- 1Department of Biosciences, Biotechnologies and Environment, University of Bari Aldo Moro, Bari, Italy
- 2Consorzio Nazionale Interuniversitario per le Scienze del Mare (CoNISMa), Roma, Italy
- 3Fondazione COISPA Ente Terzo Settore (ETS), Bari, Italy
The aim of this study is to provide new insights on the age and growth of H. dactylopterus in the north-western Ionian Sea (central Mediterranean) by using different data sets and comparing three different methods, one direct by otolith reading corroborated by two indirect methods, by means of back calculation and Length Frequency Distribution analysis (LFDa), in order to obtain robust data for use in analytical management models. A total of 1403 specimens of blackbelly rosefish were sampled from habitats explored on muddy bottoms in the depth range 106-721 m and 309 individuals caught in the cold-water coral habitat between 418 and 635 m in depth. The length-frequency distributions of blackbelly rosefish showed a polymodal pattern in both habitats explored, with medium-small individuals caught on muddy bottoms and larger ones in coral habitats and canyons. The maximum age observed in females was 13 years (315 mm TL), while in males it was 15 years (336 mm TL), and no significant difference was observed comparing age-length keys from otolith readings of females with the males. The analysis of the daily increments allowed us to validate the average length of the first year of age (approximately 90-100 mm LT) obtained from the three different methods used. No significant difference was highlighted comparing the average lengths of age class 1, calculated with the direct method and by counting the daily increments. The von Bertalanffy growth parameters were estimated for the sexes and for the whole population from otolith readings and for the entire population by LFD analysis and the back calculation method. The growth curves were comparable between the different methods and no significant difference was observed. The von Bertalanffy growth parameters estimated for the different methods were comparable (otolith readings L∞ 494 mm, k=0.06 years-1, t0=-3.00 years; back calculation method L∞ 487 mm, k=0.06 years-1, t0=-2.30 years; LFDa L∞ 415 mm, k=0.07 years-1, t0=-2.23 years), indicating a slow growth in accordance with what has already been observed in other areas of the Mediterranean.
1 Introduction
Age and growth studies are fundamental components of fisheries management. Although age and growth data are generally considered together, each component provides exclusive information on individuals and population. Age refers to some quantitative description of the length of time that an organism has lived, whereas growth is the change in body or body part size between two points in time, and growth rate is a measure of change in some metric of fish size as a function of time (DeVries and Frie, 1996). Fish age estimations and growth studies using hard parts, such as otoliths, provide basic information for understanding population dynamics and stock assessments that are essential for responsible fisheries management (Panfili et al., 2002).
The blackbelly rosefish, Helicolenus dactylopterus (Delaroche, 1809) (Osteichthyes, Scorpaeniformes), is a benthic deep-water scorpionfish found at depths of 100–1000 m both on muddy bottoms and in heterogeneous habitats such as seamounts, canyons and cold-water coral habitats. It feeds on both benthic and pelagic organisms (mainly crustaceans and fishes)) showing dietary variation with ontogentic development and in relation to the habitat in which it lives (Capezzuto et al., 2020).
It is viviparous species, and larvae and offspring are pelagic (Massutí et al., 2001; Costello et al., 2005; D’Onghia et al., 2010, 2012; Sartor et al., 2017). It is widespread both in the eastern Atlantic and in the Mediterranean Sea (Whithehead et al., 1986), where it is both common and exploited. In the Mediterranean, this teleost is a commercial species with an important economic value targeted by long-lines and gillnets, but it also regularly appears in the by-catch of bottom trawls over a wide depth range, with adult specimens mostly collected during deep-sea fishing targeting crustaceans (Fisher et al., 1987; Relini et al., 1999).
Concerning age and growth, several studies published concern both the Atlantic and the Mediterranean. In the latter, studies on the growth of blackbelly rosefish were conducted by (Massutí et al., 2000, 2001) in Iberian Mediterranean coast and in the Alboran Sea respectively; by Mili et al. (2016) in the Northern Waters of Tunisia and in Italian waters by Peirano and Tunesi (1986) and Ragonese (1989) in the Ligurian Sea; Ragonese and Reale (1995) in the Strait of Sicily; by Ungaro and Marano (1995) and Romanelli et al. (1997) in the Adriatic Sea and by D’Onghia et al. (1992, 1996) and by Sion et al. (2018, 2022) in the western Ionian Sea. A wide range of von Bertalanffy growth parameters were estimated by different authors in relation to different factors: the method used (otolith reading method and/or length frequency distribution analysis), the heterogeneity of the sample studied (fish of different size but also sampled with different types of gear), different environmental conditions, different latitudes and different fishing pressures. Despite many studies having been published, there is still no study to our knowledge focused on the validation and/or corroboration.
Poor-quality ageing data could result in inaccurate population status evaluation which in turn could lead stock collapse (Beamish and McFarlane, 1995). Age data discrepancies can be attributed to preparation methods (Smith et al., 2016), ageing standards (Carbonara and Follesa, 2019a; Hüssy et al., 2016), and sample strategies (fishing related or fishing independent data) (Coggins et al., 2013). Diverse degrees of fishing pressure (Carbonara et al., 2022) and geographical variances resulting from genetic or environmental factors (Carbonara et al., 2018; Isely et al., 1987) may explain differences in growth patterns between population, even within adjacent populations (Carbonara et al., 2022). Furthermore, otolith reader experience level may be another source of variation (Carbonara et al., 2019b). Moreover, fish distribution could be an additional source of bias. Indeed, large individuals of H. dactylopterus are often rare on muddy bottoms because they are removed by trawling. Whereas large individuals distributed in canyons and cold-water coral habitats, generally collected by bottom longline and only the presence in the sample of two population components (juvenile and adult) can provide an important contribution to resolving the existing disagreement on H. dactylopterus age and growth pattern. All these factors can compromise both precision and accuracy of age data and consequently the analysis of the level of population exploitation. For this reason, the aim of this study is to provide new insights into the age and growth of H. dactylopterus in the north-western Ionian Sea by using different data sets and comparing three different methods, a direct by otolith reading verified by means of back calculation and corroborated by Length Frequency Distribution analysis (LFDa), in order to obtain more robust data for use in analytical management models (Carbonara et al., 2023; ICES, 2020). Moreover, a validation of first annulus was carried out by Daily Growth Increments (Campana, 2001).
2 Materials and methods
2.1 Data collection
Data on H. dactylopterus have been collected in the north-western Ionian Sea, corresponding to Geographical Sub-Area (GSA) 19 (Figure 1) between Cape Otranto and Cape Passero, during two international research projects: the MEDITS experimental bottom trawl surveys (Spedicato et al., 2019) and CoralFISH bottom longline surveys (Grehan et al., 2017). Data from four MEDITS surveys (from 2013 to 2016) and three CoralFISH surveys (2010-2011) were used (Table 1). In the first project the trawlable fishing grounds were explored, while the second involved areas where trawl fishing activity does not take place due to irregular and hard bottoms, giving the opportunity of sampling larger individuals, using the longline.
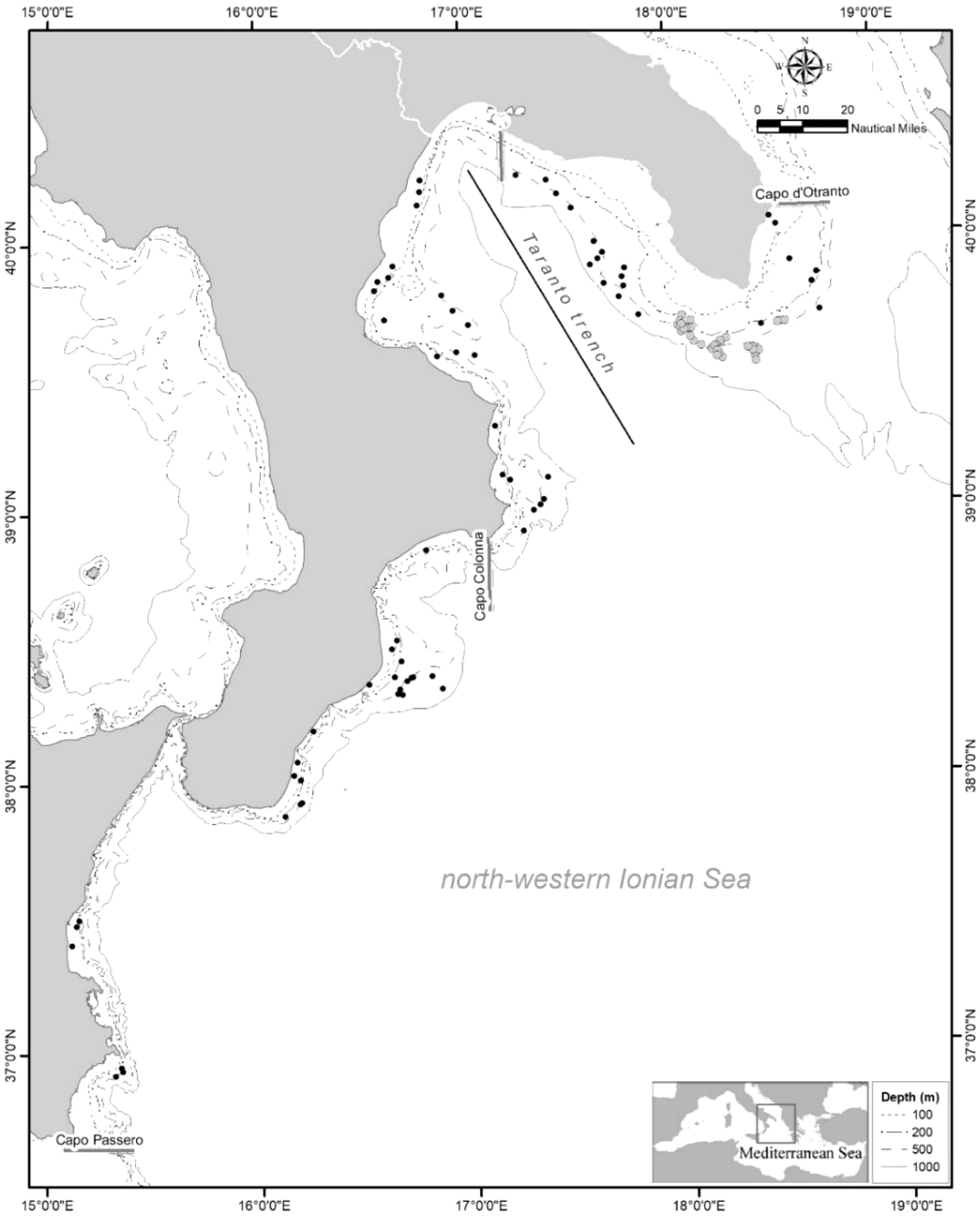
Figure 1. Study area in the north-western Ionian Sea, corresponding to GSA 19, with indication of the hauls carried out during the MEDITS (black dots) and CORALFISH (grey dots) projects.

Table 1. Number of individuals and range of total length (mm), by sex, corresponding to each sampling period.
From the sampled specimens the Total Length (TL; in millimeters) to the nearest 1 mm, Total Weight (TW) to the nearest 0.1 g, sex, and maturity stages (AAVV, 2017) were measured. A total of 1403 specimens of H. dactylopterus were sampled from muddy bottoms in the depth range 106-721 m and 309 individuals were caught in the cold-water coral habitats between 418 and 635 m depth (Table 1).
2.2 Age analysis
The sagittal otoliths were extracted from a subsample of specimens captured during the trawl and the bottom longline surveys. Both otoliths (right and left) were removed but the right one was read preferentially. All the readings were carried out in the posterior area on the distal side along the longitudinal axis joining the sulcus and the nucleus. For larger individuals, grinding was performed on the proximal surface to obtain the best reading. The sagittae were placed in a black dish with glycerine (30%) and alcohol (70%) and read by standard techniques (Morales-Nin, 1987). All otoliths showed the ring pattern common to teleost fish, opaque and transparent rings laid down around an opaque nucleus, corresponding to fast and slow growth phases (Williams and Bedford, 1974). The age and growth estimations were carried out by band count, with one opaque band followed by a transparent one assumed to be an annulus (Panfili et al., 2002). An age was assigned to each otolith according to the age estimation scheme proposed by Carbonara and Follesa (2019a) with a yearly resolution, assuming the birthday as January 1. In the sagittae the transparent bands were counted using a stereo microscope (Leica M165C, x 10 magnification), under reflected light against a dark background (Figure 2).
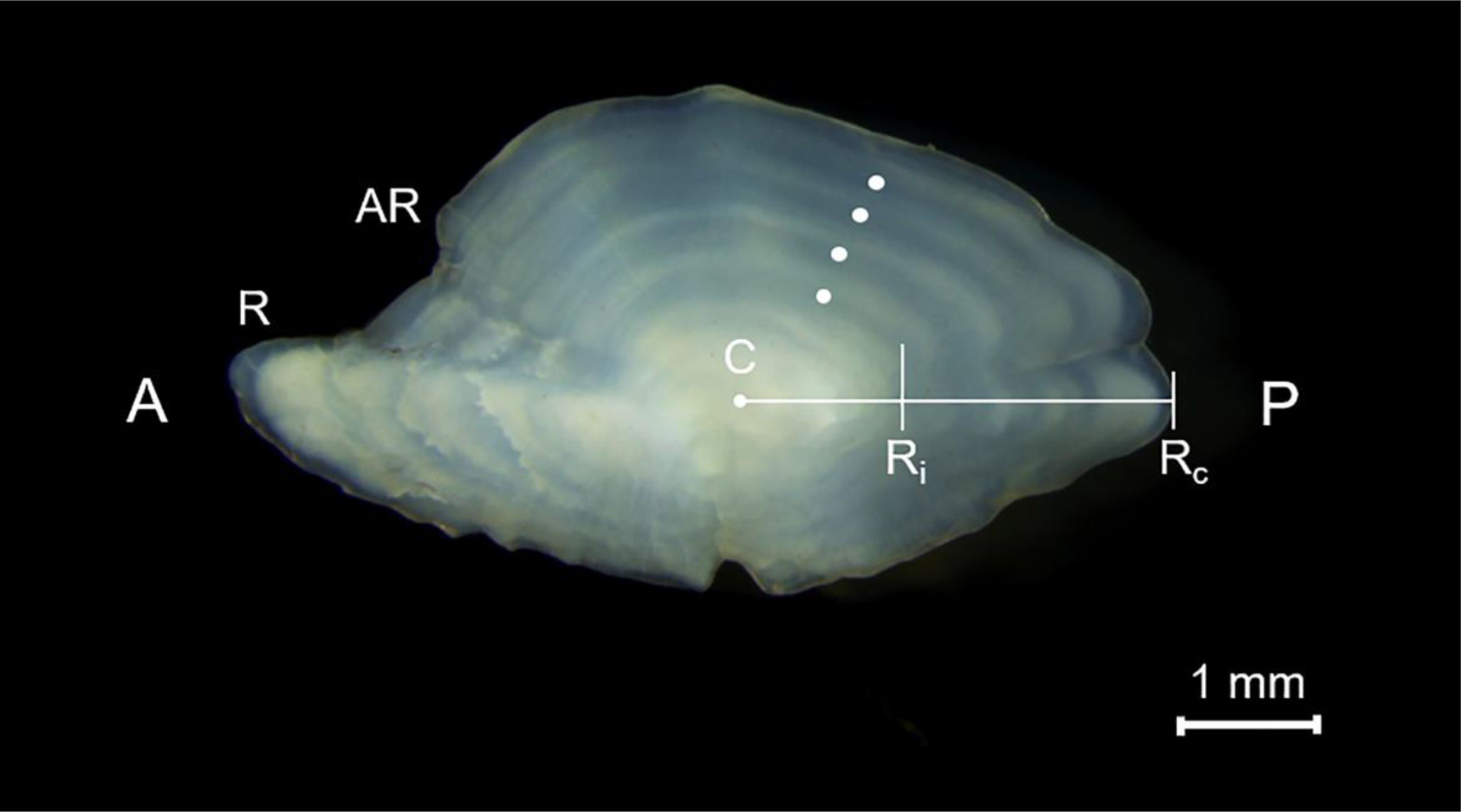
Figure 2. Right otolith of H. dactylopterus: evident opaque and transparent bands, 4-year-old (161 mm TL). A: Anterior part; P: Posterior part; R: Rostrum; AR: Anti Rostrum; Ri: Radius of the ith band (distance from the centre of the otolith to the outer margin of the annulus), Rc is the radius of the otolith at capture.
An age–length key for the females, males and the entire population from whole otolith readings was provided and for each age classes the growth rate was calculated as: Gr = (Li+1-Li)/(Li+1+Li)/2; where Li is the total length of the ith annulus and Li+1 is the total length of the (i+1)th annulus. The differences between females and males in otolith readings were assessed using the non-parametric Kolmogorov-Smirnov (KS) statistical test.
In addition, Daily Growth Increments (DGI) present in the otoliths were estimated by the direct method of annuli counting to validate the first year of age (Campana, 2001). The daily periodicity was made visible, after sanding and polishing, using a microscope (Leica DM LS2, x 800 magnification) with transmitted light (Figure 3).
Regarding the otolith readings, the precision, defined as the reproducibility (Chilton and Beamish, 1982), was measured from the average percent error (APE), the percentage agreement (PA), and the coefficient of variation (CV). The formula used to calculate APE was the following (Beamish and Fournier, 1981):
where Xij is the ith age determination of the jth fish, Xj is the average age calculated for the jth fish, and R is the number of times each fish was aged.
CV and PA within one year (+/─1 yr) were proposed by Campana, 2001:
where R is the number of times each fish is aged, Xij the i(th) age determination of the j(th) fish, Xj is the mean age calculated for the j(th) fish, and ndiff is the difference in age determination between the readings of three readers.
The direct method was corroborated with the back calculation method, in particular, the formula of Fraser-Lee (Francis, 1990) was used:
where is the total length (mm) of the fish at the time of annulus “i” formation, and is the total length (mm) of the fish at the time of otolith removed. is otolith radius (mm) from the centre of the otolith to the outer margin of the transparent ring annulus, and is the radius of the otolith at capture (mm), the distance from the centre of the otolith to the periphery; c is the intercept obtained from the linear regression of the otolith radius versus the total length. All measurements were taken in the posterior area on the distal side along the longitudinal axis joining the sulcus and the nucleus, the centre of the otolith. They were taken from the right otolith of only annuli that were clearly defined according to the criteria proposed by ICES, 2019. The mean values for age classes coming from the otoliths reading and back calculation method were tested using the Kolmogorov-Smirnov (KS) statistical test.
The Length Frequency Distribution analysis (LFD) was based on data collected in MEDITS 2010 survey. The Bhattacharya method, incorporated in FiSAT software (Gayanilo et al., 2006), was used to discriminate the normal distribution assuming that each mode in the overall size-frequency distribution represented a cohort. The separation index among different cohorts was taken into account and values < 2 indicated a large overlap between cohorts, which was considered unacceptable.
Furthermore, the von Bertalanffy growth equation was fitted to the length-at-age data obtained, for males, females and whole population from otolith readings and for entire population from LFD analysis and the back calculation method, using FiSAT computer software (Gayanilo et al., 2006). The growth curves (L∞., k and t0.) were compared using the Chen test (Chen et al., 1992).
Finally, the growth performance index (ϕ’ = log K + 2 log TL∞) (Munro and Pauly, 1983) was also estimated to compare the results of the present study with those obtained in different areas of the Mediterranean Sea and in the Atlantic Ocean.
2 Results
The length-frequency distributions of blackbelly rosefish showed a polymodal pattern in both habitats explored, with sizes between 22 and 300mm TL and total weight from 0.60 to 462.6 g from muddy bottoms and between 140 and 380mm TL and total weight from 44.30 to 700.00 g taken in the cold-water coral habitat (Figure 4). In muddy habitats most individuals were smaller than 100 mm TL and sexually indeterminate. In both surveys the males were larger than females (Table 1; Figure 4). Age estimation, through otolith reading, was carried out on a total of 834 specimens, of which 4% (N=33) were unreadable. Otoliths showed the characteristic pattern of teleosts, with transparent and opaque rings laid down around an opaque nucleus due to slow and fast growth phases. Analysis of 801 otoliths produced estimates from 0 to 15 years, with sizes between 42 and 336-mm TL (Table 2). In particular, the sagittae were however read separately by sex, 350 otoliths of the females gave estimates from 0 to 13 years (Supplementary Table S1), with sizes between 64 and 315-mm TL while for the males the age estimated was from 0 to 15 years (Supplementary Table S2), with sizes between 57 and 336-mm TL. Most of the samples belonged to the age class 0 and 1, representing about 13% (N=102) and 10% (N=78) respectively, however a large length range was recorded for each age group. A higher increase in the Growth rate (Gr), for both sexes and for the entire population, was observed in the first two age classes, while it slows down starting from the third year. Regarding the ageing precision CV and APE values were 5.14 and 4% respectively and the PA was of 82.6%, suggesting a good agreement between readers. No significant difference was found when comparing age keys from otolith readings of females with those of males (Kolmogorov-Smirnov test (D=0.17; p>0.05), the estimation of growth parameters using the von Bertalanffy model was, however, carried out by sex and for the entire sampled population.
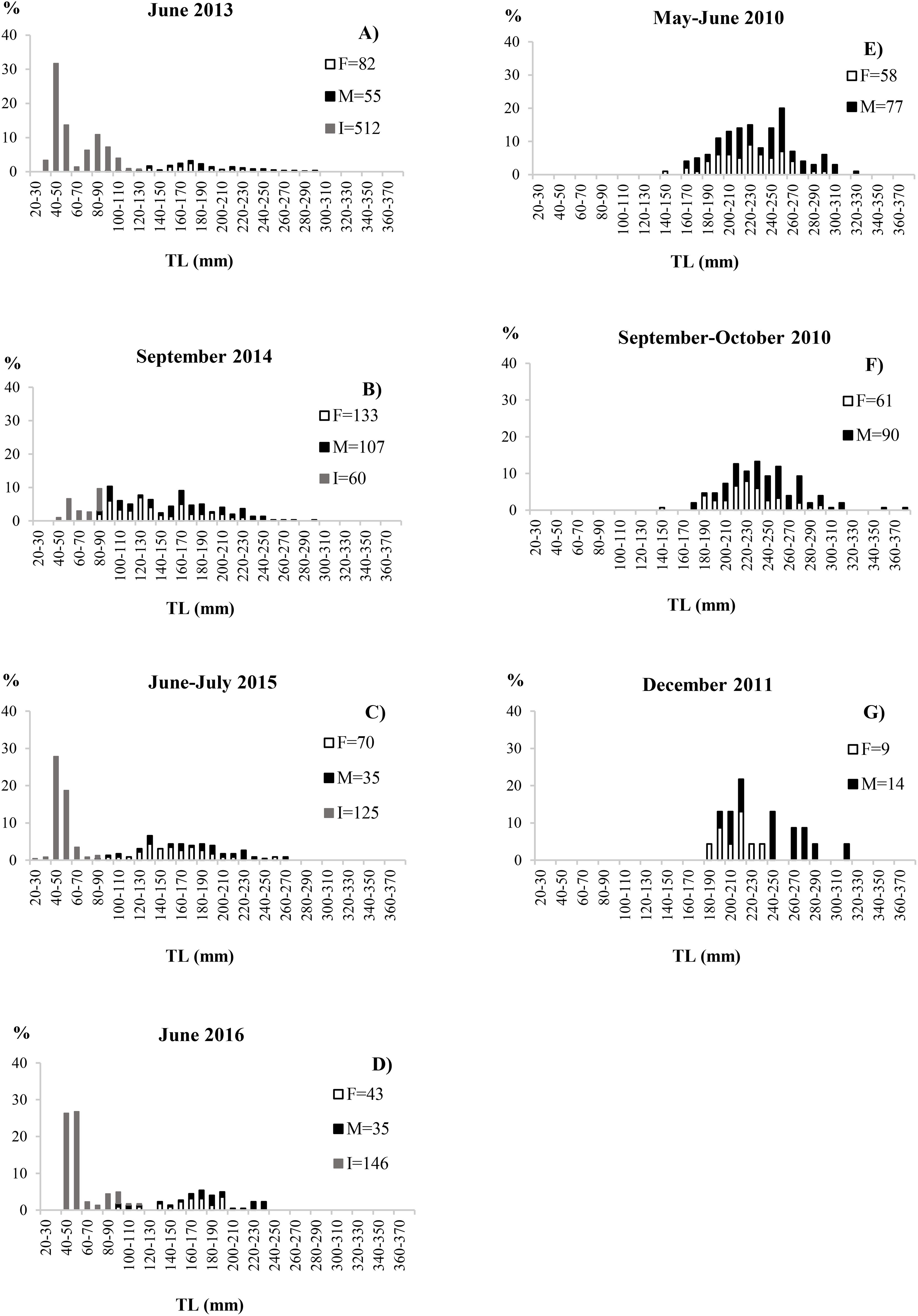
Figure 4. Length-frequency distributions of H. dactylopterus, by sex, caught on muddy bottoms (A–D) (from 2013 to 2016) and in the cold-water coral habitat (E–G) (2010-2011).
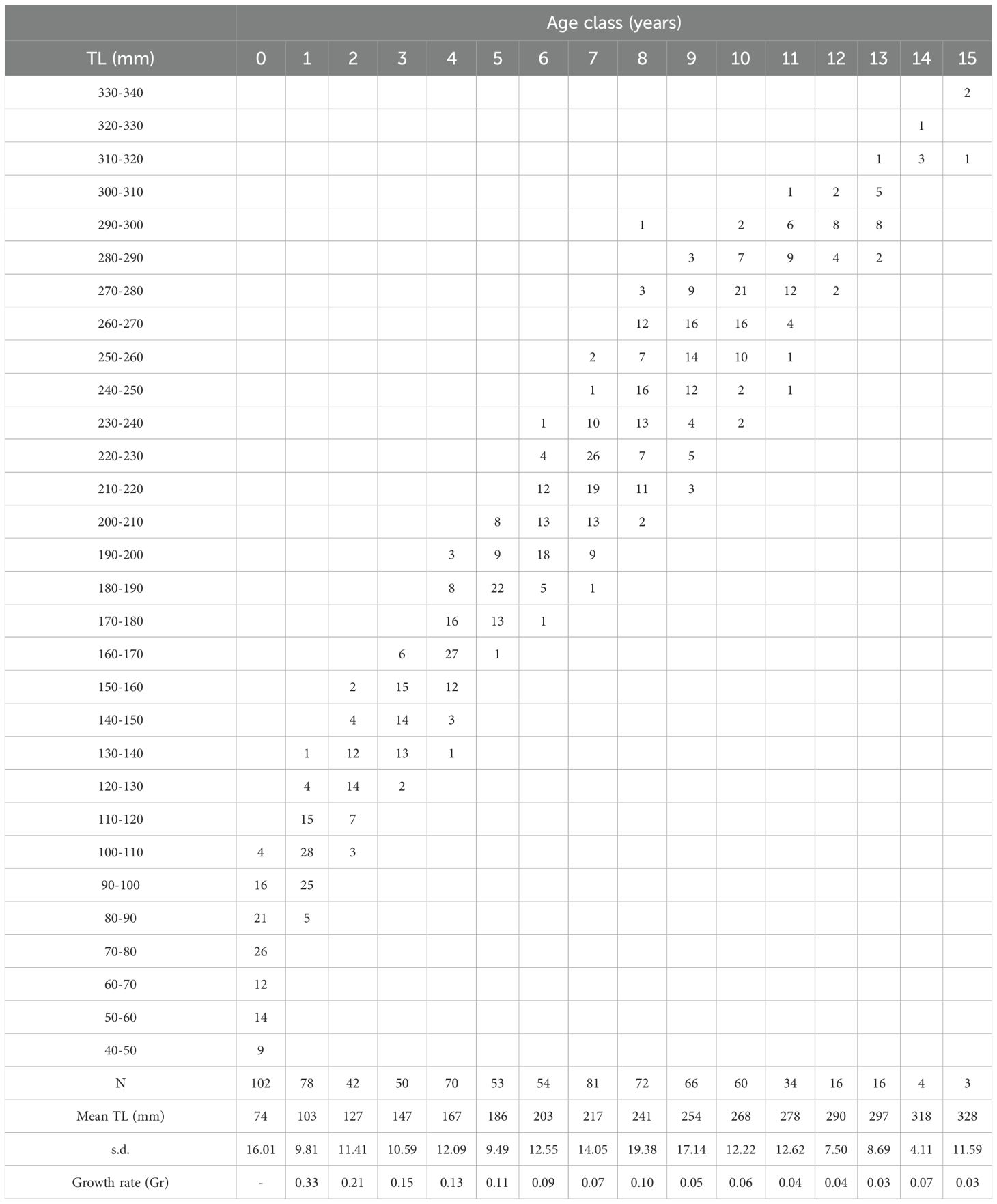
Table 2. Age-length key for the entire population of H. dactylopterus in the north-western Ionian Sea, from whole otolith readings, with number of fish (N), mean length (TL, mm), standard deviation (s.d.) and Growth rate (Gr).
Age validation of the first annulus was achieved by analysis of Daily Growth Increments (DGI). From the 61 otoliths prepared for DGI interpretation, 32 (52%) were read, while for the rest polishing and breakage problems hampered the reading. On the assumption that these increments were deposited daily, the age range determined was from 191 to 431 days old for specimens between 42 and 113mm TL (Table 3). A linear regression between TL and DGI (Figure 5) was used to validate the first annulus; the variance explained by the regression (r2) was 0.95, showing a very good correlation between the two variables. From the analysis of the daily rings, it was observed that the specimens, belonging to age class 1 (starting from 352 days), had TL between 85 and 113 mm and an average TL value of 94 mm ± 9.7 s.d. Comparing, the average lengths of age class 1, calculated both with the direct method (103 mm ± 9.81 s.d.) and by counting the daily increments (94 mm ± 9.7 s.d.) there was no significant difference (Student’s t-test, t=1.69, p>0.05).
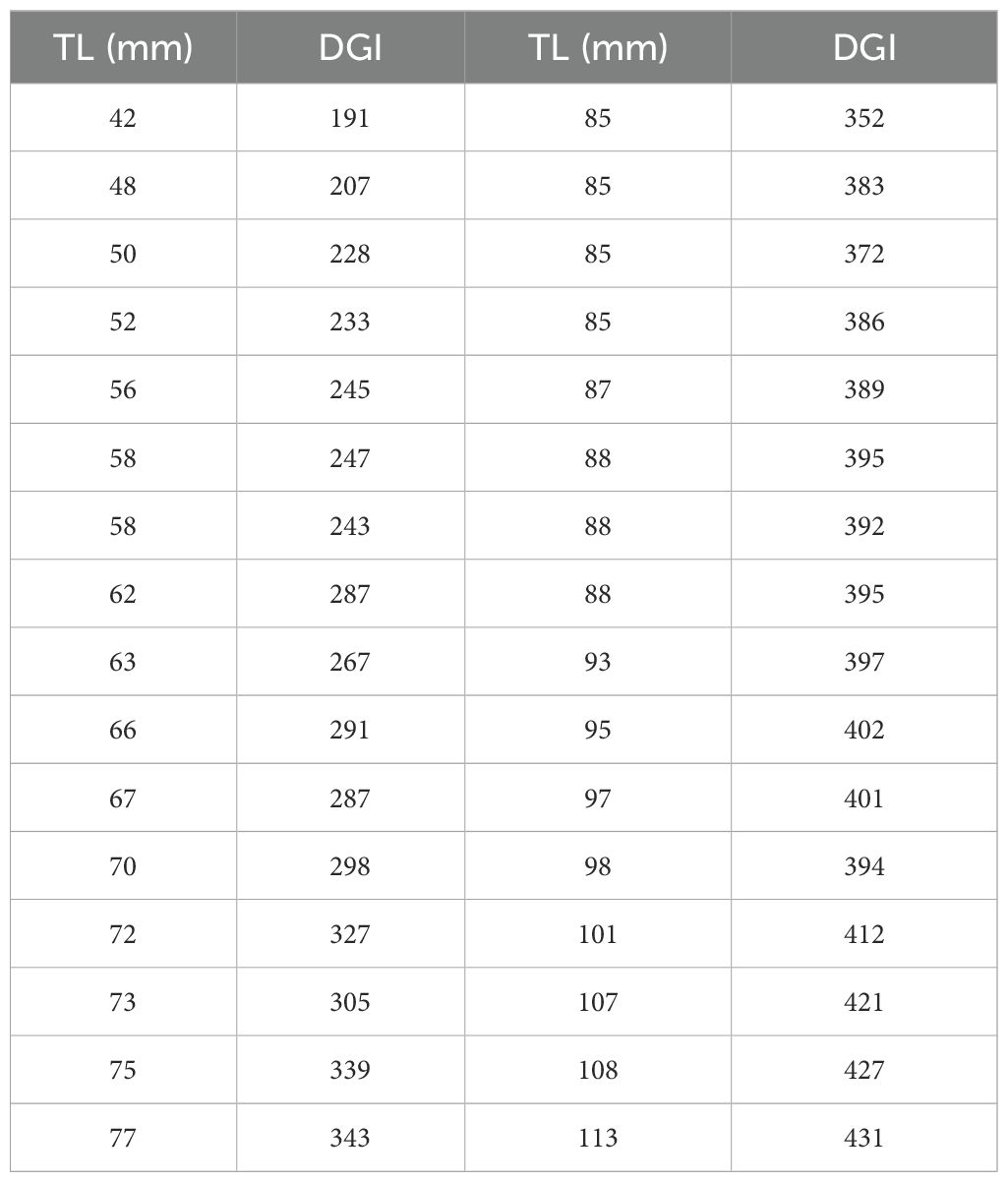
Table 3. Total lengths (TL) and number of daily increments (DGI) identified in H. dactylopterus individuals caught in the north-western Ionian Sea.
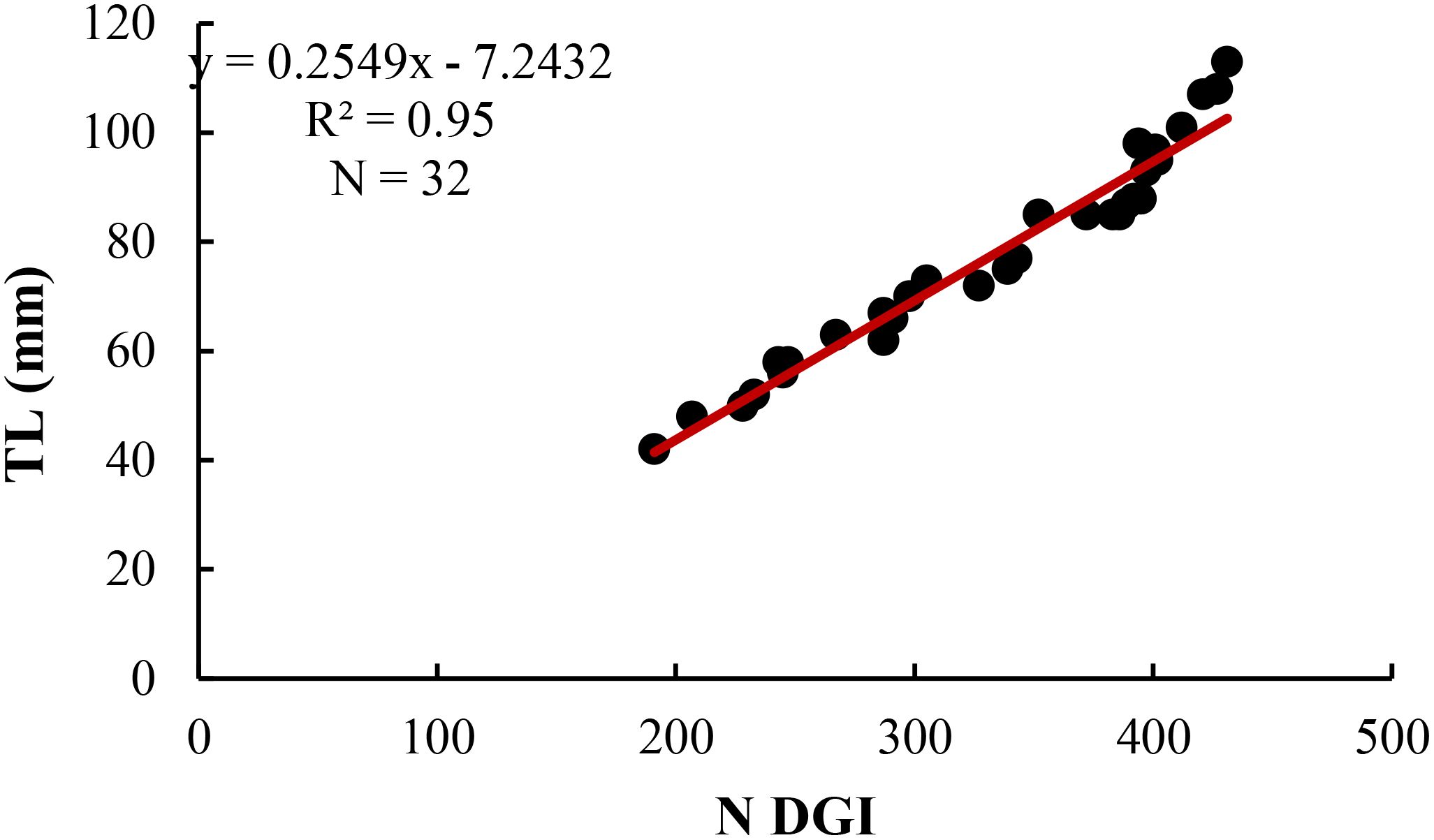
Figure 5. Regression line between total number of daily increments (DGI) and corresponding Total Length (TL) in specimens of H. dactylopterus.
Fish Total Length (TL) and otolith radius (R) were highly correlated (Figure 6); the relationship estimated using a linear model was TL = 7.3579R-17.446 (r2 = 0.96). The intercept value and the ray measurements were carried out on 746 structures and a length-age key (Table 4) was constructed through the back calculation procedure using the Fraser-Lee formula (1920). The Length Frequency Distribution analysis produced 8 modes (from 0 to 7) (Figure 7); all modal components were identified with Separation Indices (S.I.) largely greater than 2 (Gayanilo et al., 2006). The age–length keys obtained from otolith reading and the back calculation method, in which the number of age classes was comparable (Table 5), were tested using the Kolmogorov-Smirnov test showing no significant differences (D=0.18; p>0.05). The growth curves were comparable between the different methods (Fcritic = 2.42> Fobserved = 1.79; p>0.05) and no significant difference was observed. The growth parameters calculated are presented in Table 6 and the growth curves obtained with different methods are shown in Figure 8.
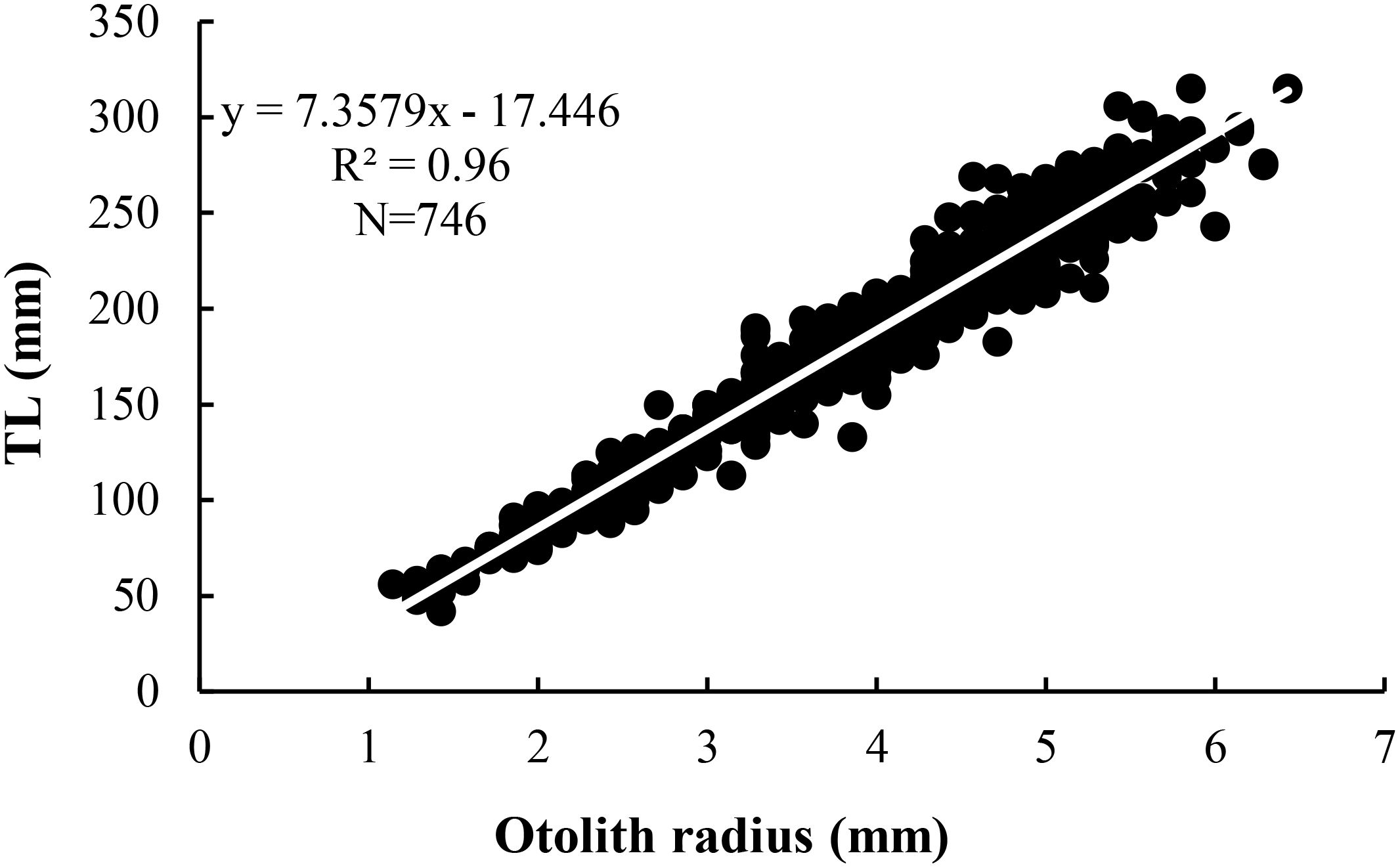
Figure 6. Regression line between otolith radius and corresponding Total Length (TL) in specimens of H. dactylopterus.
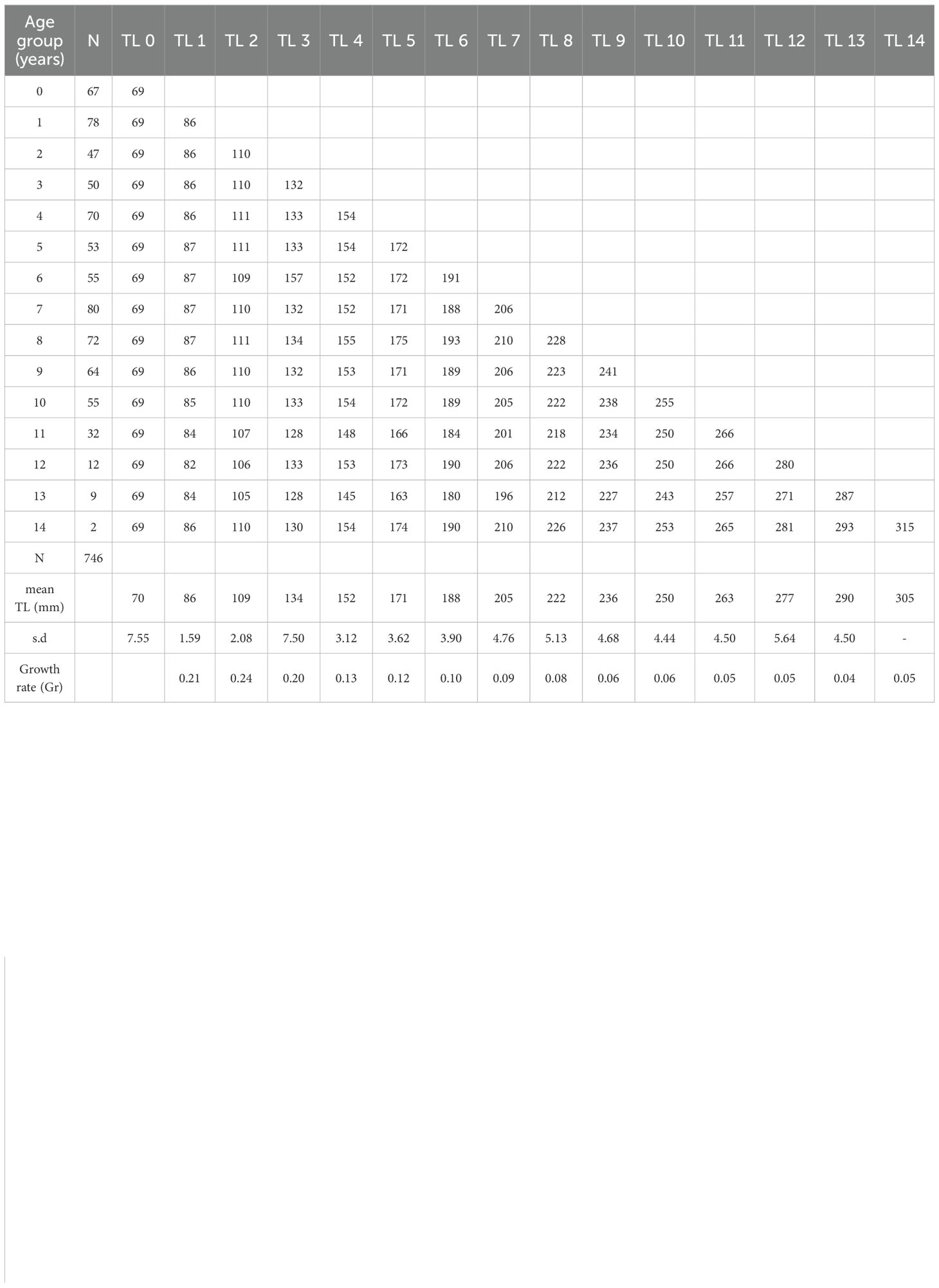
Table 4. Back calculated lengths at-age (TL) for whole population of H. dactylopterus caught in the north-western Ionian Sea.
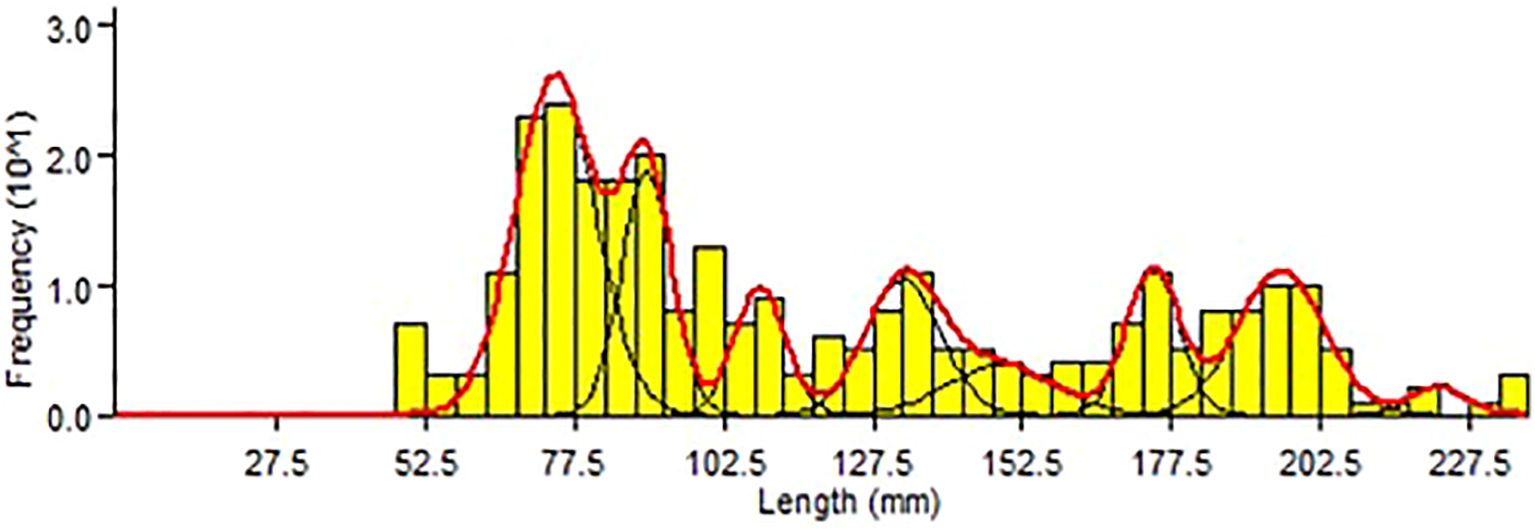
Figure 7. Length Frequency Distribution analysis (LFDa) calculated for individuals of H. dactylopterus caught during the MEDITS 2010 survey.
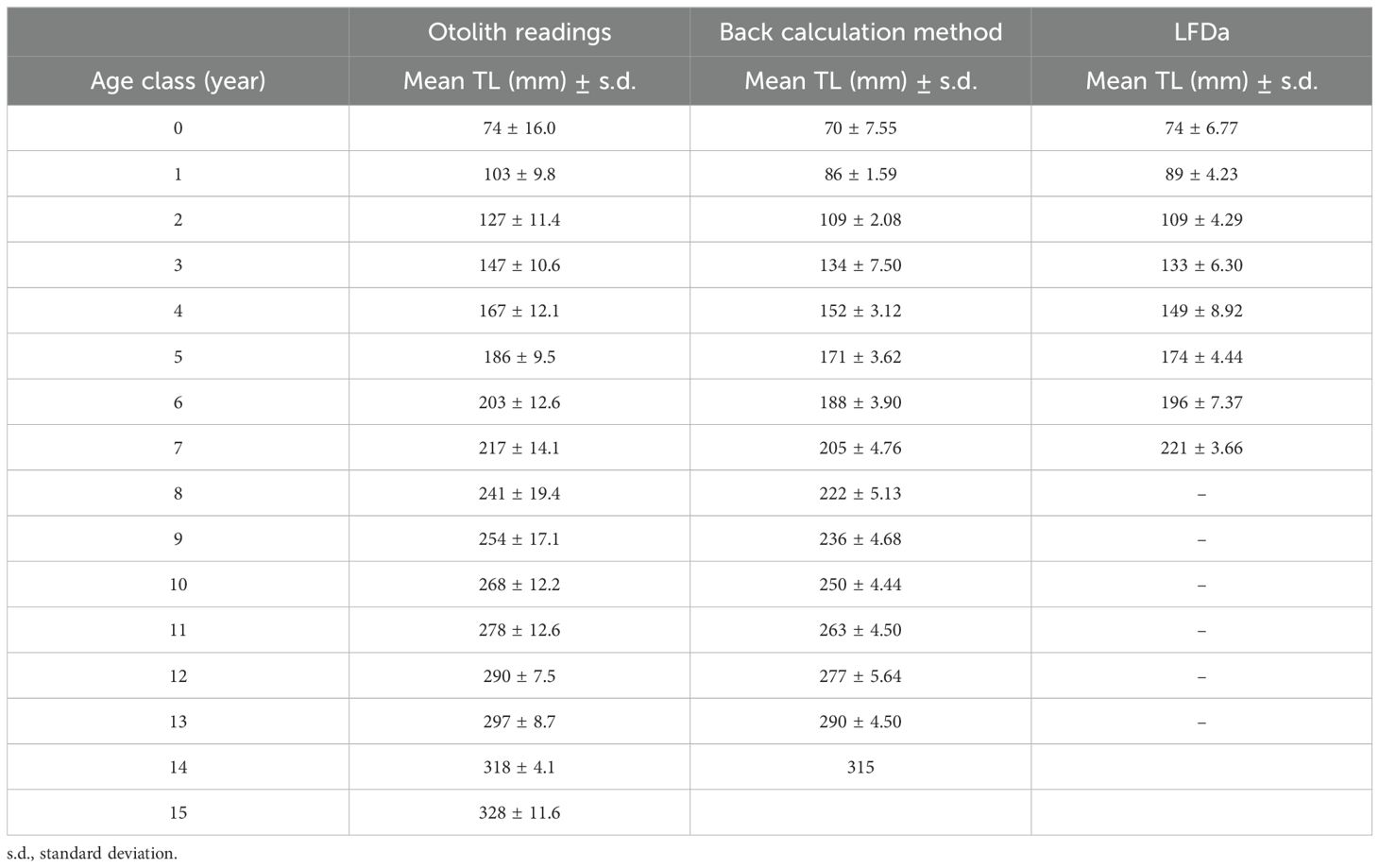
Table 5. The age-length key estimated, by three different methods, for H. dactylopterus caught in the north-western Ionian Sea.
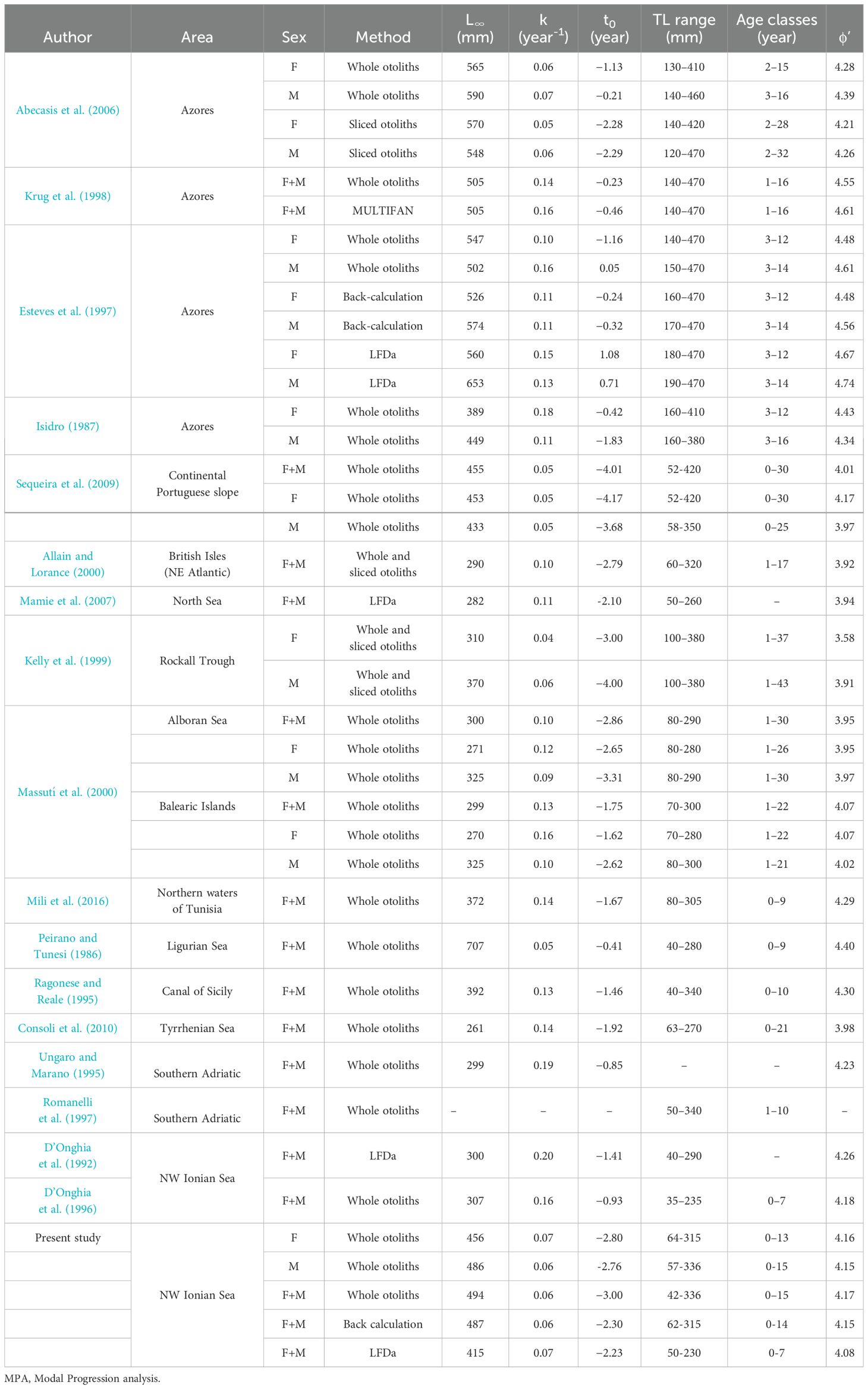
Table 6. Von Bertalanffy growth parameters (L∞, k, t0) and growth performance indexes (ϕ’) for H. dactylopterus reported in different study areas.
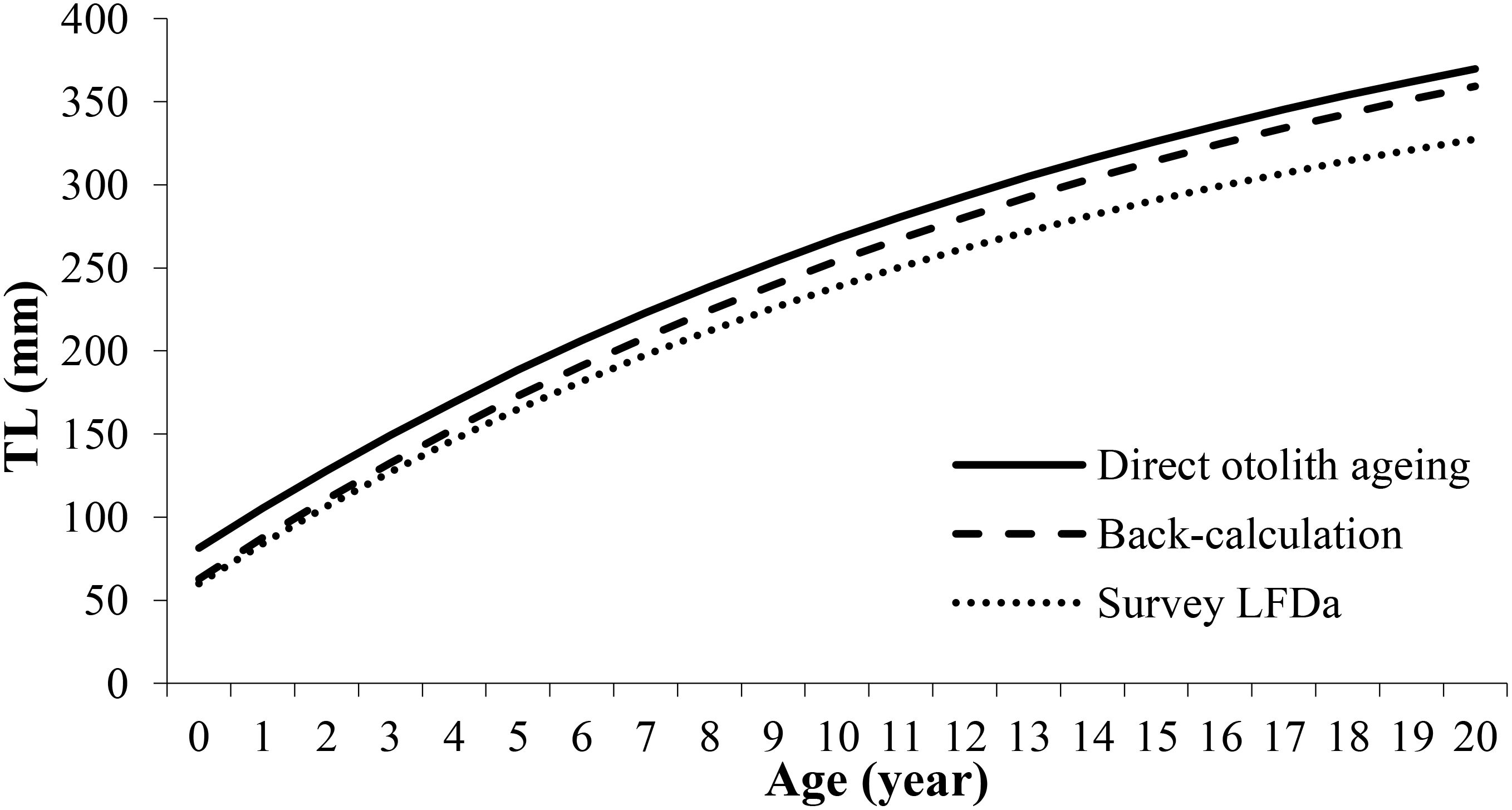
Figure 8. H. dactylopterus von Bertalanffy growth curves obtained from direct otolith readings (continuous line); back-calculation (dashed line) and Length Frequency Distribution analysis (dotted line).
4 Discussion
This study contributes to a better understanding of the of age and growth estimation of H. dactylopterus in the north-western Ionian Sea by comparing three different methods, a direct method by otolith reading verified by means of back calculation and corroborated by the Length Frequency Distribution analysis (LFDa). The three methods provided consistent results confirming the same growth pattern. Furthermore, the analysis of the daily increments allowed the validation of the first annulus to which one year of age was assigned using the direct method (reading of the otoliths).
The use of different sampling tools, in different habitats, allowed the capture of a wide range of sizes. In the north-western Ionian Sea, as in other areas of the Mediterranean, the smaller individuals of H. dactylopterus are distributed in shallower waters and, as they grow, move towards greater depths (D’Onghia et al., 1992). In habitats with muddy bottoms, however, the use of the trawl net allowed the capture, mainly, of small-medium sized individuals; on these substrates, fishing activity is widespread even at great depths, significantly affecting the structure of fish stocks and reducing the adult fraction. The size of the specimens captured was greater in coral habitats than in habitats with muddy substrates, confirming the peculiarity of coral habitats as refuge areas linked to sensitive phases of the life cycle (reproduction, nursery site and feeding) (D’Onghia, 2019). For this species there seems to be a strong association with structurally very complex seabeds such as those with cold-water corals or in canyons (D’Onghia et al., 2012, 2015; Mytilineou et al., 2014). Carbonara et al. (2020) found double biomass of H. dactylopterus in areas associated with bamboo coral (Isidella elongata) compared to areas of deep mud without any three-dimensional structure. Coral and/or canyon habitats act as source areas in which individuals, thanks to the protection offered by the habitat, can reach larger dimensions and carry out reproduction, generating new individuals who, thanks to the spill-over effect, can renew their populations in sink areas present on muddy seabeds, where organisms are continuously removed due to fishing activities (D’Onghia et al., 2016; Sion et al., 2019; Carbonara et al., 2020). However, the differences in size observed in the survey areas could be due both to the method of capture (different gear used) but also to the different fishing pressure (Massutí et al., 2000).
The length-frequency distributions of the two habitats explored showed sexual dimorphism with males larger than females, confirming what has been observed for this teleost in other areas of the Mediterranean basin (Massutí et al., 2000, 2001; Anastasopoulou et al., 2017; Sequeira et al., 2009; Mili et al., 2016). The reproductive effort, considering the bioenergetic constraints of the energy budget of the organisms, implies reduced growth rates of females (Gunderson, 1997). However, this is not always the case, in fact in other deep-sea fishes, such as the Gadiforms, the females are larger than the males. The maximum age observed in females was 13 years (315 mm TL), while in males it was 15 years (336 mm TL), but no significant difference was observed comparing age-length keys from otolith readings of females with the males (Tables 3, 4). The maximum length sampled in our sample was equal to a male of 380 mm TL, consequently one could think of an age of approximately 25 years old, calculated using the von Bertalanffy growth parameters obtained in this study, an age comparable to other areas of the Mediterranean Sea.
The problems encountered were essentially due to the otoliths of adult individuals, in which there is the difficulty in clearly distinguishing all the growth rings, even by making thin sections of the otoliths. As the greater deposition of calcium carbonate makes the separation of the first rings less evident, but also because the presence of false rings due to the slowdown in the growth speed makes the rings thinner and closer together, making the count less certain. This is confirmation of why numerous works have been published on estimating the age and growth of blackberry rosefish, but there are still numerous controversies linked mainly to the maximum age detected (Abecasis et al., 2006). However, the alternation of transparent and opaque rings in the otoliths of H. dactylopterus indicates that, despite being a bathyal species, its growth shows aspects of seasonality that are certainly not linked to the thermal regimes that remain almost constant in the deep environment. For this species, there seems to be a strong link with the seasonal fluctuations in primary productivity that occur in surface waters, and which have repercussions in terms of secondary productivity in the deep environment (Massutì et al., 1995). The deposition of the rings discussed by Massutí et al. (2000) is confirmed in the present study, in fact the first 4–8 pairs of rings were wider than the following rings, which were laid down with decreasing thickness, while in adult fish the outer rings decreased in width, becoming irregular and equally wide.
Counts of daily increments provided the validation of the first annulus. In species with a clearly interpretable otolith microstructure, daily increment counts can often be used to confirm the identity of the first annulus (Campana, 2001). The analysis of the daily increments allowed us to validate the average length of the first year of age (approximately 90-100 mm LT) obtained from the three different methods used. No significant difference was highlighted comparing the average lengths of age class 1, calculated with the direct method and by counting the daily increments. Therefore, the analysis of the daily increments, allowed the validation of the first annulus to which one year of age was assigned using the direct method (reading of the otoliths). Our results are comparable with those found by other authors for the western Mediterranean (Massutí et al., 2001; Sequeira et al., 2009). In particular, Massutí et al. (2000) reported a mean TL of 83 mm TL for the first-year class, based on DGI analysis. The growth parameters estimated for the sex and pooled, according to the von Bertalanffy model, would indicate slow growth in agreement with what has already been observed in the northern Ionian (D’Onghia et al., 1992, 1996), in the Adriatic Sea (Romanelli et al., 1997), for the Balearic Islands (Massutí et al., 2000) and for the Tyrrhenian Sea (Consoli et al., 2010). In the Mediterranean, the maximum ages observed were 21 years (270 mm TL) in the Tyrrhenian Sea (Consoli et al., 2010) and 10 years (340 mm TL) in the southern Adriatic (Romanelli et al., 1997). Moreover, this latter author reported an individual with a length of 410 mm TL with an estimated age of 25-30 years old, indicating that this species may grow to be 25 years old or more. These authors suggested that the maximum number of growth zones distinguishable on the whole otoliths is approximately 30, and that divergence on ages, estimated using whole or sectioned otoliths, usually occurs after 20-25 years of age. A large variation of results can be observed in the von Bertalanffy growth parameters for H. dactylopterus between different authors (Table 6) and this may be a consequence of the method used (otolith reading or length-frequency analysis) and of the heterogeneity of the sample studied (fish of different size distribution caught with different types of sampling gear). In relation to the growth parameters obtained for this species in the Mediterranean basin (Table 6), a longevity of over 30 years can be hypothesized, indicating a long-lived species, with numerous age-classes in the population. Differences in growth parameters reported for Mediterranean and Atlantic populations, in terms of asymptotic length, can be related to different range sizes analyzed and environmental variability, such as differences in temperature, prey availability, nutrient levels, pollution, or strong regional genetic differentiation between Atlantic and Mediterranean populations (Atkinson and Sibly, 1997), together with different degrees of fishing pressure (Schindler et al., 2000; Carbonara et al., 2022). These are all factors that commonly represent an additional source of variation in growth characteristics.
Comparing the results of the present study with those obtained on a larger spatial scale, it is possible to confirm that the size of this teleost is generally larger in the Atlantic Ocean than in the Mediterranean Sea (Table 6).
The differences of maximum age estimates are very important for the management because the exploitation of the species is correlated to its life history (Jennings et al., 1998; Denney et al., 2002). Deep-water species like the blackbelly rosefish are vulnerable to overfishing because of their biological characteristics (long life, large size, late maturity and slow growth). The accuracy and precision of age and growth data have a negative impact on the assessment of the status of stocks and the application of management measures aimed at achieving sustainable exploitation of this species in the Mediterranean basin (Carbonara et al., 2019b). If an age overestimation occurs, the stock assessment will provide an erroneous scenario with a population composed of older individuals and, consequently, affected by lower fishing mortality, while in the opposite case, fish would be younger with an overestimation of fishing mortality (Campana, 2001).
In the present study, age determination of large individuals contributes to better understanding of the growth pattern of H. dactylopterus, which is useful for future stock assessment by providing more robust scientific advice on the management of this Mediterranean stock. This agrees with Caddy (1993, 2008) who argued for the inclusion of habitat components in the fisheries management to recover and maintain overexploited or depleted fishery resources.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the study was carried out on dead animals caught in fishing activities.
Author contributions
LS: Conceptualization, Data curation, Formal analysis, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LC: Data curation, Writing – original draft, Software, Methodology, Formal analysis. GG: Writing – original draft, Software, Methodology, Formal analysis, Data curation. GD: Writing – original draft, Visualization, Validation, Supervision, Conceptualization. PC: Writing – review & editing, Visualization, Validation, Supervision, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The data used in this study were collected under the Data CollectionFramework (DCF), supported by the Italian Ministry of Agriculture, Food and Forestry Policy (MiPAAF) and by the European Commission (EU Regulations 1004/2017). Data provided are owned by the Italian Ministry of Agriculture, Food and Forestry Policy (MiPAAF).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1506180/full#supplementary-material
References
AAVV. (2017). MEDITS-Handbook. Version n_9 (MEDITS Working Group). Available online at: https://www.sibm.it/MEDITS%202011/principaledownload.htm (Accessed November, 2020).
Abecasis D., Costa A. R., Pereira J. G., Pinho M. R. (2006). Age and growth of bluemouth, Helicolenus dactylopterus (Delaroche 1809) from the Azores. Fisheries Res. 79, 148–154. doi: 10.1016/j.fishres.2006.02.010
Allain V., Lorance P. (2000). Age estimation and growth of some deepsea fish from the Northeast Atlantic Ocean. Cybium 24, 7–16. doi: 10.26028/cybium/2000-243s-001
Anastasopoulou A., Mytilineou C., Dimitriadis G., Smith C. J. (2017). “Aspects on age and growth of Helicolenus dactylopterus from the deep waters of the eastern Ionian Sea (pp.61-66),” in Fisheries Centre Research Reports, 25 (1). Institute for the Oceans and Fisheries, University of British Columbia. Eds. Pauly D., Hood L., Stergiou K. I., 99pp.
Atkinson D., Sibly R. M. (1997). Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 12, 235–239. doi: 10.1016/S0169-5347(97)01058-6
Beamish R. J., Fournier D. A. (1981). A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 38, 982–983. doi: 10.1139/f81-132
Beamish R. J., McFarlane G. A. (1995). “A discussion of the importance of ageing error, and an application to walleye Pollock: the world’s largest fishery,” in Recent developments in fish otolith research. Eds. Secor D. H., Dean J. M., et al (Univ. South Carolina Press, Columbia), 545–565.
Caddy J. F. (1993). Some future perspectives for assessment and management of Mediterranean fisheries. Sci. Mar. 57, 121–130.
Caddy J. F. (2008). The importance of “cover“ in the life histories of demersal and benthic marine resources: a neglected issue in fisheries assessment and management. Bull. Mar. Sci. 83, 7–52.
Campana S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 59, 197–242. doi: 10.1111/j.1095-8649.2001.tb00127.x
Capezzuto F., Ancona F., Calculli C., Sion L., Maiorano P., D’Onghia G. (2020). Feeding of the deep-water fish Helicolenus dactylopterus (Delaroche 1809) in different habitats: from muddy bottoms to cold-water coral habitats. Deep-Sea Res. Part I 159, 103252. doi: 10.1016/j.dsr.2020.103252
Carbonara P., Bellodi A., Massaro A., Basilone G., Casciaro L., Palmisano M., et al. (2023). Age validation of the European Anchovy (Engraulis encrasicolus Linnaeus 1758) in the central-southern Tyrrhenian Sea (West Mediterranean Sea). Regional Stud. Mar. Sci. 60 2023. doi: 10.1016/j.rsma.2023.102891
Carbonara P., Ciccolella A., De Franco F., Palmisano M., Bellodi A., Lembo G., et al. (2022). Does fish growth respond to fishing restrictions within Marine Protected Areas? A case study of the striped red mullet in the south-west Adriatic Sea (Central Mediterranean). Aquat. Conserv. 32, 417–429. doi: 10.1002/aqc.v32.3
Carbonara P., Follesa M. C. (2019a). Handbook on Fish Age Determination: A Mediterranean Experience. Studies and Reviews. No. 98 (Rome: FAO), 192.
Carbonara P., Intini S., Kolitari J., Joksimovi´c A., Milone N., Lembo G., et al. (2018). A holistic approach to the age validation of Mullus barbatus L. 1758 in the Southern Adriatic Sea (Central Mediterranean). Sci. Rep. 8, 13219. doi: 10.1038/s41598-018-30872-1
Carbonara P., Zupa W., Anastasopoulou A., Bellodi A., Bitetto I., Charilaou C., et al. (2019b). Explorative analysis on red mullet (Mullus barbatus) ageing data variability in the Mediterranean. Sci. Mar. 83S1, 271–279. doi: 10.3989/scimar.04999.19A
Carbonara P., Zupa W., Follesa M. C., Cau A., Capezzuto F., Chimienti G., et al. (2020). Exploring a deep-sea vulnerable marine ecosystem: Isidella elongata (Esper 1788) species assemblages in the Western and Central Mediterranean. Deep Sea Res. Part I: Oceanographic Res. Papers 166. doi: 10.1016/j.dsr.2020.103406
Chen Y., Jackson D. A., Harvey H. H. A. (1992). Comparison of von bertalanffy and polynomial functions in modelling fish growth data. Can. J. Fish. Aquat. Sci. 49, 1228–1235. doi: 10.1139/f92-138
Chilton D. E., Beamish R. J. (1982). Age Determination Methods for Fishes Studied by the Groundfish Program at the Pacific Biological Station (Ottawa, ON, Canada: Canadian Special Publication of Fisheries and Aquatic Sciences 60; Department of Fisheries and Oceans), 102p.
Coggins L. G., Gwinn D. C., Allen M. S. (2013). Evaluation of age- length key sample sizes required to estimate fish total mortality and growth. Trans. Am. Fish. Soc 142, 832–840. doi: 10.1080/00028487.2013.768550
Consoli P., Battaglia P., Castriota L., Esposito V., Romeo T., Andaloro F. (2010). Age, growth and feeding habits of the bluemouth rockfish, Helicolenus dactylopterus dactylopterus (Delaroche 1809) in the central Mediterranean (southern Tyrrhenian Sea). J. Appl. Ichthyology 26, 583–591. doi: 10.1111/j.1439-0426.2010.01467.x
Costello M. J., McCrea M., Freiwald A., Lundalv T., Jonsson L., Bett B. J., et al. (2005). “Rôle of cold-water Lophelia pertusa coral reefs as fish habitat in the NE Atlantic,” in Cold-Water Corals and Ecosystems. Eds. Freiwald A., Roberts M. J. (Springer, Berlin), 771–805.
D’Onghia G., Mastrototaro F., Panza M. (1996). “On the growth of rockfish, Helicolenus dactylopterus (Delaroche 1809), from the Ionian Sea,” in FAO Fish Rep, vol. 533, Suppl. (FAO, Rome), 143 ± 152.
D’Onghia G., Matarrese A., Tursi A. (1992). Biologia di Helicolenus dactylopterus (Delaroche 1809): distribuzione ed accrescimento sui fondi batiali del Mar Ionio. Oebalia 17, 129 ± 131.
D’Onghia G. (2019). “Cold-water corals as shelter, feeding and life-history critical habitats for fish species: ecological interactions and fishing impact,” in Mediterranean Cold-Water Corals: Past, Present and Future. Eds. Orejas C., Jiménez C. (Enalia Physis Environmental Research Centre (ENALIA), Nicosia, Cyprus: Springer).
D’Onghia G., Calculli C., Capezzuto F., Carlucci R., Carluccio A., Maiorano P., et al. (2016). New records of cold-water coral sites and fish fauna characterization of a potential network existing in the Mediterranean Sea. Mar. Ecol. 37, 1398–1422. doi: 10.1098/rspb.2002.2138
D’Onghia G., Capezzuto F., Cardone F., Carlucci R., Carluccio A., Chimienti G., et al. (2015). Macro- and megafauna recorded in the submarine Bari Canyon (southern Adriatic, Mediterranean Sea) using different tools. Mediterr. Mar. Sci. 16, 180–196. doi: 10.12681/mms.1082
D’Onghia G., Maiorano P., Carlucci R., Capezzuto F., Carluccio A., Tursi A., et al. (2012). Comparing deep-sea fish fauna beween Coral and Non- Coal ʺMegahabitatsʺ in the Santa Maria di Leuca cold-water coral province (Mediterranean Sea). PLoS One 7, (9). doi: 10.1371/journal.pone.0044509
D’Onghia G., Maiorano P., Sion L., Giove A., Capezzuto F., Carlucci R., et al. (2010). Effects of deep-water coral banks on the abundance and size structure of the megafauna in the Mediterranean Sea. Deep-Sea Res. Pt. II 57, 397–411. doi: 10.1016/j.dsr2.2009.08.022
Delaroche F. E. (1809). Suite du mémoire sur les espéces de poissons observées `a Iviça. Tableau des esp`eces de poissons que j’ai observées `a Iviça pendant les mois de décembre, janvier et février. Tableau des poissons que j’ai observés `a Maïorque et `a Barcelonne, mais que je n’ai point vus `a Iviça. Observations sur quelques-uns des poissons indiqués dans le précédent tableau, et descriptions des espéces nouvelles ou peu connues, 13. Annales du Muséum d’Histoire Naturelle, Paris. pp, 313–361 plates 20-25. Available online at: https://biodiversitylibrary.org/page/29092211.
Denney N. H., Jennings S., Reynolds J. D. (2002). Life-history correlates of maximum population growth rates in marine fishes. Proc. R. Soc Lond.: Biol. Sci. 269, 2229–2237. doi: 10.1098/rspb.2002.2138
DeVries D. R., Frie R. V. (1996). “Determination of age and growth,” in Fisheries Techniques, 2nd ed. Eds. Murphy B. R., Willis D.w. (American Fisheries Society, Bethesda, Md), 483–512.
Esteves E., Aníbal J., Krug H., Silva H. M. (1997). Aspects of age and growth of bluemouth, Helicolenus dactylopterus dactylopterus (Delaroche 1809) from the Azores. Arquipélago Life Mar. Sci. 15A, 83–95.
Fisher W., Bauchot M. L., Schneider M. (1987). “Poisson osseux. Fishes FAO d’identification des espéces poue les besoins de la peche,” in Mediterranee et Mer Noire. Zone de peche 37, vol. II. (Vertebrés. Publication préparée par la FAO, résultant d’un accord entre la FAO et la Commission des Communautés Européemmes (Project GCP/INT/422/EEC) financée conjointement par ces deux organisation, Rome, FAO), 891–1442 pp.
Francis R. I. C. C. (1990). Back-calculation of fish length: A critical review. J. Fish Biol. 36, 883–902.
Gayanilo F. C., Sparre P., Pauly D. (2006). “FAO-ICLARM Stock assessment tools II (FiSAT II)”. Revised version,” in User’s Guide, vol. 8. (Rome: FAO Computerized Information series (Fisheries)), 168.
Grehan A. J., Arnaud-Haond S., D’Onghia G., Savini A., Yesson C. (2017). - Towards ecosystem based management and monitoring of the deep Mediterranean, North-East Atlantic and beyond. Deep-Sea Res. Pt. II 145, 1–7. doi: 10.1016/j.dsr2.2017.09.014
Gunderson D. R. (1997). Trade-off between reproductive effort and adult survival in oviparous and viviparous fishes. Can. J. Fish. Aquat. Sci. 54, 990 ± 998. doi: 10.1139/f97-019
Hüssy K., Radtke K., Plikshs M., Oeberst R., Baranova T., Krumme U., et al. (2016). Challenging ICES age estimation protocols: lessons learned from the eastern Baltic cod stock. ICES J. Mar. Sci. 73, 2138–2149. doi: 10.1093/icesjms/fsw107
ICES. (2019). “Workshop on age estimation methods of deep-water species (WKAMDEEP2),” in ICES CM 2018/EOSG, Cadiz, Spain, 17-21 September 2018, Vol. 27. 31pp.
ICES. (2020). ICES Workshop on age validation studies of small pelagic species (WKVALPEL). ICES Sci. Rep. 2, 76 pp. doi: 10.17895/ices.pub.5966
Isely J. J., Noble R. L., Koppelman J. B., Philipp D. P. (1987). Spawning period and first-year growth of northern, Florida, and intergrade stocks of largemouth bass. Trans. Am. Fish. Soc 116, 757–762. doi: 10.1577/1548-8659(1987)116<757:SPAFGO>2.0.CO;2
Isidro E. J. (1987). “Age and growth of the bluemouth, Helicolenus dactylopterus dactylopterus (Delaroche 1809) off the Azores,” in ICES Document CM 1987/G, Vol. 63. 6 pp.
Jennings S., Reynolds J. D., Mills S. C. (1998). Life history correlates of responses to fisheries exploitation. Proc. R. Soc Lond.: Biol. Sci. 265, 333–339. doi: 10.1098/rspb.1998.0300
Kelly C. J., Connolly P. L., Bracken J. J. (1999). Age estimation, growth, maturity, and distribution of the bluemouth rockfish Helicolenus d. dactylopterus (Delaroche 1809) from the Rockall Trough. ICES J. Mar. Sci. 56, 61–74. doi: 10.1006/jmsc.1998.0426
Krug H., Rosa D., Menezes G., Pinho M. (1998). “Age and growth of some demersal species of the Azores,” in ASC 1998 - O. Conference contribution, 84 (11). doi: 10.17895/ices.pub.26807476.v1
Mamie J. C. J., Beare D. J., Jones E. G., Kienzle M., Dobby H., Heath M. R., et al. (2007). Aspects of the distribution and growth of bluemouth (Helicolenus dactylopterus, Delaroche 1809) since its invasion of the northern North Sea in 1991. Fisheries Oceanography 16, 85–94. doi: 10.1111/j.1365-2419.2006.00412.x
Massutí E., Morales Nin B., Moranta J. (2000). Age and growth of blue-mouth, Helicolenus dactylopterus (Osteichthyes: Scorpaenidae), in the western Mediterranean. Fisheries Res. 46, 165–176. doi: 10.1016/S0165-7836(00)00143-0
Massutì E., Morales-Nin B., Stefanescu C. (1995). Distribution and biology of five grenadier fish (Pisces: Macrouridae) from the upper and middle slope of the northwestern Mediterranean. Deep-Sea Res. 42, 307–330. doi: 10.1016/0967-0637(95)00003-O
Massutí E., Moranta J., Gil De Sola L., Morales Nin B., Prats L. (2001). Distribution and population structure of the rock-fish Helicolenus dactylopterus (Pisces: Scorpaenidae), in the western Mediterranean. J. Mar. Biol. Assoc. UK 81, 129–141. doi: 10.1017/S0025315401003472
Mili S., Ennouri R., Amdouni F., Chammam B., Missaoui H. (2016). Age and growth of bluemouth Helicolenus dactylopterus (Delaroche 1809) in the northern waters of Tunisia (Central Mediterranean). Greener J. Life Sci. 3, 1–12. doi: 10.15580/GJLS.2016.1.102516171
Morales-Nin B. (19871987). Métods de Determinación de la Edad en los Osteíctios en Base a Estructuras de Crecimiento. Inf. Tec. Invest. Pesq. 143, 30 p.
Munro J. L., Pauly D. (1983). A simple method for comparing the growth of fishes and invertebrates. Fishbyte 1, 5 ± 6.
Mytilineou Ch., Smith C. J., Anastasopoulou A., Papadopoulou K. N., Christidis G., Bekas P., et al. (2014). New cold-water coral occurrences in the Eastern Ionian Sea: Results from experimental long line fishing. Deep-Sea Res. II 99, 146–157. doi: 10.1016/j.dsr2.2013.07.007
Panfili J., Pontual H.d., Troadec H., Wright P. J. (2002). Manual of Fish Sclerochronology (Brest, France: Ifremer-IRD Coedition), 464 p.
Peirano A., Tunesi L. (1986). Preliminary notes on the biology of Helicolenus dactylopterus (Delaroche 1809) in the Ligurian Sea. Rapport la Commission Internationale pour l’exploration scientifique la Mer Méditerrané 30, 233–238.
Ragonese S. (1989). L'Applicazione dell'equazione di von Bertalanffy generale: il caso di Helicolenus dactylopterus (Delar.) (Pisces: Scorpaenidae) del Tirreno Settentrionale. Oebalia 15, 753–762.
Ragonese S., Reale B. (1995). Distribuzione e crescita dello Scorfano di fondale, Helicolenus dactylopterus dactylopterus (Delaroche 1809), nello Stretto di Sicilia (Mar Mediterraneo). Biol. Mar. Mediterr. 2, 269–273.
Relini G., Bertrand J., Zamboni A. (1999). - Synthesis of the knowledge on bottom fishery resources in Central Mediterranean, Italy and Corsica. Biol. Mar. Mediterr. 6, 868.
Romanelli M., Palladino S., Tarulli E., Ferretti M. (1997). Stima dell'accrescimento di Helicolenus dactylopetrus (Delaroche) in Adriatico Meridionale tramite esame dell sagittae di esemplari prelevati con reti a strascico e palangari di fondo. Biol. Mar. Medit. 4, 554–556.
Sartor P., Mannini A., Carlucci R., Massaro E., Queirolo S., Sabatini A., et al. (2017). Sintesi delle conoscenze di biologia, ecologia e pesca delle specie ittiche dei mari italiani. Biol. Mar. Mediterr. 24, 608.
Schindler D. E., Geib S. I., Williams M. R. (2000). Patterns of fish growth along a residential development gradient in north temperate lakes. Ecosystems 3, 229–237. doi: 10.1007/s100210000022
Sequeira V., Neves A., Vieira A. R., Figueiredo I., Gordo L. S. (2009). Age and growth of bluemouth, Helicolenus dactylopterus, from the Portuguese continental slope. ICES J. Mar. Sci. 66 (3), 524–531. doi: 10.1093/icesjms/fsp010
Sion L., Calculli C., Capezzuto F., Carlucci R., Carluccio A., Cornacchia L., et al. (2019). Does the Bari Canyon (Central Mediterranean) influence the fish distribution and abundance? Prog. Oceanography 170, 81–92. doi: 10.1016/j.pocean.2018.10.2015
Sion L., Capezzuto F., Carlucci R., Cornacchia L., D’Onghia G., Indennidate A., et al. (2018). Età e accrescimento di Helicolenus dactylopterus nel Mar Ionio nord occidentale. Biol. Mar. Mediterr. 25, 183–186.
Sion L., Cornacchia L., Galasso G., Capezzuto F., Carluccio A., Maiorano P., et al. (2022). “Age and growth of Helicolenus dactylopterus in the north-western Ionian Sea: comparison between direct and indirect methods,” in 2022 IEEE International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea), Milazzo, Italy. 526–529. doi: 10.1109/MetroSea55331.2022.9950960
Smith B. J., Dembkowski D. J., James D. A., Wuellner M.R. (2016). A simple method to reduce interpretation error of ages estimated from Otoliths. Open Fish Sci. J. 9, 1–7. doi: 10.2174/1874401X01609010001
Spedicato M.T., Massutí E., M´erigot B., Tserpes G., Jadaud A., Relini G., et al. (2019). The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 83S1, 9–20. doi: 10.3989/scimar.04915.11X
Ungaro N., Marano G. (1995). Analytical models for Mediterranean species: an application on the Helicolenus dactylopterus (Delaroche) resource in the lower Adriatic. Rapport la Commission Internationale pour l’exploration scientifique la Mer Méditerranée 34, 260–281.
Whitehead P. J. P., Bauchot M. L., Hureau J. C., Nielsen J., Tortonese E. (1984-86). Fishes of the North-Eastern Atlantic and the Mediterranean (FNAM) Vol. 3 (Paris: Unesco), 1212 p.
Keywords: Helicolenus dactylopterus, age and growth, back calculation, length frequency distribution analysis, cold-water coral habitats, Central Mediterranean
Citation: Sion L, Cornacchia L, Galasso G, D’Onghia G and Carbonara P (2024) Integrating data from cold-water coral habitats increase knowledge on age and growth studies: the case of Helicolenus dactylopterus (Delaroche, 1809) in the central Mediterranean. Front. Mar. Sci. 11:1506180. doi: 10.3389/fmars.2024.1506180
Received: 04 October 2024; Accepted: 06 November 2024;
Published: 04 December 2024.
Edited by:
Josipa Ferri, University of Split, CroatiaReviewed by:
Ely Sidi Beibou, Institut Mauritanien de Recherches Océanographiques et des Pêches, MauritaniaAlba Jurado-Ruzafa, Centro Oceanográfico de Canarias (CN IEO, CSIC), Spain
Mark C. Belk, Brigham Young University, United States
Copyright © 2024 Sion, Cornacchia, Galasso, D’Onghia and Carbonara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Letizia Sion, bGV0aXppYS5zaW9uQHVuaWJhLml0
 Letizia Sion
Letizia Sion Laura Cornacchia1,2
Laura Cornacchia1,2 Gianfranco D’Onghia
Gianfranco D’Onghia