- 1Southwest Fisheries Science Center, NOAA Fisheries, La Jolla, CA, United States
- 2South Atlantic Environmental Research Institute, Stanley, Falkland Islands
- 3Marine and Environmental Science Department, Hampton University, Hampton, VA, United States
- 4Laboratório de Ecologia de Mamíferos, Universidade do Vale do Rio dos Sinos, São Leopoldo, Brazil
- 5Grupo de Estudos de Mamíferos Aquáticos do Rio Grande do Sul, Torres, RS, Brazil
- 6Associação de Pesquisa e Preservação de Ecossistemas Aquáticos, Fortaleza, RS, Brazil
- 7Southern Right Whale Health Monitoring Program, Puerto Madryn, Argentina
- 8Karen C. Drayer Wildlife Health Center, School of Veterinary Medicine, University of California, Davis, Davis, CA, United States
- 9Servicio Nacional de Pesca y Acuicultura, Valparaíso, Chile
- 10Veterinary Histology and Pathology, Institute of Animal Health and Food Safety, Veterinary School, University of Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain
1 Introduction
The panzootic high pathogenicity avian influenza subtype H5N1 clade 2.3.4.4b (hereafter H5N1) has spread globally in recent years at unprecedented rates (Klaassen and Wille, 2023). This novel H5N1 differs from previous outbreaks because the barrier of transmission from birds to pinnipeds (seals and sea lions) is low (Dewar et al., 2023; Tomás et al., 2024), direct mammal to mammal transmission is likely (Restori et al., 2024; Rimondi et al., 2024; Uhart et al., 2024; Plaza et al., 2024a), and even small doses are virulent in mammals (Restori et al., 2024). During 2023 the virus spread around South America from the Pacific to the Atlantic Ocean, killing hundreds of thousands of seabirds and tens of thousands of pinnipeds (Leguia et al., 2023; Ulloa et al., 2023; Azat et al., 2024; Campagna et al., 2024; De Lima et al., 2024, Plaza et al., 2024b). Fears of H5N1 following wildlife migration routes and infecting Antarctic seabird and pinniped colonies (Boulinier, 2023; Dewar et al., 2023; Stokstad, 2024) have recently been realized by confirmed infections at several locations on the Antarctic Peninsula (AP) (Bennett Lazo et al., 2024; SCAR-AWHN, 2024; WAHIS, 2024). Given the geographic proximity of South Georgia in the southwest Atlantic (Figure 1), where Antarctic fur seals (Arctocephalus gazella) and southern elephant seals (Mirounga leonina) have been infected (Bennison et al., 2024), and the common, direct behavioral interactions with regional H5N1-infected seabird species, the risk to Antarctic pinnipeds is extremely high.
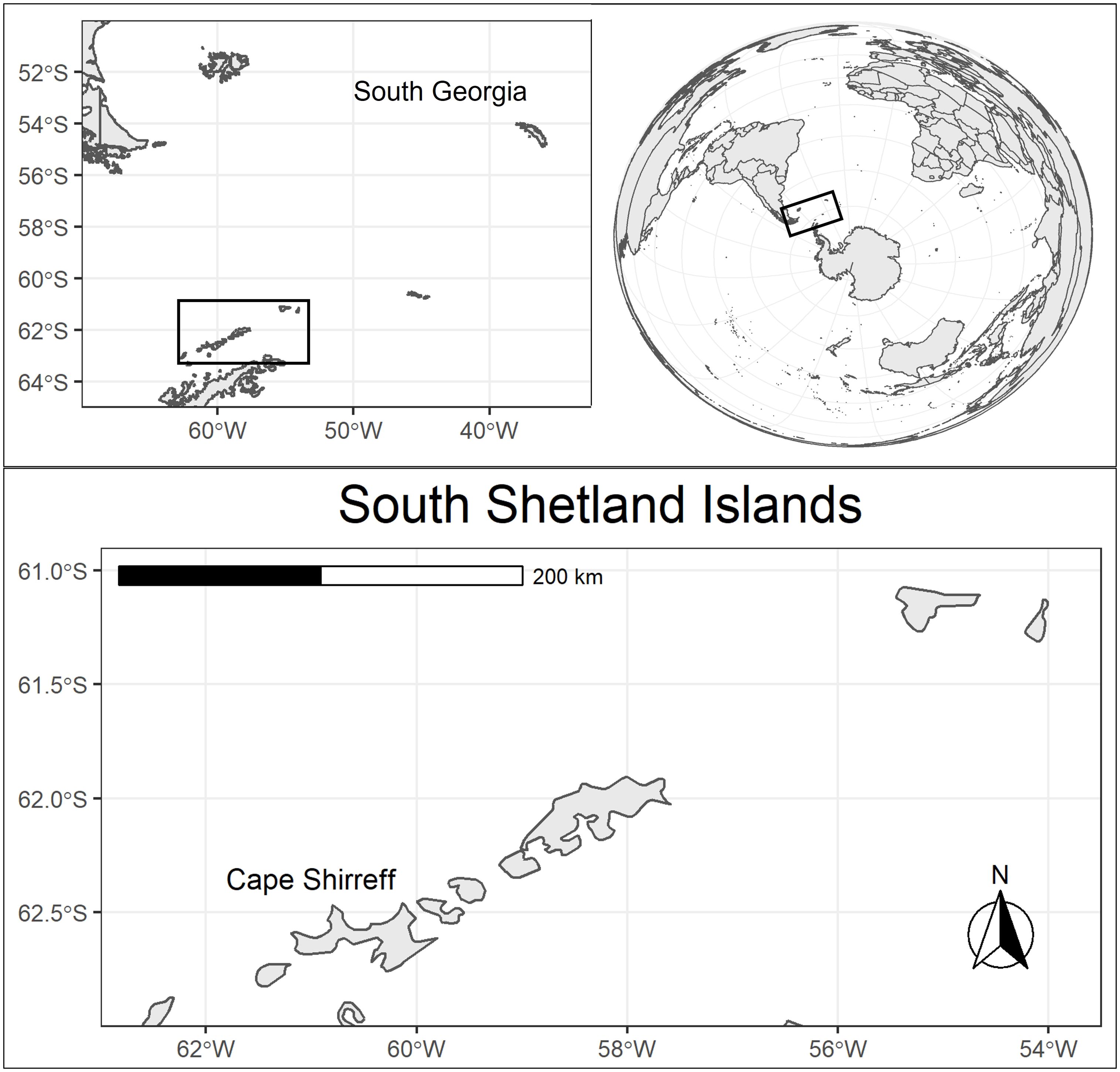
Figure 1. A regional map of the Antarctic Peninsula illustrating the proximity of H5N1-infected seabird and pinniped colonies along migratory pathways from South America via South Georgia to the Peninsula, particularly the South Shetland Island archipelago.
The vast majority of Antarctic seabirds and marine mammals depend on Antarctic krill (Euphausia superba, hereafter krill) as their main prey source (Laws, 1985). Krill is also the target of the largest commercial fishery in the Southern Ocean, which is managed by the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR, 1980). The krill fishery has grown substantially in recent decades, and has temporally and spatially concentrated its effort into small areas that overlap with krill-dependent predators (Santa Cruz et al., 2018; Watters et al., 2020). Empirical observations indicate that, given natural variability in the system, such concentrated catches pose plausible risks to predator populations (Watters et al., 2020; Krüger et al., 2021). The current krill fishery management strategy is not adaptable based on changes in the ecosystem and has not been updated for over a decade. However, CCAMLR is currently considering the adoption of management strategies that are annually adaptable and spread the allowable catch in space and time, which could alleviate risks to krill predators (Warwick-Evans et al., 2022; Watters and Hinke, 2022). Trawling vessels fishing for krill are required to use marine mammal-exclusion devices; nevertheless, pinnipeds and whales are occasionally killed incidentally (e.g., CCAMLR Secretariat, 2024). Therefore, CCAMLR is also considering time-area closures to limit interactions between the krill fishery and Antarctic seabirds and marine mammals during biologically critical periods.
Given the novel, highly-transmissible nature of H5N1 resulting in mass die-offs in marine mammals, we have grave concern about the potential negative effects on krill-dependent predators, including some already-vulnerable populations. Particularly, South Shetland Antarctic fur seals (SSAFS, Figure 2), a genetically-distinct population of the species that is experiencing a catastrophic population collapse (Krause et al., 2022). Since 2007, the abundance estimates of SSAFS have dropped extraordinarily from over 40,000 to under 1,500 animals. While there have been demographically-positive changes in the region like the substantial reduction of predation on SSAFS pups by leopard seals since 2017 (Krause et al., 2024), other high risk conservation threats remain. Immediate threats to this long-lived, low-fecundity population include competition for prey from other krill predators and the krill fishery, incidental mortality in fishing trawls, and most urgently, H5N1 mortality (Krause et al., 2024).

Figure 2. A mother–pup pair of South Shetland Antarctic fur seals (Arctocephalus gazella), a genetically-distinct population of the species that is at extreme conservation risk due to high pathogenicity avian influenza H5N1, potential incidental mortality in the krill fishery, and other factors. Photo taken in accordance with Marine Mammal Protection Act Permit No. 25786.
2 Discussion
This influenza virus alone can devastate pinniped populations within months (Leguia et al., 2023; Campagna et al., 2024; Plaza et al., 2024b), and cumulatively these threats imperil adequate SSAFS conservation and require urgent management action. To minimize the negative impacts of H5N1 on SSAFS and other small or vulnerable populations, we recommend: 1) adoption of conservation measures to reduce non-H5N1 mortality for SSAFS including adaptive krill fishery management and targeted time-area closures, 2) enhanced surveillance and testing of H5N1 in the AP, 3) real-time communication of suspected cases (SCAR-AWHN, 2024), 4) using biosecurity best practices during interactions with wildlife (WOAH, 2024), and 5) removal and proper disposal of H5N1-infected carcasses when possible (Dewar et al., 2023; Knief et al., 2024).
Author contributions
RB: Writing – review & editing, Writing – original draft, Project administration, Conceptualization. DK: Writing – review & editing, Writing – original draft, Project administration. AB: Writing – review & editing, Writing – original draft. CB: Writing – review & editing, Writing – original draft. LO: Writing – review & editing, Writing – original draft. MMU: Writing – review & editing, Writing – original draft. MU: Writing – review & editing, Writing – original draft. GW: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank J. T. Hinke and a Frontiers reviewer for reviewing a draft of this letter.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Azat C., Alvarado-Rybak M., Aguilera J. F., Benavides J. A. (2024). Spatio-temporal dynamics and drivers of highly pathogenic avian influenza H5N1 in Chile. Front. Veterinary Sci. 11, 1387040. doi: 10.3389/fvets.2024.1387040
Bennett Lazo B., Berazay B., Muñoz G., Ariyama N., Enciso N., Braun C., et al. (2024). Confirmation of Highly Pathogenic Avian Influenza (HPAI) H5N1 associated with an unexpected mortality event in south polar skuas (Stercorarius maccormicki) during 2023-2024 surveillance activities in Antarctica. bioRxiv 2024.04.10.588951. doi: 10.1101/2024.04.10.588951
Bennison A., Byrne A. M. P., Reid S. M., Lynton-Jenkins J. G., Mollett B., De Silva D., et al. (2024). Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic Region. bioRxiv, 2023.11.23.568045. doi: 10.1101/2023.11.23.568045
Boulinier T. (2023). Avian influenza spread and seabird movements between colonies. Trends Ecol. Evol. 38, 391–395. doi: 10.1016/j.tree.2023.02.002
Campagna C., Uhart M., Falabella V., Campagna J., Zavattieri V., Vanstreels R. E. T., et al. (2024). Catastrophic mortality of southern elephant seals caused by H5N1 avian influenza. Mar. Mammal Sci. 40, 322–325. doi: 10.1111/mms.13101
CCAMLR (1980). Text of the convention on the conservation of antarctic marine living resources. (Commision for the Conservation of Antarctic Marine Living Resources). Available online at: https://www.ccamlr.org/en/organisation/camlr-convention-text (Accessed April 7 2023).
De Lima R. C., Estima S. C., Tavares M., Canabarro P. L., Botta S., Dias L. A., et al. (2024). Impacts and lessons learned from the first highly pathogenic avian influenza (H5N1) outbreak in South American pinnipeds along the southern Brazilian coast. Mar. Mammal Sci., e13163. doi: 10.1111/mms.13163
Dewar M., Wille M., Gamble A., Vanstreels R. E. T., Bouliner T., Smith A., et al. (2023). The risk of highly pathogenic avian influenza in the Southern Ocean: a practical guide for operators and scientists interacting with wildlife. Antarctic Sci. 35, 407–414. doi: 10.1017/S0954102023000342
Klaassen M., Wille M. (2023). The plight and role of wild birds in the current bird flu panzootic. Nat. Ecol. Evolution. 7, 1541–1542. doi: 10.1038/s41559-023-02182-x
Knief U., Bregnballe T., Alfarwi I., Ballmann M. Z., Brenninkmeijer A., Bzoma S., et al. (2024). Highly pathogenic avian influenza causes mass mortality in Sandwich Tern Thalasseus sandvicensis breeding colonies across north-western Europe. Bird Conserv. Int. 34, e6. doi: 10.1017/S0959270923000400
Krause D. J., Bonin C. A., Goebel M. E., Reiss C. S., Watters G. M. (2022). The rapid population collapse of a key marine predator in the northern antarctic peninsula endangers genetic diversity and resilience to climate change. Front. Mar. Sci. 8, 796488. doi: 10.3389/fmars.2021.796488
Krause D. J., Brownell R. L. Jr., Bonin C. A., Woodman S. M., Shaftel D., Watters G. M. (2024). Evaluating threats to South Shetland Antarctic fur seals amidst population collapse. Mammal Rev. 54, 30–46. doi: 10.1111/mam.12327
Krüger L., Huerta M. F., Santa Cruz F., Cárdenas C. A. (2021). Antarctic krill fishery effects over penguin populations under adverse climate conditions: Implications for the management of fishing practices. Ambio 50, 560–571. doi: 10.1007/s13280-020-01386-w
Leguia M., Garcia-Glaessner A., Muñoz-Saavedra B., Juarez D., Barrera P., Calvo-Mac C., et al. (2023). Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat. Commun. 14, 5489. doi: 10.1038/s41467-023-41182-0
Plaza P. I., Gamarra-Toledo V., Euguí J. R., Lambertucci S. A. (2024a). Recent changes in patterns of mammal infection with highly pathogenic avian influenza A(H5N1) virus worldwide. Emerging Infect. Dis. 30, 444–452. doi: 10.3201/eid3003.231098
Plaza P. I., Gamarra-Toledo V., Rodríguez Euguí J., Rosciano N., Lambertucci S. A. (2024b). Pacific and Atlantic sea lion mortality caused by highly pathogenic Avian Influenza A(H5N1) in South America. Travel Med. Infect. Dis. 59, 102712. doi: 10.1016/j.tmaid.2024.102712
Restori K. H., Septer K. M., Field C. J., Patel D. R., Vaninsberghe D., Raghunathan V., et al. (2024). Risk assessment of a highly pathogenic H5N1 influenza virus from mink. Nat. Commun. 15, 4112. doi: 10.1038/s41467-024-48475-y
Rimondi A., Vanstreels R. E. T., Olivera V., Donini A., Lauriente M. M., Uhart M. M. (2024). Highly pathogenic avian influenza A(H5N1) viruses from multispecies outbreak, Argentina, August 2023. Emerging Infect. Dis. 30 (4), 812–814. doi: 10.3201/eid3004.231725
Santa Cruz F., Ernst B., Arata J. A., Parada C. (2018). Spatial and temporal dynamics of the Antarctic krill fishery in fishing hotspots in the Bransfield Strait and South Shetland Islands. Fisheries Res. 208, 157–166. doi: 10.1016/j.fishres.2018.07.020
SCAR-AWHN (2024). Scientific Committee on Antarctic Research Antarctic Wildlife Health Network (SCAR AWHN) highly pathogenic avian influenza (HPAI) database. Available online at: https://scar.org/library-data/avian-flu (Accessed June 14, 2024).
Secretariat C. (2024). Fishery Report 2023: Euphausia superba in Area 48. (Commission for the Conservation of Antarctic Marine Living Resources). Available online at: https://fishdocs.ccamlr.org/FishRep_48_KRI_2023.pdf (Accessed June 21, 2024).
Stokstad E. (2024). In Antarctica, scientists track a dangerous bird flu. Science 383, 1281. doi: 10.1126/science.adp3271
Tomás G., Marandino A., Panzera Y., Rodríguez S., Wallau G. L., Dezordi F. Z., et al. (2024). Highly pathogenic avian influenza H5N1 virus infections in pinnipeds and seabirds in Uruguay: Implications for bird–mammal transmission in South America. Virus Evol. 10 (1), veae031. doi: 10.1093/ve/veae031
Uhart M., Vanstreels R. E. T., Nelson M. I., Olivera V., Campagna J., Zavattieri V., et al. (2024). Massive outbreak of Influenza A H5N1 in elephant seals at Península Valdés, Argentina: increased evidence for mammal-to-mammal transmission. bioRxiv 2024.05.31.596774. doi: 10.1101/2024.05.31.596774
Ulloa M., Fernández A., Ariyama N., Colom-Rivero A., Rivera C., Nuñez P., et al. (2023). Mass mortality event in South American sea lions (Otaria flavescens) correlated to highly pathogenic avian influenza (HPAI) H5N1 outbreak in Chile. Veterinary Q. 43, 1–10. doi: 10.1080/01652176.2023.2265173
WAHIS (2024). World Organization for Animal Health Information System (WAHIS). Available online at: https://wahis.woah.org/#/event-management,events5649and5599 (Accessed June 21, 2024).
Warwick-Evans V., Constable A. J., Dalla Rosa L., Secchi E., Seyboth E. (2022). Using a risk assessment framework to spatially and temporally spread the fishery catch limit for Antarctic krill in the west Antarctic Peninsula: A template for krill fisheries elsewhere. Front. Mar. Sci. 9, 1015851. doi: 10.3389/fmars.2022.1015851
Watters G. M., Hinke J. T. (2022). Conservation in the Scotia Sea in light of expiring regulations and disrupted negotiations. Conserv. Biol. 36, e13925. doi: 10.1111/cobi.13925
Watters G. M., Hinke J. T., Reiss C. S. (2020). Long-term observations from Antarctica demonstrate that mismatched scales of fisheries management and predator-prey interaction lead to erroneous conclusions about precaution. Sci. Rep. 10, 2314. doi: 10.1038/s41598-020-59223-9
WOAH (2024). World Organisation for Animal Health (WOAH), Practical guide for authorised field responders to HPAI outbreaks in marine mammals. Available online at: https://www.woah.org/app/uploads/2024/02/practicalguide-forauthorisedfieldresponders-hpaimarinemammals-feb24-1.pdf (Accessed June 1, 2024).
Keywords: high pathogenicity avian influenza, HPAI, H5N1, South Shetland Antarctic fur seals, Arctocephalus gazella, CCAMLR, krill predator
Citation: Brownell RL Jr, Krause DJ, Baylis AMM, Bonin CA, Oliveira LR, Uhart MM, Ulloa M and Watters GM (2024) Avian influenza H5N1 threatens imperiled krill-dependent predators in Antarctica. Front. Mar. Sci. 11:1453737. doi: 10.3389/fmars.2024.1453737
Received: 23 June 2024; Accepted: 22 August 2024;
Published: 12 September 2024.
Edited by:
Salvatore Siciliano, Fundação Oswaldo Cruz (Fiocruz), BrazilReviewed by:
Lucas Krüger, Instituto Antártico Chileno (INACH), ChileCopyright © 2024 Brownell, Krause, Baylis, Bonin, Oliveira, Uhart, Ulloa and Watters. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Douglas J. Krause, ZG91Z2xhcy5rcmF1c2VAbm9hYS5nb3Y=
†These authors have contributed equally to this work
 Robert L. Brownell Jr
Robert L. Brownell Jr Douglas J. Krause
Douglas J. Krause Alastair M. M. Baylis
Alastair M. M. Baylis Carolina A. Bonin
Carolina A. Bonin Larissa R. Oliveira
Larissa R. Oliveira Marcela M. Uhart
Marcela M. Uhart Mauricio Ulloa
Mauricio Ulloa George M. Watters
George M. Watters