- 1LR18ES41-Département de Biologie, Faculté des Sciences, Université de Tunis El Manar, Tunis, Tunisia
- 2Departamento de Biología Animal, Universidad de Málaga, Malaga, Spain
- 3Institut de Systématique, Évolution, Biodiversité, Muséum National d’Histoire Naturelle, Paris, France
- 4Institute of Marine Sciences (ISMAR), Consiglio Nazionale delle Ricerche, Bologna, Italy
- 5Stazione Zoologica ‘Anton Dohrn’, Napoli, Italy
Sea bottom sampling of the Skerki Bank in the Strait of Sicily updates the census of marine molluscs inhabiting a biogeographically key sector of the Mediterranean Basin. One single dredge haul at 112-m depth yielded 110 molluscan species belonging to Gastropoda, Bivalvia, and Scaphopoda, of which 41 are new records for Tunisian jurisdictional waters, including three gastropod species new to science. Nearly all the species are shared with the fauna inhabiting the adjacent Italian waters, and most of them are reported also from Malta. We stress that sites characterized by a relevant species richness, diagnosed by hosting more than 100 species, inclusive of rare taxa, are important areas for conservation in need of adequate management.
1 Introduction
The completion of an accurate and up-to date checklist of the fauna and flora within a country’s territory and jurisdictional water has emerged as a valuable tool for planning and environmental management. Efforts in this direction also point to biases among taxonomic groups and among geographical areas of the country.
Molluscs are one of the best-known groups of marine invertebrates, accounting for roughly one quarter of the benthic species (Appeltans et al., 2012) and, therefore, can be taken as a fair proxy to global species richness of an area. In the Mediterranean Sea, Coll et al. (2010) listed 2,113 species of molluscs. This number should be increased by ca. 105 species (MolluscaBase eds., 2024) to account for new records and new species descriptions since then, with many more under description constantly adding to the remarkably high diversity of the basin’s mollusc fauna (Sabelli and Taviani, 2014). At a regional level, species counts in areas where the fauna is well known are well above 1,000 species (Mediterranean Spain: 1,481 species of which 1,245 in the Alboran Sea and Strait of Gibraltar and 1,165 in Eastern Spain and the Baleares, Gofas et al., 2017; Italy: 1,777 species of which 1,398 species in Sector 3, which comprises the southern Tyrrhenian and the Sicily Channel, Renda et al., 2022; Aegean and eastern Mediterranean Turkey: 1,003 species, Öztürk et al., 2014; Malta: 1,273 species, of which 1,227 listed by Cachia et al., 2019, and updates summarized in Cachia, 2024), whereas current species counts for marine molluscs in Tunisian waters are only 459 species in the latest official report on marine biodiversity in Tunisia (Ministère de l’Environnement et du Développement Durable, 2019). The World Register of Marine Species (MolluscaBase eds., 2024), although incomplete, takes into account some sources ignored in the aforementioned report, e.g., Cecalupo et al. (2008) and lists so far 767 marine molluscan species with a published record in Tunisia (458 of them in the Western Mediterranean part, i.e., north of Cap Bon). There is no reason for which mollusc species richness in this area, located in the central part of the Mediterranean with a great diversity of habitats and an important centre of endemism in the Gulf of Gabès (Aissaoui et al., 2017), should be so markedly lower than in the rest of the Mediterranean. Therefore, we hypothesize that these low numbers are due to a gap of knowledge rather than to a genuine poverty.
Cruise CS96 of R/V Urania in the Strait of Sicily (or Sicily Channel) was designed to systematically sample banks, seamounts, and troughs throughout the strait from subtidal to bathyal depths with special focus on biogenic carbonates and deep-water corals (Taviani and Remia, 2001; Zibrowius and Taviani, 2005), and also on the extensive rhodolith beds in the deep circalittoral. On the occasion, 25 stations were sampled in the area of the Skerki Bank, a submarine elevation situated off the NW coast of Tunisia, in the middle of the Strait of Sicily as a submarine prolongation of the North African “Maghrebides” fold–thrust belt. It comprises several summits of which the shallowest (Keith Reef) reaches only 0.30 m from the surface (Cebrian and Requena, 2015) and has been a hazard to navigation since the Antiquity (Weitemeyer and Döhler, 2009). The only published account on CS96 Mollusca is dedicated to Polyplacophora (Dell’Angelo et al., 1998). Here, we report on a circalittoral station on Skerki Bank whose richness in mollusc shells significantly increases the census of Mollusca in Tunisian waters, and the Mediterranean Sea as a whole. We also comment on some elusive and poorly known taxa recorded in this survey.
2 State of the art
The first contribution to the Tunisian marine molluscan fauna was that of MacAndrew (1851), who reported 135 species from a dredge haul off Zembretta (35 fathoms), as part of the first scientific programme targeted to the biogeography of European molluscs. Jeffreys (1870) reported on several dredge hauls by Capt. Spratt (most of them in Greece, and one 140 miles east of Malta, 310 f) and on another set of dredge hauls by Capt. Nares in “Pantellaria, 40 fathoms; south of Syracuse, 40 f.; the Adventure Bank, between Sicily and Tunisia, 60 f.; Tunis Bay, 30 f.; and off that coast, 50 to 100 f. (…)”, but mentioned that these will be distinguished by “Med.” in the text. In the species list proper pp. 66–86, the exact sites are not indicated and can only be inferred from the depth range. Therefore, all the entries (152 in total) marked “Med.” with a depth range including 30, 50, or 100 fathoms regard Tunisian waters.
Another important early source for records of deep-water molluscs on the Tunisian northern coast is the expeditions of R/V “Porcupine” (1870) with 135 species listed (Jeffreys, 1878–1885; Sykes, 1904–1925), many of them shared with Capt. Nares’ samples. Another expedition by R/V “Shearwater” (1871) visited the Tripoli coast, Adventure Bank south of Sicily and Skerki Bank (Carpenter, 1872), but work mostly focused on currents and next to nothing was contributed to the fauna. A confusing point in the Porcupine reports is the mention of Ras el Amouch (always misspelled “Rasel Amouch”) intercalated between “Benzert Road” and “Tunis Bay” stations; more definitely, Jeffreys (1874) wrote “Rasel Amouch, North coast of Tunis”. However Ras el Amouch (36°38′N, 2°24′E) is located near the Algerian town of Tipasa, West of Algiers, and was assumed as such by workers dealing with Algeria, e.g., Seurat (1930), who wrote “le Porcupine a dragué, au large de Ras-el-Amouch, une des pointes du Chenoua, un Bryozoaire…”. Therefore, species recorded only from this station will not be treated as already recorded for Tunisian waters.
Altogether, Jeffreys’ and Sykes’ papers recorded 223 molluscan species for the north coast of Tunisia (plus six brachiopod species). Most (ca. 90%) of the names used by Jeffreys have changed (different genus, synonymy…), but for 204 of them (also 90%), it can be understood unambiguously which species was meant. Jeffreys was among the best molluscan taxonomists of his time; the records contained in those works are to be considered reliable, will be treated herein as already recorded from Tunisia, and are registered as such in the “Distribution” entries of the World Register of Marine Species (MolluscaBase eds., 2024). Only for 19 cases, the species mentioned is ambiguous because the named taxon has now been split into several, without a clue to which one was intended.
This pioneer work did not escape Pallary (1914) who incorporated many of those records in his “Liste des Mollusques du Golfe de Tunis”. Although he acknowledged Jeffreys as a source for his list, he seldom indicated which particular record came from Jeffreys (1870), but since his other sources drew from shallow water habitats, it can be assumed that his records of deep-water species are merely a quotation of the previous ones.
In the XXth century, R/V “Calypso” was chartered by the French government for several scientific cruises, including one on the Sicily Channel (Pérès and Picard, 1956). This cruise sampled several locations including Skerki Bank, Sentinelle Bank, and the surroundings of Galite Island between 45 m and 380 m depth. The molluscs were studied by Mars (1958) who recorded 70 species from four dredge hauls, of which 20 were new records for Tunisian waters and 7 are ambiguous as to which species was meant. Another important contribution in the XXth century was that of Rosso (1979) in the context of offshore oil exploration, using R/V “Catherine Laurence” of the CNRS marine lab at Villefranche in 1976. This report, however, regards only the well-explored continental shelf south of Cap Bon and contributed only a couple of new records to the Tunisian malacofauna. Fekih and Gougerot (1977) published an extensive list of Gastropoda from the Gulf of Tunis, but again, their original material was mostly beach drift and did not document deep-water species.
While the Gulf of Gabès has been given reasonable attention (Monterosato, 1880; Dautzenberg, 1883; Pallary, 1904-1906; Ghisotti, 1972; Cecalupo et al., 2008; Aissaoui et al., 2017), reports on the deeper part of the Northern coast are extremely scanty and we do not know of any recently published work going into details of the circalittoral malacofauna.
3 Materials and methods
Cruise CS96 took place in December 1996–January 1997 onboard R/V Urania. The sea bottom was sampled by means of a large-volume (60 L) modified Van Veen grab and a cylindrical rock dredge (see Bonfitto et al., 1994). Station CS 282 (Figure 1) was particularly productive in collecting mollusc shells, obtained by means of rock dredge on 07/01/1997 between 37°53′05.8″, 10°49′42.7″, ca. 112-m depth (start) and 7°53′38.8″, 10°48′39.3″, 70 m (end). A bulk sample was washed onboard, rinsed in freshwater, and dried at room temperature. Picking of mollusc shells was carried out under the stereomicroscope in a laboratory.
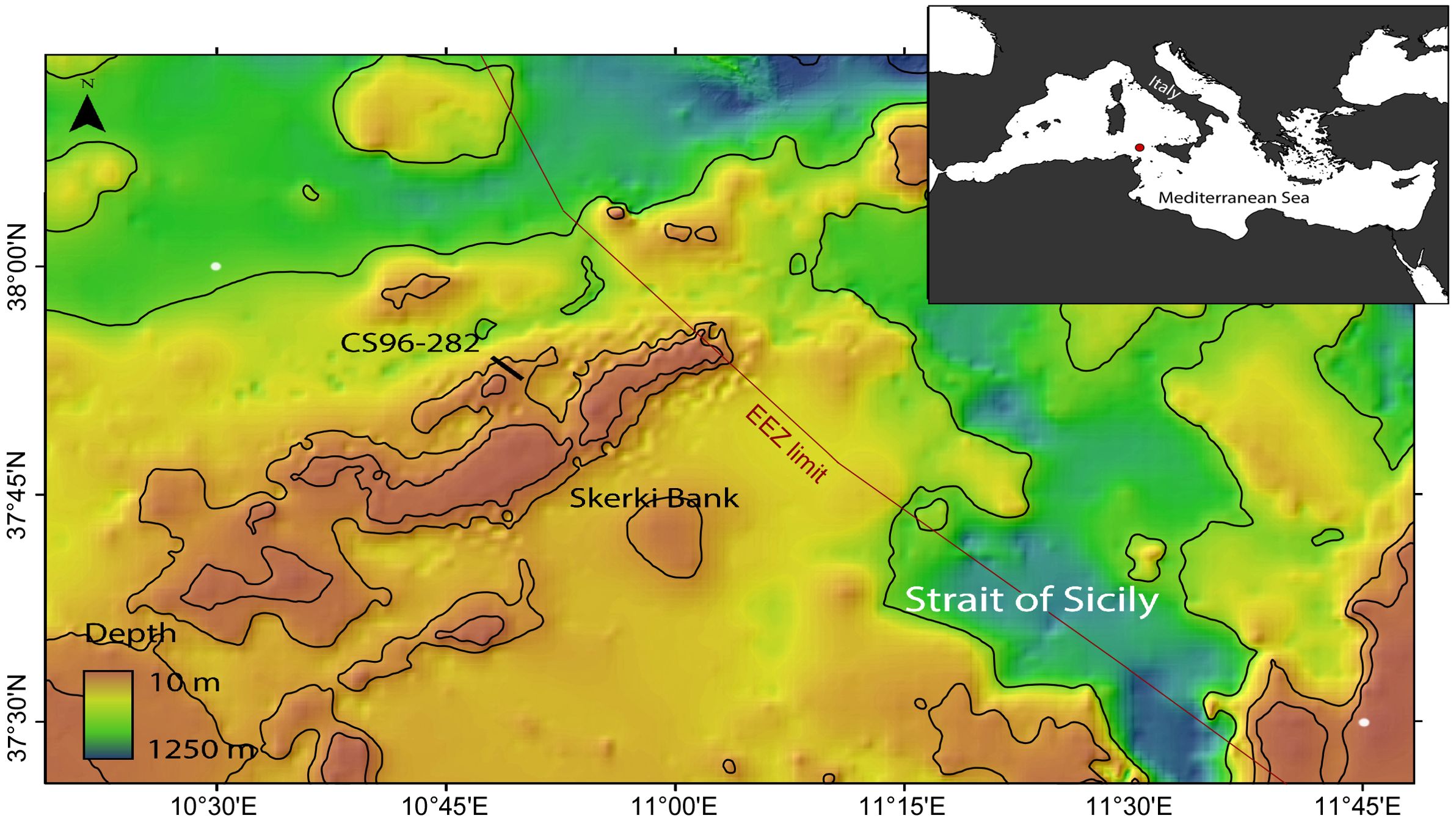
Figure 1. Map of the Strait of Sicily showing the Skerki Bank with location of the dredging station discussed in the paper (line); isobaths 100 m, 200 m, and 500 m from EMODnet Digital Bathymetry (DTM) 2022; inset, situation within the Mediterranean Sea: courtesy of Giorgio Castellan (CNR).
For preparing illustrations, shells were cleaned by soaking for ca. 15 min in a 10% aqueous solution of sodium lauryl sulphate (a pH-neuter detergent) and then mildly sonicated in water. Light photographs were made using a Nikon DXM camera mounted on a Nikon SMZ1000 stereomicroscope, taking a series of views focused on distinct planes which were assembled using CombineZ stacking software (Hadley, 2006). SEM micrographs were made using a JEOL SM 6490LV scanning electron microscope at University of Málaga. For this, specimens were dried, mounted on conductive copper tape, and coated for SEM observation.
The CS96 collection of bio-geological samples is currently stored in the Institute of Marine Sciences (ISMAR-CNR), Bologna. The type material is hosted in the Museum of Evolution (formerly Zoology) of the University of Bologna (code MZUB), and some paratypes in the Museum National d’Histoire Naturelle of Paris (code MNHN).
The accepted names for the identified species were checked against MolluscaBase (MolluscaBase eds., 2024).
4 Results
4.1 The faunal list
The list of species found in this sample amounts to 110 species, of which nine could not be identified further than the genus level. There are 41 records (Figures 2A–N, 3A–R, 4A–P, 5A–J, 6A–L, 7A–J, 8A–N) new to Tunisian waters, and of these three are believed to represent new species which are described in the taxonomic notes. Only shells were found, no living specimens. Asperarca secreta La Perna, 1998, was the most abundant species, and the family Arcidae (which comprises epifaunal species) was represented by seven species. Neolepton sulcatulum, Acar clathrata, Granulina melitensis, Skeneoides exilissima, and Anatoma sp. were also abundant. The complete list is presented in Table 1.

Figure 2. Species of gastropods (subclass Vetigastropoda) found on Skerki Bank (CS96 sta. 282) and new to the Tunisian fauna. (A, B) Emarginula pustula Thiele, 1913 (3.0 mm). (C, D) Emarginula tenera Locard, 1891 (2.1 mm). (E, F) Anatoma sp. (1.5 mm). (G) Anatoma sp., SEM micrograph in apertural view. (H) apical view, same shell as E-F. (I, J) details of apical whorl and protoconch, same shell. (K, L) Scissurella nauarchorum n. sp., Holotype (0.7 mm). (M) SEM micrograph of the protoconch of a paratype. (N) SEM micrograph of another paratype. Scale bars are 100 µm.
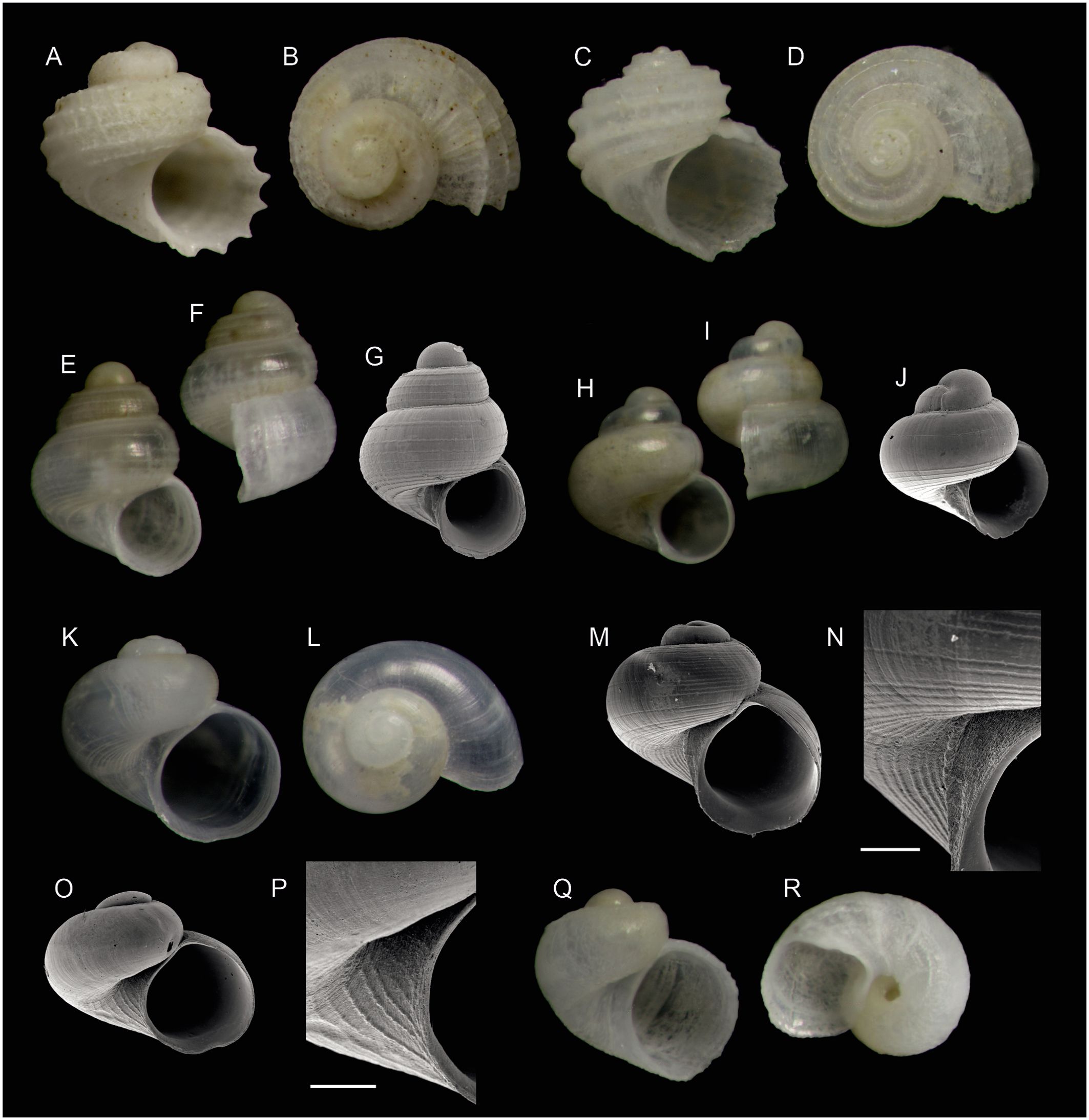
Figure 3. Species of gastropods (subclass Vetigastropoda, continued) found on Skerki Bank (CS96 sta. 282) and new to the Tunisian fauna. (A, B) Parviturbo fenestratus (Chaster, 1896) (1.1 mm). (C, D) Pseudorbis granulum (Brugnone, 1873)(1.2 mm). (E, F) Lissotesta gittenbergeri (van Aartsen & Bogi, 1988) (0.7 mm). (G) Same shell as (E), SEM micrograph. (H, I) Lissotesta turrita (Gaglini, 1987) (0,7 mm). (J) Lissotesta turrita, SEM micrograph of another shell (0.6 mm). (K, L) Dikoleps semistriata n. sp., holotype (0.9 mm). (M, N) Dikoleps semistriata n. sp., SEM micrograph of the holotype and detail of the umbilical area. (O, P) Dikoleps templadoi Rubio, Dantart & Luque, 2004, SEM micrograph and detail of the umbilical area. (Q, R) Rugulina monterosatoi (van Aartsen & Bogi, 1987) (0.7 mm). Scale bars are 100 µm.

Figure 4. Species of gastropods (subclass Caenogastropoda) found on Skerki Bank (CS96 sta. 282) and new to the Tunisian fauna. (A, B) Alvania zetlandica (Montagu, 1816)(2.8 mm). (C, D) Talassia dagueneti (de Folin, 1873)(1.9 mm). (E, F) Curveulima beneitoi Peñas & Rolán, 2006 (1.2 mm). (G, H) Vitreolina cionella (Monterosato, 1878)(1.1 mm). (I, J) Epitonium finitimum (Monterosato, 1890) (2.2 mm). (K, L) Punctiscala cerigottana (Sturany, 1896) (2.8 mm). (M, N) Murexsul aradasii (Monterosato, 1883) (4.0 mm). (O, P) Chauvetia recondita (Brugnone, 1873) (4.9 mm).
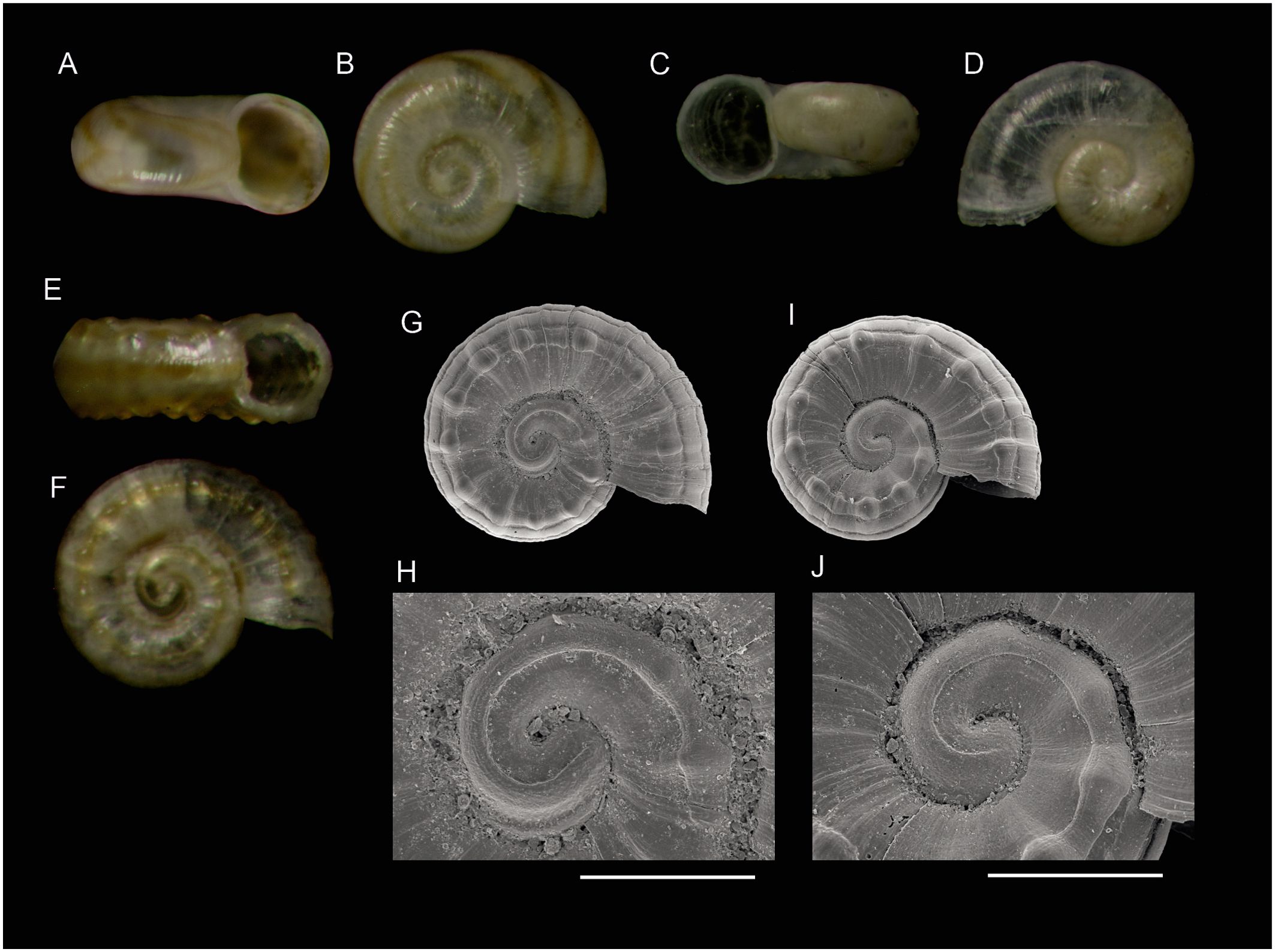
Figure 5. Species of gastropods (subclass Heterobranchia, family Omalogyridae) found on Skerki Bank (CS96 sta. 282) and new to the Tunisian fauna. (A, B) Omalogyra simplex (O. G. Costa, 1861)(0.7 mm). (C, D) Retrotortina fuscata Chaster, 1896 (0.7 mm). (E, F) Ammonicera consilii n. sp., holotype (0.5 mm). (G, H) Ammonicera consilii n. sp., SEM micrograph of the holotype (0.5 mm) and closeup of the protoconch. (I, J) Ammonicera consilii n. sp., paratype (0.4 mm), SEM micrograph and closeup of the protoconch. Scale bars are 100 µm.
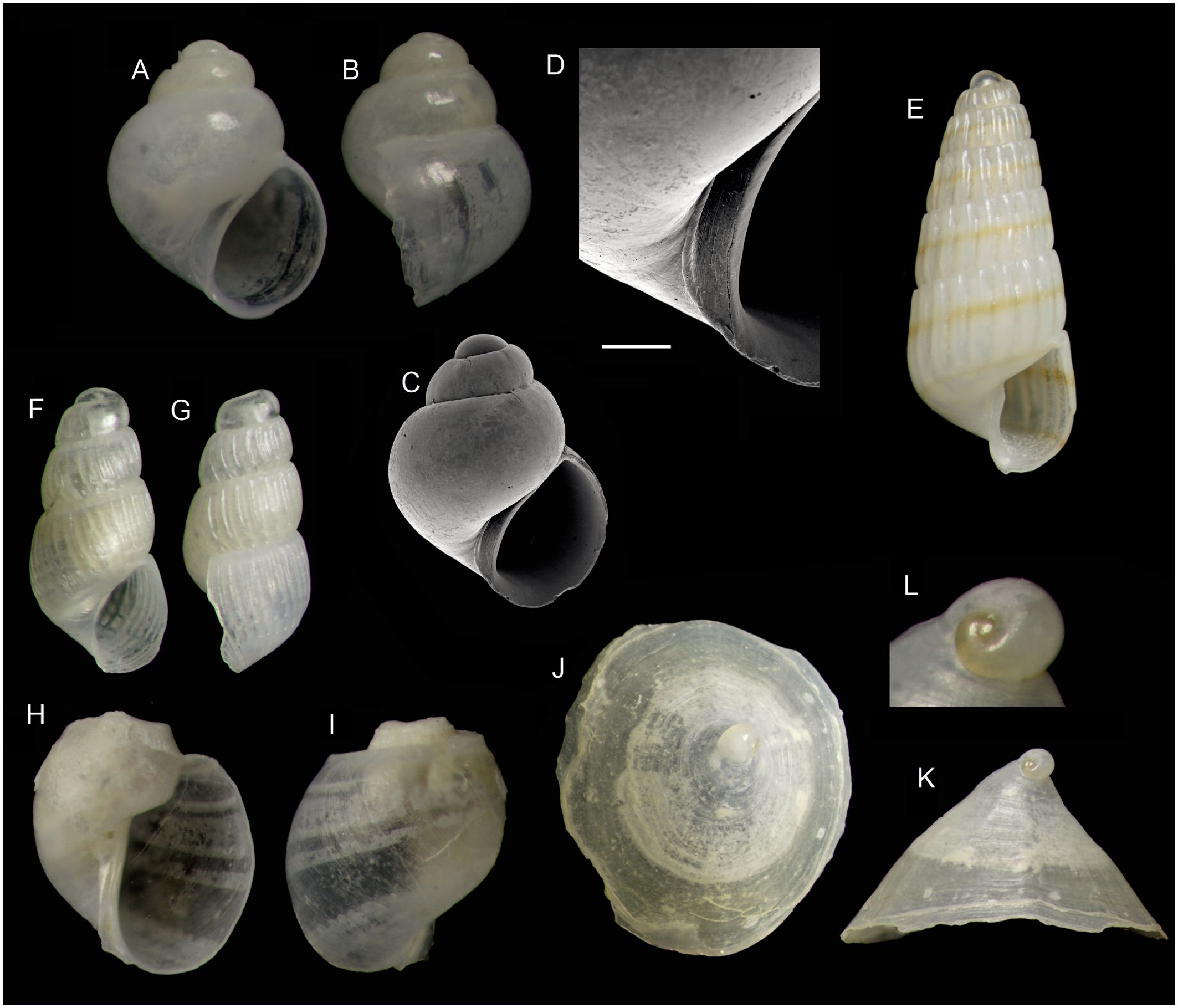
Figure 6. Species of gastropods (subclass Heterobranchia) found on Skerki Bank (CS96 sta. 282) and new to the Tunisian fauna.(A, B) Rissoella angeli Manousis, 2021 (1.0 mm). (C, D) same specimen, SEM micrograph and detail of the umbilical area. (E) Odostomella bicincta (Tiberi, 1868) (3.0 mm). (F, G) Parthenina rinaldii (Micali & Nofroni, 2004) (1.3 mm). (H, I) Colpodaspis pusilla M. Sars, 1870 (1.1 mm). (J–L) Tylodina duebenii Lovén, 1846, and detail of the heterostrophic protoconch. Scale bar is 100 µm.
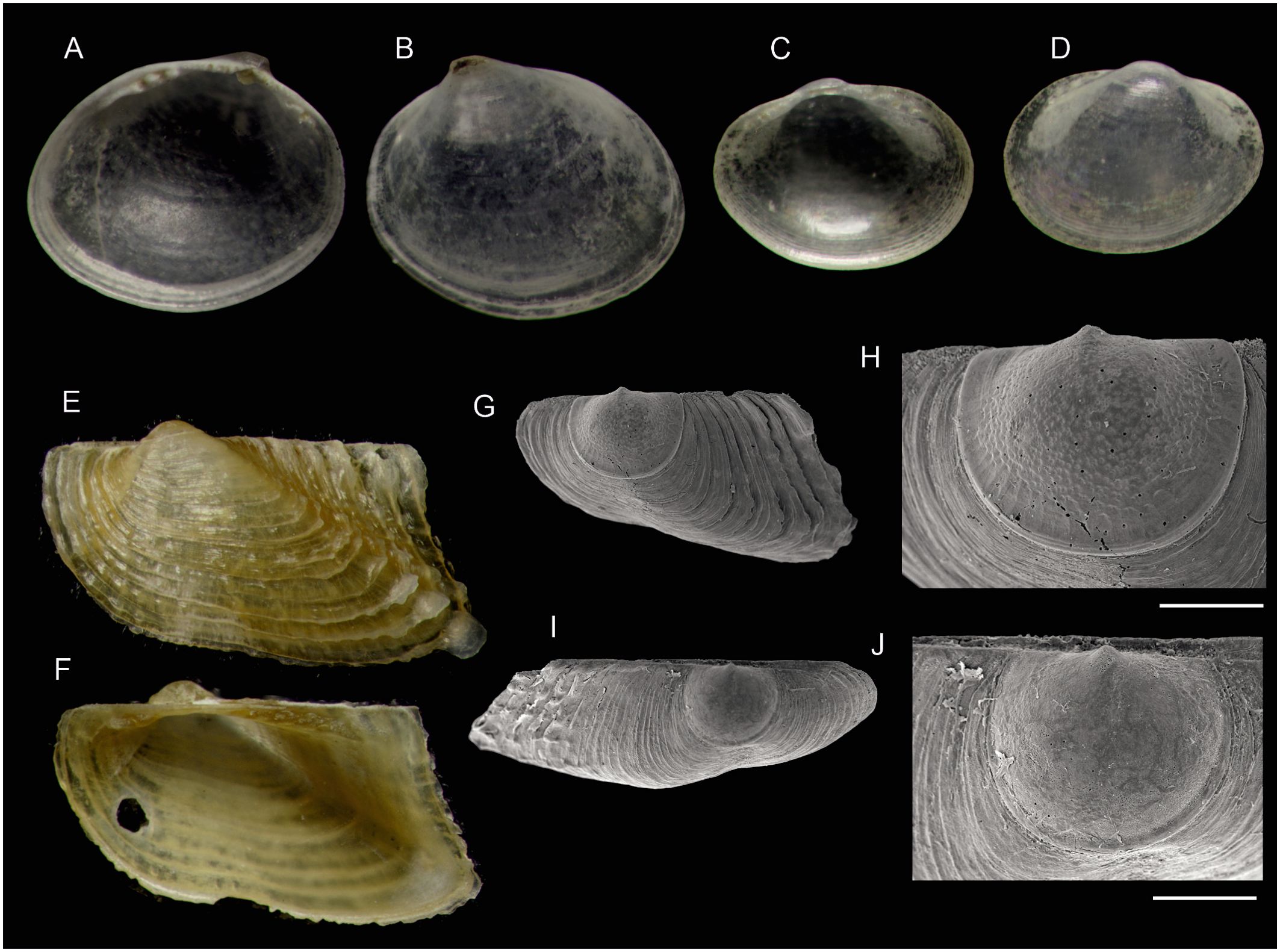
Figure 7. Species of bivalves (subclasses Protobranchia and Pteriomorphia) found on Skerki bank (CS96 sta. 282) and new to the Tunisian fauna. (A, B) Nucula perminima (Monterosato, 1875) (1.2 mm). (C, D) Microgloma cf. guilonardi (Hoeksema, 1993) (0.8 mm). (E, F) Asperarca secreta La Perna, 1998 (2.2 mm). (G, H) Asperarca secreta, SEM micrograph of a left valve and detail of the prodissoconch. (I, J) Juvenile Asperarca nodulosa (O. F. Müller, 1776), SEM micrograph of a left valve from sta. 282 and detail of the prodissoconch, for comparison. Scale bars are 100 µm.

Figure 8. Species of bivalves (subclass Pteriomorphia, continued, and subclass Heterodonta) found on Skerki bank (CS96 sta. 282) and new to the Tunisian fauna. (A, B) Crenella arenaria Monterosato, 1875 (1.6 mm). (C, D) Heteranomia squamula (Linnaeus, 1758) (1.6 mm). (E, F) Notolimea clandestina Salas, 1994 (1.0 mm). (G–J) Kelliopsis jozinae van Aartsen & Carrozza, 1997 (1.6 mm). (K–N) Neolepton sulcatulum (Jeffreys, 1859), inside and outside of right valve and detail of the hinge of both valves (1.8 mm).
4.2 Taxonomic notes and descriptions of new species
Anatoma sp.
A small species belonging to the genus Anatoma is represented in our material by more than 50 shells (Figures 2E–J). It differs from A. aspera (Philippi, 1844), the most common Mediterranean species of the genus, in being half the size (c. 1.5 mm in diameter vs. 3 mm) with a less elevated profile, in having one whorl less (2 ⅓ vs. 3 ¾) and in having the selenizone placed very high on the first teleoconch whorl. Anatoma micalii Geiger, 2012, is similar in size and shape to the Skerki species but differs markedly in having a very raised selenizone and a much coarser sculpture on the upper part of the last whorl (30–40 coarse axial ribs vs. 60–70 fine riblets). Anatoma tenuisculpta G. Seguenza, 1880, as figured in Nappo et al. (2024), is definitely not this species; it is larger with a much finer sculpture and with equal density of ribs above and below the selenizone. Conversely, Anatoma cf. tenuisculpta as figured in Romani et al. (2018) from Lastovo, Croatia, is more similar to our Anatoma sp. with adapical ribs fewer than abapical, but somewhat larger. The Skerki specimens possibly represent an undescribed species, but because the configuration of the protoconch, the early teleoconch prior to the selenizone, and the umbilicus are similar to those in A. aspera and A. micalii, we refrain from describing a new taxon in the absence of further evidence, e.g., molecular data.
Scissurella nauarchorum n. sp.
urn:lsid:zoobank.org:act:88BCF841-A739-4641-854A-2D2993F30A88
Type material. Holotype: Tunisia • 1 shell (diameter 0.5 mm, Figures 2K, L); Skerki Bank; 37°53′05.8″N, 10°49′42.7″E; 112 m; R/V “Urania” CS96 sta. 282; MZUB 60351. Paratypes: Tunisia • 2 shells (Figure 2M); same data as for holotype; MZUB 60352 • 1 shell (Figure 2N); same data as for holotype; MNHN IM-2012-25392.
Description: Shell minute, with a very flat spire, reaching 0.9 mm in diameter with 2 ½ whorls. Protoconch with a little more than one whorl, exposed part 170 μm in diameter, with a blunt spiral keel on its central part and with 30–35-min axial threads between the suture and the keel, bulging at their intersection with the keel to form a spiral row of knobs, and then thinning out and extending somewhat towards the nucleus; those knobs on the summit of the keel become more crowded and finally fused on the final part of the protoconch. Protoconch–teleoconch limit clearly demarcated by a very fine rim.
Teleoconch I (prior to the onset of the selenizone) slightly more than one whorl, with ca. 13–15 very strong and raised axial ribs, thinning out adapically before reaching the suture; the intervening spaces approximately five times as broad as the ribs and ornamented with minute, irregularly anastomosing axial threads; no spiral sculpture.
Teleoconch II 0.25 whorls, with one more axial rib and weak axial threads, no spiral sculpture. Shoulder angled down from the coiling axis. Base rounded, with the 13–15 axial ribs continued, fine irregular threads between axial ribs. Umbilicus open with marginal carina, weak axial lines on base. Selenizone, slit above periphery, moderately keeled. Aperture subquadratic, edge slightly projecting. Animal unknown.
Etymology: From the Latin nauarchus (ship commander). This species honours the veteran Captains of CNR research vessels “Bannock” and “Urania” (Emanuele Gentile, Pasquale Guida, Nicolangelo Lembo, Vincenzo Lubrano, Nicola Scotto di Carlo) who over the years where on duty for the missions to explore the deep Mediterranean Sea.
Remarks and comparisons: This minute species most resembles Scissurella azorensis Nolt, 2008, which was originally described from the Azores, and later reported from several Mediterranean localities by Micali and Geiger (2015) and Raveggi et al. (2021). Both are distinguished from the very similar Sinezona semicostata Burnay et Rolán, 1990, originally described from the Cape Verde islands and also reported from the Mediterranean, by having less numerous and stronger axial ribs on the teleoconch, and ca. 30 axial threads of which the middle part is bulging to form small beads aligned in the spiral direction along a crest (Figure 2M), instead of with 14–16 strong, parallel-sided ribs interrupted adapically on Sinezona semicostata.
Our shells from Skerki are deemed conspecific with those from Linosa (Italian part of the Sicily Channel) figured by Micali and Geiger (2015) and from the Adriatic Sea (Tremiti Is.) by Raveggi et al. (2021). The protoconchs illustrated in Nolt (2008) (figure 1, lower row) show distinct riblets along the crest only and a broad smooth band, whereas both in our specimens from Skerki (we have two more protoconchs viewed in SEM) and in the specimen illustrated by Micali and Geiger, the nodes on the crest are coalescent and, from there, there are fine riblets running in the direction of the opposite suture; therefore the surface is not smooth. Teleoconch sculpture also differs: even the specimen with coarsest sculpture illustrated in Nolt (2008) has ca. 25 ribs on the last teleoconch whorl, and those tend to fade towards the umbilicus, whereas the specimen from Linosa in Micali and Geiger has at most 14 ribs, the specimen from Tremiti in Raveggi et al. has ca. 15 ribs and our holotype has 13. Therefore, we consider that the Mediterranean Scissurella “azorensis” should be recognized as a distinct species. Scissurella azorensis is not recorded from the well-explored Alboran Sea, and it does not make sense that this would be the only shared scissurellid species between the Central Mediterranean and the Azores, situated some 6,000 km apart.
Parviturbo fenestratus (Chaster, 1896)
This species (Figures 3A, B) was originally believed to be endemic to the Strait of Gibraltar. It has been reported from southern Italy (Giannuzzi-Savelli et al., 1994: fig. 364), but that figure is reproduction of figure 1B in Warén (1992) representing a specimen from Ceuta; therefore, that report is unsupported. Nevertheless, Warén (1992) also reported 17 shells collected by R/V “Porcupine” in 1870 on Adventure Bank, 167 m, in the Italian part of the Sicily Channel. Parviturbo elegantulus (Philippi, 1844), also reported from southern Italy, is larger (1.7 mm) with more numerous spiral cords and the keel surrounding the umbilicus less pronounced. Pseudorbis granulum (Brugnone, 1873) (Figures 3C, D) is similar in size and shape to Parviturbo but completely lacks axial sculpture. Giacobbe et al. (2022) claimed that Mediterranean records of Parviturbo fenestratus could be misidentified, but we are confident in our identification: Parviturbo sertum Tabanelli, Bertaccini, Bertamini, Bongiardino, Gardella and Petracci, 2019, differs in having almost twice as many axial riblets (ca. 60 on the last whorl in the holotype, vs. 32 in the syntype of P. fenestratus illustrated by Giacobbe et al. and ca. 30 in our illustrated specimen).
Dikoleps semistriata n. sp.
urn:lsid:zoobank.org:act:0668476C-EBF2-4487-AAAA-55A6B1630647
Type material. Holotype: Tunisia • 1 shell (diameter 0.9 mm, Figures 3K–N); Skerki Bank; 37°53′05.8”N, 10°49′42.7”E; 112 m; R/V “Urania” CS96 sta. 282; MZUB 60353. Paratypes: Tunisia • 10 shells; same data as for holotype; MZUB 60354 • 10 shells; same data as for holotype; MNHN IM-2012-25393.
Description: Shell minute, thin and translucent, whitish, of 0.91 mm of maximum diameter and 0.89 mm of maximum height (h/d = 0.97), with 2 ½ convex whorls separated by a deep suture. Protoconch of ¾ whorl and ca. 235 μm of diameter, smooth. Teleoconch of about two whorls, sculptured on the last whorl with approximately 30 spiral grooves and flexuous growth lines; grooves irregularly spaced on the middle part of the last whorl, some of them interrupted to give way to a smooth surface, those surrounding the umbilicus more marked and equal to interspaces. Aperture rounded, slightly angled adapically, prosocline, comprising approximately 60%–65% of height; body whorl approximately 95% of shell height. Umbilicus deep and narrow, delimited by a thick ridge.
Etymology: the name alludes to the incomplete extent of striation on the adapical part of the shell.
Remarks and comparisons: Only three species of Dikoleps are reported to have a spiral sculpture on the adapical part of the shell: the Atlantic Dikoleps cutleriana (Clark, 1849), which is twice as large and has ca. 30 closely set cordlets regularly covering the entire teleoconch, and two Mediterranean species, Dikoleps marianae Rubio, Dantart and Luque, 1998, originally described from the Alboran Sea, and D. rolani Rubio, Dantart and Luque, 1998, originally described from the Catalan coast. All three species and also D. semistriata n. sp. share the character of having the umbilicus delimited by a thick, beaded spiral cord. Dikoleps marianae is similar in size to the Skerki specimens but has ca. 40 closely and regularly set cordlets on the teleoconch, separated by broad interspaces within which a granular microsculpture can be seen (Rubio et al., 1998: fig. 19). The Skerki specimens are unique in having instead spiral grooves separated by broader interspaces, some grooves interrupted on the last whorl so as to leave still broader smooth interspaces between the remaining ones. Dikoleps rolani has never been recorded after its original description. In the words of Rubio et al. (1998), it differs from D. marianae mainly by its more depressed spire, teleoconch sculpture with fine spiral grooves mainly concentrated on the basis and around the umbilicus, and by its wider umbilicus; these differences also hold in comparison with D. semistriata n. sp. The specimen (0.9 mm in diameter) from Siracusa, Sicily, illustrated by Giannuzzi-Savelli et al. (1994: fig. 353b) as D. cutleriana is probably the present species, but the resolution of the light photograph does not allow to see the striation pattern which could distinguish it from D. marianae.
Curveulima beneitoi Peñas and Rolán, 2006
This species (Figures 4E, F) was originally described from the Alboran Sea but has now been reported from southern Italy (Scuderi et al., 2023). Among the many small eulimids recorded in the Mediterranean Sea, it is readily recognized by having the first three whorls at a slight angle with the following ones.
Ammonicera consilii n. sp.
urn:lsid:zoobank.org:act:24A44455-5DCD-4F33-A7D9-0058A01F5B38
Type material. Holotype: Tunisia • 1 shell (diameter 0.5 mm, Figure 5E–H); Skerki Bank; 37°53’05.8”N, 10°49’42.7”E, 112 m; R/V “Urania” CS96 sta. 282; MZUB 60357. Paratypes: Tunisia • 2 shells; same data as for holotype; MZUB 60358 • 1 shell (Figures 5I, J); same data as for holotype; MNHN IM-2012-25394.
Description: Shell minute, planispiral, reaching 0.6 mm in diameter with two whorls. Protoconch with less than one whorl, with a continuous transition to teleoconch. Protoconch sculpture with a broad ridge occupying ca. 1/3 of the exposed part of the whorl, bordering the suture; this ridge sharply delimited from a broad flat surface surrounding the nucleus. Under very high magnification, this ridge appears corrugated with indistinct scars, predominantly oriented in the spiral direction. Teleoconch of a little more than one whorl. Periphery rounded with narrow spiral sulci visible only under the SEM. Adapical and abapical surfaces demarcated from the periphery by one such sulcus, and bearing a flat, well-defined nodose spiral cord in prolongation of the ridge on the protoconch and, between this cord and the suture, a broad depression on which the nodes are prolonged by ill-defined folds which do not reach the suture; the nodose cord is situated at the outer 2/3 of the whorl in apical and abapical views, and the interval between nodes on this cord is equivalent to the diameter of the nodes. Aperture rounded, simple. Colour cream, with three brown lines, one on the periphery and one on the nodose cord of each side.
Etymology: This taxon is dedicated to the Italian National Research Council (CNR, Consiglio Nazionale delle Ricerche; in Latin Consilium) for its pivotal role to promote to investigation of the Mediterranean Sea from lagoons down to its deepest parts, also providing continuous support to research vessels since the ‘60s of the past century.
Remarks and comparisons: For a long time, the genus Ammonicera was believed to be represented in the Mediterranean by only two species, Ammonicera rota (Forbes and Hanley, 1850) with coarse “radial” (actually, axial as opposed to spiral) ribs and A. fischeriana (Monterosato, 1869) with ribs attenuated or absent. This view was challenged by Oliver and Rolán (2015) who recognized seven Mediterranean species, distinguished by details which mostly require observation under the SEM for a correct identification. While in most gastropods there is a radical change in the sculpture from protoconch to teleoconch, in Ammonicera, the protoconch sculpture is not demarcated from that of the teleoconch and its features may be continued, making it difficult to pinpoint the limit. In the taxonomic treatment of Oliver and Rolán (2015), the sculpture of the protoconch is a feature of utmost importance, with a single narrow spiral groove on A. rota and two narrow grooves in A. fischeriana and others. Based on this and other characters, all previous Mediterranean records of “Ammonicera rota” are reassigned to newly described species and the distribution of the “real” Ammonicera rota restricted to the Atlantic, from Norway to the strait of Gibraltar. Compared with Ammonicera consilii n. sp., Ammonicera columbretensis Oliver and Rolán, 2015, differs in having a single, definite narrow groove in the protoconch (vs. a broad ridge surrounding a depressed area), in having distinct “radial” ribs which reach the suture, and a marked keel overrun by those ribs, situated very close to the periphery (vs. situated at the outer 2/3); the interval between ribs at their intersection with the spiral cord is twice the thickness of the ribs (vs. subequal). The colour is also different, uniformly reddish brown (vs. with three lines). In Ammonicera andresi Oliver and Rolán, 2015, the protoconch was described as having two spiral grooves, but as shown in figure 18 C, E, F of Oliver and Rolán (2015), it is better described as having two narrow ridges, one in the middle part of the whorl and another one running along the suture, each of which are bordered in the inner side by a sharp, very narrow groove (vs. a single thick ridge on A. consilii n. sp.). The teleoconch sculpture is also very different in A. andresi, with the spiral cords and “radial” ribs very narrow, forming, in the words of Oliver and Rolán, a pattern “reminiscent of the slots of a roulette”. Ammonicera nodulosa Oliver and Rolán, 2015, differs in the protoconch which has a pattern of two narrow spiral grooves similar to those of A. fischeriana, and in the sculpture which comprises much more narrow cords and ribs, the ribs separated by twice their breadth and markedly continued over the periphery. Ammonicera arrondoi Oliver and Rolán, 2015, is distinguished by the protoconch which has two narrow grooves, the outer one partly concealed by the suture and distinctly partitioned by minute lamellae; the teleoconch sculpture is somewhat similar to that of A. consilii n. sp., but the nodes along the cord are smaller and more numerous (more than 20 on the last whorl vs. 13–15). Of the older names reported in the synonymy of A. rota, Ammonicerina pulchella O. G. Costa, 1861, was described by Costa (1861: 71, pl. 12 figures 1a, b) from “rocce coralligene delle coste settentrionali dell’ Affrica,” which could be Skerki bank, or the coast between Tabarka and La Calle, where red coral was exploited at his time. Without a neotype designated and examined under SEM, it is not possible from the figure to recognize a particular species with certainty, but the indication of “color rosso vivace quasi sanguigno” suggests an older name for A. columbretensis, if any. Homalogyra rota ssp. margaritifera Fekih and Gougerot, 1977, is also difficult to identify, but the position of the sculpture elements on the drawing, with a thin keel on the median part of the whorls instead of the row of large, flat beads in A. consilii n. sp. precludes that the could be conspecific and suggests that ssp. margaritifera represents a coarsely sculptured form of Ammonicera fischeriana.
We are convinced that the specimen from Capraia (no indication of depth) figured by Palazzi (1988: figure 9, 0.6 mm in diameter according to scale bar) belongs to Ammonicera consilii n. sp.; although not quite distinct on the image, the broad ridge merging into the nodulous spiral cord can be recognized, and the nodulous cord is positioned on the outer 1/3 of the whorl like on our specimens from Skerki.
Rissoella angeli Manousis, 2021
This rather nondescript shell (Figures 6A–D) resembles superficially some small rissooideans such as Obtusella intersecta (Wood, 1857) also present in the sample, and other small heterobranchs in the genus Rissoella such as R. opalina (Jeffreys, 1848). The distinct ridge bordering the umbilicus readily distinguishes it from other Rissoella species. This species was reported from the Southern Aegean Sea by Manousis et al. (2021) and Villari and Scuderi (2022); this is the first report outside the type locality.
Microgloma cf. guilonardi (Hoeksema, 1993)
Originally described from the North Sea, this species has been recorded from Malta (Cachia, 1995) and the Italian Tyrrhenian Sea (Hoeksema, 2000) but the authenticity of the Mediterranean occurrences is disputed. La Perna (2003) noted that “Microgloma” guilonardi has few elongated lamellar teeth, similar to those of Phaseolus Monterosato, 1875, and considered the Mediterranean records as based on misidentifications. Admittedly, our specimens (Figures 7C, D) have more teeth (four posterior, three anterior) than the North Sea specimens. They are certainly conspecific with the species reported by Cachia (1995) and Hoeksema (2000), but their identification calls for further studies on taxonomy and distribution.
Asperarca secreta La Perna, 1998
This species (Figures 7E–H) is the most abundant in this Skerki bank sample. It is distinguished from the widespread Asperarca nodulosa (O. F. Müller, 1776) by its size which hardly exceeds 4 mm in adult stage (La Perna, 1998), by the colour pattern with a whitish quadrant in the anterior 1/3 and above all by the prodissoconch, which is larger and bears a distinct peripheral rim (compare Figures 7G, H with Figures 7I, J).
Notolimea clandestina (Salas, 1994)
This is one of the smallest extant bivalves (Figures 8E, F), originally believed to be endemic to the Strait of Gibraltar, but later found in distinct localities of Western Europe (Hoeksema and Janse, 2002) where it is probably subfossil. It was described again from the Sicily Channel as Limopsis sebastianoi Cecalupo, 1995, from nearby island of Lampedusa in Italian waters.
5 Discussion
There are few occasions in the Mediterranean Sea where the faunal list of a single sample amounts to more than 100 species. Examples are found in deep bioclastic gravel bottoms of the Alboran Sea: 156 species on Djibouti Bank (R/V “Cornide de Saavedra” haul BT04, 36°21.06′N, 03°58.07′E, 365-m depth, Gofas et al., 2014b), 173 species on the Alboran platform (R/V “Isla de Alboran” BV21, 36°00.40′N, 02°55.32′W, 101 m, unpublished). Most of the species reported herein are small, 12 of them not even surpassing 1 mm in adult stage, but this is a general feature of Mollusca (Bouchet et al., 2002) and does not reflect a trend in this particular area. Altogether, this sample from the CS96 cruise raises the census of Tunisian marine molluscs from 767 to 808, clearly giving the perspective of further increase when the deeper part of the jurisdictional waters become properly explored.
Our sample is a thanatocoenosis (dead shell assemblage); therefore, the potential presence of species no longer living in the Mediterranean cannot be discarded a priori. Where such shelly assemblages contain subfossil components, those are commonly of Pleistocene glacial age and pertain to deeper water situations, and at times also contain taxa still living currently in the Mediterranean Basin. For instance, this is likely the case for shell assemblages reported previously from the Mediterranean, including the Strait of Sicily (e.g., Taviani et al., 2023; Amati et al., 2024, with references therein). However, we are convinced that our circalittoral assemblage, apparently deprived of any species unambiguously attributable to any fossil deposit, is largely representing the living community inhabiting the Skerki Bank. Clearly, there is a need for extensive biological surveys in these Tunisian waters to collect living specimens for anatomical description and genetic characterization, as well as habitat assessment.
Of the 101 identified species, including the new records, virtually all are shared with the Italian fauna (Renda et al., 2022). Even among the new species, at least two, and possibly three (see notes above), were reported from Italian waters under another name. This indicates a great homogeneity in the fauna of the Sicily Channel as a whole, where areas like Adventure Bank in the northern part of the channel are expected to provide similar habitats and are within reach for dispersal even by the species with poor dispersal capacity. The number of species shared with the Maltese fauna (Cachia et al., 2019; Cachia, 2024) is also high, as could be expected considering the vicinity and presence of similar habitats. More striking is the presence of species such as Parviturbo fenestratum and Notolimea clandestina, once believed to be endemic of the Strait of Gibraltar and present in the Sicily Channel without any report in intermediate locations. The habitat factor, with strong bottom currents and extensive occurrence of deep hard bottoms and coarse bioclastic gravels devoid of mud, may explain those apparently disjoint distributions.
The absence of alien species is noteworthy, taking into account that there are 35 species (i.e., 5% of the total marine Mollusca) known as aliens in the Tunisian fauna (MolluscaBase eds., 2024). We do not know of any proved example of aliens being present in a species-rich deep shelf environment such as that sampled here, and this may reflect the resilience of a well-structured habitat, as well as the inadequacy of the introduced species, most of them proper to shallow waters or lagoons.
A renewal of interest in taxonomic studies and marine biodiversity is currently in the international agenda of many countries at a global scale. The production of taxonomic lists of marine taxa, the presence of charismatic taxa, the connectivity, and the occurrence of new or rare species are key elements for subsequent actions to eventually promote measures at governing at best the marine habitats up to their protection in the Mediterranean Sea (e.g., Boero et al., 2017; Danovaro et al., 2020; Fanelli et al., 2021; Schiaparelli et al., 2021).
Skerki Bank shares many features with the offshore Natura 2000 site “Alboran platform” studied by one of us (Gofas et al., 2014a). The astounding species richness, more in the circalittoral bioclastic gravels than in the rhodoliths beds proper, with an unusual concentration of species usually deemed “extremely rare”, make these areas remarkable. Another natural value shared between Alboran and Skerki is the presence of large populations of the red coral Corallium rubrum Linnaeus, 1758, which is the object, legally or not, of a sustained exploitation (Jaziri et al., 2017).
We believe that the richness in rare species (including the taxa here described as new) warrants that the Skerki Bank be considered for adequate consideration of its unique biological heritage by the Tunisian authorities for the proper governance of this key sector of the Mediterranean Sea.
Data availability statement
All data regarding new records and species are included in this paper. Illustrations of these and other specimens will be posted on the World Register of Marine Species www.marinespecies.org, and specimens will be deposited at Museo di Zoologia, Università di Bologna, where they will be available for study upon request.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MA: Data curation, Conceptualization, Writing – review & editing. SG: Investigation, Writing – original draft, Methodology, Writing – review & editing. MT: Supervision, Methodology, Writing – review & editing, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for open-access article processing charges is provided by the research group RNM141 of Junta de Andalucía, Spain.
Acknowledgments
We thank the Captain, Officers, Crew, and scientific staff aboard RV Urania during cruise CS96 for their efficient cooperation at sea. Alessandro Remia (Ismar-CNR) is acknowledged for his key support to all phases of cruise preparation and execution. Giorgio Castellan kindly prepared Figure 1. This paper is in memory of Nicola Scotto di Carlo (“Scottone”) doyen of the Italian scientific seafaring. This is Ismar-CNR, Bologna, scientific contribution n. 2093, and pertains to the Biodiversity Science Gateway of the National Biodiversity Future Center funded by the Italian recovery and resilience Plan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer LR declared a shared affiliation with the author SG to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aissaoui C., Galindo L. A., Puillandre N., Bouchet P. (2017). The nassariids from the Gulf of Gabès revisited (Neogastropoda, Nassariidae). Mar. Biol. Res. 13, 370–389. doi: 10.1080/17451000.2016.1273528
Amati B., Oliverio M., Taviani M. (2024). Rissoidae (Mollusca, Gastropoda, Rissooidea) from taphocoenotic assemblages of the Strait of Sicily (Mediterranean Sea) with the description of two new species. Zootaxa 5405, 526–544. doi: 10.11646/ZOOTAXA.5432.1.2
Appeltans W., Ahyong S., Anderson G., Angel M., Artois T., Bailly N., et al. (2012). The magnitude of global marine species diversity. Curr. Biol. 22, 2189–2202. doi: 10.1016/j.cub.2012.09.036
Boero F., Foglini F., Fraschetti S., Goriup P., MacPherson E., Planes S., et al. (2017). CoCoNet: towards coast to coast networks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential. Sci. Res. Inform. Technol. 6(supplement), 1–95. doi: 10.2423/i22394303v6SpI
Bonfitto A., Bigazzi M., Fellegara I., Impiccini R., Gofas S., Oliverio M., et al. (1994). Rapporto scientifico sulla crociera DP’91 (Margine orientale della Sardegna, Mar Mediterraneo). Boll. Malacol. 30, 129–140.
Bouchet P., Lozouet P., Maestrati P., Heros V. (2002). Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc 75, 421–436. doi: 10.1046/j.1095-8312.2002.00052.x
Boyer F., Renda W., Pagli A. (2021). About the identity of Granulina pusaterii Smriglio & Mariottini 2003 (Volutoidea: Granulinidae) from the Tunisian Plateau. Ege J. Fish. Aquat. Sc. 38, 461–466. doi: 10.12714/egejfas.38.4.07
Cachia C. (1995). On the occurrence of Phaseolus guilonardi Hoeksema 1993 (Fam. Phaseolidae Scarlato & Starobogatov 1971) in the Mediterranean. Boll. Malacol. 31, 37–38.
Cachia C. (2024). Updates to the marine malacofauna (Mollusca: Gastropoda) of Malta. Bull. Natl. Mus. Nat. Hist. Malta 1, 17–29.
Cachia C., Mifsud C., Sammut C. I., Sammut P. M. (2019). An annotated and bibliographical check-list of the marine Mollusca of the Maltese Islands Vol. xi (Żejtun, Malta: Sunland Printers), 266 pp.
Carpenter W. B. (1872). Report on scientific researches carried on during the months of August, September, and October 1871, in H.M. Surveying ship ‘Shearwater’. Proc. R. Soc Lond. 20, 535–644. doi: 10.1098/rspl.1871.0100
Cebrian D., Requena S.(2015). Sicily Channel/Tunisian Plateau: Topography, circulation and their effects on biological components (Tunis: UNEP-MAP-RAC/SPA). Available online at: https://www.rac-spa.org/sites/default/files/doc_open_seas/sicily_Tunisian_oceanography_abnj.pdf (Accessed June 1, 2024).
Cecalupo A., Buzzurro G., Mariani M. (2008). Contributo alla conoscenza della macrofauna del Golfo di Gabès (Tunisia). Quad. Civ. Staz. Idrobiol. Milano 31, 1–173. pl. 1-92.
Coll M., Piroddi C., Steenbeek J., Kaschner K., Ben Rais Lasram F., Aguzzi J., et al. (2010). The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PloS One 5, e11842. doi: 10.1371/journal.pone.0011842
Costa O. G. (1861). Microdoride mediterranea; o, Descrizione de poco ben conosciuti od affatto ignoti viventi minuti e micoscropici del Meditterraneo. Tomo primo Vol. xviii (Napoli: Stamperia dell’Iridei), 80 pp. 13 pl.
Danovaro R., Fanelli E., Canals M., Ciuffardi T., Fabri M.-C., Taviani M., et al. (2020). Towards a marine strategy for the deep Mediterranean Sea: Analysis of current ecological status. Mar. Policy 112, 103781. doi: 10.1016/j.marpol.2019.103781
Dell’Angelo B., Lombardi C., Taviani M. (1998). Chitons (Mollusca, Polyplacophora) collected during cruise CS96 in the Strait of Sicily. Giorn. Geol. 60, 235–252.
Fanelli E., Bianchelli S., Foglini F., Canals M., Castellan G., Güell-Bujons Q., et al. (2021). Identifying priorities for the protection of deep Mediterranean Sea ecosystems through an integrated approach. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.698890
Fekih M., Gougerot L. (1977). Liste commentée des gastéropodes testacés marins recueillis dans les dépots littoraux actuels du Golfe de Tunis. Bull. Inst. Natl. Sci. Techn. Océanogr. Pêche Salammbo 3, 165–232.
Giacobbe S., Renda W., Appolloni M. (2022). First contextualized marine record of Parviturbo sertum Tabanelli et al. 2019 (Gastropoda: Skeneidae) and comparison with the congeneric Mediterranean species. J. Conchol. 44, 213–226.
Giannuzzi-Savelli R., Pusateri F., Palmeri A., Ebreo C. (1994). Atlante delle conchiglie marine del Mediterraneo Vol. 1 (Archaeogastropoda) (Roma: Edizioni de “La Conchiglia”), 125 pp.
Gofas S., Goutayer J., Luque Á. A., Salas C., Templado J. (2014a). Espacio Marino de Alborán. Proyecto LIFE+ INDEMARES (Madrid: Fundación Biodiversidad del Ministerio de Agricultura, Alimentación y Medio Ambiente), 129 pp.
Gofas S., Luque Á. A., Templado J., Salas C. (2017). A national checklist of marine Mollusca in Spanish waters. Sci. Mar. 81, 241–254. doi: 10.3989/scimar.04543.21A
Gofas S., Salas C., Rueda J. L., Canoura J., Farias C., Gil J. (2014b). Mollusca from a species-rich deep-water Leptometra community in the Alboran Sea. Sci. Mar. 78, 537–553. doi: 10.3989/scimar.04097.27A
Hadley A. (2006). Combine ZP public domain image processing software. Available online at: https://web.archive.org/web/20160221032141/http://www.hadleyweb.pwp.blueyonder.co.uk/ (Accessed March 15, 2024).
Hoeksema D. F. (2000). A new record of Microgloma guilonardi (Hoeksema 1993) from the Mediterranean (Bivalvia, Protobranchia, Nuculanidae). Basteria 64, 3–4.
Hoeksema D. F., Janse C. (2002). Microgloma pusilla (Jeffreys 1879) (Bivalvia, Protobranchia, Nuculanidae) and Notolimea clandestina Salas 1994 (Bivalvia, Pteriomorpha, Limidae), new for the southern North Sea Basin. Basteria 66, 193–196.
Jaziri S., Costantini F., Rugiu L., Abbiati M., Jarboui O. (2017). Does historical harvesting affect colony size distribution and genetic diversity in Corallium rubrum (Linnaeus 1758)? Evidences from the Southern Mediterranean commercial banks. Hydrobiologia 784, 211–224. doi: 10.1007/s10750-016-2875-7
Jeffreys J. G. (1870). Mediterranean Mollusca. Ann. Mag. Nat. Hist. 6, 65–86. doi: 10.1080/00222937008696204
Jeffreys J. G. (1874). Some remarks on the Mollusca of the Mediterranean. Rep. Brit. Assoc. Adv. Sci. 1873, 111–116.
Jeffreys J. G. (1878-1885). On the Mollusca procured during the H. M. S. “Lightning” and “Porcupine” expedition. Proc. Zool. Soc Lond. doi: 10.1111/j.1469-7998.1878.tb07976.x. Part 1 (1878), 393-416, pls 22-23.; Part 4 (1881), 922-952, pls 70-71 [1882]; Part 5 (1882), 656-687, pls 49-50 [1883]. Part 6 (1883), 88-115 pls 19-20; Part 7 (1884), 111-149, pls 9-10; Part 8 (1884), 341-372, pls 26-28; Part 9 (1885), 27-63, pls 4-6.
La Perna R. (1998). On Asperarca Sacco 1898 (Bivalvia, Arcidae) and two new Mediterranean species. Boll. Malacol. 33, 11–18.
La Perna R. (2003). The Quaternary deep-sea protobranch fauna from the Mediterranean: composition, depth-related distribution and changes. Boll. Malacol. 39, 17–34.
MacAndrew R. (1851). Notes on the distribution and range in depth of Mollusca and other marine animals observed on the coast of Spain, Portugal, Barbary, Malta, and Southern Italy in 1849. Rep. Brit. Assoc. Adv. Sci. 20, 264–304.
Manousis T., Zaminos G., Samara E., Mbazios G., Galinou-Mitsoudi S. (2021). Rissoella angeli n. sp. (Gastropoda: Heterobranchia: Rissoellidae) and additional new records of molluscs for E. Mediterranean and the Hellenic Seas. Xenophora Taxon. 31, 51–70.
Mars P. (1958). Etudes sur le seuil Siculo-Tunisien. 4. Mollusques testacés. Ann. Inst. Océanogr. 34, 127–143.
Micali P., Geiger D. L. (2015). Additions and corrections to the Scissurellidae and Anatomidae (Gastropoda-Vetigastropoda) of the Mediterranean Sea, with first record of Sinezona semicostata Burnay et Rolán. Biodiv. J. 6, 703–708.
Ministère de l’Environnement et du Développement Durable (2019). 4ème rapport national sur la diversité biologique (Tunis: Ministère de l’Environnement et du Développement Durable), 136 pp.
MolluscaBase eds (2024). MolluscaBase. Available online at: https://www.molluscabase.org (Accessed June 1, 2024).
Monterosato T. A. (1880). Notizie intorno ad alcune conchiglie delle coste d’Africa. Bull. Soc Malacol. Ital. 5, 213–233.
Nappo A., Cilia D. P., Cardona S. (2024). Additions to the marine mollusc fauna of the Maltese Archipelago, with observations on the nomenclature and biogeography of rare or poorly known Central Mediterranean species. Boll. Malacol. 60, 46–79. doi: 10.53559/BollMalacol.2023.16
Nolt J. M. (2008). A new species of Scissurella from the Azores with discussions on Sinezona semicostata Burnay & Rolan 1990 and Sinezona cingulata (O.G. Costa 1861) (Gastropoda: Vetigastropoda: Scissurellidae). Zootaxa 1678, 51–62. doi: 10.11646/ZOOTAXA.1678.1.3
Oliver J. D., Rolán E. (2015). The genus Ammonicera (Heterobranchia, Omalogyridae) in the Eastern Atlantic. 1: the species of the Iberian Peninsula. Iberus 33, 45–95. doi: 10.5281/zenodo.4586242
Öztürk B., Doğan A., Bitlis-Bakir B., Salman A. (2014). Marine molluscs of the Turkish coasts: an updated checklist. Turk. J. Zool. 38, 832–879. doi: 10.3906/zoo-1405-78
Pallary P. (1904-1906). Addition à la faune malacologique du Golfe de Gabès. J. Conchyl. 52, 212–248. pl. 7; 54(2), 77-124, pl. 4.
Pérès J.-M., Picard J. (1956). Etudes sur le seuil Siculo-Tunisien. I. Recherches sur les peuplements benthiques du seuil Siculo-Tunisien. Ann. Inst. Océanogr. 32, 233–264.
Raveggi A., Scaperrotta M., Bartolini S., Romani L. (2021). Contributo alla conoscenza della malacofauna marina delle isole adriatiche. 3. Nota su i micromolluschi conchiferi rinvenuti sulle coste delle isole di Caprara e San Domino (Arcipelago delle Tremiti, Mar Adriatico Centro-Occidentale). Alleryana 39, 16–29.
Renda W., Amati B., Bogi C., Bonomolo G., Capua D., Dell’Angelo B., et al. (2022). The new Checklist of the Italian Fauna: marine Mollusca. Biogeographia – J. Integrat. Bioeogr. 37, uc1005. doi: 10.21426/b637156028
Romani L., Raveggi A., Scaperrotta M., Bartolini S. (2018). Contributo alla conoscenza della malacofauna marina delle isole adriatiche. 1. Nota sui micromolluschi marini conchiferi rinvenuti sulla costa settentrionale dell’isola di Lastovo [Lagosta](Croazia, Mar Adriatico Sud-Orientale). Alleryana 36, 1–22.
Rosso J. C. (1979). “Mollusques testacés (Macrofaune),” in La Mer Pélagienne. Etude sédimentologique et écologique du Plateau Tunisien et du Golfe de Gabès. Eds. Burollet P. F., Clairefond P., Winnock E., Géol Médit 6, 143–170.
Rubio F., Dantart L., Luque A. A. (1998). Two new species of Dikoleps (Gastropoda, Skeneidae) from the Mediterranean coast of Spain. Iberus 16, 81–93. doi: 10.5281/zenodo.4651565
Sabelli B., Taviani M. (2014). “The making of the Mediterranean molluscan biodiversity,” in The Mediterranean Sea: Its history and present challenges. Eds. Goffredo S., Dubinsky Z. (Springer Science+Business Media, Dordrecht, Heidelberg, New York and London), 285–306. doi: 10.1007/978-94-007-6704-1_16
Schiaparelli S., Stanić R., Canese S., Montagna P., Taviani M. (2021). “The rare Mediterranean endemic gastropod Heliacus jeffreysianus (Tiberi 1867) host of the endangered Savalia savaglia coral: A case for urgent protection,” in Imperiled: The Encyclopedia of Conservation. Eds. DellaSala D. A., Goldstein M. I. (Elsevier, Amsterdam), 710–714. doi: 10.1016/B978-0-12-821139-7.00057-X
Scuderi D., Villari A., Reitano A. (2023). Another new eulimid (Gastropoda Eulimidae) from the Mediterranean Sea: Vitreolina micalii n. sp. Biodiv. J. 14, 505–512. doi: 10.31396/Biodiv.Jour.2023.14.3.505.512
Seurat L. G. (1930). Exploration zoologique de l’Algérie de 1830 à 1930 (Paris: Masson et Cie), 709 pp.
Sykes E. R. (1904-1925). On the Mollusca procured during the “Porcupine” Expeditions 1869-1870. Supplemental notes. Proc. Malacol. Soc Lond. 6, 23–40. doi: 10.1093/oxfordjournals.mollus.a066021. (Part I, 1904); 6, 322-332 (Part II, 1905); 7, 173-190 (Part III, 1906); 9, 331-348 (Part IV, 1911); 16, 181-193 (Part V, 1925).
Taviani M., Remia A. (2001). I coralli del buio: archivi climatici degli oceani passati. Ric. Futuro 18, 28–30.
Taviani M., Sosso M., Dell’Angelo B. (2023). Chitons from deep-water mollusk-rich deposits in the Southwestern Adriatic Sea (Mollusca, Polyplacophora). Diversity 15, 359. doi: 10.3390/d15030359
Villari A., Scuderi D. (2022). Mainly Mediterranean Rissoellidae (Heterobranchia Acteonimorpha)? with the description of Rissoella camillae n. sp. Biodiv. J. 13, 717–728. doi: 10.31396/Biodiv.Jour.2022.13.3.717.728
Warén A. (1992). New and little known “Skeneimorph” gastropods from the Mediterranean Sea and the adjacent Atlantic Ocean. Boll. Malacol. 27, 149–248.
Weitemeyer C., Döhler H. (2009). Traces of roman offshore navigation on Skerki Bank (Strait of Sicily). Int. J. Nautic. Archaeol. 38, 254–280. doi: 10.1111/j.1095-9270.2009.00228.x
Zibrowius H., Taviani M. (2005). “Remarkable sessile fauna associated with deep coral and other calcareous substrates in the Strait of Sicily, Mediterranean Sea,” in Cold-water Corals and Ecosystems. Eds. Freiwald A., Roberts J. M. (Springer, Berlin and Heidelberg), 807–819. doi: 10.1007/3-540-27673-4_42
Keywords: Mediterranean, rare species, checklist, species richness, taxonomy
Citation: Antit M, Gofas S and Taviani M (2024) Skerki Bank (Strait of Sicily) is a hotspot of molluscan biodiversity: multiple new records for Tunisian waters and description of three new species. Front. Mar. Sci. 11:1451567. doi: 10.3389/fmars.2024.1451567
Received: 19 June 2024; Accepted: 11 September 2024;
Published: 16 October 2024.
Edited by:
Austin Gallagher, Beneath the Waves, Inc., United StatesReviewed by:
Luigi Romani, Muséum National d’Histoire Naturelle, FranceJulian Evans, University of Malta, Malta
Copyright © 2024 Antit, Gofas and Taviani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serge Gofas, c2dvZmFzQHVtYS5lcw==
 Mouna Antit
Mouna Antit Serge Gofas
Serge Gofas Marco Taviani
Marco Taviani