- 1College of Food and Biological Engineering, Bengbu University, Bengbu, China
- 2College of Ocean and Earth Sciences, Xiamen University, Xiamen, China
- 3State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, China
- 4Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao Marine Science and Technology Center, Qingdao, China
- 5National Engineering Research Center of Marine Facilities Aquaculture, Zhejiang Ocean University, Zhoushan, China
- 6College of Fishery Economics, Tangshan Maritime Institute, Tangshan, China
This study explores the effects of norfloxacin (NOR) on oxidative damage, iron (Fe) transport, energy metabolism, and immunotoxicity in the intestine of large yellow croaker under Fe stress. The fish were subjected to Fe (180 μg/L), low-dose NOR (1.8 μg/L, LNOR), high-dose NOR (180 μg/L, HNOR), Fe plus LNOR, and Fe plus HNOR for 60 days. These results demonstrated that Fe alone exposure increased malondialdehyde (MDA), protein carboxylation (PC), and mortality rate, and impaired intestinal tissue, which was related to the increment of Fe accumulation. Compared to Fe alone exposure, Fe plus LNOR exposure decreased MDA, PC, and mortality rate, and alleviated intestinal malformations by improving Fe transport, energy metabolism, anti-inflammatory response, and protein folding protective effect, and reducing pro-inflammatory response, indicating that LNOR had an antagonistic effect on Fe toxicity. Compared to Fe alone exposure, Fe plus HNOR exposure elevated MDA, PC, and mortality rate, and deteriorated intestinal malformations by inhibiting Fe excretion, energy metabolism, anti-inflammatory response, and protein folding protective effect, and enhancing pro-inflammatory response, indicating a synergetic effect between HNOR and Fe stress. These findings suggested that NOR had a dose-dependent effect on Fe-toxicity to large yellow croaker, which contributes to revealing the molecular mechanisms behind their interaction and its ecological implications.
1 Introduction
Fish are being subjected to a large number of environmental metal pollutants as a consequence of the aquaculture industry, industrial effluent, and anthropogenic activity. Among these contaminants, essential metals have a dual effect on the physiological functions of fish, which has already aroused widespread concern (Chandrapalan and Kwong, 2021). Iron (Fe) is required for a wide range of biological functions including energy production, oxygen transport, antioxidant defense, immune function, and DNA synthesis and repair (Moreira et al., 2020). However, high-concentration Fe may induce toxic effects on aquatic animals (Shahjahan et al., 2022). Previous studies indicated that Fe concentrations in Indian rivers range from 0.002 to 14.44 mg/L (Singh et al., 2019). The adverse effects of Fe exposure on the growth, histology, physiological function, and stress tolerance of aquatic organisms have been well documented (Sayadi et al., 2020). Similarly, the increasing presence of antibiotics like NOR in aquatic ecosystems poses significant risks to non-target organisms, even at low concentrations (Xu et al., 2021). Fish may be simultaneously threatened by metal and antibiotic pollutants. Previous studies have shown that more stable complexes can be formed between metal cations and electron donors of antibiotics by complex reactions (Ajewole et al., 2021). Thus, it is reasonable to assume that antibiotics can influence the toxicity effects of metal exposures on aquatic animals, as observed by Chinese mitten crab (Eriocheir sinensis) co-exposed to cadmium and oxytetracycline (Zeng et al., 2024).
The intestine is the primary absorption site of hazardous substances in fish, which involves maintaining material homeostasis (Dawood, 2021). The intestine also plays a vital role in energy metabolism and immunity, which is closely linked to stress tolerance (Lin et al., 2020). Many studies on the intestine have shown that NOR exposure significantly inhibits the metabolism and cellular defense of large yellow croaker (Larimichthys crocea) (Wang et al., 2020b) and impairs the immune function of carp (Cyprinus carpio) (Zhao et al., 2021). Our researches have shown that metal pollutants like copper and zinc have adverse effects on the antioxidant defense and inflammation in the intestine of large yellow croaker (Zeng et al., 2018, 2019a). Thus, the intestine is a vital target organ for assessing the ecotoxicological effects of metal and antibiotic contaminations on fish.
Despite evidence that metal and antibiotic pollutants can interact to affect aquatic organisms, there is limited research on how NOR affects Fe-induced toxicity in fish. Given the growing concern over environmental contamination and its potential effects on marine biodiversity, this study seeks to fill this gap by examining how NOR modulates Fe toxicity in the intestine of the large yellow croaker (Larimichthys crocea Richardson, 1846), focusing on key indicators of oxidative stress, energy metabolism, immune function, and protein folding protective effect. To that end, the large yellow croaker, which is widely distributed in the southeast coastal regions in China, was chronically exposed to Fe (180 μg/L) and/or NOR (1.8 μg/L and 180 μg/L) for 60 d. The main indicators of measurement included (i) Fe homeostasis indicators [Fe bioaccumulations, and Fe transport related gene expressions like divalent metal ion transporter 2 (DMT2), transferrin (Tf), ferritin (Fer), ferroportin (Fpn), and metal regulatory transcription factor 1 (Mtf1)]; (ii) oxidative stress indicators (malondialdehyde (MDA), protein carboxylation (PC), survival rate, and intestinal histomorphology observation); (iii) energy metabolism indicators (adenosine triphosphate (ATP) and adenosine monophosphate (AMP) contents, and ATP synthase (F-ATPase), succinate dehydrogenase (SDH), and mitochondria electron transport chain (ETC) complexes I to IV (CI-IV) activities); (iv) immunotoxicity indicators [gene expressions of pro-inflammatory (interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ)], anti-inflammatory [transforming growth factor-β (TGF-β2, TGF-β3) and interleukin-10 (IL-10)], nuclear factor kappa B (NF-κB), and inhibitor of nuclear factor kappa-B kinase subunit alpha (IκBα), and activities of lysozyme (LZM) and alkaline phosphatase (AKP)]; and (v) protein folding (heat stress protein (HSP60, HSP70, and HSP90) gene expressions).
2 Materials and methods
2.1 Preparation of Fe and NOR solutions
FeCl3·6H2O (AR, Shanghai Sinopharm Group Corporation, Shanghai, China) was dissolved in the ultrapure water to obtain 180 mg/L Fe mother solution. NOR (C16H18FN3O3, purity > 98%) and dimethyl sulfoxide (DMSO, AR) were purchased from Sigma-Aldrich Chemical Co., Ltd. (United States). 180 mg/L NOR stock solution was obtained by blending NOR with DMSO solvent. The final working solutions of DMSO: NOR were 0.01% (v/v). Our previous research has confirmed that DMSO concentration at 0.01% (v/v) does not affect the metabolism and antioxidant defense of large yellow croaker (Zeng et al., 2023). Fe and NOR mother solutions were prepared every week, and stored in amber bottles at 4°C in a refrigerator. Individual working solutions were obtained by adding the shaken mother liquors to filtered natural seawater in the cisterns before they were used.
2.2 Fe and NOR exposures
Large yellow croaker was obtained from an aquaculture farm (Ningde, China), and was maintained in 80-L circular fiberglass tanks in a static aquarium system for the 2-week acclimatization. After acclimation, 600 uniform-sized fish (body weight of 9.32 ± 0.98 g) were transferred to 24 fiberglass tanks with 25 fish per tank. Then these tanks were randomly assigned to 6 groups and each group had 4 replicates: control group, Fe group, low-concentration NOR group (LNOR group), high-concentration NOR group (HNOR group), Fe plus low-concentration NOR group (Fe plus LNOR group) and Fe plus high-concentration NOR group (Fe plus HNOR group). To make the Fe3+ particles in suspension, each tank was fitted with a water pump to generate a mild water circulation. Commercial feed (lipid 6%, crude protein 48%, and 80 mg iron/kg diet) was provided to fish at a rate of 2% body, twice every day. Based on our previous research methods (Zeng et al., 2020), using a log probit analysis program to obtain 96-h lethal concentration (LC50) of Fe, which was about 8.96 mg/L. In this study, fish were exposed to 180 μg/L Fe (approximately 2% of the 96-h LC50). There were two NOR concentrations. Low-concentration NOR (1.8 μg/L) was close to contaminant concentration in the natural environment, and high-concentration NOR (180 μg/L) contributed to revealing the toxic effects of NOR on fish. The water-quality parameters were detected twice every week: salinity 25.85 ± 1.03, pH 7.52 ± 0.46, temperature 22.53 ± 1.93°C, dissolved oxygen 7.74 ± 0.82 mg/L, total alkalinity (indicated by CaCO3) 124.76 ± 8.37 mg/L, photoperiod regime 12L:12D and total ammonia < 0.06 mg/L. To maintain relatively constant Fe and NOR concentrations, 100% water was changed every day by adding desired aquaculture water from the cisterns. To adjust the water pH of Fe treatment groups to the control group, adding diluted KOH to neutralize the decrease of water pH caused by the formation of Fe (OH)3. After 60 days of Fe and NOR exposures, fish were counted to assess mortality. 4 fish in each tank were anesthetized with benzocaine (Sigma), the intestine was collected, washed with sterile PBS, frozen in liquid nitrogen, and then kept at -80°C refrigerator until later molecular and biochemical analysis. Based on our previous research data (Zeng et al., 2018), the absorption of substances mainly presented in the proximal intestine, and antioxidant defense and immunotoxicity mainly occurred in the distal intestine when large yellow croaker was exposed to polluted environments. Thus, the proximal intestine was used for analyzing Fe and NOR contents, Fe homeostasis, and energy metabolism, and the distal intestine was used for measuring immune function. We assured that all the protocols were adhered to the ethical and moral principles of Chinese experimental animals.
2.3 Concentration measurements of Fe and NOR
According to Zeng et al. (2020), HNO3 (110°C for 72 h) was used to digest tissues or water samples, which were diluted for Fe determination with inductivity-coupled plasma mass spectrometry (ICP-MS, Agilent Technologies, USA). The quantification limits of Fe in the tissues and water were 2.3 ng/L dry weight and 0.8 ng/L wet weight, respectively. Recovery of Fe ranged from 95 to 104%. Using a solid-phase extraction method to obtain NOR samples from tissues or water, which were measured by high-performance liquid chromatography/mass spectrometry (HPLC-MS, Shimadzu, Japan), following the method described by Zeng et al. (2023). The detection limits of NOR in the tissues or water were 7.6 ng/L dry weight and 3.3 ng/L wet weight, respectively. The recovery of spiked samples ranged from 91.68 to 94.32%. All the Fe and NOR analyses were carried out in duplicate.
2.4 Intestinal histomorphology observation
Histomorphology observation was carried out according to the methods from our previous research (Zeng et al., 2019b). Briefly, using 4% paraformaldehyde to fix the proximal intestine sample at 4°C for 48 h, graded ethanol concentrations for dehydration, and xylene for transparency before samples were embedded in paraffin wax. 4 μm sections of intestinal tissues were stained with hematoxylin/eosin (H&E) after drying. To calculate the fold height, muscular thickness, and tissue area of intestinal sections, 12 microscope fields for each sample were observed under a light microscope with Image Pro-Plus 6.0 software (Media Cybernetics, USA). The observation data from individual section was merged into the overall result.
2.5 Biochemical analysis
According to our previous study, intestinal samples were homogenized for biochemical (Zeng et al., 2016). Briefly, intestinal samples were homogenized in 9-fold volumes of ice-cold physiological salt solution. After the homogenates were centrifuged, the supernatant was collected and stored at -80 until being measured for biochemical analysis. MDA, PC, ATP, and AMP contents, and FATPase, SDH, CI-IV, LZM, and AKP activities were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, China), which were based on the manufacturer protocol. The activities of enzymes were represented as units (U)/mg protein. Protein content was analyzed by the Bradford method (Bradford, 1976). These measurements were carried out in triplicates.
2.6 Gene expression analysis
Following our previous study (Zeng et al., 2016), TRIzol reagent (Invitrogen, USA), Revert Aid First Strand cDNA synthesis kit (Thermo Fisher Scientific, USA), and SYBR® Premix Ex Taq™ kit (Takara, Japan) were used for RNA isolation, cDNA synthesis and quantitative PCR (qPCR), respectively. The thermal program included: initial denaturation at 95°C for 1 min, followed by 45 cycles of 95°C for 5 s, 57°C for 10 s, and 72°C for 30 s. All reactions were performed in duplicate and each reaction was verified to contain a single product of the correct size using agarose gel electrophoresis. The primer sequences are shown in Table 1. Using β-actin as a housekeeping gene. The 2−ΔΔCt method was applied to determine the relative expression levels of genes (Pfaffl, 2001).
2.7 Statistical analysis
Experimental data was expressed as the means ± SEM (n = 4). Before statistical analyses, the normality of distribution and homogeneity of variances were analyzed by the Kolmogorov-Smirnov test and Bartlett’s test, respectively. One-way ANOVA and Duncan’s multiple range test were used to analyze the significance among groups. All the statistical analyses were carried out using SPSS V.20 software, and significant levels were set at P < 0.05.
3 Results
3.1 Fe and NOR contents
Compared to the control, Fe exposure elevated Fe content; LNOR exposure did not affect Fe content; HNOR exposure decreased Fe content (Figure 1). Fe plus LNOR exposure had no impact on Fe content, and Fe plus HNOR exposure boosted Fe content when compared to Fe alone exposure (Figure 1).

Figure 1. Effects of Fe and NOR on the Fe (A) and NOR (B) contents in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
Compared to the control, Fe exposure had no effect on NOR content; both LNOR and HNOR exposures increased NOR content (Figure 1). Compared to Fe alone exposure, both Fe plus LNOR and Fe plus HNOR exposures enhanced NOR content (Figure 1).
3.2 MDA and PC contents, and survival rate
Compared to the control, Fe and HNOR exposures increased MDA and PC contents, and reduced survival rate; LNOR exposure did not affect MDA and PC contents, and survival rate (Figure 2). Compared to Fe alone exposure, Fe plus LNOR exposure inhibited MDA and PC contents, and elevated survival rate; while Fe plus HNOR exposure increased PC and MDA contents, and reduced survival rate (Figure 2).
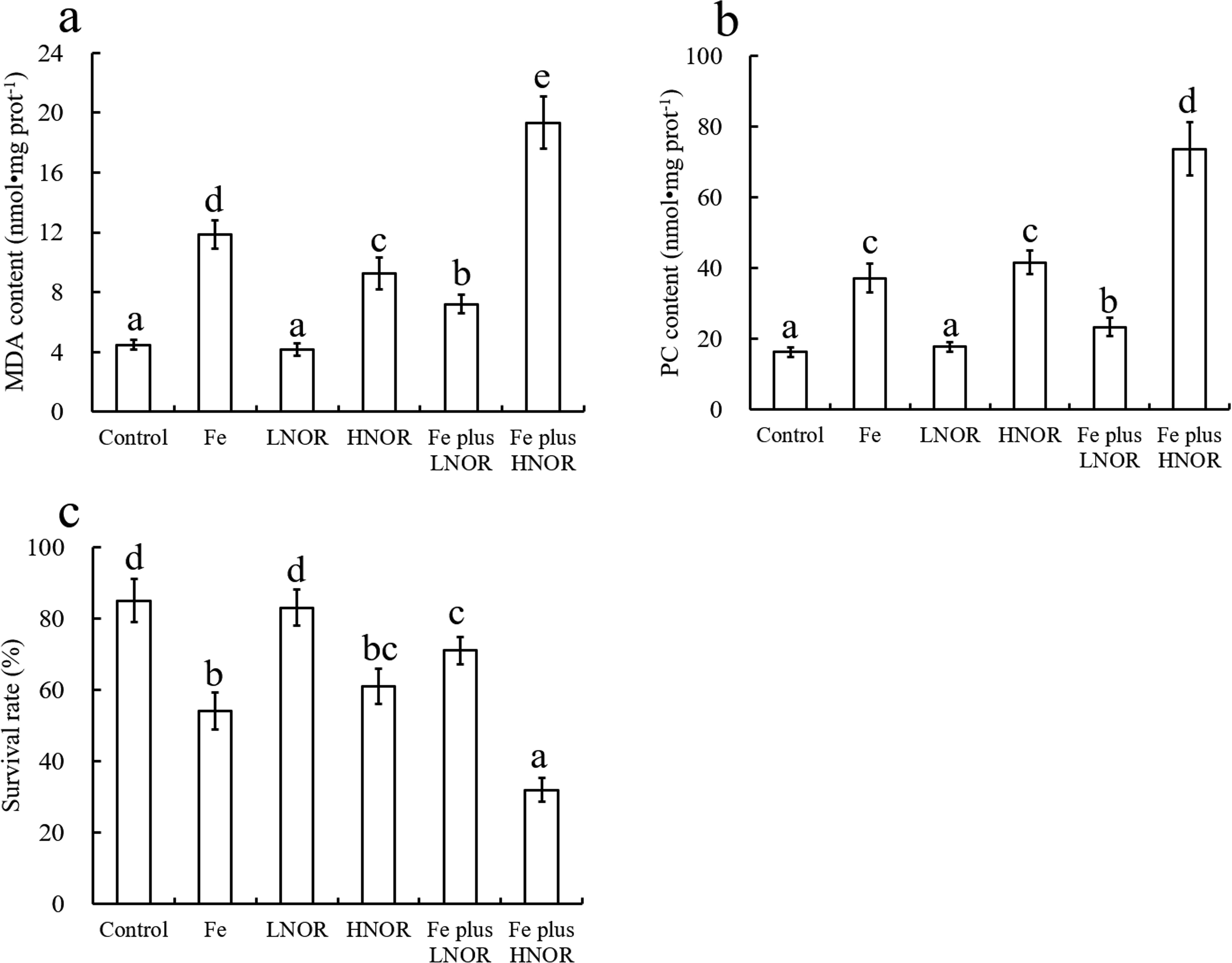
Figure 2. Effects of Fe and NOR on the MDA (A) and PC (B) contents, and survival rate (C) in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
3.3 Histological observation
Normal intestinal histomorphology was observed in the control group (Figure 3). The villi were partially dissolved and necrotic in the Fe group and LNOR group, and slightly disorganized in the Fe group (Figure 3). Fe and LNOR exposures reduced fold height, muscular thickness and tissue area compared to the control (Figure 4). Serious malformation of tissue architect was observed in the HNOR group, including significantly shortened fold height, muscular thickness and tissue area (Figures 3, 4). No significant changes in the intestinal histomorphology between Fe plus LNOR and the control groups, except for slightly shortened fold height and tissue area (Figures 3, 4). Compared to Fe alone exposure, Fe plus LNOR exposure elevated fold height, muscular thickness, and tissue area (Figure 4). Further aggravation of deformed intestinal histomorphology was observed in the Fe plus HNOR group compared to that in the HNOR group (Figure 3).
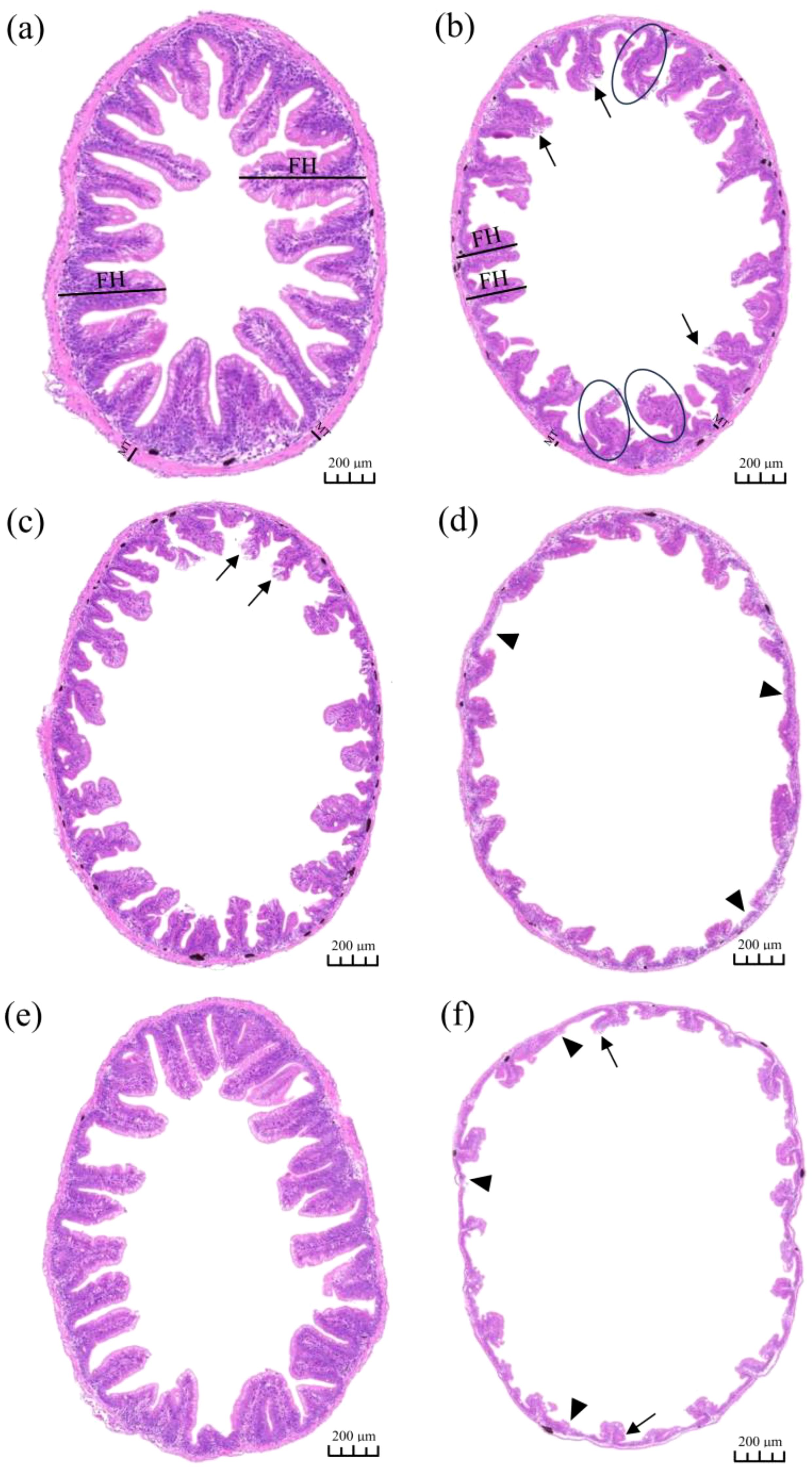
Figure 3. Effects of Fe and NOR on the intestinal morphology of large yellow croaker. (A) control group, the intestinal morphology was normal; (B) Fe group, the villi were partial dissolution and necrosis (black arrows), slightly disorganized (circle), and significantly shortened; (C) LNOR group, the villi were partial dissolution and necrosis (black arrows), and significantly shortened; (D) HNOR group, the villi were severely atrophic (triangle); (E) Fe plus LNOR group, the villi were slightly shortened; (F) Fe plus HNOR group, the villi were partial dissolution and necrosis (black arrows), and severely atrophic (triangle). FH, fold height; MT, muscular thickness; tissue area (purple zone).
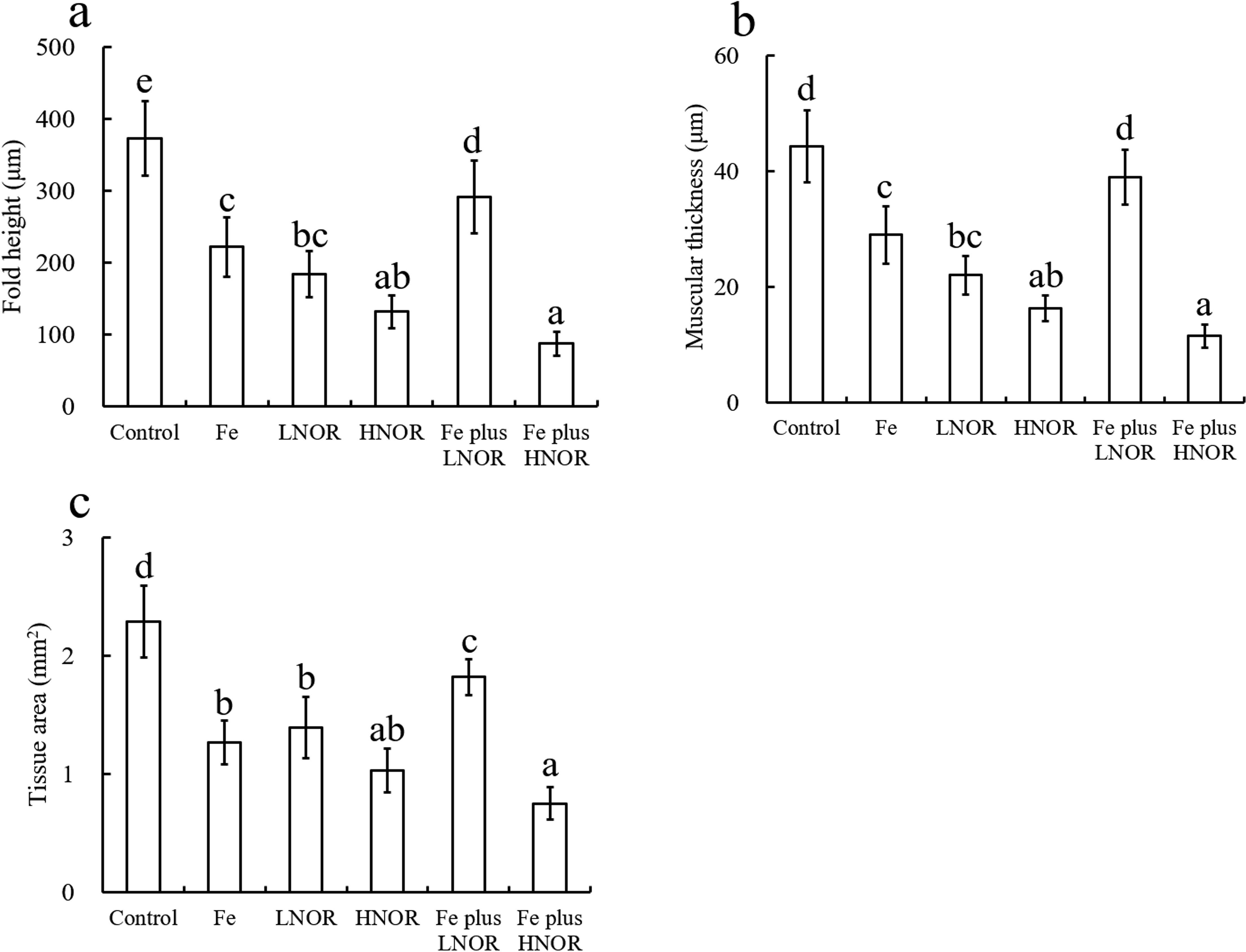
Figure 4. Effects of Fe and NOR on the fold height (A), muscular thickness (B), and tissue area (C) in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
3.4 Fe transport
Compared to the control, Fe exposure up-regulated gene expressions of Fe transport; LNOR exposure did not affect mRNA levels of Fe transport; HNOR exposure reduced DMT2, Fer1, and Fpn mRNA levels (Figure 5). Compared to Fe alone exposure, Fe plus LNOR exposure up-regulated DMT2, Fer1, Fer2, Fpn and Mtf1 gene expressions, and down-regulated Ff gene expression; Fe plus HNOR exposure increased DMT2, Tf, Fer1, Fer2 and Mtf1 mRNA levels, and reduced Fpn mRNA level (Figure 5).
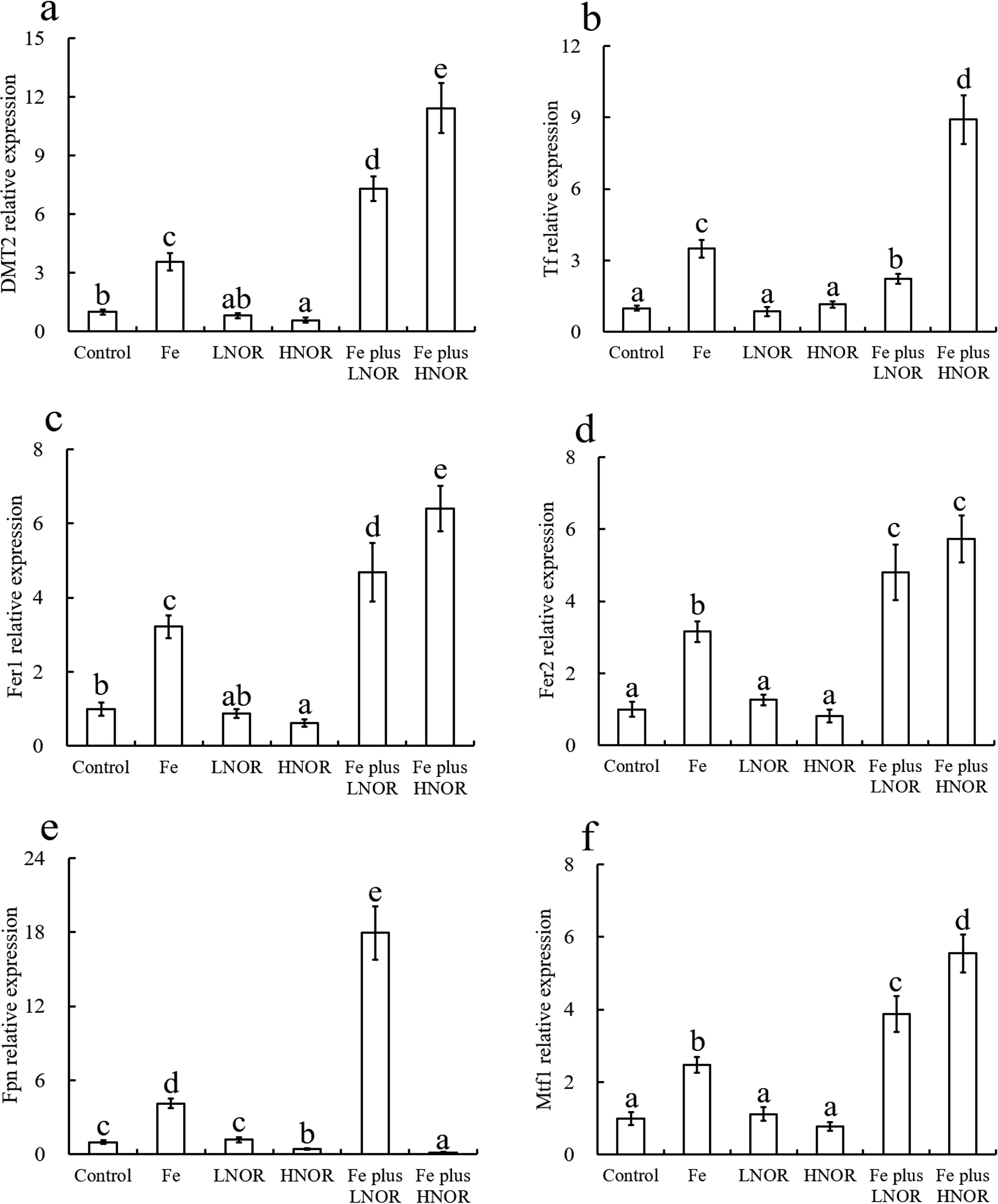
Figure 5. Effects of Fe and NOR on the Fe transport-related gene expressions (A–F) in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
3.5 Energy metabolism
Compared to the control, Fe exposure decreased ATP content, F-ATPase, CI, CIII, and CIV activities, and ATP: AMP and CI: CII ratios; LNOR exposure reduced ATP content, SDH, F-ATPase, and CIII activities; HNOR exposure inhibited ATP content, SDH, F-ATPase and CI-CIV activities, and ATP: AMP and CI: CII ratios, and enhanced AMP content (Figures 6–8).
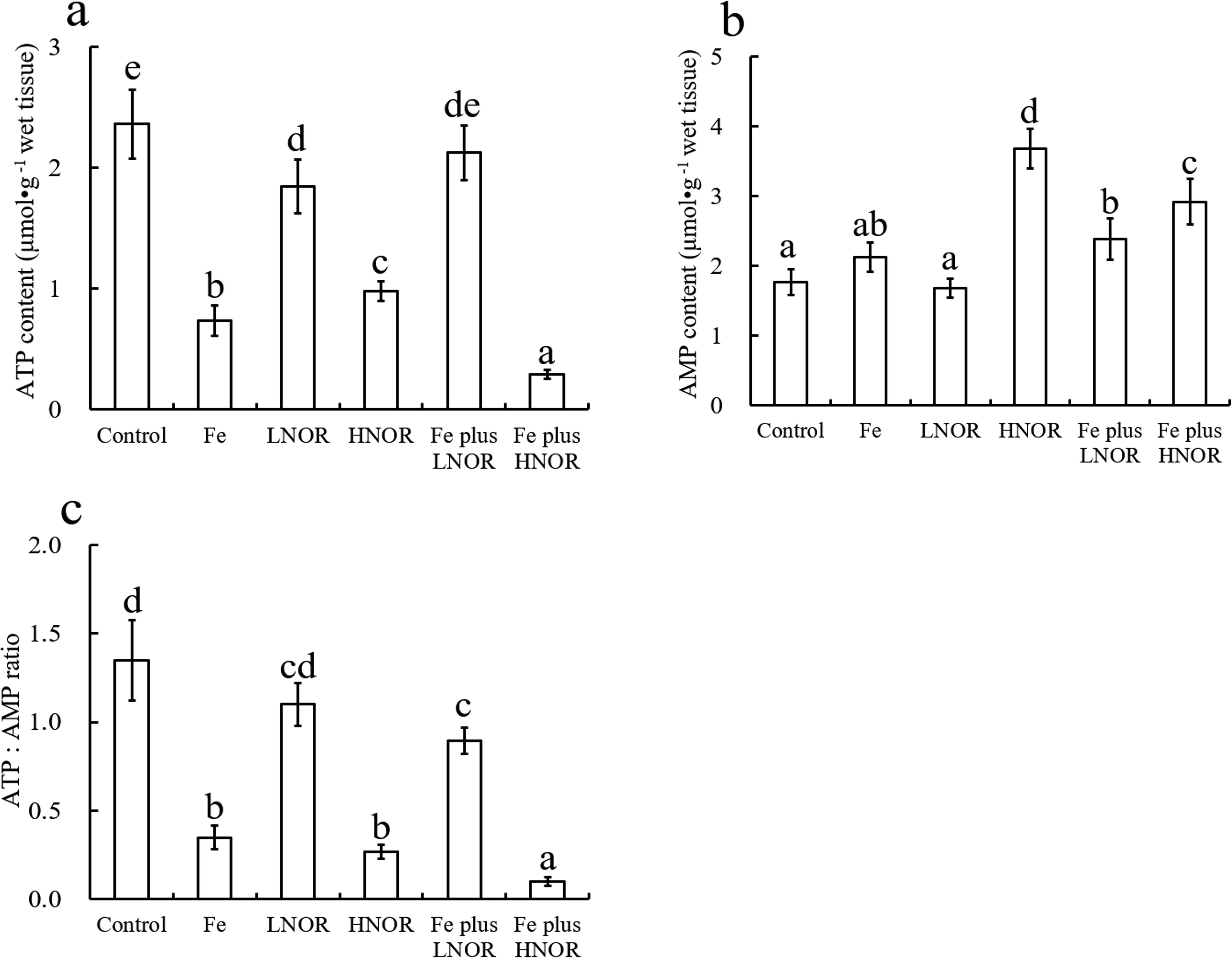
Figure 6. Effects of Fe and NOR on the ATP (A) and AMP (B) contents, and ATP: AMP ratio (C) in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).

Figure 7. Effects of Fe and NOR on the F-ATPase (A) and SDH (B) activities in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
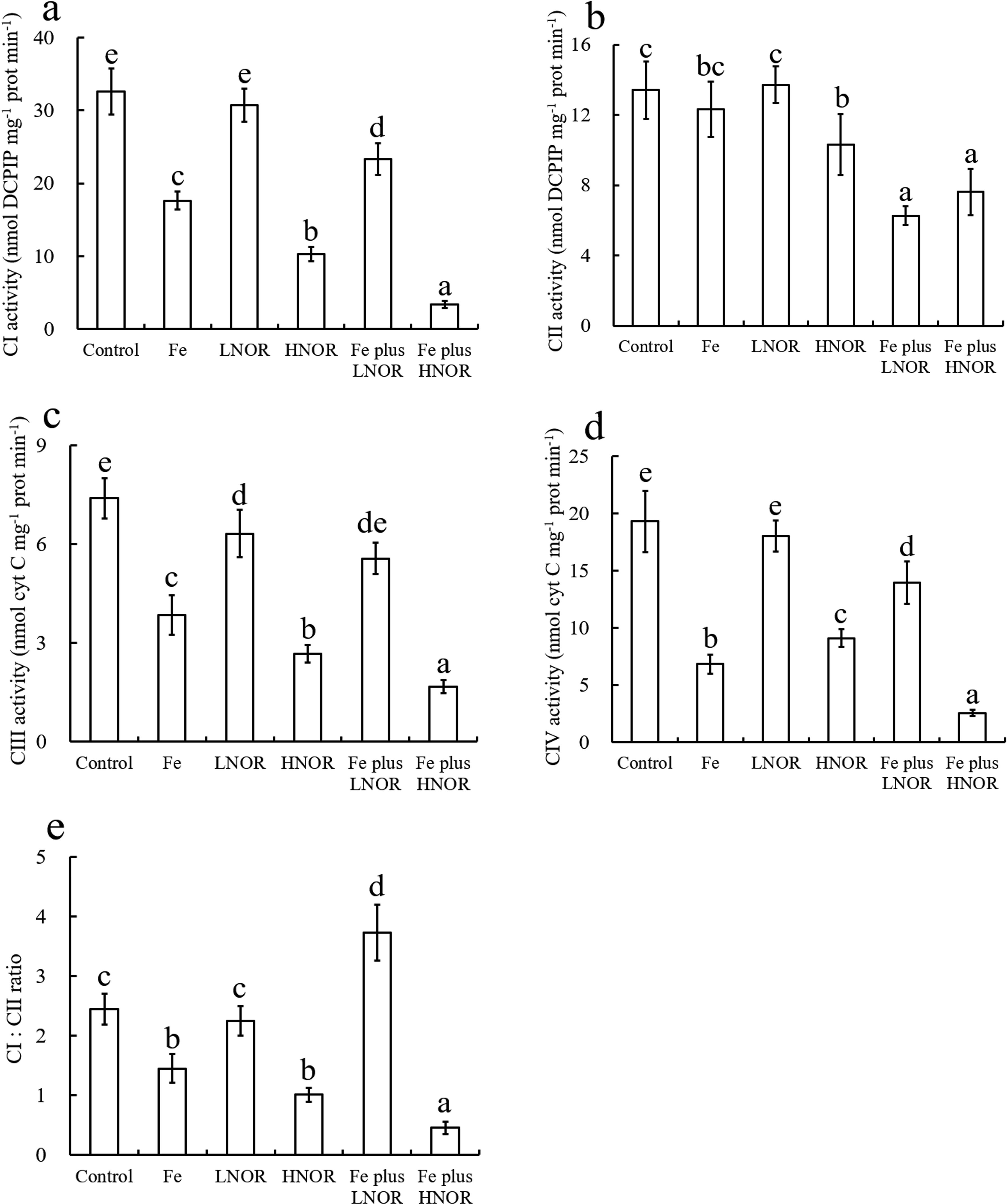
Figure 8. Effects of Fe and NOR on the CI-CIV activities (A-D), and CI: CII ratio (E) in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
Compared to Fe alone exposure, Fe plus LNOR exposure enhanced ATP content, F-ATPase, CI, CIII, and CIV activities, and ATP: AMP and CI: CII ratios, and inhibited SDH and CII activities; Fe plus HNOR exposure decreased ATP content, F-ATPase, SDH and CI-CIV activities, and ATP: AMP and CI: CII ratios, and increased AMP content (Figures 6–8).
3.6 Immunotoxicity
Compared to the control, Fe exposure up-regulated NF-κB and pro-inflammatory gene expressions, and down-regulated TGF-β2, IL-10, and IκBα mRNA levels, and ZLM and AKP activities; LNOR exposure increased IL-8, IκBα and anti-inflammatory gene expressions, and ZLM and AKP activities; HNOR exposure enhanced NF-κB and pro-inflammatory gene expressions, and inhibited IκBα and anti-inflammatory mRNA levels, and ZLM and AKP activities (Figures 9–11).
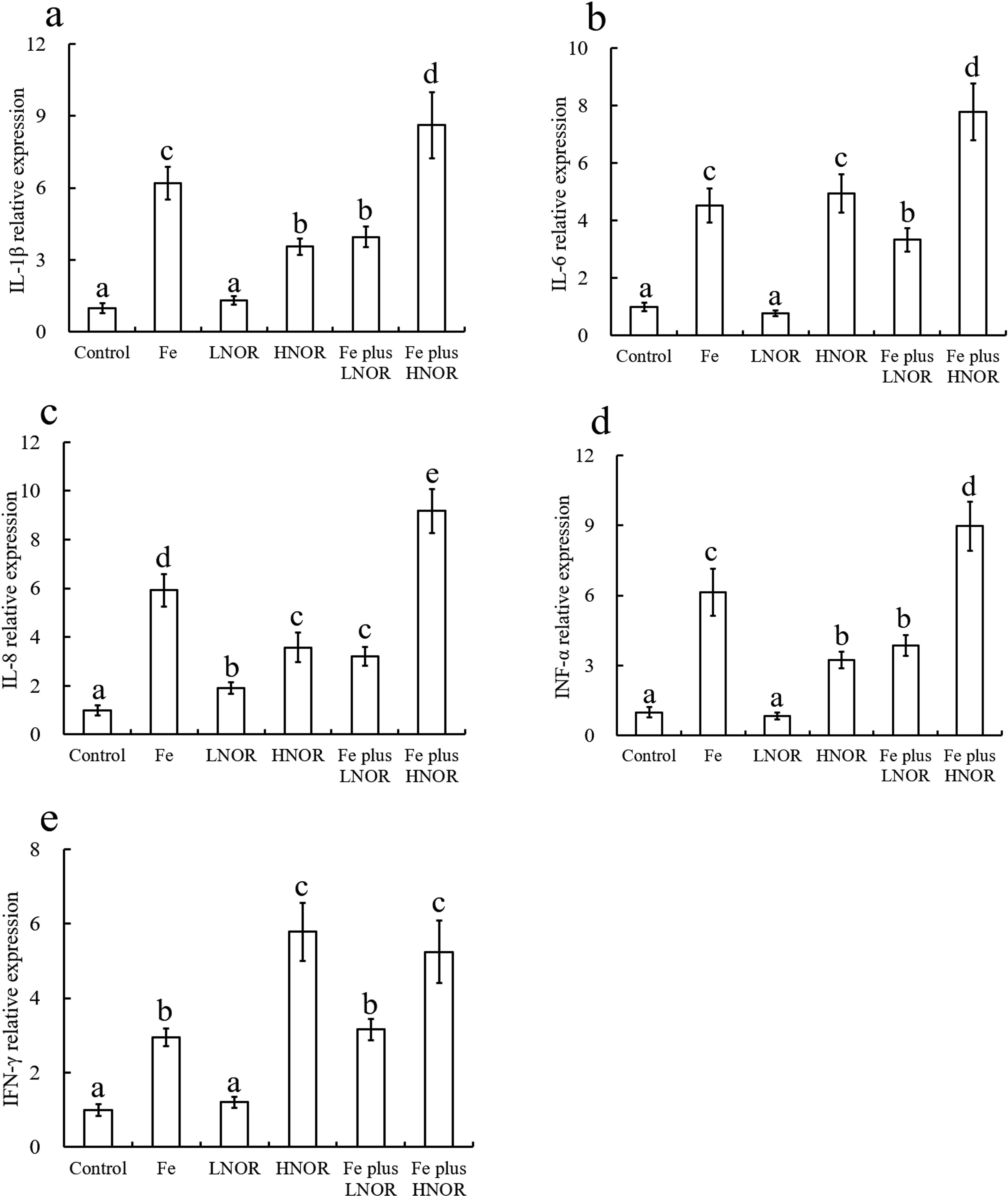
Figure 9. Effects of Fe and NOR on the pro-inflammatory gene expressions (A–E) in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
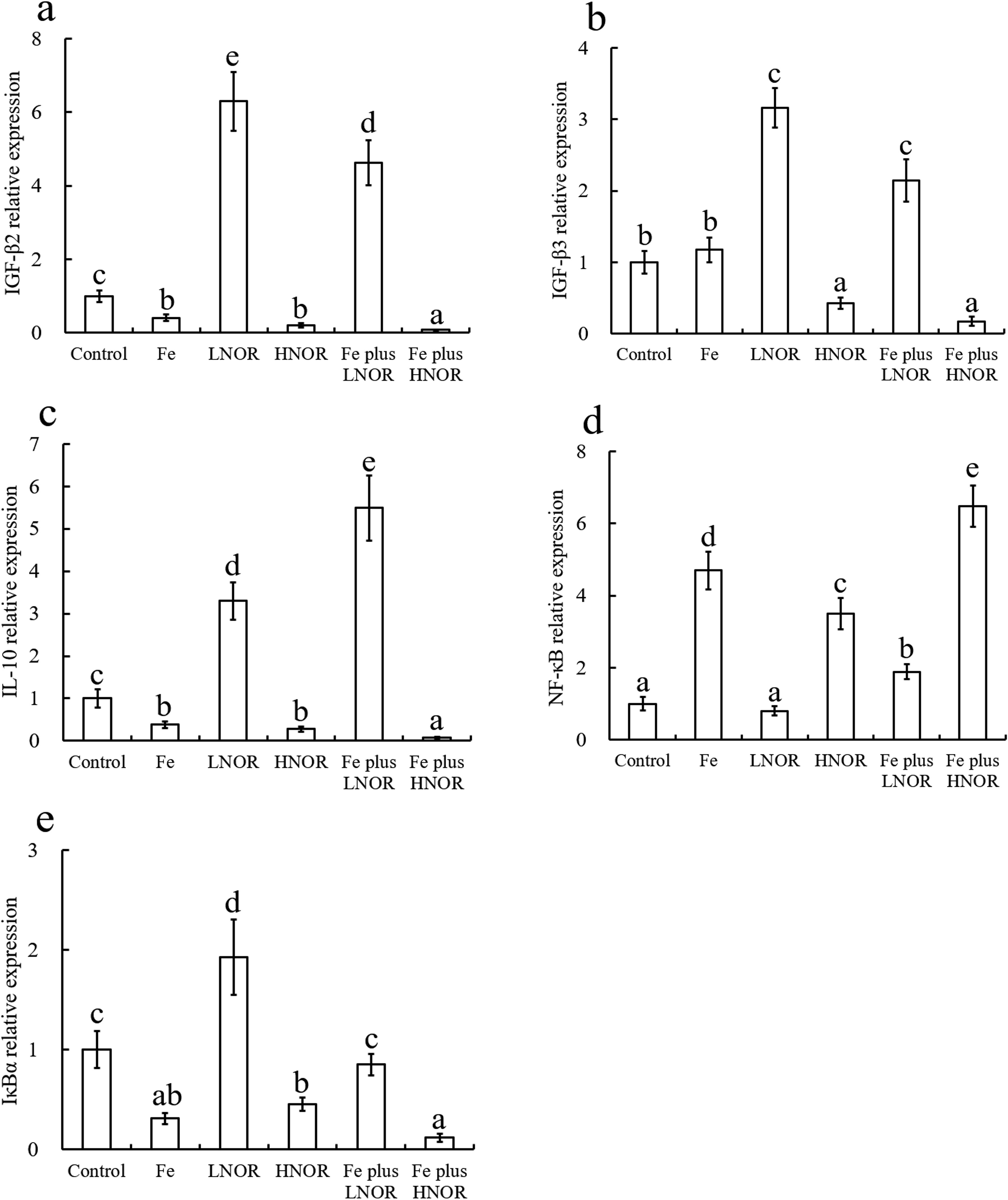
Figure 10. Effects of Fe and NOR on the gene expressions of anti-inflammatory (A-C), NF-κB (D) and IκBα (E) in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).

Figure 11. Effects of Fe and NOR on the ZLM (A) and AKP (B) activities in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
Compared to Fe alone exposure, Fe plus LNOR exposure reduced IL-1β, IL-6, IL-8, TNF-α, and NF-κB mRNA levels, and boosted IκBα and anti-inflammatory gene expressions, and ZLM and AKP activities; Fe plus HNOR exposure up-regulated NF-κB and pro-inflammatory gene expressions, and down-regulated anti-inflammatory gene expressions, and ZLM and AKP activities (Figures 9-11).
3.7 HSP gene expressions
Compared to the control, Fe exposure had no effect on HSP60 gene expression, and up-regulated HSP70 and HSP90 mRNA levels; LNOR exposure increased HSP90 gene expression; HNOR exposure enhanced HSP60, HSP70, and HSP90 mRNA levels (Figure 12).
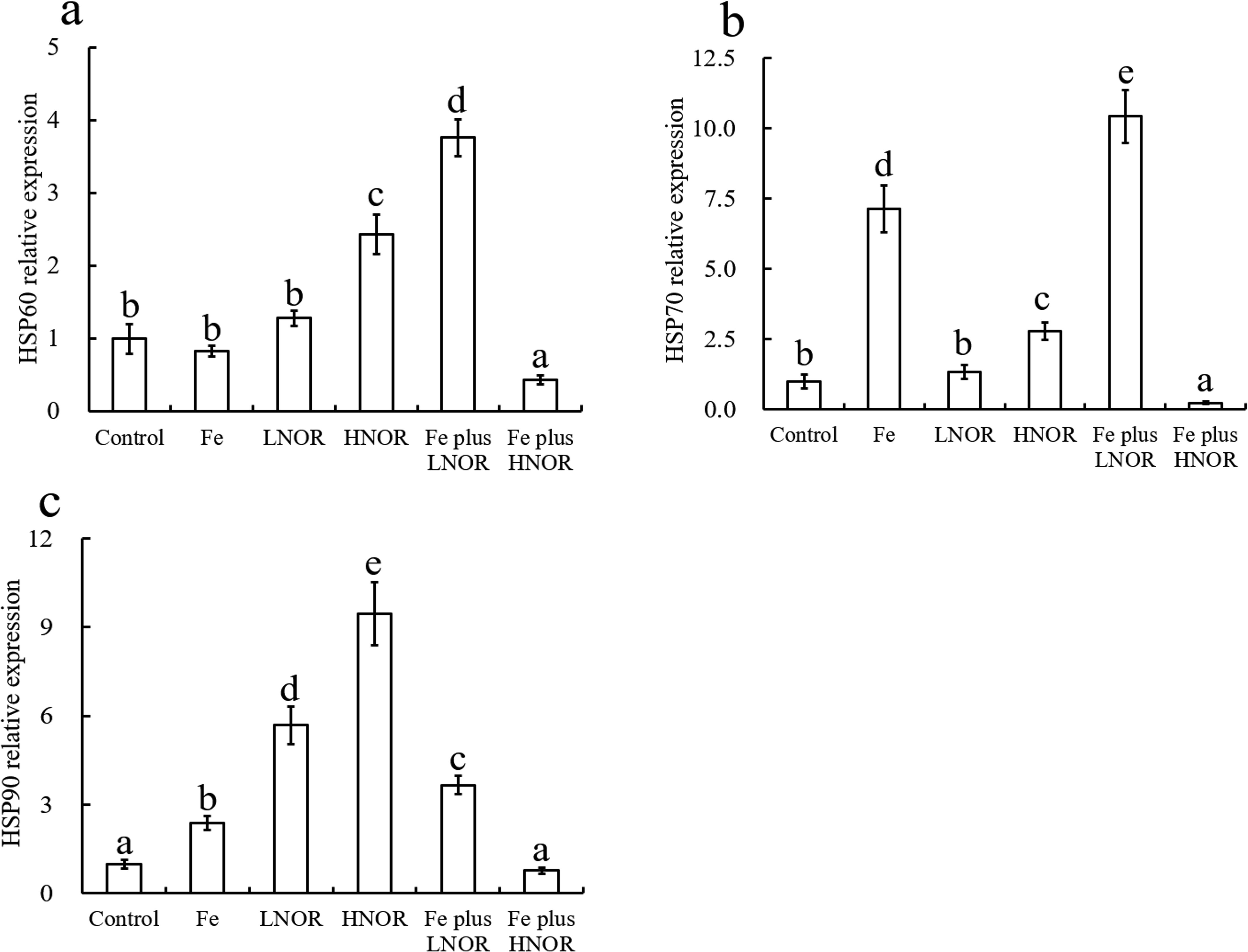
Figure 12. Effects of Fe and NOR on the HSP gene expressions (A–C) in the intestine of large yellow croaker. The vertical bars represent the mean ± SEM (n = 4). Different letters indicate significant differences among groups (P < 0.05).
Compared to Fe alone exposure, Fe plus LNOR exposure up-regulated HSP60, HSP70, and HSP90 mRNA levels; Fe plus HNOR exposure down-regulated HSP60, HSP70, and HSP90 gene expressions (Figure 12).
4 Discussion
Fe and NOR are widely distributed in the marine environment. The environmental risks of Fe and NOR mixture are different from single Fe or NOR itself (Ajewole et al., 2021). However, the underlying mechanism regarding the effects of NOR on fish exposed to Fe is largely unknown. The present study revealed for the first time that Fe toxicity was affected by NOR in a concentration-dependent manner, which had close relationships with Fe homeostasis, energy metabolism, immunotoxicity, and protein folding protective effect of HSPs.
4.1 Oxidative stress mechanisms in Larimichthys crocea
MDA and PC are the biomarkers of oxidative damage. In this study, Fe exposure increased MDA and PC, lowered the survival rate, and damaged the intestinal structure. These changes highlighted the deleterious effects of Fe on organisms. This result is consistent with blackfish (Capoeta fusca) under Fe stress (Sayadi et al., 2020). Fe3+ was reduced to Fe2+ during entering into fish, which easily induced the Fenton reaction, resulting in excessive free radical species (ROS) generation. This caused cellular oxidative damage, even ferroptosis.
LNOR did not affect MDA, PC, and survival rate, suggesting that fish have strong adaptability to low-dose NOR. Sehonova et al. (2019) reported that oxidative damage to zebrafish (Danio rerio) caused by NOR decreased with prolonged exposure time. However, LNOR had an adverse impact on intestinal histomorphology, indicating the intestine was particularly susceptible to antibiotic pollution. The toxicity effect of LNOR on the intestine might be partly related to the destruction of microflora balance, which inhibited normal metabolism function such as digestion and absorption, leading to the reduced development of the intestine, as reflected by the reduced intestinal fold height, muscular thickness, and tissue area (Sun et al., 2019; Wang et al., 2020b). Previous studies showed that long-term exposure to antibiotics could impair the intestinal structure of Nile tilapia (Limbu et al., 2018). Unlike LNOR, HNOR imposed toxicity effects on fish, as reflected by the enhanced MDA and PC, the decreased survival rate, and the deteriorating intestinal malformation structure. 180 μg/L NOR may exceed the limit of stress tolerance for large yellow croaker, indicating that NOR induced toxicity effects in a dose-dependent manner. Similar observations were reported in zebrafish embryos that norfloxacin nicotinate had a concentration-dependent effect on hatching rate, body length, abnormality, and mortality (Liang et al., 2020). Thus, NOR concentration is an important factor affecting toxic effects on organisms.
Fe plus HNOR exposure increased MDA and PC, reduced survival rate, and aggravated intestinal malformation compared to Fe alone exposure, showing a synergistic effect between HNOR and Fe exposures. This may be partly related to the increment of Fe accumulation through complex reactions between iron cations and carbonyl/carboxylate groups of NOR. Interestingly, LNOR reduced MDA and PC, alleviated intestinal malformation, and increased survival rate when fish were subjected to Fe, suggesting that LNOR mitigated Fe-induced toxic effects on fish. Although LNOR impaired intestinal structure, LNOR could reduce the reproduction and growth of bacteria, and enhance the adaptability of fish to Fe pollution. A similar result was observed in Crucian carp that Cu-induced oxidative damage was affected by diclofenac in a dose-dependent effect (Xie et al., 2020).
4.2 Fe homeostasis mechanisms in Larimichthys crocea
DMT2, Tf, Fer, and Fpn are important for maintaining intracellular Fe homeostasis (Chandrapalan and Kwong, 2020). Fe3+ in the non-hem protein should be catalyzed into Fe2+ before being absorbed into intestinal epithelial cells by DMT2, then is transported to another side of epithelial cells by Fpn1, last is transported to target organs by Tf or is stored in the form of Fer (Vogt et al., 2021). In this study, Fe exposure increased DMT2, Tf, Fer1, Fer2, and Fpn gene expressions. Fe absorption resulted in the increment of Fe accumulation. Enhancing Fe utilization, storage, and excretion may be a vital adaptive response to deal with Fe imbalance (Xu et al., 2023).
HNOR reduced DMT2, Fer1, and Fpn gene expressions, indicating the inhibition of Fe absorption, storage, and discharge, resulting in the reduction of intestinal Fe content, which was mainly sourced from feed. This might be related to the reduction of intestinal absorption area (Jiao et al., 2022). Although LNOR reduced fold height, muscular thickness, and tissue area, but did not affect Fe transport-related gene expressions and Fe content, suggesting that the reduced intestinal absorption area in the LNOR group was fully qualified to maintain Fe equilibrium (Chandrapalan and Kwong, 2020).
Compared to Fe alone exposure, Fe plus LNOR exposure enhanced DMT2 (2.05 times) and Fpn1 (4.35 times) mRNA levels, and inhibited Tf, Fer1, and Fer2 gene expressions. Fpn1 is the only protein for eliminating Fe in the cells (Xia et al., 2021). Fe secretion was more than Fe absorption, which contributed to the reduction of Fe-toxicity by boosting Fe emissions. Fe plus HNOR exposure elevated DMT2, Tf, Fer1, and Fer2 gene expressions, and remarkably reduced Fpn1 mRNA level when compared to Fe alone exposure. The imbalance between Fe uptake and efflux resulted in a significant increment of Fe bioaccumulation (Nemeth and Ganz, 2021).
4.3 Energy metabolism mechanisms in Larimichthys crocea
The coupling of tricarboxylic acid cycle (TAC) and ETC can efficiently provide a large amount of energy for organelles through oxidative phosphorylation (OXPHOS) (Ryan, 2018). Fe is the coenzyme of SDH, aconitase, and acetyl CoA, which are essential components of TAC. Fe is also the essential ingredient of ETC complexes, including iron-sulfur (Fe-S) centers of CI-CIII, and cytochrome C oxidase of CIV, which play vital roles in driving protons from the matrix into mitochondrial intermembranous space to generate ATP during the process of OXPHOS (Read et al., 2021). In this study, the Fe group had no impact on SDH and CII activities, but inhibited F-ATPase, CI, CIII, and CIV activities, suggesting Fe stress reduced energy metabolism efficiency, mirroring the reduction of ATP content. Previous studies indicated that a moderate dose of Fe can elevate ETC enzyme activities, which promotes ATP synthesis by driving proton-motive force (Afshari et al., 2021). However, excessive Fe may impair Fe-S centers and cytochrome C oxidase, which has adverse effects on the affinities of ETC enzymes with substrates by modifying the quantities and/or conformation of enzymes (Niemuth et al., 2020). In addition, the reduction of CIV activity signified providing less proton motive force for the F-ATPase enzyme, leading to the reduction of OXPHOS (Nesci et al., 2021). Thus, the inefficiency of mitochondrial ETC caused by Fe stress compromised energy metabolism.
LNOR had no impact on most of ETC enzyme activities, but inhibited SDH and F-ATPase activities, resulting in the decrement of energy metabolism efficiency. The detrimental effect of LNOR on TAC enzymes might be related to the reduction of intestinal absorption area and microflora maladjustment, which inhibited nutrition absorption and metabolism (Yukgehnaish et al., 2020). HNOR reduced the global activities of TAC and ETC enzymes, which hindered energy metabolism. The main reason may involve the destruction of ETC and intestinal structure caused by HNOR-induced oxidative stress, except for the decreased nutrition absorption area and microflora imbalance (Yang et al., 2020). A similar result was observed in C. carpio that environmental NOR concentration impaired intestinal function by enhancing oxidative damage (Zhao et al., 2021).
Compared to Fe alone exposure, Fe plus LNOR exposure boosted CI, CIII, CIV, and F-ATPase activities, and reduced CII and SDH activities, which contributed to the improvement of energy metabolism, mirroring the enhancement of ATP: AMP ratio. The positive effect of LNOR on energy metabolism was partly associated with the decrement of free Fe content through complexation reactions (Miller et al., 2019). The increment of CI not only enhanced the respiratory flux through the ETC, but also reduced ROS production (Zhao et al., 2019a). CII took part in the ATP synthesis by producing high-energy molecules FADH2 catalyzed by SDH, which was unable to transfer the protons from the cellular matrix into mitochondria (Vercellino and Sazanov, 2022). Thus, CII is a pathway with relatively inefficient ATP synthesis compared to CI (Markevich et al., 2020). As a sensitive indicator for energy metabolism efficiency, CI: CII was enhanced in the LNOR plus Fe group compared to that in the Fe alone group, which strengthened the evidence of variation in the energy metabolism (Nesci and Lenaz, 2021).
Compared to Fe alone exposure, Fe plus HNOR exposure reduced the global activities of TAC and ETC enzymes, indicating a synergistic effect of HNOR and Fe exposure on energy metabolism. A possible reason for this phenomenon was that the insoluble complex formed by NOR and Fe3+ covered the surface of gills and intestinal mucosa (Shumoy and Raes, 2021), which impeded fish respiration and nutrient absorption, leading to a decrement in aerobic metabolism. The fact was proved by the decrement of ATP content, and the increment of AMP content. In addition, Fe and HNOR co-exposure might damage the mitochondrial ETC, which reduces the transformation efficiency of respiration-generated proton gradient into ATP generation (Nolfi-Donegan et al., 2020).
4.4 Immunotoxicity mechanisms in Larimichthys crocea
The tolerance to stress highly depends on the immune system (Shi et al., 2023). Fe is an essential element for the immune function of aquatic animals, which participates in the development and formation of immune organs, proliferation and differentiation of immune cells, and secretion of humoral immune factors (Nairz et al., 2014). Proinflammatory factors (IL-1β, IL-6, IL-8, TNF-α, and IFNγ), anti-inflammatory factors (TGF-β2, TGF-β3, and IL-10), LZM and AKP enzymes play important roles in the immune response (Liu et al., 2024). In this study, Fe exposure increased the pro-inflammatory gene expressions, inhibited TGF-β2 and IL-10 gene expressions, and LZM and AKP activities, indicating that Fe stress intensified inflammatory response, and suppressed immune function. Previous studies indicated that excessive Fe impaired the phagocytic function of neutrophils, and weakened the killing ability of lymphocytes (Lang et al., 2022). Enhancing Fe significantly increased the pro-inflammatory factor (TNF-α, IL-1β, and IL-6) gene expressions, resulting in a deleterious effect on the health of Pelteobagrus fulvidraco (He et al., 2024). The main reason may be that pathogens synthesized and secreted high-affinity Fe carriers to compete with the host for Fe sources, thereby promoting the growth and reproduction of pathogens (Lemos and Balado, 2020), while interfering with the host’s Fe homeostasis (Galy et al., 2024). Thus, excessive Fe might enhance the pathogenicity of bacteria.
In this study, LNOR increased anti-inflammatory gene expressions, and LZM and AKP activities, but did not have a significant effect on most of the pro-inflammatory gene expressions. The improvement of non-specific immunity may reflect a compensatory mechanism for protecting organisms against LNOR (Imperatore et al., 2023). HNOR increased pro-inflammatory gene expressions, and reduced anti-inflammatory gene expressions, and LZM and AKP activities, suggesting that HNOR enhanced inflammatory response and inhibited innate immunity of fish (Ren et al., 2020). The inequality between pro-inflammatory and anti-inflammatory responses may result in immunotoxicity. Previous studies indicated that different concentrations of norfloxacin nicotinate have different effects on the immune system of D. rerio (Liang et al., 2020). A moderate amount of antibiotic stimulates immune responses to inhibit the growth and reproduction of bacteria, but high-dose antibiotic seriously disrupts the balance of gut microbiota, which impairs the innate immune barrier function (Limbu et al., 2018; Zhao et al., 2019b). NOR exposure significantly reduced the abundance and diversity of gut microbiota in large yellow croaker, thereby inhibiting cellular defense function (Wang et al., 2020b). NOR exposure also disrupted the gut microbiota homeostasis in C. carpio, which damaged the intestinal barrier and other immune functions (Zhao et al., 2021). The intimate relationship between intestinal microbiota and immunity function can be further investigated through omics techniques.
Compared to Fe alone exposure, Fe plus LNOR exposure increased anti-inflammatory mRNA levels, LZM and AKP activities, and reduced IL-1β, IL-8, and INF-α mRNA levels, suggesting that LNOR relieved inflammatory reactions, and improved the non-specific immune of fish under Fe stress. The main reason may be that LNOR increased Fe storage and efflux by enhancing Fer and Fpn mRNA levels, and reduced cellular Fe utilization by inhibiting Tf gene expression, leading to a decrease in free iron, which contributed to inhibiting the growth of bacterial (Ueda and Takasawa, 2018). Fer, Tf, and Fpn1 not only participate in Fe equilibrium, but also regulate the immune response. Previous studies have demonstrated that bacterial infection up-regulates the Fer mRNA level of Mylopharygodon piceus (Chen et al., 2016), which contributes to reducing the utilization of free iron by pathogens through increasing Fe storage. Ferrous sulfate reduces Fpn1 gene expression, and increases IL-1β, IL-8, and TNF-α mRNA levels in the liver of Siniperca chuatsi, indicating a close relationship between Fe elimination and immune function (Shen et al., 2019). Increasing Fer gene expression of Nile tilapia (Oreochromis niloticus) (Yin et al., 2018) and reducing Tf gene expression of Procambarus clarkia (Yang et al., 2019) can inhibit pathogen replication, which contributes to the enhancement of host tolerance to pathogenic infections. Further research is needed to investigate the relationship between Fe homeostasis and immunity through gene silencing and over-expression. Thus, Fe homeostasis is closely related to the host’s immunological function (Ding et al., 2020).
Compared to Fe alone exposure, Fe plus HNOR exposure increased proinflammatory gene expressions, and reduced anti-inflammatory gene expressions, LZM and AKP activities, suggesting that HNOR intensified inflammatory response, and suppressed the immune function of fish under Fe stress (Wang and Lu, 2022). Fe plus HNOR exposure may provide more Fe for pathogens by inhibiting Fe secretion, and enhancing Fe absorption and transport, as reflected by the reduced Fpn mRNA level, and the increased DMT2 and Tf mRNA levels. What’s more, Fe and HNOR co-exposure may impair the integrity of intestinal structure, including mitochondria ETC (Niemuth et al., 2020; Zeng et al., 2021). The abnormal mitochondria, as the potential source of ROS production, might enhance mitochondrial membrane hyperpolarization, and further increase the accumulation of ROS, which in turn aggravates mitochondrial dysfunction that creates a vicious cycle (Hernansanz-Agustín and Enríquez, 2021). Ultimately, excessive or prolonged oxidative stress could lead to the decline of immunity or even cell death.
4.5 Protein folding protective effects of HSPs
Heat shock proteins (HSPs) are highly conserved, and are widely distributed in eukaryotic organisms. As a molecular chaperone, HSPs prevent other protein misfolding to facilitate repair when aquatic animals are under environmental stresses, such as heavy metals and antibiotics (Jeyachandran et al., 2023). Thus, HSPs are crucial for maintaining protein balance (Garbuz et al., 2019; Zhang et al., 2022). In this study, Fe exposure had no impact on HSP60 gene expression, but increased HSP70 and HSP90 mRNA levels. FeS, as a cofactor of DNA polymerase and helicase, plays a role in DNA replication and repair processes (Puig et al., 2017). In addition, Fe is also a coenzyme of glutamate synthase and ribonucleic acid reductase, which participates in the synthesis of proteins and nucleic acids (Zhou et al., 2021). However, excessive Fe could destroy biomolecules by generating ROS. The increased HSP70 and HSP90 could help organisms restore the original structure of proteins. HSP60, HSP70, and HSP90 have different sensitivities to environmental pollutants (Rosenzweig et al., 2019; Taghavizadeh Yazdi et al., 2021), and their protective effects on organisms also vary (Kumar et al., 2022).
LNOR did not affect HSP60 and HSP70 mRNA levels, but significantly increased HSP90 gene expression. This result is inconsistent with the fact that sulfamethoxazole up-regulated the HSP70 mRNA level of O. niloticus (Hu et al., 2021). The enhancement of HSP90 gene expression endowed the fish with protective effects against LNOR-induced toxicity effects (Oksala et al., 2014). The standpoint was confirmed by the improvement of energy metabolism and immune response, and no change in ROS content. HNOR significantly increased HSP60 (2.43 times), HSP70 (2.78 times), and HSP90 (9.44 times) gene expressions, suggesting that NOR had a concentration-dependent effect on HSP gene expressions. In addition, the increment of HSP90 gene expression was greater than those of HSP60 and HSP70, suggesting that HSP90 was more sensitive to NOR than HSP60 and HSP70. Previous studies indicated that the HSP90 gene of Cherax quadricarinatus was more quickly and effectively activated by pathogenic bacteria than HSP60 and HSP70 (Wang et al., 2020a). Compared to HSP60 and HSP70, HSP90 may be more critical to the immune response.
Fe plus LNOR increased HSP gene expressions compared to Fe alone exposure, suggesting that LNOR prompted a compensatory mechanism for protecting organisms against Fe-induced oxidative damage, except for the improvement of energy metabolism and immune response (Sun et al., 2022). Compared to Fe alone exposure, Fe plus HNOR reduced HSP60 (51.83%), HSP70 (3.37%), and HSP90 (32.79%) gene expressions, which contribute to worsening the detrimental effect of Fe on fish. The variation degree of HSP70 mRNA level was greater than those in HSP60 and HSP90, indicating that HSP70 is more sensitive to the co-exposure of Fe and NOR. Previous studies indicated that relative to other HSPs, HSP70 is more suitable for predicting cadmium toxicity (Taghavizadeh Yazdi et al., 2021). Due to the HSP70 expression level depending on ATP content (Jeyachandran et al., 2023), the effects of HNOR on the HSP70 expression level may be related to the reduced energy metabolism of fish exposed to Fe, which needs further investigation.
5 Conclusion
These findings suggested that NOR and Fe co-exposure had different toxic effects on large yellow croaker from Fe alone exposure. LNOR exposure enhanced Fe transport, energy metabolism, anti-inflammatory, and protein folding protective effect, and inhibited pro-inflammatory when fish exposed to Fe pollutants, resulting in the reduction of MDA, PC and mortality rate, and the alleviation of intestinal malformations, indicating LNOR exposure mitigated Fe-induced toxic effects to fish. HNOR exposure reduced Fe excretion, energy metabolism, anti-inflammatory, and protein folding protective effect, and increased pro-inflammatory when fish was under Fe stress, leading to the increment of MDA, PC and mortality rate, and the deterioration of intestinal malformations, suggesting that HNOR exposure aggravated Fe-induced toxic effects to fish. Antibiotic concentration should be taken into consideration when the environmental risk and fate of metal pollutants in aquaculture water are to be assessed. The underlying mechanisms of the interactive effects of antibiotics and metals on aquatic animals are worthy for further studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the ethical guidelines of the Xiamen University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LZ: Funding acquisition, Supervision, Visualization, Writing – review & editing. YW: Conceptualization, Formal analysis, Methodology, Writing – original draft. CA: Funding acquisition, Supervision, Writing – review & editing. BL: Formal analysis, Methodology, Writing – original draft. MY: Methodology, Writing – original draft. HZ: Data curation, Methodology, Writing – original draft. FL: Data curation, Investigation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Scientific Research Foundation for High-level Talent, Bengbu University (2024YYX11QD); Anhui Province University Outstanding Young Teacher Training Project (YQYB2023056); Natural Science Foundation of Fujian Province (2022J01027); Enterprise Cooperation Project “Research on Key Technologies for Breeding and Artificial Breeding of Scylla paramamosain”(00014166); Fujian Province Key Laboratory of Special Aquatic Formula Feed (TMKJZ2306).
Acknowledgments
We would like to be grateful for the support from the public service platform of instruments and equipment of the College of Ocean and Earth Sciences in Xiamen University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afshari A., Sourinejad I., Gharaei A., Johari S. A., Ghasemi Z. (2021). The effects of diet supplementation with inorganic and nanoparticulate iron and copper on growth performance, blood biochemical parameters, antioxidant response and immune function of snow trout Schizothorax zarudnyi (Nikolskii 1897). Aquaculture 539, 736638. doi: 10.1016/j.aquaculture.2021.736638
Ajewole O. A., Ikhimiukor O. O., Adelowo O. O. (2021). Heavy metals (Cu and Zn) contamination of pond sediment and co-occurrence of metal and antibiotic resistance in Escherichia coli from Nigerian aquaculture. Int. J. Environ. Stud. 78, 773–784. doi: 10.1080/00207233.2020.1804741
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1006/abio.1976.9999
Chandrapalan T., Kwong R. W. M. (2020). Influence of dietary iron exposure on trace metal homeostasis and expression of metal transporters during development in zebrafish. Environ. pollut. 261, 114159. doi: 10.1016/j.envpol.2020.114159
Chandrapalan T., Kwong R. W. M. (2021). Functional significance and physiological regulation of essential trace metals in fish. J. Exp. Biol. 224, jeb238790. doi: 10.1242/jeb.238790
Chen G., Zhang C., Wang Y., Guo C., Sang F., Wang C., et al. (2016). Identification and characterization of a ferritin gene involved in the immune defense response of scallop Chlamys farreri. Fish Shellfish Immun. 55, 1–9. doi: 10.1016/j.fsi.2016.04.128
Dawood M. A. (2021). Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev. Aquacult. 13, 642–663. doi: 10.1111/raq.12492
Ding H., Yu X., Feng J. (2020). Iron homeostasis disorder in piglet intestine. Metallomics 12, 1494–1507. doi: 10.1039/d0mt00149j
Galy B., Conrad M., Muckenthaler M. (2024). Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Bio. 25, 133–155. doi: 10.1038/s41580-023-00648-1
Garbuz D. G., Zatsepina O. G., Evgen’ev M. B. (2019). The major human stress protein Hsp70 as a factor of protein homeostasis and a cytokine-like regulator. Mol. Biol. 53, 176–191. doi: 10.1134/s0026893319020055
He K., Long X., Jiang H., Qin C. (2024). The differential impact of iron on ferroptosis, oxidative stress, and inflammatory reaction in head-kidney macrophages of yellow catfish (Pelteobagrus fulvidraco) with and without ammonia stress. Dev. Comp. Immunol. 157, 105184. doi: 10.1016/j.dci.2024.105184
Hernansanz-Agustín P., Enríquez J. A. (2021). Generation of reactive oxygen species by mitochondria. Antioxidants 10, 415. doi: 10.3390/antiox10030415
Hu F., Dong F., Yin L., Wang H., Zheng M., Fu S., et al. (2021). Effects of sulfamethoxazole on the growth, oxidative stress and inflammatory response in the liver of juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 543, 736935. doi: 10.1016/j.aquaculture.2021.736935
Imperatore R., Orso G., Facchiano S., Scarano P., Hoseinifar S. H., Ashouri G., et al. (2023). Anti-inflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio. Aquaculture 563, 738878. doi: 10.1016/j.aquaculture.2022.738878
Jeyachandran S., Chellapandian H., Park K., Kwak I. S. (2023). A review on the involvement of heat shock proteins (extrinsic chaperones) in response to stress conditions in aquatic organisms. Antioxidants 12, 1444. doi: 10.3390/antiox12071444
Jiao L., Dai T., Lu J., Tao X., Jin M., Sun P., et al. (2022). Excess iron supplementation induced hepatopancreas lipolysis, destroyed intestinal function in Pacific white shrimp Litopenaeus vannamei. Mar. pollut. Bull. 176, 113421. doi: 10.1016/j.marpolbul.2022.113421
Kumar V., Roy S., Behera B. K., Das B. K. (2022). Heat shock proteins (Hsps) in cellular homeostasis: a promising tool for health management in crustacean aquaculture. Life 12, 1777. doi: 10.3390/life12111777
Lang L., Zhang Y., Yang A., Dong J., Li W., Zhang G. (2022). Macrophage polarization induced by quinolone antibiotics at environmental residue level. Int. Immunopharmacol. 106, 108596. doi: 10.2139/ssrn.3982289
Lemos M. L., Balado M. (2020). Iron uptake mechanisms as key virulence factors in bacterial fish pathogens. J. Appl. Microbiol. 129, 104–115. doi: 10.1111/jam.14595
Liang X., Wang F., Li K., Nie X., Fang H. (2020). Effects of norfloxacin nicotinate on the early life stage of zebrafish (Danio rerio): developmental toxicity, oxidative stress and immunotoxicity. Fish Shellfish Immun. 96, 262–269. doi: 10.1016/j.fsi.2019.12.008
Limbu S. M., Zhou L., Sun S. X., Zhang M. L., Du Z. Y. (2018). Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 115, 205–219. doi: 10.1016/j.envint.2018.03.034
Lin G., Zheng M., Li S., Xie J., Fang W., Gao D., et al. (2020). Response of gut microbiota and immune function to hypoosmotic stress in the yellowfin seabream (Acanthopagrus latus). Sci. Total Environ. 745, 140976. doi: 10.1016/j.scitotenv.2020.140976
Liu C., Pan K., Xu H., Song Y., Qi X., Lu Y., et al. (2024). The effects of enrofloxacin exposure on responses to oxidative stress, intestinal structure and intestinal microbiome community of largemouth bass (Micropterus salmoides). Chemosphere 348, 140751. doi: 10.2139/ssrn.4575692
Markevich N. I., Galimova M. H., Markevich L. N. (2020). Hysteresis and bistability in the succinate-CoQ reductase activity and reactive oxygen species production in the mitochondrial respiratory complex II. Redox Biol. 37, 101630. doi: 10.1016/j.redox.2020.101630
Miller K. A., Vicentini F. A., Hirota S. A., Sharkey K. A., Wieser M. E. (2019). Antibiotic treatment affects the expression levels of copper transporters and the isotopic composition of copper in the colon of mice. P. Natl. A. Sci. India B. 116, 5955–5960. doi: 10.1073/pnas.1814047116
Moreira A. C., Mesquita G., Gomes M. S. (2020). Ferritin: an inflammatory player keeping iron at the core of pathogen-host interactions. Microorganisms 8, 589. doi: 10.3390/microorganisms8040589
Nairz M., Haschka D., Demetz E., Weiss G. (2014). Iron at the interface of immunity and infection. Front. Pharmacol. 5, 152. doi: 10.3389/fphar.2014.00152
Nemeth E., Ganz T. (2021). Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int. J. Mol. Sci. 22, 6493. doi: 10.3390/ijms22126493
Nesci S., Algieri C., Trombetti F., Ventrella V., Fabbri M., Pagliarani A. (2021). Sulfide affects the mitochondrial respiration, the Ca2+-activated F1FO-ATPase activity and the permeability transition pore but does not change the Mg2+-activated F1FO-ATPase activity in swine heart mitochondria. Pharmacol. Res. 166, 105495. doi: 10.1016/j.phrs.2021.105495
Nesci S., Lenaz G. (2021). The mitochondrial energy conversion involves cytochrome c diffusion into the respiratory supercomplexes. BBA-Bioenergetics 1862, 148394–148396. doi: 10.1016/j.bbabio.2021.148394
Niemuth N. J., Zhang Y., Mohaimani A. A., Schmoldt A., Laudadio E. D., Hamers R. J., et al. (2020). Protein Fe–S centers as a molecular target of toxicity of a complex transition metal oxide nanomaterial with downstream impacts on metabolism and growth. Environ. Sci. Technol. 54, 15257–15266. doi: 10.1021/acs.est.0c04779.s001
Nolfi-Donegan D., Braganza A., Shiva S. (2020). Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 37, 101674. doi: 10.1016/j.redox.2020.101674
Oksala N. K. J., Ekmekçi F. G., Özsoy E., Kirankaya Ş., Kokkola T., Emecen G., et al. (2014). Natural thermal adaptation increases heat shock protein levels and decreases oxidative stress. Redox Biol. 3, 25–28. doi: 10.1016/j.redox.2014.10.003
Pfaffl M. W. (2001). A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 29, e45. doi: 10.1093/nar/29.9.e45
Puig S., Ramos-Alonso L., Romero A. M., Martínez-Pastor M. T. (2017). The elemental role of iron in DNA synthesis and repair. Metallomics 9, 1483–1500. doi: 10.1039/c7mt00116a
Read A. D., Bentley R. E., Archer S. L., Dunham-Snary K. J. (2021). Mitochondrial iron–sulfur clusters: structure, function, and an emerging role in vascular biology. Redox Biol. 47, 102164. doi: 10.1016/j.redox.2021.102164
Ren Z., Wang S., Cai Y., Wu Y., Tian L., Liao J., et al. (2020). Antioxidant capacity, non-specific immunity, histopathological analysis and immune-related genes expression in Nile tilapia Oreochromis niloticus infected with Aeromonas schubertii. Aquaculture 529, 735642. doi: 10.1016/j.aquaculture.2020.735642
Rosenzweig R., Nillegoda N. B., Mayer M. P., Bukau B. (2019). The Hsp70 chaperone network. Nat. Rev. Mol. Cell Bio. 20, 665–680. doi: 10.1038/s41580-019-0133-3
Ryan M. T. (2018). Mitochondria – The energy powerhouses. Semin. Cell Dev. Biol. 76, 130–131. doi: 10.1016/j.semcdb.2017.09.038
Sayadi M. H., Mansouri B., Shahri E., Tyler C. R., Shekari H., Kharkan J. (2020). Exposure effects of iron oxide nanoparticles and iron salts in blackfish (Capoeta fusca): acute toxicity, bioaccumulation, depuration, and tissue histopathology. Chemosphere 247, 125900. doi: 10.1016/j.chemosphere.2020.125900
Sehonova P., Tokanova N., Hodkovicova N., Kroupova H. K., Tumova J., Blahova J., et al. (2019). Oxidative stress induced by fluoroquinolone enrofloxacin in zebrafish (Danio rerio) can be ameliorated after a prolonged exposure. Environ. Toxicol. Phar. 67, 87–93. doi: 10.1016/j.etap.2019.02.002
Shahjahan M., Taslima K., Rahman M. S., Al-Emran M. D., Alam S. I., Faggio C. (2022). Effects of heavy metals on fish physiology–a review. Chemosphere 300, 134519. doi: 10.1016/j.chemosphere.2022.134519
Shen Y., Zhao Z., Zhao J., Chen X., Cao M., Wu M. (2019). Expression and functional analysis of hepcidin from mandarin fish (Siniperca chuatsi). Int. J. Mol. Sci. 20, 5602. doi: 10.3390/ijms20225602
Shi F., Yao M., Huang Y., Chen Z., Xiao J., Zhan F., et al. (2023). Effects of antibiotics on immunity and apoptosis on grass carp liver and hepatocytes. J. Environ. Chem. Eng. 11, 110168. doi: 10.1016/j.jece.2023.110168
Shumoy H., Raes K. (2021). Dissecting the facts about the impact of contaminant iron in human nutrition: a review. Trends Food Sci. Tech. 116, 918–927. doi: 10.1016/j.tifs.2021.08.038
Singh M., Barman A. S., Devi A. L., Devi A. G., Pandey P. K. (2019). Iron mediated hematological, oxidative and histological alterations in freshwater fish Labeo rohita. Ecotox. Environ. Safe. 170, 87–97. doi: 10.1016/j.ecoenv.2018.11.129
Sun J., Liu Z., Quan J., Li L., Zhao G., Lu J. (2022). Protective effects of different concentrations of selenium nanoparticles on rainbow trout (Oncorhynchus mykiss) primary hepatocytes under heat stress. Ecotox. Environ. Safe. 230, 113121. doi: 10.1016/j.ecoenv.2021.113121
Sun Y., Tang L., Liu Y., Hu C., Zhou B., Lam P. K. S., et al. (2019). Activation of aryl hydrocarbon receptor by dioxin directly shifts gut microbiota in zebrafish. Environ. pollut. 255, 113357. doi: 10.1016/j.envpol.2019.113357
Taghavizadeh Yazdi M. E., Amiri M. S., Nourbakhsh F., Rahnama M., Forouzanfar F., Mousavi S. H. (2021). Bio-indicators in cadmium toxicity: role of HSP27 and HSP70. Environ. Sci. pollut. R. 28, 26359–26379. doi: 10.1007/s11356-021-13687-y
Ueda N., Takasawa K. (2018). Impact of infammation on ferritin, hepcidin and the management of iron defciency anemia in chronic kidney disease. Nutrients 10, 1173. doi: 10.3390/nu10091173
Vercellino I., Sazanov L. A. (2022). The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Bio. 23, 141–161. doi: 10.1038/s41580-021-00415-0
Vogt A. C. S., Arsiwala T., Mohsen M., Vogel M., Manolova V., Bachmann M. F. (2021). On iron metabolism and its regulation. Int. J. Mol. Sci. 22, 4591. doi: 10.3390/ijms22094591
Wang X., Hu M., Gu H., Zhang L., Shang Y., Wang T., et al. (2020b). Short-term exposure to norfloxacin induces oxidative stress, neurotoxicity and microbiota alteration in juvenile large yellow croaker Pseudosciaena crocea. Environ. pollut. 267, 115397. doi: 10.1016/j.envpol.2020.115397
Wang Q., Huang C., Liu K., Lu M., Dan S. F., Xu Y. (2020a). Cloning and expression of three heat shock protein genes in the gills of Cherax quadricarinatus responding to bacterial challenge. Microb. Pathogenesis 142, 104043. doi: 10.1016/j.micpath.2020.104043
Wang P., Lu Y. Q. (2022). Ferroptosis: a critical moderator in the life cycle of immune cells. Front. Immunol. 13. doi: 10.3389/fimmu.2022.877634
Xia X., Cheng Z., He B., Liu H., Liu M., Hu J., et al. (2021). Ferroptosis in aquaculture research. Aquaculture 541, 736760. doi: 10.1016/j.aquaculture.2021.736760
Xie Z., Luan H., Zhang Y., Wang M., Cao D., Yang J., et al. (2020). Interactive effects of diclofenac and copper on bioconcentration and multiple biomarkers in crucian carp (Carassius auratus). Chemosphere 242, 125141. doi: 10.1016/j.chemosphere.2019.125141
Xu P. C., Song C. C., Tan X. Y., Zhao T., Zhong C. C., Xu J. J., et al. (2023). Characterization of fifteen key genes involved in iron metabolism and their responses to dietary iron sources in yellow catfish Pelteobagrus fulvidraco. J. Trace Elem. Med. Bio. 80, 127301. doi: 10.1016/j.jtemb.2023.127301
Xu L., Zhang H., Xiong P., Zhu Q., Liao C., Jiang G. (2021). Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: a review. Sci. Total Environ. 753, 141975. doi: 10.1016/j.scitotenv.2020.141975
Yang H., Liu Z., Jiang Q., Xu J., An Z., Zhang Y., et al. (2019). A novel ferritin gene from Procambarus clarkii involved in the immune defense against Aeromonas hydrophila infection and inhibits WSSV replication. Fish Shellfish Immun. 86, 882–891. doi: 10.1016/j.fsi.2018.12.022
Yang C., Song G., Lim W. (2020). A review of the toxicity in fish exposed to antibiotics. Comp. Biochem. Phys. C. 237, 108840. doi: 10.1016/j.cbpc.2020.108840
Yin X., Mu L., Bian X., Wu L., Li B., Liu J., et al. (2018). Expression and functional characterization of transferrin in Nile tilapia (Oreochromis niloticus) in response to bacterial infection. Fish Shellfish Immun. 74, 530–539. doi: 10.1016/j.fsi.2018.01.023
Yukgehnaish K., Kumar P., Sivachandran P., Marimuthu K., Arshad A., Paray B. A., et al. (2020). Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Rev. Aquacult. 12, 1903–1927. doi: 10.1111/raq.12416
Zeng L., Ai C., Zhang J., Pan Y. (2019a). Toxicological effects of waterborne Zn on the proximal and distal intestines of large yellow croaker Larimichthys crocea. Ecotox. Environ. Safe. 174, 324–333. doi: 10.1016/j.ecoenv.2019.02.088
Zeng L., Ai C. X., Zheng J. L., Zhang J. S., Li W. C. (2019b). Cu pre-exposure alters antioxidant defense and energy metabolism in large yellow croaker Larimichthys crocea in response to severe hypoxia. Sci. Total Environ. 687, 702–711. doi: 10.1016/j.scitotenv.2019.06.047
Zeng L., Li W. C., Zhang H., Cao P., Ai C. X., Hu B., et al. (2021). Hypoxic acclimation improves mitochondrial bioenergetic function in large yellow croaker Larimichthys crocea under Cu stress. Ecotox. Environ. Safe. 224, 112688. doi: 10.1016/j.ecoenv.2021.112688
Zeng L., Wang Y. H., Ai C. X., Zhang J. S. (2018). Differential effects of β-glucan on oxidative stress, inflammation and copper transport in two intestinal regions of large yellow croaker Larimichthys crocea under acute copper stress. Ecotox. Environ. Safe. 165, 78–87. doi: 10.1016/j.ecoenv.2018.08.098
Zeng L., Wang Y. H., Ai C. X., Zhang B., Zhang H., Liu Z. M., et al. (2024). Differential effects of oxytetracycline on detoxification and antioxidant defense in the hepatopancreas and intestine of Chinese mitten crab under cadmium stress. Sci. Total Environ. 930, 172633. doi: 10.2139/ssrn.4717821
Zeng L., Wang Y. H., Song W., Ai C. X., Liu Z. M., Yu M. H., et al. (2023). Different effects of continuous and pulsed Benzo [α] pyrene exposure on metabolism and antioxidant defense of large yellow croaker: Depend on exposure duration. Ecotox. Environ. Safe. 263, 115370. doi: 10.1016/j.ecoenv.2023.115370
Zeng C. X., Zhang J. S., Li W. C. (2020). Pre-hypoxia exposure inhibited copper toxicity by improving energy metabolism, antioxidant defence and mitophagy in the liver of the large yellow croaker Larimichthys crocea. Sci. Total Environ. 708, 134961. doi: 10.1016/j.scitotenv.2019.134961
Zeng L., Zheng J. L., Wang Y. H., Xu M. Y., Zhu A. Y., Wu C. W. (2016). The role of Nrf2/Keap1 signaling in inorganic mercury induced oxidative stress in the liver of large yellow croaker Pseudosciaena crocea. Ecotox. Environ. Safe. 132, 345–352. doi: 10.1016/j.ecoenv.2016.05.002
Zhang H., Gong W., Wu S., Perrett S. (2022). Hsp70 in redox homeostasis. Cells 11, 829. doi: 10.3390/cells11050829
Zhao R. Z., Jiang S., Zhang L., Yu Z. B. (2019a). Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 44, 3–15. doi: 10.3892/ijmm.2019.4188
Zhao X. L., Li P., Zhang S. Q., He S. W., Xing S. Y., Cao Z. H., et al. (2021). Effects of environmental norfloxacin concentrations on the intestinal health and function of juvenile common carp and potential risk to humans. Environ. pollut. 287, 117612. doi: 10.1016/j.envpol.2021.117612
Zhao Y., Liu H., Wang Q., Li B. (2019b). The influence of three antibiotics on the growth, intestinal enzyme activities, and immune response of the juvenile sea cucumber Apostichopus japonicus Selenka. Fish Shellfish Immun. 84, 434–440. doi: 10.1016/j.fsi.2018.10.022
Keywords: iron exposure, norfloxacin, NF-κB, HSP, Larimichthys crocea
Citation: Zeng L, Wang Y-H, Ai C-X, Liu B, Yu M-H, Zhang H and Li F (2025) Concentration-dependent effect of norfloxacin on iron toxicity in the intestine of large yellow croaker Larimichthys crocea (Richardson, 1846). Front. Mar. Sci. 11:1530807. doi: 10.3389/fmars.2024.1530807
Received: 19 November 2024; Accepted: 24 December 2024;
Published: 22 January 2025.
Edited by:
Heba H. Abdel-Kader, National Institute of Oceanography and Fisheries (NIOF), EgyptReviewed by:
Edison Barbieri, Agência de Agronegócio e Tecnologia de São Paulo (APTA), BrazilTolga Akdemir, Recep Tayyip Erdoğan University, Türkiye
Copyright © 2025 Zeng, Wang, Ai, Liu, Yu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zeng, emVuZ2xpbjYxNUAxMjYuY29t; Chun-Xiang Ai, Y2h1bnhhaUB4bXUuZWR1LmNu
 Lin Zeng
Lin Zeng Yong-Hong Wang1
Yong-Hong Wang1 Chun-Xiang Ai
Chun-Xiang Ai