
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 15 January 2025
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1461982
This article is part of the Research TopicChallenges in Fishery Assessment MethodologiesView all 12 articles
The northeast Atlantic (NEA) mackerel (Scomber scombrus) is a commercially significant species, with expansive spawning migrations occurring along the continental shelf of northwestern Europe. To identify the main variables influencing the spatial distribution of mackerel eggs, this study analyzed data from egg surveys conducted by the Working Group on Mackerel and Horse Mackerel Egg Surveys (WGMEGS) of the International Council for the Exploration of the Sea (ICES). To achieve this objective, a Random Forest model was used to predict the presence of mackerel eggs based on temporal, geographical, and environmental variables. Applying the Random Forest model to the survey data revealed that the main variables affecting mackerel spawning were the bottom depth, latitude, temperature, and salinity. Subsequently, Quotient Analysis was used to determine the optimal ranges of the key variables identified as influencing mackerel spawning. The results demonstrated a clear preference for spawning at depths between 100 m and 200 m, as well as a consistent preference for the area between 43° and 44° North, corresponding to the Cantabrian Sea. Furthermore, the results indicated that mackerel exhibited a considerable range of temperature tolerance throughout the spawning process, with a preference for cooler waters in the Western area in recent years. Salinity seems to have an effect on spawning at salinities between 35.0 ppm to 35.5 ppm, but results were imprecise. These results contribute to our understanding of how environmental and geographical variables influence the spawning behavior of NEA mackerel.
Atlantic mackerel (Scomber scombrus) is a pelagic species found on both sides of the North Atlantic, with two distinct stocks: the western stock in the Northwest Atlantic (NWA) and the eastern stock in the Northeast Atlantic (NEA) (Sette, 1950; Jamieson and Smith, 1987). In the NEA, mackerel ranges from Morocco to northern Norway, whereas in the NWA, the mackerel range extends from North Carolina to Newfoundland. It has been suggested after genetic and tagging studies that limited connectivity exists between these populations (Nesbø et al., 2000; Uriarte and Lucio, 2001; Tenningen et al., 2011). Mackerel have a fully pelagic life cycle, with eggs and larvae floating passively in currents. They form large schools, increasing their dispersal capacity (Lockwood, 1988; Jansen and Gislason, 2013), and have no swim bladder, so they move continuously to prevent sinking, yet can rapidly change their depth (DFO, 1997).
Traditionally, NEA mackerel has been considered as a single stock, divided into three spawning components: The North Sea, the Western and the Southern components (Jansen and Gislason, 2013). These authors concluded that these components cannot be considered independent because of the reproductive exchange and overlapping ranges outside the spawning season. Despite the lack of complete separation, the International Council for the Exploration of the Sea (ICES) continues to categorize NEA mackerel into the three components mentioned above (Figure 1), now defined as spawning areas rather than stock components (ICES, 2013, 2023). This interconnected metapopulation highlights the complexity of the distribution of NEA mackerel in the Northeast Atlantic (Van Beveren et al., 2019).
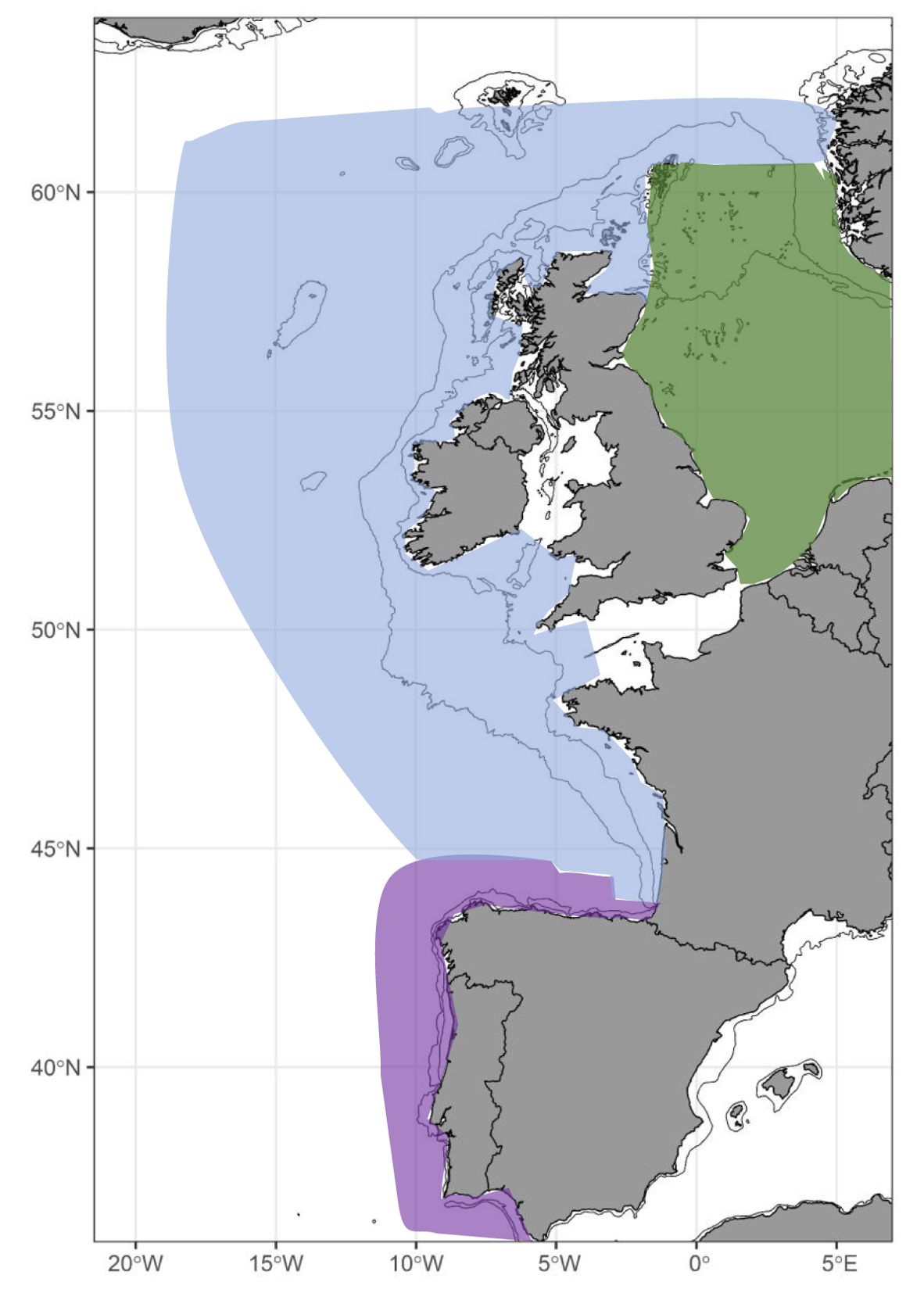
Figure 1. The spawning area of Northeast Atlantic mackerel is subdivided into three distinct spawning grounds, designated by the colors purple (the Southern area), blue (the Western area) and green (the North Sea area).
NEA mackerel exhibit remarkable migratory behavior, undertaking extensive journeys between spawning, feeding and overwintering grounds (Ono et al., 2022; Jansen et al., 2012). This process is driven by both environmental and biological factors. In late winter, mackerel migrate along the northwestern European continental shelf to reach spawning grounds, after which many move northward to the Norwegian and Nordic Seas to feed on abundant food resources. At the end of the feeding season, they form large overwintering shoals along the northern continental shelf of the North Sea (Iversen, 2002). Temperature is a key driver of these migrations and influences the timing, routes, growth, mortality and location of spawning grounds (Jansen et al., 2012; Gødo et al., 2004), particularly through its effects on shelf-edge currents, which are critical for larval dispersal and recruitment (Bruge et al., 2016). Additional factors such as maturation, body condition, size, food availability, turbidity and ocean currents also influence their movements (Hughes et al., 2014; Bruge et al., 2016). Although geographical variables may play a role in the distribution of spawning grounds, evidence for density-dependent habitat selection is limited (Brunel et al., 2018).
In recent decades, the distribution of NEA mackerel has shifted northward and eastward, which is closely linked to rising ocean temperatures (Astthorsson et al., 2012; Bruge et al., 2016). This shift has been particularly noticeable in spawning area, where an expansion towards the north and northwest has been observed (ICES, 2021; dos Santos Schmidt et al., 2024). This is illustrated in Supplementary Figure 1, which shows the distribution of mackerel egg production over the time series. These changes are believed to be driven by climate change and oceanographic factors, highlighting the need to understand the environmental mechanisms that influence mackerel spawning (Chust et al., 2023).
This study aimed to investigate how environmental and geographical variables influence the spatial and temporal distribution of mackerel eggs, which are indicators of spawning activity. By analyzing key variables, this study aimed to better understand the presence or absence of mackerel eggs and determine the specific ranges within which these variables affect their distribution. To achieve this, two methodologies, Quotient Analysis and Random Forest, were employed, allowing for a comprehensive characterization of mackerel spawning in the Northeast Atlantic. This approach provides valuable insights into the environmental drivers of mackerel spawning and contributes to a deeper understanding of how changing ocean conditions may affect future reproductive patterns.
The data for this study were obtained from mackerel and horse mackerel egg surveys in the Northeast Atlantic. These surveys were coordinated by the Working Group on Mackerel and Horse Mackerel Egg Surveys (WGMEGS) of ICES. This survey has been conducted every third year since 1977 and its methodology and design have remained largely unchanged since 1992. The survey covered the spawning season of mackerel from January to July and from Gibraltar to Iceland. Sampling was strategically distributed in space and time to ensure a comprehensive coverage of the mackerel spawning season. The main objective of these WGMEGS surveys was to calculate an index of spawning stock biomass (SSB) for NEA mackerel. To achieve this, mackerel egg surveys have developed a methodology for the spatial and temporal distribution of sampling. The mackerel egg survey was designed to collect ichthyoplankton samples from predefined rectangles measuring 0.5° × 0.5°. Each rectangle contained at least one sample that was considered to be representative of the entire area. The narrow continental shelf of the northern Iberian Peninsula (the Southern area) required a modified sampling grid of 0.25° x 1° to ensure an accurate representation of the ichthyoplankton population of the region (Figure 2). Sampling covered the upper 200 m of the water column or within 5 m of the bottom (ICES, 2019).
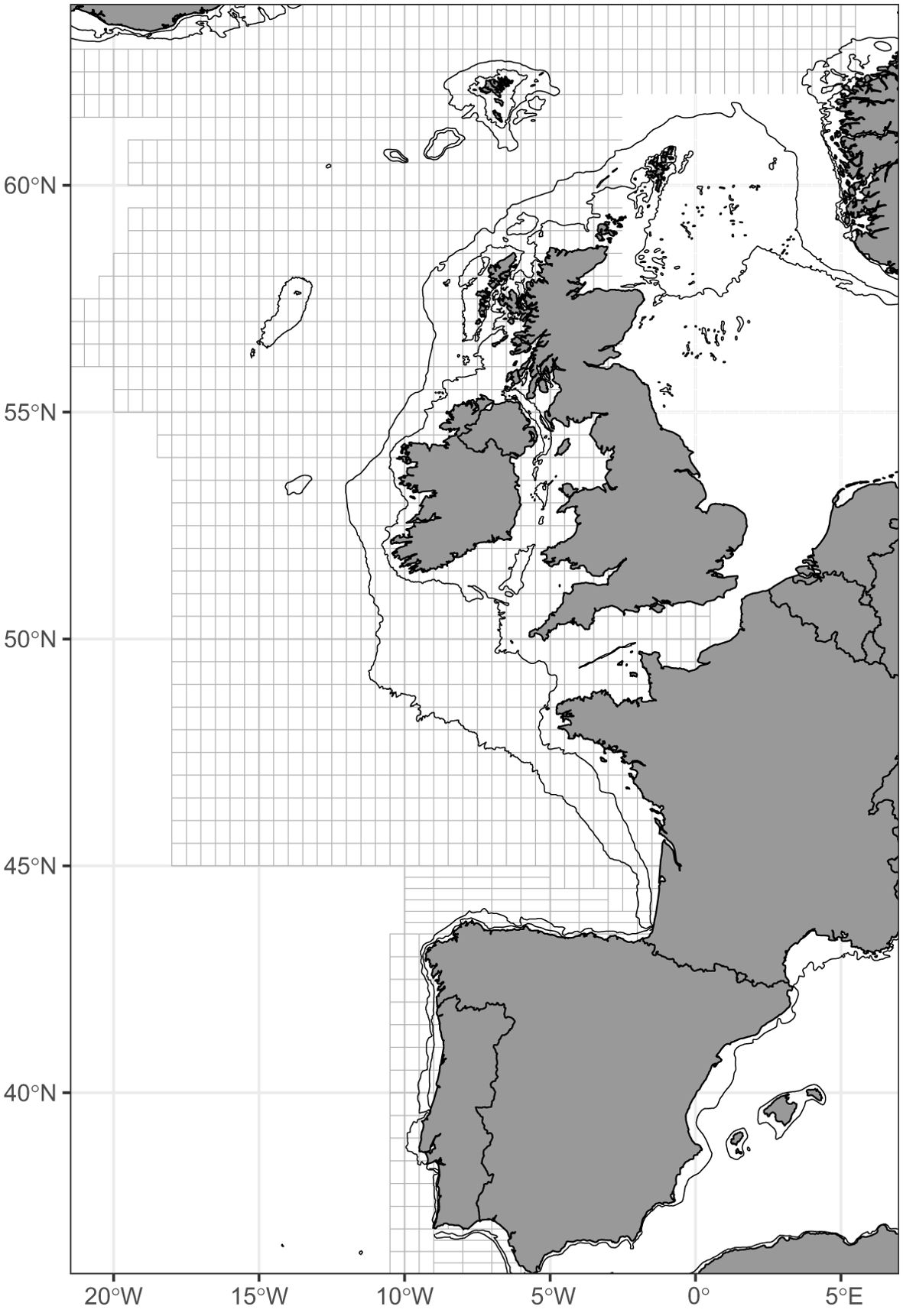
Figure 2. Survey design for Southern and Western areas of mackerel egg survey in the Northeast Atlantic.
Ichthyoplankton samples were obtained using Gulf VII or Bongo nets deployed in double oblique tows along latitudinal transects in the Western area of the study area. Samples were collected at 0.5° intervals until no mackerel eggs were found (ICES, 2019). The spawning season was divided into multiple sampling periods, and the spawning area was surveyed to estimate the spatio-temporal distribution and abundance of mackerel eggs (ICES, 2019; Brunel et al., 2018). Following the collection of ichthyoplankton samples, mackerel eggs were collected and classified into five distinct developmental stages according to the methodology described by Lockwood et al. (1977). In addition to ichthyoplankton samples, environmental and geographical data, including temperature, salinity, bottom depth, sampling depth, location, time, and date, were recorded at each station. The densities of stage I mackerel eggs in each sample were standardized to the number of eggs per square meter. The focus on stage I eggs, representing the earliest stage post-fertilization, in WGMEGS surveys provides a more precise estimate of egg production because of the lower likelihood of displacement by ocean currents or natural mortality. In this study, only stage I mackerel eggs (i.e., recently spawned eggs) were used.
The Random Forest model (RF), introduced by Breiman (2001), is an ensemble machine learning technique that combines multiple decision trees (CARTs) for accurate predictions. It can handle large data sets and multiple variables, but is considered a “black box” because of its complexity. In this study, a RF classification model was used to analyze the variables affecting the presence of stage I mackerel eggs, indicating spawning activity. A distinctive feature of the RF model is its capacity to assess the relative importance of the predictor variables in relation to the response variable. This allows the model to not only make accurate predictions but also to identify the most influential variables affecting the outcome (importance). Such insights are valuable for understanding the variables that have a greater impact on model predictions. The performance of the RF model in predicting mackerel spawning distribution was assessed by comparing its predictions with observed data. Various evaluation metrics, detailed in Supplementary Table 1 were used to measure the accuracy and effectiveness of the model, providing a complete evaluation of the predictive capabilities of the RF model.
To further understand predictive behavior of the RF model, we employed Partial Dependence Plots (PDP) to visualize and interpret the results produced by the RF model. This demonstrates how the prediction of the target variable is affected by variations in a specific input variable, while simultaneously averaging the effects of all other variables. This facilitates identification of whether the relationship is linear, monotonic, or complex. In this study, PDPs were used to graphically represent the relationship between specific variables and the predicted probabilities of mackerel egg presence based on annual RF model outputs.
To develop the RF model, predictor variables representing the temporal, spatial, and environmental variables were included. The variables used were derived from the WGMEGS survey, comprising data on sea temperature and salinity at depths of 5 m and 20 m, maximum sampling depth, geographic location, time and month. Furthermore, data from the MARESPEC database (http://www.marspec.org) were included, specifically slope of the seabed (Slope), bottom depth (Bdepth), seabed complexity (Roughness), and distance to the coast (Distance) (Table 1). To avoid multicollinearity and overfitting, highly correlated predictor variables were excluded before applying the RF model (Supplementary Figure 2). To avoid multicollinearity and overfitting, highly correlated variables were excluded before applying the RF model (Supplementary Figure 2). Consequently, the following variables were excluded: sea temperature at 5 m, salinity at 5 m, maximum sampling depth, roughness and longitude. Numerical variables were standardized (mean = 0, standard deviation = 1) and categorical variables were converted to a numerical format using dummy variables.
The RF model used in this study consisted of 1,000 trees. The “tuneRF” algorithm determined the number of predictor variables for each node split, assessed using the Gini impurity measure. A spatial bootstrap approach with 1,000 repetitions, utilizing the R package “spatialsample,” was used to train each tree. The model was evaluated by the out-of-bag error metric (OOB), with lower values indicating better performance. The RF model classified instances as ‘non-egg’ only if the probability exceeds 75%. Predictive performance was assessed using 10-fold cross-validation, considering the absence of mackerel eggs as positive and the presence as negative. The egg survey data were split into a training set (80%) and a test set (20%).
RF was limited to data from surveys conducted since 2001 because of significant missing data for predictor variables in earlier surveys. NEA mackerel spawning area has recently expanded northward, particularly in the Western area, while the Southern area remains stable. Accordingly, the analysis focused on the Western area due to notable changes in spawning patterns. All statistical analyses were performed using the R (version 4.2.1; R Core Team, 2020). Random Forest was performed using the R packages RandomForest and tidymodels. Partial Dependence Plot was performed using the R packages pdp, spatialsample, DALEX and DALEXtra.
Single-parameter Quotient Analysis (QA) is an exploratory technique that is used to investigate the selection of NEA mackerel spawning habitat by assessing environmental and geographical variables. QA analyzes fish egg abundance in relation to these variables, offering insights into preferred spawning habitats (Van der Lingen and Huggett, 2003; Drapeau and van der Lingen, 2005). This method has been widely applied to studies of pelagic fish, such as anchovy, sardine, and tuna, to examine preferences based on temperature, salinity, and depth (Ibaibarriaga et al., 2007; Bernal et al., 2007; Arrizabalaga et al., 2015). Using QA, this study identified the optimal environmental and geographical variables influencing mackerel spawning preferences. The analysis focused on the key variables identified as most important by the RF model. Each year, separate QA were carried out comparing the combined Southern and Western areas with the Western area alone. This dual strategy aimed to account for the northward expansion of NEA mackerel spawning and improve the understanding of habitat preferences by comparing QA results from different areas.
To calculate the quotient, the variable of interest was initially categorized into intervals of equal size. Subsequently, the proportion of stations within each category was compared to the overall abundance within that category by utilizing a designated quotient, denoted as Qi. The estimated results for each class were calculated based on observed proportions.
The notation Ni represents the number of stations and Ai denotes the total abundance of each class.
The “shachar” R package (Bernal et al., 2007; http://sourceforge.net/projects/ichthyoanalysis/), was utilized to calculate the quotient values and confidence intervals for the null hypothesis of an even distribution (quotient = 1) using a resampling procedure with 1000 iterations. This assessed the statistical significance of quotient values that were significantly different of 1. Preference values were defined as covariate values for which the quotient was significantly above 1 (above the upper confidence interval). Avoidance values were defined as covariate values for which the quotient was significantly below 1 (below the lower confidence interval). The tolerance range included values that did not show significant avoidance or preference.
The RF model demonstrated strong predictive performance, with an OOB error ranging between 0.12 to 0.16 across all years (Supplementary Table 2). The Accuracy ranged from 75% to 91% and its F1 score (performance of the model) ranged from 0.69 to 0.86. The roc_auc ranged between 0.84 to 0.94, indicating good to very good discrimination (Table 2). The RF model identified the main predictors of mackerel egg presence, showing consistent variable importance across years (Figure 3). The most important variables were bottom depth (Bdepth), latitude (Lat), temperature at 20 m (Temp20), and salinity at 20 m (Sal20). Initially, distance to the coast (Distance) was identified as an important variable, but its importance has declined in recent years. In contrast, the importance of monthly variables increased significantly in the last few years of the study, with May (Month_X5) demonstrating the most notable increase, followed by a more modest increment in February (Month_X2) (Table 3).
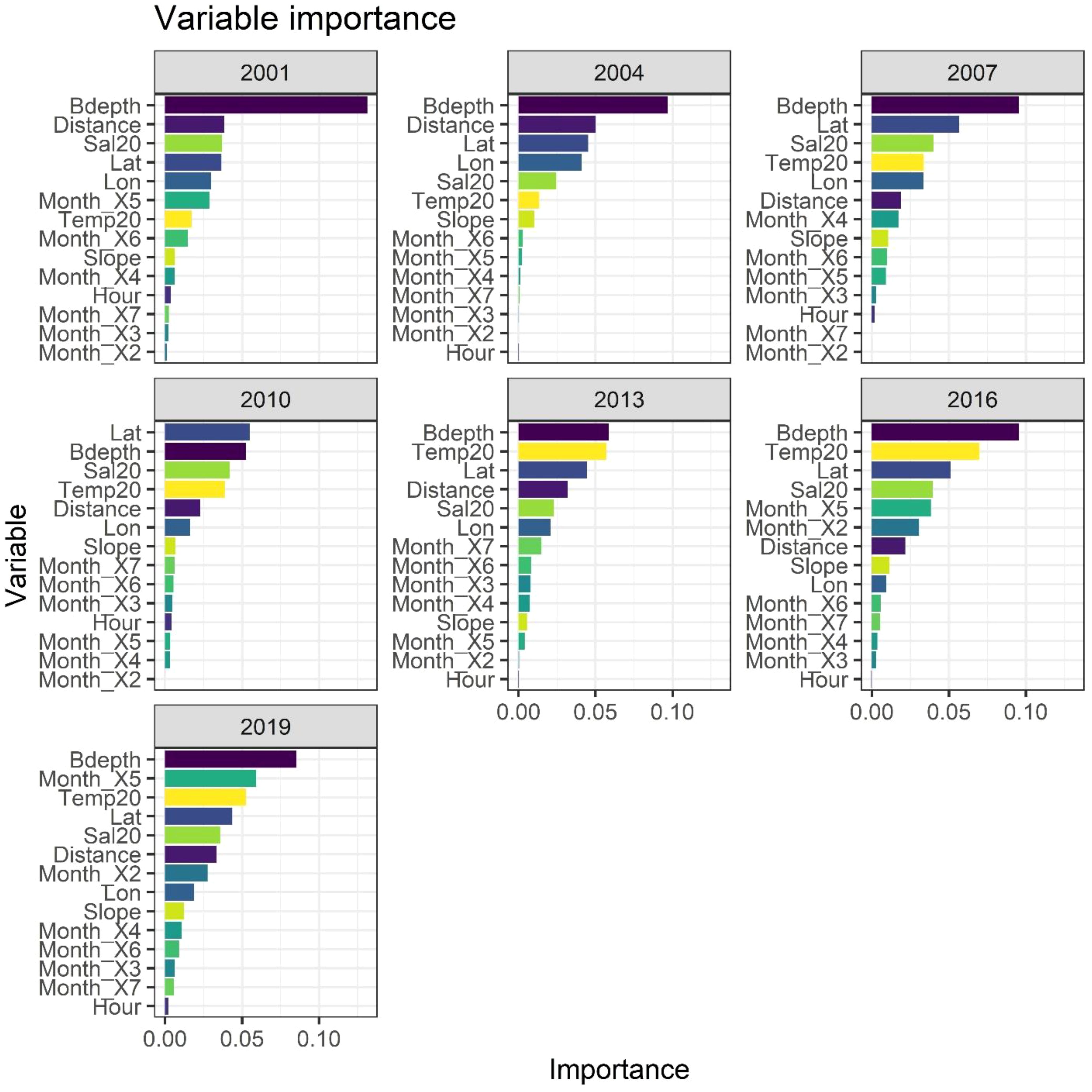
Figure 3. The variable importance plots of variables predicting the absence of stage I mackerel eggs for each year. Variable importance was determined using a Random Forest classification model.
The influence of the identified variables on the presence of eggs was further explored using PDP (Figure 4). This figure illustrates the relationship between the main variables identified in the RF model (bottom depth, distance from the coast, latitude, salinity and temperature) and the probability of mackerel egg presence. The PDP indicated the probability of detecting mackerel eggs increased with bottom depth, reaching its highest values between 100 m to 200 m. Thereafter, the probability of finding eggs declined significantly, and at depths exceeding 1000 m, the likelihood of detecting mackerel eggs fell below 50% across most survey years. For the variable distance from the coast, the probability of egg presence remained slightly above 50% for distances of up to 400 km from the coast from 2001 to 2013, although no consistent pattern was observed for this variable in recent surveys (2016 and 2019). The influence of latitude was observed to exhibit variability, with probabilities increasing at latitudes north of 48° North between 2004 and 2010, shifting to above 55° North in 2013. However, this pattern was not significant in the surveys conducted in 2001, 2016, and 2019. Temperature was found to have a significant impact, with stations with temperatures between 8°C and 11°C showing a higher likelihood of egg presence from 2010 to 2016. Similarly, salinity levels between 35.0 ppm to 35.5 ppm were found to be consistently associated with increased probabilities of egg presence, emphasizing the importance of this variable in defining suitable spawning habitats.

Figure 4. Partial dependence plots (PDP) for each year (2001-2019), based on the of Random Forest results, showing the mean marginal influence of the main explanatory variables (Bathy: bottom depth, Distance: distance to the coast, LatDeg: latitude, Sal20m: salinity at 20 m, Temp20m: temperature at 20 m). Each plot represents the influence of each variable while keeping the other variables constant. The marks on the x-axis represent the distribution of data. (A) PDP results for 2001, (B) 2004, (C) 2007, (D) 2010, (E) 2013, (F) 2016, and (G) 2019.
The efficacy of the RF model in predicting the distribution of mackerel eggs was evaluated by comparing its predictions with the empirical observations. The observed egg densities at the sampling stations were superimposed onto probability maps of the model to indicate the presence or absence of eggs. The Supplementary Material presents Supplementary Figures 3–9, which demonstrate a high level of correspondence between the predicted and observed data, confirming the reliability of the model.
QA was employed to discern patterns of preferences, tolerances, and avoidances associated with the spawning mackerel. This technique facilitated a comprehensive examination of key variables such as bottom depth, latitude, salinity, and temperature, identified by the RF model as critical to understanding mackerel behavior. Figures 5–8 summarize the QA results for the key variables over time. The QA of both the combined Southern and Western areas and the Western area alone showed some variation in favorable mackerel spawning conditions over the years.
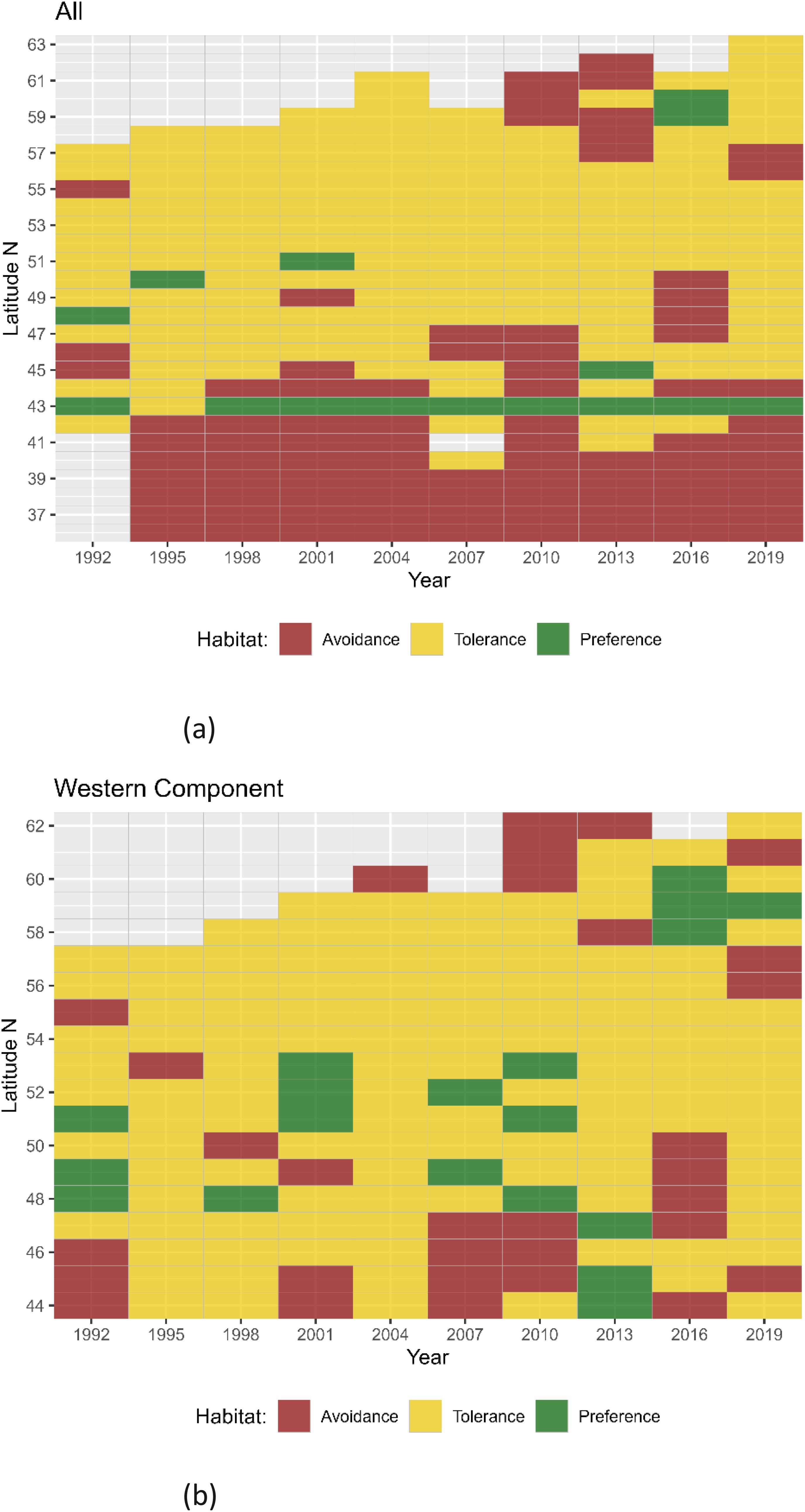
Figure 5. Results of Quotient Analysis of mackerel spawning by latitude (in 1 grade classes) for each survey year. Green squares indicate significant preferences (quotient > 1), red squares indicate significant avoidance (quotient < 1) and yellow squares indicate tolerance ranges. Light gray shaded squares denote data not available. (A) Results for the combined Southern and Western areas and (B) results for the Western area alone.
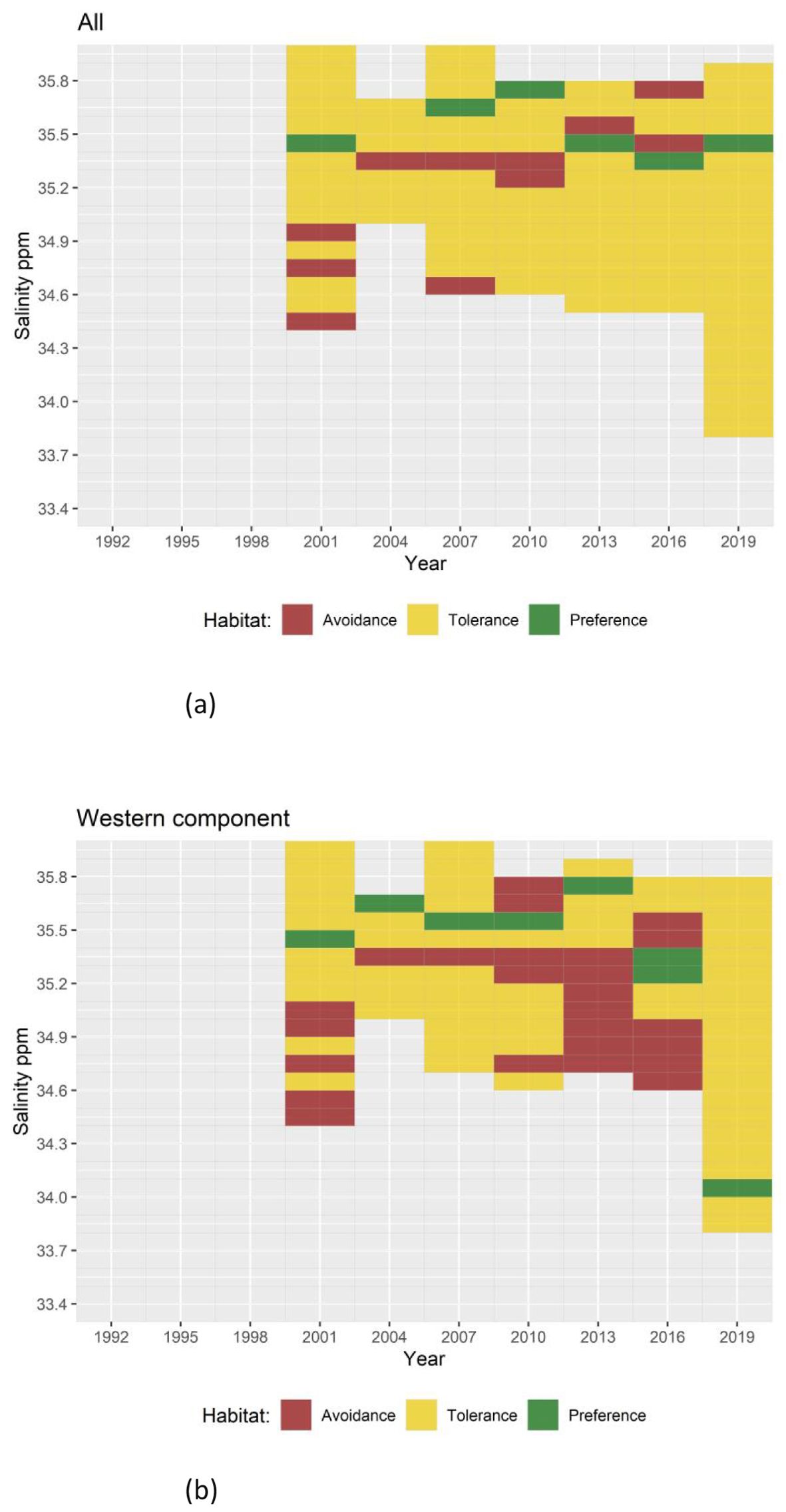
Figure 6. Results of Quotient Analysis of mackerel spawning by salinity (in 0.1 ppm classes) in each survey year. Green squares indicate significant preferences (quotient > 1), red squares indicate significant avoidance (quotient < 1) and yellow squares indicate tolerance ranges. Light gray shaded squares denote data not available. (A) Results for the combined Southern and Western areas and (B) results for the Western area alone.
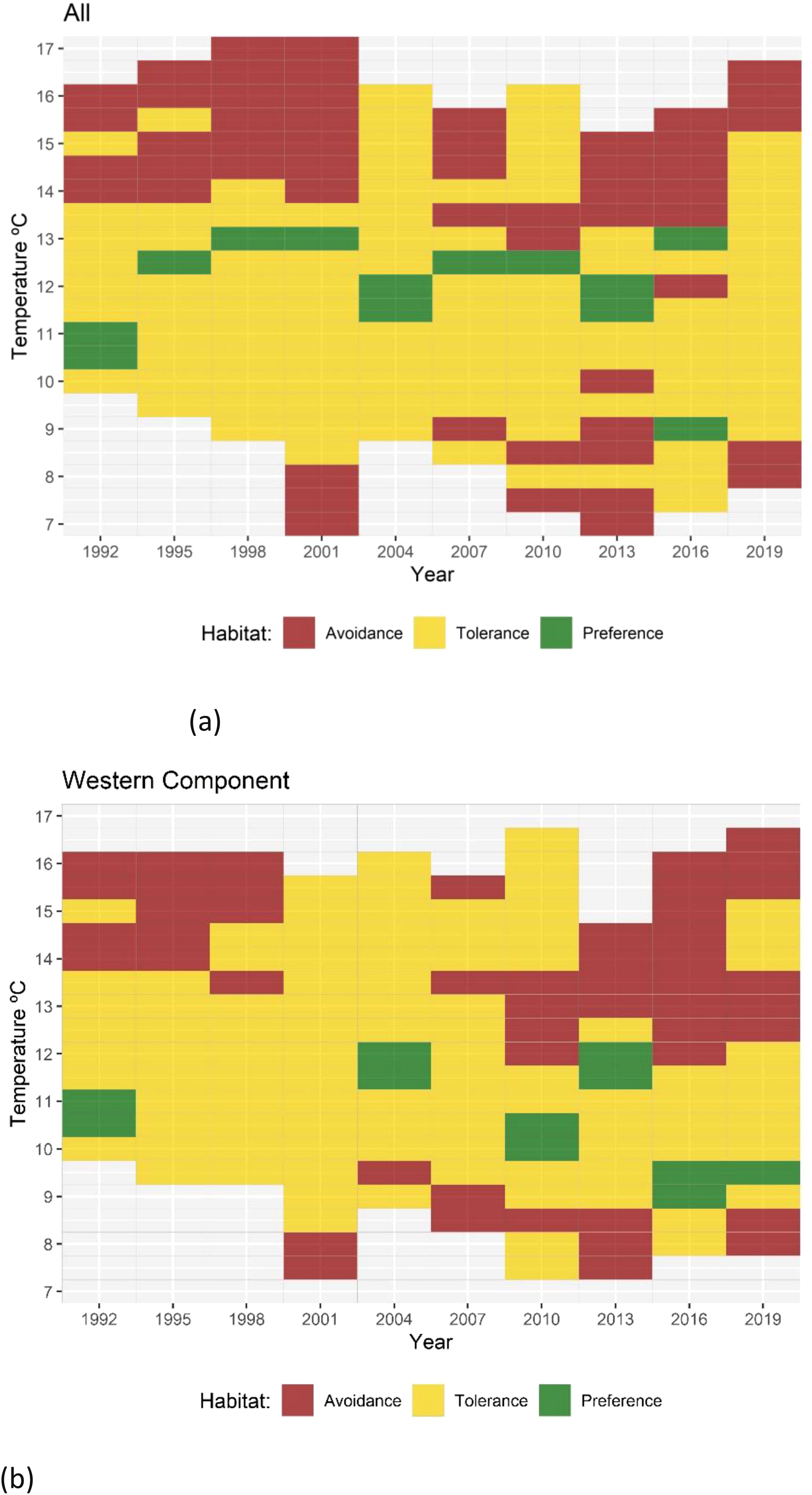
Figure 7. Results of Quotient Analysis of mackerel spawning by temperature (in 0.5°C classes) for each survey year. Green squares indicate significant preferences (quotient > 1), red squares indicate significant avoidance (quotient < 1) and yellow squares indicate tolerance ranges. Light gray shaded squares denote data not available. (A) Results for the combined Southern and Western areas and (B) results for the Western area alone.
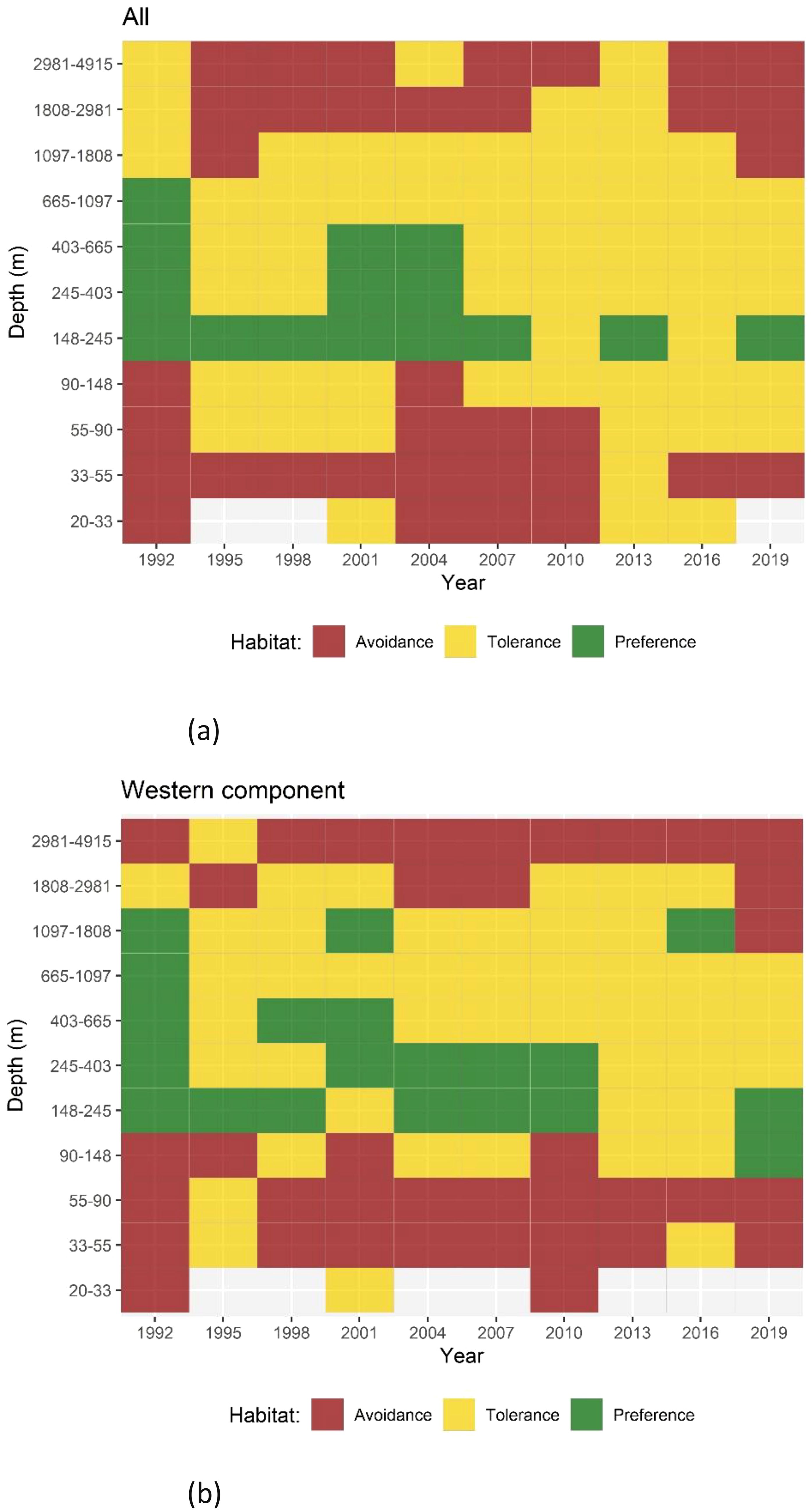
Figure 8. Results of Quotient Analysis for mackerel spawning by log-transformed depth (in 0.5 classes) for each survey. Green squares indicate significant preferences (quotient > 1), red squares indicate significant avoidance (quotient < 1) and yellow squares indicate tolerance ranges. Light gray shaded squares denote data not available. (A) Results for the combined Southern and Western areas and (B) results for the Western area alone.
QA for latitude classified the variable into 1 grade class. The results of the QA conducted on the combined Southern and Western n areas consistently demonstrated a preference towards the area between 43° and 44° North, which corresponds to the Cantabrian Sea (Figure 5A). Fluctuations in both the preference and avoidance latitudes were observed throughout the time series. In recent years, the Western area (Figure 5B) has shown an expansion in the range of tolerance towards higher latitudes, which could be linked to an increase in the mackerel spawning area. In addition to latitude, salinity levels were also found to be a significant variable in defining mackerel spawning areas, as evidenced by the RF results. Salinity was categorized into 0.1 ppm classes; however, no consistent pattern was observed. In certain years, there was a preference for a salinity level of 35.4 ppm, while in other years this specific salinity showed avoidance (Figure 6). Temperature was categorized into 0.5°C classes and showed a general preference for temperatures between 11.5°C to 13.0°C in the combined Southern and Western areas, while temperatures above 13.5°C and below 8.5°C were typically avoided (Figure 7A). In 2016, an unexpected preference for 9.0°C was observed, which may indicate adaptation to environmental changes. In contrast, no discernible temperature preference range was identified in 2019. In the Western area, no discernible pattern of temperature tolerance could be identified, but, a narrowing of tolerance range and a preference for cooler waters has been observed in recent years (Figure 7B).
A logarithmic transformation was applied to the observed bottom depth, which was subsequently categorized into classes of 0.5. QA showed a preference for depths near the 200 m isobath in the combined Southern and Western areas. However, this preference has become less evident in recent years (Figure 8A). Depths below 50 m and above 1800 m were generally avoided. In the Western area (Figure 8B), the most frequent preference depth range was between 150 m to 400 m in most years. However, this pattern has not been discernible in the recent years.
Supplementary Figures 10 and 11 in Supplementary Material present QA plots for the key variables identified as the most influential in the presence of mackerel eggs. The Figures show data for each year analyzed separately for the combined Southern and Western areas and for the Western area alone.
The results are synthesized in Table 4. This table presents the principal variables identified by the RF model, together with an interpretation based on the PDP and the ranges of preference or avoidance determined by QA.
This study offers insights into the spawning distribution of NEA mackerel, revealing the complex interplay between geographic and environmental variables. By integrating the Random Forest model and Quotient Analysis, we present a comprehensive framework for the analysis of spawning dynamics in mackerel. The RF model exhibited high predictive accuracy, identifying the relative importance of variables in predicting egg presence without assuming linear relationships. In contrast, QA provided detailed insights into the specific preferences and avoidances of mackerel eggs with regard to the ranges of predicted variables. These results challenge the traditional notion that pelagic species are primarily driven by environmental factors (Munk et al., 2009; Alvarez-Berastegui et al., 2014). Geographical variables, particularly bottom depth, were identified as relevant in this study, indicating that spatial characteristics had a significant influence. These results demonstrate the complex and interdependent relationship between geographical and environmental variables, providing insight into the mechanisms driving the distribution of mackerel spawning.
The increasing importance of temporal variables in recent years, particularly May and, to a lesser extent, February, has added complexity to our understanding of mackerel spawning dynamics by influencing interactions between environmental variables (e.g., temperature and salinity) and geographical variables (e.g., bottom depth and latitude). These temporal shifts influenced interactions between environmental variables, such as temperature and salinity, and geographical variables, such as bottom depth and latitude, thereby improving the model used to predict spawning patterns. This interaction is evidenced by the northwestward expansion of the mackerel spawning area since 2010, which initially affected the entire spawning season, but since 2013, spawning expansion has consistently begun in May, with eggs detected at greater depths. The anomalous profile observed in 2013 was characterized by an observed expansion of spawning area from May onwards and according to RF results, temporal variables had a negligible influence on egg presence. In contrast, in 2016 and 2019, the temporal variables (mainly May) were found to have a significant influence on egg presence. This shift suggests that hydrodynamic mechanisms, such as ocean currents and eddies or broader oceanographic changes, such as warming, are the underlying drivers of these patterns. Supporting these interpretations, previous studies, including those by Nissling and Larsson (2018) and Hüssy et al. (2012), identified temperature and salinity as critical determinants of spawning success, while others, such as Ganias (2009) and Lima et al. (2022), highlighted the influence of seasonal shifts in ocean currents and wind patterns. The results of this study indicated that complex and interdependent mechanisms regulate mackerel spawning, with temporal variables emerging as significant factors influencing adaptation to oceanographic changes.
According to the QA results, NEA mackerel showed strong preferences for spawning at specific depths and latitudes. Regarding latitudinal distribution, QA consistently identified a concentration of spawning activity towards the area between 43° and 44° North, which corresponds to the Cantabrian Sea, across the majority of years included in the analysis. In addition, a marked northward shift of spawning grounds towards western Scotland has been observed in recent years. These findings align with those of Brunel et al. (2018), who documented significant egg densities in regions north of the Iberian Peninsula and west of Scotland and Ireland, that were influenced by both geographical and environmental variables. However, studies by Chust et al. (2023) and Beare and Reid (2002) have emphasized a broader northward shift in spawning grounds without identifying specific latitudinal hotspots, focusing instead on temperature-driven patterns. Regarding depth preferences, QA analysis showed a clear preference for spawning near the 200m isobath, indicating the importance of bathymetric features in determining spawning habitats. These results are in accordance with those of Brunel et al. (2018), who reported high egg densities at depths of approximately 250 m. In contrast, mackerel spawning in the Northwest Atlantic tends to occur in shallower waters, usually less than 120 m in depth, according to Mbaye et al. (2020). This divergence highlights regional differences in spawning behavior between the Northeast and Northwest Atlantic populations. These contrasting results underscore the complexity of mackerel spawning dynamics and suggest that depth and latitude preferences vary depending on the regional environmental conditions and temporal variables.
Although temperature has traditionally been viewed as the primary influence on mackerel spawning dynamics, this study reveals a more nuanced interaction between temperature, geographic features, and temporal shifts (Bruge et al., 2016; Hughes et al., 2014), influencing both the timing and location of spawning. The studies by Jansen et al. (2012) and dos Santos Schmidt et al. (2024) indicate that mackerel migrate and spawn within optimal thermal ranges, with warming trends driving a poleward shift in spawning areas. This study, however, demonstrates that spawning dynamics are influenced not only by temperature but also by geographical variables and the interaction between environmental and geographic variables. This result is in accordance with the findings of Brunel et al. (2018); Mbaye et al. (2020), and Richardson et al. (2020), which emphasize the existence of a more complex multi-driver system in the distribution of spawning grounds. This challenges the traditional single-driver paradigm and illustrates the adaptive strategies of mackerel in response to global environmental changes.
In this study, QA showed that the preferred temperature range for mackerel spawning in the Northeast Atlantic was between 11.5°C to 13.0°C. This result is in close accordance with the findings of Brunel et al. (2018) (11°C to 15°C) and Ibaibarriaga et al. (2007) (12°C to 13°C), supporting the consistency between studies. However, regional variations were evident when compared to the Northwest Atlantic mackerel, where Richardson et al. (2020) documented a broader preference of 8°C to 12°C and Mbaye et al. (2020) identified an optimal range of 13°C to 14°C. QA have also shown a wider range of temperature tolerance for spawning and a preference for colder waters in the Western area in recent years. This suggests that mackerel can adapt to changing environmental conditions. This adaptability challenges the traditional view that temperature is the primary variable and highlights the importance of considering the cumulative effects of additional variables, such as ocean currents. For example, dos Santos Schmidt et al. (2024) suggested that changes in the North Atlantic Current might have allowed spawning grounds to move northwards. Salinity is another environmental variable that can potentially affect mackerel spawning, although its effects are less consistent. In the present study, the salinity preference of the fish ranged from 35.3 ppm to 35.5 ppm, a value that is similar to that observed by Brunel et al. (2018). The aforementioned authors observed that salinities below 34.5 ppm had no significant impact on spawning, whereas higher salinities (approximately 35.5 ppm) were associated with more favorable spawning conditions. Thus, mackerel spawning may be linked to specific salinity levels. This study adds to the evidence that salinity affects spawning behavior. However, further studies are required to confirm these findings.
This study integrates geographical and environmental variables that influence NEA mackerel spawning distribution. By employing the Random Forest model and Quotient Analysis, we were able to identify the main drivers of mackerel egg presence and delineate preference and avoidance ranges in spawning for each key variable. These complementary methods enhance our understanding of the multifactorial dynamics of mackerel spawning and offer a comprehensive habitat assessment framework. These results highlight the importance of geographical variables such as bottom depth and latitude, which have been shown to be important in the selection of spawning grounds. The observed preference for areas near the 200 m isobath and latitudinal hotspots in the Cantabrian Sea, coupled with the noted northward shift of spawning grounds towards waters to the west of Scotland in the last few years, underlines the significant influence of geographical variables in determining reproductive behavior. These geographical variables interact dynamically with environmental variables such as temperature and salinity to further define habitat suitability. Temperature was identified as a key environmental variable, with a preferred range of 11.5°C to 13.0°C for NEA mackerel spawning However, the study also indicated a broader temperature tolerance, suggesting the potential for adaptive responses to environmental changes. Salinity, which has a narrower preferred range of 35.3 ppm to 35.5 ppm, was found to have less impact but nevertheless remains significant.
Publicly available datasets were analyzed in this study. This data can be found here: ICES Eggs and Larvae Database. ICES, Copenhagen, Denmark. https://www.ices.dk/data/data-portals/Pages/Eggs-and-larvae.aspx.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the analysis used retrospectively collected data.
GC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author would like to thank the ICES Working Group on Mackerel and Horse Mackerel Egg Surveys (WGMEGS) for providing data on mackerel eggs.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1461982/full#supplementary-material
Alvarez-Berastegui D., Ciannelli L., Aparicio-Gonzalez A., Reglero P., Hidalgo M., López-Jurado J. L., et al. (2014). Spatial scale, means and gradients of hydrographic variables define pelagic seascapes of bluefin and bullet tuna spawning distribution. PloS One 9, e109338. doi: 10.1371/journal.pone.0109338
Arrizabalaga H., Dufour F., Kell L., Merino G., Ibaibarriaga L., Chustd G., et al. (2015). Global habitat preferences of commercially valuable tuna. Deep Sea Res. Part II Top. Stud. Oceanogr. 113, 102–112. doi: 10.1016/j.dsr2.2014.07.001
Astthorsson O. S., Valdimarsson H., Gudmundsdottir A., Oskarsson G. J. (2012). Climate-related variations in the occurrence and distribution of mackerel (Scomber scombrus) in Icelandic waters. ICES J. Mar. Sci. 69, 1289–1297. doi: 10.1093/icesjms/fss084
Beare D. J., Reid D. G. (2002). Investigating spatio-temporal change in spawning activity by Atlantic mackerel between 1977 and 1998 using generalized additive models. ICES J. Mar. Sci. 59, 711–724. doi: 10.1006/jmsc.2002.1207
Bernal M., Stratoudakis Y., Coombs S., Angelico M., Lago de Lanzós A., Porteiro C., et al. (2007). Sardine off the European Atlantic coast: Characterization of and spatio-temporal variability in spawning habitat. Prog. Oceanography 74, 210–227. doi: 10.1016/j.pocean.2007.04.018
Bruge A., Alvarez P., Fontán A., Cotano U., Chust G. (2016). Thermal niche tracking and future distribution of atlantic mackerel spawning in response to ocean warming. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00086
Brunel T., van Damme C. J. G., Samson M., Dickey-Collas M. (2018). Quantifying the influence of geography and environment on the northeast Atlantic mackerel spawning distribution. Fisheries Oceanography 27, 159–173. doi: 10.1111/fog.2018.27.issue-2
Chust G., Taboada F. G., Alvarez P., Ibaibarriaga L. (2023). Species acclimatization pathways: latitudinal shifts and timing adjustments to track ocean warming. Ecol. Indic. 146, 109752. doi: 10.1016/j.ecolind.2022.109752
DFO (1997). Atlantic mackerel: integrated fisheries management plan 1997-1999 (Department of Fisheries and Oceans). Available at: http://www.dfo-mpo.gc.ca/FM/rm/mgmtplans (Accessed January 4, 2025).
Drapeau L., van der Lingen C. D. (2005). “Predicting spawning habitat location of anchovy and sardine in the southern Benguela using remotely- sensed data,” in Report of the SPACC meeting on small pelagic fish spawning habitat dynamics and the daily egg production method (DEPM), vol. 22 . Eds. Castro L. R., Fréon P., Lingen C. D. v. d., Uriarte A. (GLOBEC Rep), 6–8.
dos Santos Schmidt T., Slotte A., Olafsdottir A. H., Nøttestad L., Jansen T., Jacobsen J. A., et al. (2024). Poleward spawning of Atlantic mackerel (Scomber scombrus) is facilitated by ocean warming but triggered by energetic constraints. ICES J. Mar. Sci. fsad098. doi: 10.1093/icesjms/fsad098
Ganias K. (2009). Linking sardine spawning dynamics to environmental variability. Estuarine Coast. Shelf Sci. 84, 402–408. doi: 10.1016/j.ecss.2009.07.004
Gødo O. R., Hjellvik V., Iversen S. A., Slotte A., Tenningen E., Torkelsen T. (2004). Behavior of mackerel schools during summer feeding migration in the Norwegian Sea, as observed from fishing vessel sonars. ICES J. Mar. Sci. 61, 1093–1099. doi: 10.1016/j.icesjms.2004.06.009
Hughes K. M., Dransfeld L., Johnson M. P. (2014). Changes in the spatial distribution of spawning activity by north-east Atlantic mackerel in warming seas: 1977–2010. Mar. Biol. 161, 2563–2576. doi: 10.1007/s00227-014-2528-1
Hüssy K., Coad J. O., Farrell E. D., Clausen L. W., Clarke M. W. (2012). Sexual dimorphism in size, age maturation, and growth characteristics of boarfish (Capros aper) in the Northeast Atlantic. ICES J. Mar. Sci. 69, 1729–1735. doi: 10.1093/icesjms/fss156
Ibaibarriaga L., Irigoien X., Santos M., Motos L., Fives J. M., Franco C., et al. (2007). Egg and larval distributions of seven fish species in northeast Atlantic waters. Fisheries Oceanography 16, 284–293. doi: 10.1111/j.1365-2419.2007.00430.x
ICES (2013). Report of the working group on widely distributed stocks (WGWIDE), 27 august– 2 september 2013 Vol. 15 (Copenhagen, Denmark: ICES Headquarters), 950. ICES CM 2013/ACOM.
ICES (2019). Manual for mackerel and horse mackerel egg surveys, sampling at sea. Ser. ICES Survey Protoc. SISP 6, 82. doi: 10.17895/ices.pub.5140
ICES (2021). ICES Working Group on Mackerel and Horse Mackerel Egg Surveys (WGMEGS: outputs from 2020 meeting). ICES Sci. Rep. 3, 11. doi: 10.17895/ices.pub.78
ICES (2023). Working group on widely distributed stocks (WGWIDE). ICES Sci. Rep. 5, 82. doi: 10.17895/ices.pub.24025482
Iversen S. A. (2002). Changes in the perception of the migration of Northeast Atlantic mackerel during the last 100 years. ICES Mar. Sci. Symp. 215, 382–390. doi: 10.17895/ices.pub.8877
Jamieson A., Smith P. J. (1987). Atlantic mackerel (Scomber scombrus L.) stocks and genes: a review. J. Cons. ICES J. Mar. Sci. 44, 66–72. doi: 10.1093/icesjms/44.1.66
Jansen T., Campbell A., Kelly C. J., Payne M. (2012). Temperature, migration and fisheries of North East Atlantic mackerel (Scomber scombrus) in autumn and winter. PLoS18 One 7, e51541. doi: 10.1371/journal.pone.0051541
Jansen T., Gislason H. (2013). Population structure of Atlantic mackerel (Scomber scombrus). PloS One 8, (5). doi: 10.1371/journal.pone.0064744
Lima A. R., Garrido S., Riveiro I., Rodrigues D., Borges R., Angélico M. M., et al. (2022). Seasonal approach to forecast the suitability of spawning habitats of a temperate small pelagic fish under a high-emission climate change scenario. Front. Mar. Sci. 1523. doi: 10.3389/fmars.2022.956654
Lockwood S. J. (1988). The mackerel – its biology, assessment and the management of a fishery (Farnham, Surrey, England: Fishing News Books Ltd.), Vol. 181, ISBN: 0-85238-156-5.
Lockwood S. J., Nichols J. H., Coombs S. H. (1977). The development rates of mackerel (Scomber scombrus L.) eggs over a range of temperature. ICES CM 13, 8.
Mbaye B., Doniol-Valcroze T., Brosset P., Castonguay M., Van Beveren E., Smith A., et al. (2020). Modelling Atlantic mackerel spawning habitat suitability and its future distribution in the northwest Atlantic. Fish. Oceanogr. 29 , 84–99. doi: 10.1111/fog.12456
Munk P., Fox C. J., Bolle L. J., van Damme C. J., Fossum P., Kraus G. (2009). Spawning of North Sea fishes linked to hydrographic features. Fisheries Oceanography 18, 458–469. doi: 10.1111/j.1365-2419.2009.00525.x
Nesbø C. L., Rueness E. K., Iversen S. A., Skagen D. W., Jakobsen K. S. (2000). Phylogeography and population history of Atlantic mackerel (Scomber scombrus L.): a genealogical approach reveals genetic structuring among the eastern Atlantic stocks. Proc. R. Soc. London. Ser. B Biol. Sci. 267, 281–292. doi: 10.1098/rspb.2000.0998
Nissling A., Larsson R. (2018). Population specific sperm production in European flounder Platichthys flesus: adaptation to salinity at spawning. J. Fish Biol. 93, 47–52. doi: 10.1111/jfb.2018.93.issue-1
Ono K., Slotte A., Hølleland S., Mackinson S., Jónsson S. Þ., Jacobsen J. A., et al. (2022). Space-time recapture dynamics of PIT-tagged Northeast Atlantic mackerel (Scomber Scombrus) reveal size-dependent migratory behaviour. Provisionally accepted. Front. Mar. Sci. 9, 983962. doi: 10.3389/fmars.2022.983962
R Core Team. (2020). R: A language and environment for statistical computing. (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/.
Richardson D. E., Carter L., Curti K. L., Marancik K. E., Castonguay M. (2020). Changes in the spawning distribution and biomass of Atlantic mackerel (Scomber scombrus) in the western Atlantic Ocean over 4 decades. Fishery Bull. 118, 120–134. doi: 10.7755/FB.118.2.2
Sette O. E. (1950). Biology of the atlantic mackerel (Scomber scombrus) of north america. Pt. II. Migration Habits Fish. Bull. U.S. 51, 212–358.
Tenningen M., Slotte A., Skagen D. (2011). Abundance estimation of Northeast Atlantic mackerel based on tag recapture data – A useful tool for stock assessment? Fisheries Res. 107, 68 –674. doi: 10.1016/j.fishres.2010.10.009
Uriarte A., Lucio P. (2001). Migration of adult mackerel along the Atlantic European shelf edge from a tagging experiment in the south of the Bay of Biscay in 1994. Fish. Res. 50, 129–139. doi: 10.1016/S0165-7836(00)00246-0
Van Beveren E., Duplisea D. E., Brosset P., Castonguay M. (2019). Assessment modelling approaches for stocks with spawning components, seasonal and spatial dynamics, and limited resources for data collection. PloS One 14, e0222472. doi: 10.1371/journal.pone.0222472
Van der Lingen C. D., Huggett J. A. (2003). “The role of ichthyoplankton surveys in recruitment research and management of South African anchovy and sardine,” in The big fish bang: proceedings of the 26th annual larval fish conference. Eds. Browman H. I., Skiftesvik A. B. (Institute of Marine Research, Bergen, Norway), 303–341.
Keywords: Atlantic mackerel, spawning, random forest, egg survey, quotient analysis
Citation: Costas G (2025) Analysis of the spatio-temporal variability of spawning mackerel in the Northeast Atlantic. Front. Mar. Sci. 11:1461982. doi: 10.3389/fmars.2024.1461982
Received: 09 July 2024; Accepted: 18 December 2024;
Published: 15 January 2025.
Edited by:
Pablo Presa, University of Vigo, SpainReviewed by:
Victor Manuel Tuset, Instituto de Oceanografía y Cambio Global (IOCAG), SpainCopyright © 2025 Costas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gersom Costas, Z2Vyc29tLmNvc3Rhc0BpZW8uY3NpYy5lcw==
†ORCID: Gersom Costas, orcid.org/0000-0001-6604-3757
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.