Abstract
As one of the typical coastal ecosystems, seagrass bed has important ecological service functions. In order to enrich the basic data of multispecies tropical seagrass beds, the main controlling factors affecting the community status of seagrass were identified. In this study, the species, distribution and community characteristics of seagrass in Wenchang were investigated at five stations in 2023, and Spearman correlation analysis and Redundancy analysis were used to investigate the relationship between seagrass and environmental characteristics. The results showed that there were 7 species of seagrass belonging to 5 genera in 2 families along Wenchang coast, including Enhalus acoroides, Thalassia hemprichii, Cymodocea rotundata, Cymodocea serrulata, Halophila ovalis, Halophila minor and Halodule uninervis. The distribution of seagrass beds in Wenchang showed an obvious trend of degradation. Except for the relatively stable of seagrass beds in the central part of Wenchang, the seagrass beds on both the north and south sides decreased significantly, and the coverage of seagrass beds decreased from 24.31% in 2016 to 21.0% in 2020, and further decreased to 20.67% in 2023. The data showed that the coverage and aboveground biomass of seagrass were significantly positively correlated with temperature, and the density of seagrass was significantly positively correlated with DO, but significantly negatively correlated with COD. In addition, increased nutrient salts in the water column could negatively affect seagrass bed. In order to promote the sustainable development of seagrass ecosystem and enhance the stability of seagrass habitat, it is suggested to rationally plan the aquaculture scale of the surrounding area; flexibly manage the rake snail, rake clam and other fishing activities, appropriately reduce the frequency of mining; and scientifically plan marine engineering to reduce the damage to seagrass bed.
1 Introduction
As one of the most important coastal ecosystems in the world, seagrass has high productivity and important ecosystem service value (Scott et al., 2018). It can not only purify water quality, but also fix sediment (McKenzie et al., 2021). It is also a food source, habitat and shelter for a variety of marine organisms (Jiang et al., 2020). In addition, seagrass bed is one of the most efficient carbon sequestration systems on earth, holding more than 10% of the ocean’s carbon reserves (Howard et al., 2020). However, with the interference of human activities and the intensification of environmental pressure, the degradation of seagrass bed has increased globally (Short et al., 2011).
The growth and distribution of seagrass are affected by a variety of environmental factors, including temperature, light, salinity and nutrient (Lee et al., 2007; Suykerbuyk et al., 2018). Suitable water temperature and light conditions have important effects on the photosynthesis and growth of seagrass (Collier and Waycott, 2014; Bertelli and Unsworth, 2018), while excessive nutrient content and suspended matter concentration may lead to deterioration of water quality, which affects the growth and development of seagrass (Bertelli et al., 2020). Therefore, exploring the relationship between seagrass and environmental factors is of great significance for understanding the stability and vulnerability of seagrass ecosystems.
Wenchang is located in the northeastern part of Hainan Province, which is rich in seagrass resources and is the largest seagrass distribution area in Hainan Province (Wang et al., 2012). According to data records, there were eight species of seagrass belonging to six genera along Wenchang seagrass distribution area, including Enhalus acoroides, Thalassia hemprichii, Cymodocea rotundata, Cymodocea serrulata, Syringodium isoetifolium, Halophila ovalis, Halophila minor and Halodule uninervis (Wang et al., 2012). In recent years, the growth of seagrass bed in Wenchang has been affected to varying degrees, with the phenomenon of seagrass degradation has gradually emerged (Chen et al., 2015; Xu et al., 2022). With the reduction of seagrass habitats, Thalassodendron ciliatum and Halophila decipiens, which were historically recorded in Hainan, were not found in the corresponding distribution areas during surveys in the 21st century (Zheng et al., 2013). The degradation and reduction of seagrass bed area has seriously threatened the species diversity of seagrass. Therefore, in-depth understanding of the community status and ecological characteristics of the seagrass bed in Wenchang and analysis of the main factors affecting the degradation of the seagrass bed can not only enhance the public’s attention to the conservation of seagrass, but also provide the scientific basis for the protection, restoration and effective management of seagrass beds in Wenchang.
2 Materials and methods
2.1 Study area
Wenchang is a tropical oceanic monsoon climate with sufficient sunshine and rainfall. The unique climate environment provides superior conditions for the development of seagrass. In recent years, seagrass habitats along the coast of Wenchang have been degraded and reduced, and seagrass beds that were originally distributed in sheets have gradually been distributed in patches or scattered. A total of five survey stations were deployed in this survey, covering Longlou Town (HC01), Yelin Bay (HC02), Gangdong Village (HC03), Changpi Port (HC04) and Fengjiawan (HC05) in Wenchang from north to south (Figure 1).
Figure 1

Investigation stations of seagrass in Wenchang, Hainan.
2.2 Survey method
During May and June 2023, seagrass beds would be surveyed and samples collected during the ebb tide period when seagrass was exposed to the surface. According to the distribution of seagrass, survey sections were arranged perpendicular to the shoreline of each survey station, from the intertidal zone to the subtidal zone, through the distribution of seagrass beds. The section layout covers the upper limit, lower limit and distribution center of seagrass. Transects parallel to the shoreline were laid on each section according to the upper limit, center and lower limit of seagrass distribution, and three 25cm×25cm quadrats were randomly placed on each transect. Each survey station has 3 transects and 9 quadrats. A total of 5 survey stations with 45 quadrats were set up in the study area. The investigation and analysis methods were carried out according to the《Technical guideline for investigation and assessment of coastal ecosystem—Part 6: Seagrass bed》(T/CAOE 20.6-2020).
Using the 25cm×25cm quadrat frame, the species of seagrass in the quadrat were identified on site, and the coverage of seagrass was estimated according to the distribution of seagrass in each quadrat. All seagrass plants in the quadrat were removed completely during sampling, washed and brought back to the laboratory for determination and analysis. Species were identified in each quadrat, and species were combined for shoot counts and biomass. Then all the seagrass plants in the quadrat were divided into aboveground and underground parts, dried at 60 °C to constant weight, and the biomass of seagrass per unit area (g/m2) was measured.
The temperature (Temp), pH, salinity (Sal) and dissolved oxygen (DO) of seawater were measured on-site using the DZB-712F portable multi-parameter water quality analyzer in the areas where transects were laid out at each survey station, and water samples for laboratory testing were collected. The water samples were then transported to the laboratory for determination of and chemical oxygen demand (COD), nitrate (NO3-), nitrite (NO2-), total nitrogen (TN), active phosphate (PO43-), total phosphorus (TP) and active silicate (SiO32-). The collection, transportation and analysis of seawater samples were carried out in accordance with《The requirements of the specification for marine monitoring》(GB 17378-2007) and《The specification for marine investigation》(GB 12763-2007).
2.3 Methods for assessing the community status of seagrass bed
According to the《Technical guideline for investigation and assessment of coastal ecosystem—Part 6: Seagrass bed》(T/CAOE 20.6-2020), the community status of seagrass bed in Wenchang was assessed, and the community characteristics of seagrass were assigned according to the degree of change (Table 1).
Table 1
| Indicator | Stable | Damaged | Severely damaged |
|---|---|---|---|
| Change in area | ≥-10% | ≥-30%~<-10% | <-30% |
| Change in coverage | ≥-10% | ≥-30%~<-10% | <-30% |
| Change in density | ≥-10% | ≥-30%~<-10% | <-30% |
| Assigned index | 50 | 30 | 10 |
Assessment indicators and assigned values of seagrass beds.
The change in the coverage of seagrass (V1) was calculated according to the Equation 1:
Where V1 is the rate of change in the coverage of seagrass, unit is percentage; . is the monitoring average of seagrass coverage; and is the historical reference data.
The change in the density of seagrass (V2) was calculated according to the Equation 2:
Where V2 is the rate of change in the density of seagrass, unit is percentage; is the monitoring average of seagrass density; and is the historical reference data.
The assessment index (IV) of seagrass bed was calculated according to the Equation 3:
Where IV is the seagrass bed assessment index; Vi is the value assigned to the ith seagrass bed assessment indicator; and q is the total number of seagrass bed assessment indicators.
When 37≤IV≤50, the seagrass bed is stable; when 30≤IV<37, the seagrass bed is damaged; when 10≤IV<30, the seagrass bed is severely damaged.
2.4 Data processing
In this study, SPSS 26.0 software was used to process and analyze the data. Based on the 2023 survey data of seagrass resources in Wenchang at five stations, combined with the data of the “Marine Biodiversity Survey of Hainan Province” from 2016 to 2020, the community status of seagrass beds was assessed, and the assessment map of seagrass community status along Wenchang was drawn by ArcGIS 10.2 Kriging interpolation. Spearman correlation analysis and Redundancy analysis were used to analyze the potential relationship between community characteristics of seagrass and environmental factors, and the significance level was P<0.05. Smaller sample size may lead to significant relationships by chance. Therefore, we will report all statistical results, including both significant and non-significant findings, in the hope of providing preliminary insights for further research.
3 Results and analysis
3.1 Current status of seagrass bed resources
3.1.1 Composition of seagrass species
Seven species of seagrasses belonging to two families and five genera were surveyed along the coast of Wenchang in 2023. They were Enhalus acoroides, Thalassia hemprichii, Cymodocea rotundata, Cymodocea serrulata, Halophila ovalis, Halophila minor and Halodule uninervis. The survey area is rich in seagrass species, accounting for 70% of the seagrass species in Hainan Island. Among all surveyed stations, HC02 had the highest number of seagrass species (5 species) and HC01 had the lowest number (1 specie). T. hemprichii was present in all surveyed stations, followed by E. acoroides with a higher frequency (Table 2).
Table 2
| Seagrass species | Survey area | ||||
|---|---|---|---|---|---|
| HC01 | HC02 | HC03 | HC04 | HC05 | |
| Enhalus acoroides | –– | –– | + | + | + |
| Thalassia hemprichii | + | + | + | + | + |
| Cymodocea rotundata | –– | + | –– | –– | –– |
| Cymodocea serrulata | –– | + | –– | –– | –– |
| Halophila ovalis | –– | + | –– | + | –– |
| Halophila minor | –– | –– | –– | + | –– |
| Halodule uninervis | –– | + | –– | –– | –– |
| Number of species | 1 | 5 | 2 | 4 | 2 |
Distribution of seagrass species.
“+” indicates that the distribution of the species is surveyed in the region, and “––” indicates that the distribution of the species is not surveyed in the region.
3.1.2 Community characteristics of seagrass
The coverage of seagrass beds in Wenchang ranges from 10.22% to 42.78%, with HC02 being the largest, followed by HC03 (Figure 2A). The mean density of seagrass bed was 334.58 ind/m2, and the mean density of HC02 was significantly higher than that of the other four stations (P<0.05) (Figure 2B). Aboveground biomass ranged from 32.05 g/m2 to 142.38 g/m2, and the average aboveground biomass was 92.72 g/m2, with the overall pattern of HC02>HC03>HC04>HC05>HC01 (Figure 2C). The underground biomass ranged from 105.05 g/m2 to 514.06 g/m2, and the average underground biomass was 274.85 g/m2, with no significant difference among different stations (P>0.05) (Figure 2D).
Figure 2
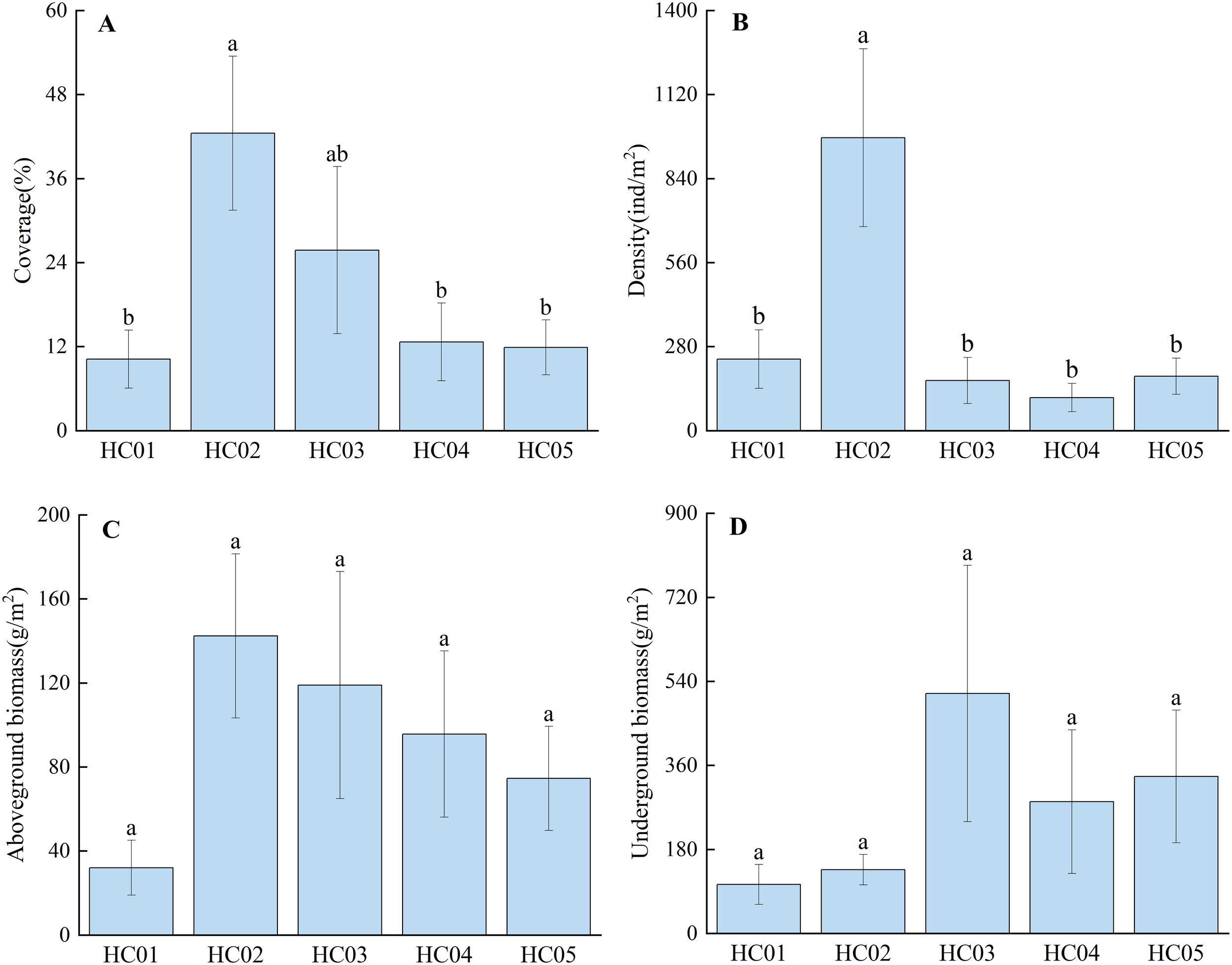
Community characteristics of seagrass. (A) coverage; (B) density; (C) aboveground biomass; (D) underground biomass. The sample sizes for the community characteristic of seagrass in each station is 9; error bars denote the standard error of the mean; different lowercase letters (a, b) in the same graph indicate significant differences (P<0.05).
3.1.3 Assessment of seagrass community status
In this study, combined with the survey data of seagrass beds along Wenchang from 2016 to 2020, the community characteristics of seagrass beds were assigned and evaluated (Table 3). The results of the study showed that HC01 and HC05 seagrass beds were severely damaged with an assessment index (IV) of 10, which was consistent with the high level of habitat destruction of seagrass beds in the region. HC03 and HC04 seagrass beds were assessed as damaged with an index (IV) of 30, which may be related to the high density of local aquaculture activities and frequent fishing activities. HC02 seagrass beds had an assessment index (IV) of 50, which was a stable condition with abundant and well-established seagrass species. It could be seen that the community status of seagrass beds in the central part of the coastal of Wenchang were better than that of the north and south sides (Figure 3).
Table 3
| Station | Coverage (%) | Assignment value for change in coverage | Density (ind/m2) | Assignment value for change in density | IV | ||
|---|---|---|---|---|---|---|---|
| C0 | |||||||
| HC01 | 21.00 | 10.22 | 10 | 590.70 | 238.22 | 10 | 10 |
| HC02 | 14.35 | 42.78 | 50 | 362.00 | 976.00 | 50 | 50 |
| HC03 | 25.55 | 25.78 | 50 | 857.00 | 167.11 | 10 | 30 |
| HC04 | 11.25 | 12.67 | 50 | 586.00 | 110.22 | 10 | 30 |
| HC05 | 25.00 | 11.89 | 10 | 471.00 | 181.33 | 10 | 10 |
Assessment of seagrass community status.
is the monitoring average of seagrass coverage, and is the historical reference data; is the monitoring average of seagrass density; and is the historical reference data. The lower IV of the seagrass bed, the more serious the damage condition of seagrass bed.
Figure 3
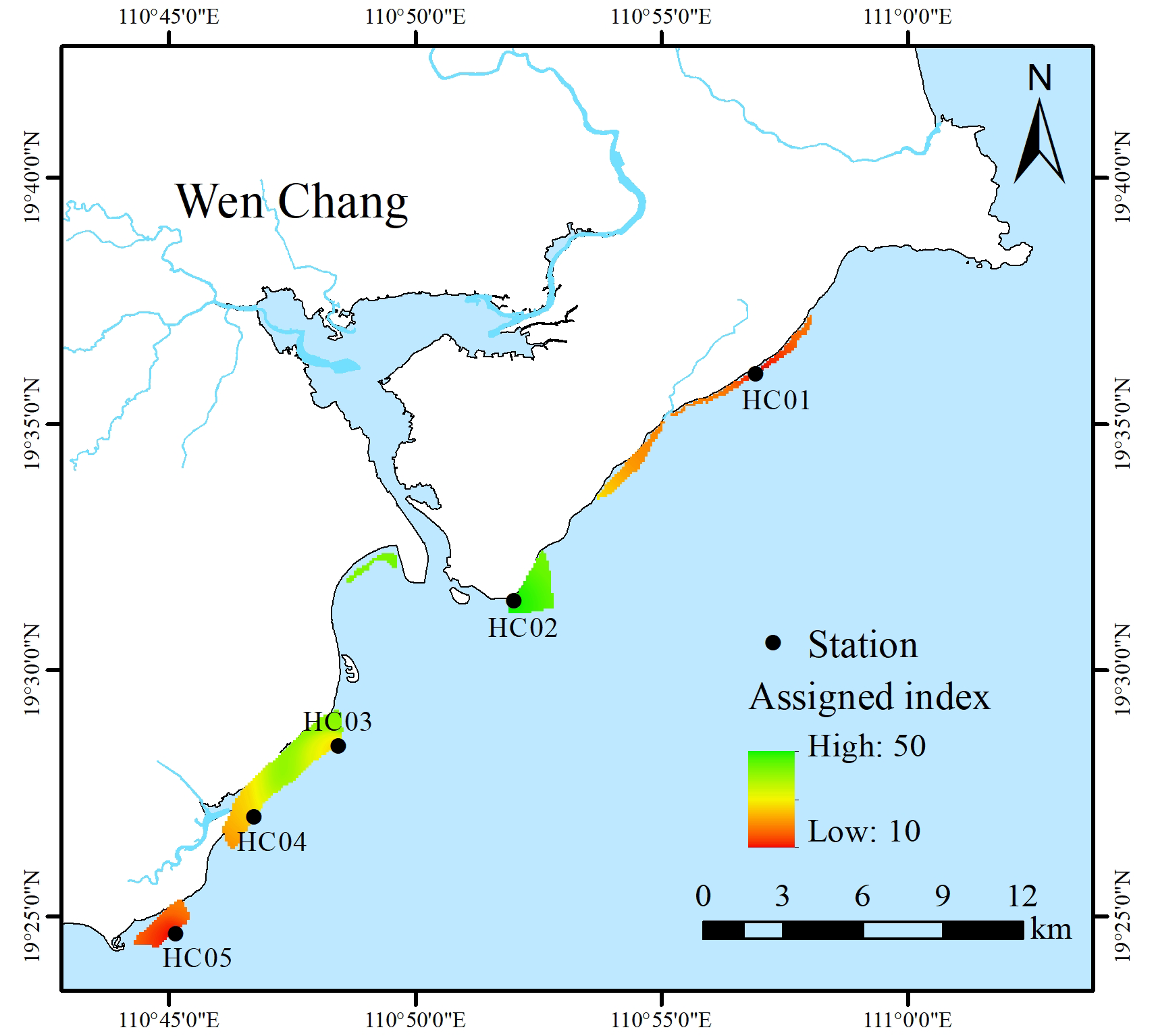
Community status of seagrass beds in Wenchang.
3.2 Environmental factors of seagrass bed
The seawater temperature at each station ranges from 30.8°C to 31.6°C, the salinity ranges from 32.6‰ to 33.3‰, and the pH ranges from 7.97 to 8.62 (Table 4). The DO content of seawater ranged from 6.34 mg/L to 6.78 mg/L, and the COD concentration ranged from 0.48 mg/L to 0.91 mg/L, which met the first water quality standard. The contents of NO3- and NO2- were the highest in HC01 and the lowest in HC05. The content of SiO32- was the highest in HC05 and the lowest in HC02. The content of PO43- ranged from 0.002 mg/L to 0.107 mg/L, and the content of PO43- varied greatly among different stations. The highest contents of total nitrogen and total phosphorus were found in HC04, which were 1.12 mg/L and 1.22 μ mol/L respectively.
Table 4
| Environmental factor | HC01 | HC02 | HC03 | HC04 | HC05 |
|---|---|---|---|---|---|
| Temp (°C) | 30.8 ± 0.00 | 31.6 ± 0.00 | 31.4 ± 0.00 | 30.9 ± 0.00 | 30.9 ± 0.00 |
| pH | 8.27 ± 0.00 | 8.05 ± 0.00 | 8.28 ± 0.00 | 7.97 ± 0.00 | 8.62 ± 0.00 |
| Sal (‰) | 33.3 ± 0.00 | 33.2 ± 0.00 | 33.1 ± 0.00 | 32.6 ± 0.00 | 32.7 ± 0.00 |
| DO (mg/L) | 6.62 ± 0.00 | 6.78 ± 0.00 | 6.34 ± 0.00 | 6.52 ± 0.00 | 6.52 ± 0.00 |
| COD (mg/L) | 0.91 ± 0.02 | 0.48 ± 0.06 | 0.71 ± 0.12 | 0.74 ± 0.02 | 0.67 ± 0.01 |
| NO3- (mg/L) | 0.60 ± 0.00 | 0.12 ± 0.04 | 0.44 ± 0.23 | 0.49 ± 0.29 | 0.07 ± 0.03 |
| NO2- (mg/L) | 0.53 ± 0.00 | <0.001 | <0.001 | 0.23 ± 0.10 | <0.001 |
| TN (mg/L) | 0.71 ± 0.00 | 0.84 ± 0.61 | 0.89 ± 0.01 | 1.12 ± 0.06 | 0.57 ± 0.11 |
| SiO32- (mg/L) | 0.38 ± 0.01 | 0.37 ± 0.02 | 0.55 ± 0.09 | 0.41 ± 0.02 | 0.63 ± 0.12 |
| PO43- (mg/L) | 0.014 ± 0.00 | 0.002 ± 0.00 | 0.107 ± 0.04 | 0.091 ± 0.01 | 0.034 ± 0.00 |
| TP (μ mol/L) | 0.32 ± 0.13 | 0.97 ± 0.28 | 0.99 ± 0.02 | 1.22 ± 0.27 | 1.16 ± 0.43 |
Environmental characteristics of seagrass bed.
The sample sizes of environmental characteristic in each station is 3; “±” denotes the standard error of the mean.
3.3 Relationship between community characteristics of seagrass and environmental factors
3.3.1 Spearman correlation analysis
Spearman correlation analysis was conducted on the community characteristics of seagrass and environmental factors in Wenchang (Figure 4), and the results showed that there was a strong correlation between the community characteristics of seagrass and environmental factors. The coverage and aboveground biomass of seagrass were significantly positively correlated with temperature (P<0.05). The density of seagrass was positively and negatively correlated with salinity and pH, respectively. There was a significant positive correlation between the density of seagrass and DO content (P<0.05). The coverage and density of seagrass were significantly negatively correlated with COD (P<0.05). The coverage, density, and biomass of seagrass were negatively correlated with NO3- and NO2-. The density of seagrass was negatively correlated with the TN and TP content. The density of seagrass was significantly negatively correlated with SiO32- and PO43- (P<0.05).
Figure 4
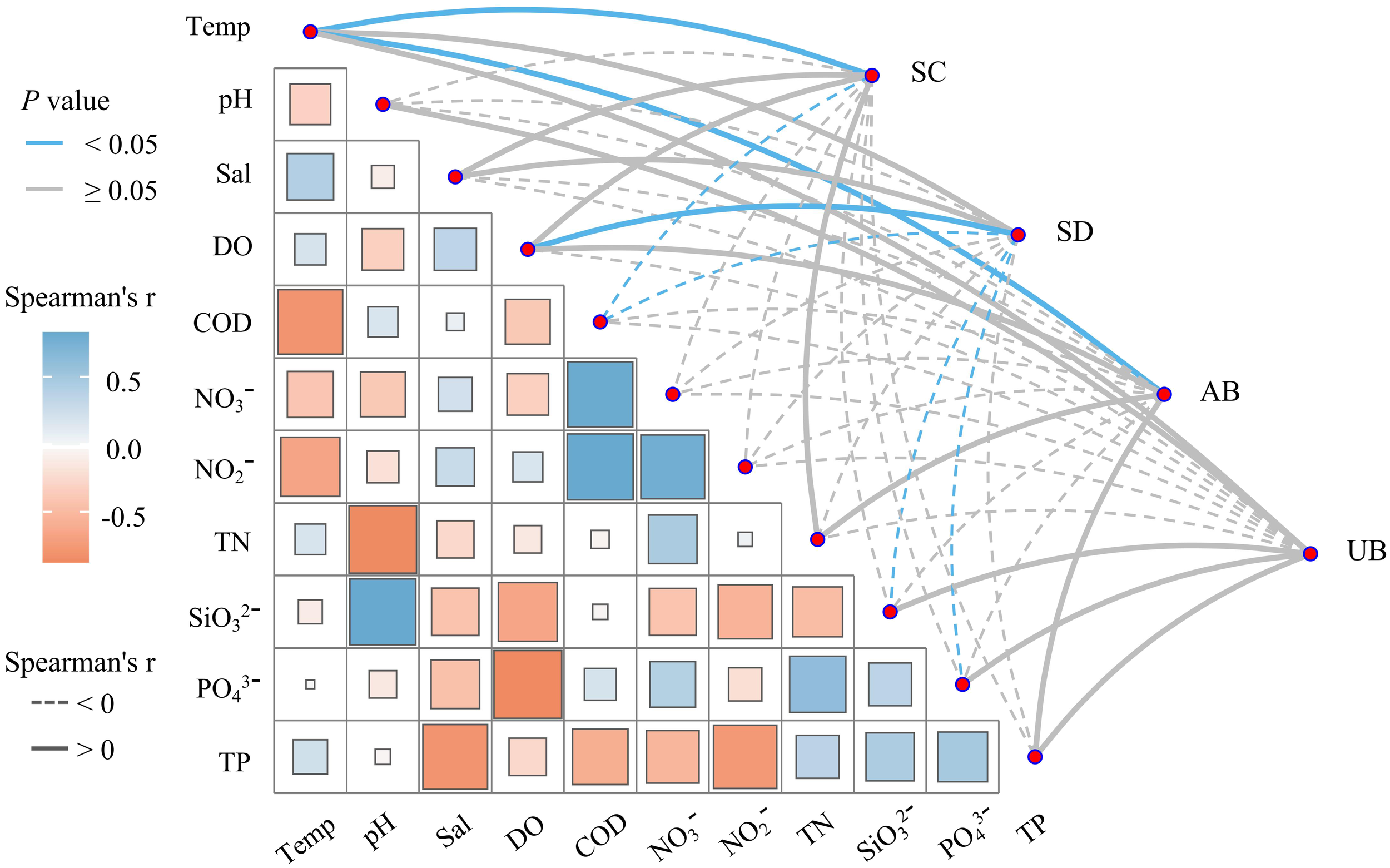
Correlation analysis between seagrass community characteristics and environmental factors.
3.3.2 Redundancy analysis
Redundancy analysis was applied to seagrass bed, in which environmental factors were used as explanatory variables and seagrass community characteristics were used as response variables. The eigenvalues of the first and second spindles were 0.8716 and 0.1235, respectively, which explained 99.51% of the variation in seagrass indicators, and the adjusted variance explanation was 98.1% (Figure 5). The interpretation rates of DO, temperature and COD were 66.9%, 31.3% and 1.3%, respectively. Among them, the coverage and aboveground biomass of seagrass were significantly positively correlated with temperature, while the density of seagrass was significantly positively correlated with DO, but significantly negatively correlated with COD. In addition, the density, coverage, and aboveground biomass of seagrass were relatively close to each other in the diagram, there was a positive correlation between them.
Figure 5
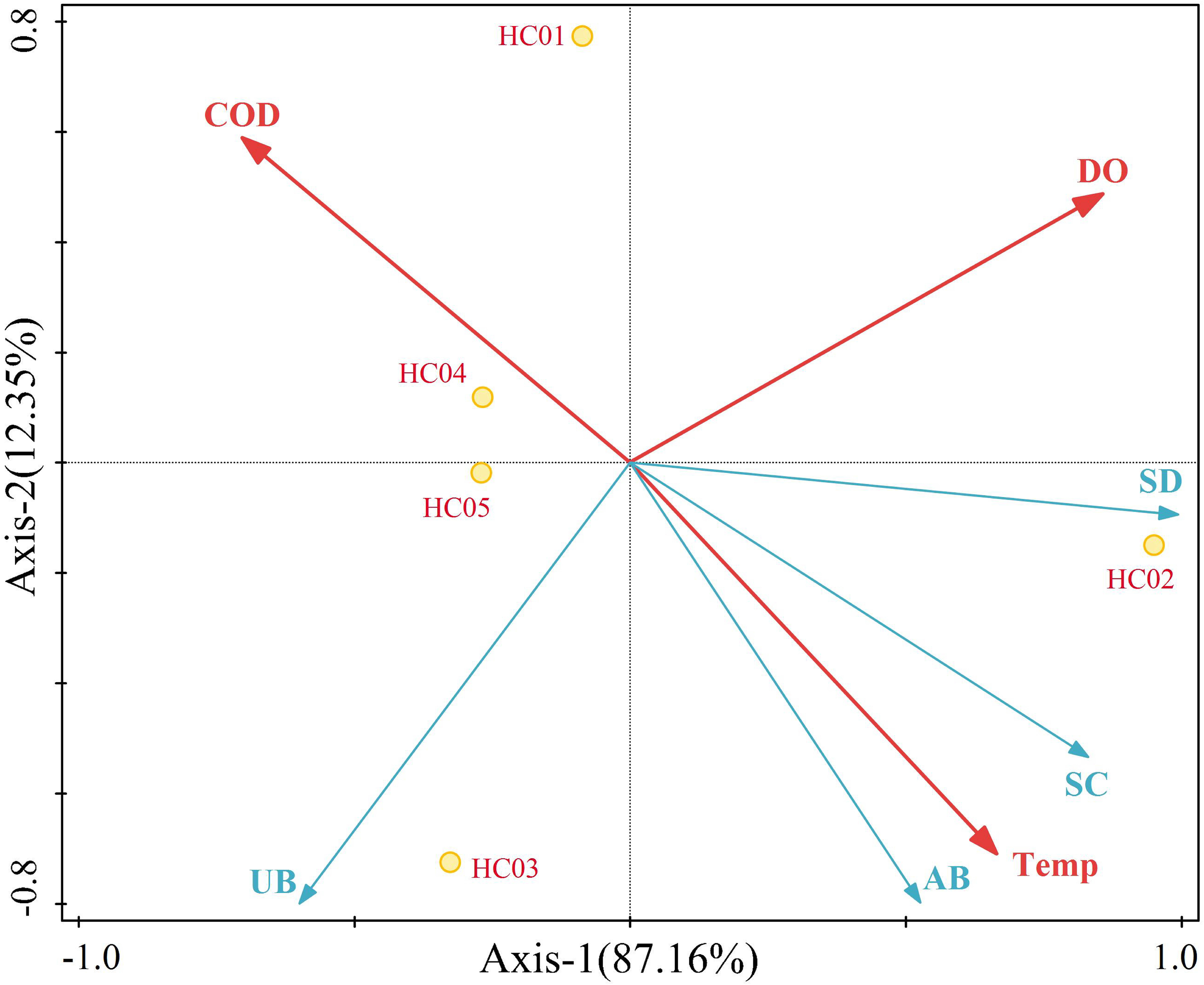
Redundancy analysis between seagrass community characteristics and environmental factors.
4 Discussion
4.1 Analysis of the community status of seagrass beds in Wenchang
In this survey of seagrass in Wenchang, 7 species of seagrass were found in 2 families and 5 genera. Compared with previous surveys (Wang et al., 2012; Zhu et al., 2017), S. isoetifolium was not found in this survey. Historical records S. isoetifolium was mainly distributed in the Fengjiawan. Fengjiawan is a sewage outlet for many cultured shrimp ponds, and the active phosphate and total phosphorus in this area are at a high level. Under the condition of nutrient enrichment, the seagrass ecosystem will produce a large amount of organic matter, resulting in low oxygen in the ecosystem, reducing the utilization rate of phosphate in the seagrass, and thus limiting the metabolism and energy transfer process of the seagrass (Han et al., 2017). In addition, a large-scale water intake project has been constructed along the coast of Fengjiawan, and the layout of water intake pipes would destroy the habitat of seagrass and change the dynamic characteristics of water flow, including the speed and direction of water flow, which may affect the seed dispersal and the selection of developmental sites of seagrass. Compared with E. acoroides and T. hemprichii from Fengjiawan, S. isoetifolium belongs to small seagrass, which was more sensitive to environmental changes and difficult to survive in harsh environments (Cai et al., 2017).
The survey found that the average coverage of seagrass bed in Wenchang was 20.67%, which was similar to the survey results in 2020 (21%) (Xu et al., 2022). Studies have shown that the average density of seagrass bed in Hainan Province reached 1753.39 ind/m2 from 2004 to 2009, among which the average density of seagrass bed in Changpi Port was 585.85 ind/m2 (Wang et al., 2012), whereas the average density of seagrass beds in Changpi Port in the present survey was only 110.22 ind/m2. The estuary of the Changpi Port was a navigable area, and the navigation of fishing vessels cut seagrass while stirring up the substrate of the seagrass beds, caused irreversible damage to seagrass habitats (Neckles et al., 2005). In addition, fishing activities such as raking snails and clams were relatively common along the whole coast of Wenchang, especially in Gangdong Village, Changpi Port and Fengjiawan. High-intensity fishing activities dug seagrasses out by the roots, which directly caused the reduction of seagrass resources (Chen et al., 2020).
The average biomass of T. hemprichii in Longlou Town was the smallest because the morphology of T. hemprichii was generally small and the defect of stems and leaves was serious. Field investigations have found that algae were widely distributed in this region, which not only competed with seagrass for habitat and nutrient resources, but also shaded seagrass and inhibit its photosynthesis (Huang et al., 2017). In addition, the low DO and high COD environment caused by algae blooms would also have a negative impact on the growth and reproduction of seagrass (Liu et al., 2016). Seagrass in the Yelin Bay was mainly found in the reef area, where seagrass was distributed in a wide variety of species. It was mainly related to the clearer water and slower currents at coral reef margins, which was conducive to the photosynthesis of seagrass. However, the growth of seagrass in Yelin Bay was under some threat due to coastal erosion and a few fishing activities (Guo et al., 2009). By compared with the historical survey data of seagrass bed in Wenchang, the average coverage, density and biomass of seagrass bed showed a decreasing trend, indicated that the seagrass in Wenchang was in a serious decline.
4.2 Effects of environmental characteristics on seagrass
Gene and environmental factors are the main causes of species differences, and the same species may also show certain differences in performance under different environments (Palacio-Lόpez et al., 2015). We used Spearman correlation analysis and Redundancy analysis to analyze the relationship between environmental factors and the community characteristics of seagrass. Since our survey stations were only five, this may limit the strength of our interpretation of the results. Therefore, we report all statistical findings in our results, including significant and non-significant findings, with a view to providing initial insights for further research. Among them, Spearman correlation analysis showed that seagrass was sensitive to environmental changes, and its community characteristics were affected by many environmental factors. RDA analysis indicated that the coverage and aboveground biomass of seagrass were significantly positively correlated with temperature, while the density of seagrass was significantly positively correlated with DO, but significantly negatively correlated with COD.
4.2.1 Effect of temperature on seagrass
Seagrass is broadly thermophilic plants but it is very sensitive to changes in temperature (Jiang et al., 2012). In this study, the temperature of seawater was significantly and positively correlated with the coverage and aboveground biomass of seagrass. At suitable temperatures, higher temperatures have a positive effect on the enzymatic mechanism of photosynthesis (Egea et al., 2018). Different seagrass species have different sensitivity to temperature. The optimum temperature for photosynthesis in tropical and subtropical seagrass is between 27°C and 33°C, and the optimum growth temperature of temperate seagrass is between 11.5°C and 26°C (Liu W. et al., 2017). The temperature of seawater in the investigated area ranged from 30.8°C to 31.6°C, which was a suitable temperature for photosynthesis of tropical seagrass, and the coverage and aboveground biomass of seagrass increased with the increase of seawater temperature. However, data from Odense, Denmark, showed that Zostera marina, a temperate seagrass, has significantly lower stem survival rate as well as leaf growth at 27°C (Höffle et al., 2011). In the context of global climate change, even if the impact of temperature change on seagrass is relatively slow, the impact on seagrass bed cannot be ignored. The temperature above or below the appropriate temperature for seagrass may reduce photosynthesis, and its increased respiration rate can break the balance between photosynthesis and respiration, which in turn affects the synthesis and storage of non-structural carbohydrates (Han et al., 2023).
4.2.2 Effects of DO and COD on seagrass
DO plays the role of oxidant in the chemical purification process of water, and abundant DO content can effectively accelerate the degradation rate of organic matter and improve the purification capacity of seawater (He et al., 2012). The study in Zhelin Bay showed that the NH3-N in aquaculture area was higher than that in non- aquaculture area, while the DO content was at a lower level, indicating that the large amount of NH3-N produced by culture activities would consume more DO (Du et al., 2006). It has been demonstrated that the reduction of DO content caused by aquaculture will lead to excess organic matter remaining in the sediment, and then inhibit the growth of the erect stems of seagrasss (Marbà et al., 2006). In addition, during the investigation of seagrass beds in Liusha Bay, it was found that the biomass of seagrass was significantly positively correlated with the concentration of DO (Zhong et al., 2019). In this study, DO content was significantly and positively correlated with the coverage of seagrass, indicating that the aquaculture activity and marine engineering around Wenchang not only affected nutrient salt in the water and sediment, but also caused hypoxia in the water. Anoxic environment will increase the energy demand of seagrass photosynthesis, which will affect the material circulation of seagrass ecosystem.
During the investigation of the seagrass bed in Liusha Bay, it was found that H. ovalis was suitable for living in the sea with the high concentration of COD (Zhong et al., 2019). However, in this study, the concentration of COD was significantly negatively correlated with the coverage and density of seagrass. On the one hand, the high COD values are usually accompanied by water eutrophication, which increases the primary productivity of algae and the activity of microorganisms (Liu S. et al., 2017). When strongly stimulated, the microorganisms in the rhizosphere of seagrass accelerates the oxygen consumption and the anaerobic metabolic processes of the bacterial community, thereby releasing toxic by-products such as sulfide and methane (Terrados et al., 1999). On the other hand, higher COD will cause hypoxia in the water and increase the energy demand for photosynthesis in seagrass, which affecting the absorption of nutrients by seagrass, and ultimately impacting the cycle of nutrient in the seagrass ecosystem (Liu W. et al., 2017).
4.2.3 Effects of other environmental factors on seagrass
Influenced by the input of freshwater from the terrestrial domain, there are obvious spatial and seasonal differences in the salinity of seagrass bed in the coastal waters (Wu et al., 2017). Long-term low salinity environment could reduce the leaf growth rate of T. hemprichii (Jiang et al., 2013). On the contrary, within an appropriate salinity, the increase of salinity can promote the growth of seagrass and increase the biomass of seagrass (Zhong et al., 2019). This was consistent with the conclusion in this study that salinity was positively correlated with the density of seagrass. However, it has also been shown that photosynthesis and respiration of seagrass is often inhibited in the environments with extreme high salinity, and that the increase in salinity leads to a decrease in chlorophyll content and enzyme activities in the plant, which in turn affects its growth and physiological processes (Kahn and Durako, 2006). Whether the seagrass bed is in a high salinity or low salinity environment, large amounts of energy are required to maintain the osmotic pressure of intracellular and the integrity of cell membrane, thus affecting the growth and metabolic processes of the seagrass bed (Atkinson and Smith, 1983).
Nutrient salt is one of the main factors limiting the growth and reproduction of seagrass (Lee et al., 2007). Under the condition of nutrient deficiency, reduced nutrient salt is the main environmental factor limiting the development of seagrass. However, under the condition of eutrophication, excessive concentration of the nutrient will have a negative impact on the growth of seagrass (Burkholder et al., 2007). In this study, the density of seagrass was significantly and negatively correlated with SiO32- and PO43-. The coastal area of Wenchang is densely populated, and the aquaculture area of shrimp ponds has been increasing year by year. Among them, the aquaculture scale from Gangdong Village to Fengjiawan has been maintained at a high level (Zhang et al., 2022). The discharge of domestic sewage and aquaculture wastewater introduce significant inputs of nutrients such as nitrogen and phosphorus to seagrass bed. A small amount of nitrogen and phosphorus input can provide nutrients for the growth of seagrass, but excess nutrients can cause the outbreak of algae, thus affecting the growth of seagrass (Han et al., 2016). Furthermore, in the eutrophic environment, seagrass needs to continuously consume carbohydrates to meet their carbon requirements (Alexandre et al., 2015), and extracellular enzyme activities associated with seagrass cellulose as well as lignin decomposition are significantly increased, which accelerates the decomposition of organic matter, which in turn negatively impacts seagrass beds (Liu et al., 2022).
Studies have shown that seagrass exposed to alkaline environment will be inhibited in growth with increasing pH (Invers et al., 1997). The overall water environment in Wenchang was weakly alkaline, and the coverage and density of seagrass were negatively correlated with pH, indicated that the change of pH caused by exogenous pollution could negatively affect the seagrass. The productivity and carbon sequestration capacity of seagrass increased with the decrease of pH, which was mainly related to the concentration of HCO3- in seawater, and the increase of HCO3- concentration was conducive to promoting the photosynthesis of seagrass (Palacios and Zimmerman, 2007; Liu et al., 2020).
5 Conclusion
In 2023, a total of seven species of seagrasses belonging to two families and five genera were investigated in the Wenchang of Hainan Province, Among them, T. hemprichii was the most widely distributed, followed by E. acoroides. According to the historical data, the species, coverage, density and biomass of seagrass beds in Wenchang showed a decreasing trend. The community status of seagrass beds in the central part of Wenchang were relatively stable, while the seagrass beds in the north and south were damaged.
Through the study on the community status and environmental characteristics of seagrass in Wenchang, it was found that the seagrass was sensitive to environmental changes, which the coverage and aboveground biomass of seagrass were significantly positively correlated with temperature, and the density of seagrass was significantly positively correlated with DO, but significantly negatively correlated with COD. In addition, due to the influence of fishery production, aquaculture activities and marine engineering, the increase of nutrient in the seawater of Wenchang will have a negative impact on the seagrass bed. In order to further analyze the effects of environmental changes on seagrass, we will expand the scope of our research and increase the frequency of monitoring, and strengthen the study on the growth and physiological level of seagrass from the interaction of multiple factors, to understand the long-term changes of seagrass in different environments, and explore the mechanism of its response to the external environmental changes.
In view of the degradation of seagrass resources in Wenchang, it is hoped that all sectors of society should pay attention to the conservation of seagrass, in particular, by rationally planning the scale of aquaculture; flexibly managing fishery activities such as raking snails and clams, appropriately reducing the frequency of digging; scientifically planning the construction of water intake projects, so as to reduce the damage to the seagrass bed.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MF: Conceptualization, Writing – original draft, Writing – review & editing. JJ: Data curation, Investigation, Writing – original draft. DW: Supervision, Writing – review & editing. GF: Funding acquisition, Supervision, Writing – review & editing. YS: Writing – review & editing. HW: Writing – review & editing. DZ: Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We acknowledge the financial support from the Comprehensive Survey of Natural Resources in “Hai Cheng Wen” Coastal Zone (DD20230414), the Comprehensive Survey of Natural Resources in Huizhou-Shanwei Coastal Zone (DD20230415).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alexandre A. Hill P. W. Jones D. L. Santos R. (2015). Dissolved organic nitrogen: A relevant, complementary source of nitrogen for the seagrass Zostera marina. Limnol. Oceanogr.60, 1477–1483. doi: 10.1002/lno.10084
2
Atkinson M. J. Smith S. V. (1983). C: N: P ratios of benthic marine plants. Limnol. Oceanogr.28, 568–574. doi: 10.4319/lo.1983.28.3.0568
3
Bertelli C. M. Creed J. C. Nuuttila H. K. Unsworth R. K. F. (2020). The response of the seagrass Halodule wrightii Ascherson to environmental stressors. Estuarine Coast. Shelf Sci.238, 106693. doi: 10.1016/j.ecss.2020.106693
4
Bertelli C. M. Unsworth R. K. F. (2018). Light stress responses by the eelgrass, zostera marina (L). Front. Environ. Sci.6. doi: 10.3389/fenvs.2018.00039
5
Burkholder J. M. Tomasko D. A. Touchette B. W. (2007). Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol.350, 46–72. doi: 10.1016/j.jembe.2007.06.024
6
Cai Z. Chen S. Wu Z. Liang D. Yin F. Tong Y. et al . (2017). Distribution differences and environmental effects of seagrasses between Bays and Lagoons of Hainan Island. Trans. Oceanol. Limnol.3), 74–84. doi: 10.13984/j.cnki.cn37-1141.2017.03.011
7
Chen S. Pang Q. Cai Z. Wu Z. Shen J. Wang D. et al . (2020). Analysis of distribution characteristics, health status, and influencing factors of seagrass bed in Lian lagoon, Hainan Island. Mar. Sci.44, 57–64. doi: 10.11759/hykx20200426004
8
Chen S. Wang D. Wu Z. Zhang G. Li Y. Tu Z. et al . (2015). Discussion of the change trend of the seagrass beds in the east coast of Hainan Island in nearly a decade. Mar. Environ. Sci.34, 48–53. doi: 10.13634/j.cnki.mes20150109
9
Collier C. J. Waycott M. (2014). Temperature extremes reduce seagrass growth and induce mortality. Mar. pollut. Bulletin.83, 483–490. doi: 10.1016/j.marpolbul.2014.03.050
10
Du H. Huang C. Dong Q. (2006). Distribution of dissolved oxygen in zhelin Bay and its relationship with nutrients. J. oceanogr. Taiwan strait.25, 188–193. doi: 10.3969/j.issn.1000-8160.2006.02.006
11
Egea L. G. Jiménez-Ramos R. Vergara J. J. Hernández I. Brun F. G. (2018). Interactive effect of temperature, acidification and ammonium enrichment on the seagrass Cymodocea nodosa. Mar. Pollut. Bulletin.134, 14–26. doi: 10.1016/j.marpolbul.2018.02.029
12
Guo Z. Huang D. Huang Z. Qi S. Yu X. (2009). Study on investigation and evolution of seagrass bed in Yelin Bay of Hainan Province. Mar. Environ. Sci.28, 706–709. doi: 10.3969/j.issn.1007-6336.2009.06.026
13
Han Q. Soissons L. M. Bouma T. J. van Katwijk M. M. Liu D. (2016). Combined nutrient and macroalgae loads lead to response in seagrass indicator properties. Mar. pollut. Bulletin.106, 174–182. doi: 10.1016/j.marpolbul.2016.03.004
14
Han Q. Soissons L. M. Liu D. van Katwijk M. M. Bouma T. J. (2017). Individual and population indicators of Zostera japonica respond quickly to experimental addition of sediment-nutrient and organic matter. Mar. pollut. Bulletin.114, 201–209. doi: 10.1016/j.marpolbul.2016.08.084
15
Han Q. Zeng W. Ye J. Qiu C. Shi Y. Zhao M. (2023). Characteristics of morphological and physiological indicators of Thalassia hemprichii and environmental factors in Xincun Bay, Haiman Island. Prog. Fishery Sci.44, 225–238. doi: 10.19663/j.issn2095-9869.20230330001
16
He B. Wei M. Li Z. (2012). Relations of water self-purify ability with water dynamics, biological and chemical factor in sea grass bed ecosystem in Tieshan Bay. Mar. Environ. Sci.31, 662–666, 673.
17
Höffle H. Thomsen M. S. Holmer M. (2011). High mortality of Zostera marina under high temperature regimes but minor effects of the invasive macroalgae Gracilaria vermiculophylla. Estuarine Coast. Shelf Sci.92, 35–46. doi: 10.1016/j.ecss.2010.12.017
18
Howard J. L. Lopes C. C. Wilson S. S. McGee-Absten V. Carrión C. I. Fourqurean J. W. (2020). Decomposition rates of surficial and buried organic matter and the lability of soil carbon stocks across a large tropical seagrass landscape. Estuaries Coasts44, 846–866. doi: 10.1007/s12237-020-00817-x
19
Huang C. Zhang J. Jiang Z. Huang X. (2017). Nutrients uptake processes of seagrass and its competition with epiphytic algae. J. Fisheries Res.39, 222–228. doi: 10.14012/j.cnki.fjsc.2017.03.009
20
Invers O. Romero J. Pérez M. (1997). Effects of pH on seagrass photosynthesis: a laboratory and field assessment. Aquat. Botany59, 185–194. doi: 10.1016/s0304-3770(97)00072-7
21
Jiang Z. Huang D. Fang Y. Cui L. Zhao C. Liu S. et al . (2020). Home for marine species: Seagrass leaves as vital spawning grounds and food source. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00194
22
Jiang Z. Huang X. Zhang J. (2012). Effect of environmental stress on non-structural carbohydrates reserves and transfer in seagrasses. Acta Ecologica Sinica32, 6242–6250. doi: 10.5846/stxb201108311275
23
Jiang Z. Huang X. Zhang J. (2013). Effect of nitrate enrichment and salinity reduction on the seagrass Thalassia hemprichii previously grown in low light. J. Exp. Mar. Biol. Ecol.443, 114–122. doi: 10.1016/j.jembe.2013.02.034
24
Kahn A. E. Durako M. J. (2006). Thalassia testudinum seedling responses to changes in salinity and nitrogen levels. J. Exp. Mar. Biol. Ecol.335, 1–12. doi: 10.1016/j.jembe.2006.02.011
25
Lee K. S. Park S. R. Kim Y. K. (2007). Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. J. Exp. Mar. Biol. Ecol.350, 144–175. doi: 10.1016/j.jembe.2007.06.016
26
Liu P.-J. Ang S.-J. Mayfield A. B. Lin H.-J. (2020). Influence of the seagrass Thalassia hemprichii on coral reef mesocosms exposed to ocean acidification and experimentally elevated temperatures. Sci. Total Environment700, 134464. doi: 10.1016/j.scitotenv.2019.134464
27
Liu W. Han. Q. Tang Y. Sun X. (2017). Review of nutrient enrichment and global warming effects on seagrasses. Chin. J. Ecol.36, 1087–1096. doi: 10.13292/j.1000-4890.201704.027
28
Liu S. Jiang Z. Wu Y. Zhang J. Zhao C. Huang X. (2017). Mechanisms of sediment carbon sequestration in seagrass meadows and its responses to eutrophication (in Chinese). Chin. Sci. Bulletin.62, 3309–3318. doi: 10.1360/N972017-00376
29
Liu S. Jiang Z. Zhang J. Wu Y. Lian Z. Huang X. (2016). Effect of nutrient enrichment on the source and composition of sediment organic carbon in tropical seagrass beds in the South China Sea. Mar. pollut. Bulletin.110, 274–280. doi: 10.1016/j.marpolbul.2016.06.054
30
Liu S. Trevathan-Tackett S. M. Jiang Z. Cui L. Wu Y. Zhang X. et al . (2022). Nutrient loading decreases blue carbon by mediating fungi activities within seagrass meadows. Environ. Res.212, 113280. doi: 10.1016/j.envres.2022.113280
31
Marbà N. Santiago R. Díaz-Almela E. Álvarez E. Duarte C. M. (2006). Seagrass (Posidonia oceanica) vertical growth as an early indicator of fish farm-derived stress. Estuarine Coast. Shelf Sci.67, 475–483. doi: 10.1016/j.ecss.2005.11.034
32
McKenzie L. J. Yoshida R. L. Aini J. W. Andréfouet S. Colin P. L. Cullen-Unsworth L. C. et al . (2021). Seagrass ecosystem contributions to people’s quality of life in the Pacific Island countries and territories. Mar. pollut. Bulletin.167, 203–223. doi: 10.1016/j.marpolbul.2021.112307
33
Neckles H. A. Short F. T. Barker S. Kopp B. S. (2005). Disturbance of eelgrass Zostera marina by commercial mussel Mytilus edulis harvesting in Maine: dragging impacts and habitat recovery. Mar. Ecol. Prog. Series285, 57–73. doi: 10.3354/MEPS285057
34
Palacio-López K. Beckage B. Scheiner S. Molofsky J. (2015). The ubiquity of phenotypic plasticity in plants: a synthesis. Ecol. Evolution5, 3389–3400. doi: 10.1002/ece3.1603
35
Palacios S. Zimmerman R. (2007). Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Mar. Ecol. Prog. Series344, 1–13. doi: 10.3354/meps07084
36
Scott A. L. York P. H. Duncan C. Macreadie P. I. Connolly R. M. Ellis M. T. et al . (2018). The role of herbivory in structuring tropical seagrass ecosystem service delivery. Front. Plant Sci.9. doi: 10.3389/fpls.2018.00127
37
Short F. T. Polidoro B. Livingstone S. R. Carpenter K. E. Bandeira S. Bujang J. S. et al . (2011). Extinction risk assessment of the world’s seagrass species. Biol. Conserv.144, 1961–1971. doi: 10.1016/j.biocon.2011.04.010
38
Suykerbuyk W. Govers L. van Oven W. G. Giesen K. Giesen W. B. J. T. de Jong D. J. et al . (2018). Living in the intertidal: Desiccation and shading reduce seagrass growth but high salinity or population of origin have no additional effect. PeerJ.6, 52341. doi: 10.7717/peeri.5234
39
Terrados J. Duarte C. Kamp-Nielsen L. Agawin N. S. Gacia E. Lacap D. et al . (1999). Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquat. Botany65, 175–197. doi: 10.1016/s0304-3770(99)00039-x
40
Wang D. Wu Z. Chen C. Lan J. Wu R. Chen X. et al . (2012). Distribution of seagrass resources and existing threat in Hainan Island. Mar. Environ. Sci.31, 34–38. doi: 10.3969/j.issn.1007-6336.2012.01.008
41
Wu Y. Jiang Z. Liu S. Zhang J. Lian Z. Huang X. (2017). Effects of salinity on the photodegradation of chromophoric dissolved organic matter (CDOM) released by seagrass Enhalus acoroides and Thalassia hemprichii leaf litter. Chin. J. Ecol.36, 687–694. doi: 10.13292/j.1000-4890.201703.012
42
Xu B. Zhang J. Lang S. Chen S. Wu Z. Wang D. (2022). Ecological status and degradation factors in seagrass beds along the coast of Wenchang, Hainan. J. Appl. Oceanogr.41, 614–624. doi: 10.3969/J.ISSN.2095-4972.2022.04.007
43
Zhang J. Xu B. Lang S. Chen S. Wu Z. (2022). Resource changes of macroalgae and analysis of their influencing actors in Wenchang coast of Hainan province. Trans. Oceanol. Limnol.4, 9–138. doi: 10.13984/j.cnki.cn37-1141.2022.03.018
44
Zheng F. Qiu G. Fan H. Zhang W. (2013). Diversity, distribution and conservation of Chinese seagrass species. Biodiversity Sci.21, 517–526. doi: 10.3724/SP.J.1003.2013.10038
45
Zhong C. Sun K. Liao Y. Qi S. Chen Q. Yin Q. et al . (2019). Distribution status of seagrass and its relationship with different habitat types in Liusha bay of Guangdong province. Mar. Environ. Sci.38, 521–527. doi: 10.13634/j.cnki.mes.2019.04.006
46
Zhu Z. Ma K. Fang Z. Cai Z. Chen S. (2017). Distribution of seagrass resources of Eucheuma nature reserve of Hainan province and its protection suggestions. Guangdong Agric. Sci.44, 90–98. doi: 10.16768/j.issn.1004-874X.2017.04.014
Summary
Keywords
seagrass beds, community characteristics, environmental characteristics, community status, assessment index
Citation
Fu M, Jiang J, Wang D, Fu G, Song Y, Wang H and Zhang D (2024) Assessment of the community status of seagrass bed and its relationship with environmental characteristics in Wenchang, Hainan Island, China. Front. Mar. Sci. 11:1433104. doi: 10.3389/fmars.2024.1433104
Received
15 May 2024
Accepted
12 August 2024
Published
29 August 2024
Volume
11 - 2024
Edited by
Charitha Bandula Pattiaratchi, University of Western Australia, Australia
Reviewed by
Shiquan Chen, Hainan Academy of Ocean and Fisheries Sciences, China
Jennifer Li Ruesink, University of Washington, United States
Updates
Copyright
© 2024 Fu, Jiang, Wang, Fu, Song, Wang and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dacheng Wang, wangdacheng@mail.cgs.gov.cn; Guowei Fu, fuguowei@mail.cgs.gov.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.