
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 16 January 2025
Sec. Marine Ecosystem Ecology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1515098
Introduction: Non-native species are widely recognized as threats to biodiversity, ecosystems, and the services they provide to humans. The Mediterranean Sea has a high biodiversity of endemic species and is a hot spot of biological invasions. One of the most recent threats to Mediterranean ecosystems is the invasion of the Atlantic blue crab Callinectes sapidus.
Methods: The occurrences of the crab throughout the Mediterranean coastline were indexed from citizen science through the Global Biodiversity Information Facility. Using spatial analysis and linear mixed models, we investigated geomorphology (i.e., water depth and coastal wetlands extension), water physical variables (i.e., salinity and winter and summer water temperature), water quality variables (i.e., chlorophyll-a, nitrate and orthophosphate) and anthropogenic factors (i.e., ship density and population size) potentially affecting the blue crab occurrence along the coast.
Results: Our results showed that nitrate, as an indicator of riverine nutrient loading, and water depth, as an indicator of slope of the bottom, were the most influential variables in explaining the occurrences of blue crabs. Water temperature and salinity had lesser impacts; anthropogenic factors, such as the density of commercial marine traffic and human population size had no effect on blue crab occurrence.
Discussion: These results sug gest that benthic primary production and shallow water drive blue crab occurrences along the Mediterranean coasts. Even considering data limitations and gaps, our large-scale findings contribute to a broader understanding of the factors that drive blue crab invasion success which, in turn, can inform management actions and outline research needs.
The global redistribution of species is resulting in alterations to food webs, development and structure of ecological communities, and ecosystem structure and function (Cheng et al., 2022; Simberloff et al., 2013; Tsirintanis et al., 2022). The introduction and establishment of non-native species is due to a range of processes, including accidental and intentional introductions of species, global transportation of humans and products, and species range extensions with changing abiotic conditions, such as temperature and rainfall patterns (Christianson and Eggleston, 2021; Kelly and Goulden, 2008; Ruiz et al., 2000; Sardain et al., 2019; Sorte et al., 2010). Despite numerous efforts to prevent and control the introduction and establishment of non-native species, the rate of species invasions is increasing worldwide (Seebens et al., 2017; Sardain et al., 2019).
Not all introduced species become invasive or produce negative effects of the same magnitude. Only a subset of non-native species becomes established in a new area by reproducing and forming self-sustaining populations. Furthermore, only a subset of these established non-native species becomes invasive, spreading rapidly over significant distances from their introduction sites and having harmful effects on the economy, environment, or human health (Blackburn et al., 2011; IUCN, 2000). Sometimes introduced species exist for years to decades before becoming invasive. For example, the non-native mussel Brachidontes pharaonis has been present in the Mediterranean Sea since the 1940s without displaying any apparent negative effects until the1990s, when its populations experienced an increase in many eastern Mediterranean areas. This led to the mussel becoming dominant in shallow rocky reef platforms, with high densities, outcompeting native species and significantly influencing the composition of the community (Sará et al., 2008).
The Mediterranean Sea is a hotspot of biological invasions with approximately 1,000 non-native species estimated to have been introduced into its ecoregion since at least 1900 (Galil, 2008; Zenetos et al., 2012). Over half of these species have successfully established viable populations (Zenetos and Galanidi, 2020). Various factors can contribute to both the increased presence and invasiveness of non-native species in the Mediterranean Sea. For example, the artificial connection of the Suez Canal has facilitated the movement of marine organisms between the Red Sea and the Mediterranean, as well as the general increase in maritime traffic and climate change (Galil, 2008).
Among non-native species affecting the Mediterranean Sea is the Atlantic blue crab Callinectes sapidus Rathbun, 1896 (hereafter blue crab), which was reported to negatively impact local fisheries through predation and entanglement in fishing nets (Tsirintanis et al., 2022). The species was first recorded in the late 1940s along the Egyptian coast, probably introduced into the region through the ballast water of transoceanic ships. Since 2006, the species has rapidly expanded its range into the western Mediterranean, with occurrences observed in the Adriatic Sea and along the eastern coast of Spain (Mancinelli et al., 2021). Genetic analysis of blue crabs in the Mediterranean Sea suggests that the species was initially introduced from a single source area corresponding to the Atlantic coast of North America, and subsequent introductions among crab populations from Mediterranean areas may have occurred (González-Ortegón et al., 2022; Vecchioni et al., 2022). In recent years, the blue crab population has increased significantly throughout the Mediterranean, further affecting the native fauna and local economies (Castriota et al., 2024). Blue crabs are omnivores with a wide range of prey, including plants and various animals such as mollusks, crustaceans, and fish (Mancinelli et al., 2016, 2017; Prado et al., 2022). Predation is likely the main mechanism through which the blue crab has contributed to decline in several native aquatic species, as observed with the green crab and Spanish toothcarp in Spain (Clavero et al., 2022), but also with other invasive species such as the case of apple snail in the Ebro River (Céspedes et al., 2024). Its aggressive behavior and the wide range of prey were also considered the causes of reduced commercial fish and clam catches from fisherman, with higher economic losses (Cannarozzi et al., 2023; Hamiche and Aksissou, 2024; Marchessaux et al., 2023b; Milori and Ruci, 2021).
Like many estuarine-dependent species, the blue crab exhibits a complex life history. In coastal ecosystems where it is endemic, a larval stage is released by egg-bearing female crabs in high salinity estuarine areas connected to the ocean, followed by an ~ 30-day larval period after which the larvae metamorphose into a megalopal (postlarval) stage that settles in complex estuarine habitats such as seagrass and shallow detrital habitats (Lipcius et al., 2007 and references therein). As blue crabs grow, they exhibit ontogenetic habitat shifts from complex benthic habitats to foraging on unstructured estuarine bottoms (Etherington and Eggleston, 2000). Sub-adult and adult male crabs generally inhabit relatively low salinity waters, whereas females inhabit relatively high salinity waters. Prior to her final molt to maturity, female crabs mate with males in the mesohaline zones of estuaries, followed by female migration down-estuary to the spawning grounds (Hines, 2007). The degree to which blue crab life history follows this same pattern in the Mediterranean Sea, generated from studies along the U.S. east and gulf coasts, is unclear.
The high adaptability to environmental conditions, from marine to freshwater habitats, and the broad diet which include plants, detritus, polychaetes, molluscs, crustaceans, and fish, help make the blue crab a successful invader (Carrozzo et al., 2014; Prado et al., 2022, 2024). Salinity, water temperature and structurally complex benthic habitats could be key factors involved in the successful colonization of blue crabs. The Mediterranean Sea provides a supporting environment for larvae offshore, as well as structurally complex benthic habitat such as the seagrass Posidonia oceanica and Zostera noltei for megalopae and early juveniles inshore, as well as abundant food resources such as bivalves in estuaries for sub-adults and adults (Mao and Xia, 2024; Marchessaux et al., 2022; Prado et al., 2024; Weatherall et al., 2018). Shallow water depths that contain structurally complex benthic habitats may be especially important to blue crabs by providing access to food and a refuge from predators (Dittel et al., 1995). In addition, proximity to shipping promotes the introduction of invasive species via ballast water and hull fouling (Costello et al., 2022) and has probably played a central role in transporting larvae and favouring multiple arrivals of blue crabs in an area (Marchessaux et al., 2023a).
Water quality can impact blue crabs in several ways. In general, high levels of nutrient concentrations, such as phosphorus and nitrogen, due to human activities (i.e., cultural eutrophication) is thought to have negative effects on blue crab populations by increasing the spread of hypoxic and anoxic bottom waters, and can stimulate water column algal production which, in turn, reduces water clarity leading to a loss of seagrasses that are important blue crab nursery habitat (Kemp et al., 2005). For example, hypoxic and anoxic water volume are correlated positively with nitrate loading (Kemp et al., 2005). Conversely, microbenthic biomass as a source of prey for blue crabs is sometimes positively correlated with algal production (Kemp et al., 2005). To help resolve this dichotomy (Caddy, 1993; 2000) proposed a conceptual model of eutrophication effects on fish and crabs that follow a sequence of three stages: (1) nutrient-enhanced production of demersal and pelagic species (more food), (2) decline of demersal fish but continued increase in pelagic fish species (benthic habitat loss), and (3) a general decline in total fish production under conditions of broadly deteriorating water and habitat quality. Thus, numerous factors need consideration in assessing crab invasion success. Although models predict the distribution of blue crab native and non-native range as a function of environmental predictors such as water temperature (e.g., Costa et al., 2023), a gap remains in the identification of environmental drivers that promote the presence of blue crabs in invaded areas. This study aims to fill this gap by examining the potential relationships between the occurrence of blue crabs at the scale of the Mediterranean Sea, and a large set of physical, social, and ecological explanatory variables available from on-line sources. In particular, we hypothesize that higher occurrences of blue crabs may occur with (1) increasing water temperature that could enhance growth and (2) proximity to shipping, which could serve as a source of larvae and favor the invasion. Results of this study may be viewed as a first step in assessing large scale drivers of blue crab occurrence and identifying key data gaps, both of which are useful for managers targeting exploitation of blue crabs for emerging fisheries (Mancinelli et al., 2017a) and for a deeper understanding of the blue crab’s biology and ecology in the Mediterranean Sea.
The study area includes the coastline of Mediterranean Sea, a semi-enclosed basin connected to the Atlantic Ocean by the Strait of Gibraltar. The average depth is around 1500 meters, ranging from 0 to 5270 m, with the deepest point found in the eastern Mediterranean Sea, in the Calypso Deep, in the Ionian Sea. The Mediterranean Sea borders Europe, Africa, and Asia with a Mediterranean climate with mild to cool, rainy winters and warm to hot, dry summers. A wide variety of climatic and anthropogenic pressures affect the area (Durrieu de Madron et al., 2011). For example, thermal anomalies due to rising water temperature caused mass mortality events among marine species (Crisci et al., 2011). Human activities exert significant stress on Mediterranean marine ecosystems, including (i) coastal development, which alter natural habitats, (ii) increased input of nutrients due to farming, commercial and industrial activities, (iii) non-native species introduction through shipping, aquaculture and the Suez Canal opening, and (iv) overfishing, which depletes fish stocks and alters community structures (Katsanevakis et al., 2014; Korpinen et al., 2021).
Occurrences of Atlantic blue crab were obtained by extracting data from the Global Biodiversity Information Facility (GBIF.org, 2024). The whole dataset of blue crab in the GBIF was filtered to include only data with georeferenced information obtained from human observations of living organisms recorded between 2021 and 2023 along the Mediterranean coasts. These data were contributed by citizen science volunteers who recorded sightings in the citizen science platform iNaturalist (https://www.inaturalist.org/). Data from citizen science plays a critical role in monitoring biodiversity, especially at large-scales (Cardoso et al., 2017; Lucy et al., 2016) and in monitoring biological invasions (Encarnação et al., 2021; Pocock et al., 2024). To ensure data accuracy, observations were cross-checked to avoid duplicate species occurrences. While comprehensive datasets on the occurrence of Atlantic blue crab in Mediterranean Sea are available covering its historical detection in the early 1900s (Castriota et al., 2024; Mancinelli et al., 2021), we chose to extract data directly from the GBIF, because it provided updated occurrence data at the scale of the entire Mediterranean Sea for the recent period 2021-2023. This choice of data aligned the blue crab occurrence data with environmental and human activities data over the same period. Consequently, recent blue crab occurrence data inevitably lack information on the status of older occurrences (which in Mediterranean started from 1937 according to Mancinelli et al., 2021, as well as possible crab locations not captured in our dataset. However, the overlap between our data and the more comprehensive datasets provided by Castriota et al. (2024) and Mancinelli et al. (2021) showed that our data cover a significant proportion of the areas where blue crabs have been historically recorded (Supplementary Figure 1).
NetCDF environmental data of water temperatures (°C), salinity (P.S.U.), nitrate (mg·m-3), orthophosphate (mmol·m-3) and chlorophyll-a (mg·m-3) for the whole Mediterranean Sea was retrieved from the database of CMEMS (Copernicus Marine Environment Monitoring Service, available at https://data.marine.copernicus.eu/products, Supplementary Figures 1A–F). Monthly water temperatures and salinity data were acquired from 2022 to 2023 with resolution of 0.083°x0.083° (E.U. Copernicus Marine Service Information (CMEMS), 2024b). Monthly data of nitrate, orthophosphate and chlorophyll-a were acquired from 2022 to 2023 with resolution of 0.25°x0.25° (E.U. Copernicus Marine Service Information (CMEMS), 2024a). Summer and winter water temperature means annual salinity, phosphate, chlorophyll-a and nitrate means (Supplementary Figures 2A–F, respectively) were calculated for the surface water using QGIS software (QGIS.org, 2024).
Data on water depth (Supplementary Figure 2G) and the density of commercial ships (hereafter, ship density) in the Mediterranean Sea were derived from the European Marine Observation and Data Network (EMODnet, 2014). Ship density data for 2022 was extracted in a GeoTIFF format, representing the annual number of ship route crossings in 1x1km cells of a grid covering all Mediterranean Sea waters (Supplementary Figure 2H). Data on coastal wetlands (Supplementary Figure 2I) was derived in a GeoTIFF format with a resolution of 0.008°x 0.008°from Lehner and Döll, (2004).
Human population data were collected from the Socioeconomic Data and Applications Center (CIESIN, C. University, 2018) in a GeoTIFF format and represent the population size with resolution of 0.0083°x0.0083° (Supplementary Figure 2J). The most recently available data on human population size was for 2020. They are included in the analysis to investigate both the potential linkage to cultural eutrophication and whether observations in blue crab occurrence data might be biased by human abundance (i.e., more people more observations).
For each 50 km of coastline, the total number of observed occurrences of blue crabs was calculated. We assumed that higher numbers of observed occurrences along 50 km of coast are expected in areas with higher abundance of blue crab. This assumption is necessary since density or abundance data are not available in GBIF for C. sapidus, and studies with quantitative data on this species are not available for the entire Mediterranean Sea. A good approximation between the number of observations in GBIF and the abundance of a species in the world has already been used and verified for bird species (Callaghan et al., 2023). However, to account for potential bias due to the higher numbers of crab observations occurring in higher densities of humans, we also calculated the proportion of blue crab occurrences relative to local human population size (hereafter “proportional occurrence”).
Water physical variables (i.e, water temperature, salinity), water quality variables (i.e., nitrate, orthophosphate, chlorophyll-a), water depth and human activities (i.e, shipping density and population count) data were paired with each coastal stretch of 50 km where blue crabs occurred using QGIS software (QGIS.org, 2024). To do this, for each 50 km coastal stretch, the average values of environmental and shipping density data were calculated up to 5 km offshore of the coastline. Similarly, for each 50 km coastal stretch, the total population and the extension of coastal wetlands present in 25 km onshore (on the land side) were calculated. At the end of this process, for each coastal stretch of 50 km of length, the total number of blue crab occurrences, the averages of summer and winter water temperatures (°C), annual salinity (P.S.U.), annual nitrate (mg·m-3), annual orthophosphate (mmol·m-3) and annual chlorophyll-a (mg·m-3), water depth, ship density and total population were obtained (Figure 1; Table 1).

Figure 1. Data collection scheme (not to scale): the total number of observed occurrences of blue crabs was calculated for 50 km of coastline. In the area of 5 km offshore of the 50 km stretch, the mean values of summer and winter temperatures, annual salinity, annual nitrate, annual orthophosphate, annual chlorophyll-a, ship density and water depth were calculated. In the area of 25 km onshore of the 50 km stretch, the total amount of population and the total extension of coastal wetlands were calculated.
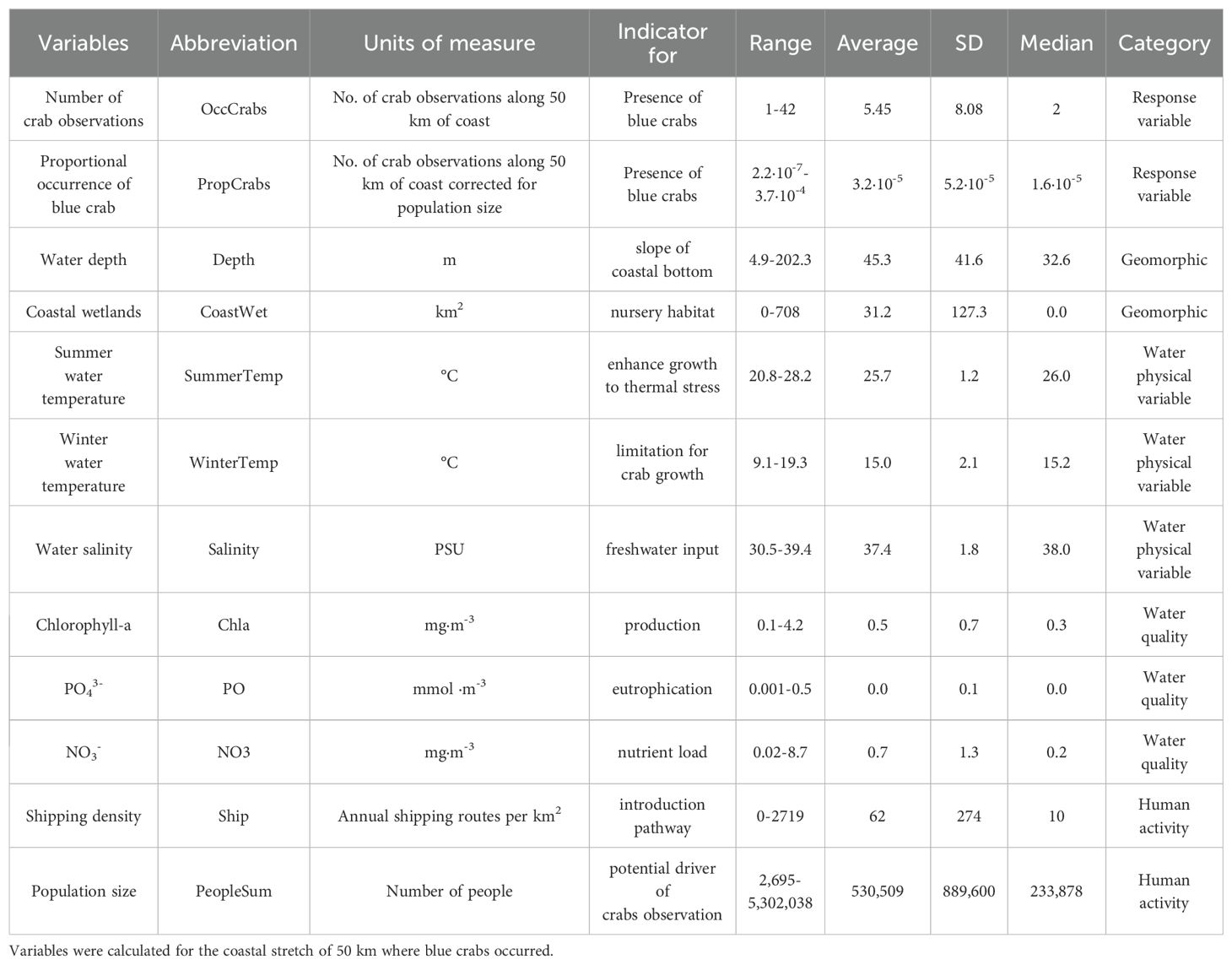
Table 1. Variables considered: abbreviations, units, indicator represented, range, average, standard deviation (SD), median and category of these are shown.
All analyses were performed in RStudio software (R Studio Team, 2024). Linear mixed-effect (LME) models were used to test the association of geomorphic, water physical variables, water quality variables and human activities with blue crab occurrences and proportional occurrence. To avoid strongly correlated variables in the models (rs>0.7), the correlations between variables were investigated through Spearman correlation using corrplot R package (Wei and Simko, 2017). The country of the observation was included as a random factor to account for nested observations. The explanatory variables were standardized by centering and scaling the values (Bates et al., 2015). Response variables (i.e., blue crab occurrences and proportional occurrence) were log-transformed with log10(x+1). Models were validated by checking residual patterns in the “DHARMa” R package (Zuur and Ieno, 2016; Hartig, 2021). The lme model was fitted using the R package ‘lme4’ (Bates et al., 2015). The best model was selected based on the Akaike Information Criterion (AIC; Akaike, 1974) corrected for small sample sizes (AICc; Hurvich and Tsai, 1993) using AICcmodavg R package (Mazerolle, 2019). The selection of the best model was based on Akaike weights (models with large Akaike weights have strong support) and low AICc values (Snipes and Taylor, 2014). Explanatory variables with p-values<0.157 were retained in the best models as they still hold explanatory power and can describe true relationships (Sutherland et al., 2023). The Variance Inflation Factor (VIF<4) of the explanatory variables used in the selected model allowed assessment of the presence of collinear variables (Zuur et al., 2009), using “car” R package (Fox & Weisberg, 2020).
To estimate the variance explained by each of the fixed and random effects of the best models selected, the marginal and conditional R2 values were calculated for each linear mixed model (Stoffel et al., 2021). The marginal R2 gives an estimate of the variance explained by each fixed effect relative to the total variance in the response, whereas the conditional R2 gives an estimate of the variance explained by fixed effects and random effects together, which better reflects the heterogeneity of the variables. The 95% confidence intervals were estimated for the marginal and conditional R2 using 1000 parametric bootstrap iterations (Stoffel et al., 2021). Marginal and conditional R2 were calculated with “partR2” R package (Stoffel et al., 2021).
Among geomorphic variables, the water depth of the coastal strip extending 5 km from the coastline where the blue crab was reported ranged from 4.9 to 202.3 m, with a mean depth of 45.3 ± 41.6 m. Onshore, coastal wetlands within 25 km of the coastline extended from 0 to 708 km², with a mean area of 31.2 ± 127.2 km² (Table 1). Among water physical variables, summer water temperatures ranged from 20.8 to 28.2°C, with an average value of 25.7 ± 1.2°C. Winter water temperatures ranged from 9.1 to 19.3°C, with an average value of 15.0 ± 2.1°C. Water salinity ranged from 30.5 to 39.4 PSU, with an average value of 37.4 ± 1.8 PSU (Table 1). Water quality variables showed a wide range of values with highest values in the northern Adriatic Sea (Table 1; Supplementary Figure 2). The shipping density (i.e., annual shipping routes per km2) ranged from 0 to 2719.18 annual shipping routes per km2, with an average of 62.1 annual shipping routes per km2. Human population size around 25 km onshore of the 50 km stretch ranged from 2695 to 5,302,038 people with an average value of 530,509 people (Table 1). Means and medians were similar for 4 variables: summer and winter water temperatures, water salinity and chlorophyll-a. The medians for the other 8, including crab occurrence variables, were less than means, indicating skewed distributions to the right.
From 2021 to 2023, the blue crab occurrences were reported along the Mediterranean coast from 13 countries, in a total of 102 stretches of 50 km each where crab occurrences ranged from 1 to 42, with highest values in northern Italy, Albania, Greece and Spain (>26 observations·50 km-1; Figure 2A). Proportional blue crab occurrences exhibited comparable patterns, with a more pronounced invasion in the eastern Adriatic Sea and Greece (Figure 2B). The number of blue crab occurrences is higher along the coasts of Europe than along the coasts of North Africa and Eurasia (Figure 2).
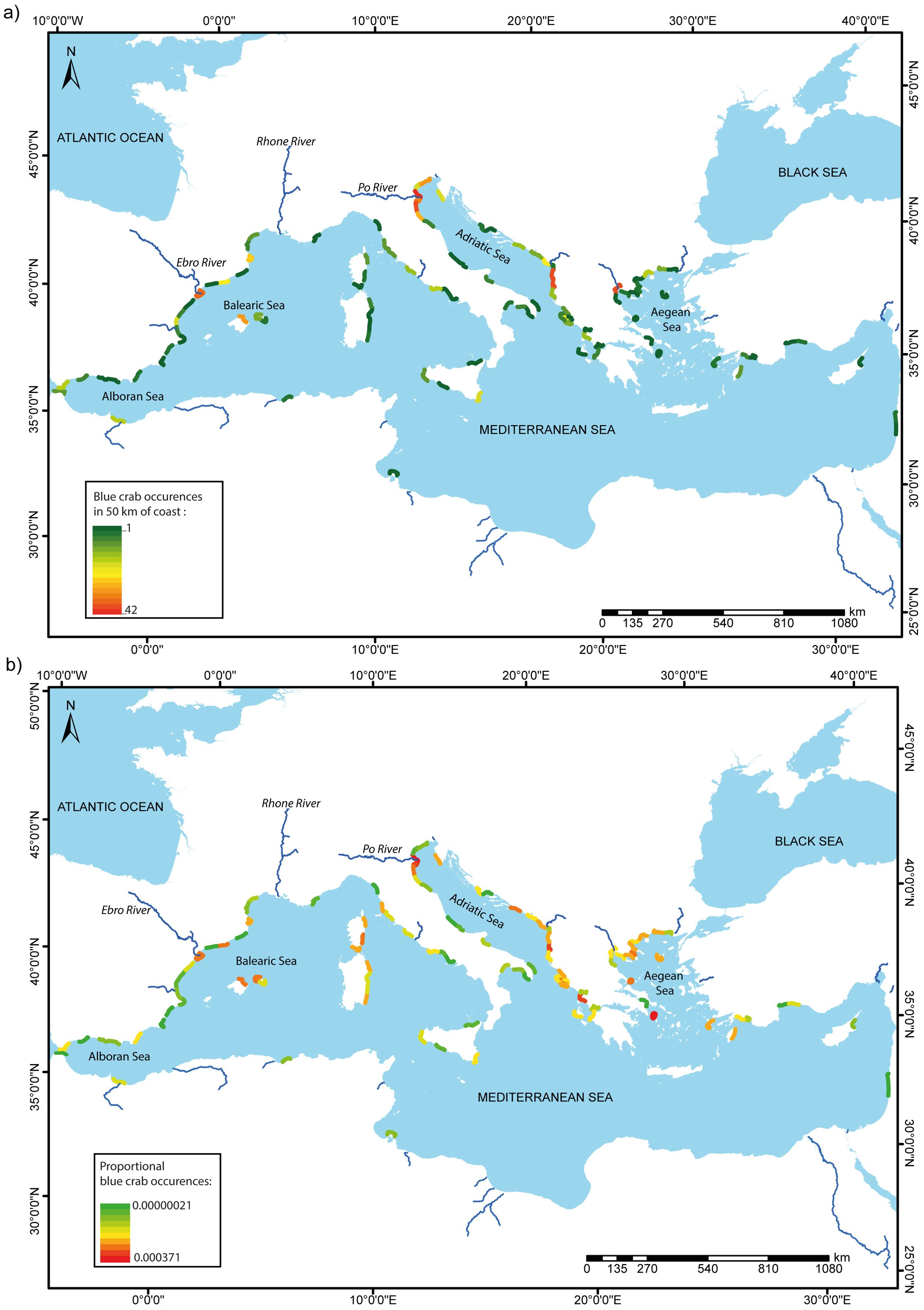
Figure 2. Map of blue crab (A) occurrence and (B) proportional occurrence in 50 km of coast from 2021 to 2023 of the Atlantic blue crab Callinectes sapidus along Mediterranean coasts. Main rivers cited in the text are also shown.
There was correlation among some of the independent variables. Water quality variables (i.e., Chla, NO3 and PO4) were negatively correlated between WinterTemp, Salinity and Depth, whereas they were positively correlated with PeopleSum and CoastWet (Supplementary Figure 2). CoastWet resulted negatively related to Salinity and Depth (rs=-0.28 and rs =-0.30 P-values<0.05, respectively; Supplementary Figure 3). Shipping density showed a positive correlation with PeopleSum (rs=0.19, P-values<0.05; Supplementary Figure 3).
The best LME model retained from the AIC model selection process (Mod3) indicated that blue crab occurrence (response variable) increased significantly with decreasing depth, and increasing nitrate concentrations (Table 2A, Figure 3A; Supplementary Table 1A). The partitioning of R2 showed that NO3 was the variable with the highest value of conditional and marginal R2 explanatory ability (conditional R2: 0.32, IC: 0.19–0.46, marginal R2: 0.21, IC: 0.07-0.36) followed by water depth (conditional R2: 0.16, IC: 0.00–0.20, marginal R2: 0.03, IC: 0.00–0.22; Figure 4A).
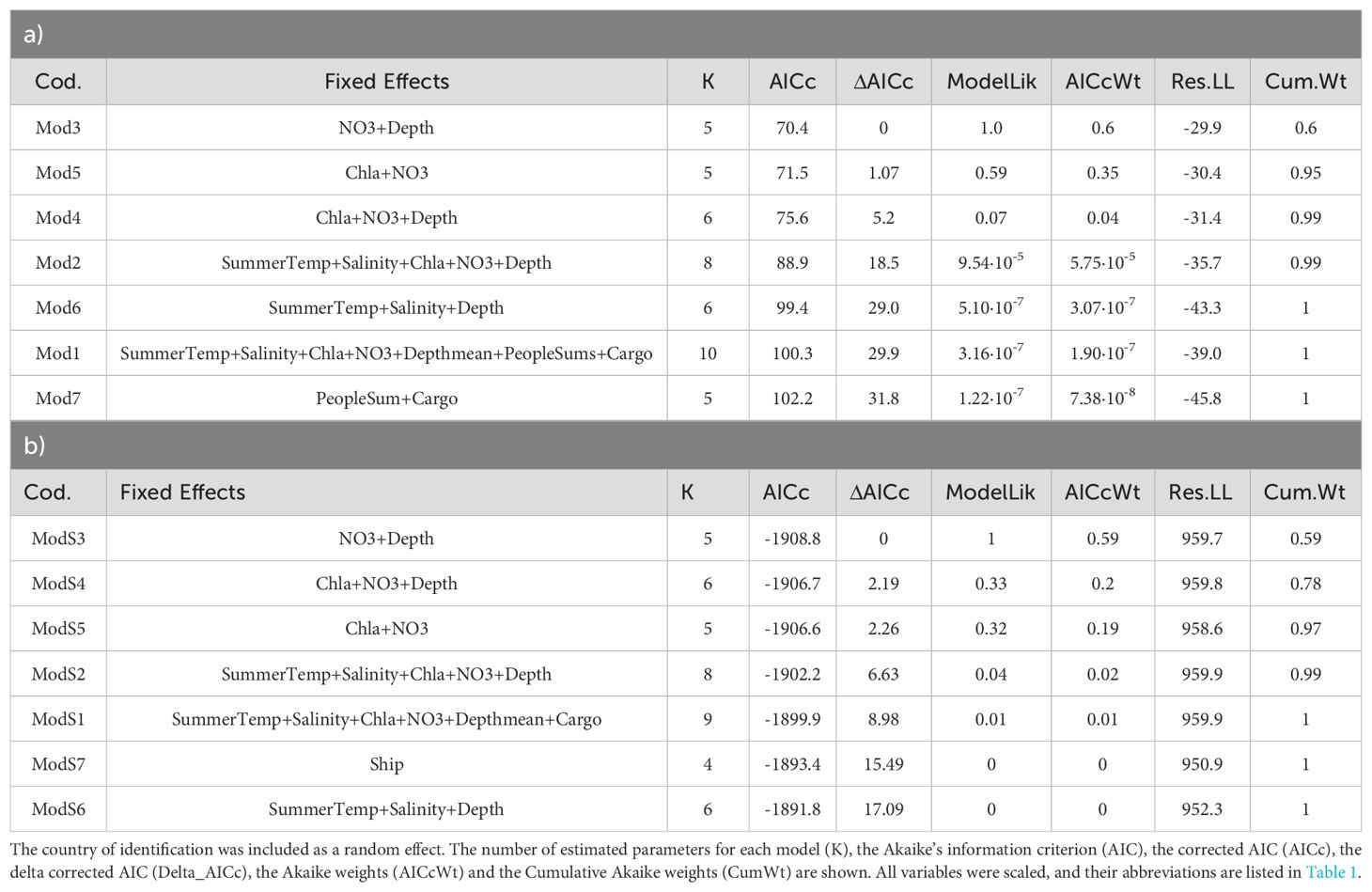
Table 2. Summary of AIC results for LME models selection correlating C. sapidus occurrences A) and C. sapidus proportional occurrences (occurrences corrected by human population size) B) and explanatory variables.
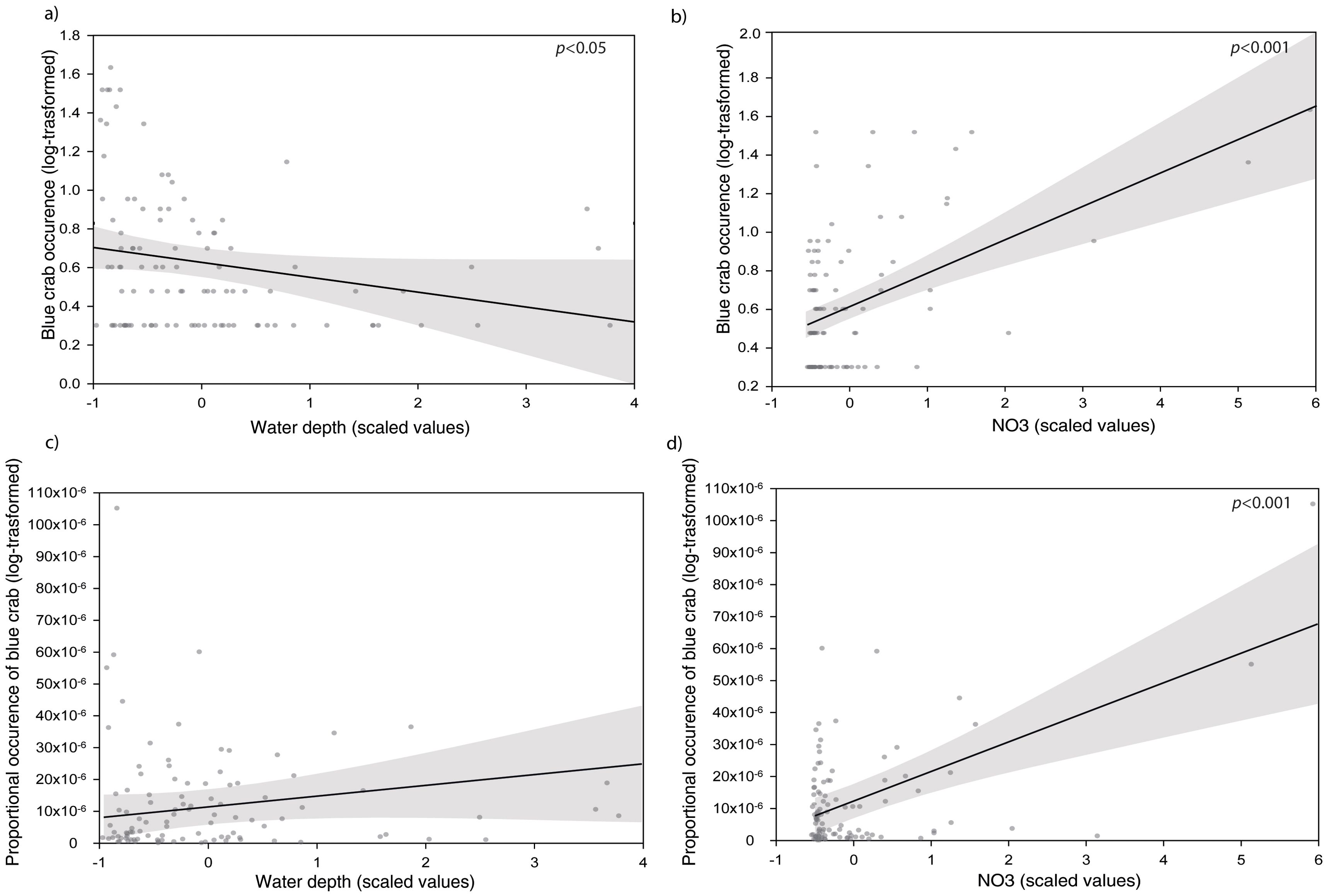
Figure 3. Predictor effect plots of (A) water depth and (B) NO3 (scaled values) on the blue crab occurrence (log-transformed values) in the Mediterranean Sea from the best model (Mod3) selected by AIC model selection and predictor effects plots of (C) water depth (scaled values) and (D) NO3 (scaled values) on log-transformed proportional occurrence of blue crabs (occurrence corrected by population size) from the best model (ModS3).
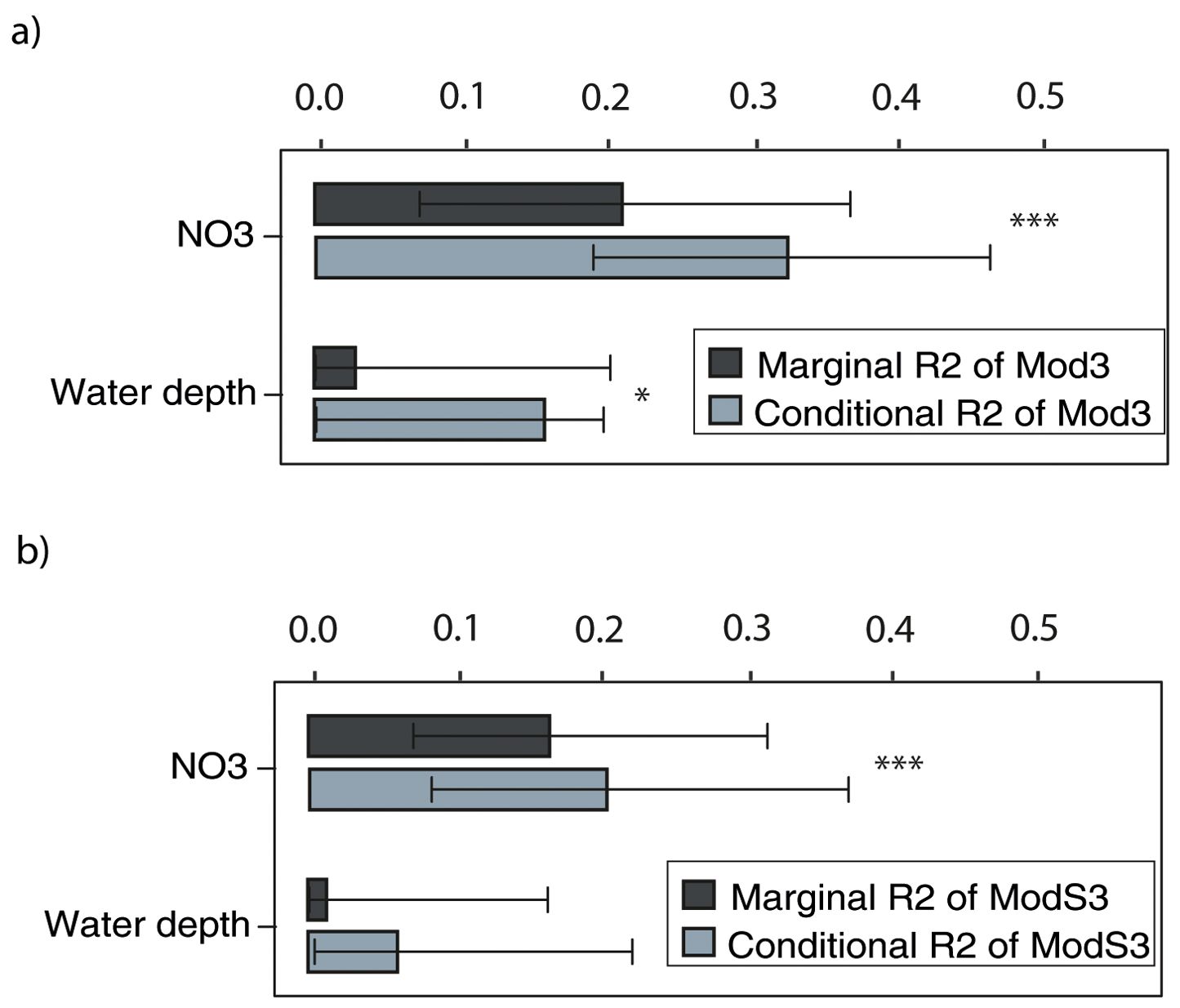
Figure 4. Marginal (dark grey bar) and conditional (light grey bar) R2 and the confidence intervals at 95% for predictors of the best model selected by AIC model selection for blue crab occurrence (Mod3) (A) and blue crab proportional occurrence (occurrence corrected by population size; ModS3) (B). Significance levels (*p < 0.05, ***p < 0.001) for all the explanatory variables included in the best model are also shown.
Models with the combination of nitrate, chlorophyll and water depth were less plausible (Mod4 and Mod5; ΔAICc=5.2 and ΔAICc=1.07, respectively, Table 2A, followed by models with only environmental variables (Mod2; ΔAICc>18.5; Table 2A), with all variables (Mod 1, ΔAICc=29.90; Table 2A) and with only physical parameters (Mod 6; ΔAICc=29.0; Table 2A). The model including only human activities (Mod7) was the least plausible (ΔAICc=32.8; Table 2A).
AIC model selection analysis using proportional occurrence of blue crabs as response variable produced similar results, retaining the model with depth and nitrate concentration as the best model (ModS3; Table 2B). According to ModS3, the proportional occurrence of blue crabs showed a positive relationship with both variables, although depth resulted in lower explanatory ability than nitrates (Figures 3B, 4B; Supplementary Table 1B). The partitioning of R2 showed that NO3 was the variable with the highest value of conditional and marginal R2 in explaining the proportional occurrence of blue crab (conditional R2: 0.20, IC: 0.08–0.37, marginal R2: 0.16, IC: 0.06–0.31) followed by water depth (conditional R2: 0.05, IC: 0.00–0.24, marginal R2: 0.01, IC: 0.00–0.17; Figure 4B).
For the first time, this study examined potential relationships between the occurrence of invasive blue crabs at the scale of the Mediterranean Sea, and a large set of physical, social, and ecological explanatory variables available from on-line sources. We found that nitrate concentration, as an indicator of nutrient load, and water depth (although more weakly), as an indicator of slope of the coastal bottom, were the main variables retained in the best models explaining the occurrences of blue crab and its proportional occurrences along the coast of Mediterranean Sea. Contrary to what we expected, water temperature and salinity had lesser impacts. The direct indicators of anthropogenic activity (i.e., human populations and ship density) were not retained in the best model explaining the blue crab occurrences. Particularly, human population size was not a significant factor statistically affecting blue crab occurrence, suggesting that that the number of blue crab observations was not dependent on the number of people in the 50 x 25 Km grid, at least within the broad context of this study (however, see below).
Blue crab occurrences (also corrected by human population size) from 2021 to 2023 were predominantly concentrated along the European coastline with few reported occurrences along African shores, mainly located in Marocco, Algeria and Tunisia. The highest blue crab occurrences in Spain, Greece and Italy coincided with areas with large rivers that flow into the sea or create estuarine lagoons. These large river outlets and coastal wetlands provide brackish environments which appear to be relevant in the spatial variation of blue crabs. These habitats provide nutrient loads which promote high primary production and more food availability for blue crab, as discussed below. Also, shallow waters and salinity gradients are suitable for the blue crab reproduction (Hines, 2007). First occurrences and large spreading of blue crab populations were in fact reported in such Mediterranean brackish environments such as the Ebro Delta in Spain (Castejón and Guerao, 2013; Clavero et al., 2022), Venice Lagoon (Nehring, 2011) and Po River Delta in Italy (Manfrin et al., 2015), Gulf of Thessaloniki in Greece (Kampouris et al., 2020; Nehring, 2011), estuary of Oued Z’hor in Algeria (Benabdi et al., 2019) and Bizerte Lagoon in Tunisia (Shaiek et al., 2021).
Compared to extensive, historical occurrence data provided by Mancinelli et al. (2021) and Castriota et al. (2024), we found a lack of occurrences from 2021 to 2023 in regions where blue crabs have been previously documented but where they are still likely. For example, blue crab occurrences were reported in Egypt (Abdel Razek et al., 2016; Mehanna et al., 2019), Gulf of Lions in France (Labrune et al., 2019), western part of Sardinia in Italy (Culurgioni et al., 2020) and Malta (Vella et al., 2023). This suggests a lack of citizen reporting rather than absence of blue crab populations in these areas, and represents a limitation of this and similar data. Nevertheless, this discrepancy was limited to few areas, and our analysis covered almost all regions where blue crabs have been reported previously. Consequently, we inferred that while all specific regions may not be represented, our results were generally robust for interrelationships among variables.
Although the blue crab life cycle (e.g., larval recruitment, juvenile growth, reaching of maturity and reproduction) is known to be influenced by seasonal temperature variations, as demonstrated by studies on the U.S. Atlantic coast (Hines, 2007), neither winter nor summer water temperature was associated with higher blue crab occurrences in this study, suggesting that other factors primarily influence large-scale crab distribution. On the other hand, the blue crab exhibits a large thermal adaptability ranging between 0-40°C with an optimum value at 24°C along a Thermal Performance Curve (Marchessaux et al., 2022), and dormancy induced below 9°C (Brylawski and Miller, 2006). During the summer season, optimal water temperatures for blue crab metabolism were reached throughout the Mediterranean, but maximum tolerance levels were not exceeded, at least at the Mediterranean scale in this study. This suggests that warm water temperature alone is unlikely to limit or prevent population growth in this region. Similarly, also lower temperature reached in winter appear to not be an obstacle for blue crab populations growth as global warming will likely reduce severe winter in the region (Shaltout and Omstedt, 2014). Furthermore, increasing water temperature under global change, rather than playing a direct role in limiting the expansion of the blue crab, may enhance eutrophication-related phenomena such as bottom hypoxia and anoxia. In fact, on July 28, 2024, in the northern part of the Po Delta, in the locality of Boccasette, municipality of Porto Tolle, province of Rovigo in Italy, a massive die-off of blue crabs was registered, linked to the deoxygenation of the coastal bottom waters (ANSA, 2023). Such events, although sporadic and localized, could have a significant impact on the survival and reproduction of the crabs and requires further investigation.
Another important factor influencing the life cycle of blue crab is salinity. This species is adapted to a wide range of salinities, with its abundances varying along salinity gradients depending on sex and developmental stage (Hines, 2007). Salinity plays a key role in the blue crab reproduction, as the brackish conditions of estuaries and lagoons are essential for courtship and mating (Epifanio, 2019). However, our large-scale dataset limits our ability to explore finer-scale relationships that might explain local blue crab distribution and abundance patterns, such as the dynamics of estuarine systems. Local outlet geomorphology, river discharge and salinity distributions affect crab biology (e.g., abundances and distribution of sexes and reproductive stages). Unfortunately, this detail is unavailable from GBIF data.
The importance of estuaries and brackish lagoons in determining the presence of blue crabs is indirectly supported by our analysis, which shows an increase in blue crab populations associated with nutrient loads and shallow waters, conditions typical of these estuarine environments (Mancinelli et al., 2017a). We found that blue crab occurrences were generally higher in areas with shallower depths, such as the northern Adriatic Sea. An exception was observed in terms of proportional occurrences, as the depth was retained in the best model with, a weak and positive association. This was probably due to the lower human population density in deeper areas of the Mediterranean Sea, such as around islands and close to the Calypso Deep, its deepest point, rather than an actual increase in blue crab populations in deeper waters. To our knowledge, the only blue crab found in deeper Mediterranean waters was a female collected at 220 meters depth off the northern part of Imbroz Island, in the eastern Mediterranean Sea (Daban et al., 2016). In their native range, blue crabs exhibit depth-specific habitat uses, with females migrating to deeper waters for egg development and juveniles preferring shallower, vegetated areas (Hines, 2007; Eggleston et al., 2015; Ogburn and Habegger 2015). While our study did not differentiate between sex or life stage, it is evident that shallow waters, resulting from a gentle slope of bottom, can support blue crab populations in the Mediterranean by providing suitable habitat for various life stage. Shallow waters contain seagrass beds, oyster reefs, or intertidal sand flats that support dense populations of shellfish which, in turn, provide food and refuge habitat for blue crabs at various life stages (Cheng et al., 2022). Moreover, depth may play another important role in determining trophic availability. For example, shallow areas support relatively high benthic primary and secondary production in estuarine systems (e.g., Kemp et al., 2005), thereby providing food resources for blue crabs. Interestingly, nitrate concentration and depth were the most important predictors of blue crab occurrence. In general, phytoplanktonic chlorophyll is widely regarded as an indicator of coastal primary production, given its role as the foundation of the pelagic food web and its direct and indirect influence on benthic primary production. However, the density of blue crabs is more likely to be determined by benthic primary and secondary production, particularly in shallow waters where a consistent amount of solar radiation reaches the bottom. In these conditions, water column nitrate is the most important nutrient source for benthic diatoms and other micro- and macroalgae. This contribution to benthic primary production is not retained directly by chlorophyll a level in the water column; however, it is of paramount importance to the richness of the benthic consumers and thus to the abundance of the blue crab. Under this hypothesis, it was not surprising that the best model selected by AIC was explained by depth and nitrate concentration which, in turn, were related to the presence of river estuaries and lagoons, essential ecosystems for blue crab growth and reproduction. Excessive nitrate concentrations, which can indicate anthropogenic activities like nitrogen loads from diffuse agricultural sources and wastewater point sources (e.g., Viaroli et al., 2018), could provide less favorable conditions for blue crab populations, particularly when dissolved oxygen concentrations suffer.
Human population and shipping density were not main factors in explaining variation in blue crab occurrences. Thus, within the scales of observation, the blue crab occurrences were independent of the human population size and shipping in each area. The negligible influence of human population density on blue crab occurrences was further supported by similar results obtained using population size-corrected data (i.e., proportional occurrence). On the other hand, the low genetic diversity within Mediterranean blue crab populations suggests that the Mediterranean invasion was not a gradual process, but it likely resulted from a few isolated colonization events involving a small number of individuals (Schubart et al., 2023).
Shipping and ballast waters might have played a role in the initial phases of the blue crab invasion, providing continuous introduction of blue crab larvae within and across the Mediterranean Sea, potentially determining the pattern of distribution (Marchessaux et al., 2022). However, the subsequent spread in the Mediterranean has likely been influenced by other factors such as water mass dynamics and habitat connectivity, and suitable environmental conditions (Jeschke and Strayer, 2005).
The results from this study provide a large-scale perspective on spatial variation of invasive blue crab relative distribution and abundance patterns, and the possible mechanisms driving these patterns. The results provide an initial, large-scale picture of the current state of the invasion and suggest that shallow waters and availability of food can promote invasion success by blue crabs, whereas increasing temperatures may not impede its spread in the short-term. Our results suggest that the regions of the Mediterranean Sea provide ample benthic production and available habitats such as seagrass beds and shallow estuarine bottom fueled by high nutrient loads and benthic production to support trophic demands by blue crab populations. Other regions may not be so hospitable. These hypotheses remain to be tested, and management strategies to prevent the spread of the blue crab and to mitigate its negative impacts on the biodiversity and fisheries of the region should focus on the spatial crab hotspots identified in this study.
This study also highlights the importance of, and caveats related to the use of Citizen science data and large-scale environmental monitoring for ecological research on species invasions. Citizen science data requires validation, which calls for the collection of data from local, standardized monitoring programs. Such programs are scattered spatially but could become a reference for global datasets such as the one used here. Detailed studies of the distribution of Atlantic blue crabs in the Mediterranean can validate the possibility of using citizen science occurrence data as a proxy for their abundance (Callaghan et al., 2023). Finally, when analyzing species invasions, which are likely to be driven by narrow gradients of environmental parameters, data resolution and, collaborative scientific research and management programs are critical. Therefore, global-scale datasets collected at low resolution should be integrated with higher-resolution data in the most informative areas, which are transitional environments such as estuaries, deltas, and lagoons, often not even covered by large-scale datasets. This would immediately allow for deeper ecological analysis and capture relevant patterns in areas critical to invasive species’ reproductive cycle and invasion success. A comprehensive study of the blue crab invasion across the Mediterranean Sea can promote, for example, the development of common strategies to address the issue.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
AG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. GC: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. DE: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. RC: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors express their gratitude to the EcosistER Project under the National Recovery and Resilience Plan (NRRP) Mission 4 Component 2 Investment 1.5 - Call for tender No. 3277 of 30/12/2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU (Project code ECS00000033) within WP3—Biotic and abiotic marine resources of the Spoke 5—Circular economy and blue economy.
The authors would like to thank the reviewers for their valuable comments and to Vassilis Aschonitis and Niccolò Colombani for their helpful support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1515098/full#supplementary-material
Abdel Razek F. A., Ismaiel M., Ameran M. A. A. (2016). Occurrence of the blue crab Callinectes sapidus, Rathbun 1896, and its fisheries biology in Bardawil Lagoon, Sinai Peninsula, Egypt. Egypt. J. Aquat. Res. 42, 223–229. doi: 10.1016/j.ejar.2016.04.005
Akaike H. (1974). A new look at the statistical model identification. IEEE Trans. Automat. Control 19, 716–723.
ANSA (2023)Moria di granchi blu su costa veneta, 'uccisi dalla mucillagine'. In: In italian. Available online at: https://www.ansa.it/veneto/notizie/2024/07/29/moria-di-granchi-blu-su-costa-veneta-uccisi-dalla-mucillagine_d680aaa7-6cbf-4a1f-8bd8-f1c05ac804fc.html (Accessed August 01, 2024).
Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. doi: 10.18637/jss.v067.i01
Benabdi M., Belmahi A. E., Grimes S. (2019). First record of the atlantic blue crab callinectes sapidus rathbun 1896 (Decapoda: Brachyura: Portunidae) in Algerian coastal waters (Southwestern Mediterranean). Bioinvasions. Rec. 8, 119–122. doi: 10.3391/BIR.2019.8.1.13
Blackburn T. M., Pyšek P., Bacher S., Carlton J. T., Duncan R. P., Jarošík V., et al. (2011). A proposed unified framework for biological invasions. Trends Ecol. Evol. 26, 333–339. doi: 10.1016/j.tree.2011.03.023
Brylawski B. J., Miller T. J. (2006). Temperature-dependent growth of the blue crab (Callinectes sapidus): A molt process approach. Can. J. Fish. Aquat. Sci. 63, 1298–1308. doi: 10.1139/F06-011
Caddy J. F. (1993). Toward a comparative evaluation of human impacts on fishery ecosystems of enclosed and semi-enclosed seas. Rev. Fish. Sci. 1, 57–95. doi: 10.1080/10641269309388535
Caddy J. F. (2000). Marine catchment basin effects versus impacts of fisheries on semi-enclosed seas. ICES J. Mar. Sci. 57 (3), 628–640. doi: 10.1006/jmsc.2000.0739
Callaghan C. T., Borda-de-Água L., van Klink R., Rozzi R., Pereira H. M. (2023). Unveiling global species abundance distributions. Nat. Ecol. Evol. 7, 1600–1609. doi: 10.1038/s41559-023-02173-y
Cannarozzi L., Paoli C., Vassallo P., Cilenti L., Bevilacqua S., Lago N., et al. (2023). Donor-side and user-side evaluation of the Atlantic blue crab invasion on a Mediterranean lagoon. Mar. Pollut. Bull. 189, 114758. doi: 10.1016/j.marpolbul.2023.114758
Cardoso A. C., Tsiamis K., Gervasini E., SChade S., Taucer F., Adriaens T., et al. (2017). Citizen science and open data: a model for invasive alien species in europe. Res. Ideas. Outcomes. 3, e14811. doi: 10.3897/rio.3.e14811
Carrozzo L., Potenza L., Carlino P., Costantini M. L., Rossi L., Mancinelli G. (2014). Seasonal abundance and trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in a Mediterranean coastal habitat. Rendiconti. Lincei. 25, 201–208. doi: 10.1007/s12210-014-0297-x
Castejón D., Guerao G. (2013). A new record of the American blue crab, Callinectes sapidus rathbun 1896 (Decapoda: Brachyura: Portunidae), from the mediterranean coast of the Iberian Peninsula. Bioinvasions. Rec. 2, 141–143. doi: 10.3391/bir.2013.2.2.08
Castriota L., Falautano M., Perzia P. (2024). When nature requires a resource to be used—The case of callinectes sapidus: distribution, aggregation patterns, and spatial structure in northwest europe, the mediterranean sea, and adjacent waters. Biol. (Basel). 13, 279. doi: 10.3390/biology13040279
Céspedes V., Bernardo-Madrid R., Picazo F., Vilà M., Rubio C., García M., et al. (2024). Massive decline of invasive apple snail populations after blue crab invasion in the Ebro River, Spain. Biol. Invasions. 26, 2387–2395. doi: 10.1007/s10530-024-03334-1
Cheng S. L., Tedford K. N., Smith R. S., Hardison S., Cornish M. R., Castorani M. C. N. (2022). Coastal vegetation and bathymetry influence blue crab abundance across spatial scales. Estuaries. Coasts. 45, 1701–1715. doi: 10.1007/s12237-021-01039-5
Christianson K. A., Eggleston D. B. (2021). Testing ecological theories in the Anthropocene: alteration of succession by an invasive marine species. Ecosphere 12, e03471. doi: 10.1002/ecs2.3471
CIESIN (Center for International Earth Science Information Network of Columbia University) (2018). Documentation for the Gridded Population of the World, Version 4 (GPWv4), Revision 11 Data Sets (Palisades NY: NASA Socioeconomic Data and Applications Center (SEDAC). doi: 10.7927/H45Q4T5F
Clavero M., Franch N., Bernardo-Madrid R., López V., Abelló P., Queral J. M., et al. (2022). Severe, rapid and widespread impacts of an Atlantic blue crab invasion. Mar. Pollut. Bull. 176, 113479. doi: 10.1016/j.marpolbul.2022.113479
Costa E. F. S., Encarnação J., Teodósio M. A., Morais P. (2023). Aquatic species shows asymmetric distribution range shifts in native and non-native areas. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1158206
Costello K. E., Lynch S. A., McAllen R., O’Riordan R. M., Culloty S. C. (2022). Assessing the potential for invasive species introductions and secondary spread using vessel movements in maritime ports. Mar. Pollut. Bull. 177, 113496. doi: 10.1016/j.marpolbul.2022.113496
Crisci C., Bensoussan N., Romano J. C., Garrabou J. (2011). Temperature anomalies and mortality events in marine communities: Insights on factors behind differential mortality impacts in the NW Mediterranean. PloS One 6, e23814. doi: 10.1371/journal.pone.0023814
Culurgioni J., Diciotti R., Satta C. T., Camedda A., de Lucia G. A., Pulina S., et al. (2020). Distribution of the alien species callinectes sapidus (Rathbun 1896) in sardinian waters (western mediterranean). Bioinvasions. Rec. 9, 65–73. doi: 10.3391/bir.2020.9.1.09
Daban İ.B., Cengiz Ö., Tuncer S. (2016). Further range expansion of the blue crab Callinectes sapidus (Rathbun 1896) (Crustacea: Decapoda: Brachyura) in Turkish waters, Northern Aegean Sea: insight into distribution depth. Cah. Biol. Mar. 57, 175–178.
Dittel A. I., Hines A. H., Ruiz G. M., Ruffin K. K. (1995). Effects of shallow water refuge on behavior and density-dependent mortality of juvenile blue crabs in Chesapeake Bay. Bull. Mar. Sci. 57, 902–916.
Durrieu de Madron X., Guieu C., Sempéré R., Conan P., Cossa D., D’Ortenzio F., et al. (2011). Marine ecosystems’ responses to climatic and anthropogenic forcings in the Mediterranean. Prog. Oceanogr. 91, 97–166. doi: 10.1016/j.pocean.2011.02.003
Eggleston D. B., Millstein E., Plaia G. (2015). Timing and route of migration of mature female blue crabs in a tidal estuary. Biol. Lett., 1120140936. doi: 10.1098/rsbl.2014.0936
EMODnet (2014). European marine observation data network (EMODnet) (www.emodnet.eu), financed by the european union under regulation (EU) no 508/2014 of the european parliament and of the council of 15 may 2014 on the european maritime and fisheries fund.
Encarnação J., Teodósio M. A., Morais P. (2021). Citizen science and biological invasions: A review. Front. Environ. Sci. 8. doi: 10.3389/fenvs.2020.602980
Epifanio C. E. (2019). Early life history of the blue crab callinectes sapidus: A review. J. Shellfish. Res. 38, 1–22. doi: 10.2983/035.038.0101
Etherington L. L., Eggleston D. B. (2000). Large-scale blue crab recruitment: linking postlarval transport, post-settlement planktonic dispersal, and multiple nursery habitats. Mar. Ecol. Prog. Ser. 204, 179–198. doi: 10.3354/meps204179
E.U. Copernicus Marine Service Information (CMEMS) (2024a). Global Ocean Biogeochemistry Analysis and Forecast. Available online at: https://marine.copernicus.eu/access-data (Accessed April 02, 2024).
E.U. Copernicus Marine Service Information (CMEMS) (2024b). Global Ocean Physics Analysis and Forecast. Available online at: https://marine.copernicus.eu/access-data (Accessed April 02, 2024).
Fox J., Weisberg S. (2019). An r companion to applied regression. 3rd ed. (Thousand Oaks CA: Sage). Available at: https://www.john-fox.ca/Companion/.
Galil B. S. (2008). Alien species in the Mediterranean Sea - Which, when, where, why?. Hydrobiologia. 606, 105–116. doi: 10.1007/s10750-008-9342-z
GBIF.org (2024). GBIF Occurrence Downloaded on 09-May 2024. Available online at: https://www.gbif.org/ (Accessed May 09, 2024).
González-Ortegón E., Berger S., Encarnação J., Chairi H., Morais P., Teodósio M. A., et al. (2022). Free pass through the pillars of hercules? Genetic and historical insights into the recent expansion of the atlantic blue crab callinectes sapidus to the west and the east of the strait of Gibraltar. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.918026
Hamiche F. Z., Aksissou M. (2024). The invasive blue crab Callinectes sapidus Rathbun 1896 (Decapoda, Portunidae) is rapidly expanding its distributional range in the northwestern Mediterranean coast of Morocco. Biodivers. Data J. 12, e115875. doi: 10.3897/BDJ.12.e115875
Hartig F. (2021). DHARMa: Residual diagnostics for hierarchical (multilevel/mixed) regression models. R Package Version 0.4.7. Available online at: http://florianhartig.github.io/DHARMa/ (Accessed May 02, 2024).
Hines A. H. (2007). “Chapter 14: ecology of juvenile and adult blue crabs,” in The Blue Crab: Callinectes Sapidus. Eds. Kennedy S. V., Cronin L. E. (College Park, MD: Maryland Sea Grant Program), 565–654.
Hurvich C. M., Tsai C. (1993). A corrected akaike information criterion for vector autoregressive model selection. J. Time Anal. 14 (3), 271–279. doi: 10.1111/j.1467-9892.1993.tb00144.x
IUCN (2000). IUCN Guidelines for the Prevention of Biodiversity Loss Caused by Alien Invasive Species APPROVED BY THE 51 ST MEETING OF THE IUCN COUNCIL, GLAND SWITZERLAND, FEBRUARY 2000 International Union for Conservation of Nature. (Gland Switzerland: IUCN Council).
Jeschke J. M., Strayer D. L. (2005). Invasion success of vertebrates in Europe and North America. Glob. Chang. Biol. 102, 7198–7202. doi: 10.1111/j.1365-2486.2006.01213.x
Kampouris T. E., Kouroupakis E., Batjakas I. E. (2020). Morphometric relationships of the global invader callinectes sapidus rathbun 1896 (Decapoda, brachyura, portunidae) from papapouli lagoon, NW Aegean Sea, Greece. with notes on its ecological preferences. Fishes 5, 5. doi: 10.3390/fishes5010005
Katsanevakis S., Coll M., Piroddi C., Steenbeek J., Lasram F. B. R., Zenetos A., et al. (2014). Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 1. doi: 10.3389/fmars.2014.00032
Kelly A. E., Goulden M. L. (2008). Rapid shifts in plant distribution with recent climate change. Proc. Nat. Acad. Sci. USA. 105, 11823–11826. doi: 10.1073/pnas.0802891105
Kemp W. M., Boynton W. R., Adolf J. E., Boesch D. F., Boicourt W. C., Brush G., et al. (2005). Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar Ecol Prog Ser 303, 1–29. doi: 10.3354/meps303001
Korpinen S., Laamanen L., Bergström L., Nurmi M., Andersen J. H., Haapaniemi J., et al. (2021). Combined effects of human pressures on Europe’s marine ecosystems. Ambio 50, 1325–1336. doi: 10.1007/s13280-020-01482-x
Labrune C., Amilhat E., Amouroux J. M., Jabouin C., Gigou A., Noël P. (2019). The arrival of the american blue crab, callinectes sapidus rathbun 1896 (Decapoda: Brachyura: Portunidae), in the gulf of lions (mediterranean sea). Bioinvasions. Rec. 8, 876–881. doi: 10.3391/bir.2019.8.4.16
Lehner B., Döll P. (2004). Development and validation of a global database of lakes, reservoirs and wetlands. J. Hydrol. (Amst). 296, 1–22. doi: 10.1016/j.jhydrol.2004.03.028
Lipcius R., Eggleston D., Heck K. Jr., Seitz R., van Montfrans J. (2007). “Ecology of postlarval and young juvenile crabs,” in The blue crab, Callinectes sapidus. Eds. Kennedy. V., Cronin L. (College Park, MD: Maryland Sea Grant College), 535–564.
Lucy F. E., Roy H., Simpson A., Carlton J. T., Hanson J. M., Magellan K., et al. (2016). INVASIVESNET towards an international association for open knowledge on invasive alien species. Manage. Biol. Invasions. 7, 131–139. doi: 10.3391/mbi.2016.7.2.01
Mancinelli G., Bardelli R., Zenetos A. (2021). A global occurrence database of the Atlantic blue crab Callinectes sapidus. Sci. Data 8, 1–10. doi: 10.1038/s41597-021-00888-w
Mancinelli G., Chainho P., Cilenti L., Falco S., Kapiris K., Katselis G., et al. (2017a). The Atlantic blue crab Callinectes sapidus in southern European coastal waters: Distribution, impact and prospective invasion management strategies. Mar. Pollut. Bull. 119, 5–11. doi: 10.1016/j.marpolbul.2017.02.050
Mancinelli G., Glamuzina B., Petric M., Carrozzo L., Glamuzina L., Zotti M., et al. (2016). The trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): A first assessment using stable isotopes. Mediterr. Mar. Sci. 17, 634–643. doi: 10.12681/mms.1724
Mancinelli G., Guerra M. T., Alujević K., Raho D., Zotti M., Vizzini S. (2017b). Trophic flexibility of the Atlantic blue crab Callinectes sapidus in invaded coastal systems of the Apulia region (SE Italy): A stable isotope analysis. Estuarine. Coast. Shelf. Sci. 198, 421–431. doi: 10.1016/j.ecss.2017.03.013
Manfrin C., Turolla E., Chung J. S., Giulianini P. G. (2015). First occurrence of Callinectes sapidus (Rathbun 1896) within the Sacca di Goro (Italy) and surroundings. Check. List. 11, 1640. doi: 10.15560/11.3.1640
Mao M., Xia M. (2024). Modeling blue crab (Callinectes sapidus) larval transport and recruitment dynamics in a shallow lagoon-inlet-coastal ocean system. J. Geophys. Res. Oceans. 129, e2023JC020785. doi: 10.1029/2023JC020785
Marchessaux G., Bosch-Belmar M., Cilenti L., Lago N., Mangano M. C., Marsiglia N., et al. (2022). The invasive blue crab Callinectes sapidus thermal response: Predicting metabolic suitability maps under future warming Mediterranean scenarios. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1055404
Marchessaux G., Chevalier C., Mangano M. C., Sarà G. (2023a). Larval connectivity of the invasive blue crabs Callinectes sapidus and Portunus segnis in the Mediterranean Sea: A step toward improved cross border management. Mar. Pollut. Bull. 194 (Part A), 115272. doi: 10.1016/j.marpolbul.2023.115272
Marchessaux G., Mangano M. C., Bizzarri S., M’Rabet C., Principato E., Lago N., et al. (2023b). Invasive blue crabs and small-scale fisheries in the Mediterranean sea: Local ecological knowledge, impacts and future management. Mar. Policy 148, 105461. doi: 10.1016/j.marpol.2022.105461
Mazerolle M. (2019). Model selection and multimodel inference based on (Q)AIC(c) version 2.2-2, 1–212. Available at: https://Cran.r-Project.Org/Web/Packages/AICcmodavg/AICcmodavg.Pdf.
Mehanna S. F., Mohammed, Desouky G., Farouk A. E. (2019). Population dynamics and fisheries characteristics of the Blue Crab Callinectes sapidus (Rathbun 1896) as an invasive species in Bardawil Lagoon, Egypt. J. Aquatic Biol. Fisheries 23, 599–611. doi: 10.21608/ejabf.2019.34459
Milori E., Ruci S. (2021). State of blue crab callinectes sapidus in the lagoon of orikum in Albania. J. Earth Environ. Sci. Res. 3, 1–9. doi: 10.47363/JEESR/2021(3)157
Nehring S. (2011). “Invasion history and success of the american blue crab callinectes sapidus in european and adjacent waters,” in In the Wrong Place - Alien Marine Crustaceans: Distribution, Biology and Impacts, vol. 6 . Eds. Galil B., Clark P., Carlton J. (Springer, Dordrecht). Springer Series in Invasion Ecology.
Ogburn M. B., Habegger L. C. (2015). Reproductive status of callinectes sapidus as an indicator of spawning habitat in the south atlantic bight, USA. Estuaries Coasts 38, 2059–2069. doi: 10.1007/s12237-015-9962-2
Pocock M. J. O., Adriaens T., Bertolino S., Eschen R., Essl F., Hulme P. E., et al. (2024). Citizen science is a vital partnership for invasive alien species management and research. iScience 27, 108623. doi: 10.1016/j.isci.2023.108623
Prado P., Baeta M., Mestre E., Solis M. A., Sanhauja I., Gairin I., et al. (2024). Trophic role and predatory interactions between the blue crab, Callinectes sapidus, and native species in open waters of the Ebro Delta. Estuar. Coast. Shelf. Sci. 298, 108638. doi: 10.1016/j.ecss.2024.108638
Prado P., Ibáñez C., Chen L., Caiola N. (2022). Feeding habits and short-term mobility patterns of blue crab, callinectes sapidus, across invaded habitats of the ebro delta subjected to contrasting salinity. Estuaries. Coasts. 45, 839–855. doi: 10.1007/s12237-021-01004-2
QGIS.org (2024). QGIS geographic Information System (QGIS Association). Available onlne at: https://www.qgis.org. (Accessed April 01, 2024).
R Studio Team. (2024). RStudio: Integrated Development for R. (Boston, MA: RStudio, PBC). Available online at: http://www.rstudio.com/. (Accessed April 24, 2024).
Ruiz G. M., Fofonoff P. W., Carlton J., Wonham M. J., Hines A. H. (2000). INVASION OF COASTAL MARINE COMMUNITIES IN NORTH AMERICA: Apparent patterns, processes, and biases. Annu. Rev. Eeal. Syst. 31, 481–531. doi: 10.1146/annurev.ecolsys.31.1.481
Sará G., Romano C., Mazzola A. (2008). A new lessepsian species in the western Mediterranean (Brachidontes pharaonis Bivalvia: Mytilidae): density, resource allocation and biomass. Mar. Biodivers. Rec. 1, e8. doi: 10.1017/s175526720600087x
Sardain A., Sardain E., Leung B. (2019). Global forecasts of shipping traffic and biological invasions to 2050. Nat. Sustain. 2, 274–282. doi: 10.1038/s41893-019-0245-y
Schubart C. D., Deli T., Mancinelli G., Cilenti L., Gil Fernández A., Falco S., et al. (2023). Phylogeography of the Atlantic Blue Crab Callinectes sapidus (Brachyura: Portunidae) in the Americas versus the Mediterranean Sea: Determining Origins and Genetic Connectivity of a Large-Scale Invasion. Biology. 12, 35. doi: 10.3390/biology12010035
Seebens H., Blackburn T. M., Dyer E. E., Genovesi P., Hulme P. E., Jeschke J. M., et al. (2017). No saturation in the accumulation of alien species worldwide. Nat. Commun. 8, 1–9. doi: 10.1038/ncomms14435
Shaiek M., El Zrelli R., Crocetta F., Mansour L., Rabaoui L. (2021). On the occurrence of three exotic decapods, callinectes sapidus (Portunidae), portunus segnis (portunidae), and trachysalambria palaestinensis (penaeidae), in northern Tunisia, with updates on the distribution of the two invasive portunids in the mediterranean sea. Bioinvasions. Rec. 10, 158–169. doi: 10.3391/bir.2021.10.1.17
Shaltout M., Omstedt A. (2014). Recent sea surface temperature trends and future scenarios for the Mediterranean Sea. Oceanologia 56, 411–443. doi: 10.5697/oc.56-3.411
Simberloff D., Martin J. L., Genovesi P., Maris V., Wardle D. A., Aronson J., et al. (2013). Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 28, 58–66. doi: 10.1016/j.tree.2012.07.013
Snipes M., Taylor D. C. (2014). Model selection and akaike information criteria: an example from wine ratings and prices. Wine Economics Policy 3, 3–9. doi: 10.1016/j.wep.2014.03.001
Stoffel M. A., Nakagawa S., Schielzeth H. (2021). partR2: Partitioning R2 in generalized linear mixed models. PeerJ 9, 1–17. doi: 10.7717/peerj.11414
Sorte C. J. B., Williams S. L., Carlton J. T. (2010). Marine range shifts and species introductions: Comparative spread rates and community impacts. Global Ecol. Biogeogr. 19, 303–316. doi: 10.1111/j.1466-8238.2009.00519.x
Sutherland C., Hare D., Johnson P. J., Linden D. W., Montgomery R. A., Droge E. (2023). Practical advice on variable selection and reporting using akaike information criterion. Proc. R. Soc B., 29020231261. doi: 10.1098/rspb.2023.1261
Tsirintanis K., Azzurro E., Crocetta F., Dimiza M., Froglia C., Gerovasileiou V., et al. (2022). Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions. 17, 308–352. doi: 10.3391/ai.2022.17.3.01
Vecchioni L., Russotto S., Arculeo M., Marrone F. (2022). On the occurrence of the invasive Atlantic blue crab Callinectes sapidus Rathbun 1896 (Decapoda: Brachyura: Portunidae) in Sicilian inland waters. Natural History. Sci. 9, 43–46. doi: 10.4081/nhs.2022.586
Vella A., Giarrusso E., Monaco C., Mifsud C. M., Darmanin S. A., Raffa A., et al. (2023). New records of callinectes sapidus (Crustacea, portunidae) from Malta and the san leonardo river estuary in sicily (Central mediterranean). Diversity (Basel). 15, 679. doi: 10.3390/d15050679
Viaroli P., Soana E., Pecora S., Laini A., Naldi M., Fano E. A., et al. (2018). Space and time variations of watershed N and P budgets and their relationships with reactive N and P loadings in a heavily impacted river basin (Po river, Northern Italy). Sci. Total. Environ. 639, 1574–1587. doi: 10.1016/j.scitotenv.2018.05.233
Weatherall T. F., Scheef L. P., Buskey E. J. (2018). Spatial and temporal settlement patterns of blue crab (Callinectes sapidus and Callinectes similis) megalopae in a drought-prone Texas estuary. Estuar. Coast. Shelf. Sci. 214, 89–97. doi: 10.1016/j.ecss.2018.09.017
Wei T., Simko V. (2021). R package “corrplot”: Visualization of a Correlation Matrix. Available online at: https://github.com/taiyun/corrplot. (Accessed May 02, 2024).
Zenetos A., Galanidi M. (2020). Mediterranean non indigenous species at the start of the 2020s: Recent changes. Mar. Biodivers. Rec. 13, 10. doi: 10.1186/s41200-020-00191-4
Zenetos A., Gofas S., Morri C., Rosso A., Violanti D., García Raso J. E., et al. (2012). Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 13, 328–352. doi: 10.12681/mms.327
Zuur A. F., Ieno E. N. (2016). A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 7, 636–645. doi: 10.1111/mee3.2016.7.issue-6
Keywords: Atlantic blue crab, invasive species, non-native species, ecological determinants, invasion drivers, nutrients, water temperature, water depth
Citation: Gavioli A, Castaldelli G, Eggleston DB and Christian RR (2025) From ecological to anthropogenic factors: unraveling the drivers of blue crab Callinectes sapidus occurrence along the Mediterranean coasts. Front. Mar. Sci. 11:1515098. doi: 10.3389/fmars.2024.1515098
Received: 23 October 2024; Accepted: 23 December 2024;
Published: 16 January 2025.
Edited by:
Alberto Basset, University of Salento, ItalyReviewed by:
Ettore Nepote, Marche Polytechnic University, ItalyCopyright © 2025 Gavioli, Castaldelli, Eggleston and Christian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Gavioli, Z3Zsbm5hQHVuaWZlLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.