- Department of Marine Science and Fisheries, College of Agricultural and Marine Sciences, Sultan Qaboos University, Muscat, Oman
Larval connectivity relies on the ability of coral larvae to disperse into the environment following ocean currents. At short timescales, larval connectivity plays a key role in the resilience of coral reefs, as it determines their capacity to regain structure and function after major disturbances. At longer time scales, larval connectivity controls the distribution and, ultimately, the biogeography of species. We used a Lagrangian stochastic model to simulate the transport routes of coral larvae released from the major reef communities of the Arabian Sea and Gulf of Oman. The model used surface currents from two independent global circulation models, and we simulated 120 scenarios, covering four years and three larval competency models. Additionally we determine mean flow fields and LCS structures based on 20 years of reanalysis data from a third model. Connectivity values—the proportion of larvae successfully transported from their natal reef to another reef—varied significantly across reefs and years due to mesoscale variability in ocean currents, yet both circulation models produced similar overall patterns of connectivity. The general flow of larvae was from northwest to southeast in the Gulf of Oman, and from southwest to northeast in the Arabian Sea. The exchange of larvae across Ras Al-Hadd between the coral communities of the Arabian Sea and those of the Gulf of Oman is very low. Local retention (self-seeding) was the most important larval source for most reefs (mean = 32.3% for spawning corals and 70.8% for brooding corals). All reefs received larvae from at least one other reef and several received larvae from as many as five other reefs. ANOVA indicated significant differences between brooding and spawning coral larvae, and between reefs. Differences between years depended on the reef or reproduction type. Some reefs (Daymaniyat Islands in the Gulf of Oman and Mirbat in the Arabian Sea) could be considered sources of larvae, as they proportionally produced more larvae that later settled successfully than the other reefs. The limited connectivity between the Gulf of Oman and Arabian Sea supports their biogeographic distinction based on species distribution.
1 Introduction
Over large temporal scales, reef-building scleractinian corals have relatively narrow habitat requirements: shallow waters to ensure photosynthesis of zooxanthellae, rocky substrates for attachment, and a range of temperatures suitable for maintaining the symbiotic relationship between coral and zooxanthellae. In the Northern Arabian Sea and the Gulf of Oman, in particular, these conditions are uncommon, and only a few locations show significant development of diverse coral communities and a few true coral reefs. Ten of these areas with well-developed coral communities were identified in the literature, although sparse coral communities can be observed between these areas: eight in the Sultanate of Oman (Claereboudt, 2006a) and one along the north shore of the Gulf of Oman in Chabahar Bay, Iran (Maghsoudlou: Pers. Com.) and one along the coast of Yemen near Mukallah (Benzoni et al., 2003). Areas devoid of corals are typically characterized by long stretches of coastlines lined by beaches or salt marshes (Sabkha).
Recent classifications of biogeographic regions of the world’s marine ecosystems based on community compositions (Spalding et al., 2007) have identified three eco-regions in the northwestern Indian Ocean: the Arabo-Persian Gulf, the Gulf of Oman (also known as the Sea of Oman), and the Western Arabian Sea. In particular, the Gulf of Oman and the Western Arabian Sea, differ in their coral community fauna (Sheppard and Sheppard, 1991). Endemic species such as the clownfish Amphiprion omanensis, the parrotfish Scarus zufar (Randall and Hoover, 1995), the abalone Haliotis mariae (Geiger and Owen, 2012), and the coral Porites decasepta (Claereboudt, 2006b) have a restricted distribution along the coral communities of the Arabian Sea coast of Oman. In addition, several genera of scleractinian corals (Leptoria, Goniastrea) or species (Pavona minuta, Fungia scabra, and Alveopora sp.), octocorals (Tubipora musica, Lobophyton spp.), and the hydrozoan Millepora sp., common in the Gulf of Aden (DeVantier et al., 2004), are present along the Arabian Sea coast of Oman (Sheppard and Sheppard, 1991); however, they do not seem to cross the limits between the Arabian Sea and the Gulf of Oman at Ras-Al-Hadd (Claereboudt, 2006a). A similar sharp biotic transition between the Gulf of Oman and the Arabian Sea has also been described for macrophyte (Schils and Wilson, 2006), holothuroid (Claereboudt and Al-Rashdi, 2011), and fish communities (Burt et al., 2011).
Most marine species have a potentially wide dispersive stage in the form of pelagic larvae which are transported by ocean currents. Boundaries between biogeographic regions based on differences in species records are ideal places to identify oceanographic barriers to gene flow (Baums et al., 2006). Such oceanographic barriers have been identified, for instance, in coastal convergence zones in California (Dawson, 2001), Puerto Rico at the Mona Passage separating the Eastern and Western Caribbean regions (Baums et al., 2006), and more recently in the East Pacific between Polynesia and the west coast of the Americas (Wood et al., 2014). In Oman, remotely sensed images of both sea surface temperature and ocean color show a distinct front and its associated eddies in the surface water masses located northwest and southeast of the Ras-Al-Hadd headland (Ayouche et al., 2021), suggesting the possibility of such an oceanographic barrier.
Several recent studies have detailed dispersal patterns of reef community organisms in the Red Sea (Raitsos et al., 2017; Wang et al., 2019) and the Arabo-Persian Gulf (Riegl et al., 2018), showing a general pattern of local recruitment with occasional and variable long-distance dispersal. For the Arabian Sea and Gulf of Oman, Torquato and Møller (2022) simulated the dispersal of fish larvae and determined a biogeographic break at Ras Al-Hadd.
The aim of this study was to evaluate the level of connectivity among coastal coral communities in the northern Arabian Sea and the Gulf of Oman and to test the hypothesis of limited dispersal of coral larvae across the Ras-Al-Hadd headland. Using publicly available circulation data (US Navy NCOM, HYCOM consortium models, and GLORYS reanalysis fields), we modeled coral larvae trajectories released from known coral communities in the Arabian Sea and Gulf of Oman, and calculated Lagrangian Coherent Structures of mean flow fields. The model trials were designed to answer the following questions: (1) What are the relative levels of connectivity between the Arabian Sea and Gulf of Oman coral communities? (2) How variable are these connectivity patterns across years? (3) Can the modeled connectivity pattern explain the steep change in community composition observed for several taxa across Ras-Al-Hadd?
2 Materials and methods
2.1 Study region
This study focuses on the Northern Arabian Sea and Gulf of Oman (15°N–26°N and 51°E–65°E). Geologically, the northern Arabian Sea forms a relatively deep basin, partially isolated from the Indian Ocean by rising chains of seamounts, including the Murray Ridge and Owen Fracture Zone. The shelf is narrow along most of Oman’s coastline, except between the Hallaniyat Islands and Masirah and northwest of Muscat (Figure 1). The oceanography of the region is dominated by the seasonal alternation of large-scale upwelling, with a northeast current during the summer monsoon (June–September) and a southwest current during the winter monsoon (November to March) (Burkill, 1999). This general circulation pattern is altered by numerous mesoscale eddies, some of which persist over long periods of time (Tang et al., 2002; L’Hégaret et al., 2013) with potential long-term ecological consequences (Zhang et al., 2014). Because of the large yearly variability in the surface velocity field (Piontkovski et al., 2019), it is important to account for this mesoscale variability in the model by (a) extending the spawning period to approximately five weeks around the suspected spawning time (March–April), and (b) conducting multiple replicate spawning events over a four-year period. In the Gulf of Oman, spawning was recorded only once, between March 5 and 17, 2003, near Muscat (personal observation). In April–May 2013–2014, Howells et al. (2014) reported a multi-species event in South Musandam, while the only reported spawning period in the Arabian Sea extends from February to April (DeVantier et al., 2004) at Socotra.
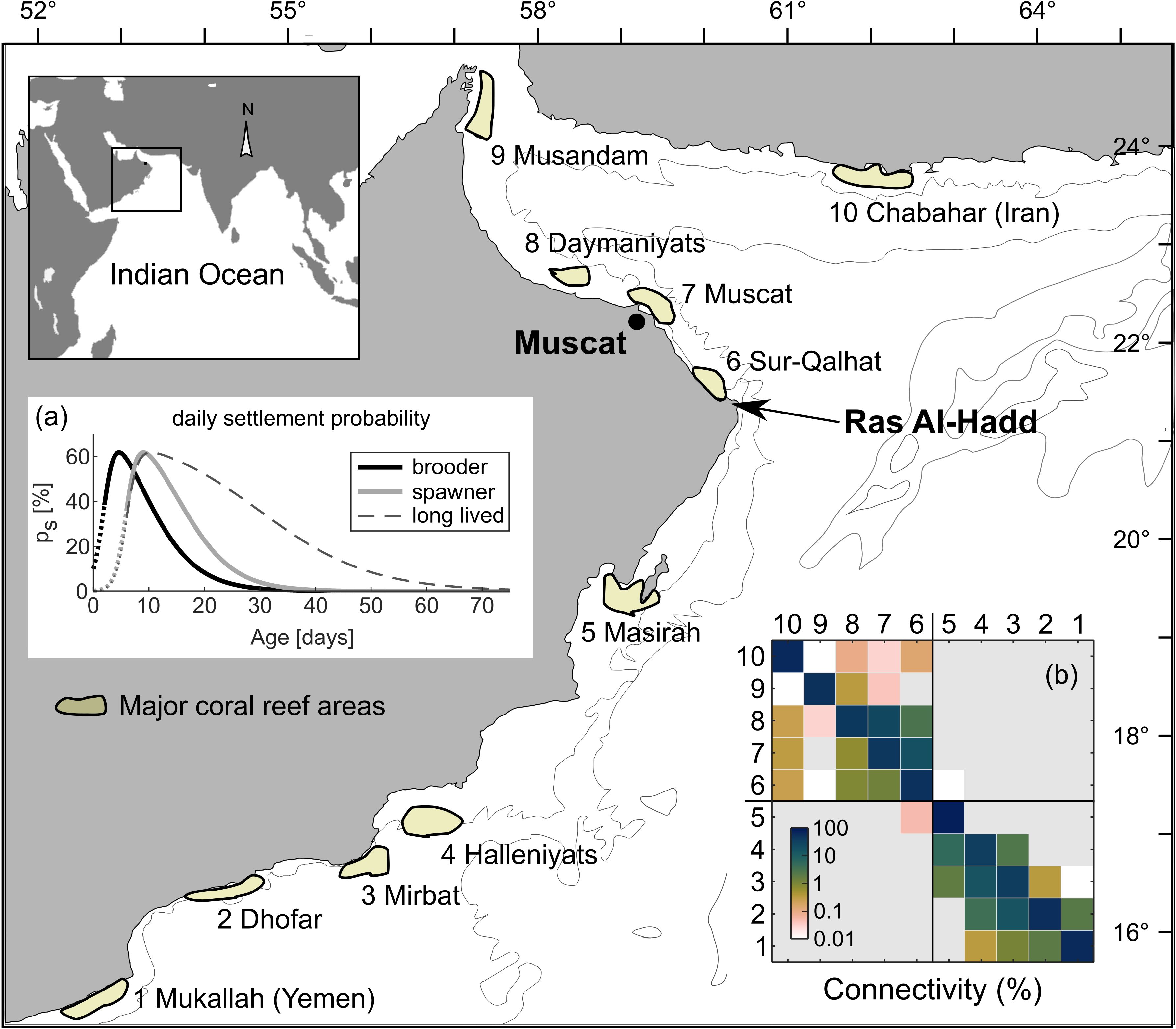
Figure 1. The geographic focus of the study was the Northern Arabian Sea and the Gulf of Oman (14°N –26°N and 51°E –65°E). Areas with significant coral communities were marked with colored polygons and labeled according to their geographic names. Insert (A) shows the settlement competency functions for three types of simulations: brooding, spawning, and long-lived coral larvae. Insert (B) shows the overall connectivity (%) between reef areas in logarithmic color coding. The matrix represents the average of 96 standard simulations (except for the long-lived simulations).
Although sparse coral colonies occasionally occur along most of the region’s coastline, well-developed communities occur only in a few areas in the Gulf of Oman and Arabian Sea. Some of these areas are quite large (tens of kilometers), but they are separated by extensive areas unsuitable for coral development (sandy-muddy beaches, Sabkha) and can thus be considered distinct. Here, we considered 10 coral-rich systems: five in the Arabian Sea and five in the Gulf of Oman (Figure 1).
2.2 Hydrodynamic models
The results of two independent hydrodynamic models were used to simulate the surface currents of the Gulf of Oman and the Arabian Sea: the NCOM (1/8°, Navy Coastal Ocean Model) model developed by the US Navy (Kara et al., 2006; Barron et al., 2007), and the HYCOM (1/12°, Hybrid Coordinate Ocean Model) (Bleck, 2002; Chassignet et al., 2007). For the NCOM model, daily real-time nowcasts of surface current velocities were interpolated every 2 h. More details and data can be accessed at https://www.ncei.noaa.gov/products/weather-climate-models/global-navy-coastal-ocean. For the HYCOM model, the 3-hourly reanalysis fields of surface currents were interpolated hourly. Additional information and data are available at https://hycom.org/data/glbu0pt08/expt-19pt1 (Fox et al., 2002; Cummings, 2005; Cummings and Smedstad, 2013). Both models are based on similar fundamental flow descriptions, but differ in their numerical implementation, discretization scheme, resolution, parameterizations, and data assimilation. The replication of dispersal modeling using these two models allows the assessment of the influence of uncertainties in flow fields. Direct validations of the modeled surface currents for the area and period considered in this study were not available. A study using these models, but applying ensemble metrics, was conducted in the Gulf of Mexico and suggested that both models produce adequate Lagrangian trajectories and dispersal patterns (Wei et al., 2016). The HYCOM model, extensively used in the Arabian Sea (McClean, 2015), was globally validated by Zhang et al. (2023), who found that it generally conforms to ocean current characteristics, although it exhibits regional differences in accuracy, particularly in the Gulf Stream and other major currents.
To visualize the general circulation pattern and enhance the robustness of our results, we used currents from an additional reanalysis product: the 1/12° Global Ocean Physics Reanalysis (GLORYS12V1). We analyzed 20 years (2002–2021) of monthly mean flow fields in March and April. The depth layers from the surface to 20 m were averaged to account for vertical flow variations in the upper water column. The general circulation in March and April was determined by calculating the 20-year average for each month. These flow fields were further used to calculate the Lagrangian coherent structures, as discussed in Section 2.5.
2.3 Larval dispersal
Larval dispersal was simulated offline using both circulation models for the same period. To evaluate the extent to which coral and other larvae disperse under realistic oceanographic conditions, we used the same particle-tracking dispersal models (Lagrangian stochastic model, LSM) with slightly different parameterizations for the two circulation models to account for the differences in their spatial resolutions.
The individual particle (larva) velocity was calculated as follows:
where Ua is the daily (NCOM) or 3-hourly (HYCOM) horizontal ocean surface velocity and ur is a random component added to the horizontal velocity vector using:
where R is a real random uniform deviation between +1 and −1 and Kh is the imposed explicit Lagrangian horizontal diffusion of the form:
where l is the unresolved sub-grid scale of the flow model (≈ 13 km for the NCOM model and ≈ 9 km for the HYCOM model) and is the turbulent dissipation rate (Peliz et al., 2007). We used 2-hour time steps (Δt) on the 1/8° NCOM circulation data and 1-hour time steps for the simulations running on the 1/12° HYCOM model.
Coral larvae were assumed to belong to one of three types: spawned larvae with relatively long larval durations, brooded larvae with comparatively shorter pelagic larval durations, and long-lived larvae with characteristics of spawned larvae but with larval settlement competency extended to 70 days. Larval behavior was modeled assuming that coral larvae drift passively in the upper layer of the ocean after being released into the water column by the parent colony. Settlement was modeled as follows: larvae were allowed to develop during a pre-competency period estimated to be 48 h for brooding corals and six days for spawning corals (Blanco-Martin, 2000). After this initial pre-competency period, the larvae settled only if they encountered an appropriate substrate in their current-driven trajectories. The maximum competency period lasted 15 days for spawners (Miller and Mundy, 2003) and eight days for brooders (Blanco-Martin, 2000). After this period of high competency, larvae progressively lost their ability to settle successfully until the end of the pelagic larval duration (PLD), which was set at 40 days in this series of simulations (70 days in the long PLD trials). The settlement behavior was parameterized as the daily probability of a successful settlement and modeled according to the following equation:
where Age is the age of the larva (days), Tc is the duration of the pre-competence period (days), Tk (days) indicates the end of the competence period (the period after which the settlement probability ps starts to decline), and β is the shape coefficient (0.2 in all simulations) of the settlement function, which alters the rate at which larvae lose competency after the competency period (Figure 1A). From then on, settled larvae, having left the pool of drifting pelagic larvae, were considered recruits, and their geographic positions were recorded.
Land boundaries were considered reflective and coral habitats suitable for settlement were parameterized as a series of 10 closed polygons covering the actual coral reef areas of the region, with a buffer zone extending offshore around the coral communities. The size of the buffer zone was set to the length of the unresolved sub-grid scale of the model: 13 km and 9 km for the NCOM and HYCOM models, respectively. Larvae were released evenly across the reef areas, including those close to the borders of the polygons, to ensure that some larvae could always leave their source reefs and become exposed to the mesoscale flow field. Corrections for mortality or predation were not introduced into the model. Thus, successful recruitment in this model was solely driven by transport settlement and did not include any peri- or post-settlement processes (competition, growth, mortalities) or behavior beyond competency, such as swimming or vertical migration.
In the Lagrangian transport simulations, we advected larvae within the surface layer of the water column. Being rich in lipids, coral larvae are likely to be naturally buoyant, at least for the first 30 days after spawning (Figueiredo et al., 2012). Torquato and Møller (2022) also found that while vertical migration influenced the transport of fish larvae through the straits of Bab el Mandeb and Hormuz, it had little impact on larval connectivity in the Arabian Sea and Gulf of Oman. Furthermore, the vertical migration behavior of coral larvae is species- and region-specific, and is influenced by environmental conditions (e.g., Fagerström et al., 2022). To our knowledge, no quantified descriptions of vertical migration behavior are available for the coral larvae in our study area. Incorporating uncertain vertical migration patterns would introduce further complexity and uncertainty into our findings.
Stokes drift might play a role in the advection of larvae on the surface. Dobbelaere et al. (2024) found that hurricanes can alter general dispersal patterns through their combined effects on waves and currents. However, the March/April period in our study region corresponds to the pre-monsoon season, with only moderate wind and sea-state conditions. Therefore, we did not include the Stokes drift in our simulations. Integrating the complex effects of Stokes drift on different larvae (Fuchs et al., 2018) requires wave modeling coupled with species-specific reactions to wave stresses and vertical migration dynamics, which is beyond the scope of this study.
The resolution of the models we used was coarser than the typical size of individual reefs. At the 2,000 km scale and within our study region, higher-resolution flow data, particularly with advanced data assimilation schemes, are not readily available. Despite resolution limitations, which do not fully resolve coastal scales, these models represent the most comprehensive data available for capturing broad oceanic currents in our study area. Therefore, they are suitable for our main objective of understanding the general patterns of larval movement over large geographical scales.
To address the uncertainty in the flow fields, arising in part from unresolved sub-grid processes, and to test the sensitivity of our results to resolution and other model parameters, we used two different circulation models for Lagrangian transport. We conducted transport simulations for four distinct years and observed several spawning events each year.
2.4 Simulation trials
Six spawning events (2,000 particles each) of both spawning and brooding corals were generated in each of the 10 coral reef areas on six regularly spaced dates (every 9 days) within the estimated spawning period in March–April of each year. To resolve inter-annual variability, current fields from four successive years (2005, 2006, 2007, and 2008) were used in both NCOM and HYCOM flow fields. Successful recruitment was measured as the relative number of larvae settling in a suitable habitat during the 40-day duration of each spawning event. A connectivity matrix was built for each of the 96 individual events (4 years × 6 dates × 2 larval types), and summary matrices were calculated for each year. Three summary values were extracted from these 96 matrices: (A) a local retention index, calculated as the relative proportion of larvae released and settling in the same reef area (the diagonal values of the connectivity matrix); (B) a success index, which is the proportion of larvae released in one location and settling anywhere (the sum of each row in the connectivity matrix); and (C) a subsidy index, which represents the proportion of larvae born on a reef and settling on another reef (i.e., the difference between B and A) (Cowen et al., 2006).
An additional series of simulations was run, using the NCOM circulation model only, for broadcast spawning corals with a competence period extended to 40 days and a survival period of 70 days to simulate the reported extreme larval duration of some scleractinian species (Wilson and Harrison, 1998; Nozawa and Harrison, 2007).
2.5 Lagrangian coherent structures
Lagrangian coherent structures (LCS) are useful for identifying regions of flow separation and transport barriers relevant to larval connectivity (Chaput et al., 2022). We used this method as an additional approach to enhance the robustness of our results. We calculated the Finite-Time Lyapunov Exponent (FTLE) for both mean monthly flow fields of the GLORYS model (c.f. 2.2). A regular 1/24° particle grid was forward-advected for 20 days using a time step of 10 min. After advection, the FTLE field was derived by analyzing the deformation of the particle grid. The deformation gradient tensor between the final and initial particle positions is defined as:
where the subscripts 0 and 1 indicate the start and end positions, respectively. B is the right Cauchy-Green deformation tensor. The FTLE is calculated as FTLE = log(λ_max), where λ_max is the largest eigenvalue of B. Ridges of high values in the resulting FTLE field, computed through forward-time integration, correspond to the repelling LCS. These ridges highlight regions where the particle trajectories diverge strongly, indicating transport barriers.
2.6 Analysis
The summary indices of connectivity (success, local retention, and subsidy indices) were analyzed using a nested model of analysis of variance (ANOVA) with Reef (different reef areas) nested within the region (Arabian Sea vs. Gulf of Oman) as Factor 1, Region as Factor 2, Year (2005, 2006, 2007, 2008) as Factor 3, and Reproduction Strategy (Brooding vs. Spawning) as Factor 4. All factors were considered fixed except for Reef, which was treated as random. An transform was applied to all indices to normalize the 0–1 proportion values (Zar, 1984). All statistical analyses were carried out using the R package (R Core Team, 2017).
3 Results
Dispersal patterns indicated that local retention was predominant in most of the 10 communities (diagonal values in Figure 2). Both circulation models yielded similar results, with an average of 32.3% of larvae from spawning corals released from any reef settling in the same reef area using the NCOM model and 29.7% using the HYCOM model. For brooding corals with a shorter pelagic phase, the percentage of local retention increased to an average of 70.8% and 79% in the NCOM and HYCOM models, respectively (Table 1), with a simultaneous decrease in between-reef connectivity (subsidy index; Table 1).
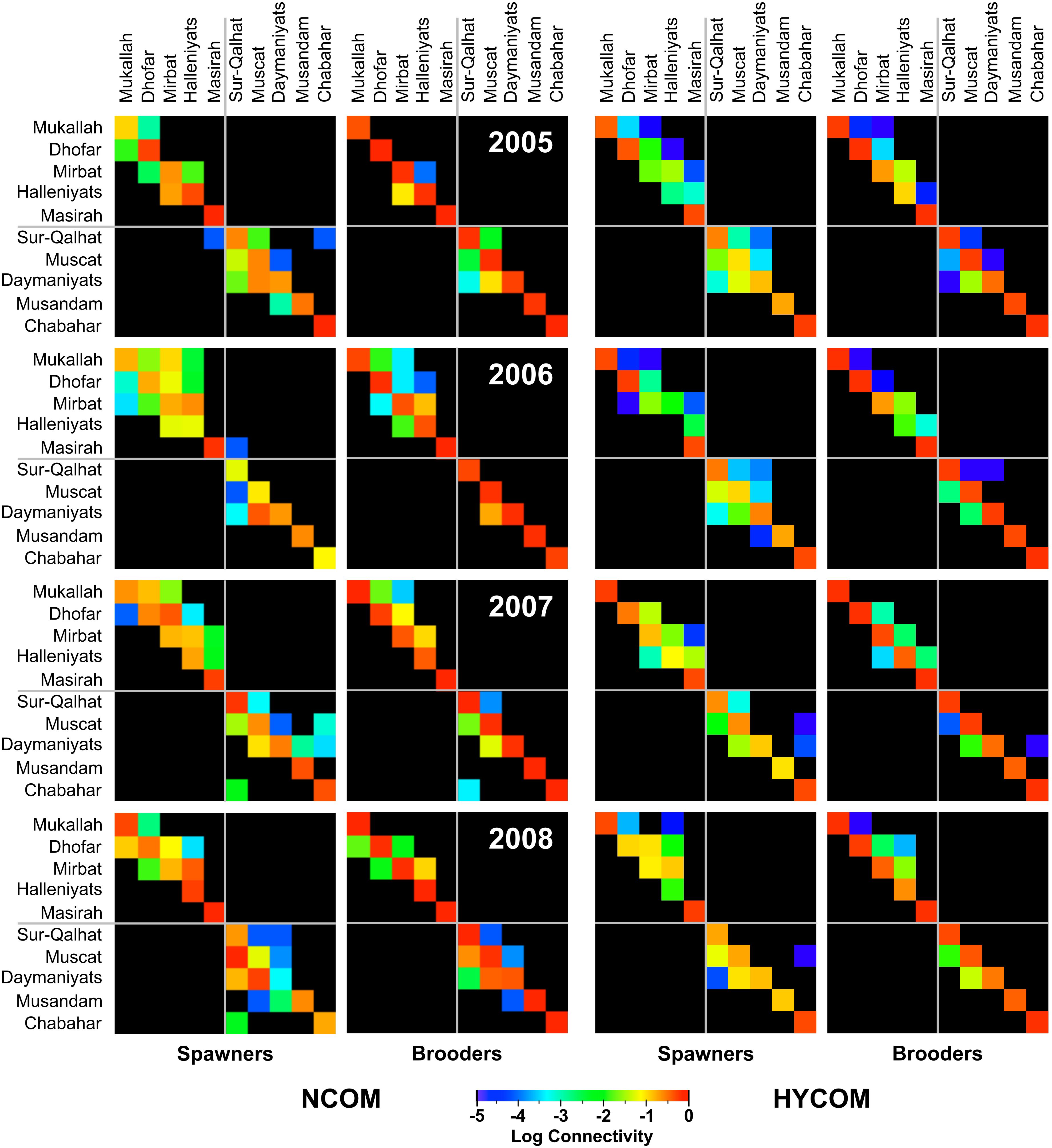
Figure 2. Connectivity matrices for all 10 coral communities. Shown are 16 matrixes (2 × 4 × 2), for the two hydrodynamic models (NCOM and HYCOM), the four studied years (2005–2008) and distinguished between spawning and brooding corals. Each matrix was built by observing the tracks of all larvae released from each reef community on six dates in March and April of each year. Each cell in this matrix represents the proportion (0–1) of larvae that are released from the community listed on the y-axis (‘from’ community) and settle in the community listed on the x-axis (‘to’ community). The diagonal values from top-left to bottom-right of each matrix correspond to local retention (self-seeding). Colors follow a logarithmic scale with high connectivity in red, low connectivity in blue, and no observed connectivity in black. The gray lines subdivide each matrix between the Gulf of Oman and Arabian Sea.
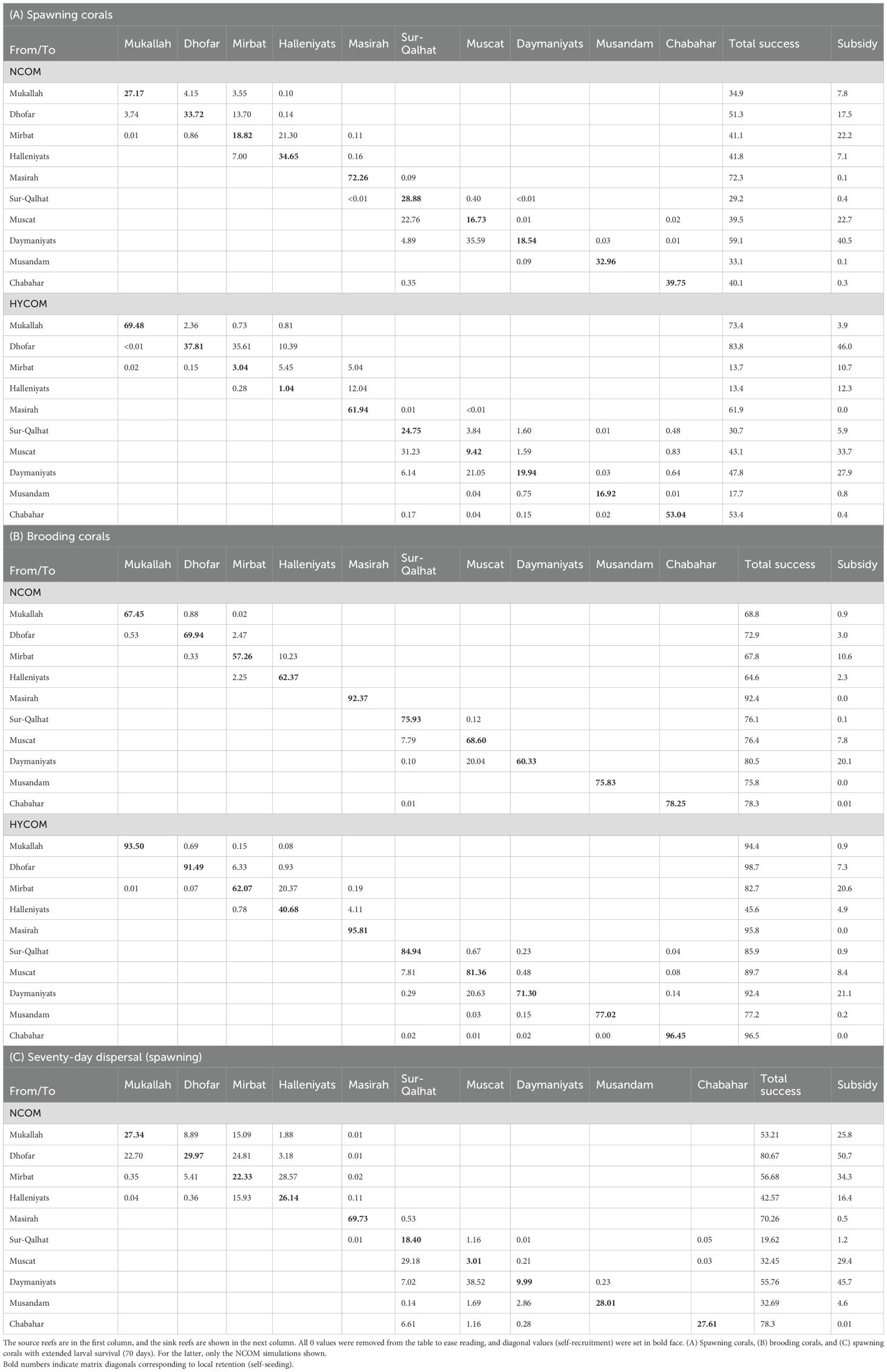
Table 1. Overall connectivity (% of larvae released in one reef area and successfully settling) was calculated over six replicated spawning events in four different years (48,000 larvae from each reef area) during a 2-month period beginning on 3 March, using both the NCOM and HYCOM circulation models.
However, there were some exceptional connections where transport between reefs was significant; for instance, many of the larvae settling in Muscat originated from the Daymaniyat Islands (21%–35%), and the Halleniyat Islands received a considerable input of larvae from coral communities further south: the Mirbat reef area in the NCOM model and the Dhofar reef area in the HYCOM model (Table 1). Connectivity matrices for brooders and spawners showed similar patterns of connectivity, favoring local retention or short-distance connectivity, with high values along or near the diagonal of the connectivity matrix (Table 1; Figure 2). The connectivity matrices were not symmetrical; generally, the connectivity values for reefs situated east of the larval source in the Gulf of Oman and north of the source in the Arabian Sea were larger than those in the opposite direction, defining a general direction of larval flow: southwest in the Gulf of Oman and northeast in the Arabian Sea. Globally, these dispersal patterns corresponded to two main modes: (A) an eddy mode, where larvae left the coastal zone through one of the numerous nearshore eddies common along both the Gulf of Oman and the Arabian Sea during the pre-summer monsoon period, resulting in low connectivity (Figure 3A); and (B) an alongshore mode, where larvae followed an alongshore current until encountering either parts of the same reef complex originating from or another reef system located downstream (Figure 3B). A few larvae successfully rode eddies from Oman to settle in Chabahar, Iran, particularly in 2007 (Table 1; Figure 2).
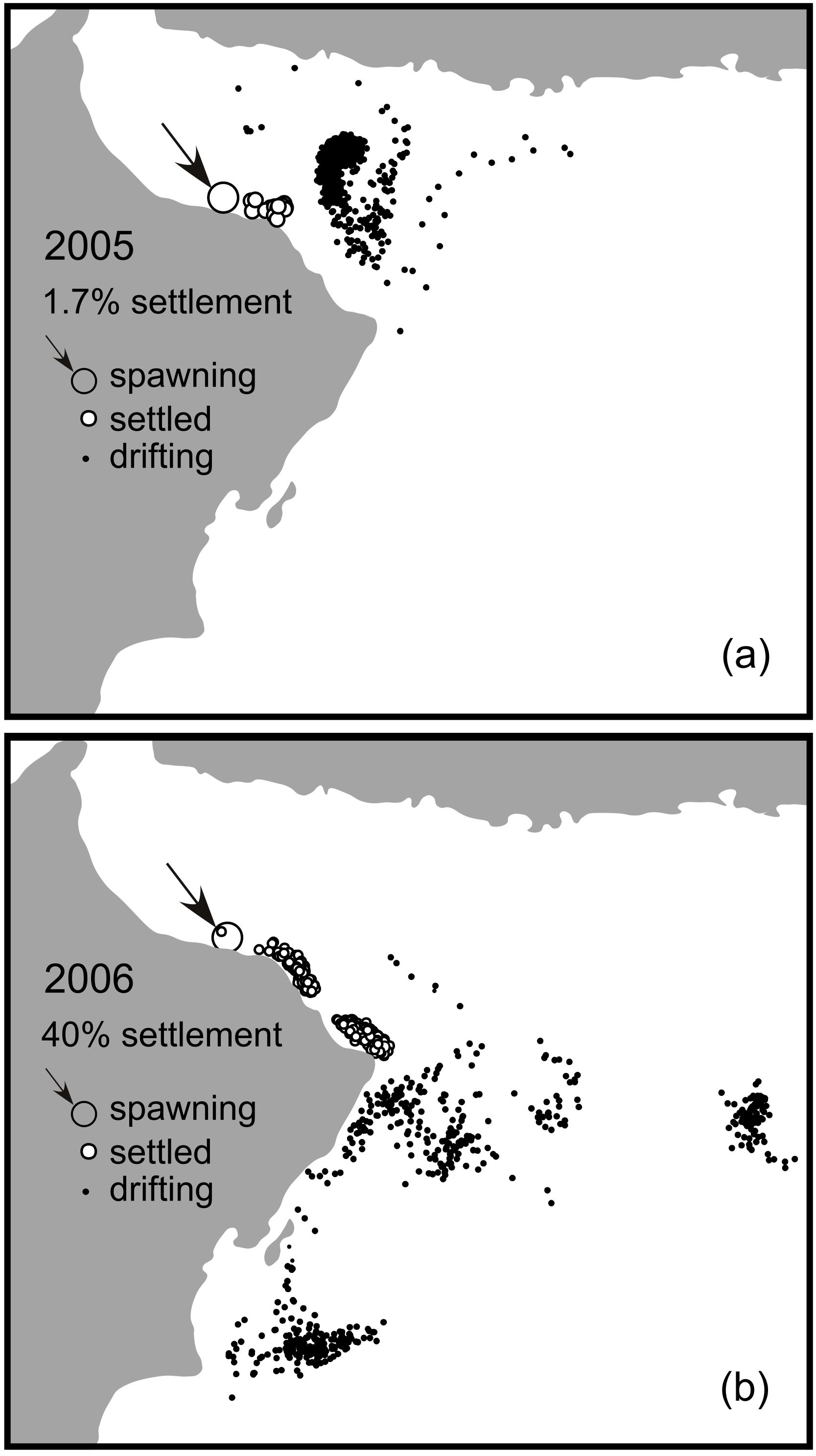
Figure 3. Two examples of dispersal patterns. The figures represent the position of the drifting larvae at the end of the 40-day simulation for two spawning events initiated on the Daymaniyat Islands reefs (arrows and largest disks) on the same date in two different years: 3 March 2005 and 2006. Larvae that settled successfully are represented by white circles, whereas larvae that still drifted at the end of the simulation (40 days) are represented by small black dots. In (A) larvae rapidly entered a series of eddies and remained offshore for most of their competence period (eddy-dispersal pattern). In (B), the larvae followed a coastal current, leading to high connectivity between the origin and downstream coral communities (alongshore dispersal pattern).
Although there were variable exchanges of larvae between reefs within both the Gulf of Oman and Arabian Sea (non-diagonal values in connectivity matrices), there were no or only minimal exchanges of larvae between the two sub-regions, and most cells in the upper-right and lower-left quadrants of the connectivity matrix were empty (Table 1; Figure 2). Using the NCOM model, only two spawning dates in 2005 and 2006 resulted in the successful transport of a few larvae from Sur-Qalhat to Masirah (1/12,000; Figure 2, 2005) and from Masirah to Sur-Qalhat (32/12,000; Figure 2, 2006). With the HYCOM model, the two regions remained completely isolated, as not a single larva crossed the Ras-Al-Hadd headland and successfully settled.
Overall reproductive success, quantified by the proportion of larvae settling successfully after a spawning event, varied between reefs and nearly doubled in brooders compared with spawners (Table 1) in both circulation models. Some coral communities produced significantly more successful larvae than other communities. For instance, the Masirah–Bar Al-Hikman and Daymaniyat Islands reefs showed average successful transport rates of 60% and 70%, respectively, whereas corals in the Sur-Qalhat and Musandam reefs had only an average of 30%. The subsidy index (proportion of successful larvae exported to other reefs) also varied considerably between the reefs (Table 1). In the Daymaniyat Islands reefs, 33–40% of the larvae were exported to four other reefs in the Gulf of Oman. In Mirbat and Dhofar in the Arabian Sea, 11%–46% of the larvae were exported to other reefs. However, for coral communities in Musandam or Masirah–Bar Al-Hikman, fewer than 1% of the larvae were exported to other reef areas. The subsidy index was considerably reduced for brooding corals because of the much shorter pelagic larval stage, although the same reefs appeared to be good suppliers of larvae (Daymaniyat Islands and Mirbat) (Table 1).
The correlation (Spearman rank) between the connectivity matrices observed from the two circulation models was 0.79 and 0.80 for spawning and brooding corals, respectively, suggesting that both circulation models captured essentially the same surface features of the oceans.
When pelagic larval duration was increased from 40 days to 70 days, overall larval success increased from 43.9% to 52.2%, but overall inter-reef connectivity, as measured by the subsidy index, nearly doubled. Simultaneously, the local retention remained very similar (32%) to that measured for the spawning corals. The number of reefs directly interconnected by successful larval transport, quantified by the number of non-blank cells in the connectivity matrix, increased from 34 to 43 (Table 1) out of a possible 100 with an extended larval duration. However, the connectivity between the Gulf of Oman and Arabian Sea did not increase, as the lower-left and upper-right quadrants of the connectivity matrix remained nearly empty.
The overall level of connectivity varied considerably between years (Figure 2). Using the NCOM model in 2007, for instance, there were more inter-reef connections (8) for spawners in the Gulf of Oman than in other years using both the NCOM and HYCOM models. In 2005, according to both circulation models, connectivity in the Arabian Sea was lower than that in other years, particularly for brooding species (Figure 2).
Quantitatively, the variability in successful larval transport in space (reef within sea), time (year), and reproductive type was tested using a multiway ANOVA on the three calculated recruitment indices: local retention index, larval success index, and subsidy index (Table 2). The two hydrodynamic models are treated separately. The Reproduction Type factor (brooding corals vs. spawning corals: RT) was highly significant and affected both the local retention index (p <0.0001) and the overall success index (p <0.0001), but not the subsidy index. The factor Year was only marginally significant for the successful transport of larvae in the NCOM model (p = 0.0388) but showed a very significant interaction with the Reproduction Strategy (RT × Y: p <0.0001) for all three indices in both circulation models (Table 2). The Reef factor (nested within the sea area) had a significant effect (p <0.0001) on all connectivity indices in both models, but the Sea factor itself was not significant (p >0.3). The subsidy index (relative abundance of larvae settling outside their release reef area) showed no significant factors, except for Reef and its interaction terms (p <0.001, Table 2), although this analysis must be interpreted cautiously because of the high number of zeroes (100% local retention or no larvae exported) in the dataset. A comparison of the ANOVA tables from both hydrodynamic models suggests strong coherence in the results. The main discrepancy was observed in several significant complex interactions (RT × R, S × RT × Y, and RT × R × Y) in the HYCOM model, which were not significant in the NCOM model (Table 2).
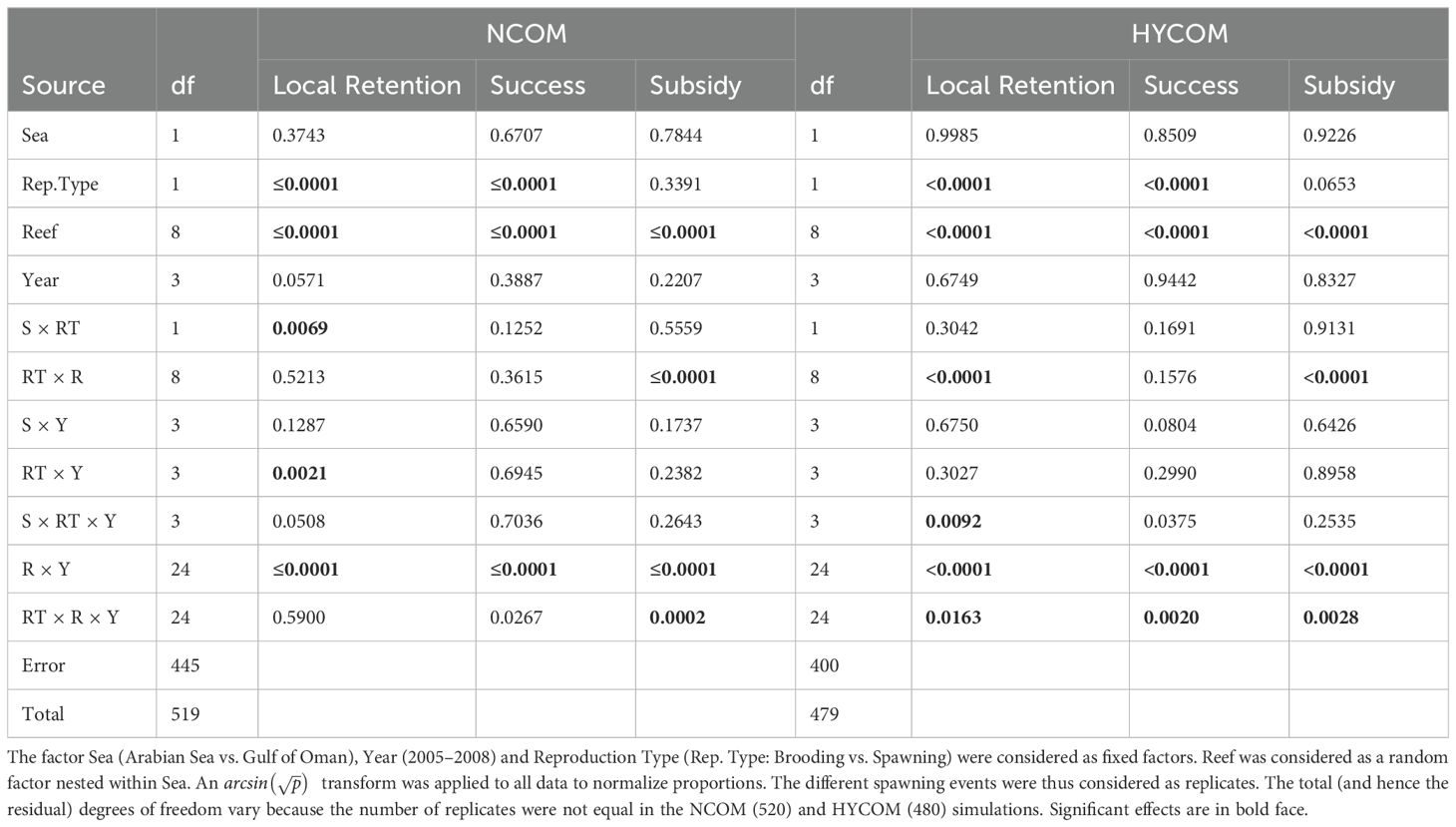
Table 2. Summary of the p-values observed for the three ANOVAs of Local Retention Index, Successful Transport Index and Subsidy Index.
Connectivity can also be examined along the column of the connectivity matrix to determine the origin of larvae settling in a given reef. These data should only be interpreted qualitatively, as the actual number of larvae originating from a reef varies dramatically with the size and composition of the different source reef areas, whereas our simulation released 2,000 larvae from each reef for each spawning event. All reefs potentially received larvae from at least one other reef and many received larvae from as many as five other reefs (Table 1; Figure 2).
In summary, the matrix shown in Figure 1 represents the overall connectivity between reef areas based on an average of 96 standard simulations (excluding long-lived). Several key observations can be made, ranked in decreasing order of significance: connectivity across Ras Al-Hadd is very low; self-seeding is strongest for all reefs; the main directions of larval transport are southeast in the Gulf of Oman and northeast in the Arabian Sea; connectivity in the Arabian Sea is more unidirectional and, despite longer distances, slightly higher than in the Gulf of Oman; and the three reefs around Muscat have limited exchange of larvae with the Iranian reef in Chabahar, with higher export from the Omani reefs.
Figure 4 shows the mean circulation patterns for March and April, the months when larval dispersal was most likely. The flow fields illustrate the long-term average conditions. Ridges of the FTLE, displayed in grayscale, indicate regions of repelling LCS, indicating transport barriers. In both months, there is a pronounced cyclonic circulation in the central Gulf of Oman, with southeastward flow along the Oman coast. While FTLE ridges appear within the Gulf of Oman, exchange seems possible, particularly between the Daymaniyat Islands, Muscat, and the Iran Reef near Chabahar. Immediately southeast of Ras Al-Hadd, a pronounced cross-shore LCS barrier is evident in both months, which is linked to a cyclonic eddy. Larger differences between the two months were observed in the Arabian Sea. In March, the flow fields exhibited a dispersed and less-organized pattern along the coast. The flow was relatively weaker and scattered, especially near the reefs along the southern coast, such as Mukallah, Dhofar, and Mirbat. In contrast, in April, the early onset of the southwest monsoon was apparent, driving a pronounced and organized northward and eastward current structure along the coast. These patterns suggest that, in the Arabian Sea, April likely experiences more efficient transport and dispersal, whereas March may have higher retention near the coastline.
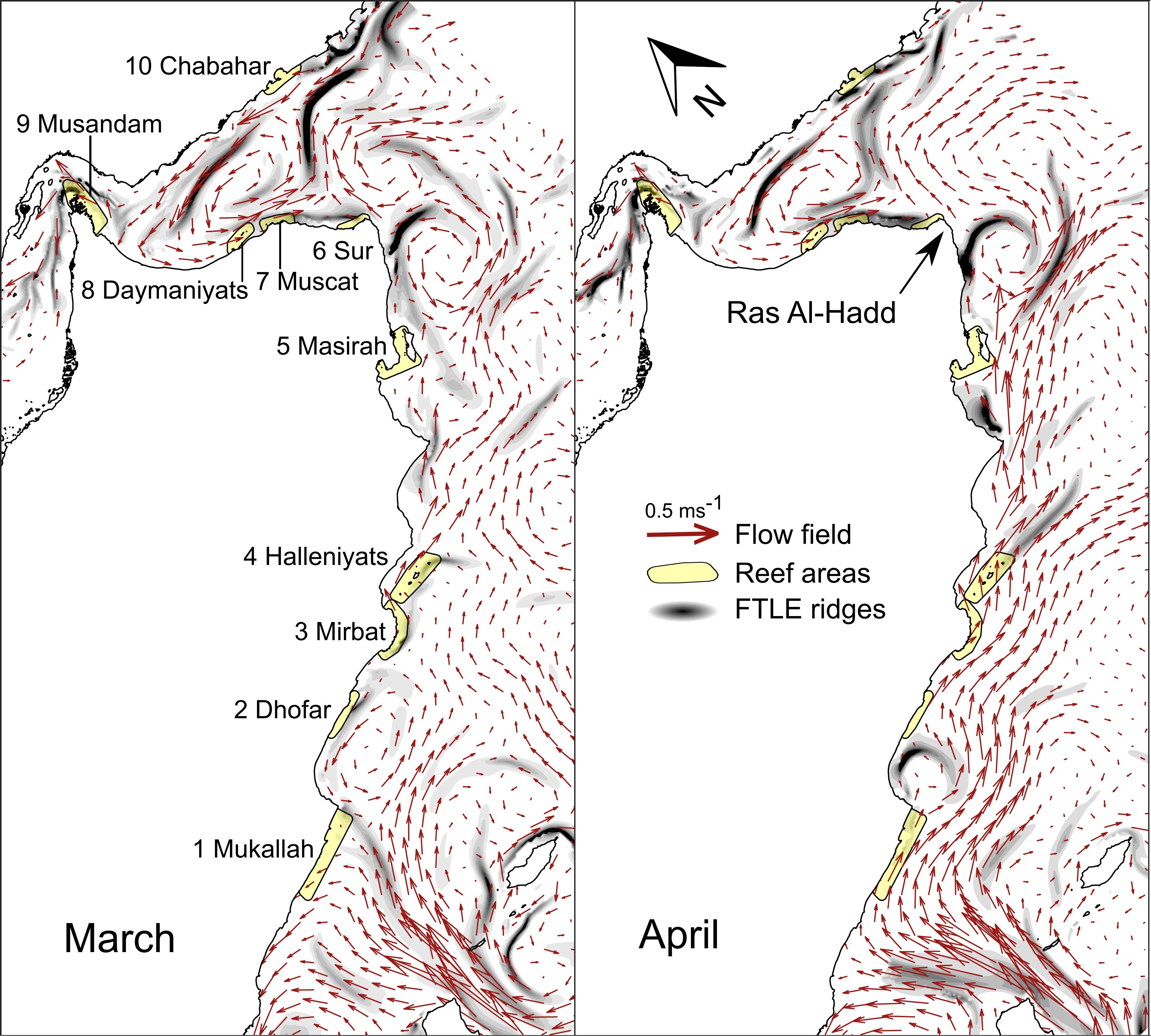
Figure 4. General circulation patterns and FTLE ridges for March (left) and April (right). The flow fields are the averages of the 20-year monthly mean currents derived from the GLORYSv1 dataset. For clarity, the vectors were displayed at one-fourth of the actual resolution (1/3° instead of 1/12°). The FTLE ridges were calculated using 20-day forward advection within the mean flow fields and represent general repelling LCS, indicating transport barriers. The maps were rotated by 45° for a better fit.
4 Discussion
Ecologists and conservationists are interested in the patterns of larval exchange between distant communities, as these help define potential conservation management strategies (Roberts, 1997) or explain the biogeographical distribution of species (Barber et al., 2000).
In the present simulation study, the two circulation models suggested that connectivity was very limited between communities separated by more than 150 km–200 km. This value is consistent with estimates from the Caribbean Sea (Cowen et al., 2006) and Pacific Ocean (Treml et al., 2008), and within the range predicted by Kinlan and Gaines (2003) for invasive marine invertebrates. The same limited dispersal capabilities have also been observed on a global scale (Wood et al., 2014). Although long-term gene flow seems possible through stepping stone effects on the Omani shores of the Gulf of Oman, low dispersal occurred when offshore eddies transported many larvae north, out of the coastal areas, thus decreasing the chances of recruitment in a reef area within the competency period. Although some of these larvae were entrained in large eddies and reached the Iranian coastline, many failed to settle successfully because of the relative lack of suitable substrates along the Iranian shore, which is dominated by sandy or muddy areas. Others were trapped in the rotating eddy or exceeded the period of competency conducive to a successful settlement. A small proportion of larvae successfully crossed the Gulf of Oman from north to south, riding one of these eddies and enabling a low level of connectivity between Iranian and Omani reefs. Along the Arabian Sea, mesoscale eddies are interspersed with characteristic long jets and filaments able to export coastal waters and passive drifters hundreds of kilometers offshore (Kindle and Arnone, 2001), particularly before and during the SW monsoon. The eddies themselves, on the other hand, can accumulate larvae offshore near their centers (Wolanski and Hamner, 1988), limiting further dispersal, but at the same time compromising the return of competent larvae to inshore coral communities.
The important role of surface eddies and surface currents in shaping coral community structure was recently demonstrated in the Red Sea by comparing genetic distances in coral reef fish and oceanic connectivity modeled from passive particle tracking (Raitsos et al., 2017). In the Mona Passage in Puerto Rico, the development of eddies at the time of coral spawning resulted in a lack of connectivity (Baums et al., 2006).
The dispersal patterns generated by our simulations suggest the existence of two large, ecologically isolated coral metapopulations: one in the Gulf of Oman and the other along the Arabian Sea coast of Oman, thus strengthening the hypothesis of two distinct biogeographical regions based on species distribution (Spalding et al., 2007). Torquato and Møller (2022) tested the hypothesis that phylogeographical patterns of coral-dependent fish species around the Arabian Peninsula are driven by ocean circulation, larval behavior, and seascape features. They identified the Ras Al Hadd zone as a significant biogeographical break that restricts larval exchange between the Gulf of Oman and the Arabian Sea.
For algal communities, temperature cycles (and more importantly, nutrient input cycles) associated with the SW monsoon seasonal upwelling contribute to this biogeographic distinctiveness (Schils and Wilson, 2006). However, the same argument is less compelling for coral distribution patterns. Coral communities are thought to be negatively affected by the “pseudo high-latitude effect” (Sheppard and Salm, 1988) of the Arabian monsoonal upwelling, resulting in weakly developed reefs and reef frameworks and a dominance, at least seasonally, of large macroalgae, which compete with the corals for light and space. However, species richness is unexpectedly higher along the Arabian Sea coast than along the Gulf of Oman (Sheppard and Sheppard, 1991; Salm, 1993; Claereboudt, 2006a), which contradicts the empirical relationship of reduced diversity at lower average temperatures (Rosen, 1971). It appears that the hydrodynamic barrier observed at Ras-Al-Hadd may decrease the arrival of species from the Arabian Sea into the Gulf of Oman, thereby reducing overall biological diversity and resulting in what has been described as an incomplete “mixing” of the Indian Ocean fauna (Sheppard and Sheppard, 1991; Salm, 1993).
The reason for this incomplete biological mixing may be the limited time (ca. 15,000 years) since the last major change in sea level in the region, combined with the physical distances between suitable substrates (Sheppard et al., 1992) and hydrodynamic separation. Species can move from the more diverse communities in the southern Arabian Sea (Socotra, Somalia) toward the north, following the main NE flow of the March–May coastal currents, and using the various coral communities along the Arabian Sea coast of Oman as stepping stones. As the distances between these hard substrate communities often exceed several hundred kilometers, the chances of successful progression of any given species across several reefs is relatively low, which considerably limits the ecological connectivity of the metapopulations and explains the progressive impoverishment of coral fauna toward the north of the Arabian Sea. Compared with our simulations, the actual level of connectivity between Arabian Sea coral communities will likely be further reduced by the natural mortality of coral larvae and predation (Cowen et al., 2000; Graham et al., 2008) occurring during dispersal between these widely spaced communities. This may explain the progressive decrease in coral diversity in the Yemeni (Socotra) coral communities (253 spp., DeVantier et al., 2004) to the Yemeni Arabian Sea shores (77 spp., Pichon et al., 2010), northern Arabian Sea coral communities (Sheppard and Sheppard, 1991), and Gulf of Oman (112 spp., Claereboudt, 2006a).
A genetic study combined with the NCOM biophysical model applied to an endemic clownfish (A. omanensis) along the Arabian Sea coast of Oman showed that although population mixing occurred between the main northern and southern subpopulations, the proportion of migrants in each of the two subpopulations was low compared to that in locally produced recruits (Simpson et al., 2014). In a similar study, but based on a very different approach, the genetic structure of another clownfish endemic to the Red Sea (Amphiprion bicinctus) was shown to be strongly linked to the potential dispersal of larvae following observed circulation patterns in the Red Sea (Raitsos et al., 2017).
Exceptionally, some coral larvae could jump the hydrodynamic barrier at Ras-Al-Hadd, enter the Gulf of Oman, and then settle successfully. However, because the main direction of dispersal on the south shore of the Gulf of Oman is from west to east, the chances of colonizing the coast further eastward are further reduced.
Coral reefs are among the most threatened marine ecosystems under likely climate change scenarios (Hughes et al., 2003; Hoegh-Guldberg et al., 2007). Despite major bleaching events in the Indian Ocean and Arabian Gulf in 1998, 2002, 2004, and 2010 (Riegl et al., 2012), corals along both the Gulf of Oman and Arabian Sea survived relatively unscathed (Rezaei et al., 2004), although the 2010 event caused some bleaching near Muscat and Musandam (Pers. Observ.). Along the Arabian Sea shores, cooling of the coastal water column during summer upwelling (Savidge et al., 1990; Morrison et al., 1998) is certainly part of the reason for this survival (Coles and Wilson, 2001), but the adaptation of corals in the region to frequent and large fluctuations in temperature might also partly explain this relative insensitivity to temperature changes (Coles, 1997; Claereboudt, 2018). On the northwestern shelf of the Gulf of Oman (between Muscat and Sohar), intense internal tides regularly lift cold (sub-thermocline) water up to a few meters below the surface (Bruss et al., 2023). This process can serve as thermal protection for local coral reefs and may explain their lower bleaching rates compared to reefs in the nearby Arabo-Persian Gulf.
The low connectivity between reefs in the region, coupled with the strong local retention of larvae identified in the present model study, might favor such adaptation under continuous selective pressure and generate local populations of corals that are more resistant to temperature increases. At the same time, the limited dispersal of larvae reduces the potential of these adapted genotypes to spread to other reefs in the Indian Ocean, which could be further damaged by heat-induced mortalities.
Different reef areas appear to function as independent units in terms of recruitment because of the relatively low connectivity between most reef communities in the study area. This implies that the destruction, even partial, of a given reef community in Oman by large-scale disturbances, such as tropical cyclones (Taylor, 2009) or algal blooms (Foster et al., 2011), could considerably reduce the self-seeding process and lead rapidly to extirpation of some species, particularly when recruitment is limited (Jones et al., 2009). This is particularly important at sites that rely almost exclusively on local recruitment, such as Chabahar in Iran and Bar-Al-Hikman-Masirah or Musandam in Oman. Such sites should be considered of high conservation value, particularly if they harbor endemic or rare species and should thus receive a higher level of protection to balance the lack of connectivity (Almany et al., 2009). In the present simulations, some coral communities played an important role as larval providers (Daymaniyat Island in the Gulf of Oman and Mirbat in the Arabian Sea) to other reefs during the modeled dispersal events, and should also receive a higher level of protection (Roberts et al., 2006). The Daymaniyat Islands became Oman’s first marine National Nature Reserve in 1998 and received some protection; however, all other coral reefs in the region (with the exception of the recently proclaimed Bar-Al-Hikman Nature Reserve) remain nearly unprotected. Recommendations on the proportion of protection for a given habitat vary widely (10%–50%; Jones et al., 2007), and theoretically, the total area needed for effective biodiversity conservation should increase with decreasing connectivity (Roberts et al., 2006).
To increase the robustness of our results, we determined general circulation patterns as mean flow fields for the considered periods from a third state-of-the-art long-term dataset (GLORYS) and calculated the corresponding LCS. The general flow patterns and transport barriers support the findings of the Lagrangian simulations. Connectivity across Ras al Hadd is restricted, and the main directions of larval transport are southeast in the Gulf of Oman and northeast in the Arabian Sea.
The resolution of the models we use is coarser than the typical size of individual reefs. Nonetheless, the general connectivity patterns that we determined remained consistent between all models. Saint-Amand et al. (2023) suggested that reef management recommendations based on Lagrangian connectivity models should be made only at scales coarser than the model resolution. The main focus of this study was to assess large-scale connectivity, and we only derived conclusions on that scale. We further considered our connectivity estimates conservative. Low model resolution and advection of larvae with a surface flow field may overestimate transport distances and connectivity, while underestimating local retention and self-recruitment. Despite this, we identified barriers and observed a generally high local retention and self-recruitment. One of our main results confirms the restricted connectivity between the Arabian Sea and Gulf of Oman around the Ras Al Hadd Cape. By similar dynamics, the Gulf of Oman is separated from the summer upwelling of the Arabian Sea and experiences significantly higher summer temperatures (Piontkovski and Chiffings, 2014), to which local corals have adapted. The near complete isolation of the Gulf of Oman reefs is biologically important as the inability to “export” these heat adapted corals may have profound implications for Indian Ocean coral response to climate changes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MC: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. GB: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by SQU grant IG/AGR/FISH/17/01 and ONR-G grant N629092112008.
Acknowledgments
The authors wish to acknowledge the data provided as an open source by the HYCOM, NCOM and GLORYS consortia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almany G. R., Conolly S. R., Heath D. D., Hogan J. D., Jones G. P., McCook L. J., et al. (2009). Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs 28, 339–351. doi: 10.1007/s00338-009-0484-x
Ayouche A., De Marez C., Morvan M., L’Hegaret P., Carton X., Le Vu B., et al. (2021). Structure and dynamics of the Ras al Hadd oceanic dipole in the Arabian Sea. Oceans 2, 105–125. doi: 10.3390/oceans2010007
Barber P. H., Palumbi S. R., Erdmann M. V., Moosa M. K. (2000). A marine Wallace’s line. Nature 406, 692–693. doi: 10.1038/35021135
Barron C. N., Kara A. B., Rhodes R. C., Rowley C., Smedstad L. F. (2007). “Validation test report for the 1/8 global navy coastal ocean model nowcast/forecast system,” in Naval Research Laboratory Stennis Space Center, Oceanography Division, Vol. 149 (Naval Research Laboratory).
Baums I. B., Paris C. B., Chérubin L. M. (2006). A bio-oceanographic filter to larval dispersal in a reef-building coral. Limnology Oceanography 51, 1969–1981. doi: 10.4319/lo.2006.51.5.1969
Benzoni F., Bianchi C. N., Morri C. (2003). Coral communities of the northwestern Gulf of Aden (Yemen): Variation in framework building related to environmental factors and biotic conditions. Coral Reefs 22, 475–484. doi: 10.1007/s00338-003-0342-1
Blanco-Martin B. (2000). Influence of larval competence periods on coral larval settlement and reef connectivity: A modelling approach. In: Proceedings of the 9th International Coral Reef Symposium. 1, 57–62.
Bleck R. (2002). An oceanic general circulation model framed in hybrid isopycnic-Cartesian coordinates. Ocean Model. 4, 55–88. doi: 10.1016/S1463-5003(01)00012-9
Bruss G., Queste B., Font E., Hall R. (20232023). Stratification and internal tides on the Al-Batinah shelf. Book Abstracts: Conf. Appl. Coast. Res. 10, 44.
Burkill P. H. (1999). ARABESQUE: an overview. Deep-Sea Res. II 46, 529–547. doi: 10.1016/S0967-0645(98)00116-7
Burt J. A., Feary D. A., Bauman A. G., Usseglio P., Cavalcante G. H., Sale P. F. (2011). Biogeographic patterns of reef fish community structure in the northeastern Arabian Peninsula. ICES J. Mar. Sci. 68, 1875. doi: 10.1093/icesjms/fsr129
Chaput R., Sochala P., Miron P., Kourafalou V. H., Iskandarani M. (2022). Quantitative uncertainty estimation in biophysical models of fish larval connectivity in the Florida Keys. ICES J. Mar. Sci. 79, 609–632. doi: 10.1093/icesjms/fsac021
Chassignet E. P., Hurlburt H. E., Smedstad O. M., Halliwell G. R., Hogan P. J., Wallcraft A. J., et al. (2007). The HYCOM (HYbrid Coordinate Ocean Model) data assimilative system. J. Mar. Syst. 65, 60–83. doi: 10.1016/j.jmarsys.2005.09.016
Claereboudt M. R. (2006a). Reef corals and coral reefs of the Gulf of Oman (Muscat: Historical Association of Oman - Al-Roya).
Claereboudt M. R. (2006b). Porites decasepta Nov. Sp.: A new species of scleractinian coral (Scleractinia, Poritidae) from the Arabian Sea coast of Oman. Zootaxa. 1188 (1), 55–62. doi: 10.11646/zootaxa.1188.1.5
Claereboudt M. R. (2018). Monitoring the vertical thermal structure of the water column in coral reef environments using divers of opportunity. Current Trends in Oceanography and Marine Sciences. 107. doi: 10.29011/CTOMS-107.100007
Claereboudt M. R., Al-Rashdi K. M. (2011). Shallow-water sea cucumber inventory in the Sultanate of Oman. SPC Beche-de-mer Information Bulletin. 31, 25–29.
Coles S. L. (1997). Reef corals occurring in a highly fluctuating temperature environment at Fahal Island, Gulf of Oman (Indian Ocean). Coral Reefs. 16 (4), 269–272. doi: 10.1007/s003380050084
Coles S. L., Wilson S. C. (2001). Environmental factors affecting reef corals in Oman: A comparison to the Indo-Pacific region.
Cowen R. K., Lwiza K. M. M., Sponaugle S., Paris C. B., Olson D. B. (2000). Connectivity of marine populations: Open or closed? Science 287, 857–859. doi: 10.1126/science.287.5454.857
Cowen R. K., Paris C. B., Srinivasan A. (2006). Scaling of connectivity in marine populations. Science 311, 522–527. doi: 10.1126/science.1122039
Cummings J. A. (2005). Operational multivariate ocean data assimilation. Q. J. R. Meteorological Soc. 131, 3583–3604. doi: 10.1256/qj.05.105
Cummings J. A., Smedstad O. M. (2013). “Variational data assimilation for the global ocean,” in Data Assimilation for Atmospheric, Oceanic and Hydrologic Applications, vol. II . Eds. Park S. K., Xu L. (Springer), 303–343. doi: 10.1007/978-3-642-35088-7_13
Dawson M. N. (2001). Phylogeography in coastal marine animals: A solution from California? J. Biogeography 28, 723–736. doi: 10.1046/j.1365-2699.2001.00572.x
DeVantier L., De’Ath G., Klaus R., Al-Moghrabi S. A., Abdulaziz M., Reinicke G. B., et al. (2004). Reef-building corals and coral communities of the Socotra Archipelago, a zoogeographic “crossroads” in the Arabian Sea. Fauna Arabia 20, 117–168.
Dobbelaere T., Dekens A., Saint-Amand A., Alaerts L., Holstein D. M., Hanert E. (2024). Hurricanes enhance coral connectivity but also superspread coral diseases. Global Change Biol. 30, e17382. doi: 10.1111/gcb.17382
Fagerström V., Broström G., Larsson A. I. (2022). Turbulence affects larval vertical swimming in the cold-water coral Lophelia pertusa. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1062884
Figueiredo J., Baird A. H., Cohen M. F., Flot J. F., Kamiki T., Meziane T., et al. (2012). Ontogenetic change in the lipid and fatty acid composition of scleractinian coral larvae. Coral Reefs 31, 613–619. doi: 10.1007/s00338-012-0874-3
Foster K. A., Foster G., Tourenq C., Shuriqi M. K. (2011). Shifts in coral community structures following cyclone and red tide disturbances within the Gulf of Oman (United Arab Emirates). Mar. Biol., 1–14. doi: 10.1007/s00227-010-1622-2
Fox D. N., Teague W. J., Barron C. N., Carnes M. R., Lee C. M. (2002). The modular ocean data assimilation system (MODAS). J. Atmospheric Oceanic Technol. 19, 240–252. doi: 10.1175/1520-0426(2002)019<0240:TMODAS>2.0.CO;2
Fuchs H. L., Gerbi G. P., Hunter E. J., Christman A. J. (2018). Waves cue distinct behaviors and differentiate transport of congeneric snail larvae from sheltered versus wavy habitats. Proc. Natl. Acad. Sci. 115 (32), E7532–E7540. doi: 10.1073/pnas.1804558115
GLORYS12V1 Global ocean physics reanalysis. In: E.U. Copernicus Marine Service Information (CMEMS) (Marine Data Store (MDS) (Accessed Dec 2024).
Graham E. M., Baird A. H., Connolly S. R. (2008). Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27, 529–539. doi: 10.1007/s00338-008-0361-z
Hoegh-Guldberg O., Mumby P. J., Hooten A. J., Steneck R. S., Greenfield P., Gomez E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Howells E. J., Abrego D., Vaughan G. O., Burt J. A. (2014). Coral spawning in the Gulf of Oman and relationship to latitudinal variation in spawning season in the northwest Indian Ocean. Sci. Rep. 4, 7484–7486. doi: 10.1038/srep07484
Hughes T. P., Baird A. H., Bellwood D. R., Card M., Connolly S. R., Folke C., et al. (2003). Climate change, human impacts and the resilience of coral reefs. Science 301, 929–933. doi: 10.1126/science.1085046
Jones G. P., Almany G. R., Russ G. R., Sale P. F., Steneck R. S., van Oppen M. J. H., et al. (2009). Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28, 307–325. doi: 10.1007/s00338-009-0469-9
Jones G. P., Srinivasan M., Almany G. R. (2007). Population connectivity and conservation of marine biodiversity. Oceanography 20, 100–111. doi: 10.5670/oceanog.2007.33
Kara A. B., Barron C. N., Martin P. J., Smedstad L. F., Rhodes R. C. (2006). Validation of interannual simulations from the 1/8° global Navy Coastal Ocean Model (NCOM). Ocean Model. 11, 376–398. doi: 10.1016/j.ocemod.2005.01.003
Kindle J. C., Arnone R. A. (2001). A review of the surface circulation of the northern Arabian Sea. Ocean Sci. 2, 113–122.
Kinlan B. P., Gaines S. D. (2003). Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84, 2007–2020. doi: 10.1890/01-0622
L’Hégaret P., Lacour L., Carton X., Roullet G., Baraille R. (2013). A seasonal dipolar eddy near Ras Al Hamra (Sea of Oman). Ocean Dynamics 63, 633–659. doi: 10.1007/s10236-013-0616-2
McClean J. L. (2015). Monsoon variability in the Arabian Sea from global 0.08 deg HYCOM simulations (San Diego, La Jolla, United States: University of California).
Miller K., Mundy C. N. (2003). Rapid settlement in broadcast spawning corals: implications for larval dispersal. Coral Reefs 22, 99–106. doi: 10.1007/s00338-003-0290-9
Morrison J. M., Codispoti L. A., Gaurin S., Jones B., Manghnani V., Zheng Z. (1998). Seasonal variation of hydrographic and nutrient fields during the US JGOFS Arabian Sea Process Study. Deep Sea Res. Part II 45, 2101–2153. doi: 10.1016/S0967-0645(98)00063-0
Nozawa Y., Harrison P. L. (2007). Effects of elevated temperature on larval settlement and post-settlement survival in scleractinian corals, Acropora solitaryensis and Favites chinensis. Mar. Biol. 152, 1181–1185. doi: 10.1007/s00227-007-0765-2
Peliz A., Marchesiello P., Dubert J., Marta-Almeida M., Roy C., Queiroga H. (2007). A study of crab larvae dispersal on the western Iberian shelf: physical processes. J. Mar. Syst. 68, 215–236. doi: 10.1016/j.jmarsys.2006.11.007
Pichon M., Benzoni F., Chaineu C., Dutrieux E. (2010). Field guide to the hard corals of the southern coast of Yemen (Parthenope, Paris: Biotope).
Piontkovski S. A., Al-Tarshi M. H., Al-Ismaili S. M. (2019). Inter-annual variability of mesoscale eddy occurrence in the western Arabian Sea. Int. J. Oceans Oceanography 13, 1–23.
Piontkovski S. A., Chiffings T. (2014). Long-term changes of temperature in the Sea of Oman and the Western Arabian Sea. Int. J. Oceans Oceanography 8, 53–72.
Raitsos D. E., Brewin R. J. W., Zhan P., Dreano D., Pradhan Y., Nanninga G. B., et al. (2017). Sensing coral reef connectivity pathways from space. Sci. Rep. 7, 1–10. doi: 10.1038/s41598-017-08729-w
Randall J. E., Hoover J. P. (1995). Scarus zufar, a new species of parrotfish from southern Oman with comments on endemism of the area. Copeia 3, 685–688. doi: 10.2307/1446765
Rezaei H., Wilson S., Claereboudt M., Riegl B. (2004). Coral reef status in the ROPME sea area: Arabian/Persian Gulf, Gulf of Oman and Arabian Sea. In Wilkinson C. (Ed.), Status of coral reefs of the world: 2004, 1, 155–171. Australian Institute of Marine Science.
R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Available at: https://www.R-project.org/.
Riegl B., Johnston M., Purkis S., Howells E., Burt J., Steiner S. C. C., et al. (2018). Population collapse dynamics in Acropora downingi, an Arabian/Persian Gulf ecosystem-engineering coral, linked to rising temperature. Global Change Biol. 24, 2447–2462. doi: 10.1111/gcb.2018.24.issue-6
Riegl B., Purkis S. J., Al-Cibahy A., Al-Harthi S., Grandcourt E., Al-Sulaiti K., et al. (2012). “Coral bleaching and mortality thresholds in the SE Gulf: Highest in the world,” in Coral Reefs of the Gulf. Eds. Burt J. A., Bartholomew A., Feary D. A., 95–105.
Roberts C. M. (1997). Connectivity and management of Caribbean coral reefs. Science 278, 1454–1457. doi: 10.1126/science.278.5342.1454
Roberts C. M., Reynolds J. D., Côté I. M., Hawkins J. P. (2006). “Redesigning coral reef conservation,” in Coral reef conservation. Eds. Côté I. M., Reynolds J. D. (Cambridge University Press), 517–537.
Rosen B. R. (1971). The distribution of reef coral genera in the Indian Ocean. Symposium Zoological Soc. London 28, 263–299.
Saint-Amand A., Lambrechts J., Hanert E. (2023). Biophysical models resolution affects coral connectivity estimates. Sci. Rep. 13, 9414. doi: 10.1038/s41598-023-36158-5
Savidge G., Lennon J., Matthews A. J. (1990). A shore-based survey of upwelling along the coast of Dhofar region, southern Oman. Continental Shelf Res. 10, 259–275. doi: 10.1016/0278-4343(90)90022-E
Schils T., Wilson S. C. (2006). Temperature threshold as a biogeographic barrier in northern Indian Ocean macroalgae. J. Phycology 42, 749–756. doi: 10.1111/j.1529-8817.2006.00242.x
Sheppard C. R. C., Price A., Callum R. (1992). “Marine biogeography of the Arabian region,” in Marine ecology of the Arabian region: Pattern and processes in extreme tropical environments. Eds. Sheppard C. R. C., Price A., Callum R. (Academic Press), 221–240.
Sheppard C. R. C., Salm R. V. (1988). Reef and coral communities of Oman, with a description of a new coral species (Order Scleractinia, Genus Acanthastrea). J. Natural History 22, 263–279. doi: 10.1080/00222938800770201
Sheppard C. R. C., Sheppard A. L. S. (1991). Corals and coral communities of Arabia. Fauna Saudi Arabia 12, 1–171.
Simpson S. D., Harrison H. B., Claereboudt M. R., Planes S. (2014). Long-distance dispersal via ocean currents connects Omani clownfish populations throughout entire species range. PloS One 9. doi: 10.1371/journal.pone.0107610
Spalding M. D., Fox H. E., Allen G. R., Davidson N., Ferdaña Z. A., Finlayson M. A. X., et al. (2007). Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. Bioscience 57, 573–583. doi: 10.1641/B570707
Tang D. L., Kawamura H., Luis A. J. (2002). Short-term variability of phytoplankton blooms associated with a cold eddy in the northwestern Arabian Sea. Remote Sens. Environ. 81, 82–89. doi: 10.1016/S0034-4257(01)00334-0
Taylor O. (2009). “The impact of Cyclone Gonu on selected coral-rich areas of the Gulf of Oman including indications of recovery at the Daymaniyat Islands,” in Indian Ocean tropical cyclones and climate change. Ed. Charabi Y. (Springer Netherlands), 289–293.
Torquato F., Møller P. R. (2022). Physical–biological interactions underlying the connectivity patterns of coral-dependent fishes around the Arabian Peninsula. J. Biogeography 49, 483–496. doi: 10.1111/jbi.14318
Treml E. A., Halpin P. S., Urban D. L., Pratson L. F. (2008). Modeling population connectivity by ocean currents: A graph-theoretic approach for marine conservation. Landscape Ecol. 23, 19–36. doi: 10.1007/s10980-007-9138-y
Wang Y., Raitsos D. E., Krokos G., Gittings J. A., Zhan P., Hoteit I. (2019). Physical connectivity simulations reveal dynamic linkages between coral reefs in the southern Red Sea and the Indian Ocean. Sci. Rep. 9, 16598. doi: 10.1038/s41598-019-53126-0
Wei M., Jacobs G., Rowley C., Barron C. N., Hogan P., Spence P., et al. (2016). The performance of the US Navy’s RELO ensemble, NCOM, HYCOM during the period of GLAD at-sea experiment in the Gulf of Mexico. Deep Sea Res. Part II 129, 374–393. doi: 10.1016/j.dsr2.2013.09.002
Wilson J. R., Harrison P. L. (1998). Settlement-competency periods of larvae of three species of scleractinian corals. Mar. Biol. 131, 339–345. doi: 10.1007/s002270050327
Wolanski E., Hamner W. H. (1988). Topographically controlled fronts in the ocean and their biological influence. Science 241, 177–181. doi: 10.1126/science.241.4862.177
Wood S., Paris C. B., Ridgwell A., Hendy E. J. (2014). Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Global Ecol. Biogeography 23, 1–11. doi: 10.1111/geb.2014.23.issue-1
Zhang Z., Wang W., Qiu B. (2014). Oceanic mass transport by mesoscale eddies. Science 345, 322–324. doi: 10.1126/science.1252418
Keywords: coral, larval dispersal, Lagrangian modeling, Arabian Sea, Gulf of Oman
Citation: Claereboudt MR and Bruss G (2025) Short distances dominate connectivity patterns of coral communities in the North-West Arabian Sea. Front. Mar. Sci. 11:1494563. doi: 10.3389/fmars.2024.1494563
Received: 11 September 2024; Accepted: 30 December 2024;
Published: 24 January 2025.
Edited by:
Yehuda Benayahu, Tel Aviv University, IsraelReviewed by:
Miguel Angel Ahumada-Sempoal, University of the Sea, MexicoEmmanuel Hanert, Université Catholique de Louvain, Belgium
Copyright © 2025 Claereboudt and Bruss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerd Bruss, Z2VyZEBzcXUuZWR1Lm9t
 Michel R. Claereboudt
Michel R. Claereboudt Gerd Bruss
Gerd Bruss