
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 11 July 2024
Sec. Aquatic Physiology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1441426
This article is part of the Research Topic Endocrine Regulation and Physiological Adaptation of Stress Response in Aquatic Organisms, Volume II View all 13 articles
 Tingting Shu1,2*
Tingting Shu1,2* Jing Yang1,2
Jing Yang1,2 Zhaoxi Yu1,2
Zhaoxi Yu1,2 Kan Xiao1,2
Kan Xiao1,2 Hongtao Huang1,2
Hongtao Huang1,2 Lingquan Dai1,2
Lingquan Dai1,2 Zhan Yin3
Zhan Yin3 Wei Jiang1,2*
Wei Jiang1,2*Ecological operation of hydraulic engineering is essential for the conservation of fishery resources. Water velocity is known to affect the spawning of fishes delivering drifting eggs. This study aims to explore the effects of water velocity stimulation on the ovarian maturation and antioxidant capacity of adult grass carp (Ctenopharyngodon idellus) through laboratory experiments in order to understand the physiological mechanism underlying the response of natural reproduction to ecological flows. We examined the histology, sex hormones and vitellogenin (VTG) concentrations of ovary, and the transcripts of key genes in the hypothalamus-pituitary-gonad (HPG) axis, as well as the antioxidant activities of ovary and liver in grass carp. The results showed that although there was no discernible difference on the ovarian development characteristics of grass carp under water velocity stimulation, estradiol, testosterone, progesterone, 17α,20β-dihydroxy-4-pregnen-3-one (17α,20β-DHP), and VTG concentrations were elevated, which was related to the transcriptional regulation of the HPG axis genes. The gene expression levels (gnrh2, fshβ, lhβ, cgα, hsd20b, hsd17b3, and vtg) in the HPG axis were significantly elevated under water velocity stimulation, while those of hsd3b1, cyp17a1, cyp19a1a, hsd17b1, star, and igf3 were suppressed. In addition, appropriate water velocity stimulation could enhance body health status by increasing the activities of antioxidant enzymes in the ovary and liver. The results of this study provide the fundamental knowledge and data support for ecological operation of hydropower projects and river ecological restoration.
The Three Gorges Dam (TGD), located in the middle stretch of the Yangtze River, is the world’s largest hydropower project and plays a crucial role in harnessing and exploiting the river’s power (Tang et al., 2016). However, the operation of the TGD not only significantly alters the hydrological processes of rivers but also threatens aquatic habitats both upstream and downstream of the dam site, thereby contributing to the degradation of riverine ecosystems (Zhang et al., 2021). In detail, the regulation of reservoirs homogenizes the flow processes of rivers and weakens or eliminates the natural flood peaks, thus leading to a decrease in fish eggs (She et al., 2023).
Fish spawning activity is likely influenced by a variety of environmental factors, including water velocity, water temperature, and dissolved oxygen. By influencing hormone synthesis and secretion, these environmental factors affect the gonadal development of fish (Liu et al., 2021). In particular, water velocity has been recognized to affect the spawning of fishes delivering drifting eggs in rivers (Chen et al., 2021a). In order to mitigate the adverse effects of dam operations on fish spawning, it is necessary to establish specific eco-hydrological processes to stimulate fish spawning (Wang et al., 2020).
The four major Chinese carps (FMCC), including black carp (Mylopharyngodon piceus), grass carp (Ctenopharyngodon idellus), silver carp (Hypophthalmichthys molitrix), and bighead carp (Hypophthalmichthys nobilis), which are highly sensitive to hydrological processes, represent the most economically important fishes in China. The FMCC population would migrate to the spawning sites and start spawning in response to high-flow pulses from March to June, while the construction and operation of TGD alter the natural hydrological rhythm and hinder fish migration (Zhang et al., 2023). Therefore, incorporating ecological flow into the operation scheme of TGD would be a mitigation measure to protect the spawning of FMCC. It has been demonstrated that implementing controlled man-made floods as part of the TGD operation enhances the reproductive success of FMCC in downstream regions (Xiao et al., 2022). Since 2011, several attempts have been organized to promote the spawning behavior of FMCC in order to mitigate the decline in FMCC from the Yangtze River. It was found that the water velocity that induces FMCC spawning ranged from 1.11 to 1.49 m/s (Cao et al., 2022), with an optimal flow velocity of 1.31 m/s was identified for the spawning of FMCC in rivers (Chen et al., 2021a). Although water velocity plays a crucial role in the reproduction of FMCC, there is a notable scarcity of research on the physiological mechanism underlying the response of natural reproduction to ecological flows.
In this study, we took adult grass carp as target species to assess the effects of water velocity stimulation on the ovarian maturation and antioxidant capacity through laboratory experiments. Histology, sex hormones and vitellogenin (VTG) concentrations of ovary, and the transcripts of key genes in the hypothalamus-pituitary-gonad (HPG) axis, as well as antioxidant activities of ovary and liver in grass carp were measured. The findings of this study will provide a theoretical basis for ecological operation.
In the present study, five-year-old sexually mature grass carp were purchased from Tengda Ecological Agriculture Development Co., Ltd. in Zhijiang City, Hubei Province, China. The average weight of female grass carp was 4.66 ± 0.75 kg (n = 60) while male grass carp was 4.50 ± 0.82 kg (n = 60). Before the experiment, all fish were acclimatized in the laboratory facility for one week. Next, a total of 120 healthy grass carp were allocated into five groups randomly with three replicates per group (fifteen 20,000 L PVC circular tanks in total, 4 females and 4 males in each tank). During the acclimatized and experimental period, the fish were fed in excess duckweed twice daily at 9:00 and 16:00. Water temperature, pH, and dissolved oxygen was maintained at 23 ± 1°C, 7.0−7.5, and 6.0−6.5 mg/L, respectively, and the light conditions followed a natural light/dark cycle (approximately 12/12 h).
Two hormones or agents were used in this study: luteinizing hormone-releasing hormone analogue (LHRH-A2) and domperidone (DOM), purchased from Ningbo Second Hormone Factory (Ningbo, China), and dissolved in physiological saline.
Research showed that an optimal water velocity of 1.3 m/s was identified for the spawning of FMCC in rivers, and no spawning activity was observed in flume at a velocity of 0.8 m/s (Chen et al., 2021a). Therefore, we set water velocities ranging from 0.8 to 1.3 m/s to explore the influence of water velocity on the gonad development in fish. Two submerged pumps (SHIMGE, China) were installed to accelerate the flow of water in a PVC circular tank with an inner diameter of 4.5 m and a water depth of 0.9 m (Figure 1A). Since the water velocity at the center of the tank was quite different from that at other locations, an isolation net with a diameter of 1.0 m was placed at the center to limit the swimming area of the grass carp. Twelve measuring points were arranged around the tank, and the velocity at a depth of 0.45 m was measured using a portable current meter (LS300-A, China) (Figure 1B). In artificial propagation, LHRH-A2 and DOM, which efficiently induce ovulation in females, have been utilized as oxytocic drugs in Cyprinid fishes (Hu et al., 2020; Zhong et al., 2021). Thus, hormone injection was chosen as the positive control. In detail, the experiment consisted of five groups: a control group with no water velocity (negative control), a low water velocity group at 0.8 m/s, a graded water velocity group with velocities increasing from 0.8 to 1.3 m/s gradually, a high water velocity group at 1.3 m/s, and a hormone injection group where females were injected with 2 mg/kg DOM and 2.5 μg/kg LHRH-A2 at the base of the pectoral fin, with a half dose for males (positive control), labeled NS, DS, ZS, GS, and JS, respectively (Figure 2). Flow stimulation was carried out from 8:00 to 11:00 and 15:00 to 18:00 every day for five days. The experiment was conducted at Hubei Key Laboratory of Three Gorges Project for Conservation of Fishes.
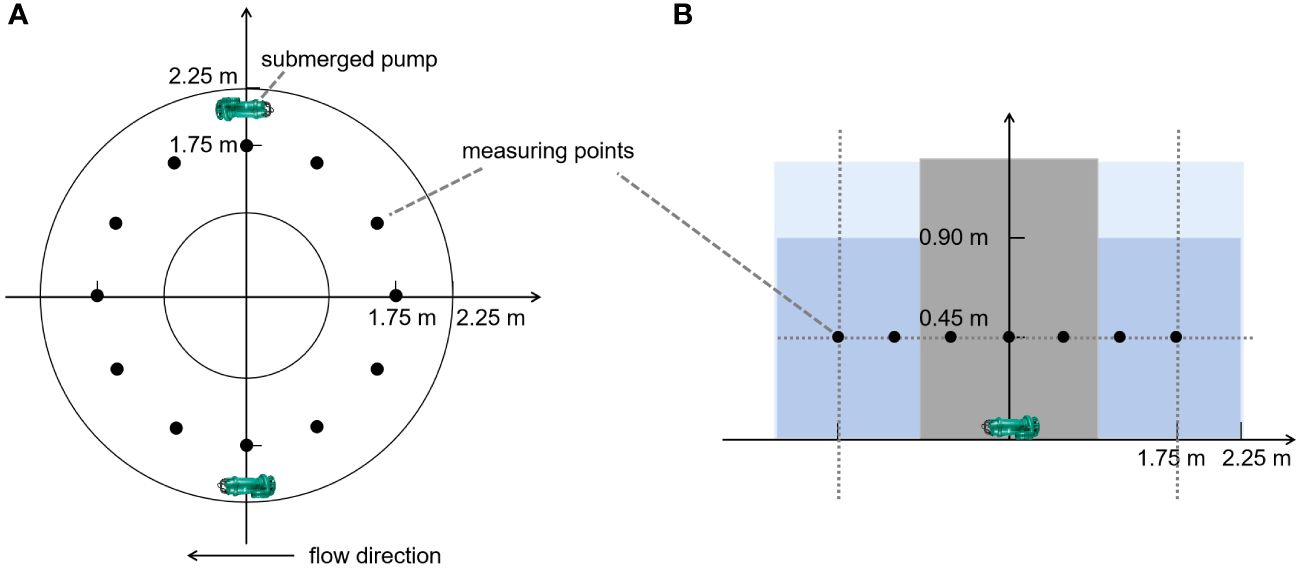
Figure 1 Schematic diagram of the experiment system for water velocity stimulation. (A) Vertical view of circular tank. (B) Front view of circular tank.
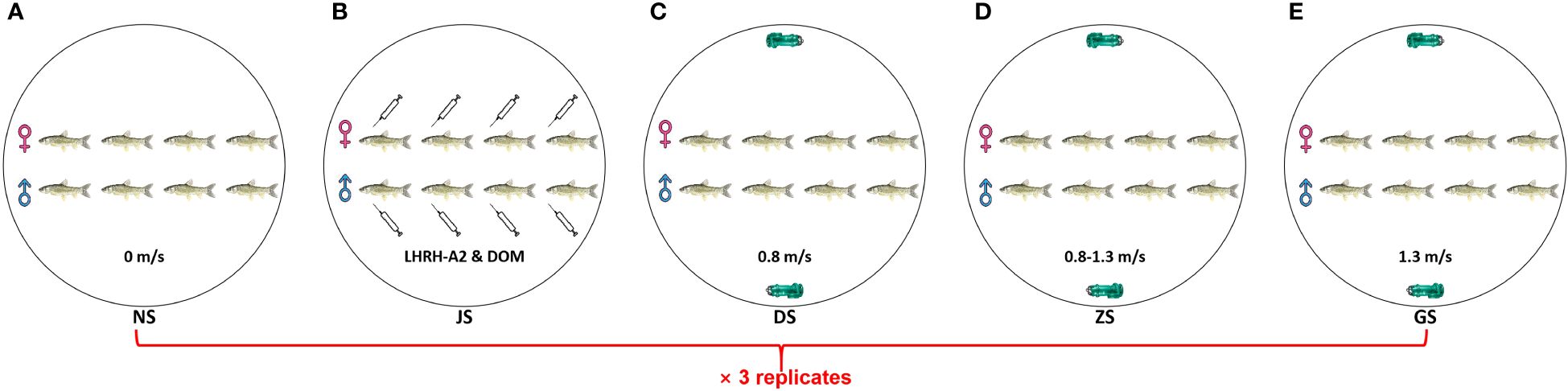
Figure 2 Schematic diagram of five experiment groups. (A) NS group. (B) JS group. (C) DS group. (D) ZS group. (E) GS group.
At the end of the experiment, females were anesthetized by immersion in a benzocaine solution at a concentration of 200 mg/L according to the previous procedure (Shu et al., 2023). The fish were then slaughtered, and their hypothalami, pituitaries, ovaries, and livers were promptly dissected. One part of each ovary was put into Bouin’s solution for subsequent histological analysis, while the hypothalami, pituitaries, livers, and the remaining ovary tissue were frozen immediately in liquid nitrogen, then transferred and stored at −80°C for the subsequent determination of various parameters.
The ovary slides were performed following the methods described previously (Lau et al., 2016). Briefly, ovary tissues of grass carp were put into Bouin’s solution for 24 h, then dehydrated through graded ethanol solutions, infiltrated with xylene, embedded in paraffin, and subsequently sliced into 5 μm thick sections for hematoxylin-eosin (H&E) staining. These sections were examined using a Nikon Eclipse Ni-U microscope (Nikon, Japan) for histopathological analysis. Scale bars are provided in the lower left corner of each image.
Superoxide enzyme (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), total antioxidant capacity (T-AOC), and peroxidase (POD) activities, as well as malondialdehyde (MDA) concentration in the ovaries and livers of grass carp were determined according to the kit instructions (Nanjing Jiancheng, China) via spectrophotometric analysis with a microplate reader. The serial numbers of SOD, GSH-Px, CAT, T-AOC, and POD enzyme activity kits, as well as MDA concentration kit were A001–3-2, A005–1-2, A007–1-1, A015–2-1, A084–1-1, and A003–1-2, respectively. The 0.1 g ovary or liver samples were isolated and homogenized in 900 μL of 0.9% sodium chloride solution (1:9 w/v) in a TGrinder H24R Tissue Homogenizer (TIANGEN, China) at 4°C, and then centrifuged at 2,000 rpm for 10 min. The supernatants were collected and allocated for antioxidant index analysis.
The concentrations of sex hormones [estradiol, testosterone, progesterone, and 17α,20β-dihydroxy-4-pregnen-3-one (17α,20β-DHP)] and VTG in ovaries were determined using commercial ELISA kits (Shanghai mlbio, China). The serial numbers of estradiol, testosterone, progesterone, 17α,20β-DHP, and VTG ELISA kits were ml003452, ml025781, ml003449, ml625990, and ml103464, respectively. The 0.1 g ovary samples were isolated and then mixed with 1 mL PBS before homogenization at 4°C. Following homogenization, the sex hormones and VTG were extracted with an organic solvent four times according to the manufacturer’s protocols. The layers were separated by vortexing and centrifugation, and then the organic phase was immediately transferred into a new tube and evaporated at 30°C under a gentle stream of nitrogen. Finally, the extracts were dissolved in 200 µL ELISA buffer, and the sex hormone and VTG concentrations were determined according to the manufacturer’s protocols.
Total RNA extraction, reverse transcription, and quantitative real-time PCR (qRT-PCR) were carried out according to our recent study (Shu et al., 2023). Briefly, total RNA was extracted from hypothalamus, pituitary, ovary samples by TRIzol reagent (Ambion, America). The integrity and quality of extracted RNA were assessed by agarose gel electrophoresis, meanwhile, the concentration was measured using NanoDrop One (Thermo Scientific, America). Then, EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen, China) was used to reverse transcribe 1.5 μg RNA to synthesize cDNA. qRT-PCR was conducted using the TransStart® Tip Green qPCR SuperMix (TransGen, China) and StepOnePlus™ real-time system (ABI, America).
Partial sequences of gonadotropin-releasing hormone 2 (gnrh2), gonadotropin-releasing hormone 3 (gnrh3), follicle stimulating hormone beta polypeptide (fshβ), luteinizing hormone beta polypeptide (lhβ), glycoprotein hormones alpha polypeptide (cgα), cytochrome P450-mediated side-chain cleavage enzyme (cyp11a1), 3-beta-hydroxysteroid dehydrogenase 1 (hsd3b1), cytochrome P450c17 (cyp17a1), ovarian cytochrome P450 aromatase (cyp19a1a), 20-beta-hydroxysteroid dehydrogenase (hsd20b), 17-beta-hydroxysteroid dehydrogenase 1 (hsd17b1), 17-beta-hydroxysteroid dehydrogenase 3 (hsd17b3), steroidogenic acute regulatory protein (star), vitellogenin (vtg), and insulin-like growth factor 3 (igf3) were obtained from the previous transcriptome of grass carp (Shu et al., 2023). Based on our previous study on the assessment of internal control genes (Shu et al., 2023), β-actin was used as a reference gene. All specific primers used for qRT-PCR were either designed by Primer-BLAST in National Center for Biotechnology Information (NCBI) or obtained from previous studies and synthesized commercially, and were confirmed approximately 100% effective. The sequences of the primers are listed in Table 1. Each sample was run in triplicate and the relative gene expression was normalized to β-actin compared to the control group and calculated using the 2-ΔΔCT method (Schmittgen and Livak, 2008).
Statistical analysis was analyzed in GraphPad Prism 8.0 software (GraphPad software, America). All results are presented as mean ± standard deviation (SD) for each experimental group. Differences were assessed using one-way ANOVA followed by Fisher’s least significant difference (LSD) test for multiple comparisons. For all statistical comparisons, P < 0.05 was identified as statistically significant.
To evaluate the effect of water velocity on the ovarian development characteristics of grass carp, we performed dissections and histological analysis of females to observe the ovarian morphology and the development of each oocyte types by H&E staining. Histological examination revealed that the oocyte type at different developmental stages existed in ovaries of all sampled female grass carp, including primary growth oocytes (PGs), pre-vitellogenic oocytes (PVs), and full-grown oocytes (FGs), primarily composed of full-grown oocytes (Figure 3). After the hormone injection and 5-day water velocity stimulation, respectively, the ovarian development characteristics were similar to those of the control group. To be specific, during this stage, the nuclear membrane vanished, and the nucleoplasm and cytoplasm started to integrate, accompanied by the yolk protein granules of a large size filling the entire oocyte, and zona radiata proteins form the inner layer of the envelope surrounding the oocyte, indicating the fulfillment of vitellogenesis. In maturing and growing oocytes, the zona radiata is overlaid with follicle cells (granulosa and theca cells) (Arukwe and Goksoyr, 2003; von Schalburg et al., 2023). The cortical alveoli were mainly present in the cytoplasm (Lubzens et al., 2017; Zhang et al., 2017). No discernible difference was observed in the histological sections among the five groups.
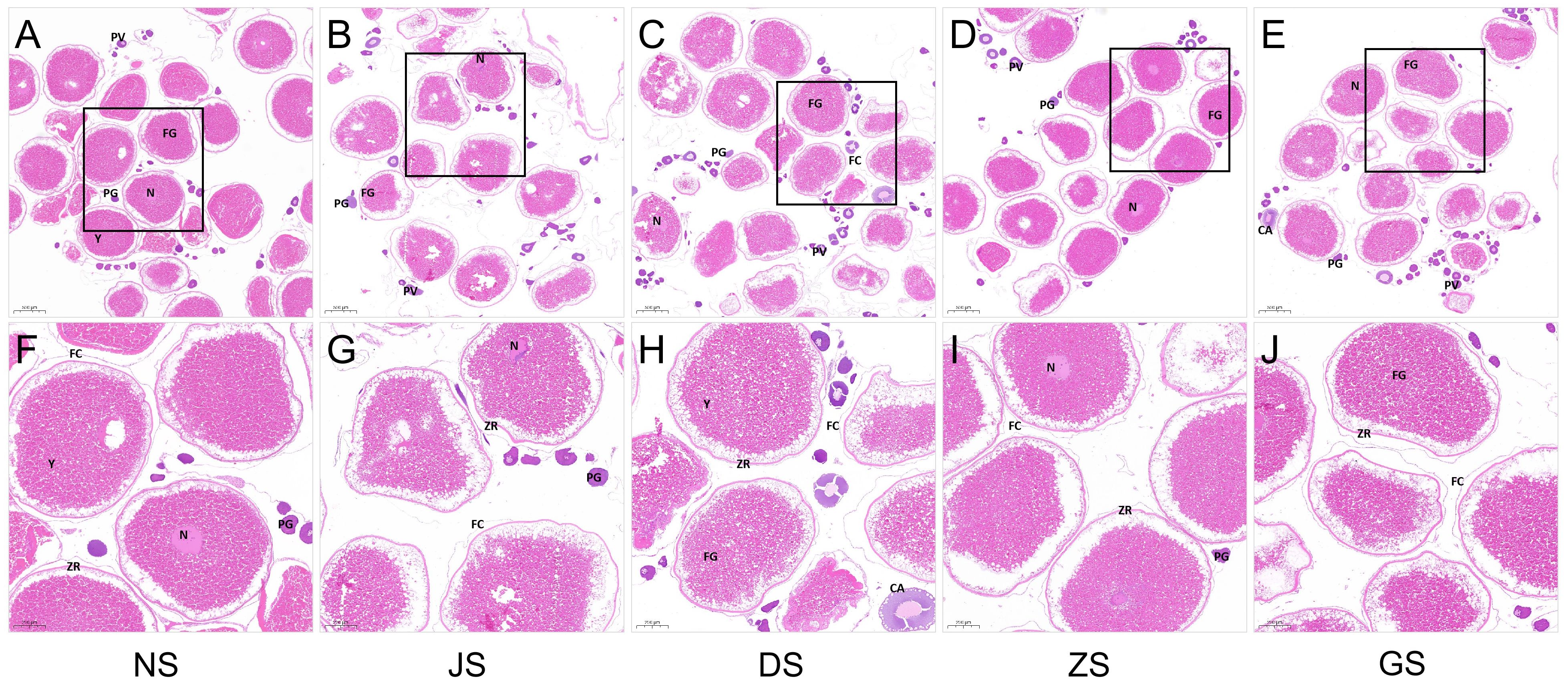
Figure 3 Histological sections of the ovarian status of grass carp (n = 6 for each group). (A, F) NS group. (B, G) JS group. (C, H) DS group. (D, I) ZS group. (E, J) GS group. PG, primary growth follicle; PV, previtellogenic follicle; FG, full-grown follicle; N, nucleus; Y, yolks; FC, follicle cells; ZR, zona radiata; CA, cortical alveoli. Scale bars: (A–E): 500 μm; (F–J): 200 μm.
A total of four sex hormones, including estradiol, testosterone, progesterone, and 17α,20β-DHP, as well as VTG concentrations, were detected in the ovary tissue. The estradiol concentration in the NS group was observed as 0.3649 ± 0.0146 pmol/g, which significantly increased to 0.5318 ± 0.0195 pmol/g and 0.5016 ± 0.0415 pmol/g in the JS and ZS groups, respectively. The estradiol concentrations in the DS and GS groups were slightly increased, although they were not statistically significant. Besides, the distribution of ovary estradiol concentration was significantly higher in the JS group than the distribution in the DS group (Figure 4A). The testosterone concentrations in the JS, DS, ZS, and GS groups ranged from 2,444 to 2,781 pg/g, which was significantly higher than that in the NS group (1905 ± 88.15 pg/g). However, no significant differences in testosterone concentrations were detected between the hormone injection and water velocity stimulation groups (Figure 4B). The progesterone concentrations in the NS, JS, DS, ZS, and GS groups were 71.43 ± 6.13, 87.13 ± 4.68, 84.22 ± 3.23, 130.2 ± 3.17, 105.1 ± 2.39 ng/g, respectively. Although progesterone concentrations were higher in the JS, DS, ZS, and GS groups compared to the NS group, significant differences were detected only in the ZS and GS groups. Moreover, the ZS and GS groups showed significantly higher progesterone concentrations than the JS group (Figure 4C). The 17α,20β-DHP concentrations in the JS and GS groups were 135.6 ± 6.396, 134.1 ± 7.626 ng/g, respectively, which showed obviously higher levels than that in the NS group (100.2 ± 8.276 ng/g), wherein no significant differences in 17α,20β-DHP concentrations were observed among the NS, DS, and ZS groups. And hormone injection and water velocity stimulation groups display no significant differences in 17α,20β-DHP concentrations (Figure 4D). The VTG concentration reached 3152 ± 47.04 ng/g in the NS group and was highest at 4267 ± 112.3 ng/g in the ZS group. The distribution of ovary VTG concentration was significantly higher in the ZS group than the distribution in the NS and JS groups. No significant differences were observed among the NS, JS, DS, and GS groups (Figure 4E).
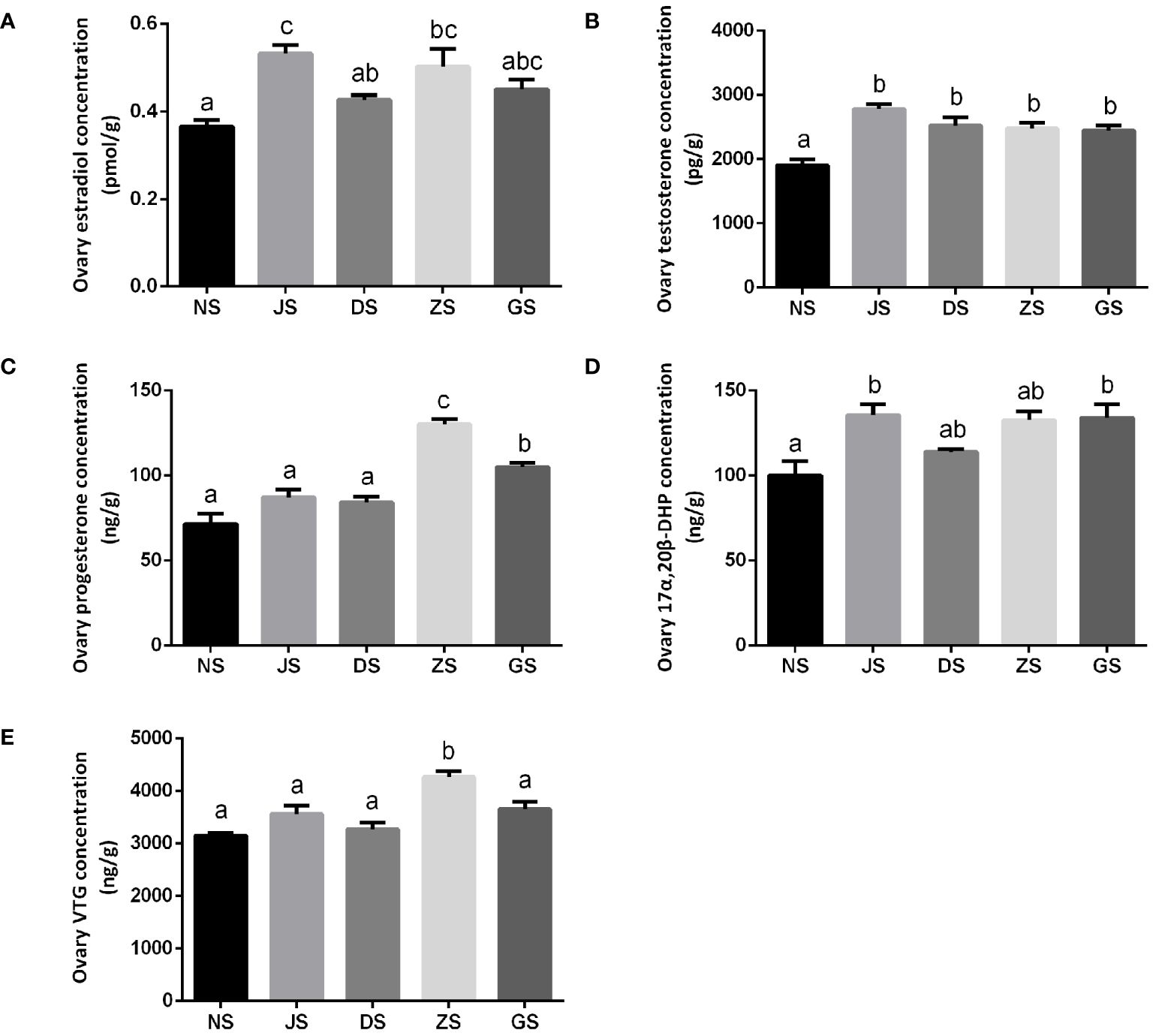
Figure 4 The sex hormones and VTG measurements (n = 6 for each group). Ovary concentrations of estradiol (A), testosterone (B), progesterone (C), 17α,20β-DHP (D), and VTG (E) in the NS, JS, DS, ZS, and GS groups. The letters in the bar charts represent significant differences.
The relative mRNA expression levels of key genes along the HPG axis were systematically examined using qRT-PCR. Hormone injection and water velocity stimulation significantly upregulated the transcript levels of gnrh2 in the hypothalamus (Figure 5A), accompanied by elevated expressions of fshβ, lhβ, and cgα in the pituitary, compared with those in the NS group (Figures 5C–E). Moreover, the transcription of hsd20b in the ovary was markedly upregulated in response to hormone injection and water velocity stimulation when compared to the control group (Figure 5J). Specifically, the transcriptions of gnrh2, fshβ, lhβ, cgα, and hsd20b in the JS group increased by 14.4-, 457.5-, 3.1-, 4.6-, and 1.4-fold, respectively, while in fish stimulated by water velocity, the levels were elevated up to 2.0-, 1854.0-, 4.2-, 10.8-, and 1.5-fold, respectively (Figures 5A, C–E, J). Accordingly, apart from hypothalamus gnrh2 and ovary hsd20b (Figures 5A, J), water velocity stimulation caused significantly greater levels of mRNA for pituitary fshβ, lhβ, and cgα than hormone injection (Figures 5C–E). However, there were no significant differences in gene expression of gnrh3 in the hypothalamus (Figure 5B), as well as cyp11a1 in the ovary of female grass carp among the five groups (Figure 5F). The expressions of hsd3b1, cyp17a1, cyp19a1a, hsd17b1, and igf3 in the ovary were significantly downregulated following hormone injection and water velocity stimulation in comparison with the control group (Figures 5G–I, K, O). Additionally, no significant differences were observed for ovary hsd3b1, hsd17b1, and igf3 between the hormone injection and water velocity stimulation groups (Figures 5G, K, O), while the transcriptions of cyp17a1 and cyp19a1a were significantly higher in the DS, ZS, and GS groups compared to the JS group (Figures 5H, I). Although the gene expression levels of hsd17b3, star, and vtg in the ovary of female grass carp did not show significant differences between the JS and NS groups (Figures 5L–N), water velocity stimulation led to a notable increase in hsd17b3 transcript levels in the DS group and elevated vtg levels in the ZS and GS groups (Figures 5L, N), while reducing the expression of star mRNA at all velocities (Figure 5M).
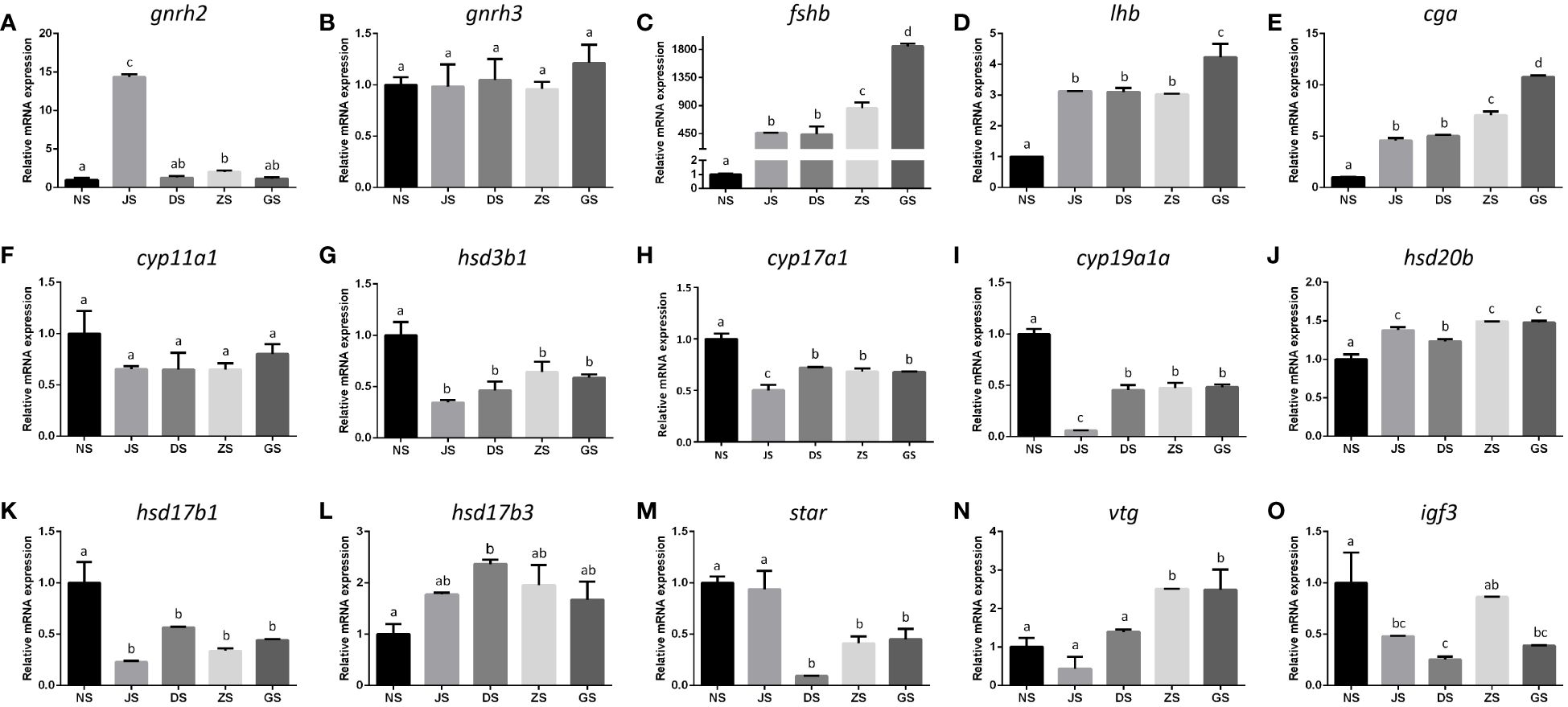
Figure 5 The mRNA expression levels of reproductive hormone genes in the HPG axis. All mRNA levels were calculated as the fold expression relative to β-actin (n = 6 for each group). gnrh2 (A) and gnrh3 (B) gene expression in the hypothalamus. fshβ (C), lhβ (D), and cgα (E) gene expression in the pituitary. cyp11a1 (F), hsd3b1 (G), cyp17a1 (H), cyp19a1a (I), hsd20b (J), hsd17b1 (K), hsd17b3 (L), star (M), vtg (N), and igf3 (O) gene expression in the ovary. The letters in the bar charts represent significant differences.
We examined the antioxidant enzyme capacities in the ovary of female grass carp. Compared with the NS group, ovary SOD and CAT activities were significantly higher in the JS, ZS, and GS groups. However, ovary SOD and CAT activities in the DS group were not different from those in the NS group (Figures 6A, B). Ovary POD activity in the NS group was obviously lower than that in the treated groups (Figure 6C). Additionally, no significant differences in ovary SOD and POD activities were observed between the hormone injection and water velocity stimulation groups (Figures 6A, C). A significant increase in ovary CAT activity was detected in the ZS group compared to the other experimental groups (Figure 6B). No significant differences in ovary GSH-Px activity were found among the five groups (Figure 6D). The ZS group had the highest ovary T-AOC activity, but there was a non-significant increase compared to the NS group. Overall, various types of water velocity stimulation had no effect on ovary T-AOC activity compared to the control group, but ovary T-AOC activity significantly increased in the ZS group compared to the JS group (Figure 6E). Ovary MDA concentration in the GS group was significantly lower than that in the NS and JS groups, while there were no significant differences in ovary MDA concentration among the NS, JS, DS, and ZS groups (Figure 6F).
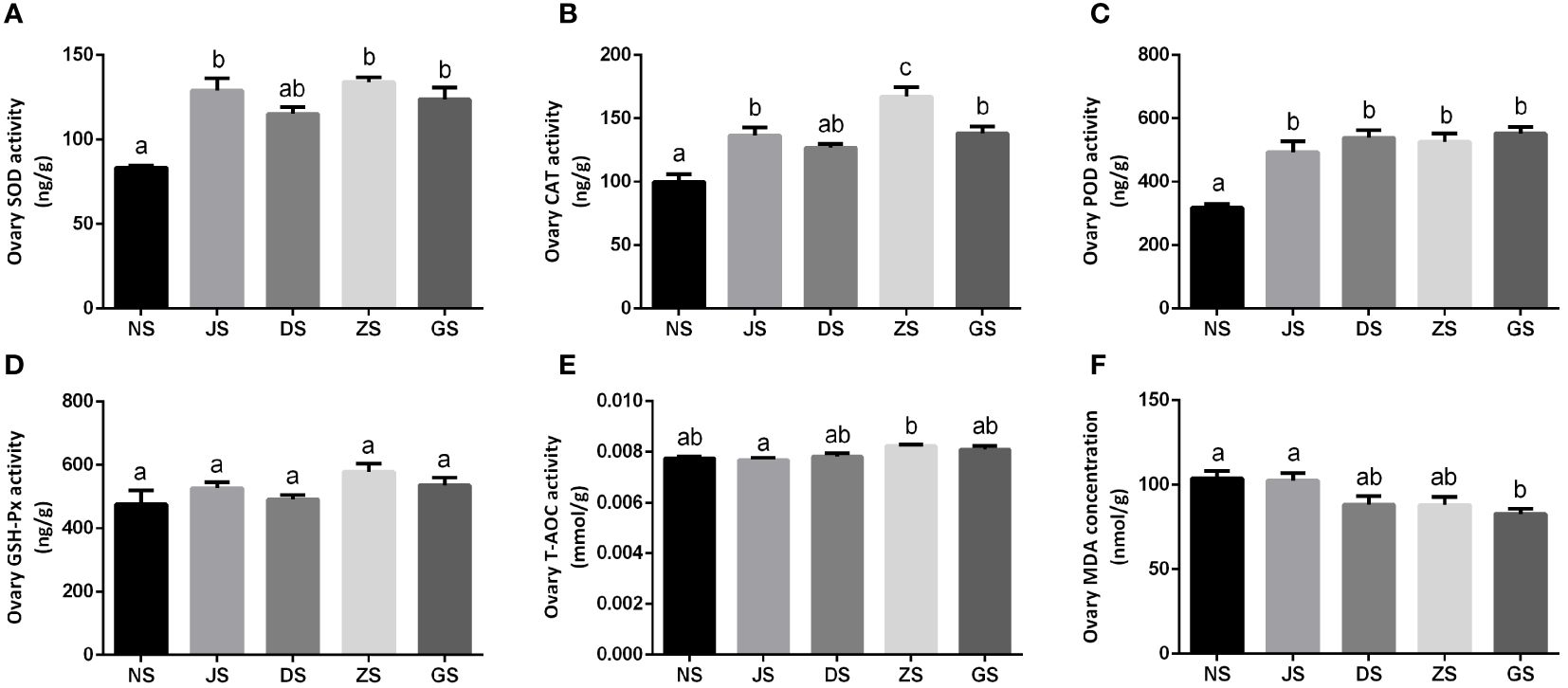
Figure 6 The ovary antioxidant enzyme activities measurements (n = 6 for each group). Ovary activities of SOD (A), CAT (B), POD (C), GSH-Px (D), T-AOC (E), and MDA (F) in the NS, JS, DS, ZS, and GS groups. The letters in the bar charts represent significant differences.
Meanwhile, hepatic antioxidant enzyme capacities were also measured in female grass carp. Hepatic SOD activity in the ZS and GS groups increased significantly when compared to the NS group, while the values did not show significant differences among the NS, JS, and DS groups (Figure 7A). There were few changes in hepatic CAT activity among all treatments compared to the NS group, except for the ZS group (Figure 7B). Changes in hepatic POD activity in the JS and ZS groups were also not obvious when compared to the NS group. However, hepatic POD activity in the DS and GS groups increased significantly (Figure 7C). No significant changes in hepatic SOD, CAT, and POD activities were detected between the hormone injection and water velocity stimulation groups (Figures 7A–C). Hepatic GSH-Px activity was significantly higher in the JS and ZS groups than in the NS group, while no significant differences were found among the NS, DS, and GS groups. Moreover, the JS group showed significantly higher hepatic GSH-Px activity than the DS group (Figure 7D). Hepatic T-AOC activity was significantly higher in all treatments relative to the NS group, but no significant differences were observed between the hormone injection and water velocity stimulation groups for hepatic T-AOC activity (Figure 7E). However, hepatic MDA concentration was not significantly affected by hormone injection and water velocity stimulation (Figure 7F).
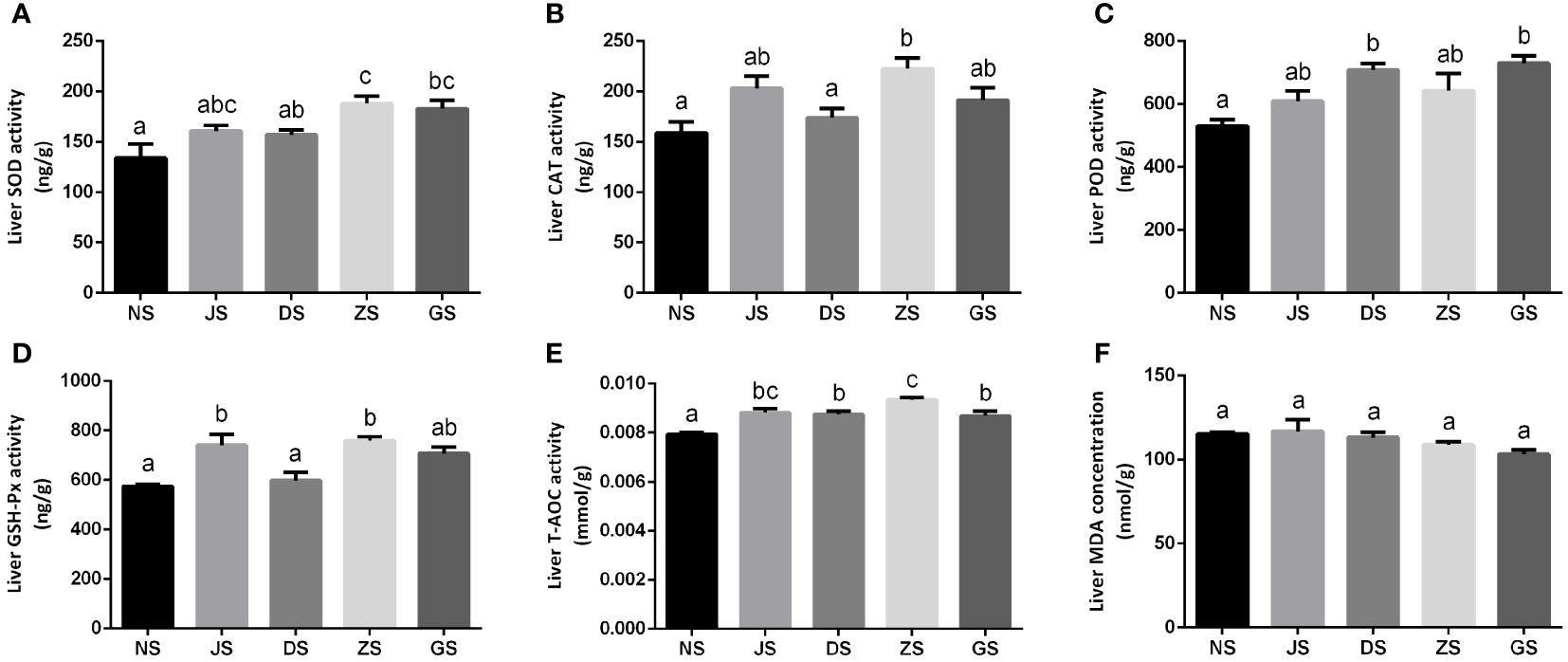
Figure 7 The hepatic antioxidant enzyme activities measurements (n = 6 for each group). Hepatic activities of SOD (A), CAT (B), POD (C), GSH-Px (D), T-AOC (E), and MDA (F) in the NS, JS, DS, ZS, and GS groups. The letters in the bar charts represent significant differences.
We explored the effects of water velocity stimulation on the ovarian maturation and antioxidant capacity of adult grass carp through laboratory experiments. Our research provided evidence that a certain water velocity is necessary for the ovarian maturation of grass carp. Regrettably, no spawning activity was observed. In the natural spawning sites of Yangtze River, there are complicated hydrological conditions and habitat characteristics. It has been well known that environmental conditions, such as warm temperatures, long photoperiod, and rising discharge, are considered to be crucial factors in controlling the reproductive cycles in teleost. In particular, a rising discharge serves as the primary cue for the spawning of FMCC (Chen et al., 2021a). Apparently, more research is needed to fully understand how environmental conditions influence grass carp spawning.
Estradiol, testosterone, progesterone, and 17α,20β-DHP, along with VTG concentrations, are commonly utilized as important biomarkers to assess gonad development and maturation of fish in various studies (Gadekar, 2014; Tucker et al., 2020). VTG, a large-molecular-weight glycolipoprotein synthesized in the liver and transported to egg cells, has a significant effect on the development of oocytes (Liu et al., 2022). Estradiol can stimulate VTG synthesis and secretion from the liver to developing oocytes via the bloodstream (Ghiasi et al., 2023). Testosterone, serving as the androgenic precursor, is converted to estradiol under the action of aromatase, inducing maturation processes of post-vitellogenic oocytes (Golmoradizadeh et al., 2021; Wang et al., 2023). Progesterone, a precursor of 17α,20β-DHP in the steroidogenic pathway, has been demonstrated to trigger final oocyte maturation and ovulation in fish species (Jéhannet et al., 2023). 17α,20β-DHP, recognized as the main maturation inducing hormone (MIH), plays a crucial role in the final maturation of oocytes and the induction of spawning in fish (Barcellos et al., 2001). Our study showed that a suitable water velocity stimulation (ZS group) could significantly enhance the concentrations of estradiol, testosterone, progesterone, and 17α,20β-DHP, as well as VTG in the ovaries of grass carp compared to the control group, indicating that water velocity stimulation could promote gonadal development and maturation. This agreed well with previous study, which showed that flow stimulation had a positive effect on the hormone rate of change for estradiol and testosterone (Liu et al., 2021). Furthermore, it has been proven that during ovarian development in the European eel (Anguilla anguilla), the estrogen receptor in the liver binds with estradiol and facilitates VTG synthesis (Morini et al., 2020). In female grass carp, water velocity may accelerate the accumulation of yolk by promoting VTG expression.
We utilized qRT-PCR to investigate the transcriptional changes of major genes associated with the HPG axis pathway, specifically concerning gonadotropin-releasing hormone (GnRH), gonadotropins (GTHs), and sex hormones. GnRH, an essential neuropeptide produced in the hypothalamus, plays a crucial role in regulating the levels of pituitary GTHs, referring to follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in vertebrates. Several studies have reported that two GnRH variants, known as GnRH2 and GnRH3, could stimulate pituitary FSH and LH secretion in teleost (Li et al., 2022). In the present study, although the transcript level of gnrh3 showed little difference, hormone injection and graded water velocity stimulation could significantly induce hypothalamus gnrh2 mRNA expression in female grass carp. In goldfish (carassius auratus), GnRH2 elicited a stronger LH secretion compared to GnRH3 in sexually mature, pre-spawning fish (Khakoo et al., 1994; Murthy and Peter, 1994). Similarly, recent studies in grass carp pituitary cells, GnRH2 was found to significantly induce fshβ and lhβ mRNA expression (Li et al., 2022). Moreover, the gnrh2 deficiency leads to decreased oocyte quality in female zebrafish (Danio rerio) (Marvel et al., 2019). These results indicated that GnRH2 may play an important role in ensuring the integrity of reproduction.
Gonadotropins are complex heterodimers consisting of a common α-glycoprotein subunit (CGα) and a hormone-specific β subunit (FSHβ or LHβ), which are bound together by non-covalent interactions. We found that both hormone injection and water velocity stimulation could significantly induce pituitary-releasing hormones gene (fshβ, lhβ, and cgα) mRNA expression. In Red Seabream (Pagrus major), the cgα mRNA expression was high during vitellogenesis and at the spawning phase but sharply declined in the regressed phase (Gen et al., 2000). Besides, the mRNA expression levels of fshβ and lhβ showed a significant increase in the pituitary gland of mature tiger puffer (Takifugu rubripes) (Zahangir et al., 2021). In grass puffer (Takifugu niphobles), there was a sharp increase in the transcription of fshβ during the mature stage, while the transcription of lhβ peaked during the spawning period (Yamanoue et al., 2009). Studies haves shown that the development of both ovary and testis in fshβ-deficient zebrafish was significantly retarded, and lhβ-deficient females failed to spawn, rendering them infertile (Zhang, 2015). Therefore, we speculate that water velocity stimulation might play an important role in stimulating GTHs secretion and sexual maturation in female grass carp.
Vertebrate reproduction is controlled by sex hormones. Upon stimulation of GTHs, biologically active sex hormones such as estradiol, testosterone, progesterone, and 17α,20β-DHP are abundantly produced in gonads, primarily derived from cholesterol (Tenugu et al., 2021). Moreover, sex hormones can regulate the transcription of gonadotropin subunit genes, either by acting on the GnRH-releasing hypothalamus or directly through the pituitary gonadotrope cells. Genes such as cyp11a1, hsd3b1, cyp17a1, cyp19a1a, hsd20b, hsd17b1, hsd17b3, and star are involved in the steroidogenesis pathway and play crucial roles during gonadal development. In this study, our data showed that hsd20b and hsd17b3 mRNA expressions were significantly upregulated in response to water velocity stimulation. In Nile tilapia (Oreochromis niloticus), elevated expression of hsd20b in post-vitellogenic immature follicles during final oocyte maturation strongly suggests its crucial role in mediating the final gamete maturation (Senthilkumaran et al., 2002). High expression of hsd17b3 at vitellogenesis was observed in zebrafish during ovarian follicular development (Ings and van der Kraak, 2006). However, cyp17a1, cyp19a1a, and hsd17b1 mRNA expressions were significantly downregulated in the ovary under water velocity stimulation in this study and our previous study (Shu et al., 2023). Similar patterns were found in half-smooth tongue sole (Cynoglossus semilaevis), with reduced mRNA levels of cyp17a1, cyp19a1a, and hsd17b1 during oocyte maturation (Dong et al., 2021). Studies also have reported that the transcription of cyp19a1a was found to be high during the vitellogenic phase, while a significant decline was observed during the final oocyte maturation in Nile tilapia (Yoshiura et al., 2003). In addition, significant downregulation of hsd3b1 and star were observed following water velocity stimulation. In zebrafish, the expressions of star, hsd3b1, cyp17a1, hsd17b1, and cyp19a1a were decreased in mature follicles (Ings and van der Kraak, 2006). Taken together, our data demonstrated that water velocity stimulation promoted the synthesis of sex hormones, which in turn activated the HPG axis pathway to ensure ovarian development and maturation in grass carp. However, the molecular mechanisms need to be further determined.
For oxidative stress regulation, it has been reported that reactive oxygen species (ROS) refer to highly oxidizing compounds, ions, and free radicals (Ma et al., 2023). Under normal physiological conditions, ROS maintain a dynamic balance between constantly being generated and removed to alleviate oxidative stress by antioxidant defense system in organisms, which helps maintain long-term health (Liao et al., 2023). SOD, CAT, POD, and GSH-Px are typical enzymes in the biological antioxidant defense system that play a crucial part in scavenging ROS within the body, thereby providing protection for cell membranes and nucleic acids from oxidative damage (Tse et al., 2004; Li et al., 2023). T-AOC reflects the body’s ability to compensate for external stimuli and indicates the status of free radical metabolism (Tan et al., 2016; Kong et al., 2021). In our study, water velocity stimulation significantly enhanced the activities of hepatic SOD, CAT, POD, GSH-Px, and T-AOC, as well as ovary SOD, CAT, and POD in female grass carp. This phenomenon is consistent with the results obtained from a previous study on exercise training in juvenile qingbo (Spinibarbus sinensis) (Yu et al., 2014). The study by Liu et al. also shows similarities to this result (Liu et al., 2023). Furthermore, MDA is one of the most important decomposition products of membrane lipid peroxidation, and the MDA concentration can serve as a reliable bioindicator of the degree of cellular damage (Zhou et al., 2016). In this study, it was found that high water velocity stimulation significantly reduced the MDA concentration in the ovary, indicating that fish in the GS group possessed stronger antioxidant capacity. Similar to the results of the present experiment, previous work in juvenile largemouth bass (Micropterus salmoides) also reported that the MDA content of the high flow group was significantly lower than that of the middle flow and low flow groups (Chen et al., 2021b). This could be attributed to the fact that with the increase in water velocity, the fish’s energy consumption increases and its metabolism is accelerated, thereby metabolizing oxygen-free radicals more quickly. These findings revealed that water velocity stimulation can significantly increase antioxidant enzyme activity in female grass carp, decrease the level of MDA, inhibit lipid peroxidation, and protect against damage caused by oxidative stress.
In summary, our comprehensive study demonstrates that a suitable water velocity stimulation (ZS group) can promote ovarian development and maturation by elevating sex hormones and VTG concentrations, as well as regulating the expression of HPG axis genes. It also can enhance the antioxidant activity in the ovary and liver of grass carp. We recommend graded water velocity (0.8−1.3 m/s, water velocity increased from 0.8 m/s to 1.3 m/s gradually) as an appropriate water velocity, which is suitable for gonadal development. This study provides important evidence for understanding the response of fish’s natural reproduction to ecological flows.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The animal study was approved by Animal Ethics Committee of Institute of Hydrobiology, Chinese Academy of Sciences (Approval ID: IHBLL2017035; Approval Date: January 11, 2017). The study was conducted in accordance with the local legislation and institutional requirements.
TS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JY: Conceptualization, Project administration, Writing – review & editing. ZXY: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. KX: Investigation, Methodology, Resources, Validation, Writing – review & editing. HH: Investigation, Methodology, Writing – review & editing. LD: Funding acquisition, Writing – review & editing. ZY: Supervision, Writing – review & editing. WJ: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by National Key Research and Development Program of China (Grant No. 2022YFC3204200), Hubei Provincial Natural Science Foundation of China (Grant Nos. 2022CFB738 and 2024AFA036), Hubei Provincial Chenguang Project for Young Scientific and Technological Talents (Grant No. 202350), and Key Laboratory of Breeding Biotechnology and Sustainable Aquaculture, Chinese Academy of Sciences (Grant No. 2023FB01).
We would like to thank Dezhi Zhang, Yang Li, Binzhong Wang, Zixuan Hu, Pei Chen, and Wei Wang from Chinese Sturgeon Research Institute, China Three Gorges Corporation for the support of the culture system in this research work.
Authors TS, JY, ZXY, KX, HH, LD, and WJ are employed by China Three Gorges Corporation.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arukwe A., Goksoyr A. (2003). Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: oogenetic, population, and evolutionary implications of endocrine disruption. Comp. Hepatol. 2, 4. doi: 10.1186/1476-5926-2-4
Barcellos L. J. G., Wassermann G. F., Scott A. P., Woehl V. M., Quevedo R. M., Ittzés I., et al. (2001). Steroid profiles in cultured female jundia, the siluridae Rhamdia quelen (Quoy and Gaimard, Pisces Teleostei), during the first reproductive cycle. Gen. Comp. Endocrinol. 121, 325–332. doi: 10.1006/gcen.2001.7603
Cao Y., Wang C., Qian D. (2022). Influence of cascaded hydropower development on high and low flows in the spawning grounds of the four major Chinese carps. J. Hydroecology 43, 18–26. doi: 10.15928/j.1674–3075.202011130323
Chen Q., Zhang J., Chen Y., Mo K., Wang J., Tang L., et al. (2021a). Inducing flow velocities to manage fish reproduction in regulated rivers. Engineering 7, 178–186. doi: 10.1016/j.eng.2020.06.013
Chen Z. L., Ye Z. Y., Ji M. D., Zhou F., Ding X. Y., Zhu S. M., et al. (2021b). Effects of flow velocity on growth and physiology of juvenile largemouth bass (Micropterus salmoides) in recirculating aquaculture systems. Aquaculture Res. 52, 3093–3100. doi: 10.1111/are.15153
Dong Y., Lyu L., Zhang D., Li J., Wen H., Shi B. (2021). Integrated lncRNA and mRNA Transcriptome Analyses in the Ovary of Cynoglossus semilaevis Reveal Genes and Pathways Potentially Involved in Reproduction. Front. Genet. 12. doi: 10.3389/fgene.2021.671729
Gadekar G. P. (2014). Studies on the seasonal histomorphological changes in the ovary of Indian major carp, Labeo Rohita (HAM.). Bioscan 9, 1037–1042.
Gen K., Okuzawa K., Senthilkumaran B., Tanaka H., Moriyama S., Kagawa H. (2000). Unique expression of gonadotropin-I and -II subunit genes in male and female red seabream (Pagrus major) during sexual maturation. Biol. Reprod. 63, 308–319. doi: 10.1095/biolreprod63.1.308
Ghiasi S., Falahatkar B., Sajjadi M. (2023). Effect of dietary flaxseed meal on growth, blood biochemistry, reproductive hormones and oocyte development in previtellogenic Siberian sturgeon (Acipenser baerii Brandt 1869). Anim. Feed Sci. Technol. 295. doi: 10.1016/j.anifeedsci.2022.115546
Golmoradizadeh A., Noori A., Amiri B. M. (2021). Physiological effects of the lunar cycle on the spawning of a coral reef fish, Abudefduf Vaigiensis: in vivo and in vitro trait. Coral Reefs 40, 1757–1767. doi: 10.1007/s00338-021-02183-x
Hu W. H., Huang P. P., Xiong Y., Guo W. J., Wang Y. H., Fan Q. X., et al. (2020). Synergistic combination of exogenous hormones to improve the spawning and post-spawning survival of female yellow catfish. Front. Genet. 11. doi: 10.3389/fgene.2020.00961
Ings J. S., van der Kraak G. J. (2006). Characterization of the mRNA expression of StAR and steroidogenic enzymes in zebrafish ovarian follicles. Mol. Reprod. Dev. 73, 943–954. doi: 10.1002/mrd.20490
Jéhannet P., Palstra A. P., Meijerhof M., Schipper H., Giménez I. N., Dirks R. P., et al. (2023). The induction of oocyte maturation and ovulation in the European eel (Anguilla Anguilla): in vitro and in vivo comparison of progesterone with 17α,20β-dihydroxy-4-pregnen-3-one. Front. Physiol. 14. doi: 10.3389/fphys.2023.1207542
Khakoo Z., Bhatia A., Gedamu L., Habibi H. R. (1994). Functional specificity for salmon gonadotropin-releasing hormone (GnRH) and chicken GnRH-II coupled to the gonadotropin release and subunit messenger ribonucleic acid level in the goldfish pituitary. Endocrinology 134, 838–847. doi: 10.1210/en.134.2.838
Kong Y. D., Li M., Chu G. S., Liu H. J., Shan X. F., Wang G. Q., et al. (2021). The positive effects of single or conjoint administration of lactic acid bacteria on Channa argus: Digestive enzyme activity, antioxidant capacity, intestinal microbiota and morphology. Aquaculture 531. doi: 10.1016/j.aquaculture.2020.735852
Lau E. S.-W., Zhang Z., Qin M., Ge W. (2016). Knockout of Zebrafish ovarian aromatase gene (cyp19a1a) by TALEN and CRISPR/Cas9 leads to all-male offspring due to failed ovarian differentiation. Sci. Rep. 6. doi: 10.1038/srep37357
Li P. J., Chen X. Y., Hou D. Q., Chen B., Peng K., Huang W., et al. (2023). Positive effects of dietary Clostridium butyricum supplementation on growth performance, antioxidant capacity, immaunity and viability against hypoxic stress in largemouth bass. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1190592
Li W., Du R. X., Xia C. H., Zhang H. Y., Xie Y. Y., Gao X. W., et al. (2022). Novel pituitary actions of GnRH in teleost: The link between reproduction and feeding regulation. Front. Endocrinol. 13. doi: 10.3389/fendo.2022.982297
Liao Z. H., Liu Y. T., Wei H. L., He X. S., Wang Z. Q., Zhuang Z. X., et al. (2023). Effects of dietary supplementation of Bacillus subtilis DSM 32315 on growth, immune response and acute ammonia stress tolerance of Nile tilapia (Oreochromis niloticus) fed with high or low protein diets. Anim. Nutr. 15, 375–385. doi: 10.1016/j.aninu.2023.05.016
Liu R. C., Li K., Wang G. X., Jiang Z. X., Ba X. B., Liu L. P. (2022). Effect of swimming on the induction of vitellogenin in conger eel (Conger myriaster). Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.887074
Liu H., Yin X. A., Qiu X., Qin J., Yang W., Zhang J. (2021). Coupled influence of flow velocity and water temperature on grass carp swimming behaviour and gonad development. Hydrological Processes 35. doi: 10.1002/hyp.14052
Liu M., Yuan J., Lian Q., Ni M., Gu Z. (2023). Different water flow rates on the growth performance, antioxidant capacity, energy metabolism and tissue structure of Micropterus salmoides under an in-pond recirculating aquaculture system. Acta Hydrobiologica Sin. 47, 25–36. doi: 10.7541/2022.2021.0167
Lubzens E., Bobe J., Young G., Sullivan C. V. (2017). Maternal investment in fish oocytes and eggs: The molecular cargo and its contributions to fertility and early development. Aquaculture 472, 107–143. doi: 10.1016/j.aquaculture.2016.10.029
Ma F., Ma B. H., Zhang B. X., He Y. D., Wang Y. (2023). Disturbance of oxidation/antioxidant status and histopathological damage in tsinling lenok trout under acute thermal stress. Trop. Anim. Health Production 55. doi: 10.1007/s11250-023-03705-1
Marvel M. M., Spicer O. S., Wong T. T., Zmora N., Zohar Y. (2019). Knockout of Gnrh2 in zebrafish (Danio rerio) reveals its roles in regulating feeding behavior and oocyte quality. Gen. Comp. Endocrinol. 280, 15–23. doi: 10.1016/j.ygcen.2019.04.002
Morini M., Lafont A. G., Maugars G., Baloche S., Dufour S., Asturiano J. F., et al. (2020). Identification and stable expression of vitellogenin receptor through vitellogenesis in the European eel. Animal 14, 1213–1222. doi: 10.1017/S1751731119003355
Murthy C. K., Peter R. E. (1994). Functional evidence regarding receptor subtypes mediating the actions of native gonadotropin-releasing hormones (GnRH) in goldfish, carassius auratus. Gen. Comp. Endocrinol. 94, 78–91. doi: 10.1006/gcen.1994.1062
Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Senthilkumaran B., Sudhakumari C. C., Chang X. T., Kobayashi T., Oba Y., Guan G. J., et al. (2002). Ovarian carbonyl reductase-like 20β-hydroxysteroid dehydrogenase shows distinct surge in messenger RNA expression during natural and gonadotropin-induced meiotic maturation in Nile tilapia. Biol. Reprod. 67, 1080–1086. doi: 10.1095/biolreprod67.4.1080
She Z. Y., Tang Y. M., Chen L. H., Nong X. Z., Li X. F. (2023). Determination of suitable ecological flow regimes for spawning of four major Chinese carps: A case study of the Hongshui River, China. Ecol. Inf. 76. doi: 10.1016/j.ecoinf.2023.102061
Shu T. T., Chen Y., Xiao K., Huang H. T., Jia J. Y., Yu Z. X., et al. (2023). Effects of short-term water velocity stimulation on the biochemical and transcriptional responses of grass carp (Ctenopharyngodon idellus). Front. Physiol. 14. doi: 10.3389/fphys.2023.1248999
Tan X. H., Lin H. Z., Huang Z., Zhou C. P., Wang A. L., Qi C. L., et al. (2016). Effects of dietary leucine on growth performance, feed utilization, non-specific immune responses and gut morphology of juvenile golden pompano (Trachinotus ovatus). Aquaculture 465, 100–107. doi: 10.1016/j.aquaculture.2016.08.034
Tang Q., Bao Y. H., He X. B., Fu B. J., Collins A. L., Zhang X. B. (2016). Flow regulation manipulates contemporary seasonal sedimentary dynamics in the reservoir fluctuation zone of the Three Gorges Reservoir, China. Sci. Total Environ. 548, 410–420. doi: 10.1016/j.scitotenv.2015.12.158
Tenugu S., Pranoty A., Mamta S.-K., Senthilkumaran B. (2021). Development and organisation of gonadal steroidogenesis in bony fishes - A review. Aquaculture Fisheries 6, 223–246. doi: 10.1016/j.aaf.2020.09.004
Tse H. M., Milton M. J., Piganelli J. D. (2004). Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: Implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radical Biol. Med. 36, 233–247. doi: 10.1016/j.freeradbiomed.2003.10.029
Tucker E. K., Zurliene M. E., Suski C. D., Nowak R. A. (2020). Gonad development and reproductive hormones of invasive silver carp (Hypophthalmichthys molitrix) in the Illinois River. Biol. Reprod. 102, 647–659. doi: 10.1093/biolre/ioz207
von Schalburg K. R., Gowen B. E., Christensen K. A., Ignatz E. H., Hall J. R., Rise M. L. (2023). The late-evolving salmon and trout join the GnRH1 club. Histochem. Cell Biol. 160, 517–539. doi: 10.1007/s00418-023-02227-z
Wang L., Chen Q. W., Zhang J. Y., Xia J., Mo K. L., Wang J. (2020). Incorporating fish habitat requirements of the complete life cycle into ecological flow regime estimation of rivers. Ecohydrology 13. doi: 10.1002/eco.2204
Wang X. B., Li Y. Y., Hu J. B., Zhang Y. Y., Zhang M., Wang G. L., et al. (2023). Effects of different photoperiods on growth and ovarian development and maturation of silver pomfret Pampus argenteus. J. Fish Biol. 103, 59–72. doi: 10.1111/jfb.15413
Xiao Y., Deng J. H., Yang S. F., Hu J., Wang L., Li W. J. (2022). Study on the spawning habitat suitability of four major Chinese carps in the fluctuating backwater area of the Three Gorges Reservoir. Ecol. Indic. 143. doi: 10.1016/j.ecolind.2022.109314
Yamanoue Y., Miya M., Matsuura K., Miyazawa S., Tsukamoto N., Doi H., et al. (2009). Explosive speciation of Takifugu: another use of fugu as a model system for evolutionary biology. Mol. Biol. Evol. 26, 623–629. doi: 10.1093/molbev/msn283
Yoshiura Y., Senthilkumaran B., Watanabe M., Oba Y., Kobayashi T., Nagahama Y. (2003). Synergistic expression of Ad4BP/SF-1 and cytochrome P-450 aromatase (ovarian type) in the ovary of Nile tilapia, Oreochromis niloticus, during vitellogenesis suggests transcriptional interaction. Biol. Reprod. 68, 1545–1553. doi: 10.1095/biolreprod.102.010843
Yu L., Li X., Yi J., Huang Z., Chen D., Wang Z. (2014). Effects of different water velocities on the free radical metabolism of juvenile Spinibarbus sinensis. J. Fishery Sci. China 21, 101–107. doi: 10.3724/SP.J.1118.2014.00101
Zahangir M. M., Matsubara H., Ogiso S., Suzuki N., Ueda H., Ando H. (2021). Expression dynamics of the genes for the hypothalamo-pituitary-gonadal axis in tiger puffer (Takifugu rubripes) at different reproductive stages. Gen. Comp. Endocrinol. 301. doi: 10.1016/j.ygcen.2020.113660
Zhang Z. (2015). Genetic Analysis of Gonadotropins and Their Receptors in the Zebrafish. Hongkong, China: The Chinese University of Hongkong.
Zhang W. W., Jia Y. F., Wang F., Du Q. Y., Chang Z. J. (2017). Identification of differentially-expressed genes in early developmental ovary of Yellow River carp (Cyprinus carpio var) using Suppression Subtractive Hybridization. Theriogenology 97, 9–16. doi: 10.1016/j.theriogenology.2017.04.017
Zhang P., Qiao Y., Grenouillet G., Lek S., Cai L., Chang J. B. (2021). Responses of spawning thermal suitability to climate change and hydropower operation for typical fishes below the Three Gorges Dam. Ecol. Indic. 121. doi: 10.1016/j.ecolind.2020.107186
Zhang Y., Zhang J., Zhang L. C., Hu K. L., Wang Y., Ji Y. (2023). Balancing economic and ecological benefits for hydro-junction operation based on the ecological flow from the four major Chinese carps: a case study from Xinjiang River, China. Environ. Res. Commun. 5. doi: 10.1088/2515-7620/acd912
Zhong H., Hu J., Zhou Y. (2021). Transcriptomic evidence of luteinizing hormone-releasing hormone agonist (LHRH-A) regulation on lipid metabolism in grass carp (Ctenopharyngodon idella). Genomics 113, 1265–1271. doi: 10.1016/j.ygeno.2020.09.043
Keywords: water velocity, ovarian maturation, sex hormones, oxidative stress, grass carp
Citation: Shu T, Yang J, Yu Z, Xiao K, Huang H, Dai L, Yin Z and Jiang W (2024) Influences of water velocity on ovarian maturation and antioxidant capacity in adult grass carp (Ctenopharyngodon idellus). Front. Mar. Sci. 11:1441426. doi: 10.3389/fmars.2024.1441426
Received: 31 May 2024; Accepted: 20 June 2024;
Published: 11 July 2024.
Edited by:
Yi-Feng Li, Shanghai Ocean University, ChinaReviewed by:
Sofia Priyadarsani Das, National Taiwan Ocean University, TaiwanCopyright © 2024 Shu, Yang, Yu, Xiao, Huang, Dai, Yin and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Shu, MTg3NzgwMDQ4NThAMTYzLmNvbQ==; Wei Jiang, amlhbmdfd2VpNkBjdGcuY29tLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.