- 1Centre for Sustainable Aquatic Ecosystems, Harry Butler Institute, Murdoch University, Murdoch, WA, Australia
- 2Australian Institute of Marine Science, Indian Ocean Marine Research Centre, University of Western Australia, Crawley, WA, Australia
- 3Environmental and Conservation Sciences, Murdoch University, Murdoch, WA, Australia
- 4Edith Cowan University, School of Science, Joondalup, WA, Australia
- 5Flinders Accelerator for Microbiome Exploration, College of Science and Engineer, Flinders University, Bedford Park, SA, Australia
- 6Oceans Institute, University of Western Australia, Crawley, WA, Australia
Very little is known about the reproductive behaviours of whale sharks (Rhincodon typus). Here, we describe field observations of courtship behaviour by a whale shark at Ningaloo Reef, Western Australia. We witnessed and recorded following and biting behaviours by a sexually mature male of a smaller female. Following and biting are common events during courtship and copulation of other species of elasmobranchs. Our observations are consistent with earlier reports of courting behaviours in whale sharks provided by fishers, the pilot of a light plane and observations of courtship by a sexually mature male towards females held in aquaria.
Introduction
Whale sharks (Rhincodon typus) aggregate in coastal waters at tropical and warm temperate locations around the world (Araujo et al., 2022). Although these near-shore aggregations provide the opportunity to access these animals for research, they are typically dominated by juvenile males (with some exceptions (see summaries: Norman et al., 2017; Araujo et al., 2022). For this reason, research on the ecology of the species tends to be based on data that are biased towards this subset of the population. Our knowledge of adult whale sharks, particularly their reproductive and behavioural ecology, is largely derived from chance observations of sharks caught by fisheries or from animals held in aquaria (Pierce et al., 2021). For example, the only pregnant shark that has ever been documented was harvested by a (now closed) Taiwanese fishery in 1994 (Joung et al., 1996; Hsu et al., 2012). This individual showed that the species is ovoviviparous and contained 300 young in various stages of development (Joung et al., 1996). Large female whale sharks with distended abdomens thought to be indicative of pregnancy have been recorded in the waters of Holbox Island, Mexico (Ramírez-Macías et al., 2012) Baja California Peninsula, Mexico (Ramírez-Macías et al., 2007), the Archipelago of São Pedro and São Paulo, Brazil (Macena and Hazin, 2016), the Galapagos Archipelago, Ecuador (Acuña-Marrero et al., 2014; Hearn et al., 2016), and at St. Helena, Central South Atlantic (Perry et al., 2020). However, hormone testing and ultrasound examination of these females in the Galapagos found them to be sexually mature, but not pregnant (Matsumoto et al., 2023). Thus, to date, the female captured in Taiwan remains the sole doumented example of pregnancy in the species.
Mating and courting behaviours of whale sharks have been observed in the wild at two locations. In the Atlantic Ocean, aggregations of whale sharks occur in the waters surrounding St Helena Islands and can include sexually mature males and females (Perry et al., 2020). Fishers have provided anecdotal evidence of male whale sharks in this aggregation swimming alongside females and rotating ventral side up in a ‘belly to belly’ position below the female, probably to insert their claspers (Perry et al., 2020). Researchers also reported what was presumed to be courtship behaviour, where a male followed a female and nudged the caudal fin of the female (Perry et al., 2020). At Ningaloo Reef, Western Australia, the pilot of a light plane photographed a larger (estimated size 9 m) whale shark attempting to grasp the pectoral fins of a smaller (estimated size 6.5 m) individual, and orient ventral-side-up below the smaller shark. As this was observed from the air, it could not be confirmed that the interaction involved a male and female shark (Gudgeon, 2019). Within aquaria, mating attempts by mature males towards unreceptive, immature females have shown the same processes of body inversions, biting of females, and the additional insight of clasper flexion (Pierce et al., 2021).
Given the rarity of observations of reproductive behaviour in whale sharks, any reports provide valuable insights into the ecology of this Endangered species (International Union for the Conservation of Nature Red List; Pierce and Norman, 2016). Here, we describe observations of what is assumed to be courtship behaviour between a mature male shark and a female of indeterminate sexual maturity in the waters off Ningaloo Reef in Western Australia.
Methods
Annual two-week field expeditions in early May (the peak of the seasonal aggregation of whale sharks) occurred at Ningaloo Reef, Western Australia, from 2009-2024 (Supplementary Table S1). A light plane was used to spot whale sharks and to direct our research vessel towards a sighting. Once in the vicinity of the shark, the research team entered the water to record body dimensions of sharks with stereo cameras (Sequeira et al., 2016; Meekan et al., 2020), take images for photo identification (Speed et al., 2007; Lester et al., 2020), use ultrasound scanners for internal images (Meekan et al., 2024), collect eDNA (Dugal et al., 2022), deploy satellite tags to understand movement behaviour (Sleeman et al., 2010; Thums et al., 2013; Meekan et al., 2015; D’Antonio et al., 2024) and collect tissue biopsies and parasitic copepods for trophic studies (Marcus et al., 2019; Meekan et al., 2022; Osorio et al., 2023). When we could obtain a clear view of the pelvic fins, we recorded the sex of each shark based on the presence or absence of claspers (Awruch et al., 2008). We recorded males as mature when their claspers were calcified and extended beyond the trailing edge of the pelvic fins (Norman and Stevens, 2007). We determined female sexual maturity based on total length (> 10 m; Nozu et al., 2015; Meekan et al., 2020; Matsumoto et al., 2023). If we were unable to observe the pelvic fins before the shark departed from surface waters the sex was recorded as unknown.
A sex ratio of males to females was calculated from the annual sightings data. Repeated sightings of the same sharks within each season of field work (identified by the presence of a recent scar from a tissue biopsy and by photo-identification) and sharks of unknown sex (approximately 20% of individuals) were excluded from this analysis.
Results
On the 14th of May 2024, our research vessel (Isurus; 7 m length) was directed by the spotter plane to a whale shark sighted at the surface offshore of the reef slope of Ningaloo Reef in waters ~ 40 m deep (22° 04’ 80” S 113° 52’ 51” E). Researchers entered the water at 13:15 AWST to sample a 7 m (total length) female whale shark. After length and photo-ID measurements were collected, a second whale shark, identified as a male, appeared and swam 2-3 m behind the caudal fin of the female. Photo-ID and length measurements of this male shark were also collected. The calcified claspers (Figure 1A) and size of the male (8.5 m) suggested that it was an adult (Norman and Stevens, 2007; Meekan et al., 2020). The behaviour of the male following the female was recorded by video (GoPro V. 11; Supplementary Video S1A; Figure 1B). One researcher also sampled the skin microbiome of the male (see Doane et al., 2023 for methods), during which time it maintained a close trajectory behind the female shark and appeared to be indifferent to the activity of the researcher (Supplementary Video S1B). Once the sampling was complete, researchers followed the male whale shark, which had remained swimming close to the caudal fin of the female. The male was observed to open its mouth and lunge forward towards the caudal fin of the female. The male shark increased its swimming speed and lunged again at the caudal fin of the female, this time making contact and briefly biting the tail (Supplementary Video S1C). The female responded by rapidly pivoting with pectoral fins pointing downwards to face the male (Supplementary Video S1C; Figure 1C). After a brief pause in forward movement, the female again turned rapidly, with contact occurring between the snout of the male and the caudal fin of the female (Figure 1D). The female then rapidly descended to depth followed by the male (Supplementary Video S1C). At this point, the researchers lost sight of the sharks from the surface.
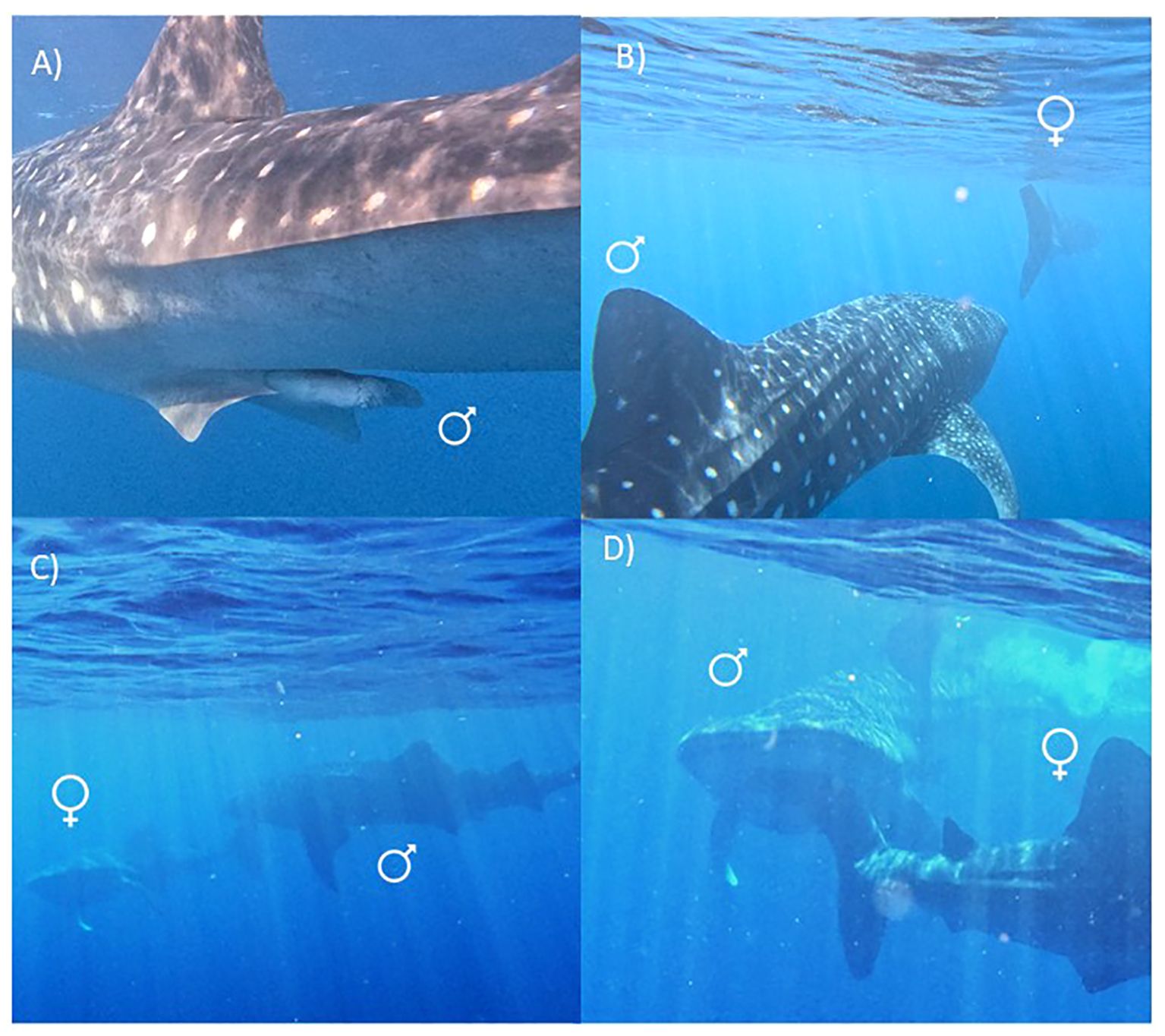
Figure 1. Whale sharks (Rhincodon typus) engaging in possible courtship behaviors at Ningaloo Reef. (A) Calcified clasper of the male shark. (B) An ~8.5 m male whale shark following closely behind a ~7 m female whale shark. (C) The female shark (left) rapidly pivoting after being bitten on the caudal fin by the male (right). (D) The caudal fin of the female whale shark (right) bending as it makes contact with the snout of the male shark (left). Key: ♀ = female, ♂ = male.
We recorded the sex of 1047 whale sharks at Ningaloo Reef across 2014-2024 (Supplementary Table S1). The mean sex ratio during this time was one female to three males (Figure 2).
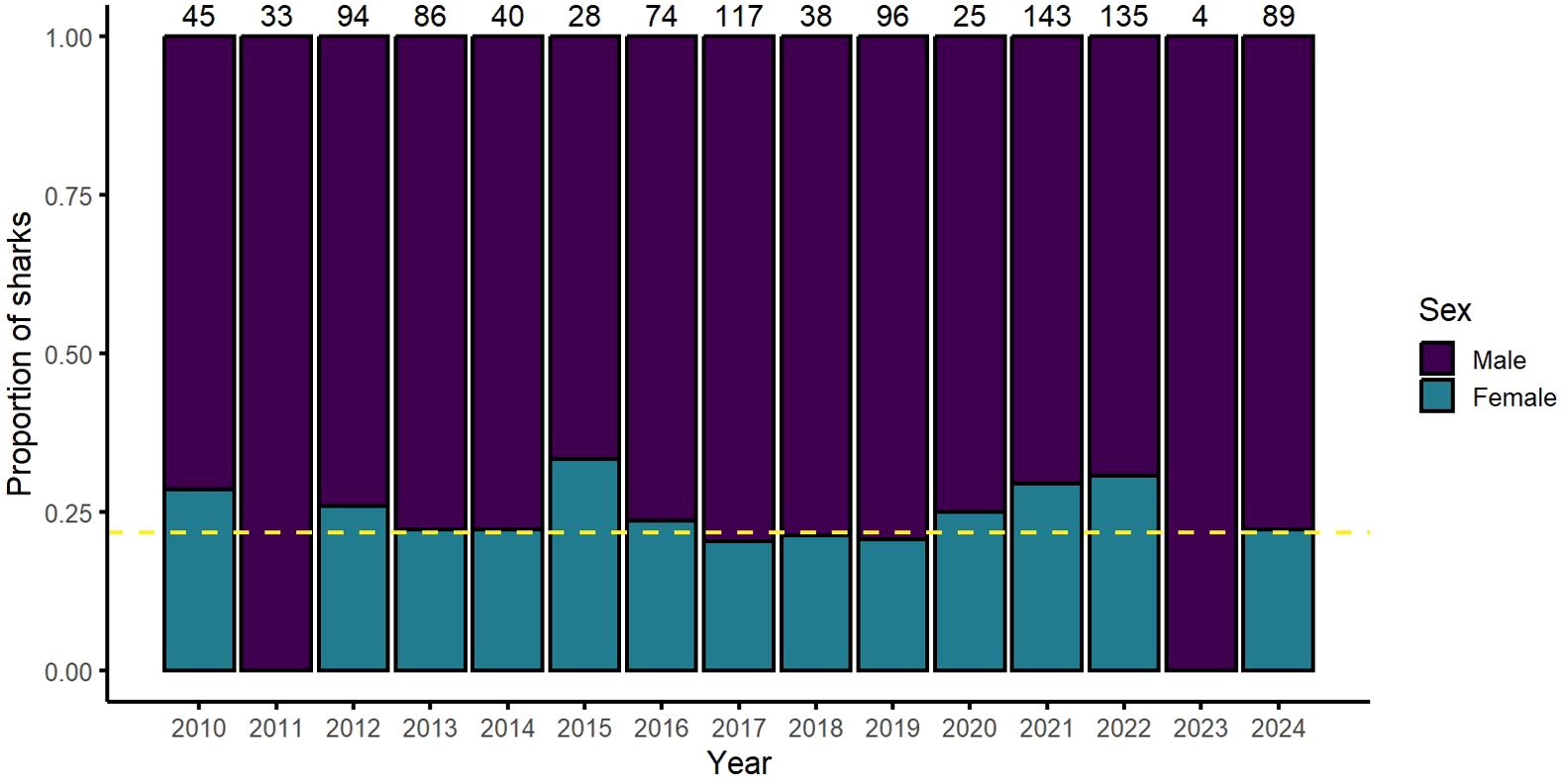
Figure 2. The proportionate sex of whale sharks (Rhincodon typus) encountered on annual research expeditions (see Supplementary Table S1) from 2010-2024. The yellow dashed line (x = 0.22) represents the mean sex ratio across the sampling period. Numbers along the top represent the number of individual whale sharks where sex was identified per year.
Supplementary Video S1 available at: https://youtu.be/PhLx_7NHGCQ
Discussion
The pre-copulatory behaviours of male sharks commonly include the following and biting of females (Parsons et al., 2008). Our observations of whale sharks at Ningaloo are very similar to pre-copulatory behaviours witnessed in this species in aquaria and reported by fishers in the waters around St Helena Island (Macena and Hazin, 2016; Gudgeon, 2019; Perry et al., 2020; Pierce et al., 2021). Similar behaviours have also been reported for many other species, including scalloped hammerhead, Sphyrna lewini (Salinas-de-León et al., 2017), oceanic white tip, Carcharhinus longimanus (Talwar et al., 2023), tiger, Galeocerdo cuvier (Rangel et al., 2023) and basking Cetorhinus maximus (Curtis et al., 2024) sharks. Notably, male zebra sharks (Stegostoma fasciatum) – the closest living relative to whale sharks – have also been observed biting the tails of female zebra sharks in an effort to slow the movement of the female down, likely so they can position themselves for clasper insertion (Birt et al., 2019). The consistency of our observations with earlier reports of courtship in whale sharks and a wide variety of other species is evidence that we witnessed pre-copulatory behaviour.
At Ningaloo Reef, the behaviours we observed and those reported by Gudgeon (2019) did not appear to result in successful mating. Although it was possible that mating could have occurred after the sharks that we followed descended to deep water, the female appeared to actively resist the attention of the male. This is not surprising as the females in our study and Gudgeon (2019) report were unlikely to be sexually mature, based on their small sizes (6 - 7 m). These are much smaller than sizes at which maturity for female whale sharks is thought to occur (~ 10 - 12 m; Nozu et al., 2015; Meekan et al., 2020; Matsumoto et al., 2023). Ningaloo (and many other locations) is male dominated, a pattern consistent across several decades (Meekan et al., 2006; Norman and Stevens, 2007). Studies between 1992 – 2004 estimated that only 15% (Norman and Stevens, 2007) and 17% (Meekan et al., 2006) of the sharks sighted at Ningaloo Reef were females, with a sex ratio of approximately 1:5 females to males. More recent data collected on our sampling trips between 2010-2024 found that on average, 22% of the sharks we encountered were females, a ratio of approximately 1:3 females to males (Figure 2). As the sex ratio of males and females at birth is roughly 1:1, as it is for most other species of sharks (Schmidt et al., 2010), this implies that some female whale sharks may be actively avoiding aggregation sites.
Segregation of habitats by size and sex is typical of many populations of elasmobranchs (Speed et al., 2010; Klimley et al., 2023; Wearmouth and Sims, 2010; Sims, 2005) and for juvenile female whale sharks, the energetic costs of unwanted attention from males may be one reason that there is a strong male bias at aggregation sites. This idea is consistent with social factor hypotheses (Wearmouth and Sims, 2008) as drivers of spatial patterns of sexual segregation in elasmobranchs. However, a variety of other hypotheses have been proposed to account for this spatial phenomenon (Wearmouth and Sims, 2008), which is very common in elasmobranchs (Wearmouth and Sims, 2010). Of these, the predation-risk hypothesis involves sharks segregating to avoid intra-species depredation from the larger sex and cannibalism of young (Wearmouth and Sims, 2008) and is obviously not applicable for a species that is a filter-feeder. Similarly, the thermal niche-fecundity hypothesis, which suggests that females choose different thermal habitats to increase reproductive success (Wearmouth and Sims, 2008) and the activity budget hypothesis, which argues that sexual dimorphisms and/or resource allocation into reproduction leads to different habitat requirements (Wearmouth and Sims, 2008) are also unlikely, given that the females at Ningaloo are mostly immature (with no mature length females recorded in our data) and have been observed feeding with males. Although these hypotheses can be discounted, it is still possible that there is some other advantage for females that choose to forage offshore (the foraging selection hypothesis; Wearmouth and Sims, 2008), beyond the avoidance of unwanted attention by males in inshore environments. Determining if this is the case will require observations of foraging of both male and female sharks in offshore habitats, a task that remains a major logistical challenge.
Intra-species competition and aggression could provide an alternate explanation for the biting behaviour we witnessed. This seems unlikely however, as no aggression seems to occur among whale sharks that are occasionally seen feeding in groups of two-to-three individuals at the water surface at Ningaloo Reef. These sharks typically circle around each other in a ‘yin-yang’ pattern (Gudgeon, 2019). Furthermore, cameras deployed on five whale sharks at Ningaloo Reef (see Barry et al., 2023) recorded no antagonistic interactions in 23 hours of footage, and no aggression has been reported in the literature.
Although the observed behavioural interaction reported here did not likely culminate in mating, our observations suggest that pre-copulatory behaviors of whale sharks resemble those of many other species of shark. Such records do not just expand our understanding of reproductive behaviors but may also provide insights into the potential drivers of sexual segregation reported in populations of whale sharks at many coastal aggregations.
Data availability statement
The data used in this study is available at: https://apps.aims.gov.au/metadata/view/57d5b0e7-c0f7-4e3d-bb3e-4ecb6ca8638f.
Ethics statement
The animal study was approved by Animal ethics approval was provided by permit 2019/RA/3/100/1715 of the University of Western Australia. All aspects of the field work program were approved by the management agency, the Western Australia Department of Biodiversity, Conservation and Attractions. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. EL: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. MD: Conceptualization, Data curation, Investigation, Writing – review & editing. LF: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. MT: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. AG: Investigation, Methodology, Supervision, Writing – review & editing. MM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Santos Ltd. the Australian Institute of Marine Science, The Jock Clough Marine Foundation, and The Minderoo Foundation. Santos Ltd. was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. CB was supported by an Australian Government Research Training Program (RTP) Scholarship.
Acknowledgments
The authors acknowledge the Baiyungu, Thalanyji and Yinigurdira peoples as the Traditional Owners of Nyinggulara (Ningaloo) Reef and its surrounding waters on which this work was undertaken. The authors recognize their connection to Country and pay our respects to their Elders past and present. The authors thank Kim Brooks and the many researchers and volunteers who contributed to annual research trips.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1507072/full#supplementary-material
Supplementary Video S1 available at: https://youtu.be/PhLx_7NHGCQ.
Supplementary Table 1 | Annual sampling effort of whale shark (Rhincodon typus) sex at Ningaloo Reef across 2010-2024.
References
Acuña-Marrero D., Jiménez J., Smith F., Doherty P. F. Jr., Hearn A., Green J. R., et al. (2014). Whale shark (Rhincodon typus) seasonal presence, residence time and habitat use at Darwin Island, Galapagos Marine Reserve. PLoS One 9, e115946. doi: 10.1371/journal.pone.0115946
Araujo G., Agustines A., Bach S. S., Cochran J. E., de la Parra-Galván E., de la Parra-Venegas R., et al. (2022). Improving sightings-derived residency estimation for whale shark aggregations: A novel metric applied to a global data set. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.775691
Awruch C. A., Frusher S. D., Pankhurst N. W., Stevens J. D. (2008). Non-lethal assessment of reproductive characteristics for management and conservation of sharks. Mar. Ecol. Prog. Ser. 355, 277–285. doi: 10.3354/meps07227
Barry C., Gleiss A. C., Ferreira L. C., Thums M., Davis R. W., Fuiman L. A., et al. (2023). Predation of baitfishes associated with whale sharks at Ningaloo Reef. Mar. Biol. 170, 140. doi: 10.1007/s00227-023-04291-4
Birt M., Bond T., Taylor M., McLean D., Langlois T. (2019). Chasing tail: putative pre-copulatory behaviour in free-living zebra sharks (Stegostoma fasciatum). Matters. (ISSN: 2297-8240) doi: 10.19185/matters.201903000016
Curtis T. H., Robinson J., Pratt H. L. Jr., Skomal G. B., Whitney N. M. (2024). Novel pre-copulatory behavior in basking sharks observed by drone. J. Fish Biol. 105(4), 1041-1361. doi: 10.1111/jfb.15858
D’Antonio B., Ferreira L. C., Meekan M., Thomson P. G., Lieber L., Virtue P., et al. (2024). Links between the three-dimensional movements of whale sharks (Rhincodon typus) and the bio-physical environment off a coral reef. Movement Ecol. 12, 10. doi: 10.1186/s40462-024-00452-2
Doane M. P., Reed M. B., McKerral J., Farias Oliveira Lima L., Morris M., Goodman A. Z., et al. (2023). Emergent community architecture despite distinct diversity in the global whale shark (Rhincodon typus) epidermal microbiome. Sci. Rep. 13, 12747. doi: 10.1038/s41598-023-39184-5
Dugal L., Thomas L., Jensen M. R., Sigsgaard E. E., Simpson T., Jarman S., et al. (2022). Individual haplotyping of whale sharks from seawater environmental DNA. Mol. Ecol. Resour. 22, 56–65. doi: 10.1111/1755-0998.13451
Gudgeon K. (2019). Whale sharks seen mating on Ningaloo Reef, exciting scientists (Pilbara, Australia: ABC Pilbara). Available online at: https://www.abc.net.au/news/2019-06-12/whale-sharks-seen-mating-on-ningaloo-reef/11199336?utm_campaign=abc_news_web&utm_content=link&utm_medium=content_shared&utm_source=abc_news_web (Accessed 07/10 2024).
Hearn A. R., Green J., Román M., Acuña-Marrero D., Espinoza E., Klimley A. (2016). Adult female whale sharks make long-distance movements past Darwin Island (Galapagos, Ecuador) in the Eastern Tropical Pacific. Mar. Biol. 163, 1–12. doi: 10.1007/s00227-016-2991-y
Hsu H., Joung S., Liu K. (2012). Fisheries, management and conservation of the whale shark Rhincodon typus in Taiwan. J. Fish Biol. 80, 1595–1607. doi: 10.1111/j.1095-8649.2012.03234.x
Joung S.-J., Chen C.-T., Clark E., Uchida S., Huang W. Y. (1996). The whale shark, Rhincodon typus, is a livebearer: 300 embryos found in one ‘megamamma’supreme. Environ. Biol. Fishes 46, 219–223. doi: 10.1007/BF00004997
Klimley A. P., Porcher I. F., Clua E. E., Pratt H. L. (2023). A review of the behaviours of the Chondrichthyes: a multi-species ethogram for the chimaeras, sharks, and rays. Behaviour 160, 967–1080. doi: 10.1163/1568539X-bja10214
Lester E., Meekan M. G., Barnes P., Raudino H., Rob D., Waples K., et al. (2020). Multi-year patterns in scarring, survival and residency of whale sharks in Ningaloo Marine Park, Western Australia. Mar. Ecol. Prog. Ser. 634, 115–125. doi: 10.3354/meps13173
Macena B. C., Hazin F. H. (2016). Whale shark (Rhincodon typus) seasonal occurrence, abundance and demographic structure in the mid-equatorial Atlantic Ocean. PLoS One 11, e0164440. doi: 10.1371/journal.pone.0164440
Marcus L., Virtue P., Nichols P. D., Ferreira L. C., Pethybridge H., Meekan M. G. (2019). Stable isotope analysis of dermis and the foraging behavior of whale sharks at Ningaloo Reef, Western Australia. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00546
Matsumoto R., Murakumo K., Nozu R., Acuña-Marrero D., Green J. R., Pierce S. J., et al. (2023). Underwater ultrasonography and blood sampling provide the first observations of reproductive biology in free-swimming whale sharks. Endangered Species Res. 50, 125–131. doi: 10.3354/esr01226
Meekan M. G., Bradshaw C. J., Press M., McLean C., Richards A., Quasnichka S., et al. (2006). Population size and structure of whale sharks Rhincodon typus at Ningaloo Reef, Western Australia. Mar. Ecol. Prog. Ser. 319, 275–285. doi: 10.3354/meps319275
Meekan M. G., Fuiman L., Davis R., Berger Y., Thums M. (2015). Swimming strategy and body plan of the world's largest fish: implications for foraging efficiency and thermoregulation. Front. Mar. Sci. 2. doi: 10.3389/fmars.2015.00064
Meekan M. G., Taylor B. M., Lester E., Ferreira L. C., Sequeira A. M. M., Dove A. D. M., et al. (2020). Asymptotic growth of whale sharks suggests sex-specific life-history strategies. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.575683
Meekan M., Thompson F., Brooks K., Matsumoto R., Murakumo K., Lester E., et al. (2024). Internal organs and body tissues of free-swimming whale sharks (Rhincodon typus) imaged using underwater ultrasound. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1285429
Meekan M., Virtue P., Marcus L., Clements K., Nichols P., Revill A. (2022). The world's largest omnivore is a fish. Ecology. 103(12). doi: 10.1002/ecy.3818
Norman B. M., Holmberg J. A., Arzoumanian Z., Reynolds S. D., Wilson R. P., Rob D., et al. (2017). Undersea constellations: the global biology of an endangered marine megavertebrate further informed through citizen science. BioScience 67, 1029–1043. doi: 10.1093/biosci/bix127
Norman B. M., Stevens J. D. (2007). Size and maturity status of the whale shark (Rhincodon typus) at Ningaloo Reef in Western Australia. Fisheries Res. 84, 81–86. doi: 10.1016/j.fishres.2006.11.015
Nozu R., Murakumo K., Matsumoto R., Nakamura M., Ueda K., Sato K. (2015). Gonadal morphology, histology, and endocrinological characteristics of immature female whale sharks, Rhincodon typus. Zoological Sci. 32, 455–458. doi: 10.2108/zs150040
Osorio B. J., Skrzypek G., Meekan M. (2023). Parasitic copepods as biochemical tracers of foraging patterns and dietary shifts in whale sharks (Rhincodon typus Smith 1828). Fishes 8, 261. doi: 10.3390/fishes8050261
Perry C. T., Clingham E., Webb D. H., de la Parra R., Pierce S. J., Beard A., et al. (2020). St. Helena: An important reproductive habitat for whale sharks (Rhincodon typus) in the central south Atlantic. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.576343
Pierce S., Norman B. (2016). Rhincodon typus. (The IUCN red list of threatened species), T19488A2365291. doi: 10.2305/IUCN.UK.2016-1.RLTS.T19488A2365291.en
Ramírez-Macías D., Meekan M., de la Parra-Venegas R., Remolina-Suárez F., Trigo-Mendoza M., Vázquez-Juárez R. (2012). Patterns in composition, abundance and scarring of whale sharks Rhincodon typus near Holbox Island, Mexico. J. fish Biol. 80, 1401–1416. doi: 10.1111/j.1095-8649.2012.03258.x
Ramírez-Macías D., Vázquez-Juárez R., Galván-Magaña F., Munguía-Vega A. (2007). Variations of the mitochondrial control region sequence in whale sharks (Rhincodon typus) from the Gulf of California, Mexico. Fisheries Res. 84, 87–95. doi: 10.1016/j.fishres.2006.11.038
Rangel B. S., Afonso A. S., Bettcher V., Bucair N., Andres N., Veras L. B., et al. (2023). Evidence of mating scars in female tiger sharks (Galeocerdo cuvier) at the Fernando de Noronha Archipelago, Brazilian Equatorial Atlantic. Environ. Biol. Fishes 106, 107–115. doi: 10.1007/s10641-022-01380-z
Salinas-de-León P., Hoyos-Padilla E., Pochet F. (2017). First observation on the mating behaviour of the endangered scalloped hammerhead shark Sphyrna lewini in the Tropical Eastern Pacific. Environ. Biol. Fishes 100, 1603–1608. doi: 10.1007/s10641-017-0668-0
Schmidt J. V., Chen C.-C., Sheikh S. I., Meekan M. G., Norman B. M., Joung S.-J. (2010). Paternity analysis in a litter of whale shark embryos. Endangered Species Res. 12, 117–124. doi: 10.3354/esr00300
Sequeira A. M., Thums M., Brooks K., Meekan M. G. (2016). Error and bias in size estimates of whale sharks: implications for understanding demography. R. Soc. Open Sci. 3, 150668. doi: 10.1098/rsos.150668
Sims D. W. (2005). Differences in habitat selection and reproductive strategies of male and female sharks. Sexual segregation vertebrates 20, 127–147.
Sleeman J., Meekan M. G., Fitzpatrick B., Steinberg C., Ancel R., Bradshaw C. J. (2010). Oceanographic and atmospheric phenomena influence the abundance of whale sharks at Ningaloo Reef, Western Australia. J. Exp. Mar. Biol. Ecol. 382, 77–81. doi: 10.1016/j.jembe.2009.10.015
Speed C. W., Field I. C., Meekan M. G., Bradshaw C. J. (2010). Complexities of coastal shark movements and their implications for management. Mar. Ecol. Prog. Ser. 408, 275–293. doi: 10.3354/meps08581
Speed C. W., Meekan M. G., Bradshaw C. J. (2007). Spot the match–wildlife photo-identification using information theory. Front. zoology 4, 1–11. doi: 10.1186/1742-9994-4-2
Talwar B. S., Bond M. E., Williams S., Brooks E. J., Chapman D. D., Howey L. A., et al. (2023). Reproductive timing and putative mating behavior of the oceanic whitetip shark Carcharhinus longimanus in the eastern Bahamas. Endangered Species Res. 50, 181–194. doi: 10.3354/esr01231
Thums M., Meekan M., Stevens J., Wilson S., Polovina J. (2013). Evidence for behavioural thermoregulation by the world's largest fish. J. R. Soc. Interface 10, 20120477. doi: 10.1098/rsif.2012.0477
Wearmouth V. J., Sims D. W. (2008). Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv. Mar. Biol. 54, 107–170. doi: 10.1016/S0065-2881(08)00002-3
Keywords: courtship, reproduction, elasmobranch, sexual segregation, mating activity, intra-specific aggression, sex ratio, marine megafauna
Citation: Barry C, Lester E, Doane MP, Ferreira LC, Thums M, Gleiss AC and Meekan MG (2025) Love bites? Pre-copulatory behaviours of whale sharks (Rhincodon typus) at Ningaloo Reef, Western Australia. Front. Mar. Sci. 11:1507072. doi: 10.3389/fmars.2024.1507072
Received: 07 October 2024; Accepted: 02 December 2024;
Published: 03 January 2025.
Edited by:
J. Marcus Drymon, Mississippi State University, United StatesReviewed by:
Jose Julian Tavera, University of the Valley, ColombiaArturo Tripp, National Polytechnic Institute (IPN), Mexico
Copyright © 2025 Barry, Lester, Doane, Ferreira, Thums, Gleiss and Meekan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine Barry, Y2hyaXN0aW5lLmJhcnJ5QG11cmRvY2guZWR1LmF1
†ORCID: Christine Barry, orcid.org/0000-0002-1486-9749
Emily Lester, orcid.org/0000-0003-2207-5225
Micheal P. Doane, orcid.org/0000-0001-9820-2193
Luciana C. Ferreira, orcid.org/0000-0001-6755-2799
Michele Thums, orcid.org/0000-0002-8669-8440
Adrian C. Gleiss, orcid.org/0000-0002-9960-2858
Mark G. Meekan, orcid.org/0000-0002-3067-9427
 Christine Barry
Christine Barry Emily Lester
Emily Lester Micheal P. Doane
Micheal P. Doane Luciana C. Ferreira
Luciana C. Ferreira Michele Thums
Michele Thums Adrian C. Gleiss
Adrian C. Gleiss Mark G. Meekan6†
Mark G. Meekan6†