
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 28 June 2024
Sec. Aquatic Physiology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1444210
This article is part of the Research Topic Endocrine Regulation and Physiological Adaptation of Stress Response in Aquatic Organisms, Volume II View all 13 articles
This study aimed to evaluate the impacts of wild and breeding juvenile rice field eel under conditions of ammonia and heat stress. The growth performance (FBW, WGR, SGR, and FCR) of 360 wild (24.22 ± 0.30 g) and 360 breeding (24.16 ± 0.27 g) strains was significantly hindered by ammonia and heat stress. The inhibitory effects were more obvious when the two stresses were combined. The growth performance and survival rates of the breeding strains outperformed that of the wild strains under identical stress conditions, this was explained by the expression of the growth-related gene (gh). They have increased the enzyme activity (CAT and GSH-Px) and expression of immune-related genes (cat, gpx3, and hsp90α) in response to oxidative stress. However, the results of certain indicator enzymes indicate the presence of oxidative damage in their tissues. The presence of an inflammatory response in the tissues was suggested by the up-regulation of genes associated with pro-inflammatory cytokines (il-1β and il-8) and the down-regulation of genes related to anti-inflammatory cytokines (il-10). Additionally, the presence of tissue damage was shown by the up-regulation of genes connected to apoptosis (cas2, cas8, and cas9) and the down-regulation of genes connected to tight junctions (zo-1). Nevertheless, it is noteworthy that breeding strains exhibited superior adaptability to ammonia and heat stress in comparison to wild strains.
Rice field eel (Monopterus albus) is a common freshwater fish in Asian countries that taxonomically belongs to Osteichthyes, Synbranchiformes, and Synbranchidae. Due to its delicious taste, rich nutrition, and officinal value, eel is considered to be one of the most economically valuable fish in China, with a yield up to 334,215 tons in 2022. However, this yield is far from meeting consumer demands. One of the important reasons to restrict this yield is the scarcity of breeding fry and the abuse of wild fry. Meanwhile, the efficiency of breeding and wild fry has been the focus of debate in the industry.
In China, the traditional net-cage farming mode has been adopted by more than 95% of eel farmers. Due to their open farming environment, the eels are highly vulnerable to extreme climate changes, such as high temperatures and typhoons. Moreover, with the increase in rearing density, ammonia in water will become one of the main threats to eel rearing, may exhibit toxic effects on fish, and can result in widespread fatalities (Ip and Chew, 2010; Bucking, 2017). In order to break the restrictions of natural conditions, a novel method of recycled water rearing was proposed. However, since few studies have focused on the physiological effects of environmental factors on M. albus larvae, the popularization of this technology may be subject to certain resistance.
Water temperature is one of the important environmental factors affecting the growth of aquatic animals (Rebl et al., 2013; Reid et al., 2019; Wu et al., 2021). A latest study demonstrated that the breeding strains of eel larvae showed the best growth performance under a temperature of 34°C (Mao et al., 2024). Nevertheless, the wild strains do not encounter such elevated water temperatures in their natural habitat. Excessively high-water temperatures can have devastating consequences for aquatic organisms, leading to reduced growth and increased mortality in fish (Dominguez et al., 2004; Zhang et al., 2014). Moreover, high water temperatures may also cause other environmental problems, such as the occurrence of ammonia, nitrogen, and nitrite, because part of the beneficial bacteria will be inactivated at high temperatures, making it harder for residual erbium and feces to be degraded (Li et al., 2022). When exposed to a certain concentration of nitrite, aquatic animals often show significant oxidative stress and even exhibit inflammatory responses (Li et al., 2020). In addition, prior research has demonstrated that elevated concentrations of ammonia in the water column hinder the development and immune response of aquatic species (Kim et al., 2015; Cui et al., 2022; Ou et al., 2022). However, no studies have focused on the effects of nitrite on eel larvae. In aquaculture, high temperatures are often accompanied by multiple negative environmental factors. In this study, a comparative study was conducted to detect the differences in growth performance, antioxidant, immune, and apoptosis indices between wild and breeding strains of eel larvae under the combined exposure of heat and ammonia. These results will provide a reference for the factory farming of swamp eels.
Wild strains of juvenile eels (WS) obtained from nearby rice farms or ponds in their natural habitat were transported to be temporarily reared under laboratory conditions for one week to continuously check the fish’s health status. During this period, aeration of the water was maintained constantly, and one-third of the water volume was replaced on a daily basis. Over the next two weeks, WS were adapted to the feeding experiment until commercial feed (Hubei Zhaoliang Biotechnology Co., Ltd., Hubei, China) could be consumed. After two weeks of acclimatization, a total of 360 healthy fish with a similar weight (24.22 ± 0.30 g) were selected for the experiment. The fish were placed in a random and equal manner throughout 12 cylindrical tanks (r = 0.5 m, h = 0.3 m), with 30 fish per tank.
Breeding strains of juvenile eels (BS) were obtained from the Zhuanghang Comprehensive Experiment Station of the Shanghai Academy of Agricultural Sciences, and acclimation was completed according to the same method. Similarly, a total of 360 healthy fish with a similar weight (24.16 ± 0.27 g) were selected for distribution in 12 cylindrical tanks.
The test site was a greenhouse at the Zhuanghang Comprehensive Experiment Station of the Shanghai Academy of Agricultural Sciences, which had a thermostatic heating system. The initial temperature of the experimental group was 26°C, which corresponds to room temperature, and this was used as a constant water temperature for the control and unheated groups. The heat stress (HS) groups were set at 34°C and warmed at 1°C/h (the error is within ± 0.1°C). Ammonium chloride (NH4Cl) is a compound used as a source of ammonia to attain the appropriate concentration of ammonia in a solution. The actual concentration of nitrite-N in test solutions is measured using spectrophotometry. The ammonia concentration in the ammonia stress (AS) groups was maintained at 12.0 ± 0.5 mg/L. Eels in 12 tanks from WS were divided into four experimental groups (WS-CT, WS-HS, WS-AS, and WS-HS+AS), and each group consisted of three replicates. Eels from BS follow this method as (BS-CT, BS-HS, BS-AS, and BS-HS+AS).
For the WS and BS feeding experiments, growth performance was accomplished over an eight-week period, where the temperature was maintained at the same stress temperature (the error is within ± 0.3°C). The fish were manually fed at 16:00 each day with an amount of food equal to 4-5% of their body weight. Any food that was not eaten was collected within 30 min of feeding. It was then dried until it achieved a constant weight and finally weighed to determine the amount of food consumed. During the feeding trial, the aeration of the water was maintained constantly, and the rest of the water conditions were kept constant (dissolved O2 ≥ 5.8 mg/L, pH 7.2 ± 0.2). The tank’s water quality is regularly assessed six times a day using a portable water quality analyzer (model HQ40D, HACH, Colorado, USA) to measure water temperature, pH levels, and dissolved oxygen. After feeding was completed, the concentration of nitrite-N in the water was measured spectrophotometrically and adjusted to the same concentration as the preset concentration within two hours.
After fasting for 24 h, the survival rate of each tank was counted, and the weight and length of each fish were measured accurately. Then, a total of 144 fish were randomly selected (six fish per tank), after being anesthetized with 100 mg/L of MS-222 (Shanghai Reagent Corp., Shanghai, China), the liver and viscera were accurately weighed. A total of 72 fish were randomly selected (three fish per tank), the muscle, intestine, and liver tissues were stored at -80°C after being snap-frozen in liquid nitrogen.
The growth performance indices in this experiment were calculated using the following formula:
Intestine and liver tissues from three fish in each tank were rinsed with ice-cold phosphate buffered saline (PBS) and then sliced into minute fragments. Tissue homogenates were obtained by utilizing a freshly made ice-cold saline solution (1:10 w/v). Afterwards, the homogenate was centrifuged at a speed of 4,000 rpm for 10 min at a temperature of 4°C. The liquid portion that settled at the top, known as the supernatant, was collected and stored for further measurement.
Commercially available kits (Nanjing Jiancheng Biotechnic Institute, Nanjing, China) were utilized to quantify the biochemical indicators of antioxidant enzyme (superoxide dismutase, catalase, and glutathione peroxidase) and biochemical indicators of body injury (alkaline phosphatase, acid phosphatase, and malondialdehyde) in the liver and intestine. Digestive enzymes (amylase, trypsin, and lipase) in the intestine were determined in the same way.
Total RNA was extracted from intestine and liver tissues from three fish in each tank by Trizol reagent (Sigma, Burlington, USA). The reverse transcription was performed following the instructions provided by the cDNA synthesis kit (TaKaRa, Kusatsu, Japan). The gene sequence was acquired from GenBank, and qPCR primers (Table 1) were produced by Sangon Biotech Co., Ltd. (Shanghai, China).
The Roche LightCycler®480 II Real-Time System (Roche, Basel, Switzerland) was used to perform qPCR, and the SYBR Green PCR Master Mix Kit (TaKaRa, Kusatsu, Japan) was utilized for this purpose. Each reaction system consisted of 10 µl of SYBR mix, 6.4 µl of ddH2O, 0.8 µl of forward primer, 0.8 µl of reverse primer, and 2 µl of cDNA as the template. A two-step PCR reaction procedure was used: pre-denaturation at 95°C for 30 s, 40 cycles of denaturation at 95°C for 5 s, and annealing at 60°C for 20 s. Following each qPCR experiment, a melting curve analysis was conducted on the products to verify their specificity. The relative abundance of target gene mRNA was calculated by R=2−ΔΔCt, β-actin was utilized as a calibration standard to standardize the expression of the target genes, each sample was replicated three times.
The data were calculated using Microsoft Excel and analyzed using SPSS 22.0 software. Differences in the same strain under different stress conditions were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s test. Validation of differences between wild and breeding strains under the same stress conditions using independent-sample t-tests. The results were reported as means ± SD (standard deviation), and statistical significance was assessed using a P-value< 0.05.
Table 2 displays the growth performance of each group of eels in WS and BS under varying stress conditions. Under the same strain, the survival rate was lowest under the combined stress condition, followed by the ammonia stress condition, and highest under the heat stress condition. Under identical stress conditions, the survival rate of BS was higher than that of WS.
Between WS and BS, the effects of stress conditions on growth performance were in ascending order: HS, AS, and HS+AS. But BS showed better growth performance in FBW, SR, WGR, SGR, and FCR compared to WS under the same stress conditions. HSI under combined stress showed a significant decrease (P< 0.05) compared to single-factor stress.
The hepatic SOD activity exhibited a decreasing trend in response to stress (Figure 1A), while the activities of CAT and GSH had an increasing trend, with both significantly elevated (P< 0.05) under combined stress (Figure 1B, C). There was no significant change (P > 0.05) in the activity of SOD in the intestine (Figure 1D), but the activities of CAT and GSH exhibited an overall increasing trend, and both increased significantly (P< 0.05) under combined stress (Figure 1E, F).
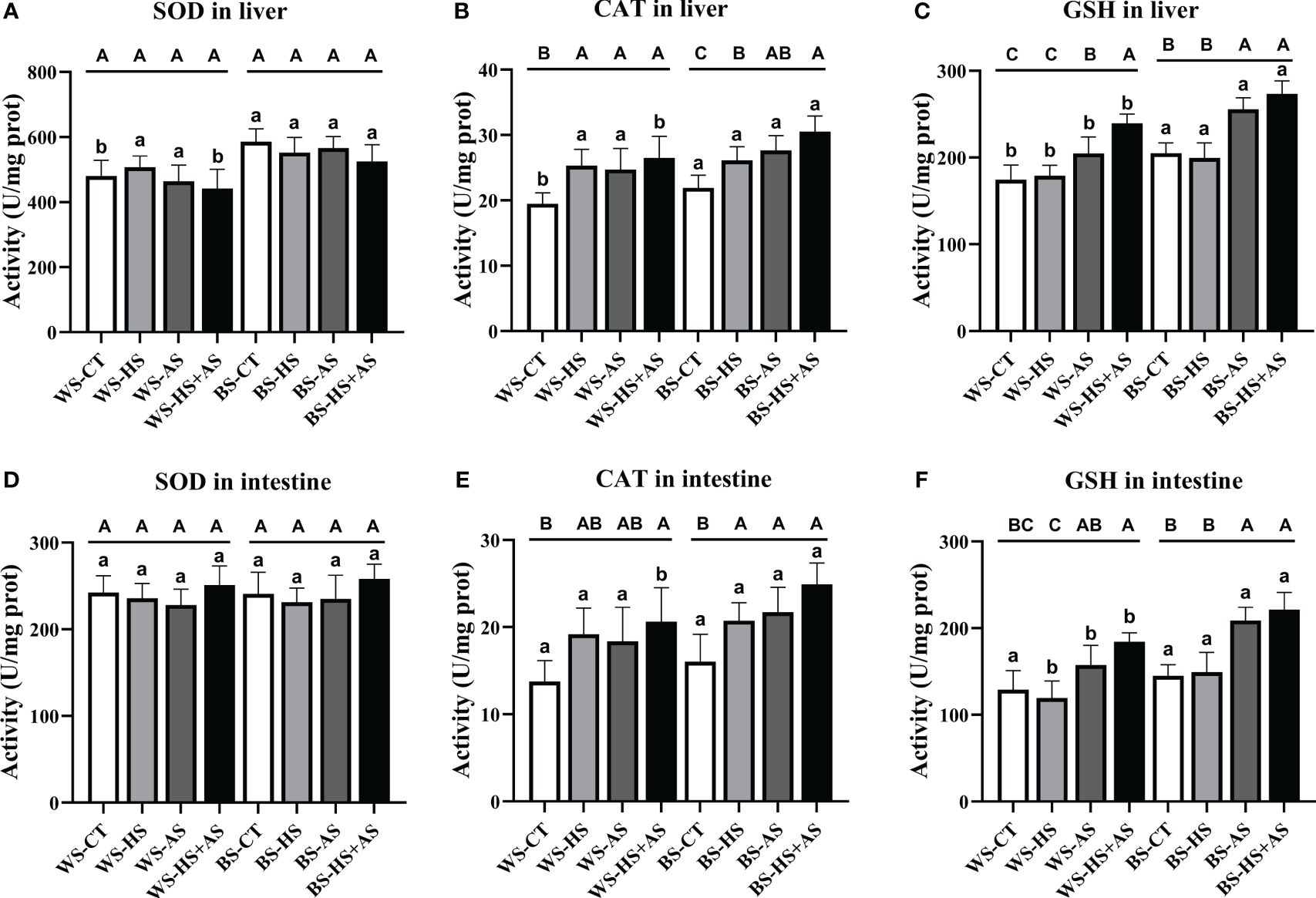
Figure 1 Effect of varying stress conditions on the antioxidant enzyme activity of M. albus after 8 weeks. Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH). (A-C) SOD, CAT, and GSH activity in the liver, (D-F) SOD, CAT, and GSH activity in the intestine. Different letters indicate significant differences (P > 0.05), while the same letters indicate no significant differences (P > 0.05). Lowercase letters indicate differences between wild and breeding strains under the same stress conditions, uppercase letters indicate differences between stress conditions under the same strain.
The liver and intestine activities of AKP and ACP showed different trends of decrease, with the lowest levels observed under combined stress in the WS and ammonia stress in the BS (Figure 2A, B, D, E). The levels of MDA showed an increase in both the liver and the intestine, and in general, the BS exhibited lower activity compared to the WS (Figure 2C, F).

Figure 2 Effect of varying stress conditions on the injury-indicating enzyme activity of M. albus after 8 weeks. Alkaline phosphatase (AKP), acid phosphatase (ACP), and malondialdehyde (MDA). (A-C) AKP, ACP, and MDA activity in the liver, (D-F) AKP, ACP, and MDA activity in the intestine. Different letters indicate significant differences (P < 0.05), while the same letters indicate no significant differences (P > 0.05). Lowercase letters indicate differences between wild and breeding strains under the same stress conditions, uppercase letters indicate differences between stress conditions under the same strain.
The mRNA expression of gh in muscle exhibited a significant decrease (P< 0.05) in both WS and BS, with both reaching a minimal level under combined stress (Figure 3).
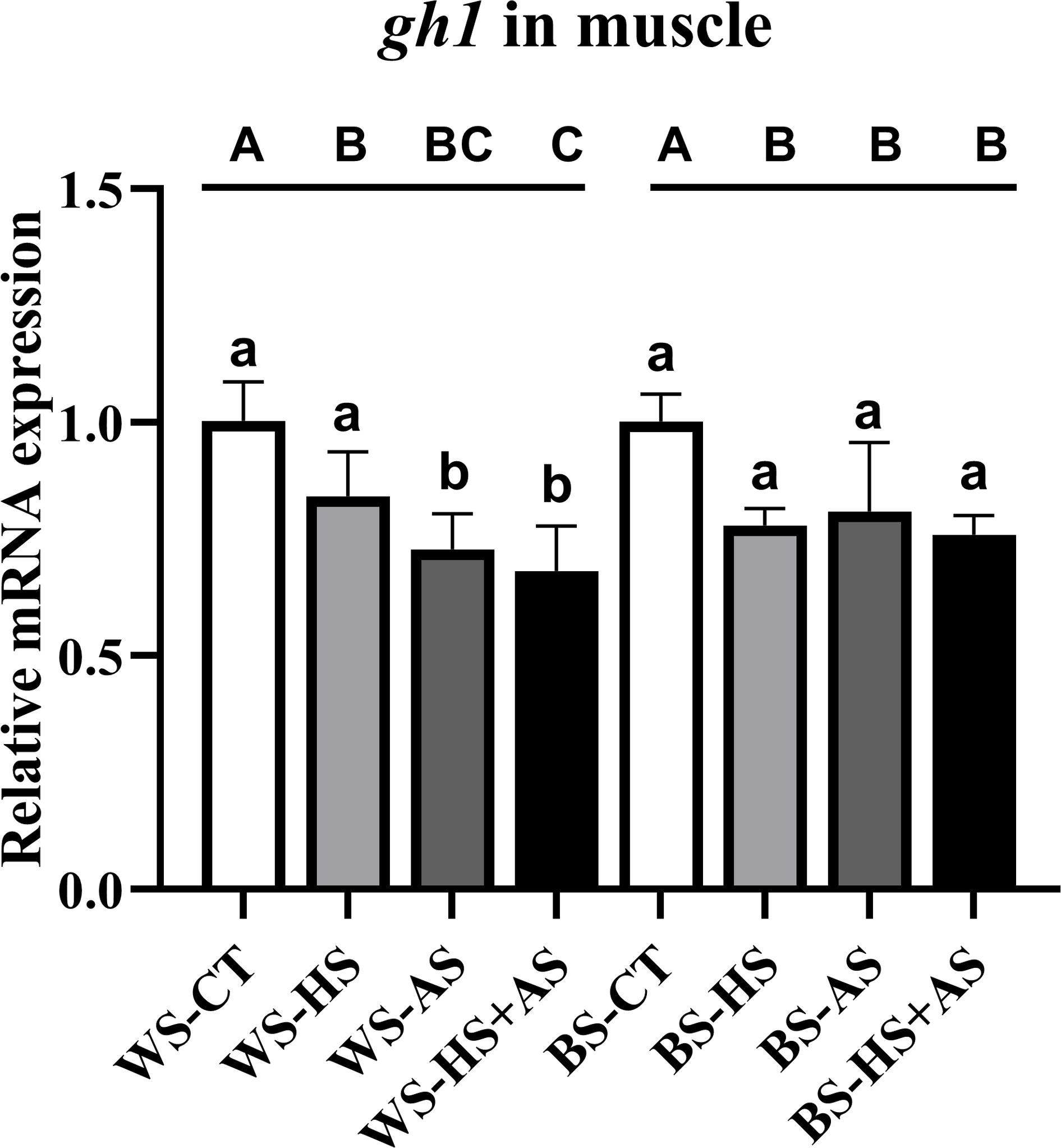
Figure 3 Effect of varying stress conditions on the growth-related gene expression of M. albus after 8 weeks. Different letters indicate significant differences (P < 0.05), while the same letters indicate no significant differences (P > 0.05). Lowercase letters indicate differences between wild and breeding strains under the same stress conditions, uppercase letters indicate differences between stress conditions under the same strain.
The levels of mRNA expression of genes (il-1β, il-8, il-10) associated with immunity in the liver and intestine were measured to investigate intestinal health under varying stress conditions. Across all stress conditions, the expression of il-1β and il-8 was markedly elevated under the same strain. However, the increase in BS expression was not as pronounced as the increase in WS expression under the same stress conditions (Figure 4A, B, D, E). Conversely, the levels of il-10 expression exhibited a declining pattern; furthermore, when subjected to identical stress circumstances, the decline in BS expression was less pronounced compared to WS (Figure 4C, F).
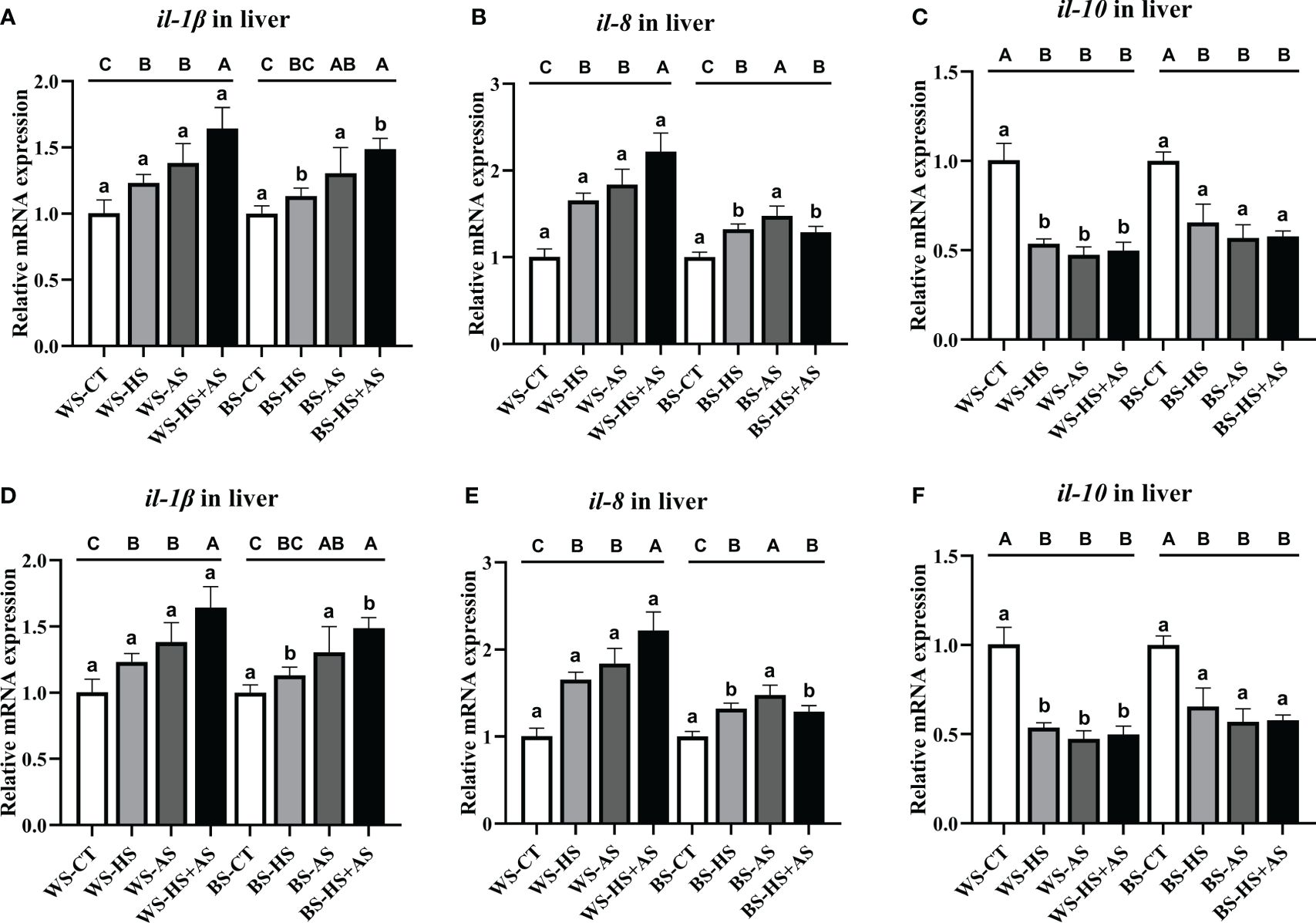
Figure 4 Effect of varying stress conditions on the immune-related gene expression of M. albus after 8 weeks. Interleukin-1 β (il-1β), interleukin-8 (il-8), and interleukin-10 (il-10). (A-C) Relative mRNA expression of il-1β, il-8, and il-10 in the liver, (D-F) Relative mRNA expression of il-1β, il-8, and il-10 in the intestine. Different letters indicate significant differences (P < 0.05), while the same letters indicate no significant differences (P > 0.05). Lowercase letters indicate differences between wild and breeding strains under the same stress conditions, uppercase letters indicate differences between stress conditions under the same strain.
The analysis of antioxidant-related gene expression revealed that, with the exception of cat in the liver and sod1 in the liver and intestine, all genes (hsp90α and gpx3) exhibited elevated expression to varying extents, with the maximum expression observed under combined stress (Figure 5).

Figure 5 Effect of varying stress conditions on the antioxidant-related gene expression of M. albus after 8 weeks. Heat shock protein 90-α (hsp90α), superoxide dismutase 1 (sod1), catalase (cat), and glutathione peroxidase 3 (gpx3). (A–D) Relative mRNA expression of hsp90α, sod1, cat, and gpx3 in the liver, (E–H) Relative mRNA expression of hsp90α, sod1, cat, and gpx3 in the intestine. Different letters indicate significant differences (P < 0.05), while the same letters indicate no significant differences (P > 0.05). Lowercase letters indicate differences between wild and breeding strains under the same stress conditions, uppercase letters indicate differences between stress conditions under the same strain.
Under each stress condition, the expression of cas2, cas8, and cas9 in the liver and intestine was up-regulated. In contrast, zo-1 showed a down-regulated expression pattern (Figure 6). In general, the patterns of expression between WS and BS were similar, with no notable discrepancies.
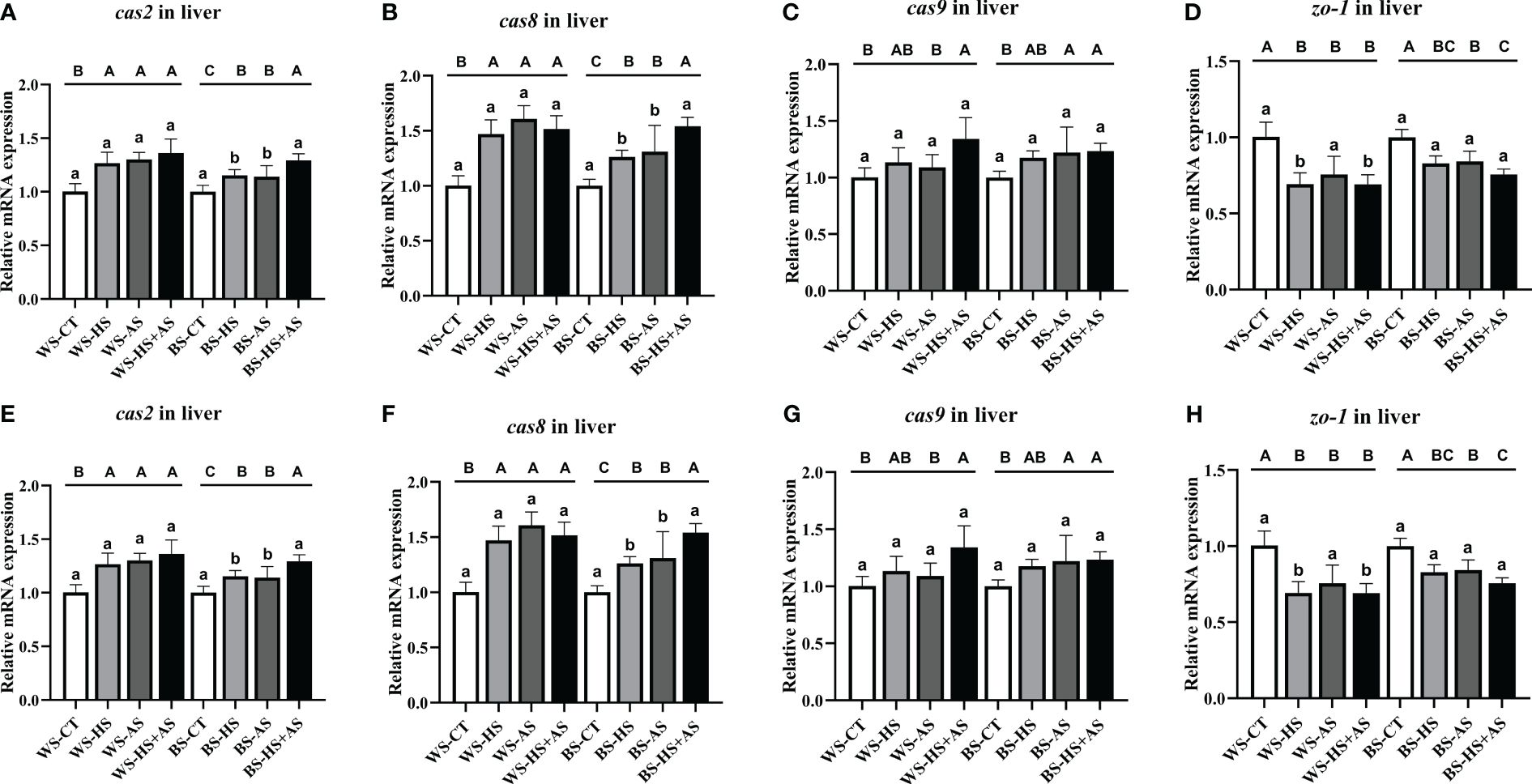
Figure 6 Effect of varying stress conditions on the apoptosis-related gene expression of M. albus after 8 weeks. Caspase 2 (cas2), caspase 8 (cas8), caspase 9 (cas9), and tight junction protein-1 (zo-1). (A–D) Relative mRNA expression of cas2, cas8, cas9, and zo-1 in the liver, (E-H) Relative mRNA expression of cas2, cas8, cas9, and zo-1 in the intestine. Different letters indicate significant differences (P < 0.05), while the same letters indicate no significant differences (P > 0.05). Lowercase letters indicate differences between wild and breeding strains under the same stress conditions, uppercase letters indicate differences between stress conditions under the same strain.
Aquatic organisms might experience growth inhibition or death when exposed to ammonia and elevated temperatures for an extended period (Paust et al., 2011; Chen et al., 2019; Dettleff et al., 2022). Hence, it is crucial to investigate the growth performance of fish under such stress conditions and elucidate the underlying factors contributing to it.
The current study shows that after eight weeks of feeding experiments, the FBW, WGR, and SGR of eels experienced a substantial decrease when subjected to either HS or AS stress individually. Furthermore, this suppression was further intensified when the eels were exposed to combined stress (HS+AS) together. This was verified by its impact on survival, as both WS and AS exhibited elevated death rates when exposed to the combined exposure. This phenomenon could be attributed to the reduction in the rate of food intake experienced by the fish when subjected to stressful circumstances, a finding that has been corroborated in other fish species, including yellow catfish (Pelteobagrus fulvidraco) (Wang et al., 2024), tra catfish (Pangasianodon hypophthalmus) (Lee et al., 2023), and largemouth bass (Micropterus salmoides) (Du et al., 2024). This phenomenon can be more intuitively explained by the significant decrease in the expression of growth-related genes in muscle.
Tissue damage has been identified as the cause of impaired one of growth performance in aquatic species exposed to heat stress and ammonia stress in the water column. Aquatic species experienced altered metabolic, immunological, and oxidative processes, leading to decreased growth and elevated rates of death and morbidity (Kolarevic et al., 2013; Zhang et al., 2019; Esam et al., 2022). Oxidative stress in fish is a reaction to external environmental conditions, including temperature, low oxygen levels, salinity, ammonia concentration, and other stressors. It serves as a crucial detoxification mechanism for aquatic species in response to environmental stresses (Birnie-Gauvin et al., 2017; Zhang et al., 2020). Reactive oxygen species (ROS) are products produced under physiological or pathological conditions, and the balance of ROS is thought to be relevant in apoptosis, control of signaling, and maintaining homeostasis in vivo (Ye et al., 2023). In typical circumstances, oxygen-free radicals are normal products of tissue metabolism, but oxidative stress arises when the formation of ROS surpasses the cellular antioxidant capability, resulting in structural harm caused by ROS (Horssen et al., 2008).
SOD, CAT, and GSH-Px play a crucial role in combating ROS by transforming them into harmless metabolites, thereby efficiently avoiding the buildup of ROS (Rahimnejad et al., 2020; Zhu et al., 2023). Our investigation revealed that the activities of CAT and GSH in the liver and intestine were elevated in response to oxidative stress induced by various stressors, but there was no significant change in SOD. The explanation was elucidated through studies on the expression of related antioxidant genes. The organism up-regulated the expression of cat and gpx4 in response to long-term environmental stress. The decrease in sod expression was likely caused by the inhibitory effects of ammonia and heat stress, which were especially noticeable under combined stress. Heat shock proteins are biomarkers of a stress response that carry out distinct protective roles in all living species (Wan et al., 2020). hsp90α, as a member of the heat shock protein family, can enhance immunological and antioxidant abilities in reaction to environmental stress by activating both innate and adaptive cellular immunity (Hoter et al., 2018). This gene’s expression is also increased in the liver and intestines, indicating its role in the body’s antioxidant response. AKP and ACP, functioning as biomarker enzymes, are crucial components of lysosomal enzymes. They are widely present in tissues and play a significant role as hydrolytic enzymes in immune defense, and their activity levels reflect the strength of tissue immunity (Song et al., 2006; Elabd et al., 2016). MDA also functions as a biomarker enzyme, and its concentration can indirectly indicate the antioxidant capability of lipids (Kanner and Lapidot, 2001). The findings of our study demonstrated that the levels of AKP and ACP were decreased to different extents in both the liver and intestine, whereas MDA was dramatically elevated, indicating the occurrence of oxidative stress in the tissues. Remarkably, BS outperformed WS in its ability to counteract oxidative damage under the same stress conditions.
The expression of mRNAs associated with intestinal and liver immunity in eels further revealed that environmental stress can modify immune responses. Activation of inflammatory cytokines induces a range of pro-inflammatory cytokines (il-1β and il-8) (Luissint et al., 2016). In innate immunity, these cytokines are recruited by macrophages, neutrophils, and lymphocytes to infected tissues and ultimately trigger inflammation as a response to pathogen infection (Yusuf et al., 2020). il-1β is primarily released by activated macrophages and activates diverse pro-inflammatory transcription factors in various target cells, leading to the release of other inflammatory molecules (Zhu et al., 2013). il-8, a chemokine, primarily stimulates the movement of neutrophils towards the gastrointestinal mucosa as a result of inflammation, damage, and infection (Lemme-Dumit et al., 2022). il-10 has demonstrated efficacy in suppressing the production of pro-inflammatory chemicals, hence safeguarding the integrity of the intestinal mucosal barrier, known as an anti-inflammatory cytokine (Zhao et al., 2020). The results of our study showed a large increase in the expression of il-1β and il-8 in both the liver and intestine under various stress conditions; conversely, the expression of il-10 showed a considerable decrease. This indicates that the tissue experiences an inflammatory response when exposed to the environment. zo-1 is a tight junction protein, plays a vital role in preserving the structural integrity and optimal functioning of intact epithelial cells (Turner, 2009). Caspase activity serves as a reliable indication of apoptosis in fish cells (Zhang et al., 2023). cas2, cas8, and cas9 have significant functions in the processes of apoptosis and immune response (Fu et al., 2020; Cui et al., 2023). The current work demonstrates that the expression of cas2, cas8, and cas9 was increased in response to ammonia and heat stress, whereas the expression of zo-1 was decreased. This suggests a potential association between apoptotic pathways that rely on caspase activity and these two types of stress.
In summary, the presence of ammonia and heat stress seemed to inhibit the growth performance of both wild and breeding eels; these inhibitory effects were more noticeable when combined with stress. In response to oxidative stress, they have upregulated the activity and gene expression of immune-related enzymes, while the results of some indicator enzymes suggest the occurrence of oxidative damage in their tissues. The presence of an inflammatory response in the tissues was suggested by the up-regulation of genes associated with pro-inflammatory cytokines and the down-regulation of genes related to anti-inflammatory cytokines. Furthermore, the up-regulation of genes connected to apoptosis revealed tissue damage. Nevertheless, it is worth noting that breeding strains exhibited superior adaptability to ammonia and heat stress in comparison to wild strains.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
The animal study was approved by Statute of Experimental Animal Ethics Committee of Shanghai Academy of Agricultural Sciences with Approval Number SAASPZ0520016. The study was conducted in accordance with the local legislation and institutional requirements.
ML: Writing – original draft, Visualization, Investigation, Formal Analysis, Data curation, Conceptualization. WH: Validation, Investigation, Data curation, Conceptualization, Writing – review & editing. YZ: Writing – original draft, Formal Analysis, Data curation. QY: Writing – review & editing, Investigation, Conceptualization. HY: Writing – review & editing, Investigation, Conceptualization. WL: Writing – review & editing, Project administration, Investigation, Funding acquisition, Conceptualization. WZ: Writing – review & editing, Supervision, Resources, Investigation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shanghai Municipal Agricultural Commission (grant number 2022-02-08-00-12-F01187); and the China Agriculture Research System of MOF and MARA (grant number CARS-46).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Birnie-Gauvin K., Costantini D., Cooke S. J., Willmore W. G. (2017). A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish 18, 928–942. doi: 10.1111/faf.12215
Bucking C. (2017). A broader look at ammonia production, excretion, and transport in fish: a review of impacts of feeding and the environment. J. Comp. Physiol. B 187, 1–18. doi: 10.1007/s00360-016-1026-9
Chen K., Tang T., Song Q., Wang Z., He K., Liu X., et al. (2019). Transcription analysis of the stress and immune response genes to temperature stress in ostrinia furnacalis. Front. Physiol. 10. doi: 10.3389/fphys.2019.01289
Cui T., Liu P., Chen X., Liu Z., Wang B., Gao C., et al. (2023). Identification and functional characterization of caspases in turbot (Scophthalmus maximus) in response to bacterial infection. Fish Shellfish Immun. 137, 108757. doi: 10.1016/j.fsi.2023.108757
Cui Y., Zhao N., Wang C., Long J., Chen Y., Deng Z., et al. (2022). Acute ammonia stress-induced oxidative and heat shock responses modulated by transcription factors in Litopenaeus vannamei. Fish Shellfish Immun. 128, 181–187. doi: 10.1016/j.fsi.2022.07.060
Dettleff P., Zuloaga R., Fuentes M., Gonzalez P., Aedo J., Manuel Estrada J., et al. (2022). High-temperature stress effect on the red cusk-eel (Geypterus Chilensis) liver: transcriptional modulation and oxidative stress damage. Biology-Basel 11, 990. doi: 10.3390/biology11070990
Dominguez M., Takemura A., Tsuchiya M., Nakamura S. (2004). Impact of different environmental factors on the circulating immunoglobulin levels in the Nile tilapia. Oreochromis niloticus. Aquacult. 241, 491–500. doi: 10.1016/j.aquaculture.2004.06.027
Du J., Xie Y., Li M., Zhu T., Lei C., Song H., et al. (2024). Effects of chronic heat stress on growth performance, liver histology, digestive enzyme activities, and expressions of HSP genes in different populations of Largemouth bass (Micropterus salmoides). Aquacult. Rep. 35, 101972. doi: 10.1016/j.aqrep.2024.101972
Elabd H., Wang H.-P., Shaheen A., Yao H., Abbass A. (2016). Feeding Glycyrrhiza glabra (liquorice) and Astragalus membranaceus (AM) alters innate immune and physiological responses in yellow perch (Perca flavescens). Fish Shellfish Immun. 54, 374–384. doi: 10.1016/j.fsi.2016.04.024
Esam F., Khalafalla M. M., Gewaily M. S., Abdo S., Hassan A. M., Dawood M. A. O. (2022). Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotox Environ. Safe 231, 113187. doi: 10.1016/j.ecoenv.2022.113187
Fu S., Ding M., Wang J., Yin X., Zhou E., Kong L., et al. (2020). Identification and functional characterization of three caspases in Takifugu obscurus in response to bacterial infection. Fish Shellfish Immun. 106, 252–262. doi: 10.1016/j.fsi.2020.07.047
Horssen J., Schreibelt G., Drexhage J., Hazes T., Dijkstra C. D., van der Valk P., et al. (2008). Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radical Bio Med. 45, 1729–1737. doi: 10.1016/j.freeradbiomed.2008.09.023
Hoter A., El-Sabban M. E., Naim H. Y. (2018). The HSP90 family: structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 19, 2560. doi: 10.3390/ijms19092560
Ip Y. K., Chew S. F. (2010). Ammonia production, excretion, toxicity, and defense in fish: a review. Front. Physiol. 1. doi: 10.3389/fphys.2010.00134
Kanner J., Lapidot T. (2001). The stomach as a bioreactor: Dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radical Bio Med. 31, 1388–1395. doi: 10.1016/S0891-5849(01)00718-3
Kim S.-H., Kim J.-H., Park M.-A., Hwang S. D., Kang J.-C. (2015). The toxic effects of ammonia exposure on antioxidant and immune responses in Rockfish, Sebastes schlegelii during thermal stress. Environ. Toxicol. Phar. 40, 954–959. doi: 10.1016/j.etap.2015.10.006
Kolarevic J., Selset R., Felip O., Good C., Snekvik K., Takle H., et al. (2013). Influence of long term ammonia exposure on Atlantic salmon (Salmo salar L.) parr growth and welfare. Aquac. Res. 44, 1649–1664. doi: 10.1111/j.1365-2109.2012.03170.x
Lee Y.-C., Huang Y.-T., Chang C.-C., Lin Y.-H. (2023). Physiological responses to hypothermal stress of Tra catfish (Pangasianodon hypophthalmus) subjected to different feeding rates. Aquacult. Rep. 33, 101809. doi: 10.1016/j.aqrep.2023.101809
Lemme-Dumit J. M., Doucet M., Zachos N. C., Pasetti M. F. (2022). Epithelial and neutrophil interactions and coordinated response to shigella in a human intestinal enteroid-neutrophil coculture model. Mbio 13, 00944–00922. doi: 10.1128/mbio.00944-22
Li Y., Liu Z., Li M., Jiang Q., Wu D., Huang Y., et al. (2020). Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense. J. Hazard Mater. 398, 122990. doi: 10.1016/j.jhazmat.2020.122990
Li Y., Xiang Y., Jiang Q., Yang Y., Huang Y., Fan W., et al. (2022). Comparison of immune defense and antioxidant capacity between broodstock and hybrid offspring of juvenile shrimp (Macrobrachium nipponense): Response to acute ammonia stress. Anim. Genet. 53, 380–392. doi: 10.1111/age.13182
Luissint A.-C., Parkos C. A., Nusrat A. (2016). Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151, 616–632. doi: 10.1053/j.gastro.2016.07.008
Mao Y., Lv W., Huang W., Yuan Q., Yang H., Zhou W., et al. (2024). Effects on growth performance and immunity of Monopterus albus after high temperature stress. Front. Physiol. 15. doi: 10.3389/fphys.2024.1397818
Ou H., Liang J., Liu J. (2022). Effects of acute ammonia exposure on oxidative stress, endoplasmic reticulum stress and apoptosis in the kuruma shrimp (Marsupenaeus japonicus). Aquacult. Rep. 27, 101383. doi: 10.1016/j.aqrep.2022.101383
Paust L. O., Foss A., Imsland A. K. (2011). Effects of chronic and periodic exposure to ammonia on growth, food conversion efficiency and blood physiology in juvenile Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 315, 400–406. doi: 10.1016/j.aquaculture.2011.03.008
Rahimnejad S., Yuan X.-Y., Liu W.-B., Jiang G.-Z., Cao X.-F., Dai Y.-J., et al. (2020). Evaluation of antioxidant capacity and immunomodulatory effects of yeast hydrolysates for hepatocytes of blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immun. 106, 142–148. doi: 10.1016/j.fsi.2020.06.019
Rebl A., Verleih M., Koebis J. M., Kuehn C., Wimmers K., Koellner B., et al. (2013). Transcriptome profiling of gill tissue in regionally bred and globally farmed rainbow trout strains reveals different strategies for coping with thermal stress. Mar. Biotechnol. 15, 445–460. doi: 10.1007/s10126-013-9501-8
Reid G. K., Gurney-Smith H. J., Marcogliese D. J., Knowler D., Benfey T., Garber A. F., et al. (2019). Climate change and aquaculture: considering biological response and resources. Aquacult. Env. Interac. 11, 569–602. doi: 10.3354/aei00332
Song Z.-f., Wu T.-x., Cai L.-s., Zhang L.-j., Zheng X.-d. (2006). Effects of dietary supplementation with clostridium butyricum on the growth performance and humoral immune response in Miichthys miiuy. J. Zhejiang Univ-Sc B 7, 596–602. doi: 10.1631/jzus.2006.B0596
Turner J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809. doi: 10.1038/nri2653
Wan Q., Song D., Li H., He M.-l. (2020). Stress proteins: the biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduct Tar 5, 125. doi: 10.1038/s41392-020-00233-4
Wang S., Li X., Zhang M., Li M. (2024). Effects of dietary sodium acetate on growth, intestinal microbiota composition, and ammonia tolerance of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 581, 740480. doi: 10.1016/j.aquaculture.2023.740480
Wu Y., Zhou Z., Pan Y., Zhao J., Bai H., Chen B., et al. (2021). GWAS identified candidate variants and genes associated with acute heat tolerance of large yellow croaker. Aquaculture 540, 736696. doi: 10.1016/j.aquaculture.2021.736696
Ye Y., Zhu B., Yun J., Yang Y., Tian J., Xu W., et al. (2023). Comparison of antioxidant capacity and immune response between low salinity tolerant hybrid and normal variety of Pacific white shrimp (Litopenaeus vannamei). Aquacult. Int. 32, 1879–1894. doi: 10.1007/s10499-023-01248-8
Yusuf A., Huang X., Chen N., Apraku A., Wang W., Cornel A., et al. (2020). Impact of dietary vitamin c on plasma metabolites, antioxidant capacity and innate immunocompetence in juvenile largemouth bass, Micropterus salmoides. Aquacult. Rep. 17, 100383. doi: 10.1016/j.aqrep.2020.100383
Zhang C., Hu Q.-Y., Feng L., Wu P., Liu Y., Kuang S.-Y., et al. (2023). Isalo scorpion Cytotoxic peptide (IsCT) improved the physical barrier of the intestine on on-growing grass carp (Ctenopharyngodon idella). Aquaculture 577, 739895. doi: 10.1016/j.aquaculture.2023.739895
Zhang C.-N., Tian H.-Y., Li X.-F., Zhu J., Cai D.-S., Xu C., et al. (2014). The effects of fructooligosaccharide on the immune response, antioxidant capability and HSP70 and HSP90 expressions in blunt snout bream (Megalobrama amblycephala Yih) under high heat stress. Aquaculture 433, 458–466. doi: 10.1016/j.aquaculture.2014.07.007
Zhang T., Yan Z., Zheng X., Wang S., Fan J., Liu Z. (2020). Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (Corbicula fluminea). Fish Shellfish Immun. 99, 514–525. doi: 10.1016/j.fsi.2020.02.046
Zhang W., Xia S., Zhu J., Miao L., Ren M., Lin Y., et al. (2019). Growth performance, physiological response and histology changes of juvenile blunt snout bream, Megalobrama amblycephala exposed to chronic ammonia. Aquaculture 506, 424–436. doi: 10.1016/j.aquaculture.2019.03.072
Zhao J., Wang H., Yang H., Zhou Y., Tang L. (2020). Autophagy induction by rapamycin ameliorates experimental colitis and improves intestinal epithelial barrier function in IL-10 knockout mice. Int. Immunopharmacol. 81, 105977. doi: 10.1016/j.intimp.2019.105977
Zhu L.-y., Nie L., Zhu G., Xiang L.-x., Shao J.-z. (2013). Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 39, 39–62. doi: 10.1016/j.dci.2012.04.001
Keywords: Monopterus albus, ammonia stress, heat stress, growth, antioxidant, immunity
Citation: Li M, Huang W, Zhao Y, Yuan Q, Yang H, Lv W and Zhou W (2024) Effects of long-term ammonia and heat stress on growth performance, antioxidant and immunity of wild and breeding juvenile rice field eel (Monopterus albus). Front. Mar. Sci. 11:1444210. doi: 10.3389/fmars.2024.1444210
Received: 05 June 2024; Accepted: 17 June 2024;
Published: 28 June 2024.
Edited by:
Yiming Li, Fishery Machinery and Instrument Research Institute, ChinaReviewed by:
Lanmei Wang, Freshwater Fisheries Research Center (CAFS), ChinaCopyright © 2024 Li, Huang, Zhao, Yuan, Yang, Lv and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Lv, d3dsdjE5ODZAc2luYS5jb20=; Wenzong Zhou, d3p6aG91NTA1QHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.