- 1Focal Species Conservation Program, Ocean and Science Department, Konservasi Indonesia, Jakarta, Indonesia
- 2Independent Researcher, Kaimana, Indonesia
- 3Research Center for Oceanography, National Research and Innovation Agency, Jakarta, Indonesia
- 4Independent Researcher, Boyolali, Indonesia
- 5Sahul Papua Ecoregion, Konservasi Indonesia, Sorong, Indonesia
- 6Sunda Banda Ecoregion, Konservasi Indonesia, Denpasar, Indonesia
- 7Marine Program, Conservation International Asia-Pacific, Auckland, New Zealand
A comprehensive understanding of cetacean ecology is crucial for conservation and management. In 2018, Kaimana was identified as an Important Marine Mammal Area (IMMA) due to the regular presence of feeding aggregations of Australian humpback dolphins (Sousa sahulensis), Pacific bottlenose dolphins (Tursiops aduncus) and Bryde's whales (Balaenoptera edeni). Despite this, information on cetacean ecology in the Kaimana region is currently lacking. Notably, no cetacean surveys have been undertaken in Kaimana since it was officially recognized as an IMMA. We monitored food-provisioning interactions between lift-net fisheries and cetaceans from May 2021 to March 2023 to examine cetacean sightings, abundance and feeding associations. Five species were positively identified, including a new record of Killer whales (Orcinus orca). Our findings suggest a strong association between T. aduncus and lift-net fisheries, where they have been observed feeding on anchovies from outside the net in the morning. While other species were also observed, their presence was less frequent. Furthermore, year-round sightings of S. sahulensis, B. edeni, and T. aduncus during the study period indicate that these species are resident in this region. Our results suggest that Kaimana fulfills a second IMMA sub-criterion (small and resident populations of these three species) that was not previously noted in the original IMMA assessment.
1 Introduction
Cetaceans, comprised of whales, dolphins, and porpoises, play a vital role in the complex structure and function of coastal ecosystems through a variety of mechanisms over both ecological and evolutionary time (Kiszka et al., 2015) and are generally considered as a crucial indicator of ocean health (Cossaboon et al., 2019; Fossi et al., 2020). Their role in top-down population control of some prey species (Williams et al., 2004; MacLeod et al., 2007), nutrient cycling (Gilbert et al., 2023), and carbon sequestration (Sheehy et al., 2022; Pearson et al., 2023) is critical for the stability of these ecosystems. Through their extensive movements, cetaceans contribute to nutrient redistribution via their excrement, fostering a dynamic environment that supports diverse marine life (Doughty et al., 2016). Unfortunately, various human activities and their impacts, including fishing operations (Reeves et al., 2005; Read, 2008; Reeves et al., 2013), military sonar exercises (Barlow and Gisiner, 2005; Henderson et al., 2014), ship strikes (Pennino et al., 2017), habitat loss and degradation (Weir and Pierce, 2013), and the accumulation of marine debris (Williams et al., 2011) and other pollutants, pose threats to cetacean populations and their habitats. Therefore, monitoring cetacean populations, particularly within their critical habitats, is imperative. This effort is not only crucial for comprehending the status of cetacean populations, but also provides essential insights into the overall health of marine ecosystems (Wells et al., 2004).
Indonesia has a diverse range of cetacean species, with 34 different species recorded to date (Mustika et al., 2015). Indonesia was a major whaling area for large whales during the Yankee whaling era, which lasted from the 18th to the early 20th centuries (Townsend, 1935; Sahri et al., 2020a). However, research on cetaceans in the country has been scarce, especially in remote areas (Ender et al., 2014). Although some information regarding Indonesian cetaceans is available in unpublished internal reports, there is still a significant gap in knowledge of the ecology of these animals, hindering the government from developing effective conservation plans (Sahri et al., 2020b).
In 2018, a regional workshop on Important Marine Mammal Areas for the Northeast Indian Ocean and Southeast Asian Seas (NIO-SEAS) was conducted with the primary objectives of identifying and delineating Important Marine Mammal Areas (IMMAs) in the region. IMMAs pinpoint specific habitat areas crucial for one or more marine mammal species, with the potential for delineation and conservation management (Tetley et al., 2022). Scientific experts identified these areas during regional workshops and assessed the areas against eight criteria that encapsulate essential aspects of marine mammal biology, ecology, and population structure (Tetley et al., 2022). Kaimana, along with 29 other areas, was approved as an IMMA in the NIO-SEAS region (IUCN Marine Mammal Protected Areas Task Force, 2019). At the time of its proposal, Kaimana IMMA was only recognized as important habitat for aggregation sites and feeding areas of ‘Vulnerable’ Australian humpback dolphins (Sousa sahulensis), ‘Near Threatened’ Indo-Pacific bottlenose dolphins (Tursiops aduncus), and ‘Least Concern’ Bryde’s whales (Balaenoptera edeni). Moreover, the Kaimana IMMA is also home for ‘Vulnerable’ dugongs (Dugong dugon), although it remains unclear regarding how this species utilizes the area for its key life-cycle activities (IUCN-MMPATF, 2022a).
An early cetacean survey in the Kaimana IMMA was initiated in 2009 and subsequently continued in 2015, prior to the recognition of this region as an IMMA. During a dedicated nine-day Marine Mammal Rapid Ecological Assessment conducted in Triton Bay (Kahn, 2009), six marine mammal species were identified, including the Bryde’s whale (Balaenoptera edeni), Indo-Pacific bottlenose dolphin (Tursiops aduncus), Australian humpback dolphin (Sousa sahulensis), Pantropical spotted dolphins (Stenella attenuata), Spinner dolphin (Stenella longirostris), and the Dugong (Dugong dugon). In more recent cetacean surveys in Kaimana IMMA, specifically in Arguni Bay located in North Kaimana (Wijaya, 2015), a comprehensive 90-day visual observation effort revealed the presence of 64 groups of Australian humpback dolphins and 40 groups of bottlenose dolphins (Tursiops sp.). It is important to note that, to date, no cetacean surveys have been conducted in Kaimana IMMA after its official recognition in 2018. This indicates a substantial gap in our understanding, particularly given the critical need for continuous population monitoring, especially through time-series studies. The establishment of a cetacean survey initiative is vital for sustaining ongoing conservation endeavors and achieving a thorough insight into the ecological trends of cetacean populations, particularly following the recognition of the Kaimana IMMA.
Line transect distance sampling and other dedicated cetacean surveys are predominantly intended to generate accurate population abundance estimates, necessitating substantial financial resources and extensive coverage (Buckland et al., 2001; Sahri et al., 2020c). In contrast, opportunistic observations provide a more cost-effective alternative by focusing on aggregation sites where cetaceans are consistently observed, including in areas frequently associated with fisheries operations (Goetz et al., 2015; Tobeña et al., 2016). Instead of focusing on estimating population numbers, this study takes another approach to provide baseline data on cetacean presence and interactions with fisheries.
This study leveraged the food-provisioning interactions between lift-net fisheries (locally known as “bagan”) and cetaceans in Kaimana from May 2021 to March 2023. The primary objective of this survey was to provide ecological insights into cetacean diversity, feeding associations, as well as the seasonal variations of cetacean sightings and relative abundance. We also discussed the advantages and limitations of using observation platforms from boat lift-net fisheries for cetacean data collection.
2 Materials and methods
2.1 Site description
Kaimana is situated in the southwestern part of West Papua, Indonesia, and is is an important part of the Bird's Head Seascape MPA Network and the Bird’s Head Seascape (BHS) (Setyawan et al., 2022). This area is located at the heart of the Coral Triangle, renowned for its extraordinary coral reef and marine biodiversity, marking it as a top conservation priority (White et al., 2014). Furthermore, it maintains a continuous shallow-water ecological connection to the Australian continent, through the Sahul Shelf. The Sahul Shelf extends northwest from northern Australia towards Timor under the Timor Sea, while another segment spans from Australia’s north coast under the Arafura Sea to West Papua, New Guinea (Lohman et al., 2011; Vernon et al., 2009). Thus, biogeographically, Kaimana shares greater affinities with Northern Australia and Papua New Guinea rather than the larger islands of eastern Indonesia, evidenced by the presence of coastal species, such as the Australian humpback dolphin (Lohman et al., 2011; Beasley et al., 2016).
The Kaimana IMMA contains of diverse habitats, including expansive riverine, coastal, and estuarine mangroves in Arguni Bay, as well as coral islands and deep, narrow oceanic passages in the Iris and Namatota Straits of Triton Bay (Figure 1). Additionally, the Seram Sea Canyon, with a depth of 2,000 meters, is located approximately 10 km southwest of Triton Bay. The Kaimana region, spanning 597,000 hectares, has been designated as a multiple-use Locally Managed Marine Area (IUCN Management Category VI) in 2008.
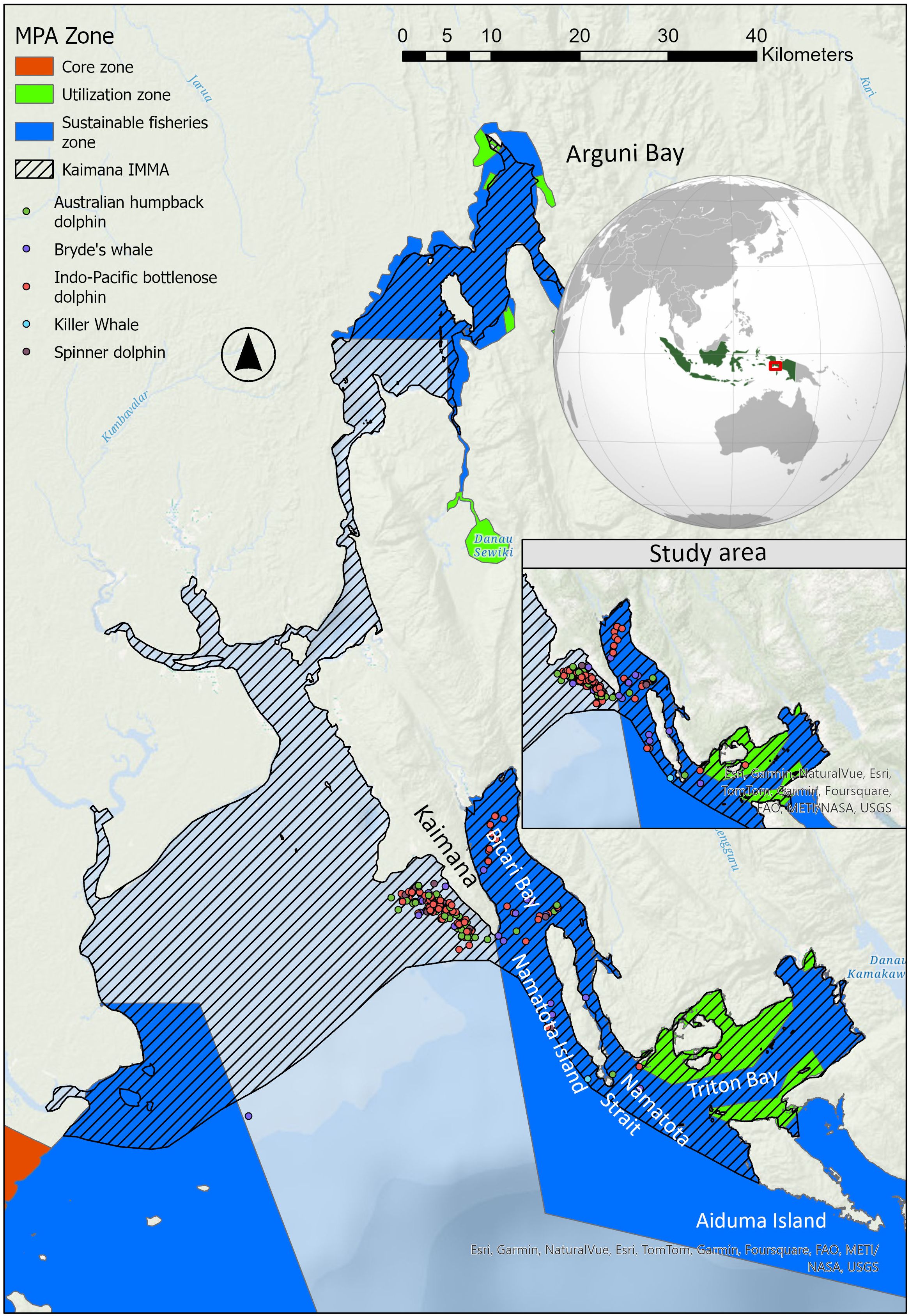
Figure 1. Study area of cetacean observations at the boat lift net fishery in Kaimana Important Marine Mammal Area during the study period.
2.2 Data collection
Observations of cetaceans within the Kaimana IMMA were conducted from May 2021 to March 2023. These surveys were conducted alongside whale shark (Rhincodon typus) monitoring, and the focus of each survey was to document occurrences of whale sharks and cetaceans around the lift-net fishery (Figure 2). Cetacean observations took place between 5:00 AM and 6:30 PM. The lift-net fishery typically wrapped up operations around 5–6 AM. However, when the catch was abundant, not all the fish-filled nets were hauled up and stored in cool boxes, with some nets left submerged and still holding fish, which would often attract whale sharks and dolphins. Observations took place throughout the day.

Figure 2. Multi-species interactions between lift net fishery operations with megafauna including whale sharks and cetaceans in Kaimana IMMA, where these species opportunistically forage. (A, D, F) Indo-Pacific bottlenose dolphin, (B) Bryde’s whale, (C) Australian humpback dolphin, (E) Whale shark and Indo-Pacific bottlenose dolphin.
The survey focused on opportunistic sightings around lift-net fisheries operating in various locations, including Kaimana City, Bicari Bay, Namatota Island, Triton Bay, and Aiduma Island (Figure 1). During the observations, key ecological data were systematically gathered, including trip ID, date, GPS coordinates, sighting location, survey duration (effort), sighting time, species identification, species behavior, and an approximate estimation of abundance. Furthermore, feeding behavior is defined as the direct observation of a dolphin feeding on anchovies surrounding a lift-net fishing platform, whereas non-feeding behavior is described as a dolphin’s behavior solely cruising the observation area without engaging in feeding activities.
The lift-net fishery utilizes artificial lights and lift nets as its main gear and technique (Sianipar, 2022). Bright lights were employed at night to attract baitfish such as anchovies, sergestid shrimps, and other small pelagic species (Salman et al., 2015). Consequently, this practice attracts higher tropic level animals such as whale sharks and cetaceans to aggregate around the lift-net fishing boat, where they have been observed feeding on anchovies from outside the net. This unique interaction was first documented in Cenderawasih Bay in 2006 (Sianipar, 2022) and has since been observed in various locations across Indonesia, including Kaimana, Talisayan-Berau in East Kalimantan, and Saleh Bay in West Nusa Tenggara (Djunaidi et al., 2019; Sianipar, 2022). This interaction has created additional opportunities for cetacean observations, aside from whale shark monitoring.
2.3 Data analysis
Sightings per unit effort (SPUE) and abundance per unit effort (APUE) serve as metrics for quantifying the frequency of sightings and the abundance of individuals, respectively, within a specific area over the study period. The calculations involved dividing the number of sightings (for SPUE) or the total count of individuals (for APUE) by the duration of the observation period conducted on the respective day.
The used of SPUE and APUE establishes a standardized measurement that considers the effort invested during observations. Through the standardization of data based on the time allocated to observe, these metrics provide a more precise representation of the sighting cetacean abundance. This standardization process accommodated variations in observation durations, facilitating meaningful comparisons across different observation sessions. As a result, these metrics contributed to a more dependable assessment of the ecology of species in the studied area. The nonparametric Kruskal-Wallis H test, followed by post-hoc Dunn’s test were employed to assess whether statistically significant differences exist in SPUE and APUE across various factors such as species and seasonal variations. Additionally, the Mann-Whitney U test was used to assess whether the presence of species was linked to feeding behavior in the proximity of lift net fishery activities. Based on our observations at the lift-net fishery platforms, cetacean presence generally falls into two categories. The first involves direct sightings of cetaceans feeding around the lift-net area, which we categorize as feeding behavior. The second category includes non-feeding observations, such as traveling, aerials, spyhopping, and lobtailing. These behaviors suggest that the dolphins may be in the area for play or social interaction rather than feeding.
3 Results
From May 2021 to March 2023, a total of 111 days were spent conducting cetacean observations in the Kaimana IMMA. During these surveys, the average duration of cetacean search activities was (mean ± SD) 5.55 ± 1.39 hours/day. The year 2022 was the peak of the survey effort, with 69 survey days and an average search effort of 5.44 ± 0.95 hour/day. This maximum effort resulted in the documentation of approximately 185 cetacean sightings, with a total of 2,585 individuals recorded. In contrast, 2023 was the lowest year in survey effort, lasting only 15 days with an average search effort of 6.44 ± 1.85 hour/day, documenting 24 cetacean sightings with a total of 387 individuals (Figure 3). The number of months during which observations were conducted varied significantly between years, leading to the differences in survey effort and sightings. Cetacean sightings demonstrated daily fluctuations, occurring between the hours of 5:45 a.m. and 4:45 p.m., with a significant peak between 6-9 AM, during which fully 210 of the 265 sighting events (79.24%) occurred. Cetacean sightings then dropped off significantly from 10 AM onwards.

Figure 3. Summary of monthly cetacean survey efforts (A) survey days, (B) mean and SD of search effort, (C) cetacean sightings, and (D) cumulative individual recorded in Kaimana Important Marine Mammal Area. Northwest monsoon (NWM): December–February, Transition 1 (intermonsoon period): March–May, Southeast monsoon (SEM): June–August, Transition 2 (intermonsoon period): September–November.
3.1 Species diversity
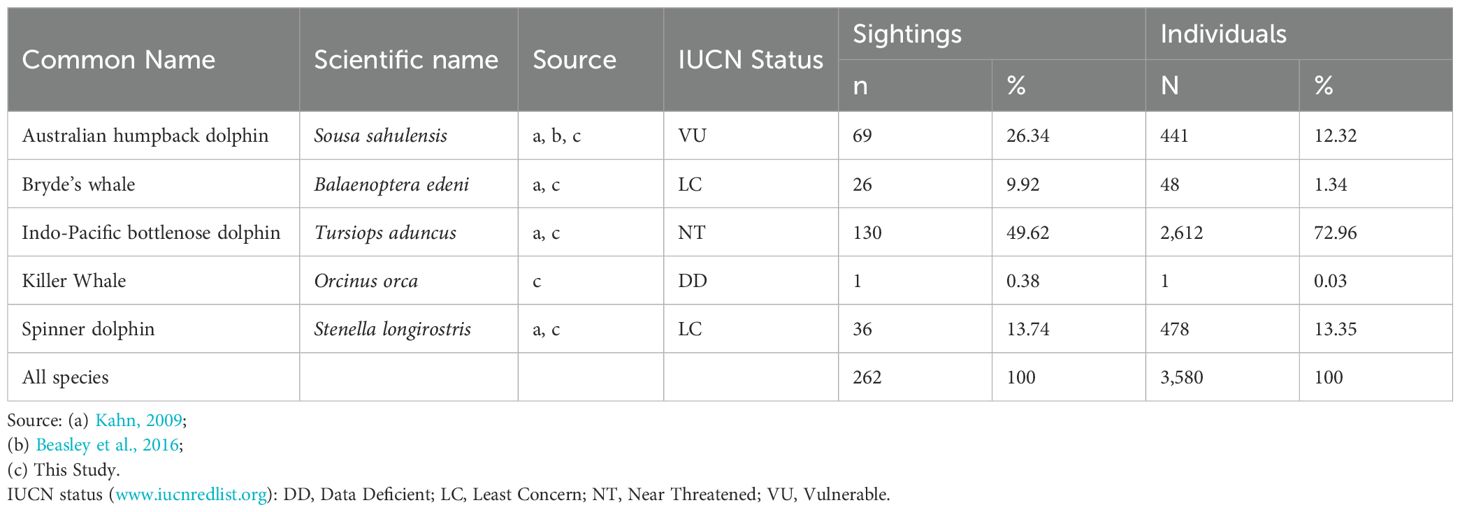
Five species were positively identified in the Kaimana IMMA during this study (Figure 4), including Bryde’s whale (Balaenoptera edeni), Killer whale (Orcinus orca), Australian humpback dolphin (Sousa sahulensis), Spinner dolphin (Stenella longirostris), and Indo-Pacific bottlenose dolphin (Tursiops aduncus). Of the five species, killer whales represented a new record, having never before been reported from the Kaimana IMMA. These species were listed as “Least Concern” (two species), “Data Deficient” (one species), “Near Threatened” (one species), and “Vulnerable” (one species) in the IUCN Red List of Threatened species, as detailed in Table 1. The most frequently sighted species was the Indo-Pacific bottlenose dolphin (130 sightings) representing 49.62% of all cetacean sightings, with 2,612 individuals recorded, accounting for 72.96% of all individuals observed.

Figure 4. Five cetacean species that were identified during the study period. (A) Killer whale (Orcinus orca), (B) Spinner dolphin (Stenella longirostris), (C) Bryde’s whale (Balaenoptera edeni), (D) Australian Humpback dolphin (Sousa sahulensis), (E) Indo-Pacific bottlenose dolphin (Tursiops aduncus).
3.2 Feeding behavior
The only species demonstrating a higher occurrence of feeding behavior than non-feeding behavior was the Indo-Pacific bottlenose dolphin (Z = 2.67, U = 4,302.5, p < 0.05; Table 2). In contrast, Spinner dolphins predominantly engaged in non-feeding behavior, as indicated by the statistically significant findings (Z = -2.27, U = 208.5, p < 0.05). Meanwhile, the remaining 3 species showed no statistically significant difference in the prevalence of feeding and non-feeding behaviors (Table 2).
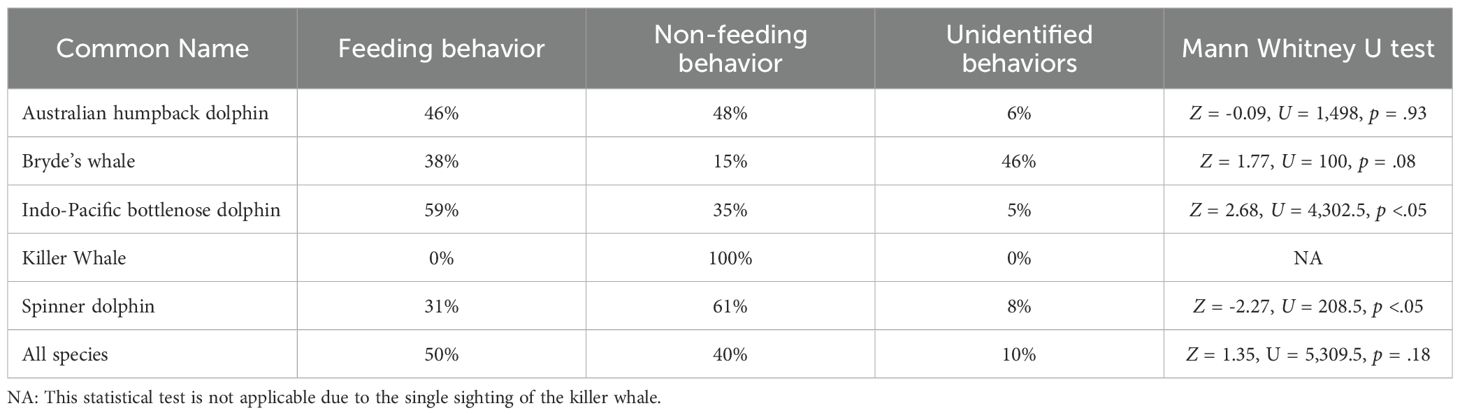
Table 2. Summary of statistical tests assessing feeding and non-feeding behaviors observed in cetacean sightings over the study period.
3.3 Sightings and abundance per unit effort
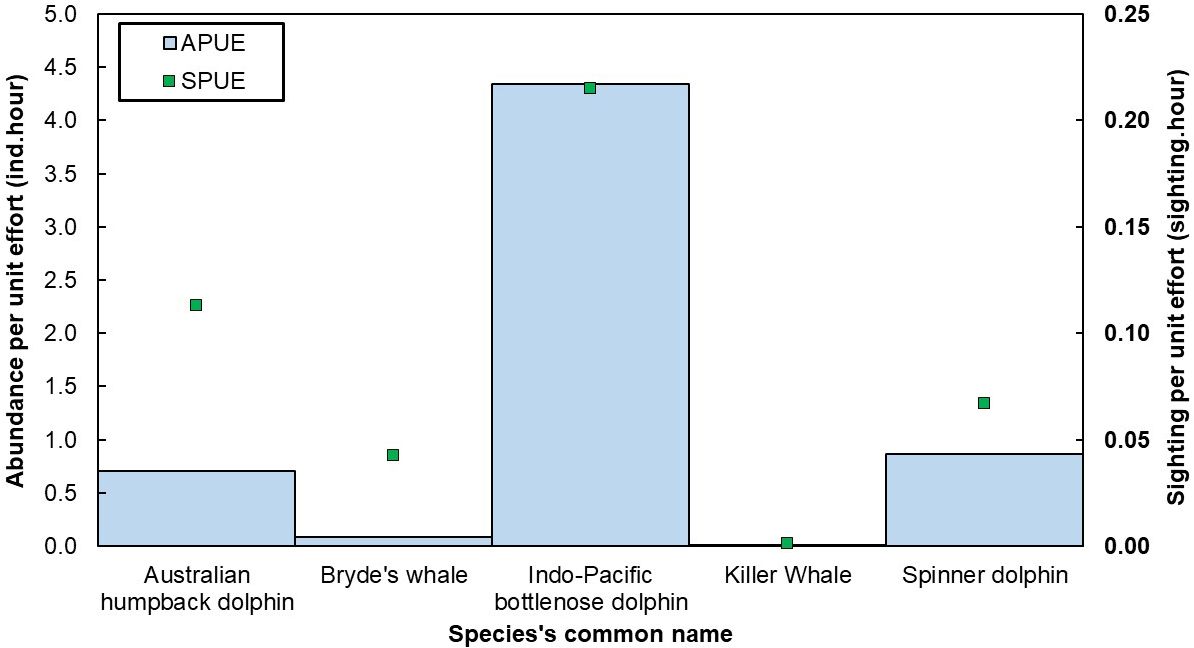
Sightings per unit effort (SPUE) of cetaceans demonstrated that the Indo-Pacific bottlenose dolphin had the highest average (χ2 = 185.7, df = 4, p < 0.001) number of sightings per hour (mean ± SE, 0.22 ± 0.01, Figure 5). Similar with the SPUE, Indo-Pacific bottlenose dolphin also had the highest APUE (χ2 = 230.4, df = 4, p < 0.001) in individuals per hour (4.30 ± 0.36). The post-hoc Dunn’s test highlighted that the Indo-Pacific bottlenose dolphin and Killer whale exhibited the most considerable mean rank differences in SPUE and APUE, with a value of 230.44 (z = 12.50) and 255.10 (z = 13.83), respectively. Additionally, a statistically significant positive relationship was observed between SPUE and APUE, with an r2 of 0.61, F(1,110) = 169.65, and p < 0.001.
3.4 Seasonal trends
The cetacean observations conducted using opportunistic platforms from the boat lift net fishery in the Kaimana IMMA have consistently shown that cetaceans were regularly sighted in the region (Figure 6). The highest SPUE occurred during the Transition 1 intermonsoon period (March-May; mean ± SE, 0.51 ± 0.06), followed by the Transition 2 intermonsoon period (September-November; 0.46 ± 0.05), northwest monsoon (0.40 ± 0.04), and southeast monsoon (0.23 ± 0.04; Figure 6A). However, there was no statistically significant difference in SPUE among the different seasons (χ2 = 7.05, df= 3, p = 0.07).
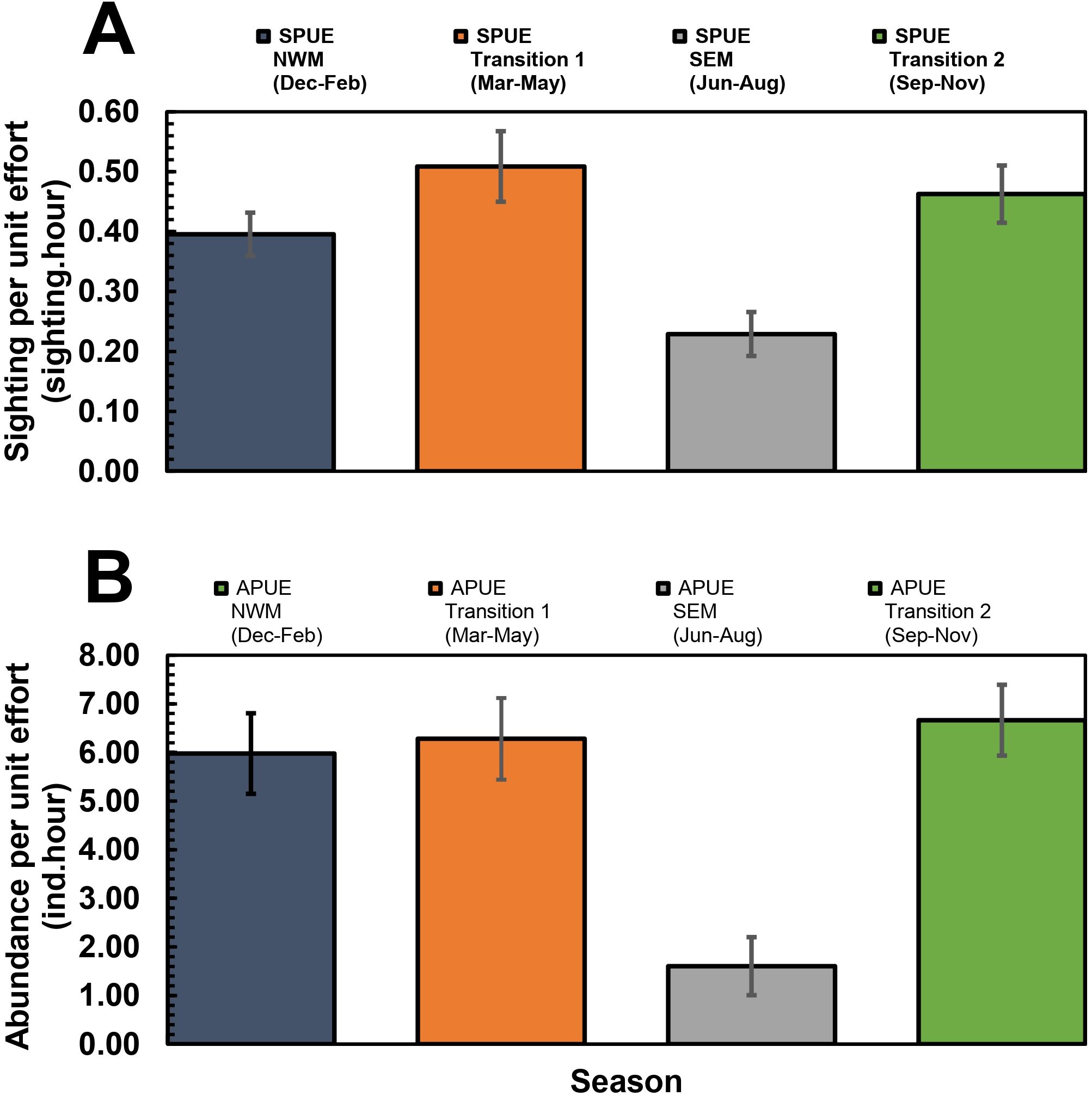
Figure 6. Seasonal trends in cetacean sightings (SPUE; A) and relative abundance (APUE; B) in Kaimana IMMA. The error bars represent the standard error. NWM, northwest monsoon; SEM, southeast monsoon.
In contrast to SPUE, the Kruskal-Wallis H test indicated that there was a significant difference in APUE among the different seasons, (χ2 = 10.44, df= 3, p = 0.015), suggesting the differences during the Southeast monsoon. The post-hoc Dunn’s test indicated that the mean ranks of individuals per hour in the southeast monsoon and Transition 2 intermonsoon period (Mean Rank difference = -42.84, z= 3.21), Transition 1 intermonsoon period and the southeast monsoon (Mean Rank difference = 37.87, z= 2.85), and northwest monsoon and southeast monsoon (Mean Rank difference = 35.77, z= 2.67) were significantly different. The highest APUE, measured in individuals per hour, was observed during the Transition 2 (6.66 ± 0.73), followed by Transition 1 (6.28 ± 0.84), northwest monsoon (5.98 ± 0.83), and Southeast monsoon (1.60 ± 0.60).
Our examination of seasonal patterns demonstrates the year-round presence of Australian humpback dolphins, Bryde’s whales, and Indo-Pacific bottlenose dolphins, although some species show fluctuations in SPUE and APUE across different seasons. Spinner dolphins and Killer whales were notable for being the only two species recorded consistently across different seasons and had statistically no significant difference in both SPUE (χ2 = 4.33, df = 3, p = 0.228) and (χ2 = 2.39, df =3, p = 0.495), respectively, both APUE (χ2 = 4.47, df = 3, p = 0.215) and (χ2 = 2.39, df = 3, p = 0.495), respectively, across different seasons (Figure 7).
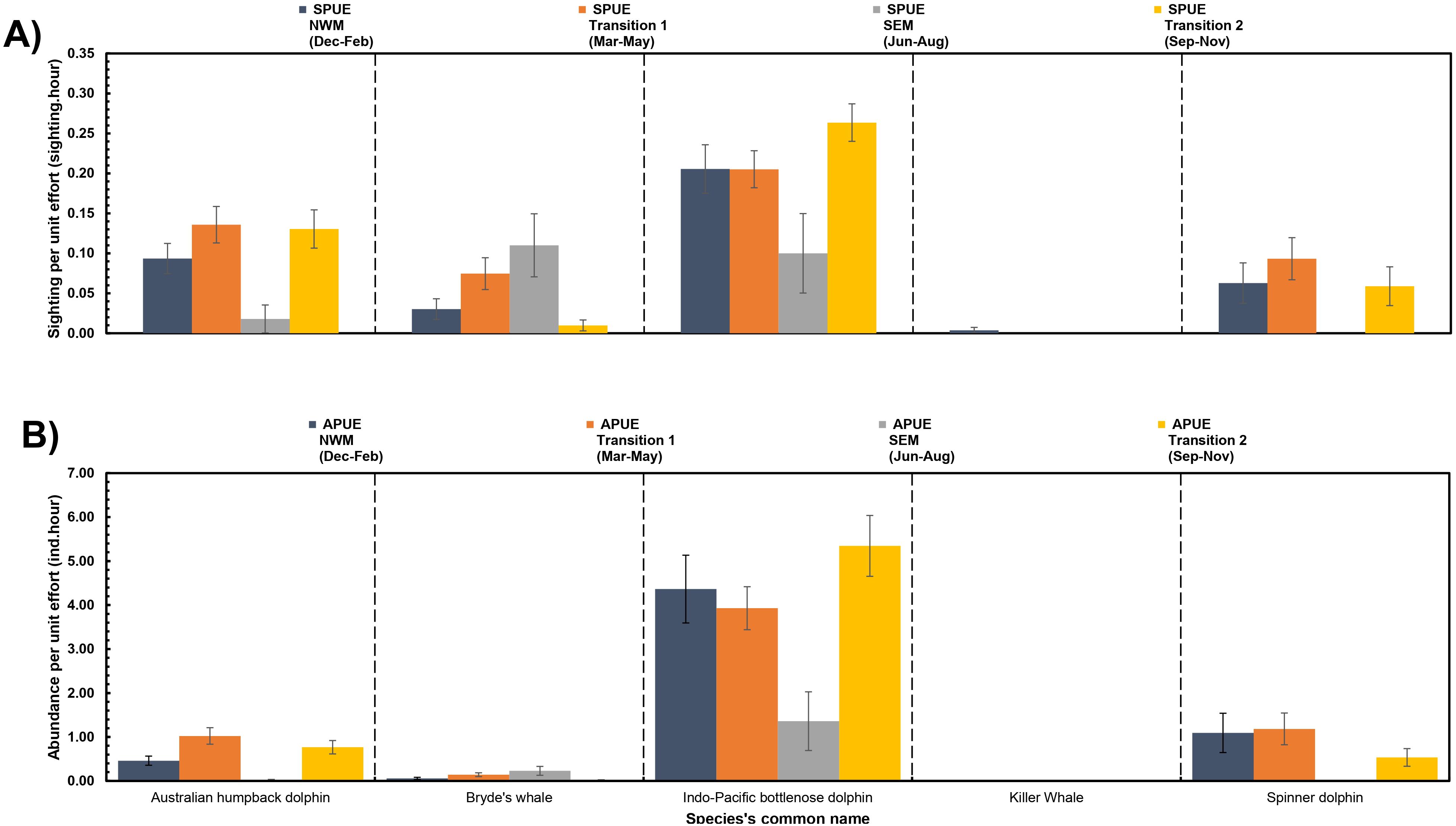
Figure 7. Seasonal trends in cetacean sightings (SPUE; A) and relative abundance (APUE; B) for each species in Kaimana IMMA. The error bars represent the standard error. NWM: northwest monsoon, SEM: southeast monsoon.
Although there were no significant differences in SPUE for Australian humpback dolphins (χ2 = 6.74, df = 3, p = 0.081), a significant difference was observed in APUE (χ2 = 9.55, df = 3, p = 0.023), particularly during the transition from Transition 1 intermonsoon period (1.02 ± 0.19 individuals per hour) to the southeast monsoon (0.02 ± 0.02 individuals per hour). Meanwhile, there was a significant difference in SPUE for Indo-Pacific bottlenose dolphin among seasons (χ2 = 8.02, df = 3, p = 0.046), but there was no significant difference in APUE (χ2 = 7.22, df = 3, p = 0.065). Lastly, only Bryde’s whale showed significant differences in both SPUE (χ2 = 14.21, df = 3, p = 0.003) and APUE (χ2 = 14.7, df = 3, p = 0.01), particularly between Transition 1 and Transition 2, as well as the southeast monsoon and Transition 2.
4 Discussion
This is the first study in Asia that uses lift net fishing boats as platforms for cetacean observations in order to give insights into cetacean diversity and feeding behaviors, as well as variations in sightings and relative abundance. We recognize that relying on lift-net fisheries as observation platforms may introduce bias into certain aspects of cetacean ecology discussed in this study. For instance, species diversity may be underrepresented, as observations were restricted to locations where these platforms operate, predominantly in coastal areas, potentially excluding oceanic species. Additionally, adverse weather conditions limiting fishing activities also restricted survey efforts, leading to an incomplete understanding of temporal patterns of cetacean presence. Therefore, these patterns should be interpreted as specific to cetacean interactions with lift-net fisheries. Further independent cetacean observation studies are necessary to better understand their occurrence within the Kaimana IMMA.
Over the study period, Indo-pacific bottlenose dolphins were the most sighted species around lift net fishery operated in Kaimana IMMA. Cetacean aggregations linked to the lift net fishery in Kaimana IMMA were found to be most sighted in the front of Kaimana city compared to Bicari Bay, Namatota, and Triton Bay (Figure 1). Kaimana IMMA is a significant area for the aggregation and feeding activities of the Australian humpback dolphin, Bryde’s whale, and Indo-Pacific bottlenose dolphin (IUCN-MMPATF, 2022a). Specifically, our study found that the Indo-pacific bottlenose dolphin had a strong relationship with lift net fisheries as shown from their feeding activity around the lift net, especially in the morning. Furthermore, this study offers a novel perspective that Kaimana IMMA met another sub-criterion not previously reported in the initial IMMA assessment i.e., related to small and resident populations (sub-criterion B1 in IMMA assessment; IUCN Marine Mammal Protected Areas Task Force (2022); Tetley et al., 2022). Australian humpback dolphins, Bryde's whales, and Indo-Pacific bottlenose dolphins were consistently recorded in this region in every season and every year of the study period; although further research is needed to confirm their individual residency through mark-recapture using photo-identification (Chilvers and Corkeron, 2003; Fearnbach et al., 2012; Boyd and Punt, 2021).
4.1 Species diversity
The Kaimana region in the southern Bird Head Seascape is recognized as a global marine biodiversity hotspot (Mangubhai et al., 2012). Characterized by numerous islands separated by deep passages, such as the Iris and Namatota Straits in Triton Bay, this region experiences strong tidal currents (Kahn, 2009), which is likely partly responsible for the high abundance of cetacean species in Triton Bay. However, compared to other regions in Indonesia, marine mammal diversity in the Kaimana IMMA (spanning 2,173 sq km) does not meet the criteria for an area with high diversity of cetacean species (criterion D2 in IMMA assessment; Tetley et al., 2022). In total, only six cetacean species (Spinner dolphins, Bryde's whales, Australian humpback dolphins and Indo-Pacific bottlenose dolphins from this study, as well as the dugong and Pantropical spotted dolphin, not recorded in this study) were previously reported from this region (Kahn, 2009). This low diversity may explain why Kaimana IMMA was primarily recognized as an aggregation and feeding area, rather than a diversity habitat for marine mammals in IMMA assessment.
In contrast, other regions met the sub-criteria of species diversity including the Wakatobi and Adjacent Waters IMMA (26,815 sq km) with 11 species (IUCN-MMPATF, 2022b), Savu Sea and Surrounding Areas IMMA (160,512 sq km) with 24 species (IUCN-MMPATF, 2022c), Berau and East Kutai District - Kalimantan IMMA (19,470 sq km) with 25 species (IUCN-MMPATF, 2022d), and Southern Bali Peninsula and adjacent slope IMMA (2,239 sq km) with 18 species (IUCN-MMPATF, 2022e). Interestingly, Bintuni Bay IMMA (5,558 sq km), the nearest IMMA that is located approximately 400 km from Kaimana IMMA, also does not meet the sub-criteria for species diversity (IUCN-MMPATF, 2022f). Bintuni Bay has a similar species composition to Kaimana IMMA, including Australian humpback dolphins, Spinner dolphins, Indo-Pacific bottlenose dolphins, and Bryde’s whales, but also has the common bottlenose dolphin, which is not reported in Kaimana IMMA (IUCN-MMPATF, 2022f).
The small size of the Kaimana IMMA may explain its lower species diversity compared to other IMMAs in Indonesia. Kaimana IMMA is relatively small compared to other IMMAs mentioned above, except for Southern Bali Peninsula and adjacent slope IMMA. Larger areas tend to encompass a greater variety of habitats, ranging from coastal zones to open ocean, and these diverse habitats can support a wider array of cetacean species with different ecological requirements (Gnone et al., 2023). Additionally, larger areas may exhibit greater environmental heterogeneity, including variations in bathymetry, temperature, and oceanographic features (Sahri et al., 2021). These diverse environmental conditions contribute to the availability of different niches, enabling various cetacean species to coexist (Breen et al., 2016). Moreover, larger areas often support more complex ecosystems, with a greater abundance and variety of prey species (Bluhm and Gradinger, 2008; Laidre et al., 2008). This abundance of prey can attract and sustain a higher diversity of cetaceans, as different species adapt to feed on specific types of prey (Lambert et al., 2014; Putra and Mustika, 2020; Receveur et al., 2022). Conversely, smaller areas may have limited resources and habitat types, which can result in a more restricted range of cetacean species and prey competition. Unlike Kaimana IMMA that also has small size, the Southern Bali Peninsula and Adjacent Slope IMMA has higher species diversity. This condition could be strongly influenced by the complex oceanographic features, including steep bathymetric gradients, undersea canyons, and seasonal upwelling in the Southern Bali that facing the Indian Ocean directly (Ningsih et al., 2013; Purba and Khan, 2019; Mustika et al., 2021a; IUCN-MMPATF, 2022e).
Despite the presence of open ocean environments around Kaimana, such as the Ceram Sea Canyon just south of Triton Bay, where the canyon sharply ascends from depths exceeding 2000 meters to less than 100 meters near Aiduma Island (Kahn, 2007), this specific area does not fall within the Kaimana IMMA boundary (Figure 1). Interestingly, the Aiduma region represents a significant ‘deep-sea yet near-shore habitat’ (Kahn, 2007), characterized by extreme bathymetric gradients, strong currents, periodic ocean fronts, and upwelling events (Purba and Khan, 2019). Notably, this region generally maintains cooler temperatures than that of in Raja Ampat (Mangubhai et al., 2012). Therefore, conducting research beyond the boundaries of the Kaimana IMMA may uncover the existence of other cetacean species, particularly those associated with oceanic environments. Observations from lift-net fisheries may be limited in capturing oceanic cetacean species, as most fishing operations occur in coastal areas. Therefore, independent and systematic surveys encompassing both coastal and oceanic habitats are recommended for future studies.
4.2 Rare species
From five species that were positively identified in Kaimana IMMA during the study period (Figure 2), the Killer whale is the only species that was only recorded once during the study period (Table 1). Although killer whales are the most cosmopolitan of all cetaceans and may be the second-most widely ranging mammal species on the planet, they are most common in cold-water areas of high marine productivity, particularly at higher latitudes and within about 800 km off continents (Dahlheim and Heyning 1999, Forney et al., 2006). In tropical water such as Indonesia, the density of killer whales is low, and potentially only 0-0.10 whales per 100 sq km due to limited foraging opportunities and anthropogenic threats (Forney et al., 2006; Ford, 2009). Therefore, this species is rarely found in Indonesia including in Kaimana IMMA.
The presence of killer whales in Indonesia has been documented, both through stranding cases and through direct observation in the wild. According to Mustika et al. (2022) who analyzed cetacean stranding data in Indonesia between 1995 and 2022, only three Killer whale cases out of 568 records were reported in East Nusa Tenggara, North Sulawesi, and East Java, and those three cases were reported only recently in 2020-2021. Other species, such as the Sperm whale, which is commonly found in Indonesian seas, have been reported to have a significant stranding rate in Indonesia (Mustika et al., 2022). Observations of killer whales in the wild have been documented in Ceram (Soede et al., 2019), Savu Sea (Barnes, 1996; Mustika, 2006), Bali (Mustika, 2011), Wakatobi (Sahri et al., 2020c), Banda and Timor Seas (Sivasubramaniam, 1964; Leatherwood et al., 1991), Gorontalo (Mustika et al., 2021b), and Raja Ampat (Ender et al., 2014). The latter is located close to Kaimana IMMA (± 600 km). From 2006 to 2011, boat surveys in Raja Ampat recorded killer whale sightings in January, April, May, July, September, October, and November (Ender et al., 2014). However, killer whales were documented in Kaimana only in February. This time difference in the killer whale occurrence may reflect a regional movement pattern for killer whales, given their absence from recorded sightings in Raja Ampat during that month. Further research utilizing biotelemetry is necessary to confirm this movement pattern, particularly to determine whether killer whales are present in Kaimana exclusively between February and March, a period when they are not observed in Raja Ampat. It is possible that this observed pattern is not due to differences in killer whale movements between Raja Ampat and Kaimana but rather because, in February, killer whales may be in Kaimana’s coastal areas, where lift-net fishing platforms operate. Alternatively, they might also occupy Kaimana’s oceanic habitats, which are currently not monitored due to the limitations of the observation method used in this study.
4.3 Common species
Triton Bay has been previously recognized as a particularly important habitat for Bryde’s whales (Kahn, 2009). Local villagers were familiar with Bryde’s whales, which are known to migrate in a predictable path between Bicari and Triton Bays via the Namatota Strait (Kahn, 2009; Figure 1). Similar surveys in East Indonesia, such as those in North Sulawesi, Sangihe-Talaud, Komodo, Solor-Alor, Derawan, Bali-Lombok, and the Solomon Islands, found few encounters of Bryde’s whales (Kahn and Pet, 2003), except in the Raja Ampat Islands, where they were consistently found year-round (Kahn, 2007). Triton Bay was therefore observed as a significant Bryde’s whale ‘hotspot’ for Indonesia as a whole, as their relative abundance is exceptionally high and whale sightings are common throughout the year (Kahn, 2009). Bryde’s whales have been reported to have high residency in several areas, including in the Gulf of California (Tershy, 1992; Viloria-Gómora et al., 2021), where they rarely travel in groups and frequently feed alone or in small groups, and in the Gulf of Mexico (Rosel and Wilcox, 2014; Soldevilla et al., 2017), where they have a small population and a restricted range. Bryde’s whales do not migrate seasonally, as most other baleen whale species do, moving from warm water breeding sites in winter to cold water and extremely productive feeding grounds in summer (Moore et al., 2019). The fact that they do not show migration is observed in Gulf of Mexico (Sirovic et al., 2014) and the Hauraki Gulf in New Zealand (Tezanos-Pinto et al., 2017), although certain populations do exhibit seasonal movements and range expansions that are likely influenced by environmental conditions and prey availability (Best, 2001).
Australian humpback dolphins and Indo-Pacific bottlenose dolphin were also consistently sighted in Triton Bay (Kahn, 2009). Dedicated land-based observations were conducted in Arguni Bay, north Kaimana during January-April 2015, where 64 Australian humpback dolphin groups and 40 Indo-Pacific bottlenose dolphin groups were confirmed (Wijaya, 2015; Beasley et al., 2016). Arguni Bay was subsequently considered a hotspot for both species, given the consistent sightings throughout the survey period.
Australian humpback dolphins tend to be resident species with strong site fidelity (Parra and Cagnazzi, 2016). The residency of Australian humpback dolphins is likely influenced by a combination of historical genetic factors, such as bottlenecks and founder events, and ecological factors, including habitat preferences and resource partitioning (Parra et al., 2018). In Australia, the species shows a pattern of isolation by distance, with gene flow restrictions occurring over distances of 382-509 km, indicating that populations are largely resident and do not mix over large distances (Parra et al., 2018).
Moreover, Indo-Pacific bottlenose dolphins exhibit varying degrees of residency depending on the location. While some populations show high site fidelity and are considered residents with stable population sizes, including off the coast of Queensland, Australia, and Amakusa-Shimo-shima Island, Japan (Chilvers and Corkeron, 2003; Haughey et al., 2021), others display a mix of resident and transient behaviors or form large, fluid communities that may extend over broad geographic ranges (Haughey et al., 2020). Indo-Pacific bottlenose dolphins tend to be resident due to typical social structure with significant dyadic associations and stable social groups, and often isolated with weak interconnections (Bonneville et al., 2021).
Using lift-net fishing platforms as observation sites in the Kaimana IMMA can be an effective approach for studying residency patterns and the dominance of species that frequent coastal areas and interact with lift-net fisheries. This method is particularly useful in stable fishing areas such as Kaimana City and Bicari Bay.
4.4 Seasonal patterns in Bryde’s whale sightings and abundance
Only Bryde’s whales had sufficient records to investigate their seasonal patterns. Bryde’s whale sightings and abundance reveal seasonal fluctuations in both SPUE and APUE in Kaimana IMMA, peaking during the Transition 1 intermonsoon period and the southeast monsoon. This seasonal pattern may be related to the oceanographic dynamics in Kaimana. Although Bryde’s whales can be observed year-round, local villagers are aware that these whales follow a predictable migratory route between Bicari Bay (where lift-net fisheries operate) and Triton Bay via the Namatota Strait (Kahn, 2009; Figure 1). However, since most observations in this study were concentrated in Bicari Bay rather than the Namatota Strait or Triton Bay, it is likely that during other seasons (Transition 2 and NWM), Bryde’s whales may be present in these areas.
This area exhibits strong seasonality in sea surface temperature fluctuations, particularly during the SEM, marked by cold-water upwellings (Mangubhai et al., 2012). These upwellings coincide with increased chlorophyll-a and primary productivity in Kaimana’s coastal and marine waters, extending southward to the Arafura Sea (Gordon, 2005). The banana prawn fishery that operated in the front of Kaimana City is similarly influenced by this periodical upwelling. The optimal fishing season of banana prawn fishery was reported from July to September, with peak of yields occurring in August to November and the highest catch rate occurring in August (Panggabean, 2018; Pane et al., 2023). The increase in banana prawn catches in Kaimana in the May to October time frame is likely related to the upwelling that occurs at this time, when lowest temperatures are recorded in August (Mangubhai et al., 2012).
Bryde’s whales show seasonal variations in their feeding patterns, with higher feeding activity observed in summer and autumn when schooling fish are concentrated in the area (Tershy, 1992; Penry et al., 2011). Bryde’s whales have a diverse diet consisting mainly of schooling fish, with a particular preference for anchovies and sardines, and they also consume krill in certain regions and seasons (Tershy, 1992; Murase et al., 2007). The wide-ranging diet of Bryde's whales indicates flexibility and adaptability in their feeding strategies to accommodate the availability of prey in different environments.
4.5 Feeding associations with the lift-net fishery
Our findings demonstrate that the Indo-Pacific dolphin is the only species that has a strong association with lift net fishery feeding behavior. Although other species have been seen feeding around the lift net fishery, they were not as common as the Indo-Pacific bottlenose dolphin. This could be attributed to the species’ strong preference for coastal habitats (Vargas-Fonseca et al., 2018, 2020; Bonneville et al., 2021), which overlap with the areas where lift-net fisheries operate in Kaimana. In contrast, spinner dolphins, often found around lift nets exhibiting non-feeding behavior, are oceanic species with expansive foraging ranges. This range potentially exposes them to a greater diversity of prey (Perrin et al., 1999; Perrin, 2009) and leads them to coastal zones primarily for daytime rest, as highlighted in other research (Thorne et al., 2012; Sahri et al., 2020c). This explains why most spinner dolphins observed were not feeding (Table 2).
Lift net fisheries operations began in Kaimana in 2000, primarily at the mouth of Bicari Bay, with the target of selling anchovies and other baitfish to markets and supplying the pole and line fishing industry in the Kaimana area (Sianipar, 2022). The seemingly high productivity of the bay draws year-round operations to the area. They generally rely on artificial lighting and lift nets as their major equipment and technique. Bright lights are used at night to catch baitfish like anchovies and other small pelagic species. In a productive night, the fishermen manage 3-4 hauls per night, with the last haul completed just before sunrise at 5.30-6 AM, when whale sharks and dolphins are frequently spotted near the lift net fishery. The process of capturing many baitfish attracts whale sharks and dolphins to the fishing vessel, and they frequently feed on the collected fish before being caught in the net and aggressively sucking (in the case of whale sharks) the collected baitfish from the outside of the net. Because the lift net fishing vessel moved relatively little during the operation, its location has become a regular foraging place for whale sharks and dolphins.
This interaction has created unique opportunities for the establishment of provisioning-based whale shark and dolphin watching industries. Fishermen and tour operators use anchovies as bait to attract and encourage them to remain close to the lift-net fishery boat. This suggests that both sharks and cetaceans likely have a natural preference for hunting anchovies. The coexistence of passive provisioning through the presence of lift-net fisheries and active feeding driven by tourism has led to the development of distinctive behavioral and ecological adaptations in their foraging strategies (Sianipar, 2022).
4.6 Management implications and future research directions
It is worth noting that no cetacean surveys have been conducted in Kaimana IMMA since its official recognition in 2018. Thus, this study fills a significant gap in our understanding, underlining the significance of continual monitoring through time-series observations for sustaining ongoing conservation efforts and gaining a full understanding of the ecological trends of cetacean populations. Our study demonstrates that observing cetaceans from lift net fishery platforms is important because it offers insight into how cetaceans interact with fisheries. However, we acknowledge that the current study approach has limits in terms of area coverage and observation period, both of which were dependent on lift net fisheries operation season. Thus, establishing an independent survey to cover unbiased locations and times of cetacean observation may provide new perspectives into cetacean ecology, including new species records in Kaimana IMMA. Since this study confirms year-round sightings of Bryde’s whales, Australian humpback dolphins, and Indo-Pacific bottlenose dolphins, suggesting these species may represent resident populations (Kahn, 2009), further mark-recapture studies and genetic research are necessary to validate and provide robust evidence of their population structure.
Our studies discovered that most interactions between lift net fisheries and cetaceans occur outside the Kaimana MPA. Thus, it is critical to ensure that fisheries management measures under the provincial government are implemented outside the MPA to ensure the sustainability of anchovies, which are important not only for the community and pole and line industries, but also as a food source for the population of Bryde’s whale, Indo-Pacific bottlenose dolphin, and Australian humpback dolphins. There is currently very little information available on anchovy stock assessment in Kaimana. We recommend undertaking further research on stock assessment of anchovies to better understand the extent of exploitation in this region, which will then be utilized as a baseline for fisheries management measures.
Banana prawn, an important fisheries commodity in Kaimana with a significant ecological link to Bryde’s whale ecology, is currently overfished in Kaimana (Pane et al., 2023). Although we do not yet have direct evidence that Banana prawn is one of the food sources for Bryde’s whales in Kaimana, the increased sighting and abundance of Bryde’s whales during the peak season for Banana shrimp suggests that the two species are ecologically related and may be associated with predator-prey interaction. Further study on stable isotopes is needed to confirm this predator-prey interaction (Niño-Torres et al., 2014). Nonetheless, fisheries management needs to regulate the efforts of trammel net fishers to avoid further fishing pressure in Kaimana waters.
Apart from implementing fisheries management measures, maintaining the mangrove ecosystem in Kaimana is very important to ensure the sustainability of anchovy and banana prawn stocks. Mangroves act as essential nursery grounds, providing food and shelter for juvenile prawns and anchovies (Sukardjo, 2004), which contributes to their growth and survival. Juvenile banana prawns (Penaeus merguiensis), utilize mangrove estuaries as nursery areas, with the highest survival rates and abundance observed in mangrove-lined habitats, suggesting a preference for these environments during early life stages (Stapels, 1980; Vance et al., 1990; Vance and Rothlisberg, 2020).
Kaimana is well-known for having some of the most extensive mangrove forests in the BHS (Alongi, 2007; Mangubhai et al., 2012). Unfortunately, these mangrove forests in Kaimana are currently being converted/deforested at an estimated average rate of 5 ha per annum during the period from 1996-2020 (Konservasi Indonesia unpublished data, reanalysis from Bunting et al., 2022) due to the logging activities for firewood and tambelo, residential expansion, land conversion for fish drying areas, rubbish dumps, and expansion of water bodies for boat access and aquaculture areas (Konom et al., 2019). Weak enforcement of government regulations, such as granting building permits, and private land use in mangrove forest areas due to low land prices, as well as local people’s ignorance of the mangrove forest benefits, are the primary causes of land conversion (Yabana et al., 2021). Despite the relatively small degree of loss in the mangrove ecosystem, this shows that growing human activity is nonetheless causing significant negative impacts. This is a warning indicator that mitigating actions must be implemented to prevent ongoing and significant damage to the mangrove ecosystems of Kaimana.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because the study was purely observational.
Author contributions
MP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YM: Data curation, Validation, Writing – review & editing. AS: Validation, Writing – review & editing. ES: Validation, Writing – review & editing. SH: Validation, Writing – review & editing. AH: Project administration, Supervision, Validation, Writing – review & editing. HP: Data curation, Validation, Writing – review & editing. NH: Project administration, Supervision, Validation, Writing – review & editing. ME: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The financial support from Conservation International and Konservasi Indonesia donors, including the Allchin Family’s Sunbridge Foundation, the Wolcott Henry Foundation, and MAC3 Impact Philanthropies, is acknowledged with profound gratitude for their generous support of this project.
Acknowledgments
The authors express sincere gratitude to the Government of Indonesia, in particular the Ministry of Marine Affairs and Fisheries. Special appreciation is extended to the Kaimana MPA management authority for graciously hosting and facilitating this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alongi D. M. (2007). “5.4 mangrove forests of Papua,” in Marshall A. J., Beehler B. M. Eds. The Ecology of Papua (part two). Singapore: Periplus Editions. vol. 5. , 824–857.
Barlow J., Gisiner R. (2005). Mitigating, monitoring and assessing the effects of anthropogenic sound on beaked whales. J. Cetacean Res. Manage. 7, 239–249. doi: 10.47536/jcrm.v7i3.734
Barnes R. H. (1996). Sea hunters of Indonesia: Fishers and weavers of Lamalera (New York: Oxford University Press).
Beasley I., Jedensjö M., Wijaya G. D., Anamiato J., Kahn B., Kreb D. (2016). Observations on Australian humpback dolphins (Sousa sahulensis) in waters of the Pacific Islands and New Guinea. Adv. Mar. Biol. 73, 219–271. doi: 10.1016/bs.amb.2015.08.003
Best P. (2001). Distribution and population separation of Bryde’s whale Balaenoptera edeni off southern Africa. Mar. Ecol. Prog. Ser. 220, 277–289. doi: 10.3354/MEPS220277
Bluhm B. A., Gradinger R. (2008). Regional variability in food availability for Arctic marine mammals. Ecol. Appl. 18, S77–S96. doi: 10.1890/06-0562.1
Bonneville C. D., Derville S., Luksenburg J. A., Oremus M., Garrigue C. (2021). Social structure, habitat use and injuries of Indo-Pacific bottlenose dolphins (Tursiops aduncus) reveal isolated, coastal, and threatened communities in the South Pacific. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.606975
Boyd C., Punt A. E. (2021). Shifting trends: Detecting changes in cetacean population dynamics in shifting habitat. PloS One 16, e0251522. doi: 10.1371/journal.pone.0251522
Breen P., Brown S., Reid D., Rogan E. (2016). Modelling cetacean distribution and mapping overlap with fisheries in the northeast atlantic. Ocean Coast. Manage. 134, 140–149. doi: 10.1016/j.ocecoaman.2016.09.004
Buckland S. T., Anderson D. R., Burnham K. P., Laake J. L., Borchers D. L., Thomas L. (2001). Introduction to Distance Sampling (Oxford: Oxford University Press).
Bunting P., Rosenqvist A., Hilarides L., Lucas R. M., Thomas N., Tadono T., et al. (2022). Global mangrove extent change 1996–2020: Global mangrove watch version 3.0. Remote Sens. 14, 3657. doi: 10.3390/rs14153657
Chilvers B., Corkeron P. (2003). Abundance of Indo-Pacific bottlenose dolphins, Tursiops aduncus, off Point Lookout, Queensland, Australia. Mar. Mammal Sci. 19, 85–095. doi: 10.1111/J.1748-7692.2003.TB01094.X
Cossaboon J. M., Hoh E., Chivers S. J., Weller D. W., Danil K., Maruya K. A., et al. (2019). Apex marine predators and ocean health: Proactive screening of halogenated organic contaminants reveals ecosystem indicator species. Chemosphere 221, 656–664. doi: 10.1016/j.chemosphere.2019.01.050
Dahlheim M. E., Ridgway S. H. (1999). “Killer whale,” in The second book of dolphins and the porpoises handbook of marine mammals. Eds. Ridgway S. H., Harrison R. (London: Academic Press), 281–322.
Djunaidi A., Jompa J., Bahar A., Sianipar A., Hasan A. W., Alaydrus I. S., et al. (2019). “Potential tourism development for whale shark (Rhincodon typus) watching in eastern Indonesia,” in IOP Conference Series: Earth and Environmental Science, vol. 253. (Bristol: IOP Publishing), 012043. doi: 10.1088/1755-1315/253/1/012043
Doughty C. E., Roman J., Faurby S., Wolf A., Haque A., Bakker E. S., et al. (2016). Global nutrient transport in a world of giants. Proc. Natl. Acad. Sci. 113, 868–873. doi: 10.1073/pnas.1502549112
Ender A. I., Mangubhai S., Wilson J. R., Muljadi A. (2014). Cetaceans in the global centre of marine biodiversity. Mar. Biodivers. Records 7, e18. doi: 10.1017/S1755267214000207
Fearnbach H., Durban J., Parsons K., Claridge D. (2012). Photographic mark–recapture analysis of local dynamics within an open population of dolphins. Ecol. Appl. 22, 1689–1700. doi: 10.1890/12-0021.1
Ford J. K. (2009). “Killer whale: Orcinus orca,” in Encyclopedia of marine mammals (Amsterdam: Academic Press), 650–657. doi: 10.1016/B978-0-12-373553-9.00150-4
Forney K. A., Wade P. R., Estes J. (2006). Worldwide distribution and abundance of killer whales. Whales Whaling Ocean Ecosyst. 145, 162.
Fossi M. C., Baini M., Simmonds M. P. (2020). Cetaceans as ocean health indicators of marine litter impact at global scale. Front. Environ. Sci. 8. doi: 10.3389/fenvs.2020.586627
Gilbert L., Jeanniard-du-Dot T., Authier M., Chouvelon T., Spitz J. (2023). Composition of cetacean communities worldwide shapes their contribution to ocean nutrient cycling. Nat. Commun. 14, 5823. doi: 10.1038/s41467-023-41532-y
Gnone G., Bellingeri M., Airoldi S., Gonzalvo J., David L., Di-Méglio N., et al. (2023). Cetaceans in the Mediterranean sea: encounter rate, dominant species, and diversity hotspots. Diversity 15, 321. doi: 10.3390/d15030321
Goetz S., Read F. L., Ferreira M., Portela J. M., Santos M. B., Vingada J., et al. (2015). Cetacean occurrence, habitat preferences and potential for cetacean–fishery interactions in Iberian Atlantic waters: results from cooperative research involving local stakeholders. Aquat. Conserv.: Mar. Freshw. Ecosyst. 25, 138–154. doi: 10.1002/aqc.2481
Gordon A. L. (2005). Oceanography of the Indonesian seas. Oceanography-Washington Dc-Oceanogr. Soc. 18, 13. doi: 10.5670/oceanog.2005.18
Haughey R., Hunt T., Hanf D., Rankin R., Parra G. (2020). Photographic Capture-Recapture Analysis Reveals a Large Population of Indo-Pacific Bottlenose Dolphins (Tursiops aduncus) With Low Site Fidelity off the North West Cape, Western Australia. Front. Mar. Sci. 6, 781. doi: 10.3389/fmars.2019.00781
Haughey R., Hunt T. N., Hanf D., Passadore C., Baring R., Parra G. J. (2021). Distribution and habitat preferences of Indo-Pacific bottlenose dolphins (Tursiops aduncus) inhabiting coastal waters with mixed levels of protection. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.617518
Henderson E. E., Smith M. H., Gassmann M., Wiggins S. M., Douglas A. B., Hildebrand J. A. (2014). Delphinid behavioral responses to incidental mid-frequency active sonar. J. Acoustic. Soc. America 136, 2003–2014. doi: 10.1121/1.4895681
IUCN Marine Mammal Protected Areas Task Force (2019). Important marine mammal area regional workshop for the North East Indian ocean and South East Asian seas: final report of the third IMMA workshop, Kota Kinabalu, Sabah, Malaysia, 12-16 March 2018.
IUCN Marine Mammal Protected Areas Task Force (2022). Guidance on the identification of Important Marine Mammal Areas (IMMAs). IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force, 74.
IUCN-MMPATF (2022a). Kaimana, West Papua IMMA Factsheet (IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force). https://www.marinemammalhabitat.org/factsheets/kaimana-west-papua/.
IUCN-MMPATF (2022b). Wakatobi and Adjacent Waters IMMA Factsheet (IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force). https://www.marinemammalhabitat.org/factsheets/wakatobi-adjacent-waters/.
IUCN-MMPATF (2022c). Savu Sea and Surrounding Areas IMMA Factsheet (IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force). https://www.marinemammalhabitat.org/factsheets/savu-sea-surrounding-area/.
IUCN-MMPATF (2022d). Berau and East Kutai District, Kalimantan IMMA Factsheet (IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force). https://www.marinemammalhabitat.org/factsheets/berau-east-kutai-district/.
IUCN-MMPATF (2022e). Southern Bali Peninsula and Adjacent Slope IMMA Factsheet (IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force). https://www.marinemammalhabitat.org/factsheets/southern-bali-peninsula-slope/.
IUCN-MMPATF (2022f). Bintuni Bay IMMA Factsheet (IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force). https://www.marinemammalhabitat.org/factsheets/bituni-bay-west-papua/.
Kahn B. (2007). Marine mammals of the Raja Ampat Islands: Visual and Acoustic Cetacean Survey & Training Program. Bali: Apex Environmental, 57, Technical Report AE07/01 to Conservation International - Indonesia Program.
Kahn B. (2009). Marine mammal survey and training in Triton Bay, West Papua, Indonesia: management implications for resident Bryde’s whales. Unpublished technical report to Conservation International Indonesia. Bali: Apex Environmental.
Kahn B., Pet J. (2003). “Long-term visual and acoustic cetacean surveys in komodo national park, indonesia 1999–2001: Management implications for large migratory marine life,” in Aquatic protected areas, what works best and how do we know? proceedings and publications of the world congress on aquatic protected areas 2002. Eds. Beumer J. P., Grant A., Smith D. C. (Australian Society for Fish Biology), 625–637.
Kiszka J. J., Heithaus M. R., Wirsing A. J. (2015). Behavioural drivers of the ecological roles and importance of marine mammals. Mar. Ecol. Prog. Ser. 523, 267–281. doi: 10.3354/meps11180
Konom N. H., Cabuy R. L., Wanma A. O. (2019). Identifikasi kerusakan areal hutan mangrove akibat aktivitas penduduk di daerah Airtiba Kabupaten Kaimana. Jurnal kehutanan papuasia 5, 153–163. doi: 10.46703/jurnalpapuasia.Vol5.Iss2.148
Laidre K. L., Stirling I., Lowry L. F., Wiig Ø., Heide-Jørgensen M. P., Ferguson S. H. (2008). Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol. Appl. 18, S97–S125. doi: 10.1890/06-0546.1
Lambert C., Mannocci L., Lehodey P., Ridoux V. (2014). Predicting cetacean habitats from their energetic needs and the distribution of their prey in two contrasted tropical regions. PloS One 9, e105958. doi: 10.1371/journal.pone.0105958
Leatherwood S., McDonald D., Prematunga W. P., Girton P., Ilangakoon A., McBrearty D. (1991). “Records of the “blackfish” (killer, false killer, pilot, pygmy killer and melon-headed whales) in the Indian Ocean 1772-1986,” in Cetaceans and cetacean research in the Indian Ocean sanctuary: 33-65.— Marine Mamma l Technical Report Number 3. Eds. Leatherwood S., Donovan G. P. (United Nations Environment Programme, Nairobi).
Lohman D. J., de Bruyn M., Page T., von Rintelen K., Hall R., Ng P. K. L., et al. (2011). Biogeography of the indo-australian archipelago. Annu. Rev. Ecol. Evol. Syst. 42, 205–226. doi: 10.1146/annurev-ecolsys-102710-145001
MacLeod R., MacLeod C. D., Learmonth J. A., Jepson P. D., Reid R. J., Deaville R., et al. (2007). Mass-dependent predation risk and lethal dolphin–porpoise interactions. Proc. R. Soc. B: Biol. Sci. 274, 2587–2593. doi: 10.1098/rspb.2007.0786
Mangubhai S., Erdmann M. V., Wilson J. R., Huffard C. L., Ballamu F., Hidayat N. I., et al. (2012). Papuan Bird’s Head Seascape: Emerging threats and challenges in the global center of marine biodiversity. Mar. pollut. Bull. 64, 2279–2295. doi: 10.1016/j.marpolbul.2012.07.024
Moore S. E., Haug T., Víkingsson G. A., Stenson G. B. (2019). Baleen whale ecology in arctic and subarctic seas in an era of rapid habitat alteration. Prog. Oceanogr. 176, 102118. doi: 10.1016/j.pocean.2019.05.010
Murase H., Tamura T., Kiwada H., Fujise Y., Watanabe H., Ohizumi H., et al. (2007). Prey selection of common minke (Balaenoptera acutorostrata) and Bryde’s (Balaenoptera edeni) whales in the western North Pacific in 2000 and 2001. Fish. Oceanogr. 16, 186–201. doi: 10.1111/J.1365-2419.2006.00426.X
Mustika P. L. K. (2006). Marine mammals in the Savu Sea (Indonesia): Indigenous knowledge, threat analysis and management options. Ph. D. thesis. Townsville: James Cook University.
Mustika P. L. K. (2011). Towards sustainable dolphin watching tourism in Lovina, Bali, Indonesia. Ph.D. thesis. Townsville: James Cook University.
Mustika P. L. K., High K. K., Putra M. I. H., Sahri A., Ratha I. M. J., Prinanda M. O., et al. (2022). When and where did they strand? the spatio-temporal hotspot patterns of cetacean stranding events in indonesia. Oceans 3 (4), 509–526. doi: 10.3390/oceans3040034
Mustika P. L. K., Sadili D., Sunuddin A., Kreb D., Sarmintohadi R. I., Suprapti D., et al. (2015). Rencana Aksi Nasional Konservasi Cetacea Indonesia Periode I: 2016-2020. Direktorat Konservasi dan Keanekaragaman Hayati Laut, Ditjen Pengelolaan Ruang Laut, Kementerian Kelautan dan Perikanan Indonesia. Jakarta.
Mustika P. L. K., Williams R., Kadarisman H. P., Purba A. O., Maharta I. P. R. F., Rahmadani D., et al. (2021a). A rapid assessment of the marine megafauna biodiversity around South Bali, Indonesia. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.606998
Mustika P. L. K., Wonneberger E., Erzini K., Pasisingi N. (2021b). Marine megafauna bycatch in artisanal fisheries in Gorontalo, northern Sulawesi (Indonesia): An assessment based on fisher interviews. Ocean Coast. Manage. 208, 105606. doi: 10.1016/j.ocecoaman.2021.105606
Ningsih N. S., Rakhmaputeri N., Harto A. B. (2013). Upwelling variability along the southern coast of Bali and in Nusa Tenggara waters. Ocean Sci. J. 48, 49–57. doi: 10.1007/s12601-013-0004-3
Niño-Torres C. A., Jorge Urbán R., Olavarrieta T., Blanco-Parra M. D. P., Hobson K. A. (2014). Dietary preferences of Bryde’s whales (Balaenoptera edeni) from the Gulf of California: A δ13C, δ15N analysis. Mar. Mammal Sci. 30, 1140–1148. doi: 10.1111/mms.12081
Pane A. R. P., Pradisty N. A., Widiyastuti H., Fauzi M., Mardlijah S., Hanintyo R., et al. (2023). Exploitation status and spawning potential ratio of banana prawn (Penaeus merguiensis) after trawling ban in Kaimana, West Papua. Region. Stud. Mar. Sci. 61, 102884. doi: 10.1016/j.rsma.2023.102884
Panggabean A. S. (2018). Catch rate and population parameters of banana prawn Penaeus merguiensis in Kaimana waters, West Papua, Indonesia. Aquacult. Aqua. Conserv. Legislation 11, 1378–1387. Available at: http://www.bioflux.com.ro/docs/2018.1378-1387.pdf
Parra G., Cagnazzi D. (2016). Conservation status of the Australian humpback dolphin (Sousa sahulensis) using the IUCN red list criteria. Adv. Mar. Biol. 73, 157–192. doi: 10.1016/bs.amb.2015.07.006
Parra G., Cagnazzi D., Jedensjö M., Ackermann C., Frère C., Seddon J., et al. (2018). Low genetic diversity, limited gene flow and widespread genetic bottleneck effects in a threatened dolphin species, the Australian humpback dolphin. Biol. Conserv. 220, 192–200. doi: 10.1016/J.BIOCON.2017.12.028
Pearson H. C., Savoca M. S., Costa D. P., Lomas M. W., Molina R., Pershing A. J., et al. (2023). Whales in the carbon cycle: can recovery remove carbon dioxide? Trends Ecol. Evol. 38, 238–249. doi: 10.1016/j.tree.2022.10.012
Pennino M. G., Arcangeli A., Prado Fonseca V., Campana I., Pierce G. J., Rotta A., et al. (2017). A spatially explicit risk assessment approach: Cetaceans and marine traffic in the Pelagos Sanctuary (Mediterranean Sea). PloS One 12, e0179686. doi: 10.1371/journal.pone.0179686
Penry G., Cockcroft V., Hammond P. (2011). Seasonal fluctuations in occurrence of inshore Bryde’s whales in Plettenberg Bay, South Africa, with notes on feeding and multispecies associations. Afr. J. Mar. Sci. 33, 403–414. doi: 10.2989/1814232X.2011.637617
Perrin W. F. (2009). Spinner dolphin: Stenella longirostris. In Encyclopedia of marine mammals (Amsterdam: Academic Press), 1100–1103.
Perrin W., Dolar M., Robineau D. (1999). Spinner dolphins (Stenella longirostris) of the Western Pacific and Southeast Asia: pelagic and shallow-water forms. Mar. Mammal Sci. 15, 1029–1053. doi: 10.1111/J.1748-7692.1999.TB00876.X
Purba N. P., Khan A. M. (2019). Upwelling session in Indonesia waters. World News Natural Sci. 25, 72–83.
Putra M. I. H., Mustika P. L. K. (2020). Incorporating in situ prey distribution into foraging habitat modelling for marine megafauna in the Solor waters of the Savu Sea, Indonesia. Aquat. Conserv.: Mar. Freshw. Ecosyst. 30, 2384–2401. doi: 10.1002/aqc.3379
Read A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. Mammal. 89, 541–548. doi: 10.1644/07-MAMM-S-315R1.1
Receveur A., Allain V., Menard F., Lebourges Dhaussy A., Laran S., Ravache A., et al. (2022). Modelling marine predator habitat using the abundance of its pelagic prey in the tropical South-Western Pacific. Ecosystems 25, 757–779. doi: 10.1007/s10021-021-00685-x
Reeves R. R., Berggren P., Crespo E. A., Gales N., Northridge S. P., di Sciara G. N., et al. (2005). Global priorities for reduction of cetacean bycatch. WWF Report. (IUCN), 29 pp. Retrieved from https://www.wwf.eu/?21057/Report-Global-Priorities-for-Reduction-of-Cetacean-Bycatch.
Reeves R. R., McClellan K., Werner T. B. (2013). Marine mammal bycatch in gillnet and other entangling net fisheries 1990 to 2011. Endangered Species Res. 20, 71–97. doi: 10.3354/esr00481
Rosel P., Wilcox L. (2014). Genetic evidence reveals a unique lineage of Bryde’s whales in the northern Gulf of Mexico. Endangered Species Res. 25, 19–34. doi: 10.3354/ESR00606
Sahri A., Mustika P. L. K., Dewanto H. Y., Murk A. J. (2020b). A critical review of marine mammal governance and protection in Indonesia. Mar. Policy 117, 103893. doi: 10.1016/j.marpol.2020.103893
Sahri A., Mustika P. L. K., Purwanto P., Murk A. J., Scheidat M. (2020c). Using cost-effective surveys from platforms of opportunity to assess cetacean occurrence patterns for marine park management in the heart of the Coral Triangle. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.569936
Sahri A., Putra M. I. H., Mustika P. L. K., Kreb D., Murk A. J. (2021). Cetacean habitat modelling to inform conservation management, marine spatial planning, and as a basis for anthropogenic threat mitigation in Indonesia. Ocean Coast. Manage. 205, 105555. doi: 10.1016/j.ocecoaman.2021.105555
Sahri A., Putra M. I., Mustika P. L., Murk A. J. (2020a). A treasure from the past: Former sperm whale distribution in Indonesian waters unveiled using distribution models and historical whaling data. J. Biogeogr. 47, 2102–2116. doi: 10.1111/jbi.13931
Salman S., Sulaiman M., Alam S., Anwar A., Syarifuddin S. (2015). Proses penangkapan dan tingkah laku ikan bagan petepete menggunakan lampu LED. Jurnal Teknologi Perikanan dan Kelautan 6, 169–178. doi: 10.24319/jtpk.6.169-178
Setyawan E., Erdmann M., Gunadharma N., Gunawan T., Hasan A., Izuan M., et al. (2022). A holistic approach to manta ray conservation in the Papuan Bird’s Head Seascape: Resounding success, ongoing challenges. Mar. Policy 137, 104953. doi: 10.1016/j.marpol.2021.104953
Sheehy J. M., Taylor N. L., Zwerschke N., Collar M., Morgan V., Merayo E. (2022). Review of evaluation and valuation methods for cetacean regulation and maintenance ecosystem services with the joint cetacean protocol data. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.872679
Sianipar A. B. (2022). Regionally contrasting movement behaviour of sub-adult whale sharks in Indonesia. Perth: Murdoch University.
Sirovic A., Bassett H., Johnson S., Wiggins S., Hildebrand J. (2014). Bryde’s whale calls recorded in the Gulf of Mexico. Mar. Mammal Sci. 30, 399–409. doi: 10.1111/MMS.12036
Sivasubramaniam K. (1964). Predation of tuna longline catches in the Indian Ocean, by killer-whales and sharks. Bull. Fish. Res. Station Ceylon. 17, 221–236.
Soede L. P., Natasasmita D., Mahendra I. G., Rizki W. (2019). “Marine mammals interactions with tuna fishing activities in Indonesian seas,” in IOP Conference Series: Earth and Environmental Science, vol. 399. (Bristol: IOP Publishing), 012128. doi: 10.1088/1755-1315/399/1/012128
Soldevilla M., Hildebrand J., Frasier K., Dias L., Martinez A., Mullin K., et al. (2017). Spatial distribution and dive behavior of Gulf of Mexico Bryde’s whales: potential risk of vessel strikes and fisheries interactions. Endangered Species Res. 32, 533–550. doi: 10.3354/ESR00834
Stapels D. (1980). Ecology of juvenile and adolescent banana prawns, Penaeus merguiensis, in a mangrove estuary and adjacent off-shore area of the Gulf of Carpentaria. I. Immigration and settlement of post larvae. Mar. Freshw. Res. 31, 635–652. doi: 10.1071/MF9800635
Sukardjo S. (2004). Fisheries Associated with Mangrove Ecosystem In Indonesia: A View from a Mangrove Ecologist (Biotropia: The Southeast Asian Journal of Tropical Biology). doi: 10.11598/btb.2004.0.23.20
Tershy B. (1992). Body size, diet, habitat use, and social behavior of balaenoptera whales in the gulf of California. J. Mammal. 73, 477–486. doi: 10.2307/1382013
Tetley M. J., Braulik G. T., Lanfredi C., Minton G., Panigada S., Politi E., et al. (2022). The important marine mammal area network: a tool for systematic spatial planning in response to the marine mammal habitat conservation crisis. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.841789
Tezanos-Pinto G., Hupman K., Wiseman N., Dwyer S., Baker C., Brooks L., et al. (2017). Local abundance, apparent survival and site fidelity of Bryde’s whales in the Hauraki Gulf (New Zealand) inferred from long-term photo-identification. Endangered Species Res. 34, 61–73. doi: 10.3354/ESR00839
Thorne L. H., Johnston D. W., Urban D. L., Tyne J., Bejder L., Baird R. W., et al. (2012). Predictive modeling of spinner dolphin (Stenella longirostris) resting habitat in the main Hawaiian Islands. PloS One 7, e43167. doi: 10.1371/journal.pone.0043167
Tobeña M., Prieto R., Machete M., Silva M. A. (2016). Modeling the potential distribution and richness of cetaceans in the Azores from fisheries observer program data. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00202
Townsend C. H. (1935). The distribution of certain whales as shown by logbook records of american whaleships. Zoologica 19, 1–50. doi: 10.2307/j.ctvh9vv3t.4
Vance D., Haywood M., Staples D. (1990). Use of a mangrove estuary as a nursery area by postlarval and juvenile banana prawns, Penaeus merguiensis de Man, in Northern Australia. Estuar. Coast. Shelf Sci. 31, 689–701. doi: 10.1016/0272-7714(90)90020-R
Vance D., Rothlisberg P. (2020). The biology and ecology of the banana prawns: Penaeus merguiensis de Man and P. indicus H. Milne Edwards. Adv. Mar. Biol. 86 1, 1–139. doi: 10.1016/bs.amb.2020.04.001
Vargas-Fonseca O. A., Kirkman S. P., Conry D., Rishworth G. M., Cockcroft V., Pistorius P. A. (2018). Distribution and habitat use of Indo-Pacific bottlenose dolphins Tursiops aduncus along the south coast of South Africa. Afr. J. Mar. Sci. 40, 439–450. doi: 10.2989/1814232X.2018.1547221
Vargas-Fonseca O. A., Kirkman S. P., Oosthuizen W. C., Bouveroux T., Cockcroft V., Conry D. S., et al. (2020). Abundance of Indo-Pacific bottlenose dolphins (Tursiops aduncus) along the south coast of South Africa. PloS One 15, e0227085. doi: 10.1371/journal.pone.0227085
Veron J. E. N., Devantier L. M., Turak E., Green A. L., Kininmonth S., Stafford-Smith M., et al. (2009). Delineating the coral triangle. Galaxea J. Coral Reef Stud. 11 (2), 91–100. doi: 10.3755/galaxea.11.91
Viloria-Gómora L., R. J., Leon-Lopez B., Romero-Vivas E. (2021). Geographic variation in Bryde’s whale be4 calls in the gulf of California: an insight to population dynamics. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.651469
Weir C. R., Pierce G. J. (2013). A review of the human activities impacting cetaceans in the eastern tropical Atlantic. Mammal Rev. 43, 258–274. doi: 10.1111/j.1365-2907.2012.00222.x
Wells R. S., Rhinehart H. L., Hansen L. J., Sweeney J. C., Townsend F. I., Stone R., et al. (2004). Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system. EcoHealth 1, 246–254. doi: 10.1007/s10393-004-0094-6
White A. T., Aliño P. M., Cros A., Fatan N. A., Green A. L., Teoh S. J., et al. (2014). Marine protected areas in the coral triangle: progress, issues, and options. Coast. Manage. 42, 87–106. doi: 10.1080/08920753.2014.878177
Wijaya G. M. (2015). Marine mammals observation di perairan teluk arguni (Jakarta: KrisEnergy (Udan Emas) B.V).
Williams R., Ashe E., O’Hara P. D. (2011). Marine mammals and debris in coastal waters of British Columbia, Canada. Mar. pollut. Bull. 62, 1303–1316. doi: 10.1016/j.marpolbul.2011.02.029
Williams T. M., Estes J. A., Doak D. F., Springer A. M. (2004). Killer appetites: assessing the role of predators in ecological communities. Ecology 85, 3373–3384. doi: 10.1890/03-0696
Keywords: important marine mammal area, lift net fishery, predator-prey interaction, cetacean sighting and abundance, temporal trend
Citation: Putra MIH, Malaiholo Y, Sahri A, Setyawan E, Herandarudewi SMC, Hasan AW, Prasetio H, Hidayat NI and Erdmann MV (2025) Insights into cetacean sightings, abundance, and feeding associations: observations from the boat lift net fishery in the Kaimana important marine mammal area, Indonesia. Front. Mar. Sci. 11:1431209. doi: 10.3389/fmars.2024.1431209
Received: 11 May 2024; Accepted: 29 November 2024;
Published: 10 January 2025.
Edited by:
Xuelei Zhang, Ministry of Natural Resources, ChinaReviewed by:
Alejandro Daniel Buren, Argentine Antarctic Institute (IAA), ArgentinaSaifullah Arifin Jaaman, University of Malaysia Terengganu, Malaysia
Copyright © 2025 Putra, Malaiholo, Sahri, Setyawan, Herandarudewi, Hasan, Prasetio, Hidayat and Erdmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mochamad Iqbal Herwata Putra, aXFiYWxoZXJ3YXRhQGdtYWlsLmNvbQ==
†Present address: Edy Setyawan, Elasmobranch Institute Indonesia, Denpasar, Bali, Indonesia
 Mochamad Iqbal Herwata Putra
Mochamad Iqbal Herwata Putra Yance Malaiholo2
Yance Malaiholo2 Achmad Sahri
Achmad Sahri Edy Setyawan
Edy Setyawan Hanggar Prasetio
Hanggar Prasetio Mark V. Erdmann
Mark V. Erdmann