- 1Department of Aquaculture, Zhejiang Ocean University, Zhoushan, China
- 2Zhejiang Provincial Engineering Technology Research Center of Marine Biomedical Products, School of Food and Pharmacy, Zhejiang Ocean University, Zhoushan, China
Introduction: Screening excellent bacterial strains for fermentation is the key to improving the nutritional value and bioavailability of soybean meal (SBM). This study investigated the application of Bacillus subtilis-fermented soybean meal (FSBM) on the feed of Pacific white shrimp (Litopenaeus vannamei).
Methods: FSBM was used to replace 0%, 25%, 50%, 75%, and 100% fish meal, and the feeding trial was lasted for 8 weeks (initial weight: 0.9 g).
Results and discussion: The amino acid profile in the whole shrimp body was tested. FSBM substitution only significantly reduced the content lysine in whole shrimp body, but increased the content of arginine. Fatty acid data showed that the content of n-6 PUFAs in whole shrimp was significantly increased by FSBM substitution. In muscle, FSBM substitution significantly reduced the content of MUFAs, but increased the content of PUFAs including C18:3n-3, C18:2n-6 and C20:4n-6. No hepatopancreas structure modifications appeared in the 25% group compared with the control group. Subsequently, we investigated the response patterns of different organs to FSBM substitution from antioxidant, endoplasmic reticulum stress and immunity. A high proportion of FSBM significantly reduced the content of GSH in hemolymph and hepatopancreas, while increased the mRNA expression of cat. FSBM substitution did not affect the activities of antioxidant enzymes in the intestine. However, the mRNA expression level of hsp70 in the intestine was significantly reduced by FSBM. In terms of immunity, the mRNA expression levels of lgbp and penaeidin in the hepatopancreas showed a significant linear increase trend. In muscle, high proportion of FSBM significantly increased the mRNA expression of imd. FSBM substitution did not significantly affect the expression of immune genes in the intestine. In terms of endoplasmic reticulum stress, FSBM substitution significantly increased the mRNA expression of eif2α in the hepatopancreas. In muscle, FSBM substitution inhibited the mRNA expression of bip. In the intestine, FSBM replacing 75% of fish meal significantly increased the mRNA expression of bip and ire1. In summary, this study indicated that when the fish meal content account for 40% in diets (dry weight), the screened Bacillus subtilis-FSBM can replace 25% of fish meal protein without reducing the antioxidant and immune abilities of shrimp.
1 Introduction
Pacific white shrimp (Litopenaeus vannamei) is one of the world’s most important crustacean species. In 2023, the world’s aquaculture output reached 5.6 million tons, mainly from countries such as Ecuador, China, India, and Vietnam (Zhi et al., 2023). Undoubtedly, it has become an important measure to ensure people’s food security. The downturn in China’s shrimp market price allowed industries and farmers to further reduce costs. In the process of shrimp breeding, feed is the main source of cost, especially the fish meal in the diet (Chen et al., 2024). The rising prices and shortage of resources seriously limit the development of shrimp farming. Therefore, it is crucial to find alternatives to fish meal and reduce farming costs without affecting the growth of shrimp.
Soybean meal (SBM), a by-product of soybean oil extraction, has been applied as protein source in aquatic feed (Kari et al., 2023). However, anti-nutritional factors (ANFs) limit the application of SBM in aquatic feed (Qi et al., 2023). Recently, it is a trend to use modern molecular biology and microbiology technologies to improve SBM under the background of interdisciplinary integration. Among them, fermentation technology is gradually applied to improve the nutritional value of SBM (Cao et al., 2023). Fermented soybean meal (FSBM) is widely used in shrimp feed as a substitute for fish meal and SBM protein (Abd El-Naby et al., 2023; Wang et al., 2024). The key to fermentation lies in stable processes, controllable conditions and excellent bacteria (Barragán et al., 2016; Kårlund et al., 2020). At present, Lactobacillus and Bacillus subtilis are the main strains used in fermentation (Shrestha et al., 2010; Zhang et al., 2014). The improvement of the nutritional value of SBM by fermentation is reflected in two aspects. On the one hand, the ANFs in SBM, including antigen proteins, ureases and phytic acid, are degraded during the fermentation process (Zheng et al., 2017); On the other hand, the microbial fermentation process also endows SBM with abundant bioactive substances. Wang et al. (2023) found that fermenting SBM with mixed strains containing Enterococcus faecium, Bacillus velezensis and Saccharomyces boulardii can significantly increase the content of small peptides in FSBM. SBM fermented by Bacillus subtilis is rich in hydrolytic enzymes such as α-amylase, proteases and β-glucosidase (Dai et al., 2017). Our laboratory has screened a type of Bacillus subtilis (copyright number: CN115197876A; preservation number: CGMCCNo.24836), which relies on traditional strain selection techniques. Through efficient mutagenesis breeding technology combined with high-throughput screening methods, we have developed a Bacillus subtilis strain that can degrade non starch polysaccharides and antigen proteins. On this basis, the hydrolysis ability of non starch polysaccharides and antigen proteins of Bacillus subtilis was optimized through iterative evolution to obtain probiotic strains with high protein and peptide production capacity. In the process of mutation breeding screening, Bacillus subtilis with high accumulation of negative metabolites are selectively avoided to obtain probiotic strains of Bacillus subtilis with low viscosity of fermentation products and no ammonia taste. However, further exploration is needed to determine the effectiveness of this FSBM in shrimp feed.
The standard to evaluate the effect of FSBM in shrimp feed mainly focuses on the growth performance, health status and tissue structure of shrimp. Although FSBM has multiple functional ingredients, its utilization ratio in shrimp feed is still limited. The basic nutritional characteristics of FSBM is similar with SBM, and there are also problems such as amino acid imbalance (Chen et al., 2023). Excessive addition can affect the health status of various organs in shrimp, such as inducing oxidative stress (Abd El-Naby et al., 2023) and endoplasmic reticulum stress (ER stress) (Xie et al., 2020), affecting muscle tissue growth and intestinal health (Cheng et al., 2020). Hence, the present study evaluated the utilization value of FSBM in shrimp feed by detecting the induction patterns of three main tissues, including hepatopancreas, muscle and intestine. We hope to provide alternative protein sources for shrimp feed.
2 Materials and methods
2.1 Experimental diets
This experiment was equipped with five groups of feed with isonitrogenous (37.31%) and isolipidic (6.47%) element. Bacillus subtilis selected in our lab was used to ferment SBM. In brief, following a prolonged period of incubation, 5*109 CFU of Bacillus subtilis was dissolved in 100 mL of water and introduced into 100 g of sterile SBM. SBM was put in a shaker right away, and keeping fermented for 24 h at 37°C, 100 rpm, and 80% humidity. The FSBM was then put in an oven and dried for 6–7 h at 50°C, maintaining 8–10% humidity. FSBM replaced 0%, 25%, 50%, 75% and 100% of fish meal protein, respectively, which named CON, 25F, 50F, 75F and 100F. The details and composition of feed were presented in the Table 1. Before the feed preparation, various raw materials crushed through the ultra -micro crushing machine and passed through the 60 -mesh screen. The ingredients were pelleted into a diameter of 0.8 mm-1.0 mm using a twin-screw extruder (Guangzhou Huagong Optical Mechanical & Electrical Technology CO. LTD, F-75Q). The screw rotation speed was 15–17 rpm. The pelletizing speed was 30–40 rpm and the extrusion pressure was under 400 Kgf. The pellet was dried in an oven at 45°C for 24 h. The dried feed was stored in a sealed plastic bag in a -20°C refrigerator for later use.
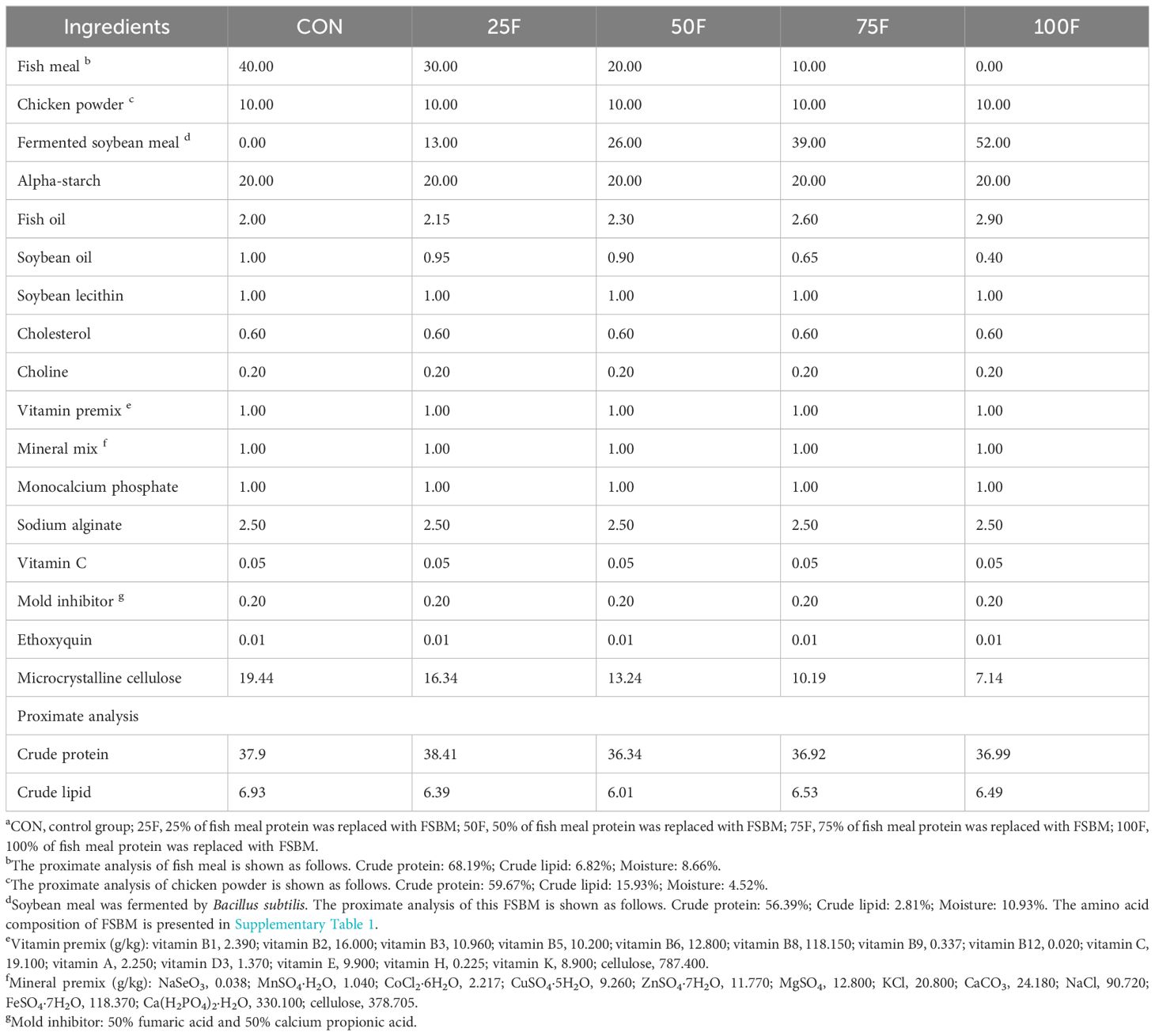
Table 1 The experimental diets’ formulation and chemical content a (% dry matter).
2.2 Feeding procedure and sampling
The experimental shrimp were purchased from Zhejiang Yize Aquaculture Co., Ltd. (Taizhou, Zhejiang, China). After 14 d of laboratory adaptation, healthy and active shrimp (initial weight: 0.9 g) were randomly assigned to 15 tanks (water volume: 300 L), with 20 shrimp per tank and 3 replicates per treatment. During the experiment, the feeding frequency of shrimp was maintained at 4 times a day (06:00, 11:00, 16:00, 22:00), and the feeding amount was determined by body weight with a daily ratio of 5% to 8% which can maintain satiety and ensure that all feed was consumed by shrimp. The aquaculture water quality should be maintained within the optimal range for shrimp feeding and growth (temperature: 24–26°C; nitrite < 0.1 mg/L; dissolved oxygen level ≥ 6 mg/L; total ammonia nitrogen < 0.5 mg/L; pH: 7.8–8.3; salinity: 29–31 ‰).
After 8-week feeding trial, all shrimp were subjected to a 24 h fasting treatment. Six shrimp were randomly selected from each tank, and the hepatopancreas, muscle and intestine were collected. All the above samples were frozen in a liquid nitrogen tank and stored at -80°C until use. 4% paraformaldehyde was used to fix the hepatopancreas for subsequent histological observation.
2.3 Biochemical analysis and histology
Before the biochemical indices were detected, hepatopancreas, muscle and intestine samples were homogenized with saline solution at a ratio of 1:9 (w: v). After centrifugation at 2,500 rpm for 10 min, the supernatant was collected for use. The contents of total protein (TP), reduced glutathione (GSH), malondialdehyde (MDA) total cholesterol (T-CHO) and total triglyceride (TG) and the activities of superoxide dismutase (SOD), total antioxidant capacity (T-AOC), glutamic oxalacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) were determined by the commercially available kit (Nanjing Jiancheng Bioengineering Institute, China). The analysis of TP content was conducted with Coomassie Brilliant Blue method. For GSH measurement, reduced GSH reacts with dithiodinitrobenzoic acid (DTNB) to produce a yellow compound that can be quantitatively measured by colorimetric analysis at 405nm. MDA can form a red product with thiobarbituric acid (TBA), with a maximum absorption peak at 532nm. Hence, the MDA content was analyzed with TBA method. CHO generates a red pyrodhumin compound under the catalysis of cholesterase, cholesterol oxidase and peroxidase, and the content of CHO was measured using a microplate reader at 500 nm. The TG content was measured with GPO-PAP (Glycerol-3-Phosphate Oxidase and Perioxidase) method. SOD activity was measured using WST-1 method and T-AOC activity was analyzed with ABTS method. The GOT and GPT activities were measured with a microplate reader at 505 nm.
For hepatopancreas histological observation, after 24 h of fixation with paraformaldehyde, ethanol was used for stepwise dehydration and embedded in paraffin. The slices were got using a microtome (5–8 μm) and stained with hematoxylin eosin (H&E) staining method. Finally, the stained sections were photographed and observed under the microscope (Olympus BX53, Japan).
2.4 Amino acid composition and fatty acid profile analysis
The amino acid analysis was measured according to the published method with some modifications (Yang et al., 2022a). In brief, for amino acid profile in the diets and shrimp body, the samples were placed at the bottom of an ampoule bottle. 6 M hydrochloric acid was added into the ampoule bottle and then nitrogen blowing for 10 min. Subsequently, the ampoule bottle was sealed and then nitrified at 110°C for 24 h. Then, the samples were diluted 100 times and mixed with acetonitrile. The mixture was centrifuged and the supernatant was mixed with 0.1 N hydrochloric acid, followed by measurement using a liquid chromatography (Agilent, 1260 Infinity II, USA).
Transesterification was made with boron trifluoride and methanol and profile of metal-esters were performed in 7890B gas chromatography (Agilent Technologies Inc., USA) with an external standard method. The gas chromatography method was set as follows (Liu et al., 2021): the flow rate of the carrier gas (nitrogen) was 1 mL/min. The split ratio was 100:1, the injector temperature was 250°C, the pressure was 20.363 psi, the overall flow rate was 104 mL/min, and the septum purge flow was 3 mL/min. The oven temperature increased at a rate of 20°C/min from 70°C to 150°C, then increased at a rate of 6°C/min to 180°C, increased at a rate of 20°C/min to 220°C and remained there for 6 min, and increased at a pace of 20°C/min to 250°C and remained there for 11.5 min. The air flow, hydrogen (H2), and makeup flow (N2) were 300 mL/min, 30 mL/min, and 25 mL/min, respectively, and the detector’s temperature was 300°C. The fatty acid content is shown as a percentage of the overall fatty acid content.
2.5 Real-time polymerase chain reaction analysis
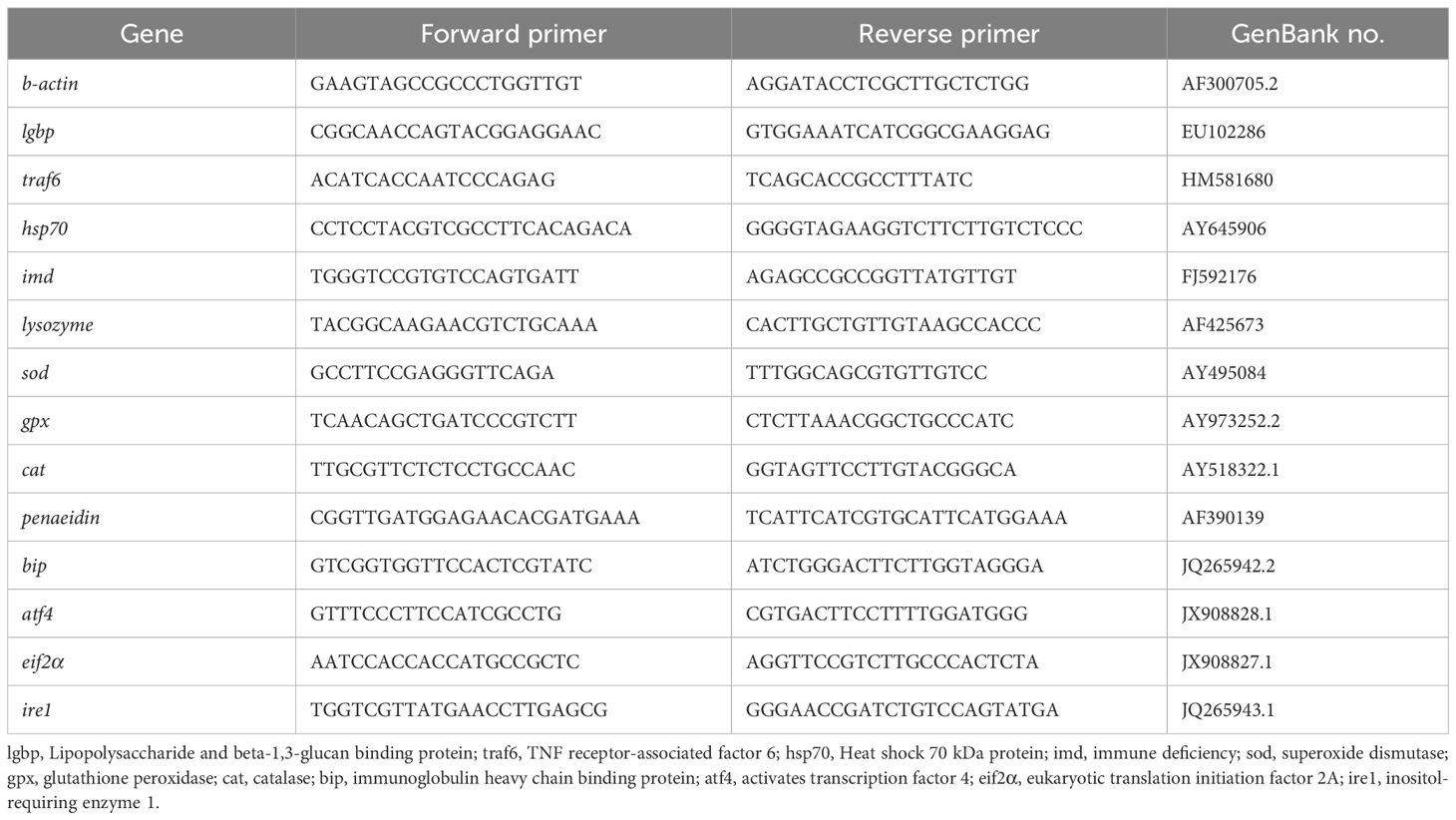
The RNA of hepatopancreas, muscle and intestine were extracted with TRIzol reagent. In brief, 100 mg of tissues were extracted with 1 mL of TRIzol. Immediately, the homogenate was centrifuged for 10 min at 4°C, 12–000 g. Chloroform was added to the supernatant, and the mixture was incubated on ice for 3 min before being centrifuged for 15 min at 4°C and 12–000 g. Subsequently, the supernatant was combined with isopropanol. The mixture was stewing for 10 min and centrifuged for 10 min at 4°C, 12, 000 g. Finally, the RNA sediment was washed twice with 75% ethanol and dissolved in RNAse-free water. The extracted RNA was reverse transcribed into cDNA with HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, China). SYBR qPCR Master Mix (Vazyme) was used to conduct RT-qPCR. The primers were designed according to the published sequences in NCBI and are presented at Table 2. β-actin was selected as the housekeeping gene in the present study. The relative expression of genes were calculated using the comparative cycle threshold (CT) method (2-△△CT method) (Livak and Schmittgen, 2001).
2.6 Statistical analysis
The data are presented as the mean ± standard error of the mean (S. E. M.). Linear and quadratic patterns was analyzed using SPSS 23.0 software followed by Tukey’s tests to compare differences between the means. For the feeding experiment, the n = 3. For amino acid and fatty acid analysis, the n = 3. For enzyme activity detection and gene expression analysis, the n = 4. P < 0.05 defined statistical significance.
3 Results
3.1 Amino acid composition
After 8-week feeding trial, the survival rate of shrimp in all groups were all above 90% and were not affected by dietary treatment. Firstly, we tested the amino acid composition in feed and shrimp. Results showed that the amino acid profile in feed was affected by FSBM substitution. For essential amino acids (EAAs), the content of amino acids showed a significant linear downward trend with the increase of FSBM substitution ratio, except for phenylalanine (Table 3). Compared with the FM group (control group), 25% FSBM substitution only significantly reduced the content of threonine. However, when the proportion of FSBM replacing fish meal exceeded 50%, the contents of nine EAAs were significantly lower than those in the FM group (Table 3). For non-essential amino acids (NEAAs), FSBM substitution significantly increased the content of proline with a linear pattern and significantly decreased the content of glycine with a linear pattern. When the proportion of FSBM replacing fish meal exceeded 50%, the content of serine was significantly lower than that in the FM group (Table 3). In the whole shrimp body, with the increase of FSBM substitution ratio, the content of lysine showed a significant linear downward trend (Table 4). On the contrary, FSBM substitution increased the content of arginine with a significant linear pattern (Table 4).
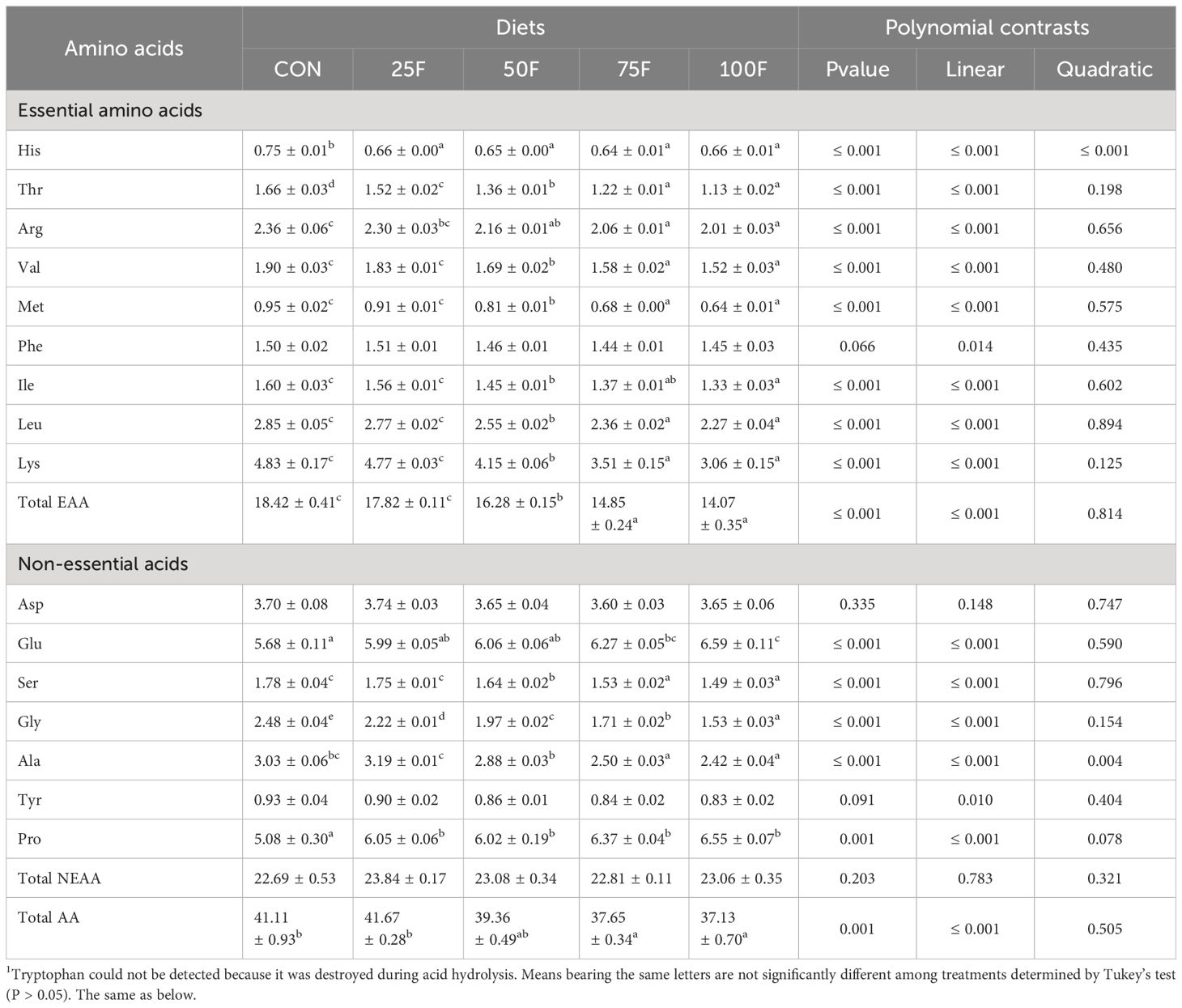
Table 3 Amino acids profile of experimental diets for Pacific white shrimp (% dry matter, n=3, mean± S.E.M.) 1.
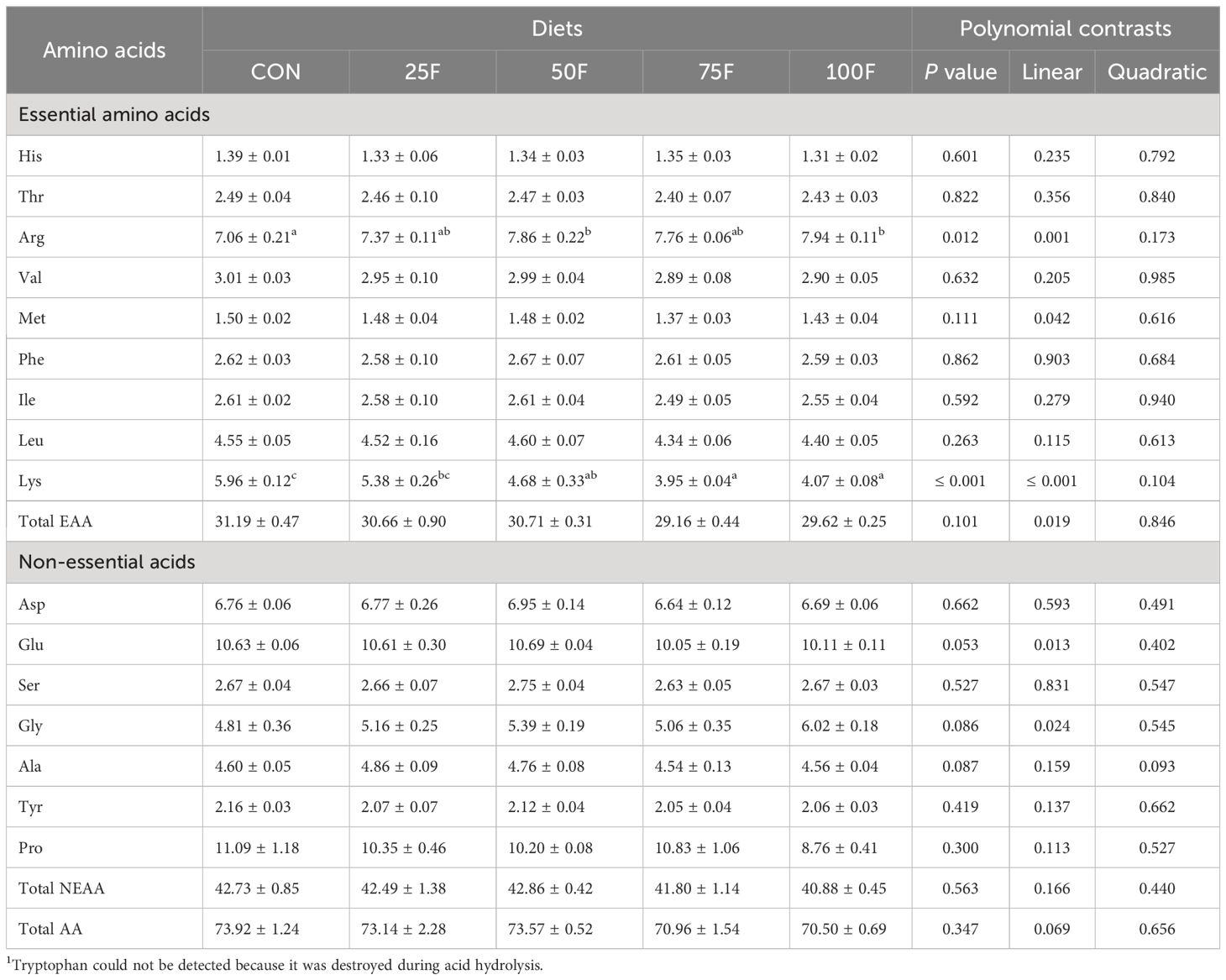
Table 4 Amino acids profile of the whole shrimp body fed with different diets (% dry matter) (n = 3, mean ± S.E.M.) 1.
3.2 Fatty acid profile in the feed, muscle and whole shrimp body
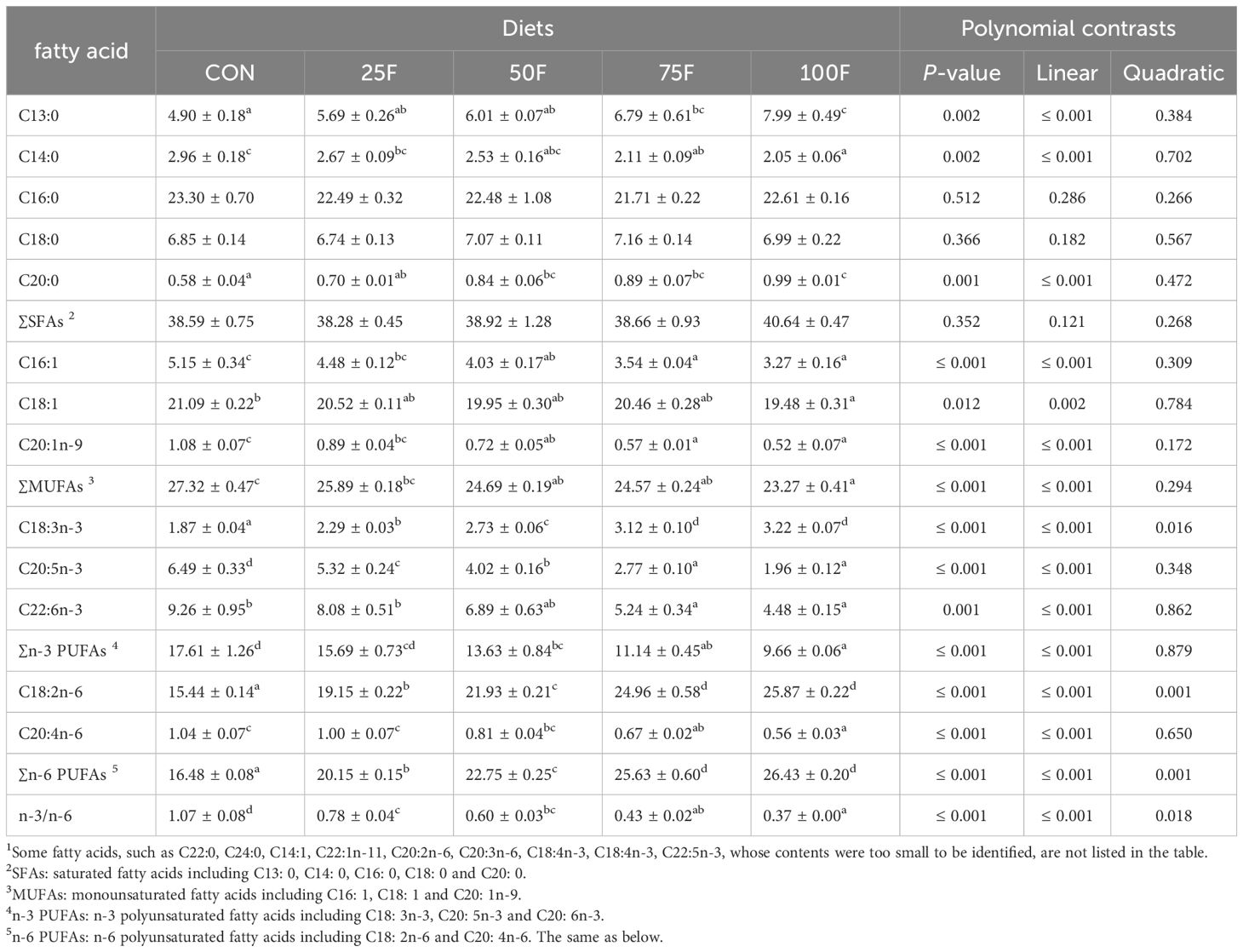
With the increase of FSBM substitution ratio, the levels of saturated fatty acids (SFAs) including C13:0 and C20:0 in diets showed an increasing trend in significantly linear pattern (Table 5). On the contrary, the level of C14:0 exerted a decreasing trend in significantly linear pattern with the increase of FSBM substitution ratio (Table 5). FSBM substitution significantly decreased the contents of monounsaturated fatty acids (MUFAs) including C16:1, C18:1 and C20:1n-9 with a linear pattern (Table 5). For n-3 polyunsaturated fatty acids (n-3 PUFAs), FSBM substitution significantly increased the content of C18:3n-3 with a linear and quadratic pattern while significantly decreased the contents of C20:5n-3 and C22:6n-3 with a linear pattern (Table 5). In addition, FSBM substitution significantly increased the content of C18:2n-6 with a linear pattern (Table 5). In the whole body, with the increase of FSBM substitution ratio, the contents of total MUFAs showed a trend of first increasing and then decreasing with a significant linear pattern (Table 6). However, compared with the FM group, there was no significantly difference in total MUFA contents among these FSBM substitution groups (Table 6). The contents of C18:3n-3 showed a trend of first decreasing and then increasing with a significant linear pattern (Table 6). FSBM substitution significantly enhanced the levels of C18:2n-6 and C20:4n-6 with a linear pattern (Table 6). Hence, FSBM substitution significantly decreased the ratio of n-3 PUFAs and n-6 PUFAs with a linear pattern. In the muscle, with the increase of FSBM substitution ratio, the content of C14:0 showed a trend of first increasing and then decreasing with a significant linear pattern (Table 7). FSBM substitution significantly decreased the levels of C16:1 and C18:1 with a linear pattern, while increased the levels of C18:3n-3, C18:2n-6 and C20:4n-6 (Table 7). Hence, FSBM substitution significantly decreased the ratio of n-3 PUFAs and n-6 PUFAs with a linear pattern (Table 7), which was consistent with the trend in the whole body.
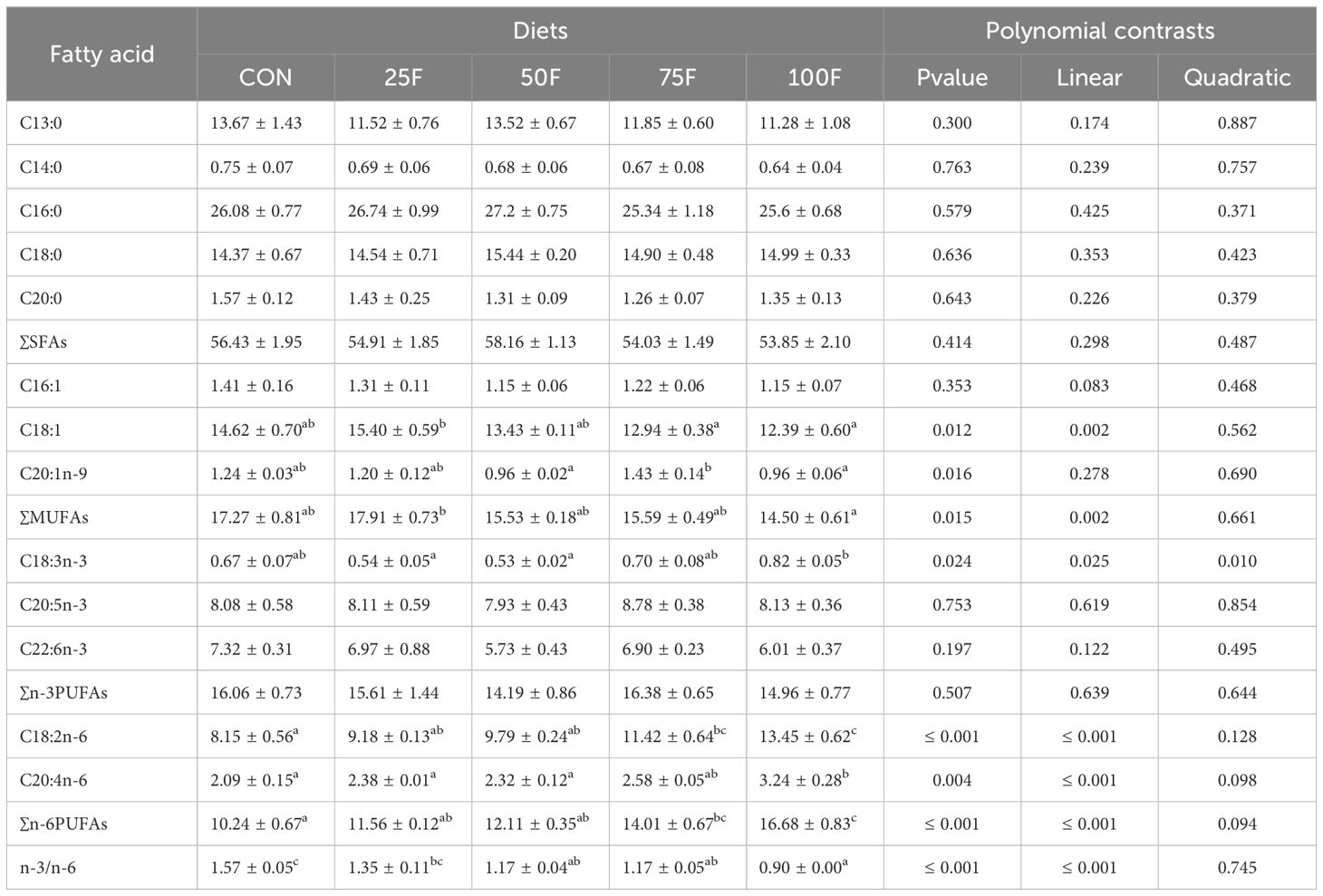
Table 6 Fatty acid profile of the whole shrimp body in different diets (% total fatty acids; n = 3, mean ± S.E.M.).
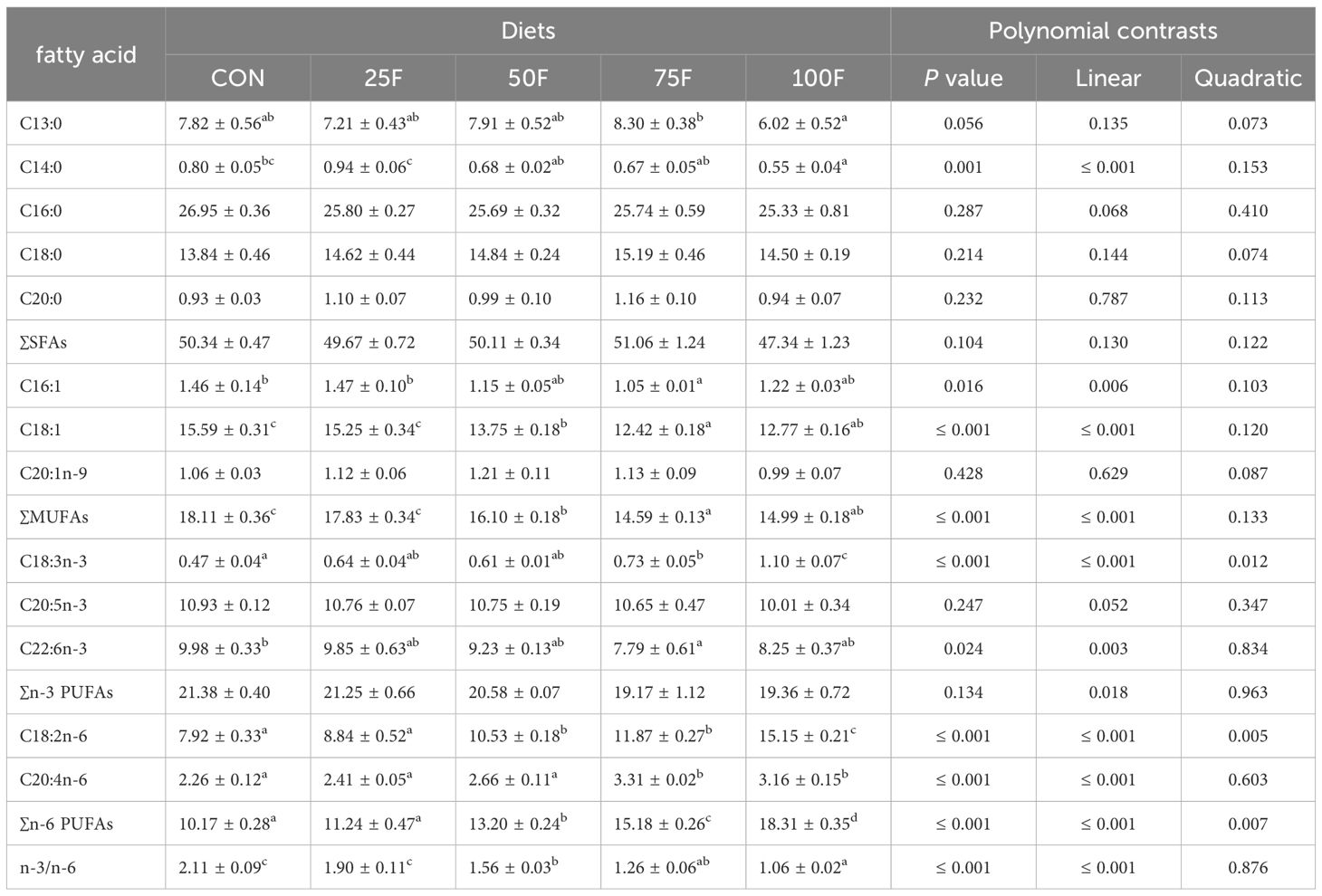
Table 7 Fatty acid profile of muscle in the shrimp fed with different diets (% total fatty acids; n = 3, mean ± S.E.M.) .
3.3 Histology of hepatopancreas
Compared with the FM group, 25% FSBM substitution increased the number of secretory cells (B cells), which indicated that 25% FSBM substitution enhanced the digestive and absorptive capacity (Figure 1). In addition, the structure of the various types of cells in the 25FSBM group was complete and clear, and the arrangement was neatly arranged, indicating that no damage to shrimp hepatopancreas cells was damaged (Figure 1). However, in the 50FSBM and 100FSBM groups, the arrangement of hepatic corpuscles was not tight and the gap increased (Figure 1). In the 75FSBM group, the basement membrane was shed and the tubular lumen was enlarged. Hence, we indicated that no structure modifications appeared in the 25% group compared with the control group, but the hepatopancreas were damaged if the replacement ratio increased till 50% and more (Figure 1).
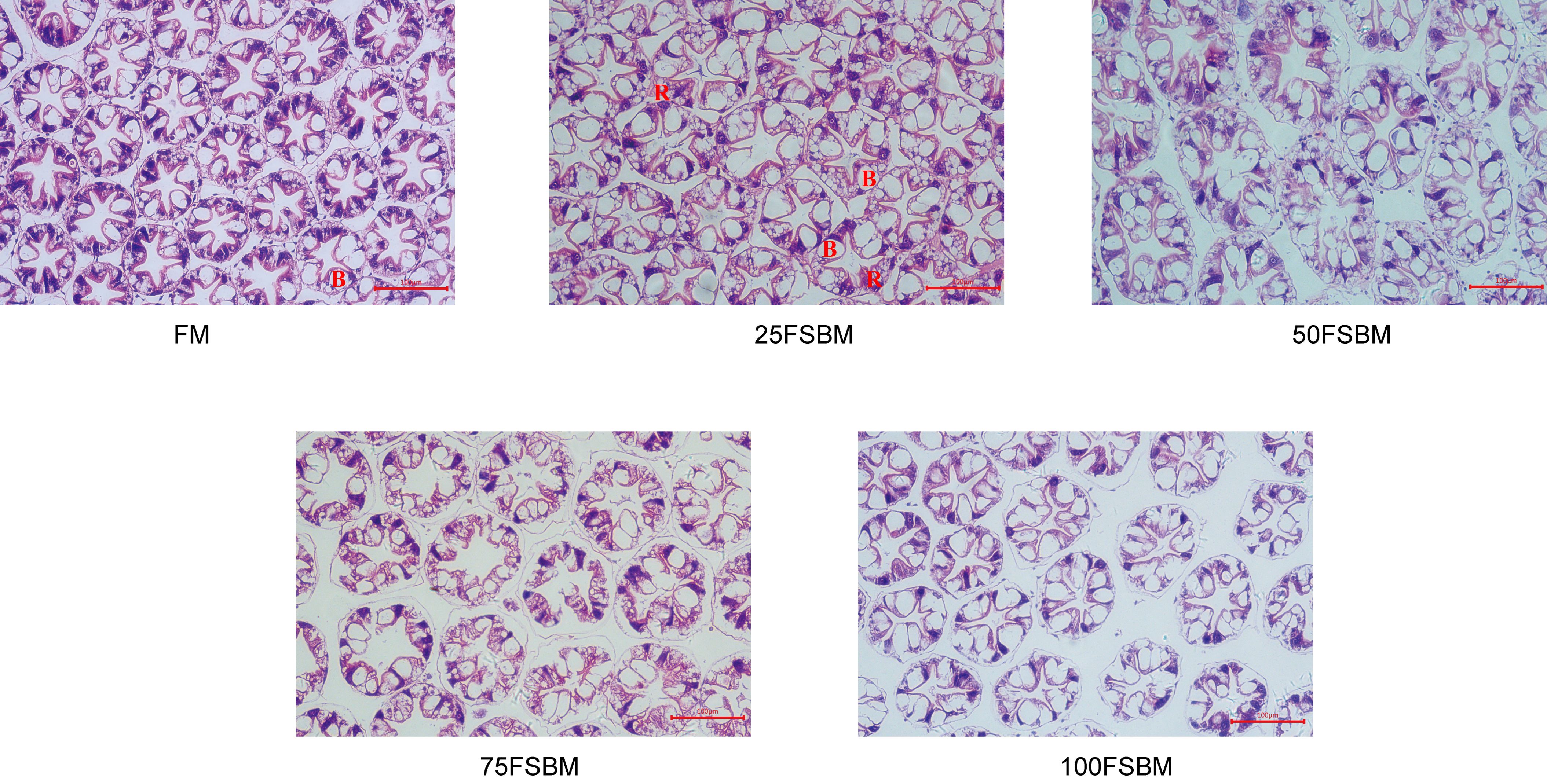
Figure 1 The effects of FSBM substitution on the structure of hepatopancreas in Pacific white shrimp. Scale bar, 100 μm. B cells (Blasenzellen). R cells (Restzellen).
3.4 Physiological and biochemical indicators in hemolymph, hepatopancreas, muscle and intestine
In the hemolymph, compared with the control group, FSBM substitution had no significant effects on the activities of GPT, GOT and T-AOC (Figures 2A, B, E). Correspondingly, as the proportion of FSBM substitution increased, the activity of SOD showed a trend of first increasing and then decreasing with a significant linear and quadratic pattern, but there was no significant difference compared to the control group (Figure 2D, Table 8). When FSBM replaced more than 50% of fish meal, the content of GSH significantly decreased with a significant linear pattern (Figure 2C, Table 8). We also tested the effects of FSBM substitution on total cholesterol (T-CHO) and triglyceride (TG) contents in hemolymph. Results showed that the response of these two lipid substances to FSBM substitution was consistent. 25% FSBM substitution did not affect the contents of T-CHO and TG. However, when the substitution ratio exceeded 50%, the content of T-CHO and TG significantly decreased with a significant linear pattern (Figures 2F, G, Table 8).
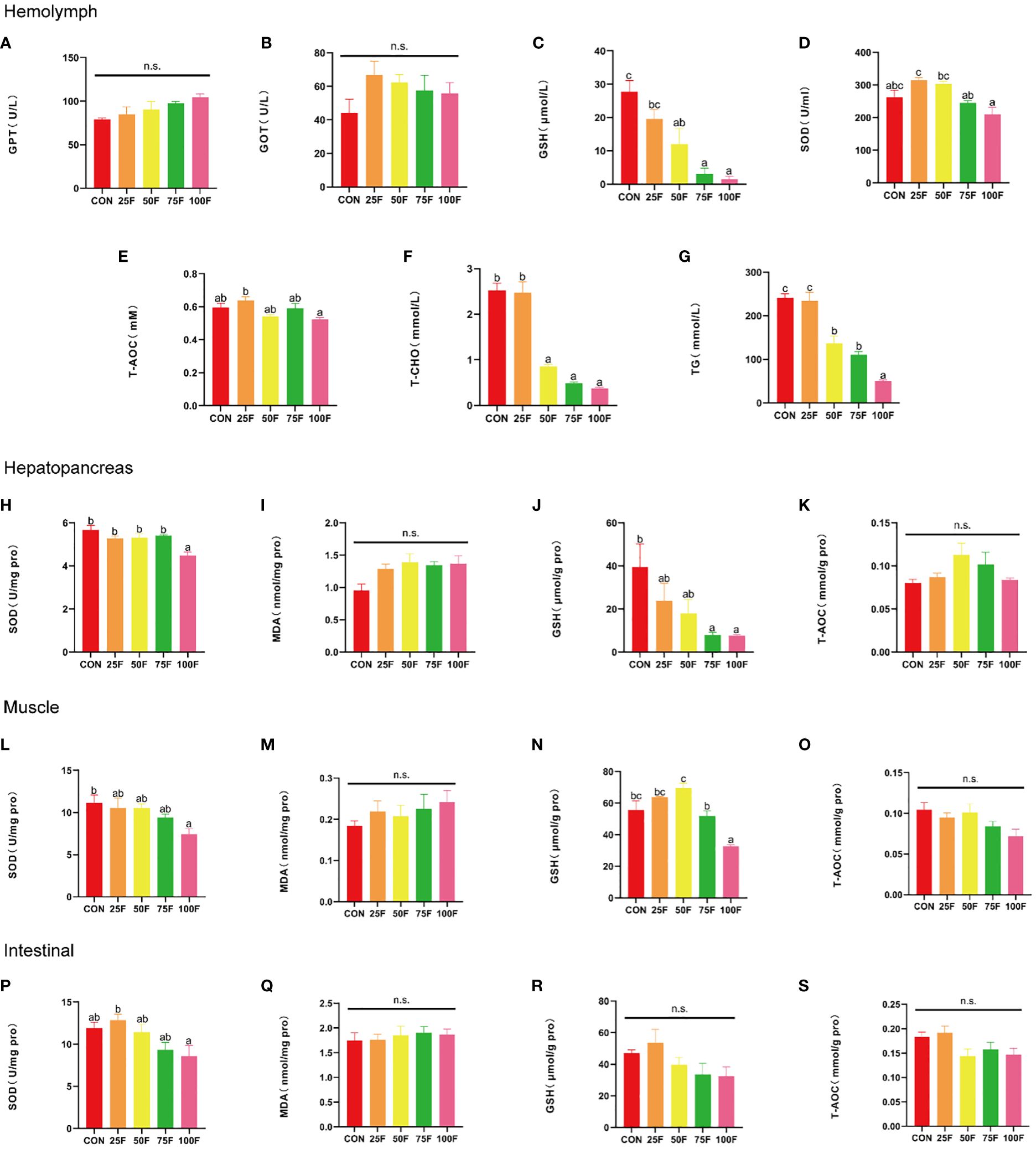
Figure 2. The effects of FSBM substitution on the antioxidant indices in different tissues and the contents of T-CHO and TG in the hemolymph (n = 4). The results are presented as the mean ± S. E. M. and were analyzed with Tukey’s tests [bars bearing the same letters are not significantly different among treatments (P > 0.05)]. The same as below. (A) GPT (glutamic-pyruvic transaminase) activity in the hemolymph; (B) GOT (glutamic oxalacetic transaminase) activity in the hemolymph; (C) GSH (reduced glutathione) content in the hemolymph; (D) SOD (superoxide dismutase) activity in the hemolymph; (E) T-AOC (total antioxidant capacity) activity in the hemolymph; (F) T-CHO (total cholesterol) content in the hemolymph; (G) TG (triacylglycerol) content in the hemolymph; (H) SOD activity in the hepatopancreas; (I) MDA (malondialdehyde) content in the hepatopancreas; (J) GSH content in the hepatopancreas; (K) T-AOC activity in the hepatopancreas; (L) SOD activity in the muscle; (M) MDA content in the muscle; (N) GSH content in the muscle; (O) T-AOC activity in the muscle; (P) SOD activity in the intestinal; (Q) MDA content in the intestinal; (R) GSH content in the intestinal; (S) T-AOC activity in the intestinal. n. s., not significant.
In the hepatopancreas, FSBM substitution had no significant effects on the activity of T-AOC and the content of MDA (Figures 2I, K). On the contrary, the activity of SOD and the content of GSH decreased gradually as the proportion of FSBM substitution increased with a significant linear pattern (Figures 2H, J, Table 8). However, compared with the control group, only group 100F significantly inhibited the activity of SOD (Figure 2H). The contents of GSH in group 75F and 100F were significantly lower than that in the control group (Figure 2J).
In the muscle, the content of MDA and the activity of T-AOC were not affected by FSBM substitution (Figures 2M, O). The trend of SOD activity in the muscle under FSBM substitution was similar with that in the hepatopancreas and only 100F significantly decreased the activity of SOD (Figure 2L). The content of GSH showed a trend of increasing first and then decreasing with both linear and quadratic patterns (Figure 2N, Table 8).
In the intestine, FSBM substitution had no significant effects on the contents of GSH and MDA and the activity of T-AOC (Figures 2Q-S). With the increasing of FSBM substitution ratio, the activity of SOD showed a trend of first increasing and then decreasing with a significant linear pattern (Figure 2P, Table 8).
3.5 Expression of antioxidant and immune related genes in tissues
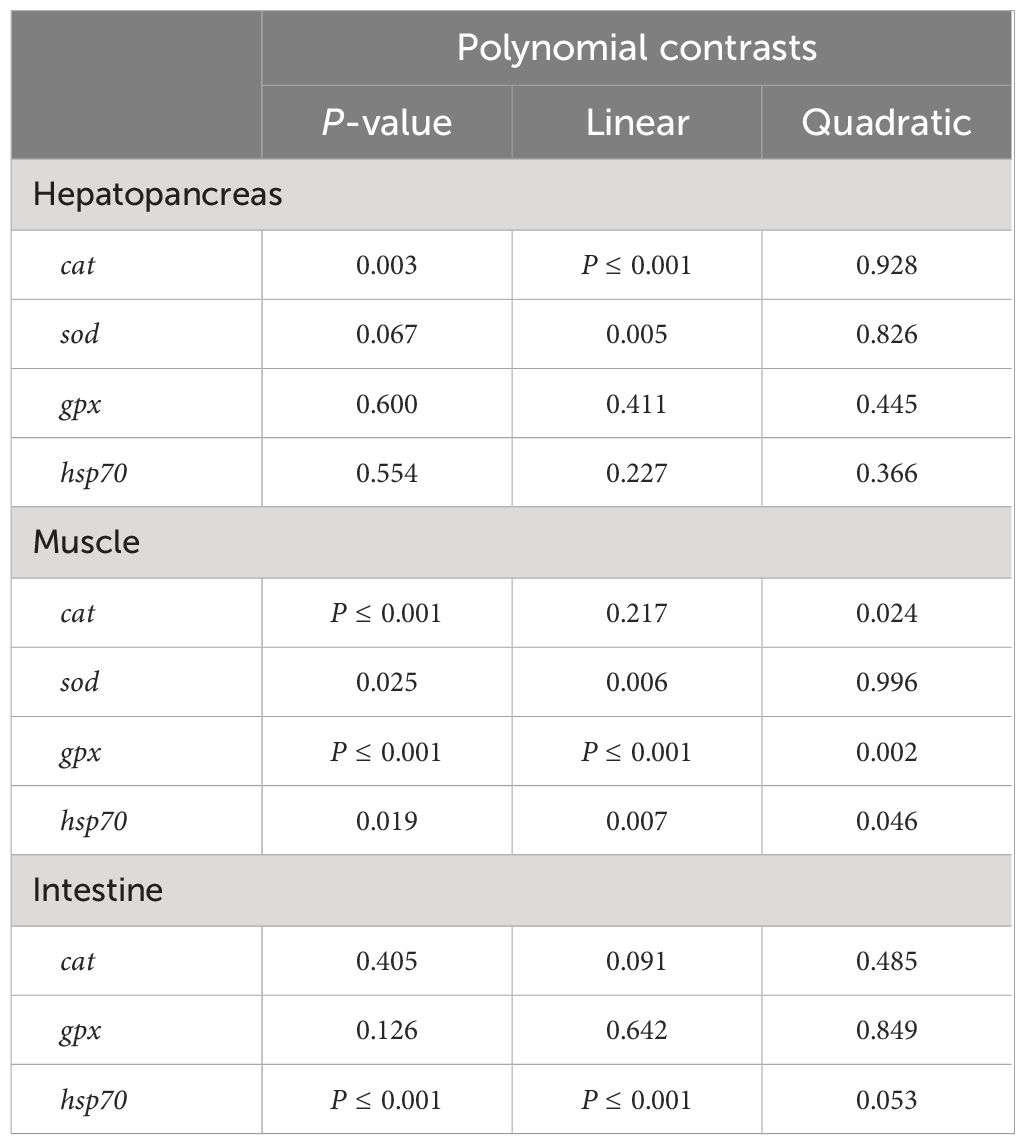
In the hepatopancreas, there was no significant correlation between the substitution of FSBM and the expression levels of hsp70 (heat shock 70 kDa protein) and gpx (glutathione peroxidase) (Figures 3B-C). With the increase of FSBM substitution ratio, the mRNA expression of sod (superoxide dismutase) showed an increasing tendency without statistical difference, but underwent a significant linear relationship on the whole (Figure 3A, Table 9). The tendency of cat (catalase) expression under FSBM substitution was similar with sod expression. However, compared with the control group, 75F and 100F significantly increased the mRNA expression of cat (Figure 3D). In the muscle, compared with the control group, 75F significantly enhanced the mRNA expression of cat and sod (Figures 3E, H). When FSBM substitution ratio exceeded 50%, the mRNA expression of gpx was significantly higher than that in the control group with a linear pattern (Figure 3G, Table 9). As the FSBM substitution ratio increased, the mRNA expression of hsp70 in muscle showed a decreasing tendency with a significant linear and quadratic pattern (Figure 3F, Table 9). In the intestine, FSBM substitution did not affect the mRNA expression of gpx and cat (Figures 3I, K). However, FSBM substitution significantly inhibited the mRNA expression of hsp70 with a linear pattern (Figure 3J, Table 9).
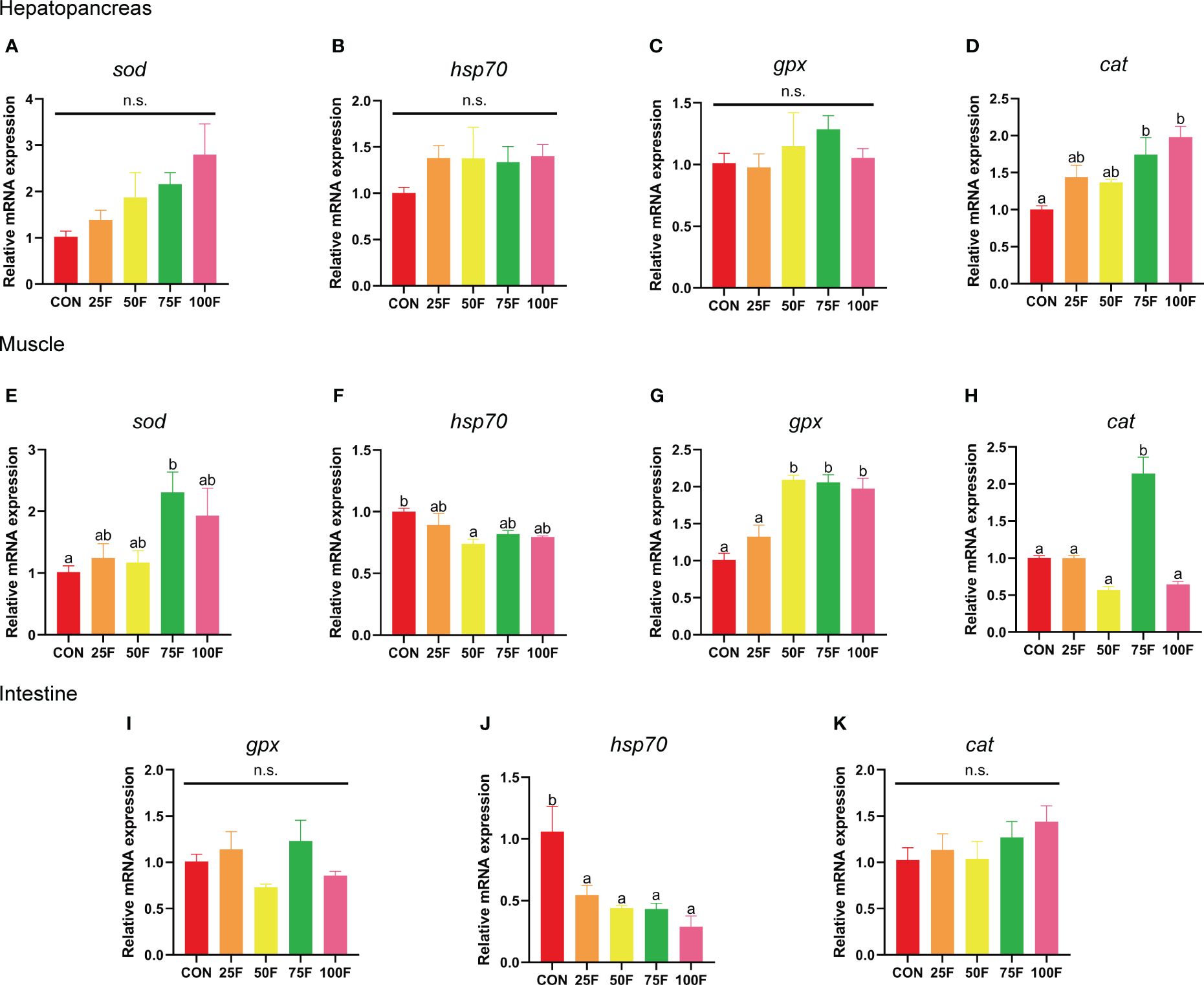
Figure 3 The effects of FSBM substitution on the mRNA expression of antioxidant enzymes in different tissues of shrimp (n=4). (A-D) mRNA expression of antioxidant enzymes in hepatopancreas. (E-H) mRNA expression of antioxidant enzymes in muscle. (I-K) mRNA expression of antioxidant enzymes in intestine. n. s., not significant.
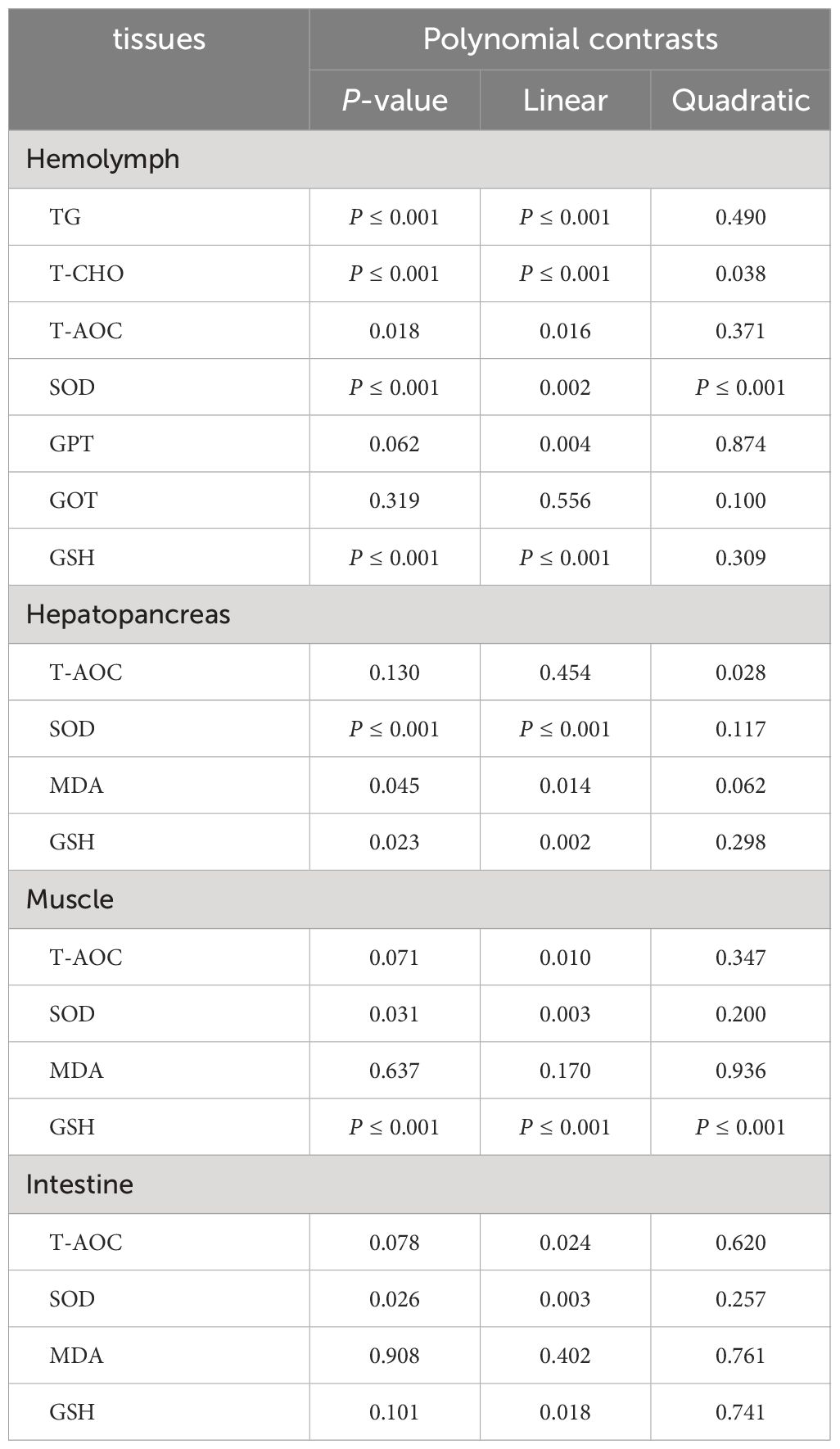
Table 8 Polynomial contrasts of antioxidant indices in different tissues and T-CHO and TG in the hemolymph.
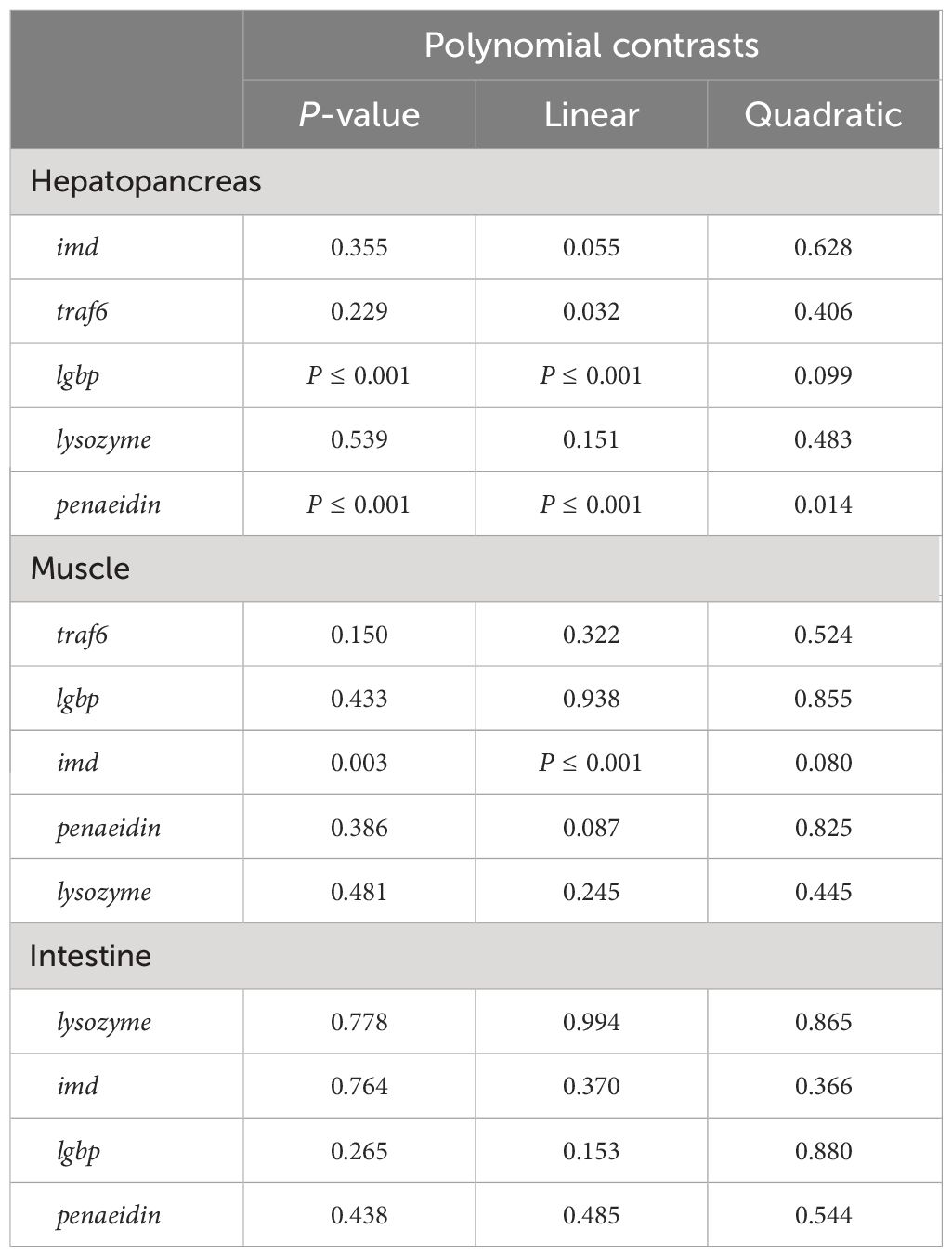
We also detected the effect of FSBM substitution on the mRNA expression of genes related with innate immunity in tissues. Results indicated that the mRNA expression of lgbp (lipopolysaccharide and beta-1,3-glucan binding protein) and penaeidin in the hepatopancreas showed significantly linear increase with increasing substitution ratio, but the difference was that when the FSBM substitution ratio exceeded 50%, the mRNA expression level of lgbp was significantly higher than that of the control group, while only in the 100F group did the mRNA expression level of penaeidin be significantly higher than that of the control group (Figures 4D, E, Table 10). FSBM substitution did not affect the mRNA expression of lysozyme and imd (immune deficiency) in the hepatopancreas (Figures 4A, C). Although there was no significant correlation between the substitution of FSBM and the expression levels of traf6 (tnf receptor-associated factor 6), it showed a significantly linear increase tendency (Figure 4B, Table 10). In the muscle, FSBM substitution did not affect the mRNA expression of traf6, lysozyme, lgbp and penaeidin (Figures 4F, H-J). However, 75F and 100F significantly increased the mRNA expression of imd with a significant linear pattern (Figure 4G, Table 10). The mRNA expression of penaeidin, imd, lysozyme and lgbp in the intestine were not affect by FSBM substitution (Figures 4K-N).
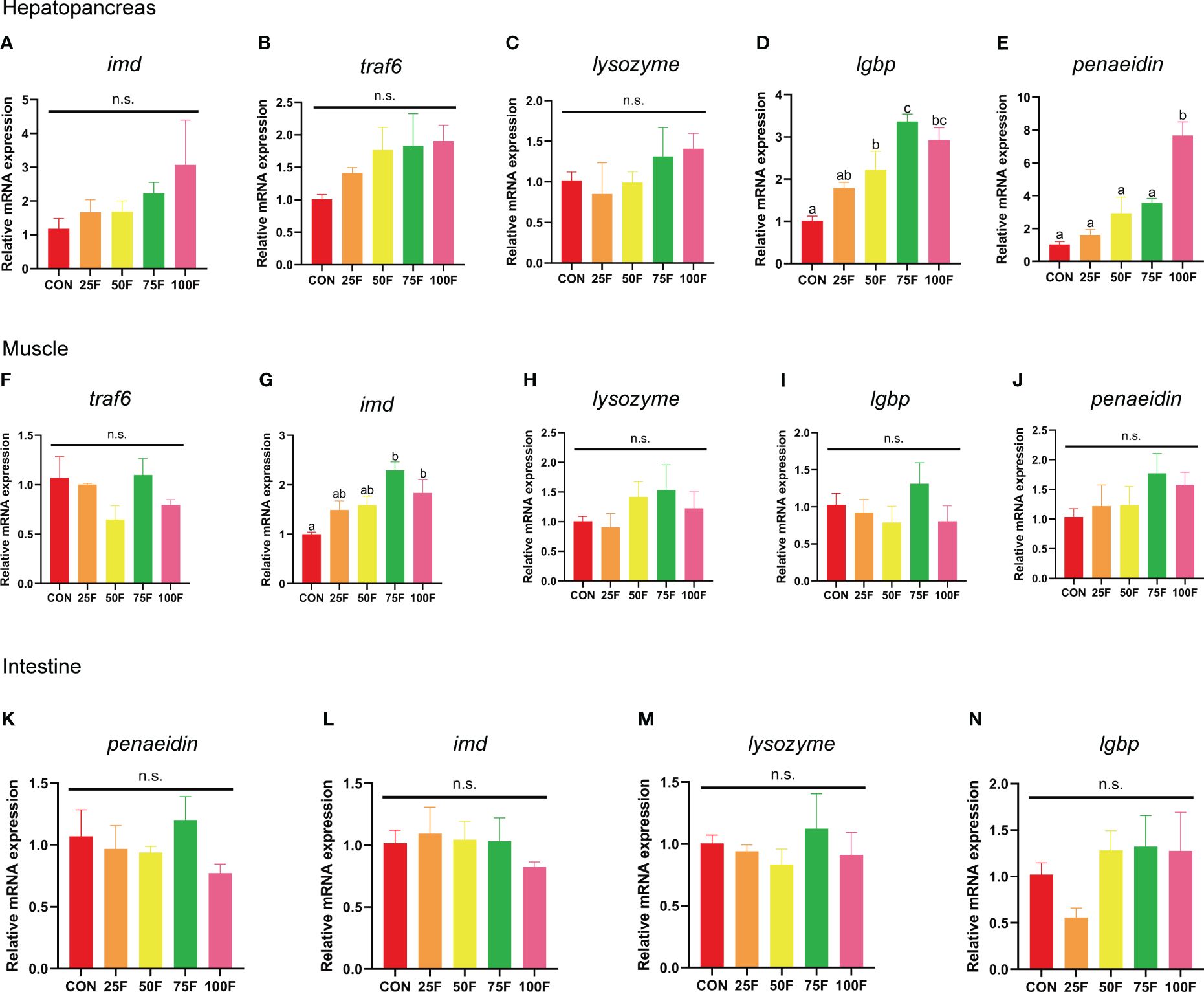
Figure 4 The effects of FSBM substitution on the mRNA expression of immunity genes in different tissues of shrimp (n=4). (A–E) mRNA expression of immunity genes in hepatopancreas. (F–J) mRNA expression of immunity genes in muscle. (K–N) mRNA expression of immunity genes in intestine. n. s., not significant.
3.6 The mRNA expression of ER stress-related genes in tissues
In the hepatopancreas, FSBM substitution did not significantly affected the expression of atf4 (activates transcription factor 4) and ire1 (inositol-requiring enzyme 1) (Figures 5B, D). However, FSBM substitution significantly enhanced the mRNA expression of eif2α (eukaryotic translation initiation factor 2A) with a linear and quadratic pattern (Figure 5C, Table 11). Compared with the control group, only 25F significantly increased the mRNA expression of bip (immunoglobulin heavy chain binding protein) (Figure 5A). In the muscle, compared with the control group, FSBM substitution did not significantly affect the expression of eif2α and atf4, but significantly inhibited the mRNA expression of bip with a linear and quadratic pattern (Figures 5E, F, H, Table 11). The mRNA expression of ire1 in 50F was significantly lower than that in the control group, while 75F significantly enhanced the mRNA expression of ire1 (Figure 5G). In the intestine, FSBM substitution did not affect the mRNA expression of atf4 and eif2α (Figures 5I, J). When the FSBM substitution ratio exceeded 75%, the mRNA expression of bip was significantly increased with a linear pattern (Figure 5L, Table 11). Compared with the control group, 75F significantly enhanced the mRNA expression of ire1 (Figure 5K).
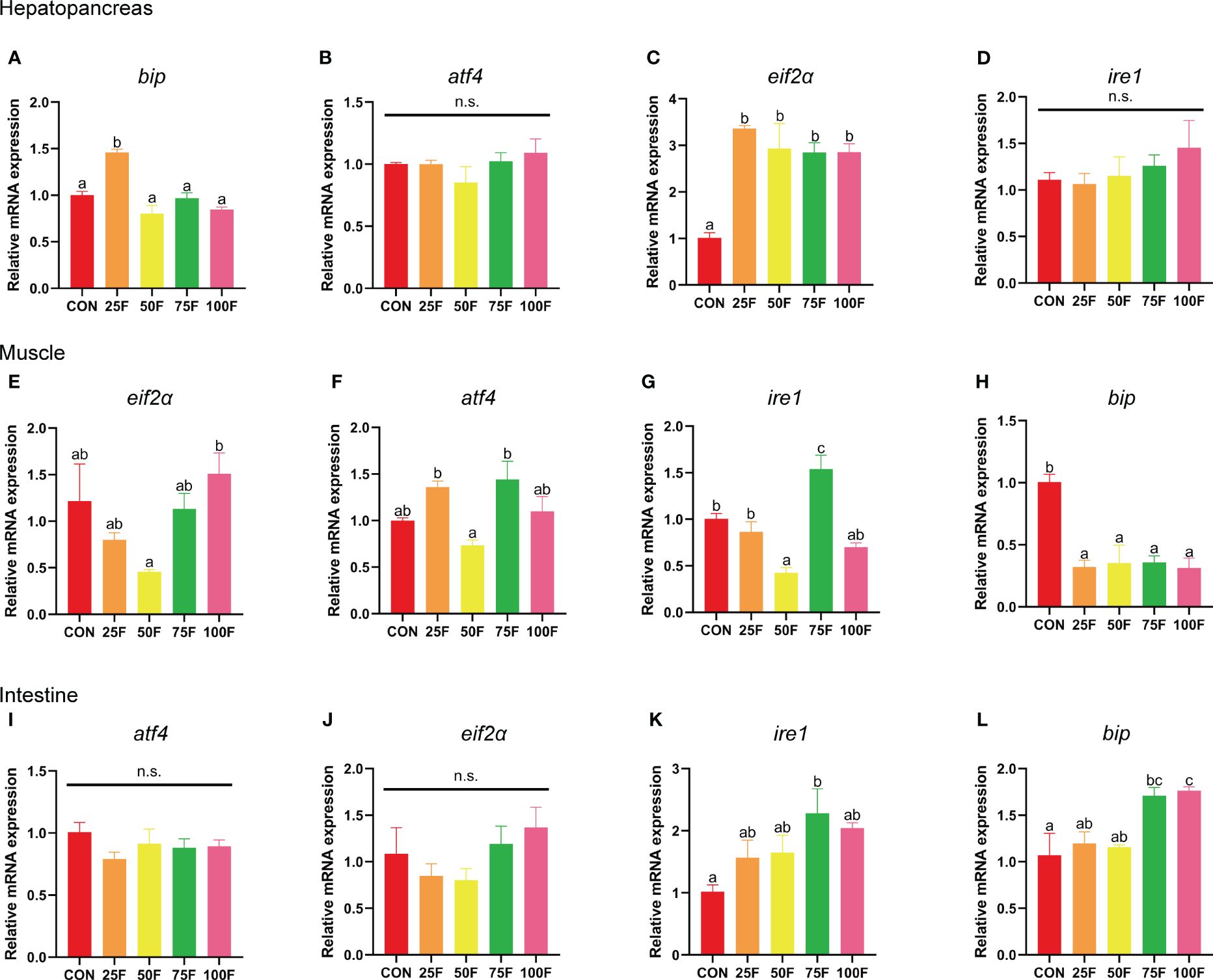
Figure 5 The effects of FSBM substitution on the mRNA expression of endoplasmic reticulum stress-related genes in different tissues of shrimp (n=4). (A–D) mRNA expression of endoplasmic reticulum stress-related genes in hepatopancreas. (E–H) mRNA expression of endoplasmic reticulum stress-related genes in muscle. (I–L) mRNA expression of endoplasmic reticulum stress-related genes in intestine. n. s., not significant.
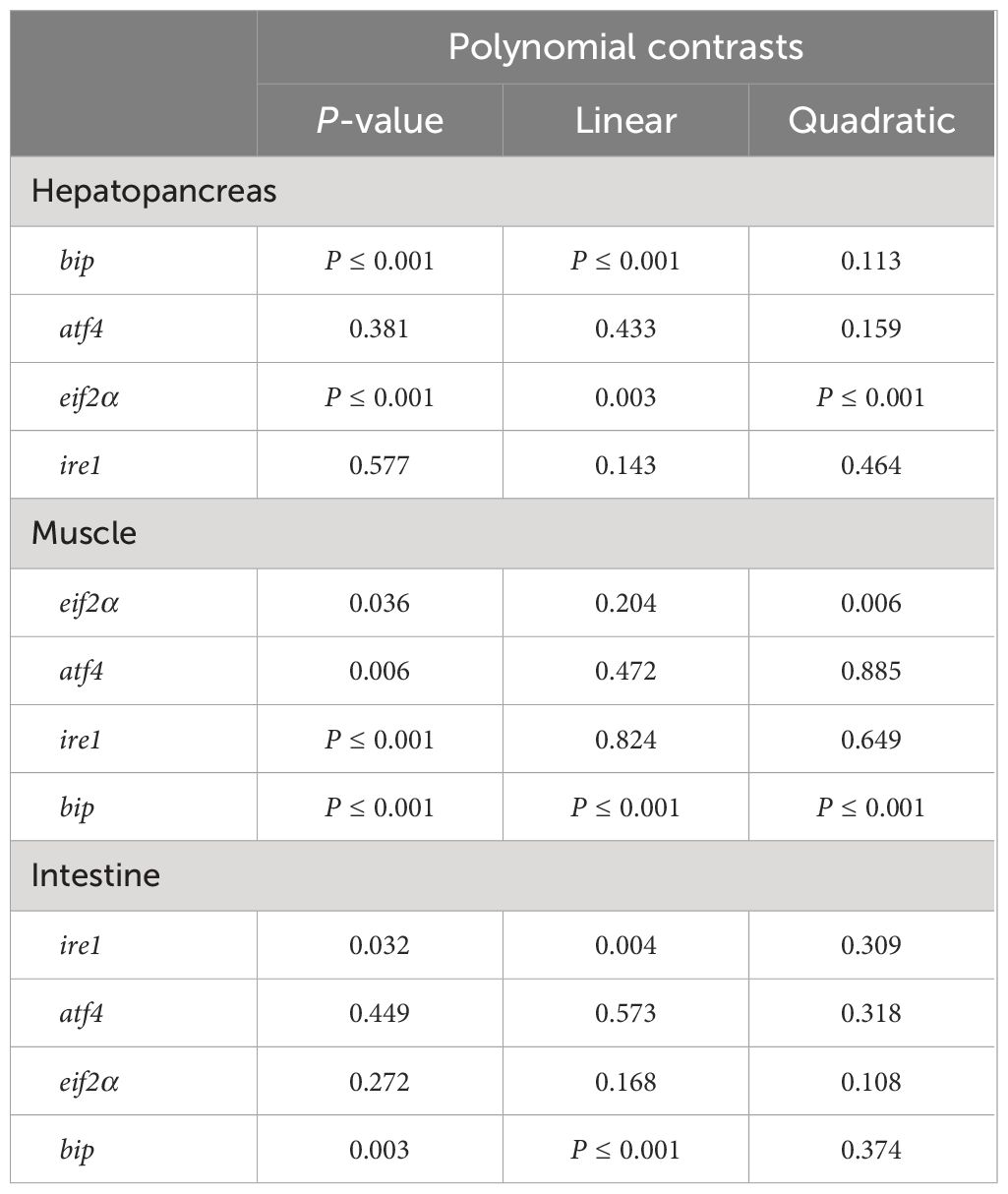
Table 11 Polynomial contrasts of endoplasmic reticulum stress-related gene expression in different tissues.
4 Discussion
The purpose of this study is to explore the application value of SBM fermented by a self-developed probiotic strain of Bacillus subtilis with non starch polysaccharides and antigen protein hydrolysis ability in the feed of Litopenaeus vannamei. Shrimp, like other aquatic animals, do not actually need protein; instead, they need a well-balanced combination of essential and nonessential amino acids provided by protein sources (Council et al., 2011). Hence, the amino acid composition of whole shrimp under FSBM substitution was first detected. We found that FSBM substitution significantly reduced the content of lysine in whole shrimp. Lysine is abundant in animal-based protein, but lacking in plant-based protein (Gorissen et al., 2018). Therefore, lysine is also known as the first limiting amino acid in the process of replacing fish meal with plant protein in aquaculture (Sá et al., 2021). The short of lysine is one of the reasons that affect the utilization efficiency of plant-based protein. Previous study has shown that fermentation technology can increase the content of lysine in SBM (Yang et al., 2018). However, it still cannot fill the gap between FSBM and fish meal. On the contrary, the content of arginine in whole shrimp body gradually increased with the increase of FSBM substitution proportion in the present study. Similar results have also been confirmed in African catfish (Clarias gariepinus) (Kari et al., 2022). Arginine is considered as an essential amino acid unique to aquatic animals (Zhou et al., 2012), and the content of arginine in SBM is already high. Perhaps plant protein regulated the gene expression and enzyme activity of arginine metabolism, but the specific mechanism still needs to be further explored. Overall, with the substitution of FSBM, the amino acid composition of the whole shrimp is relatively stable.
Protein sources in the feed not only affect the protein metabolism of aquatic animals, but also have an impact on lipid metabolism (Torrecillas et al., 2021). In the present study, we revealed the effect of FSBM replacing fish meal on shrimp lipid metabolism from two aspects. On the one hand, when the FSBM replacement ratio exceeded 50%, the content of TG and T-CHO in the hemolymph significantly decreased. Similar results were also confirmed in Jannathulla’s study (Jannathulla et al., 2018). they indicated that Aspergillus niger-fermented SBM replacing fish meal significantly reduced the content of T-CHO and TG in the hemolymph of shrimp, which may be attributed to the lipid-lowering effect of plant-based proteins. However, Yue et al. (2012) found that using SBM and peanut meal to replace fish meal did not change the lipid metabolism indicators of the hemolymph. On the other hand, we also tested the effect of FSBM on the fatty acid composition of shrimp. As the proportion of FSBM substitution increased, the contents of α-linolenic acid, linoleic acid and n-6 PUFAs in the whole shrimp body and muscle were increased. However, the content of DHA in muscle was reduced. Both of the above phenomena reflected the difference in fatty acids between protein raw materials. Soybeans are rich in α- linolenic acid (Asekova et al., 2014) but lacking in DHA. There is relatively little research on the effect of FSBM replacing fish meal on the fatty acid composition in aquatic animals. This study just filled this gap and provided new ideas for optimizing the application strategy of FSBM in aquatic feed.
During the aquaculture process, nutrient availability is one of the main factors inducing oxidative stress in aquatic animals (Ciji and Akhtar, 2021). Therefore, antioxidant indicators in different tissues were detected. As a representative species of open circulation system, the hemolymph of shrimp plays an important role in nutrient transport, innate immune response, and stress resistance (Tassanakajon et al., 2013; Irani et al., 2022). In the present study, when the proportion of FSBM exceeded 50%, the content of GSH significantly decreased. GSH is a key substrate for GPX to play catalyzing function, which is synthesized from glycine, cysteine, and glutamate (Ruiz-Ramírez et al., 2014). The previous study in our lab indicated that the lower content of glycine in FSBM may be the reason for the decrease in GSH in largemouth bass (Chen et al., 2023). Recently, the function of glycine in aquatic feed is gradually being explored, and studies in hybrid striped bass have also emphasized the growth promoting effect of glycine in SBM-based diets (He et al., 2023; Li et al., 2023). We speculate that dietary glycine supplementation may enhance the utilization efficiency of FSBM in aquaculture. In addition, from the activities of SOD and T-AOC, 25% FSBM substitution enhanced antioxidant capacity, although there is no significant difference. The hepatopancreas is important organ in shrimp and responsible for digestion, absorption, storage and metabolism (Liang et al., 2022). However, hepatopancreas is also easily disturbed by oxidative stress (Yu et al., 2023). In this study, the activity of SOD significantly decreased after fish meal protein was 100% replaced by FSBM, and the content of GSH also showed a similar trend to that of hemolymph. In addition, the mRNA expression level of cat significantly increased after FSBM replaced over 75% of fish meal protein. The above indicators also proved that a high proportion of FSBM replacing fish meal caused oxidative stress in the hepatopancreas, which can be reflected by the gradual increase in the content of lipid peroxidation product MDA, although there was no significant difference. Oxidative stress in the hepatopancreas is an important factor limiting the proportion of plant-based protein sources, as oxidative stress can easily damage the structure of the hepatopancreas, thereby affecting their normal function (Yang et al., 2022b). In this study, histological sectioning results showed that when the FSBM substitution ratio exceeded 50%, the hepatopancreas structures were damaged, with severe vacuolization and detachment of glandular ducts from the basement membrane. The conclusion that a high proportion of plant-based proteins damage the structure of the hepatopancreas has also been confirmed in Lin’s study (Lin and Chen, 2022). Compared with hepatopancreas, there is less research on oxidative stress in muscle. However, it is undeniable that as an edible part of shrimp, ensuring the normal growth and development of muscles is crucial for consumers. Oxidative stress can easily cause damage to muscle growth (Kozakowska et al., 2015; Huang et al., 2022), which is reflected in sparse muscle fiber density and shortened sarcomere length. The intestine of shrimp plays critical role of digesting and absorbing nutrients. Therefore, the health of the intestine affects the growth status of shrimp. Here, we found that FSBM substitution had little effect on the antioxidant enzyme activity in the intestine, but significantly reduced the gene expression of hsp70. Hsp70 can protect the proteome from stress interference and is also known as the intestinal gatekeepers due to its interaction with gut microbiota (Liu et al., 2014). Actually, microbiomics is often used to investigate the effects of FSBM on the intestinal health of aquatic animals (Cheng et al., 2020; Wei et al., 2021). Probiotics used for fermentation may affect the colonization and abundance of gut microbiota. Therefore, we will introduce microbiology to further explore the impact of FSBM on the intestinal health of shrimp in the future.
The execution of basic cellular functions requires regulated protein folding homeostasis. The endoplasmic reticulum (ER) is an active organelle that achieves this function by folding and modifying secreted and membrane proteins (Kettel and Karagöz, 2024). Reactive oxygen species (ROS) produced in the oxidative stress can accelerate ER dysfunction (Tse et al., 2016). Hence, we explored the effect of FSBM substitution on the mRNA expression of genes associated with ER stress. Endoplasmic reticulum chaperone BIP (also known as GRP78) is essential for protein folding and quality control in the endoplasmic reticulum lumen (Liu et al., 2023). However, in the present study, the response of bip in the intestine and muscle to FSBM substitution showed an opposite trend. Chen et al. (2021) demonstrated that tunicamycin (TM) could induced ER stress in the liver of large yellow croaker (Larimichthys crocea) and enhanced the mRNA expression of bip. Hence, the elevation of bip expression in the intestine of shrimp indicated that high proportion of FSBM induced ER stress. However, further research is needed to investigate the mechanisms by which FSBM reduced the expression of bip in muscle. In addition, the response of eif2α in different tissues under FSBM substitution also varied acutely. With the increase of FSBM substitution proportion, the mRNA expression of eif2α showed a trend of decreasing first and then increasing. However, all FSBM substitution group enhanced the mRNA expression of eif2α. Eif2α has been proved to protect tissues against ER stress (Yu et al., 2021). Actually, the expression of some specific genes does not represent whether the ER stress has really occurred, because it is a complete response. Hence, further exploration is needed in the future to investigate the mechanism of FSBM in regulating ER stress.
The health status of the body is a balance of various opposites, including stress and anti-stress, immunity and anti-immunity. When this balance is disrupted, the immunity system is destroyed and inflammation is occurred. Emerging evidence has shown that nutritional stimulation is an important factor affecting the immune status of the aquatic animal (Dawood, 2021), especially anti-nutritional factors (ANFs) in SBM. Although fermentation techniques can reduce ANFs in SBM, excessive FSBM has also been shown to reduce the immunity of farmed animals (Zhang et al., 2021). Invertebrates do not have the adaptive immune system and innate immunity system (including hemocytes and antimicrobial peptides) plays a crucial role in resisting external immune stimuli (recognition, cell–cell communication, lysis of foreign cells, encapsulation, phagocytosis, and cytotoxicity) (Ballarin et al., 2021; Lanz-Mendoza and Contreras-Garduño, 2022). Hence, the mRNA expression of immunity-related genes was examined in the present study. FSBM substitution did not significantly affect the expression of immune genes in the muscle and intestine. However, a high proportion of FSBM substitution activated lgbp and penaeidin in the hepatopancreas. Lgbp is a kind of pattern recognition proteins (PRPs) which is responsible for activation of the prophenoloxidase system and plays essential roles in host immunological defense (Zhang et al., 2016). Previous studies have mainly emphasized its role in resisting bacterial infection (Zhang et al., 2016). Recently, there have been reports of its protective role in resisting external stress (Chen et al., 2015). However, its activation in nutritional stress is the first time reported in the present study. Penaeidin is an antimicrobial peptide isolated from shrimp hemolymph, which is activated under bacteria and virus infection, especially in inflammation conditions (Song and Li, 2014). Studies in the aquatic animals found that probiotics in feed can positively regulate the expression of penaeidin and enhance immunity (Pooljun et al., 2020; Tseng et al., 2023). However, Bacillus subtilis in this study did not affect its expression during a low-proportion FSBM replacement, whereas a sharp rise in the total FSBM replacement group suggested that the hepatopancreas might be diseased. Due to the high temperature treatment during the production process, the finished feed may contain no active Bacillus subtilis. Co-feeding Bacillus subtilis may be a better method to exert the probiotic effect of subtilis.
In summary, through the responses to FSBM in different tissues, the screened Bacillus subtilis is a bioconverter of low value biomass (SBM) into high value feed ingredient, which can replace up to 25% dietary fish meal without affecting antioxidant capacity and immunity. Moreover, 25% FSBM substitution enhanced hepatopancreas structure. Considering the shortage of fish meal resources affecting the development of shrimp industry, this FSBM has clear application prospects.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of Zhejiang Ocean University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SC: Formal analysis, Methodology, Writing – original draft. JD: Formal analysis, Investigation, Methodology, Writing – review & editing. YC: Funding acquisition, Resources, Writing – review & editing. QC: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. FD: Software, Validation, Visualization, Writing – review & editing. CW: Investigation, Methodology, Writing – review & editing. YS: Software, Validation, Writing – review & editing. JW: Conceptualization, Project administration, Resources, Writing – review & editing. TH: Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Ten-thousand Talents Plan of Zhejiang Province (No. 2022R52021), “Pioneer” and “Leading Goose” R&D Program of Zhejiang (No: 2022C04020) and Zhoushan Science and Technology Project (No: 2023C41008; No: 2023C41009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1449066/full#supplementary-material
References
Abd El-Naby A. S., Eid A., Gaafar A. Y., Sharawy Z., Khattaby A., El-sharawy M. S., et al. (2023). Overall evaluation of the replacement of fermented soybean to fish meal in juvenile white shrimp, Litopenaeus vannamei diet: growth, health status, and hepatopancreas histomorphology. Aquacult. Int., 1–19.
Abd El-Naby A. S., Eid A., Gaafar A. Y., Sharawy Z., Khattaby A., El-sharawy M. S., et al. (2023). Overall evaluation of the replacement of fermented soybean to fish meal in juvenile white shrimp, Litopenaeus vannamei diet: growth, health status, and hepatopancreas histomorphology. Aquacult. Int. 32, 1665–1683. doi: 10.1007/s10499-023-01234-0
Asekova S., Chae J.-H., Ha B.-K., Dhakal K. H., Chung G., Shannon J., et al. (2014). Stability of elevated α-linolenic acid derived from wild soybean (Glycine soja Sieb. & Zucc.) across environments. Euphytica 195, 409–418. doi: 10.1007/s10681-013-1004-1
Ballarin L., Karahan A., Salvetti A., Rossi L., Manni L., Rinkevich B., et al. (2021). Stem cells and innate immunity in aquatic invertebrates: Bridging two seemingly disparate disciplines for new discoveries in biology. Front. Immunol. 12, 688106. doi: 10.3389/fimmu.2021.688106
Barragán L. P., Figueroa J., Durán L. R., González C. A., Hennigs C. (2016). Fermentative production methods, Biotransformation of Agricultural Waste and By-Products (Elsevier), 189–217. doi: 10.1016/B978-0-12-803622-8.00007-0
Cao Y., Xu M., Lu J., Cai G. (2023). Simultaneous microbial fermentation and enzymolysis: a biotechnology strategy to improve the nutritional and functional quality of soybean meal. Food Rev. Int. 40, 1–16. doi: 10.1080/87559129.2023.2255892
Chen Y.-Y., Chen J.-C., Lin Y.-C., Yeh S.-T., Huang C.-L. (2015). White shrimp Litopenaeus vannamei that have received Gracilaria tenuistipitata extract show early recovery of immune parameters after ammonia stressing. Mar. Drugs 13, 3606–3624. doi: 10.3390/md13063606
Chen Q., Fang W., Cui K., Chen Q., Xiang X., Zhang J., et al. (2021). Endoplasmic reticulum stress induces hepatic steatosis by transcriptional upregulating lipid droplet protein perilipin2. FASEB J. 35, e21900. doi: 10.1096/fj.202100739RR
Chen Y., Mitra A., Rahimnejad S., Chi S., Kumar V., Tan B., et al. (2024). Retrospect of fish meal substitution in Pacific white shrimp (Litopenaeus vannamei) feed: Alternatives, limitations and future prospects. Rev. Aquacult. 16, 382–409. doi: 10.1111/raq.12843
Chen Q., Wang C., Sun Y., Chen Y., Chen S., Han T., et al. (2023). Excessive substitution of fish meal with fermented soybean meal induces oxidative stress by impairing glutathione metabolism in largemouth bass (Micropterus salmoides). Antioxidants 12, 2096. doi: 10.3390/antiox12122096
Cheng A. C., Yeh S. P., Hu S. Y., Lin H. L., Liu C. H. (2020). Intestinal microbiota of white shrimp, Litopenaeus vannamei, fed diets containing Bacillus subtilis E20-fermented soybean meal (FSBM) or an antimicrobial peptide derived from B. subtilis E20-FSBM. Aquacult. Res. 51, 41–50. doi: Abd El-Naby
Ciji A., Akhtar M. S. (2021). Stress management in aquaculture: A review of dietary interventions. Rev. Aquacult. 13, 2190–2247. doi: 10.1111/raq.12565
Council N. R., Earth D.o., Studies L., Fish C. (2011). Shrimp, Nutrient requirements of fish and shrimp (National academies press).
Dai C., Ma H., He R., Huang L., Zhu S., Ding Q., et al. (2017). Improvement of nutritional value and bioactivity of soybean meal by solid-state fermentation with Bacillus subtilis. Lwt 86, 1–7. doi: 10.1016/j.lwt.2017.07.041
Dawood M. A. (2021). Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquacult. 13, 642–663. doi: 10.1111/raq.12492
Gorissen S. H., Crombag J. J., Senden J. M., Waterval W. H., Bierau J., Verdijk L. B., et al. (2018). Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50, 1685–1695. doi: 10.1007/s00726-018-2640-5
He W., Posey E. A., Steele C. C., Savell J. W., Bazer F. W., Wu G. (2023). Dietary glycine supplementation enhances postweaning growth and meat quality of pigs with intrauterine growth restriction. J. Anim. Sci. 101, skad354. doi: 10.1093/jas/skad354
Huang X., Huang Z., Sun L., Qiu M., Deng Q., Fang Z., et al. (2022). Protective mechanisms of three antioxidants against T-2 toxin-induced muscle protein deterioration in shrimp. J. Sci. Food Agricult. 102, 4883–4891. doi: 10.1002/jsfa.11851
Irani M., Rajabi Islami H., Nafisi Bahabadi M., Hosseini Shekarabi S. P. (2022). Effect of density on performance and some hemolymph antioxidant parameters of white-leg shrimp (Litopenaeus vannamei) in a biofloc system. Aquat. Physiol. Biotechnol. 10, 41–62. doi: 10.22124/JAPB.2022.21352.1451
Jannathulla R., Dayal J. S., Ambasankar K., Muralidhar M. (2018). Effect of Aspergillus Niger fermented soybean meal and sunflower oil cake on growth, carcass composition and haemolymph indices in Penaeus vannamei Boon. Aquaculture 486, 1–8. doi: 10.1016/j.aquaculture.2017.12.005
Kari Z. A., Kabir M. A., Dawood M. A., Razab M. K. A. A., Ariff N. S. N. A., Sarkar T., et al. (2022). Effect of fish meal substitution with fermented soy pulp on growth performance, digestive enzyme, amino acid profile, and immune-related gene expression of African catfish (Clarias gariepinus). Aquaculture 546, 737418. doi: 10.1016/j.aquaculture.2021.737418
Kari Z. A., Mat K., Kabir M. A., ZAL W. A., MUNIR M. B., WEI L. S., et al. (2023). Soybean by-product: As an alternative to fish meal as protein source for aquaculture industry. J. Sustainabil. Sci. Manag. 18, 177–200. doi: 10.46754/jssm
Kårlund A., Gómez-Gallego C., Korhonen J., Palo-Oja O. M., El-Nezami H., Kolehmainen M. (2020). Harnessing microbes for sustainable development: food fermentation as a tool for improving the nutritional quality of alternative protein sources. Nutrients 12, 1020. doi: 10.3390/nu12041020
Kettel P., Karagöz G. E. (2024). ENDOPLASMIC RETICULUM: Monitoring and maintaining protein and membrane homeostasis in the endoplasmic reticulum by the unfolded protein response. Int. J. Biochem. Cell Biol. 172, 106598. doi: 10.1016/j.biocel.2024.106598
Kozakowska M., Pietraszek-Gremplewicz K., Jozkowicz A., Dulak J. (2015). The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J. Muscle Res. Cell Motility. 36, 377–393. doi: 10.1007/s10974-015-9438-9
Lanz-Mendoza H., Contreras-Garduño J. (2022). Innate immune memory in invertebrates: Concept and potential mechanisms. Dev. Comp. Immunol. 127, 104285. doi: 10.1016/j.dci.2021.104285
Li X., He W., Wu G. (2023). Dietary glycine supplementation enhances the growth performance of hybrid striped bass (Morone saxatilis♀× Morone chrysops♂) fed soybean meal-based diets. J. Anim. Sci. 101, skad345. doi: 10.1093/jas/skad345
Liang Z., Chen T., Yang F., Li S., Zhang S., Guo H. (2022). Toxicity of chronic waterborne zinc exposure in the hepatopancreas of white shrimp Litopenaeus vannamei. Chemosphere 309, 136553. doi: 10.1016/j.chemosphere.2022.136553
Lin Y.-H., Chen Y.-T. (2022). Lactobacillus spp. fermented soybean meal partially substitution to fish meal enhances innate immune responses and nutrient digestibility of white shrimp (Litopenaeus vannamei) fed diet with low fish meal. Aquaculture 548, 737634. doi: 10.1016/j.aquaculture.2021.737634
Liu H., Dicksved J., Lundh T., Lindberg J. E. (2014). Heat shock proteins: intestinal gatekeepers that are influenced by dietary components and the gut microbiota. Pathogens 3, 187–210. doi: 10.3390/pathogens3010187
Liu Z., Liu G., Ha D. P., Wang J., Xiong M., Lee A. S. (2023). ER chaperone GRP78/BiP translocates to the nucleus under stress and acts as a transcriptional regulator. Proc. Natl. Acad. Sci. 120, e2303448120. doi: 10.1073/pnas.2303448120
Liu T., Xu H., Han T., Wang J., Yin F., Wang C. (2021). Effect of dietary egg yolk lecithin levels on survival, growth, lipid metabolism, and antioxidant capacity of early juvenile green mud crab Scylla paramamosain. Aquaculture 540, 736706. doi: 10.1016/j.aquaculture.2021.736706
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Pooljun C., Daorueang S., Weerachatyanukul W., Direkbusarakom S., Jariyapong P. (2020). Enhancement of shrimp health and immunity with diets supplemented with combined probiotics: application to Vibrio parahaemolyticus infections. Dis. Aquat. Organisms. 140, 37–46. doi: 10.3354/dao03491
Qi N., Zhan X., Milmine J., Sahar M., Chang K.-H., Li J. (2023). Isolation and characterization of a novel hydrolase-producing probiotic Bacillus licheniformis and its application in the fermentation of soybean meal. Front. Nutr. 10, 1123422. doi: 10.3389/fnut.2023.1123422
Ruiz-Ramírez A., Ortiz-Balderas E., Cardozo-Saldaña G., Diaz-Diaz E., El-Hafidi M. (2014). Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. 126, 19–29. doi: 10.1042/CS20130164
Sá A. G. A., Silva D. C. D., Pacheco M. T. B., Moreno Y. M. F., Carciofi B. A. M. (2021). Oilseed by-products as plant-based protein sources: Amino acid profile and digestibility. Future Foods 3, 1020. doi: 10.1016/j.fufo.2021.100023
Shrestha A. K., Dahal N. R., Ndungutse V. (2010). Bacillus fermentation of soybean: A review. J. Food Sci. Technol. Nepal. 6, 1–9. doi: 10.3126/jfstn.v6i0.8252
Song Y.-L., Li C.-Y. (2014). Shrimp immune system-special focus on penaeidin. J. Mar. Sci. Technol. 22, doi: 10.6119/JMST-013-0813-1.
Tassanakajon A., Somboonwiwat K., Supungul P., Tang S. (2013). Discovery of immune molecules and their crucial functions in shrimp immunity. Fish. Shellfish. Immunol. 34, 954–967. doi: 10.1016/j.fsi.2012.09.021
Torrecillas S., Montero D., Carvalho M., Benitez-Santana T., Izquierdo M. (2021). Replacement of fish meal by Antarctic krill meal in diets for European sea bass Dicentrarchus labrax: Growth performance, feed utilization and liver lipid metabolism. Aquaculture 545, 737166. doi: 10.1016/j.aquaculture.2021.737166
Tse G., Yan B. P., Chan Y. W., Tian X. Y., Huang Y. (2016). Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis. Front. Physiol. 7, 208400. doi: 10.3389/fphys.2016.00313
Tseng K.-C., Huang H.-T., Huang S.-N., Yang F.-Y., Li W.-H., Nan F.-H., et al. (2023). Lactobacillus plantarum isolated from kefir enhances immune responses and survival of white shrimp (Penaeus vannamei) challenged with Vibrio alginolyticus. Fish. Shellfish. Immunol. 135, 108661. doi: 10.1016/j.fsi.2023.108661
Wang C., Qiu X., Hou R., Liu J., Li L., Mao X. (2023). Improvement of soybean meal quality by one-step fermentation with mixed-culture based on protease activity. Innovative. Food Sci. Emerging. Technol. 85, 103311. doi: 10.1016/j.ifset.2023.103311
Wang P., Wang S., Zhu C., Sun Y., Yan Q., Yi G. (2024). Monascus purpureus M-32 fermented soybean meal improves the growth, immunity parameters, intestinal morphology, disease resistance, intestinal microbiota and metabolome in Pacific white shrimp (Litopenaeus vannamei). Anim. Nutr. 17, 283-296. doi: 10.1016/j.aninu.2024.03.009
Wei C., Wang X., Li C., Zhou H., Liu C., Mai K., et al. (2021). Replacement of fishmeal with Shewanella sp. MR-7 fermented soya bean meal in Pacific white shrimp. Aquacult. Res. 52, 2110–2120. doi: 10.1111/are.15063
Xie S., Liu Y., Tian L., Niu J., Tan B. (2020). Low dietary fish meal induced endoplasmic reticulum stress and impaired phospholipids metabolism in juvenile pacific white shrimp, Litopenaeus vannamei. Front. Physiol. 11, 1024. doi: 10.3389/fphys.2020.01024
Yang S., Luo J., Huang Y., Yuan Y., Cai S. (2022b). Effect of sub-lethal ammonia and nitrite stress on autophagy and apoptosis in hepatopancreas of Pacific whiteleg shrimp Litopenaeus vannamei. Fish. Shellfish. Immunol. 130, 72–78. doi: 10.1016/j.fsi.2022.08.069
Yang H., Rahman M. M., Li X., Sharifuzzaman S., Leng X. (2022a). Dietary leucine requirement of juvenile largemouth bass (Micropterus salmoides) based on growth, nutrient utilization and growth-related gene analyses. Aquaculture 555, 738207. doi: 10.1016/j.aquaculture.2022.738207
Yang A., Zuo L., Cheng Y., Wu Z., Li X., Tong P., et al. (2018). Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food Funct. 9, 1899–1909. doi: 10.1039/C7FO01824J
Yu Q., Fu Z., Huang M., Xu C., Wang X., Qin J. G., et al. (2021). Growth, physiological, biochemical, and molecular responses of Pacific white shrimp Litopenaeus vannamei fed different levels of dietary selenium. Aquaculture 535, 736393. doi: 10.1016/j.aquaculture.2021.736393
Yu Y., Hu L., Tian D., Yu Y., Lu L., Zhang J., et al. (2023). Toxicities of polystyrene microplastics (MPs) and hexabromocyclododecane (HBCD), alone or in combination, to the hepatopancreas of the whiteleg shrimp, Litopenaeus vannamei. Environ. pollut. 329, 121646. doi: 10.1016/j.envpol.2023.121646
Yue Y. R., Liu Y. J., Tian L. X., Gan L., Yang H. J., Liang G. Y. (2012). Effects of replacing fish meal with soybean meal and peanut meal on growth, feed utilization and haemolymph indexes for juvenile white shrimp Litopenaeus vannamei, Boone. Aquacult. Res. 43, 1687–1696. doi: 10.1111/are.2012.43.issue-11
Zhang S., Shi Y., Zhang S., Shang W., Gao X., Wang H. (2014). Whole soybean as probiotic lactic acid bacteria carrier food in solid-state fermentation. Food Control. 41, 1–6. doi: 10.1016/j.foodcont.2013.12.026
Zhang W., Tan B., Deng J., Dong X., Yang Q., Chi S., et al. (2021). Effects of high level of fermented soybean meal substitution for fish meal on the growth, enzyme activity, intestinal structure protein and immune-related gene expression and intestinal flora in juvenile pearl gentian grouper. Aquacult. Nutr. 27, 1433–1447. doi: 10.1111/anu.13281
Zhang X., Zhu Y.-T., Li X.-J., Wang S.-C., Li D., Li W.-W., et al. (2016). Lipopolysaccharide and beta-1, 3-glucan binding protein (LGBP) stimulates prophenoloxidase activating system in Chinese mitten crab (Eriocheir sinensis). Dev. Comp. Immunol. 61, 70–79. doi: 10.1016/j.dci.2016.03.017
Zheng L., Li D., Li Z. L., Kang L. N., Jiang Y. Y., Liu X. Y., et al. (2017). Effects of Bacillus fermentation on the protein microstructure and anti-nutritional factors of soybean meal. Lett. Appl. Microbiol. 65, 520–526. doi: 10.1111/lam.12806
Zhi L., Zihong Z., Yudi H., Piao C., Xianmei G., Bin L., et al. (2023). Origin and farming pattern authentication of wild-caught, coast-pond and freshwater farming white shrimp (Litopenaeus vannamei) in Chinese market using multi-stable isotope analysis of tail shell. Food Control. 148, 109646. doi: 10.1016/j.foodcont.2023.109646
Keywords: antioxidant activity, endoplasmic reticulum stress, fermented soybean meal, immunity, Litopenaeus vannamei
Citation: Chen S, Dai J, Chen Y, Chen Q, Dong F, Wang C, Sun Y, Wang J and Han T (2024) Effects of Bacillus subtilis-fermented soybean meal replacing fish meal on antioxidant activity, immunity, endoplasmic reticulum stress and hepatopancreas histology in Pacific white shrimp (Litopenaeus vannamei). Front. Mar. Sci. 11:1449066. doi: 10.3389/fmars.2024.1449066
Received: 14 June 2024; Accepted: 09 July 2024;
Published: 22 July 2024.
Edited by:
Lei Wang, Anhui Normal University, ChinaReviewed by:
Dong Han, Chinese Academy of Sciences (CAS), ChinaAntonio Luca Langellotti, University of Naples Federico II, Italy
Junyan Jin, Chinese Academy of Sciences (CAS), China
Copyright © 2024 Chen, Dai, Chen, Chen, Dong, Wang, Sun, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Chen, Y2hlbnFpYW5nQHpqb3UuZWR1LmNu; Tao Han, Z29vZGhhbnRhb0BnbWFpbC5jb20=
 Songming Chen1
Songming Chen1 Jieyu Dai
Jieyu Dai Qiang Chen
Qiang Chen Yulong Sun
Yulong Sun Jiteng Wang
Jiteng Wang Tao Han
Tao Han