Abstract
The effects of stony coral tissue loss disease (SCTLD) in the Dutch Caribbean, specifically in Bonaire, have not been documented since its first sighting in March 2023. By comparing the results of the 2023 surveys with data from previous surveys conducted over the past 9 years, this study quantifies the overall decline in coral cover and investigates the spatial variability of SCTLD’s impact across different subregions of the reef on the leeward side of Bonaire. In the year 2023, a crucial period in the initial phase of the progression of SCTLD, significant reduction in coral coverage was observed, with six key reef-building coral species showing significant vulnerability. Importantly, this research identifies specific subregions that have been disproportionately affected. The insights gained from this study are important for the potential development of specific conservation and restoration strategies for Bonaire, underscoring the necessity for ongoing ecosystem monitoring to safeguard the future of highly sensitive coral reefs in a changing ocean. By advancing our understanding of SCTLD dynamics, this research contributes to the global effort to preserve coral reef ecosystems in the face of emerging coral diseases.
1 Introduction
Coral reefs are among the most biologically diverse and productive ecosystems on Earth, supporting an incredible array of marine life (Reaka-Kudla, 1997; Moberg and Rönnbäck, 2003). These vibrant underwater habitats play a crucial role in maintaining biodiversity, sustaining fisheries, protecting coastlines from erosion, driving tourism, and serve as a source for medical discoveries (Bertness et al., 2001; Newman et al., 2015; Brathwaite et al., 2022; Uyarra et al., 2008; Abdelhafez et al., 2019). However, coral reef systems have experienced significant declines over recent decades due to climate change-induced bleaching, ocean acidification, hurricanes, diseases, and several anthropogenic stressors (Høegh-Guldberg et al., 2007; Precht et al., 2016; Graham et al., 2014; Kjerfve et al., 2021; Mallela and Crabbe, 2009; Aronson and Precht, 2001).
The Caribbean Sea, in particular, is regarded as a coral disease ‘hotspot’, with numerous well-documented outbreaks in the last decades (Van Woesik and Randall, 2017; Thurber et al., 2020; Gladfelter, 1982). Coral diseases often arise due to a combination of biological stressors, such as bacteria, fungi, and viruses, as well as non-biological factors like elevated sea surface temperatures, ultraviolet radiation, and pollutants (Rosenberg and Ben-Haim, 2002; Jones et al., 2021). Over the past decade, the incidence of coral diseases has rapidly escalated, leading to significant mortality among reef-building corals (Precht et al., 2016; Costa et al., 2021). This trend is often linked to declining water quality from anthropogenic pollutants and rising sea surface temperatures, which facilitate the growth and spread of pathogenic particles (Merselis et al., 2018; Rosenberg and Ben-Haim, 2002). However, pinpointing definitive causes remains challenging due to the complexity of the interactions between multiple environmental stressors (Jones et al., 2021).
Coral diseases such as black-band, red-band, and yellow-blotch/band typically manifest as discolored bands or lesions on coral tissues, slowly degrading them and exposing the white skeleton underneath (Rosenberg and Ben-Haim, 2002). More virulent diseases like white band, white plague, white pox, and Stony Coral Tissue Loss Disease (SCTLD) cause large patches of coral tissue to deteriorate rapidly, leading to colonization by algae and invertebrates and often resulting in the irreversible decline of entire coral colonies (Aronson and Precht, 2001; Cróquer et al., 2021).
Initially characterized as a form of white-plague disease causing rapid tissue loss (Precht et al., 2016), SCTLD is now recognized by distinct simultaneous lesions that often manifest centrally within coral colonies (Cróquer et al., 2021; Florida Department of Environmental Protection, 2018). While the exact cause of SCTLD remains under investigation, viral-like particles (VLPs) have been associated with the disease in some studies. For instance, Work et al. (2021) identified VLPs associated with endosymbiont pathology in Florida corals affected by SCTLD, but VLPs were also found in unaffected corals, suggesting they may not be specific to this disease. Additionally, microbiome studies have revealed a variety of bacterial groups enriched in SCTLD lesions, pointing to a potential role for bacteria in disease progression (Meyer et al., 2019; Becker et al., 2021; Clark et al., 2021; Rosales et al., 2022, 2023). Further evidence supporting bacterial involvement comes from experiments using antibiotics, which have shown some efficacy in halting lesion progression (Aeby et al., 2019; Neely et al., 2020; Shilling et al., 2021; Walker et al., 2021; Forrester et al., 2022). Together, these findings highlight the complexity of SCTLD’s etiology, with both viral-like particles and bacteria being investigated as contributing factors.
This disease differs from other coral diseases in its extensive impact, affecting at least 20 species of Caribbean corals, including key reef-building species such as Orbicella annularis and Orbicella faveolata (Precht et al., 2016; Muller et al., 2020; Costa et al., 2021; Álvarez-Filip et al. 2019; 2022; Hernández-Delgado and Weil 2019). However, preliminary surveys and observations by STINAPA (Stichting Nationale Parken Nederlandse Antillen), a local NGO responsible for managing Bonaire’s National Parks, as well as training surveys conducted as part of this study, indicate that Orbicella annularis and Orbicella faveolata have not yet shown signs of SCTLD infection in Bonaire, and its colonies remained unaffected during the study period (STINAPA Bonaire National Parks Foundation, 2024). Since its detection in Florida in 2014, SCTLD has spread rapidly across more than 25 countries and territories, causing widespread ecological disruption, as documented by the Atlantic and Gulf Rapid Reef Assessment (AGGRA) SCTLD spread map (Atlantic and Gulf Rapid Reef Assessment (AGGRA), 2024). SCTLD has proven to be a significant threat to coral reefs, sometimes infecting up to 81% of the hard corals present on the reef, with its impact varying among species (Precht et al., 2016). SCTLD spreads swiftly via water currents (Dobbelaere et al., 2020), and research suggests that its dissemination could involve potential vectors such as reef sediments (Studivan et al., 2020), as well as the movement of vessels and ballast water (Studivan et al., 2022; Rosenau et al., 2021).
Bonaire’s coral reefs, often considered some of the most robust and vibrant in the Caribbean, have been highly valued for their biodiversity and as a prime destination for diving tourism, contributing significantly to the island’s economy (Parsons and Thur, 2007; Uyarra et al., 2008). Despite their shown resilience, Bonaire’s reefs have experienced challenges from environmental stressors, including climate-induced bleaching and various disease outbreaks (Lessios, 2016; De Bakker et al., 2017; De Bakker et al., 2019; Aronson and Precht, 2001; Gladfelter, 1982). Coral diseases, such as white band disease, have historically devastated local coral populations, particularly the acroporid species (Bak and Criens, 1981; Gladfelter, 1982). Additionally, a severe decline in the sea urchin Diadema antillarum led to reduced grazing, resulting in algal overgrowth, further stressing the coral ecosystem (Lessios, 2016).
SCTLD is a highly destructive coral disease that spreads rapidly, causing extensive mortality in susceptible species. Highly vulnerable species, such as Meandrina meandrites and Colpophyllia natans, often display early signs of infection, which can escalate to rapid tissue loss and death (Florida Department of Environmental Protection, 2018). Other SCTLD-sensitive species, such as Dendrogyra cylindrus and Dichocoenia stokesi, were not included in the analyses due to their sparse or virtually absent populations in Bonaire. The six SCTLD-sensitive species analyzed in this research—Meandrina meandrites, Eusmilia fastigiata, Montastraea cavernosa, Pseudodiploria strigosa, Diploria labyrinthiformis, and Colpophyllia natans—were selected because they represented nearly all observed SCTLD-like symptoms and recently dead coral. Notably, half of these species are brain corals, which are typically thermally resilient and less affected by bleaching events. This targeted focus allowed us to assess the disease’s effects on the most visibly impacted coral populations in Bonaire’s reef systems.
STINAPA first documented the arrival of SCTLD in Bonaire in March 2023 (STINAPA Bonaire National Parks Foundation, 2024). Since then, the disease has spread rapidly across the island’s reef systems, even reaching remote northern areas within nine months. According to a map created by STINAPA, SCTLD has significantly impacted Bonaire’s coral populations, particularly the six reef-building species analyzed in this study (see Figure 1; STINAPA Bonaire National Parks Foundation, 2024).
Figure 1

The six SCTLD-sensitive coral species on Bonaire, covered with tissue loss lesions. (A)Pseudodiploria strigosa; (B)Diploria labytinthiformis; (C)Colpophyllia natans; (D)Eusmilia fastigiata; (E)Meandrina meandrites; (F)Montastraea cavernosa.
Among the six SCTLD-sensitive species, the smooth brain coral Pseudodiploria strigosa, once known for its resilience in the Dutch Caribbean. now faces a significant threat. Previously regarded as a ‘winner’ due to its ability to withstand environmental stressors and its expansion over recent decades (De Bakker et al., 2016), P. strigosa is now at risk from SCTLD, marking a potential shift from a resilient survivor to a vulnerable species (Álvarez‐Filip et al., 2022; Florida Department of Environmental Protection, 2018). Additionally, slow-growing, thermally resistant brain corals—despite their inherent resilience—are frequently among the first to be afflicted, with some large colonies succumbing within weeks of infection (Álvarez-Filip et al., 2022; Florida Department of Environmental Protection, 2018). The decline of highly susceptible species, such as these brain corals, could jeopardize their long-term local persistence (Precht et al., 2016). Furthermore, patterns of susceptibility and prevalence vary regionally; species previously considered less vulnerable in one area have shown significant declines in another, suggesting that environmental, physical, and ecological factors play a critical role in SCTLD’s effects (Muller et al., 2020).
Although SCTLD’s presence in Bonaire is recent, its rapid spread underscores an urgent need for research, particularly given the limited documentation of coral disease outbreaks in the Dutch Caribbean. Understanding the disease’s early impact is crucial, as SCTLD progresses swiftly and can cause significant changes in a short period. This study seeks to fill a critical knowledge gap by providing the first systematic assessment of SCTLD’s effects on SCTLD-sensitive coral species in Bonaire, a region with unique reef characteristics compared to other parts of the Caribbean. Specifically, it establishes baseline data on SCTLD within its first year on Bonaire, quantifying its impact and assessing changes in population dynamics, spatial distribution, and overall density of six SCTLD-sensitive species. By analyzing how these species respond to the disease across different subregions and quantifying declines since the 2023 outbreak, this research aims to offer valuable insights into the early phase of SCTLD’s influence. The findings will be crucial in understanding the trajectory of the disease, informing conservation strategies, and contributing to the broader knowledge of SCTLD’s impact on coral reef ecosystems in the Caribbean.
2 Materials and methods
2.1 Study site and experimental design
The survey spans 115 sites located every 500m along the west coast of Bonaire (see Supplementary Table 1; Figure 1). These sites are divided into different subregions based on carbonate accretion and proximity to anthropogenic stressors, homogenously distributed (De Bakker et al., 2019). The 7 subregions are categorized as: South, Other, City Limits, Klein Bonaire, ND, BOPEC, and North (Figure 2).
Figure 2

The 7 sub-regions defined in De Bakker et al., 2019 on the leeward side of Bonaire. The map was generated using the ‘leaflet’ package in R (Cheng et al., 2023).
The surveys are part of a larger initiative by Wageningen Marine Research, under the supervision of Dr. EM, which is dedicated to conducting a long-term analysis of the leeward side coral reefs of the Bonairian island. This initiative aims to monitor the evolving dynamics of these ecosystems in light of climatic (Meesters and Bak, 1993) and anthropogenic stressors (Meesters et al., 2022). The monitoring program has conducted surveys on these same 115 georeferenced sites every two to three years since 2014, with the most recent dataset collected in 2023, marking the fourth round of sampling.
A four-member team followed a systematic protocol. Surveys were done from the shore, or from the boat when inaccessible from shore. Boat days were based on weather forecasts and boat/captain availability. On average 3 to 4 sites were surveyed each day between 9:00 and 17:00 in the months of September to November 2023. Due to the exceptional circumstances surrounding the arrival of SCTLD in March, dive sites 71 to 104 were forbidden for recreational SCUBA diving to help slow the spread of the disease. However, STINAPA granted us permission to conduct scientific surveys in these areas. Additionally, to prevent further spread of the disease in these areas, our diving gear and equipment were thoroughly disinfected after each survey conducted in the dive sites where SCTLD was reported previously (STINAPA Bonaire National Parks Foundation, 2024).
2.2 In the water protocol
Data was collected at each site by deploying two transect lines, each laid out by a pair of divers parallel to the reef front along the isobath (Hill and Wilkinson, 2004). Surveys were conducted at two depth zones: the Drop-off zone (10-15m depth) and the Lower Terrace zone (5m depth). A weight marker at 10m depth indicated the starting point of the transect. The weight marker used in the transects is placed temporarily during each survey and is not permanent to avoid environmental damage. The same transects were revisited during each monitoring period, guided by GPS coordinates and site markers, in accordance with the standardized monitoring protocol established by Wageningen Marine Research. One pair of divers conducted the survey to the left (L), while the other pair surveyed to the right (R), both parallel to the shore.
Once the transect lines were laid out at the weight-marked starting points, both pairs of divers could begin their respective tasks. The first surveyor rolled out the transect line along the 30-meter transect, while the second surveyor ensured it followed the 10-meter isobath and did not get stuck. Once the transect line was securely in place, the second surveyor began data collection at the 20-meter mark and continued to the end of the transect at the 30-meter mark. Images were captured every 50 cm on alternating sides (left and right) to avoid overlap, resulting in 40 photographs per transect, including both left and right sides. The 20-meter starting point was chosen to ensure consistency with previous surveys and to minimize potential edge effects from habitat transitions or site-specific conditions. These photographs were taken from approximately 50 cm above the substrate. Additionally, rugosity was estimated by measuring the height of the nearest coral colony (living or dead), and quadrats (0.25 x 0.25 meters) were placed every 2 meters in the last 10 meters of the transect for further analysis, including the percentage of crustose coralline algae, cyanobacteria, macroalgae, and turf (Figure 3).
Figure 3

Transect lines left and right containing quadrats. L is left and R is right. Dotted line in the middle contains every meter in between the two transects from 20 to 0 meters. Squares are quadrats of 0.25 x 0.25 m.
Tissue loss and bleaching were not directly assessed as part of the standardized monitoring protocol, which focuses on coral density and composition. However, qualitative observations noted that coral bleaching peaked in November, primarily affecting Orbicella and Agaricia species, while SCTLD-sensitive corals such as M.cavernosa, E.fastigiata, M.meandrites, and other brain corals were less affected. Additionally, SCTLD-like symptoms, other coral disease symptoms, and recently dead SCTLD-sensitive corals were frequently observed during most surveys, unlike other coral species like Orbicella, which showed fewer instances of recent mortality. These observations were not quantitatively analyzed due to logistical challenges and the rapid progression of the disease but were documented to provide context for understanding potential changes in coral cover.
Pictures were taken with an Olympus Tough tg-6 camera, equipped with a Weefine WFL-02 wide-angle lens (52 mm).
2.3 Data processing
To accurately assess and compare benthic organisms, the images underwent several processing steps. Benthic images were edited using Adobe Lightroom (Adobe Systems Inc, 2024) by cropping them to fit 50cm by 50cm squares and enhancing different parameters of the images. This process involved adjusting the colors, brightness, and sharpness to enhance clarity and detail, ensuring the images were as clear and sharp as possible for accurate organism classification. The CoralNet AI was chosen as it is an advanced image analysis resource, for the classification of benthic organisms (Beijbom et al., 2015). Upon upload, CoralNet AI’s deep neural networks facilitated the annotation process, identifying and categorizing benthic features within the images (using benthos list from the Supplementary Table 2). Each automated annotation was manually verified. This method offers a more efficient and accurate assessment compared to broad group classifications obtained from simple visual estimation. Each photo was divided into a regular 5 x 5 grid with 1 randomly generated annotation point per grid cell to ensure systematic and comprehensive coverage of the entire image area. To avoid the dark edges of the photos, which could introduce errors or inconsistencies in the analysis, the annotation area was adjusted to cover 10% to 90% of both the X and Y axes.
2.4 Data analysis
In the analysis, we concentrated on comparing the densities of M.meandrites, C.natans, P.strigosa, D.labyrinthiformis, E.fastigiata, and M.cavernosa. These species were chosen based on their high vulnerability to SCTLD, as documented in previous studies (Precht et al., 2016; Costa et al., 2021), and their prevalence among corals displaying SCTLD-like symptoms or recently observed mortality in Bonaire during the study period. Together, these species contributed approximately to 15% of the total hard coral cover in Bonaire, making them ecologically significant and highly visible indicators of SCTLD’s impact.
While other studies highlight additional species impacted by SCTLD, such as Orbicella annularis (Costa et al., 2021), preliminary surveys conducted as part of this study, along with observations from STINAPA, found no evidence of infection in these species during the study period. Additionally, species like Dendrogyra cylindrus and Dichocoenia stokesi, known to be sensitive to SCTLD in other regions, were excluded due to their sparse or virtually absent populations on Bonaire. By focusing on the six most affected species, we aimed to capture the disease’s localized impact on Bonaire’s reefs while acknowledging the need for broader investigations into less common or unaffected species to understand SCTLD’s full ecological scope.
A 4th root square transformation was applied to stabilize the variance and improve the symmetry of the data distribution (Supplementary Figures 3, 4). Mean values and 95% confidence intervals of back-transformed values were used to evaluate the spatial distribution of these SCTLD-sensitive species across seven subregions (De Bakker et al., 2019). Trend lines were fitted using linear models (least-squares regression) to analyze temporal changes in coral cover, with gray shaded areas representing 95% confidence intervals. These intervals, computed in R (R Core Team, 2023) and visualized using the ggplot2 package (Wickham, 2016), provide a visual representation of variability and help identify significant patterns where they do not overlap.
Our assessment considered varying depths of 10 and 5 meters and transects across four datasets spanning the years 2014, 2017, 2020, and 2023. The mapping of subregions, defined by sites (Supplementary Table 1), was crucial for understanding the spatial dynamics of coral health. We used R programming tools to calculate and aggregate the coral cover data, adjusting for factors such as transect orientation (left/right) and depth categorization (5/10 meters depth).
This methodology was replicated at the species level, which provided insight into individual species trends, despite the interpretive challenges posed by the high prevalence of zero cover at numerous sites when SCTLD-sensitive coral species are not aggregated.
In this study, we took a straightforward approach to analyzing coral cover data. Using linear models and 95% confidence intervals, we’ve been able to spot important patterns and differences in the coral reef’s condition.
3 Results
3.1 Overall trends
The analysis of coral cover on Bonaire’s reefs from 2014 to 2023 reveals a significant decline in SCTLD-sensitive coral species for 2018 and 2023 datasets, with mean cover dropping from over 3% in 2014 to just about 1% by 2023 (Figure 4). By 2023, significant reductions are observed in City Limits and Klein Bonaire, with declines recorded across all depths in seven out of eight comparisons. Total coral cover, which includes SCTLD-sensitive species, shows similar declines, particularly in 2023, with significant reductions in Klein Bonaire and non-significant changes in most other subregions, reflecting the disproportionate contribution of SCTLD-sensitive species to overall coral loss.
Figure 4

Aggregate mean cover of SCTLD-sensitive coral species on Bonaire from 2014 to 2023 displaying a continuous decline throughout the period. The 95% confidence intervals illustrate variability in the data.
The following sections explore these species-specific and subregional patterns in greater detail.
3.2 Individual species trends
The analysis of SCTLD-sensitive coral species from 2014 to 2023 shows a significant decline across all species, with 2023 often marking the lowest mean coral cover for most species (Figure 5).
Figure 5
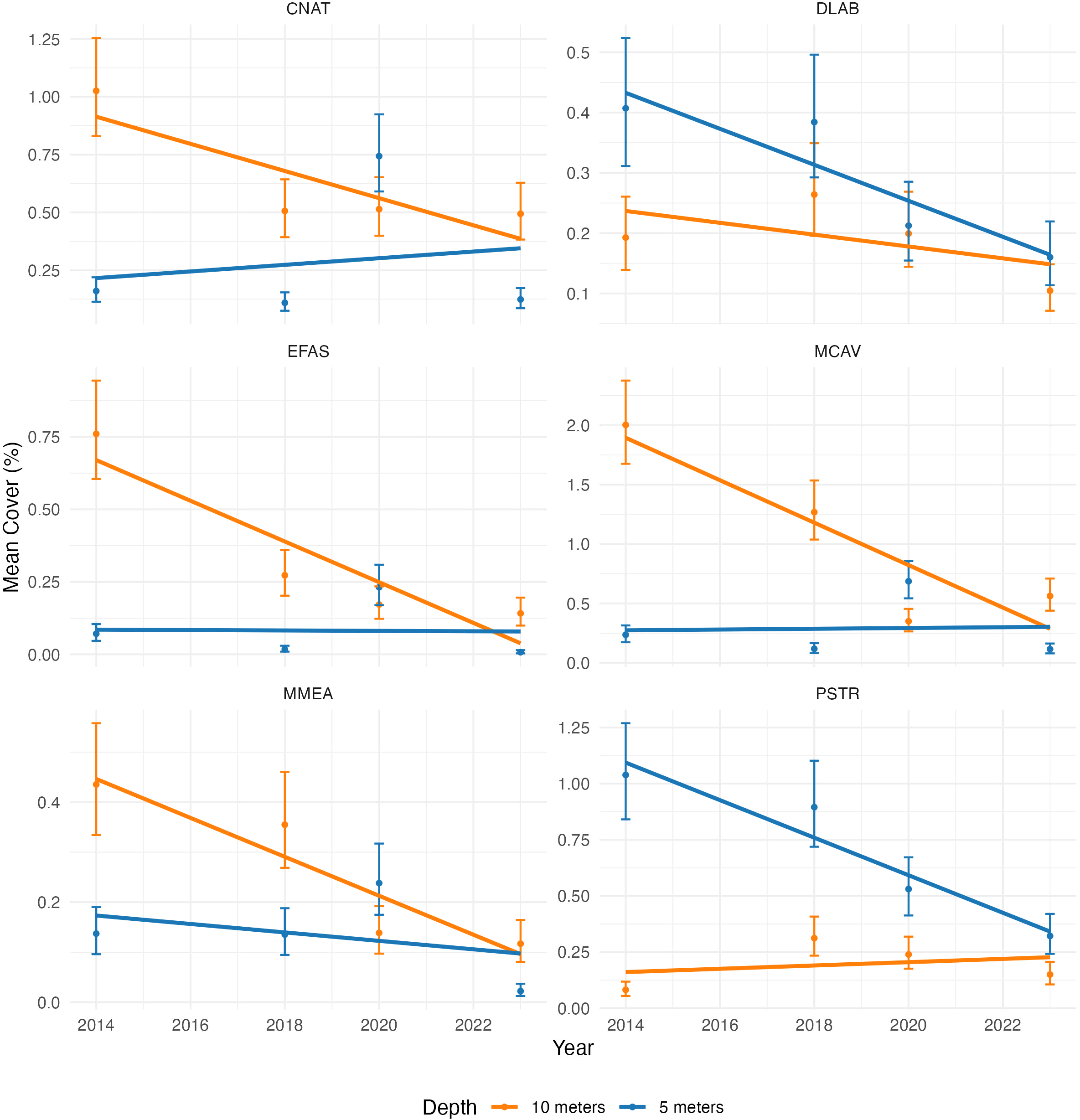
Comparative decline in overall mean coral cover for the six SCTLD-sensitive coral species at Depths of 5 Meters (Orange) and 10 Meters (Blue) from 2014 to 2023. Significant differences are shown when the 95% CI doesn’t overlap.
C. natans (CNAT), predominantly found at 10 meters, exhibits a significant decline in mean cover between 2014 and 2018, stabilizing at approximately 0.5% in 2023. At 5 meters, mean cover values significantly increase in 2020 (from almost 0% to 0.75%), followed by a significant decline to near-zero values by 2023. D. labyrinthiformis (DLAB), predominantly found at 5 meters, shows a significant reduction in mean cover at both depths from 2014 to 2023, with a significant decline observed at 5 meters in 2020. By 2023, mean cover at both depths is at its lowest level. E. fastigiata (EFAS), predominantly found at 10 meters, exhibits a significant decline in mean cover values in 2018 and again in 2023, with mean cover at both 5 and 10 meters reaching their lowest levels by 2023. M. cavernosa (MCAV), predominantly found at 10 meters, shows a significant decline in mean cover at both 5 and 10 meters from 2014, with a significant increase at 5 meters to 0.7% after 2018, followed by a significant decline to 0.1% by 2023. M. meandrites (MMEA), predominantly found at 10 meters, show a significant decline in mean cover at both depths, with a significant drop observed at 10 meters in 2020 and at 5 meters in 2023, where mean cover approaches 0%. P. strigosa(PSTR), predominantly found at 5 meters, experienced a decrease in mean cover values at both depths until recent years but exhibits a significant decline by 2023 at 5 meters, with sharp drops observed in 2020 and again in 2023.
These results indicate a widespread decline in coral cover, with 2023 showing the lowest values for most species and depth combinations. Notably, declines in C. natans, M. cavernosa, and E. fastigiata appear to have started before the arrival of SCTLD in Bonaire, suggesting that other stressors may have contributed to their vulnerability. Aggregating these species and analyzing regional trends will provide deeper insights into the overall impact of SCTLD across Bonaire’s reefs.
3.3 Regional trends
3.3.1 SCTLD-sensitive coral cover
The regional analysis of SCTLD-sensitive coral cover in 2023 reveals a heterogeneous pattern of decline. A significant reduction in SCTLD-sensitive coral cover is observed in the City Limits and Klein Bonaire subregions for all depths and transects, with the majority of transects indicating values of nearly 0% cover (Figures 6, 7). In contrast, when most regions showed a downward trend for both depths, the North subregion displayed stable SCTLD-sensitive coral cover levels between 2014 and 2023 (Figure 8). The other subregions also suggest declines in SCTLD-sensitive coral cover in 2023, although these decreases were not often significant (see Supplementary Figures 10–13).
Figure 6

Trend analysis of SCTLD-sensitive coral species cover in the City Limits region from 2014 to 2023, with separate lines representing depths of 5 meters (5 L/R) and 10 meters (10 L/R). Each point is accompanied by 95% confidence intervals illustrating variability.
Figure 7

Trend analysis of SCTLD-sensitive coral species cover in the Klein Bonaire region from 2014 to 2023, with separate lines representing depths of 5 meters (5 L/R) and 10 meters (10 L/R). Each point is accompanied by 95% confidence intervals illustrating variability.
Figure 8

Trend analysis of SCTLD-sensitive coral species cover in the North region from 2014 to 2023, with separate lines representing depths of 5 meters (5 L/R) and 10 meters (10 L/R). Each point is accompanied by 95% confidence intervals illustrating variability.
3.3.2 Total coral cover
The findings on individual species trends reveal a variable but generally declining pattern in coral cover between 2020 and 2023. However, most of these differences are not significantly more pronounced since the arrival of SCTLD in Bonaire.
Nonetheless, SCTLD-sensitive coral cover values showed spatial heterogeneity, with City Limits and Klein Bonaire the most impacted subregions (Figures 6, 7).
When examining total coral cover in City Limits and Klein Bonaire, a decline is also visible, which may be related to the patterns observed in SCTLD-sensitive species cover (Figures 9, 10). While six out of seven subregions of Bonaire showed no significant difference in total coral cover over the past year(s) (see Supplementary Figures 6–9; Figures 9, 11), Klein Bonaire’s coral community experienced a significant decrease in mean coral cover in 2023, suggesting a more serious issue affecting the reef system (Figure 10). In the City Limits subregion, a decline in total coral cover was evident from 2014 to 2023, with the most pronounced reduction (not significant) occurring in the final year (Figure 9). Spatial patterns of decline, as visualized in Figures 12 and 13, suggest that these impacts may not yet be uniformly distributed across all subregions, with less pronounced changes in the North and the highest decrease in coral cover in the Kralendijk area.
Figure 9
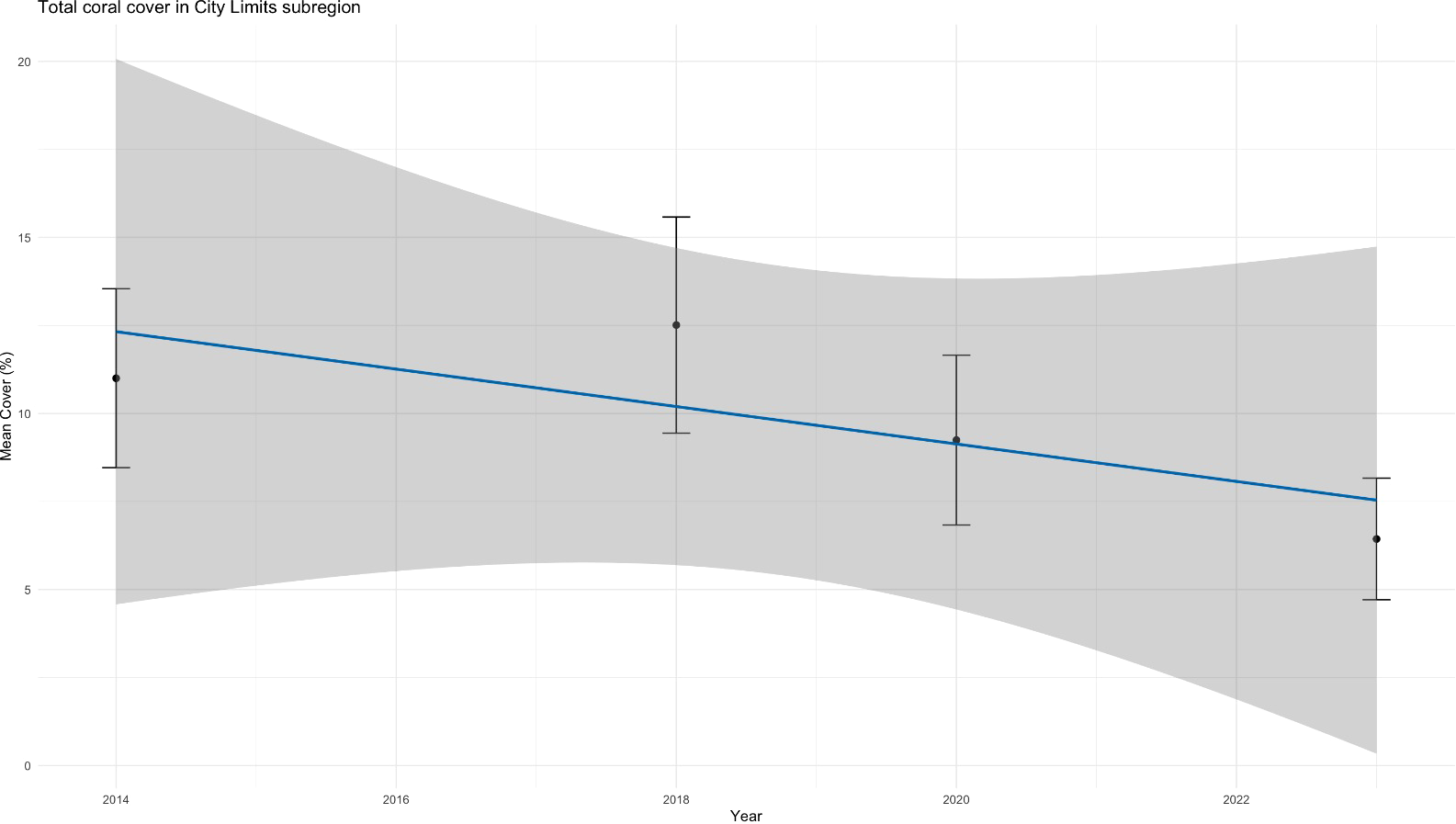
Total coral cover in City Limits subregion from 2014 to 2023, illustrating a significant decline in 2023 compared to earlier years, with the difference being marginally significant compared to 2020.
Figure 10

Total coral cover in Klein Bonaire subregion from 2014 to 2023, illustrating a significant drop between 2023 and all other years, with 95% confidence intervals reflecting the variation within the data.
Figure 11

Total coral cover in Other subregion from 2014 to 2023, illustrating a relatively stable trend over time at sampled depths, with 95% confidence intervals reflecting the variation within the data.
Figure 12

Color map showing the relative decrease in SCTLD-sensitive species coral cover values by subregions between 2023 and 2020. The map was generated using the ‘leaflet’ package in R.
Figure 13

Color map showing the relative changes in total coral cover values by subregions between 2023 and 2020. The map was generated using the ‘leaflet’ package in R.
These results underscore the urgency of addressing the declines in coral cover happening in the year 2023 by identifying potential contributing factors. As we move into discussing the implications of these results, the focus will shift to understanding SCTLD’s impact, especially in the North region where its presence has been minimal during the survey timeframe (STINAPA Bonaire National Parks Foundation, 2024).
4 Discussion
Caribbean coral reefs are facing numerous threats, and SCTLD adds to an already extensive list of coral stressors identified in the region (Gladfelter, 1982; Lessios, 2016). However, SCTLD’s impact is particularly severe, as it affects a broader range of species and exhibits higher prevalence than other diseases, especially in vulnerable coral species. This can lead to rapid ecological shifts and significant degradation of coral reef ecosystems across the Caribbean (Precht et al., 2016). Early detection and monitoring are critical in understanding SCTLD’s spread and impact, as observed in the Virgin Islands and the Bahamas, where its arrival resulted in widespread mortality and severe declines in coral cover (Brandt et al., 2021; Dahlgren et al., 2021).
This study highlights the early impact of SCTLD on Bonaire’s coral reefs, revealing significant species declines and spatial variations that emphasize the complex influence of both disease and environmental stressors. The species analysis shown in Figure 5 suggests that while all examined coral species were likely impacted by SCTLD since its arrival, declines for certain species—such as C. natans, M. cavernosa, and E. fastigiata—may have begun before 2023, indicating that pre-existing stressors, likely made them more vulnerable to the disease (Van Der Geest et al., 2020; Eakin et al., 2019; Meesters et al., 2022; Álvarez-Filip et al., 2022). These historical stressors align with events like the 2014–2017 mass bleaching, which weakened coral populations and likely reduced resilience to subsequent stressors, including SCTLD. Previous studies suggest that corals resistant to bleaching may exhibit compromised immune responses, potentially increasing their susceptibility to subsequent diseases (Merselis et al., 2018). Chronic stressors, including sediment runoff, pollution, and warming sea surface temperatures, further weakened coral vulnerability, particularly for brain corals like C. natans and P. strigosa, which are otherwise thermally resilient (Merselis et al., 2018; Rosenberg and Ben-Haim, 2002). These trends suggest that SCTLD likely added to existing vulnerabilities, accelerating coral declines observed in Bonaire. While bleaching and SCTLD prevalence are not directly linked, the cumulative impact of past stressors likely reduced coral resilience to SCTLD.
The arrival of SCTLD in Bonaire, much like in other regions of the Caribbean, likely exacerbated the decline in coral cover, with all six SCTLD-sensitive species showing consistently low values in 2023, underscoring their susceptibility. This aligns with previous studies that have shown intermediate to high sensitivity among these species to SCTLD, resulting in steep coral cover declines after outbreaks (Williamson et al., 2022; Brandt et al., 2021). The pronounced susceptibility of species like E. fastigiata and M. meandrites, along with the decline of D. labyrinthiformis and P. strigosa at both depths, reflects the widespread impact of SCTLD on coral populations. By 2023, these species reached their lowest recorded cover values within just 6 to 9 months of SCTLD’s arrival in Bonaire’s waters. However, the individual species analysis alone is insufficient to draw definitive conclusions. While SCTLD likely played a significant role in the observed overall low values observed in 2023, attributing these trends solely to the disease is challenging, as many species exhibited declines prior to its documented arrival, likely driven by other stressors.
The spatial analysis reveals that SCTLD’s impact is not uniformly distributed across Bonaire’s coral reefs. Declines in SCTLD-sensitive species were most pronounced in City Limits and Klein Bonaire, which experienced acute drops in coral cover that closely align with SCTLD spread patterns mapped by STINAPA (STINAPA, 2024). In contrast, SCTLD’s impact in the North subregion during the survey period was minimal, suggesting either a lower prevalence of SCTLD or delayed disease progression rather than inherent ecological resistance. Bonaire’s geography, particularly the limited water flow connecting northern reefs with affected areas in the south and central regions, may have slowed SCTLD’s spread.
Declines in total coral cover observed in Klein Bonaire and City Limits also mirror the patterns seen in SCTLD-sensitive species, further underscoring the potential influence of SCTLD. Similar trends have been documented elsewhere in the Caribbean, where SCTLD has caused substantial coral cover losses (Precht et al., 2016; Brandt et al., 2021). However, the lack of widespread declines in total coral cover across other subregions suggests that losses are primarily concentrated among SCTLD-sensitive species, while other coral species have remained relatively stable. While this pattern aligns with the expected impact of SCTLD, further investigation is necessary to rule out additional localized stressors.
Color maps comparing changes in SCTLD-sensitive and total coral cover between 2020 and 2023 (Figures 12, 13) reveal a gradient of coral cover decline radiating from the Kralendijk area toward outer regions. This pattern likely reflects heightened human activity near the port as well as the spread of SCTLD from its initial detection point, moving progressively along reefs over time. Similar patterns of SCTLD transmission have been reported in Florida and the US Virgin Islands, where waterborne currents played a key role in disease spread (Dobbelaere et al., 2020; Muller et al., 2020; Brandt et al., 2021). These findings emphasize the intersection of disease dynamics, physical factors, and anthropogenic stressors in shaping coral reef health (Brandt et al., 2021; Costa et al., 2021; De Bakker et al., 2016; Van Der Geest et al., 2020).
In summary, the species and spatial analyses indicate that SCTLD is a significant factor in the coral decline observed in Bonaire, though pre-existing stressors likely heightened species vulnerability. The steep declines in City Limits and Klein Bonaire call for urgent conservation actions, such as antibiotic treatments (Forrester et al., 2022). These findings not only shed light on SCTLD’s impact in Bonaire but also contribute to the broader understanding of coral diseases across the Caribbean. Continued monitoring and research are essential to unravel the complex interactions between SCTLD, human activity, and environmental factors affecting SCTLD-sensitive coral species.
While this study provides critical insights into the early impact of SCTLD on Bonaire’s coral reefs, certain limitations must be acknowledged. The use of photographic records to estimate coral cover introduces potential biases, as coral density, human bias and the presence of outliers could affect results. Additionally, high variability in species density across regions and depths, along with the skewed nature of the data (heavily inflated with zeros and low proportions), poses challenges for standard statistical models. Although the analysis strongly indicates changes in coral cover, formal hypothesis testing was not applied due to these constraints. Therefore, the results should be interpreted cautiously but remain indicative of SCTLD’s likely role in driving these declines.
These factors are particularly important when comparing differences across species (see Figure 5), where very low densities require careful interpretation. Pre-existing stressors, such as past bleaching events and pollution, complicate efforts to isolate SCTLD’s specific impact during its recent outbreak. The overlap of stressors such as the 2014–2017 bleaching events and other environmental stressors, presents a challenge in disentangling the specific contributions of SCTLD to coral mortality. As previously noted, corals resistant to bleaching may exhibit immune suppression, leaving them more vulnerable to coral diseases like SCTLD (Merselis et al., 2018; Eakin et al., 2019). This overlap between several stressors highlights the difficulty of interpreting disease-related coral cover changes over time and emphasizes the need for further studies to understand SCTLD’s role in the broader context of coral reef degradation.
Furthermore, SCTLD’s selective impact on a subset of coral species complicates correlating disease prevalence with coral cover changes. While the direct causes of coral mortality cannot be fully disentangled due to the absence of colony-level tracking, observations during surveys indicated that corals presenting SCTLD-like lesions or signs of recent mortality belonged to the six SCTLD-sensitive species analyzed. Many of the recently dead colonies exhibited skeletons that were still visible and free from algal overgrowth, indicating very recent mortality. Nearby colonies, or living portions of the same colony, often displayed SCTLD-like lesions, further supporting the likelihood that SCTLD was the primary cause of mortality. The significant declines observed in SCTLD-sensitive species, particularly in Klein Bonaire and City Limits, strongly align with the documented emergence of SCTLD. These patterns suggest that SCTLD acted as a major driver of recent mortality, compounded by pre-existing stressors such as bleaching and anthropogenic impacts.
We leveraged linear trend models and 95% confidence intervals to track changes in coral cover over time. While significant declines were observed in 2023 in several cases (Figures 5–7, 10), more sophisticated analyses could be required to fully interpret these trends and reduce p-values given by the linear models, offering a more comprehensive understanding of the data (See P. strigosa in Figure 5; additional examples in Supplementary Figures 6–13). For example, in the five less-impacted subregions, changes in total coral cover were often not significant. This may be due to the relatively small contribution of SCTLD-sensitive species to overall coral cover, as even notable declines in these species may not be reflected on broader metrics. Incorporating more advanced models in future analyses could help detect statistical differences and changes that may go unnoticed, providing a deeper understanding of SCTLD’s impact across all subregions.
The full extent of SCTLD’s impact has yet to be determined, as it only reached the northern part of Bonaire several months after its first sighting in the Kralendijk area (STINAPA Bonaire National Parks Foundation, 2024). Currently, it affects fewer coral species than anticipated (Precht et al., 2016; Brandt et al., 2021; Costa et al., 2021), with some species that have been severely impacted in other regions showing resistance in Bonaire. Regional differences in SCTLD’s progression and species resilience, as observed in other studies, warrant further investigation, particularly for key reef-building species such as O. faveolata and O. annularis, which have seemingly not yet shown signs of infection by SCTLD in Bonaire (Brandt et al., 2021; Costa et al., 2021; Eaton et al., 2021; STINAPA Bonaire National Parks Foundation, 2024). Additionally, species like Dendrogyra cylindrus, Dichocoenia stokesi, and Porites spp., while highly susceptible to SCTLD elsewhere, are found in very low densities in Bonaire and were therefore almost absent from our dataset. This highlights the importance of focusing on locally dominant species to better understand SCTLD’s ecological impact in Bonaire.
Ongoing monitoring is essential to track SCTLD’s progression among the six highly SCTLD-sensitive coral species and to detect any emerging symptoms in other species. Additionally, future research should explore other stressors impacting these species, such as thermal stress, pollution, and runoff (Eakin et al., 2019; Meesters et al., 2022; Van Der Geest et al., 2020). While slow-growing brain corals are typically thermally resistant, they are not immune to SCTLD, which has reduced their populations and weakened their resilience to climate change (Álvarez-Filip et al., 2022).
For example, P. strigosa, once showing signs of recovery (De Bakker et al., 2016), has suffered significant declines in 2023, likely due to SCTLD, highlighting a concerning shift in reef diversity. The population dynamics of P. strigosa and other SCTLD-sensitive species will require close attention and monitoring to assess their long-term viability.
Our findings, which detail the immediate and mid-term effects of SCTLD on key coral species, contribute to the broader understanding of stressors affecting Bonaire’s reefs and inform targeted restoration efforts. Future research could expand on these results by correlating coral mortality with water quality parameters, offering deeper insights into SCTLD’s spread and the influence of human activity.
5 Conclusion
This study provides the first systematic assessment of the impact of Stony Coral Tissue Loss Disease (SCTLD) on Bonaire’s SCTLD-sensitive coral species, capturing the disease’s early effects on local reefs. The research revealed a significant decline in coral cover across multiple species, particularly in 2023, marking SCTLD’s detrimental influence on Bonaire’s reefs. Importantly, the decline was not uniformly distributed, with certain subregions like City Limits and Klein Bonaire experiencing the most severe reductions, while others, such as the North, exhibited a degree of resilience.
The findings emphasize the vulnerability of key reef-building species, M. meandrites, E. fastigiata, M. cavernosa, P. strigosa, C. natans, and D. labyrinthiformis, which have shown significant susceptibility to SCTLD. This highlights the broader ecological concern of the disease, particularly for species previously recognized for their resilience to other stressors like thermal stress. The spatial variability observed underscores the potential need for targeted conservation efforts that take into account regional differences in coral health and disease prevalence.
Moving forward, long-term monitoring is essential to track the continued progression of SCTLD and to identify potential resilience mechanisms within certain coral species. Additionally, further research should explore the role of environmental and anthropogenic factors, such as water quality and human activity, in exacerbating or mitigating the disease’s spread. This understanding will be critical for developing effective management strategies aimed at preserving Bonaire’s coral reefs.
Ultimately, the health of these ecosystems is not only an ecological priority but also crucial for the island’s economy and community well-being. This research contributes to the broader global effort to protect coral reefs in the face of emerging diseases, offering valuable insights for both scientific understanding and conservation practices.
Statements
Data availability statement
The datasets generated for this study are included in the article and Supplementary Material. Additional raw data, such as Excel sheets with coral cover percentages, will be made available upon reasonable request to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. VR: Data curation, Methodology, Writing – original draft, Writing – review & editing. OV: Data curation, Methodology, Writing – original draft, Writing – review & editing. SN: Data curation, Methodology, Writing – original draft, Writing – review & editing. EM: Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was carried out by Wageningen Marine Research and co-financed by the Ministry of Agriculture, Fisheries, Food Security for the purposes of Policy Support Research Theme ‘E2 Natuur-inclusieve landbouw, visserij en waterbeheer in Caribisch Nederland’ (project no. BO-43-117-002).
Acknowledgments
Professor Appy Sluijs of Utrecht University is thanked for his role as the internal supervisor of this project. The fieldwork was supported by Dive Friends Bonaire through discounted tank rentals, and most importantly STINAPA Bonaire, which provided a boat, a captain, and funding for our surveys. Volunteers Alain Hoffman and Richard Schwerdtfeger, are also thanked for their assistance and availability to help during surveys.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1512371/full#supplementary-material
References
1
Abdelhafez O. H. Fahim J. R. Desoukey S. Y. Kamel M. S. Abdelmohsen U. R. (2019). Recent updates on corals from nephtheidae. Chem. Biodiversity16. doi: 10.1002/cbdv.201800692
2
Adobe Systems Inc (2024). Adobe lightroom desktop (Version 2024). Available online at: https://www.adobe.com/products/photoshop-lightroom.html (Accessed November 02, 2023).
3
Aeby G. S. Ushijima B. Campbell J. E. Jones S. Williams G. J. Meyer J. L. (2019). Pathogenesis of a tissue loss disease affecting multiple species of corals along the florida reef tract. Front. Mar. Sci.6, 678. doi: 10.3389/fmars.2019.00678
4
Álvarez-Filip L. Estrada-Saldívar N. Pérez-Cervantes E. Molina-Hernández A. González-Barrios F. J. (2019). A rapid spread of the stony coral tissue loss disease outbreak in the Mexican Caribbean. PeerJ7, e8069. doi: 10.7717/peerj.8069
5
Álvarez-Filip L. González-Barrios F. J. Pérez-Cervantes E. Molina-Hernández A. Estrada-Saldívar N. (2022). Stony coral tissue loss disease decimated Caribbean coral populations and reshaped reef functionality5, 440. doi: 10.1038/s42003-022-03398-6
6
Aronson R. B. Precht W. F. (2001). White-band disease and the changing face of Caribbean coral reefs (Dordrecht, Netherlands: Kluwer Academic Publishers), 25–38. doi: 10.1007/978-94-017-3284-0_2
7
Atlantic and Gulf Rapid Reef Assessment (AGGRA) . (2024) AGGRA protocols and data. Available at: https://www.agrra.org (Accessed January 10, 2024).
8
Bak R. P. M. Criens S. R. (1981). Survival after fragmentation of colonies of Madracis mirabilis, Acropora palmata, and Agaricia agaricites (Scleractinia) and the subsequent impact of a coral disease. Proc. 4th Int. Coral Reef Symposium2, 221–227.
9
Becker C. C. Ushijima B. Häse C. C. (2021). Bacterial associations with stony coral tissue loss disease in Florida corals. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.000675
10
Beijbom O. Edmunds P. J. Roelfsema C. M. Smith J. E. Kline D. I. Neal B. P. et al . (2015). Towards automated annotation of benthic survey images: variability of human experts and operational modes of automation. PloS One10, e0130312. doi: 10.1371/journal.pone.0130312
11
Bertness M. D. Gaines S. D. Hay M. E. (2001). Marine community ecology. Available online at: https://ci.nii.ac.jp/ncid/BA50743656 (Accessed January 10, 2024).
12
Brandt M. E. Ennis R. S. Meiling S. S. Townsend J. E. Cobleigh K. Glahn A. et al . (2021). The emergence and initial impact of stony coral tissue loss Disease (SCTLD) in the United States Virgin Islands. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.715329
13
Brathwaite A. Clua E. Roach R. Pichon N. (2022). Coral reef restoration for coastal protection: Crafting technical and financial solutions. J. Environ. Manage.310, 114718. doi: 10.1016/j.jenvman.2022.114718
14
Cheng J. Karambelkar B. Xie Y. (2023). leaflet: Create interactive web maps with the JavaScript ‘Leaflet’ library (Version 2.1.2) [R package] (CRAN). Available at: https://cran.r-project.org/package=leaflet (Accessed January 15 2024).
15
Clark A. S. Guy C. M. Voss J. D. (2021). Disease-associated microbiomes of stony coral tissue loss disease-affected, bleached, and healthy corals in the Florida Keys. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.667
16
Costa S. V. Hibberts S. J. Olive D. A. Budd K. Long A. E. Meiling S. S. et al . (2021). Diversity and Disease: The effects of coral diversity on prevalence and impacts of stony coral tissue loss disease in Saint Thomas, U.S. Virgin Islands. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.682688
17
Cróquer A. Weil E. Rogers C. S. (2021). Similarities and differences between two deadly Caribbean coral diseases: white plague and stony coral tissue loss disease. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.709544
18
Dahlgren C. P. Sherman K. D. Lang J. C. Kramer P. R. (2021). Stony coral tissue loss disease in the caribbean: Current status and management. Nat. Conservancy.
19
De Bakker D. M. Meesters E. H. Bak R. P. M. Nieuwland G. Van Duyl F. C. (2019). Long-term shifts in coral communities on shallow to deep reef slopes of curaçao and bonaire: The rise of octocorals as major reef builders. Front. Mar. Sci.6, 66. doi: 10.3389/fmars.2019.00066
20
De Bakker D. M. Meesters H. Bak R. P. M. Nieuwland G. Van Duyl F. C. (2016). Long-term shifts in coral communities on shallow to deep reef slopes of Curaçao and Bonaire: Are there any winners? Front. Mar. Sci.3. doi: 10.3389/fmars.2016.00247
21
De Bakker D. M. Van Duyl F. C. Bak R. P. M. Nugues M. M. Nieuwland G. Meesters H. (2017). 40 Years of benthic community change on the Caribbean reefs of Curaçao and Bonaire: the rise of slimy cyanobacterial mats. Coral Reefs36, 355–367. doi: 10.1007/s00338-016-1534-9
22
Dobbelaere T. Muller E. M. Gramer L. J. Holstein D. M. Hanert E. (2020). Coupled Epidemio-Hydrodynamic modeling to understand the spread of a deadly coral disease in Florida. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.591881
23
Eakin C. M. Sweatman H. Brainard R. E. (2019). The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs38, 539–545. doi: 10.1007/s00338-019-01844-2
24
Eaton K. R. Landsberg J. H. Kiryu Y. Peters E. C. Muller E. M. (2021). Measuring stony coral tissue loss disease induction and lesion progression within two intermediately susceptible species, Montastraea cavernosa and Orbicella faveolata. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.717265
25
Florida Department of Environmental Protection . (2018). Case definition: Stony coral tissue loss disease (SCTLD). Available at: https://floridadep.gov/sites/default/files/Copy%20of%20StonyCoralTissueLossDisease_CaseDefinition%20final%2010022018.pdf (Accessed January 10, 2024).
26
Forrester G. E. Arton L. Horton A. Nickles K. Forrester L. M. (2022). Antibiotic treatment ameliorates the impact of stony coral tissue loss disease (SCTLD) on coral communities. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.859740
27
Gladfelter W. B. (1982). White-band disease in acropora palmata: Implications for the structure and growth of shallow reefs. Bull. Mar. Sci.32, 639–643.
28
Graham N. A. J. Chong-Seng K. M. Huchery C. Januchowski–Hartley F. A. Nash K. L. (2014). Coral reef community composition in the context of disturbance history on the great barrier reef, Australia. PloS One9, e101204. doi: 10.1371/journal.pone.0101204
29
Hernández-Delgado E. A. Weil E. (2019). Spread of the new coral disease “SCTLD” into the Caribbean: implications for Puerto Rico. Reef Encounter, pp. 38–43. Available at: https://www.academia.edu/41578839/Spread_of_the_new_coral_disease_SCTLD_into_the_Caribbean_implications_for_Puerto_Rico.
30
Hill J. Wilkinson C. (2004). Methods for ecological monitoring of coral reefs: A resource for managers. Available at: https://www.aims.gov.au/docs/research/monitoring/reef/monitoring-methods.html (Accessed January 2024).
31
Høegh-Guldberg O. Mumby P. J. Hooten A. J. Steneck R. S. Greenfield P. F. Gomez E. D. et al . (2007). Coral reefs under rapid climate change and ocean acidification. Science318, 1737–1742. doi: 10.1126/science.1152509
32
Jones N. P. Kabay L. Lunz K. S. Gilliam D. S. (2021). Temperature stress and disease drives the extirpation of the threatened pillar coral, Dendrogyra cylindrus, in southeast Florida. Sci. Rep.11, 1–8. doi: 10.1038/s41598-021-93111-0
33
Kjerfve B. McField M. Thattai D. Giró A. (2021). Coral reef health in the Gulf of Honduras in relation to fluvial runoff, hurricanes, and fishing pressure. Mar. pollut. Bull.172, 112865. doi: 10.1016/j.marpolbul.2021.112865
34
Lessios H. A. (2016). The great diadema antillarum die-off: 30 years later. Annu. Rev. Mar. Sci.8, 267–283. doi: 10.1146/annurev-marine-122414-033857
35
Mallela J. Crabbe M. J. C. (2009). Hurricanes and coral bleaching linked to changes in coral recruitment in Tobago. Mar. Environ. Res.68, 158–162. doi: 10.1016/j.marenvres.2009.06.001
36
Meesters E. H. Bak R. (1993). Effects of coral bleaching on tissue regeneration potential and colony survival. Mar. Ecol. Prog. Ser.96, 189–198. doi: 10.3354/meps096189
37
Meesters H. De Hart M. Dogruer G. (2022). An assessment of sand quality and potential impacts on corals at the Chogogo Dive and Beach Resort artificial beach. (Den Helder, Netherlands: Wageningen Marine Research). doi: 10.18174/579171
38
Merselis D. G. Lirman D. Rodríguez-Lanetty M. (2018). Symbiotic immuno-suppression: is disease susceptibility the price of bleaching resistance? PeerJ6, e4494. doi: 10.7717/peerj.4494
39
Meyer J. L. Castellanos-Gell J. Aeby G. S. Häse C. C. Ushijima B. Paul V. J. (2019). Microbial community shifts associated with the ongoing stony coral tissue loss disease outbreak on the florida reef tract. Front. Microbiol.10, 2244. doi: 10.3389/fmicb.2019.02244
40
Moberg F. Rönnbäck P. (2003). Ecosystem services of the tropical seascape: interactions, substitutions and restoration. Ocean Coast. Manage.46, 27–46. doi: 10.1016/s0964-5691(02)00119-9
41
Muller E. M. Sartor C. Alcaraz N. I. Van Woesik R. (2020). Spatial epidemiology of the stony-coral-tissue-loss disease in florida. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00163
42
Neely K. L. Macaulay K. A. Hower E. K. Dobler M. A. (2020). Efficacy of topical antibiotics in treating corals affected by stony coral tissue loss disease. Sci. Rep.10, 12479. doi: 10.1038/s41598-020-69321-5
43
Newman S. P. Meesters H. Dryden C. Williams S. M. Sánchez C. Mumby P. J. et al . (2015). Reef flattening effects on total richness and species responses in the Caribbean. J. Anim. Ecol.84, 1678–1689. doi: 10.1111/1365-2656.12429
44
Parsons G. R. Thur S. M. (2007). Valuing changes in the quality of coral reef ecosystems: A Stated preference study of SCUBA Diving in the Bonaire National Marine Park. Environ. Resource Economics40, 593–608. doi: 10.1007/s10640-007-9171-y
45
Precht W. F. Gintert B. Robbart M. L. Fura R. Van Woesik R. (2016). Unprecedented Disease-Related coral mortality in southeastern Florida. Sci. Rep.6, 1–9. doi: 10.1038/srep31374
46
R Core Team . (2023). R: A language and environment for statistical computing (Version 4.3.1) [Computer software] (R Foundation for Statistical Computing). Available at: https://www.R-project.org/ (Accessed January 10, 2024).
47
Reaka-Kudla M. L. (1997). “The global biodiversity of coral reefs: A comparison with rainforests,” in Biodiversity II: Understanding and protecting our biological resources. Eds. Reaka-KudlaM. L.WilsonD. E.WilsonE. O. (Washington, D.C.: Joseph Henry Press), 83–108.
48
Rosales S. M. Miller M. W. Williams D. E. (2022). Differential microbial community responses to stony coral tissue loss disease in corals of the Florida Reef Tract. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.00148
49
Rosales S. M. Thurber R. V. Muller E. M. (2023). Longitudinal analysis of microbiomes from corals infected with stony coral tissue loss disease. ISME J.17, 350–362. doi: 10.1038/s41396-022-01276-y
50
Rosenau N. A. Gignoux-Wolfsohn S. Everett R. A. Miller A. W. Minton M. S. Ruiz G. M. (2021). Considering commercial vessels as potential vectors of stony coral tissue loss disease. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.709764
51
Rosenberg E. Ben-Haim Y. (2002). Microbial diseases of corals and global warming. Environ. Microbiol.4, 318–326. doi: 10.1046/j.1462-2920.2002.00302.x
52
Shilling E. N. Muller E. M. Baker A. C. (2021). Evaluation of the antibiotic amoxicillin as a treatment for stony coral tissue loss disease in corals. Sci. Rep.11, 1219. doi: 10.1038/s41598-020-80357-3
53
STINAPA Bonaire National Parks Foundation (2024). Bonaire national marine park. Available online at: https://stinapabonaire.org/bonaire-national-marine-park/ (Accessed December 15, 2023).
54
Studivan M. S. Baptist M. Molina V. Riley S. C. First M. R. Soderberg N. et al . (2022). Transmission of stony coral tissue loss disease (SCTLD) in simulated ballast water confirms the potential for ship-born spread. Sci. Rep.12, 1–11. doi: 10.1038/s41598-022-21868-z
55
Studivan M. S. Hatch W. I. Voss J. D. (2020). Reef sediments as a potential vector for stony coral tissue loss disease. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.614700
56
Thurber R. V. Mydlarz L. D. Brandt M. Harvell D. Weil E. Raymundo L. et al . (2020). Deciphering coral disease dynamics: integrating host, microbiome, and the changing environment. Front. Ecol. Evol.8. doi: 10.3389/fevo.2020.575927
57
Uyarra M. C. Watkinson A. R. Côté I. M. (2008). Managing dive tourism for the Sustainable use of coral reefs: Validating diver perceptions of attractive site features. Environ. Manage.43, 1–16. doi: 10.1007/s00267-008-9198-z
58
Van Der Geest M. Meesters H. Mücher S. (2020). Impact of terrestrial erosion on coral reef health at Bonaire: a plea for nature-inclusive “watershed-to-reef” based coastal management. Netherlands: Wageningen Marine Research. doi: 10.18174/524688
59
Van Woesik R. Randall C. J. (2017). Coral disease hotspots in the Caribbean. Ecosphere8, 1–8. doi: 10.1002/ecs2.1814
60
Walker B. K. Pitts K. Dodge R. E. (2021). Mitigating stony coral tissue loss disease through an adaptive management framework. Ecol. Appl.31, e02391. doi: 10.1002/eap.2391
61
Wickham H. (2016). ggplot2: Elegant graphics for data analysis (Springer-Verlag). doi: 10.1007/978-3-319-24277-4
62
Williamson O. M. Dennison C. E. O’Neil K. L. Baker A. C. (2022). Susceptibility of Caribbean brain coral recruits to stony coral tissue loss disease (SCTLD). Front. Mar. Sci.9. doi: 10.3389/fmars.2022.821165
63
Work T. M. Aeby G. S. Maragos J. E. (2021). Viral-like particles are associated with endosymbiont pathology in Florida corals affected by stony coral tissue loss disease. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.750658
Summary
Keywords
coral reef decline, Bonaire coral health, coral disease outbreak, stony coral tissue loss disease (SCTLD), Dutch Caribbean Marine Conservation, Wageningen Marine Research
Citation
Pepe BA, Van der Roest V, Vlam O, Nouse S and Meesters E (2025) Assessing the impact of stony coral tissue loss disease on coral cover on Bonaire’s Leeward side. Front. Mar. Sci. 11:1512371. doi: 10.3389/fmars.2024.1512371
Received
16 October 2024
Accepted
11 December 2024
Published
10 January 2025
Volume
11 - 2024
Edited by
William F. Precht, Bio-Tech Consulting, United States
Reviewed by
Erin Papke, University of North Carolina Wilmington, United States
Jason Croop, Consultant, Miami, United States
Updates
Copyright
© 2025 Pepe, Van der Roest, Vlam, Nouse and Meesters.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernardo A. Pepe, Bernardo.a.pepe@outlook.com; Erik Meesters, erik.meesters@wur.nl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.