Abstract
Coastal urbanization has significantly degraded coral reef habitats worldwide, often driving shifts from coral to algal dominance. Quantifying fish herbivory, a key ecological process mitigating such transitions, is essential for understanding reef health, functioning, and resilience. This study examined herbivory rates (bites multiplied by fish biomass) across five fish functional groups (detritivores, croppers, browsers, scrapers, and excavators) in relation to coral reef conditions along a gradient of urban influence in the Spermonde Archipelago, Indonesia. Herbivory rates generally increased from inshore to offshore sites, with notable differences among functional groups. Cropper and scraper herbivory varied significantly across sites, while detritivore and excavator rates were consistent. Browser herbivory was only observed at the most offshore site, highlighting potential vulnerability of the browsing function near urban centers. Environmental factors influenced herbivory rates in distinct ways. Detritivore herbivory was higher on reefs with lower rugosity, likely due to increased sediment accumulation on flatter substrates. Herbivory rates of all herbivorous fish, and of croppers, scrapers and excavators individually, were strongly correlated with the organic matter content of turf algae sediments, underscoring the importance of food quality in shaping herbivory dynamics. Experimental manipulation of turf algae sediments (clearing vs. control) did not affect herbivory rates, suggesting that the effects of sediment accumulation are not the main driver of herbivory patterns at the studied sites. Preserving functional and taxonomic diversity among herbivorous fish is critical for maintaining reef resilience amidst increasing urbanization and local stressors.
1 Introduction
The marine and coastal environment supports essential economic activities, including fishing, energy production, tourism, and transport. Consequently, approximately 40% of the global population resides within 100 km of the coastlines (IOC/UNESCO, IMO, FAO, UNDP, 2011), despite coastal areas covering only a small portion (<15%) of the planet’s surface (Cohen and Small, 1998; UNEP, 2006). Most of these coastal communities live in the tropical belt (Spalding et al., 2023), including Southeast Asia (SEA). This region includes the Coral Triangle, a 6 million km2 area spanning Indonesia, Malaysia, the Philippines, Papua New Guinea, Timor Leste, and the Solomon Islands, which is a global center of marine biodiversity (Veron et al., 2009).
The Coral Triangle supports a diverse community of marine biota, including 55% of Indo-Pacific reef fish species (Allen, 2008). Many reef fish are ecologically, economically, and socially important for ecosystems and coastal communities. Herbivorous fish, which primarily feed on plant and algal material, including detritus (Crossman et al., 2001; Tebbett et al., 2024a), can prevent coral overgrowth by algae, facilitate coral recruitment by clearing settlement substrate, and thereby maintain reef health and resilience (Bellwood et al., 2004; Mumby and Harborne, 2010). Coastal communities throughout the region also depend economically and socially on reef fish resources for fisheries and livelihoods (Burke et al., 2012; Estradivari et al., 2022). Indonesia, the Philippines, and Malaysia are among the world’s top 20 capture fisheries producers, contributing 12% of global fisheries production (FAO, 2020), approximately 30% of which are reef-associated fishes and invertebrates (Geronimo and Cabral, 2013).
Since the 1950s, coastal areas worldwide, including the Coral Triangle, have rapidly urbanized (Blackburn et al., 2013; Heery et al., 2018). Coastal urbanization often involves extensive land reclamation, land-use change, population expansion, and coastal activities such as fisheries, tourism, and shipping (Heery et al., 2018; Zweifler et al., 2021). These urbanization impacts can harm coral reefs. For instance, over-accumulating sediments can smother coral reefs, excessive polluted runoff can contaminate marine waters, dredging and land-use change can damage coastal ecosystems, and overfishing can disrupt ecological dynamics. Coastal urbanization increasingly damages inshore reefs, often triggering overgrowth by algae or other alternative taxa that outcompete corals (Baum et al., 2015; Bauman et al., 2017; Cleary et al., 2014; Heery et al., 2018; Teichberg et al., 2018). In the long term, it reduces the capacity of reefs to provide essential ecosystem functions and services (Barros et al., 2021; Poquita-Du et al., 2019).
Herbivory by reef fishes, the activity of feeding on algae and detritus, plays a significant role in controlling algal growth. Without this control, algae can outcompete corals for space and resources, disrupting the functioning of coral reef ecosystems (Green and Bellwood, 2009; Tebbett and Bellwood, 2019). Fish herbivory is a critical ecological process for maintaining coral reef health and preventing phase shifts from coral to algae dominance (Done, 1992; Hughes, 1994). Different functional groups of herbivorous fish play distinct roles in maintaining reef health. Detritivores, like the surgeonfish Ctenochaetus striatus, use bristle-like teeth to feed on detritus and turf algae (Purcell and Bellwood, 1993), supporting organic matter recycling (Wilson et al., 2003). Croppers/grazers, hereafter croppers, including some surgeonfish and rabbitfish, use lined teeth to crop turf algae, inhibiting algae growth and promoting coral recruitment and survival (Tebbett et al., 2022). Browsers, such as unicornfish and rabbitfish, consume macroalgae, controlling their abundance and preventing coral overgrowth by macroalgae (Green and Bellwood, 2009). Scrapers and excavators, primarily parrotfish, possess fused beak-like jaws to scrape the epilithic algal matrix, removing turf, macroalgae, detritus, and parts of the calcareous reef matrix (Bonaldo and Bellwood, 2009; Green and Bellwood, 2009). The primary distinction between the two is in their feeding behaviors and ecological impacts. Scrapers take rapid bites that scrape the surface without significant excavation, thereby controlling algal growth without substantially altering the substratum (Bellwood and Choat, 1990; Bonaldo and Bellwood, 2009). Conversely, excavators take powerful bites that gouge the substratum, leaving visible scars. They contribute to bioerosion and create space for new coral recruits (Bellwood and Choat, 1990; Bonaldo and Bellwood, 2009).
Understanding fish herbivory dynamics, especially on reefs near large coastal cities, is important given the strong environmental pressures these systems face. Coastal urbanization often increases nutrient inputs, sedimentation, and pollution, which can promote algal growth and reduce coral cover (Wolanski et al., 2009), affecting fish herbivory. On degraded turbid reefs, herbivory may diminish when turf algae become sediment-laden and have low organic content (Goatley and Bellwood, 2012; Gordon et al., 2016; Tebbett et al., 2017). Conversely, nutrient enrichment promotes macroalgal growth and increases herbivorous fish feeding (Burkepile and Hay, 2009). Nevertheless, high macroalgal cover can overwhelm the ability of herbivorous fish to feed (Hoey and Bellwood, 2011; Williams et al., 2001). This can result in lower overall herbivory rates on degraded reefs than healthier reefs with higher coral cover (Chong-Seng et al., 2014; Paddack et al., 2006), hindering coral recovery. Additionally, intense fishing of specific fish groups may further exacerbate herbivory loss by depleting key species or disrupting fish populations within reef ecosystems (Bellwood et al., 2004; Roberts, 1995).
Understanding the complex dynamics of algae, coral, and herbivory interactions in urban reefs is vital for effective management and supporting coral reef resilience to cope with ongoing disturbances. This is critically important because over 85% of reefs in the Coral Triangle are severely threatened by diverse human activities, including coastal development (Burke et al., 2012). Comprehensive data on fish herbivory can help managers design targeted measures to reduce stressors, restore degraded reefs, improve resilience, or guide fisheries management. As the Coral Triangle is a global conservation priority area (Jenkins and Van Houtan, 2016), managers must implement practical solutions that fit local conditions and contexts.
The Spermonde Archipelago (South Sulawesi, Indonesia) is representative of the conditions that prevail in many parts of the Coral Triangle, making it an ideal backdrop for a cross-shelf fish herbivory study. Since the 1950s, the Archipelago has experienced the effects of the rapid urbanization of Makassar, a waterfront provincial city of over 1.4 million inhabitants (BPS, 2022), and surrounding satellite cities along the South Sulawesi coastlines. Environmental influences from the mainland include sewage input, agricultural runoff, pollution, and sedimentation from land reclamation. Additionally, intensive and destructive fishing further pressures reefs (Destructive Fishing Watch, 2003; Munsi et al., 2017; Pet-Soede and Erdmann, 1998). Notably, these factors create a pronounced inshore-to-offshore gradient in water quality, sedimentation, and coral reef conditions across the Archipelago (Plass-Johnson et al., 2018a; Polónia et al., 2015; Teichberg et al., 2018).
In this study, we leverage this human-induced cross-shelf gradient to investigate the herbivory rates of five fish functional groups across a continuum of coral reef conditions at increasing distances from the South Sulawesi mainland. Specifically, we test (1) whether herbivory rates are higher on coral-dominated than on algae-dominated reefs and (2) whether sediments entrapped within turf algae affect fish herbivory. To address these questions, we quantified reef conditions, measured fish herbivory rates, and conducted turf algae sediment-removal experiments at eight sites along a coastal urbanization gradient.
2 Methods
2.1 Study sites
The Spermonde Archipelago lies in central Indonesia within the Coral Triangle, between the southern arc of Sulawesi and the Makassar Strait in South Sulawesi Province. It comprises ca. 70 islands, of which around 50 are inhabited (Ferse et al., 2014, Figure 1). Coastal urbanization of Makassar started in the 17th century during the Dutch colonial period (Knaap and Sutherland, 2004) and accelerated after the 1950s, driven by economic growth and migration (Hugo, 1982). The city then expanded into suburbs, prompting substantial land-use changes and land expansions along coastal areas (Surya et al., 2020, 2021). Since 2017, a massive land reclamation project has created 157 ha for the “Center Point of Indonesia,” featuring business centers, housing, public facilities, and green spaces surrounding riverine outflows (Langkoke et al., 2022).
Figure 1

Map of the Spermonde Archipelago, Indonesia, and the eight study sites.
The rapid development in Makassar and its satellite cities has caused high river contamination, and most of these effluents reach the Archipelago as runoff from major rivers in the Makassar and Maros districts (Figure 1). Habitat alteration, including coastal reclamation projects, has substantially increased nearshore sedimentation (Langkoke et al., 2022). Recent studies indicate that the inshore waters are polluted (Faizal, 2022; Lestari et al., 2021; Retnaningdyah et al., 2019; Tahir et al., 2018), with several water quality variables exceeding Indonesian standards. Elevated nutrient concentrations also reach offshore, where eutrophic waters expanded tenfold – up to nearly 10 km from the mainland – between 1996 and 2020 (Faizal, 2022).
To examine inshore-to-offshore urbanization effects, eight study sites were selected with increasing distances from the mainland (perpendicular to the South Sulawesi mainland shoreline): Lae-Lae (1LL, 1 km from mainland), Samalona (2SA, 7 km), Barang Lompo (3BL, 17 km), Bonetambung (4BO, 22 km), Badi (5BA, 23 km), Lumu-Lumu (6LU, 31 km), Karang Kassi (7KS, 39 km), and Kapoposang (8KP, 62 km, Figure 1). All sites, except 7KS which is a shallow reef bank, are on fringing reefs around inhabited islands. We chose the northwest side of each island or reef bank for surveys and experiments because this side has better reef development than other sides (Plass-Johnson et al., 2015), except at 8KP, where transects were laid on the northeast side for logistical reasons. Site 8KP lies within the Kapoposang marine protected area (MPA), located in a traditional fisheries zone that allows small-scale fishing. Field data collection was conducted in August 2022.
2.2 Benthic and turf algae sediment assessments and fish herbivory experiment
Four 1×1-m PVC quadrats were placed at 4–7 m depth at each site (Figure 2a), except for 1LL, where they were positioned at 3 m due to poor visibility. Given the diverse habitat characteristics across inshore-to-offshore gradients, quadrats were placed with two considerations: relatively flat substrates (slope <30 degrees) and having representative turf algal cover, which was ranging from 5% at 8KP to 40% at 1LL. Nine overlapping ~35×35-cm close-up images of the area enclosed within the quadrat were taken from above the quadrat grid and merged into a 1x1-m image using the automatic photo merge feature in Adobe Photoshop 2023®. Furthermore, half of each quadrat (0.5×1 m) was carefully covered with a weighted transparent plastic sheet to serve as a control area.
Figure 2
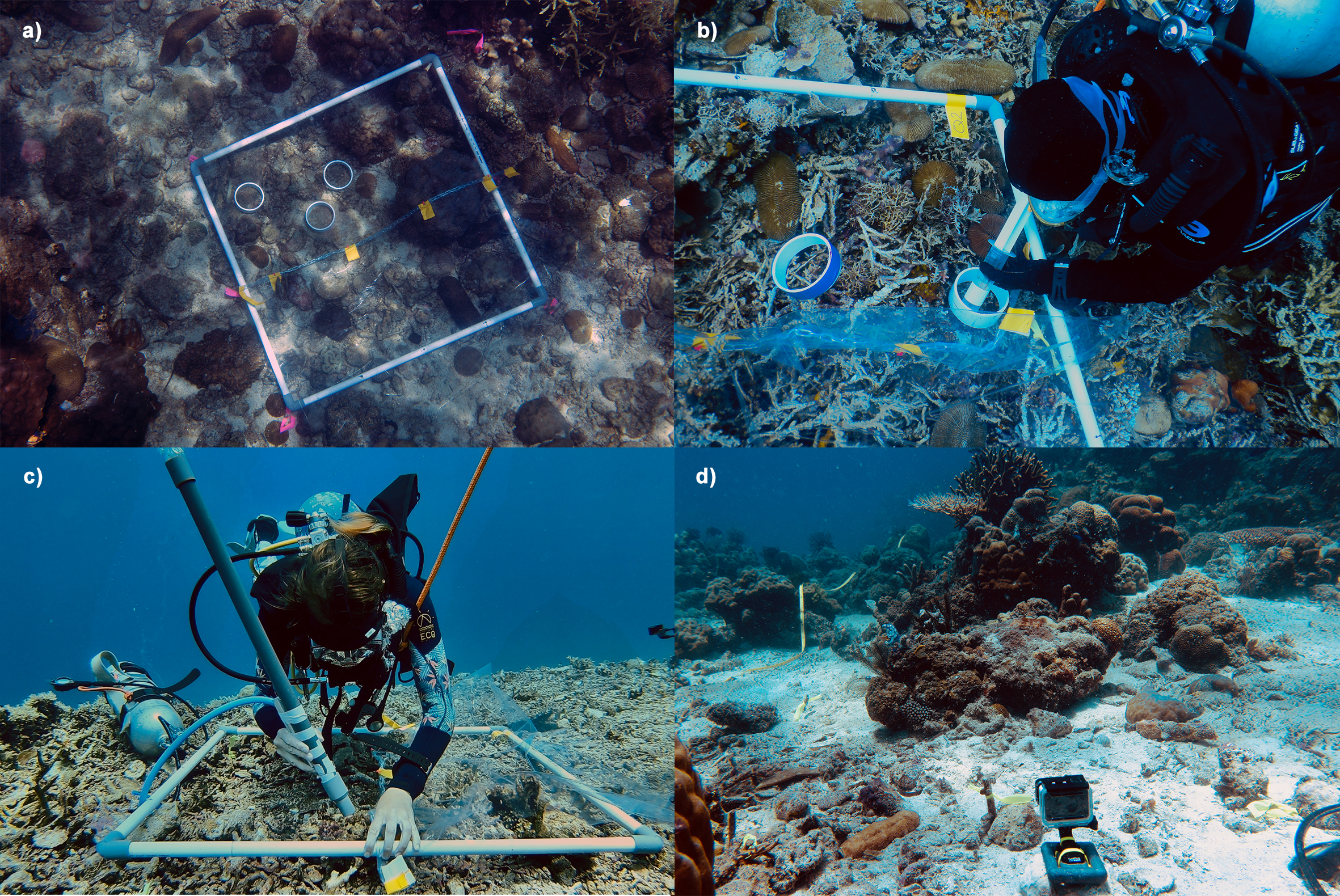
Experimental procedure to test the effect of turf algae sediments on fish herbivory. (a) 1x1 m quadrat divided into two sections for treatment (sediment removed, open area) and untreated areas (control, area covered with plastic), (b) three PVC rings were placed on the treatment area, and turf algae sediments within the ring were carefully collected with syringes, (c) entire algal turf sediments in the treatment area were cleaned with a portable suction device, (d) a GoPro camera recorded the entire quadrat area for at least 1 hour (Images: Marleen Stuhr (a, c, d), Andi M. A. Pratama (b)).
Turf algae sediment loads (g/m2) and composition (inorganic/organic) were measured in the treatment area using three 10-cm diameter PVC rings placed on turf algae mats (Figure 2a). Sediment, which had settled within turf algal and may include bound detritus, within each ring was carefully suctioned using four to eight 200-mL syringes without damaging the algae (Figure 2b). After collection, the rings were removed. The entire treatment area was vacuumed with a SCUBA tank-powered suction device to create a “sediment-free” area (Figure 2c). This manipulation aimed to compare fish herbivory on substrata with and without sediments/detritus following Tebbett and Bellwood (2019). A high-resolution video camera (GoPro Hero 3 or 6) was positioned one to two meters from each quadrat, with four markers on the quadrat edges and two at the center of each horizontal/vertical frame within view. Subsequently, the camera recorded continuously for at least one hour after careful removal of the plastic sheet and quadrat (Figure 2d). Fish herbivory observations occurred between 11:00 and 14:00, coinciding with peak feeding activity for many herbivorous fishes (Bonaldo and Bellwood, 2008; Khait et al., 2013).
In the laboratory, very fine sediments were removed by sieving samples through a 58 µm mesh, as these sediments frequently became suspended in the water column and could be expelled from the syringe tips during data collection, potentially compromising the reliability of sampling for this fraction. Sediments larger than 58 µm were washed three times with distilled water, then filtered through pre-weighed filter paper (Whatman 41, pore-size 25 µm) using a peristaltic vacuum pump. The samples were dried for one hour at 105°C in a drying oven to yield total particle loads, with weights measured using an analytical digital scale (Mettler Toledo ME204E). To separate inorganic and organic sediment fractions, the total particle load was combusted at 650°C for four hours to oxidize organic matter (Zhang and Wang, 2014). Weight loss upon combustion indicated organic sediment, while the residuals represented inorganic sediment.
We used Coral Point Count with Excel extensions (CPCE; Kohler and Gill, 2006) to calculate the percentage of ten benthic categories (live corals, turf algae, macroalgae, soft corals, crustose coralline algae, cyanobacteria, sponges, other living organisms, recently dead coral, and sand) in each quadrat. Each benthic image was examined with 100 uniformly distributed data points, resulting in 50 points per treatment (with and without sediment removal). Benthic groups were classified by the topmost layer; e.g., dead coral covered by cyanobacteria was categorized as cyanobacteria. The turf algae category included short productive algal turfs (SPAT), long sediment-laden algal turfs (LSAT), and mixed turf algae assemblages. Differentiation was limited by image resolution, especially for images from inshore reefs. Only live corals, turf algae, and macroalgae data were analyzed due to their relevance to this study.
Herbivorous fish species are often classified into functional groups based on their contributions to herbivory (Ladds et al., 2018). We focused on five functional groups: detritivores, croppers, browsers, scrapers, and excavators, reflecting their distinct diets, feeding mechanisms, and ecological roles. Given the high diversity of herbivorous species recorded in the surveys (49 species), we followed the general functional group classification from www.datamermaid.org (accessed July 2023, Supplementary Table S1), which includes an extensive species list compiled from FishBase (Froese and Pauly, 2010). As herbivorous fishes may feed on various algae and microorganisms (Choat et al., 2002), potential overlap exists among the functional groups. Therefore, while functional groups are used as the unit of analysis, a closer look at individual species is included to provide deeper insights into their ecological roles in urbanized reef systems.
Due to poor water visibility at 1LL, small herbivorous fish (<5cm TL) were excluded at all sites to avoid identification bias. These small individuals contributed <1% of total recorded bites, making their impact negligible. The first and last 15 minutes of each video were discarded to eliminate diver disturbance effects during quadrat setup and recording. Herbivory rates were quantified over 30 minutes per video, totaling 14.5 hours across 29 quadrats at eight study sites. Four quadrats were filmed per site except at 2SA (n=3) and 3BL (n=2) due to camera malfunctions.
For each feeding fish, we recorded the species, functional group, estimated total length (TL, cm), bite count, and feeding location (treatment/control). These data were converted into mass-standardized bite rates, hereafter referred to as “herbivory rates” for simplicity, by multiplying the biomass of each fish (kg) by its bite count (Hoey and Bellwood, 2009; Plass-Johnson et al., 2015). Fish biomass was estimated via length-weight relationships (Kulbicki et al., 2005) using conversion parameters from FishBase (Froese and Pauly, 2010). Herbivory rates were expressed as “bites × kg/30 mins.” Fish species identification, TL, and bite counts were visually estimated from the videos by a trained observer (PLK) using the quadrat width as a reference.
2.3 Reef rugosity and roving fish biomass observations
We estimated reef rugosity and roving fish biomass within three 50 m transects at 7–9 m depth, slightly deeper than the quadrats (see 2.2), to minimize disturbance to fish herbivory during the experiment. Rugosity was measured using the chain intercept method (Hill and Wilkinson, 2004), where a 20 m chain was laid along the transect tape following the reef contour. A rugosity index (C) was calculated as C=1-d/l, where d is the horizontal distance the chain covered, and l is its fully extended length. A higher index indicates greater reef structure complexity. Roving fish surveys were conducted with underwater visual censuses (UVCs) along 5×50 m transects, with three replicates per site (Hill and Wilkinson, 2004). A trained observer (PLK) recorded detritivore, cropper, browser, scraper, and excavator fishes ≥5 cm TL within 2.5 m of either side of the tape. Fish identity and size (TL to the nearest cm) were recorded, and biomass was calculated using the length-weight conversion parameters described above.
2.4 Statistical analyses
We conducted a two-step analysis to test whether the composition of herbivory differed among sites and treatments. First, we used herbivory rate data from all five functional groups and performed a two-way analysis of similarity (ANOSIM, Clarke and Warwick, 1994) with treatments nested within study sites. Herbivory rates were fourth root-transformed, and Bray-Curtis similarity was used to measure the distance between herbivory rates on 9,999 permutations. The two-way ANOSIM showed significant differences in herbivory rates among sites (p-value <0.001, R=0.409, Supplementary Table S1) but no differences between treatments.
In the second step, we identified functional groups with different herbivory rates among sites. Kruskal-Wallis tests were used for detritivore herbivory rates due to the presence of only one species, while one-way ANOSIM was applied to cropper, scraper, and excavator rates. Browser herbivory rates were excluded, as they occurred at only one site. Treatment and control area data were averaged at the replicate level to avoid pseudoreplication, as the first analysis detected no effect of treatment. Herbivory rates were fourth root-transformed, and Bray-Curtis similarity was calculated before ANOSIM analysis. When statistical differences were observed, pairwise analysis and similarity percentages (SIMPER, Clarke, 1993; Clarke and Warwick, 1994) were used to identify sites with different herbivory rates and discriminating species driving the overall pattern. For both analysis steps, replicates with no herbivory data were removed to increase the analytical power, as the statistical methods were sensitive to zero values. Therefore, the analysis focused on sites where herbivory activity was recorded. While this approach provided a clearer picture of how the distance of the reef site to the mainland affects these rates, in some cases, this resulted in a low sample size.
Last, we employed multiple linear regression for detritivores and biota and/or environmental matching (BIO-ENV) analysis (Clarke and Ainsworth, 1993) for croppers, scrapers, excavators, and all herbivorous fish combined to determine the environmental and ecological factors influencing the herbivory rates. This analysis used six reef parameters (i.e., percentage of organic matter in turf algae sediments, turf algae, macroalgae, and live coral cover, reef rugosity, and total roving herbivorous fish biomass). We used the percentage of organic matter in turf algae sediment, calculated by dividing the organic sediment loads by total (inorganic + organic) sediment loads, considering that this variable is one of the best predictors of fish herbivory (Tebbett et al., 2024b). Due to differing replicate numbers, analyses were conducted in two steps: first, using the percentage of organic matter in turf algae, macroalgae, and live coral cover variables at the replicate (quadrat) level; second, using reef rugosity and UVC’s total fish biomass variables at the site level. For the second step, given the low sample size (between five and eight) and to increase statistical power, the biomass of roving herbivorous fish was aggregated across all functional groups to obtain total roving biomass, thereby reducing the number of independent variables.
Prior to analysis, multicollinearity among independent variables was assessed using Spearman’s rank correlation. Since no variables showed high collinearity (Spearman’s ρ < 0.8), all were retained. Herbivory rates were fourth-root transformed, while macroalgae and organic matter percentages were log-transformed to reduce skewness. After transformations, all variables were standardized to have a mean of zero and a standard deviation of one. Linear regression assumptions were checked using diagnostic tests, including Levene’s test for homoscedasticity, the Shapiro-Wilk test for normality, and QQ plots for residuals.
For the BIO-ENV analysis, a Bray-Curtis similarity matrix was calculated for herbivory rates of croppers, scrapers, excavators, and all herbivorous fish combined. BIO-ENV was then performed, applying Spearman’s rank correlation and a permutation test with 9,999 iterations to assess statistical significance. Directions were visualized through principal component analysis (PCA) when significant relationships were detected.
Given the exploratory nature of this study and the limited sample size, a significance threshold of p < 0.1 was used to identify potential patterns and relationships for further investigation. Some analyses (Kruskal-Wallis, linear regression, and PCA) were performed using R 4.2.3 (R Core Team, 2023), while other analyses (ANOSIM, SIMPER, and BIO-ENV) were done using PRIMER v7 (Clarke and Gorley, 2015).
3 Results
3.1 Benthic reef condition
Benthic reef condition showed spatial patterns along an inshore-offshore gradient (Figure 3). Across the Spermonde Archipelago, percentage of organic matter within turf algae (mean ± SE = 29.0 ± 8.7 g/m2), reef rugosity (0.325 ± 0.009) and live coral cover (16.9 ± 3.3%) generally increased from inshore to offshore reefs, while inorganic and organic algal turf sediment loads (125 ± 64.7 g/m2 and 14.6 ± 6.4 g/m2, respectively), turf algae (22.8 ± 5.5%) and macroalgae (9.4 ± 4.5%) covers decreased. The nearest inshore site (1LL) was characterized as an algae-dominated reef, indicated by having 75% turf and macroalgal cover and 2% live coral cover, with Sargassum and Padina species thriving well at this site. In contrast, the offshore reefs (6LU to 8KP) displayed the lowest turf algae sediment loads (inorganic <1–14 g/m2; organic 2.7–3.7 g/m2), highest live coral cover (21–31%), and highest reef rugosity (0.298–0.407), indicating better overall reef condition compared to other study sites.
Figure 3

Environmental and ecological variables indicating reef conditions across eight study sites in the Spermonde Archipelago. (a) turf algae sediment composition, (b) percentage of organic matter in turf algae sediment, (c) reef rugosity, (d) benthic cover of turf algae (left panel), macroalgae (middle panel), and live coral (right panel). Wide view (top) and close-up (bottom) images of habitat conditions and representative turf algae assemblages across the spatial gradient, (e) Lae-lae (1LL, 1 km from mainland) was dominated by silt substrate with a long, dense algae assemblage and thick sediment loads, (f) Barang Lompo (3BL, 17 km) had a mixed-bottom substrate of sand, live corals, and dead corals covered by dense algae assemblage with medium sediment loads, (g) Lumu-lumu (6LU, 31 km) and (h) Kapoposang (8KP, 62 km) had mixed substrates dominated by live corals, rubble and dead corals covered by shorter, sparser turf algae assemblage with very low sediment loads. Study sites are ordered by distance from the mainland, from 1LL (1 km) to 8KP (62 km). Wide-view images (top) by Estradivari and close-up images (bottom) by Andi M. A. Pratama.
A shift in turf algae composition was observed from inshore to offshore reefs. The substrate at 1LL was dominated by silt, and long, dense, sediment-laden turf algae could be found covering dead corals (Figure 3e). Site 3BL (Figure 3f), in contrast, had a mixture of sand, live coral, and dead coral substrate, and the turf algae formed a dense assemblage with medium sediment loads. Meanwhile, mid-to-offshore reefs displayed a range of turf algae characteristics: from very sparse, short turf algae (Figure 3g) on relatively flat, smooth substrates to slightly longer, denser mixtures with cyanobacteria, small fleshy algae, or other microorganisms (Figure 3h) on rubbles or broken dead corals. Further, mid-to-offshore turf algae consistently had low sediment loads (Figure 3a).
3.2 Roving herbivorous reef fish community
We recorded 12,750 herbivorous fishes from 45 species across five families (Acanthuridae, Pomacanthidae, Pomacentridae, Labridae, and Siganidae) in 24 visual census transects at eight sites (Supplementary Table S1). The average biomass of all herbivorous fish species ranged from 29.4 ± 22.7 (1LL) to 861.5 ± 202.1 (8KP) kg/ha, with an average of 389.5 ± 90.1 kg/ha (Figure 4a). These species were categorized by their feeding habits into detritivores (3 species), croppers (21 species), browsers (4 species), scrapers (13 species), and excavators (4 species, Figure 4). Fish biomass varied among functional groups, with browsers and detritivores showing the lowest biomass (31.9 ± 14.7 and 32.2 ± 14.6 kg/ha, respectively), while croppers and excavators exhibited the highest (138.9 ± 37.6 and 118.0 ± 34.8 kg/ha, respectively, Figure 4a). Biomass generally increased offshore for all groups except scrapers, which decreased with distance from the mainland (Figure 4). However, sites 6LU and 7KS showed reduced biomass for croppers, scrapers, and excavators, with browsers absent at 7KS (Figure 4a). A heatmap analysis (Figure 4b) revealed key species with high biomass within each functional group. For example, Siganus virgatus, S. vulpinus, and Pomacentrus species were major contributors among croppers, Ctenochaetus striatus among detritivores, and Chlorurus bleekeri among excavators. Almost half of the species recorded during the visual censuses (bold texts, Figure 4B) fed within the quadrats. The site closest to the mainland (1LL) exhibited the lowest fish diversity and biomass, with only two cropper and one browser species (Dischistodus prosopotaenia, Siganus virgatus, and S. canaliculatus) present (Figures 4a, b). Similarly, browsers were not observed at all at 2SA.
Figure 4

Roving fish biomass (kg/ha) across the Spermonde Archipelago recorded during the visual censuses. (a) Boxplots of roving fish biomass of five functional groups, i.e., detritivores, croppers, browsers, scrapers, excavators, and all herbivorous fish combined. Note that the y-axis range for all herbivorous fish (bottom right panel in a) differs from other panels. (b) Shade plot of the fourth root transformed fish biomass of 45 herbivore species from five functional groups. The scale intensity keys have units back-transformed to the original fish biomass measurements. The names of fish species in bold indicate that these were recorded feeding within the quadrats. Study sites are ordered based on distance from the mainland, from 1LL (1 km) to 8KP (62 km).
3.3 Fish herbivory rates
A total of 25 herbivorous fish species from five functional groups were observed feeding within the quadrats: a detritivore (1 species), croppers (12 species), browsers (2 species), scrapers (8 species), and excavators (2 species, Figure 5a). All species recorded performing herbivory were found roving in the surrounding reefs (Figure 4b), except for four cropper species (Figure 5b, species names with asterisk). We recorded 1,538 individual fish taking 12,956 bites within the quadrats, ranging from 1 to 116 bites per fish. The mean herbivory rates across the Spermonde Archipelago were 27.1 ± 22.9 bites.kg.30 mins-1, ranging from 0.6 (1LL) to 187.1 (7KS) bites.kg.30 mins-1. Excavators had the highest average herbivory rate (53.7 ± 47.9 bites.kg.30 mins-1), followed by scrapers (19.3 ± 6.5 bites.kg.30 mins-1), and browsers with the lowest rate (1.2 bites.kg.30 mins-1). High average herbivory rates by excavators were primarily driven by exceptionally high rates at 7KS, with values more than 25 times higher than those at other sites. At all sites except 7KS, scraper herbivory rates (<1–40 bites.kg.30 mins-1) were generally higher than those of excavators (2-11 bites.kg.30 mins-1). Herbivory rates for all functional groups typically increased from nearshore to offshore sites, though site-level variations were noted (Figure 5a). Cropper and excavator herbivory rates increased offshore, but cropper rates dropped at 6LU, while excavator rates showed a substantial increase at 7KS. Herbivory by detritivores, scrapers, and excavators was not observed at 1LL, 3BL (except scrapers), and 5BA, and browsers were only found feeding at 8KP.
Figure 5
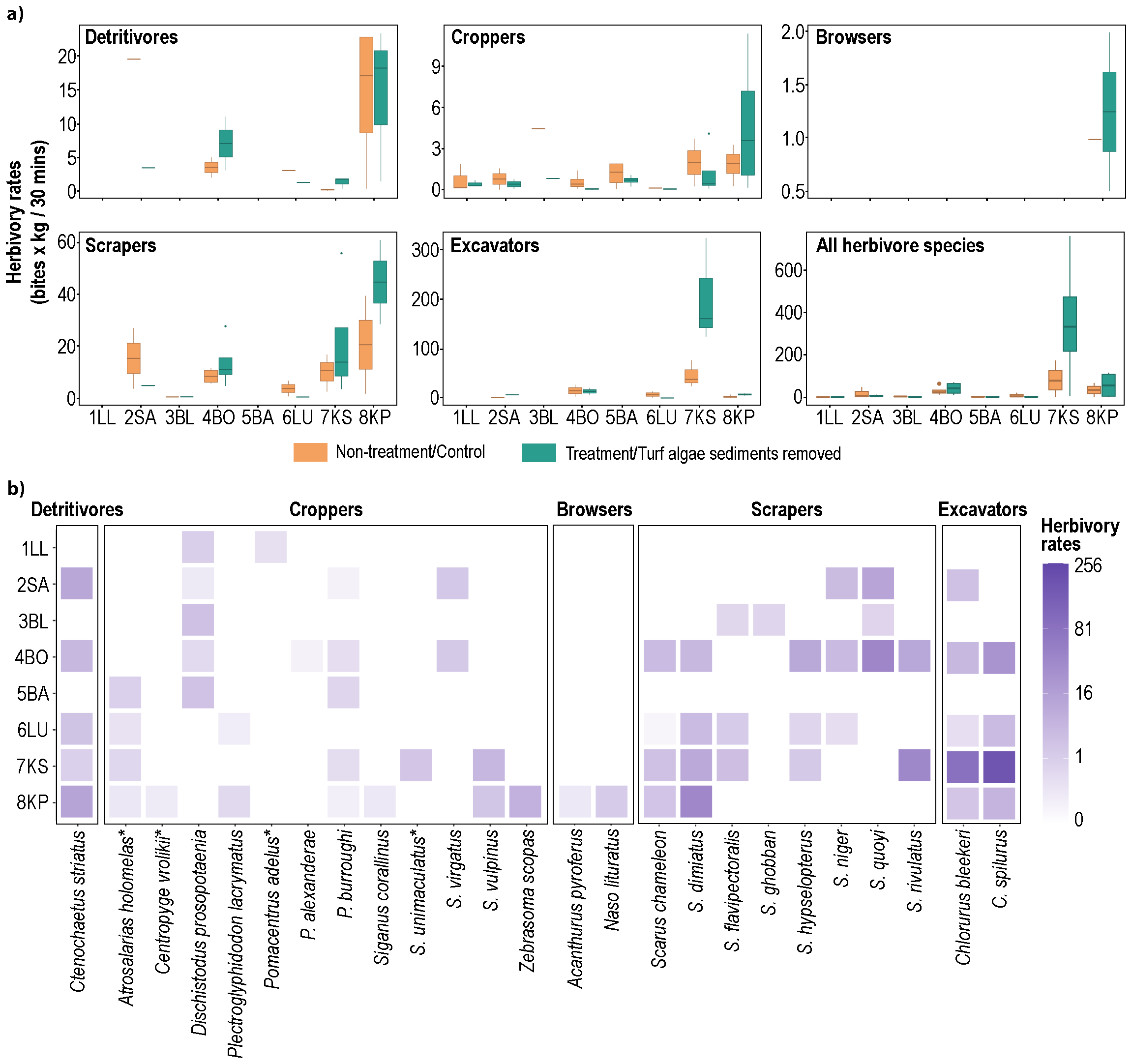
Fish herbivory rates across the Spermonde Archipelago. (a) Boxplots of herbivory rates of five fish functional groups, i.e., detritivores, croppers, browsers, scrapers, excavators, and all herbivorous fish combined, with treatment (sediments removed from turf algae, green bars) and no treatment (control, orange bars). Note that the y-axes differ between panels. (b) Shade plot of the fourth root transformed herbivory rates of 25 species from five functional groups. The scale intensity keys have units back-transformed to the original herbivory rate measurements. Fish species with an asterisk (*) indicate the species were not observed during the visual censuses. Study sites are ordered based on distance from the mainland, from 1LL (1 km) to 8KP (62 km).
The herbivory rates of all herbivore species differed significantly among sites (ANOSIM sites, p-value=0.001, R=0.409), but not between treatments (Supplementary Table S2). Further analysis showed cropper and scraper herbivory rates contributed to site-specific differences (ANOSIM cropper, p-value <0.001, R=0.389; ANOSIM scraper, p-value <0.001, R=0.434, Supplementary Tables S3, S4), while detritivore and excavator rates were similar across sites (Supplementary Table S5). Pairwise analysis showed that several sites had different herbivory rate compositions. For example, cropper herbivory rates were significantly lower at 3BL and 5BA compared to 7KS and 8KP (Supplementary Table S3), and scraper rates were higher at 7KS than at 2SA (Supplementary Table S3).
Due to significant differences in cropper and scraper herbivory rates, we conducted SIMPER analysis to identify the primary discriminating species contributing significantly to group dissimilarity (noted by the highest average dissimilarity/Av.Diss in SIMPER results). Five cropper species (Astrosalarias homomelas, Dischistodus prosopotaenia, Pomacentrus burroughi, Siganus virgatus, and Zebrasoma scopas, Supplementary Table S6) and three scraper species (Scarus dimidiatus, S. quoyi, and S. rivulatus, Supplementary Table S7) primarily drove these differences. The distribution and herbivory activity of these discriminating species reflected environmental gradients from nearshore to offshore. For example, nearshore reefs were mainly influenced by the cropper D. prosopotaenia (1LL and 3BL) and the scraper S. quoyi (2SA), while the outermost site (8KP) exhibited unique discriminating species due to their high herbivory rates there, i.e., Z. scopas and S. dimidiatus. The remaining discriminating species mostly drove the differentiation of herbivory rates in the middle reef zone.
The composition of herbivory rates was affected by several reef variables that differed for each functional group (Table 1). Reef rugosity was linked to detritivore herbivory rates (Multiple linear regression, p-value = 0.07). Overall, herbivory rates of the three functional groups and all herbivorous fish were consistently influenced by the percentage of organic matter in turf algae sediments (BIO-ENV results, p-value < 0.1, Table 1, Supplementary Tables S8–S11, Supplementary Figure S12). In addition, croppers were influenced by reef rugosity and roving fish biomass (BIO-ENVcropper, p-value = 0.08), scrapers by turf algal cover (BIO-ENVscraper, p-value = 0.02), and all herbivorous fish combined by macroalgae and live coral covers (BIO-ENVallherb, p-value = 0.02). The PCA results revealed distinct relationship directions between herbivory rates of different fish functional groups and environmental variables (Supplementary Figure S12). For example, the percentage of organic matter in turf algae sediment was positively associated with excavator herbivory rates, particularly at 7KS, and negatively associated with cropper herbivory rates at 8KP.
Table 1
| Functional groups/reef variables | t-value | Sample statistic (ρ) | p-value |
|---|---|---|---|
| Detritivores | |||
|
Percentage of organic matter within turf algae sediment
Turf algae Macroalgae Live corals Reef rugosity Roving fish biomass |
0.727 0.364 -1.031 -0.695 -3.599 1.951 |
0.49 0.73 0.34 0.51 0.07 0.19 |
|
| Croppers | |||
|
Percentage of organic matter within turf algae sediment
Reef rugosity, Roving fish biomass |
0.355
0.393 |
<0.01
0.08 |
|
| Scrapers | |||
|
Turf algae, Percentage of organic matter within turf algae sediment
Reef rugosity |
0.320
0.302 |
0.02
0.32 |
|
| Excavators | |||
|
Percentage of organic matter within turf algae sediment
Reef rugosity |
0.253
0.379 |
0.09
0.41 |
|
| All herbivorous fish | |||
|
Percentage of organic matter within turf algae sediment,
Macroalgae, Live corals Reef rugosity, Roving fish biomass |
0.235
0.195 |
0.02
0.32 |
|
Reef variables affecting variation in herbivory rates of detritivores, croppers, scrapers, excavators, and all herbivorous fish combined.
Two different statistical analyses were used, i.e., multiple linear regression for herbivory rates of detritivores and BIO-ENV analysis for the herbivory rates of croppers, scrapers, excavators, and all herbivorous fish combined. This table shows only the best results with the highest correlation value for the BIO-ENV analysis. Significant relationships are highlighted in bold. Browser herbivory rates were excluded from the analysis because their feeding was observed at only one site (8KP).
4 Discussion
The Spermonde Archipelago is one of the few well- and long-studied reef areas within the Coral Triangle. It serves as a model system for understanding the effects of coastal urbanization and long-term human activities on reefs, pressures that are becoming increasingly frequent in many coastal areas around the Coral Triangle (Burke et al., 2012; Heery et al., 2018). Although our study is not the first fish herbivory study in the Archipelago (see Husain et al., 2013; Plass-Johnson et al., 2015, 2016, 2018b), it offers insights related to a crucial knowledge gap on the natural feeding of herbivorous fish across a wide range of herbivore species, functional groups, and distance from the urbanization center that links to coral reef condition and turf algae sediment loads.
4.1 The effects of coastal urbanization-related factors on coral reef conditions and fish herbivory rates
Coral reef conditions in the Spermonde Archipelago vary with distance from the mainland. The inshore reef (1LL) showed high turf and macroalgal cover and elevated turf algae sediment loads, indicating poor reef health. Conditions gradually improved from 2SA to 5BA, with increasing rugosity, live coral cover, and decreasing turf algae sediment loads and macroalgal cover. Offshore reefs (6LU to 8KP) had higher hard coral cover and rugosity, suggesting healthier conditions. Roving herbivorous fish biomass generally increased offshore, peaking at middle-to-offshore sites, although roving scraper biomass decreased offshore. These findings align with earlier studies (see Edinger et al., 1998; Edinger and Risk, 2000; Plass-Johnson et al., 2018a; Teichberg et al., 2018), highlighting poor water quality from urbanization remains the primary factor shaping reef conditions and assemblages (Becking et al., 2006; Cleary et al., 2005; Girard et al., 2022; Kegler et al., 2017; Plass-Johnson et al., 2016; Polónia et al., 2015; Teichberg et al., 2018). Fish herbivory rates also varied across functional groups and distances from the mainland. Reefs closer to the mainland (1LL to 5BA) had lower herbivory rates, particularly for detritivores and croppers, suggesting higher susceptibility to algal overgrowth. Reefs further offshore exhibited increased herbivory, especially for scrapers and excavators. Several functional groups were absent in inshore reefs, and discrepancies were noted between fish species observed in the UVC surveys and those feeding in video recording. Such patterns are likely driven by poor water quality resulting from urbanization and island-based human activities, which shape fish herbivory composition through environmental filtering. This process selectively limits species capable of functioning under specific environmental conditions, e.g., high turbidity, while reducing the feeding activity of others (Bejarano et al., 2017).
Coastal urbanization factors affect herbivory composition differently across functional groups. Detritivore and excavator herbivory rates remained consistent across sites, suggesting stable roles in the urban Spermonde reef ecosystem. Although diet composition was not examined, past studies indicate many herbivores can adapt or alter their diets in response to environmental changes (Bonaldo and Bellwood, 2008; Gordon et al., 2016; Lin et al., 2021; Plass-Johnson et al., 2018b). For instance, Ctenochaetus striatus adjusts its diet based on coral cover and algal availability (Lin et al., 2021). Although detritivore herbivory stability is crucial for removing detritus and sediments settled within turf algae, the presence of only one detritivore species in a reef system impacted by urbanization and sedimentation highlights low functional redundancy and increased vulnerability to disturbance (e.g., Bellwood et al., 2003; Bonaldo and Bellwood, 2009). If C. striatus is functionally lost due to disease, predation, fishing, or severe environmental changes, it could lead to over-accumulation of detritus and sediments settled within turf algae, which thus can smother the corals. This detritus removal role may be fulfilled by other fish, such as parrotfish, known for their herbivore-detritivore function in sediment production and reworking on reefs (Perry et al., 2015). Excavators also maintained relatively high herbivory rates across spatial gradients, which may influence reef dynamics in complex ways. While excavators can help control algal growth and facilitate coral recruitment by clearing substrate (Bellwood and Choat, 1990; Bonaldo and Bellwood, 2009), they may also inadvertently damage small, newly settled corals or larger colonies during feeding (Huertas et al., 2021; Charendoff et al., 2023; Rempel et al., 2024). Their net effect is, therefore, context-dependent, shaped by spatial scale, coral resilience, and local conditions.
Current and past observations in the Archipelago (Plass-Johnson et al., 2015) show browsing by Naso species mainly at reefs further offshore (i.e., 5BA and 8KP, with observations in this study restricted to the latter). This contrasts with the Great Barrier Reef, where browsers like Naso unicornis significantly remove macroalgae in nearshore areas (Hoey and Bellwood, 2011; Lefévre and Bellwood, 2011). However, Plass-Johnson et al. (2014, 2015) also recorded other species, classified in different functional groups in this study, feeding on offered macroalgae, such as Siganus virgatus, S. corallinus, Zebrasoma scopas (croppers), Scarus flavipectoralis (scraper), and Bolbometopon muricatum (excavator), indicating dietary flexibility across species. Although these species may help curb macroalgae, observing browser herbivory only at 8KP remains concerning, given browser species were present at all sites except 2SA and 7KS (Figure 3b). Browsers can be selective, often favoring specific macroalgae even when less abundant (Kelly et al., 2016), and are influenced by the chemical properties of plants (Meyer et al., 1994). For example, N. lituratus avoids green algae like calcified Halimeda spp (Meyer et al., 1994), which are more common on inshore reefs of the Spermonde Archipelago. Inshore macroalgae may also contain chemical compounds from polluted runoff, potentially deterring browsers from feeding on them. Additionally, intensive and destructive fishing throughout the Archipelago may negatively impact herbivorous fish and herbivory. In a global review, Edwards et al. (2014) reported a 50% drop in herbivorous fish biomass in fished areas, with browsers most affected.
A few species drove differences in cropper and scraper herbivory rates. Their distributions differed across the spatial gradient, likely influenced by ecological factors such as habitat differences, food resource availability, and environmental conditions. For instance, the territorial damselfish D. prosopotaenia was among the five cropper species distinguishing 1LL from all sites except 8KP. This farmerfish defends its territory against other herbivorous fishes (Hoey and Bellwood, 2009; Plass-Johnson et al., 2018b), ensuring a consistent food supply despite environmental changes.
Furthermore, S. rivulatus was a key driver of scraper herbivory rate differences in the middle-to-offshore Archipelago (4BO and 7KS). This species demonstrates feeding flexibility across turf algae sediment sources, although it favors a high-quality epilithic algal matrix (EAM, sensuWilson et al., 2003) with fine sediments and high nutritional content (Bonaldo and Bellwood, 2008; Gordon et al., 2016). The middle-to-offshore Archipelago (4BO and 7KS), less affected by coastal pollution, sedimentation, and oceanic waves, likely provides ideal conditions for S. rivulatus, with 7KS having the highest organic-to-inorganic sediment ratio in turf algae. Despite high S. rivulatus herbivory rates, this species was not observed in the UVCs, possibly due to methodological differences in measuring herbivory versus roving biomass and local conditions during data collection. Noises from frequent boat traffic at 4BO and blast fishing at 7KS, which were heard multiple times during data collection, may have affected fish movement and feeding patterns. Moreover, some species may be susceptible to diver presence and only be detected using video observations (Mallet et al., 2014).
The surgeonfish Z. scopas distinguished 8KP from other sites, where its herbivory was uniquely recorded, consistent with findings from Plass-Johnson et al. (2015). This species is likely sensitive to urban influence and, therefore, was most abundant and active feeding at the furthest site from the mainland. Its high herbivory rates at 8KP may stem from a flexible diet and capacity to exploit diverse microhabitats and lower-quality food resources (Robertson et al., 1979). In the competitive environment of 8KP, with high roving herbivore biomass and intense herbivory, Z. scopas thrive perhaps by consuming algae that other species do not efficiently consume. This adaptability enables Z. scopas to maintain high feeding rates despite competition. Preserving species with such dietary flexibility is vital for ecological balance and for reducing the risk of phase shifts to algal dominance in urbanized reefs like the Spermonde Archipelago.
4.2 Factors influencing herbivory rates
Our study provides preliminary insights into environmental factors influencing herbivory rates across functional groups. For detritivores, herbivory rates were higher on lower-rugosity reefs. Syafruddin, Estradivari et al. (in review) reported increased sediment accumulation on flatter substrates in the Spermonde Archipelago, potentially favoring detritivore herbivory. For croppers, scrapers, excavators, and all herbivorous fish combined, herbivory rates were consistently associated with the percentage of organic matter within turf algae sediments, although the nature and strength of these associations varied across groups (Supplementary Figure S12), underscoring the importance of food quality. Other variables also mattered: cropper herbivory was correlated with reef rugosity and roving fish biomass, scraper rates with turf algal cover, and combined herbivory with macroalgal and live coral cover. These findings highlight the complex dynamics shaping herbivory in urban reefs. However, the limited sample size necessitates cautious interpretation, and further studies with larger datasets are needed to confirm these patterns.
None of the functional groups showed a clear relationship between herbivory rates and roving fish biomass, except for croppers. This may reflect our limited sample size, which could have obscured potential patterns or local factors shaping these relationships. Visual observations revealed fewer fish feeding within quadrats than recorded in visual censuses, particularly in the middle zone (3BL to 6LU) where fish biomass peaked, yet herbivory rates varied or were even absent. While higher fish biomass often predicts greater herbivory, this relationship can be non-linear or non-existent due to factors such as species and size composition (Kindinger et al., 2024), habitat features (Jones and Andrew, 1990), coral cover, reef complexity (Williams et al., 2001, Verges et al., 2011), local fishing pressures, environmental conditions (Edwards et al., 2014; Plass-Johnson et al., 2015), and management (Humphries et al., 2014). Environmental filtering may prevent species from feeding under certain conditions despite their presence (Bejarano et al., 2017). In the Spermonde reef system, heavy fishing, destructive fishing methods, intense coastal anthropogenic activities (e.g., boat traffic), and urban influences from the mainland likely shape fish grazing behavior and herbivory across the Archipelago.
Unexpectedly, we found no significant differences in herbivory rates between sediment-cleared and control areas, suggesting that settled sediment within turf algae did not directly or immediately affect herbivory at our experimental scale. This contrasts with earlier studies reporting substantial herbivory increases (up to 225%) following sediment removal (Bellwood and Fulton, 2008; Goatley and Bellwood, 2012). Several factors may explain this discrepancy. First, sediment loads within turf algae in the Archipelago were generally below the 250 g/m2 threshold at which herbivory is inhibited (Tebbett et al., 2024b), except at 1LL (Figure 3a). This implies that most naturally occurring sediment loads were insufficient to suppress herbivory; hence settled sediment removal had minimal impact. Second, turf algae assemblages might influence outcomes. For example, in Fiji, a mixture of cyanobacteria in turf algae reduced herbivory by up to 50% (Ford et al., 2021). While we observed inshore-offshore variations in turf algae mixtures, our photo quadrats lacked the resolution to confirm assemblage diversity. Habitat characteristics likely also influenced the results. Inshore reefs like 1LL had silt-dominated substrates with patchy corals and macroalgae (Figure 3e), making sediment clearance challenging without disturbing silt. Offshore reefs (Figures 3g, h) had mixed rubble substrates with holes and encrusting organisms, complicating sediment removal. Previous studies recommend sampling flat, smooth surfaces free of sediment-retaining pits or macroalgae (see Purcell, 2000; Tebbett et al., 2017), but standardizing surfaces across habitats was unfeasible. These challenges probably limited sediment removal efficacy, explaining the lack of observable effects on herbivory.
Our findings revealed associations between specific benthic and fish community characteristics and herbivory rates; however, unobserved factors also likely shape these patterns. This is evident from unexpected absences or reductions in herbivory: no detritivore, scraper, or excavator herbivory at 1LL, 3BL (except scrapers), and 5BA; no browser herbivory except at 8KP; and anomalously low rates at 6LU compared to nearby 7KS. The lack of recorded herbivory at the nearshore site 1LL aligns with its degraded reef and poor water quality. However, notably low herbivory at 3BL, 5BA, and 6LU, despite herbivorous fish presence at these sites (Figure 3a) and in video footage, is noteworthy. While earlier studies documented herbivory at these sites when macroalgae were offered (Plass-Johnson et al., 2014, 2015), this study focused on natural feeding. Thus, no herbivory observed in certain quadrats or reefs does not necessarily indicate a complete lack of herbivory across the entire reef. Rather, selective feeding behavior and localized factors, including anthropogenic pressures, appear to affect fish distribution and feeding activity.
The 3BL, 5BA, and 6LU islands are among the most densely populated in the Spermonde Archipelago, each hosting 1,900-4,700 people on 8–21 ha islands (BPS, 2022). These communities significantly impact coral reef ecosystems through fishing, boat traffic, and waste discharge. Most coastal communities are fishers, targeting marine biota indiscriminately, including herbivorous fishes like parrotfish (Tresnati et al., 2019, 2020). Many fishers also engage in illegal, destructive fishing practices using explosives, cyanide, and bottom trawls (Munsi et al., 2017; Suarthawan et al., 2022), due to weak enforcement, mild sanctions, and black-market access for materials and fish products (Radjawali, 2012; Renggong et al., 2022; Zaelany, 2019). While destructive fishing is more common at outer, remote, or uninhabited islands (Suarthawan et al., 2022), it also occurs around inhabited islands (Jompa et al., 2005; Zaelany, 2019; ES, unpublished data), though its frequency, distribution, and severity are unknown. During data collection at 3BL, 5BA, and 6LU, we frequently heard blasts from nearby reefs and encountered small-scale fishing near transects. At 5BA, our site was adjacent to a boat mooring area where fishers often fished while awaiting their boats. Local pressures, including human predation, noise pollution from blast fishing and boats, and untreated waste discharge, likely disrupt herbivorous fish distribution, density, and behavior, causing patchy feeding or avoidance behaviors (Ferrier-Pagès et al., 2021; Januchowski-Hartley et al., 2011; McCormick et al., 2018). Impacts on 3BL and 5BA may be amplified compared to 6LU because of closer proximity to the mainland, where urbanization effects are more pronounced and may deter effective feeding. Further research is needed to disentangle these factors and inform strategies for maintaining key ecological functions in reef ecosystems.
4.3 Management implications
Distinct coral reef conditions and herbivory patterns across a spatial gradient from the urban center and functional groups underscore the need for targeted management. From an area-based management perspective, nearshore reefs (1LL to 5BA) require intensive interventions to mitigate urban impacts and support herbivore communities. This is crucial as many herbivorous fishes are sensitive to sediment loads and turf algae composition. Effective strategies should focus on improving water quality and protecting vital habitats to maintain herbivorous fish diversity and functionality. Integrating urban runoff controls, sedimentation management, and broader coastal zone measures can address both land-based and marine stressors, sustaining reef resilience under growing urban pressures.
For mid-to-offshore reefs, improving the newly established Liukang Tupabiring Pangkep marine protected area (MPA, 63,407 ha, established in 2022, Figure 1) and revitalizing over a dozen community-based conservation areas (Glaser et al., 2010, 2021; Jompa et al., 2023) is essential for habitat protection. Implementing Kapoposang MPA (50,000 ha, established in 1996) for offshore reefs appeared effective in maintaining diverse herbivorous fish populations, although better reef conditions may also reflect better water quality, which can strongly influence benthic composition (Fabricius et al., 2005; De’ath and Fabricius, 2010) and herbivorous fish biomass (Russ et al., 2015). This synergy is evident at 8KP, within Kapoposang MPA, where higher coral cover and rugosity coincide with lower turf algal and macroalgal covers, diverse fish species, and high herbivory rates. In urban Spermonde reefs, MPAs can play a crucial role by providing opportunities to integrate active restoration and sediment management strategies and buffering additional human impacts. MPAs also protect fish species and habitats resilient to sediment-rich and turbid conditions while supporting fish larval supply to degraded reefs. Studies indicate that outer zone reefs, including Kapoposang Island, benefit from a more substantial larval supply from other regions due to the Indonesian Throughflow (Reuter et al., 2021; Treml et al., 2012), enhancing connectivity with other reef systems in the Archipelago.
Protecting diverse herbivore functional groups is essential for reef resilience to withstand ongoing disturbances (Bellwood et al., 2004, 2006; Donovan et al., 2023), especially since coastal urbanization stressors affect fish herbivory differently across functional groups (e.g., lack of herbivory by detritivores, scrapers, and excavators in some inshore-to-mid reefs, and consistently low herbivory by croppers across all sites). Moreover, many large-bodied herbivorous fishes, such as Scarus and Chlolurus species, are heavily fished and over-exploited in the Archipelago (Fatihah et al., 2021; Tresnati et al., 2019, 2020). Additionally, illegal and destructive fishing methods, including bombs and cyanide, further devastate live coral and degrade reef structures, disproportionately reducing herbivorous fish biomass and herbivory rates (Edwards et al., 2014; McManus et al., 2000). Such impacts compromise reef resilience and increase the risk of ecosystem shifts towards algae-dominated states, hindering coral recovery and compromising overall reef health.
Maintaining browser, scraper, and excavator populations is particularly critical in nearshore reefs (2BA to 5BA) to prevent or reverse phase shifts to algal dominance. While all herbivorous species are functionally important for reef health, conservation efforts focusing on these specific functional groups may be most effective in preserving the ecological balance of nearshore reefs. It is important that the quota-based fisheries regulation (Government Regulation No. 11/2023), recently introduced by the national government, is adopted and effectively implemented by the provincial government. This regulation, which includes fishing zones, quota systems, and vessel and landing port management, is crucial for promoting sustainable fisheries in the Archipelago. However, it is essential to ensure the inclusion of fisheries management for herbivores, particularly those targeted by fishers, in this regulation. Besides this, strengthening enforcement, applying heavier sanctions against illegal and destructive fishing practices, and facilitating social approaches to encourage environmentally friendly fishing gear are paramount for sustaining the overall fish population, including key herbivores.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Leibniz Center for Tropical Marine Research (ZMT). The study was conducted in accordance with the local legislation and institutional requirements. The study was conducted under research permit No. 98/SIP/IV/FR/7/2022, which was issued by Indonesia's National Research and Innovation Agency.
Author contributions
E: Conceptualization, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Data curation. AP: Data curation, Investigation, Writing – review & editing. GS: Data curation, Investigation, Writing – review & editing. PLK: Data curation, Investigation, Writing – review & editing. MS: Investigation, Visualization, Writing – review & editing. AT: Investigation, Writing – review & editing. M: Data curation, Writing – review & editing. DR: Writing – review & editing. RA-R: Writing – review & editing, Supervision. SB: Supervision, Writing – review & editing. OP: Supervision, Writing – review & editing. CW: Supervision, Writing – review & editing. SF: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the European Union’s Horizon 2020 research and innovation program (4D-REEF, grant agreement No. 813360) and the Russell E. Train Education for Nature Program (EFN) of the World Wildlife Fund (grant number: EF16945).
Acknowledgments
We thank Willem Renema, Chantal Cornelissen (Naturalis Biodiversity Center), and Jamaluddin Jompa (Hasanuddin University) for their leadership in coordinating the fieldwork. We also gratefully acknowledge the valuable contributions of Stefanie Bröhl, Michael Schmid, Andreas Kunzmann, Ulrich Pint, Christian Brandt (Leibniz Centre for Tropical Marine Research, ZMT), Nenni Asriani, Ahmad Sahlan, A. Agung Asnur, Ismul Musyawirah, Nuthy Nhasya Riana, Nurul Mutmainnah, and Rio Edwin Patiung Randa (Hasanuddin University), who provided substantial support in the fieldwork preparation, data collection, and analysis processes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1359139/full#supplementary-material
References
1
Allen G. R. (2008). Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 18, 541–556. doi: 10.1002/aqc.v18:5
2
Barros Y. Lucas C. C. Soares M. O. (2021). An urban intertidal reef is dominated by fleshy macroalgae, sediment, and bleaching of a resilient coral (Siderastrea stellata). Mar. Pollut. Bull.173, 112967. doi: 10.1016/j.marpolbul.2021.112967
3
Baum G. Januar H. I. Ferse S. C. A. Kunzmann A. (2015). Local and regional impacts of pollution on coral reefs along the thousand islands north of the megacity Jakarta, Indonesia. PloS One10, 1–26. doi: 10.1371/journal.pone.0138271
4
Bauman A. G. Hoey A. S. Dunshea G. Feary D. A. Low J. Todd P. A. (2017). Macroalgal browsing on a heavily degraded, urbanized equatorial reef system. Sci. Rep.7 (1), 8352. doi: 10.1038/s41598-017-08873-3
5
Becking L. E. Cleary D. F. R. Voogd N. J. Renema W. de Beer M. van Soest R. W. M. et al . (2006). Beta diversity of tropical marine benthic assemblages in the Spermonde Archipelago, Indonesia. Mar. Ecol.27, 76–88. doi: 10.1111/j.1439-0485.2005.00051.x
6
Bejarano S. Jouffray J. B. Chollett I. Allen R. Roff G. Marshell A. et al . (2017). The shape of success in a turbulent world: wave exposure filtering of coral reef herbivory. Funct. Ecol.31, 1312–1324. doi: 10.1111/1365-2435.12828
7
Bellwood D. R. Choat J. H. (1990). A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. In: BrutonM. N. (eds) Alternative life-history styles of fishes. Developments in environmental biology of fishes (Dordrecht: Springer) 10. doi: 10.1007/978-94-009-2065-1_11
8
Bellwood D. R. Fulton C. J. (2008). Sediment-mediated suppression of herbivory on coral reefs: Decreasing resilience to rising sea levels and climate change? Limnol. Oceanogr.53, 2695–2701. doi: 10.4319/lo.2008.53.6.2695
9
Bellwood D. R. Hoey A. S. Ackerman J. L. Depczynski M. (2006). Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Global Change Biol.12, 1587–1594. doi: 10.1111/j.1365-2486.2006.01204.x
10
Bellwood D. R. Hoey A. S. Choat J. H. (2003). Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol. Lett.6, 281–285. doi: 10.1046/j.1461-0248.2003.00432.x
11
Bellwood D. R. Hughes T. P. Folke C. Nyströ M. (2004). Confronting the coral reef crisis. Nature429, 827–833. doi: 10.1038/nature02691
12
Blackburn S. Marques C. A. De Sherbinin A. Modesto F. (2013). Mega-urbanisation on the coast: global context and key trends in the twenty-first century. In PellingM.BlackburnS.. Megacities and the Coast: Risk, Resilience and Transformation. (Oxon: Routledge), 1–21.
13
Bonaldo R. M. Bellwood D. R. (2008). Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser.360, 237–244. doi: 10.3354/meps07413
14
Bonaldo R. M. Bellwood D. R. (2009). Dynamics of parrotfish grazing scars. Mar. Biol.156, 771–777. doi: 10.1007/s00227-009-1129-x
15
BPS (2022). Provinsi Sulawesi Selatan Dalam Angka 2022. Badan Pusat Statistik, Sulawesi Selatan.
16
Burke L. Reytar K. Spalding K. Perry A. (2012). “Reefs at risk revisited in the Coral Triangle,” in The Nature Conservancy. World-Fish Center, International Coral Reef Action Network (UNEP World Conservation Monitoring Centre and Global Coral Reef Monitoring).
17
Burkepile D. E. Hay M. E. (2009). Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar. Ecol. Prog. Ser.389, 71–84. doi: 10.3354/meps08142
18
Charendoff J. A. Edwards C. B. Pedersen N. E. Petrovic V. Zgliczynski B. Sandin S. A. et al . (2023). Variability in composition of parrotfish bite scars across space and over time on a central Pacific atoll. Coral. Reefs.42, 905–918. doi: 10.1007/s00338-023-02392-6
19
Choat J. H. Clements K. D. Robbins W. D. (2002). The trophic status of herbivorous fishes on coral reefs 1: Dietary analyses. Mar. Biol.140, 613–623. doi: 10.1007/s00227-001-0715-3
20
Chong-Seng K. M. Nash K. L. Bellwood D. R. Graham N. A. J. (2014). Macroalgal herbivory on recovering versus degrading coral reefs. Coral. Reefs.33, 409–419. doi: 10.1007/s00338-014-1134-5
21
Clarke K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol.18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
22
Clarke K. R. Ainsworth M. (1993). A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser.92, 205. doi: 10.3354/meps092205
23
Clarke K. R. Gorley R. N. (2015). PRIMER v7: User Manual/Tutorial (Devon, United Kingdom: PRIMER-E Plymouth).
24
Clarke K. R. Warwick R. M. (1994). Similarity-based testing for community pattern: the two-way layout with no replication. Mar. Biol.118, 167–176. doi: 10.1007/BF00699231
25
Cleary D. F. R. Becking L. E. De Voogd N. J. Renema W. De Beer M. Van Soest R. W. M. et al . (2005). Variation in the diversity and composition of benthic taxa as a function of distance offshore, depth and exposure in the Spermonde Archipelago, Indonesia. Estuarine. Coast. Shelf. Sci.65, 557–570. doi: 10.1016/j.ecss.2005.06.025
26
Cleary D. F. R. Polónia A. R. M. Renema W. Hoeksema B. W. Wolstenholme J. Tuti Y. et al . (2014). Coral reefs next to a major conurbation: A study of temporal change, (1985-2011) in coral cover and composition in the reefs of Jakarta, Indonesia. Mar. Ecol. Prog. Ser.501, 89–98. doi: 10.33.54/meps10678
27
Cohen J. E. Small C. (1998). Hypsographic demography: the distribution of human population by altitude. Proceedings of the National Academy of Sciences.95 (24), 14009–14014.
28
Crossman D. J. Choat J. H. Clements K. D. Hardy. T. Mcconochie J. (2001). Detritus as food for grazing fishes on coral reefs. Limnol. Oceanogr.46, 1596–1605. doi: 10.4319/lo.2001.46.7.1596
29
De’ath G. Fabricius K. (2010). Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl.20, 840–850. doi: 10.1890/08-2023.1
30
Destructive Fishing Watch (2003). Profile of Destructive Fishing. Destructive Fishing Watch Indonesia and Coral Reef Rehabilitation and Management Program, Jakarta. 99.
31
Done T. J. (1992). Phase shifts in coral reef communities and their ecological significance. Hydrobiologia247, 121–132. doi: 10.1007/BF00008211
32
Donovan M. K. Counsell C. W. W. Donahue M. J. Lecky J. Gajdzik L. Marcoux S. D. et al . (2023). Evidence for managing herbivores for reef resilience. Proc. R. Soc. B.: Biol. Sci.290, 20232101. doi: 10.1098/rspb.2023.2101
33
Edinger E. N. Jompa J. Limmon G. V. Widjatmoko W. Risk M. J. (1998). Reef degradation and coral biodiversity in Indonesia: Effects of land-based pollution, destructive fishing practices and changes over time. Mar. Pollut. Bull.36, 617–630. doi: 10.1016/S0025-326X(98)00047-2
34
Edinger E. N. Risk M. J. (2000). Reef classification by coral morphology predicts coral reef conservation value. Biol. Conserv.92, 1–13. doi: 10.1016/S0006-3207(99)00067-1
35
Edwards C. B. Friedlander A. M. Green A. G. Hardt M. J. Sala E. Sweatman H. P. et al . (2014). Global assessment of the status of coral reef herbivorous fishes: evidence for fishing effects. Proc. R. Soc. B.: Biol. Sci.281, 20131835. doi: 10.1098/rspb.2013.1835
36
Estradivari A.-B. Amkieltiela D. A. Handayani C. N. Sjahruddin F. F. Agung M. F. Campbell S. J. et al . (2022). Marine conservation in the Sunda banda seascape, Indonesia. Mar. Policy138, 104994. doi: 10.1016/j.marpol.2022.104994
37
Fabricius K. De’ath G. McCook L. Turak E. Williams D. (2005). Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar. Pollut. Bull.51, 384–398. doi: 10.1016/j.marpolbul.2004.10.041
38
Faizal A. (2022). Decadal remote-sensing analysis of eutrophic indication in the Spermonde Islands, Indonesia. Jurnal. IPTEKS. PSP.9, 1–11. doi: 10.20956/jipsp.v9i1.20061
39
FAO . (2020). The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome. doi: 10.5860/choice.50-5350
40
Ferrier-Pagès C. Leal M. C. Calado R. Schmid D. W. Bertucci F. Lecchini D. et al . (2021). Noise pollution on coral reefs? — A yet underestimated threat to coral reef communities. Mar. Pollut. Bull.165, 112129. doi: 10.1016/j.marpolbul.2021.112129
41
Fatihah I. Umari M. T. Kudsiah H. Yasir I. Yanti A. Rahmani P. Y. et al . (2021). “Uncontrolled fishing of dusky parrotfish scarus niger (Forsska) spermonde islands, makassar strait, indonesia,” in IOP Conference Series: Earth and Environmental Science (IOP Publishing). 860, 012019.
42
Ferse S. C. A. Glaser M. Neil M. Schwerdtner Máñez K. (2014). To cope or to sustain? Eroding long-term sustainability in an Indonesian coral reef fishery. Regional. Environ. Change14, 2053–2065. doi: 10.1007/s10113-012-0342-1
43
Ford A. K. Visser P. M. van Herk M. J. Jongepier E. Bonito V. (2021). First insights into the impacts of benthic cyanobacterial mats on fish herbivory functions on a nearshore coral reef. Sci. Rep.11, 7147. doi: 10.1038/s41598-021-84016-z
44
Froese R. Pauly D. (2010). FishBase (Fisheries Centre, University of British Columbia).
45
Geronimo R. C. Cabral. R. B. (2013). “Fish production in the Coral Triangle: Status, trends, and challenges,” in Economics of Fisheries and Aquaculture in the Coral Triangle (Consultant’s Report Submitted to the Asian Development Bank, Manila, Philippines), 5–29.
46
Girard E. B. Estradivari Ferse S. Ambo-Rappe R. Jompa J. Renema W. (2022). Dynamics of large benthic foraminiferal assemblages: A tool to foreshadow reef degradation? Sci. Total. Environ.811, 151396. doi: 10.1016/j.scitotenv.2021.151396
47
Glaser M. Adrianto L. Breckwoldt A. Buhari N. Deswandi R. Ferse S. et al . (2021). “The governance of coastal and marine social-ecological systems: Indonesia and beyond,” in Science for the Protection of Indonesian Coastal Ecosystems (SPICE) (Bremen: Elsevier), 407–443. doi: 10.1016/B978-0-12-815050-4.00008-0
48
Glaser M. Baitoningsih W. Ferse S. C. A. Neil M. Deswandi R. (2010). Whose sustainability? Top-down participation and emergent rules in marine protected area management in Indonesia. Mar. Policy34, 1215–1225. doi: 10.1016/j.marpol.2010.04.006
49
Goatley C. H. R. Bellwood D. R. (2012). Sediment suppresses herbivory across a coral reef depth gradient. Biol. Lett.8, 1016–1018. doi: 10.1098/rsbl.2012.0770
50
Gordon S. E. Goatley C. H. R. Bellwood D. R. (2016). Low-quality sediments deter grazing by the parrotfish Scarus rivulatus on inner-shelf reefs. Coral. Reefs.35, 285–291. doi: 10.1007/s00338-015-1374-z
51
Green A. L. Bellwood D. R. (2009). Monitoring functional groups of herbivorous reef fishes as indicators of coral reef resilience – A practical guide for coral reef managers in the Asia Pacific region. IUCN working group on Climate Change and Coral Reefs. IUCN, Gland, Switzerland. 70.
52
Heery E. C. Hoeksema B. W. Browne N. K. Reimer J. D. Ang P. O. Huang D. et al . (2018). Urban coral reefs: Degradation and resilience of hard coral assemblages in coastal cities of East and Southeast Asia. Mar. Pollut. Bull.135, 654–681. doi: 10.1016/j.marpolbul.2018.07.041
53
Hill J. Wilkinson C. (2004). Methods for Ecological monitoring of coral reef: A Resource for Managers, Version 1. Townsville. Aust. Inst. Mar. Sci. vi + 117.
54
Hoey A. S. Bellwood D. R. (2009). Limited functional redundancy in a high diversity system: Single species dominates key ecological process on coral reefs. Ecosystems12, 1316–1328. doi: 10.1007/s10021-009-9291-z
55
Hoey A. S. Bellwood D. R. (2011). Suppression of herbivory by macroalgal density: A critical feedback on coral reefs? Ecol. Lett.14, 267–273. doi: 10.1111/j.1461-0248.2010.01581.x
56
Huertas V. Morais R. A. Bonaldo R. M. Bellwood D. R. (2021). Parrotfish corallivory on stress-tolerant corals in the Anthropocene. PloS One16, e0250725. doi: 10.1371/journal.pone.0250725
57
Hughes T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science265, 1547–1551. doi: 10.1126/science.265.5178.1547
58
Hugo G. J. (1982). Circular migration in Indonesia. Population and Development Review, 59–83.
59
Humphries A. T. McClanahan T. R. McQuaid C. D. (2014). Differential impacts of coral reef herbivores on algal succession in kenya. Mar. Ecol. Prog. Ser.504, 119–132. doi: 10.3354/meps10744
60
Husain A. A. A. Nessa M. N. Jompa J. Rani C. Buhari N. Marasabessy A. Z. et al . (2013). The dynamics of benthic algae among herbivorous coral reef fishes. World Appl. Sci. J.26, 01–06. doi: 10.5829/idosi.wasj.2013.26.nrrdsi.26001/6/
61
IOC/UNESCO IMO FAO UNDP (2011). A Blueprint for Ocean and Coastal Sustainability. Paris: IOC/UNESCO.
62
Januchowski-Hartley F. A. Graham N. A. J. Feary D. A. Morove T. Cinner J. E. (2011). Fear of fishers: Human predation explains behavioral changes in coral reef fishes. PloS One6 (8), e22761. doi: 10.1371/journal.pone.0022761
63
Jenkins C. N. Van Houtan K. S. (2016). Global and regional priorities for marine biodiversity protection. Biol. Conserv.204, 333–339. doi: 10.1016/j.biocon.2016.10.005
64
Jompa J. Moka W. Yanuarita D. (2005). Kondisi Ekosistem Perairan Kepulauan Spermonde: Keterkaitannya dengan Pemanfaatan Sumberdaya Laut di Kepulauan Spermonde. South Sulawesi: Hasanuddin University.
65
Jompa J. Putri A. P. Moore A. M. Tamti H. Haerani S. (2023). The transference of marine protected area management authority in Indonesia: Problems encountered, consequences and ways to move forward. Mar. Policy155, 105756. doi: 10.1016/j.marpol.2023.105756
66
Jones G. P. Andrew N. L. (1990). Herbivory and patch dynamics on rocky reefs in temperate australasia: the roles of fish and sea urchins. Aust. J. Ecol.15 (4), 505–520. doi: 10.1111/j.1442-9993.1990.tb01474.x
67
Kegler H. F. Lukman M. Teichberg M. Plass-Johnson J. Hassenrück C. Wild C. et al . (2017). Bacterial community composition and potential driving factors in different reef habitats of the Spermonde Archipelago, Indonesia. Front. Microbiol.8. doi: 10.3389/fmicb.2017.00662
68
Kelly E. L. A. Eynaud Y. Clements S. M. Gleason M. Sparks R. T. Williams I. D. et al . (2016). Investigating functional redundancy versus complementarity in Hawaiian herbivorous coral reef fishes. Oecologia182, 1151–1163. doi: 10.1007/s00442-016-3724-0
69
Khait R. Obolski U. Hadany L. Genin A. (2013). Food selectivity and diet switch can explain the slow feeding of herbivorous coral-reef fishes during the morning. PloS One8 (12), e82391. doi: 10.1371/journal.pone.0082391
70
Kindinger T. L. Adam T. C. Baum J. K. Dimoff S. A. Hoey A. S. Williams I. D. (2024). Herbivory through the lens of ecological processes across pacific coral reefs. Ecosphere15 (2), e4791. doi: 10.1002/ecs2.4791
71
Knaap G. J. Sutherland H. (2004). Monsoon traders: ships, skippers and commodities in eighteenth-century Makassar (Leiden: KITLV Press Leiden).
72
Kohler K. E. Gill S. M. (2006). Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci.32, 1259–1269. doi: 10.1016/j.cageo.2005.11.009
73
Kulbicki M. Guillemot N. Amand M. (2005). A general approach to length-weight relationships for New Caledonian lagoon fishes. Cybium29 (3), 235–252.
74
Ladds M. A. Sibanda N. Arnold R. Dunn M. R. (2018). Creating functional groups of marine fish from categorical traits. PeerJ6, e5795. doi: 10.7717/peerj.5795
75
Langkoke R. Bt Ismail N. A Bahar A. M. Thamrin M. Putra A. (2022). Coastline and land use changes by remote sensing analysis at Tanjung Bunga west coastal of Makassar South Sulawesi Indonesia. Int. J. Eng. Sci. Appl. IJEScA.9, 2022.
76
Lefévre C. D. Bellwood D. R. (2011). Temporal variation in coral reef ecosystem processes: Herbivory of macroalgae by fishes. Mar. Ecol. Prog. Ser.422, 239–251. doi: 10.3354/meps08916
77
Lestari H. A. Samawi M. F. Faizal A. Moore A. M. Jompa J. (2021). Physical and chemical parameters of estuarine waters around South Sulawesi. Indonesian. J. Geogr.53, 373–387. doi: 10.22146/ijg.67831
78
Lin X. Hu S. Liu Y. Zhang L. Huang H. Liu S. (2021). Disturbance-mediated changes in coral reef habitat provoke a positive feeding response in a major coral reef detritivore, Ctenochaetus striatus. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.682697
79
Mallet D. Wantiez L. Lemouellic S. Vigliola L. Pelletier D. (2014). Complementarity of rotating video and underwater visual census for assessing species richness, frequency and density of reef fish on coral reef slopes. PloS One9, e84344. doi: 10.1371/journal.pone.0084344
80
McCormick M. I. Allan B. J. M. Harding H. Simpson S. D. (2018). Boat noise impacts risk assessment in a coral reef fish but effects depend on engine type. Sci. Rep.8 (1), 3847. doi: 10.1038/s41598-018-22104-3
81
McManus J. W. Menez L. A. B. Kesner-Reyes K. N. Vergara S. G. Ablan M. C. (2000). Coral reef fishing and coral-algae phase shifts: implications for global reef status. ICES. J. Mar. Sci.57, 572–578. doi: 10.1006/jmsc.2000.0720
82
Meyer K. D. Paul V. J. Sanger H. R. Nelson S. G. (1994). Effects of seaweed extracts and secondary metabolites on feeding by the herbivorous surgeonfish Naso lituratus. Coral Reefs. 13, 105–112. doi: 10.1007/BF0030077
83
Mumby P. J. Harborne A. R. (2010). Marine reserves enhance the recovery of corals on Caribbean reefs. PloS One5 (1), e8657. doi: 10.1371/journal.pone.0008657
84
Munsi L. Demmalino E. B. Neil M. Jompa J. (2017). Main drivers and alternative solutions for destructive fishing in South Sulawesi-Indonesia: lessons learned from Spermonde Archipelago, Taka Bonerate, and Sembilan Island. Sci. Int. (Lahore).29, 159–164.
85
Paddack M. J. Cowen R. K. Sponaugle S. (2006). Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral. Reefs.25, 461–472. doi: 10.1007/s00338-006-0112-y
86
Perry C. T. Kench P. S. O’Leary M. J. Morgan K. M. Januchowski-Hartley F. (2015). Linking reef ecology to island building: Parrotfish identified as major producers of island-building sediment in the Maldives. Geology43, 503–506. doi: 10.1130/G36623.1
87
Pet-Soede L. Erdmann M. V. (1998). Blast Fishing in Southwest Sulawesi, Indonesia. Jakarta: ICLARM.
88
Plass-Johnson J. G. Bednarz V. N. Hill J. M. Jompa J. Ferse S. C. A. Teichberg M. (2018b). Contrasting responses in the niches of two coral reef herbivores along a gradient of habitat disturbance in the Spermonde Archipelago, Indonesia. Front. Mar. Sci.5. doi: 10.3389/fmars.2018.00032
89
Plass-Johnson J. G. Ferse S. C. A. Jompa J. Wild C. Teichberg M. (2015). Fish herbivory as key ecological function in a heavily degraded coral reef system. Limnol. Oceanogr.60, 1382–1391. doi: 10.1002/lno.10105
90
Plass-Johnson J. G. Ferse S. C. Wild C. Teichberg M. (2014). Observation of macroalgal browsing in juvenile humphead parrotish, Bolbometopon muricatum, in the Spermonde Archipelago, Indonesia. Bull. Mar. Sci.90, 763–764. doi: 10.5343/bms.2014.1006
91
Plass-Johnson J. G. Heiden J. P. Abu N. Lukman M. Teichberg M. (2016). Experimental analysis of the effects of consumer exclusion on recruitment and succession of a coral reef system along a water quality gradient in the Spermonde Archipelago, Indonesia. Coral. Reefs.35, 229–243. doi: 10.1007/s00338-015-1369-9
92
Plass-Johnson J. G. Teichberg M. Bednarz V. N. Gärdes A. Heiden J. P. Lukman M. et al . (2018a). Spatio-temporal patterns in the coral reef communities of the Spermonde Archipelago 2012-2014, II: Fish assemblages display structured variation related to benthic condition. Front. Mar. Sci.5. doi: 10.3389/fmars.2018.00036
93
Polónia A. R. M. Cleary D. F. R. de Voogd N. J. Renema W. Hoeksema B. W. Martins A. et al . (2015). Habitat and water quality variables as predictors of community composition in an Indonesian coral reef: A multi-taxon study in the Spermonde Archipelago. Sci. Total. Environ.537, 139–151. doi: 10.1016/j.scitotenv.2015.07.102
94
Poquita-Du R. C. Quek Z. B. R. Jain S. S. Schmidt-Roach S. Tun K. Heery E. C. et al . (2019). Last species standing: loss of Pocilloporidae corals associated with coastal urbanization in a tropical city state. Mar. Biodivers.49, 1727–1741. doi: 10.1007/s12526-019-00939-x
95
Purcell S. W. (2000). Association of epilithic algae with sediment distribution on a windward reef in the northern Great Barrier Reef, Australia. Bull. Mar. Sci.66, 199–214.
96
Purcell S. W. Bellwood D. R. (1993). A functional analysis of food procurement in two surgeonfkh species, Acanthurus nigtofuscus and Ctenochaetus striatus (Acanthuridae). Environ. Biol. Fishes.37, 139–159.
97
Radjawali I. (2012). Examining local conservation and development: Live reef food fishing in Spermonde Archipelago, Indonesia. Rev. Gestão. Costeira. Integrada.12, 545–557. doi: 10.5894/rgci337
98
R Core Team (2023). R: A language and environment for statistical computing (R Foundation for Statistical Computing). Available at: https://www.R-project.org/ (Accessed December 3, 2024).
99
Rempel H. S. Bodwin K. N. Burkepile D. E. Adam T. C. Altieri A. H. Barton E. M. et al . (2024). Ecological drivers of parrotfish coral predation vary across spatial scales. Mar. Ecol. Prog. Ser.740, 145–160. doi: 10.3354/meps14633
100
Renggong R. Hamid A. H. Yulia Y. (2022). Investigating law enforcement for coral reef conservation of the Spermonde Archipelago, Indonesia. Asian J. Conserv. Biol.11, 3–11. doi: 10.53562/ajcb.61904
101
Retnaningdyah C. Hakim L. Mana Sikana A. Hamzah R. (2019). Keterkaitan Aktivitas Manusia dengan Kualitas Ekosistem Perairan Pantai di Kepulauan Spermonde, Makassar, Sulawesi Selatan. Biotropika.: J. Trop. Biol.7 (3), 129–135. doi: 10.21776/ub.biotropika.2019.007.03.6
102
Reuter H. Breckwoldt A. Dohna T. Ferse S. Gärdes A. Glaser M. et al . (2021). “Coral reef social-ecological systems under pressure in Southern Sulawesi,” in Science for the Protection of Indonesian Coastal Ecosystems (SPICE) (Bremen: Elsevier), 143–199). doi: 10.1016/B978-0-12-815050-4.00005-5
103
Roberts C. M. (1995). Effects of fishing on the ecosystem structure of coral reefs. Conserv. Biol.9, 988–995. doi: 10.1046/j.1523-1739.1995.09050988.x
104
Robertson D. R. Polunin N. V. C. Leighton K. (1979). The behavioral ecology of three Indian Ocean surgeonfishes (Acanthurus lineatus, A. leucosternon and Zebrasoma scopas): their feeding strategies, and social and mating systems. Env. Biol. Fish.4, 125–170. doi: 10.1007/BF00005448
105
Russ G. R. Questel S.-L. A. Rizzari J. R. Alcala A. C. (2015). The parrotfish–coral relationship: refuting the ubiquity of a prevailing paradigm. Mar. Biol.162, 2029–2045. doi: 10.1007/s00227-015-2728-3
106
Spalding A. K. Grorud-Colvert K. Allison E. H. Amon D. J. Collin R. de Vos A. et al . (2023). Engaging the tropical majority to make ocean governance and science more equitable and effective. NPJ Ocean. Sustainabil.2 (1), 8. doi: 10.1038/s44183-023-00015-9
107
Suarthawan I. G. Dirawan G. D. Mandra M. A. S. (2022). Fishermen’s perceptions of destructive fishing in the Pangkep regency, South Sulawesi, Indonesia. Int. J. Fish. Aquat. Stud.10, 178–182. doi: 10.22271/fish.2022.v10.i2c.2669
108
Surya B. Hadijah H. Suriani S. Baharuddin B. Fitriyah A. T. Menne F. et al . (2020). Spatial transformation of a new city in 2006-2020: Perspectives on the spatial dynamics, environmental quality degradation, and socio-Economic sustainability of local communities in Makassar city, Indonesia. Land9 (9), 324. doi: 10.3390/LAND9090324
109
Surya B. Salim A. Hernita H. Suriani S. Menne F. Rasyidi E. S. (2021). Land use change, urban agglomeration, and urban sprawl: A sustainable development perspective of Makassar city, Indonesia. Land10 (6), 556. doi: 10.3390/land10060556
110
Tahir A. Werorilangi S. Mulana Isman F. Zulkarnaen A. Massinai A. Faizal A. (2018). Short-term observation on marine debris at coastal areas of Takalar District and Makassar City, South Sulawesi, Indonesia. Jurnal. Ilmu. Kelautan.4. doi: 10.20956/jiks.v4i2.7061
111
Tebbett S. B. Bellwood D. R. (2019). Algal turf sediments on coral reefs: what’s known and what’s next. Mar. Pollut. Bull.149, 110542. doi: 10.1016/j.marpolbul.2019.110542
112
Tebbett S. B. Bellwood D. R. Bassett T. Cuttler M. V. W. Moustaka M. Wilson S. K. et al . (2024b). The limited role of herbivorous fishes and turf-based trophic pathways in the functioning of turbid coral reefs. Rev. Fish. Biol. Fish. 34 (1), 439–460. doi: 10.1007/s11160-023-09823-1
113
Tebbett S. B. Bennett S. Bellwood D. R. (2024a). A functional perspective on the meaning of the term ‘herbivore’: patterns versus processes in coral reef fishes. Coral. Reefs. 43 (2), 219–232. doi: 10.1007/s00338-023-02378-4
114
Tebbett S. B. Goatley C. H. R. Bellwood D. R. (2017). The effects of algal turf sediments and organic loads on feeding by coral reef surgeonfishes. PloS One12 (1), e0169479. doi: 10.1371/journal.pone.0169479
115
Tebbett S. B. Siqueira A. C. Bellwood D. R. (2022). The functional roles of surgeonfishes on coral reefs: past, present and future. Rev. Fish. Biol. Fish.32, 387–439). doi: 10.1007/s11160-021-09692-6
116
Teichberg M. Wild C. Bednarz V. N. Kegler H. F. Lukman M. Gärdes A. A. et al . (2018). Spatio-temporal patterns in coral reef communities of the Spermonde Archipelago 2012-2014, I: Comprehensive reef monitoring of water and benthic indicators reflect changes in reef health. Front. Mar. Sci.5. doi: 10.3389/fmars.2018.00033
117
Treml E. A. Roberts J. J. Chao Y. Halpin P. N. Possingham H. P. Riginos C. (2012). Reproductive output and duration of the pelagic larval stage determine seascape-wide connectivity of marine populations. Integr. Comp. Biol.52, 525–537. doi: 10.1093/icb/ics101
118
Tresnati J. Yasir I. Aprianto R. Yanti A. Rahmani P. Y. Tuwo A. (2019). Long-Term monitoring of parrotfish species composition in the catch of fishermen from the Spermonde Islands, South Sulawesi, Indonesia. IOP. Conf. Series.: Earth Environ. Sci.370 (1), 012015. doi: 10.1088/1755-1315/370/1/012015
119
Tresnati J. Yasir I. Yanti A. Rahmani P. Y. Aprianto R. Tuwo A. (2020). Multi years catch composition and abundance of Parrotfish landed at Makassar Fisheries Port. IOP. Conf. Series.: Earth Environ. Sci.473. doi: 10.1088/1755-1315/473/1/012059
120
UNEP . (2006). Marine and coastal ecosystems and human well-being: A synthesis based on the findings of the Millennium Ecosystem Assessment. Nairobi: UNEP.
121
Verges A. Vanderklift M. A. Doropoulos C. Hyndes G. A. (2011). Spatial patterns in herbivory on a coral reef are influenced by structural complexity but not by algal traits. PloS One6 (2), e17115. doi: 10.1371/journal.pone.0017115
122
Veron J. E. N. Devantier L. M. Turak E. Green A. L. Kininmonth S. Stafford-Smith M. et al . (2009). Delineating the Coral Triangle. Galaxea. J. Coral. Reef. Stud.11, 91–100. doi: 10.3755/galaxea.11.91
123
Williams I. D. Polunin N. V. C. Hendrick V. J. (2001). Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar. Ecol. Prog. Ser.222, 187–196. doi: 10.3354/meps222187
124
Wilson S. K. Bellwood D. R. Choat J. H. Furnas M. J. (2003). Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr. Mar. Biol.41, 279–310.
125
Wolanski E. Martinez J. A. Richmond R. H. (2009). Quantifying the impact of watershed urbanization on a coral reef: Maunalua Bay, Hawaii. Estuarine. Coast. Shelf. Sci.84, 259–268. doi: 10.1016/j.ecss.2009.06.029
126
Zaelany A. A. (2019). Fish-bombing fisherman from Pulau Barang Lompo, South Sulawesi Province: Corruption and policy for reducing destructive fishing. J. Indonesian. Soc. Sci. Humanities.9, 71–79. doi: 10.14203/jissh.v9i1.148
127
Zhang H. Wang J. J. (2014). “Loss on ignition,” in Soil Test Methods From the Southeastern United States. Eds. SikoramF. J.MooreK. P. (Southern Extension and Research Activity Information Exchange Group - 6), 155–157.
128
Zweifler A. O’Leary M. Morgan K. Browne N. K. (2021). Turbid coral reefs: past, present and future — A review. Diversity13, 1–23. doi: 10.3390/d13060251
Summary
Keywords
herbivory rates, coastal development, turbid reefs, turf algae sediments, herbivorous fish, Makassar
Citation
Estradivari, Pratama AMA, Syafruddin G, Kanna PL, Stuhr M, Torres AF, Munawwarah, Ramos DA, Ambo-Rappe R, Bejarano S, Puebla O, Wild C and Ferse SCA (2025) Coastal urbanization-related stressors affect fish herbivory in the Spermonde Archipelago, Indonesia. Front. Mar. Sci. 12:1359139. doi: 10.3389/fmars.2025.1359139
Received
20 December 2023
Accepted
07 February 2025
Published
06 March 2025
Volume
12 - 2025
Edited by
Fraser Januchowski-Hartley, Nova Southeastern University, United States
Reviewed by
Lida Teneva, Independent Researcher, Sacramento, United States
Linda Eggertsen, Federal University of Santa Maria, Brazil
Updates
Copyright
© 2025 Estradivari, Pratama, Syafruddin, Kanna, Stuhr, Torres, Munawwarah, Ramos, Ambo-Rappe, Bejarano, Puebla, Wild and Ferse.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Estradivari, estradivari@leibniz-zmt.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.