
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Mar. Sci. , 09 January 2025
Sec. Coastal Ocean Processes
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1481734
 Shubham Krishna1,2*
Shubham Krishna1,2* Carsten Lemmen2
Carsten Lemmen2 Serra Örey3,4,5
Serra Örey3,4,5 Jennifer Rehren4
Jennifer Rehren4 Julien Di Pane6
Julien Di Pane6 Moritz Mathis2
Moritz Mathis2 Miriam Püts4
Miriam Püts4 Sascha Hokamp7
Sascha Hokamp7 Himansu Kesari Pradhan2,8
Himansu Kesari Pradhan2,8 Matthias Hasenbein9
Matthias Hasenbein9 Jürgen Scheffran7
Jürgen Scheffran7 Kai W. Wirtz2
Kai W. Wirtz2Coastal ecosystems are increasingly experiencing anthropogenic pressures such as climate warming, CO2 increase, metal and organic pollution, overfishing, and resource extraction. Some resulting stressors are more direct like pollution and fisheries, and others more indirect like ocean acidification, yet they jointly affect marine biota, communities, and entire ecosystems. While single-stressor effects have been widely investigated, the interactive effects of multiple stressors on ecosystems are less researched. In this study, we review the literature on multiple stressors and their interactive effects in coastal environments across organisms. We classify the interactions into three categories: synergistic, additive, and antagonistic. We found phytoplankton and bivalves to be the most studied taxonomic groups. Climate warming is identified as the most dominant stressor which, in combination, with other stressors such as ocean acidification, eutrophication, and metal pollution exacerbate adverse effects on physiological traits such as growth rate, fitness, basal respiration, and size. Phytoplankton appears to be most sensitive to interactions between warming, metal and nutrient pollution. In warm and nutrient-enriched environments, the presence of metals considerably affects the uptake of nutrients, and increases respiration costs and toxin production in phytoplankton. For bivalves, warming and low pH are the most lethal stressors. The combined effect of heat stress and ocean acidification leads to decreased growth rate, shell size, and acid-base regulation capacity in bivalves. However, for a holistic understanding of how coastal food webs will evolve with ongoing changes, we suggest more research on ecosystem-level responses. This can be achieved by combining in-situ observations from controlled environments (e.g. mesocosm experiments) with modelling approaches.
Coastal ecosystems are exposed to a plethora of direct and indirect anthropogenic stressors such as climate warming, eutrophication, metal pollution, hypoxia, pH and salinity changes, and overfishing (Halpern et al., 2007; Crain et al., 2008; Griffen et al., 2016; Gissi et al., 2021). These stressors are inducing serious and irreversible changes in marine and coastal food webs (Carrier-Belleau et al., 2021), and they do not act in isolation but instead simultaneously (Griffen et al., 2016). It has been suggested that by 2050 about 90% of the global ocean will be impacted by exposure to multiple stressors (Henson et al., 2017). Interactions between stressors trigger responses in species and communities that are often different from the effects of the individual stressors (Crain et al., 2008; Jackson et al., 2016).
To understand the changes in coastal ecosystems and to improve marine and coastal management, a better knowledge of stressor interactions is required (Gladstone-Gallagher et al., 2023; Gissi et al., 2021). The interactive effect of multiple stressors could be the sum of the individual responses, more than that, or less. When the combined effect of multiple stressors is equal to their expected cumulative sum, such an effect is called additive. When this is not the case, it is termed multiplicative or non-additive (both used interchangeably) (Pirotta et al., 2022). A non-additive effect could, in turn, be synergistic or antagonistic. When the net effect of interactions is greater than the sum of individual stress responses, the response is called synergistic (Crain et al., 2008; Jackson et al., 2016). On the contrary, in an antagonistic interaction, the effect intensity of the combined response is less than that of the cumulative single-stressor effects.
Occurrences of additive and non-additive interactions have been reported in coastal ecosystems (Crain et al., 2008; Villar-Argaiz et al., 2018). For example, synergistic responses to elevated nutrient concentrations and metal loadings have been found in diverse plankton groups (Bundy et al., 2003; Wiegner et al., 2003; Simboura et al., 2016; Abbate et al., 2017). Negative synergistic effects have been reported in benthic organisms (such as crabs and bivalves) on exposure to ocean acidification (OA), salinity, temperature, and metal pollution (Stueckle, 2008; Miller et al., 2014; Brooks and Crowe, 2019). The combined effects of heat stress and OA have been far more adverse than their individual effects in a variety of mussel species (Vihtakari et al., 2013; Rodrigues et al., 2015). Likewise, the adverse effects of overfishing can be intensified by climate warming in top predatory fish species (Ainley and Blight, 2009). Stressor interactions also lead to antagonistic responses in coastal ecosystems. For example, it has been shown that elevated CO2 resulted in higher chlorophyll biomass under low irradiance, thereby compensating for light stress (Neale et al., 2014; Heiden et al., 2018). Similarly, low pH has been shown to have mitigated the negative effect of high temperature on egg volume in an estuarine fiddler crab (Pardo and Costa, 2021).
In spite of the significant role of stressor interactions in driving ecosystem and community-level responses, investigations of these effects are largely disconnected from the implementation of conservation and management policies for coastal systems (Gladstone-Gallagher et al., 2023; Côté et al., 2016). Over the last decades, research and management focused on eutrophication problems in coastal environments (Smith and Schindler, 2009). The non-additive effects of eutrophication with other stressors such as climate warming, pollutants, hypoxia and changes in salinity have posed a multidimensional problem (Hewitt et al., 2016; Henson et al., 2017; Thrush et al., 2021). First, the intricacy of interactive effects makes it very difficult to elucidate the net response of an ecosystem in ever-changing environments (Carrier-Belleau et al., 2021). Secondly, the occurrence of several stressors triggers differential responses across organisms. Thirdly, critical stressor combinations vary between taxonomic groups.
In this study, we address the aforementioned research gaps. With a focus on high-latitude coastal systems, we systematically review the existing studies on the interactive effects of multiple stressors in coastal habitats to answer the following important questions: 1) What are the critical stressors for triggering non-additive responses in coastal ecosystems? 2) What are the main stressor combinations for different taxonomic groups with respect to inducing negative synergistic effects? 3) What are the research gaps in multi-stressor studies? From our findings, we provide valuable insights to scientists and stakeholders with respect to the advancement of multi-stressor research and for management of coastal ecosystems.
A systematic literature search for scholarly articles relating to the topic was performed using Clarivate’s Web of Science (WoS) advanced search and Google Scholar with similar functions. The search included a topical search for multiple stressors, compound, cumulative or interactive effects at the coast or on the shelf, classified as an article or review. We implemented the following search query: “TS=(((“multipl* stress*”) OR (“compound* effect”) OR (“cumulat* effect”) OR (“interact* effect”)) AND (coast* or shelves or shelf)) AND DT=(“ARTICLE” OR “REVIEW”)”
Abstracts of all obtained records were prescreened independently by two researchers for false positives that should be discarded as not relating to the topic, e.g. articles relating to management and policy, society and socio-ecological systems, single stressors, freshwater systems, marshes or seismology. The review papers contained in the remaining records were screened for references to primary relevant literature that was not captured by the automated search. Citations within review papers that were identified as relevant after screening their abstract were included in the subsequent full-text analysis. All remaining and manually added full texts were distributed for detailed evaluation among the author team. We employed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA, Moher et al. (2009)] approach for our analysis. The systematic search, based on queries and keywords, on WoS and Google Scholar yielded 814 papers that investigated the interactive effects of multiple stressors in marine ecosystems. All of these papers were screened, first by an automated script and then manually, to select the studies that focused only on high-latitude coastal ecosystems, which reduced the number of papers from 814 to 400 (Figure 1). Out of these, a few were review or synthesis papers which were discarded from the analysis, and the remaining were distributed amongst the co-authors to review and fill up the “Summary Table” (Table 1). In total, we could identify 198 studies in which non-additive or additive effects were reported (Figure 1). All further analyses were performed based on the information provided in this table.
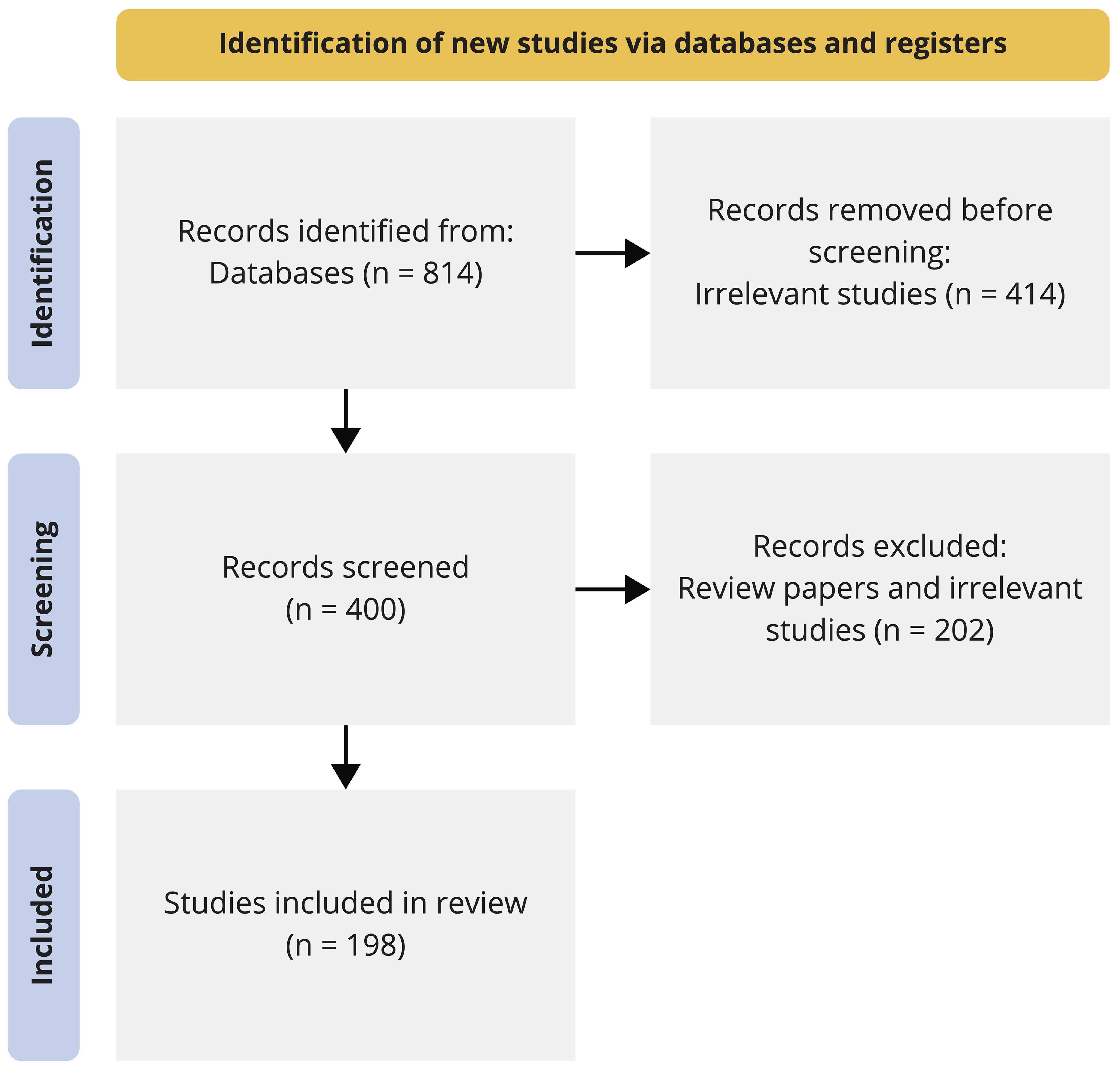
Figure 1. Schematic representation of our applied Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) scheme to identify and screen studies from the databases.

Table 1. The format of the Summary Table which was provided to all co-authors to include metadata information.
To quantify the magnitude of interactive effects (synergistic = SYN, additive = ADD, and antagonistic = ANT) for stressor combinations, we implemented a fuzzy coding/scoring method where scores between 1 to 3 were assigned for the reported responses. For example, if a study reports only a synergistic effect for a given stressor combination, a score of 3 is assigned for SYN and a score of 0 for ANT and ADD. If two types of effects (e.g. SYN and ANT or ADD and SYN) are reported then a score of 1.5 is given to each and 0 to the third effect which is missing. And, if all three effects (SYN, ANT, and ADD) could be identified then a score of 1 is assigned to all. For a given stressor pair, the sum of all three effects is always 3 (SYN + ANT + ADD = 3). If there are more than two stressor combinations, then the same procedure is followed for the respective stressor pairs resulting from that particular combination (e.g. 4 stressor combinations yield 6 stressor pairs). The final score for a given effect (SYN or ADD or ANT) is then calculated by adding up their individual scores identified from different stressor combinations. The detailed schematic of this scheme is illustrated in Figure 2.
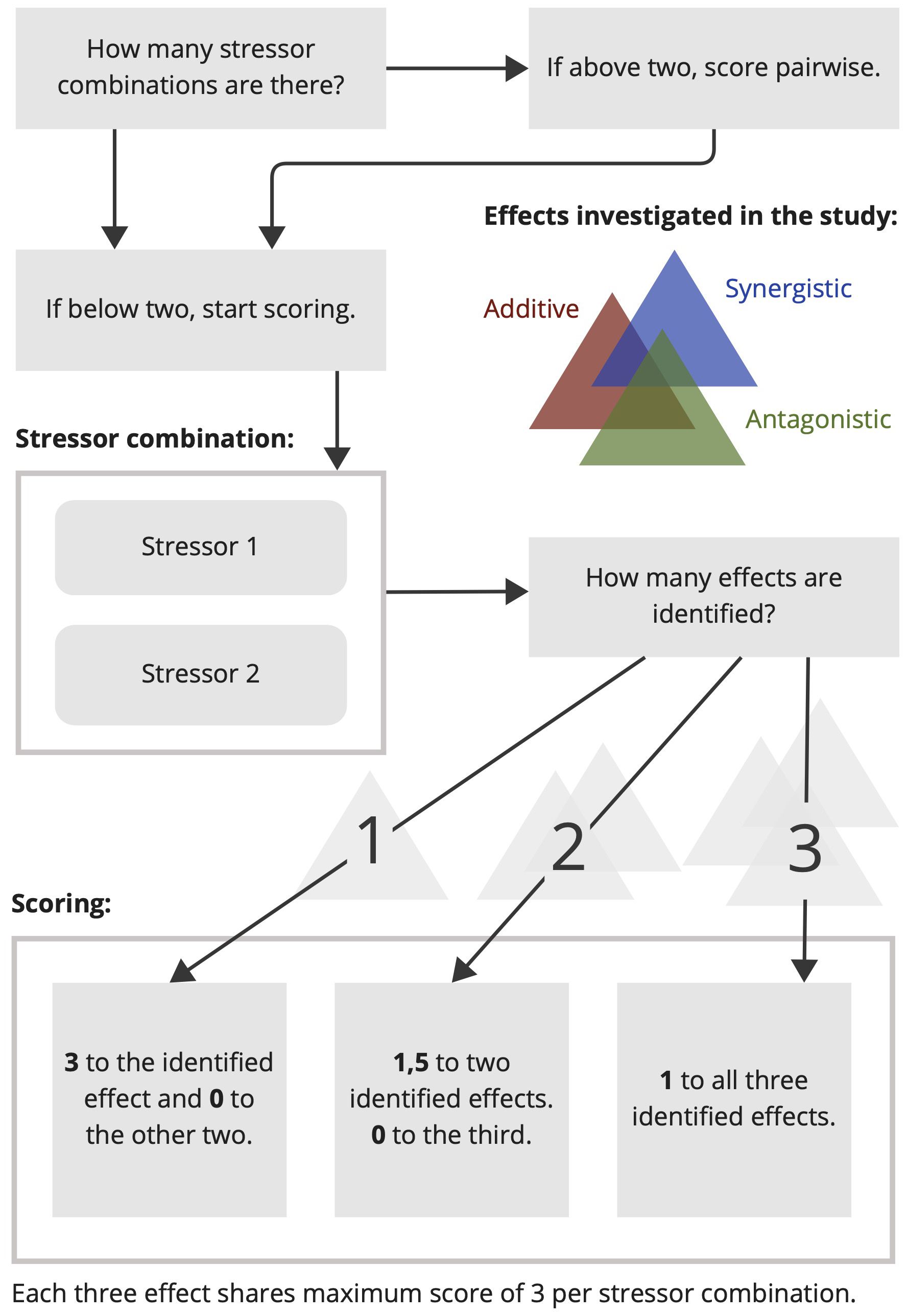
Figure 2. Schematic of score distributions for the synergistic (blue), antagonistic (green), and additive (dark red) effects corresponding to stressor pairs. The sum of all three effects is always 3 for a given stressor pair.
Interactive effects are reported at different levels of the coastal ecosystem; the majority (n=109, 55%) of studies focused on the species or organism level, 36% (n=71) on the community level, and 9% (n=18) on the entire ecosystem. The types of effects reported can be classified as synergistic, antagonistic, or additive. Most of the studies either reported additive (n = 62) or synergistic (n = 68) effects, and a few (n=15) reported both (Figure 3). These findings are indicative of the intensification or compounding of responses to multi-stressor exposure in coastal ecosystems. Whereas ANT effects are identified in only 20 studies (10% of the total). A handful of studies (n = 5) reported both ADD and ANT effects. Likewise, only 12 papers identified both SYN and ANT effects. Lastly, a few (n = 16) reported all three effects.
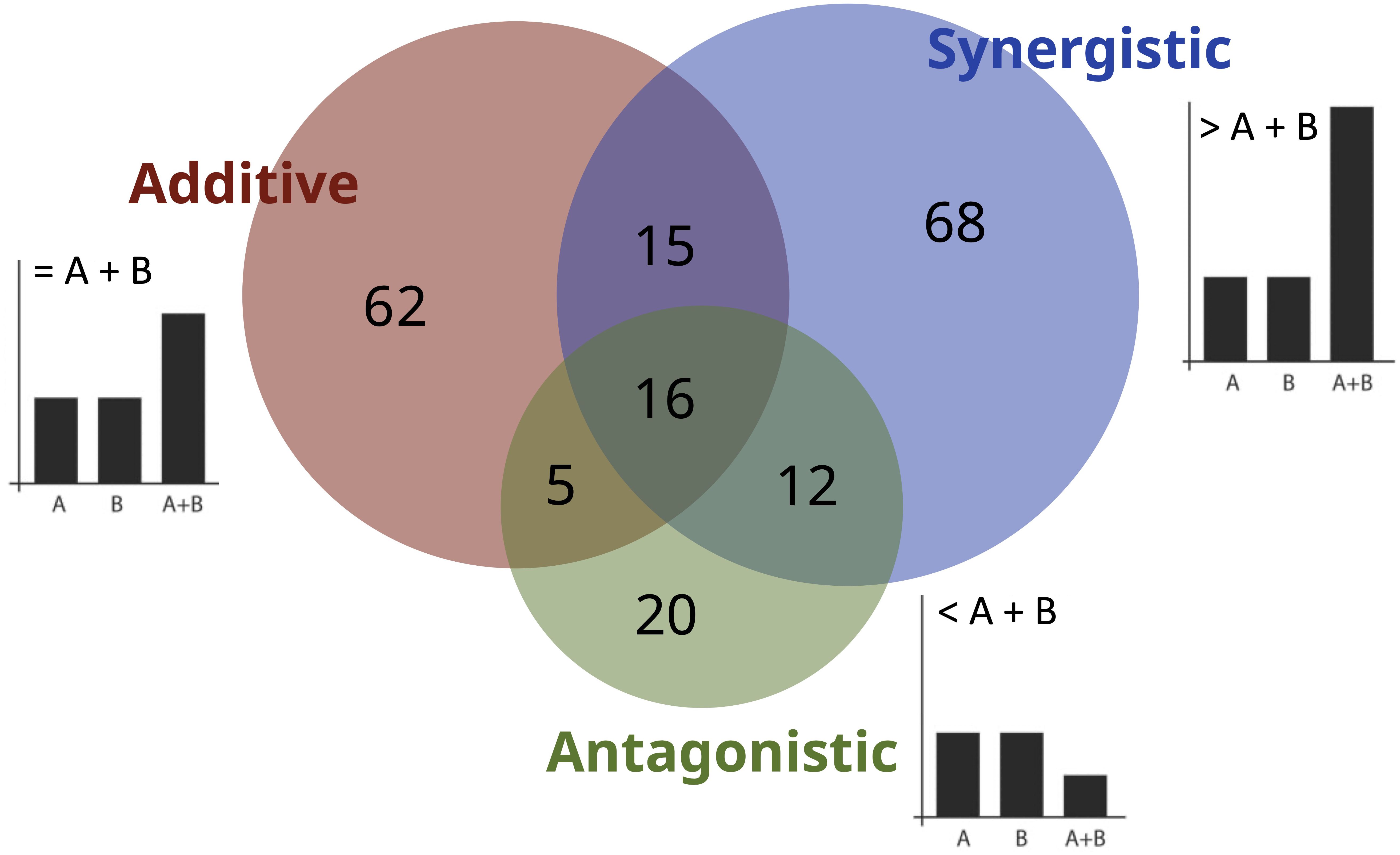
Figure 3. Venn diagram showing the number of studies which reported Synergistic, Antagonistic and Additive effects in coastal ecosystems (across taxonomic groups). The overlaps show the number of studies reporting two or more interactive effects.
Next, we identified the most investigated stressors that affect various taxonomic groups and communities in coastal ecosystems. The most frequent individual stressors in a multi-stressor constellation are temperature (Temp, 25%, n=50), followed by nutrient loading (Nut, 17%, n=33) and toxic metals/pollution (Metal) and ocean acidification (OA, together 28%, n=56) (Figure 4). While high turbidity and salinity stress are moderately studied (both 11%), hypoxia (DO) and physical/mechanical disturbances (Dist) are the least studied stressors. Quantification of the interactive effects (SYN, ANT, and ADD) for the stressor combinations of the most reported stressors is shown in Figure 5. The combinations of acidification– eutrophication (OA and Nut) and metal pollution–eutrophication (Metal and Nut) have mostly been reported as synergistic (with >50% score), whereas acidification–metal pollution (OA and Metal) has been reported mostly as additive (∼ 70% score). The Warming–acidification (Temp and OA) combination is expressed equally as additive, synergistic and antagonistic.
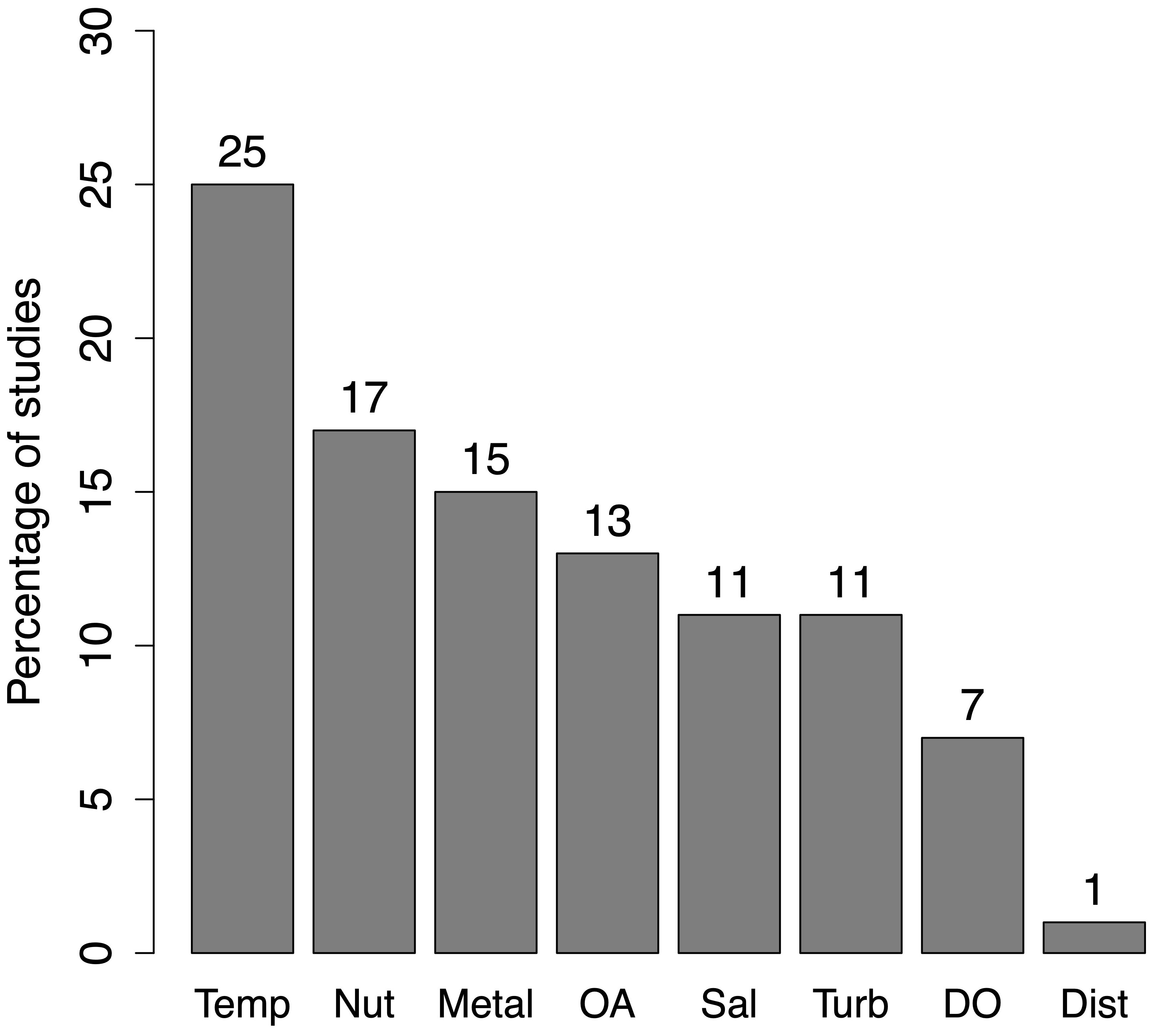
Figure 4. Most studied stressors, across species and communities, in coastal ecosystems (in terms of percentage), identified from our review. Temp, Temperature/warming; Nut, Nutrient pollution/Eutrophication; Metal, Metal pollution; OA, Ocean acidification; Sal, Salinity; DO, Dissolved oxygen; Turb, Turbidity; Dist, Physical disturbances.
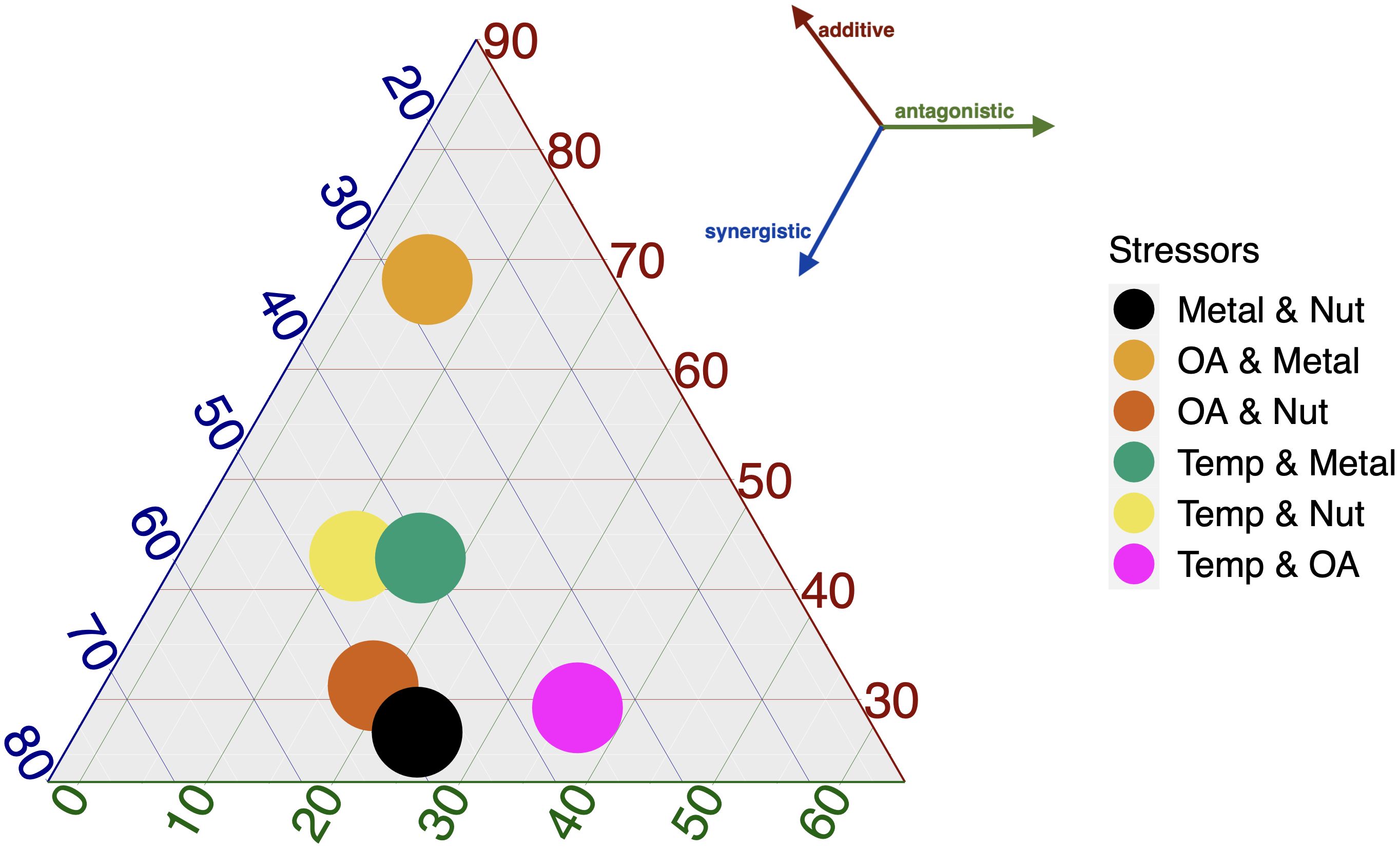
Figure 5. The above ternary plot shows the percentage score for the reported interactive effect (Synergistic, Antagonistic, Additive) corresponding to the different stressor combinations (Metal & Nut, OA & Metal, OA & Nut, Temp & Metal, Temp & Nut, Temp & OA) that are indicated by the colors of solid circles. The score (0 to 100%) for the synergistic effect increases towards the left vertex of the triangle as indicated by the blue corner lines. Likewise, the scores for the antagonistic (the green lines) and the additive (the red corner lines) effects increase towards the right and top vertexes, respectively. For an explanation of the stressor abbreviations see Figure 4. The effects shown here are aggregated across species, community and ecosystem levels in coastal environments.
The above results provide aggregated information about the critical stressors in coastal ecosystems. However, the relevance of these stressors can vary depending on organism type, trophic level, and trophic structure. Therefore, we identified the key stressors that elicit interactive effects at the species and community levels. Temperature is, by far, the most critical stressor at the species level. One-third of the studies (33%) investigating interactive effects in individual species have identified temperature as the most prominent stressor (Figure 6). However, this is not the case at the community level, where warming, nutrient pollution and metal contamination emerge as equally dominant stressors, followed by high turbidity, low pH, and salinity stress (all above 10%, Figure 6). While, hypoxia remains heavily understudied (<4%).
At the species level, the combination of metal and nutrient pollution predominantly drives synergistic effects, whereas warming and eutrophication equally trigger both SYN and ADD responses. (Figure 7A). In contrast, OA combined with other stressors appears to invoke differential responses depending on species type, as evident by the lack of a dominant effect in stressor combinations involving OA (Figure 7A). At the community level, climate warming and ocean acidification have the highest likelihood of generating synergistic responses (Figure 7B).
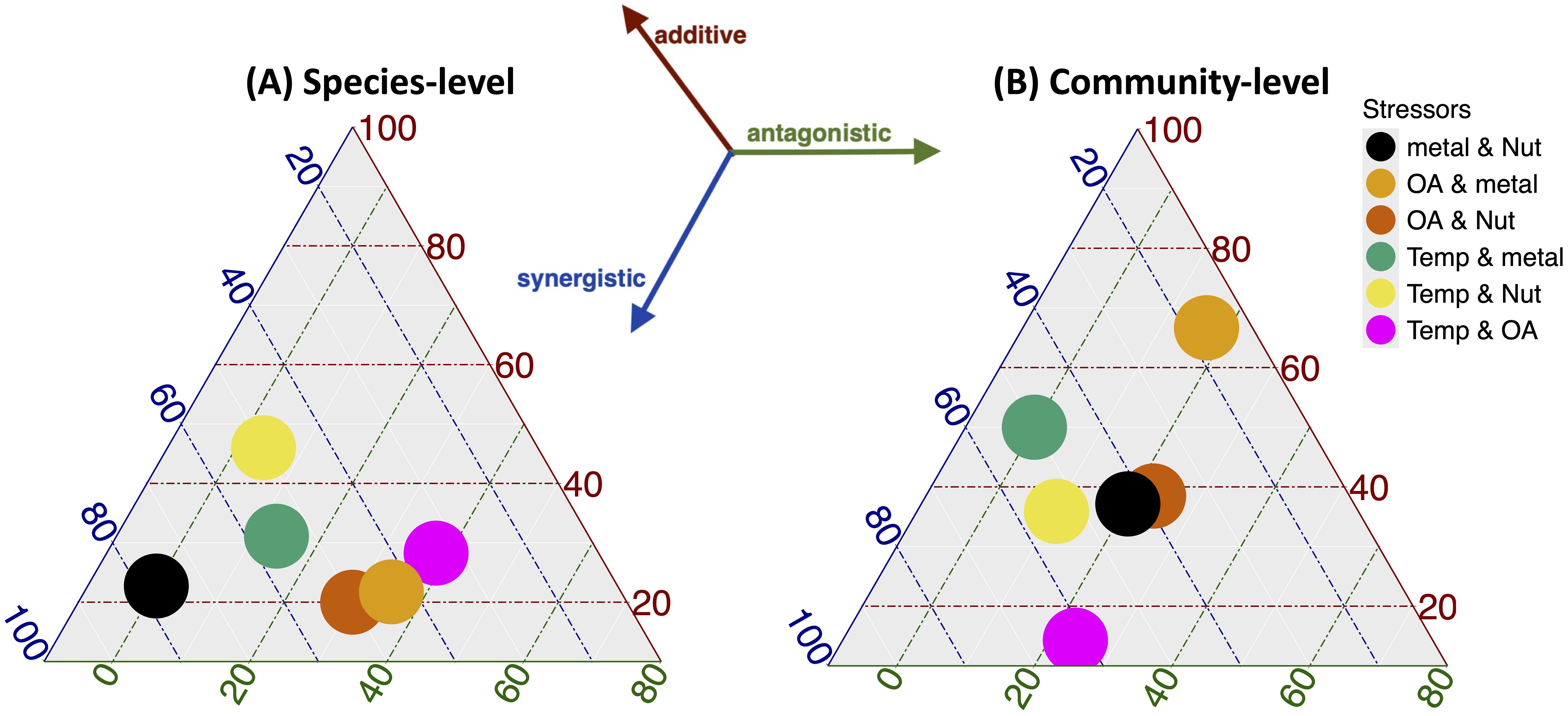
Figure 7. The above ternary plots show the percentage score for the reported interactive effect (Synergistic, Antagonistic, Additive) corresponding to the different stressor combinations at the level of individual species (A) and at community-level (B).
Subsequently, we identified the most studied organisms and their respective traits for the major stressor combinations. Bivalves and phytoplankton are the most studied taxonomic groups, followed by seagrass and fish (Figure 8). This difference could be partially attributed to research constraints arising from the complexity of studied organisms or systems. At higher body size the fraction of manipulative experiments decreases. For smaller organisms, such as phytoplankton, zooplankton, and bivalves, more than 50% of studies are performed in controlled laboratory setups, whereas this fraction falls below 50% for fish and decapods and below 20% for seagrass (Figure 9). Larger organisms are preferably studied in their natural and quasi-natural habitats. Likewise, in-situ experiments are preferred for studying ecosystem-level effects of multiple stressors.
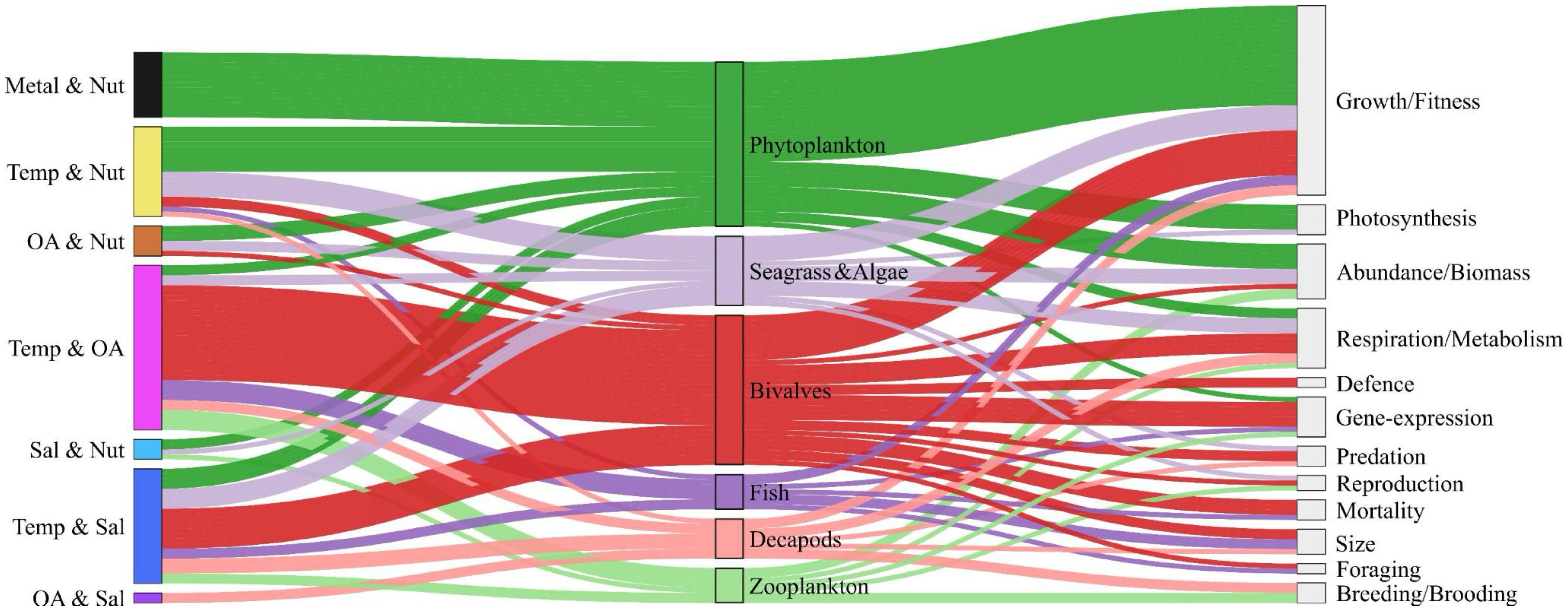
Figure 8. Illustration of relationships between stressor combinations, taxonomic groups, and traits. The lines highlight stressor combinations impacting different organisms and traits consequently affected. Colors of lines correspond to the organisms and their widths represent the frequency of studies reporting each specific connection, highlighting the most frequently observed relationships.
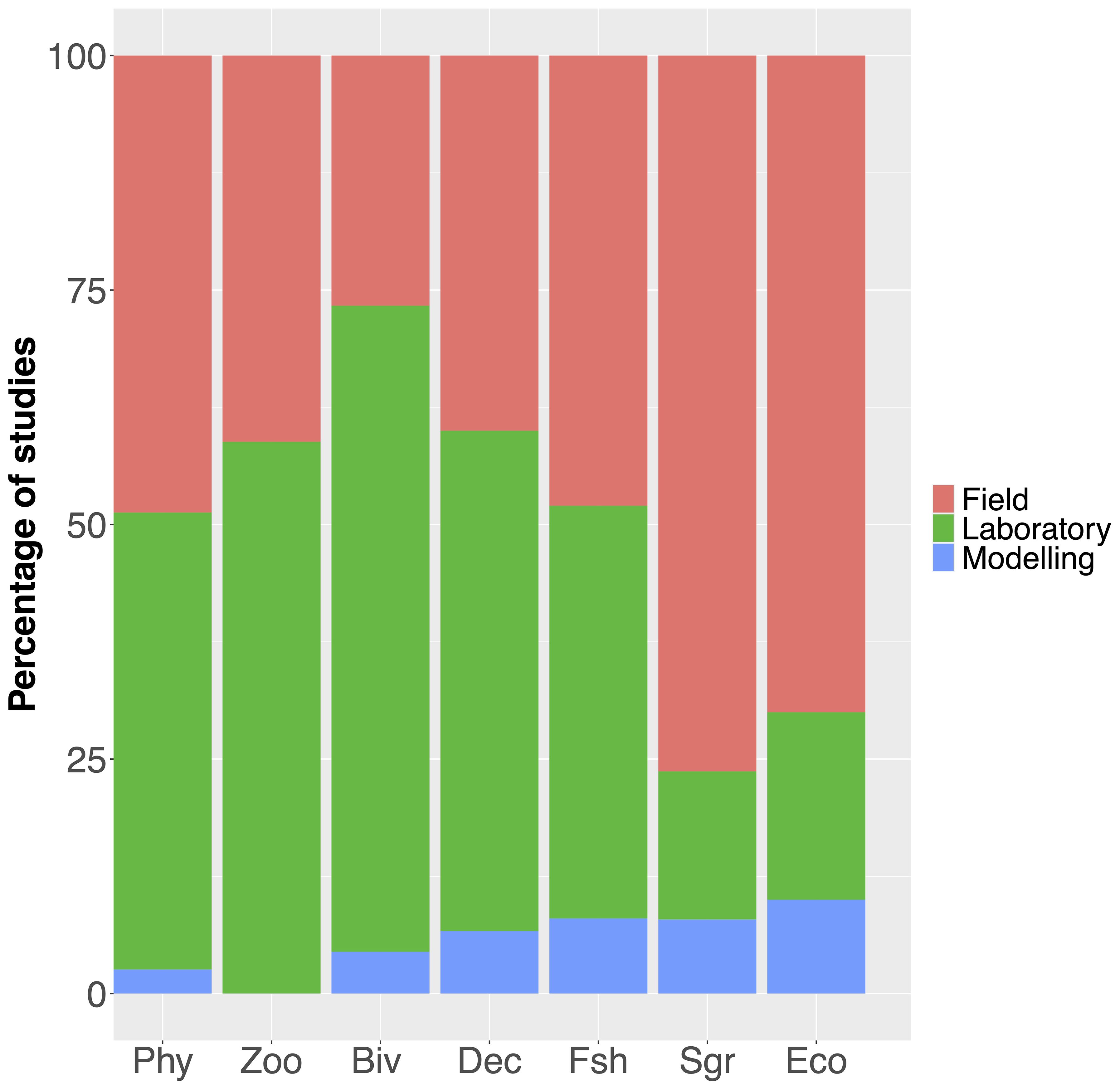
Figure 9. Study types (field, laboratory, and modelling) expressed in terms of percentage corresponding to different taxonomic groups (of increasing complexity) and to ecosystems. Phy, Phytoplankton; Zoo, Zooplankton; Biv, Bivalves; Dec, Decapods; Fsh, Fish; Sgr, Seagrass; Eco, Ecosystem.
Metal pollution, eutrophication, and climate warming seem to be the most dominant stressor combination for phytoplankton, strongly affecting photosynthesis and growth dynamics in algal species (Figures 8, 10). For bivalves, OA and Temp are the most critical stressors in driving non-additive effects (Figures 8, 10). A wide range of traits are affected by the combination of OA and Temp stressors in bivalves, including fitness, defense, abundance, and metabolism (Figure 8). Likewise in fish species, Temp, OA and Sal stressors affect a broad range of traits. For seagrass, the combinations of eutrophication, warming, and salinity stress are equally critical, affecting their growth and metabolism (Figure 8).
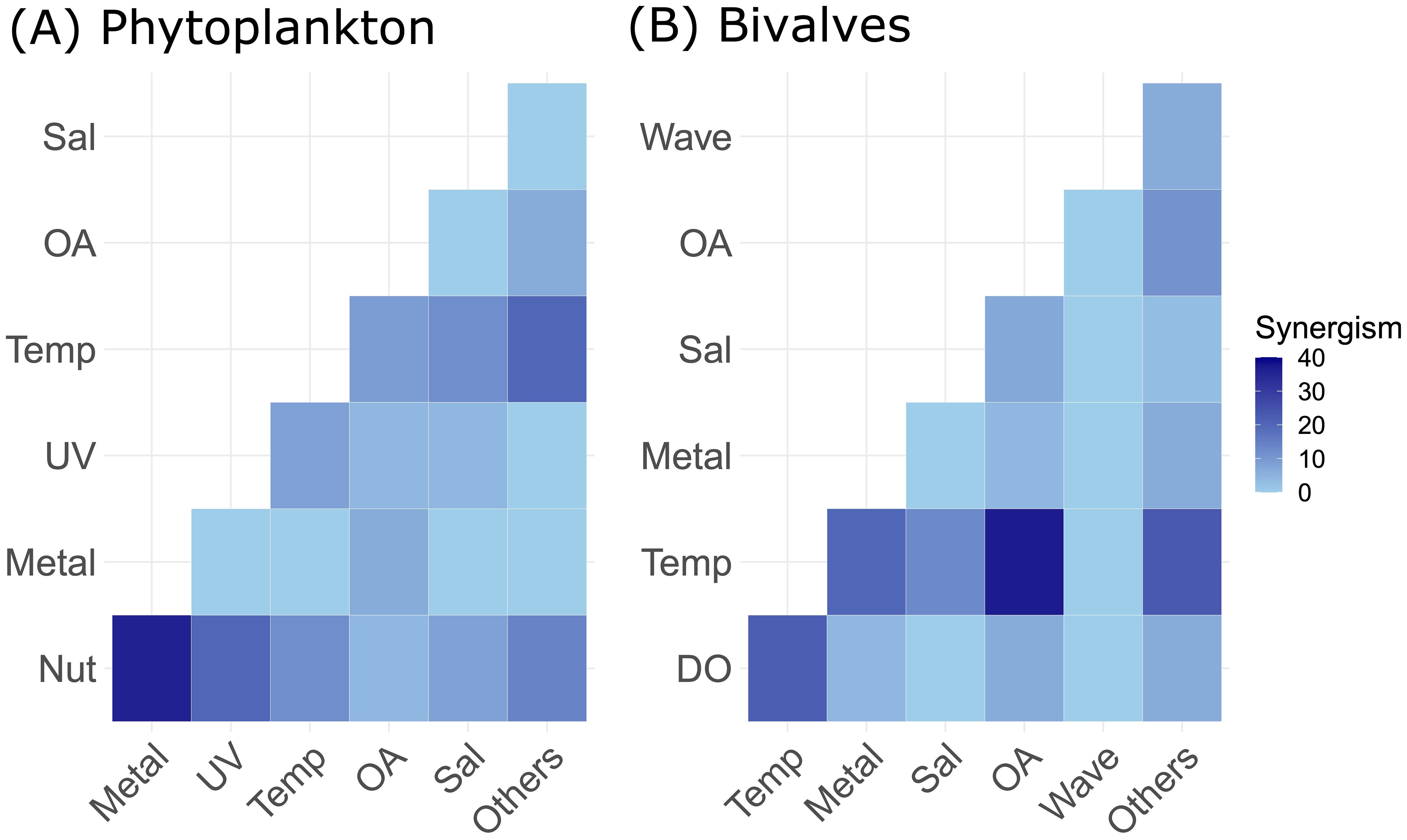
Figure 10. Critical stressor combination for phytoplankton (A) and bivalves (B) based on stressor-pair scores. A dark blue tone indicates a high score for synergism and light blue a low score. For an explanation of the stressor abbreviations see Figure 4.
As bivalves and phytoplankton are the most studied taxonomic groups, we investigated which combinations of stressors are most likely to generate synergistic responses. We identified them by a fuzzy coding approach, where stressor combinations were given scores for synergism. For phytoplankton, the metal-nutrient (Metal and Nut) combination got the highest score (Figure 8), as exposure to nutrient and metal pollution typically instigates synergistic responses at the physiological level in autotrophs. Likewise, the temperature-acidification (Temp and OA) stressor pair got the highest score for bivalves (Figure 8), indicating that the combination of warming and ocean acidification is likely to synergistically affect their physiological rates.
We identified climate warming, OA, eutrophication, and metal pollution as the most critical stressor combinations for coastal ecosystems. All of these stressors directly follow human activities on land that strongly influence coastal environments (Sandifer and Sutton-Grier, 2014; He and Silliman, 2019). Warming, eutrophication and metal pollution negatively affect the ecological health of coastal systems in isolation and their interactive effects are often even more adverse (Skei et al., 2000; Cloern, 2001; Marcus, 2004; Church et al., 2006; Lin et al., 2020). For example, several sites in the Bohai Sea (China) have been identified as high-risk areas for autotrophs, crustacean, fish and bivalves due to the compounding effects of the elevated nutrient and metal concentrations (Lin et al., 2020). For phytoplankton, the metal/nutrient stressor combination appears to be most critical and is discussed below.
A full range of physiological effects can result from the interaction of eutrophication and metal pollution in phytoplankton, ranging from acute toxicity to sub-lethal or positive effects. In addition, shifts in community structure have been observed (Solan and Whiteley, 2016). Toxic metals have inhibitory effects on the germination of phytoplankton in eutrophic coastal waters (Lu et al., 2017). It has been reported that the combined effect of metal and nutrient stressors results in significant changes in phytoplankton community structure leading to a shift from bigger diatoms to smaller and harmful cyanobacteria and dinoflagellates (Riedel et al., 2003; Song et al., 2022). Metals, such as copper and cadmium, inhibit the growth of large algal species (e.g. diatom), even in nutrient-replete conditions, by suppressing the uptake of enzymes and causing cell leakage. The shift to harmful algal species results in a reduction of food quality for secondary producers and thereby affects the trophodynamics in the coastal food web. Nutrient enrichment in many coastal waters can considerably enhance trace metal uptake in phytoplankton, which leads to bioaccumulation of metals at the higher trophic levels (Wang and Dei, 2001). Likewise, eutrophication-mediated increase in organic matter production and remineralization stimulates microbial mercury methylation which results in bioaccumulation of neurotoxic monomethyl mercury in phytoplankton (Soerensen et al., 2016). Metals like cadmium, nickel and copper are shown to make coastal areas more heterotrophic by increasing ecosystem respiration (Wiegner et al., 2003; Sundbäck et al., 2007; Nikulina and Dullo, 2009). However, some trace metals such as iron, silicon and copper could stimulate primary production in eutrophic coastal and estuarine waters by increasing the cellular uptake rates of macronutrients (Paerl, 1997; Zhang, 2000). Sometimes, the mechanisms employed by algal species to deal with metal and nutrient contamination can have negative consequences for other species or for the entire ecosystem. For example, the diatom Pseudo-nitzschia produces toxic domoic acid in response to copper-stressed conditions in nutrient-rich coastal waters, which is linked to amnesic shellfish poisoning (Maldonado et al., 2002). Regardless of the intensification or reduction in primary production or adaptation of a defense mechanism, the synergistic effects of metal and nutrients on phytoplankton are significant; and as autotrophs constitute the base of coastal food webs, changes in their growth, defense or community-structure dynamics can greatly impact organisms at higher trophic levels and the flow of energy and matter.
Eutrophication and metal pollution mediate other stressors too, such as hypoxia. In large parts of the coastal ocean, eutrophication and metal pollution lead to hypoxia, which in turn stimulate metal eco-toxicity. For example, hypoxia increases the bioavailability of manganese in sediment, potentially increasing its toxicity for pelagic and benthic organisms (Mustafa et al., 2012; Eriksson et al., 2013).
Bivalves are the second most studied taxonomic group after phytoplankton in the multistressor literature. The most critical interactive stressor for bivalves is ocean acidification combined with climate warming. The sensitivity to acidification is a consequence of the production of calcium carbonate shells (Gazeau et al., 2013); the sensitivity to warming is metabolic stimulation with higher temperature, up to a critical threshold (CTmax) at which physiological process rates start to decline again (Schulte et al., 2011; Marshall et al., 2011; Giomi and Pörtner, 2013). Vihtakari et al. (2013) suggested that warming affects larval development, whereas acidification affects the reproductive capacity of adults.
A moderate increase in water temperature can counteract the growth effects of reduced pH in M. galloprovincialis by allowing more active feeding time Kroeker et al. (2014), and thus constituting an antagonistic interaction. Mostly, however, the combined effect of OA and warming in bivalves has been observed as negatively synergistic: Thomsen et al. (2013), e.g., showed that heat shock proteins are downregulated under elevated pCO2, amplifying heat stress experienced by Mytilus edulis. Many traits may be affected by the combination of warming and acidification: for both M. edulis and Mytilus galloprovincialis growth rate, shell size, and acid-base buffering capacity were found to decrease (Gazeau et al., 2014; Fitzer et al., 2015).
The occurrence of further stressors along with warming and acidification exacerbates the negative synergistic effects. Adding hypoxia impairs the fitness of marine mussels by reducing the activity of digestive enzymes (Khan et al., 2020). Likewise, an increased frequency of extreme climatic events has been reported to impact bivalve species at 12 coastal regions around the Mediterranean (Rodrigues et al., 2015).
Multi-stressor effects on bivalve species propagate to the entire coastal ecosystem via the ecosystem services provided, foremost the habitat creation and water quality improving filtration services, but also food provisioning. The presence of bivalves prevents the proliferation of harmful algal blooms (Richard et al., 2022). Nutritional composition and thus the commercial value of oysters and mussels decrease under combined acidification and warming stress (Tate et al., 2017).
Effects of different stressor levels on populations in orthogonal experimental designs are difficult to examine, particularly when many stressors are involved Griffen et al. (2016). It is costly in terms of time and resources and the inclusion of different stressor intensities may complicate the experimental design (King et al., 2022).
We identified only a few studies (only 8%) which investigated the interactive effects of multiple stressors on ecosystem-level dynamics in coastal waters. This has been also noted by Crain et al. (2008) in their review of non-additive effects of human stressors in marine systems, pointing out the existing bias towards studying single species in ex-situ setups. Experimental determination of complex interactions in coastal environments is challenging (Carrier-Belleau et al., 2021), and it is difficult to measure stressor effects at the community or ecosystem level in natural settings (Adams, 2003; Elliott and Quintino, 2007; Borja, 2014; Wake, 2019).
As the interactive effects at every trophic level vary, depending on factors such as stressor magnitude and exposure duration, measurements of multiple endpoints have to be considered while designing the experiments on an ecosystem scale (King et al., 2022). A first step would start with individual stressor studies across a wide range of intensities to understand responses and then examine combinations of multiple stressors across a smaller range of stressor levels to explore interactions between them. This approach would help to identify critical stressors for multiple stressor experiments. Another difficulty in studying interactive effects across ecosystems is the variable response time of different taxa to a variety of stressors (Griffen et al., 2016; Turschwell et al., 2022). Furthermore, the time at which stressor response is measured can also affect the classification of the interaction type (Garnier et al., 2017). While studying and quantifying the direct cumulative effects of multiple stressors themselves is challenging, their indirect effects further complicate the problem. This has implications for coastal management, as they do not follow typical cause-and-effect pathways but significant modify ecosystem responses (Adams, 2005; Gladstone-Gallagher et al., 2023). Thus, it has been suggested to focus on both the direct and indirect effects of stressor interactions for effective stressor management (Gladstone-Gallagher et al., 2023).
Despite the complications in measuring ecosystem responses to multiple stressors, some efforts have been made in this direction. With the advent of mesocosm experiments, it has become possible to study ecosystem-level responses and effects of climatic and anthropogenic stressors in quasi-natural habitats (Stewart et al., 2013). For example, the mesocosm experiments of Pansch and Hiebenthal (2019) facilitated assessment for a whole range of effects of multiple stressors (temperature, salinity, pH, light) on benthic ecosystems and communities. Given the large number of stressor combinations and species in coastal ecosystems, it will remain unfeasible to fully understand multi-factorial stressor effects by means of observational experiments. Therefore, other approaches such as modelling and expert opinions have been proposed by Halpern et al. (2007); Griffen et al. (2016); Stelzenmüller et al. (2024). For modelling, a 3-tiered approach has been proposed. First, understand mechanistically the stressor effects at the individual level Griffen et al. (2016), and then scale these to population-level responses and, finally, assess the risks for communities across ecosystems. This practice has been adopted by some modelling studies (Pörtner, 2012; Cornwall and Eddy, 2015; Queirós et al., 2015). Other researchers propose that combining methods can improve understanding such as aligning experiments from the beginning with the models they aim to inform (Hodgson and Halpern, 2019).
Although we found that the majority of multi-stressor studies focus on species-level effects, these are largely restricted to phytoplankton and bivalves. Other taxonomic groups, such as zooplankton, fish and benthic organisms, which constitute important trophic linkages in the coastal food webs, are underrepresented (Figure 8). It is difficult to manage physiologically complex and larger organisms in manipulative experiments and to track variable responses to the same stressors by different species of the same group, e.g. in fish. Most of the multi-stressor studies performed on seagrass are in-situ experiments where co-variations in environmental boundary conditions make it hard to unravel non-additive interactions (Stockbridge et al., 2020). As a consequence, we could not identify a trend in reported interactive effects for seagrass or fish. Intra-specific responses in higher organisms (such as fish) vary depending on stressor magnitude and duration (Barton, 2002). Stressor-driven changes at higher trophic levels trigger cascading effects that impact the food web dynamics in coastal ecosystems (Pinnegar et al., 2000; Scheffer et al., 2005; Murphy et al., 2020). The same applies to the coupling of pelagic and benthic ecosystems. In our analysis, we found that only a few studies focused on benthic polychaetes and those who did mostly reported synergistic effects (having negative consequences) in response to multiple stressors. Benthic polychaetes are powerful ecosystem engineers as they contribute to nutrient recycling, carbon storage and oxygenation of sediments (Meadows et al., 2012; Griffiths et al., 2017).
Thus, the holistic understanding of how coastal ecosystems will evolve with ongoing changes requires knowledge of how different trophic levels will respond to multiple stressors. Therefore, it is important to diversify the target taxonomic group in multi-stressor studies or experiments. Our review also disclosed that relevant stressors such as hypoxia, turbidity, and invasive species, are understudied. Hypoxia has been identified as one of the most critical stressors for coastal ecosystems, negatively affecting pelagic and benthic organisms (Howarth et al., 2011). Other stressors such as warming, eutrophication and ocean acidification create feedback loops with hypoxia and together they intensify the stress on coastal food webs (Conley et al., 2009; Carstensen et al., 2014). Hypoxia, particularly, increases respiratory stress in benthic fauna (such as arthropods and bivalves) and in fish, which can trigger cascading effects (Grantham et al., 2004; Bograd et al., 2008; Hughes et al., 2015). Likewise, fishing, introduction of invasive species and turbidity have been reported to alter trophodynamics (Scheffer et al., 2005; Lehtiniemi et al., 2005; Bruno and Cardinale, 2008). Thus, more experimental and modelling efforts should be put together to investigate the non-additive effects of these stressors at species and community levels in coastal ecosystems.
In our review analysis, we identified climate warming, eutrophication, ocean acidification, and metal pollution, or combinations thereof, as the most critical stressors triggering non-additive responses in coastal ecosystems. Thus, from a management perspective, particular attention should be placed on mitigating these stressors in coastal systems. Phytoplankton and bivalves are the most studied taxonomic groups in multi-stressor experiments and observations. They are, however, sensitive to different stressor combinations. Simultaneous exposure to metal pollution and high nutrient concentrations invokes synergistic responses in phytoplankton, often with negative effects on their physiology. For bivalves, climate warming and ocean acidification appear to be the most critical stressor combination, with adverse effects on physiological and morphological traits. Organisms at different trophic levels, or belonging to different ecosystem components (such as micro- and macrobenthos), are sensitive to different stressor combinations. Consequently, interactive effects could induce radical changes in trophodynamics and food web structure as climate and human-induced changes in coastal ecosystems continue to intensify. For a holistic understanding of the cumulative effects of multiple stressors in coastal ecosystems, however, we suggest that more research focus should be placed on studying community and ecosystem-level responses. This can be achieved by combining in-situ observations (e.g. mesocosm experiments) with modelling approaches. In addition, other relevant taxonomic groups such as zooplankton, fish and benthic polychaetes demand more research.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
SK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CL: Data curation, Writing – review & editing. SÖ: Data curation, Visualization, Writing – review & editing. JR: Data curation, Writing – review & editing. JP: Data curation, Writing – review & editing. MM: Data curation, Writing – review & editing. MP: Data curation, Writing – review & editing. SH: Data curation, Writing – review & editing. HP: Data curation, Writing – review & editing. MH: Data curation, Writing – review & editing. JS: Data curation, Writing – review & editing. KW: Data curation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The funding for this study comes from the projects: Multiple Stressors on North Sea Life (MuSSeL) funded by German Federal Ministry of Education and Research BMBF (grant number: 03F0862A at Hereon, 03F0862B at BSH, 03F0862C at Hochschule Bremerhaven, 03F0862D at Thünen-Institut, 03F0862E at University of Hamburg), and CALIPSO (grant number: P11701-01) and BIOcean5D (grant number: P11370) at NOC, Southampton.
We are grateful to Cedric Meunier and Ingrid Kroencke for their valuable input in the development of this paper.
Authors CL, MM, HP, and KW were employed by company Helmholtz-Zentrum Hereon. Author JP was employed by company Electricité de France.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbate M. C. L., Molinero J. C., Guinder V. A., Perillo G. M., Freije R. H., Sommer U., et al. (2017). Time-varying environmental control of phytoplankton in a changing estuarine system. Sci. Total Environ. 609, 1390–1400. doi: 10.1016/j.scitotenv.2017.08.002
Adams S. M. (2003). Establishing causality between environmental stressors and effects on aquatic ecosystems. Hum. Ecol. Risk Assess. 9, 17–35. doi: 10.1080/713609850
Adams S. M. (2005). Assessing cause and effect of multiple stressors on marine systems. Mar. pollut. Bull. 51, 649–657. doi: 10.1016/j.marpolbul.2004.11.040
Ainley D. G., Blight L. K. (2009). Ecological repercussions of historical fish extraction from the southern ocean. Fish Fisheries 10, 13–38. doi: 10.1111/j.1467-2979.2008.00293.x
Barton B. A. (2002). Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 42, 517–525. doi: 10.1093/icb/42.3.517
Bograd S. J., Castro C. G., Di Lorenzo E., Palacios D. M., Bailey H., Gilly W., et al. (2008). Oxygen declines and the shoaling of the hypoxic boundary in the california current. Geophysical Res. Lett. 35, 35–12. doi: 10.1029/2008GL034185
Borja A. (2014). Grand challenges in marine ecosystems ecology. Frontiers in Marine Science. 1, 1. doi: 10.3389/fmars.2014.00001
Brooks P. R., Crowe T. P. (2019). Combined effects of multiple stressors: New insights into the influence of timing and sequence. Front. Ecol. Evol. 7, 387. doi: 10.3389/fevo.2019.00387
Bruno J. F., Cardinale B. J. (2008). Cascading effects of predator richness. Front. Ecol. Environ. 6, 539–546. doi: 10.1890/070136
Bundy M., Breitburg D. L., Sellner K. G. (2003). The responses of patuxent river upper trophic levels to nutrient and trace element induced changes in the lower food web. Estuaries 26, 365–384. doi: 10.1007/BF02695974
Carrier-Belleau C., Drolet D., McKindsey C. W., Archambault P. (2021). Environmental stressors, complex interactions and marine benthic communities’ responses. Sci. Rep. 11, 4194. doi: 10.1038/s41598-021-83533-1
Carstensen J., Andersen J. H., Gustafsson B. G., Conley D. J. (2014). Deoxygenation of the baltic sea during the last century. Proc. Natl. Acad. Sci. 111, 5628–5633. doi: 10.1073/pnas.1323156111
Church T., Sommerfield C., Velinsky D., Point D., Benoit C., Amouroux D., et al. (2006). Marsh sediments as records of sedimentation, eutrophication and metal pollution in the urban delaware estuary. Mar. Chem. 102, 72–95. doi: 10.1016/j.marchem.2005.10.026
Cloern J. E. (2001). Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 210, 223–253. doi: 10.3354/meps210223
Conley D. J., Carstensen J., Vaquer-Sunyer R., Duarte C. M. (2009). “Ecosystem Thresholds with Hypoxia,” in Eutrophication in Coastal Ecosystems: Towards Better Understanding and Management Strategies Selected Papers from the Second International Symposium on Research and Management of Eutrophication in Coastal Ecosystems (Springer, Nyborg, Denmark), 21–29.
Cornwall C. E., Eddy T. D. (2015). Effects of near-future ocean acidification, fishing, and marine protection on a temperate coastal ecosystem. Conserv. Biol. 29, 207–215. doi: 10.1111/cobi.2015.29.issue-1
Côté I. M., Darling E. S., Brown C. J. (2016). Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B: Biol. Sci. 283, 20152592. doi: 10.1098/rspb.2015.2592
Crain C. M., Kroeker K., Halpern B. S. (2008). Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x
Elliott M., Quintino V. (2007). The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. pollut. Bull. 54, 640–645. doi: 10.1016/j.marpolbul.2007.02.003
Eriksson S. P., Hernroth B., Baden S. P. (2013). “Stress Biology and Immunology in Nephrops Norvegicus,” in Advances in Marine Biology, vol. 64. (Amsterdam, Academic Press: Elsevier), 149–200.
Fitzer S. C., Vittert L., Bowman A., Kamenos N. A., Phoenix V. R., Cusack M. (2015). Ocean acidification and temperature increase impact mussel shell shape and thickness: problematic for protection? Ecol. Evol. 5, 4875–4884. doi: 10.1002/ece3.2015.5.issue-21
Garnier A., Pennekamp F., Lemoine M., Petchey O. L. (2017). Temporal scale dependent interactions between multiple environmental disturbances in microcosm ecosystems. Global Change Biol. 23, 5237–5248. doi: 10.1111/gcb.2017.23.issue-12
Gazeau F., Alliouane S., Bock C., Bramanti L., López Correa M., Gentile M., et al. (2014). Impact of ocean acidification and warming on the mediterranean mussel (Mytilus galloprovincialis). Front. Mar. Sci. 1, 62. doi: 10.3389/fmars.2014.00062
Gazeau F., Parker L. M., Comeau S., Gattuso J.-P., O’Connor W. A., Martin S., et al. (2013). Impacts of ocean acidification on marine shelled molluscs. Mar. Biol. 160, 2207–2245. doi: 10.1007/s00227-013-2219-3
Giomi F., Pörtner H.-O. (2013). A role for haemolymph oxygen capacity in heat tolerance of eurythermal crabs. Front. Physiol. 4, 110. doi: 10.3389/fphys.2013.00110
Gissi E., Manea E., Mazaris A. D., Fraschetti S., Almpanidou V., Bevilacqua S., et al. (2021). A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 755, 142564. doi: 10.1016/j.scitotenv.2020.142564
Gladstone-Gallagher R. V., Thrush S. F., Low J. M., Pilditch C. A., Ellis J. I., Hewitt J. E. (2023). Toward a network perspective in coastal ecosystem management. J. Environ. Manage. 346, 119007. doi: 10.1016/j.jenvman.2023.119007
Grantham B. A., Chan F., Nielsen K. J., Fox D. S., Barth J. A., Huyer A., et al. (2004). Upwelling-driven nearshore hypoxia signals ecosystem and oceanographic changes in the northeast Pacific. Nature 429, 749–754. doi: 10.1038/nature02605
Griffen B. D., Belgrad B. A., Cannizzo Z. J., Knotts E. R., Hancock E. R. (2016). Rethinking our approach to multiple stressor studies in marine environments. Mar. Ecol. Prog. Ser. 543, 273–281. doi: 10.3354/meps11595
Griffiths J. R., Kadin M., Nascimento F. J., Tamelander T., Törnroos A., Bonaglia S., et al. (2017). The importance of benthic–pelagic coupling for marine ecosystem functioning in a changing world. Global Change Biol. 23, 2179–2196. doi: 10.1111/gcb.2017.23.issue-6
Halpern B. S., Selkoe K. A., Micheli F., Kappel C. V. (2007). Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv. Biol. 21, 1301–1315. doi: 10.1111/j.1523-1739.2007.00752.x
He Q., Silliman B. R. (2019). Climate change, human impacts, and coastal ecosystems in the anthropocene. Curr. Biol. 29, R1021–R1035. doi: 10.1016/j.cub.2019.08.042
Heiden J. P., Thoms S., Bischof K., Trimborn S. (2018). Ocean acidification stimulates particulate organic carbon accumulation in two antarctic diatom species under moderate and high natural solar radiation. J. Phycology 54, 505–517. doi: 10.1111/jpy.2018.54.issue-4
Henson S. A., Beaulieu C., Ilyina T., John J. G., Long M., Séférian R., et al. (2017). Rapid emergence of climate change in environmental drivers of marine ecosystems. Nat. Commun. 8, 14682. doi: 10.1038/ncomms14682
Hewitt J. E., Ellis J. I., Thrush S. F. (2016). Multiple stressors, nonlinear effects and the implications of climate change impacts on marine coastal ecosystems. Global Change Biol. 22, 2665–2675. doi: 10.1111/gcb.2016.22.issue-8
Hodgson E. E., Halpern B. S. (2019). Investigating cumulative effects across ecological scales. Conserv. Biol. 33, 22–32. doi: 10.1111/cobi.13125
Howarth R., Chan F., Conley D. J., Garnier J., Doney S. C., Marino R., et al. (2011). Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 9, 18–26. doi: 10.1890/100008
Hughes B. B., Levey M. D., Fountain M. C., Carlisle A. B., Chavez F. P., Gleason M. G. (2015). Climate mediates hypoxic stress on fish diversity and nursery function at the land–sea interface. Proc. Natl. Acad. Sci. 112, 8025–8030. doi: 10.1073/pnas.1505815112
Jackson M. C., Loewen C. J., Vinebrooke R. D., Chimimba C. T. (2016). Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Global Change Biol. 22, 180–189. doi: 10.1111/gcb.2016.22.issue-1
Khan F. U., Hu M., Kong H., Shang Y., Wang T., Wang X., et al. (2020). Ocean acidification, hypoxia and warming impair digestive parameters of marine mussels. Chemosphere 256, 127096. doi: 10.1016/j.chemosphere.2020.127096
King O. C., van de Merwe J. P., Campbell M. D., Smith R. A., Warne M. S. J., Brown C. J. (2022). Interactions among multiple stressors vary with exposure duration and biological response. Proc. R. Soc. B 289, 20220348. doi: 10.1098/rspb.2022.0348
Kroeker K. J., Gaylord B., Hill T. M., Hosfelt J. D., Miller S. H., Sanford E. (2014). The role of temperature in determining species’ vulnerability to ocean acidification: a case study using mytilus galloprovincialis. PloS One 9, e100353. doi: 10.1371/journal.pone.0100353
Lehtiniemi M., Engström-Öst J., Viitasalo M. (2005). Turbidity decreases anti-predator behaviour in pike larvae, esox lucius. Environ. Biol. Fishes 73, 1–8. doi: 10.1007/s10641-004-5568-4
Lin H., Li H., Yang X., Xu Z., Tong Y., Yu X. (2020). Comprehensive investigation and assessment of nutrient and heavy metal contamination in the surface water of coastal Bohai sea in China. J. Ocean Univ. China 19, 843–852. doi: 10.1007/s11802-020-4283-x
Lu X., Wang Z., Guo X., Gu Y., Liang W., Liu L. (2017). Impacts of metal contamination and eutrophication on dinoflagellate cyst assemblages along the Guangdong coast of southern China. Mar. pollut. Bull. 120, 239–249. doi: 10.1016/j.marpolbul.2017.05.032
Maldonado M. T., Hughes M. P., Rue E. L., Wells M. L. (2002). The effect of fe and cu on growth and domoic acid production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnology Oceanography 47, 515–526. doi: 10.4319/lo.2002.47.2.0515
Marcus N. (2004). An overview of the impacts of eutrophication and chemical pollutants on copepods of the coastal zone. Zoological Stud. 43, 211–217. Available at: https://zoolstud.sinica.edu.tw/Journals/43.2/211.pdf.
Marshall D. J., Dong Y.-w., McQuaid C. D., Williams G. A. (2011). Thermal adaptation in the intertidal snail Echinolittorina malaccana contradicts current theory by revealing the crucial roles of resting metabolism. J. Exp. Biol. 214, 3649–3657. doi: 10.1242/jeb.059899
Meadows P. S., Meadows A., Murray J. M. (2012). Biological modifiers of marine benthic seascapes: Their role as ecosystem engineers. Geomorphology 157, 31–48. doi: 10.1016/j.geomorph.2011.07.007
Miller S. H., Zarate S., Smith E. H., Gaylord B., Hosfelt J. D., Hill T. M. (2014). Effect of elevated pco2 on metabolic responses of porcelain crab (Petrolisthes cinctipes) larvae exposed to subsequent salinity stress. PloS One 9, e109167. doi: 10.1371/journal.pone.0109167
Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P (2009). Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann. Internal Med. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Murphy G. E., Romanuk T. N., Worm B. (2020). Cascading effects of climate change on plankton community structure. Ecol. Evol. 10, 2170–2181. doi: 10.1002/ece3.v10.4
Mustafa S. A., Davies S. J., Jha A. N. (2012). Determination of hypoxia and dietary copper mediated sub-lethal toxicity in carp, Cyprinus carpio, at different levels of biological organisation. Chemosphere 87, 413–422. doi: 10.1016/j.chemosphere.2011.12.037
Neale P. J., Sobrino C., Segovia M., Mercado J., León P., Cortés M., et al. (2014). Effect of co2, nutrients and light on coastal plankton. i. abiotic conditions and biological responses. Aquat. Biol. 22, 25–41. doi: 10.3354/ab00587
Nikulina A., Dullo W.-C. (2009). Eutrophication and heavy metal pollution in the flensburg fjord: a reassessment after 30 years. Mar. pollut. Bull. 58, 905–915. doi: 10.1016/j.marpolbul.2009.01.017
Paerl H. W. (1997). Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnology Oceanography 42, 1154–1165. doi: 10.4319/lo.1997.42.5_part_2.1154
Pansch C., Hiebenthal C. (2019). A new mesocosm system to study the effects of environmental variability on marine species and communities. Limnology Oceanography: Methods 17, 145–162. doi: 10.1002/lom3.10306
Pardo J. C., Costa T. M. (2021). Multiple-stressor effects of warming and acidification on the embryonic development of an estuarine fiddler crab. Estuarine Coast. Shelf Sci. 254, 107296. doi: 10.1016/j.ecss.2021.107296
Pinnegar J., Polunin N., Francour P., Badalamenti F., Chemello R., Harmelin-Vivien M.-L., et al. (2000). Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ. Conserv. 27, 179–200. doi: 10.1017/S0376892900000205
Pirotta E., Thomas L., Costa D. P., Hall A. J., Harris C. M., Harwood J., et al. (2022). Understanding the combined effects of multiple stressors: A new perspective on a longstanding challenge. Sci. Total Environ. 821, 153322. doi: 10.1016/j.scitotenv.2022.153322
Pörtner H.-O. (2012). Integrating climate-related stressor effects on marine organisms: unifying principles linking molecule to ecosystem-level changes. Mar. Ecol. Prog. Ser. 470, 273–290. doi: 10.3354/meps10123
Queirós A. M., Fernandes J. A., Faulwetter S., Nunes J., Rastrick S. P., Mieszkowska N., et al. (2015). Scaling up experimental ocean acidification and warming research: from individuals to the ecosystem. Global Change Biol. 21, 130–143. doi: 10.1111/gcb.2014.21.issue-1
Richard M., Bec B., Bergeon L., Hébert M., Mablouké C., Lagarde F. (2022). Are mussels and oysters capable of reducing the abundances of Picochlorum sp., responsible for a massive green algae bloom in thau lagoon, France? J. Exp. Mar. Biol. Ecol. 556, 151797.
Riedel G. F., Sanders J. G., Breitburg D. L. (2003). Seasonal variability in response of estuarine phytoplankton communities to stress: Linkages between toxic trace elements and nutrient enrichment. Estuaries 26, 323–338. doi: 10.1007/BF02695971
Rodrigues L. C., Van Den Bergh J. C., Massa F., Theodorou J. A., Ziveri P., Gazeau F. (2015). Sensitivity of mediterranean bivalve mollusc aquaculture to climate change, ocean acidification, and other environmental pressures: findings from a producer survey. J. Shellfish Res. 34, 1161–1176. doi: 10.2983/035.034.0341
Sandifer P. A., Sutton-Grier A. E. (2014). Connecting stressors, ocean ecosystem services, and human health. Natural Resour. Forum 38, 157–167. doi: 10.1111/narf.2014.38.issue-3
Scheffer M., Carpenter S., de Young B. (2005). Cascading effects of overfishing marine systems. Trends Ecol. Evol. 20, 579–581. doi: 10.1016/j.tree.2005.08.018
Schulte P. M., Healy T. M., Fangue N. A. (2011). Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702. doi: 10.1093/icb/icr097
Simboura N., Pavlidou A., Bald J., Tsapakis M., Pagou K., Zeri C., et al. (2016). Response of ecological indices to nutrient and chemical contaminant stress factors in Eastern Mediterranean coastal waters. Ecol. Indic. 70, 89–105. doi: 10.1016/j.ecolind.2016.05.018
Skei J., Larsson P., Rosenberg R., Jonsson P., Olsson M., Broman D. (2000). Eutrophication and contaminants in aquatic ecosystems. AMBIO: A J. Hum. Environ. 29, 184–194. doi: 10.1579/0044-7447-29.4.184
Smith V. H., Schindler D. W. (2009). Eutrophication science: where do we go from here? Trends Ecol. Evol. 24, 201–207. doi: 10.1016/j.tree.2008.11.009
Soerensen A. L., Schartup A. T., Gustafsson E., Gustafsson B. G., Undeman E., Björn E. (2016). Eutrophication increases phytoplankton methylmercury concentrations in a coastal sea a Baltic Sea case study. Environ. Sci. Technol. 50, 11787–11796. doi: 10.1021/acs.est.6b02717
Solan M., Whiteley N. (2016). Stressors in the Marine Environment: Physiological and Ecological Responses; Societal Implications (Oxford University Press, Oxford: Oxford University Press).
Song Y., Guo Y., Liu H., Zhang G., Zhang X., Thangaraj S., et al. (2022). Water quality shifts the dominant phytoplankton group from diatoms to dinoflagellates in the coastal ecosystem of the Bohai Bay. Mar. pollut. Bull. 183, 114078. doi: 10.1016/j.marpolbul.2022.114078
Stelzenmüller V., Rehren J., Örey S., Lemmen C., Krishna S., Hasenbein M., et al (2024). Framing future trajectories of human activities in the German North Sea to inform cumulative effects assessments and marine spatial planning. Journal of Environmental Management 349, 119507.
Stewart R. I., Dossena M., Bohan D. A., Jeppesen E., Kordas R. L., Ledger M. E., et al. (2013). Mesocosm experiments as a tool for ecological climate-change research. Adv. Ecol. Res. 48, 71–181. doi: 10.1016/B978-0-12-417199-2.00002-1
Stockbridge J., Jones A. R., Gillanders B. M. (2020). A meta-analysis of multiple stressors on seagrasses in the context of marine spatial cumulative impacts assessment. Sci. Rep. 10, 11934. doi: 10.1038/s41598-020-68801-w
Stueckle T. A. (2008). An Evaluation of the Non-Target Effects of Mosquito Control Pesticides on Uca Pugnax Physiology, Limb Regeneration and Molting Processes (West Virginia University, Morgantown, USA: West Virginia University).
Sundbäck K., Petersen D. G., Dahllöf I., Larson F. (2007). Combined nutrient–toxicant effects on a shallow-water marine sediment system: sensitivity and resilience of ecosystem functions. Mar. Ecol. Prog. Ser. 330, 13–30. doi: 10.3354/meps330013
Tate R. D., Benkendorff K., Ab Lah R., Kelaher B. P. (2017). Ocean acidification and warming impacts the nutritional properties of the predatory whelk, dicathais orbita. J. Exp. Mar. Biol. Ecol. 493, 7–13. doi: 10.1016/j.jembe.2017.03.006
Thomsen J., Casties I., Pansch C., Körtzinger A., Melzner F. (2013). Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Global Change Biol. 19, 1017–1027. doi: 10.1111/gcb.2013.19.issue-4
Thrush S. F., Hewitt J. E., Gladstone-Gallagher R. V., Savage C., Lundquist C., O’Meara T., et al. (2021). Cumulative stressors reduce the self-regulating capacity of coastal ecosystems. Ecol. Appl. 31, e02223. doi: 10.1002/eap.v31.1
Turschwell M. P., Connolly S. R., Schäfer R. B., De Laender F., Campbell M. D., Mantyka-Pringle C., et al. (2022). Interactive effects of multiple stressors vary with consumer interactions, stressor dynamics and magnitude. Ecol. Lett. 25, 1483–1496. doi: 10.1111/ele.14013
Vihtakari M., Hendriks I. E., Holding J., Renaud P. E., Duarte C. M., Havenhand J. N. (2013). Effects of ocean acidification and warming on sperm activity and early life stages of the mediterranean mussel (Mytilus galloprovincialis). Water 5, 1890–1915. doi: 10.3390/w5041890
Villar-Argaiz M., Medina-Sánchez J. M., Biddanda B. A., Carrillo P. (2018). Predominant nonadditive effects of multiple stressors on autotroph c: N: P ratios propagate in freshwater and marine food webs. Front. Microbiol. 9, 69. doi: 10.3389/fmicb.2018.00069
Wake B. (2019). Experimenting with multistressors. Nat. Climate Change 9, 357–357. doi: 10.1038/s41558-019-0475-z
Wang W.-X., Dei R. C. (2001). Effects of major nutrient additions on metal uptake in phytoplankton. Environ. Pollut. 111, 233–240. doi: 10.1016/S0269-7491(00)00071-3
Wiegner T. N., Seitzinger S. P., Breitburg D. L., Sanders J. G. (2003). The effects of multiple stressors on the balance between autotrophic and heterotrophic processes in an estuarine system. Estuaries 26, 352–364. doi: 10.1007/BF02695973
Keywords: climate-stressors, anthropogenic-stressors, climate-change, global-change, non-additive-effects, coastal-foodweb, coastal-management
Citation: Krishna S, Lemmen C, Örey S, Rehren J, Pane JD, Mathis M, Püts M, Hokamp S, Pradhan HK, Hasenbein M, Scheffran J and Wirtz KW (2025) Interactive effects of multiple stressors in coastal ecosystems. Front. Mar. Sci. 11:1481734. doi: 10.3389/fmars.2024.1481734
Received: 16 August 2024; Accepted: 05 December 2024;
Published: 09 January 2025.
Edited by:
Giandomenico Foti, Mediterranea University of Reggio Calabria, ItalyReviewed by:
Patricia G. Cardoso, University of Porto, PortugalCopyright © 2025 Krishna, Lemmen, Örey, Rehren, Pane, Mathis, Püts, Hokamp, Pradhan, Hasenbein, Scheffran and Wirtz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shubham Krishna, c2h1YmhhbS5rcmlzaG5hQG5vYy5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.