- 1Coastal Oregon Marine Experiment Station, Oregon State University, Newport, OR, United States
- 2Fuvahmulah Dream Non Governmental Organization (NGO), Fuvahmulah, Maldives
- 3Miyaru - Shark Programme, NGO, Fuvahmulah, Maldives
- 4Nature Friends of Maldives, NGO, Fuvahmulah, Maldives
The reproductive state of wild, free-swimming tiger sharks (Galeocerdo cuvier), was assessed using underwater ultrasonography at a diving site in Fuvahmulah, a Maldivian atoll within the central Indian Ocean. The presence of embryos were observed in 93% of the adult sharks (26/28) and two distinct embryonic size groups were observed within the subset of scanned adult females. The results suggest that the observed dive site functions as a gestation ground and builds upon previous work that emphasizes the importance of dive sites for the collection of biological data for shark conservation and management.
Introduction
Recent studies have demonstrated that a myriad of benefits result from healthy shark populations (e.g. Dedman et al., 2024; Heithaus et al., 2022). Despite the importance of sharks, the management and conservation of these predators have lagged behind their terrestrial counterparts (Dedman et al., 2024). Understanding the reproductive biology, including where gravid sharks spend their time gestating, is critical to the conservation of sharks as their general life characteristics (slow growth, low fecundity, long-lived) make this group of fish particularly susceptible to both anthropogenic and environmental stressors (e.g. Nowicki et al., 2021). Thus, establishing effective site-based management strategies is critical to safeguard these key life-history stages (Gallagher et al., 2021). Non-lethal approaches to studying reproduction in sharks (and other elasmobranchs) have negated the need to sacrifice the animals (e.g. Hammerschlag and Sulikowski, 2011; Sulikowski and Hammerschlag, 2023), which has created opportunities to better manage threatened species. One such approach has been the development of a portable, high-definition underwater ultrasound that has been used to determine if sharks are gravid in situ (e.g. Carrier et al., 2003; Hoyos-Padilla et al., 2023).
Diving with sharks has a global footprint and has become the main activity for the sustainable use of this group of animals (Gallagher and Huveneers, 2018). In the Maldives, local businesses associated with tourists who visit the country for shark diving generate an annual revenue of over $113 million US dollars (Zimmerhackel et al., 2018, 2019). Tiger sharks (Galeocerdo cuvier) are one of the most sought-after species for visitors seeking to dive with sharks in the Maldives (Ministry of Tourism, Republic of Maldives, 2022). The island of Fuvahmulah located on the Equatorial Channel became a globally renowned destination facilitating close encounters with these apex predators. Here, over 30 tiger sharks have been regularly documented year-round, during a single, shallow dive at Tiger Harbor (Araujo et al., 2024).
Observational studies at shark diving tourism sites are important because they can provide fishery-independent scientific information on changes in shark populations, monitor their health over time, and detect anomalies in local faunal patterns or even new species within a geographical area (e.g. Parmegiani et al., 2023; Fallows et al., 2013). This is especially true for tiger sharks as data from local dive operations (Vossgaetter et al., 2024) suggest that individual females are habitual to Tiger Harbor, returning every year and spending considerable time at the location. However, it is unknown where the tiger sharks migrate from, or where they migrate to, during disappearances from this location which generally last between 3-4 months (Vossgaetter et al., 2024).
Given that such aggregations have the potential to become important population monitoring sites, particularly when individuals can be easily and reliably identified (Pierce et al., 2018), the ability to link reproductive state to the observed animals becomes a critical tool in their conservation. To better elucidate the potential function of the aggregation site observed in Tiger Harbor, a portable, high-definition underwater ultrasound was used to determine if any of the free-swimming tiger sharks were gravid at this location (Hoyos-Padilla et al., 2023).
Materials and methods
Sampling occurred within the port of Fuvahmulah, a Maldivian atoll within the Indian Ocean. Dates of sampling were between May 2 and May 12, 2024 at a baited dive site named Tiger Harbor (Figure 1). Water depth was seven meters and the substrate consisted of primarily sandy bottom where the diving occurred. Dive times ranged from 30- 60 minutes. An IBEX Aquanaut (Figure 2; Hoyos-Padilla et al., 2023) was used to scan the abdominal cavity of free-swimming tiger sharks. Each shark that swam past the divers with the ultrasound was identified from a database initiated by Fuvahmulah Dive School in 2017 and currently co-administered with Pelagic Divers Fuvahmulah (Vossgaetter et al., 2024). The Aquanaut was equipped with a 60 mm curved linear array 2.5 to 5 MHz transducer (model eCL3) capable of a 50 cm scan depth which was connected to the unit with a 3 m cord. A video of the scanning event was recorded for each shark as it moved past the transducer and each video recording of the scanning event was time-stamped and later coordinated with the internal clock on the ultrasound to link a video of the gravid female to her recorded embryos. As in Hoyos-Padilla et al. (2023), scanning was performed primarily on the lateral surface from the pectoral to the pelvic fin in either transverse or longitudinal orientation to obtain cross-sectional and lengthwise images, respectively. Stills from the video were then used to measure (via proprietary software pre-installed on the Aquanaut) embryo diameter (cm) along the transverse axis (Sulikowski and Hammerschlag, 2023). Free-swimming female shark size was determined following the methods of May et al. (2019) where objects of established lengths were used as references to estimate the size of the shark from video files obtained during each dive. Based on a published mean size-at-maturity of 3.32m total length (TL) in the Indian Ocean, female sharks that were estimated >3.32 m were considered adults and females estimated ≤3 m were considered subadults (e.g Varghese et al., 2017). Data were statistically analyzed using R v.4.4.1 (one-sided, two sample t-test and Brunner-Munzel test). All data were tested for normality prior to parametric analysis, and all tests were considered significant at p ≤ 0.05.
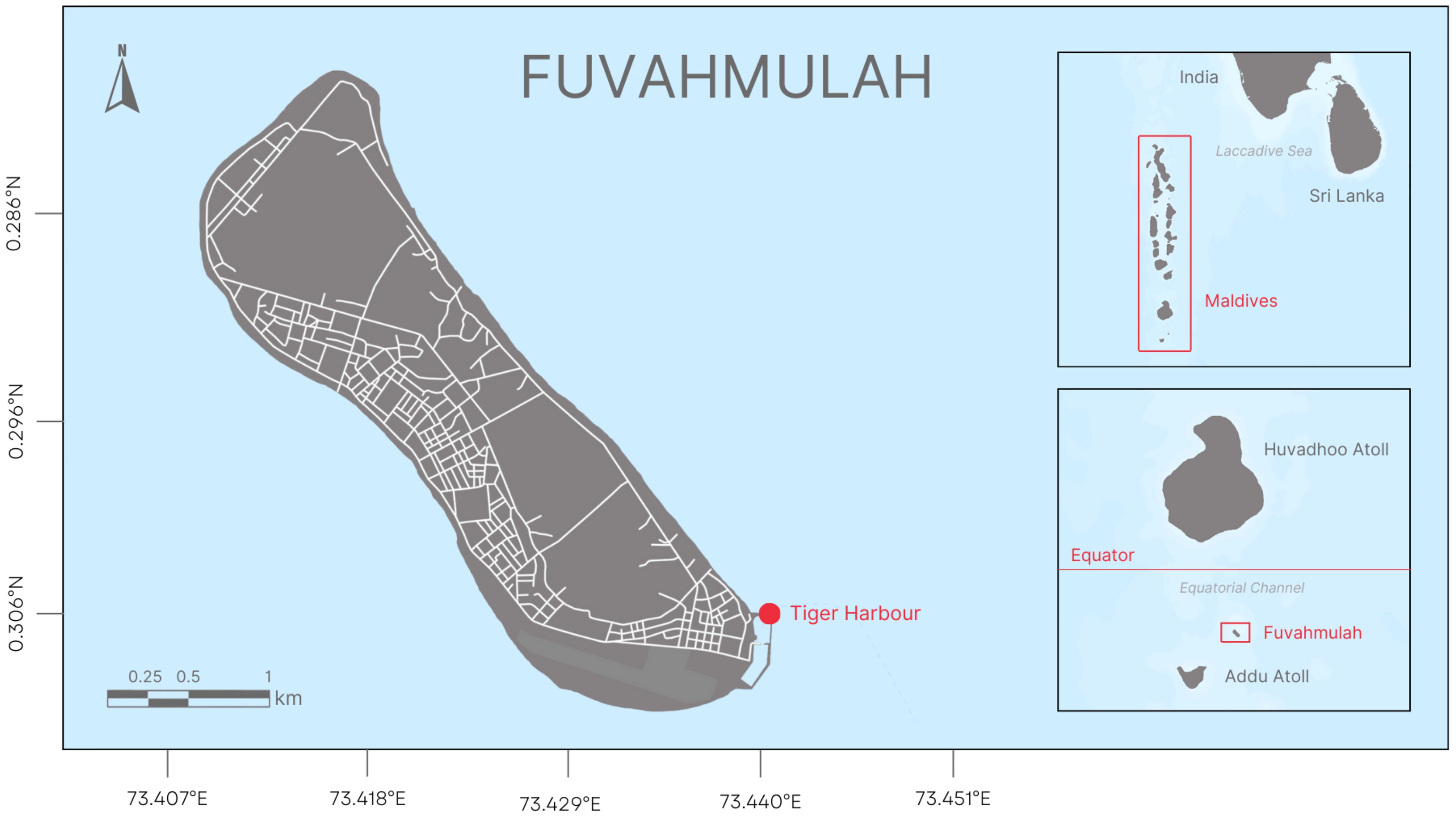
Figure 1. Location of the study area within the port of Fuvahmulah, a Maldivian atoll within the Indian ocean.

Figure 2. Representative distance from and lateral scanning of a free swimming pregnant tiger shark. Using the Aquanaut underwater ultrasound (E.I Medical Imaging, Inc). Scanning was conducted with a 60 mm curved linear array 2.5 to 5 MHz transducer. Scanning produced either a cross section or lengthwise orientation of embryos within the uterus.
Results
A total of 69 female sharks were observed (n=44 adult; n=25 sub-adult) as part of this study, with 36 sharks swimming at a distance that could be scanned. Of those 36 sharks, 32 were identified as adult individuals (greater than 3.32 m TL). The transducer was in contact with 28 adult sharks for a minimum of 4 seconds. In addition, over the course of this study, each of these sharks made several passes, and multiple scans of each shark were obtained. Despite repeated passes, transducer contact was limited in four sharks by their rapid movement which reduced transducer contact to under 2 seconds. No male sharks were observed over the study period. Based on the analysis of the Aquanaut video, 26 of the 28 scanned sharks (93%) were identified as gravid. Proprietary software measurements from still images indicated the measured embryos fell into two discrete groups (Student’s t-test, p-value < 0.01); group one (n=19) measured 8.0 cm (± 0.5 cm) in TL (Figure 3A), and group two (n= 7) measured 18.4 cm (± 1.3 cm) in TL (Figure 3B). The average size of adult females scanned was 3.79 m TL ± 0.1m) and no size differences existed between the gravid sharks carrying pups in each embryo group (Brunner-Munzel test, p-value > 0.5).

Figure 3. (A–C) Representative transverse ultrasound images of tiger shark Galeocerdo cuvier embryos measured using E.I Medical Imaging proprietary software. Embryo’s separated into two discreet groups (students t test, p-value < 0.01); (A) group one (n=19) measured 8.2 cm (0.5 cm) in total length (TL) and (B) group two (n= 7) measured 18.4 cm (1.4 cm) in total length (TL). (C) represents the scan from a non pregnant tiger shark. Arrow points to uterus.
Discussion
This study represents the first quantified aggregation of gravid female tiger sharks within the Indian Ocean and builds off previous work conducted at Tiger Harbor that suggested a proportion of tiger sharks may be gravid based on visual observation (Vossgaetter et al., 2024). The current study not only confirms this observation but was able to identify that 93% of scanned tiger sharks were gravid. This finding builds on the work of Hoyos-Padilla et al. (2023) by confirming high definition underwater ultrasounds can be used to identify the stage of gestation in free-swimming sharks. The statistical difference in embryonic size (10.4 cm TL) was an unexpected finding from the current study. While the reason for this observation is outside the scope of the current work, a potential explanation could be a product of asynchronous reproduction (e.g. Hoffmayer et al., 2013) since no differences existed within TL of gravid females within embryonic groups. While TL differences have been observed between tiger shark litters from different geographic regions (i.e Driggers et al., 2008) that scenario seems unlikely as the differences in embryo size observed herein occurred at the same temporal and spatial periodicity. However, given that tiger sharks’ neonates have been found to range in size at birth (from 45 to 90 cm in TL; Whitney and Crow, 2007), another explanation could be found in the considerable variability in habitat use and movements of tiger sharks (e.g. Hammerschlag et al., 2022). Accordingly, tiger sharks observed herein could be exposed to different environmental parameters (with varied nutritional conditions prior to arriving at the dive site (Rangel et al., 2022) which could impact embryo growth. Long-term monitoring of gravid females within the studies site as well as the use of electronic tracking of movement should be considered for future studies as such data would help inform the embryonic size difference.
Sampling size limitations of gravid females at various stages of gestation have limited the understanding of the reproductive cycle of tiger sharks and it remains one of the most challenging aspects of the species’ life history (Holland et al., 2019). As such, both biennial (e.g Castro, 2009) and triennial cycles (e.g., Whitney and Crow, 2007) have been postulated with suggested gestation periods of 12 and up to 16 months, respectively. While our data set is also limited, the proportion of pregnant sharks observed herein is unique in that way as it represents the highest recorded for the species (Whitney and Crow, 2007) and is more indicative of a biennial or possibly annual reproductive cycle. Similar to Sulikowski et al. (2016), the results suggest that Tiger Harbor may function as a refuge habitat like Tiger Beach in the Bahamas, functioning as a gestation ground where gravid females can benefit from year-round calm warm waters, potentially reducing the gestation period by accelerating embryo development (e.g. Jirik and Lowe, 2012). In addition, the Fuvahmulah supports a local tuna fishery for yellowfin Thunnus albacares and skipjack tuna Katsuwonus pelamis (Vossgaetter et al., 2024). While not quantified, these behaviors could provide gravid females with a consistent, low effort food source in the form of both discards and depredation events. Similar behaviors have been documented in a variety of other species of pregnant elasmobranchs (e.g.Brunnschweiler et al., 2018; Heim et al., 2021). Given the unique nature of the findings presented here, further research is required to better understand the relationship between habitat use and the reproductive status of female tiger sharks within Maldivian waters. Additionally, the findings of the current study have conservation implications, as the implementation of commercial long-line fishing is being considered in Maldivian waters. Fishing activities in areas with aggregations of gravid females would pose threats to the viability and health of local and regional populations (Sulikowski and Hammerschlag, 2023). Given the findings herein and those of Vossgaetter et al. (2024), year round fishing bans in this dive area should be considered to protect this critical life history stage. Regardless, the results of the current study build upon previous works (e.g. Hoyos-Padilla et al., 2023) that emphasizes the importance of ecotourism sites for the collection of biological elasmobranch data, conservation, and management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft. NP: Data curation, Formal analysis, Investigation, Writing – original draft. AA: Conceptualization, Data curation, Formal analysis, Writing – original draft. LV: Data curation, Investigation, Writing – original draft. FB: Data curation, Writing – original draft. HH: Methodology, Writing – original draft. AI: Funding acquisition, Writing – original draft. TI: Funding acquisition, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Fuvahmulah Dive Center and Pelagic Divers Fuvahmulah.
Acknowledgments
The project was facilitated by Miyaru - Shark Programme. Fieldwork and previous tiger shark identification data were provided through courtesies extended to us by Fuvahmulah Dive School and Pelagic Divers Fuvahmulah. This research was conducted under the Maldivian Scientific Research Permit: NRP2024/28 and Oregon State IACUC number 2023-0373. For field and dive support, special thanks to Ibrahim Siyan, Abdullah Niyaz, Hassan Saeed, Ibrahim Ziyadh, Mohamed Shiham, Irushaad Ahmed, Ahmed Iyaas, and Irushadh Ahmed. Thanks are extended to Kirsty Ballard and Lauren Horstmeyer for review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Araujo G., Scotts G., Zareer I. H. (2024). Review of Shark Diving Practices at Fuvahmulah, Maldives (Ocean Country Partnership Programme).
Brunnschweiler J. M., Payne N. L., Barnett A. (2018). Hand feeding can periodically fuel a major portion of bull shark energy requirements at a provisioning site in Fiji. Anim. Conserv. 21, 31–35. doi: 10.1111/acv.2018.21.issue-1
Carrier J. C., Murru F. L., Walsh M. T., Pratt H. L. (2003). Assessing reproductive potential and gestation in nurse sharks (Ginglymostoma cirratum) using ultrasonography and endoscopy: an example of bridging the gap between field research and captive studies. Zoo Biol. 22, 179–187. doi: 10.1002/zoo.10088
Castro J. I. (2009). Observations on the reproductive cycles of some viviparous North American sharks. Aqua 15, 205–222.
Dedman S., Moxley J. H., Papastamatiou Y. P., Braccini M., Caselle J. E., Chapman D. D., et al. (2024). Ecological roles and importance of sharks in the Anthropocene Ocean. Science 385, 6708. doi: 10.1126/science.adl2362
Driggers W. B., Ingram G. W., Grace M. A., Gledhill C. T., Henwood T. A., Horton C. N., et al. (2008). Pupping areas and mortality rates of young tiger sharks Galeocerdo cuvier in the western North atlantic ocean. Aquat. Biol. 2, 161–170. doi: 10.3354/ab00045
Fallows C., Gallagher A. J., Hammerschlag N. (2013). White sharks (Carcharodon carcharias) scavenging on whales and its potential role in further shaping the ecology of an apex predator. PloS One 8, e60797. doi: 10.1371/journal.pone.0060797
Gallagher A. J., Huveneers C. P. (2018). Emerging challenges to shark-diving tourism. Mar. Pol. 96, 9–12. doi: 10.1016/j.marpol.2018.07.009
Gallagher A. J., Shipley O. N., van Zinnicq Bergmann M. P., Brownscombe J. W., Dahlgren C. P., Frisk M. G., et al. (2021). Spatial connectivity and drivers of shark habitat use within a large marine protected area in the Caribbean, The Bahamas Shark Sanctuary. Fron. Mar. Sci. 1223. doi: 10.3389/fmars.2020.608848
Hammerschlag N., McDonnell L. H., Rider M. J., Street G. M., Hazen E. L., Natanson L. J., et al. (2022). Ocean warming alters the distributional range, migratory timing, and spatial protections of an apex predator, the tiger shark (Galeocerdo cuvier). Global Change Biol. 28, pp.1990–2005. doi: 10.1111/gcb.16045
Hammerschlag N., Sulikowski J. (2011). Killing for conservation: the need for alternatives to lethal sampling of apex predatory sharks. Endanger. Species Res. 14, 135–140. doi: 10.3354/esr00354
Heim V., Dhellemmes F., Smukall M. J., Gruber S. H., Guttridge T. L. (2021). Effects of food provisioning on the daily ration and dive site use of great hammerhead sharks, Sphyrna mokarran. Front. Mar. Sci. 8, 628469. doi: 10.3389/fmars.2021.628469
Heithaus M. R., Dunn R. E., Farabaugh N. F., Lester E., Madin E., Meekan M. G., et al. (2022). “Advances in our understanding of the ecological importance of sharks and their relatives,” in Biology of sharks and their relatives (Boca Raton, Florida: CRC Press), 487–521.
Hoffmayer E. R., Driggers W. B., Jones L. M., Hendon J. M., Sulikowski J. A. (2013). Variability in the reproductive biology of the Atlantic sharpnose shark in the Gulf of Mexico. Mar. Coast. Fish. 5, 1, 139–1, 151. doi: 10.1080/19425120.2013.783518
Holland K. N., Anderson J. M., Coffey D. M., Holmes B. J., Meyer C. G., Royer M. A. (2019). A perspective on future tiger shark research. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00037
Hoyos-Padilla E. M., Casanova-Santamaría I., Loria-Correa J. C., Sulikowski J. (2023). The successful use of a submersible ultrasound to confirm pregnancy on free swimming bull sharks, Carcharhinus leucas, in a provisioned shark site. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1193563
Jirik K. E., Lowe C. G. (2012). An elasmobranch maternity ward: female round stingrays Urobatis halleri use warm, restored estuarine habitat during gestation. J. Fish Biol. 80, 1227–1245. doi: 10.1111/j.1095-8649.2011.03208.x
May C., Meyer L., Whitmarsh S., Huveneers C. (2019). Eyes on the size: accuracy of visual length estimates of white sharks, Carcharodon carcharias. R. Soc Open Sci. 6, 190456. doi: 10.1098/rsos.190456
Ministry of Tourism, Malé, Republic of Maldives (2022).Maldives visitor survey September 2022. Available online at: https://www.tourism.gov.mv/en/downloads/visitor_survey (Accessed Sep 27, 2024).
Nowicki R. J., Thomson J. A., Fourqurean J. W., Wirsing A. J., Heithaus M. R. (2021). Loss of predation risk from apex predators can exacerbate marine tropicalization caused by extreme climatic events. J. Anim. Ecol. 90, 2041–2052. doi: 10.1111/1365-2656.13424
Parmegiani A., Gobbato J., Seveso D., Galli P., Montano S. (2023). First record of the bull shark Carcharhinus leucas (Valenciennes 1839) from the Maldivian archipelago, central Indian Ocean. J. Fish Biol. 103, 1242–1247. doi: 10.1111/jfb.v103.5
Pierce S. J., Holmberg J. A., Kock A. L., Marshall A. D. (2018). “Photographic identification of sharks,” in Shark research: emerging technologies and applications for the field and laboratory. Eds. Carrier J. C., Heithaus M. R., Simpfendorfer C. A. (CRC Press, Boca Raton, FL), 220–234.
Rangel B. S., Moreira R. G., Rider M. J., Sulikowski J. A., Gallagher A. J., Heithaus M. R., et al. (2022). Physiological state predicts space use of sharks at a tourism provisioning site. Anim. Behav. 191, 149–163. doi: 10.1016/j.anbehav.2022.07.004
Sulikowski J. A., Hammerschlag N. (2023). A novel intrauterine satellite transmitter to identify parturition in large sharks. Sci. Adv. 9, eadd634. doi: 10.1126/sciadv.add6340
Sulikowski J. A., Wheeler C. R., Gallagher A. J., Prohaska B. K., Langan J. A., Hammerschlag N. (2016). Seasonal and life-stage variation in the reproductive ecology of a marine apex predator, the tiger shark Galeocerdo cuvier, at a protected female-dominated site. Aquat. Biol. 24, 175–184. doi: 10.3354/ab00648
Varghese S. P., Unnikrishnan N., Gulati D. K., Ayoob A. E. (2017). Size, sex and reproductive biology of seven pelagic sharks in the eastern Arabian Sea. J. Mar. Biol. Ass. 97, 181–196. doi: 10.1017/S0025315416000217
Vossgaetter L., Dudeck T., Crouch J., Cope M., Ivanova T., Siyan I., et al. (2024). Non-invasive methods characterise the world’s largest tiger shark aggregation in Fuvahmulah, Maldives. Sci. Rep. 14, 21998. doi: 10.1038/s41598-024-73079-3
Whitney N. M., Crow G. L. (2007). Reproductive biology of the tiger shark (Galeocerdo cuvier) in Hawaii. Mar. Biol. 151, 63–70. doi: 10.1007/s00227-006-0476-0
Zimmerhackel J. S., Kragt M. E., Rogers A. A., Ali K., Meekan M. G. (2019). Evidence of increased economic benefits from shark-diving tourism in the Maldives. Mar. Pol. 100, 21–26. doi: 10.1016/j.marpol.2018.11.004
Keywords: emerging technologies, gestation ground, conservation, management, ecotourism
Citation: Sulikowski JA, Perisic N, Askin A, Vossgaetter L, Bocchi F, Hussain Ali Didi H, Inah A and Ivanova T (2024) Identification of the first gestational ground for tiger sharks (Galeocerdo cuvier) in the Central Indian Ocean using a high-definition submersible ultrasound. Front. Mar. Sci. 11:1500176. doi: 10.3389/fmars.2024.1500176
Received: 22 September 2024; Accepted: 11 October 2024;
Published: 14 November 2024.
Edited by:
David Yurkowski, Fisheries and Oceans Canada (DFO), CanadaReviewed by:
Peter Gausmann, Ruhr University Bochum, GermanyNatascha Wosnick, Federal University of Paraná, Brazil
Copyright © 2024 Sulikowski, Perisic, Askin, Vossgaetter, Bocchi, Hussain Ali Didi, Inah and Ivanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James A. Sulikowski, SmFtZXMuU3VsaWtvd3NraUBvcmVnb25zdGF0ZS5lZHU=
 James A. Sulikowski
James A. Sulikowski Nathan Perisic
Nathan Perisic Arzucan Askin
Arzucan Askin Lennart Vossgaetter
Lennart Vossgaetter Filippo Bocchi
Filippo Bocchi Hamna Hussain Ali Didi3
Hamna Hussain Ali Didi3