- 1Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, La Paz, BCS, Mexico
- 2Beneath The Waves, Boston, MA, United States
- 3Ocean Safaris, Monterey Bay, CA, United States
- 4Wander Out Expeditions, Honolulu, HI, United States
- 5Conexiones Terramar Asociación Civil (A.C.), La Paz, Baja California Sur, Mexico
- 6Protección y Conservación Pelágica Asociación Civil (A.C.), Ciudad de México, Mexico
Killer whales (Orcinus orca) are the apex predator in global oceans, and as such they are afforded access to prey species at all trophic levels and sizes. Due to their enhanced cognitive abilities, they are frequent predators of other ocean giants, including large sharks. Observations of these predator-prey interactions are rare globally; however, records appear to be increasing in recent years, possibly due to increased access to surveillance. Here we present reports of killer whales hunting and preying on the world’s largest fish species, the whale shark (Rhincodon typus), by collating and analyzing photo and video footage collected from four unique predation events spanning six years (2018 – 2024) in the southern Gulf of California. Across all events, orcas displayed a seemingly approach to collaboratively hunting and killing whale sharks, characterized by focusing on attacking the pelvic area (claspers and pelvic fins) which exsanguinates the prey and allows access to the lipid-rich liver. Photo identification of the killer whales revealed that an individual adult male “Moctezuma” was engaged in three of the four events, and the females involved in event four had previously been sighted with him. We suggest the potential existence of a specialized pod of elasmobranch-hunting killer whales occurring in the Gulf of California.
Introduction
Gigantism is the attainment of very large body sizes and is an eco-evolutionary phenomena that provides large consumers with increased ecological influence and competitive superiority (Vermeij, 2016; Pyenson and Vermeij, 2016). In marine ecosystems, gigantism is correlated with areas of high productivity, leading to the evolution of large, filter-feeding mammals such as the great whales, which comprise the largest living animals. The attainment of large body sizes also makes hunting and consumption challenging and energetically costly for most higher-order predators.
Killer whales (Orcinus orca) are the apex marine predator, and are distributed in all oceans and most seas, especially in areas characterized by high productivity. They feed on a wide variety of prey species such as marine mammals (Vargas Bravo et al., 2020), sea turtles (Pitman and Dutton, 2004), cephalopods (Hanson and Walker, 2014), bony and cartilaginous fish (Guerrero-Ruiz et al., 2007; Higuera Rivas et al., 2023). Observed variation in biological and ecological characteristics (including body size, color patterning, vocalization, social structure, and foraging strategies) led to the description of several killer whales’ “ecotypes” (de Bruyn et al., 2012). Three ecotypes of orcas co-occur in the northeastern Pacific: (1) ‘resident,’ which prey predominantly on bony fish, (2) ‘offshore,’ which is considered a cartilaginous fish ecotype, and (3) ‘transient’, which prey on marine mammals (Ford et al., 1998; Baird, 2000; Dahlheim et al., 2008). In the Eastern Tropical Pacific (ETP), an area that spans from Mexico to Peru, a new orca ecotype has been proposed, which appears to display a more generalized diet consisting of whales, dolphins, sea turtles, and bony fishes (Ortega-Ortiz et al., 2023). However, killer whales from the Gulf of California (hereafter “GoC”) have also been observed feeding on elasmobranchs (sharks and rays), and there have not been any subsequent revisions to the ETP ecotype (Guerrero-Ruiz et al., 1998; Higuera Rivas et al., 2023). Recently, the photo identification of certain individuals of killer whales that hunt elasmobranchs in the GoC has demonstrated that the feeding of sharks and rays appears to be specialized to same individuals (Higuera Rivas et al., 2023; Ayres et al., 2024) such as the adult male orca of this pod, identified as “Moctezuma,” first sighted in 1992 by Armando Jaramillo (Black et al., 1997). Within the group of targeted elasmobranch prey for killer whales in the GoC are Munk´s pygmy devil rays (Mobula munkiana; Higuera Rivas et al., 2023), other Mobula species (Guerrero-Ruiz et al., 2007), Carcharhinid sharks (e.g., Carcharhinus leucas; Ayres et al., 2024), Lamniform sharks (authors personal observation) and the whale shark (Rhincodon typus; Fertl et al., 1996; O’Sullivan and Mitchell, 2000; Guerrero-Ruiz et al., 2007; Ortega-Ortiz et al., 2023). However, proper documentation of these events remains limited.
Whale sharks are the largest member of the major clade Chondrichthyes, and at sizes reaching 18 meters, they are the largest fish of the planet (Vermeij, 2016). As with other examples of marine gigantism, the presence of whale sharks in the GoC is recorded seasonally in relation to planktonic blooming events at the following aggregation sites: Bahía de Los Ángeles (Ramírez-Macías et al., 2012), La Paz Bay (Ketchum et al., 2013), and Nayarit (Ramírez-Macías et al., 2015). La Paz Bay represents a rich feeding ground for juvenile whale shark (between 3 and 7 m TL; mostly males) from October to May (Ramírez-Macías et al., 2012). In this life-stage, the whale shark may be more vulnerable to predation from predators such as large sharks (Kukuyev, 1995; Fitzpatrick et al., 2006). In the GoC, killer whales have been anecdotally observed as predators of the whale shark once before (O’Sullivan and Mitchell, 2000) however, these types of interactions were not described in detail. To fill these knowledge gaps and confirm the presence of this predator-prey interaction, here we describe in detail, for the first time, killer whale predation events on the largest fish, the whale shark. We captured and analyzed observational photo and video data spanning four separate whale shark hunting events in the GoC and compared these existing photos identification of killer whales hunting on various elasmobranch species in the GoC (Higuera Rivas et al., 2023; Ayres et al., 2024). These data resulted in the genesis of a killer whale behavioral framework for cooperative and collaborative foraging on whale sharks, thereby presenting new information to support potential regional killer whale foraging specialization on elasmobranch fishes in the GoC, while elevating our understanding of the repertoire of hunting skills and predatory capabilities in killer whales.
Material and results
Predation events occurred between 2018 and 2024 in the southern GoC in the months of April, May, and June (Figure 1). To identify individual killer whales, present at each event, we captured and analyzed photographs of the dorsal fin and other distinctive features such as scars and/or nicks (Bigg et al., 1987). When photographs were not taken or available, stills from video footage taken during the events were exported and analyzed. The sex of the adult male was confirmed by the size and shape of the dorsal, pectoral and caudal fins, while the sex of the adult females was confirmed by analyzing images of their size and dorsal fins. When possible, the sex of the whale shark was determined by the presence of claspers in the ventral area (Norman and Stevens, 2007). Maturity stage of whale shark was established by the estimated total length < 8 m and, when possible, by observing clasper morphology (Norman and Stevens, 2007). Total length (TL) of killer whales and whale sharks were both estimated by comparing the animals with the known lengths of boats present at the events.
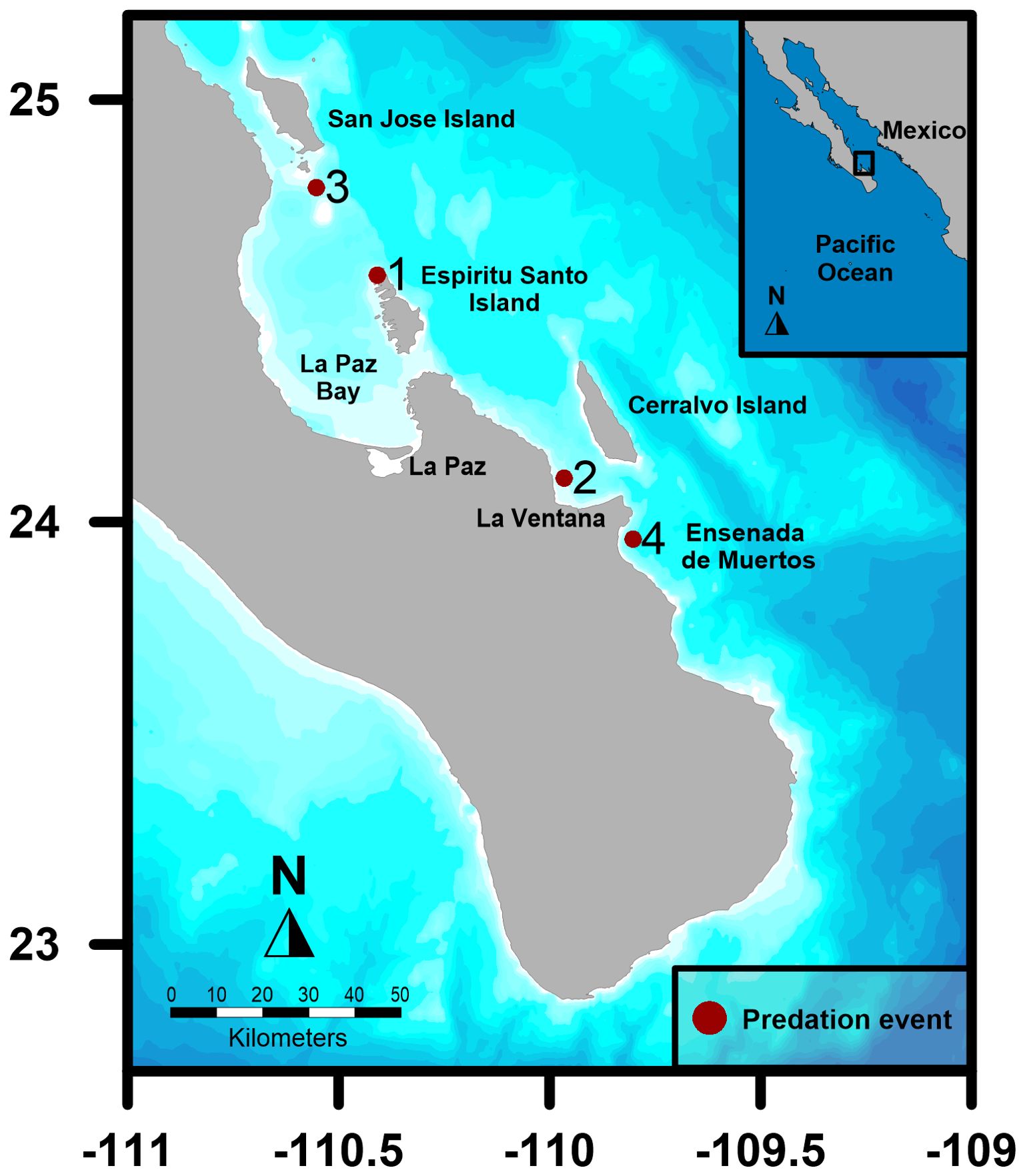
Figure 1. Map detailing the four killer whale’ predation events on whale sharks in the southern Gulf of California, Baja California Sur (the Mexican Pacific inset).
Event 1: On 13 May 2018, at 1200 hrs., tourists from two different tourism operator boats recorded their own topside videos of an adult male killer whale (estimated 8 m TL) approaching a juvenile whale shark (estimated 5 m TL) on the west side of Isla Partida, north of Espiritu Santo Archipelago (24°35.117’N &110°24.526’W; Figure 1), in southern GoC. The focal killer whale was identified as “Moctezuma”, a large adult male that was also identified and observed in 2018 feeding on Munk´s pygmy devil rays (Mobula munkiana) (Higuera Rivas et al., 2023). The weather was sunny, and the sea state was calm, with no swell. The first topside video (Supplementary Video S1) shows the killer whale “Moctezuma” approaching the whale shark (Figure 2A) that was still moving and upside down at the surface. This first observer’s boat approached the whale shark that presented several abrasions and bruises on the ventral side of its body, predominantly located between the pectoral fins, and a large wound near the pelvic fins was observed to be bleeding (Figure 2B). Topside video recorded from the second tourist (Supplementary Video S2) recorded the shark being pushed under the water by the orca’s mouth (Figure 2C). A few moments later, the shark reappeared on the surface with the pectoral fin outside the water (Figure 2D) while the killer whale was seen swimming in the radius of the shark. The killer whale then pushed the shark under the water, disappearing for an instant. The shark was then observed flapping the superior lobule of the caudal fin on the surface (Figure 2E) attempting to avoid contact with the killer whale while restoring equilibrium in the water column. Moctezuma’s dorsal fin appeared on the surface after a brief moment while the superior lobule of the caudal fin of the shark was outside the water (Figure 2F). (video available here: https://www.facebook.com/watch/?v=2056047654635366). The mammal continued ramming the shark on the surface, attempting to turn it upside down, provoking a large splash in the area (Figure 2G). Successively, a large slick of blood and floating tissue appeared in the water surface, attracting several seabirds such as yellow-footed seagulls (Larus livens) and frigatebirds (Fregata magnificens) which started feeding on the offal (Figure 2G). The killer whale disappeared for one minute, then was spotted taking several breaths while the tourist boats continued to observe the event (Figure 2H). The killer whale then disappeared entirely from the site of the attack, and the whale shark carcass could no longer be located.

Figure 2. Sequence of killer whale attack on whale shark on 13 May 2018: (A) Adult male killer whale, identified as “Moctezuma,” approaching the seemingly moribund whale shark; (B) the shark is left with at least one large bleeding wound, after being hit repeatedly, (C) the whale shark is pushed under the surface by the killer whale, (D) the whale shark is pushed on the surface again and its pectoral fins are outside the water for a moment, (E) the caudal fin of the whale shark is slapping on the surface while (F) the killer whale is behind the shark, (G) ramming it repeatedly. (H) Tourist boats observe “Moctezuma” disappearing after the attack (Photo credit: Eduardo Miranda).
Event 2: On 20 June 2021, at approximately 1500 hrs, in the proximity of La Ventana (24°6.245’N & 109°57.891’W; Figure 1) a tourist recorded a topside and an underwater video of at least six killer whales in the proximity of a motionless whale shark. The weather was overcast but sea state was calm, with no swell. Videos were recorded with a GoPro (Hero 4) inside an underwater housing. The topside video (Supplementary Video S3) shows the adult male killer whale “Moctezuma” and five other killer whales (two adult females, two juveniles, one calf), in the vicinity of the juvenile whale shark (estimated 6 m TL), which was observed to be weakly swimming at the surface (Figure 3A). From the topside video, two killer whales were seen approaching the whale shark, which was oriented upside-down. A portion of the shark’s head was exposed out of the water. The killer whales surrounded the shark, while tourist swimmers approached the group of animals. Seconds later, the shark disappeared under the water and a killer whale was seen passing in front of the swimmers, only to disappear and leave the scene after this pass. Underwater images extrapolated from the video shows the whale shark was left upside-down by the killer whale (Figures 3B, C). The whale shark was seen bleeding profusely from an area on the ventral side of its body (Supplementary Video S4).

Figure 3. Sequence of the second attack occurred on 20 June 2021. (A) Moctezuma, the adult male of the pod approaches the upside-down whale shark. (B) Underwater image of the shark oriented upside-down, with the killer whale investigating. (C) The shark remains upside-down with its mouth open, seemingly left to bleed out. Photo credits Guillermo Aceves Salazar.
Event 3: On 27 April 2023, near San Francisquito Island (24°47.553 & 110°33.121’W; Figure 1), at approximately 1600 hrs, a pod of killer whales was seen on the surface, exhibiting potential hunting behavior. The weather was sunny, and the sea state was calm, with no swell. Top side (Figure 4D) photographs of the killer whales were taken with a Nikon D500 with a 70-200 mm and a 2x teleconverter. The photo identification of captured imagery confirmed the presence of the adult male “Moctezuma” (Figure 4C). The underwater footage, recorded with a GoPro (Hero 10) displayed a juvenile male whale shark (estimated 6 m TL) evasively swimming while it was being approached by a sole killer whale that swam underneath. Seconds later, a second killer whale was observed attempting to bite first the clasper (Figure 4A) and then the pelvic fin (Figure 4B) of the whale shark, opening a bleeding wound in the ventral area of the shark. The killer whale then surfaced to breathe, leaving the shark (which appeared dead at this point), which ultimately sank deep the water column, out of view. Immediately afterwards, large pieces of fresh animal tissue (presumably from the whale shark) were seen floating in the water where the whale shark had last been seen.
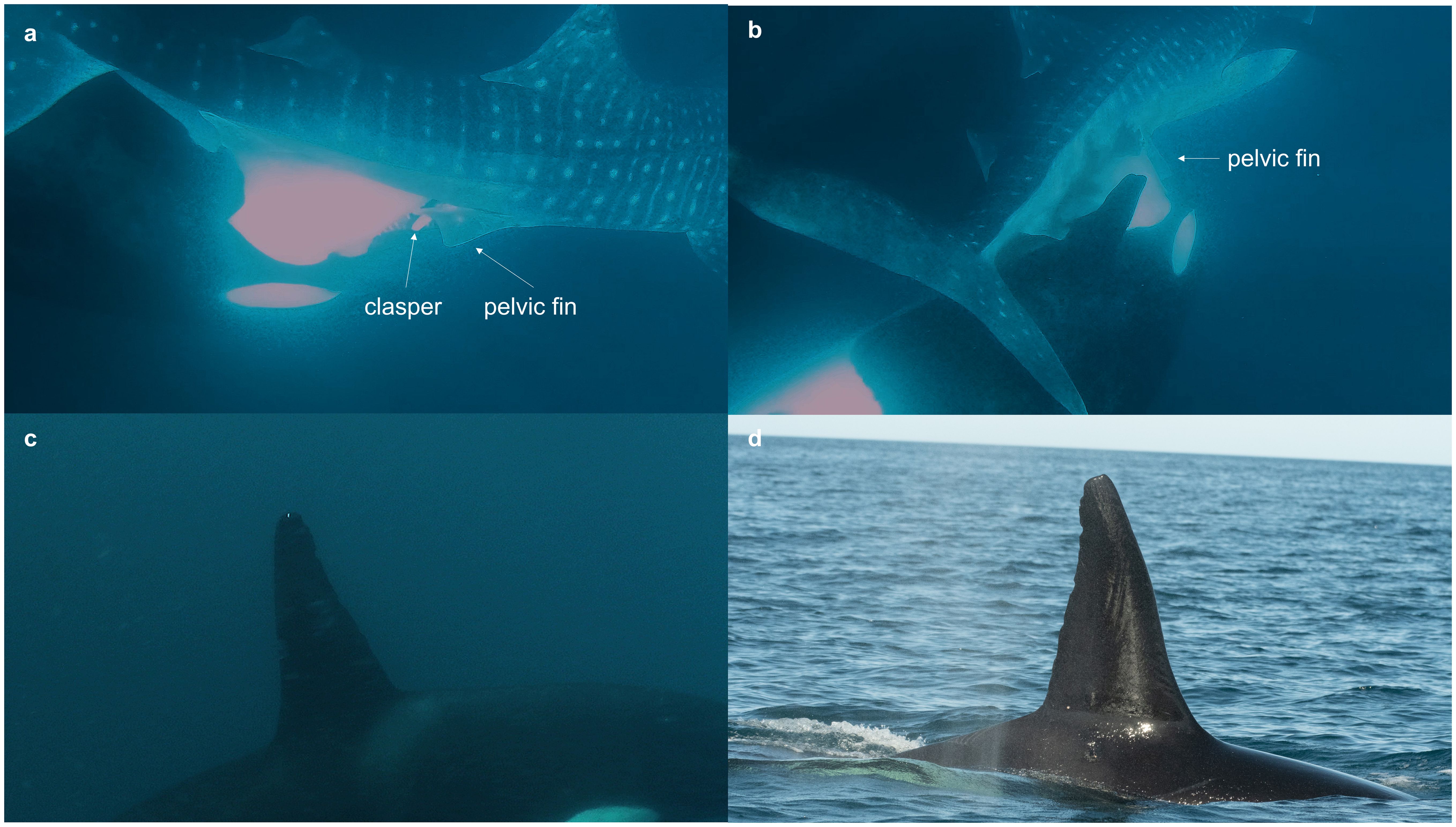
Figure 4. Sequence of killer whale attack on whale shark on 27 April 2023. (A) killer whale biting the clasper (B) and the pelvic fin of the whale shark. (C, D) “Moctezuma”, the adult male of the pod is identified as one of the killer whales involved in the attack. Photo credits: James Moskito.
Event 4: On 26 May 2024 in Ensenada de Muertos (23°57.617’N & 109°48.104’W) at approximately 1400 hrs, a group of five killer whales (four adult females, one juvenile) were encountered and observed to be swimming in circles, indicating socialization around a subsurface item of interest. The weather was sunny, and the sea state was calm, with no swell. The predation event was filmed on a full-frame mirrorless camera (Canon R5, 4K resolution at 120 frames per second) by one diver, on a DJI Osmo 4 (4K resolution at 120fps) by a second diver and on a GoPro Hero 12 (4K resolution at 120fps) by a third. In the underwater video footage from the Canon R5 camera, a single whale shark (~ 5 m TL) was seen at a depth of approximately 10 m, with two adult killer whales focused on the pelvic fins (Figure 5A). The whale shark was brought up shallower (~ 5 m depth) by the two killer whales and turned on its side, when a third killer whale hit the ventral side of the shark, head on with force (Figure 5B). One of the initial killer whales then proceeded to hit the head of the whale shark, this time side-on (Figure 5C). Two further killer whales entered the hunt (one adult and the juvenile). The adult killer whale hit the ventral side of the whale shark with her lower jaw while the juvenile swam toward the pelvic fins of the whale shark (Figure 5D). Blood was seen pouring out from the ventral side of the shark clearly from the cloaca. The juvenile killer whale then nudged the whale shark to the right of the pelvic fins (Figure 5E). The whale shark is then left momentarily as the killer whales surface for air (Figure 5F). The whale shark appeared to remain alive throughout the sequence, as its caudal fin could be seen moving slowly side to side until the end of the video footage (Canon R5). The video from the DJI Osmo continued to film, documenting one of the killer whales taking a breath to then swim down and use her jaw to hit the lateral side of the head, pushing the whale shark deeper. A second killer whale followed, joining the other to then position the whale shark on its side. Two of the other adult killer whales follow: one swimming rapidly (the fastest of the event) and hitting the ventral side of the head of the whale shark, producing an audible impact. This strong blow caused the whale shark to spin and lose equilibrium, flipping over several times from the lateral to the ventral side up. The killer whales and whale shark disappeared deeper below and out of view and the video footage stopped. Approximately ten minutes after the footage captured by the Canon R5 footage and the DJI, the GoPro Hero 12 footage showed the whale shark motionless apparently dead at the surface, ventral side up and with a large laceration starting from the pelvic region to the abdomen (Figure 6A). This was the last moment the whale shark was seen whole, as the orcas then dragged the shark deeper and out of sight. Approximately ten minutes later several of the killer whales were observed with whale shark carrion in their mouths (Figure 6B), and many small, unidentifiable parts of the whale shark were seen floating in the water column, confirming the predation was successful and at least partially consumed.

Figure 5. Sequence of killer whale attack on whale shark on 26 May 2024. (A) Predation event where two of the killer whales brought the whale shark to the surface, (B) killer whale hits the ventral side of the whale shark head-on, (C) another killer whale hits the shark with the side of its head, (D) another killer whale hits the ventral side of the whale shark, (E) the whale shark is brought shallower to the surface and one orca is biting near the pelvic area; and (F) killer whales surface to breath before taking whale shark down and delivering a rapid, final blow. Photo credits: Kelsey C Williamson.
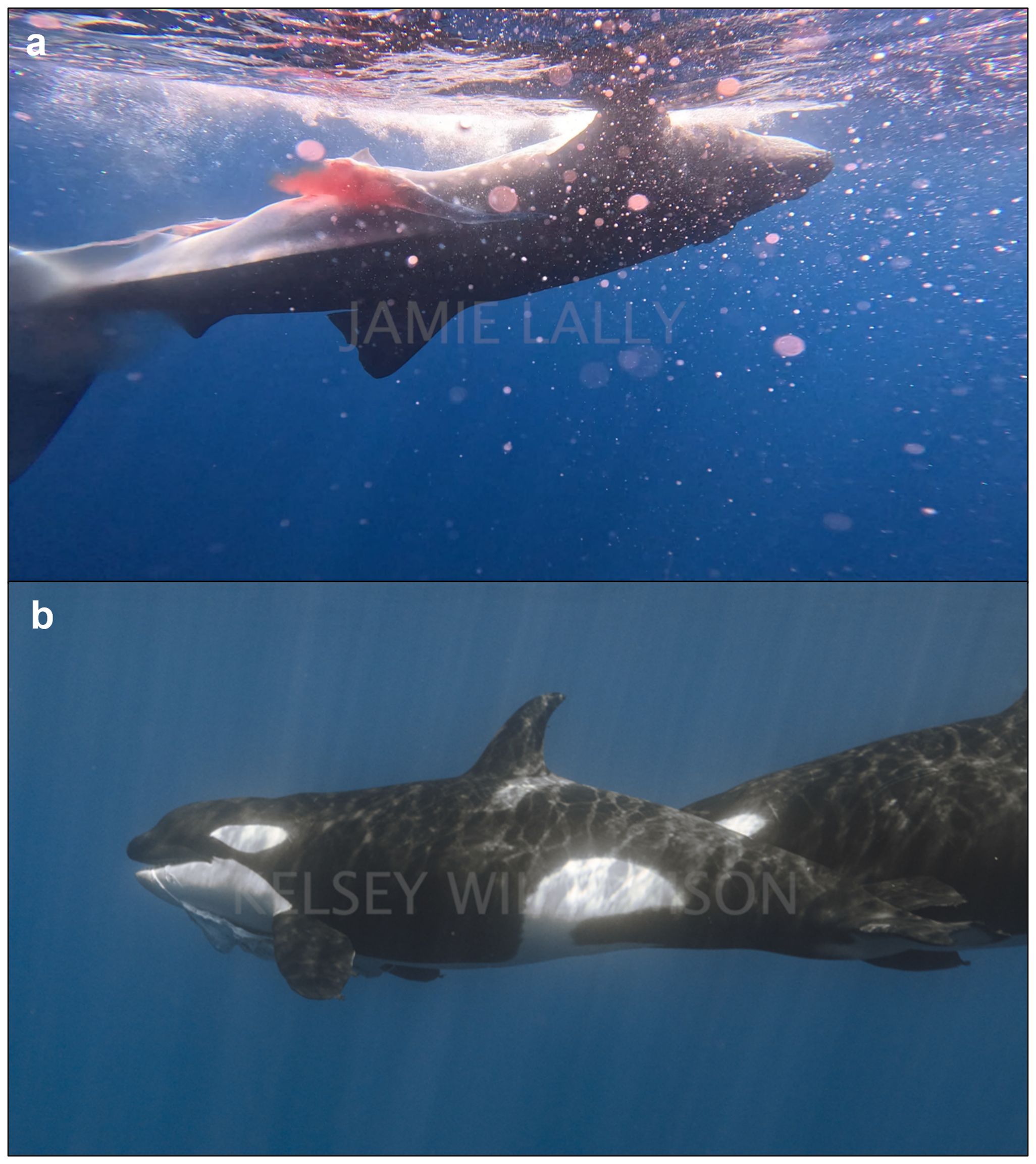
Figure 6. Stills extracted from video footage recorded during the attack on whale shark on 26 May 2024. (A) Dead juvenile whale shark with large lateral laceration, and clear evidence of blood and trauma from the ventral side of the individual (Photo credit: Jamie Lally) (B) An adult killer whale female with whale shark carrion in mouth (Photo credit: Kelsey C Williamson).
Discussion
We detailed and presented reports of killer whale predation on the largest fish, the whale shark. Across all four events, several behavioral consistencies were observed that provided insights into an apparent killer whale strategy for hunting and killing gigantic prey such as whale sharks. In all the events, the whale sharks were observed profusely bleeding from the ventral side of the body. According to the events, killer whales displayed a cooperative and coordinate hunting behavior which included repeatedly hitting the whale shark at high speed to stun and immobilize it, manipulate and positioning the shark ventral side up and biting the shark in the exposed ventral region, to desanguinate the shark through the cloaca and allow access to the organs for consumption. Despite the killer whale’s interest in accessing the internal organs of the whale sharks, we did not observe any direct consumption of the livers (which is observed in other regions, Towner et al., 2022), as the whale sharks were either already killed or they sank out of view. Previous report on killer whale hunting whale shark in the Mexican Pacific outlines how the shark was repeatedly struck in the ventral region by two killer whales, until it was killed and subsequently consumed (Ortega-Ortiz et al., 2023). The publication also made reference to additional instances of killer whale predation on whale sharks in Bahía de Los Ángeles (Mexico), but no report of these events is available in the literature (Ortega-Ortiz et al., 2023).
Killer whales have the highest brain to body mass ratio of any studied apex predator (Waugh and Thewissen, 2021), suggesting enhanced cognitive abilities, which may allow them to overcome the challenges of hunting elasmobranch prey, including ocean giants such a whale sharks. Our report demonstrates that killer whales in the GoC exhibit hunting techniques which are specialized and adapted according to the target species; these techniques include cooperative chasing and disabling schooling fish, and large cetaceans (Dahlheim and Heyning, 1999; Baird, 2000), stranding pinnipeds such as Otaria byronia and Mirounga leonina (Lopez and Lopez, 1985; Guinet, 1992); “karate chopping” thresher (Alopias vulpinus) and smooth hammerhead sharks (Sphyrna zygaena) (Visser, 2005); chasing and tossing sevengill sharks (Notorhynchus cepedianus) in Patagonia (Reyes and García-Borboroglu, 2004); corralling Munk´s pygmy devil rays into a ball (Higuera Rivas et al., 2023). In South Africa, killer whales use a special hunting technique which consists in causing a large tear across the pectoral girdle of broad-nose sevengill sharks (Notorynchus cepedianus) to access the liver and feed only on this organ (Engelbrecht et al., 2019). In South Africa, killer whales have been observed hunting and targeting the liver of juvenile white sharks (Towner et al., 2023, 2024). However, it is still unclear what strategy killer whales use to access the liver of that prey (Towner et al., 2024).
The predation events in our study clearly show how the killer whales deliver impactful hits focusing on the ventral side of the head of the whale shark, followed by body positioning and then biting of the ventral side of the whale shark (cloaca, claspers, pelvic fins). We hypothesize that the claspers and/or pelvic fins is the entry area for killer whale to access the whale shark rich-lipid liver, which comprises the majority of the sharks’ body weight. The right liver lobe of a juvenile dead whale shark from the GoC (TL 548 cm) measured 152 cm TL and 38 cm at its widest area. The left lobe of the liver measured 151 cm TL and 39 cm in its wider area (Pancaldi et al., 2021). The importance of the shark’s liver to killer whale’s diets is related to its high caloric content, since it has been suggested that its consumption provides additional energy benefits (Jorgensen et al., 2019). The ventral portion of the whale shark is likely the least-protected areas of the body, as the dorsal side of the whale shark’s body consists of a thin and dense external layer of denticles that overlie a layer of connective tissue (Becerril-García et al., 2021). In adult whale sharks, the connective tissue can be more than 20 cm thick (Meekan et al., 2015) making juvenile individuals the easiest prey to target (where the connective tissue is about 5 cm thick). In addition, in the circulatory system of sharks, the aorta in the dorsal area is protected by a dense thickness of muscles. On the contrary, the same artery in the ventral area is not protected by muscle; it enters the liver and passes under the intestines and the spiral valve (Sato, 2012; Brill and Chin Lai 2015). This type of anatomy turns the ventral part of a shark into an extremely vulnerable area where deep bleeding can be caused by bites from large predators.
Whale sharks are a protected species, listed as “Endangered” by the International Union for the Conservation of Nature since 2016. Mexico protects this species through two regulations (DOF 2006, 2010) that forbid capture and trading; in addition, specific management plans are implemented in the main aggregation areas such as La Paz Bay, Bahía de Los Angeles, and Cancún where the observation and swimming with this species is an important tourist attraction. Whale sharks aggregate in La Paz Bay between October to May and the population in this area is estimated to be between 50 and 100 individuals, with 70% males, and almost 100% are juveniles (between 2 and 7 m TL; Ramírez-Macías et al., 2012). Between April and May, whale sharks start to leave La Paz Bay toward other aggregation areas of the GoC (Ramírez-Macías et al., 2012) and Nayarit (Ramírez-Macías et al., 2015) which exposes them to possible killer whale predation and aligns with the timing of all four predation events. In addition, the size of this species of shark, its low swimming speed, lack of large teeth and agility in the water convert it as easy prey for killer whales.
In the last decade the rising tourism in certain regions of the world has increased the possibility of recording seemingly rare events involving killer whales (Johnson et al., 2019, Dixon et al., 2023, Ayres et al., 2024). In this sense, citizens become scientists by contributing their sightings and footage to allow researchers to piece together information about species populations and behaviors (Silvertown, 2009). In this study all four predation events were filmed by members of the public, in addition to scientists. In recent years, the advancement in technology and the affordability and access to cameras that can shoot in high resolution has also aided in better captures during these opportunistic sightings which is particularly important for photo-identification.
The high-quality pictures and repeated observations of the adult male allowed the identification of “Moctezuma” as the male killer whale involved in the whale shark attacks during event one, two and three. Since 2018, “Moctezuma” and several other killer whales have been observed in the GoC hunting elasmobranch species, including the Munk’s pygmy devil ray and the pelagic stingray (Higuera Rivas et al., 2023), bull sharks (Ayres et al., 2024), in addition to other species of sharks (Higuera-Rivas, personal observation). In particular, the female killer whales observed during the event four have previously been observed to be associated with “Moctezuma” (Higuera-Rivas, personal observation) and could be relatives and/or part of the same pod. In this sense, it is possible that Moctezuma has acquired the ecological and behavioral information for hunting elasmobranchs and ocean giants such as whale sharks, along with other members of the same pod, although further study would be needed to prove this claim.
Although there is not an ecotype assigned to the killer whales of the GoC region, there is a hypothesis that these killer whales belong to a generalist ecotype specialized in hunting marine mammals, turtles, and teleost fishes (Ortega-Ortiz et al., 2023). However, elasmobranchs were not considered as part of the prey items in this ecotype. It remains unclear if the “Moctezuma pod” is strictly an elasmobranch-eating specialist pod, but additional studies utilizing stable isotopes would help refine killer whale ecotype differentiation in the highly dynamic ocean basin of the GoC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements. The images were obtained and contributed by local members of the public and scientists and the data was purely observational. Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
Author contributions
FP: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Conceptualization. KA: Writing – review & editing, Writing – original draft, Methodology. AG: Writing – review & editing, Writing – original draft. JM: Writing – review & editing, Writing – original draft. KW: Writing – review & editing, Writing – original draft. JH: Writing – review & editing, Writing – original draft, Visualization, Supervision, Methodology, Investigation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Jamie Lally from Baja Wild Encounters and Guillermo Aceves Salazar for providing us with their images and we thank KW and JM for contributing their footage and accounts of the predation events. We also thank Abel Trejo Ramírez for creating the map in Figure 1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1448254/full#supplementary-material
References
Ayres K. A., Gallagher A. J., Higuera Rivas J. E. (2024). Orca (Orcinus orca) and shark predator-prey interactions within Cabo Pulmo National Park in the Gulf of California, Mexico. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1407379
Baird R. W. (2000). “The killer whale: Foraging specializations and group hunting in Cetacean societies: Field studies of dolphins and whales. Eds. Mann J., Connor R. C., Tyack P. L., Whitehead H. (Chicago: The University of Chicago Press and London: :The University of Chicago Press Ltd.), 127–153.
Becerril-García E. E., Pancaldi F., Cruz-Villacorta A. A., Rivera-Camacho A. R., Aguilar-Cruz C. A., Whitehead D. A., et al. (2021). General descriptions of the dermis structure of a juvenile whale shark Rhincodon typus from the Gulf of California. J. Fish Biol. 99, 1524–1528. doi: 10.1111/jfb.14827
Bigg M. A. (1987). Killer whales: a study of their identification, genealogy and natural history in British Columbia and Washington State.
Black N. A., Schulman-Janiger A., Ternullo R. L., Guerrero-Ruiz M. (1997). Killer Whales of California and Western Mexico: A Catalog of Photo-Identified Individuals. U.S. department of commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service Southwest Fisheries Science Center. NOAA-TM-NMFS-SWFSC-247. Available online at: https://swfsc-publications.fisheries.noaa.gov/publications/TM/SWFSC/NOAA-TM-NMFS-SWFSC-247.PDF (Accessed September 1997).
Brill R. W., Chin Lai N. (2015). “Elasmobranch cardiovascular system,” in Fish Physiology, vol. 34, Part B . Eds. Shadwick R. E., Farrell A. P., Brauner C. J. (Elsevier: Academic Press), 1–8. doi: 10.1016/B978-0-12-801286-4.00001-0
Dahlheim M. E., Heyning J. E. (1999). “Killer whale Orcinus orca (Linneaus 1758),” in Handbook of marine mammals: Vol. 6, The second book of dolphins and porpoises. Eds. Ridgway S. H., Harrison R. (Academic Press), 281–322.
Dahlheim M. E., Schulman-Janiger A., Black N., Ternullo R., Ellifrit D., Balcomb K. C. III (2008). Eastern temperate North Pacific offshore killer whales (Orcinus orca): Occurrence, movements, and insights into feeding ecology. Mar. Mammal Sci. 24, 719–729. doi: 10.1111/j.1748-7692.2008.00206.x
de Bruyn P. J. N., Tosh C. A., Terauds A. (2012). Killer whale ecotypes: is there a global model? Biol. Rev. 88, 62–80. doi: 10.1111/j.1469-185X.2012.00239.x
Dixon O. F., Gallagher A. J., Towner A. V. (2023). Novel aerial observations of a group of killer whales Orcinus orca in The Bahamas. Front. Mar. Sci. 10, 1265064.
Engelbrecht T. M., Kock A. A., O’Riain M. J. (2019). Running scared: when predators become prey. Ecosphere 10, e02531. doi: 10.1002/ecs2.2531
Fertl D., Acevedo-Gutierrez A., Darby F. L. (1996). A report of killer whales (Orcinus orca) feeding on a carcharhinid shark in Costa Rica. Mar. Mammal Sci. 12, 606–611. doi: 10.1111/j.1748-7692.1996.tb00075.x
Fitzpatrick B., Meekan M., Richards A. (2006). Shark attacks on A whale shark (Rhincodon typus) at Ningaloo Reef, Western Australia. Bull. Mar. Sci. 78, 397–402(6).
Ford J. K. B., Ellis G. M., Barrett-Lennard L. G., Morton A. B., Palm R. S., Balcomb III, K. C. (1998). Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 76, 1456–1471. doi: 10.1139/z98-089
Guerrero-Ruiz M., Gendron D., Urban R. J. (1998). Distribution, movements and communities of killer whales (Orcinus orca) in the Gulf of California, Mexico. Rep. Int. Whaling Commission 48, 537–543.
Guerrero-Ruiz M., Urbán R. J., Gendron D., Rodriguez M. E. (2007). Prey items of killer whales in the Mexican Pacific (Paper SC/59/SM14). Presented to the International Whaling Commission Scientific Committee (Anchorage, Alaska), 6 pp.
Guinet C. (1992). Comportement de chasse des orques (Orcinus orca) autour des Isles Crozet [Hunting behavior of killer whales (Orcinus orca) around the Crozet Islands. Can. J. Zool. 70, 1656–1667. doi: 10.1139/z92-231
Hanson M. B., Walker W. A. (2014). Trans-Pacific consumption of cephalopods by North Pacific killer whales (Orcinus orca). Aquat. Mammals 40, (3) 274. doi: 10.1578/AM.40.3.2014.274
Higuera Rivas J. E., Hoyos-Padilla E. M., Elorriaga-Verplancken F. R., Rosales-Nanduca H., Rosenthal R., Urbán R. J. (2023). Orcas (Orcinus orca) use different strategies to prey on rays in the Gulf of California. Aquat. Mammals 2023, 49(1). doi: 10.1578/AM.49.1.2023
Johnson A. F., Gonzales C., Townsel A., Cisneros-Montemayor A. M. (2019). Marine ecotourism in the Gulf of California and the Baja California Peninsula: Research trends and information gaps. Sci. Mar. 83, 177–185. doi: 10.3989/scimar.04880.14A
Jorgensen S. J., Anderson S., Ferretti F., Tietz J. R., Chapple T., Kanive P., et al. (2019). Killer whales redistribute white shark foraging pressure on seals. Sci. Rep. 9, 6153. doi: 10.1038/s41598-019-39356-2
Ketchum J. T., Galván-Magaña F., Klimley A. P. (2013). Segregation and foraging ecology of whale sharks, Rhincodon typus, in the southwestern Gulf of California. Environ. Biol. Fishes 96, 779–795. doi: 10.1007/s10641-012-0071-9
Kukuyev E. I. (1995). The new finds in recently born individuals of the whale shark Rhincodon typus (Rhiniodontidae) in the Atlantic Ocean. J. Ichthyol. 36, 203–205.
Lopez J. C., Lopez D. (1985). Killer whales (Orcinus orca) of Patagonia, and their behavior of intentional stranding while hunting nearshore. J. Mammal. 66, 181–183. doi: 10.2307/1380981
Meekan M. G., Fuiman L. A., Davis R., Berger Y., Thums M. (2015). Swimming strategy and body plan of the world’s largest fish: implications for foraging efficiency and thermoregulation. Front. Mar. Sci. 2. doi: 10.3389/fmars.2015.00064
Norman B. N., Stevens J. D. (2007). Size and maturity status of the whale shark (Rhincodon typus) at Ningaloo reef in Western Australia. Fish. Res. 84, 81–86. doi: 10.1016/j.fishres.2006.11.015
O’Sullivan J. B., Mitchell T. (2000). “A fatal attack on a whale shark Rhincodon typus, by killer whales Orcinus orca off Bahía de Los Angeles, Baja California,” in American Society of Ichthyologists and Herpetologists 80th Annual Meeting/American Elasmobranch Society (ASIH/AES) (Abstracts), 16th Annual Meeting, La Paz, Mexico.
Ortega-Ortiz C. D., Lazcano-Pacheco C., Llamas-González M., Meza-Yáñez R., Ruano-Cobian S., López-Luna D. G., et al. (2023). Expanding information on the prey items and hunting tactics of the eastern tropical pacific killer whale (Orcinus orca) ecotype. Examines Mar. Biol. Oceanogr. 6, 1–8. doi: 10.31031/EIMBO.2023.06.000635
Pancaldi F., Marmolejo-Rodríguez A. J., Soto-Jiménez M. F., Murillo-Cisneros D. A., Becerril-García E. E., Whitehead D. A., et al. (2021). Trace elements in the whale shark Rhincodon typus liver: an indicator of the health status of the ecosystem base (plankton). Latin Am. J. Aquat. Res. 49, 359–364. doi: 10.3856/vol49-issue2-fulltext-2535
Pitman R. L., Dutton P. H. (2004). Killer whale predation on a leatherback turtle in the Northeast Pacific. Pacific Sci. 58, 497–498. doi: 10.1353/psc.2004.0034
Pyenson N. D., Vermeij G. J. (2016). The rise of ocean giants: maximum body size in Cenozoic marine mammals as an indicator for productivity in the Pacific and Atlantic Oceans. Biol. Lett. 12, 20160186. doi: 10.1098/rsbl.2016.0186
Ramírez-Macías D., Murillo Olmeda R., Vázquez-Haikin A., Luja V., Mata R. (2015). Monitoreo de tiburón ballena en Nayarit, temporada 2014-2015 (In Spanish). Acta Pesquera 2, 6–16. Available at: https://revistas.cimateuan.education/openjs/index.php/aprevista/article/view/102.
Ramírez-Macías D., Vázquez-Haikin A., Vázquez-Juárez R. (2012). Whale shark Rhincodon typus populations along the west coast of the Gulf of California and implications for management. Endangered Species Res. 18, 115–128. doi: 10.3354/esr00437
Reyes L. M., García-Borboroglu P. (2004). Killer whale (Orcinus orca) predation on sharks in Patagonia, Argentina: a first report. Aquat. Mammals 30, 376–379. doi: 10.1578/AM.30.3.2004.376
Sato K. (2012). Biology of sharks and their relatives, second edition. Mar. Biol. Res. 9, 227. doi: 10.1080/17451000.2012.745005
Silvertown J. (2009). A new dawn for citizen science. Trends Ecol. Evol. 24, 467–471. doi: 10.1016/j.tree.2009.03.017
Towner A., Micarelli P., Hurwitz D., Smale M. J., Booth A. J., Stopforth C., et al. (2024). Further insights into killer whales Orcinus orca preying on white sharks Carcharodon carcharias in South Africa. Afr. J. Mar. Sci. 46, 1–5. doi: 10.2989/1814232X.2024.2311272
Towner A. V., Kock A. A., Stopforth C., Hurwitz D., Elwen S. H. (2023). Direct observation of killer whales preying on white sharks and evidence of a flight response. Ecology 104. doi: 10.1002/ecy.3875
Towner A. V., Watson R. G. A., Kock A. A., Papastamatiou Y., Sturup M., Gennari E., et al. (2022). Fear at the top: killer whale predation drives white shark absence at South Africa’s largest aggregation site. Afr. J. Mar. Sci. 44, 139–152. doi: 10.2989/1814232X.2022.2066723
Vargas-Bravo M. H., Elorriaga-Verplancken F. R., Olivos-Ortiz A., Morales-Guerrero B., Liñán-Cabello M. A., Ortega-Ortiz C. D. (2020). Ecological aspects of killer whales from the Mexican Central Pacific coast: Revealing a new ecotype in the Eastern Tropical Pacific. Mar. Mammal Sci., 1–16. doi: 10.1111/mms.12748
Vermeij G. J. (2016). Gigantism and its implications for the history of life. PlosOne. 11, e0146092. doi: 10.1371/journal.pone.0146092
Visser I. N. (2005). First observations of feeding on thresher (Alopias vulpinus) and hammerhead (Sphyrna zygaena) sharks by killer whales (Orcinus orca), which specialize on elasmobranchs as prey. Aquat. Mammals 31, 83–88. doi: 10.1578/AM.31.1.2005.83
Keywords: orca, predation, hunting strategy, whale shark, Mexico
Citation: Pancaldi F, Ayres KA, Gallagher AJ, Moskito J, Williamson KC and Higuera Rivas JE (2024) Killer whales (Orcinus orca) hunt, kill and consume the largest fish on Earth, the whale shark (Rhincodon typus). Front. Mar. Sci. 11:1448254. doi: 10.3389/fmars.2024.1448254
Received: 13 June 2024; Accepted: 23 September 2024;
Published: 29 November 2024.
Edited by:
Nathan Jack Robinson, Fundación Oceanográfica, SpainReviewed by:
Alexander J. Werth, Hampden–Sydney College, United StatesRob Harcourt, Macquarie University, Australia
Diego Horacio Rodriguez, National Scientific and Technical Research Council (CONICET), Argentina
Copyright © 2024 Pancaldi, Ayres, Gallagher, Moskito, Williamson and Higuera Rivas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jesús Erick Higuera Rivas, amVyaWNraHJAZ21haWwuY29t
 Francesca Pancaldi
Francesca Pancaldi Kathryn A. Ayres2
Kathryn A. Ayres2 Austin J. Gallagher
Austin J. Gallagher Jesús Erick Higuera Rivas
Jesús Erick Higuera Rivas