- Jiangxi Fisheries Research Institute, Nanchang, China
A 56-days feeding experiment was conducted to evaluate the effect of dietary protein levels on fish growth, gonad development, and physiological biochemistry of female Pengze crucian carp (Carassius auratus var. Pengze) broodstock. Three isoenergetic diets were formulated, and the crude protein levels were 26.85%, 35.73% and 44.38%, the lipid levels were 7.39%, 7.92% and 7.91%, respectively. The results showed that the weight gain rate (WGR), the specific growth rate (SGR) and the protein efficiency ratio (PER) of the female Pengze crucian carp broodstock increased significantly and the feed conversion ratio (FCR) decreased significantly when the dietary protein level was 35.73% (P < 0.05). The WGR, the SGR and the PER were significantly higher in the 35.73% and 44.38% protein groups than in the 26.85% protein group (P<0.05). Increasing dietary protein levels led to a significant increase in the gonad index and the egg menstrual length of the female Pengze crucian carp broodstock (P<0.05). The higher protein levels resulted in a significant increase in total amino acids, total essential amino acids, and total nonessential amino acids (P<0.05). The varying ovaries of each group of total saturated fatty acids, total highly unsaturated fatty acids, and total polyunsaturated fatty acids all increased significantly with increasing protein levels (P<0.05). Furthermore, the aspartate transaminase (AST), low-density lipoprotein cholesterol (LDLC), and malondialdehyde (MDA) contents in plasma were significantly lower in the 35.73% and 44.38% groups than in the 26.85% group (P < 0.05). The 35.73% group exhibited the highest plasma superoxide dismutase (SOD) activity,. The plasma vitellogenin (VTG), estradiol 2 (E2), and progesterone (PROG) in plasma of female Pengze crucian carp broodstock were significantly higher in the 35.73% and 44.38% protein groups than in the 26.85% protein group (P<0.05). The results showed that the addition of an appropriate amount of protein to the feed can significantly improve the growth, gonadal development, and health of female Pengze crucian carp. In this study, the effect of 35.73% protein concentration was better than in the other two groups.
1 Introduction
The high-quality seedlings required for modern aquaculture are primarily dependent on the reproduction and seedling cultivation of broodstock under artificial breeding conditions. Therefore, conducting nutritional research on broodstock to enhance their reproductive performance and offspring quality has become an important area of research in aquatic animal nutrition. However, due to the complex biological mechanisms involved, such as gonadal maturation, broodstock nutrition remains one of the least studied areas (Coldebella et al., 2010; Asadi et al., 2021). Relevant studies have shown that the physiological processes and nutritional needs of breeding animals differ significantly from the nutritional needs of young animals growing. For freshwater fish, nutritional supply during the reproductive stage is crucial for the development of broodstock gonads, gamete quality, and larvae growth. Factors such as the sexual maturity of broodstock, gonadal development, egg diameter, fertilization rate, hatching rate, quality, and survival rate of fry are all closely related to feed nutritional status (Izquierdo et al., 2001; Coldebella et al., 2010).
Proteins are essential in fish nutrition, not only supporting bodily functions, but also serving as a crucial energy source that significantly impacts egg quality, especially during the development of yolk oocytes and ovaries (Chen et al., 2022). However, fish have limited ability to synthesize protein de novo, so most of the required protein must be provided through their diet (Ibim and Sikoki, 2014). Research on Nile tilapia has shown that higher protein levels in the feed can reduce the spawning interval and enhance reproductive efficiency (El-Sayed et al., 2003). Similarly, higher protein levels can enhance yellowtail (Seriola quinqueradiata) reproductive performance, leading to increased total egg weight and egg count (Santiago et al., 1991). Investigated the effects of different protein levels in isocaloric diets on Nile tilapia (Oreochromis niloticus) eggs revealed that fish fed higher protein levels grew faster, entered the vitellogenesis stage earlier, and showed faster egg growth and maturation, resulting in significantly larger egg diameters compared to those fed lower protein levels (Gunasekera et al., 1995). Studies on Xiphophorus helleri (Poeciliidae) (Chong et al., 2004), Labeo rohita (Hamilton) (Khan et al., 2005), Poecilia sphenops (Valenciennes) (Pandey et al., 2016), greater amberjack (Seriola dumerili, Risso, 1810) (Sarih et al., 2018), and yellow catfish (Pelteobagrus fulvidraco) (Chen et al., 2022), have also confirmed that appropriate dietary protein levels can improve egg hatching rates, while insufficient dietary protein levels can reduce broodstock fertility (Izquierdo et al., 2001).
Pengze crucian carp (Carassius auratus var. Pengze) is a newly discovered species in China that has been successfully bred by artificial means using wild crucian carp. Recent research has focused on the genetic diversity of crucian carp resources in China, as well as the origins and evolution of different crucian carp species (Pan et al., 2016). It has been observed that Pengze Crucian carp exhibits significant genetic variation and has great potential for breeding (Du et al., 2014). Proper nutrition for broodstock is essential for successful breeding, and it is important to understand and meet the specific nutritional requirements of the species. Studies have shown that a dietary protein level of 35.1% results in optimal growth for juvenile Pengze crucian carp (Ding et al., 2022). However, the nutritional needs of broodstock differ from those of juvenile fish, and the nutritional status of the broodstock directly impacts the health of their offspring. Currently, there is a lack of research focusing on the nutritional needs of female Pengze crucian carp broodstock both domestically and internationally. Therefore, it is crucial to conduct research on protein levels for female Pengze crucian carp broodstock. Drawing from the recommended protein levels for freshwater fish growth outlined by the NRC in 2011 (NRC, 2011), which fall between 29% and 40%, this study aimed to investigate the effects of high, medium, and low protein levels on growth, gonad development, and physiological and biochemical aspects of female Pengze crucian carp. This research is intended to establish a foundation for future studies on the mechanisms of gonad development and the seed cultivation of female Pengze crucian carp.
2 Materials and methods
2.1 Experimental diets
Three isoenergetic diets were formulated in which fish meal and soybean meal were used as protein sources, fish oil was used as a lipid source, and corn starch was used to adjust the protein gradient (Table 1). Dietary protein levels were 26.85%, 35.73% and 44.38%, lipid levels were 7.39 %, 7.92% and 7.91%, respectively. All ingredients were ground through an 80 mesh screen, weighed according to the proportion of the formula, and then mixed with oil and water to make sinking granular diets with a diameter of 2 mm. The pellets were dried and stored at -20°C until use.
2.2 Fish and feeding management
The female Pengze crucian carp broodstock used in this experiment was purchased from Jiujiang Liangsheng Ecological Agriculture Development Co., Ltd. All fish were reared in a circulating water system for four weeks to adapt to the breeding environment and feed specifications. Before initiating the feeding trial, the fish were fasted for 24 h. A total of 135 healthy and energetic female Pengze crucian carp broodstocks (mean initial weight: 253.78 ± 5.34 g) were collected and divided into 3 groups, each group having 3 parallels. Each parallel consisted of 15 fish. The circular breeding barrel used in the experiment had a size of ¢800 mm×650 mm, and each parallel had a breeding cycle of 56 days. During the trial, the fish were fed to apparent satiation, twice a day at 9:00 and 17:00. Throughout the test period, the water quality was monitored. The water temperature was maintained at 20 ± 2°C, the dissolved oxygen concentration was maintained above 7 mg/L, the pH was maintained at 7.53 ± 0.12, and the ammonia nitrogen and nitrite concentrations were not allowed to exceed 0.1 mg/L, measured by handheld water quality analyzer (Octadem, W-II, USA). The photoperiod followed the natural period.
2.3 Sample collection and chemical analysis
When the feeding trial ended, the fish were fasted for 24 h prior to sampling. All the fish in each barrel were weighed, and the total number of fish was counted. After the fish were anaesthetized with MS-222 (Sigma, St. Louis, MO, USA, 120 mg/L), six fish per barrel were randomly collected and stored (-20°C) to determine the approximate body composition. Another six fish were randomly selected from each barrel to determine their body weight and body length for calculating the condition factor (CF). Blood was collected from the caudal vein of the female fish with a sterile 1.0 ml syringe and stored in a sodium heparin anticoagulant tube. The samples were then rested at 4°C for 12 h and centrifuged for 10 min at 4000×r·min-1 (4°C) to test biochemical indexes and hormone content. Then, the fish were placed on a dissection tray for dissection, and the complete female fish gonads were removed, and the fish were weighed to calculate the gonad somatic index. The gonad tissues from the fish were sampled and fixed in 4% formaldehyde for the determination of egg diameter, and 20 eggs were measured per female. All the samples were stored at -80°C.
After fixation of the female gonads with 4% paraformaldehyde, the sections were subjected to a series of steps, including immersion in xylene I for 20 minutes, xylene II for 20 minutes, anhydrous ethanol I for 5 minutes, anhydrous ethanol II for 5 minutes, and 75% alcohol for 5 minutes, followed by washing with tap water. Subsequently, the sections were subjected to hematoxylin staining for 3-5 minutes, differentiated in hydrochloric acid aqueous solution, reversed to blue in ammonia aqueous solution, and finally washed with water. Dehydration in 85% and 95% gradient alcohol was followed by staining with eosin staining solution for 5 minutes. The slices were then treated with anhydrous ethanol I for 5 minutes, anhydrous ethanol II for 5 minutes, anhydrous ethanol III for 5 minutes, dimethyl for 5 minutes, and xylene II for 5 minutes to achieve transparency before being sealed with neutral gum. The process was concluded with microscopic examination, image acquisition, and analysis.
The levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), and low-density lipoprotein cholesterol (LDLC) in pooled serum were determined by an automatic chemistry analyser (Hitachi 7600, Tokyo, Japan). All indexes of plasma hematological parameters and enzyme activities, including alanine transaminase (ALT, C009-2-1), aspartate transaminase (AST, C010-2-1), superoxide dismutase (SOD, A001-3-2), malondialdehyde (MDA, A003-1-2), lysozyme (LZM, A050-1-1), alkaline phosphatase (ALP, A059-2-2), Acid phosphatase(ACP, A060-1-1), Estradiol 2(E2, H102-1-1), progesterone (PROG, H089-1-1), and Vitellogenin (VTG, H362-1), were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The fatty acids were determined with reference to GB T5009.168-2016, and separated on gas chromatograph Agilent 7890 (Agilent Technologies, USA) with ULTRA-2 capillarycolumn and with flame-ionization detector (FID). In the course of analysis, use chloroform-methanol (2:1, v/v) extracted fat, then esterified by adding KOH-methanol (0.5 mol/L) and boron trifluoride-methanol (1:3, v/v), and the reaction was thermally reacted for 3 min, and then cooled down, and then extracted by n-hexane for detection on the machine. The amino acids were determined with reference to GB T5009.124-2016 (acid hydrolysis method), and the samples were hydrolyzed by 6 mol/L hydrochloric acid solution at 110°C for 24h, and then analyzed with LA8080 amino acid auto analyzer (Hitachi, Japan).
2.4 Statistical analyses
All data were subjected to analysis of variance using SPSS 25.0 (SPSS Inc., Chicago, IL, USA) for Windows. One-way analysis of variance (ANOVA) was used to determine whether there were significant differences between treatments. The results are expressed as the mean ± S.D. Tukey’s multiple range test was used to detect significant differences between groups, and the significance level was established at P <0.05.
3 Results
3.1 Growth performance
The growth performance results for female Pengze crucian carp broodstock fed different levels of protein are listed in Table 2. The weight gain rate (WGR), specific growth rate (SGR) and protein efficiency ratio (PER) of the female Pengze crucian carp broodstock significantly increased and feed conversion ratio (FCR) significantly decreased when the dietary protein level was 35.73% (P < 0.05). However, with further increases to 44.38% in the dietary protein level, no significant increases were observed (P > 0.05).
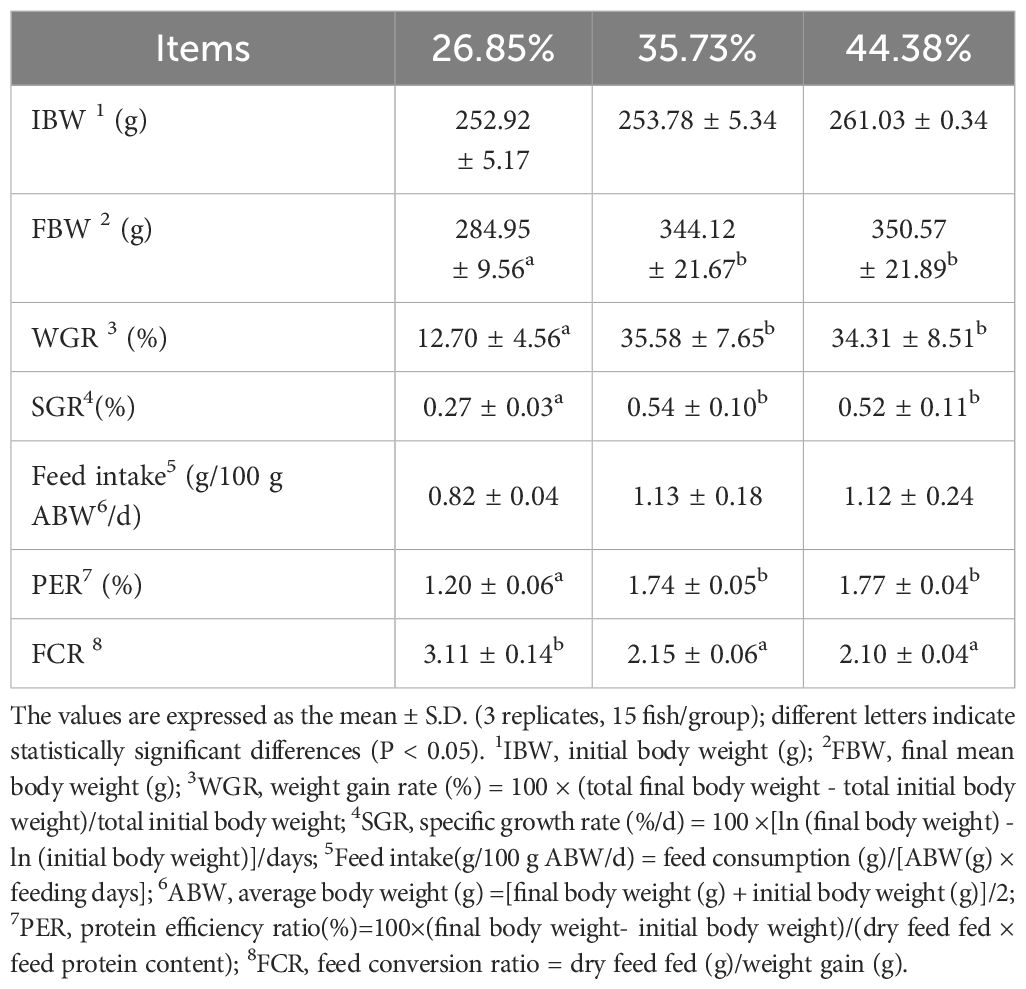
Table 2 Growth performance of female Pengze crucian carp broodstock fed the experimental diets with varying protein levels.
3.2 Ovarian morphological parameters and ovarian histology
As shown in Table 3, the 26.85% group had the lowest CF, GSI and egg diameter, and the GSI and egg diameter were significantly lower than those of the 35.73% and 44.38% groups (P < 0.05), while the group with the highest dietary protein (44.38% group) had the highest total egg production (P < 0.05). Hematoxylin and eosin staining of the ovarian tissue of female Pengze crucian carp (Figure 1) revealed that as the dietary protein level increased, the number of nuclei gradually decreased, the number of oocytes significantly increased, and the number of yolk granules significantly increased, indicating increased developmental maturity.

Table 3 Ovarian morphological parameters of female Pengze crucian carp broodstocks fed the experimental diets with varying protein levels.
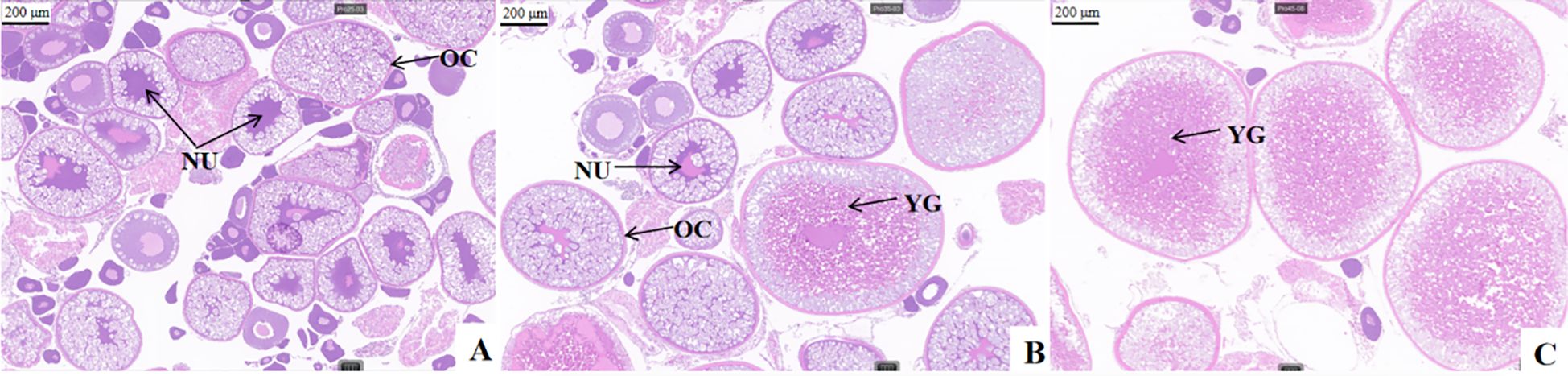
Figure 1 Ovarian histology of female Pengze crucian carp broodstock fed the experimental diets with varying protein levels. (A–C) were fed with 25%, 35% and 45% dietary protein, respectively, in the female Pengze crucian carp ovarian tissue sections. NU, nucleus; OC, oocytes; YG, yolk granules, 200 μm.
3.3 Amino acid composition of ovaries
Table 4 shows the amino acid compositions and contents of the ovaries of female Pengze crucian carp broodstocks with different dietary protein levels. Seventeen common amino acids were detected in each group. The glutamic acid content was the highest, followed by the alanine, leucine, glycine, aspartic, and lysine acid contents. The contents of amino acids, total essential amino acids (∑EAA), total nonessential amino acids (∑NEAA), and total amino acids (∑AA) increased significantly with increasing dietary protein levels (P < 0.05), except for histidine, phenylalanine, methionine, tryptophan, aspartic acid and glycine acid in the 44.38% protein group, which were not significantly different from those in the 35.73% protein group (P > 0.05).
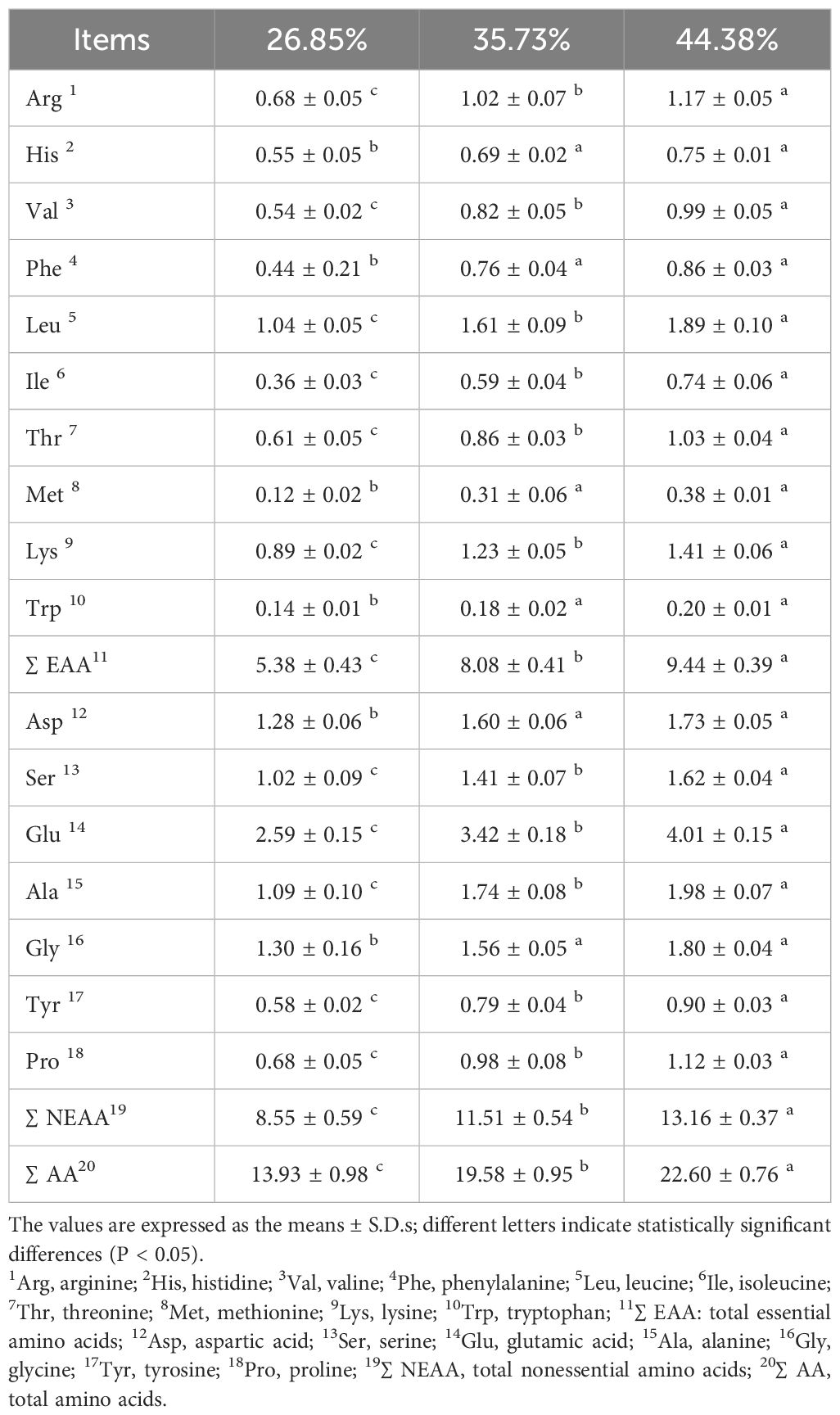
Table 4 Amino acid composition of the ovaries of female Pengze crucian carp broodstock fed the experimental diets with varying protein concentrations (g/100 g, dry weight).
3.4 Fatty acid composition of the ovaries
A total of 7 saturated fatty acids (SFAs), 4 monounsaturated fatty acids (MUFAs) and 8 polyunsaturated fatty acids were detected in the female Pengze crucian carp broodstock (Ovarian) at different protein levels (Table 5). The contents of C20:4n6 (ARA), C20:5n3 (EPA), C22:6n3 (DHA), total saturated fatty acids (∑SFA), total highly unsaturated fatty acids (∑HUFAs), total polyunsaturated fatty acids (∑PUFAs), total n-3 polyunsaturated fatty acids (∑n-3PUFAs), and total n-3 polyunsaturated fatty acids/total n-6 polyunsaturated fatty acids (∑n-3/∑n-6) increased significantly with increasing dietary protein levels, and at 44.38% protein level, the peak was reached (P < 0.05). However, total monounsaturated fatty acids (∑MUFAs) and total n-6 polyunsaturated fatty acids (∑n-6PUFAs) decreased significantly with increasing dietary protein levels among any of the treatments (P < 0.05). C16:0 was the dominant fatty acid within the SFAs, while C18:1n9c was the most abundant fatty acid within the MUFAs. C18:2n6c was the dominant fatty acid within the PUFAs.

Table 5 Fatty acid composition of the ovaries of female Pengze crucian carp broodstock fed the experimental diets with varying protein concentrations (% total fatty acids).
3.5 Serum physiological and biochemical parameters
Serum biochemical indexes are presented in Table 6. AST, LDLC, and MDA levels were significantly lower in the 35.73% and 44.38% groups than in the 26.85% group (P < 0.05). Additionally, there was a significant increase in SOD activity with increasing dietary protein levels (P < 0.05). The 35.73% group exhibited the highest SOD activity, while the 44.38% group showed a significant decrease (P < 0.05). Moreover, the MDA content in the 35.73% group was significantly lower than in the 26.85% group (P < 0.05). However, as the protein level increased to 44.38%, the MDA content tended to increase (P > 0.05). On the other hand, ALT, TC, TG, HDLC, LZM, AKP, and ACP did not differ significantly between the groups (P > 0.05).
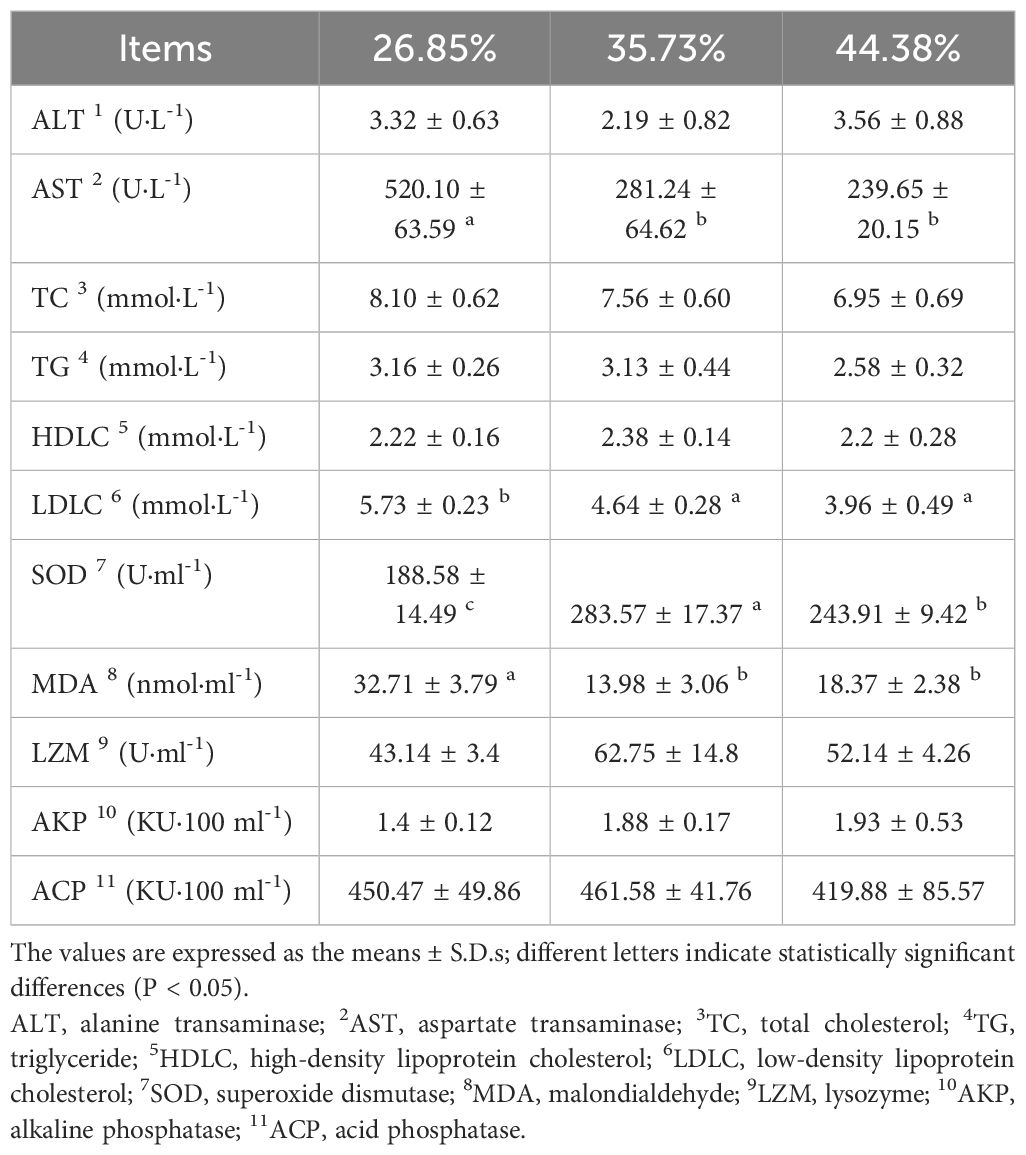
Table 6 Plasma biochemical indexes and antioxidant activity indexes of female Pengze crucian carp broodstock fed formulated diets with varying protein levels.
3.6 Serum hormone content
As shown in Table 7. The contents of E2, PROG and VTG first significantly increased and then slowly increased with increasing dietary protein levels. The contents of E2, PROG and VTG at the 35.73% and 44.38% protein levels were significantly higher than those at the 26.85% protein level (P < 0.05). The 44.38% protein level was slightly greater than the 35.73% protein level, but the difference was not significant (P >0.05).
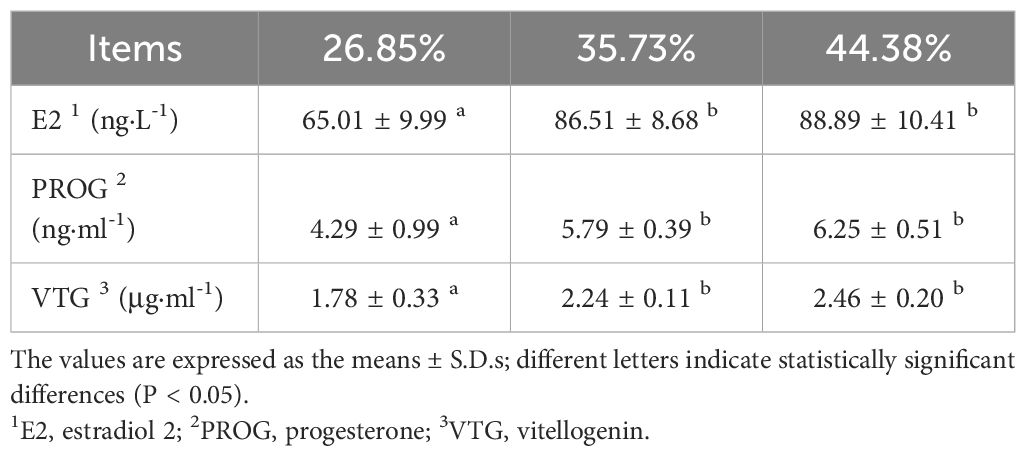
Table 7 Plasma hormone contents of female Pengze crucian carp broodstock fed formulated diets with varying protein levels.
4 Discussion
Numerous studies have demonstrated the significant impact of dietary protein on the gonadal development of broodstock, particularly in relation to body size. For instance, research on Nile tilapia (Oreochromis niloticus) revealed that a high-protein diet facilitated the passage of developmental stages more easily and led to increased oocyte maturation, highlighting the influence of feed on tilapia growth (Marinez et al., 2014). Similarly, investigations of female climbing perch (Anabas testudineus) indicated that a high-protein diet exhibited greater body weight and produced more yolk-containing oocytes, potentially resulting in higher spawning rates (Satheesh et al., 2023). The present study revealed a notable increase in weight gain rate (WGR), specific growth rate (SGR) and protein efficiency ratio (PER) with increasing dietary protein levels from 26.85% to 44.38% (P< 0.05). However, with the increase in dietary protein levels, the feed conversion ratio (FCR) exhibited a pattern of initially decreasing and then stabilizing at a lower level. This phenomenon has also been observed in previous studies on the protein requirements of giant grouper (Epinephelus lanceolatus) (Gao et al., 2019), grass carp (Ctenopharyngodon idellus) (Wang et al., 2022), giant grouper (Epinephelus lanceolatus) (Gao et al., 2019), and mandarin fish (Siniperca scherzeri) (Sankian et al., 2019). The improved fish growth rate and the reduced feed coefficient can be attributed to the higher protein content in the feed. The enhanced protein supply stimulates growth and minimizes feed wastage (McGoogan and Gatlin, 2000). In conclusion, a dietary protein level of 26.85% minimally affected the growth of the female Pengze crucian carp broodstock, while a level of 44.38% did not further enhance growth. Therefore, for optimal growth considerations, the protein requirement for female Pengze crucian carp broodstock can reach 35.73%.
Proteins play a crucial role as energy sources for the development of embryos and larvae. Adequate energy reserves are essential for the maturation of gonads in broodstock. The process of egg formation requires substantial energy and material resources to ensure the provision of nutrient stores for the eggs (Lochmann et al., 2007). Gonads must closely align with specific ecological conditions to develop and enhance effectively. Egg size and parental body size are interconnected during development (Camille et al., 2011; Jonsson and Jonsson, 2014). This study demonstrated that increased dietary protein levels could promote reproductive performance and resulted in larger body size and higher gonad index, egg length, and egg production of female Pengze crucian carp and influenced the maturity of ovarian development. This effect can be attributed to increased protein intake providing more raw materials for vitellogenin synthesis in gonadal tissue, thereby accelerating oocyte growth and gonadal development. Similar results were observed in studies involving Asian catfish (Clarias batrachus) (Singh et al., 2009) bagrid catfish (Mystus nemurus Cuv. & Val.) broodstock (Abidin et al., 2006) and tilapia (Oreochromis niloticus) (Marinez et al., 2014) broodstock. Some research has indicated that a 20% protein level in the diet can limit body size, egg maturation, and reproductive capacity in tilapia, although successfully hatched fry quality remains unaffected. Furthermore, some experiments revealed that increasing protein levels did not significantly affect body length of hatched larvae, suggesting that the growth rate, protein content, and water content of fry are not correlated with the protein level in the diet (Gunasekera et al., 1996; Marinez et al., 2014). This experiment also revealed that an increase in protein level had no significant effect on the body length of the hatched larvae, which was consistent with the above results. Similar results have been found in other fish studies (Milton and Arthington, 1983). In summary, a high-protein diet significantly promoted the gonadal development of female Pengze crucian carp.
Embryo and prehatchling development is dependent on yolk nutrition, which provides essential nutrients for growth and development. Fish protein needs are met through amino acids, which are crucial for various physiological processes (Asadi et al., 2021). Dietary amino acids have been shown to have an impact on hormone production and gonadal development in aquatic animals (Chen et al., 2007). Increased dietary protein levels have been found to elevate essential and non-essential amino acid levels of yellow river carp (Cyprinus carpio haematopterus) (Wang et al., 2023). Consistent with the results of this study, as the dietary protein level increased, the content of essential and non-essential amino acids in the ovarian tissue increased significantly. Research suggests that arginine supplementation can increase serum ketone concentrations in Chinese mitten crab (Eriocheir sinensis) (Qi et al., 2019), while the addition of 0.1% tryptophan to the diet can stimulate early maturation of male ayu fish spermatozoa and early ovulation in female fish (Akiyama et al., 1996). After the lysine content in the gilthead seabream feed decreased, the protein content in the yolk decreased, resulting in a low survival rate of the larvae. The results of the present study indicated that dietary protein significantly affected amino acid composition (such as Arg, Trp, Lys, Leu, and Met). In the ovary, most amino acids increased significantly with increasing dietary protein levels. In summary, high protein levels in diet can significantly promote the deposition of amino acids in the ovaries, thereby significantly improving the ovarian development of female Pengze crucian carp broodstock.
During gonad development, fat plays a crucial role as a cell membrane material, supporting the growth of oocytes. Fatty acids not only affect the synthesis of steroid hormones but also act as a source of energy through oxidation, which is essential for gonad development (Morais et al., 2014). Fat is utilized in the early stages of fish development primarily through the egg itself, providing energy for embryonic growth. Studies on various aquatic species, such as Japanese flounder (Paralichthys olivaceus) (Furuita et al., 2000), Siberian sturgeon (Acipenser baeri) (Luo et al., 2015) and female hilsa (Tenualosa ilisha) (Kumar et al., 2023), have demonstrated the importance of specific fatty acids, such as ARA, EPA, and DHA, in promoting gonad development. Research indicates that higher levels of these fatty acids result in improved gonad development and offspring quality, underscoring their significant role in reproductive success (Zhou et al., 2017). The regulatory impact of PUFAs on fish reproductive performance is complex, with studies emphasizing the influence of LC-PUFAs and sex steroid hormones on processes such as yolk formation and ovarian maturation (Peng et al., 2015). During gonad development, LC-PUFAs are transported from adipose tissue to the liver, where they stimulate the synthesis of vitellogenin, a key protein (Bransden et al., 2007). This synthesis relies not only on sufficient levels of LC-PUFAs but also on the presence of sex steroid hormones (Lubzens et al., 2010). Studies have shown that higher n-3 LC-PUFAs in ovaries can improve reproductive success in both male and female Siberian sturgeon (Acipenser baerii), improving the quality of their offspring (Luo et al., 2017). Similarly, research on Japanese flounder (Paralichthys olivaceus) has indicated that LC-PUFAs can boost reproductive performance and reduce larval deformities (Furuita et al., 2000). This experiment demonstrated a positive correlation between protein levels and concentrations of certain fatty acids in the ovaries. This indicates that high-protein diets can promote the accumulation of unsaturated fatty acids, thus supporting ovarian development in female Pengze crucian carp broodstock.
Fish blood is intricately linked to the metabolism, nutritional status, and diseases (Li et al., 2024). High-density lipoprotein and low-density lipoprotein are crucial in the transportation of cholesterol and triglycerides, and their levels reflect the body’s ability to transport these substances to some extent (Deng et al., 2010). In this study, the concentrations of low-density lipoproteins in the 35.73% and 44.38% groups were significantly lower than those of the 26.85% group. This finding aligns with previous research on hybrid abalone(Haliotis discus hannai♀ × H. fulgens♂) (Ma et al., 2022) and grouper (Epinephelus malabaricus) (Chen and Tsai, 1994). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are crucial aminotransferases in fish liver (Foroud et al., 2021). Normally, its serum activity is low but increases significantly with liver damage or disease (Nyblom et al., 2004). In this study, AST levels were significantly lower in the 35.73% and 44.38% groups than in the 26.85% group. ALT did not differ significantly, but tended to decrease with increasing protein levels in the diet, suggesting a potential impact on hepatopancreatic function in the crucian carp broodstock. More comprehensive research is necessary to validate these findings. SOD has been shown to inhibit inflammatory factors in fish, eliminate free radicals, and improve immune function (Cristóbal and Esteban, 2021; Heng et al., 2021; Ribeiro de Souza et al., 2021). Additionally, MDA is often used as a parameter to measure oxidative damage (Lu et al., 2020). This study revealed a significant increase in SOD activity in the serum of female Pengze crucian carp with increasing dietary protein levels, followed by a significant decrease. In contrast, the MDA content initially decreased significantly and then increased. These findings are consistent with previous studies on common carp (Cyprinus carpio) (Ebrahimi et al., 2020) and yellow river carp (Cyprinus carpio haematopterus) (Wang et al., 2023). The results suggest that an appropriate level of dietary protein can enhance the antioxidant capacity of female Pengze crucian carp broodstock, but excessive protein intake can lead to damage.
Teleost fish are commonly used as a model for investigating the impact of sex steroid hormones and the diversity of their receptors in serum. These hormones are essential to promoting the development and maturation of oocytes through meiosis and are mainly produced in granulosa and theca cells located outside follicle cells (Nagahama and Yamashita, 2008). Vitellogenin (VTG) is a crucial component of the fish reproductive process. It is produced primarily in the liver or hepatopancreas and then transported to the developing oocyte through the bloodstream. Once it reaches the oocyte, the VTG undergoes a transformation process that results in the production of mature lecithin proteins, which are vital nutrients and a source of energy for the growth and development of offspring. VTG can also be synthesized in the ovary and stored within the developing oocyte, serving as an important source of nutrients and energy during embryonic development (Jiang et al., 2023). Estradiol (E2) plays a key role in stimulating vitellogenin synthesis in the liver during the stage of egg cell growth. After yolk accumulation is complete, the follicle’s focus shifts to producing progesterone (PROG) in the steroid hormone pathway, which then stimulates sexual behavior in fish. Studies have shown that trace amounts of progesterone can be detected in the environment during estrus and play a role in oocyte development (Liu et al., 2012). Progesterone is produced as an intermediate product during the development of oocytes through the cleavage of prostaglandins by P450-scc and 3β-HSD, eventually forming estradiol through enzymatic action (Senthilkumaran et al., 2003). The change in steroid hormone production categories during egg cell development is likely due to the conversion of specific enzymes involved in steroid hormone synthesis. This study revealed that VTG, E2, and PROG concentrations were significantly higher at 35.73% and 44.38%, respectively, than at 26.85%, with 44.38% showing a slightly higher concentration than 35.73%, but the difference was not significant. Therefore, maintaining appropriate dietary protein levels can enhance vitellogenin accumulation in the hepatopancreas and ovaries, providing essential support for gonad development.
5 Conclusions
In conclusion, the present study revealed that dietary protein is important in broodstock nutrition for both the growth and reproductive performance of female fish. The 35.73% and 44.38% of the protein groups showed better growth performance and gonadal development than the 26.85% protein group. However, the protein level of 44.38% did not improve significantly compared to 35.73%, but the cost of the culture increased. Our results suggest that a diet containing 35.73% protein concentration was better than the other two groups.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Animal Care and Use Committee of Jiangxi Fisheries Research Institute. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JX: Writing – original draft, Writing – review & editing. FL: Writing – original draft. LD: Writing – review & editing. YY: Methodology, Writing – original draft. WH: Data curation, Writing – original draft. YF: Writing – review & editing. WC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Key Research and Development Program of Jiangxi (20203BBF63045), China Agriculture Research System (CARS-46), the earmarked fund for Jiangxi Agriculture Research System (JXARS-03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abidin M. Z., Hashim R., Chien A. C. S. (2006). Influence of dietary protein levels on growth and egg quality in broodstock female bagrid catfish (Mystus nemurus Cuv. & Val.). Aquaculture Res. 37, 416–418. doi: 10.1111/j.1365-2109.2005.01382.x
Akiyama T., Shiraishi M., Yamamoto T., Unuma T. (1996). Effect of dietary tryptophan on maturation of ayu Plecoglossus altivelis. Fisheries Sci. 62, 776–782. doi: 10.2331/fishsci.62.776
Asadi M., Kenari A. ,. A., Esmaeili M. (2021). Restricted- protein feeding strategy decreased the protein consumption without impairing growth performance, flesh quality and non-specific immune parameters in rainbow trout (Oncorhynchus mykiss). Aquaculture 531, 735946. doi: 10.1016/j.aquaculture.2020.735946
Bransden M. P., Battaglene S. C., Goldsmid R. M., Dunstan G. A., Nichols P. D. (2007). Broodstock condition, egg morphology and lipid content and composition during the spawning season of captive striped trumpeter, Latris lineata. Aquaculture 268, 2–12. doi: 10.1016/j.aquaculture.2007.04.026
Camille A. L. L., David B., Broddi R. H., Bjarni K. K., Skúli S. (2011). The importance of egg size and social effects for behaviour of arctic charr juveniles. Ethology 117, 664–674. doi: 10.1111/eth.2011.117.issue-8
Chen D. W., Zhang M., Shrestha S. (2007). Compositional characteristics and nutritional quality of Chinese mitten crab (Eriocheir sinensis). Food Chem. 103, 1343–1349. doi: 10.1016/j.foodchem.2006.10.047
Chen H. Y., Tsai J. C. (1994). Optimal dietary protein level for the growth of juvenile grouper, Epinephelus malabaricus, fed semipurified diets. Aquaculture 119, 265–271. doi: 10.1016/0044-8486(94)90181-3
Chen Z., Fei S., Duan Y., Liu C., Liu H. K., Han D., et al. (2022). Effects of dietary protein level on the growth, reproductive performance, and larval quality of female yellow catfish (Pelteobagrus fulvidraco) broodstock. Aquaculture Rep. 24, 101102. doi: 10.1016/j.aqrep.2022.101102
Chong A. S. C., Ishak S. D., Osman Z., Hashim R. (2004). Effect of dietary protein level on the reproductive performance of female swordtails Xiphophorus helleri (Poeciliidae). Aquaculture 234, 381–392. doi: 10.1016/j.aquaculture.2003.12.003
Coldebella I. J., Neto J. R., Mallmann C. A., Veiverberg C. A., Bergamin G. T., Pedron F. A., et al. (2010). The effects of different protein levels in the diet on reproductive indexes of Rhamdia quelen females. Aquaculture 312, 137–144. doi: 10.1016/j.aquaculture.2010.12.021
Cristóbal E. R., Esteban M.Á. (2021). Wound-induced changes in antioxidant enzyme activities in skin mucus and in gene expression in the skin of Gilthead seabream (Sparus aurata L.). Fishes 6, 15–15. doi: 10.3390/fishes6020015
Deng J., Mai K., Ai Q., Zhang W., Wang X., Tan B., et al. (2010). Interactive effects of dietary cholesterol and protein sources on growth performance and cholesterol metabolism of Japanese flounder (Paralichthys olivaceus). Aquaculture Nutr. 16, 419–429. doi: 10.1111/j.1365-2095.2009.00681.x
Ding L. Y., Chen W. J., Fu H. Y., Xiao J., Fu Y. L., Ma J. J. (2022). Estimation of the optimum dietary protein to lipid ratio in juvenile Pengze crucian carp (Carassius auratus Var. Pengze). Aquaculture Nutr. 2022, 2485134. doi: 10.1155/2022/2485134
Du M., Niu B. Z., Liu Y. H., Li Y. S. (2014). RAPD analysis in two cultured populations of Carassius auratus from South lake and Xiangshuihe reservoir. Freshw. Fisheries 44, 24–28 + 94. doi: 10.13721/j.cnki.dsyy.2014.03.004
Ebrahimi A., Akrami R., Najdegerami E. H., Ghiasvand Z., Koohsari H. (2020). Effects of different protein levels and carbon sources on water quality, antioxidant status and performance of common carp (Cyprinus carpio) juveniles raised in biofloc based system. Aquaculture 516, 734639. doi: 10.1016/j.aquaculture.2019.734639
El-Sayed A. F. M., Mansour C. R., Ezzat A. A. (2003). Effects of dietary protein level on spawning performance of Nile tilapia (Oreochromis niloticus) broodstock reared at different water salinities. Aquaculture 220, 619–632. doi: 10.1016/S0044-8486(02)00221-1
Foroud Y., Mehdi S., Hossein M. M., Afshin A. B. (2021). Efficacy of vitamin E with or without probiotic, astaxanthin or rosemary extract on growth performance, survival, haematological parameters, antioxidant activity and liver enzymes in rainbow trout (Oncorhynchus mykiss). Aquaculture Res. 52, 5606–5616. doi: 10.1111/ARE.15436
Furuita H., Tanaka H., Yamamoto T., Shiraishi M., Takeuchi T. (2000). Effects of n-3 HUFA levels in broodstock diet on the reproductive performance and egg and larval quality of the Japanese flounder, Paralichthys olivaceus. Aquaculture 187, 387–398. doi: 10.1016/S0044-8486(00)00319-7
Gao Y. J., Lu S. D., Wu M. J., Yao W., Jin Z. B., Wu X. Y. (2019). Effects of dietary protein levels on growth, feed utilization and expression of growth related genes of juvenile giant grouper (Epinephelus lanceolatus). Aquaculture 504, 369–374. doi: 10.1016/j.aquaculture.2019.02.023
Gunasekera R. M., Shim K., Lam T. (1995). Effect of dietary protein level on puberty, oocyte growth and egg chemical composition in the tilapia, Oreochromis niloticus (L.). Aquaculture 134, 169–183. doi: 10.1016/0044-8486(95)00028-Z
Gunasekera R. M., Shim K., Lam T. (1996). Effect of dietary protein level on spawning performance and amino acid composition of eggs of Nile tilapia, Oreochromis niloticus. Aquaculture 146, 121–134. doi: 10.1016/S0044-8486(96)01365-8
Heng N., Gao S., Chen Y., Wang L., Li Z., Guo Y., et al. (2021). Dietary supplementation with natural astaxanthin from Haematococcus pluvialis improves antioxidant enzyme activity, free radical scavenging ability, and gene expression of antioxidant enzymes in laying hens. Poultry Sci. 100, 101045. doi: 10.1016/j.psj.2021.101045
Ibim A. T., Sikoki F. D. (2014). Effect of protein level on gonadal development of the African catfish. J. Biol. Agricul. and Healthcare 4, 54–56.
Izquierdo M. S., Fernández P. H., Tacon A. G. J. (2001). Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197, 25–42. doi: 10.1016/S0044-8486(01)00581-6
Jiang K., Fang X., Li Y. L., Qiu G. F. (2023). Genome-wide identification, phylogeny, expression and eyestalk neuroendocrine regulation of vitellogenin gene family in the freshwater giant prawn Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 340, 114306. doi: 10.1016/j.ygcen.2023.114306
Jonsson B., Jonsson N. (2014). Early environment influences later performance in fishes. J. fish Biol. 85, 151–188. doi: 10.1111/jfb.12432
Khan M. A., JAFRI A. K., Chadha N. K. (2005). Effects of varying dietary protein levels on growth, reproductive performance, body and egg composition of rohu, Labeo rohita (Hamilton). Aquaculture Nutr. 11, 11–17. doi: 10.1111/j.1365-2095.2004.00318.x
Kumar P., Chandan N. K., Biswas G., Mandal B., Hussain T., Kailasam M., et al. (2023). Fatty acid mobilization and histological changes at different maturation stages of female hilsa. Tenualosa ilisha Aquaculture 576, 739887. doi: 10.1016/j.aquaculture.2023.739887
Li S. P., Zhou D. L., Pang D. R., Li Q. R., Li Q., Wang H., et al. (2024). Effects of Ramulus mori oligosaccharides on growth performance, serum physiological and biochemical parameters, and immunomodulation in largemouth bass (Micropterus salmoides). Aquaculture 589, 741008. doi: 10.1016/j.aquaculture.2024.741008
Liu S., Ying G. G., Zhou L. J., Zhang R. Q., Chen Z. F., Lai H. J. (2012). Steroids in a typical swine farm and their release into the environment. Water Res. 46, 3754–3768. doi: 10.1016/j.watres.2012.04.006
Lochmann S. E., Goodwin K. J., Lochmann R. T., Stone N. M., Clemment T. (2007). Volume and lipid, fatty acid, and amino acid composition of golden shiner eggs during a spawning season. North Am. J. Aquaculture 69, 116–126. doi: 10.1577/A05-094.1
Lu Z. Y., Feng L., Jiang W. D., Wu P., Liu Y., Kuang S. Y., et al. (2020). Mannan oligosaccharides improved growth performance and antioxidant capacity in the intestine of on-growing grass carp (Ctenopharyngodon idella). Aquaculture Rep. 17, 100313. doi: 10.1016/j.aqrep.2020.100313
Lubzens E., Young G., Bobe J., Cerdà J. (2010). Oogenesis in teleosts: how fish eggs are formed. Gen. Comp. Endocrinol. 165, 367–389. doi: 10.1016/j.ygcen.2009.05.022
Luo L., Ai L. C., Li T. L., Xue M., Wang J., Li W. T., et al. (2015). The impact of dietary DHA/EPA ratio on spawning performance, egg and offspring quality in Siberian sturgeon (Acipenser baeri). Aquaculture 437, 140–145. doi: 10.1016/j.aquaculture.2014.11.036
Luo L., Ai L. C., Liang X. F., Hu H. X., Xue M., Wu X. F. (2017). n-3 Long-chain polyunsaturated fatty acids improve the sperm, egg, and offspring quality of Siberian sturgeon (Acipenser baerii). Aquaculture 473, 266–271. doi: 10.1016/j.aquaculture.2017.02.021
Ma Y. B., Zou W. G., Ai C. X., You W. W., Liu S. T., Luo X., et al. (2022). Evaluation of optimal dietary protein levels for juvenile hybrid abalone under three temperatures: growth performance, body composition, biochemical responses, and antioxidant capacity. Aquaculture Nutr. 2022, 7008746. doi: 10.1155/2022/7008746
Marinez M. D. O., Tainá R., Tamira M. O., Dênio G. S. D. O., Mariana M. D., Rilke T. F. D. F., et al. (2014). Effects crude protein levels on female Nile tilapia (Oreochromis niloticus) reproductive performance parameters. Anim. Reprod. Sci. 150, 62–69. doi: 10.1016/j.anireprosci.2014.08.006
McGoogan B. B., Gatlin D. M. (2000). Dietary manipulations affecting growth and nitrogenous waste production of red drum, Sciaenops ocellatus. Aquaculture 182, 271–285. doi: 10.1016/S0044-8486(99)00260-4
Milton D., Arthington A. H. (1983). Reproductive biology of Gambusia affinis holbrooki Baird and Girard, Xiphophorus helleri (Gunther) and X. maculatus (Heckel) (Pisces; poeciliidae) in Queensland, Australia. J. Fish Biol. 23, 23–41. doi: 10.1111/j.1095-8649.1983.tb02879.x
Morais S., Mendes A. C., Castanheira M. F., Coutinho J., Bandarra N., Dias J., et al. (2014). New formulated diets for Solea Senegalensis broodstock: Effects of parental nutrition on biosynthesis of long-chain polyunsaturated fatty acids and performance of early larval stages and juvenile fish. Aquaculture 432, 374–382. doi: 10.1016/j.aquaculture.2014.04.033
Nagahama Y., Yamashita M. (2008). Regulation of oocyte maturation in fish. Development Growth differentiation 50, S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x
NRC (2011). National Research Council (NRC) Committee on the Nutrient Requirements of Fish and Shrimp, Nutrient Requirements of Fish and Shrimp (Washington, D.C., USA: National Academies Press).
Nyblom H., Berggren U., Balldin J., Olsson R. (2004). High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol alcoholism 39, 336–339. doi: 10.1093/alcalc/agh074
Pan Z. J., Zhao H. T., Zhu C. K., Chen H., Zhao P. F., Cheng Y. (2016). Genetic diversity analysis of crucian carp (Carassius auratus) based on cyt b and D-loop-containing region around Hongze Lake. Environ. Biol. Fishes 104, 1401–1420. doi: 10.1007/s10641-021-01175-8
Pandey A., Kaur V. I., Srivastava A., Datta S. N., Singh A. (2016). Effect of formulated feeds with different nutrient levels on growth and reproductive performance of molly, poecilia sphenops (Valenciennes). Anim. Nutr. Feed Technol. 16, 61–70. doi: 10.5958/0974-181X.2016.00006.8
Peng S. M., Gao Q. X., Shi Z. H., Zhang C. J., Wang J. G., Yin F., et al. (2015). Effect of dietary n-3 LC-PUFAs on plasma vitellogenin, sex steroids, and ovarian steroidogenesis during vitellogenesis in female silver pomfret (Pampus argenteus) broodstock. Aquaculture 444, 93–98. doi: 10.1016/j.aquaculture.2015.03.031
Qi C. L., Wang X. D., Han F. L., Jia Y. Y., Lin Z. D., Wang C. L., et al. (2019). Arginine supplementation improves growth, antioxidant capacity, immunity and disease resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 93, 463–473. doi: 10.1016/j.fsi.2019.07.082
Ribeiro de Souza R., Duarte de Oliveira Paiva P., Ricardo de Souza A., Reis da Silva R., Corrêa da Silva D. P., Valquíria dos Reis M., et al. (2021). Morpho-anatomical changes and antioxidant enzyme activity during the acclimatization of Genipa americana. Acta Physiologiae Plantarum 43, 93. doi: 10.1007/s11738-021-03263-9
Sankian Z., Khosravi S., Kim Y. O., Lee S. M. (2019). Dietary protein requirement for juvenile mandarin fish, Siniperca scherzeri. J. World Aquaculture Soc. 50, 34–41. doi: 10.1111/jwas.12569
Santiago C. B., Camacho A. S., Laron M. A. (1991). Growth and reproductive performance of bighead carp (Aristichthys nobilis) reared with or without feeding in floating cages. Aquaculture 96, 109–117. doi: 10.1016/0044-8486(91)90143-U
Sarih S., Djellata A., Roo J., Hernández C. C. M., Fontanillas R., Rosenlund G., et al. (2018). Effects of increased protein, histidine and taurine dietary levels on egg quality of greater amberjack (Seriola dumerili, Risso 1810). Aquaculture 499, 72–79. doi: 10.1016/j.aquaculture.2018.09.011
Satheesh M., Gour H. P., Parimal S., Subrata D., Dilip K. S., Prasanta J., et al. (2023). Dietary protein requirement of female climbing perch, Anabas testudineus (Bloch 1792) broodstock. Anim. Feed Sci. Technol. 305, 115778. doi: 10.1016/j.anifeedsci.2023.115778
Senthilkumaran B., Yoshikuni M., Nagahama Y. (2003). A shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. Mol. Cell. Endocrinol. 215, 11–18. doi: 10.1016/j.mce.2003.11.012
Singh R. K., Desai A. S., Chavan S. L., Khandagale P. A. (2009). Effect of water temperature on dietary protein requirement, growth and body composition of Asian catfish, Clarias batrachus fry. J. Thermal Biol. 34, 8–13. doi: 10.1016/j.jtherbio.2008.08.005
Wang S. H., Tian J. W., Jiang X. N., Li C. T., Ge Y. L., Hu X. S., et al. (2023). Effects of Different Dietary Protein Levels on the Growth Performance, Physicochemical Indexes, Quality, and Molecular Expression of Yellow River Carp (Cyprinus carpio haematopterus). Animals: an Open Access J. MDPI 13, 1237. doi: 10.3390/ani13071237
Wang X. Y., Liu G. Q., Xie S. Q., Pan L., Tan Q. S. (2022). Growth and meat quality of grass carp (Ctenopharyngodon idellus) responded to dietary protein (Soybean meal) level through the muscle metabolism and gene expression of myosin heavy chains. Front. Nutr. 9. doi: 10.3389/fnut.2022.833924
Zhou H., Leng X. Q., Tan Q. S., Du H., Wu J. P., Liang X. F., et al. (2017). Identification of key nutrients for gonadal development by comparative analysis of proximate composition and fatty/amino acid profile in tissues and eggs of Chinese sturgeon (Acipenser sinensis Gray 1835). J. Appl. Ichthyol. 33, 885–891. doi: 10.1111/jai.2017.33.issue-5
Keywords: Carassius auratus var. Pengze, broodstock, protein level, ovarian, gonad development
Citation: Xiao J, Long F, Ding L, Yao Y, Wu W, Fu Y and Chen W (2024) Effects of three different protein levels on the growth, gonad development, and physiological biochemistry of female Pengze crucian carp (Carassius auratus var. Pengze) broodstock. Front. Mar. Sci. 11:1459412. doi: 10.3389/fmars.2024.1459412
Received: 04 July 2024; Accepted: 24 July 2024;
Published: 08 August 2024.
Edited by:
Xueshan Li, Jimei University, ChinaReviewed by:
Zhijie Dan, Jiangsu Ocean University, ChinaYe Yuan, Shantou University, China
Kenneth Prudence Abasubong, University of South Bohemia, Czechia
Copyright © 2024 Xiao, Long, Ding, Yao, Wu, Fu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyun Ding, ZGluZ2xpeXVuMjAwOEAxNjMuY29t
 Jun Xiao
Jun Xiao Fan Long
Fan Long Liyun Ding
Liyun Ding Yuan Yao
Yuan Yao