- 1College of Marine Technology, Faculty of Information Science and Engineering, Ocean University of China/Laboratory for Regional Oceanography and Numerical Modeling, Qingdao Marine Science and Technology Center, Qingdao, China
- 2School of Life and Environmental Sciences, the University of Sydney, Sydney, NSW, Australia
- 3Sanya Oceanographic Institution, Ocean University of China/SANYA Oceanographic Laboratory, Sanya, China
Coral bleaching events have become more frequent in recent years due to the impact of widespread marine heatwaves. The Coral Reef Watch (CRW) program, part of the National Oceanic and Atmospheric Administration (NOAA), assesses bleaching risk by considering measures of daily coral heat stress (Hotspot, HS) and accumulated heat stress (Degree Heating Week, DHW). However, there is a mismatch between coral bleaching alerts through satellite monitoring and records of coral bleaching in the South China Sea (SCS) and its surrounding seas in the historical database. Through comparison with field records of bleaching events in the SCS, this study examined the optimization of the DHW under a fixed or variable HS threshold, evaluating the accuracy of coral bleaching monitoring through a range of evaluation indices, including the Peirce Skill Score (PSS) and the Area Under the Curve (AUC). Our results show that when the DHW index was calculated based on the current operational HS threshold (1°C), reducing the DHW threshold from 4°C to 1.86°C-weeks significantly improved PSS from 0.17 to 0.66, and AUC from 0.58 to 0.83. Further, by optimizing both HS and DHW, evaluation statistics were further improved, with the PSS increasing to 0.71 and the AUC increasing to 0.85. While both methods could significantly optimize the operational bleaching alert level for the SCS, the results suggest that optimization of both the HS and DHW thresholds is better than optimizing DHW alone. As marine heatwaves become more frequent, accurately predicting when and where coral bleaching is likely to occur will be critical to improving the estimation of regional coral stress due to climate change and for understanding coral reefs’ response to recurrent bleaching events.
1 Introduction
In recent years, extreme weather induced by climate change has caused many irreversible effects, including increases in coral bleaching and mortality (IPCC, 2022). With high biodiversity and primary productivity, coral reef ecosystems are among the most important in the ocean (Moberg and Folke, 1999). Corals are highly sensitive to environmental factors such as temperature, salinity, light, and water turbidity, which can cause the breakdown of the symbiotic relationship between coral polyps and zooxanthellae, resulting in coral bleaching (Hoegh-Guldberg, 1999). Continuous, extremely high temperatures are considered to be the primary cause of large-scale coral bleaching events (Douglas, 2003). In recent decades, frequent and intense marine heatwave events worldwide have led to multiple coral bleaching events and large-scale coral mortality (Hughes et al., 2017, 2018; Eakin et al., 2019; Skirving et al., 2019; Virgen-Urcelay and Donner, 2023). Live coral cover in the South China Sea (SCS) has significantly declined since the 1960s, and live coral cover in some areas of the SCS has been reduced to less than 10% in 2020 (Huang, 2021).
Long-term research has shown that thermal stress indicators based on sea surface temperature (SST) data can effectively alert large-scale coral bleaching by monitoring the thermal stress state in which corals are exposed (Liu et al., 2003; Heron et al., 2014). Historical field research found that severe mass coral bleaching can be predicted when the water temperature exceeds the average of the warmest historical month by more than 1°C (Goreau, 1990; Williams and Bunkley-Williams, 1990; Hayes and Goreau, 1991; Goreau, 1991; Goreau et al., 1993). Corals face a risk of bleaching when the thermal anomaly exceeds 1-2°C (Glynn and D'croz, 1990). Based on these studies, Goreau and Hayes (1994) proposed the concept of Hotspot (HS), suggesting that during the warm season, coral bleaching can be monitored by positive anomalous SST (Hotspot, in °C). In 1995, Gleeson et al. proposed the Degree Heating Weeks (DHW) indicator, which represents the cumulative bleaching heat pressure corals face by accumulating heat exceeding a certain temperature over several weeks, i.e. an accumulation of the magnitude of HS values (DHW, °C-weeks). In 2000, the National Oceanic and Atmospheric Administration’s (NOAA) Coral Reef Watch (CRW) program began using HS and DHW as key indicators to measure and monitor coral bleaching thermal stress (Liu et al., 2005). HS and DHW products are the short-term thermal anomaly in 1 day and the heat stress accumulation over 12 weeks, respectively (Liu et al., 2003). Based on regional coral biological experiments (Glynn and D'croz, 1990; Berkelmans and Willis, 1999) and field observational reports, CRW defined the combination of ecologically significant coral bleaching alert thresholds as an HS threshold of 1°C and DHW threshold of 4°C-weeks; when both HS and DHW thresholds are reached, the minimum bleaching alert level is reached and ecologically significant bleaching is expected (Liu et al., 2003). The magnitude of DHW is independent of how quickly heat accumulates, so a 4°C HS lasting 1 week versus a 1°C HS lasting 4 weeks both have a DHW of 4°C-weeks.
An evaluation by Donner (2011) showed that the false negative rate, i.e. coral bleaching occurring but not predicted, of global coral bleaching prediction under CRW thresholds was 60%. Similarly, for the SCS and surrounding waters, CRW thresholds for coral bleaching alerts may be too conservative. For example, coral bleaching was observed in both Spratly and Paracel Islands in the SCS during field surveys, while the DHW did not reach 4°C-weeks (Li et al., 2011; Zuo et al., 2015). Therefore, although the global coral bleaching heat stress monitoring product suite developed by CRW has been instrumental in monitoring and managing coral bleaching, many bleaching events continue to go undetected.
To increase bleaching detection accuracy, several studies have attempted to modify the thermal stress threshold in the coral bleaching alert model. DeCarlo (2020) found that removing the 1°C HS filter threshold used in the calculation of DHW, while reducing the DHW cumulative time window to 9 weeks, improved the accuracy of bleaching detection. In addition to modifying the DHW calculation threshold and cumulative window, Kumagai et al. (2018) found that the optimal filtering threshold in the best generalized linear model based on DHW was 0.68°C and the bleaching warning threshold was 2.07°C-weeks in the Ryukyu Islands.
Further, some methods directly optimize the DHW judgment threshold. van Hooidonk and Huber (2009) evaluated the DHW thresholds for different regions based on the Peirce Skill Score (PSS) and found that the optimal DHW thresholds varied by region. Qin et al. (2023) found that the optimized threshold of DHW for coral bleaching in the SCS region was 3.32°C-weeks, and for severe coral bleaching events, the optimized threshold of DHW was 4.52°C-weeks. Finally, Whitaker et al. (2024) revised the global bleaching threshold to a 0.4°C HS filter threshold with an 11-week cumulative window and 3°C-weeks DHW. Almost all past global and regional studies show that lower thermal stress thresholds improve the accuracy of coral bleaching alerts.
The conservative thresholds in current satellite monitoring of coral bleaching were developed primarily to detect severe, global-scale bleaching events (Liu et al., 2014). While widespread bleaching events in recent years have significantly impacted coral ecosystems (Goreau et al., 2005; Goreau and Hayes, 2024), field temperature records indicate that local features, such as upwelling, can lower water temperatures and thus reduce coral bleaching (Riegl et al., 2019). This underscores the need to reassess thermal stress thresholds to account for local thermal histories and better reflect regional coral bleaching patterns. Further, in the development of HS and DHW thresholds, biological experiments supporting their thresholds were separately considered, without considering their combination, where the role of HS and its impact on the calculation of DHW has not been thoroughly evaluated in previous studies. In the CRW bleaching watch system, the bleaching alert levels are triggered only when both HS and DHW values surpass their respective thresholds. In this process, the HS threshold is used to calculate the DHW value, which means only heating above the HS threshold will be accumulated in the DHW. Thus, determining an accurate HS threshold is critical for improving coral bleaching alerts.
Therefore, based on historical coral bleaching survey records in the SCS and its surrounding seas, this study aimed to find the most accurate coral bleaching thermal stress alert thresholds by optimizing DHW thresholds independently with a fixed 1°C HS threshold (i.e. the current CRW threshold), and synergistically optimizing both HS and DHW thresholds. Improved monitoring of coral bleaching thermal stress will better support coral reef practitioners in their management and response to coral bleaching events. Further, as there is evidence that corals can develop resilience to increased temperature through exposure to recurrent heatwaves (Brown and Barott, 2022), accurately identifying coral bleaching thresholds that result in bleaching is critical.
2 Materials and methods
2.1 Study region and coral bleaching event data
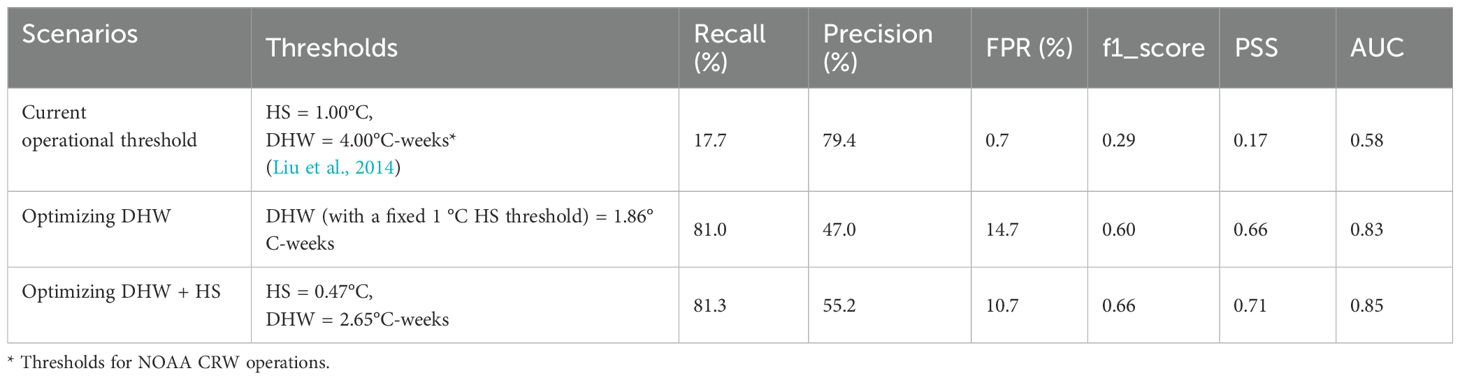
This study focused on the SCS and its surrounding seas (105°E to 125°E, 0 to 25°N). The study area is located in the western Pacific Ocean, adjacent to the western boundary of the Coral Triangle, and covers more than 3 million square kilometers (Morton and Blackmore, 2001). With almost 600 known coral species, the SCS and its surrounding seas are an important part of global coral reef ecosystems (Huang et al., 2015).
To ensure that the thermal stress indicators accurately reflect the thermal stress corals experienced in historical studies, the quality of the historical coral bleaching event database is critical. The primary source of coral bleaching event data for this study is version 2.0 of the Historical Coral Bleaching Event Database compiled by Virgen-Urcelay and Donner (2023), which covers global coral bleaching events from 1963 to 2017. Data sources include scientific literature and conference archives, the Reefbase database, and bleaching event information provided by scientists and related organizations, in addition to NOAA CRW internal database records. The database was geo-corrected and spatially interpolated for the geographic coordinates of each coral bleaching event, using a 0.05°×0.05° grid layer to define reef locations. The 0.05° spatial resolution matches the spatial resolution of the SST data used in this study, ensuring that the corresponding thermal stress index can be calculated at each event coordinate location. In addition, 28 coral bleaching records from other sources were added to the database for the study areas by referencing pieces of Chinese literature (Yu et al., 2006; Tang et al., 2010; Chen et al., 2012; Li et al., 2012; Zuo et al., 2015; Huang, 2021; Liu et al., 2021; Meng et al., 2022). This resulted in a total of 3513 events between the time range of 1981-2020, the spatial range of 0°-25°N, 105°-125°E, including 2342 non-bleaching events and 1176 bleaching events of differing severity. The geographical location of the study area and the distribution of coral bleaching records are shown in Figure 1.
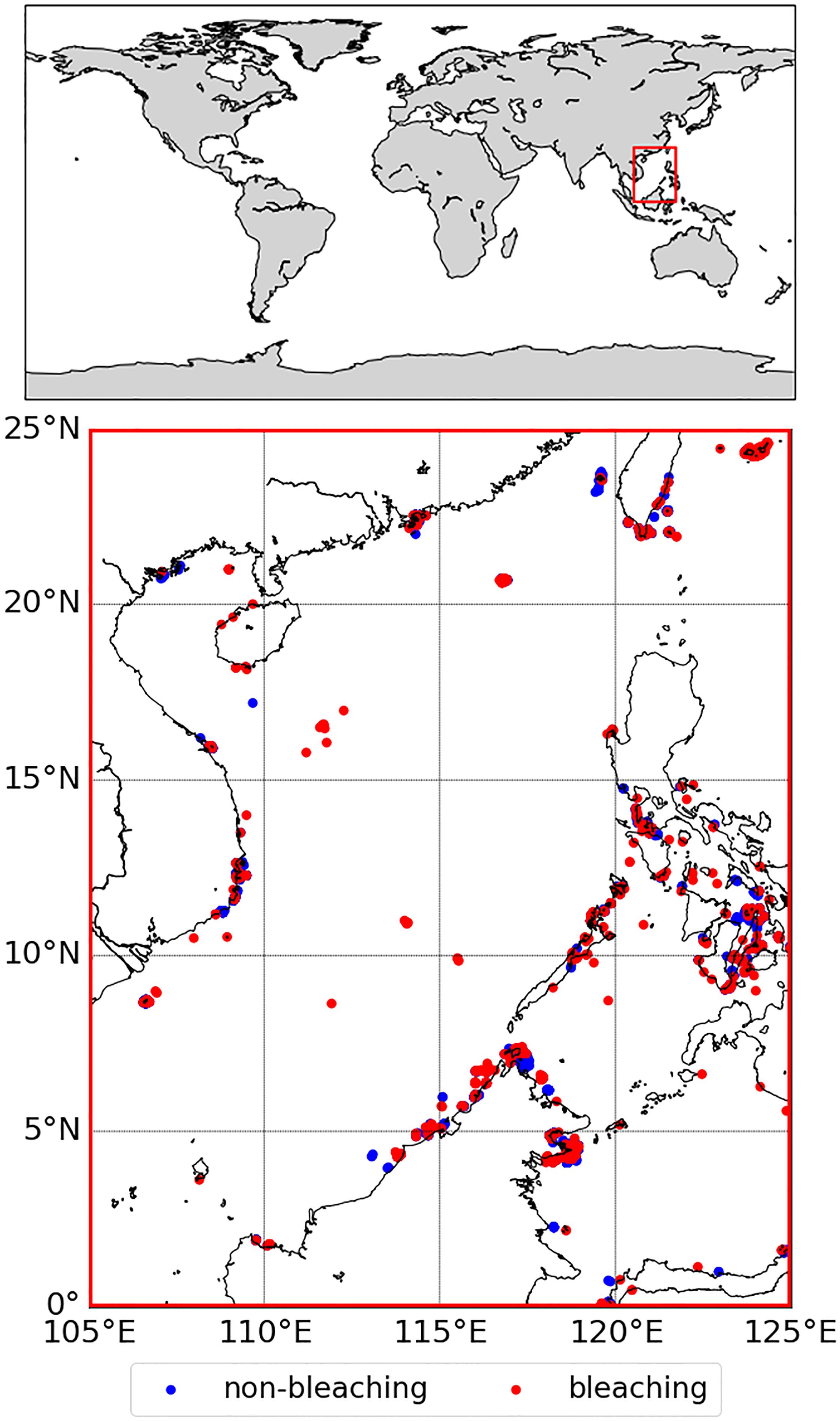
Figure 1. Study area and location of all coral bleaching records, with blue dots representing records of non-bleaching and red dots representing records of bleaching events.
All historical event records in the database have been through a strict 4-step quality control to ensure the accuracy of subsequent analysis results. (1) Event quality control: For the study of thermal stress monitoring of coral bleaching, the first step is to ensure that all bleaching events are dominated by thermal stress, thus all bleaching events caused by non-thermal stress marked in the database (Virgen-Urcelay and Donner, 2023) were excluded. In addition, when calculating the required DHW, it is necessary to know the specific location of the event, the survey month, and the degree of bleaching, so this study excluded events with grades less than “AA” (Virgen-Urcelay and Donner, 2023) to ensure that all events can obtain the corresponding survey month information. This step excluded 175 records. (2) Exclusion of mild bleaching events: Coral bleaching rates of 10% or less are generally considered to not be ecologically significant (Lachs et al., 2021). Further, mild bleaching events are likely to be associated with observational errors (Virgen-Urcelay and Donner, 2023). Therefore, in this study, 583 records of minor bleaching events (1%< coral bleaching rate ≤10%) were excluded from the database to reduce the impact of potential observational errors. (3) Water depth control: The influence of thermal stress on coral communities is tightly linked to water depth, and the change in water depth will affect the influence of thermal stress and light radiation on coral bleaching (Oliver et al., 2009; Perez-Rosales et al., 2021). In order to mitigate the error caused by depth on coral bleaching, only records of coral bleaching events in shallow water areas are analyzed in this study. With reference to the depth threshold adopted by Williamson et al. (2022) in monitoring environmental stress on coral reefs, this study excluded 58 records of events with unknown water depth or water depth >20 m. (4) Merging or removing spatiotemporal coincident events: Since the spatial resolution of the SST data source is 0.05°, some coral reef areas that are close in space and surveyed at the same time are likely to have both coral bleaching and non-bleaching records. There may also be multiple records of bleaching at different depths in the same location. To address conflicting or repeated events, groups of events that occurred simultaneously and had a spatial difference of less than 0.05° were consolidated into a single bleaching event record if the proportion of bleaching events exceeded 80%. If the proportion of both bleaching and non-bleaching events did not exceed 80%, all events in that group were excluded. Through this step, this study can effectively reduce contradicting events and false replication, in turn reducing the spatial deviation of the event distribution. This step removed 437 non-bleaching records and 84 bleaching records. After quality control, the database had a total of 2182 events, including 1877 non-bleaching events and 305 bleaching events.
2.2 SST dataset and climatology
This study used version 3.1 of the CoralTemp daily global 5 km SST product, which provides data from 1985 onwards. The data sources of CoralTemp include the 5 km daily global satellite SST product produced by NESDIS (National Environmental Satellite, Data and Information Service) (Maturi et al., 2017) and the Operational SST and Sea Ice Analysis (OSTIA) product produced by the Met Office (Roberts-Jones et al., 2012). CoralTemp overlaps and merges different data sources to ensure its internal data consistency.
Since reef-building corals typically grow in waters near their thermal limit (Glynn and D'croz, 1990), the thermal stress of coral bleaching is usually calculated based on the local mean sea temperature during their hottest period, the maximum monthly mean (MMM). In this study, the MMM of the study area was calculated using CoralTemp from 1985 to 2019 by first calculating the local long-term monthly mean SST for 12 months, and then selecting the maximum among the 12 long-term monthly mean SST. Although the period of climatological calculation is extended (1985-2019) compared to the climatological period of NOAA CRW (1985-2012), this study still follows the climatological protocol version 3 of NOAA CRW, by shifting the midpoint of the climatology to 1985-1990 plus 1993 (Heron et al., 2014, 2015; Liu et al., 2017) to provide consistency between different versions of the coral bleaching thermal stress monitoring model. The following formula is used to recentralize the climatological baseline:
where T represents the climatological temperature, slope is the long-term change rate of the monthly mean SST, and t is the time center. The subscript refers to the year, 85-19 represents 1985 to 2019, and 85-93 represents 1985 to 1990 plus 1993 (CRW’s temperature baseline), which are the two time periods before and after the recentralization.
2.3 Thermal stress indicators
HS can reflect the positive outlier of the daily SST relative to the MMM climatological basis, in °C. HS is calculated as follows, where the subscript, daily, represents each day (Liu et al., 2014):
Although HS can reflect the abnormal short-term SST increase to corals, studies have found that in addition to the influence of short-term thermal stress, long-term heat accumulation stress is also an important factor leading to coral bleaching, and cumulative thermal stress can more effectively reflect the process of large-scale coral bleaching (Berkelmans and Willis, 1999). As shown in formula (3), the DHW index is the cumulative of HS in the last 12 weeks (84 days), and the unit is °C-weeks. It takes into account both the magnitude of the thermal anomaly and the exposure time, and represents the amount of cumulative thermal stress to corals by a single number (Liu et al., 2014):
2.4 Calculation of HS and DHW for historical events
Coral bleaching events commonly exhibit varying degrees of temporal lag. Therefore, the direct calculation of the DHW of the coral bleaching survey month may not accurately reflect the actual thermal stress experienced during the coral bleaching period. This study will refer to the local climatological hottest month for each coral bleaching event to capture the accurate month in which bleaching occurred. If the bleaching event was recorded before the hottest month, the monthly maximum DHW of the survey month will be calculated. Conversely, if the bleaching event was recorded in the hottest month or the month after the hottest month, the monthly maximum DHW of the hottest month will be calculated to represent the level of thermal stress experienced during the coral bleaching event. For non-bleaching events, the historical record means that at the time of the survey, the thermal stress level did not cause bleaching conditions or that the bleached coral had recovered. Therefore, this study refers to the survey time recorded in the database, using the maximum DHW for the survey month to represent the thermal stress level that did not cause coral bleaching. Meanwhile, the HS for each event is the maximum value for the period in which the maximum DHW was obtained.
2.5 Performance evaluation
In this study, we aim to optimize new HS and DHW thresholds for the SCS and surrounding seas by investigating the classification ability of HS and DHW indicators against historical coral bleaching events for two different scenarios. Table 1 shows the definition of coral bleaching alert models under the two scenarios examined. Scenario one uses the current, fixed HS threshold of 1°C when calculating DHW, therefore only considering the optimization of DHW when alerting coral bleaching stress. Scenario two considers the optimization of both HS and DHW to alert bleaching stress. Each scenario includes four levels of thermal stress: ‘No stress’, ‘Watch’, ‘Bleaching warning’ and ‘Bleaching alert’ (Table 1). Each event would be classified as a bleaching event when the DHW (scenario 1) or HS + DHW (scenario 2) reached the “Bleaching alert” level. Conversely, the event is classified as non-bleaching if it did not reach the “Bleaching alert” level.
To test the coral bleaching monitoring capabilities of different threshold combinations, a performance evaluation of a binary classification model is required for each event. For each coral bleaching event, there were four possible coral bleaching alert outcomes: the database records may reach the bleaching alert level and successfully detect bleaching (true positive, TP), or may not be successfully detected and missed (false negative, FN). On the other hand, the unbleached event recorded in the database may not reach the bleaching alert level and successfully detect no bleaching (true negative, TN), or the unbleached event may reach the bleaching alert level and result in a false positive (FP). All records were classified as above and assessed according to the two scenarios outlined in Table 1. The HS and DHW are used to assess whether the events reached the bleaching alert level in two scenarios.
To comprehensively optimize thresholds, this study adopted multiple evaluation indices that are widely used in the assessment of coral bleaching threshold models (van Hooidonk and Huber, 2009; Kumagai et al., 2018; Lachs et al., 2021).
Recall, also known as the true positive rate (TPR), is based on all bleaching events and judges the probability of correct detection of all bleaching events:
Precision represents the percentage of actual bleaching events out of all events detected as bleaching:
Ideally, higher values for each indicator imply better performance, but the reality is that the relationship between the two indicators is usually positive and negative, so to comprehensively consider the bleaching detection capability (TP) of the model, the f1 score (f1_score) is introduced for evaluation:
The proportion of false detection events in all non-bleaching events is the false positive rate (FPR):
The PSS (Peirce, 1884) metric is defined as the difference between the TPR and the FPR values. It provides a comprehensive assessment of bleaching monitoring.
Since the proportion of non-bleaching events in the database of this study is significantly higher than that of bleaching events, it is easy to be affected by non-bleaching events when evaluating the models, resulting in the bias of the evaluation results. In order to evaluate the quality of the models more objectively, this study considered the area under the curve (AUC) (Hanley and McNeil, 1982) as the standard for the final evaluation of the quality of the threshold models. The receiver operating characteristic (ROC) curve is a curve with FPR as the horizontal coordinate and TPR as the vertical coordinate. The benefit of this evaluation curve is that it can objectively evaluate the quality of the model regardless of the unbalanced distribution of samples. AUC refers to the area under the ROC curve. An AUC of 0.5 indicates almost random classification, while an AUC closer to 1 indicates a better model effect (Hanley and McNeil, 1982). Therefore, we used AUC as the final indicator for model evaluation in this paper.
As the maximum AUC is used here to determine the optimal threshold, when assessing the DHW with a fixed HS threshold of 1°C (scenario one), the DHW thresholds can assume the values of 0 to 8°C-weeks where we consider an interval of 0.01°C-weeks. The best DHW threshold corresponds to the maximum AUC. For the assessment of DHW under a variable HS threshold (scenario two), the HS thresholds are tested from 0 to 1.5°C, considering an interval of 0.01°C, and the DHW thresholds are tested from 0 to 8°C-weeks, with an interval of 0.01°C-weeks. We chose this range because beyond that the evaluation metrics no longer change. This process results in a threshold matrix of 151×801, where each point represents a combination of thresholds. For each coral survey record in the database, the corresponding monthly maximum DHW under 151 HS thresholds was calculated separately. The optimized HS and DHW were determined using the intercept between the highest AUC and the HS and DHW metrics.
2.6 Comparing performance under different threshold combinations
The third global mass coral bleaching event influenced by two consecutive El Niño events in 2014-2016, led to the death of many corals with far-reaching impacts on the marine environment (Eakin et al., 2019). Records of bleaching events in the global historical coral bleaching database confirmed that corals also experienced extensive coral bleaching during 2014-2016. To demonstrate the improvement that optimization of thresholds has on coral bleaching alert results, the differences between CRW thresholds and optimized thresholds were visualized for the SCS and surrounding seas during this global event.
3 Results
3.1 DHW with a fixed 1°C HS threshold to monitor coral bleaching
Currently, the HS operational threshold for DHW calculation is 1°C (Liu et al., 2003). If the optimization of HS is not considered, i.e., the HS threshold of 1°C is used to calculate DHW, the evaluation of the bleaching monitoring performance of DHW is shown in Figure 2. As shown in Figure 2, FPR and Recall decrease with increasing DHW. A lower DHW threshold increases the Recall value, improving the classification of bleaching events. However, this also leads to a higher rate of misclassification for non-bleaching events.

Figure 2. Evaluation indices of coral bleaching thermal stress monitoring performance for different DHW (Degree Heating Weeks) thresholds when the HS (Hotspot) threshold = 1°C, The blue region is the region with AUC (the area under the curve) > 0.8 and the dotted line is the maximum value of the f1_score (the comprehensive bleaching detection capability) versus PSS (Peirce Skill Score) in this region.
Regarding the overall evaluation metrics, PSS and f1_score initially increase, then sharply decrease, followed by a gradual rise as the DHW threshold changes. This trend is primarily driven by the early rapid decline in the false positive rate (FPR), which gradually improves the accuracy of the classification model. In the light blue region with AUC > 0.8, maximum AUC, f1_score, and PSS at DHW = 1.86°C-weeks. However, the recall value drops sharply when the DHW exceeds about 2°C-weeks, which means that further increasing the DHW threshold will significantly reduce the correct classification of bleaching events. In the plateau following the initial rise and subsequent sharp decline, the classification of non-bleaching events continues to improve, eventually reaching an FPR of 0, indicating that all non-bleaching events were correctly classified. At this stage, however, the number of unclassified bleaching events is more than 60% with the increase in PSS and f1_score more greatly influenced by the large number of non-bleaching events. Since the AUC is not affected by the unbalanced distribution of the source data, the AUC remains at a low level.
3.2 Monitoring of coral bleaching through optimized HS and DHW
This study adjusted the current operational HS threshold and comprehensively assessed the combined impact of both optimized HS and DHW on monitoring coral bleaching thermal stress. The changes in different HS and DHW combinations are shown in Figure 3. Due to the interaction of HS and DHW, the evaluation metrics do not change continuously. Recall is higher at lower HS and DHW thresholds. Additionally, for non-bleaching events, the overall level of FPR remains low, with the maximum value at the origin being only 43.2%. The thermal stress of the non-bleaching events recorded in the survey is mostly low or zero. The best evaluation values for PSS and f1_score are concentrated in the middle of their respective value ranges, and the threshold changes of HS and DHW have a synergistic effect on the final evaluation results of the model.
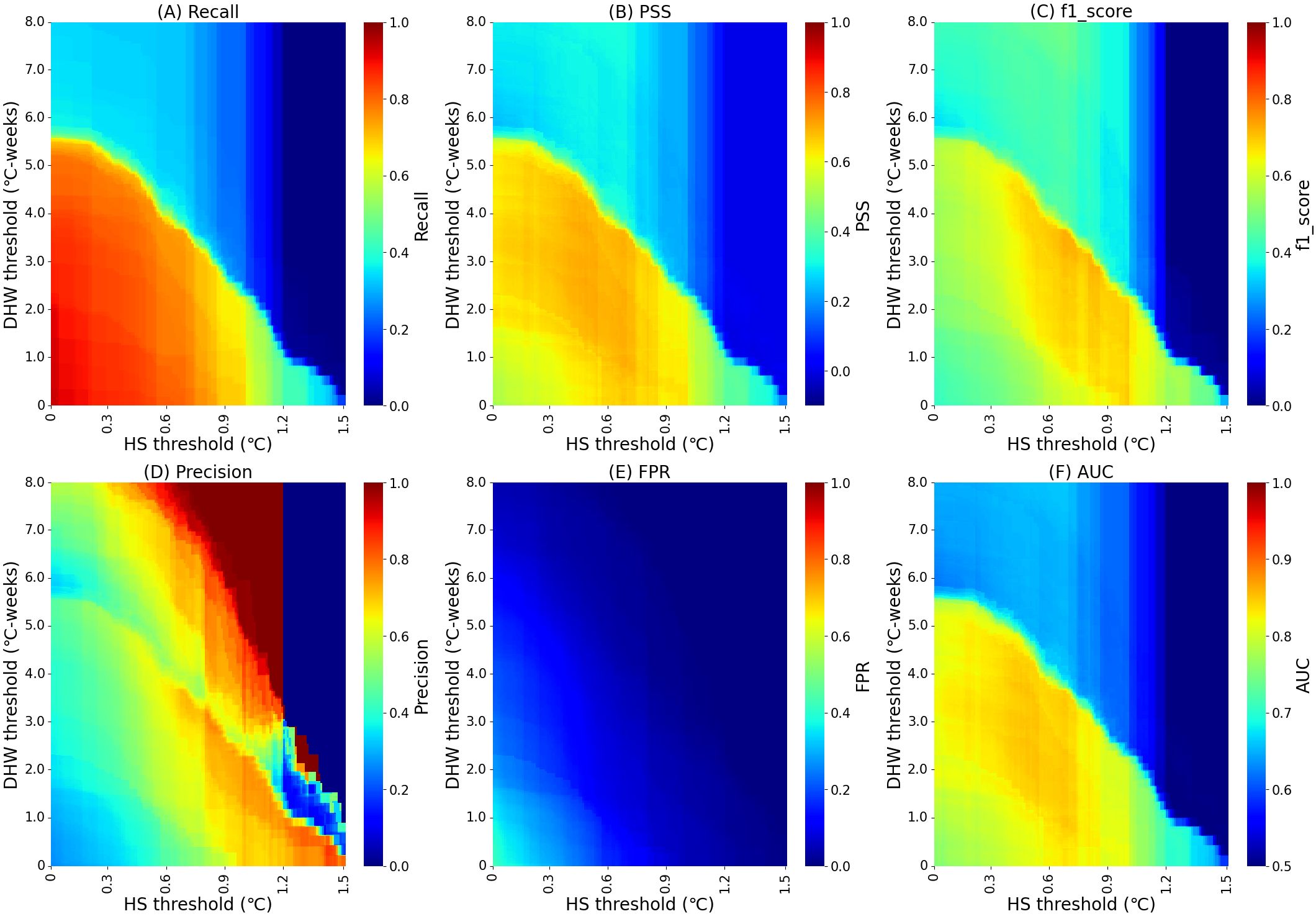
Figure 3. Performance evaluation of HS (Hotspot) + DHW (Degree Heating Weeks) coral bleaching thermal stress monitoring across all indices: (A) Recall (the probability of correct detection of all bleaching events), (B) PSS (Peirce Skill Score), (C) f1_score (the comprehensive bleaching detection capability), (D) Precision (actual bleaching events out of all events detected as bleaching), (E) FPR (the false positive rate), (F) AUC (the area under the curve).
Figure 4A shows that models with high classification ability and large AUC are mainly concentrated in an irregular interval where the HS threshold is less than 1.2°C and the DHW threshold is less than 6°C-weeks. As shown in Figure 4B, when AUC is further restricted to > 0.8, a better combination of thresholds appears in the middle region. Deviating from this range will degrade the model’s performance. Finally, Figure 4C shows that the maximum AUC value of 0.853 occurs at HS=0.47°C and DHW=2.65°C-weeks. The optimal threshold of HS is significantly lower than 0.99°C, while the optimal threshold of DHW is slightly higher than 2°C-weeks, reflecting the interaction between the two thermal stress evaluation indices.
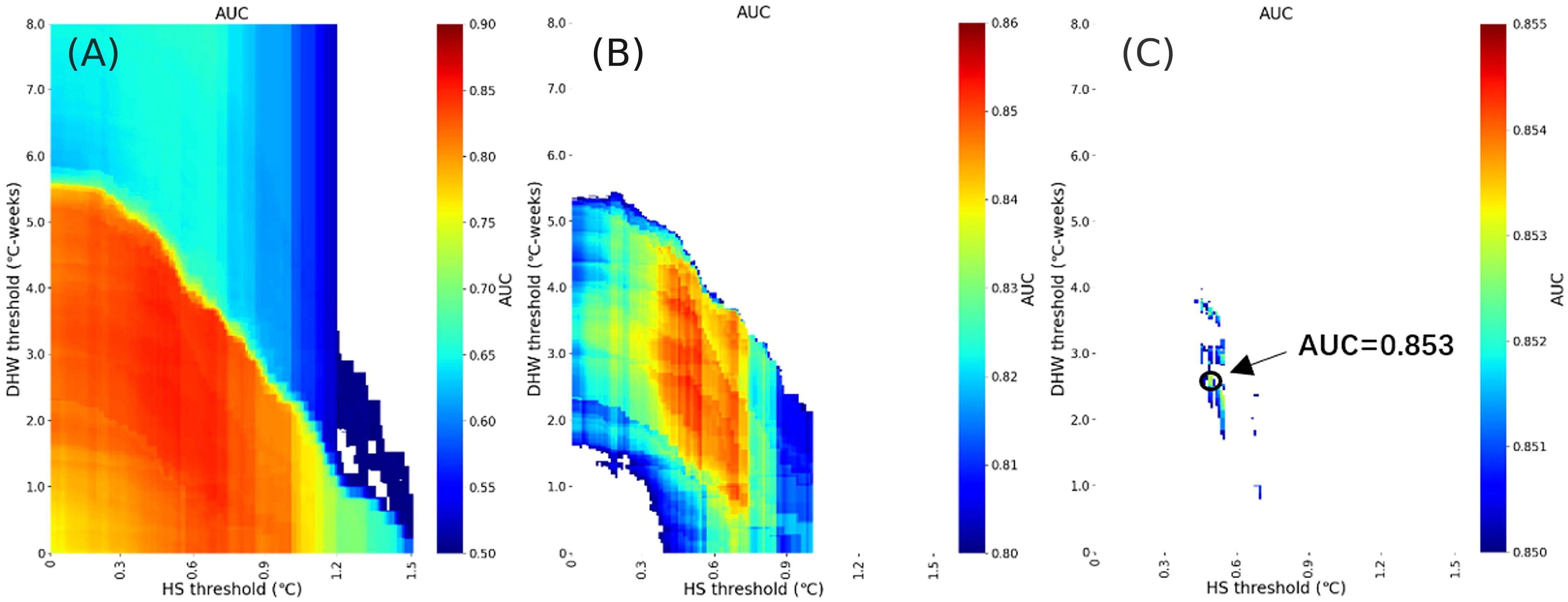
Figure 4. Achieving the highest AUC (the area under the curve) by optimizing HS (Hotspot) and DHW (Degree Heating Weeks) by gradually narrowing the AUC range: (A) 0.50≤AUC ≤ 0.90, (B) 0.80≤AUC ≤ 0.86, (C) 0.85≤AUC ≤ 0.855. The arrow indicates the point where the maximum AUC is located, highest AUC=0.853.
3.3 Evaluating and comparing performance under different threshold combinations
By assessing two different threshold optimization methods, the optimal thresholds obtained in the SCS and surrounding seas vary. Table 2 provides a comparison of each evaluation metric alongside the current operational threshold. All evaluation results of AUC significantly improved after optimizing the classification threshold based on real historical events, as compared to the current operational threshold (Liu et al., 2014).
The best DHW threshold calculated with an HS threshold of 1°C is 1.86°C-weeks for coral bleaching monitoring, which is significantly lower than the current operational DHW threshold of 4°C-weeks. The evaluation results based on historical events show that using a lower DHW threshold can significantly increase the recall of regional bleaching event monitoring from 17.7% to 81.0%.
Threshold optimization considering both HS and DHW resulted in the highest performance. Table 2 shows the synergistic threshold optimization of both HS and DHW indices. The new combination of regional optimization thresholds is HS = 0.47°C and DHW = 2.65°C-weeks. After optimizing the HS and DHW, the recall rate increased from 17.7% (considering NOAA's thresholds) to 81.3%, and the AUC increased from 0.58 to 0.85. Furthermore, optimizing both DHW and HS thresholds reduces the FPR for coral bleaching detection to 10.7%, compared to 14.7% when optimizing DHW alone.
3.4 Optimization thresholds during a period of widespread bleaching
In the NOAA CRW results shown in Figures 5A-C, accurate detection of bleaching events (true positive bleaching, green dots) occurred only in Japan, the Paracel Islands, and one site in northern Malaysia, while all other sites were underestimated (false negative bleaching, blue dots). After optimizing the thresholds, as shown in Figures 5D-F, all bleaching events were accurately detected apart from two sites in northern Malaysia in 2014, where bleaching events were still underestimated.
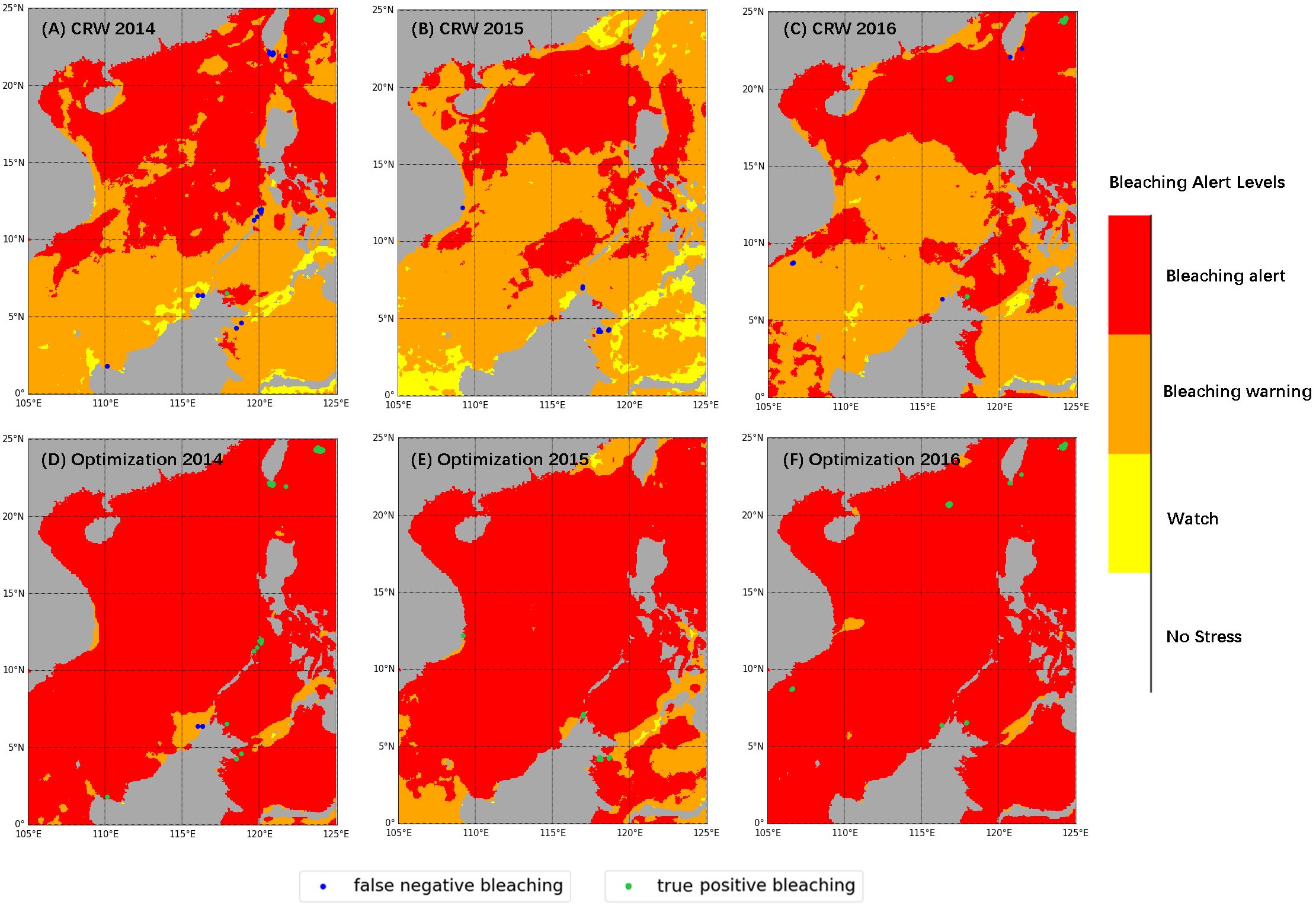
Figure 5. Annual maximum bleaching alert levels and bleaching records detecting results for 2014-2016, with (A–C) NOAA CRW (the National Oceanic and Atmospheric Administration, Coral Reef Watch program) thresholds (HS [Hotspot]= 1.00°C, DHW [Degree Heating Weeks]= 4.00°C -weeks), and (D–F) optimal thresholds (HS = 0.47°C, DHW = 2.65°C-weeks), respectively. Blue dots represent coral bleaching records that were not correctly detected and green dots represent records that were correctly detected.
4 Discussion
4.1 Optimizing DHW vs. optimizing both HS and DHW
The results demonstrate that the current global threshold setting in the SCS and surrounding seas can be optimized in its application. Previous studies showed that the addition of DHW, which reflects long-term heat accumulation based on HS, better balances model performance and improves the accuracy of monitoring coral bleaching events (Gleeson and Strong, 1995). By adding the optimized DHW, our model was greatly improved in monitoring and predicting bleaching events in the SCS region. The best DHW threshold calculated with an HS threshold of 1°C is 1.86°C-weeks. This result is similar to the 2.07°C-weeks threshold calculated for Japanese coral reefs (Kumagai et al., 2018). Table 2 demonstrates that the combined optimization of HS and DHW yields superior results compared to optimizing DHW alone. This is because when the HS threshold is fixed at 1°C, any heat below 1°C is excluded from the DHW cumulative calculations. Prolonged exposure to low heat stress can also cause coral bleaching (Berkelmans, 2002). By lowering the HS threshold, we gain valuable insights into potential bleaching risks that would otherwise remain undetected, and what could be considered low stress scenarios are still able to induce bleaching.
4.2 Importance of increasing the accuracy of coral bleaching detection
By comparing data from coral bleaching survey databases, this study determined the optimal regional thermal stress threshold for bleaching through different optimization methods. This approach to evaluating optimal thresholds can be broadly applied to other regions. It is important to understand the temperature conditions that lead to bleaching. Accurate bleaching alerts are extremely important for coral reef managers, providing them with appropriate time to respond, especially as local conditions can magnify coral mortality after heatwaves (Donovan et al., 2021). Further, accurate records of conditions that cause bleaching can help us to understand the impacts of exposure to repeated marine heat waves, and whether corals become more resilient after experiencing multiple thermal stressors. The results indicate that the current operational coral bleaching thermal stress monitoring threshold misses a large number of coral bleaching events in the SCS and surrounding seas.
After synergistic optimization of both HS and DHW, every evaluation index improved. Reducing HS and DHW thresholds could significantly correct the current underestimation of coral bleaching monitoring (Recall from 17.7% to 81.3%, AUC from 0.58 to 0.85), while keeping the false non-bleaching detection rate FPR of coral bleaching events at a low level (10.7%). Regional collaborative optimization of HS and DHW leads to a better classification model than optimizing them separately. This is further supported by examining bleaching alerts during a period of widespread coral bleaching (2014-2016) in the SCS and its surrounding seas.
4.3 Optimization of thresholds during a period of widespread bleaching
The definitions of the threshold models for optimal optimization and NOAA CRW are shown in Table 3. The new regional threshold combinations calculated here (HS = 0.47°C, DHW = 2.65°C-weeks) are similar to the latest optimized bleaching thresholds (HS = 0.4°C, DHW = 3°C-weeks) presented by Whitaker et al. (2024) for the globe. Considering these thresholds, the FPR for thermal stress coral bleaching reduces from 14.7% to 10.7%. This reduction in false bleaching alerts remarkably improves coral bleaching monitoring. When both threshold models were applied simultaneously during a period of widespread coral bleaching in the SCS and its surrounding seas from 2014 to 2016, we find that the CRW thermal thresholds underestimate most of the study region. Thus, the optimization of the thermal thresholds improves the accuracy of coral bleaching alerts widely.
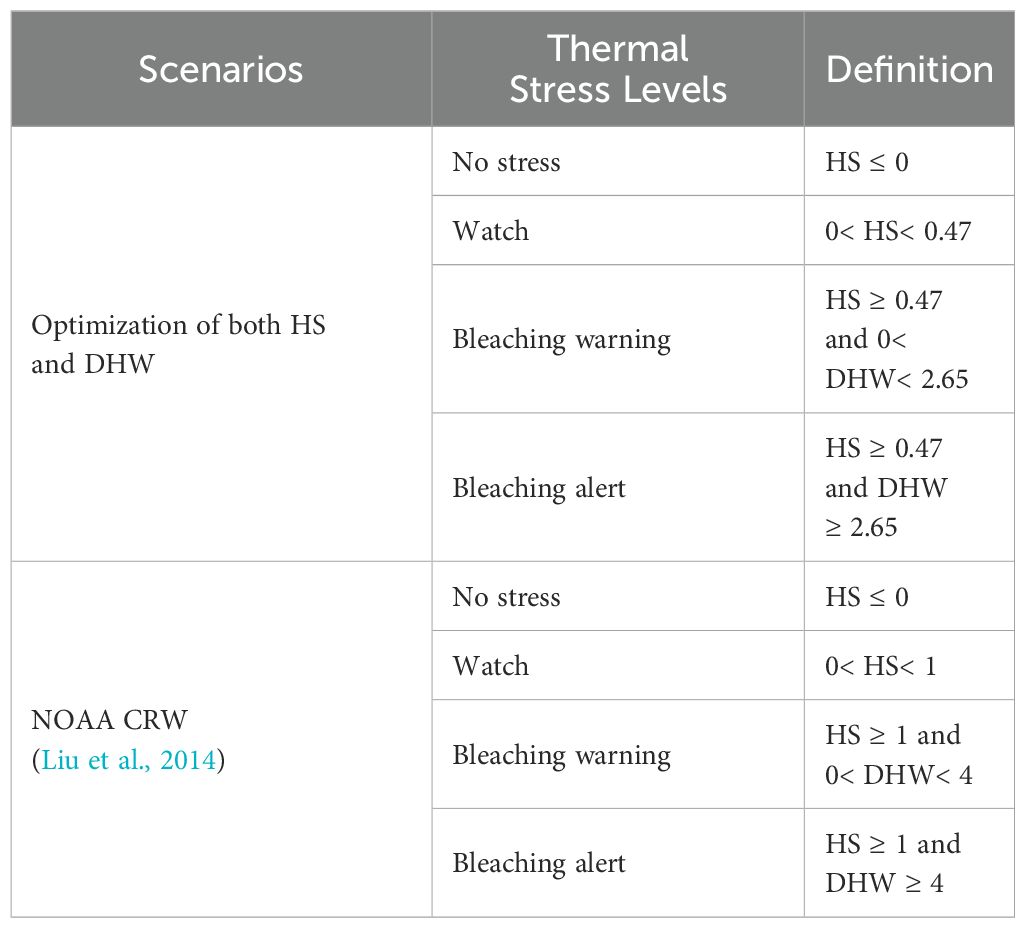
Table 3. Comparison of regionally optimized SCS (the South China Sea) and NOAA CRW (the National Oceanic and Atmospheric Administration, Coral Reef Watch program) for coral bleaching thermal stress alerts.
Our results show that corals in the SCS and its surrounding seas may experience moderate or severe bleaching at thermal thresholds below the current CRW thresholds. The lowering of heat stress thresholds may facilitate the monitoring of coral bleaching that occurs during weak marine heatwaves (Lachs et al., 2021). On a global scale, extensive regional coral bleaching has caused many negative impacts on coral reefs (Goreau and Hayes, 2024). Considering the optimization thresholds shown in Table 3 will help to provide a more accurate reference for historical analysis.
4.4 Limitations of remotely sensed SST
The optimal detection accuracy for coral bleaching events, indicated by a Recall of 81.3% (Table 2, under threshold combination HS = 0.47°C, DHW = 2.65°C-weeks), reveals that approximately 18.7% of bleaching events remain undetected. To improve future monitoring, it will be crucial to integrate additional environmental stress parameters to consider a multi-parameter model for coral bleaching. Additionally, limitations in remotely sensed SST data, as highlighted by Leichter et al. (2006), may obscure important temperature variations in certain coral reefs. For example, coral atolls often exhibit significant temperature differences between the inner reef and deeper waters due to depth and thermal exchange (Colin and Johnston, 2020). Utilizing higher-resolution SST data alongside in situ temperature measurements would more effectively capture these dynamics, enabling a clearer assessment of whether remotely sensed data accurately represents the study area.
5 Conclusions
Through repeated exposure to heat waves, corals and their symbiotic algae may increase their heat tolerance through plasticity or through association with more heat-tolerant symbionts (Lachs et al., 2023). Thus, to accurately monitor coral bleaching, it is imperative to continually update and refine the alert threshold based on real-world events. This will more accurately account for cumulative heat stress events on coral reef communities, allowing an understanding of whether there have been increases or decreases in coral reef resilience. Furthermore, accurately linking thermal stress with coral bleaching will be critical in identifying climate refugia (McWhorter et al., 2022). Future research should incorporate additional remotely sensed environmental factors to enhance the accuracy of regional coral bleaching monitoring.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
BL: Conceptualization, Formal analysis, Methodology, Writing – original draft. SF: Methodology, Writing – review & editing. LG: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research was supported by the Hainan Province Science and Technology Special Fund (ZDYF2024GXJS260 & SOLZSKY2024006), the Hainan Provincial Natural Science Foundation of China (122CXTD519), the Fundamental Research Funds for the Central Universities (202261010), the China Scholarship Council (202306330058), the Australian Research Council (grant number DE220100555), a Westpac Research Fellowship from the Westpac Scholars Trust and a Horizon Fellowship from the University of Sydney.
Acknowledgments
The authors would like to acknowledge the NOAA Coral Reef Watch (https://coralreefwatch.noaa.gov) for providing the version 3.1 daily global 5 km satellite SST product CoralTemp, and Virgen-Urcelay et al. for providing the high-resolution global mass coral bleaching dataset Version 2.0.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Berkelmans R. (2002). Time-integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 229, 73–82. doi: 10.3354/meps229073
Berkelmans R., Willis B. L. (1999). Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18, 219–228. doi: 10.1007/s003380050186
Brown K. T., Barott K. L. (2022). The costs and benefits of environmental memory for reef-building corals coping with recurring marine heatwaves. Integr. Comp. Biol. 62, 1748–1755. doi: 10.1093/icb/icac074
Chen B., Chen Y., Huang H. (2012). Satellite remote sensing monitoring coral bleaching during the 2010 bleaching event at Parcel Islands, South China Sea. Proceedings of 2012 International Conference on Earth Science and Remote Sensing (ESRS 2012). USA: Information Engineering Research Institute, 30, 776–783.
Colin P. L., Johnston T. S. (2020). Measuring temperature in coral reef environments: Experience, lessons, and results from Palau. J. Mar. Sci. Eng. 8, 680. doi: 10.3390/jmse8090680
DeCarlo T. M. (2020). Treating coral bleaching as weather: a framework to validate and optimize prediction skill. PeerJ 8, e9449. doi: 10.7717/peerj.9449
Donner S. D. (2011). An evaluation of the effect of recent temperature variability on the prediction of coral bleaching events. Ecol. Appl. 21, 1718–1730. doi: 10.1890/10-0107.1
Donovan M. K., Burkepile D. E., Kratochwill C., Shlesinger T., Sully S., Oliver T. A., et al. (2021). Local conditions magnify coral loss after marine heatwaves. Science 372, 977–980. doi: 10.1126/science.abd9464
Douglas A. E. (2003). Coral bleaching—-how and why? Mar. pollut. Bull. 46, 385–392. doi: 10.1016/S0025-326X(03)00037-7
Eakin C. M., Sweatman H. P., Brainard R. E. (2019). The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38, 539–545. doi: 10.1007/s00338-019-01844-2
Gleeson M. W., Strong A. E. (1995). Applying MCSST to coral reef bleaching. Adv. Space Res. 16, 151–154. doi: 10.1016/0273-1177(95)00396-V
Glynn P. W., D'croz L. (1990). Experimental evidence for high temperature stress as the cause of El Nino -coincident coral mortality. Coral reefs 8, 181–191. doi: 10.1007/BF00265009
Goreau T. J. (1991). Testimony to the National Ocean Policy Study Subcommittee of the United States Senate Committee on Commerce, Science, and Transportation, S. HRG Vol. 101-1138 (Washington, DC, USA: US Government Printing Office), 30–37.
Goreau T. J., Hayes R. L. (1994). Coral bleaching and ocean “hot spots”. Ambio-Journal Hum. Environ. Res. Manage. 23, 176–180.
Goreau T. J., Hayes R. L. (2024). 2023 Record marine heat waves: coral reef bleaching HotSpot maps reveal global sea surface temperature extremes, coral mortality, and ocean circulation changes. Oxford Open Climate Change 4, kgae005. doi: 10.1093/oxfclm/kgae005
Goreau T. J., Hayes R. L., Clark J. W., Basta D. J., Robertson C. N. (1993). “Elevated sea surface temperatures correlate with Caribbean coral reef bleaching,” in A Global Warming Forum: Scientific, Economic, and Legal Overview. Ed. Geyer R. A. (CRC Press, Boca Raton, FL, USA), 225–255.
Goreau T. J., Hayes R. L., McAlllister D. (2005). Regional patterns of sea surface temperature rise: implications for global ocean circulation change and the future of coral reefs and fisheries. World Resource Rev. 17, 350–370.
Hanley J. A., McNeil B. J. (1982). The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36. doi: 10.1148/radiology.143.1.7063747
Hayes R. L., Goreau T. J. (1991). The tropical coral reef ecosystem as a harbinger of global warming, Proc 2nd International conference on global warming. World Resour. Rev. 3, 306–322.
Heron S. F., Liu G., Rauenzahn J. L., Christensen T. R. L., Skirving W. J., Burgess T. F. R., et al. (2014). Improvements to and continuity of operational global thermal stress monitoring for coral bleaching. J. Operational Oceanography 7, 3–11. doi: 10.1080/1755876X.2014.11020154
Heron S. F., Liu G., Eakin C. M., Skirving W. J., Muller-Karger F. E., Vega-Rodriguez M., et al. (2015). Climatology Development for NOAA Coral Reef Watch's 5-km Product Suite. Technical Report NESDIS 145 National Oceanic and Atmospheric Administration's National Environmental Satellite, Data, and Information Service, Coral Reef Watch, College Park, MD, 21.
Hoegh-Guldberg O. (1999). Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 50, 839–866. doi: 10.1071/MF99078
Huang D., Licuanan W. Y., Hoeksema B. W., Chen C. A., Ang P. O., Huang H., et al. (2015). Extraordinary diversity of reef corals in the South China Sea. Mar. Biodiversity 45, 157–168. doi: 10.1007/s12526-014-0236-1
Hughes T. P., Anderson K. D., Connolly S. R., Heron S. F., Kerry J. T., Lough J. M., et al. (2018). Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. doi: 10.1126/science.aan8048
Hughes T. P., Kerry J. T., Álvarez-Noriega M., Álvarez-Romero J. G., Anderson K. D., Baird A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
IPCC (2022). Climate change 2022: Impacts, adaptation, and vulnerability (Cambridge, UK and New York, NY, USA: Cambridge University Press), 9.
Kayanne H. (2017). Validation of degree heating weeks as a coral bleaching index in the northwestern Pacific. Coral Reefs 36, 63–70. doi: 10.1007/s00338-016-1524-y
Kumagai N. H., Yamano H. (2018). High-resolution modeling of thermal thresholds and environmental influences on coral bleaching for local and regional reef management. PeerJ 6, e4382. doi: 10.7717/peerj.4382
Lachs L., Bythell J. C., East H. K., Alasdair J Edwards A. J., Mumby P. J., Skirving W. J., et al. (2021). Fine-tuning heat stress algorithms to optimise global predictions of mass cxoral bleaching. Remote Sens. 13, 2677. doi: 10.3390/rs13142677
Lachs L., Donner S. D., Mumby P. J., Bythell J. C., Humanes A., East H. K., et al. (2023). Emergent increase in coral thermal tolerance reduces mass bleaching under climate change. Nat. Commun. 14, 4939. doi: 10.1038/s41467-023-40601-6
Leichter J. J., Helmuth B., Fischer A. M. (2006). Variation beneath the surface: quantifying complex thermal environments on coral reefs in the Caribbean, Bahamas and Florida. J. Mar. Res. 64, 4. doi: 10.1357/002224006778715711
Li X., Liu S., Huang H., Huang L., Jing Z., Zhang C. (2012). Coral bleaching caused by an abnormal water temperature rise at Luhuitou fringing reef, Sanya Bay, China. Aquat. Ecosystem Health Manage. 15, 227–233. doi: 10.1080/14634988.2012.687651
Li S., Yu K., Chen T., Shi Q., Zhang H. (2011). Assessment of coral bleaching using symbiotic zooxanthellae density and satellite remote sensing data in the Nansha Islands, South China Sea. Chin. Sci. Bull. 56, 1031–1037. doi: 10.1007/s11434-011-4390-6
Liu B., Guan L., Chen H. (2021). Detecting 2020 coral bleaching event in the northwest Hainan island using coralTemp SST and sentinel-2B MSI imagery. Remote Sens. 13, 4948. doi: 10.3390/rs13234948
Liu G., Heron S. F., Eakin C. M., Muller-Karger F. E., Vega-Rodriguez M., Guild L. S., et al. (2014). Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA Coral Reef Watch. Remote Sens. 6, 11579–11606. doi: 10.3390/rs61111579
Liu G., Skirving W. J., Geiger E. F., de la Cour J. L., Marsh B. L., Heron S. F., et al. (2017). NOAA Coral Reef Watch’s 5km satellite coral bleaching heat stress monitoring product suite version 3 and four-month outlook version 4. Reef Encounter 32, 39–45.
Liu G., Strong A. E., Skirving W. (2003). Remote sensing of sea surface temperatures during 2002 Barrier Reef coral bleaching. Eos Trans. Am. Geophysical Union 84, 137–141. doi: 10.1029/2003EO150001
Liu G., Strong A., Skirving W., Arzayus F. (2005). “Overview of NOAA Coral Reef Watch program's near real-time satellite global coral bleaching monitoring activities,” in Proc 10th Int Coral Reef Symp, Okinawa, Japan, Vol. 1. 1783–1793.
Maturi E., Harris A., Mittaz J., Sapper J., Wick G., Zhu X., et al. (2017). A new high-resolution sea surface temperature blended analysis. Bull. Am. Meteorological Soc. 98, 1015–1026. doi: 10.1175/BAMS-D-15-00002.1
McWhorter J. K., Halloran P. R., Roff G., Skirving W. J., Mumby P. J. (2022). Climate refugia on the Great Barrier Reef fail when global warming exceeds 3 C. Global Change Biol. 28, 5768–5780. doi: 10.1111/gcb.v28.19
Meng L., Huang W., Yang E., Wang Y., Xu L., Yu K. (2022). High temperature bleaching events can increase thermal tolerance of Porites lutea in the Weizhou Island. J. Oceanography 44, 87–96. doi: 10.12284/hyxb2022126
Moberg F., Folke C. (1999). Ecological goods and services of coral reef ecosystems. Ecol. economics 29, 215–233. doi: 10.1016/S0921-8009(99)00009-9
Morton B., Blackmore G. (2001). South China Sea. Mar. pollut. Bull. 42, 1236–1263. doi: 10.1016/S0025-326X(01)00240-5
Oliver J. K., Berkelmans R., Eakin C. M. (2009). Coral Bleaching in Space and Time. In: van Oppen M. J. H., Lough J. M. (eds) Coral Bleaching. Ecological Studies 205. Springer, Berlin, Heidelberg. doi: 10.1007/978-3-540-69775-6_3
Peirce C. S. (1884). The numerical measure of the success of predictions. Science 93), 453–454. doi: 10.1126/science.ns-4.93.453.b
Perez-Rosales G., Rouze H., Torda G., Bongaerts P., Pichon M., Under the Pole Consortium, et al. (2021). Mesophotic coral communities escape thermal coral bleaching in French Polynesia. R. Soc. Open Sci. 8, 210139. doi: 10.1098/rsos.210139
Qin B., Yu K., Zuo X. (2023). Study of the bleaching alert capability of the CRW and CoRTAD coral bleaching heat stress products in China's coral reefs. Mar. Environ. Res. 186, 105939. doi: 10.1016/j.marenvres.2023.105939
Riegl B., Glynn P. W., Banks S., Keith I., Rivera F., Vera-Zambrano M., et al. (2019). Heat attenuation and nutrient delivery by localized upwelling avoided coral bleaching mortality in northern Galapagos during 2015/2016 ENSO. Coral Reefs 38, 773–785. doi: 10.1007/s00338-019-01787-8
Roberts-Jones J., Fiedler E. K., Martin M. J. (2012). Daily, global, high-resolution SST and sea ice reanalysis for 1985–2007 using the OSTIA system. J. Climate 25, 6215–6232. doi: 10.1175/JCLI-D-11-00648.1
Skirving W. J., Heron S. F., Marsh B. L., Liu G., de la Cour J. L., Geiger E. F., et al. (2019). The relentless march of mass coral bleaching: a global perspective of changing heat stress. Coral Reefs 38, 547–557. doi: 10.1007/s00338-019-01799-4
Tang C., Li M., Zheng Z., Zhou X., Shi X. (2010). Analysis of SST index change trend of 5 coral heat bleaching ocean stations on Weizhou Island in recent 45 years. Trop. Geogr. 30, 577–581+586.
van Hooidonk R., Huber M. (2009). Quantifying the quality of coral bleaching predictions. Coral Reefs 28, 579–587. doi: 10.1007/s00338-009-0502-z
Virgen-Urcelay A., Donner S. D. (2023). Increase in the extent of mass coral bleaching over the past half-century, based on an updated global database. PLoS One 18, e0281719. doi: 10.1371/journal.pone.0281719
Whitaker H., DeCarlo T. (2024). Re (de) fining degree-heating week: coral bleaching variability necessitates regional and temporal optimization of global forecast model stress metrics. Coral Reefs, 1–16. doi: 10.1007/s00338-024-02512-w
Williams J. E.H., Bunkley-Williams L. (1990). The world-wide coral reef bleaching cycle and related sources of coral mortality. Atoll Res. Bull. 335, 1–71. doi: 10.5479/si.00775630.335.1
Williamson M. J., Tebbs E. J., Dawson T. P., Thompson H. J., Head C. E., Jacoby D. M. (2022). Monitoring shallow coral reef exposure to environmental stressors using satellite earth observation: the reef environmental stress exposure toolbox (RESET). Remote Sens. Ecol. Conserv. 8, 855–874. doi: 10.1002/rse2.v8.6
Yu K. F., Zhao J. X., Shi Q., Chen T. G., Wang P. X., Collerson K. D., et al. (2006). U-series dating of dead Porites corals in the South China Sea: evidence for episodic coral mortality over the past two centuries. Quaternary Geochronology 1, 129–141. doi: 10.1016/j.quageo.2006.06.005
Keywords: coral bleaching monitoring, South China Sea, sea surface temperature, degree heating week, thermal stress
Citation: Liu B, Foo SA and Guan L (2024) Optimization of thermal stress thresholds on regional coral bleaching monitoring by satellite measurements of sea surface temperature. Front. Mar. Sci. 11:1438087. doi: 10.3389/fmars.2024.1438087
Received: 25 May 2024; Accepted: 11 November 2024;
Published: 28 November 2024.
Edited by:
Eslam O. Osman, King Abdullah University of Science and Technology, Saudi ArabiaReviewed by:
Walter Rich, King Abdullah University of Science and Technology, Saudi ArabiaThomas Goreau, Global Coral Reef Alliance, United States
Copyright © 2024 Liu, Foo and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Guan, bGVpZ3VhbkBvdWMuZWR1LmNu
 Bailu Liu
Bailu Liu Shawna A. Foo
Shawna A. Foo Lei Guan
Lei Guan