overuse and mismanagement of antibiotics poses a major threat not only to human health but also to the ecosystem (Li et al., 2023; Sathicq et al., 2021). For example, marine environments are particularly vulnerable to ARG pollution, as coastal ecosystems often receive wastewater effluent and runoff containing ARGs, which can disrupt marine biodiversity (Ohore et al., 2023; Siri et al., 2023). Studies have detected ARGs in different environmental media (soil, waterbodies, sediment, and aerosols) and regions in recent years (Lu et al., 2022; Makkaew et al., 2021; Agarwal et al., 2023). The long-range transport of airborne ARGs is a major concern.
Atmospheric aerosols are generally considered important carriers of bacteria and ARGs, which may pose additional health risks together with traditional toxic substances to humans (Hu et al., 2018; Li et al., 2018; Xie et al., 2019). As potential reservoirs of ARGs, atmospheric bioaerosols released from natural and anthropogenic sources may spread to distant areas (Wooten et al., 2019; Wu et al., 2020; Zhang et al., 2018). During the transport of bioaerosols in the atmosphere, meteorological conditions and chemical components of particulate matter are proposed to affect the spread and evolution of airborne bacteria and the associated ARGs (Ouyang et al., 2020; Santarpia et al., 2013; Xu et al., 2017), whereas mobile genetic elements (MGEs), including plasmids, integrons and prophages, reportedly promote the horizontal gene transfer (HGT) of ARGs among different bacteria, which makes them widespread in the environment and further affects human health (Ben et al., 2019; Martínez et al., 2015). The transport of airborne ARGs to downwind regions has also been proposed to affect local aquatic ecosystems through dry or wet sedimentation (Ahmed et al., 2018; Zhang et al., 2022). Technically, a major challenge that persists is to effectively reduce the spread and abundance of ARGs in ambient environments (Liu et al., 2022). Moreover, in the process of long-distance transport, ARGs may be degraded and inactivated through oxidation, photolysis, and other processes (Meng and Zhao, 2012), in addition to amplification via bacterial proliferation and HGT. However, little is known about the relative importance of a series of proliferation, diffusion, and degradation processes of ARGs that occur through long-range transport.
Oceans constitute two-thirds of the Earth. In recent decades, an increasing number of people have migrated from inland areas to coastal areas for better natural and economic environments (Merkens et al., 2016; Qi et al., 2021). To satisfy the increasing demand for seafood, antibiotic-dependent mariculture has developed rapidly in developing countries (Luthman et al., 2024; Shao et al., 2021). Many studies have revealed the abundance of ARGs in different water and sediment samples (Su et al., 2020; Zago et al., 2020; Kang et al., 2022). ARGs in seawater may theoretically be released into the atmosphere via sea spray aerosols and potentially be transported to downwind marine atmospheres or the coastal atmosphere via land-sea breezes. The sea spray aerosols generated by the interaction between wind and waves are largely affected by meteorological parameters, such as the wind speed (Lewis and Schwartz, 2004). Liang et al. (2020) measured aerosols at a seaside observatory and hypothesized that the abundance and composition of airborne ARGs around the Pearl River Estuary were affected by both marine and land sources. However, knowledge of airborne ARGs in the marine atmosphere over marginal seas that are far from coastlines is virtually nonexistent. The key questions need to be addressed, 1) What types of airborne ARGs are dominantly derived from marine sources? 2) Do marine airborne ARGs amplify or decay during long-range transport?
In this study, we aimed to characterize and compare ARGs in marine and coastal atmospheric aerosols by measuring the abundance of ARGs via real-time qPCR. Multiple correlation analysis techniques have been used to explore the origins, evolution, influencing factors and long-range transport of ARGs to address the abovementioned knowledge gaps. Moreover, the inhalation exposure doses of ARGs were calculated to evaluate the risk to humans in these two environments.
2 Materials and methods
2.1 Sampling of the coastal and marine atmospheres
A total of 40 samples of total suspended particles (TSP) were collected at an observation site in the Laoshan Campus of Ocean University of China (OUC), which is located in Qingdao, a coastal megacity in northern China (36°06′N, 120°33′E), between December 2019 and June 2020 (Figure 1). This site is located at least 5 km away from large anthropogenic ARG sources, such as wastewater treatment plants, breeding farms and sea farms, and is 7 km away from the coastal line of the Yellow Sea. The nearest mariculture farm to the sampling point is a shrimp farm, located 10 km away from the sampling site. In addition, there is a dairy farm located 10 km from the sampling point. The 24 h TSP samples were collected on quartz microfiber filters (8×10 in2, GE Whatman Inc., England) via a high-volume TSP sampler (Qingdao Laoying Co., Ltd., China) at a flow rate of 1.05 m3/min.

Figure 1. Map of the coastal sampling site and the cruise route (each sample is marked on the route where it was collected; the red dot represents the coastal campus sampling site in Qingdao, the blue dot represents the location where Marine 1 and Marine 2 were sampled and the shallow and dark blue lines represent the samples from Maine 3 to Marine 13 along the cruise route).
From May to June, 2020, 13 TSP samples were collected over the Yellow Sea and Bohai Sea during the cruise onboard R/V LanHai101 (Figure 1). The samples marked Marine 1 and Marine 2 were collected at the rural port of Qingdao in the Yellow Sea. Marine 3 - Marine 9 and Marine 11 - Marine 12 samples were collected over the Yellow Sea and the Bohai Sea, respectively. Marine 10 and Marine 13 samples were collected across the Yellow Sea and Bohai Sea (Supplementary Table S1). An identical TSP sampler as that employed at the coastal site on the Laoshan Campus of OUC was placed at the front of the compass deck. For the marine samples, the sampling was performed only when the research vessel was sailing to minimize the probability of contamination from the self-vessel stack and onboard human interferences. Each marine sample was collected for approximately 20 h, considered the suspension time during vessel stop, it usually covered 24 h. Detailed descriptions of the sample pretreatment, chemical analysis (Shi et al., 2020) and DNA extraction (Jiang et al., 2015) procedures are presented in Supplementary Text S1.
2.2 Analyses of ARGs and the chemical composition of aerosols
PCR was used to detect 15 ARG types associated with 7 types of major antibiotics: tetracyclines (tetW, tetL, and tetA), sulfonamides (sul1, sul2, and sul3), macrolides (ermB and ermC), β-lactams (bla-TEM1, bla-CMY2, and bla-NDM1), quinolones (qnrB and qnrS), aminoglycosides (strB) and vancomycin (vanA) (Li et al., 2018; Lv et al., 2020; Zhao et al., 2018). However, the abundance of ARGs related to quinolones (qnrB, qnrS, and vanA) was too low to be quantified. Supplementary Table S2 lists the information about the primer sets used in the present study. In addition, to facilitate the analysis of ARGs, we also detected one mobile genetic element (MGE), intI1, because it is commonly used to assess the potential horizontal transfer of ARGs and the influence of anthropogenic sources. Additionally, the abundance of 16S rRNA was measured as it serves as a marker for bacterial abundance and helps to evaluate the proportion of bacteria carrying ARGs. A 7500 Real-Time qPCR System (Applied Biosystems Inc., America) was used to quantify the 12 detected types associated with 5 major antibiotics (excluding the undetected quinolone and vancomycin), 1 MGE (intI1) and 16S rRNA. Detailed information on the primers and qPCR conditions for each ARG is listed in Supplementary Table S2. Detailed information on the qPCR system is provided in Supplementary Text S2.
The chemical composition was analyzed via a Dionex ICS-6000 ion chromatograph. Anions (F-, Cl-, NO3-, SO42-, and PO43-) were analyzed via an AS11 column, whereas a CS12A column was used to determine cations (NH4+, Na+, K+, Ca2+, and Mg2+) (Supplementary Figure S1). The detection limits, precisions and recoveries were listed in our previous studies (Qi et al., 2018).
2.3 Meteorological and air quality data
The meteorological data at the coastal site were measured via a real-time meteorological station at the same location as those used for the TSP sampling, whereas those over the Yellow Sea and Bohai Sea were measured via an onboard real-time meteorological station (Supplementary Table S3). The air quality data and the concentrations of air pollutants were obtained from the Qingdao Environmental Protection Bureau (http://hbj.qingdao.gov.cn/slairhour.aspx) (Supplementary Table S4). According to the air quality index (AQI), the TSP samples collected at the coastal site were divided into three categories: clean Coastal-C (AQI<50), lightly polluted Coastal-L (50≤AQI<75), and polluted Coastal-P (AQI≥75).
2.4 Statistical analysis
Spearman’s rank correlation coefficients between environmental factors and ARGs were calculated via SPSS Statistics (version 19.0) (IBM, Armonk, NY, USA). The sampling route was drawn by Ocean Date View (Schlitzer, 2021). The co-occurrence and contributor networks based on Spearman’s correlation coefficients were visualized using Gephi (version 0.9.1) (Bastian et al., 2009). The heatmap of ARG abundance in different samples and Spearman’s correlation coefficients were generated via R (version 4.0.2) (Revolution Analytics, Mountain View, CA, USA) with the pheatmap package. The prcomp function in R was used for principal coordinate analysis (PCoA) of the regional differences in ARGs, and the PCoA diagram was drawn via the ggbioplot package. A redundancy analysis (RDA) between environmental factors and ARGs was performed via CANOCO (version 4.5) (Gilliam and Saunders, 2003). Welch’s t test was performed with STAMP (version 2.1.3) and was used to compare the differences in ARG abundances between coastal and marine samples at the 95% confidence level (Parks et al., 2014). The NOAA HYSPLIT-4 model (Hybrid Single-Particle Lagrangian Integrated Trajectory Model, http://ready.arl.noaa.gov/HYSPLIT.php) was used to identify and cluster the transport pathways of the coastal and marine aerosol samples; 24 h backward trajectories with starting levels of 500 m, 1000 m and 2000 m a.s.l. were calculated.
2.5 Human exposure risk
The respiratory intake of ARGs in humans exposed to the atmosphere was calculated to assess the human risk of exposure to airborne ARGs in coastal and marine environments in China. In accordance with the human health evaluation manual (Part A) and supplemental guidance for inhalation risk assessment (Part F) (USEPA, 2002), the ARG daily intake (ADI) was estimated to assess the risks posed by ARGs via inhalation and ingestion (Nie et al., 2018). The following equation was used to calculate the ADI of the target gene:
CARGs indicate the concentration of ARGs in aerosols (copies/m3). The respiratory intake targets were divided into children, adult males, and adult females. The inhalation rate (IR), average weight (BW), exposure rate (EF), exposure duration (ED) and average life expectancy time (AT) were determined according to the China Population Exposure Factor Handbook (Ministry of Ecology and Environment of the People's Republic of China, 2014), the human health evaluation manual (Part A) and supplemental guidance for inhalation risk assessment (Part F) (USEPA, 2002). Detailed descriptions of the parameters are provided in Supplementary Text S3.
3 Results
3.1 Overview of ARG abundance in marine and coastal aerosols
The heatmap of ARGs, MGE and 16S rRNA abundances in each sample is shown in Figure 2. β-lactam (bla-TEM1, bla-CMY2) and tetracycline (tetA, tetL, and tetW) resistance genes were the most detected genes, with abundance of 104 - 105 copies/m3. The abundance of intI1 varied by approximately 1.30×104 ± 1.69×104 (average ± standard deviation) copies/m3 and was lower than that of the β-lactam and tetracycline resistance genes detected in this study. Additionally, subject to β-lactam ARGs, the abundance of bla-NDM1 was approximately 2-3 orders of magnitude lower than that of bla-TEM1 and bla-CMY2. The macrolide resistance genes ermC and ermB were present at lower levels of 103 and 104 copies/m3, respectively. Even lower abundances were detected for sul2 and sul3, which were present at 103 and 101 copies/m3, respectively. However, the abundance of sul1 remained at the level of 104 copies/m3. The abundances of strB in the marine aerosols were similar to those of sul3, whereas those in the coastal aerosols were comparable to those of tetW. When the ARGs in the marine and coastal aerosols were examined separately, the proportions of bla-NDM1, bla-CMY2, sul2, and tetA were significantly greater in the marine aerosols than in the coastal aerosols (p<0.01). In contrast, the proportions of strB, sul1, sul3, tetL, and tetW were significantly greater in coastal aerosols (p<0.01).
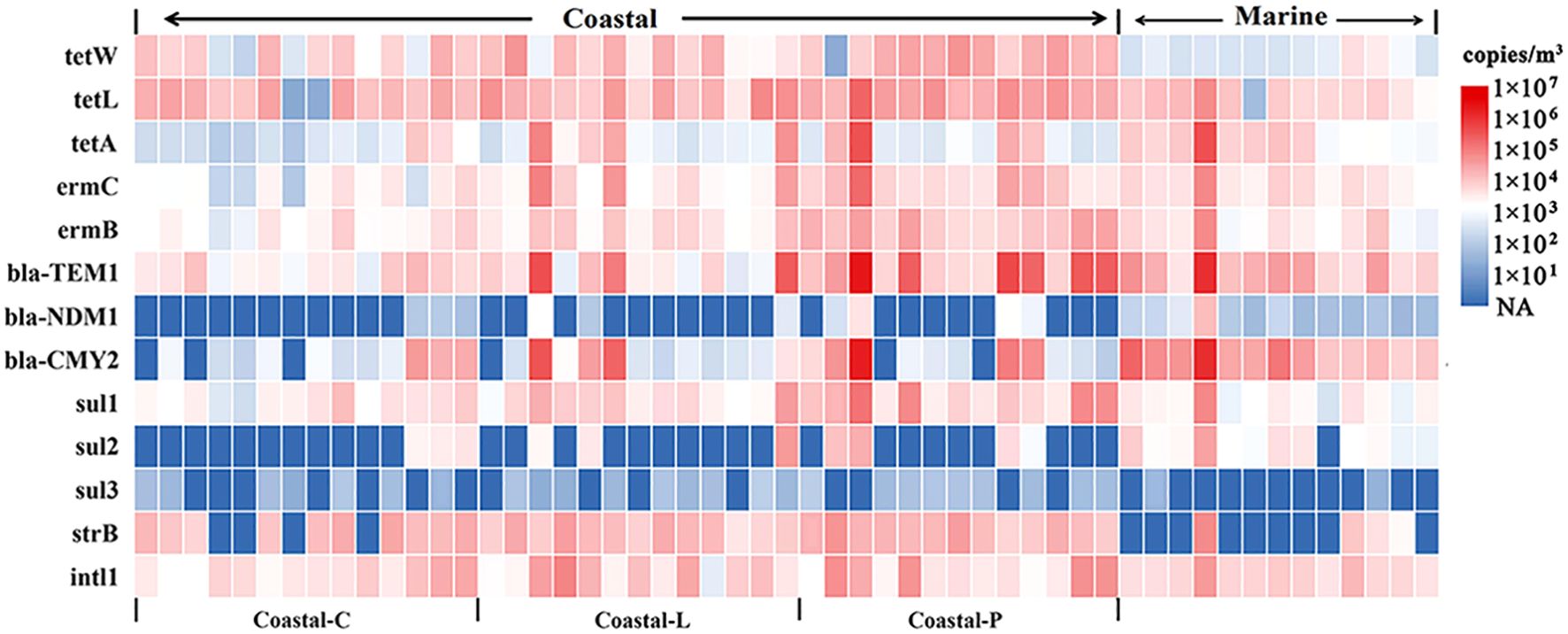
Figure 2. Abundances of ARGs and intI1 in marine and coastal aerosols (Coastal clean group (Coastal-C) (AQI<50), Coastal lightly polluted group (Coastal-L) (50≤AQI<75), and Coastal polluted group (Coastal-P) (AQI≥75)) (13 marine aerosol samples and 40 coastal aerosol samples were analyzed).
3.2 Analysis of the composition of ARGs in coastal and marine aerosols
We further compared the composition of the analyzed ARGs in coastal and marine aerosols (Figure 3). In 17 of the 40 coastal aerosol samples, the sum of the tetracycline resistance genes tetW and tetL accounted for more than 50% of the total analyzed ARG abundances (here after referred to as total ARGs). However, the percentages of these two ARGs were always less than 24% in the 13 marine aerosol samples. The sum of the β-lactam resistance genes bla-TEM1 and bla-CMY2 accounted for over 50% of the total ARGs in only 10 of the 40 coastal aerosol samples. In contrast, the percentages of those two ARGs exceeded 57% in 11 of the 13 marine aerosol samples. An appreciable percentage of strB was detected in most of the coastal aerosol samples, but it was generally negligible in the marine aerosols, except for three samples collected over the Bohai Sea. The composition patterns of ARGs in marine and coastal aerosols differ (Supplementary Figure S2). As presented in Section 3.3, RDA was conducted to establish the link between different ARGs in coastal and marine aerosols and environmental factors.

Figure 3. Proportions of different ARGs in coastal and marine aerosols (percentages of each ARG among the total ARGs; 13 marine aerosol samples and 40 coastal aerosol samples were analyzed).
Welch’s t test was then used to examine the differences in the proportion of each ARG among the total ARGs between coastal and marine aerosols. Only the differences with p<0.05 are shown in Supplementary Figure S3, where the left panel shows the mean proportion and the standard deviation. In the right panel of Supplementary Figure S3, it depicts the differences in abundance of ARGs between the groups, with error bars representing the 95% confidence intervals of the differences. The largest negative difference was observed for bla-CMY2, suggesting that bla-CMY2 may be uniquely associated with the use of antibiotics in marine mariculture. This will be discussed in Section 4.1. A moderately larger positive difference existed for tetL, strB and tetW. These three ARGs may be associated mainly with the use of antibiotics on continents. Although the remaining five ARGs were significantly different between coastal and marine aerosols, the absolute values of the differences were small. In the third case, ambient factors may overwhelm the use of antibiotics and cause differences, as analyzed in Sections 3.3 and 4.1.
In the co-occurrence network of different ARGs in coastal aerosols (Figure 4A), most ARGs were significantly associated with macrolide resistance genes, followed by β-lactam resistance genes, tetracycline resistance genes, and sulfonamide resistance genes. A significant correlation was not detected between the aminoglycoside resistance gene strB and most ARGs, which is consistent with the limited use of aminoglycoside antibiotics for decades. The macrolide resistance genes ermC and ermB were relatively low in abundance (Figure 2) but exhibited a significant correlation with most ARG types. In addition, tetW, tetA, sul1, and sul2 were significantly correlated with intI1, suggesting high potentially high activities in horizontal gene transfer of those ARGs as discussed in Section 4.1. Similarly, ermC was significantly correlated with most ARGs in marine aerosols, followed by the β-lactam resistance gene bla-CMY2, the tetracycline resistance gene tetA and the sulfonamide resistance gene sul2 (Figure 4B). In contrast to coastal samples, tetA and ermC were significantly correlated with intI1 in marine aerosols, suggesting that these two ARGs may have a high risk of horizontal gene transfer. Notably, the correlations between the ARGs associated with the same antibiotics were not necessarily stronger than those between the ARGs associated with different antibiotics.
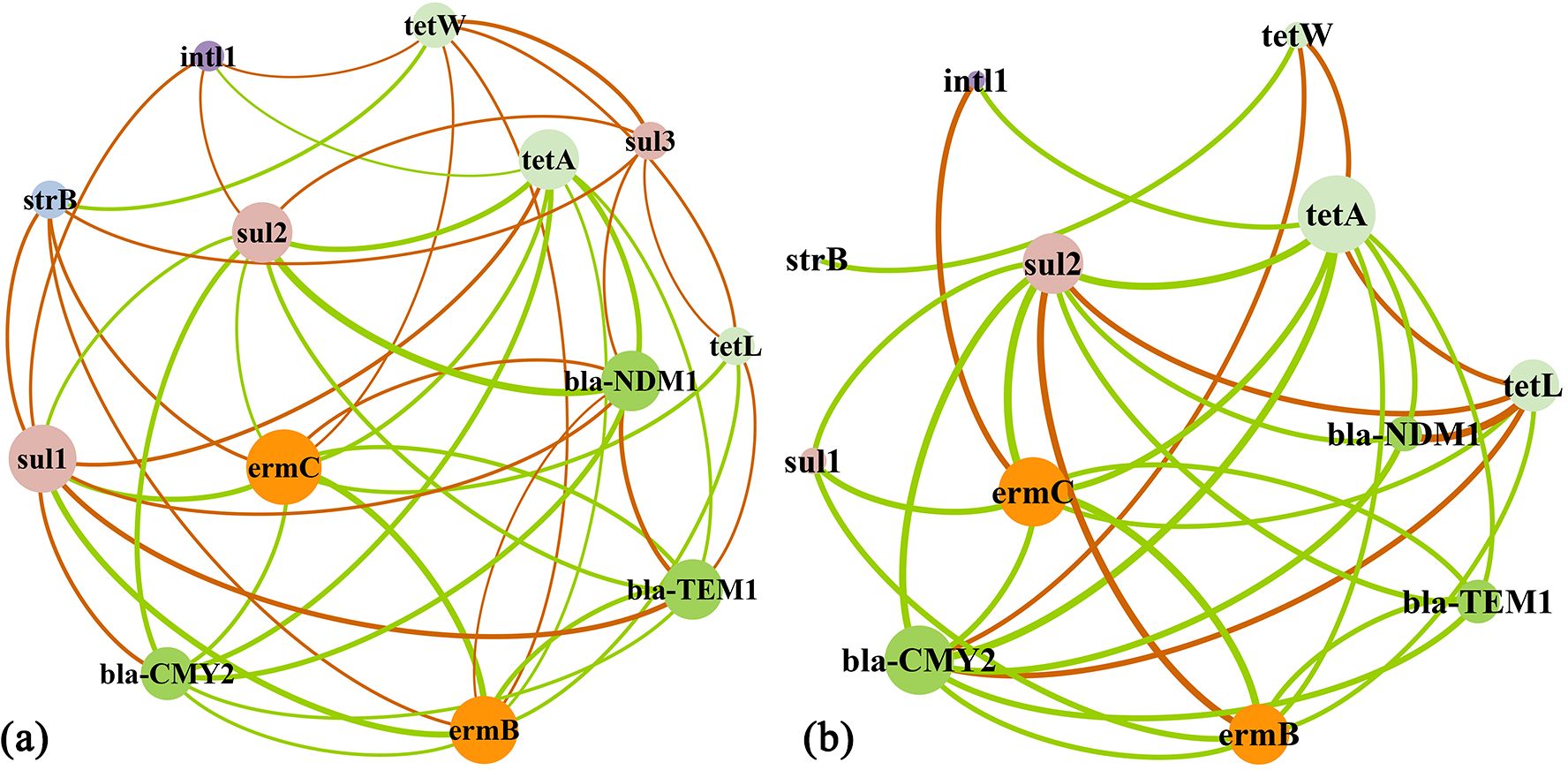
Figure 4. Co-occurrence network of different ARGs: (A) coastal aerosols and (B) marine aerosols [each connection represents a significant difference (p<0.05)]. The thickness of the line is proportional to the value of Spearman’s correlation coefficient. The colored nodes represent different ARGs, and the size of each node is proportional to the number of related genes. The green lines represent correlations existing in both coastal and marine aerosols, and the orange lines represent correlations existing only in either coastal aerosols or marine aerosols.
3.3 Relevance of the associations of different ARGs with environmental factors
Ambient conditions, including meteorological factors and air pollutants, may affect the activities of microorganisms and subsequently change the abundance of bioaerosols. RDA was thereby used to establish the link between ARGs in coastal and marine aerosols and environmental factors, particularly concentrations of those particulate ions (Figure 5). In the coastal samples, Ca2+ was strongly positively correlated with all the ARGs, whereas PO43- was strongly negatively correlated with the ARGs (Figure 5D). NO3- was strongly positively correlated with most ARGs, except for sul2, bla-NDM1 or bla-CMY2, and similar results were obtained for NH4+ and SO42- (Supplementary Figure S4C). In contrast, all detected ions, excluding Ca2+, had strong negative correlations with most ARGs, except for tetW, strB, and sul3, in the marine samples (Figure 5E). The ions in the marine samples presented positive correlations with tetW, strB and sul3 (Supplementary Figure S4D); however, their abundance was substantially lower. Secondary ions such as NH4+, NO3- and SO42- were positively correlated with most ARGs in coastal aerosols (Supplementary Figure S4C) but negatively correlated with most ARGs except tetW, strB, and sul3 in marine aerosols (Supplementary Figure S4D).
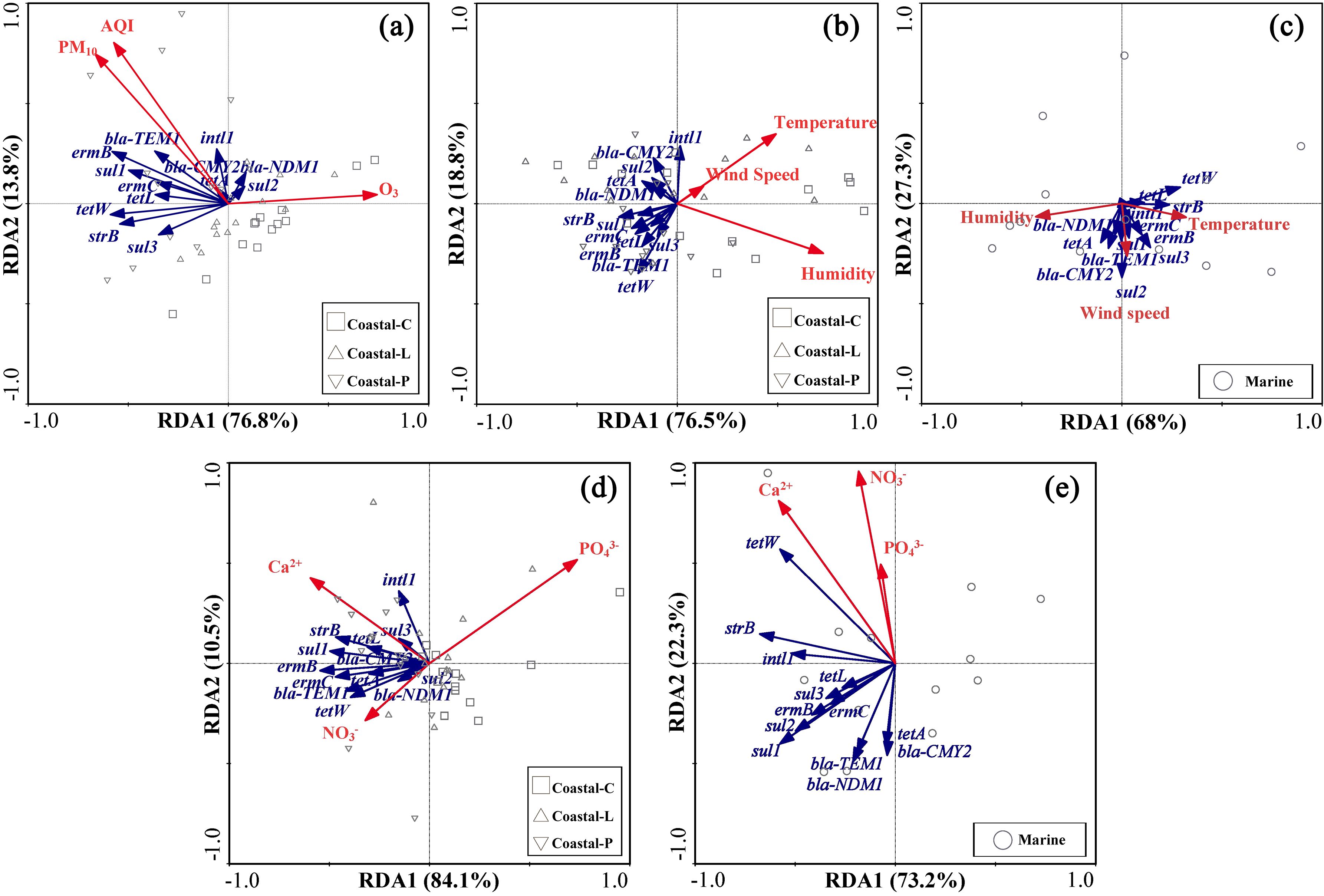
Figure 5. RDA of chemical species, meteorological factors, air pollutants and ARGs in coastal and marine aerosols: (A) chemical species in coastal aerosols, (B) chemical species in marine aerosols, (C) meteorological factors in coastal areas, (D) meteorological factors in marine atmospheres, and (E) air pollutants measured in the coastal city.
We further conducted RDA of ARGs with meteorological factors (Figures 5B, C). In coastal areas, most ARGs (except bla-CMY2) were negatively correlated with wind speed. In contrast, in marine areas, most ARGs (except tetW, tetL, and strB) were positively correlated with wind speed. The RDA also revealed a negative correlation of different ARGs with relative humidity in the coastal atmosphere. However, a consistent negative correlation of airborne ARGs with relative humidity in the marine atmosphere was not observed. For example, a positive correlation existed between tetA and relative humidity. In contrast, a negative correlation of ermC and ermB with relative humidity was identified. In addition, there were negative correlations of different ARGs with ambient temperature in the coastal atmosphere, except for intI1 and bla-CMY2. In the marine atmosphere, a positive correlation of airborne ARGs, excluding bla-NDM1 and tetA, with ambient temperature was obtained.
Moreover, RDA was performed between ARGs and air pollutants in the coastal atmosphere (Figure 5A). Most ARGs were positively correlated with the AQI, except for bla-NDM1 and sul2. Similar results were obtained when the correlations of ARGs with PM10 were analyzed. However, the correlations of ARGs with AQI were always better than those with PM10. Overall, ermB and ermC were the most sensitive ARGs to AQI and PM10. In contrast, a negative correlation was observed between most ARGs and O3.
4 Discussion
4.1 What caused the difference in the ARG profile between marine and coastal aerosols?
When the coastal samples were classified into three categories, i.e., Coastal-C, Coastal-L and Coastal-P, an additional comparison of ARGs in coastal and marine aerosols revealed that 1) the abundances of the total ARGs in the marine atmosphere were generally located between those in Coastal-C and Coastal-L; and that 2) the values were substantially lower than those in Coastal-P (Supplementary Figures S5A, S6). The number of ARG types in the marine atmosphere was greater than that in the coastal atmosphere, regardless of the pollution level (Supplementary Figure S5B). The greater number of ARG types in the marine atmosphere may imply complex sources of ARGs.
Although the sampling point is not directly adjacent to a source of anthropogenic pollution, distant pollution sources located several kilometers away or more may still influence the composition and abundance of ARGs in aerosols (Chen et al., 2022). For example, Bai et al. (2022) reported that ARGs in aerosols can disperse from farms along the wind direction, reaching environments up to 10 km away. Additionally, pollutants in seawater can be transferred into the atmosphere through sea spray aerosols and transported over long distances (Piazzola et al., 2015). Moreover, marine microorganisms have been shown to travel even thousands of kilometers through atmospheric processes (Mayol et al., 2017). These findings collectively indicate that anthropogenic activities, even those located far from the sampling site, could influence the ARGs detected in marine and coastal aerosols. This supports the potential impact of distant pollution sources on our study site.
In fact, the causes of airborne ARGs in China are highly complicated considering the large number of producers and consumers of antibiotics (Zhu et al., 2013). Macrolides, β-lactams, tetracyclines, and sulfonamides accounted for 26%, 21%, 7%, and 5%, respectively, of the 92,700 tons of 36 common antibiotics. Eighty-four percent of antibiotics are used for animals, with over half of them being excreted and entering the environment (Zhang et al., 2015). Amoxicillin, a β-lactam, is the most commonly used antibiotics, which may explain the high abundance of bla-TEM1. Although the abundance of bla-TEM1 was reasonably high at 103 - 105 copies/m3, a significant difference in its abundance was not detected between the coastal and marine aerosols in this study. The comparison results for bla-TEM1 were inconsistent with other ARGs detected in this study. Previous studies have reported high abundances of bla-TEM1 in various regions, suggesting its widespread presence. This may explain the lack of significant differences in bla-TEM1 abundance between coastal and marine aerosols in this study. For example, Wang Y. et al. (2019) reported that the abundance of bla-TEM1 at 105 copies/m3 in outdoor air largely exceeded the indoor air values of wastewater treatment plant and laboratory site in Tianjin, China. However, the values were reportedly as high as 105 - 107 copies/m3 in PM10 collected in ambient air near a municipal solid waste treatment system in Changzhou, China (Li et al., 2020). The unexpectedly high abundance of the β-lactam resistance gene bla-CMY2 in marine aerosols may be related to the extensive use of β-lactam antibiotics to treat fish vibriosis in intensive mariculture settings (Aller et al., 2005). Previous studies have reported that β-lactam resistance genes account for a relatively high proportion of ARGs in seawater and sediments from marine fish cage-culture water and non-mariculture areas (Muziasari et al., 2016; Wu et al., 2019). Moreover, Tian et al. (2023) recently reported that in wastewater treatment, β-lactam resistance genes exhibit a greater aerosolization capability than other ARGs, thus the high abundance of bla-CMY2 in seawater is likely to enter the aerosol environment through aerosolization.
Higher abundances of strB, sul1, sul3, tetL, and tetW in coastal aerosols than in marine aerosols were expected compared with the continental observations reported in the literature (Supplementary Table S5). For example, high abundances of tetW (105 - 107 copies/m3) were reported in TSP samples from the swine-feeding operations (Liu et al., 2018). The reported values were 1-3 orders of magnitude larger than those measured in the coastal atmosphere in the present study, this could be due to the campus sampling site is far from the animal husbandry farms. Typically, sulfonamide- and tetracycline resistance genes are the dominant ARGs in wastewater treatment plants and farms (Zhuang et al., 2021). For example, Wang et al. (2024) reported that the antibiotic concentrations of China’s sewage treatment plants ranked in descending order as follows: β-lactams (1462.3 ng/L) > sulfonamides (846.9 ng/L) > macrolides (676.3 ng/L) > tetracyclines (456.3 ng/L) > quinolones (351.3 ng/L), whereas the dominant ARGs included sul1, sul2 and tetW. Wang et al. (2024) also argued that strB was dominant in the bioreactors of hospital wastewater treatment facilities, which may be the source of strB in ambient aerosols. In the detected hospital aerosols, aminoglycoside, tetracycline, and chloramphenicol resistance genes were the most abundant (Kormos et al., 2022). In addition, the lower abundances of tetA in coastal aerosols than in marine aerosols have yet to be explained because of the opposite results for tetL and tetW. Both tetA and tetL are efflux pump genes, wheras tetW encodes a ribosomal protection protein (Michalova et al., 2004). The different distributions of tetA and tetW might be related to their functions but not those of tetA and tetL.
For macrolide resistance genes, although ermB and ermC had lower abundances, they were still strongly correlated with most ARGs, both in marine and coastal areas. These findings suggest these ARGs may exist on the same plasmid and thereby cause co-resistance mechanisms and multidrug resistance (Parkhill et al., 2001; Wang Y. et al., 2019). Given that MGEs are often used as vectors for HGT, relatively low intI1 abundance was argued to be conventionally due to low horizontal gene transfer risk and low anthropogenic contribution (Gao et al., 2018; Gillings et al., 2015; Sun et al., 2020).
Furthermore, the results of the RDA largely demonstrated that there were differences in the sources of ARGs, especially some specific ARGs, in the marine and coastal aerosols. In the coastal atmosphere, the positive correlation of all ARGs and Ca2+ implied that dust or soil particles were important carriers of microorganisms containing ARGs (Figure 5D). Water-soluble inorganic phosphates such as PO43- are necessary nutrients for the growth of microorganisms and may be consumed during the growth of microorganisms (Kerrnjespersen and Henze, 1993), leading to their expected negative correlation with ARGs. Han et al. (2018) also reported that water-soluble PO43- was negatively correlated with bacterial abundance. Furthermore, the positive correlation between secondary ions and ARGs might be due to the accumulation effect and/or bioaccumulation of ARGs (Sun et al., 2018; Ying et al., 2021) (Supplementary Figure S4C). However, the accumulation effect and/or bioaccumulation were not detectable for sul2, bla-NDM1 or bla-CMY2. The three ARGs also did not display significant correlations with AQI or PM10 (Figure 5A), which means that these three ARGs may be affected by other sources. In contrast, all ARGs except tetW, strB, and sul3 in marine aerosols were negatively correlated with Ca2+ and secondary ions but positively correlated with wind speed. However, tetW, strB, and sul3 exhibited the opposite pattern. Secondary ions in marine aerosols, such as NH4+, NO3- and SO42-, can be used as indicators of transported continental particulate pollutants (Chen et al., 1997, 2021; Savoie et al., 1987), which is supported by their close correlations in coastal samples (Supplementary Figure S4C). The negative correlations in marine aerosol further suggested that most marine airborne ARGs with relatively high abundances were likely derived from marine sources, e.g., sea spray aerosols enriched in organics and microorganisms, whereas the tetW, strB, and sul3 may be derived from long-range continental transport. The generation of sea spray aerosols is a strong function of sea surface wind speed (Feng et al., 2017; Prather et al., 2013; Quinn et al., 2015). The correlation with the wind speed further confirms this suspicion. Positive correlations were clearly identified in the marine aerosols, as shown in Figure 5C. Notably, negative correlations generally existed when the RDA was conducted for different ARGs in coastal samples with respect to wind speed (Figure 5B) because of the dilution effects of wind on land.
In addition, the prevalence of the human respiratory symptoms is high in dry and cold seasons, and the use of antibiotics has substantially increased, which reportedly induces the production of ARGs (Caucci et al., 2016; Suda et al., 2014). In the cold season, the accumulation of carriers of ARGs such as PM10 and TSP was always increased, this might explain the negative correlation between temperatures, humidity and ARGs (except bla-CMY2 and sul2) in coastal aerosols. The bla-CMY2 is considered an indicator of ARGs from marine sources (Muziasari et al., 2016; Wu et al., 2019). Typically, relatively high temperatures favor the growth of phytoplankton and bacteria in seawater, which leads to an increase in ARG abundance (Sato-Takabe et al., 2019; Xiong et al., 2015). In addition, O3 has been reported to damage the cell structure and biological macromolecules such as DNA (Meng and Zhao, 2012), and this mechanism might also result in a negative correlation of O3 levels with some genes. The lack of correlation of intl1 with AQI and O3 requires further investigation.
4.2 The transport of ARGs between continental and marine atmospheres
The presence of ARGs in marine aerosols has been reported in seawater and sediments (Chee-Sanford et al., 2009; Han et al., 2021; Su et al., 2017). In the present study, most ARGs in the marine aerosols presented negative correlations with secondary ions but strong positive correlations with wind speed (Figure 5). These results indicate that these ARGs are more likely to enter marine aerosols via sea spray aerosols. As some ARGs, such as bla-NDM1, bla-CMY2, sul2 and tetA, were present at significantly higher abundances in marine aerosols than in coastal aerosols (p<0.01) (Supplementary Figure S3), sea spray aerosols might be a source of those ARGs in coastal aerosols under the influence of onshore winds. In fact, ARGs may be released into the environment and exist for a long time even when ARG-containing microorganisms have already died (Crecchio et al., 2005; Hill and Top, 1998). Atmospheric aerosols with microorganisms are transported hundreds of kilometers from their sources and remain suspended in aerosols for several weeks (Li et al., 2018; Sun et al., 2020). Supplementary Figure S7 shows that the southeast winds had the highest occurrence frequency and the highest average speed in the clean coastal atmosphere, which may decrease the concentrations of PM10 and thereby reduce the abundance of continent-derived ARGs to some extent. The analysis of the 24 h backward trajectories of coastal samples also revealed consistent patterns when the trajectory data were clustered (Supplementary Figure S8). Cluster 1, accounting for most of the data, mostly originates from the Jiaodong Peninsula and contains 33% clean samples, 28% lightly polluted samples, and 39% polluted samples. Cluster 2, which traverses the Bohai Sea and northern inland areas, is composed primarily of lightly polluted samples (50%). Cluster 3, originating from southern inland regions, has similar distributions of clean (36%), lightly polluted (36%), and polluted (27%) samples. Conversely, Cluster 4, which is associated with air masses moving from the direction of the Yellow Sea without crossing inland areas, consists solely of clean samples, and Cluster 5, which tracks air masses moving from inland directions, contains exclusively polluted samples. This disparity shows the differential impact of source regions, indicating that inland sources are more likely to contribute to higher pollution levels, whereas marine influences tend to coincide with cleaner air quality days.
Based on the assumption that bla-NDM1, bla-CMY2, sul2 and tetA are derived mainly from marine sources, the ratios of the ARGs to Na+ in the marine sample were used to roughly estimate the marine contributions of ARGs in the coastal aerosols via the following equation:
Similarly, the ARGs, including strB, sul1, sul3, tetL, and tetW, in the coastal aerosol sample with the highest concentration of Ca2+ (4.3 µg/m3) were used to roughly estimate the contributions of continental ARGs in the marine aerosols via the following equation:
For bla-NDM1, bla-CMY2, tetA and sul2, ARGs from marine sources accounted for 84%->100% of the Coastal-C samples, 14-43% of the Coastal-L samples, and 7-26% of the Coastal-P samples. The ARGs derived from marine sources overwhelmingly contributed to the four ARGs in the Coastal-C samples, but their contributions substantially decreased in the lightly polluted and polluted coastal samples. Notably, ARGs derived from marine sources might proliferate or degrade during long-range transport. Their evolution cannot be accurately qualified, leading to uncertainties in the abovementioned estimates.
The calculated values for tetW, strB and sul3 from continental transport accounted for more than 100% of the measured values in the marine samples, implying overwhelming continental contributions. The percentages exceeding 100% may also reflect the uncertainties of the three ARGs during long-range transport or other factors. This discrepancy, with the estimated value exceeding the actual measurement, may be attributed to the degradation of ARGs during long-range transport. Compared with ARGs, airborne water-soluble ions are more stable and do not degrade or proliferate during atmospheric transport. In contrast, ARGs may proliferate or degrade in response to multiple factors, such as bacterial growth and decay, ultraviolet radiation, temperature fluctuations, oxidative stress, and interactions with microorganisms during atmospheric transport (Meng and Zhao, 2012). Section 4.3 provides a detailed exploration of these degradation processes contributing to the observed phenomena. The percentages were, however, only 30% for sul1 and 46% for tetL, implying that their contributions from marine sources still dominated. The abundance of airborne strB in the Bohai Sea was much greater than that in the Yellow Sea, as the Bohai Sea is encircled by continents in three directions with strong effects from continental sources of ARGs, as expected.
However, the sample from Marine 4 should be considered an exception, in which the abundances of total ARGs were significantly greater than those in other marine samples. In Marine 4, the calculated value for tetW from continental sources also accounted for >100% of the measured value, potentially due to the amplification of ARGs and other influencing factors. Surprisingly, the calculated continental source contributions for tetL, strB and sul1 accounted for only 5%, 11%, and 2%, respectively. The latter implied strong marine emissions of the three ARGs. Notably, sul3 was undetectable in the Marine 4 sample. A 24 h backward trajectory analysis was conducted to explore the possible cause of the high abundances of total ARGs in Marine 4 (Supplementary Figure S9). During the sampling period, both marine and continental air masses formed at some time points (Supplementary Figure S9B). The backward trajectories frequently passed through Yancheng and Rizhao. Within 48 h before the end of the Marine 4 sampling, Yancheng experienced severe particulate pollution. The elevated particle concentrations may provide more carriers for the spread of ARGs (Supplementary Figures S9C, D). However, this situation did not occur in Rizhao. The long-range transport of airborne ARGs in this direction may be limited.
4.3 Proliferation or degradation of ARGs in coastal and marine atmospheres
Particulate matter mass concentration may be one of the key factors that may determines ARG distribution, as microorganisms and ARGs are primarily suspended in air by adsorption to the particulate surface (Jang et al., 2018). As expected, the total ARGs were positively correlated with the mass concentrations of TSP in coastal and marine aerosols, with r=0.42 (p<0.05). Similar positive correlations were generally obtained when different ARGs were correlated with the mass concentrations of TSP. Considering the exponential growth of bacteria, the lg (ARG abundance) was normalized to the mass concentration of TSP to eliminate the accumulation effect. Thus, potential proliferation related to bacterial reproduction or the degradation of ARGs caused by photodegradation, cellular oxidative damage, and other processes in continental and marine atmospheres can be analyzed.
The normalized lg (ARG abundance) was plotted against the mass concentration of TSP in Figures 6A–J. Regardless of the coastal or marine atmosphere, the normalized lg (ARG abundance) always decreased with increasing mass concentration of TSP. Multiple factors may account for the observed phenomenon, especially bacterial proliferation, HGT, atmospheric degradation processes (e.g., oxidation by O3), and particle properties (Meng and Zhao, 2012). During the aerosol transport process, ARGs may undergo various changes. These changes include potential increases associated with the proliferation of host bacteria, as well as the contribution of HGT processes (Ben et al., 2019; Martínez et al., 2015). Conversely, in this process, ARGs may be degraded by atmospheric oxides, such as O3, and the toxic and harmful substances present in the particulate matter could also contribute to this effect (Meng and Zhao, 2012). When the degradation effect is greater than the proliferation effect during the transport of aerosols, the normalized lg (ARG abundance) decreases with increasing particle concentration. Furthermore, the source of particulate matter may also impact pollution levels, which in turn influences the concentrations of particulate matter and ARGs, as depicted in Supplementary Figures S5, S6 and S8. Additionally, larger particles, with relatively smaller specific surface areas, offer fewer sites for attachment, potentially resulting in a lower abundance of ARGs associated with these particles. To further explore whether variations in particle size contribute to these results, the ratio of PM2.5 to PM10 was examined as an indicator of the relative proportions of smaller to larger particles. There was no significant correlation between the concentration of particles and the PM2.5/PM10 ratio (p>0.05), suggesting that, within this study, the high concentration of particulate matter was not attributed to larger particle sizes. The source and degradation process of particulate matter are more likely to be the major factors leading to this result. To further investigate whether different sources are the cause of this variation, the normalized lg (ARG abundance) was plotted against the TSP mass concentration for each cluster, which was derived from distinct clustering outcomes (Supplementary Figure S10). Despite the similarity in sources, the normalized lg (ARG abundance) still decreased with increasing TSP concentration. This finding indicates that different sources are not the main reason for this phenomenon. In the coastal atmosphere, the results implied that the degradation of ARGs likely overwhelmed their proliferation with particulate matter accumulation. The same explanation was reasonably applied to the ARGs derived mainly from continents in the marine atmosphere. For those ARGs derived from sea spray aerosols in the marine atmosphere, the results were more complicated because of increasing emissions of sea-derived ARGs with increasing wind speed. Notably, the results align with the hypothesis in Section 4.2, which posits that the discrepancy between the estimate of Ca2+ and the actual abundance of ARGs can be attributed to degradation during long-range transport. Notably, the ARG types with a detection rate of less than 2/3 were not included in the normalization analysis because of large analytical errors.
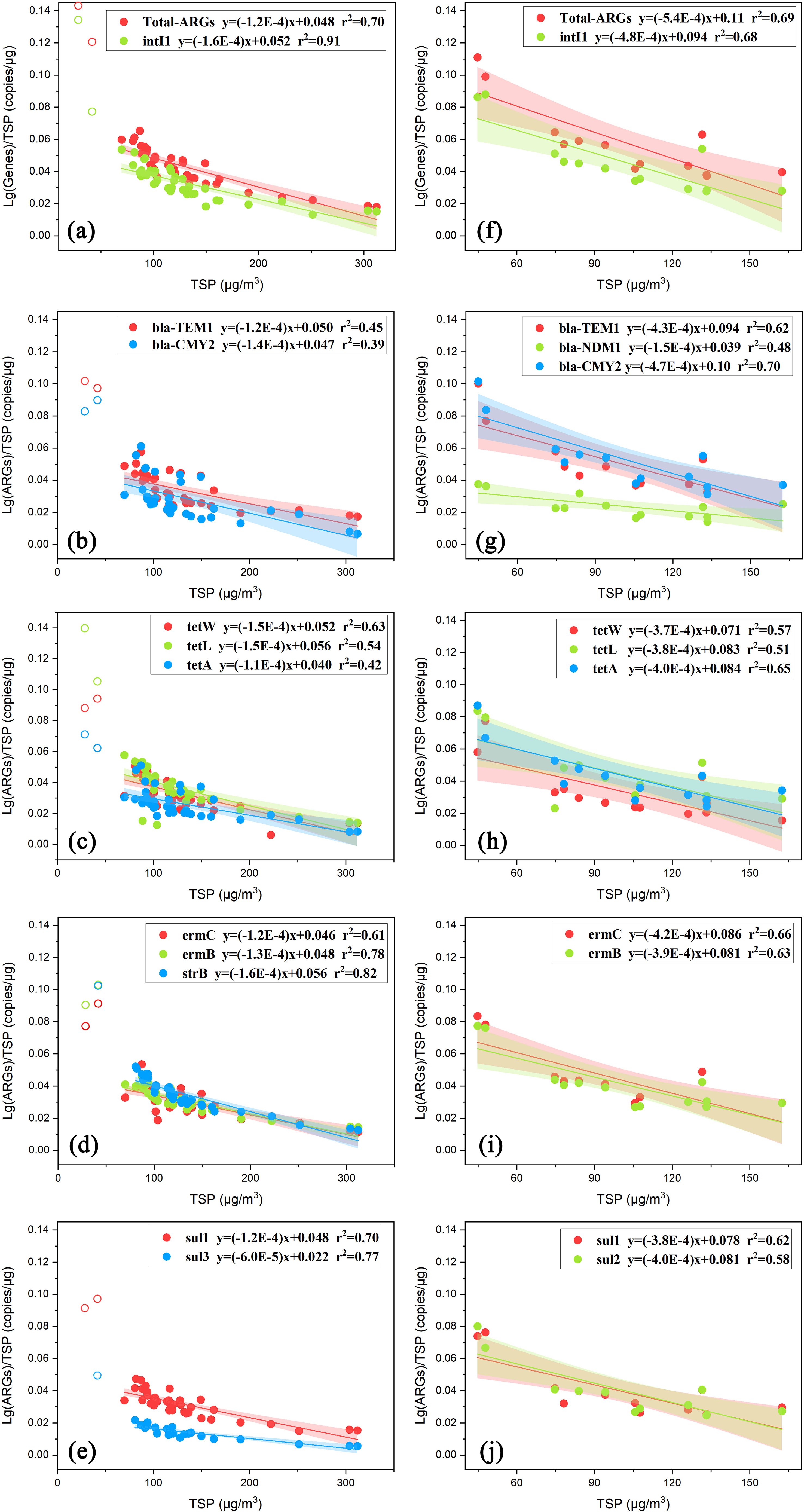
Figure 6. Linear relationship between coastal and marine lg (ARG abundance)/TSP and TSP: (A–E) results from coastal samples and (F–J) results from marine samples.
In the coastal atmosphere, the normalized lg (ARG abundance) with TSP mass concentrations less than 60 µg/m3 generally deviated because of the low concentration. Thus, only TSP mass concentrations greater than 60 µg/m3 were used to extract the regression equations and r2 values. A moderately good negative correlation was obtained between the normalized lg (total-ARG abundance) and the TSP mass concentration with r2 = 0.70 (Figure 6A). The r2 increased to 0.91 between the normalized lg (intl1 abundance) and TSP. In coastal aerosols, most ARGs showed similar degradation rates, with slopes ranging from -1.1×10-4 to -1.6×10-4, except for sul3. The slope for sul3 was only half that of the other curves, implying that either the degradation of sul3 was less sensitive to TSP accumulation or its proliferation may be stronger than that of other ARGs. Moderately good negative correlations were obtained for normalized lg (ARG abundance), including ermC, ermB, strB, sul1 and sul3, with r2 values ranging from 0.61 to 0.82. The value of r2 decreased to some extent for bla-TEM1, bla-CMY2, tetW, tetL and tetA, with the lowest values of 0.39-0.42 observed for bla-CMY2 and tetA. bla-CMY2 and tetA in the coastal atmosphere may be partially derived from sea spray aerosols, as described above, complicating their responses to TSP accumulation. Various components in aerosols may cause the degradation of ARGs. UVA ultraviolet rays, which are common in the atmosphere can also lead to bacterial death and degradation in aerosols (Wang C. et al., 2019), which may also affect ARGs. The RDA in Section 3.3 also confirmed the negative correlation between ARGs and O3, a prevalent atmospheric oxide.
For marine aerosols, the degradation rates show minimal variation, with slopes ranging from -1.5×10-4 to -4.7×10-4. Among these, most ARGs, except bla-NDM1, exhibit relatively low degradation rates. Notably, bla-NDM1 degrades more slowly, suggesting that it is better adapted to the marine environment. When the slopes and r2 values in the marine atmosphere were compared with those in the coastal atmosphere for the corresponding ARGs, the slopes of the former increased by 2- to 4-fold, except for bla-CMY2 and sul3. The larger slopes implied that either the degradation of ARGs in the marine atmosphere was more sensitive to TSP accumulation or that their proliferation may be weaker there. For bla-CMY2, the slope in the marine atmosphere was comparable to that in the coastal atmosphere and substantially smaller than others in the marine atmosphere. This unique result might be related to strong sea-derived emissions of bla-CMY2 and other complicated factors. Although the slope for sul3 in the marine atmosphere increased by 6-fold compared with that in the coastal atmosphere, the former was comparable with the slopes of other ARGs in the marine atmosphere. In addition, no clear trend in the r2 values for different ARGs was observed in the marine atmosphere compared with those in the coastal atmosphere.
4.4 Potential risks associated with airborne ARGs
Long-term exposure to opportunistic pathogens with ARGs might increase the risk of respiratory tract infections, such as cardiopulmonary diseases, which might be ineffectively treated with antibiotics (Ben et al., 2019; Liang et al., 2020). The ADI values for different populations exposed to three pollution levels were estimated to evaluate human respiratory tract exposure to airborne ARGs, and the results are shown in Figure 7. In addition, the average ADI value on an annual scale was also calculated. In this case, we first calculated the annual average abundances of different ARGs. From 2019 to 2020 in Qingdao, clean, lightly polluted and polluted days accounted for 20%, 43%, and 37%, respectively. Thus, the annual average abundance of a certain ARG equaled the sum of its abundance in each type of day multiplied by the corresponding percentage. The annual abundances were subsequently used to calculate the average ADI values for each ARG associated with different populations. As shown in Figure 7, children presented the highest respiratory inhalation, with ADI values ranging from 1.25×101 - 4.57×104 copies/kg/d during the study period, followed by adult males at 1.04×101 - 3.79×104 copies/kg/d and adult females at 9.55×100 - 3.49×104 copies/kg/d, owing to the differences in average weight and inhalation rate. In general, β-lactam resistance genes, i.e., bla-TEM1, bla-CMY2, and tetracycline resistance genes, i.e., tetL, tetA and tetW, presented high daily respiratory intake. β-lactams play a central role in the treatment of various bacteria because they are members of the antibiotic category with the greatest number of prescriptions and the largest sale volume (Klein et al., 2018; Tooke et al., 2019). As another commonly used drug for the treatment of human and animal diseases, tetracycline has good efficacy against various microorganisms, including gram-positive and gram-negative bacteria, mycoplasmas, chlamydia and many protozoan parasites (Chopra and Roberts, 2001). Although the ADI values of 1.22×100 - 3.97×104 copies/kg/d in the marine atmosphere were slightly lower than those of 4.40×100 - 9.81×104 copies/kg/d in the polluted coastal atmospheres, the values for some ARGs (such as tetA, bla-TEM1 and bla-CMY2) in the marine atmosphere were much greater than those in the clean coastal atmosphere. The higher ADI values in the marine atmosphere might impose health risks to inshore operators, such as fishermen and seafarers, considering the harsh living conditions.
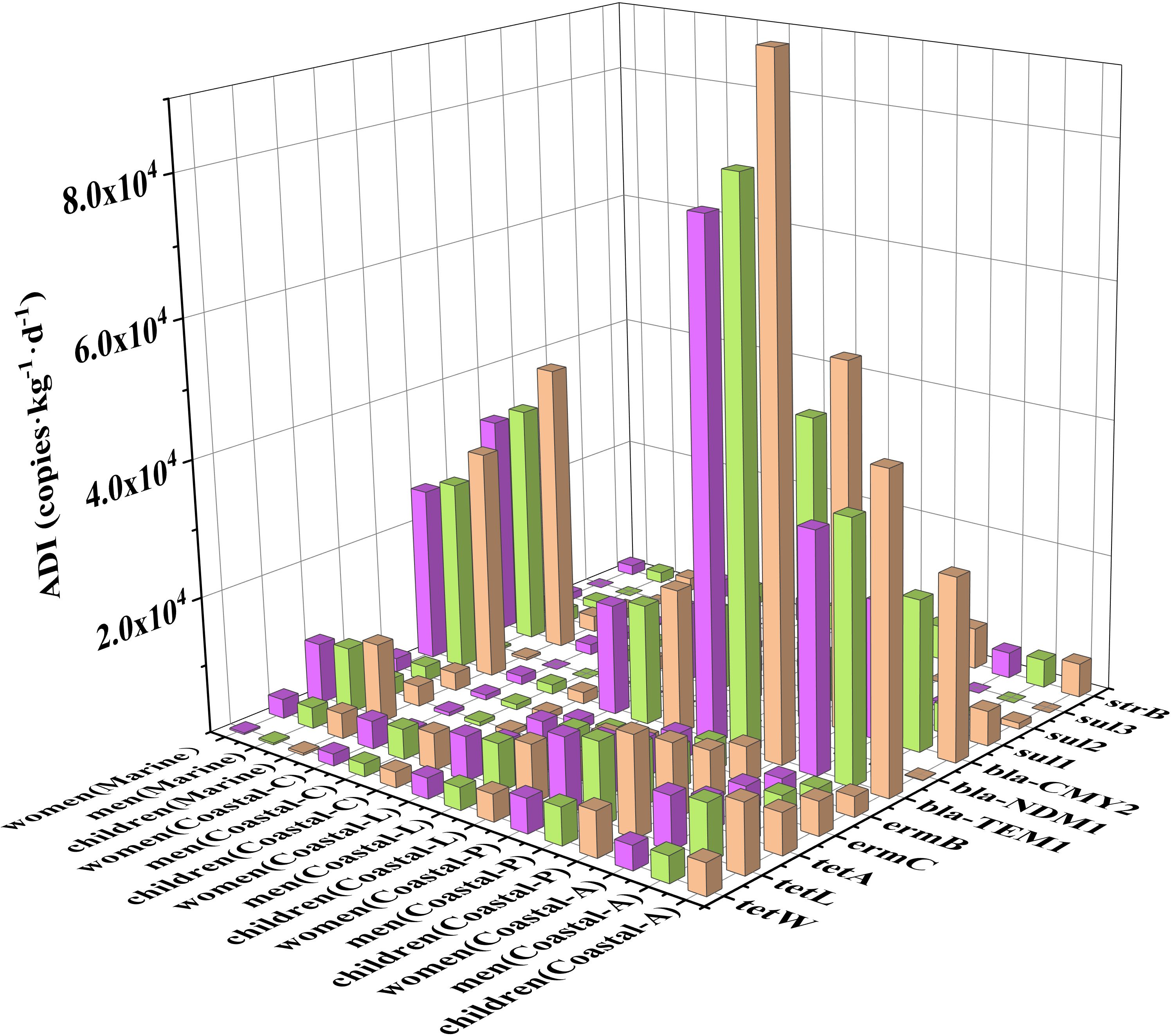
Figure 7. ADI via inhalation in coastal and marine areas (Coastal-A is the average daily intake of ARGs in Qingdao calculated by the weighted average value for each pollution condition).
When the ADI values of ARGs via inhalation were compared with those from other pathways in the literature (Supplementary Figure S11, Supplementary Table S6), the ADI values of tetracycline, macrolide and β-lactam resistance genes via inhalation exceeded those reported from drinking tap water. However, these values were much lower than those associated with the consumption of raw fruits, vegetables, and aquatic products. With respect to the sulfonamide resistance gene, the ADI values from drinking water, vegetables and aquatic products were greater than those from inhalation. However, the ADI associated with inhalation was still higher than that associated with the consumption of raw meat such as pork. Typically, people treat food and drinking water with a variety of techniques to remove bacteria and reduce the risk of disease. For example, cooking processes reportedly destroy the structure of ARGs (Claire et al., 2013). Thus, the risk of ARG exposure from drinking water and food is theoretically avoidable to some extent. Exposure to airborne ARGs is unavoidable, as people breathe constantly. The risk of ARG exposure by respiratory inhalation is almost unavoidable and should be monitored dynamically for a long time, although the ADI of inhalation is temporally lower than that of most other routes.
4.5 Challenges and future studies
The marine environment, as a major destination for human-produced pollutants, also serves as a natural reservoir for ARGs. Based on our findings, some ARGs in marine aerosols likely originate from continental areas, whereas others may originate from the marine sources. However, their specific sources and contributions remain unclear because of the lack of data and spatial-temporal samples collected in this study. In addition, our research indicates that as particulate matter accumulates, the number of ARGs per unit particle decreases, this suggests that the degradation of ARGs exceeds their amplification during the accumulation in ambient air. However, the specific mechanisms driving these changes require further investigation to improve the accuracy of ARG emission assessments and simulations.
Additionally, our study strongly suggested that mariculture may be a strong source of ARGs in ambient aerosols. However, there is still a lack of quantitative research on airborne ARGs in mariculture zones. This study also raised important issues related to marine airborne ARGs. Although β-lactam antibiotics are reportedly undetectable in marine aquaculture seawater because of their easy degradation, the abundances of bla-NDM1 and bla-CMY2 dominated over those of the other ARGs in the marine aerosols and were greater than their abundances in the coastal atmosphere. Some studies have also shown that β-lactam resistance genes dominate in seawater. This study underscores the critical necessity for further exploration into the sources and transport mechanisms of airborne ARGs in marine atmospheres, highlighting the crucial role of airborne ARGs in marine aerosols as potential sources. This finding also implies that more comprehensive analyses are needed to determine the relative importance of transmission mechanisms with respect to the use of antibiotics.
5 Conclusions
Owing to their diverse sources, the number of ARG types in marine aerosols was greater than that in coastal aerosols. However, the abundance of airborne ARGs in marine aerosols was greater than that in coastal aerosols under clean ambient conditions but lower than that in coastal aerosols under polluted ambient conditions. Some airborne ARGs, such as bla-NDM1, bla-CMY2, sul2, and tetA, observed in the marine atmosphere had significantly higher abundances than those observed in the coastal atmosphere. These ARGs are more likely to enter marine aerosols via air-sea exchanges. The rough estimates obtained here showed that bla-NDM1 and bla-CMY2 from marine sources overwhelmingly contributed to their abundances in coastal atmospheres. These findings suggest the importance of reducing the abundance and dissemination of sea-derived airborne ARGs in control strategies. Although continental transport may be an important source of airborne ARGs such as tetW, tetL, strB and sul3 in the marine atmosphere, they may be degraded more rapidly than they are in the coastal atmosphere. Marine aerosols have been shown to be a significant source of airborne ARGs in coastal atmospheres, but their contribution decreases as air pollution levels rise. Our analysis also suggested that the degradation of ARGs was likely greater than the proliferation of ARGs induced by bacterial reproduction in both marine and coastal atmospheres, leading to a detectable net degradation effect in the normalized lg (ARG abundance). In the future, it is essential to explore the composition, evolution and controlling factors of ARGs in marine aerosols from marginal seas to the open ocean so that the maximum transport distance of terrestrial airborne ARGs in the marine atmosphere can be estimated, further understand the global propagation and impact of ARGs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SJ: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. XY: Data curation, Resources, Writing – review & editing. JQ: Writing – review & editing, Methodology. XL: Resources, Writing – review & editing. HG: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number 41876125) and the Joint Program of the National Natural Science Foundation and Shandong Province of China (grant number U1906215).
Acknowledgments
Marine aerosol samples were collected onboard the R/V Lanhai 101 implementing the open research cruise NORC2020-01 supported by the NSFC Shiptime sharing project. We sincerely thank the captain and crew of the R/V Lanhai 101, the chief scientist, as well as the students and staff who joined this cruise, for their support during the work at sea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1491484/full#supplementary-material
References
Agarwal V., Yue Y., Zhang X., Feng X., Tao Y., Wang J. (2023). Spatial and temporal distribution of endotoxins, antibiotic resistance genes and mobile genetic elements in the air of a dairy farm in Germany. Environ. pollut. 336, 122404. doi: 10.1016/j.envpol.2023.122404
Ahmed W., Zhang Q., Lobos A., Senkbeil J., Sadowsky M. J., Harwood V. J., et al. (2018). Precipitation influences pathogenic bacteria and antibiotic resistance gene abundance in storm drain outfalls in coastal sub-tropical waters. Environ. Int. 116, 308–318. doi: 10.1016/j.envint.2018.04.005
Aller J. Y., Kuznetsova M. R., Jahns C. J., Kemp P. F. (2005). The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol. Sci. 36, 801–812. doi: 10.1016/j.jaerosci.2004.10.012
Bai H., He L., Wu D., Gao F., Zhang M., Zou H., et al. (2022). Spread of airborne antibiotic resistance from animal farms to the environment: dispersal pattern and exposure risk. Environ. Int. 158, 106927. doi: 10.1016/j.envint.2021.106927
Bastian M., Heymann S., Jacomy M. (2009). “Gephi: An open source software for exploring and manipulating networks,” in Proceedings of the International AAAI Conference on Web and Social Media. doi: 10.13140/2.1.1341.1520
Ben Y., Fu C., Hu M., Liu L., Wong M. H., Zheng C. (2019). Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: a review. Environ. Res. 169, 483–493. doi: 10.1016/j.envres.2018.11.040
Caucci S., Karkman A., Cacace D., Rybicki M., Timpel P., Voolaid V., et al. (2016). Seasonality of antibiotic prescriptions for outpatients and resistance genes in sewers and wastewater treatment plant outflow. FEMS Microbiol. Ecol. 92, fiw060. doi: 10.1093/femsec/fiw060
Chee-Sanford J. C., Mackie R. I., Koike S., Krapac I. G., Lin Y., Yannarell A. C., et al. (2009). Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 38, 1086–1108. doi: 10.2134/jeq2008.0128
Chen D. H., Shen Y. J., Wang J. T., Gao Y., Gao H. W., Yao X. H. (2021). Mapping gaseous dimethylamine, trimethylamine, ammonia, and their particulate counterparts in marine atmospheres of China’s marginal seas - Part 1: Differentiating marine emission from continental transport. Atmos. Chem. Phys. 21, 16413–16425. doi: 10.5194/acp-21-16413-2021
Chen L., Carmichael G. R., Hong M., Ueda H., Shim S., Song C. H., et al. (1997). Influence of continental outflow events on the aerosol composition at Cheju island, South Korea. J. Geophysical Research: Atmospheres 102, 28551–28574. doi: 10.1029/97JD01431
Chen P., Guo X. Y., Li F. X. (2022). Antibiotic resistance genes in bioaerosols: Emerging, non-ignorable and pernicious pollutants. J. Clean Prod 348, 131094. doi: 10.1016/j.jclepro.2022.131094
Chopra I., Roberts M. (2001). Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65, 232–260. doi: 10.1128/MMBR.65.2.232-260.2001
Claire V., Sigrid V. B., Eva V. M., Els V. C., Patrick B., Boudewijn C., et al. (2013). Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 10, 2643–2669. doi: 10.3390/ijerph10072643
Crecchio C., Ruggiero P., Curci M., Colombo C., Palumbo G., Stotzky G. (2005). Binding of DNA from bacillus subtilis on montmorillonite-humic acids-aluminum or iron hydroxypolymers: Effects on transformation and protection against DNase. Soil Sci. Soc Am. J. 69, 834–841. doi: 10.2136/ssaj2004.0166
Feng L. M., Shen H. Q., Zhu Y. J., Gao H. W., Yao X. H. (2017). Insight into generation and evolution of sea-salt aerosols from field measurements in diversified marine and coastal atmospheres. Scientific Reports 7, 41260. doi: 10.1038/srep41260
Gao M., Qiu T., Sun Y., Wang X. (2018). The abundance and diversity of antibiotic resistance genes in the atmospheric environment of composting plants. Environ. Int. 116, 229–238. doi: 10.1016/j.envint.2018.04.028
Gilliam F. S., Saunders N. E. (2003). Making more sense of the order: A review of CANOCO for Windows 4.5, PC-ORD Version 4 and syn-tax 2000. J. Veg. Sci. 14, 297–304. doi: 10.1111/j.1654-1103.2003.tb02155.x
Gillings M. R., Gaze W. H., Pruden A., Smalla K., Tiedje J. M., Zhu Y. (2015). Using the Class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 9, 1269–1279. doi: 10.1038/ismej.2014.226
Han Y., Wang Y., Li L., Xu G., Liu J., Yang K. (2018). Bacterial population and chemicals in bioaerosols from indoor environment: Sludge dewatering houses in nine municipal wastewater treatment plants. Sci. Total Environ. 618, 469–478. doi: 10.1016/j.scitotenv.2017.11.071
Han Q. F., Zhang X. R., Xu X. Y., Wang X. L., Yuan X. Z., Ding Z. J., et al. (2021). Antibiotics in marine aquaculture farms surrounding Laizhou Bay, Bohai Sea: Distribution characteristics considering various culture modes and organism species. Sci. Total Environ. 760, 143863. doi: 10.1016/j.scitotenv.2020.143863
Hernando-Amado S., Coquet T. M., Baquero F., Martinez J. L. (2019). Defining and combating antibiotic resistance from one health and global health perspectives. Nat. Microbiol. 4, 1432–1442. doi: 10.1038/s41564-019-0503-9
Hill K. E., Top E. M. (1998). Gene transfer in soil systems using microcosms. FEMS Microbiol. Ecol. 25, 319–329. doi: 10.1111/j.1574-6941.1998.tb00483.x
Hu J., Zhao F., Zhang X., Li K., Li C., Ye L., et al. (2018). Metagenomic profiling of ARGs in airborne particulate matters during a severe smog event. Sci. Total Environ. 615, 1332–1340. doi: 10.1016/j.scitotenv.2017.09.222
Jang G. I., Hwang C. Y., Cho B. C. (2018). Effects of heavy rainfall on the composition of airborne bacterial communities. Front. Env. Sci. Eng. 12, 12. doi: 10.1007/s11783-018-1008-0
Jiang W., Liang P., Wang B., Fang J., Lang J., Tian G., et al. (2015). Optimized DNA extraction and metagenomic sequencing of airborne microbial communities. Nat. Protoc. 10, 768–779. doi: 10.1038/nprot.2015.046
Kang H., Yu Y., Liao M., Wang Y., Yang G., Zhang Z., et al. (2022). Physiology, metabolism, antibiotic resistance, and genetic diversity of Harveyi clade bacteria isolated from coastal mariculture system in China in the last two decades. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.932255
Kerrnjespersen J. P., Henze M. (1993). Biological phosphorus uptake under anoxic and aerobic conditions. Water Res. 27, 617–624. doi: 10.1016/0043-1354(93)90171-D
Klein E. Y., Van Boeckel T. P., Martinez E. M., Pant S., Gandra S., Levin S. A., et al. (2018). Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. 115, E3463–E3470. doi: 10.1073/pnas.1717295115
Kormos D., Lin K. S., Pruden A., Marr L. C. (2022). Critical review of antibiotic resistance genes in the atmosphere. Environ. Sci.-Process Impacts 24, 870–883. doi: 10.1039/d2em00091a
Lewis E. R., Schwartz S. E. (2004). Sea salt aerosol production: mechanisms, methods, measurements and models – A critical review (DC: American geophysical Union, Washington).
Li J., Cao J., Zhu Y., Chen Q., Shen F., Wu Y., et al. (2018). Global survey of antibiotic resistance genes in air. Environ. Sci. Technol. 52, 10975–10984. doi: 10.1021/acs.est.8b02204
Li S., Ondon B. S., Ho S., Li F. (2023). Emerging soil contamination of antibiotics resistance bacteria (ARB) carrying genes (ARGs): New challenges for soil remediation and conservation. Environ. Res. 219, 115132. doi: 10.1016/j.envres.2022.115132
Li L., Wang Q., Bi W., Hou J., Xue Y., Mao D., et al. (2020). Municipal solid waste treatment system increases ambient airborne bacteria and antibiotic resistance genes. Environ. Sci. Technol. 54, 3900–3908. doi: 10.1021/acs.est.9b07641
Liang Z., Yu Y., Ye Z., Li G., Wang W., An T. (2020). Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens from typical area, Pearl river estuary and their exposure risk to human. Environ. Int. 143, 105934. doi: 10.1016/j.envint.2020.105934
Liu H., Han X., Tang G., Zhang J., Xia X. A., Zhang M., et al. (2022). Model analysis of vertical exchange of boundary layer ozone and its impact on surface air quality over the north China plain. Sci. Total Environ. 821, 153436. doi: 10.1016/j.scitotenv.2022.153436
Liu C., Zheng G., Wang L., Chen T., Shao Z., Chen L. (2018). Sources and pollution characteristics of antibiotic resistance genes and conditional pathogenic bacteria in concentrated swine feeding operation. Yingyong Shengtai Xuebao 29, 2730–2738. doi: 10.13287/j.1001-9332.201808.037
Lu J., Zhang Y. X., Wu J., Wang J. H., Zhang C., Wu J. (2022). Fate of land-based antibiotic resistance genes in marginal-sea sediment: territorial differentiation and corresponding drivers. Chemosphere 288, 132540. doi: 10.1016/j.chemosphere.2021.132540
Luthman O., Robb D. H. F., Henriksson P. J. G., Jørgensen P. S., Troell M. (2024). Global overview of national regulations for antibiotic use in aquaculture production. Aquac. Int. 32, 9253–9270. doi: 10.1007/s10499-024-01614-0
Lv B., Cui Y., Tian W., Wei H., Chen Q., Liu B., et al. (2020). Vessel transport of antibiotic resistance genes across oceans and its implications for ballast water management. Chemosphere 253, 126697. doi: 10.1016/j.chemosphere.2020.126697
Makkaew P., Kongprajug A., Chyerochana N., Sresung M., Precha N., Mongkolsuk S., et al. (2021). Persisting antibiotic resistance gene pollution and its association with human sewage sources in tropical marine beach waters. Int. J. Hyg. Environ. Health 238, 113859. doi: 10.1016/j.ijheh.2021.113859
Martínez J. L., Coque T. M., Baquero F. (2015). What is a resistance gene? Ranking risk in resistomes. Nat. Rev. Microbiol. 13, 116–123. doi: 10.1038/nrmicro3399
Mayol E., Arrieta J. M., Jimenez M. A., Martinez-Asensio A., Garcias-Bonet N., Dachs J., et al. (2017). Long-range transport of airborne microbes over the global tropical and subtropical ocean. Nat. Commun. 8, 201. doi: 10.1038/s41467-017-00110-9
Meng J., Zhao M. (2012). Reactive oxygen species and fibrosis in tissues and organs - review. J. Exp. Hematol. 20, 1284–1288.
Merkens J., Reimann L., Hinkel J., Vafeidis A. T. (2016). Gridded population projections for the coastal zone under the shared socioeconomic pathways. Glob. Planet. Change 145, 57–66. doi: 10.1016/j.gloplacha.2016.08.009
Michalova E., Novotna P., Schlegelova J. (2004). Tetracyclines in veterinary medicine and bacterial resistance to them. Vet. Med. 49, 79–100. doi: 10.17221/5681-VETMED
Ministry of Ecology and Environment of the People's Republic of China (2014). China Population Exposure Factor Handbook (Peking: China Environmental Science Press).
Muziasari W. I., Parnanen K., Johnson T. A., Lyra C., Karkman A., Stedtfeld R. D., et al. (2016). Aquaculture changes the profile of antibiotic resistance and mobile genetic element associated genes in Baltic sea sediments. FEMS Microbiol. Ecol. 92, fiw052. doi: 10.1093/femsec/fiw052
Nie D., Wu Y., Chen M., Liu H., Zhang K., Ge P., et al. (2018). Bioaccessibility and health risk of trace elements in fine particulate matter in different simulated body fluids. Atmos. Environ. 186, 1–8. doi: 10.1016/j.atmosenv.2018.05.024
Ohore O. E., Wang Y., Wei Y., Sanganyado E., Shafiq M., Jiao X., et al. (2023). Ecological mechanisms of sedimental microbial biodiversity shift and the role of antimicrobial resistance genes in modulating microbial turnover. J. Environ. Manage. 325, 116547. doi: 10.1016/j.jenvman.2022.116547
Ouyang W., Gao B., Cheng H., Zhang L., Wang Y., Lin C., et al. (2020). Airborne bacterial communities and antibiotic resistance gene dynamics in PM2.5 during rainfall. Environ. Int. 134, 105318. doi: 10.1016/j.envint.2019.105318
Parkhill J., Dougan G., James K. D., Thomson N. R., Pickard D., Wain J., et al. (2001). Complete genome sequence of a multiple drug resistant salmonella enterica serovar Typhi CT18. Nature 413, 848–852. doi: 10.1038/35101607
Parks D. H., Tyson G. W., Hugenholtz P., Beiko R. G. (2014). Stamp: Statistical analysis of taxonomic and functional profiles. Bioinformatics 30, 3123–3124. doi: 10.1093/bioinformatics/btu494
Piazzola J., Tedeschi G., Demoisson A. (2015). A model for the transport of sea spray aerosols in the coastal zone. Bound.-Layer Meteor. 155, 329–350. doi: 10.1007/s10546-014-9994-3
Prather K. A., Bertram T. H., Grassian V. H., Deane G. B., Stokes M. D., Demott P. J., et al. (2013). Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol. Proc. Natl. Acad. Sci. U. S. A. 110, 7550–7555. doi: 10.1073/pnas.1300262110
Qi W., Abel G. J., Liu S. (2021). Geographic transformation of China’s internal population migration from 1995 to 2015: Insights from the migration centerline. Appl. Geogr. 135, 102564. doi: 10.1016/j.apgeog.2021.102564
Qi J., Liu X., Yao X., Zhang R., Chen X., Lin X., et al. (2018). The concentration, source and deposition flux of ammonium and nitrate in atmospheric particles during dust events at a coastal site in northern China. Atmos. Chem. Phys. 18, 571–586. doi: 10.5194/acp-18-571-2018
Quinn P. K., Collins D. B., Grassian V. H., Prather K. A., Bates T. S. (2015). Chemistry and related properties of freshly emitted sea spray aerosol. Chem. Rev. 115, 4383–4399. doi: 10.1021/cr500713g
Santarpia J. L., Ratnesar-Shumate S., Gilberry J. U., Quizon J. J. (2013). Relationship between biologically fluorescent aerosol and local meteorological conditions. Aerosol Sci. Technol. 47, 655–661. doi: 10.1080/02786826.2013.781263
Sathicq M. B., Sbaffi T., Borgomaneiro G., Di Cesare A., Sabatino R. (2021). The meiofauna as neglected carriers of antibiotic resistant and pathogenic bacteria in freshwater ecosystems. J. Limnol 80. doi: 10.4081/jlimnol.2021.2054
Sato-Takabe Y., Hamasaki K., Suzuki S. (2019). High temperature accelerates growth of aerobic anoxygenic phototrophic bacteria in seawater. MicrobiologyOpen 8, e00710. doi: 10.1002/mbo3.710
Savoie D. L., Prospero J. M., Nees R. T. (1987). Nitrate, non-sea-salt sulfate, and mineral aerosol over the northwestern Indian Ocean. J. Geophysical Research: Atmospheres 92, 933–942. doi: 10.1029/JD092iD01p00933
Schlitzer R. (2021). Ocean Data View. Available online at: https://odv.awi.de (Accessed May 20, 2022).
Shao Y., Wang Y., Yuan Y., Xie Y. (2021). A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 798, 149205. doi: 10.1016/j.scitotenv.2021.149205
Shi J., Guan Y., Ito A., Gao H., Yao X., Baker A. R., et al. (2020). High production of soluble iron promoted by aerosol acidification in fog. Geophys. Res. Lett. 47, e2019GL086124. doi: 10.1029/2019GL086124
Siri Y., Precha N., Sirikanchana K., Haramoto E., Makkaew P. (2023). Antimicrobial resistance in southeast Asian water environments: a systematic review of current evidence and future research directions. Sci. Total Environ. 896, 165229. doi: 10.1016/j.scitotenv.2023.165229
Su Z., Huang B., Mu Q., Wen D. (2020). Evaluating the potential antibiotic resistance status in environment based on the trait of microbial community. Front. Microbiol. 11, 575707. doi: 10.3389/fmicb.2020.575707
Su H., Liu S., Hu X., Xu X., Xu W., Xu Y., et al. (2017). Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Sci. Total Environ. 607, 357–366. doi: 10.1016/j.scitotenv.2017.07.040
Suda K. J., Hicks L. A., Roberts R. M., Hunkler R. J., Taylor T. H. (2014). Trends and seasonal variation in outpatient antibiotic prescription rates in the United States 2006 to 2010. Antimicrob. Agents Chemother. 58, 2763–2766. doi: 10.1128/AAC.02239-13
Sun X., Li D., Li B., Sun S., Yabo S. D., Geng J., et al. (2020). Exploring the disparity of inhalable bacterial communities and antibiotic resistance genes between hazy days and non-hazy days in a cold megacity in northeast China. J. Hazard. Mater. 398, 122984. doi: 10.1016/j.jhazmat.2020.122984
Sun Y., Xu S., Zheng D., Li J., Tian H., Wang Y. (2018). Effects of haze pollution on microbial community changes and correlation with chemical components in atmospheric particulate matter. Sci. Total Environ. 637-638, 507–516. doi: 10.1016/j.scitotenv.2018.04.203
Tian H., Liu J., Sun J., Zhang Y., Li T. (2023). Cross-media migration behavior of antibiotic resistance genes (ARGs) from municipal wastewater treatment systems (MWTSs): Fugitive characteristics, sharing mechanisms, and aerosolization behavior. Sci. Total Environ. 893, 164710. doi: 10.1016/j.scitotenv.2023.164710
Tooke C. L., Hinchliffe P., Bragginton E. C., Colenso C. K., Hirvonen V. H. A., Takebayashi Y., et al. (2019). [amp]]Beta;-lactamases and β-lactamase inhibitors in the 21st century. J. Mol. Biol. 431, 3472–3500. doi: 10.1016/j.jmb.2019.04.002
USEPA (2002). EPA releases probabilistic risk assessment guidance for superfund (Washington, D.C: United States Environmental Protection Agency).
Wang C., Lu S., Zhang Z. (2019). Inactivation of airborne bacteria using different UV sources: Performance modeling, energy utilization, and endotoxin degradation. Sci. Total Environ. 655, 787–795. doi: 10.1016/j.scitotenv.2018.11.266
Wang Y., Wang C., Song L. (2019). Distribution of antibiotic resistance genes and bacteria from six atmospheric environments: Exposure risk to human. Sci. Total Environ. 694, 133750. doi: 10.1016/j.scitotenv.2019.133750
Wang B., Xu Z., Dong B. (2024). Occurrence, fate, and ecological risk of antibiotics in wastewater treatment plants in China: A review. J. Hazard. Mater. 469, 133925. doi: 10.1016/j.jhazmat.2024.133925
Wooten K. J., Mayer G. D., Smith P. N. (2019). Persistence of elevated concentrations of PM, affiliated pharmaceuticals, and tetracycline resistance genes downwind of feedyards. Environ. pollut. 247, 467–473. doi: 10.1016/j.envpol.2018.12.047
Wu J., Mao C., Deng Y., Guo Z., Liu G., Xu L., et al. (2019). Diversity and abundance of antibiotic resistance of bacteria during the seedling period in marine fish cage-culture areas of Hainan, China. Mar. pollut. Bull. 141, 343–349. doi: 10.1016/j.marpolbul.2019.02.069
Wu B., Qi C., Wang L., Yang W., Zhou D., Wang M., et al. (2020). Detection of microbial aerosols in hospital wards and molecular identification and dissemination of drug resistance of Escherichia coli. Environ. Int. 137, 105479. doi: 10.1016/j.envint.2020.105479
Xie J., Jin L., He T., Chen B., Luo X., Feng B., et al. (2019). Bacteria and antibiotic resistance genes (ARGs) in PM2.5 from China: Implications for human exposure. Environ. Sci. Technol. 53, 963–972. doi: 10.1021/acs.est.8b04630
Xiong W., Sun Y., Zhang T., Ding X., Li Y., Wang M., et al. (2015). Antibiotics, antibiotic resistance genes, and bacterial community composition in fresh water aquaculture environment in China. Microb. Ecol. 70, 425–432. doi: 10.1007/s00248-015-0583-x
Xu C., Wei M., Chen J., Wang X., Zhu C., Li J., et al. (2017). Bacterial characterization in ambient submicron particles during severe haze episodes at Ji’nan, China. Sci. Total Environ. 580, 188–196. doi: 10.1016/j.scitotenv.2016.11.145
Ying Q., Zhang J., Zhang H., Hu J., Kleeman M. J. (2021). Atmospheric age distribution of primary and secondary inorganic aerosols in a polluted atmosphere. Environ. Sci. Technol. 55, 5668–5676. doi: 10.1021/acs.est.0c07334
Zago V., Veschetti L., Patuzzo C., Malerba G., Lleo M. M. (2020). Shewanella algae and vibrio spp. Strains isolated in Italian aquaculture farms are reservoirs of antibiotic resistant genes that might constitute a risk for human health. Mar. pollut. Bull. 154, 111057. doi: 10.1016/j.marpolbul.2020.111057
Zainab S. M., Junaid M., Xu N., Malik R. N. (2020). Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 187, 116455. doi: 10.1016/j.watres.2020.116455
Zhang K., Liu S., Wu N., Xu W. (2022). Isotopic components and source analysis of inorganic nitrogen in coastal aerosols of the Yellow sea. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.993160
Zhang Q., Ying G., Pan C., Liu Y., Zhao J. (2015). Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 49, 6772–6782. doi: 10.1021/acs.est.5b00729
Zhang M., Zuo J., Yu X., Shi X., Chen L., Li Z. (2018). Quantification of multi-antibiotic resistant opportunistic pathogenic bacteria in bioaerosols in and around a pharmaceutical wastewater treatment plant. J. Environ. Sci. 72, 53–63. doi: 10.1016/j.jes.2017.12.011
Zhao W., Wang B., Yu G. (2018). Antibiotic resistance genes in China: occurrence, risk, and correlation among different parameters. Environ. Sci. pollut. Res. 25, ), 21467–21482. doi: 10.1007/s11356-018-2507-z
Zheng D., Yin G., Liu M., Chen C., Jiang Y., Hou L., et al. (2021). A systematic review of antibiotics and antibiotic resistance genes in estuarine and coastal environments. Sci. Total Environ. 777, 146009. doi: 10.1016/j.scitotenv.2021.146009
Zhu Y. G., Johnson T. A., Su J. Q., Qiao M., Guo G. X., Stedtfeld R. D., et al. (2013). Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. U. S. A. 110, 3435–3440. doi: 10.1073/pnas.1222743110
Keywords: marine aerosol, antibiotic resistance genes, bioaerosols, long-range transport, exposure risk
Citation: Jia S, Yao X, Qi J, Liu X and Gao H (2025) Abundance, occurrence, and degradation of airborne antibiotic resistance genes in coastal and marine atmospheres. Front. Mar. Sci. 12:1491484. doi: 10.3389/fmars.2025.1491484
Received: 05 September 2024; Accepted: 06 January 2025;
Published: 24 January 2025.
Edited by:
Tieyu Wang, Shantou University, ChinaReviewed by:
Chui Wei Bong, University of Malaya, MalaysiaChingakham Chinglenthoiba, National University of Singapore, Singapore
Copyright © 2025 Jia, Yao, Qi, Liu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiwang Gao, aHdnYW9Ab3VjLmVkdS5jbg==
 Shijie Jia
Shijie Jia Xiaohong Yao1
Xiaohong Yao1 Jianhua Qi
Jianhua Qi Xiaohuan Liu
Xiaohuan Liu Huiwang Gao
Huiwang Gao